Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 0223S62l l998-04-22
W0 97/15555 PCT/DE96/02079
ImidasolQ DQrivatives and their Use as Nitrogen Monoxide 8ynthas~
Inhibitors
The invention relates to substituted phenylimidazole
derivatives and pyridylimidazole derivatives as well as their
production and use in pharmaceutical agents, especially their use
as nitrogen monoxide synthase inhibitors.
It is known that nitrogen monoxide synthase (NOS) falls into
the category of enzymes with hemoglobin groups in the active
site, and that phenylimidazole bonds to the latter.
It has now been found that the affinity of imidazole
derivatives for NOS can be increased in a way that could not have
been predicted, if the latter have suitable substituents.
Compounds that selectively inhibit inducible human nitrogen
monoxide synthase (hiNOS) at very small concentrations (IC50) and
provide excellent action in vivo are obtained.
Selective nitrogen monoxide synthase inhibitors are suitable
as pharmaceutical agents for treating diseases of the central
nervous system, such as multiple sclerosis in all forms, dementia
such as Alzheimer's disease, HIV dementia, amyotrophic lateral
sclerosis and comparable sclerotic diseases, cerebral ischemia
and other neurodegenerative diseases. They are also suitable for
treating heart and cardiovascular diseases and sepsis or septic
shock, hypotension, ARDS (adult respiratory distress syndrome),
for treating auto-immune and/or inflammatory diseases such as
rheumatoid arthritis, osteoarthritis, insulin-dependent diabetes
mellitus (IDDM), inflammatory disease of the pelvis/intestine
(bowel disease), meningitis, glomerulonephritis, acute and
chronic liver diseases, diseases by rejection (for example, for
immunosuppression in the case of transplants such as allogenic
heart, kidney or liver transplants) or inflammatory skin diseases
such as psoriasis.
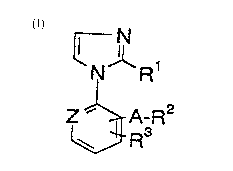
The invention relates to the new compounds of formula I
N
~N~Rl
z~A-R2
in which
ARZ can be the same or different in one or two places,
R1 means hydrogen, halogen, -SH or -S-C14 alkyl,
Z means nitrogen or -CH=,
A means a bond, straight-chain or branched C14 alkylene,
straight-chain or branched C24 alkenylene, straight-
chain or branched C24 alkinylene,
R2 means hydrogen, halogen, NOz, -C-N, C~3, -CCl3, -S(O) m~
C14 alkyl, -oR5, -S-R6, -Co-R7, -NR8R9, -CH(CN)-COOH,
-CH(CN)-COO-C16 alkyl, =C(CN)-COOH, =C(CN)-COOC1 6
alkyl, -NH-SOz-R10, -C(NHz)=NOH, -NH-CO-NH2, -NH-CS-NH2,
-NH-CS-NH(C14 alkyl), -C(=NR)-NHR11, -NH-C(=NR)-NR11R1Z,
-S-(C=NR)-NR11R1Z, a 5- or 6-membered heteroaryl radical
optionally substituted with C14 alkyl or halogen, a
phenyl or biphenyl radical optionally substituted in
one or more places with halogen, C- N, NO2, NH2, CF3,
COOH, CONH2, COOC14 alkyl, -S-C~ 4 alkyl or -O-C14 alkyl;
imidazolosulfonyl, -NH-Co-NR16R17,
R3 means hydrogen, halogen, NO2, OH, NH2 or imidazol-l-yl,
AR2 and R3 together form -O-(CH2)n-O-,
m, n mean 1 or 2,
R5 means hydrogen, a substituted or unsubstituted,
straight-chain or branched, saturated or unsaturated
hydrocarbon radical with 1-14 C atoms, which can
contain 1-2 double bonds and/or triple bonds, and in
which a -CH2 group can be replaced by oxygen, sulfur,
phenyl or C36 cycloalkyl optionally substituted with
halogen; C3 7 cycloalkyl optionally substituted with
methyl or halogen; -Co-R15, -CS-NH(C14 alkyl), -CH(NH2)-
CO016 alkyl, -Si(CH3) 2 tert-butyl, quinolin-yl, Nl -
methyl-imidazol-2-yl, thiophenyl, thiacyclohexyl,
tetrahydrofuranyl, -CS-S-C14 alkyl, -CS-CH2-S-C14
alkyl, -C (=NR) NR11R12; phenyl optionally substituted
with halogen, NO2, NH2, CF3, C - N, COOC1 4 alkyl, COOH,
CONH2, C1 4 alkylthio, C1 4 alkoxy or C1 4 alkyl;
tetrahydropyrimidine, tetrahydropyridine, pyridine,
-S~2-C1-4 alkyl, -SO2-C14 perfluoroalk
R6 has the meaning of R5,
R7 means hydrogen, C18 alkyl, C3 7 cycloalkyl, -CH2-S-C14
alkyl, -(CH2)2-S-C1 4 alkyl, -O-C1-6 alkyl, NH2, NH(C14
alkyl), N(C14 alkyl)2, -S-C14 alkyl, CF3, -C2F5, OH,
phenyl, acidic and basic L-amino acid derivatives, [(5-
nitro-2-pyridyl)amino]-ethylene amino, (2-anilino)-
ethylene amino,
R8, R9 are the same or different and mean hydrogen, C14
alkyl, C14 alkanoyl or together with the nitrogen atom
form a 5- to 6-membered saturated heterocycle, which
can contain another O, N or S atom and can be
substituted in one to two places with C~ 4 alkyl,
R10, R11, R12 and R mean hydrogen, C16 alkyl, C37 cycloalkyl,
phenyl, thienyl,
R15 means C1~7 alkyl optionally substituted with halogen,
NH2, C--N, C~ 4 alkoxy, C~ 4 alkylthio, C~ 4 alkanoyloxy,
thiophene or phenyl; C37 cycloalkyl optionally
substituted with CH3 or phenyl; C16 alkoxy, C16
alkylthio, -COOH, -COOC1-6 alkyl, NR13R14,
R13, R14 are the same or different and mean hydrogen or C~ 4
alkyl optionally substituted with halogen,
R16 R17 are the same or different and mean hydrogen, C~ 4
alkyl, which optionally is substituted with thienyl or
phenyl and in which the phenyl radical can be
substituted in one or two places with halogen,
imidazole, methylenedioxy or C18 alkylene, which is
straight-chain or branched, and in which one or two
methylene groups can be replaced by oxygen and/or
sulfur, or -NR16R17 can form a saturated 5- or 6-
membered heterocycle, which can contain another N, O or
CA 0223~621 1998-04-22
S atom and can be substituted with phenyl, pyridine,
pyridimine, pyridazine or pyrazine,
and their isomers and tautomers and physiologically compatible
salts, whereby
(1) if R1 and * mean hydrogen, and Z = -CH, _AR2 in 2-
position cannot be H, OH; Cl, Br, CF3, -CHO, -O-CO-CH3,
CH2-Br, -CH20H~ - (CH2) 2-OH, --(CH2) 2-Br, -CH=CH2~ --O-CHZ--
CH=CH2, -O-CH2-C-CH, -O-CH2-CN, -SH, -CHOH-CH3, -CH=CH-
phenyl,
(2) if F, Cl, OH, CH3 or NO2 is in 3-position of the phenyl
ring, R1, R3 and -AR2 cannot mean H or imidazole at the
same time,
(3) if R1 = H, and OCH3, OH, NH2, fluorine or CH2-NH2 is in
4-position of the phenyl ring, H, OCH3 or OH cannot be
in 2-position,
(4) if R1 = H, and NO2, Br or OH is in 2-position of the
phenyl ring, Cl or CH3 cannot be in 5-position,
(5) if R1 = H and Z is -CH=, R5 or R6 cannot be optionally
substituted benzyl.
The compounds of formula I and formula IA also comprise the
possible tautomeric forms, the E or Z iosomers, or, if a chiral
center is present, the racemates or enantiomers.
Inorganic and organic acids, such as, for example, oxalic
acid, lactic acid, citric acid, fumaric acid, acetic acid, maleic
acid, tartaric acid, phosphoric acid, HCl, HBr, sulfuric acid, p-
toluenesulfonic acid, methanesulfonic acid, i.a., are suitable
for salt formation.
The inorganic or organic bases that are known for forming
physiologically compatible salts, such as, for example, alkali
hydroxides such as sodium and potassium hydroxide, alkaline-earth
hydroxides such as calcium hydroxide, ammonia, amines such as
ethanolamine, diethanolamine, triethanolamine, N-methyl
glucamine, tris-(hydroxymethyl)-methylamine, etc., are also
suitable for salt formation.
Straight-chain or branched alkylene with 1-4 carbon atoms
contains, e.g., methylene, ethylene, propylene, butylene, 1-
methylmethylene, 1-ethylmethylene, 1-methylethylene, 1-
ethylethylene, l-methylpropylene, 1-propylmethylene, 2-
methylpropylene, i.a.
Straight-chain or branched alkenylene and alkinylene with 2-
4 carbon atoms are defined as alkenyl or alkinyl groups with 1-2
double bonds and/or triple bonds in all possible positions as
well as with all possible methyl or ethyl substitutions.
Alkyl means respectively a straight-chain or branched alkyl
group, such as, e.g., methyl, ethyl, propyl, isopropyl, n-butyl,
sec-butyl, tert-butyl, n-pentyl, sec-pentyl, tert-pentyl,
neopentyl, n-hexyl, sec-hexyl, heptyl, octyl, nonyl, decyl,
whereby 1-6 C atoms are preferred.
As hydrocarbon radical R5, alkyls, alkenyls and alkinyls are
suitable.
As alkyls, the above-described straight-chain or branched
alkyl groups are suitable.
The alkenyl and alkinyl substituents are respectively
straight-chain or branched and preferably contain 2-10 C atoms.
CA 0223~621 1998-04-22
For example, the following radicals can be mentioned: vinyl, 2-
propenyl, l-propenyl, 2-butenyl, 1-butenyl, l-butenyl, 2-butenyl,
1-methyl-1-propenyl, 2-methyl-2-propenyl, 3-methyl-2-propenyl,
ethinyl, 1-propinyl, 2-propinyl, 1-butinyl, 2-butinyl.
If R2 means a heteroaryl radical, a 5- or 6-membered
heteroaryl radical, which can contain one to two N, O or S atoms,
such as, for example, imidazole, thiophene, furan, thiazole,
pyrrole, pyridine, is suitable. The heteroaryl radical can be
substituted in one or more places with C14 alkyl or halogen.
C14 alkyl esters, for example, of histidine, arginine,
aspartic acid, glutamic acid, as well as histamine, asparagine,
glutamine, are defined as L-amino acid derivatives.
In the hydrocarbon radical, a -CH2 group can be replaced by
the radical
and/or by the cycloalkyl radical
~ X
~<
(CH~p Y
with p = O, 1, 2 or 3, and
X, Y = hydrogen or halogen.
Cycloalkyl is defined respectively as cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl or cycloheptyl.
CA 0223~621 1998-04-22
Halogen means respectively fluorine, chlorine, bromine or
iodine.
Hydrocarbon radicals R5 and R6 can be substituted at any
point with up to 3 chlorine, 3 fluorine, bromine or iodine and/or
in one to two places with OH, -O-C14 alkyl, -SH, -S-C14 alkyl,
-NH(C14 alkyl), -N(C14 alkyl)2, -NH2, -NH-CO-(CH2)2-S-C14 alkyl,
--NH-pyridin-2-yl, --C--N,-COOH, -CO-O-C1-6 alkyl, --CO-NH--(C1 4
alkyl), -CO-N-arginine, -CO-CH3, tetrahydrofuranyl, 2,2-dimethyl-
1,3-dioxolan, -S(O)m-C14 alkyl, -NH-C(=NR)-NR11R12, -C(=NH)-NH2,
-C(=NH)-C14 alkyl, -CH(NH2)-COOH, -CH(NHz)-COOC16 alkyl, C3 7
cycloalkyl, NHSO2R, phenyl, which can be substituted in one to
two places with halogen, NO2, -SH, -S-C14 alkyl, -O-C14 alkyl or
phenyl, or a 5- or 6-membered heteroaryl radical, which can
contain 1-3 nitrogen atoms and/or 1-2 sulfur atoms and can be
substituted with C14 alkyl.
Alkyl, alkenyl and alkinyl radicals R1s can be substituted
with the same or different radicals in one to two places or else
are present in perhalogenated form.
If R8, R9 together with the nitrogen atom form a 5- or 6-
membered saturated heterocycle, which can contain another O, N or
S atom and can be substituted with 1-2 C14 alkyl in one or two
places, e.g., morpholine, thiomorpholine, piperidine, piperazine,
pyrrolidine, N-methyl-piperazine or 2,6-dimethylmorpholine thus
are suitable as heterocycles.
If R16R17 together with the nitrogen atom form a saturated 5-
or 6-membered heterocycle, the above-mentioned heterocycles are
suitable.
CA 0223~62l l998-04-22
As heteroaryl radicals in R5 or R6, there can be mentioned,
for example: pyrazole, imidazole, thiophene, thiazole,
thiadiazole, pyridine, pyrimidine and triazine.
Compounds of formula I, which contain the -A-R2 substituents
in 2- and/or 4-position of the phenyl or in 3- and/or 5-position
of the pyridine, whereby -AR2 can be the same or different, can
be viewed as preferred.
Preferred are the following meanings:
R1 = hydrogen,
R2 means hydrogen, halogen, NO2, -C--N, CF3, -CCl3, -S(O)~-
C1 4 alkyl, -oR5, -S--R6, -Co-R7, -NR8R9
-NH-SOz-R10, -C(NHz)=NOH, -NH-CO-NHz, -NH-CS-NH2, NH-CO-
NH(C1 4 alkyl), --NH--CS-NH(C1 4 alkyl), --C(=NR)--NHR11, --NH--
C(=NR)-NR11R12 -S-(C=NR)-NR11R12, a 5- or 6-membered
heteroaryl radical optionally substituted with C14
alkyl or halogen, a phenyl or biphenyl radical
optionally substituted in one or more places with
halogen, C-N, NO2, NHz, CF3, COOH, CONHz, COOC14 alkyl,
--S--C14 alkyl or --O--C14 alkyl; imidazolosulfonyl, --NH--
Co_NR16R17
Especially preferred are combinations in which at least one
radical ARZ is present, in which A means a bond and RZ means an
optionally substituted heteroaryl radical or an optionally
substituted phenyl radical or
in which
A means a bond or straight-chain or branched C14 alkylene
and
CA 0223~621 1998-04-22
R2 is ORs or SR6
As preferred meanings for R5 and R6, there can be mentioned:
Hydrogen, a substituted or unsubstituted, straight-chain or
branched, saturated or unsaturated hydrocarbon radical with 1-14
C atoms, which can contain 1-2 double bonds and/or triple bonds
and in which a -CH2 group can be replaced by oxygen or sulfur,
-Si(CH3)2 tert-butyl, quinolin-yl, Nl-methyl-imidazol-2-yl,
thiophenyl, -CS-S-C14 alkyl, -CS-CH2-S-C14 alkyl, -C(=NR)NR11R12;
phenyl optionally substituted with halogen, NO2, NH2, CF3, C=N,
COOC14 alkyl, COOH, CONH2, C14 alkylthio, C14 alkoxy or C14 alkyl;
tetrahydropyrimidine, tetrahydropyridine, pyridine, --SO2-C14
alkyl, -SO2-C14 perfluoroalkyl.
Straight-chain or branched alkyl with 1-8 C atoms, straight-
chain or branched alkenyl with 2-8 C atoms, straight-chain or
branched alkinyl with 2-8 C atoms can be viewed as a preferred
meaning of -A-R2, whereby one or two of the methylene groups can
be replaced by oxygen and/or sulfur in all oxidation stages. If
a substituent is present, the latter is preferably in terminal
position. Another preferred meaning of AR2 is optionally
substituted phenyl.
The invention also relates to the use of substituted
phenylimidazole derivatives and substituted pyridylimidazole
derivatives, their isomeric and tautomeric forms and their salts
for the production of a pharmaceutical agent for treating or
preventing diseases in which the inhibition of the nitrogen
monoxide synthase is advantageous.
