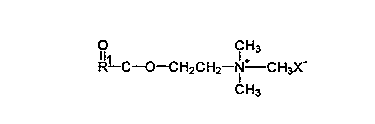Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02226568 1998-01-12
W O 97/031~4 ,. PCTnUS96111274
PROCESS FOR MAKING GRANULAR DETERGENT COMPONENT
The ~,.ese"l invention conce,.,s a process for the p,~paraliol, of a granular
deterye"t co",pone,-t and also co"ce",s granular de~ergel1t co,~ponenls
co...prising cationic su, ractar,t, in particular hydrolysable c~lionic su, r;3ct~nls
such as ~;1 .oii. .e ester.
It is known to prepare c~eter~ent powders by spray-drying calioni~
SUI raCtanlS OptiGI ~ally toyetl ~er with other su, ra~ta, Its and builders.
US-A 4 347 168, issued on August 31st, 1982 ~I;s~loses spray-drying of
alkaline crutcher mixes co"",,isin~ cationic sL"~ac~anl~. It is stated that
suitable oatiol ,ic S~ll raotanls include various choline ester quate" .a"~
ammonium hal~ s
However, .;I .oline esters are readily hyd~ olised, esl,e~ y in alkaline
conditions. Conse~ ently such powders are unsuitable for use by dry mixing
directly into co"".,er~iai granular deteryen~ coll~osilio"s which are usually
alkaline.
The object of the invention is to provide a granular deterge,)t co""~o"e,lb
which cGIll~Jrise hydrolysable cationic su,ractan~ and which are stable in
..... ,e. oial cl~tel ye. It ~ osilio.. s.
Summary of the Invention
According to the invention this object is achieved by a pr~,cess for the
pa.dtion of a granular deter~ent COIllpGllent co.."~risiny the steps of:
(i) drying an ~ eo~ ~s solution of a caliooic sl-, racldl lt to form a powder;
(ii) optiGna~ densifying the powder;
wherein the granular deter~e"t ~~ onent further co...~,rises an acid, the
~ranular detergent ~...yonent havin3 a reserve acidity of at least 1.0 9
HCI/100g, ,t~r~feraL,ly at least 3.0 9 HCI/100g, and more prererably at least
8.0 9 HCI/100g.
r~fera~ly the powder of step (i) is folll~ed by spray drying and comprises at
least 20%, ~,. ere- ably at least 50% by weight of choline ester.
CA 02226~68 1998-01-12
WO 97/03154 " PCT~US96/11274
In a first e"lL,odi",ent of the invention the powder is densified by cG",pac~ionin the presence of other detergei)l powders. In a second en,bodi,nent of the
invention the powder is densified by agglomeration in the presence of other
detergent powders. r, afer, ad detergent powders are aluminosilicAte,
car~onale, bicar~onale, silicate, sulphate, citrate, clay or mixtures thereof.
r,erer,ad acids are citric, sulphuric, hydrochloric, polycarboxylic acid, or
mixtures ll ,ereor.
Ths invention also relates to granular deters~ent components having a bulk
density of at least 500 g/l comprising:
at least 20% by weight of cationic surfactant (preferably choline ester), and
from 1% to 60% by weight of an acid (prererably from 10% to 60% by weight
of an acid selected from the group consisting of citric, sulphuric,
hydrochloric, polyca, boxylic acid, or mixtures thereofl,
and optionally, up to 79% by weight of a deterge.,l powder (prarerably from
5% to 70% by weight of dete,-gent powders selectecl from the group
CGnSiS~il 19 of aluminosili~~~te, ca, bo"ale, bicarl.onale, silicate, sulphate,
citrate, clay or mixtures thereofl,
wherein the granular deteryenl component has a reserve acidity of at least
1.0 9 HCI/100g.
