Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02235705 1998-04-23
INTEGRAL GUIDED TISSUE REGENERATION BARRIER
FOR ROOT-FORM DENTAL IMPLANTS
The present invention relates generally to the field of dental implants.
s BACKGROUND OF THE INVENTION
Predictably useful dental implants had their beginnings in Sweden in
the 1960's in work done by Per-Ingmar Branemark, who discovered that lab
animals'
bone cells would deposit mineralized bone directly on implanted titanium
objects,
thereby solidly attaching them to the surrounding bone. He is credited with
coining
io the term osseointegration, which is now in common use, to identify this
process.
Since his discovery, thousands of titanium implants of mostly screw-type
design have
been inserted in people's toothless spaces to anchor prosthetic teeth. This
implantation has been done almost exclusively by elevating the soft tissue,
drilling a
hole in bone, placing the implant in the hole, stitching the soft tissue back
over the
is implant, waiting a period of months, re-opening the soft tissue, uncovering
the
implant and attaching a stud to project through the gum. If bone augmentation
has
been necessary, yet more operatians have been required.
If one counts the extraction surgery that leads to most toothless spaces,
patients progressing from having teeth to having a toothless space, then
getting an
2o implant and fastening on a prosthetic tooth, must submit to at least three
surgeries.
The total rises to five if bone augmentation is required and done as a
separate
procedure. The number of surgeries would be reduced to one if an implant
inserted
CA 02235705 1998-04-23
-2-
on tooth removal would osseointegrate. If this could be done, patients would
expE~rience fewer painful, expensive and time-consuming surgeries and avoid
bone
loss in extraction sites, which is associated with problems of appearance,
comfort
and insufficient bone volume for eventual implants. Their periods of wearing
s temporary prosthetic replacement teeth or doing without teeth would be
shorter.
The unpredictability of success with currently available technology has
meant that only a tiny proportion of implants have been placed in tooth
sockets
immediately after teeth have been removed. Causes of failure have been
presence of
bacteria in the sockets, implant movement in the tooth socket due to the
implant
io being inadequately fixed in place, and soft tissue layers being deposited
against
titanium) preventing osseointegration. One can reduce or eliminate bacteria
from
extr<~ction sockets with surgical instruments, sterile technique and
antibiotics. Soft
tissue deposition and implant movement can be eliminated by the present
invention:
a guided-tissue-regeneration barrier integrated with root-form dental
implants.
is Placing a barrier to prevent migration of selected cell types into
selected areas to control tissue formation during healing is termed guided
tissue
regE~neration (GTR). A GTR barrier fixed around an implant could prevent
migration
of fibroblasts and epithelial cells into the healing socket, preventing
fibrous tissue or
epithelium from being created there, and allowing slower-migrating bone cells
20 (osteoblasts) to populate the socket and deposit bone throughout, including
directly
on the surface of the implant, osseointegrating it into the jaw to be an
anchor for a
prosthetic tooth.
CA 02235705 1998-04-23
-3-
A barrier securely attached to an implant could increase its stability.
The barrier would need to be malleable in order to be closely adapted to the
surface
of the bone of the alveolar crest surrounding the extraction site. It would
have to
retain the form into which it was shaped and allow circulation to be
established
s through it, in order to prevent it from becoming exposed. It would need to
be easy for
a surgeon to cut it to fit around adjoining teeth and within the limits of the
surgical
site. It could not cause an inflammatory response, so that the overlying
mucosa
sutured tightly over it would remain firm and capable of preventing it from
moving. Its
rigid connection to the implant member of the implant-barrier combination
would help
io the implant resist displacement forces applied to it.