CA 0223~621 1998 - 04 - 22
Used according to the invention as inhibitors of the
nitrogen monoxide synthase are the compounds of formula IA
N R
z~A-3R IA
in which
AR2 can be the same or different in one or two places,
R1 means hydrogen, halogen, -SH or -S-C1 4 alkyl,
Z means nitrogen or -CH=,
A means a bond, straight-chain or branched C1 4 alkylene,
straight-chain or branched C24 alkenylene, straight-
chain or branched Cz 4 alkinylene,
R2 means hydrogen, halogen, NO2, -C-N, CF3, -CCl3, -S(O) m~
C14 alkyl, -oR5, -S-R6, -Co-R7, -NR8R9, -CH(CN)-COOH,
-CH(CN)-COO-C16 alkyl, =C(CN)-COOH, =C(CN)-COOC16
alkyl, -NH-SO2-R1~, -C(NH2)=NOH, -NH-CO-NH2, -NH-CS-NH2,
-NH-CS-NH(C1 4 alkyl), -C(=NR)-NHR11, -NH-C(=NR)-NR11R12,
-S-(C=NR)-NR11R12, a 5- or 6-membered heteroaryl radical
optionally substituted with C1 4 alkyl or halogen, a
phenyl or biphenyl radical optionally substituted in
one or more places with halogen, C--N, NO2, NH2, CF3,
COOH, CONH2, COOC14 alkyl, -S-C14 alkyl or -O-C14 alkyl;
imidazolosulfonyl, -NH-Co-NR16R17,
R3 means hydrogen, halogen, NO2, OH, NH2 or imidazol-1-yl,
AR2 and R3 together form -O-(CH2)n-O-,
12
m, n mean 1 or 2,
R5 means hydrogen, a substituted or unsubstituted,
straight-chain or branched, saturated or unsaturated
hydrocarbon radical with 1-14 C atoms, which can
contain 1-2 double bonds and/or triple bonds, and in
which a -CH2 group can be replaced by oxygen, sulfur,
phenyl or C36 cycloalkyl optionally substituted with
halogen; C37 cycloalkyl optionally substituted with
methyl or halogen; -Co-R15, -CS-NH(C14 alkyl), -CH(NH2)-
CO016 alkyl, -Si(CH3)2 tert-butyl, quinolin-yl, N1-
methyl-imidazol-2-yl, thiophenyl, thiacyclohexyl,
tetrahydrofuranyl, -CS-S-C14 alkyl, -CS-CH2-S-C14
alkyl, -C(=NR)NR11R12, phenyl optionally substituted
with halogen, NO2, NH2, CF3, C-N, COOC~ 4 alkyl, COOH,
CONH2, C14 alkylthio, C~ 4 alkoxy or C~ 4 alkyl;
tetrahydropyrimidine, tetrahydropyridine, pyridine,
-SO2-C14 alkyl, -S02-C~ 4 perfluoroalkyl,
R6 has the meaning of R5,
R7 means hydrogen, C18 alkyl, C37 cycloalkyl, -CH2-S-C14
alkyl, -(CH2)2-S-C14 alkyl, -O-C16 alkyl, NH2, NH(C1 4
alkyl), -N( C~ 4 alkyl)2, -S - C~ 4 alkyl, CF3, -C2F5, OH,
phenyl, acidic and basic L-amino acid derivatives, t(5-
nitro-2-pyridyl)amino]-ethylene amino, (2-anilino)-
ethylene amino,
R8, R9 are the same or different and mean hydrogen, C14
alkyl, C14 alkanoyl or together with the nitrogen atom
form a 5- to 6-membered saturated heterocycle, which
CA 0 2 2 3 ~ 6 2 l 1 9 9 8 - 0 4 - 2 2
can contain another O, N or S atom and can be
substituted in one to two places with C14 alkyl,
R10, R11, R12 and R mean hydrogen, C16 alkyl, C37 cycloalkyl,
phenyl, thienyl,
R15 means C117 alkyl optionally substituted with halogen,
NH2, C-N, C14 alkoxy, C14 alkylthio, C~ 4 alkanoyloxy,
thiophene or phenyl; C37 cycloalkyl optionally
substituted with CH3 or phenyl; C16 alkoxy, C16
alkylthio, -COOH, -COOC1-6 alkyl, NR13R14,
R13, R14 are the same or different ~nd mean hydrogen or C14
alkyl optionally substituted with halogen,
R16 R17 are the same or different and mean hydrogen, C14
alkyl, which optionally is substituted with thienyl or
phenyl and in which the phenyl radical can be
substituted in one to two places with halogen,
imidazole, methylenedioxy or C18 alkylene, which is
straight-chain or branched and in which one or two
methylene groups can be replaced by oxygen and/or
sulfur or -NR16R17 can form a saturated 5- or 6-membered
heterocycle, which can contain another N, O or S atom
and can be substituted with phenyl, pyridine,
pyridimine, pyridazine or pyrazine,
and their isomers and tautomers and physiologically compatible
salts, whereby
R1, -A-R2 and R3 do not mean hydrogen at the same time, for
the production of a pharmaceutical agent for treating diseases
CA 0223~62l l998-04-22
14
that are triggered by the action of nitrogen monoxide at
pathological concentrations.
The compounds of formula I as well as their physiologically
compatible salts can be used as pharmaceutical agents on the
basis of their affinity to and inhibition of the action of the
nitrogen monoxide synthases and here especially the inducible
nitrogen monoxide synthase. on the basis of their profile of
action, the compounds according to the invention are suitable for
treating diseases that are caused or intensified or worsened by
excess nitrogen monoxide in the body under inducing and
pathological conditions.
The above include multiple sclerosis in all forms, dementia
such as, e.g., presenile dementia, Alzheimer's disease and HIV
dementia, Parkinson's disease, Huntington's disease, Korsakoff's
syndrome, stroke, epilepsy, sleep disturbances, schizophrenia,
depression, migraine, hypoglycemia, sepsis or septic shock,
amyotrophic lateral sclerosis and comparable sclerotic diseases,
cerebral ischemia, hypoxia and other neurodegenerative diseases,
which are associated with inflammations. They are also suitable
for treating diseases of the cardiovascular system and for
treating auto-immune and/or inflammatory diseases such as
hypotension, ARDS (adult respiratory distress syndrome),
rheumatoid arthritis, osteoarthritis, insulin-dependent diabetes
mellitus (IDDM), inflammatory disease of the pelvis/intestine
(bowel disease), meningitis, glomerulonephritis, acute and
chronic liver diseases, diseases by rejection (for example,
CA 0223~621 1998-04-22
1~
allogenic heart, kidney or liver transplants) or inflammatory
skin diseases such as psoriasis and others.
To use the compounds according to the invention as
pharmaceutical agents, the latter are put into the form of a
pharmaceutical preparation, which, in addition to the active
ingredient for enteral or parenteral administration, contains
suitable pharmaceutical, organic or inorganic inert vehicles,
such as, for example, water, gelatin, gum arabic, lactose,
starch, magnesium stearate, talc, plant oils,
polyalkyleneglycols, etc. The pharmaceutical preparations can be
present in solid form, for example, as tablets, coated tablets,
suppositories, capsules or in liquid form, for example as
solutions, suspensions or emulsions. They also optionally
contain adjuvants such as preservatives, stabilizers, wetting
agents or emulsifiers, salts for changing the osmotic pressure or
buffers.
For parenteral use, especially injection solutions or
suspensions, especially aqueous solutions of the active compounds
in polyhydroxyethoxylated castor oil, are suitable.
As vehicle systems, surface-active adjuvants, such as salts
of bile acids or animal or plant phospholipids, but also mixtures
thereof as well as liposomes or their components can also be
used.
For oral administration, especially tablets, coated tablets
or capsules with talc and/or hydrocarbon vehicles or binders,
such as for example, lactose, corn or potato starch, are
suitable. The administration can also be carried out in liquid
CA 0223~62l l998-04-22
16
form, such as, for example, as juice, to which optionally a
sweetener is added.
The dosage of the active ingredients can vary depending on
method of administration, age and weight of the patient, type and
severity of the disease to be treated and similar factors. The
daily dose is 1-2000 mg, preferably 20-500 mg, whereby the dose
can be given as a single dose to be administered once or divided
into 2 or more daily doses.
The enzyme nitrogen monoxide synthase has at least three
different isoforms: the endothelial enzyme, the brain enzyme and
the inducible NOS. The NOS-inhibitory action of the compounds of
formula IA and their physiologically compatible salts can be
determined according to the methods of Bredt and Snyder in Proc.
Natl. Acad. Sci. USA (1990) 87, 682--685.
The production of the compound according to the invention is
carried out according to methods known in the art. For example,
compounds of formula I are attained, in that
a) a compound of formula II
~kAR2
WR
ll
in which Z, AR2 and R3 have the above meaning, and Flu represents
a leaving group, is reacted with imidazole optionally substituted
with R1 in the presence of a base
or
CA 0223~621 1998 - 04 - 22
b) a compound of formula III
~N ~ R1 ~N ~' R1
Z ~ A-FIu or Z ~ A-FIu
III
in which A, Z, R1, AR2 and R3 have the above meaning and Flu
represents a leaving group, is substituted in a nucleophilic
manner in the presence of a base and/or with an organometallic
compound,
c) a compound of formula IV
N
Z ~ H
R3
lV
in which Z, R1, R3 have the above meaning, is reacted to
compounds of formula I using Aldol reactions or Wittig reactions
or by nucleophilic attack with Grignard reagents, and it is then
optionally halogenated or selectively reduced, or thioether is
CA 0223~62l l998-04-22
18
formed, or ether is cleaved, or nitriles are saponified, or acids
are esterified or amidated, or ethers or esters are synthesized
from phenolene, or amino groups are exchanged via Sandmeyer
reaction or boiled down, or sulfides are oxidized to sulfones or
sulfoxides, or sulfoxides are reduced to sulfides, or amines are
reacted or alkylated or decarboxylated to amides or sulfonamides,
or isomers or salts are formed.
In this case, Flu can mean, for example, tosylate, mesylate,
triflate, nonaflate or halogen. The aromatic compound is reacted
in the presence of bases at room temperature or elevated
temperature in aprotic solvents. Here and there, the reaction is
facilitated or the yield improved, if copper or copper salts are
added.
As bases, for example, alkali compounds such as potassium
carbonate, sodium hydroxide, alkali alcoholates such as potassium
tert-butylate and especially metal hydrides, such as sodium
hydride, are suitable. At times, the alkali compounds can also
be reacted under phase transfer conditions. If mixtures of
compounds with the substituent imidazolyl-, (2'-R1-imidazolyl-),
-RZ or -R3 are obtained in various numbers and/or in various
positions, the latter are separated in the usual way.
Solvents that are suitable for reaction are aprotic polar
solvents such as dimethylformamide, N-methylpyrrolidone or DMSO.
When the reaction is carried out in a suitable manner, both
the leaving group and an additionally present halogen atom can be
substituted.
CA 0223~621 1998-04-22
19
If the reaction is performed with organometallic compounds,
Grignard compounds optionally under transition metal catalysis,
stannyl compounds or palladium-catalyzed coupling with boric acid
derivatives are suitable.
Halogen or nitro can be introduced by bromation or
nitration, whereby optionally the reactivity of the already
present aromatic substituents can be observed. The framework
optionally must first be nitrated or halogenated before being
reacted with a heterocycle. Then, the nitro group can be
reduced, and the amino group that is produced can be exchanged
via Sandmeyer reaction or boiled down to phenol or alkylated, and
the resulting hydroxy compounds can be etherified or esterified.
By nucleophilic substitution of leaving groups, such as bromides,
thio ethers are produced; these sulfides can then be oxidized to
sulfones or sulfoxides. Nitriles are saponified and optionally
acids are esterified or amidated.
The Aldol reactions are performed according to standard
conditions with malonodinitrile, malonic acid derivatives or
other CH-acid compounds.
Using Wittig reactions or under modified Wittig-Horner
conditions, carbon double bonds are introduced in the ordinary
way.
The nucleophilic attack with Grignard reagents is carried
out in a known way in aprotic solvents such as ether or THF, for
example with alkyl or aryl magnesium bromide.
The optionally subsequent saponification of an ester group
can be carried out in a basic or acidic manner by the reaction
CA 0223~621 1998-04-22
mixture being hydrolyzed in the presence of alkali hydroxides in
ethanol or other alcohols or with the aid of acids, such as,
e.g., hydrochloric acid, at room temperature or elevated
temperature up to boiling temperature, and optionally imidazolium
salts being further processed.
The esterification of the carboxylic acid is carried out in
a way known in the art with diazomethane or the corresponding
alcohol in acid or in the presence of an activated acid
derivative. As activated acid derivatives, for example, acid
chloride, acid imidazolide or acid anhydride are suitable.
The amidation is carried out on free acids or on their
reactive derivatives, such as, for example, acid chlorides, mixed
anhydrides, imidazolides or azides by reaction with the
corresponding amines at room temperature or elevated temperature.
In addition, a nitro group or halogen, especially bromine,
can be introduced by electrophilic aromatic substitution. In
this case, mixtures are produced that can contain substituted
imidazole and can be separated in the usual way. If a nitrile is
present, the latter can be saponified according to known
processes or can be converted to the corresponding amine,
tetrazole or amidoxime.
The reduction of the nitro group or optionally the cyano
group to the amino group is carried out catalytically in polar
solvents at room temperature or elevated temperature under
hydrogen pressure. As catalysts, metals such as Raney nickel or
noble metal catalysts, such as palladium or platinum, optionally
in the presence of barium sulfate or on vehicles, are suitable.
CA 0223~621 1998 - 04 - 22
Instead of hydrogen, ammonium formate can also be used in a known
way. Reducing agents such as tin(II) chloride or titanium(III)
chloride can be used just as complex metal hydrides optionally in
the presence of heavy metal salts. The ester group can
advantageously be introduced before the reduction. Nitro groups
can also be reduced selectively in the usual way with Na2S or
sodium dithionite. The reduction with zinc in acetic acid or
ammonium chloride has proven to be of value.
If an alkylation of an amino group is desired, alkylation
can be carried out according to commonly used methods, for
example, with alkyl halides.
The introduction of the cyano group can be carried out with
the aid of the Sandmeyer reaction; for example, the diazonium
salts that are formed intermediately with nitrites from the amino
compounds can be reacted with alkali cyanides in the presence of
Cu(I) cyanide.
The introduction of the halogens chlorine, bromine or iodine
with the amino group can also be carried out, for example,
according to Sandmeyer, by the diazonium salts that are
intermediately formed with nitrites being reacted with
Cu(I) chloride or Cu(I) bromide in the presence of the
corresponding acid, such as hydrochloric acid or hydrobromic
acid, or being reacted with potassium iodide.
The introduction of an NO2 group is possible by a number of
known nitration methods. For example, nitration can be carried
out with nitronium tetrafluoroborate in inert solvents, such as
halogenated hydrocarbons or in sulfolane or glacial acetic acid.
CA 0223~621 1998-04-22
22
The introduction is also possible by, e.g., nitrating acid in
water or concentrated sulfuric acid as solvent at temperatures of
between 0~C and 30~C.
Amidoximes are produced from the corresponding nitrile with
hydroxylamine hydrochloride, e.g., in alcohol-water mixtures as
solvent.
The activated sulfonic acid derivative, such as, for
example, the sulfonic acid chloride, is reacted in the usual way
with nucleophilic N-derivatives (such as H2N(Cl4 alkyl) or H2N-
CH2coNH2 or H2N-CH2-R)-
The isomer mixtures can be separated into enantiomers or E/Zisomers according to commonly used methods, such as, for example,
crystallization, chromatography or salt formation. As an
alternative, optical isomers can also be produced from the
corresponding optically active compounds as starting material
under reaction conditions which do not cause any racemization.
Intermediate compounds can also be present as enantiomers,
diastereomers, racemates or mixtures thereof.
The production of salts is carried out in the usual way, by
a solution of the compound of formula I being mixed with the
equivalent amount of an acid or excess acid, which is optionally
in solution, and the precipitate being separated, or the solution
being worked up in the usual way.
In so far as the production of starting compounds is not
described, the latter are known or can be produced analogously to
known compounds or processes that are described here.
CA 0223~621 1998-04-22
The following examples are intended to show the structures
according to the invention and their production.
The compounds according to the invention are not limited to
the above-mentioned examples, however.
New compounds were characterized by one or more of the
following methods: melting point, mass spectroscopy, infrared
spectroscopy, nuclear-magnetic resonance spectroscopy (NMR). NMR
spectra were measured with a Bruker 300 MHz device, the
(deuterated) solvents are indicated in each case and abbreviated
as follows: CDCl3 ~chloroform), DMSO (dimethyl sulfoxide).
Shifts are indicated in delta and ppm. In addition, THF means
tetrahydrofuran, DMF means N,N-dimethylformamide, NMP means N-
methylpyrrolidone, and EE means ethyl acetate. All solvents are
of analytical quality, unless otherwise indicated. All reactions
are performed under protective gas, since these can be aqueous
solutions. Mult. means multiplet, several signals; s means
singlet; d means doublet; dd means double doublet, etc.; tr means
triplet; H means hydrogen protons; J means coupling constant; ml
means milliliter; and RT means room temperature. Melting points
are indicated in degrees Celsius and are not corrected. Yields
in percent refer to the educt, not to the conversion.
Below, the production of a few precursors or intermediate
products is described by way of example.
CA 0223~621 1998-04-22
24
Example
4-Bromo-3-bromomethylbenzonitrile
1.96 g of 4-bromo-3-methylbenzonitrile is dissolved in 15 ml
of carbon tetrachloride, mixed with 1.2 equivalents of N-
bromosuccinimide and a spatula tip full of AIBN
(azoisobutyronitrile) and refluxed under irradiation with a 500 W
lamp. After the reaction has been completed, it is concentrated
by evaporation and chromatographed on a 4 cm thick column with
hexane/EE in a gradient. The yield is 35%.
Analogously, different intermediate products are produced
from commercially available methyl aromatic compounds.
Example 2
4-Bromo-(3-methylthiomethyl)benzonitrile
1.37 g of 4-bromo-3-bromomethylbenzonitrile in 8 ml of NMP
is dissolved at 5~, and 0.42 g of sodium thiomethylate, dissolved
in 5 ml of NMP, is slowly added in drops to it at an internal
temperature of 5~. It is stirred for 2 hours at this
temperature, then for another 2 hours at room temperature. It is
dispersed between EE and water, the organic phases are washed
with brine, dried with magnesium sulfate and spun in. Column
chromatography with hexane/EE yields 79% product.
Various benzylthioethers are produced in the same way.
Biphenyl-methylthioethers are also produced in the same way;
for example, 2-bromo-5-phenylbenzyl thiomethyl ether is from 2-
bromo-S-phenylbenzyl bromide.
Example 3
CA 0223~621 1998-04-22
2-Fluoro-(3-methylthiomethyl)pyridine is produced from 2-
~luoro-3-methylpyridine as described above with a yield of 58~.
Various pyridylmethylthioethers are produced in the same way.
Exampl~ 4
2,5-Bis(methylthiomethyl)-bromobenzene
592 mg of 4-(bromomethyl)-2-bromobenzyl bromide is dissolved
in 4.81 ml of NMP and slowly mixed at room temperature with 2.2
equivalents, i.e., 267 mg, of sodium thiomethylate while being
stirred vigorously. After 12 hours, i~ is diluted with water,
extracted with ethyl acetate, the organic phase is dried with
magnesium sulfate and concentrated by evaporation. 370 mg, i.e.,
77.1%, of a slightly colored oil results.
Various aromatic thioethers and bis-thioethers are thus
produced.
Example S
2-Bromobenzyl-ethylthioether
4.62 g of crude product, which is homogeneous in NMR, is
obtained from 15 g of ortho-bromobenzyl bromide with 9 g of
sodium thioethylate, which was added in portions to the educt in
60 ml of NMP while being cooled in a water bath, after 15 hours
of stirring at room temperature and pouring onto water,
extraction with EE, drying and concentration by evaporation. The
yield is 45%.
CA 0223~621 1998-04-22
26
Analogously, various substituted benzylethylthioethers are
obtained from commercially available precursors or precursors
that can be produced in the described way.
Example 6
(2-Chloro-6-fluoro-benzyl)-methylthioether
After 1.2 equivalents of sodium thiomethylate is added after
1 hour at room temperature in 20 ml of dry NMP, 2.6 ml (20 mmol)
of 2-chloro-6-fluoro-benzyl chloride yields 2 new products. The
mixture is poured onto water, extracted with ethyl acetate, the
organic phase is dried with magnesium sulfate and concentrated by
evaporation. 59% of (2-chloro-6-fluoro-benzyl)-methylthioether
and 9.8% of (2-chloro-6-methylthio-benzyl)-methylthioether
result.
