Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02224822 1997-12-17
WO 97/40797 PCTII1S97/07326
ABSORBENT ARTICLE HAVING A RADIOPAQUE ELEMENT
FIELD OF INVENTION
This invention relates to absorbent articles which
are used in the course of administering medical care to a
patient, and particularly to an absorbent article useful in
surgical procedures and the methods for their manufacture.
BACKGROUND OF INVENTION
Heretofore the laparotomy sponge has served as the
basic absorbent article used in medical applications,
particularly surgical operations, for absorbing body fluids,
especially blood. Laparotomy sponges most commonly comprise a
plurality of layers of absorbent loosely-woven gauze which are
bound to one another along the side edges of the overlying
layers of gauze. The general theory behind the use of
multilayered gauze in laparotomy sponges is that the openness
of the weave of the gauze, plus the bulk provided by the
multiple layers permits the sponge to rapidly take up
substantial volumes of body fluids. In actual practice,
however, it has been found that and individual one of the
common laparotomy sponges takes up only a relatively small
portion of the total volume of fluids that are present in a
surgical procedure, so that in a given surgical procedure, for
example, there will be a large number of laparotomy sponges
used. In today's medical environment, laparotomy sponges are
1
CA 02224822 1997-12-17
WO 97/40797 PCT/US97/07326
disposed of after a single use, thereby bringing into question
the practice of using large numbers of single use laparotomy
sponges. Of recent, and in part due to attempts to reduce
medical care costs, the yarn counts and the denier of the
yarns employed in the weaving of the gauze employed in
laparotomy sponges has tended to be reduced in attempts to
reduce the cost of these sponges. These actions have reduced
the absorptive capacity of these sponges so that more sponges
are being required to perform a given surgical procedure than
was required when using prior sponges.
A vast majority of laparotomy sponges are used in
surgical procedures for absorbing body fluids, for wiping and
cleaning a patient, for packing body organs while the organs
I5 are exposed outside the patient's body cavity (after the
sponge has been soaked in saline solution) to protect the
organs from drying out, and for other similar uses. In many
instances, the sponges are inserted into the patient's body
cavity to aid in keeping a surgical field clear for the
surgeon to perform a surgical procedure within the body
cavity. As is well recognized in the art, a gauze laparotomy
sponge, when soaked with blood, very strikingly resembles
normal body tissue found within a patient's body cavity. As a
consequence, all surgical procedures require strict adherence
to accounting for laparotomy sponges (and other sponge types)
during a surgical procedure. Further, all laparotomy sponges
used in surgical procedures are provided with at least one
radiopaque element which is in some manner incorporated into
the structure of the sponge so as to ensure its integrity with
the sponge during use of the sponge. This radiopaque element
is intended to be readily detectable under X-ray examination.
2
CA 02224822 1997-12-17
WO 97/40797 PCTIUS97I07326
If, after completion of a surgical procedure, all sponges can a
not be accounted for, the patient is examined by X-ray
equipment in an attempt to ensure that a "missing" sponge has
not been left in the patient's body cavity. .
Heretofore, surgical towels, comprising a woven
cotton fabric, have been used in draping off a sterile field
within which a surgeon is to perform a surgical procedure.
This function of the towel is to assist in maintaining a
sterile field by ensuring that bacteria, etc. on the patient's
body are not transferred away from the body and possibly into
the patient's body cavity during the time when the body cavity
is open for performance of the surgical procedure or the like,
and to absorb any body fluids which may escape from the open
body cavity in the course of the surgical procedure. To
successfully perform these functions, the towel is commonly a
single-layered, woven, cotton fabric. The weave of the fabric
is a tight weave, that is, there is a relatively large number
of yarns per inch in the weave and the yarns are closely
packed in the weave. Because towels do not include a
radiopaque element in their structure, they are not acceptable
for use inside a patient's body cavity.
Heretofore, many attempts have been made to devise
ways to incorporate a radiopaque element in the structure of
absorbent articles that may be disposed temporarily inside a
patient's body cavity during a surgical procedure. These
attempts have included weaving one or more radiopaque yarns
into the weave pattern -of the fabric from which the absorbent
article is formed. Because radiopaque yarns are extensible
and have less breaking strength than cotton yarns, for
3
CA 02224822 1997-12-17
WO 97!40797 PCT/I1S97107326
example, they are not amenable to weaving techniques, and this
procedure has not been successful. Another attempt has been
to loosely lay a radiopague yarn into a weave pattern, or to
lay the yarn loosely between layers of gauze. Such loose
S radiopaque yarns, however, are not adequately secured within
the absorbent article and pose the danger of escape of the
yarn or a broken portion thereof into a patient's body cavity.