Ideally, it should be possible to insert implants in tooth sockets
immediately after removal of teeth with predictably successful
osseointegration, by
performing the two functions of excluding unwanted cells from the extraction
socket
and increasing the stability of the implant.
is An integrated guided-tissue-regeneration barrier for root-form dental
implants could be made either from a material which would be resorbed after it
had
served its purpose, or of a material that would remain permanently in place
around
the implant. There is, however, no non-resorbable barrier material that is
conventionally being left in place after periodontal or implant-related guided
tissue
zo regeneration. Expanded polytetrafluoroethylene, the most common GTR barrier
in
use over the last 20 years, can be left in place for only a few weeks because
of its
disadvantages. In common with other textiles, it cannot be formed into a 3-
dimE~nsional shape that it will retain, so surgeons cannot place it precisely
where they
CA 02235705 1998-04-23
-4-
want it. It penetrates the overlying gum in a high proportion of cases because
of its
tendency to revert to its original flat shape and because blood vessels cannot
penetrate it. It will not support itself over a void and must therefore be
supported by
another material placed on the bone. (It is useful as a GTR barrier despite
its
s disadvantages because it has pores small enough to prevent cellular
migration and
bec<~use it does not provoke inflammation).
Titanium foil and mesh have been tried as alternative barriers. Both
have successfully permitted bone growth) but foil shares expanded
polytetrafluoroethylene's propensity to penetrate the overlying gum, and must
be
io removed once the new bone has formed. Mesh, however, has none of the above-
mentioned disadvantages. It can be formed into complex three-dimensional
shapes,
cut i:o shape, and is rigid enough to contribute to the stability of an
implant inserted
through it. It looks to be a useful material for ensuring that osteoblasts can
migrate to
where they are wanted without having to win a race with faster-moving
fibroblasts
is and epithelial cells. Titanium mesh is being embedded and left permanently
in place
in people's jaws (and other parts of their bodies) during other types of
surgery., Like
titanium implants, it does not provoke an inflammatory response in these
situations
and is well-tolerated by the surrounding tissue. Dr John Gay and his
associates in
Toronto have used titanium mesh barriers to promote bony infill with GTR. When
2o doing re-entry surgery to remove the barriers, they have noted absence of
inflammation, intimate tissue adaptation to the mesh, and a considerable
degree of
difficulty in removing the mesh. They question whether it is really necessary
to
CA 02235705 1998-04-23
-5-
remove the titanium mesh barrier material, which they and other surgeons do
insert
permanently when doing orthognathic surgery, for example.
Titanium mesh, either on its own or bonded to a rib reinforcement
system stamped from light gauge (in the neighbourhood of 50 micron thickness)
s sheet titanium could be used to form a permanent integrated guided tissue
regE:neration barrier for root-form dental implants.
If the barrier were to be resorbable, it could be made of a rib
reinforcement system stamped from light gauge sheet titanium bonded to a
resorbable membrane such as a bioabsorbable collagen membrane made of type I
io bovine tendon collagen. The titanium reinforcement system would permit the
barrier
to be formed to the shape of the bone surrounding the implant, to retain the
shape
once formed and to be light enough to allow a surgeon to cut the barrier to
fit the
surctical site. As well, the reinforcement system would allow the barrier to
fit tightly
and securely about the implant, so as to provide an effective barrier to the
passage of
is unwanted cells right up to the implant and contribute to the positional
stability of the
impllant. Only the barrier membrane would be resorbable: the rib reinforcement
system would remain permanently.
Without a titanium reinforcement system, lateral movement of an
implant within a resorbable barrier would deform the barrier material. The
resorbable
2o material by itself would neither contribute to the stability of the implant
nor, if
deformed, remain an effective barrier immediately beside the implant, where it
is most
important to prevent migration of unwanted cell types.
CA 02235705 1998-04-23
-6-
Ideally, the integrated guided-tissue-regeneration barrier, whether with
a permanent or resorbable membrane, should be left in place after insertion,
making
re-entry surgery to remove it unnecessary. Not only would retrieval of the
barrier be
unrnecessary, but uncovering the implant would be as well. It is intended that
the full-
s thickness flap elevated to expose the bony site for integrated barrier
placement be
sutured around, not over, the implant cover screw. The cover screw would guide
the
gingival tissue to heal around it so that it would be continuously visible. It
would be
painlessly removable and replaceable without local anaesthetic when the time
came
to attach a prosthesis to the implant.
io Integrated barriers in combination with implants would be useful not
only in fresh extraction sockets, but also in toothless sites with
insufficient bone
volume. Using them, bone augmentation, implant insertion and implant exposure
could be accomplished with only two surgeries, one or two fewer than current
techniques require. The reduction would be possible because the integrated
barrier
is would not be removed. The integrated barrier would also make the operations
easier
and faster for the surgeon.