Example 7
Benzoic acids are suitably esterified as precursors. Thus,
3-bromo-4-methylbenzoic acid methyl ester is added to 24.94 g of
3-bromo-4-methylbenzoic acid with 19.35 g of potassium carbonate,
12.5 ml of iodomethane in 200 ml of acetone after 3 hours of
reflux, cooled, suctioned off, concentrated by evaporation and
column chromatography with hexane/EE with a yield of 96~.
Example 8
S-(Ortho-bromo-benzyl)-L-cysteic acid ethyl ester
2.5 g of bromobenzyl bromide is dissolved with 1 equivalent
of L--cysteic acid ethyl ester hydrochloride and 2.2 equivalents
27
of potassium carbonate in 10 ml of DMF. After 3 hours at 80~, it
is suctioned off, rewashed with EE, concentrated by evaporation
and column-chromatographed with hexane/EE. 358 mg of product is
obtained.
Bxample 9
2-(2-Bromophenyl)ethanol
15 mmol of 2-bromophenylacetic acid in 15 ml of THF is added
in drops to 15 ml of THF with 2 equivalents of lithium aluminum
hydride. Hydrogen is produced. After the end of the reaction,
it is acidified with dilute sulfuric acid, extracted with EE, the
organic phase is washed with sulfuric acid, then with water, then
with soda solution. It is dried and spun in. The yield is 81%.
In an analogous implementation, 2-(2-chlorophenyl)propanol
(the yield is 96%) and similar reduction products are produced.
~xample 10
3-(2-Chlorophenyl)propanol-tert-butyldimethylsilyl ether
At an internal temperature of 5~, 2.23 g of 3-(2-
chlorophenyl)propanol in 15 ml of NMP with 3.54 g of imidazole
and 2 equivalents of tert-butyldimethylsilyl chloride are mixed
and stirred for 1 hour. Then, stirring is continued for 12 hours
at room temperature. It is shaken out between EE and water,
washed with dilute acid, the organic phase is washed with brine,
dried with magnesium sulfate and spun in. Column chromatography
with hexane provides a 100% yield.
Other precursors are etherified analogously.
CA 0223~621 1998-04-22
28
Below are a few selected examples of the compounds according
to the invention.
Example 11
[2-(1-Imidazolyl)-phenyl]-isobutylthioether
162 mg of 2-(1-imidazolyl)-fluorobenzene in 3 ml of NMP is
stirred with 1.5 equivalents of 2-methyl-1-mercaptopropane and
1.5 equivalents of 80% sodium hydride for 4 hours at 120~. It is
diluted with water, extracted with EE, the organic phase is
washed with brine, it is dried with magnesium sulfate and
concentrated by evaporation. Column chromatography with
hexane/EE 1:1 provides a 76% yield. lH NMR (CDCl3): 7.7(1H),
7.17 to 7.5 (mult. 6H), 2.13(d 2H), 1.8(heptet lH), 0.97(d 6H).
Analogously, there are produced from the substituted
fluorobenzene, i.a.:
Example 12
[2-(1-Imidazolyl)-phenyl]-cyclohexyl ether. The yield is 93%.
[lH]-NMR (acetone d6): 7.81(1H), 7.05 to 7.37 (mult. 6H), 4.44
(mult. lH), 1.2 to 2.0 (mult. lOH).
Example 13
[2-(1-Imidazolyl)-phenyl]-(3-methylbut-1-yl)ether, yield 73%,
oil.
Example 14
[2-(1-Imidazolyl)-phenyl]-tert-butyl ether, yield: 73%. [lH]-
NMR (CDCl3): 7.82(1H), 7.15 to 7.4 (mult. 6H), l.ll(s 9H).
CA 0223~621 1998-04-22
29
Example 15
[2-(1-Imidazolyl)-phenyl]-cyclopentyl ether, yield: 49%. [lH]-
NMR (CDCl3): 7.8(1H), 6.98 to 7.33 (mult. 6H), 4.8 (mult. lH),
1.5 to 0.9 (mult. 8H).
Example 6
[2-(1-Imidazolyl)-phenyl]-(tetrahydrofuran-2-ylmethyl)ether. The
yield is 10%.
Melting point: oil.
Bxample 17
3-Chloro-2-(1-imidazolyl)-pyridine
1.48 g of 2,3-dichloropyridine is dissolved in NMP, and 0.68
g of imidazole as well as 1.66 g of dry potassium carbonate are
added to it. Then, the mixture is heated for 6 hours to 150~.
The mixture is poured onto water, extracted with ethyl acetate,
the organic phase is dried with magnesium sulfate and
concentrated by evaporation. After column chromatography with
EE, 39% product results. [lH]-NMR (CDCl3): 7.2 to 8.5 (mult.
6H).
Bxample 18
2-Nitro-4-methyl-phenyl-1-(1-imidazole)
300 mg of 3-nitro-4-fluoro-toluene is heated in 2 ml of NMP
with 132 mg of imidazole as well as 280 mg of potassium carbonate
(ground) to 100~. After the reaction has been completed, the
mixture is poured onto water, extracted with ethyl acetate, the
CA 0223~621 1998 - 04 - 22
organic phase is dried with magnesium sulfate and concentrated by
evaporation. 98.2% product results. [lH]-NMR (CDC13):
7.61(1H), 7.21(1H), 7.05(1H), 7.80(d lH), 7.52(dd J=8/1.5 Hz,
lH), 7.33(d J=8Hz lH), 2.52(s 3H).
Example 18a
[5-Chloro-2-(1-imidazolyl)-phenylmethyl]-methylthioether
consisting of [2-bromo-5-chloro-phenylmethyl]-methylthioether.
The yield is 15%.
Example 19
[3-Trifluoromethyl-2-(1-imidazolyl)-phenylmethyl]-methylthioether
448 mg of (2-fluoro-3-trifluoromethyl-benzyl)methylthioether
is heated with 1 equivalent of imidazole and 1 equivalent of
powdered potassium carbonate as well as 30 mg of copper powder
for 2 hours to 200~. It is diluted with EE, suctioned off, and
the mother liquor is concentrated by evaporation. After column
chromatography with hexane with increasing addition of EE, 240 mg
of product is obtained. The yield is 44%. [lH]-NMR (CDC13):
7.7 (mult. 2H), 7.54 (mult. 2H), 7.21 (singlet lH), 7.05(1H),
3.24(s 2H), 2.0(s 3H).
Under the above-described conditions of the reactions with
imidazole are obtained the compounds claimed from fluorine,
chlorine or bromine aromatic compounds, a few examples of which
are mentioned below.
-
CA 0223~621 1998-04-22
Example 20
1-[5-Chloro-2-(1-imidazolyl)-phenyl]-ethanol, yield: 25%, oil.
Example 21
2-Bromo-3-fluoro-phenyl-(1-imidazole), yield: 8%, melting point:
52~.
Example 22
2-Hydroxymethyl-3-fluoro-phenyl-(1-imidazole), yield: 11~, lH-
NMR (DMS0 d6): 7.96(1H), 7.5(1H), 7.1(1H), 7.25 to 7.5 (in
addition to 3H), 4.3(s 2H).
Example 23
[4-(1-Imidazolyl)-phenyl]-3-chlorophenol, yield: 80%, lH-NMR
(CDCl3): 7.7(1H), 7.22(1H), 7.09(1H), 7.30(d lH), 6.86(dd lH),
7.01(d lH).
Example 24
1-[2-(1-Imidazolyl)-4-chloro-phenyl]-ethanol, yield: 22%, lH-NMR
(DMS0 d6): 7.82(1H), 7.4(1H), 7.1(1H), 7.7 (mult. 3H), 4.5
(mult. lH), 5.3(s broad lH), 1.2(d 3H).
Example 25
2-Cyano-5-fluoro-phenyl-1-(1-imidazole), yield: 28%, lH-NMR
(CDCl3): 7.9(1H), 7.85(1H), 7.38(1H), 7.22 (mult. 3H).
32
Example 26
2-Cyano-5-(1-imidazolyl)-phenyl-1-(1-imidazole), yield: 7%, lH-
NMR (CDCl3): 7.97(1H), 7.79(1H), 7.31(1H), 7.23(1H), 6.98(1H),
6.91(1H), 7.68(d lH), 7.58(d lH), 7.40(dd lH).
Ex mple 27
2-Hydroxy-3-(1-imidazolyl)-4-methoxy-phenyl-1-(1-imidazole),
yield: 17%, melting point 253~.
Example 28
2-(1-Imidazolyl)-5-nitrophenol, yield: 12%, lH-NMR (DMS0 d6):
8.14(1H), 7.1(1H), 7.6(1H), 7.88(d lH), 7.80(dd lH), 7.69(d lH).
Ex~mple 29
2-(1-Imidazolyl)-6-methyl-phenyl]-methyl ether, yield: 99%, lH-
NMR (CDCl3): 7.2 (mult. 5H), 7.82(1H), 3.38(s 3H), 2.36(s 3H).
Example 30
2-Methoxy-4-methoxy-phenyl-1-(1-imidazole), yield: 51%, lH-NMR
(CDCl3): 6.5 to 7.8 (mult. 6H), 3.80(s 3H), 3.83(s 3H).
Example 31
2-Methyl-4-methoxy-phenyl-1-(1-imidazole), yield: 60%, melting
point: 47~.
_
:
33
Example 32
2-Methoxy-5-methoxy-phenyl-1-(1-imidazole), yield: 50%, lH-NMR
(DMS0 d6): 7.0 to 8.0 (mult. 6H), 3.78(s 3H), 3.80(s 3H).
Example 33
2-Methoxy-4-nitro-phenyl-1-(l-imidazole), yield: 7%, melting
point: 136~.
Example 34
2~Methoxy-5-(imidazol-1-yl)-phenyl-1-(1-imidazole), yield: 10%,
melting point: 116~.
Example 35
2-Methoxy-5-tert-butyl-phenyl-1-(1-imidazole), yield: 21%,
melting point: 79~.
Example 36
2-Trichloromethyl-3-fluoro-phenyl-1-(1-imidazole), yield: 7%,
melting point: oil.
Exampl~ 37
2-Nitro-5-cyano-phenyl-1-(1-imidazole), yield: 78~, melting
point: 161~, lH-NMR (CDCl3): 7.6(lH), 7.12(lH), 7.0(lH), 8.22(d
lH), 7.96(dd lH), 7.59(1H).
CA 0223~621 1998-04-22
34
Example 38
2-Nitro-5-chloro-phenyl-1-(1-imidazole), yield: 51%, lH-NMR
(CDC13): 7.05(1H), 7.22(1H), 7.63(1H), 8.0(d lH), 7.6(dd lH),
7.49(d lH,J=1.5Hz).
Example 39
2-Nitro-4-methoxy-phenyl-1-(1-imidazole), yield: 35%, melting
point: 98~.
Example 40
2-Thiomethyl-5-nitro-phenyl-1-(1-imidazole), yield: 37%, melting
point: 225~.
Bxample 41
(2-t2-(1-Imidazolyl)-phenyl]ethyl)-methylthioether, yield: 29%,
melting point: 79~.
Example 42
6-Chloro-2-(1-imidazolyl)-acetophenone, yield: 11%, lH-NMR
(CDC13): 7.74(1H), 7.13(1H), 6.1(1H), 7.2 (mult. 2H), 7.27(1H),
1.55(s 3H).
Exampl~ 43
(rac)-[2-(1-Imidazolyl)-phenylmethyl]-(l-phenyl-ethyl)-thioether,
yield: 38%, lH-NMR (CDC13): 7.1 to 7.62 (mult. 12H), 3.3 (mult.
2H), 3.89 (~uartet lH), 1.51(d 3H).
CA 0223~621 1998-04-22
Example 44
[2~ Imidazolyl)-phenylmethyl]-ethylthioether, yield: 82%, lH-
NMR (CDCl3): 7.72(1H), 7.22(1H), 7.2(1H), 7.23 to 7.5 (mult.
4H), 3.52(s 2H), 2.47 (quartet 2H), 1.2(tr3H).
Example 45
[2-(1-Imidazolyl)-phenylmethyl]-isopropylthioether, yield: 58%,
lH-NMR (CDCl3): 7.72(1H), 7.2 to 7.5 (mult. 6H), 3.53(s 2H), 2.8
(mult. lH), 1.2(lH), 1.22 each (d 3H).
Example 46
[2-(1-Imidazolyl)-phenyl]-methylthioether, yield: 65%, melting
point: 208~.
Example 47
[2-(1-Imidazolyl)-phenylmethyl]-methylthioether, yield: 59%, lH-
NMR (CDCl3): 7.72(1H), 7.22 2H(lH), 7.38 (mult. 2H), 7.45 (mult.
2H), 3.50(s 2H), 2.01(s 3H).
Example 48
2-Fluoro-4-nitro-phenyl-1-(1-imidazole), yield: 84%, lH-NMR
(CDCl3): 7.95(1H), 7.36(1H), 7.29(1H), 7.63d(dd lH), 8.2 (mult.
2H).
Ex~mple 49
2-Fluoro-phenyl-1-(1-imidazole), yield: 59%, oil.
Example 50
CA 0223~621 1998-04-22
J~
3-Chloro-4-methoxy-phenyl-1-(1-imidazole), yield: 12%, lH-NMR
(CDC13): 7.75(1H), 7.18(1H), 7.18(1H), 7.0 to 7.42 (in addition
to 3H), 3.95(s 3H).
Example 51
2-Nitro-4-chloro-phenyl-1-(1-imidazole), yield: 53%, lH-NMR
(CDC13): 7.62(1H), 7.07(1H), 7.23(1H), 8.02(d lH), 7.75(dd lH),
7.44(d lH)-
Example 52
2-Nitro-4-(1-imidazolosulfonyl)-phenyl-1-(1-imidazole), yield
65%, oil.
Example 53
2-(1-Imidazolyl)-4-bromobiphenyl, yield: 23%, lH-NMR (CDC13):
7.34 (mult. 5H), 7.08 (mult. 3H), 7.63(dd lH), 7.56(d lH),
6.8(lH)
Example 54
[4-(1-Imidazolyl)-phenyl)]-methylthioether, yield: 65%, melting
point: 93~.
Example 55
[3-(1-Imidazolyl)-phenyl)]-methylthioether, yield: 69%, lH-NMR
(CDC13): 7.85(1H), 7.1-7.4 (mult. 6H), 2.52(s 3H).
Example 56
CA 0223~621 1998-04-22
3-Chloro-4-nitro-phenyl-1-(1-imidazole), yield: 17%, lH-NMR
(CDCl3): 7.29(1H), 7.35(1H), 7.95(1H), 8.47(dd lH), 8.11d8(1H),
7.62(d lH J=1.5Hz).
Example 57
2-Bromo-phenyl-l-(l-imidazole), yield: 29%, melting point: 55~.
Example 58
3-Fluoro-4-cyano-phenyl-1-(1-imidazole), yield: 16~, lH-NMR
(CDCl3): 7.95(lH), 7.78(lH), 7.3 (mult. 4H).
Example 59
2-Fluoro-4-(1-imidazolyl)-biphenyl, yield: 11%, lH-NMR (CDCl3):
7.5 (mult. 6H), 7.27 (mult. 4H), 7.92(s lH).
Example 60
3-Methoxy-4-methoxy-phenyl-1-(1-imidazole), yield: 44%. lH-NMR
(DMSO d6): 8.12(1H), 7.64 to 7.1(5H), 3.82(s 3H), 3.87(s 3H).
Example 61
[3-(1-Imidazolyl)-benzyl)]-methylthioether,
yield: 54%. lH-NMR (CDCl3): 7.9(1H), 7.3 to 7.5 (mult. 6H),
3.72(s 2H), 2.03(s 3H).
-
38
Example 623,4 Methylenedioxyphenyl-1-(1-imidazole), yield: 42%, melting
point: 84~.
Example 63
t(2-(1-Imidazolyl)-phenyl)-1-ethyl)]-methylthioether, yield:
58%. lH-NMR (CDCl3): 7.1 to 7.62 (mult. 12H), 3.3 (mult. 2H),
3.89 (quartet lH), 1.51(d 3H).
Example 64
2-(1-Imidazolyl)-3-trifluoromethyl-pyridine, yield: 18%, lH-NMR
(CDCl3): 7.2 to 8.8 (mult. 6H).
For example, the following compounds are obtained in the
same way preferably from the corresponding substituted chlorine
aromatic compounds:
Example 65
2-(1-Imidazolyl)-3-bromopyridine. Yield: 37%. Melting point:
87.8~.
Ex~mple 66
[6-Methylthio-2-(1-imidazolyl)-phenylmethyl]-methylthioether,
yield: 72%, lH-NMR (CDCl3): 7.74(1H), 7.05 to 7.33 (mult. 5H),
3.74(s 2H), 2.57(s 3H), 2.09(s 3H).
CA 0223~621 1998-04-22
39
Exampl~ 67
[4-Chloro-2-(1-imidazolyl)-phenylmethyl]-methylthioether, yield:
9.1%. Oil.
Example 68
[5-Phenyl-2-(1-imidazolyl)-phenylmethyl]-methylthioether, yield:
16%. Melting point: 123.8~.
Example 69
[4--Methoxy-2-(1-imidazolyl)-phenylmethyl]-methylthioether, yield:
19%. lH--NMR (CDC13): 7.71(1H), 7.38(1H), 7.20(s 2H), 6.96(dd
lH), 6.78(d lH), 3.82(s 3H), 3.44(s 2H), 2.01(s 3H).
Example 70
2,6 Dichloro-4-nitro-phenyl-1-(1-imidazole), yield: 71%.
Melting point: 173.6~.
Example 71
3-[2-(1-Imidazolyl)-phen-l-yl]propyl-methylthioether, yield:
12%, lH--NMR (CDC13): 7.60(1H), 7.21(1H), 7.06(1H), 7.2 to 7.47
(mult. 4H), 2.6 (mult. 2H), 2.4 (mult. 2H), 2.02(s 3H), 1.71
(mult. 2H).
Example 72
[2-Chloro-4-(1-imidazolyl)-phenylmethyl]-methylthioether, yield:
5.1%. Oil.
