Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02228898 1998-02-0~
W O 97/09045 PCT/JP96/02319
DESCRIPTION
. USE OF A CARBOSTYRIL DERIYATIVE FOR INHIBITING CARcINoGENEsIs
FIELD OF THE lNV~N'l'ION
The present invention relates to an agent for
inhibiting carcinogenesis, specifically, it relates to
an agent for inhibiting carcinogenesis of the digestive
tract cancer. More particularly, the invention relates
to an agent for inhibiting carcinogenesis comprising, as
the active ingredient, a carbostyril derivative
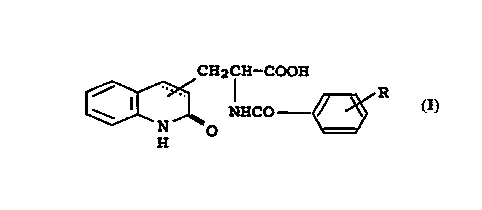
represented by the following general formula (I),
CH2CH--COOH
~ NHCO ~ (I)
[wherein R is a halogen atom (a fluorine atom, a
chlorine atom, a bromine atom or an iodine atom); the
substituted position of the substituent in the
carbostyril skeleton is 3- or 4-position in the
carbostyril skeleton; and the carbon-carbon bond between
3- and 4-positions in the carbostyril skeleton is a
single bond or a double bond]; or a salt thereof,
preferably 2-(4-chlorobenzoylamino)-3-(2-quinolon-4-
yl)propionic acid or salt thereof.
BACKGROUND ART
The carbostyril derivatives represented by the
20 general formula (I) and processes for producing the same
CA 02228898 1998-02-0~
W O 97/09045 PCT/JP96/02319
are described in Japanese Patent Publication No. 63-
35623, the usefulness of the carbostyril derivatives as
anti-gastric ulcer agents are described in Japanese
Patent Application Kokai (Laid-open) No. 3-74329, and
processes for producing those carbostyril derivatives
having optical activities are described in Japanese
Patent Application Kokai (Laid-open) No. 3-145468.
Further, inhibitory effect of carbostyril
derivatives of the present invention on reactive oxygen
metabolites is described in Japan. J. Pharmacol., Vol.
49, pp. 441-448 (1969), and the protectability of
gastric mucous membrane by carbostyril derivatives of
the present invention is described in Folia Pharmacol.
Japon., Vol. 97, pp. 371-380 (1991).
Furthermore, the usefulness of carbostyril
derivatives as agents for curing diabetes mellitus is
described in International Publication No. WO 92/21342,
the usefulness of carbostyril derivatives as agents for
protecting intestinal mucosa from disorders is described
in International Publication No. WO 94/12182, and the
usefulness of carbostyril derivatives as agents for
inhibiting reduction in somatostatin secretion is
described in International Publication No. WO 93/24043.
Hitherto, various terpenes, flavonoids and
steroids have been found as to the substances having the
activity for inhibiting carcinogenesis. However, from
a safety viewpoint, these substances have not been
practically applied yet as to agents for inhibiting
CA 02228898 1998-02-0~
WO 97/09045 PCT/JP96/OZ319
carcinogenesis. Under the circumstances, it is expected
the development of safety and effective substances for
inhibiting carcinogenesis.
DISCLOSURE OF THE lNv~NlION
The present inventors have made an extensive
study to find effective agents for inhibiting carcino-
genesis. As a result, the inventors have found the fact
that carbostyril derivatives represented by the general
formula (I) or salts thereof, particularly among of
10 these, 2--(4--chlorobenzoylamino)-3-( 2-quinolon--4-
yl)propionic acid or a salt thereof possess excellent
pharmacological activity for inhibiting carcinogenesis.
Thus, the present invention has completed by said
pharmacological finding.
In the present specification, the term
"cancers" means cancers originated from the epitheliums
existed in various parts of the body, for example,
cancers originated from epitheliums of the skin, the
tongue, the pharynx, the trachea, as well as cancers of
the digestive tracts, such as the esophagus, the
stomach, the duodenum, the small intestine and the large
intestine.
The agents for inhibiting carcinogenesis of
the present invention can be prepared into various forms
of common pharmaceutical preparations by formulating the
carbostyril derivative represented by the general
formula (I) or a salt thereof.