The range of dental surgeons providing implants with integrated
barriers immediately on extraction of teeth could encompass general dentists
capable
of the limited flap surgery required, as well as periodontists and oral
surgeons. This
2o range is wider than the range of simple implant providers, and could
potentially
increase the number of patients who could benefit from implants with
integrated GTR
barriers.
CA 02235705 1998-04-23
_7_
If priced correctly, immediate implants with integrated GTR barriers
would be found preferable to extraction and bridging, complex root canal
treatment
and toothless spaces. By being low-cost, single-surgery, short-healing-time
tooth
replacements, they would be apt to make the public more familiar with the
benefits
and relative ease of implants in general, and would therefore be apt to
increase the
number of simple implants being placed in edentulous sites.
SUPJIMARY OF THE INVENTION
According to the invention, there is provided an integrated guided-tissue-
io regeneration barrier to be attached to a root-form implant member of either
screw-in or
pre;>s-fit type. The barrier is composed of a membrane arranged to exclude
soft tissue
cell:;, a titanium reinforcement system, joining means for connecting the
membrane to
the reinforcement system and joining means for connecting the barrier to the
root-form
dental implant.
is The function of the integrated barrier is to make it possible for the
implant
portion of the implant-barrier combination to become osseointegrated after
having been
insE~rted in a tooth socket immediately after removal of a tooth. It will do
this by
decreasing the likelihood of movement of the implant in the extraction site
and by
preventing unwanted cell types fram entering and proliferating in the socket.
2o The integrated barrier is to be left permanently in place, in contrast with
non-resorbable guided-tissue-regeneration barriers used to increase bone
volume for
implant insertion.
CA 02235705 1998-04-23
_$_
The dental implant-barrier combination, though most useful in fresh
extraction sockets, could also be used in edentulous areas to induce bone
growth to
create increased bone volume where bone height or width were inadequate to
envelope
the implant.
Preferably, the barrier is composed of a light gauge sheet titanium spyder
bonded either to titanium mesh or a resorbable membrane such as a
bioabsorbable
collagen membrane made of type I bovine tendon collagen, or composed solely of
titanium mesh, all three constructions possessing biocompatibility, the
malleability
necessary to allow the barrier to be intimately adapted to the bony surface of
the
io alveolar crest and the possibility of being cut to final shape at the time
of surgery.
The barrier may be pre-formed in order for it to conform to the shape of
the bone of the maxillary or mandibular alveolar crest, thereby reducing the
amount of
manipulation needed during the surgical procedure in which it is inserted.
The means of joining the barrier membrane to the titanium reinforcement
is spyder may comprise bonding with (for example) an epoxy or cyanoacrylate
adhesive,
welding (only with a titanium mesh barrier membrane) or embedding the titanium
reinforcement spyder in the resorbable membrane during manufacturing.
The means of joining the barrier to the implant may comprise in situ
insertion of the implant through the barrier after prior adaptation of the
barrier to the
2o surrounding bone, so that the implant fits tightly in the opening provided
for it in the
barrier. Additionally, the joining means may, for press-fit implants, comprise
welding,
bonding with (for example) an epoxy or cyanoacrylate adhesive and press
fitting into a
CA 02235705 1998-04-23
_g_
groove the width of the thickness of the barrier. These additional means would
be
accomplished during the manufacturing process, before sterilization and
packaging,
rather than at the time of surgery. For screw-type implants, which must be
rotated to be
placed, the joining means may also comprise a titanium collar or ring which
could rotate
s in a race or groove in the implant, and to which the barrier could be
attached so the
implant could be screwed into place without the barrier rotating. This
additional joining
means would also be accomplished during the manufacturing process.
The above-mentioned collar could be made with a break in its
circumference to allow it to be opened enough to be snapped into place in the
groove
io meant to accept it, or the implant could be made in two pieces, with the
two being joined
after' collar and mesh were in place.
The barrier may be pre-sterilized and packaged as a separate element, to
remain so until removed from its package at the time of surgery, then cut to
final shape
and adapted to the bone surrounding the implant site, The implant-barrier
combination
is is created by the surgeon inserting the implant through the barrier after
the barrier is in
place in the surgical site.