CA 0223~621 1998-04-22
For example, the following compounds are obtained in the
same way preferably from the corresponding substituted fluorine
aromatic compounds:
Example 73
5-Nitro-2-(1-imidazolyl)-benzaldehyde. Yield: 39%. lH-NMR
(CDCl3): 9.93(s broad lH), 8.91(1H), 8.59(dd lH), 7.81s(1H),
7.68(1H), 7.37(1H), 7.30(1H).
Example 74
[4-Trifluoromethyl-2-(1-imidazolyl)-phenylmethyl]-
methylthioether, yield: 8%. lH-NMR (CDCl3): 7.20 to 7.72
(mult. 6H), 3.51(s 2H), 2.03(s 3H).
Example 75
5-Methyl-2-nitro-phenyl-1-(1-imidazole). Yield: 99%. lH-NMR
(CDCl3): 7.62(lH), 7.21(lH), 7.06(lH), 7.95(d 8Hz, lH), 7.40(dd
lH), 7.25(d lH), 2.50(s 3H).
Example 76
4-Methyl-2-nitro-phenyl-1-(1-imidazole). Yield: 98.2%8751H-NMR
(CDCl3): 7.61(1H), 7.21(1H), 7.05(1H), 7.80(d lH), 7.52(dd lH),
7.33(d 8H), 2.52(s 3H).
Example 77
t3-(Methylthiomethyl)-2-(1-imidazolyl)-phenylmethyl]-
methylthioether. Yield: 7.5%. lH-NMR (CDCl3): 7.62(1H), 7.4
(mult. 3H), 7.25(1H), 7.07(1H), 3.30(s 4H), 2.01(s 6H).
4 1
Example 78
[3-Trifluoromethyl-2-(1-imidazolyl)-phenylmethyl]-
methylthioether. Yield: 44%. lH-NMR (CDCl3): 7.7 (mult. 2H),
7.54m2(lH), 7.21 (singlet lH), 7.05(lH), 3.24(s 2H), 2.0(s 3H).
Example 79
2-Cyano-6-trifluoromethyl-phenyl-1-(1-imidazole). Yield: 43%.
H-NMR (CDCl3): 8.10(lH), 8.03(lH), 7.80(lH), 7.64(lH), 7.32(lH),
7.13(lH).
Example 80
[2-(1-Imidazolyl)-pyridine-3-methyl]-methylthioether, yield:
45%, lH-NMR (CDCl3): 8.06(lH), 7.5(lH), 7.20(lH), 8.47 ml(lH),
7.87 (mult. lH), 7.35 (mult. lH), 3.63(s 2H), 2.09(s 3H).
Example 81
5-Nitro-2-(1-imidazolyl)-phenylmethyl]-methylthioether. Yield:
20%, lH-NMR (DMS0 d6): 8.4(1H), 8.24(1H), 7.79(1H), 7.47(1H),
7.27(dd 2H).
Example 82
2-(1-Imidazolyl)-3-trifluoromethyl-phenol. Yield 69%, melting
point: oil.
Example 83
5-Fluoro-2-nitro-phenyl-1-(1-imidazole), melting point 78-84~.
CA 0223~621 1998-04-22
42
Also, the following compounds are obtained in the same way
preferably from the corresponding substituted bromine aromatic
compounds:
Examplc 84
[4-(Methylthiomethyl)-2-(1- imidazolyl)--phenylmethyl]-
methylthioether, yield: 48%. Melting point: oil.
Example 85
[5-(Methylthiomethyl) -2- (l-imidazolyl)-phenylmethyl~--
methylthioether, yield: 38%. Melting point: oil.
Example 86
[5-Cyano-2-(1-imidazolyl)-phenylmethyl]-methythioether. Yield:
34%, lH-NMR (CDCl3): 7.22 to 7.82 (mult. 6H), 3.53(s 2H), 2.09(s
3H).
Example 87
2-E-[2-(1-Imidazolyl)-phenyl]-acrylic acid ethyl ester. Yield:
33%. lH-NMR (CDCl3): 7.1 to 7.8 (mult. 8H), 6.39 (d lH,
J=16Hz), 4.22 (quartet 2H), 1.29(tr 3H).
Example 88
2-(1-Imidazolyl)-styrene. Yield: 11%. lH-NMR (CDCl3): 7.7 to
7.20 (mult. 6H), 7.10(s lH), 6.44(dd lH olef.), 5.75(d lH),
5.32(d lH).
CA 0223~621 1998-04-22
43
Example 89
[4,5--Dimethoxy--2-(1--imidazolyl)-phenylmethyl]--methylthioether.
Yield: 22%. Melting point: oil.
Example 90
6-Methyl-2-nitro-phenyl-1-(1-imidazole). Yield 31%. Melting
point: oil.
Example 91
[5-Methoxy-2-(1-imidazolyl)-phenylmethyl]-methylthioether.
Yield: 61%. lH--NMR (CDCl3): 7.63(1H), 6.84 to 7.70 (mult. 5H),
3.86(s 3H), 3.41(s 2H), 2.02(s 3H).
Example 92
[5-Bromo-2-(1-imidazolyl)-benzoic acid methyl ester. Yield:
61%. Melting point: 68~.
Example 93
S-Ethyl-thiocarboxylic acid-0-[2-(1-imidazolyl)-phenyl]--ester
400 mg of 2-(1-imidazolyl)-phenol is dissolved in 5 ml of
NMP, mixed with 1.1 equivalents of 80% sodium hydride (in oil)
and then stirred for 30 minutes at 30~. While being cooled, 1.1
equivalents of chlorothioformic acid-S-ethyl ester is introduced
and stirred for another 2 hours at room temperature. The mixture
is poured onto water, extracted with ethyl acetate, the organic
phase is dried on magnesium sulfate and concentrated by
evaporation. After column chromatography, 84% (520 mg) of
44
product results. lH-NMR (CDCl3): 7.68(1H), 7.18(1H), 7.10(1H),
7.3 to 7.5 (mult. 4H), 2.84 (quartet 2H), 1.28(tr 3H).
Bxample 94
Cyclobutanecarboxylic acid-[2-(1-imidazolyl)-phenyl]-ester
1.1 equivalents of 80% sodium hydride (in oil) is added to
0.25 g of 2-(1-imidazolyl)-phenol in 3 ml of DMF, and the mixture
is then stirred for 30 minutes at 30~. 1 equivalent of
cyclobutanecarboxylic acid chloride is slowly added and stirred
overnight at room temperature. The mixture is poured onto water,
extracted with ethyl acetate, the organic phase is dried with
magnesium sulfate and concentrated by evaporation. Column
chromatography with EE/ethanol 9:1 yields 46~ product. lH-NMR
(CDCl3): 7.1 to 7.65 (mult. 7H), 3.24 (pentet lH), 1.8 to 2.3
(mult. 6H).
Quite analogously, the esters and ethers that are mentioned
below are obtained:
Example 94a
1-t2-(1-Imidazolyl)-phenyl]-(2-methoxy-ethyl)ether, yield: 40%.
lH-NMR (DMS0 d6): 7.1 to 7.96 (mult. 7H), 3.3(s 3H), 3.65 (mult.
2H), 4.20 (mult. 2H).
Example 94b
[2-(1-Imidazolyl)-phenyl]-(methylthiomethyl)-ether, yield: 13%.
oil .
CA 0223~621 1998-04-22
Example 95
[2-(1-Imidazolyl)-phenyl]-~3-methyl-but-2-enyl)ether, yield:
62%, lH-NMR (CDC13): 7.02 to 7.8 (mult. 7H), 4.52(d 2H), 5.37
(mult. lH), 16.8(s 3H), 1.75(s 3H).
Example 96
t2-(1-Imidazolyl)-phenyl]-(2-chloro-prop-2-enyl)ether, yield:
51%. lH-NMR (CDC13): 7.92(1H), 7.4(1H), 7.05(1H), 7.1 to 7.4
(mult. 4H), 4.8(s 2H), 5.5(1H), 5.65(1H).
Bxample 97
[2-(1-Imidazolyl)-phenyl]-(E-but-2-enyl)ether, yield: 40%.
Melting point: oil.
Example 98
[2-(1-Imidazolyl)-phenyl]-allyl ether, yield: 55%, melting
point: oil.
Example 99
[2-(1-Imidazolyl)-phenyl]-(3-chloro-but-2-enyl)ether, yield:
73%.
Example 100
[2-(1-Imidazolyl)-phenyl]-([cyclopropyl]methyl)ether, yield:
25%. Melting point: oil.
CA 0223~621 1998-04-22
~o
Example 101
[2~ Imidazolyl)-phenyl]-(2,2-dichloro-2,1,1-trifluoroeth-1-
yl)ether, yield: 52%. Melting point: oil.
Example 102
[2-(1-Imidazolyl)-phenyl]-(2-neopentyl)ether, yield: 44%. lH-
NMR (acetone d6): 7.8(1H), 7.33(1H), 7.05(1H), 7.2 (mult. 3H),
3.75(s 2H), O.95(s 9H).
Example 103
t2-(1-Imidazolyl)-phenyl]-(2-methylthioeth-1-yl)ether, yield:
42~. lH-NMR (CDCl3): 7.81(1H), 7.16(1H), 7.3(1H), 7.3 (mult
lH), 7.05 (mult. 2H), 4.2 (mult. 2H), 2.82 (mult. 2H); 2.10
(mult. 3H).
Example 104
[2-(1-Imidazolyl)-phenyl]-(2-bromo-prop-2-enyl)ether, yield: 44%
melting point: oil.
Example 105
[4-Chloro-2-(1-imidazolyl)-phenyl]-(2-chloro-prop-2-enyl)ether,
yield: 47%, lH-NMR (CDCl3): 7.8(1H), 7.2 to 7.3 (mult. 4H),
6.98(1H), 4.6(s 2H), 5.45(s 2H).
Example 106
[3-Methyl-4-(1-imidazolyl)-phenyl]-(2-chloro-prop-2-enyl)ether,
yield: 49%, melting point: oil.
CA 0223~621 1998-04-22
47
Bxample 107
[2~ Imidazolyl)-phenyl]-(2,2-spirot2',2'-dichlorocyclopropyl]-
prop-1-yl)ether, yield: 51%, melting point: oil.
Ex~mple 108
[2-(1-Imidazolyl)-phenyl]-[(2,2-dichloro-3-methyl)cycloprop-1-
yl]meth-1-yl)ether, yield: 34%, oil.
Example 109
[2-(1-Imidazolyl)-phenyl]-4-[lH]quinolinyl)ether, yield: 17%,
oil.
Example 110
[2-(1-Imidazolyl)-phenyl]-(1,1-dimethyl-1-acetoxyacetic
acid)ester, yield: 69%. Melting point: oil.
Example llOa
[4-(Imidazol-1-yl)-3-methylthiomethyl-phenol]-heptanecarboxylic
acid ester, 92%.
Example 111
[2-(1-Imidazolyl)-phenyl]-cyclohexanecarboxylic acid ester,
yield: 84%, melting point: 68~.
CA 0223~621 1998-04-22
48
Example 112
[2-(1-Imidazolyl)-phenyl]-n-hexanecarboxylic acid ester, yield:
78%, lH-NMR (acetone d6): 7.7(1H), 7.07 to 7.55 (mult. 6H), 0.9
to 2.50 (mult. 13H).
Example 113
t2-(1-Imidazolyl)-phenyl]-n-heptadecanecarboxylic acid ester,
yield: 52%, melting point: 101~.
Example 114
[2-(1-Imidazolyl)-phenyl]-perfluoropropanecarboxylic acid ester,
yield: 23%, lH-NMR (CDCl3): 8.5(1H), 8(1H), 7.77(1H), 7.3
(mult. 4H).
Example 115
[2-(1-Imidazolyl)-phenyl]-N,N-dimethylcarbamic acid ester, yield:
27%, lH-NMR (CDCl3): 7.73(lH), 7.09 to 7.51 (mult. 6H), 2.85(s
3H), 3.0(s 3H).
Example 116
[2-(1-Imidazolyl)-phenyl]-N-(2-chloroethyl)-N-methylcarbamate,
yield: 55%, lH-NMR (CDCl3): 7.64(lH), 7.1 to 7.4 (mult. 6H),
3.5 (mult. 4H), 3.01(s 3H).
CA 0223~621 1998-04-22
49
Example 117
[2-(1-Imidazolyl)-phenyl]-decanoic acid ester, yield: 98%, lH-
NMR (CDCl3): 7.68(1H), 7.18(1H), 7.10(1H), 7.24 to 7.44 (mult.
4H), 2.42(dd 2H), 1.28 (mult. 14H), 0.89(tr 3H).
Example 118
t2-(1-Imidazolyl)-phenyl]-cyclopropanecarboxylic acid ester,
yield: 44%, lH-NMR (CDCl3): 7.68(1H), 7.18(lH), 7.10(1H), 7.28
to 7.48 (mult. 4H), 1.7 (mult. lH), 1.0 (mult. 4H).
Example 119
[2-(1-Imidazolyl)-phenyl]-isobutyric acid ester, yield: 97%, lH-
NMR (CDCl3): 7.68(1H), 7.18(1H), 7.10(1H), 7.20 to 7.50 (mult.
4H), 2.67 (heptet lH), l.ll(d 6H).
Example 120
[2-(1-Imidazolyl)-phenyl]-3-methylbutyric acid ester, yield:
72%, lH-NMR (CDCl3): 7.68(1H), 7.18(1H), 7.10(1H), 7.24 to 7.44
(mult. 4H), 2.31(d 2H), 2.07 (heptet lH), O.91(d 6H).
Example 121
[2-(1-Imidazolyl)-phenyl]-omega-chloro-pentanoic acid ester,
yield: 97%, melting point: 218.6~.
-
CA 0223~621 1998-04-22
Example 122
[2-(1-Imidazolyl)-phenyl]-3-phenylpropionic acid ester, yield:
96%, lH-NMR (CDCl3): 7.05 to 7.69 (mult. 6H), 2.95 (mult. 2H),
2.7 (mult. 2H).
Example 123
[2-(1-Imidazolyl)-phenyl]-(tert-butylacetic acid)ester, yield:
80%, melting point: 111~.
Example 124
[2-(1-Imidazolyl)-phenyl]-(trans-phenylcyclopropanecarboxylic
acid)ester, yield: 70%, melting point: oil.
Example 125
[2-(1-Imidazolyl)-phenyl]-([1-methylthio]acetic acid)ester,
yield: 17%, lH-NMR (CDCl3): 7.72(1H), 7.2(1H), 7.14(1H), 7.22
to 7.52 (mult. 4H), 3.3(s 2H), 2.1(s 3H).
Example 126
[2-(l~Imidazolyl)-phenyl]-oxalic acid semi-ester, yield: 42%,
melting point: oil.
Example 127
[2-(1-Imidazolyl)-phenoxy]-acetonitrile
500 mg (3.086 mmol) of 2-[2-(1-imidazolyl)-phenol is
dissolved in 15 ml of anhydrous DMF and mixed with 185.2 mg
(26.172 mmol) of an 80% NaH suspension. After 1 and 1/2 hours of
CA 0223~62l l998-04-22
51
stirring at room temperature, 404.7 mg (3.373 mmol) of
bromoacetonitrile is added, and the batch is stirred overnight at
room temperature. The mixture is added to 100 ml of H20 and
extracted 3 times with 70 ml of ethyl acetate each. The combined
organic extracts are washed twice with 50 ml of saturated NaCl
solution each, dried on NaS04, and the organic solvent is spun
off. After chromatography of the crude product on SiO2
(EE/hexane ~ EE as mobile solvent), 365.4 mg (58.8%) of the
desired product is obtained.
Example 128
4-[2-(1-Imidazolyl)-phenoxy]-butyric acid nitrile
500 mg (3.086 mmol) of 2-(1-imidazolyl)-phenol is reacted
with 499.2 mg (3.373 mmol) of 4-bromobutyric acid nitrile
analogously to Example 127. After the above-described working-up
and chromatography, 445 mg (62.7%) of product is obtained.
Example 129
5-[2-(1-Imidazolyl)-phenoxy]-pentanoic acid nitrile
500 mg (3.086 mmol) of 2-(1-imidazolyl)-phenol is reacted
with 546.4 mg (3.373 mmol) of 5-bromophentanoic acid nitrile as
described in Example 127. After working-up and chromatography,
752.9 mg (99.9%) of product is obtained.
CA 0223~621 1998-04-22
:~G
Example 130
6-[2-(1-Imidazolyl)-phenoxy]-hexanoic acid nitrile
500 mg (3.086 mmol) of 2-(1-imidazolyl)-phenol is reacted as
usual with 593.6 mg (3.373 mmol) of 6-bromohexanoic acid nitrile.
After working-up and chromatography, 750.8 mg (94.2%) of product
is obtained.
Bxample 131
7-[2-(1-Imidazolyl)-phenoxy]-heptanoic acid nitrile
500 mg (3.086 mmol) of 2-(1-imidazolyl)-phenol is reacted as
usual with 640.9 g (3.373 mmol) of 7-bromoheptanoic acid nitrile.
After working-up and chromatography, 810 mg (96.4%) of product is
obtained.
Example 132
6-[2-(1-Imidazolyl)-phenoxy]-2,2-dimethylhexanoic acid nitrile
100 mg (0.617 mmol) of 2-(1-imidazolyl)-phenol is reacted as
usual with 138.5 mg (0.679 mmol) of 2,2-dimethyl-6-bromohexanoic
acid nitrile. After working-up and chromatography, 103 mg
(58.2%) of product is obtained.
Example 133
2-t2-(1-Imidazolyl)-phenoxy]-acetic acid-tert-butyl ester
100 mg (0.617 mmol) of 2-(1-imidazolyl)-phenol is reacted
with NaH and bromoacetic acid-tert-butyl ester in DMF analogously
to Example 127. After working-up and chromatography, 84.9 mg
(49.6%) of product is obtained.
CA 0223~621 1998-04-22
53
Example 134
4-[2-(1-Imidazolyl)-phenoxy]-butyric acid ethyl ester
2 g (12.344 mmol) of 2-(1-imidazolyl)-phenol is reacted with
NaH and 4-bromobutyric acid ethyl ester in DMF analogously to
Example 127. After working-up and chromatography, 1.23 g (36%)
of product is obtained.
Example 135
5-[2-(1-Imidazolyl)-phenoxy]-pentanoic acid methyl ester
2 g (12.344 mmol) of 2-(1-imidazolyl)-phenol is reacted with
NaH and 5-bromopentanoic acid methyl ester in DMF analogously to
Example 127. After the usual working-up, 2.7 g (75.1%) of
product is isolated.