CA 02228898 1998-02-0~
W O 97/09045 PCT/JP96/02319
The pharmaceutical preparations are prepared
by formulating with commonly employed diluents or
excipients, such as fillers, extenders, binders, wetting
agents, disintegrants, surfactants, lubricants and the
like. The pharmaceutical preparations can be shaped
into various forms depending upon the curing purposes,
thus, typical examples of the forms are tablets, pills,
powders, liquid medicines, suspensions, emulsions,
granules, capsules, suppositories, injection
preparations (li~uid, suspension and the like), aerosol
preparations, syrup preparations and preparations for
external use and the like. Further, sustained release
preparations can also be prepared by formulating with
suitable resins.
For the purpose of shaping into the form of
tablets, any known carriers which are used widely in
this field can be applied, for example, excipients such
as lactose, white sugar, sodium chloride, glucose, urea,
starch, calcium carbonate, kaolin, crystalline
cellulose, silicic acid and the like; binders such as
water, ethanol, propanol, simple syrup, glucose
solution, starch solution, gelatin solution,
carboxymethyl cellulose, shellac, methyl cellulose,
potassium phosphate, polyvinyl pyrrolidone and the like;
disintegrators such as dry starch, sodium alginate, agar
powder, laminarin powder, sodium hydrogen-carbonate~
calcium carbonate, polyoxyethylene sorbitan fatty acid
esters, sodium lauryl sulfate, monoglycerides of stearic
CA 02228898 1998-02-0~
W O 97/09045 PCT/JP96/02319
s
acid, starch, lactose and the like; disintegration
inhibitors such as white sugar, stearin, cacao butter,
hydrogenated oils and the like; absorption accelerators
such as quaternary ammonium base, sodium lauryl sulfate
and the like; humectants such as glycerin, starch and
the like; adsorbents such as starch, lactose, kaolin,
bentonite, colloidal silicic acid and the like;
lubricants such as refined talc, stearic acid salts,
boric acid powder, polyethylene glycols and the like.
In case of necessity, the tablets can be prepared in the
form of common coated tablets, for example, sugar-coated
tablets, gelatin film-coated tablets, enteric film-
coated tablets, film-coated tablets, or in the form of
double-layers tablets, multiple-layers tablets and the
like.
For the purpose of shaping into the form of
pills, any known carriers which are widely used in this
field can be applied, for example, excipients such as
glucose, lactose, starch, cacao butter, hydrogenated
20 vegetable oils, kaolin, talc and the like; binders such
as arabic gum powder, tragacanth gum powder, gelatin,
ethanol and the like; and disintegrators such as
laminarin, agar-agar and the like can be exemplified.
For the purpose of shaping into the form of
25 suppositories, any known carriers which are widely used
in this field can be applied, for example, polyethylene
glycols, cacao butter, higher alcohols, esters of higher
CA 02228898 1998-02-0~
W O 97/09045 PCT/JP96/02319
alcohol, gelatin, semi-synthesized glycerides and the
like can be exemplified.
For the purpose of shaping into the form of
injection preparations, they can be prepared to
solutions, emulsions or suspensions. Generally they are
sterilized and preferably made isotonic to the blood.
In preparing the injection preparations as in the form
of solutions, emulsions or suspensions, any known
diluents which are widely used in this field can be
applied. For example, water, ethanol, propylene glycol,
ethoxylated isostearyl alcohol, polyoxylated isostearyl
alcohol, fatty acid esters of polyoxyethylene sorbitan
and the like can be exemplified. In the case of make
the injection preparations isotonic to the blood,
sufficient amount of sodium chloride, glucose or
glycerin may be contained therein. Additionally, a
dissolving adjuvant, a buffer solution, an analgesic
agent and the like which are commonly used may be
contained therein. In case of necessity, a coloring
agent, a preservatives, a perfume, a flavoring agent, a
sweetening agent and other medicines may be contained
therein.
External preparations are prepared in the form
of common pharmaceutical preparations for external use.