Detailed Description of the Invention
Choline esters:
r~f~"ed cationic surfactant are choline ester derivatives such as those
having the following formula:
l11 Cl H3
R--C--O--CH2CH2 N CH3X-
CH3
wherein R is a Cs to C30 sllaigllt chain or bra"ched chain alkyl or alkenyl,
group and X is an anion, which makes the coi"pound at least water-
dispersible, praferably selected from the group consisting of halide, methyl
CA 02226568 l998-0l-l2
W O 971031S4 , PCTnUS96/11274
sulfate, sulfate, and nitrate prererably methyl sulfate chloride bror"ide or
iodide as well as those wherein the ester linkage in the above formula is
r~pl~c~ with a reverse ester, amide or reverse amide linkage.
Particularly prere"dd exd"~,~les of this type of cationic su,ractant include
.? stearoyl choline ester quaternary an""onium halides (R1=C17 alkyl)
palmitoyl choline ester qudl~i"a,~ a"""G"ium halides (R1=C1s alkyl)
mystiroyl choline ester qualen Idl y ammonium halides (R1 =C1 3 alkyl)
lauroyl choline ester ar"",onium halides (R1=C111 alkyl) as well as coconut
and tallow choli,)e ester qualer"d,y al~monium halides (R1=C1s C17 alkyl
and C1g C13 alkyl ~specti~ely).
Additional p,efel,e~ cationic corn~Jonenl~ of the choline ester variety are
given by the structural formulas below wherein p may be from 0 to 20.
R1 o--C--(CH2)--C--~CH2CI l~ N CH3X
CH3
ICH3 O O Cl H3
X--CH3- 1--CH2--CH2--O--C--(CH2)---C--O--CH2--CH2 I CH3X
CH3 CH3
The prerel,~ ~;I,oline-derivative calionic su~ f)ces ~lisu~ssed above may
be ~r~p~red by the direct esleriri~liGn of a fatty acid of the desired chain
length with di,n~U"~la",inoetl ,anol in the presence of an acid catalyst. The
, ~actiGn product is then qudlel "i~ed with a methyl halide forming the
desire~ cationic malerial.
The choline-derived calionic ~alt:rials may also be prepared by the direct
esle,iri~liG" of a long chain fatty acid of the desired chain length togetl,er
with 2-haloethsl ,ol in the presence of an acid catalyst "~dlerial. The
r~actio" product is then used to qudle",i~e.
Trimethylamine forming the desired cationic c~r"pGnenl.
Other suitable cl loli"e esters for use herein have the formula:
CA 02226568 1998-01-12
W O 97/031S4 " PCTrUS96/11274
Ol CH3
R1_O(CH2CH20)y--(CH2~C--O--CH2--CH2 I CH3X-
CH3
O CH3
R--O(CH2CH20)y--C CH2 1 + CH3X
CH3
R~--(CHCH20)y--11--CH2 CH3
CH3
CH3 ~ CH3
11
R1 ~(CHCH20)y-(CH2)-C-O-CH2-CH2-N+-CH3X~
I
CH3
O CH3
Il I
R1 ~(CH2CH20)y-C~CH2)-C-O-CH2CH2-N+-CH3X~
CH3
O H H O CH3
Il l l 11 1
R1-O(CH2CH20)y C = C - C - C -O-CH2CH2-N~-CH3X-
CH3
CA 02226568 1998-01-12
W O 97/031S4 , PCT~US96/11274
0 CH3
Il I
R1 ~(CH2CH2CH2CH20)y C-CH2-N+-CH13X-
,, CH3
O CH3
Il I
R1~(CH2CH2CH2CH20)y-(CH2)-C-O-CH2CH2-N l-CH3X-
I
CH3
wherein t is 0 or 1, y is from 1 to 20, and R and X are as defined above.
Suitable adds include calLo~grlic and polyca,boxylic acids such as fatty
acids (C12-C18 ",onoca,L.oxylic acids), mellitic acid, citric acid, succinic
acid, oxydis~l~inic acid, carboxymethyloxysuccinic acid ethylene clidr"i"e
tel,dacelic acid, nitrilot,iacetic acid, as well as acrylic acid, maleic acid,
fumaric acid, itaconic acid, aconitic acid""es~conic acid, ~il,dconic acid,
methylenemalonic acid and polymers and copolymers Uler~or. Polymeric
polyca, I,oxylate builders are also described in US-A 3 308 067, Diehl,
issued March 7th 1967. Further acids s~ ~ital~l~ for use in the ,~, esenl
invention are sulphuric and hydrochloric acid.