Example 136
6-[2-(1-Imidazolyl)-phenoxy]-hexanoic acid ethyl ester
2 g (12.344 mmol) of 2-(1-imidazolyl)-phenol is reacted with
6-bromohexanoic acid ethyl ester and NaH in DMF as already
described (Example 127). The product is isolated with a yield of
59.2% (2.32 g).
Example 137
7-[2-(1-Imidazolyl)-phenoxy]-heptanoic acid ethyl ester
1 g (6.172 mmol) of 2-(1-imidazolyl)-phenol is reacted with
7-bromoheptanoic acid-ethyl ester and NaH in DMF according to
Example 127. After working-up, the yield is 0.416 g (31.3%).
54
Example 138
2-[4-(1-Imidazolyl)-phenoxy]-acetic acid methyl ester
4 g of 4-(imidazol-1-yl)phenol, 4.8 ml of bromoacetic acid
methyl ester and 6.5 g of cesium carbonate are added to 50 ml of
DMSO. After 1 day of stirring, the reaction mixture is added to
water and extracted with methylene chloride. The organic phase
is separated, washed with water, dried, and the solvent is drawn
off in a vacuum. After recrystallization from petroleum ether,
the title compound with a melting point of 116-117~C is obtained.
Example 139
3-[4-(1-Imidazolyl)-3--(methylthiomethyl)-phenoxy]-butyricacid
nitrile
2 equivalents of 80% sodium hydride (in oil) is added to
0.15 g of 4-(1-imidazolyl)-3-(methylthiomethyl)-phenol in 3.5 ml
of DMF, and, after being stirred briefly at room temperature,
1.08 equivalents of 4--bromobutyronitrileis slowly added.
Overnight, it is stirred at room temperature. The mixture is
poured onto water, extracted with ethyl acetate, the organic
phase is dried with magnesium sulfate and concentrated by
evaporation. Column chromatography with EE yields 81% product.
lH--NMR (CDCl3): 7.62(1H), 6.82 to 7.2 (mult. 5H), 4.14(tr 2H),
(lH), 2.62(tr 2H), 3.41(s 2H), 2.2m2(lH), 2.05(s 3H).
Analogously, there are obtained:
Example 140
1-[4-(1-Imidazolyl)-3-(methylthiomethyl)-phenyl]-prop-2-in-ether,
yield: 85%, melting point: oil.
Example 141
1-t4-(1-Imidazolyl)-3-(methylthiomethyl)-phenyl]-2-chloroprop-2-
ene ether, yield: 56% as well as 32% of the product of Example
140, melting point: oil.
Example 142
t4-(1-Imidazolyl)-3-(methylthiomethyl)-pheny]-benzyl ether,
yield: 81.6%, melting point: oil.
Bxample 143
1-[2-(1-Imidazolyl)-phenyl]-(3-methylthio)propyl ether
240 mg of 2-(1-imidazolyl)-phenol, 1 equivalent of 3-
methylthiopropan-1-ol and 1 equivalent of triphenylphosphine are
dissolved in succession in 10 ml of THF. 0.23 ml of
azodicarboxylic acid diethyl ester is slowly added in drops at an
internal temperature of 4~. After a few hours at room
temperature, it is worked up. The mixture is concentrated by
evaporation and chromatographed on a column with silica gel.
Mobile solvent: hexane/EE. The yield is 60%. lH-NMR (CDCl3):
7.79(lH), 7.4 to 7.0 (mult. 6H), 4.12m2(lH), 2.57 (mult. 2H),
2.02 (mult. 2H), 2.07(s 3H).
56
In the same way, there are obtained:
Example 144
[2-(1--Imidazolyl)-phenylmethyl]-(ortho-methylthio)phenolether,
yield: 72%, melting point: 83~.
Example 145
L-[2-(1-Imidazolyl)-benzyl alcohol]-(S-methylcysteic acid)ester,
yield: 41%, melting point: 78~.
Example 146
[2-(1-Imidazolyl)-benzyl alcohol]-(2-S-methylthioacetic
acid)ester, yield: 75%. lH-NMR (CDCl3): 7.7(lH), 7.2 (mult.
7H), 5.0(s 2H), 3.2(s 2H), 2.18(s 3H).
Example 147
[2-(1-Imidazolyl)-phenyl]-[(2,2-dimethyl-1,3-dioxolan)--4-
methyl]ether, yield: 62%, melting point: oil.
Example 148
[2-(1-Imidazolyl)-phenyl]-(3-thiacyclohex-1-yl)-ether, yield:
75%, melting point: oil.
Example 149
[2-(1-Imidazolyl)-phenyl]-(2--thiophenyl)methylether, yield:
17%, lH--NMR (CDCl3): 7.81(1H), 6.95 to 7.4 (mult. 9H), 5.25(s
2H).
CA 0223~621 1998-04-22
57
Example 150
[2-(1-Imidazolyl)-phenyl]-but-1-in-3-yl ether, yield: 26%, lH-
NMR (CDCl3): 7.81(1H), 7.07 to 7.4 (mult. 6H), 4.82 (mult. lH),
2.48 (mult. lH), 1.60(d 3H).
Example 151
l-[2-(l-Imidazolyl)-phenoxyethyl]-(2-chloroethyl)-ether, yield:
19%, lH-NMR (acetone d6): 7.92(1H), 7.05 to 7.7 (mult. 6H), 4.41
(dd 2H), 3.95(dd 2H).
Example 152
[2-(1-Imidazolyl)-phenoxy-2-eth-1-yl]-(2-hydroxyethyl)-thioether,
yield: 33%, lH-NMR (CDCl3): 7.87(1H), 7.40 to 7.00 (mult. 6H),
4.21, 3.69, 2.88, 2.61 each (tr 2H).
Exampl~ 153
[2-(1-Imidazolyl)-phenyl]-(1,3-bis-methylthiopropan-2-yl)ether,
yield: 44%, lH-NMR (CDCl3): 7.8 (mult. lH), 7.0 to 7.4 (mult.
6H), 4.25 (mult. lH), 2.8 (mult. lH), 2.1 (mult. lH).
Example 154
[4-(l-Imidazolyl)-3-methylthiomethyl-phenyl]-(2-methylthioeth-l-
yl)ether, yield: 45%, melting point: oil.
Example 155
[2-(l-Imidazolyl)-phenylmethyl]-(2-thiomethylethyl)ether, yield:
33%, lH-NMR (CDCl3): 7.72(1H), 7.20 (mult. 2H), 7.3(1H), 7.6
CA 0223~621 1998-04-22
58
(mult. 4H), 4.30(s 2H), (lH), 3.62(tr 2H), (lH), 2.70(tr 2H),
2.12(s 3H).
Example 155a
2-(1-Imidazolyl)-benzylnitrile is obtained analogously with
acetone cyanohydrin in 66% yield from 2-(1-imidazolyl)-benzyl
alcohol.
Example 156
2-Chlora-4-methoxy-phenyl-1-(1-imidazole)
378 mg of 2-amino-4-methoxy-phenyl-1-(1-imidazole) is
dissolved with 5 ml of semiconcentrated hydrochloric acid at
about 50~; sodium nitrite solution is added in portions at ice
bath temperature and stirred for 15 minutes at this temperature.
The diazonium salt solution is added in portions to 440 mg of
copper(I) chloride, which is dissolved in 4 mol of concentrated
hydrochloric acid. After a few hours, it is poured onto soda
solution, extracted with EE, suctioned off on Celite and dried
with magnesium sulfate. Concentration by evaporation and column
chromatography yield 61% of the above-mentioned compound. lH-NMR
(CDC13): 7.05 to 7.61 (mult. 5H), 6.87(1H), 3.83(s 3H).
Example 157
4-Bromo-2-fluoro-phenyl-1-(1-imidazole), yield: 32%, lH-NMR
(CDC13): 7.85(lH), 7.47(lH), 7.4(lH), 7.27 (mult. 3H).
CA 0223~621 1998-04-22
59
Example 158
3,4-Dichloro-phenyl-1-(1-imidazole), yield: 59%, lH-NMR (CDCl3):
7.85 ~mult. lH), 7.25 (mult. 3H), 7.55 (mult. 2H).
Example 159
2,5-Dichloro-phenyl-1-(1-imidazole), yield: 33%, melting point:
100~.
Example 160
2,4-Dichloro-phe~yl-1-(1-imidazole), yield: 42%, lH-NMR (DMS0
d6): 7.82 (mult. 2H), 7.55 (mult. 2H), 7.41(1H), 7.11(1H).
Example 161
4-Chloro-2-fluoro-phenyl-1-(1-imidazole), yield: 64%, lH-NMR
(CDCl3): 7.795(s lH), 7.3 (mult. 5H).
Example 162
2-(1-Imidazolyl)-3-methylthiopyridine
180 mg (1 mmol) of 2-(1-imidazolyl)-3-chloropyridine is
heated to 110~ in 2 ml of NMP with 1.5 equivalents of sodium
thiomethanolate for 4 hours. The mixture is poured onto water,
extracted with ethyl acetate, the organic phase is dried with
magnesium sulfate and concentrated by evaporation. After column
chromatography with EE, 190 mg of title compound results. Yield:
99%, lH-NMR (CDCl3): 7.19 to 8.3 (mult. 6H), 2.42(s 3H).
CA 0223~621 1998 - 04 - 22
Example 163
[2-(1-Imidazolyl)-phenyl]-ethylthioether
In 3 ml of NMP, a total of 3 equivalents of sodium
thioethylate in NMP is added successively to 223 mg of 2-bromo-
phenyl-1-(1-imidazole). After 8 hours at 120~, thin-layer
chromatography shows many products. Working-up as in Example 162
provides a yield of 51%. lH-NMR (CDCl3): 7.64(lH), 7.22(lH),
7.13(1H), 7.22 (mult. 2H), 7.4 (mult. 2H), 2.8 (quartet 2H),
1.23(tr 3H).
EXample 164
3-[3-(1-Imidazolyl)-phenyl]-prop-1-yl-methylthioether
3-[3-(1-Imidazolyl)-phenyl]-prop-1-yl-bromide (300 mg) is
dissolved in 3 ml of NMP. After 1.1 equivalents of sodium
thiomethanolate is added, it is stirred for 24 hours at room
temperature. The mixture is poured onto water, extracted with
ethyl acetate, the organic phase is dried with magnesium sulfate
and concentrated by evaporation. After chromatography (EE/hex
2:3), 27% product results. lH-NMR (CDCl3): 7.83(1H), 7.4 to 7.2
(mult. 6H), 2.8(1H), 2.54(1H), 1.95 each (mult. 2H), 2.12(s 3H).
Analogously, there is synthesized:
Example 16S
[2-(1-Imidazolyl)-phenyl]-iso-propylthioether with
thioisopropylate, yield: 79%, lH-NMR (CDCl3): 7.67(1H),
7.19(lH), 7.15(lH), 7.3 to 7.5 (mult. 4H), 3.12 (heptet lH),
1.2(d 6H).
CA 0223~621 1998 - 04 - 22
Example 16 6
~2-(1-Imidazolyl)-phenyl]-tert-butylthioether with tert-butyl
mercaptan, yield: 69%, 91H-NMR (CDCl3): 7.77(lH), 7.75(lH),
7.14 to 7.5 (mult. 5H), 1.07(s 9H).
Example 167
[2-(1-Imidazolyl)-phenyl]-(3-furanylmeth-1-yl)-thioether with
furfuryl mercaptan, yield: 27%.
Example 168
2,3-Bismethylthio-phenyl-1-(1-imidazole) with thiomethylate,
yield: 45%, lH-NMR (CDCl3): 7.7(1H), 7.2 (mult. 3H), 7.4(dd
lH), 7.08(d lH), 2.50(s 3H), 2.09(s 3H).
Example 169
[5-Nitro-2-(1-imidazolyl)-phenyl]-methylthioether with
thiomethylate, yield: 18%, lH-NMR (CDCl3): 7.7(1H), 7.28(1H),
7.18(1H), 8.1 (mult. 2H), 7.4(1H), 2.53(s 3H).
Example 17 O
[5-Chloro-2-(1-imidazolyl)-phenyl]-methylthioether with
thiomethylate, yield: 50%, lH-NMR (CDCl3): 7.1 to 7.62 (mult.
6H), 2.40(s 3H).
Example 17 oa
[5-Bromo-2-(1-imidazolyl)-benzyl]-methylthioether, yield: 75~,
oil. In addition, a few percent of [5-methylthiomethyl-2-(1-
CA 0223~621 1998-04-22
imidazolyl)-benzyl]-methylthioether is obtained. The compound is
also produced in 77% yield from [5-bromo-2-(1-imidazolyl)-
benzyl]-methylthioether with thiomethylate.
Example 17Ob
t4-Bromo-2-(1-imidazolyl)-benzyl]-methylthioether, yield: 91%,
oil.
Example 170c
[6-Nitro-2-(1-imidazolyl)-benzyl]-methylthioether, yield: 65%,
oil.
Example 17Od
[6-Chloro-2-(1-imidazolyl)-benzyl]-methylthioether, yield: 50%,
oil.
Example 170e
[3-Nitro-2-(1-imidazolyl)-benzyl]-methylthioether, yield: 15%,
oil.
Example 171
~5-Nitro-2-(1-imidazolyl)-phenyl]-ethylthioether with
thioethylate, yield: 25%, lH-NMR (CDC13): 7.7(1H), 7.25(1H),
7.18(1H), 8.21(1H), 8.09(1H), 7.4(1H), 2.99 (quartet 2H), 1.34
(tr 3H).
CA 0223~621 1998-04-22
63
Example 172
[5-Chloro-2-(1-imidazolyl)-phenyl]-ethylthioether with
thioethylate, yield: 82%, lH-NMR (CDCl3): 7.62(lH), 7.09(lH),
7.20(1H), 7.15 to 7.33 (mult. 3H), 2.83 (quartet 2H), 1.28(tr
3H).
Example 173
(1-t2-(1-Imidazolyl)-phenyl]-eth-1-yl)methylthioether with
thiomethylate, yield: 34~, lH-NMR (CDCl3): 7.1 to 7.7 (mult.
7H), 3.62 (quartet lH), 1.52(d 3H).
Example 174
(1-[2-(1-Imidazolyl)-phenyl]-eth-1-yl)-ethylthioether with
thioethylate, yield: 28%, melting point: oil.
Example 175
[2-(1-Imidazolyl)-phenyl]-(l-phenyleth-1-yl)thioether with 1-
phenylethanethiol, yield: 75%, lH-NMR (CDCl3): 7.05 to 7.55
(mult. 12H), 4.05(1H), 1.50(d 3H).
Example 176
[2-(1-Imidazolyl)-phenyl]-but-2-ylthioether with 1-methyl propyl
mercaptan, yield: 74%, lH-NMR (CDCl3): 7.66(1H), 7.20(1H),
7.15(1H), 7.54(dd lH), 7.3 (mult. 3H), 2.9 (mult. lH), 1.50(m
2H), 1.15(m 3H), O.9(tr 3H).
CA 0223~621 1998-04-22
64
Example 177
[2-(1-Imidazolyl)-phenyl]-(2-phenyleth-1-yl)thioether with 2-
phenylethanethiol, yield: 60%, lH-NMR (CDCl3): 7.69(1H), 7.1 to
7.5 (mult. llH), 3.0 (mult. 2H), 2.83 (mult. 2H).
Example 178
[2-(1-Imidazolyl)-phenyl]-cyclohexylthioether with cyclohexyl
mercaptan, yield: 80%, melting point: oil.
Bxample 179
[2-(1-Imidazolyl)-phenyl]-decylthioether with decyl mercaptan,
yield: 58%, lH-NMR (CDCl3): 7.65(1H), 7.13 to 7.45 (mult. 6H),
2.75 (mult. 2H), 0.9 to 1.6 (mult. l9H).
Example 180
[2-(1-Imidazolyl)-phenyl]-(2-N,N-dimethylaminoeth-l-yl)thioether
with N,N-dimethylaminoethanethiol, yield: 40%, melting point:
oil.
Example 181
[2-(1-Imidazolyl)-phenyl]-benzylthioether with thiomethylbenzene,
yield: 75%, melting point: 87~.
Example 182
[2-(1-Imidazolyl)-phenyl]-(2-thiophenyl)thioether with 2-
mercaptothiophene, yield: 28~. Melting point: oil.
CA 0223~621 1998-04-22
Example 183
2-[2-(1-Imidazolyl)-benzylthio]-N-methylacetamide with
methylmercaptoacetamide, yield: 44%, lH-NMR (CDCl3): 7.68(1H),
7.22(1H), 7.17(1H), 7.25 to 7.5 (mult. 4H), 3.53(s 2H), 3.14(s
2H), 2.77(d 3H).
Example 184
[2-(1-Imidazolyl)-phenylmethyl]-[lN-methyl-imidazol-2-
yl~thioether with 2-mercapto-1-methylimidazole, yield: 14%,
melting point: 226~.
Ex~lmple 185
3-[2-(1-Imidazolyl)-benzylthio]-propionic acid methyl ester with
mercaptopropionic acid methyl ester, yield: 49%, lH-NMR (CDCl3):
7.7(1H), 7.2 to 7.5 (mult. 6H), 3.55(s 2H), 3.7(s 3H), 2.7(dd
2H), 2.5(dd 2H)-
Example 1862-[2-(1-Imidazolyl)-benzylthio]-3,4,5,6-tetrahydropyrimidine
hydrobromide with 2-mercapto-tetrahydropyrimidine 1,3, yield:
35%, melting point: 269~.
Example 187
[3-(1--Imidazolyl)--4--phenyl-phenyl]methylthioetherwith sodium
thiomethylate, yield: 81%, melting point: oil.
CA 0223~621 1998-04-22
66
Example 188
[3-(1-Imidazolyl)-4-phenyl-phenyl]methylthioether with sodium
thioethylate, yield: 77%, melting point: oil.
Example 189
2-[2-(1-Imidazolyl)-benzylmercapto]-acetic acid ethyl ester with
ethyl-2-mercaptoacetate, yield: 62%, lH-NM (CDCl3): 7.7(1H),
7.2 to 7.55 (mult. 6H), 4.15 (quartet 2H), 3.69(s 2H), (lH),
3.12(s 2H), 1.28(tr 3H).