25 As to common pharmaceutical preparations for external
use are including, for example, a liquid medicine, a
medicinal oil, a lotion, a liniment, an oleoginous
ointment, an emulsion type ointment, such as O/W type
CA 02228898 l998-02-0~
W O 97/09045 PCT/JP96/02319
hydrophilic ointment and W/O type water-absorbing
ointment, a water-soluble ointment, a pasta, a plaster,
a patch, a cream, an emulsion and the like, and these
forms of pharmaceutical preparations for external use
are not restricted within the scope of these examples.
Each one of these forms of pharmaceutical preparations
for external use can be prepared by common methods.
In shaping of these external preparations,
various base materials which are widely used in this
field can be also applied. For example, at least one
oleoginous base can be used singly, or mixture o~ two or
more of them can be used widely; or at least one water-
soluble ointment base can be used singly, or mixture of
two or more of them can be used widely. Concrete
examples of these ointment base are fats and oils such
as peanut oil, sesame oil, soybean oil, safflower oil,
avogado oil, sunflower oil, corn oil, rapeseed oil,
cotton seed oil, castor oil, camellia oil, coconut oil,
olive oil, poppy seed oil, cacao butter, beef tallow,
lard, wool fat and the like; modified bases obtained by
subjecting these ~ats and oils to chemical changes such
as hydrogenation; mineral oils such as petrolatum,
paraffin, silicone oil, squalane and the like; higher
fatty acid esters such as isopropyl myristate, n-butyl
25 myristate, isopropyl linoleate, acetyl ricinoleate,
stearyl ricinoleate, propyl ricinoleate, isopropyl
ricinoleate, isobutyl ricinoleate, heptyl ricinoleate,
diethyl sebacate and diisopropyl adipate; higher
=
CA 02228898 1998-02-0~
WO 97/09045 PCT/JP96/02319
aliphatic alcohols such as cetyl alcohol and stearyl
alcohol; waxes such as bleached bees wax, spermaceti,
Japan wax, lanolin, carnauba wax, shellac wax and the
like; higher fatty acids such as stearic acid, oleic
acid, palmitic acid and the like; mixtures of mono-, di-
and tri-glycerides of saturated or unsaturated fatty
acids having 12 to 18 carbon atoms; polyhydric alcohols
such as ethylene glycols, polyethylene glycols,
propylene glycol, polypropylene glycols, glycerin, batyl
alcohol, pentaerythritol, sorbitol, mannitol and the
like; gummy substances such as arabic gum, benzoin gum,
guaiacum, tragacanth gum and the like; water-soluble
natural high molecular compounds such as gelatin,
starch, casein, dextrin, pectin, sodium pectate, sodium
alginate, methyl cellulose, ethyl cellulose,
carboxymethyl cellulose, hydroxyethyl cellulose,
hydroxypropyl cellulose, nitrocellulose, crystalline
cellulose and the like; water-soluble synthetic high
molecular compounds such as polyvinyl alcohol,
poly(vinyl methyl ether), polyvinyl pyrrolidone, sodium
polyacrylate, carboxyvinyl polymer, polyethyleneimine
and the like; nonionic, anionic, amphoteric and cationic
surfactants; ethanol, isopropanol and water, can be
exemplified.
To the pharmaceutical preparations for
external use, there can be added common additives such
as a geling agent, a preservative, an antioxidant, a
buffer solution, a pH controlling agent, a wetting
CA 02228898 l998-02-0~
W O 97/09045 PCT/JP96/02319
agent, an antiseptic agent, a coloring agent, a
flavoring agent, a pigment, a thickening agent, a metal
chelating agent and the like.
Aerosol type preparations can be prepared
generally by formulating a sterilized solution or
suspension of the carbostyril derivative of the general
formula (I) with a propellant. In case of shaping in
the form of a solution or suspension, any one of known
diluents which are commonly used in this field can also
be used, thus the diluents which are exemplified in
formulating the injection preparations can be used. As
to the propellant, any one of the propellants which are
commonly used in this field can also be used, thus,
liquefied gas propellants such as chlorofluorocarbons
like Flon-12 (general term of dichlorodifluoromethane)
or Flon-123 (general term of trifluorodichloroethane);
compressed gas propellants such as nitrogen gas, carbon
dioxide gas and the like can be exemplified. The
aerosol type preparations may further contain a common
solubilizing adjuvant, a buffering agent, and the like,
and if necessary, a coloring agent, a preservative, a
perfume, a flavoring agent, a sweetening agent may be
added thereto.