Most prer~ d is citric acid
Deter~e"l Builders/Powders.
I~lolrgallic or P-containing deter~enl builders include, but are not limited to,the alkali metal, ammGnium and alkanolam,nonium salts of pol~" I,os~hdtes
(exe."pliried by the tripolyphosph&les, py,opl,ospl,ales, and glassy
polymeric meta-ph~s~l)ales), phosphona~es, phytic acid, silicates,
ca.60nates (including bicarLotlales and sesguicA~L,Gndles), sulphates, and
alu.n;nosili~les However, non-pl,ospl)ate builders are required in some
locales. I~-lpG-ldrllly, the c~,nposilions herein function su.lJrisingly well even
in the presence of the so-called "weak" builders (as co"~par~d with
CA 02226~68 1998-01-12
W O 97/03154 ,. PCT~US96/11274
phosphates) such as citrate, or in the so-called "underbuilt" situation that
may occur with zeolite or layered silicate builders.
Examples of silicat~ builders are the alkali metal silicates, particularly thosehaving a SiO2:Na2O ratio in the range 1.6:1 to 3.2:1 and layered silicates,
such as the layered sodium silicates described in U.S. Patent 4,664,839,
issued May 12, 1987 to H. P. Rieck. NaSKS-6 is the trademark for a
crystalline layered silicate marketed by Hoechst (col"",Gnly abbreviated
herein as "SKS-6"). Unlike zeolite builders, the Na SKS-6 silicate builder
does not cG"lai" aluminum. NaSKS-6 has the delta-Na2SiOs morphology
form of layered silicate. It can be prepared by methods such as those
desuibed in German DE-A-3,417,649 and DE-A-3,742,043. SKS~ is a
highly prefer,ed layered silicate for use herein, but other such layered
silicates, such as those having the general formula NaMSixO2x+1-yH2O
wherein M is sodium or hydrogen, x is a number from 1.9 to 4, preferably 2,
and y is a number from 0 to 20, preferably 0 can be used herein. Various
other layered silicates from Hoechst include NaSKS-5, NaSKS-7 and
NaSKS-11, as the alpha, beta and gamma forms. As noted above, the
delta-Na2SiOs (NaSKS-6 form) is most prefer,ed for use herein. Other
sili~tes may also be useful such as for example magnesium silicate, which
can serve as a crispening agent in granular formulalions, as a stabilizing
agent for oxygen Ll~scl,es, and as a cGIllpooent of suds control systems.
Examples of carbonate builders are the alkaline earth and alkali metal
ca, bor,c.les as disclQsed in German Patent Application No. 2,321,001
published on November 15,1973.
Aluminosilic~t~ builders are useful in the prt:sent invention. Aluminosilicate
builders are of great i")po,lal,ce in most currently marketed heavy duty
granular cleter~e,)t compositions, and can also be a significant builder
i"y,ed;enl in liquid detergel,t formulations. Aluminosilicate builders include
those having the e,n~,i.ical formula:
MZ(zA102)y] xH2o
wherein z and y are integers of at least 6, the molar ratio of z to y is in the
rangs from 1.0 to about 0.5, and x is an integer from about 15 to about 264.
Useful aluminosilic~t~ ion exchange materials are cG""nercially available.
These aluminosilir~tes can be crystalline or amorphous in structure and can
be naturally-occurring aluminosilicates or syr,ll,elically derived. A method
for producing aluminosilic~te ion exchange materials is ~isclosed in U.S.
Patent 3,985,669, Krummel, et al, issued October 12, 1976. Prefer,ed
CA 02226~68 1998-01-12
W O 97/03154 i~ PCT~US96/11274
synthetic crystalline aluminosilicate ion exchange materials useful herei
are available under the designations Zeolite A, Zeolite P (B), Zeolite MAP
and Zeolite X. In an especially prefer,ed embodiment, the crystalline
aluminosili~t~ ion excl ,a"ye material has the formula:
Na1 2l(Alo2)1 2(Si~2)1 21-XH20
wherein x is from about 20 to about 30, especially about 27. This ",al6riai is
known as Zeolite A. Dehydrated zeolites (x = 0 - 10) may also be used
herein. r~ererably, the aluminosilicate has a particle size of about 0.1-10
",i~ o"s in dia",eter.