Example 190
[2-(1-Imidazolyl)-benzyl]-benzylthioether with benzyl mercaptan,
yield: 71%, lH-NMR (CDC13): 7.68(1H), 7.1 to 7.45 (mult. 12H),
3.68(s 2H), 3.42(s 2H).
Example 191
S-[2-(1-Imidazolyl)-benzyl]isothiourea hydrobromide
318 mg of 2-(1-imidazolyl)-benzylbromide hydrobromide is
refluxed with 153 mg of thiourea in 5 ml of ethanol for 5 hours.
After the crystals are cooled, suctioned off and washed, 250 mg
of S-~2-(1-imidazolyl)-benzyl]isothiourea hydrobromide is
obtained. Melting point: 247~. The crude product is dissolved
in saturated sodium bicarbonate solution; after a short time, the
free base S-[2-(1-imidazolyl)-benzyl]isothiourea again
precipitates. It is suctioned off and washed with water.
CA 0223~621 1998-04-22
67
A few examples illustrate the process:
Example 192
S-[2-(2-(1-Imidazolyl))-phenyleth-1-yl]isothiourea hydrobromide.
Yield: 97%, melting point: 229~.
Example 193
S-[3-(2-(1-Imidazolyl))-phenylpropyl]isothiourea hydrobromide.
Yield: 69%, lH-NMR (DMS0 d6): 9.45(lH), 9.0(lH), 8.02(lH),
7.92(lH), 7.4 to 7.7 (mult. 4H), 3.13m2(lH), 2.6 (mult. 2H), 1.8
(mult. 2H).
Example 194
N-Phenyl-S-[2-(1-imidazolyl)-benzyl]isothiourea hydrobromide.
Yield: 60%, melting point: 217~.
Example 195
S-[1-(2-(1-Imidazolyl))-phenyleth-1-yl]isothiourea hydrobromide.
Yield: 81~, melting point: 164~.
Example 196
N,N-Dimethyl-S-[2-(1-imidazolyl)-benzyl]isothiourea hydrobromide.
Yield: 87%, melting point: 183~.
Example 197
[2-(1-Imidazolyl)-benzyl]-propargylthioether
A solution of S-[2-(1-imidazolyl)-benzyl]isothiourea
hydrobromide (Example 191) is boiled in a solution of 1.5 g of
68
potassium hydroxide pellets and 3 g of water. Dichloromethane is
carefully added to 2 ml of the reaction solution, so that 2
phases are formed. 80 mg of propargyl bromide as well as 10 mg
of triethylbenzylammonium bromide are added to it and stirred for
3 hours at room temperature. It is diluted with water, extracted
with dichloromethane, the organic phase is washed with brine,
dried with magnesium sulfate and spun in.
Column chromatography with hexane/EE at a 1:1 ratio yields
50% product. lH-NMR (CDCl3): 7.7~(1H), 7.21(1H), 7.20(1H), 7.24
to 7.55 (mult. 4H), 3.71(s 2H), 3.17(d 2H), 2.27 (mult. lH).
In the same way, there are obtained:
Example 198
[2-(1-Imidazolyl)-benzyl]-(2-bromo-prop-2-enyl)thioether, yield:
68%, lH-NMR (CDCl3): 7.71(1H), 7.2 to 7.54 (mult. 6H), 5.23
ml(lH), 5.03 (mult. lH), 3.56(s 2H), 3.39(s 2H).
Example 199
S-~2-(1-Imidazolyl)-benzyl]-thiocarboxylic acid-0-ethyl ester,
yield: 45%, lH-NMR (CDCl3): 7.63(1H), 7.1(1H), 7.2(1H), 7.2 to
7.6 (mult. 4H), 3.9(s 2H), 4.29 (quartet 2H), (lH), 1.29(tr 3H).
Example l99a
(2-(1-Imidazolyl)-benzylthio)-acetone, yield: 55%, lH-NMR
(CDCl3): 7.7(1H), 7.2 to 7.5 (mult.), 3.51(s 2H), 3.16(s 2H),
2.22(s 3H).
CA 0223~621 1998-04-22
69
Example 199b
S-[2-(1-Imidazolyl)-benzyl]-N,N-dimethyl-thio-carbamate, yield:
69~, melting point: 60~.
Example 200
[2-(1-Imidazolyl)-benzyl]-(l-propen-2-yl)thioether, yield: 58%,
lH-NMR (CDCl3): 7.71(1H), 7.21(1H), 7.20(1H), 7.22 to 7.52
(mult. 4H), 5.7 (mult. lH), 5.1 (mult. 2H), 3.49(s 2H), (lH),
3.09(d 2H).
Example 201
2-(1-Imidazolyl)-benzylbromide hydrobromide
2.61 g of 2-(1-imidazolyl)-benzyl alcohol is heated for 4
hours at 140~ in 47~ HBr. It is concentrated by evaporation, and
the crude product is carefully dried. The yield is 98~. lH-NMR
(DMS0 d6): 9.51(1H), 8.10(1H), 7.98(1H), 7.79(1H), 7.6 to 7.7
(mult. 3H), 4.68(s 2H).
Phenols are obtained from methoxy aromatic compounds,
according to this example, under corresponding conditions, for
example:
Example 202
2-Hydroxy-3-methyl-phenyl-1-(1-imidazole), yield: 88~, melting
point: oil.
-
CA 0223~621 1998-04-22
Example 203
2-Hydroxy-3-(imidazol-1-yl)-4-hydroxy-phenyl-1-(1-imidazole),
yield: 77%, melting point: > 300~.
Example 204
2-Hydroxy-5-tert-butyl-phenyl-1-(1-imidazole), yield: 90%,
melting point: 198~.
Example 205
2-Hydroxy-4-(imidazol-1-yl)-phenyl-1-(1-imidazole), yield: 70%,
melting point: > 300~.
Example 206
2-Methyl-4-hydroxy-phenyl-1-(1-imidazole), yield: 85~, lH-NMR
tDMS0 d6): 9.52(1H), 6.7 to 7.7 (mult. 6H), 2.02(s 3H), ethyl-l-
bromide hydrobromide, yield: 100%, lH-NMR (CDC13): 9.55(1H), 7.5
to 8.1 (mult. 6H), 5.19 (quartet lH), 2.01(d 3H).
Example 207
2-Nitro-4-hydroxy-phenyl-1-(1-imidazole), yield: 54%, melting
point: 228-230~.
Example 208
2-Hydroxy-5-hydroxy-phenyl-1-(1-imidazole), yield: 65%, lH-NMR
(DMS0 d6): 7.9(1H), 7.03(1H), 7.4(1H), 9.2(1H), 9.33(1H), 6.65
to 6.9 (mult. 3H).
CA 0223~621 1998-04-22
71
Example 209
2-[2-(1-Imidazolyl)-phenyl]-ethylbromide hydrobromide, yield
100%, lH-NMR (DMS0 d6): 9.5(1H), 8.1(1H), 7.95(1H), 7.5(1H), 7.7
(mult. 4H), 3.70(tr 2H), 3.02(tr 2H).
Example 210
2,4-Dihydroxy-phenyl-1-(1-imidazole), yield: 65%, melting point:
195~.
Examplo 211
3-[2-(1-Imidazolyl)-phenyl]-propyl-1-bromide hydrobromide, yield:
99%, lH-NMR (DMS0 d6): 9.5(1H), 8.1(1H), 7.95(1H), 7.7 to 7.4
(mult. 4H), 3.49m2(lH), 2.64 (mult. 2H), 1.99 (mult. 2H).
Example 212
[2-(1-Imidazolyl)-phenyl]-methylthioether hydrobromide
95 mg of [2-(1-imidazolyl)-phenyl]-methylthioether is
stirred into 2 ml of 47% HBr and concentrated by evaporation. It
is dried on a bulb tube. The hydrobromide is obtained in
quantitative yield. Melting point: 176~.
Example 213
2-Hydroxyphenyl-l-(1-imidazole). Yield 96%.
Example 214
3-Chloro-4-hydroxy-phenyl-1-(1-imidazole), yield: 87%, melting
point: 216~.
CA 0223~621 1998 - 04 - 22
Example 215
3,4-Dihydroxy-phenyl-1-(1-imidazole), yield: 88%, lH-NMR (DMS0
d6): 8.0(1H), 7.5(1H), 7.1(1H), 9.2(s broad 2H), 6.92(1H), 6.8
(mult. 2H).
Example 216
4-Nitro-2-(1-imidazolyl)-benzylbromide hydrobromide, yield: 90%,
lH-NMR (DMS0 d6): 9.63(lH), 8.71(lH), 7.95 to 8.5 (mult.
4H)(lH), 4.81(s 2H).
Example 217
3-t3-(1-Imidazolyl)-phenyl]-propyl-l-bromide hydrobromide, yield:
92.6% melting point: oil.
Example 218
2-Amino-4-cyano-phenyl-1-(1-imidazole)
214 mg of 2-nitro-4-cyano-phenyl-1-(1-imidazole) with 110 mg
of ammonium chloride in 1 ml of water is mixed in a mixture of 2
ml of THF and 3 ml of ethanol. After 630 mg of zinc powder is
added, it is stirred for 3 hours at room temperature. It is
diluted with EE, suctioned off on Celite, the filtrate is washed
once with ammonium chloride solution and with brine until
neutrality is reached. Drying and concentration by evaporation
yield 93% product. Melting point: 214~.
Example 218a
t5--Amino-2-(imidazolyl)-benzyl]-methylthioether, yield 99%.
CA 0223~621 1998 - 04 - 22
Example 218b
[6-Amino-2-(1-imidazolyl)-benzyl]-methylthioether, yield 51%.
Analogously, there are produced:
Example 219
2-Amino-5-chloro-phenyl-1-(1-imidazole), yield: 95%, melting
point: 136~.
Example 220
2-Amino-4-amino-phenyl-1-(1-imidazole), yield: 54%, lH-NMR
(CDC13): 7.6(1H), 7.20(1H), 7.05(1H), 6.9 (mult. lH), 6.1 (mult.
2H).
Example 221
4-Amino-2-hydroxy-phenyl-1-(1-imidazole), yield: 69%, melting
point: 232~.
Example 222
4-Amino-[2-(1-imidazolyl)-thiophenol]-methyl ether, yield: 100%,
lH-NMR (CDCl3): 7.7(1H), 7.15(1H), 7.16(1H), 6.6(1H), 6.72(1H),
7.30(1H), 2.20(s 3H).
Example 223
4-Amino-2-fluoro-phenyl-1-(1-imidazole), yield: 95%, melting
point: 69-72~.
CA 0223~621 1998-04-22
74
Example 224
2-Amino-4-chloro-phenyl-1-(1-imidazole), yield: 100%, melting
point: 144~.
Example 225
4-Amino-3-chloro-phenyl-1-(1-imidazole~, yield: 100%, melting
point: 114~.
Example 226
2-Amino-4-methoxy-phenyl-1-(1-imidazole), yield: 100%, lH-NMR
(CDCl3): 7.8(1H), 7.30(1H), 7.1(1H), 6.99(dd lH), 6.33 (singlet
lH), 6.31 (mult. lH), 3.80(s 3H).
Example 227
3-t2-(1-Imidazolyl)-phenyl]-propionic acid ethyl ester
1.44 g of E-3-[2-(1-imidazolyl)-phenyl]-acrylic acid ethyl
ester is hydrogenated in 50 ml of ethanol at low pressure with
144 mg of 10% palladium/carbon catalyst. The reaction requires 2
hours at room temperature. It is suctioned off, concentrated by
evaporation, and 89% product is obtained. lH-NMR (CDCl3):
7.61(1H), 7.22(lH), 7.09(lH), 7.2 to 7.4 (mult. 4H), 4.09
(~uartet lH), 2.43(tr 2H), (lH), 2.83(tr 2H), 1.21(tr 3H).
A selection of other catalytic hydrogenations:
Example 228
2-Cyano-3-[2-(1-imidazolyl)-phenyl]-propionic acid ethyl ester,
yield: 48%, oil.
CA 02235621 1998-04-22
Example 229
3-t3-(1-Imidazolyl)-phenyl]-propionic acid ethyl ester, yield:
95.4~, lH-NMR (CDCl3): 7.87(lH), 7.4 to 7.2 (mult. 6H), 4.12
(quartet 2H), 3.03(tr 2H), (lH), 2.68(tr 2H), 1.22(tr 3H).
Example 230
4-(1-Imidazolyl)-3-(methylthiomethyl)-benzylamine, yield: 33%,
melting point: oil.
Example 231
2-~2-(1-Imidazolyl)-phenoxy]-ethylamine
528.4 mg (2.65 mmol) of 2-[2-(1-imidazole)-phenoxy]-
acetonitrile is mixed in 25 ml of methanol with a catalytic
amount of Raney nickel. Ammonia is now applied and hydrogenated
with hydrogen at 50~C for four hours (80 bar, autoclave). After
the reaction has been completed, the catalyst is suctioned off,
and the solvent is spun off. The residue is chromatographed on
silica gel with dichloromethane/isopropanol/ammonia as mobile
solvent. 147.1 mg (27.3%) is isolated.
Example 232
4-[2-(1-Imidazolyl)-phenoxy]-butylamine
415 mg (1.82 mmol) of the compound that is produced
according to Example 128 is reacted as described in Example 231.
After working-up and chromatography, 349.3 mg (83.8%) of amine is
obtained.
76
Example 233
5-[2-(1-Imidazolyl)-phenoxy]-pentylamine
723 mg (3 mmol) of the compound produced according to
Example 129 is reacted as described in Example 231. After
working-up and chromatography, 389.2 mg (52.9%) of amine is
obtained.
Example 234
6-[2-(1-Imidazolyl)-phenoxy]-hexylamine
720.8 mg (2.82 mmol) of the nitrile that is produced
according to Example 130 is reacted as described in Example 231.
After working-up and chromatography, 575 mg (78.7%) of amine is
obtained.
Example 235
7-[2-(1-Imidazolyl)-phenoxy]-heptylamine
780 mg (2.9 mmol) of the compound that is produced according
to Example 131 is reacted analogously to Example 231. Working-up
and chromatography yield 529.5 mg (66.8%) of amine.
Example 236
2-Cyano-E-3-[2-(1-Imidazolyl)-phenyl]-acrylic acid ethyl ester
5 mmol of cyanoacetic acid ethyl ester is boiled in toluene
with 5 mmol of 2-(1-imidazolyl)-benzaldehyde with the addition of
a small amount of acid in a water separator. After cooling,
needles precipitate, which are washed with toluene and dried at
60~. The yield is 79%, melting point: 129.5. The aldehyde can
CA 0223~621 1998-04-22
77
be obtained according to standard methods from glycolacetal by
cleavage with semiconcentrated hydrochloric acid/THF/water 1~
with a yield of 98%. The acetal is produced by aldehyde being
stirred with glycol and para-toluenesulfonic acid with a yield of
69~ or by reaction of 2-bromobenzaldehyde acetal with imidazole.
Example 237
Ortho-bromoci ~n~m; c acid ethyl ester
Ortho-bromoc; nn~m; c acid (5.22 g) is esterified with 3.05 g
of ethyl bromide, 3.86 g of potassium carbonate and 25 ml of DMF
after 2 hours at 60~. The mixture is poured onto water,
extracted with ethyl acetate; the organic phase is dried with
magnesium sulfate and concentrated by evaporation. 99% of crude
product, which is further reacted, results (Example 87).
Analogously, there are obtained
Example 238
Meta-bromocinnamic acid ethyl ester, yield 99%, oil.
Example 239
E-3-[2-(1-Imidazolyl)-phenyl]-allyl alcohol and
E-3-[2-(1-imidazolyl)-phenyl]-acrolein
In the case of the reduction of E-3-[2-(1-imidazolyl)-
phenyl]-acrylic acid ethyl ester (242 mg) with DiBAH in 5 ml of
toluene at --78~ and then for 1 hour at 0~C, a mixture of aldehyde
and allyl alcohol, which is separated chromatographically with EE
with the addition of ethanol as eluant, is obtained after
CA 0223~621 1998-04-22
78
working-up. Yield of aldehyde: 13%, lH-NMR (CDCl3): 9.57(d lH),
7.1 to 7. 8 (mult. 8H), 6.69(dd lH).
Yield of alcohol: 48%, lH-NMR (CDCl3): 7.7 to 7.07 (mult.
7H), 6.37 (mult. 2H), 4.28(dd 2H).
Analogously, there are obtained
Examp le 2 4 0
E-3-[3-(1-Imidazolyl)-phenyl~-allyl alcohol, yield: 88%, oil.
Example 241
3-[2-(1-Imidazolyl)-phenyl]-propanol and 3-[2-(1-imidazolyl)-
phenyl]-propanal consisting of 3-[2-(1-imidazolyl)-phenyl]-
propionic acid ethyl ester, yield of aldehyde: 13%, lH-NMR
~DCl3): 9=68(1H); 7=61(1H); 7=09(1H); 7=2 to 7=45 (mUlt= 5H)
2.83m2(1H), 2.57 (mult. 2H).
Alcohol: lH-NMR (CDCl3): 7.59(1H), 7.07(1H), 7.18(1H), 7.2
to 7.47 (mult. 4H), 3.55 (mult. 2H), 2.57 (mult. 2H), 1.7 (mult.
2H).
Example 2 42
3-[3-(1-Imidazolyl)-phenyl]-propanol
After extraction, the crude product is obtained with T.; Al ~4
(1.1 equivalents in THF) and 3-[3-(1-imidazolyl)-phenyl]-
propionic acid ethyl ester after reaction and pouring on sodium
tartrate in water.
Yield: 99.7~, lH-NMR (CDCl3): 7.84(1H), 7.4 to 7.2 (mult.
6H), 3.7m2(1H), 2.8m2(1H), 1.95 (mult. 2H).
CA 0223~621 1 998 - 04 - 22
Example 243
2-Fluoro-4-(1-pyrrolo)-phenyl-1-(1-imidazole)
133 mg of 4-amino-2-fluoro-phenyl-1-(1-imidazole) is
refluxed in acetic acid (1 ml) with 1 equivalent of 2,5-
dimethoxytetrahydrofuran for 2 hours. It is poured onto
saturated potassium bicarbonate solution, extracted twice with
EE, and the organic phase is washed. It is dried with magnesium
sulfate, spun in, and column chromatography is carried out with
EE. Yield: 69%, lH-NMR (CDCl3): 6.4 to 7.43 (mult. 9H), 7.8(s
broad lH).
In the same way, there are produced:
Example 243b
4-Chloro-2-pyrrolo-phenyl-1-(1-imidazole), yield 100%, melting
point: 84~.