Radiopaque yarns have also been incorporated into a loop which
is secured to one edge of an absorbent article. In this
construction, there remains the danger of all or a portion of
the yarn escaping from the loop during using of the absorbent
article. Heretofore, there has been no known successful means
for including a radiopaque element in a surgical towel, hence
surgical towels currently remain a separate supply item in
surgical procedures, as do laparotomy sponges, since these two
products presently serve different functions.
In view of the problems attending the prior art, it
is an object of the present invention to provide an absorbent
article which may be used alternatively as a drape or as a
laparotomy sponge during a surgical procedure.
It is another object to provide a novel absorbent
article useful in surgical procedures in which there is
securely incorporated a radiopaque element and wherein the
presence of the radiopaque element does not detract from the
use of the absorbent article for draping, absorption, wiping
and similar uses.
Other objects and advantages of the present
invention will be recognized by one skilled in the art and in
4
CA 02224822 1997-12-17
WO 97/40797 PCTIUS97/07326
view of the present disclosure, including the claims and
drawings in which:
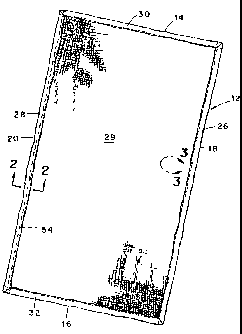
Figure 1 is a perspective representation of an
absorbent article embodying various of the features of the
present invention;
Figure 2 is a sectional view taken generally along
the line 2-2 of Figure 1;
Figure 3 is a schematic representattion of a huck
weave pattern;
Figures 4A and 4B are diagrams depicting one
embodiment of a loom setup for lproducing a huck weave pattern
of the absorbent article of the present invention; and,
Figures 5A and 5B present a schematic representation
of an apparatus for use in the manufacture of the present
absorbent article.
SUMMARY OF INVENTION
In accordance with the present invention there is
provided an absorbent article in form of a close-weave woven
single-layered fabric sheet which has incorporated therein in
a radiopaque thread embedded in multiple folds along one side
edge of the fabric sheet. The radiopaque thread preferably
is unattached to the fabric, but rather is mechanically
captured within the multiple folds. The fabric of the present
invention is woven in a pattern which produces a close or
5
CA 02224822 1997-12-17
WO 97140797 PCTlUS97/07326
tight weave, i.e. havi~ag a relatively large number of yarns
per inch of fabric length and width such that the article is
suitable for use as a draping fabric in a surgical procedure,
and further, is suitable for use in the nature of a laparotomy
sponge. Preferably the absorbent article is of a buck weave
construction of 74 X 28 with 20/1 cotton warp yarns and 8/1
cotton weft yarns thereby providing a preferred finished
nonwashed weight of a 17" X 29" fabric sheet of at least
0.1300 grams per inz. Each side edge of the fabric sheet
preferable is twice-folded inwardly of and onto a surface of
the body of the fabric sheet, each fold being about i~ inch in
width. The radiopaque thread is laid within the first fold so
that when the side edge is folded a second time, the
radiopaque thread is fully encapsulated internally of the
multiple folds. Folding of the end edges of the fabric sheet
serves to close the opposite ends of each folded side edge
thereby preventing escape of the radiopaque thread from the
open ends of that folded side edge within which the thread is
incorporated. A method for the manufacture of the absorbent
product is disclosed and claimed.
DETAILED DESCRIPTION OF INVENTION
With reference to Figure 1, there is depicted one
embodiment of an absorbent article according to the present
invention comprising a fabric sheet 12 having a length
dimension that extends between opposite ends 14 and 16 thereof
and a width dimension that extends between opposite sides 18
and 20 thereof. The fab-ric sheet is folded multiple times
inwardly along each of its side and end edges, the side edges
being designated 26 and 28 and the end edges being designated
6
CA 02224822 1997-12-17
WO 97!40797 PCT/US97/07326
30 and 32. Referring also to Figure 2, the multiple folds of
each side edge are formed by folding a first side edge portion
34 inwardly of the fabric sheet to form a first fold 36 and
thereafter folding the first fold inwardly of the fabric sheet
to form a second fold 38 and thereby define a twice-folded
side edge 26, for example, along each side edge of the fabric
sheet.