Organic det~rgel-t builders suitable for the purposes of the present
invention include, but are not resl~ ted l:o, a wide variety of polycarboxylate
compounds. As used nerein, "polycarL,~xylate" refers to compounds having
a plurality of c~r6-o~ylate groups, preferably at least 3 c ~6Oxylates.
Polycarboxylate builder can generally be addedl to the composition in acid
form, but can also be added in the form of a neutralized salt. When utilized
in salt form, alkali metals, such as sodium, potassium, and lithium, or
alkanola,.""onium salts are prere,.ed.
Incl~ded among the polycarboxylate builders are a variety of ~te~ories of
useful " ,alerials. One i~ OI lanl ~te~ory of polycarboxylate builders
~ncGr, ~p~sses the ether polycarboxylates, including oxy~lisucc~inate, as
Jisciosed in Berg, U.S. Patent 3,128,287, issued April 7, 1964, and Lamberti
et al, U.S. Patent 3,635,830, issued January 18,1972. See also '~MSITDS"
builders of U.S. Patent 4,663,071, issued to Bush et al, on May 5, 1987.
Suitable ether polycarboxylates also include cyclic compounds, particularly
alicyclic cG."pounds, such as those described in U.S. ralel,ls 3,923,679;
3,835,163; 4,158,635; 4,120,874 and 4,102,903.
Other useful d~t~rgency builders include the ether hydroxypolyca, L,oxylates,
copolymers of maleic anhydride with ethylene or vinyl methyl ether, 1, 3, 5-
trihydroxy bel,~e,-e-2, 4, 6-trisul~ho.,ic acid, and ca~boxymethyloxysuccinic
acid, the various alkali metal, a"",)G"ium and substituted ammonium salts of
polyacetic acids such as ethylenediamine tetraacetic acid and nitrilotriacetic
acid, as well as polycarboxylates such as mellitic acid, succinic acid, oxy-
disuccinic acid, polymaleic acid, benzene 1,3,5-l, iCdl ~oxylic acid,
ca, boxymethyloxysuccinic acid, and soluble salts ll ,ereor.
Citrates can also be used, especially in co-"bi"dlion with zeolite and/or
layered silicate builders. Oxydisuccinales are also especially useful in such
co m positiGns and combinations.
CA 02226~68 1998-01-12
W O 97/03154 it PCTrUS96111274
Also suitable in the detergent compositions of the present invention are the
3,3-cl;carboxy-4-oxa-1,6-hexanedio~tes and the related compounds
r~icclosed in U.S. Patent 4,566,984, Bush, issued January 28, 1986. Useful
succinic acid builders include the Cs-C20 alkyl and alkenyl succinic acids
and salts tl,ereof. A particularly prerer,ed compound of this type is do-
decenylsuccinic acid. Specific examples of succinate builders include:
laurylsucc;nale, myristylsuccinate, palmitylsuccinale, 2-dodecenylsuccinate
(preferred), 2-pentadecenylsuccinate, and the like. Laurylsuccinates are the
prerer,ed builders of this group, and are described in European Patent
Application 86200690.5/0,200,263, published November 5, 1986.
Other suitable poly~boxylates are ~isclosed in U.S. Patent 4,144,226,
Crutchfield et al, issued March 13, 1979 and in U.S. Patent 3,308,067,
Diehl, issued March 7, 1967. See also Diehl U.S. Patent 3,723,322.
Other optional additives to the granular cleter~e,)t co"")onei)t of the presenl
invention include hydrophobic s~hsl~nces such as wax and oil. Waxes are
hydrocalbo,-s which are typically derived from petroleum. Three types of
wax may be distinguished (see Kirk-Othmer, Encyclopedia of Cl ,e" ,i - ~'
Technology, 3rd Edition, Wiley,Vol. 24, pages 473 and 474): pararrin wax,
microcrystalline wax and semicrystalline wax.