Example 244
5-Chloro-2-pyrrolo-phenyl-1-(1-imidazole), yield: 94%, melting
point: 115~.
Example 245
3-Chloro-4-pyrrolo-phenyl-1-(1-imidazole), yield: 61%, lH-NMR
(CDCl3): 6.38 (mult. 2H), 6.92 (mult. 2H), 7.23 to 7.9 (mult.
6H).
CA 0223~621 1998-04-22
Example 246
1-[2-(1-Imidazolyl)-phenyl]-ethanol
1.72 g of 2-(1-imidazolyl)-benzaldehyde is reacted with 3.7
ml of methylmagnesium bromide (3M solution) in 12 ml of THF.
After 2 hours, ammonium chloride solution is added, extracted
with EE, washed with brine, dried and concentrated by
evaporation. After column chromatography with EE, the yield is
51%. lH-NMR (CDC13): 7.07 to 7.79 (mult. 7H), 4.74 (quartet
lH), 1.41(d 3H).
Example 247
2-[2-(1-Imidazolyl)-phenoxy]-acetic acid
1.3 g (4.74 mmol) of the ester that is produced according to
Example 133 is mixed with 10 ml of a mixture of KOH/CH30H/H20
(3.6 g, 24 ml, 120 ml), and it is stirred at room temperature
until saponification is completed. It is acidified with 10%
sulfuric acid and then poured over Extrelut with CH2Cl2. The
filtrate is spun in, and the residue is chromatographed on sio2
with a mobile solvent mixture that consists of
CH2Cl2/isopropanol/water. The yield is 759.2 mg (73.7%).
Example 248
4-[2-(1-Imidazolyl)-phenoxy]-butyric acid
0.600 g (2.19 mmol) of the ester that is produced according
to Example 134 is saponified analogously to Example 247. 412.1
mg (76.5%) of product is isolated.
CA 0223~621 1998-04-22
81
Example 249
5-[2-(1-Imidazolyl)-phenoxy]-pentanoic acid
1.35 g (4.92 mmol) of the ester that is produced according
to Example 135 is saponified analogously to Example 247. The
yield of product is 604.3 mg (49.1%).
Example 250
6-[2-(1-Imidazolyl)-phenoxy]-hexanoic acid
1.1 g (4.64 mmol) of the ester that is produced according to
Example 136 is saponified as described in ~xample 247 and worked
up. 784.2 mg (78.6~) is isolated.
Example 251
7-[2-(1-Imidazolyl)-phenoxy]-heptanoic acid
0.148 g (0.464 mmol) of the that is produced according to
Example 137 is saponified analogously to Example 247 and worked
up. 132.8 mg (98.5%) of product is isolated.
Example 25lb
2-[2-(1-Imidazolyl)-benzylthio]-acetic acid, yield: 81%, melting
point: 140~.
Example 252
4-(1-Imidazolyl)-3-(methylthiomethyl)phenol
1.5 mmol of 4-(1-imidazolyl)-3-
(methylthiomethyl)phenolmethylether with 5 mmol of sodium
thiomethylate is refluxed for 2 hours in DMF. It is poured onto
CA 0223~621 1998-04-22
82
saturated potassium bicarbonate solution, extracted twice with
EE, and the organic phase is washed with brine. It is dried with
magnesium sulfate, spun in and, after column chromatography with
EE/ethanol, a 64% yield is obtained. Melting point: 129~.
Analogously, there are obtained:
Example 253
3-(1-Imidazolyl)-4-(methylthiomethyl)phenol, yield: 43%, lH-NMR
(CDCl3): 7.77(1H), 7.24(1H), 7.18(1H), 7.3(1H), 7.0(1H),
6.8(1H), 3.40(s 2H), 2.02(s 3H).
Bxnmple 254
2-(lH-Imidazol-l-yl)-3-pyridinol
2-Chloro-3-phenylmethoxy-pyridine (1.4 g) and imidazole (4.3
g) are heated for 4 days in an oil bath to 120~C. The mixture is
cooled to room temperature and mixed with 5N NaOH and chloroform.
The organic phase is separated, dried and concentrated by
evaporation. The residue is purified chromatographically, and 2-
(l-imidazolyl)-3-phenylmethoxy-pyridine is obtained. 2-(1-
Imidazolyl)-3-phenylmethoxy-pyridine and 0.01 g of Pd/C are added
to 2 ml of methanol, stirred for 1 hour under hydrogen, filtered
off, and the solvent is drawn off. The title compound with a
melting point of 162-164~C is obtained.
83
Example 255
[2--(1-Imidazolyl)-benzyl]-methylsulfone
3.05 g of [2-(1--imidazolyl)-benzyl]-methylthioetheris mixed
at 5~ in 75 ml of dichloromethane with 7.08 g of meta-
chloroperbenzoic acid. It is stirred overnight and then diluted
with dichloromethane, extracted twice with lN sodium hydroxide
solution, twice with water, and the organic phase is dried with
magnesium sulfate. After spinning--in and column chromatography
with EE/ethanol, the sulfone is obtained, in addition to a little
sulfoxide, in 78% yield. Melting point: 156~. lH-NMR (CDC13):
7.71(1H), 7.70(1H), 7.52 (mult. 2H), 7.34 (mult. lH), 7.22(s 2H),
4.13(s 2H), (lH), 2.83(s 3H).
Analogously, there are obtained:
Example 256
[2--(1-Imidazolyl)-phenylmethyl]-methylsulfoxide, yield: 67%,
melting point: 94~, lH-NMR (CDCl3): 7.67(1H), 7.22(1H),
7.15(1H), 7.33 to 7.57 (mult. 4H), 3.75 (AB quartet 2H), 2.50(s
3H).
Example 257
[2--(2--(1-Imidazolyl)--phenyl)eth-1-yl]-methylsulfone, yield: 71%,
lH-NMR (CDCl3): 7.72 to 7.12 (mult. 7H), 3.02 (mult. 4H), 2.72(s
3H).
84
Example 258
[2-(1-Imidazolyl)-phenyl]-methylsulfone, yield: 67%, melting
point: 139-141~.
Example 259
[2-(1-Imidazolyl)-5-phenyl-benz-1-yl]-methylsulfone, yield: 34%,
lH-NMR (CDC13): 7.29 to 7.77 (mult. lOH), 7.91(d lH), 4.20(s
2H), 2.89(s 3H).
Example 260
[2-(1-Imidazolyl)-5-methoxy-benzyl]-methylsulfone, yield: 25%,
melting point: 115.9~.
Ex~mple 261
[2-(1-Imidazolyl)-5-cyano-benzyl]-methylsulfoxide, yield: 26%,
lH-NMR (CDC13): 7.20 to 7.9 (mult. 6H), 3.79 (mult. 2H), 2.60(s
3H).
Example 262
[2-(1-Imidazolyl)-5-phenyl-benzyl]-methylsulfoxide, yield: 77%,
lH-NMR (CDC13): 7.20 to 7.80 (mult. llH), 3.82(dd 2H), 2.56(s
3H).
Example 263
[2-(1-Imidazolyl)-5-methoxy-benzyl]-methylsulfoxide, yield:
68.8%, melting point: 103.1~.
CA 0223~621 1998-04-22
Example 264
[2-(1-Imidazolyl)-5-amido-benzyl]-methylsulfoxide, yield: 22%,
melting point: 226~.
Example 265
[2-(1-Imidazolyl)-5-cyano-benzyl]-methylsulfone, yield: 25%, lH-
NMR (CDCl3): 7.27 to 8.05 (mult. 6H), 4.20(s 2H), 2.99(s 3H).
Example 266
[2-(1-Imidazolyl)-5-amido-benzyl]-methylsulfone, yield: 36%,
melting point: 189.5~.
Example 267
[2-(2-(1-Imidazolyl)-phenyl)-et-1-yl]-methylsulfoxide, yield:
61%, lH-NMR (CDCl3): 7.72 to 7.12 (mult. 7H), 3.0(tr 2H), 2.7
(mult. 2H), 2.46(s 3H).
Example 268
[2-(1-Imidazolyl)-benzyl]-ethylsulfone, yield: 76%, lH-NMR
(CDCl3): 7.72 to 7.2 (mult. 7H), 4.03(s 2H), 2.95 (quartet 2H),
1.82(tr 3H).
Example 269
[2-(1-Imidazolyl)-benzyl]-isopropylsulfone, yield: 75%, lH-NMR
(CDCl3): 7.72 to 7.2 (mult. 7H), 4.00(s 2H), 3.07 (heptet lH),
1.45(d 6H).
86
Example 270
[2~ Imidazolyl)-benzyl]-ethylsulfoxide, yield: 77%, lH-NMR
(CDC13): 7.67 to 7.17 (mult. 7H), 3.7 (quartet 2H), 2.7 (mult.
2H), 1.28(tr 3H).
Example 271
[2-(l-Imidazolyl)-benzyl]-isopropylsulfoxide, yield: 82%, lH-NMR
(CDC13): 7.72 to 7.2 (mult. 7H), 3.6(s 2H), 2.74 (heptet lH),
1.2(dd 6H).
Example 272
[2-(2-(1-Imidazolyl)-phenoxy)-eth-l-ylmethylsulfone, yield: 31%,
lH-NMR (CDC13): 7.7(1H), 7.45 to 7.04 (mult. 6H), 4.47(dd 2H),
3.39 (mult. 2H), 2.61(s 3H).
Example 273
[2-(2-(1-Imidazolyl)-phenoxy)-eth-1-ylmethylsulfoxide, yield:
32%, lH-NMR (CDC13): 7.74(1H), 7.45 to 7.04 (mult. 6H), 4.47
(mult. 2H), 3.2(1H), 3.1(1H), 2.54(s 3H).
Example 274
t(2-(1-Imidazolyl)-pyridyl-3-meth)yl]methylsulfone, yield: 35%,
lH-NMR (CDC13): 8.62(dd lH), 8.12(dd lH), (lH), 8.00(s lH),
7.5m2(1H), 7.24(s lH), 4.29(s 2H), 2.92(s 3H).
CA 0223~621 1998-04-22
87
Example 275
[2-(2-(1-Imidazolyl)-phenyl]-isopropylsulfoxide, yield: 34%,
melting point: 91.5~.
Examplo 276
[2-(2-(l-Imidazolyl)-phenyl]-isopropylsulfone~ yield: 57%,
melting point: 80.8~.
Example 277
[2-(1-Imidazolyl)-5-hydroxy-benzyl]-methylsulfone, yield: 62.6%,
melting point: > 300~.
Example 278
[2-(1-Imidazolyl)-5-hydroxy-benzyl]-methylsulfoxide, yield: 9096,
melting point: oil.
Example 279
[3-(1-Imidazolyl)-benzyl]-methylsulfone, yield: 82%, melting
point: 144.5~.
Example 280
[3-(1-Imidazolyl)-benzyl]-methylsulfoxide, yield: 65%, lH-NMR
(CDCl3): 7.87(lH), 7.2 to 7.52 (mult. 6H), 3.99(d 2H), 2.52(s
3H).
-
CA 0223~621 1998-04-22
88
Bxample 281
[3-(1-Imidazolyl)-phenyl]-methylsulfone, yield: 56%, lH-NMR
(CDCl3): 8.01(1H), 7.94 (mult. 2H), 7.7 (mult. 2H), 7.37(lH),
7.27(1H), 3.12(s 3H).
Exampls 282
[3-(1-Imidazolyl)-benzyl]-methylsulfoxide, yield 22%, melting
point: oil.
Example 283
[2-(1-Imidazolyl)-5-(meta-pyridyl)-benzyl]-methylsulfone, yield
25%, melting point: oil.
Example 284
[2-(1-Imidazolyl)-5-(meta-pyridyl)-benzyl]-methylsulfoxide, yield
11~, melting point: oil.
Example 285
[2-(1-Imidazolyl)-phenyl]-methylsulfoxide, yield: 26%, melting
point: 147~.
Example 286
[4-(1-Imidazolyl)-phenyl]-methylsulfone, yield: 25~, lH-NMR
(acetone d6): 8.3(1H), 7.76(1H), 7.18(1H), 7.94 (mult. 2H), 8.10
(mult. 2H), 3.2(s 3H).
89
Example 287
[4-(1-Imidazolyl)-phenyl]-methylsulfoxide, yield: 60%, lH-NMR
(CDCl3): 7.23(lH), 7.32(lH), 7.92(lH), 7.58 (mult. 2H), 7.80
(mult. 2H), 2.26(s 3H).
Example 287a
t3-(Dimethylsulfon-1-yl)-4-(1-imidazolyl)-phenyl]-methylsulfone,
yield 34%, melting point: decomposition.
Exampl~ 288
[2-(1-Imidazolyl)-5-(meta-pyridyl)-benzyl]-methylthioether
~ 5-Bromo-2-(1-imidazolyl)-benzyl]-methylthioether (142 mg)
is refluxed together with 1 equivalent of diethyl-3-
pyridylborane, 10 mol% of tetrabutylammonium bromide, 57 mg of
KOH powder and 58 mg of tetrakistriphenylphosphine-palladium(~)
for 3 hours. The mixture is poured onto water, extracted with
ethyl acetate; the organic phase is dried with magnesium sulfate
and concentrated by evaporation. Column chromatography with EE
and ethanol follows. The yield is 84%. Melting point: 105~.
Bxample 289
a) [5-(Thien-2-yl)-2-(1-imidazolyl)-phenylmethyl]-
thiomethylether and [5-(thien-2-yl)-2-(1-imidazolyl)-
phenylmethyl]-methyl sulfoxide
5-Bromo-2-(1-imidazolyl)-phenylmethyl]-thiomethylether (141
mg) is mixed under protective gas in 2 ml of DME with 58 mg of
tetrakis-triphenylphosphine-palladium(O); 1.2 equivalents of 2-
CA 0223~621 1998-04-22
thiopheneboric acid (77 mg) as well as 126 mg of sodium
bicarbonate in 2 ml of water are added, and the mixture is
stirred for 4 hours at 100~. It is poured onto water, extracted
with ethyl acetate, the organic phase is dried with magnesium
sulfate and concentrated by evaporation. Column chromatography
with toluene/EE 1:1 follows. The yield totals 64%. [lH]-NMR
(CDCl3): 7.9(s broad lH), 7.6 to 7.2(m 8H), 3.48(s 2H), 2.01 (s
3H)- tlH]-NMR (CDCl3) sulfoxide: 7.78 to 7.16(m 9H), 3.78, (q,
2H), 2.53 (s 3H).
CA 0223~621 1998-04-22
Yl
Analogously, there are obtained:
b) [5-(Thien-3-yl)-2-(1-imidazolyl)-phenylmethyl]-
thiomethylether in 87% from 3-thiopheneboric acid.
Educt Product Yield
c) 2-Bromo-phenyl-l-(l- 2-Thien-2-yl-phenyl-1-(l- 71%
imidazole) imidazole)
d) 2-bromo-phenyl-l-(1- 2-thien-3-yl-phenyl-1-(1- 77%
imidazole) imidazole)
e) 3-bromo-pyridyl-2-(1- 3-thien-2-yl-pyridyl-2-(l- 97%
imidazole) imidazole)
f) 3-bromo-pyridyl-2-(1- 3-thien-3-yl-pyridyl-2-(1- 98%
imidazole) imidazole)
g) 2-bromo-3-chloro- 3-chloro-2-(thien-2-yl)- 55%
phenyl-1-(1-imidazole) phenyl-1-(1-imidazole)
h) 2-bromo-5-chloro- 5-chloro-2-(thien-2-yl)- 61%
phenyl-1-(1-imidazole) phenyl-l-(l-imidazole)
i) 2-bromo-5-chloro- 5-chloro-2-(thien-3-yl)- 94%
phenyl-l-(1-imidazole) phenyl-1-(1-imidazole)
k) 2-bromo-4-nitro- 4-nitro-2-(thien-2-yl)- 84%
phenyl-l-(l-imidazole) phenyl-1-(1-imidazole)
l) 2-bromo-4-nitro- 4-nitro-2-(thien-3-yl)- 70%
phenyl-1-(1-imidazole) phenyl-l-(1-imidazole)
m) 2-bromo-4-methyl- 4-methyl-2-(thien-2-yl)- 64
phenyl-l-(1-imidazole) phenyl-1-(1-imidazole)
n) 2-bromo-4-methoxy- 4-methoxy-2-(thien-2-yl)- 80
phenyl-1-(1-imidazole) phenyl-l-(l-imidazole)
o) 4-methoxy-2-(thien-2- 4-hydroxy-2-(thien-2-yl)- 99%
yl)-phenyl-l-(l- phenyl-l-(1-imidazole) by
imidazole) ether cleavage
p) 4-nitro-2-(thien-2- 4-amino-2-(thien-2-yl)- 79%
yl)-phenyl-1-(1- phenyl-1-(1-imidazole) by
imidazole) reduction
q) 4-nitro-2-(thien-3- 4-amino-2-(thien-3-yl)- 51%
yl)-phenyl-1-(1- phenyl-1-(1-imidazole) by
imidazole) reduction
r) 4-amino-2-(thien-2- 4-bromo-2-(thien-2-yl)- 33
yl)-phenyl-1-(1- phenyl-1-(1-imidazole)
imidazole) according to Sandmeyer
92
Example 290
3-(1-Imidazolyl)-benzamidine-hydrochloride
Hydrochloric acid is directed into a cooled solution of 0.47
g of 3-(1-imidazolyl)-benzonitrile in 5 ml of ethanol, and the
reaction mixture is stirred overnight in a sealed flask. After
the solvent is removed, the residue is dissolved in 10 ml of
ethanol, and ammonia is directed into the cooled solution. The
sealed flask is heated to 75~C in an oil bath for 2 hours. After
cooling, the title compound is filtered off.
Example 291
N-{2-t2-(1-Imidazolyl)-phenoxy]-ethyl}-quanidine-hydrochloride
100 mg (10.5 mmol) of the amine that is produced according
to Example 231 is added to 1.5 ml of DMF, mixed with equivalent
amounts of 1-H-pyrazole-carboxamidine-hydrochloride and
diisopropylethylamine and stirred until the reaction is completed
at room temperature. Since it was spun in until the dry state
was achieved, it is chromatographed on silica gel with CHzCl2,
ethanol, H20 as mobile solvent. 62.2 mg (40%) is obtained.