Alternatively, the barrier and implant are joined to create the combination,
then pre-sterilized and packaged. In this embodiment, the barrier-implant
combination
prefE~rably includes a removable handle in order for it to be manipulated
without actually
2o being touched, so that when the surgeon removes the combination from its
sterile
packaging at the time of surgery, he can cut and shape the barrier without
touching the
impl<~nt member.
CA 02235705 1998-04-23
-10-
BRIEF DESCRIPTION OF THE FIGURES
Embodiments of the invention are shown in the accompanying
drawings, in which:
s Figure 1 is a top view of an integrated guided-tissue-regeneration
barrier attached to a root-form dental implant.
Figure 2 is a side view of an integrated guided-tissue-regeneration
barrier attached to a root-form dental implant of press-fit design.
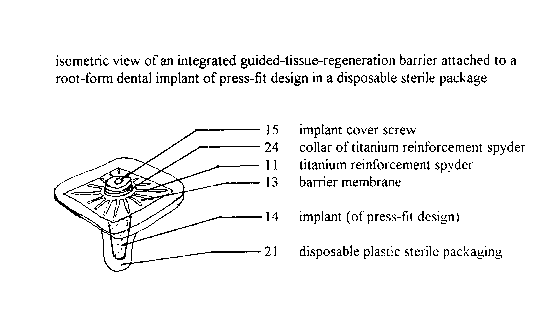
Figure 3 is an isometric view of an integrated guided-tissue-
io regE~neration barrier attached to a root-form dental implant of press-fit
design in a
disposable sterile package.
Figure 4 is a schematic view of an integrated guided-tissue-
regE~neration barrier attached to a root-form dental implant of press-fit
design inserted
in an immediate extraction socket.
is Figure 5 is a schematic view of an integrated guided-tissue-
regE~neration barrier attached to a root-form dental implant of press-fit
design inserted
in an osteotomy (drilled out) site with inadequate bone volume and the barrier
contoured to permit bone growth to fully imbed the implant.
Figure 6 is a schematic view of an osseointegrated press-fit implant with
2o an integrated guided-tissue-regeneration barrier attached to an abutment
and a
prosthetic tooth.
Figure 7 is a side view of an integrated guided-tissue-regeneration
CA 02235705 1998-04-23
-11 -
barrier attached to a screw-type implant.
Figure 8 is a side view of an integrated guided-tissue-regeneration barrier
attached to a root-form dental implant of press-fit design in a disposable
sterile
package.
s Figure 9 is a isometric view of an integrated guided-tissue-regeneration
barrier with no implant attached
Figure 10 is an isometric view of a collared integrated guided-tissue-
regeneration barrier made solely of titanium mesh.
Figure 11 is a top view of a collarless titanium mesh integrated guided-
io tissue-regeneration barrier attached to a root-form dental implant.
Figure 12 is a cutaway side view of an integrated guided-tissue-
regE:neration barrier attached to an implant of screw-in design.
In the drawings, like characters of reference indicate corresponding parts in
the different figures, as denoted here:
is
integrated guided-tissue-regeneration barrier attached to a root-form dental
implant;
11 titanium reinforcement spyder;
12 barrier;
13 barrier membrane;
14 root-form dental
implant;
15 implant cover screw;
16 tooth extraction
socket;
17 abutment;
zs 18 gum;
CA 02235705 1998-04-23
-12-
19 prosthetic tooth;
20 bone;
21 disposable plastic sterile packaging;
22 area between barrier and bone in which bony infill is desired for implant
s fixation;
24 raised collar on titanium reinforcement spyder;
26 groove in screw-type implant in which collar may rotate;
28 threads on screw-type implant;
30 disposable plastic handle.
io
DETAILED DESCRIPTION
Referring to the drawings, an implant-barrier combination 10 is comprised
of a root-form implant member 14, a barrier 12 and an implant cover screw 15.
The barrier 12 is arranged to stabilize the root-form implant member 14,
is and to act as a barrier to soft tissue cells, as described below.
Specifically, the barrier
12 acts to exclude fibroblasts and epithelial cells from a tooth extraction
socket 16. In
this embodiment, the barrier 12 is composed of titanium mesh, specifically 100
x 100
mesh. It is of note that the titanium mesh is biocompatible and malleable as
described
below.