Paraffin wax co--sisls p,incipally of normal alkanes. It is composed of 40-
90% normal pardrrins and the remainder is C18-C36 isoalkanes and
cycloalkanes. The melting point of the wax determines the actual grade and
it varies between about 46~C and 71 ~C. Average molecular weight is
bet\r een about 350 and 420. A suitable paraffin wax for use in the present
invention is BDH P~st~ ted Paraffin Wax, having a melting point of 51-55
~C
Semicrystalline and microcrystalline waxes co,~tain s~ s~nlial propoilions
of hy~hocalt,ons other than normal alkanes. Microcrystalline waxes typically
have a melting point between 60~C and 93~C. Average molecular weight is
between about 600 and 800.
A particularly pr~fe..ed microcrystalline wax for use in the present invention
is MMP 19, surplied by Shell.
CA 02226568 1998-01-12
WO 97/031S4 " PCT/US96/11274
Other waxes suitable for use in the present invention are:
Beeswax;
VeD~tAhle Wax including Candelilla; Carnauba; Japan Wax;
Ouricury Wax; Douglas-Fir Bark Wax; Rice Bran Wax; Jojoba; Castor
Wax; Bayberry Wax;
Mineral Wax including Montan Wax and Peat Waxes;
Sy, Ill ,etic Wax including Polyethylene Waxes; Fischer-Tropsch
Waxes (polymethylene) (45-106 ~C); Chemically Modified
Hydloca-6On Waxes (86-125 ~C) and Substituted Amide Waxes (very
high melting point ca 140 ~C)
The amount of wax and/or oil used in the granular deterge"l cor"ponent
should be from 0.005% to 20% by weight, pr~ferably from 0.5% to 10% by
weight and most preferably from 1% to 5% by weight of the granular
detergent cor,)pooent.
The prucess of drying as referred to herein means any process step in
which water is removed. Suitable drying processes include drum drying
vacuum drying flaking oven drying ancl spray drying. Spray drying is most
prefe" ed.
The process of densir.calion as referred to herein means any l,eal",ent of
powder which results in an i,.crease in bulk density of at least 100 g/l and
prefereal~ly of at least 200 g/l. Highly ,vlerer,ed densiricaliG" processes are
agylor"eralion and ~""~actiol). AllelnaLi~/e densification processes include
extruding, pressing, milling and pelletizing. Ginal sizing can then be
a~l .i vod by grinding and screening.
A~glo,.,eratiGi)
In a most ~,rerer,ed agglo",eralion step powdered catio,-i. su,ra~tanl is fed
into a high shear mixer such as a Loedige CB~g). At the same time deterge, .t
powder and liquid binder are fed into the mixer; the two components being
ir.li",~lely mixed. The acid maybe introduced into the agglo,..erdlioo process
as a c~,..ponent of the powdered cationic s~radanl as a col,.ponei-t of the
det~r.t powder as a col--pol-ents of the liquid binder or a co..lb;nalion of
any or all of these. The a~glo--,eralion ~.rocess is completed in a second
CA 02226~68 l998-0l-l2
W O 97/03154 " PCTrUS96/11274
mixer, such as a Loedige KM~) into which a powdered fiow aid such as
zeolite A may be added. Finaiiy the agglomerated product is p,-eferably dried
to the required finished product moisture level
Comp~ction
A highly prere"e~ co.))pac1ion process is described in detail in EP-A 0 220
024, (P&G), published on 29th April 1987. In this process base granules are
prepared by drying an ~queous slurry comprising, in the process of the
present invention, cationic surfactant. The base granules are then mixed
with detergent builder material and compacted at a pressure of ,.-ererdbly
from 140 kPa to 2 MPa: An example of suitable cGI"paction equipment is the
chilsonalor.
Test l"eU,od - Reserve acidity
1. Macerale 10 grams of granular detergenl composition comprising
cationic SUI ractanl.
2. Dissolve in deionised water and make up to 1000 mls using a volumetric
flask.