Example 2 9 la
Analogously, N-{2-[4-(1-imidazolyl)-3-methylthiomethyl-
phenoxy]-ethyl}-quanidine hydrochloride follows from the amine in
18% yield.
- . -
CA 0223~621 1998-04-22
Example 29lb
N-{4-[2-(1-Imidazolyl)-phenoxy]-butyl}-guanidine-hydrochloride
100 mg (0.43 mmol) of the amine that is produced according
to Example 232 is reacted as described in Example 290. 48.6 mg
(88.6%) of the desired compound is isolated.
Example 292
N-{5-[2-(1-Imidazolyl)-phenoxy]-pentyl}-guanidine-hydrochloride
100 mg (0.41 mmol) of the compound that is produced
according to Example 233 is reacted as described in Example 290.
57 mg (45.4%) of the desired guanidine hydrochloride is obtained.
Example 293
N-~6-[2-(1-Imidazolyl)-phenoxy]-hexyl}-guanidine-hydrochloride
100 mg (0.38 mmol) of the compound that is produced
according to Example 234 is reacted as described in Example 290.
112.7 mg (86.5%) of the desired compound is obtained.
Ex~mpl~ 294
N-~7-[2-(1-Imidazolyl)-phenoxy]-heptyl}-guanidine-hydrochloride
100 mg (0.37 mmol) of the compound that is produced
according to Example 235 is reacted as described in Example 290.
115.7 mg (89.6%) of the desired guanidine-hydrochloride is
isolated.
CA 0223~621 1998-04-22
94
Bxample 2 95
N-{6-t4-(1-Imidazolyl)-phenoxy]-hexyl}-guanidine-hydrochloride
100 mg (0.386 mmol) of 6-[4-(1-imidazolyl)-phenoxy]--
hexylamine is reacted as described in Example 290. After the
working-up, 115.2 mg (88%) of the desired compound is isolated.
Example 296
N-[2-(1-Imidazolyl)--phenyl]-N'-methyl-thiourea
0.2 g of 2-amino-phenyl-1-(1-imidazole) is dissolved in 4 ml
of THF and mixed with 86 ,~Ll of methyl isothiocyanate in 1 ml of
THF. After slight heating, the reaction is terminated. The THF
is spun off, and the residue is recrystallized from isopropanol.
First, some educt precipitates; in addition, product is obtained
with a melting point of 287~C dec. and with a yield of 27%.
Analogously, there are obtained:
Examp 1~ 2 9 7
N-[2-(1-Imidazolyl)-benzyl]-N'-methyl-thiourea, yield: 31%,
melting point: 128~.
Example 2 9 8
[2-(1-Imidazolyl)-benzyl]-methylsulfone hydrochloride
[2-(1--Imidazolyl)--benzyl]--methylsulfone(0.75 mmol) is
stirred in 6 ml of lN HCl in ether and 10 ml of ethanol for 6
hours at room temperature. Some ether is added, and it is put
into the refrigerator. Crystals are suctioned off, washed and
dried. Yield: 89%, melting point: 201~.
- ~ = -
CA 0223~62l l998-04-22
Example 299
[5-Cyano-2-(1-imidazolyl)-benzyl]-methylthioether hydrochloride,
yield: 88%, melting point: 211~ dec., lH-NMR (DMSO d6):
9.32d(s lH), 8.16(1H), 8.1 (mult. 2H), 7.8 (mult. 2H), 3.77(s
2H), 1.93(s 3H).
Analogously, hydrochlorides and hydrobromides are produced,
such as
Example 300
t2-(1-Imidazolyl)-benzyl]-methylthioether hydrobromide
150 mg of [2-(1-imidazolyl)-benzyl]-methylthioether in 3 ml
of 47% HBr is dissolved, concentrated by evaporation after 3
hours at room temperature and dried on a bulb tube in a vacuum.
Yield: 100%, lH-NMR (DMSO d6): 9.51s broad(lH), 8.1(s lH), (lH),
7.97(s lH), 7.7 to 7.50 (mult. 4H), 3.71(s 2H), 1.92(s 3H).
Other salts are also produced analogously, such as
Example 301
[2-(1-Imidazolyl)-benzyl]-methylthioether oxalate
[2-(1-Imidazolyl)-benzyl]-methylthioether (110 mg) is
stirred in 2 ml of ethanol with 1 equivalent of oxalic acid for a
total of 12 hours at room temperature; after 24 hours in a
refrigerator, it is suctioned off and dried. Yield: 85%,
melting point: 102~.
CA 0223~621 1998 - 04 - 22
Example 302
[2-(1--Imidazolyl)-benzyl]-methylsulfoneoxalate, yield: 88%,
melting point: 175~.
Example 303
[2-(2'-Bromo-imidazol--1-yl)-benzyl]-methylthioether
204 mg of t2-(imidazol-1-yl)-benzyl]-methylthioether is
stirred with 1 equivalent each of triphenylphosphine and
tetrabromomethane in 6 ml of dichloromethane. After 12 hours at
room temperature, the reaction is completed. The mixture is
concentrated by evaporation, and it is all added on a column.
After column chromatography, the title compound is obtained with
a yield of 59%. lH-NMR (CDCl3): 7.58 to 7.2 (mult. 6H), 3.35(d
lH), 3.53(d lH), l.99(s 3H).
Example 304
2--[4--(1--Imidazolyl)--phenoxy]-aceticacid--hydrochloride
100 ml of 6N HCl is mixed with 1.2 g of 2-[4-(1-imidazolyl)-
phenoxy]-acetic acid methyl ester, stirred for 4 hours and
concentrated by evaporation. The residue is treated with
ethanol, and the title compound with a melting point of 116--117~C
is obtained.
Example 305
2--[3--(1--Imidazolyl)-phenoxy]--aceticacid--hydrochloride
4 g of 3-(1-imidazolyl)--phenol, 1.8 ml of bromoacetic acid
methyl ester and 6.5 g of cesium carbonate are added to 50 ml of
97
DMS0. The mixture is stirred for 1 day, added in water and
extracted with methylene chloride. The organic phase is
separated, washed with water, dried, and the solvent is drawn
off. The residue is taken up in 100 ml of 6N HCl. After 4 hours
of stirring, it is concentrated by evaporation, and the residue
i5 treated with ethanol. The title compound with a melting point
of 206-208~C is obtained.
Example 306
2-t2-(1-Imidazolyl)-phenylmethyl]-methanesulfonamide
20 ml of methylene chloride is mixed with 1.0 g of 2-(1-
imidazolyl)-benzenemethanamine, 0.43 ml of methanesulfonyl
chloride and 1.3 ml of triethylamine. After stirring overnight,
water is added, the organic phase is separated, dried, and the
solvent is drawn off in a vacuum. After recrystallization in
alcohol, the title compound is obtained.
Example 307
[4-(1-Imidazolyl)-3methylthiomethyl-phenol]-4'-cyanophenylether
2 g of 4-(1-imidazolyl)-3-methylthiomethyl-phenol is stirred
in DMF with 1.1 g of p-fluorobenzonitrile and 1.506 g of
potassium carbonate for 7 hours at 120~. The mixture is poured
onto waterj extraeted wit~. et~yl acetate, the organiG phasQ is
dried with magnesium sulfate and concentrated by evaporation.
77.4% product results. [lH]-NMR (CDCl3): 7.7(m 3H), 7.32 to
7.0(m 7H), 3.46(s 2H), 2.03(s 3H).
CA 0223~62l l998-04-22
98
4-[4-(1-Imidazolyl)-3-methylthiomethyl-phenoxy]-benzoic acid
amide is obtained from the above in 33% yield with potassium
hydroxide in ethylene glycol.
Analogously, there are obtained:
a) from fluorobenzene, [4-(1-imidazolyl)-3-
methylthiomethyl-phenol]-phenylether in 26% yield
b) from 2-bromopyridine, [4-(1-imidazolyl)-3-
methylthiomethyl-phenol]-2-pyridylether in 7% yield
c) from 3-bromopyridine, [4-(1-imidazolyl)-3-
methylthiomethyl-phenol]-3-pyridylether in 18% yield
d) from 4-fluoronitrobenzene, [4-(1-imidazolyl)-3-
methylthiomethyl-phenol]-4'-nitrophenylether in 91% yield
e) from 2-fluorobenzonitrile, [4-(1-imidazolyl)-3-
methylthiomethyl-phenol]--2'-cyanophenylether in 81% yield
f) from 4-fluoroanisole, [4-(1-imidazolyl)-3-
methylthiomethyl-phenol]-4'-methoxyphenylether in 30% yield
g) from 4-bromobenzotrifluoride, [4-(1-imidazolyl)-3-
methylthiomethyl-phenol]--4'-trifluoromethylphenylether in 58%
yield
h) from 4-fluorobenzoic acid ethyl ester, 4-[4-(1-
imidazolyl)-3-methylthiomethyl-phenoxy]-ethylbenzoate in 17%
yield
i) 3-iodobenzotrifluoride, [4-(1-imidazolyl)-3-
methylthiomethyl-phenol]-3'-trifluoromethylphenylether in 62%
yield
CA 0223~621 1998-04-22
k) 4-hydroxymethylpyridine, [4-(1-imidazolyl)-3-
methylthiomethyl-phenol]-4-pyridylmethylether in 17% yield under
Mitsunobu conditions
l) from e) in 22% yield with potassium hydroxide in
ethylene glycol, 2-[4-(1-imidazolyl)-3-methylthiomethylphenoxy]-
benzoic acid amide
m) by reduction, t4-(1-imidazolyl)-3-methylthiomethyl-
phenol]-4'-aminophenylether from [4-(1-imidazolyl)-3-
methylthiomethyl-phenol]-4'-nitrophenylether in 59% yield.
Melting point: 203~.
Example 308
N-{[4-(1-Imidazolyl)]-(3-methylthiomethyl)-phenyl}-N'-{[4-(2-
pyridiminyl)]-(4-azapentamethylene)]-urea
110.2 mg (0.371 mmol) of triphosgene is dissolved in 2 ml of
absolute dichloromethane. 219.9 mg (1 mmol) of {[4-(1-
imidazolyl)]-(3-methylthiomethyl)}-aniline (amine 1) and 142.6 mg
(1.103 mmol) of diisopropylethylamine, dissolved in 4 ml of
dichloromethane, are added in drops to this solution under
protective gas within 30 minutes at room temperature. Then,
237.8 mg (1.003 mmol) of N-(2-pyrimidinyl)-piperazine-
dihydrochloride (amine 2) and 427.8 mg (3.309 mmol) of
diisopropylethylamine, dissolved in a mixture of 4 ml of
dimethylformamide and 4 ml of dichloromethane, are added. After
being stirred overnight, it is evaporated to the dry state, and
the residue that remains is chromatographed on silica gel (mobile
solvent dichloromethane/isopropanol or ethyl
CA 0223~621 1998-04-22
100
acetate/isopropanol). 186.6 mg (45.4%) of the desired urea
remains. Melting point 175-180~C.
IR (KBr) 3115, 1670, 1586, 1508, 1410, 1254, 1214 cm~
Example 309
N-{[4-(1-Imidazolyl)]-(3-methylthiomethyl)-phenyl}-N'-{2-[(3,4-
methylenedioxy)-phenyl]-ethyl}-urea
Production is carried out as described in Example 1 with use
of 439.9 mg (2.006 mmol) of {[4-(1-imidazolyl)]-(3-
methylthiomethyl]}-aniline (amine 1) and 404.5 g (2.006 mmol) of
{2-[(3,4-methylenedioxy)-phenyl]-ethylamine (amine 2). The base
amounts are adapted accordingly. After chromatography on silica
gel (dichloromethane/methanol or ethyl acetate as mobile
solvent), 144.8 mg (17.5%) of the desired urea is obtained,
melting point 55-60~C.
IR (KBr) 1701, 1685, 1654, 1560, 1508 cm~
Examplo 310
N,N'-Bis-[4-(1-imidazolyl)]-3-methylthiomethyl)-phenyl]-urea
219.9 mg (1.003 mmol) of [4-(1-imidazolyl)]-(3-
methylthiomethyl)]-aniline (amine 1) is reacted as described. As
amine 2, [4-(1-imidazolyl)]-(3-methylthiomethyl)]-aniline is also
used. After chromatography on silica gel (mobile solvent ethyl
acetate/methanol), the product is isolated in 41.7~ (194.5 mg)
yield, melting point 204-210~C.
IR (KBr) 3066, 2914, 2814, 1709, 1593, 1575, 1412, 1207 cm~
.
CA 0223~621 1998-04-22
101
Example 311
N-{t4-(1-Imidazolyl)]-(3-methylthiomethyl)-phenyl}-N'-(2-
thienylmethyl)-urea
219.9 mg (1.003 mmol) of {[4-(1-imidazolyl)]-(3-
methylthiomethyl)}-aniline (amine 1) is reacted as described. As
amine component 2, 2-thienylmethylamine is used. After
chromatography on silica gel (mobile solvent: ethyl acetate),
172.9 mg (52.8%) of the desired urea is obtained.
IR (KBr) 3320, 2920, 1700, 1660, 1550, 1500, 1410, 1220,
1050 cm~1
Example 312
[4-(1-Imidazolyl)]-(3-methylthiomethyl)-phenol-nonaflate
5 g (22.67 mmol) of {[4-(1-imidazolyl)]-(3-
methylthiomethyl)}-phenol is dissolved in 75 ml of
dimethylformamide and mixed at room temperature with 755.7 mg of
an 80% NaH suspension (25.19 mmol). After 30 minutes of stirring
at 60~C, 8.47 g (28.05 mmol) of perfluorobutanesulfonic acid
fluoride is added, and it is stirred for another 90 minutes at
60~C. After cooling, the reaction mixture is mixed with about 50
ml of saturated NaHC03 solution and stirred for 30 minutes at
room temperature, and the mixture is diluted with water. After
shaking three times with ethyl acetate, the combined organic
extracts are washed twice with saturated NaCl solution and dried
on sodium sulfate. After the dessicant is filtered off, the
solvent is spun in, and the remaining residue is chromatographed
CA 0223~621 1998-04-22
lU~
on silica gel (mobile solvent ethyl acetate). 11.2 g (28.2%
product) is isolated.
Bxampl~ 313
t4-(1-Imidazolyl)]-(3-methylthiomethyl)-benzoic acid methyl ester
11.2 g (22.3 mmol) of 4-(1-imidazolyl)]-(3-
methylthiomethyl)-phenol-nonaflate is dissolved in a mixture of
22.2 ml of methanol and 44.6 ml of dimethylformamide. After 4.5
g (44.6 mmol) of triethylamine, û.145 (0.646 mmol) of
palladium(II) acetate and 0.275 g (0.666 mmol) of 1,3-bis-
diphenylphosphonopropane are added, O-gas is allowed to bubble
through for 30 minutes. Under CO atmosphere, the reaction is
stirred overnight at 80~C.
The reaction mixture is added to NaCl solution. After
extraction with ethyl acetate three times, the combined organic
phases are washed twice with saturated NaCl solution. After
drying on Na2SO4 and after the dessicant is filtered off, the
solvent is spun off, and the residue is chromatographed on silica
gel (mobile solvent ethyl acetate; then
dichloromethane/methanol). The yield is 5.30 g (1.4%).
Example 314
[4-(1-Imidazolyl)]-(3-methylthiomethyl)-benzoic acid
4.3 g (16.4 mmol) of [4-(1-imidazolyl)]-(3-
methylthiomethyl)-benzoic acid methyl ester in 10 ml of methanol
is stirred with 10 ml of a 5% aqueous lithium hydroxide solution
overnight at room temperature.
CA 0223~621 1998-04-22
103
The reaction mixture is brought to pH 4-5 with 10% sulfuric
acid and then poured onto Extrelut. After elution with about 300
ml of dichloromethane and about 300 ml of methanol, the organic
extracts are concentrated by evaporation, and the residue is
chromatographed on silica gel (mobile solvent
dichloromethane/methanol). 3.36 g (82.6%) of product is
isolated, melting point 130-135~C.
Example 315
[4-(1-Imidazolyl)]-(3-methylthiomethyl)-benzoic acid-L-histidine
methyl ester amide
0.400 g (1.611 mmol) of [4-(1-imidazolyl)]-(3-
methylthiomethyl)-benzoic acid is added together with 30 ml of
dimethylformamide, 0.320 g (1.611 mmol) of L-histidine methyl
ester, 2 HCl, 0.570 g (5.639 mmol) of triethylamine and 0.569
(1.772 mmol) of TBTU and stirred overnight at room temperature.
The reaction mixture is evaporated to the dry state, and the
residue is chromatographed on silica gel (mobile solvent
dichloromethane/methanol). 0.376 g (58.4~) of product remains,
melting point 55-65~C.
Exampl~ 316
[4-(1-Imidazolyl)]-(3-methylthiomethyl)-benzoic acid-histamine-
amide
0.200 g (0.806 mmol) of [4-(1-imidazolyl)]-(3-
methylthiomethyl)-benzoic acid is mixed in 15 ml of
dimethylformamide with 0.0869 g (0.806 mmol) of histamine,
CA 0223~621 1998-04-22
104
0.0815 g (0.806 mmol) of triethylamine and 0.2763 g (0.861 mmol)
of TBTU and stirred overnight at room temperature. After
spinning-in and chromatography on silica gel (mobile solvent
dichloromethane/mçthanol), 0.2445 g (88.9%) of product is
obtained.
Exampl~ 317
[4-(1-Imidazolyl)]-(3-methylthiomethyl)-benzoic acid]-[(5-nitro-
2-pyridyl)-amino]-ethylenamide
0.150 g (0.604 mmol) of [4-(1-imidazolyl)]-(3-
methylthiomethyl)-benzoic acid is reacted under the usual
conditions with 2-(2-aminoethylamino)-5-nitropyridine. After
working-up and chromatography on silica gel (mobile solvent ethyl
acetate/ethanol or dichloromethane/isopropanol), 86 mg (34.5~) of
product remains, melting point 201-203~C.
Example 318
t4-(1-Imidazolyl)]-(3-methythiomethyl)-benzoic acid-(2-anilino)-
ethylenamide
0.150 g (0.604 mmol) of [4-(1-imidazolyl)]-(3-
methylthiomethyl)-benzoic acid is reacted as usual with (2-
anilino)-ethyl~n~;ne. After working-up and chromatography on
silica gel (mobile solvent ethyl acetate or
dichloromethane/isopropanol), 149.6 mg (67.6%) of product is
obtained, melting point 114-170~C.