The amount of the carbostyril derivative of
the general formula (I) or salt thereof to be contained
in the agent for inhibiting carcinogenesis according to
the present invention is not particularly restricted and
can be selected from a wide range, and the amount may be
CA 02228898 1998-02-0~
W O 97/09045 PCT/JP96/02319
generally selected within the range o~ 1-70% by weight,
preferably 5-50 ~ by weight.
Method for administering the agent of the
present invention is not particularly restricted except
that the case selected to the specific treating purpose.
The method is decided depending upon the form of pre-
paration, the age of patient, the distinction of sex and
other conditions, the degree of disease condition of the
patient and others. For example, tablets, pills, a
liquid medicine, a suspension, an emulsion, granules, a
syrup and capsules are administered orally. An injec-
tion preparation is intravenously administered singly or
in combination with common auxiliary solutions such as
glucose solution and/or amino acid solution, in case of
necessity, it is singly administered intramuscularly,
intradermally, subcutaneously or intraperitoneally. A
suppository is administered intrarectally. An external
preparation is coated on the diseased part.
Dosage of the agent for inhibiting carcino-
genesis of the present invention may be suitablyselected depend upon the age of patient, the distinction
of sex and other conditions, as well as the degree of
disease condition of the patient and other related
factors, and generally the amount of carbostyril
derivative of the general formula (I) or salt thereof
may be 0.6 to 50 mg per 1 kg of the body weight per day.
The desirable content of the effective ingredient in
each unit of administration form may be 10 to 1,000 mg.
CA 02228898 l998-02-0~
W O 97/09045 PCT/JP96/02319
11
EXAMPLES
The present invention will be explained more
specifically by showing Preparation Examples and Pharma-
cological Test.
Preparation Example 1
2-(4-Chlorobenzoylamino)-3-(2-quinolon-
4-yl)propionic acid 150 g
Avicel (trade name for microcrystalline
cellulose, manufactured by Asahi
Chemical Industry Co., Ltd.)40 g
Corn starch 30 g
Magnesium stearate 2 g
Hydroxypropylmethyl cellulose10 g
Polyethylene glycol 6000 3 g
Castor oil 40 g
Methanol 40 g
2-(4-Chlorobenzoylamino)-3-(2-quinolon-4-yl)-
propionic acid, Avicel, corn starch and magnesium
stearate were mixed together and ground, then the
mixture was shaped into the form of tables by using a
conventional pounder (R 10 mm) for sugar coating. The
tablets were coated with a film-coating agent consisting
of hydroxypropylmethyl cellulose, propylene glycol 6000,
caster oil and methanol, to prepare film-coated tablets.
CA 02228898 1998-02-0~
W O 97/09045 PCT/JP96/02319
12
Preparation Example 2
2-(4-Chlorobenzoylamino)-3-(2-quinolon-
4-yl)propionic acid 150.0 g
Citric acid 1.0 g
Lactose 33.5 g
Dicalcium phosphate 70.0 g
Pluronic F-68 30.0 g
Sodium lauryl sulfate 15.0 g
Polyvinyl pyrrolidone 15.0 g
Polyethylene glycol (Carbowax 1500) 4.5 g
Polyethylene glycol (Carbowax 6000) 45.0 g
Corn starch 30.0 g
Dry sodium lauryl sulfate 3.0 g
Dry magnesium stearate 3.0 g
Ethanol A sufficient
quantity
2-(4-Chlorobenzoylamino)-3-(2-quinolon-4-yl)-
propionic acid, citric acid, lactose, dicalcium
phosphate, Pluronic F-68 and sodium lauryl sulfate were
mixed together.
The mixture was sieved through a No. 60
screen. The resulting sieved mixture was wet-granulated
with an ethanol solution containing polyvinyl pyrro-
lidone, Carbowax 1500 and Carbowax 6000. In case of
necessity, ethanol was added to convert the mixture into
a paste-like mass. Corn starch was added, and mixing
operation was continued until uniform particles were
~ormed. The resulting particles were passed through a
CA 02228898 l998-02-0~
W O 97/09045 PCT/JP96/02319 13
No. 10 screen, then placed in a tray, and were dried in
an oven at 100~C for 12-14 hours. The dried particles
were sieved through a No. 16 screen. Next, dry sodium
lauryl sulfate and dry magnesium stearate were added to
the resulting particles. The mixture was compressed
into core tablets of the desired shape by using a tablet
machine.