3. Calibrale pH meter using buffers, 4.0 and 7Ø
4. Take a 50 ml aliquot of sc~tion and note pH.
5. Titrate with 0.1 N NaOH until pH = 7.
6. Note mls NaOH and apply to the following eqll~tion.
~2r~ ~eacidi~gHCl/l00g) = mlSNaOH * N * 3-65
Sample welght in aliquot
where, N = ",olari4 of NaOH sol~ ~tion,
and, sampla weight = 0.5 9
CA 02226568 1998-01-12
W O 97/031S4 " PCT~US96/11274
Examples
Example 1
Spray-dried powder Agglomerate
Choline ester 100 30
Zeolite A (hydrated) - 27
Citric Acid - 28
Polycarboxylic acid~ - 15
Pol~ca,L,oxylic acid in this example was a co-polymer of maleic and acrylic
acid with a molecular weight of about
60 000.
The choline ester powder (100%) was prepared in lab scale drying
appar~tus and suhseg~ ~ently lraaled by the following aç~glG,nerdlio.. pr~.cess.The powders of cl~oli,.e ester citric acid and zeolite are added to a Braun
food mixer. The polyca, boxylic acid is poured slowly onto the powder mix as
the blad~ is tuming. When all the liquidl binder has been added a dusting of
zeolite is added and then the agglomerates are dried in a lab scale fluid bed
dryer at 80~C to remove all the free moisture.
The resulting granular detergent component (the a~glo, . Ierale) had a
reserve acidity of 9.1 9 HCI/100g
Example 2
Spray-dried powder Agglor,lerdle
Choline ester 60 30
Zeolite A (hydrated) 40 27
Citric Acid - 28
Polycarbo,~ylic acid~ - 15
Polycarboxylic acid in this example was a co-polymer of maleic and acrylic
acid with a molecl ~ r weight of about 60 000.
CA 02226568 l998-0l-l2
W O 97/031S4 i~ PCTrUS96/11274
The choline ester powder was prepared by spray drying and subsequently
treated by the same agglol"eraliGi, process as in exa""~lo 1.
Ths resulting granular compGnent (the agglomerate) has a reserve acidity of
9.1 g HCI/100g.
Example 3
Spray-dried powder Compact
Choline ester 55 42
Zeolite A (hydrated) 44 34
Citric Acid - 24
Free water
The choline ester powder was spray-dried and s~ ~hse~ ~ently treated by the
following co""~ac~ion process .
The powder from the spray drying process is dry mixed with powdered citric
acid. The mix is then added to the die of a Ward Forsyth batch t~hl~ttirlg
press and pr~ssed for a few seconds at a pressure of around 2 MPa. The
sl~hse~uent tablet is ground up in a coffee grinder to form granules which
are then dusted with 7Polite.
The resulting granular detergei ,~ component has a reserve alkalinity of 2.3 g
G HCI1100 9.
Example 4
Aq~ ~eo~ ~s soln Oven dried Compact
powder
Choline ester 26 42 42
Zeolite A (hydrated) 34 34
Citric Acid 15 24 24
Free water 59
CA 02226568 1998-01-12
W O 97/03154 ,~ PCTAUS96/11274
Citric acid was added to aqueous solution of choline ester during its
s~ hesis. Powdered zeolite was then added to the solution and this
~ product was then dried in an oven to remove all the free water and then
ground up in a Moulinex coffee grinder. This powder was then compArtPd in
the same manner as in example 3
The resulting granular detergent cor"pGnent had a reserve alkalinity of 2.39
HCI/1 009.
Example 5
Aqueous soln Oven dried Agglomerale
powder
Choline ester 26 36 30
Zeolite A (hydrated) 30 27
Citric Acid 15 34 28
Polyca, ~oxylic acid~ - - 15
Water 59
Poly~rL,oxylic acid in this example was a co-polymer of maleic and acrylic
acid with a molecular weight of about
60 000.
Citric acid was added to aqueous sol- ~tion of choline ester during its
s~ tl ,esis. Powdered zeolite and ~dditional powdered citric acid were then
added to the ssl ~tion. This product was then dried in an oven to remove all
the free water and then ground up in a Moulinex coffee 9~ inder. This powder
was then ll ealed by the same agglomeration process as in example 1.
The resulting granular dete~ gent component had a reserve alkalinity of 9.1 g
HC111 00g.
.,