The fabric sheet of the present invention, being
intended to serve either as an absorbent for body fluids or
other fluids occasioned in the course of a surgical procedure,
or as a protective barrier about the periphery of a surgical
operative site, is constructed from an absorbent material
which also is suitable for conversion into a sheet that is
sufficiently dense as will provide suitable protection against
the transfer of bacteria through the thickness thereof when
the absorbent article is employed in a draping mode. In a
preferred embodiment, the fabric sheet is formed as a woven
fabric. The yarns employed in the weaving of the fabric
preferably are cotton yarns, but other absorbent yarn
materials or absorbent yarns formed of a combination of
materials may be employed. In any event, the yarns must be
suitable for introduction into the body cavity of a surgical
patient in the course of a surgical procedure. Among other
things, this requires that the yarns do not shed yarn
fragments during use such as might become lodged within a body
cavity and initiate the formation of granulomas. Whereas the
yarns of the resultant woven fabric may be bleached or dyed,
the dye employed must be a fast dye and the bleaching or dying
process, and/or the dye employed, must be of a type acceptable
for use in fabrics that may enter the body cavity of a
7
CA 02224822 1997-12-17
WO 97140797 PCTlUS97J07326
patient. Suitable dyes are well known in the art.
One suitable weave pattern for the construction of
the absorbent article of the present invention is depicted in
Figure 3. A typical loom setup for producing the weave pattern
of Figure 3 is depicted in Figures 4A and 4B. The depicted
weave pattern is a huck weave. In this weave pattern, each
repeat, employing an eight harness arrangement on a loom,
comprises eight warp yarns and four weft yarns. In this
pattern, there is provided one draw per eye, two per dent, and
a reed setup of 35.15 dents per inch, as depicted in Figures
4A and 4B. The warp yarns preferred in the present absorbent
article are each of a 20/1 construction, i.e. 20 denier weight
and a single yarn. The weft yarns preferred are each of 8/1
construction. Further, in a preferred embodiment, there are
78 warp yarns per inch of width of the fabric and 28 weft
yarns per inch of width of the fabric.
The radiopaque element of the present woven
absorbent product preferably comprises a thread formed from
polyvinyl chloride filled with at least about 60%, by weight,
of USP barium sulfate, as further specified in Federal
Specification DDD-P-46D, paragraphs 4.5.4 and 3.3.1, as
revised May 7, 1968. The preferred thread has a diameter of
about 0.025 inch, ~ 0.003 inch, with a yield of about 2500
feet per pound of thread. The elongation of the thread
preferably does not exceed about 3000. Less preferred, but
acceptable in certain applications, is an elongated radiopaque
element comprising a flat narrow ribbon of polyvinyl chloride
containing barium sulfate.
8
CA 02224822 1997-12-17
WO 97/40797 PCT/US97107326
A woven absorbent_product in accordance with the
present invention is about 17 inches in width and about 29
inches in length, after hemming. When cotton yarns are
employed in the manufacture of the fabric sheet, the woven
product is washed to remove oils and/or foreign matter from
the product and thereby soften the feel of the product and
enhance its rate of absorptivity. This washing of a woven
cotton absorbent product results in shrinkage of the product
by between about 5 and 10 percent. A preferred woven
absorbent product weighs about 0.1300 grams/in2 prior to
washing and about 0.1592 gm/in2 after washing.
Figures 5A and 5B depict schematically one
embodiment of an apparatus for use in the manufacture of the
present woven absorbent product. Employing the depicted
apparatus, a woven fabric sheet 50 is fed forwardly past an
element drop 52 where a length of radiopaque thread 54 is
overlaid on the top surface 56 of one side edge 26 of the
sheet. The leading end 58 of the thread is overlaid at the
leading edge 60 of the fabric sheet as the sheet is moved
forwardly beneath the element drop 52. As the fabric sheet is
further moved forwardly beneath the element drop 52, the
radiopaque thread is continued to be fed onto the top surface
of the fabric sheet. As the trailing edge 62 of the fabric
.sheet passes beneath the element drop, the thread is severed,
thereby providing a length of radiopaque thread which extends
substantially fully between the opposite ends 30 and 32 of the
fabric sheet.
The fabric sheet with the radiopaque thread disposed
along one of its side edges is fed forwardly between and
9
CA 02224822 1997-12-17
WO 97140797 PCTIUS97/07326
through first and second folders 70 and 72 whereupon each of '
the side edges 26 and 28 are folded inwardly and onto the top
surface 29 of the fabric sheet to form first folds 36 and 36'.
This first folding action captures the radiopaque thread
within the first fold as best seen in Figure 1 and 2.
Thereupon, the fabric sheet in fed forwardly between and
through third and fourth folders 74 and 76 whereupon the first
folds of each of the side edges of the fabric sheet are again
folded inwardly and onto the top surface of the fabric sheet
to form second folds 38 and 38'. This second fold serves to
cause the raw side edge 77 of the fabric sheet to be captured
inside the second fold so that no cut ends of yarns are
exposed externally of the product.