The resulting core tablets were treated with a
varnish and then talc was sprayed thereon for preventing
from moisture absorption. On the surface of resulting
core tablets, undercoat layer was coated. Sufficient
number of varnish coatings were conducted to the core
tablets so as to make them suitable for internal use.
Formation of undercoat layer and smooth coating were
conducted to make the coated tablets having completely
round and smooth sur~ace. Color coating was conducted
until the desired color sur~ace was obtained. After
drying, the coated tablets were polished to obtain
tablets of uniform gloss.
- 20 PreparatiOn Example 3
2--(4--Chlorobenzoylamino)--3-( 2--~uinolon-
4-yl)propionic acid 5.0 g
Polyethylene glycol (mol. wt.: 4000) 0.3 g
Sodium chloride 0.9 g
Polyoxyethylene sorbitan monooleate 0.4 g
Sodium metabisulfite 0.1 g
Methylparaben 0.18 g
Propylparaben 0.02 g
Distilled water for injection 10.0 ml
CA 02228898 1998-02-0~
W O 97/09045 PCT/JP96/02319
14
Parabens, sodium metabisulfite and sodium
chlo-ide were dissolved in a half volume of the above
mentioned distilled water for injection at 80~C under
stirring. The resulting solution was cooled to 40~C,
then to this solution was added 2-(4-chlorobenzoyl-
amino)-3-(2-quinolon-4-yl)propionic acid, polyethylene
glycol and polyoxyethylene sorbitan monooleate and were
dissolved. Next, to the resulting solution was added
the remaining a half volume of the distilled water to
make the solution to the final volume. Thus obtained
solution was sterilized by passing through a suitable
filter paper, to prepare the desired injection
preparation.
Pharmacological test
Effect for inhibiting carcinogenesis of ENNG-
induced cancer of the duodenum in mouse
C57/B16 strain mice of 8 week-age were used
as test animals. N-Ethyl-N'-nitro-N-nitrosoguanidine
(ENNG), which is known as a carcinogenic substance,
was administered to the mice to produce cancer of
the duodenum, then 2-(4-chlorobenzoylamino)-3-
(2-quinolon-4-yl)propionic acid (general name:
Rebamipide) was administered as test compound to
the test mice to examine the activity for inhibiting
carcinogenesis.
The test mice were classified into three
groups of A, B and C (each one of the groups is con-
CA 02228898 1998-02-0~
W O 97/09045 PCT/JP96/02319
sisting of 30 mice). The all of the test mice were
given freely the drinking water which contains ENNG
in the concentration of 100 mg/liter for 4 weeks.
Thereafter, one solid feed in which the amount
of test compound of Rebamipide is controlled to keep in
the rate of 20 mg/kg/day were given to the mice of Group
A from 5th to 16th week, and another solid feed in which
the amount of test compound of Rebamipide is controlled
to keep in the rate of 50 mg/kg/day were given to the
mice of Group B for from 5th to 16th week. Each one of
the test mice of Groups of A, B and C were given tap
water freely, then the duodenums of the test mice were
sacrificed on the 16th week after the administration of
the test compound, and the effect for inhibiting
carcinogenesis performed by the test compound were
examined.
As the result, in comparison with the
incidence of carcinogenesis of 66.7% shown by test
mice of Group C to which test compound of Rebamipide
were not administered, while the incidence of carcino-
genesis shown by test mice of Group A was 58.1% and the
incidence of carcinogenesis shown by test mice of Group
B was 45.2%, respectively. Thus, it can be said clearly
that the carcinogenesis were inhibited by administration
25 of carbostyril derivative (Rebapimide) of the present
invention. The average incidences o~ carcinogenesis
(average + SD) were 0.84 + 0.86 in Group A, 0.68 +
0.87 in Group B and 1.21 + 1.27 in Group C, respec-
CA 02228898 1998-02-o~
W097/09045 PCT/~96/02319
16
tively, thus the carcino~enesis were inhibited in the
test mice of Groups A and B which were administered with
carbostyril derivative o~ the present invention.