2o The implant member 14 of the implant-barrier combination 10 is arranged
to be inserted into tooth extraction socket 16 as described below. The details
of the
implant member 14 are not shown as these are known to persons knowledgeable in
the
art.
The dental implant-barrier combination 10 is assembled by connecting the
CA 02235705 1998-04-23
-13-
barrier 12 to the implant member 14 such that the barrier 12 extends outwardly
from the
implant member 14 as shown in Figures 1-8. In this embodiment, the barrier 12
is
bonded to the implant member 14 with an epoxy adhesive. Alternatively, the
barrier 12
may be connected to the implant member 14 by other means, for example, by
welding
s the (barrier 12 to the implant member 14. It is of note that, once
assembled, the dental
implant-barrier combination 10 may be sterilized and enclosed in sterile
packaging 21
for later use, as shown in Figures 3 and 8. This in turn will greatly reduce
the risk of
bacterial contamination and surface contamination of the titanium surfaces of
the barrier
12 and implant member 14.
io In an embodiment wherein the implant member 14 with threads 28 is
arranged to be screwed into the extraction socket 16, the barrier 12 is
attached to a
colt<~r 24, which is arranged to be fitted into a circumferential groove 26 in
the implant
mernber 14. The collar 24, together with the attached barrier 12, may rotate
in the
groove 26 freely about the implant. member 14, or alternatively may be
stationary while
Is the implant member 14 is rotated while being screwed into the extraction
socket 16.
In another embodiment shown in Figure 8, the dental implant-barrier
combination 10 may include a removable handle 30 for aiding in manipulating
the
dental implant-barrier combination 10 during surgery.
In operation, before tooth extraction, the surgeon will evaluate the site
2o clinically and view its radiograph image to determine whether the tooth is
replaceable
with an implant-barrier combination 10, and if so, what implant member 14 size
the
implant-barrier combination 10 should have and how to cut and shape the mesh
CA 02235705 1998-04-23
-14-
barrier 12. The surgeon will elevate the gum 20 about the tooth, remove the
tooth,
mechanically debride the socket 16, confirm or modify implant-barrier
combination 10
choice and cut and contour the mesh barrier 12. The barrier 12 is sufficiently
malleable that the barrier 12 may be formed into the necessary shape either
prior to
s surgery or during surgery as desired. Thus the barrier 12 may be readily
manipulated so as to conform to the desired shape, a shape which it will
retain. The
surgeon will then insert the implant member 14 in the socket 16 and press the
barrier
12 against the adjoining bone. The gum 20 will be sutured in place over the
barrier
12 and around the implant cover screw 15, the repositioned gum 20 holding the
mesh
io barrier 12 firmly in place on the bone and the barrier 12 in turn helping
to stabilize the
implant member 14 by virtue of its rigid attachment to it. . As a result of
the barrier 12
preventing migration of fibroblasts and epithelial cells into the extraction
socket 16,
osteoblasts populate the healing blood clot around the implant member 14,
achieving
oste~ointegration.
is The barrier 12 is composed of titanium mesh, is biocompatible and
causes little risk of infection, as noted above. The titanium mesh will retain
the
desired shape and circulation can be established through it. Consequently, it
will not
penetrate the overlying gum 18 as polytetrafluoroethylene and titanium foil
tend to
do. ,4s a result of its biocompatibiliy, small risk of infection, shape
retention and
2o possibility of establishment of circulation, the barrier 12 does not have
to be removed
in a subsequent surgical procedure.
Once osteointegration has occurred, an abutment 17 and a prosthetic
CA 02235705 1998-04-23
-15-
tooth 19 can be attached to the implant member 14
An alternative embodiment of the invention is shown in Figure 9 of the
dravvings, wherein an integrated guided-tissue-regeneration barrier for root-
form dental
implants is comprised of membrane material 13 and a titanium rib reinforcement
system
s 11. The titanium rib reinforcement system has a raised collar 24 of the same
inside
diameter as the outside diameter as the root-form dental implant 14 to hold
the implant
securely in place in the integrated barrier.
The membrane material may be either titanium mesh or a resorbable
material such as a bioabsorbable collagen membrane made of type I bovine
tendon
io collagen. It is joined to the reinforcement ribbing with an adhesive such
as epoxy or
cyanoacrylate.