I5 Following completion of the second folds along the
opposite side edges of the fabric sheet, the fabric sheet is
fed forwardly between and through first and second stitchers
78 and 80 where the first and second folds are stitched by
stitches 81, to the body of the fabric sheet to secure the
folds in their folded condition. In a preferred stitching
operation, the first and second folds are stitched to the body
of the fabric sheet using a cotton thread and at least eight
stitches per linear inch of stitching.
Thereafter, the fabric sheet with its opposite side
edges stitched, is fed laterally of its original forward
direction into, between and through fifth and sixth folders 82
and 84 where the ends 30 and 36 of the fabric sheet are folded
inwardly and onto the top surface of the body of the fabric
sheet to define first end folds 86 and 86'. The fabric sheet
thereupon is fed forwardly between and through seventh and
CA 02224822 1997-12-17
WO 97140797 PCT/US97107326
eighth folders 90 and 92 where the first end folds 86 and 86'
are folded inwardly and onto the top surface of the body of
the fabric sheet to form second end folds 94 and 94'. The
thus folded fabric sheet is thereupon fed between and through
third and fourth stitchers 96 and 98 where the first and
second end folds along each of the opposite ends of the fabric
sheet are stitched, by stitches 100, to the body of the fabric
sheet to secure the folds in position as described hereinabove
in connection with the formation of the side edge folds.
It will be apparent that a radiopaque element may be
included in more than one of the folded side edges of the
fabric sheet, but such is not required. Further, the
radiopaque element may be provided along an end edge of the
fabric sheet, in lieu of providing the radiopaque element
along a side edge.
Still further, the fabric sheet of the present
invention may be woven with one or more selvedge edges which
may not require folding thereof inwardly of the body of the
fabric sheet, inasmuch as selvedge edges do not include cut
ends of yarns or loose yarns.
Accordingly, the method of the present invention for
the fabrication of an absorbent product useful in medical
applications comprises the steps of (a) providing a single-
layered woven fabric sheet having a weave pattern which is
sufficiently tight as permits the fabric sheet to be used as
an effective barrier in establishing a sterile field about a
surgical site when the fabric sheet is used as surgical drape,
said woven fabric sheet including first and second sides and
11
CA 02224822 1997-12-17
WO 97!40797 PCT/US97J07326
first and second ends,-and a top surface, (b) disposing an
elongated radiopaque element on said top surface of said
fabric sheet and aligned along one of said first and second
sides or one of said first and second ends of said fabric
sheet, (c) folding that end or side of said fabric sheet along
which said radiopaque element is disposed inwardly and onto
said top surface of said fabric sheet to define a first fold
within which said radiopaque element is captured, (d)
thereafter, folding said first fold inwardly and onto said top
surface of said fabric sheet to define a second fold, and (e)
securing said first and second folds to said top surface of
said fabric sheet with said radiopaque element captured within
said folds.
Notably, the formation of the end folds also serves
to cover the opposite ends of the radiopaque element, thereby
preventing the escape or withdrawal of the radiopaque element
from its covering folds and preventing either end of the
radiopaque element from projecting from the absorbent product
where an end could present a possible source of irritation to
a patient, especially with the product is used to absorb
fluids from an incision, open wound or the like.
The absorbent product of the present invention
possesses the barrier properties required of a surgical drape
in establishing and maintaining a sterile field adjacent a
surgical site, and the absorptive properties of a surgical
sponge, employing only a single layer of woven fabric as
opposed to the multiple layers of laparotomy sponges, for
example. The presence of the radiopaque element secured along
one side edge of the absorbent product provides a means by
12
CA 02224822 1997-12-17
WO 97140797 PCTIUS97/07326
which the product can be identified through X-ray examination.'
Further, being of an elongated geometry, i.e. a thread, it is
virtually impossible for the element to become "bunched up"
and thereby escape detection by X-ray examination as occurs in
certain prior art sponges. Incorporation of the radiopaque
element into the present absorbent product effectively permits
the present product to be employed alternatively as either a
surgical drape or a surgical sponge, thereby permitting a
health care institution or facility to order and stock a
single absorbent product which can be used either as a
surgical drape or as a surgical sponge. Further, the quantity
of absorbent material present in the present product is
greater than the quantity of absorbent material of a common
surgical sponge, thereby permitting the use of fewer absorbent
products per medical procedure.
Whereas the present absorbent product has been
described herein in specific terms and embodiments, it will be
recognized by one skilled in the art that alternatives exist
in the construction and fabrication of the present product and
it is intended that the invention be limited only as set forth
in the claims appended hereto
30
13