Figure 10 shows another embodiment in which the integrated guided-
tissue-regeneration barrier is comprised solely of titanium mesh with the mesh
formed
into a raised collar around the opening for the root-form dental implant. The
titanium
is mesh is specifically 100 x 100 mesh. It is of note that the titanium mesh
is
biocompatible and malleable as described below. This embodiment would likely
be
strong enough only if bonded to the implant during the manufacturing process.
Figure 11 shows an embodiment in which the integrated guided-tissue-
regeneration barrier is composed of titanium mesh with no collar and the
opening for
2o the root-form dental implant is of a diameter slightly smaller than the
outside diameter of
the implant, but slightly larger than the diameter of a groove in the implant
designed to
have the mesh snap into it as the implant is pushed through the barrier. This
CA 02235705 1998-04-23
-16-
embodiment as well would likely be strong enough only if bonded to the implant
during
the manufacturing process.
The integrated guided-tissue-regeneration barrier 12 is arranged to
stabilize the root-form implant member 14, and to act as a barrier to soft
tissue cells, as
s described below. Specifically, the barrier membrane 13 acts to exclude
fibroblasts and
epithelial cells from a tooth extraction socket 16, as shown in Figure 4.
The implant member 14 of the implant-barrier combination is arranged to
be inserted into a tooth extraction socket 16 as described below. The details
of the
implant member 14 are not shown as these are known to persons knowledgeable in
the
io art.
The integrated guided-tissue-regeneration barrier 12 may be attached to a
root-form dental implant 14 prior to packaging, as in Figures 3 and 8, or at
the time of
surgery. Assembly is by connecting the implant member 14 to the barrier 12
such that
the barrier 12 extends outwardly from the implant member 14 as shown in
Figures 1-8.
is When the implant member 14 is inserted in the barrier at the time of
surgery, the
implant member is held in the barrier by the closeness of the fit of the
barrier collar to
the implant. In the embodiments shown in Figures 1 and 3 the implant member 14
is
bonded to the barrier 12 with an epoxy adhesive. Alternatively, the implant
member 14
may be connected to the barrier 12 by other means, for example, by welding the
barrier
20 12 to the implant member 14. It is of note that, once assembled, the dental
implant-
barrier combination 10 may be sterilized and enclosed in sterile packaging 21
for later
use, as shown in Figures 3 and 8. This in turn will greatly reduce the risk of
bacterial
CA 02235705 1998-04-23
-17-
contamination and surtace contamination of the barrier 12 and implant member
14
In an embodiment illustrated in Figures 7 and 11, wherein the implant
member 14 with threads 28 is arranged to be screwed into the extraction socket
16, the
collar 24 of the integrated guided-tissue-regeneration barrier 12 is arranged
to be fitted
s into a circumferential groove 26 in the implant member 14. The integrated
guided-
tissue-regeneration barrier 12, may rotate in the groove 26 freely about the
implant
member 14, or alternatively may be stationary while the implant member 14 is
rotated
while being screwed into the extraction socket 16.
In another embodiment shown in Figure 8, the dental implant-barrier
io combination 10 may include a removable handle 30 for aiding in manipulating
the
dental implant-barrier combination 10 during surgery.
In Oaeration with Insertion of the Implant Member in the Barrier at the Time
of Suraery
In operation, before tooth extraction, the surgeon evaluates the site
is clinically and views its radiograph image to determine whether the tooth is
replaceable with an implant-barrier combination 10, and if so, what implant
member
14 size the implant-barrier combination 10 should have and how to cut and
shape the
integrated guided-tissue-regeneration barrier 12. The surgeon then elevates
the gum
20 about the tooth, removes the tooth, mechanically debrides the socket 16,
confirms
20 or modifies implant member 14 choice and cuts and contours the integrated
guided-
tissue-regeneration barrier 12. The barrier 12 is sufficiently malleable that
it may be
formed into the necessary shape either prior to surgery or during surgery as
desired.
CA 02235705 1998-04-23
-18-
Thus the barrier 12 is readily manipulated so as to conform to the desired
shape, a
shape which it will retain. The surgeon then places the correctly shaped
integrated
guided-tissue-regeneration barrier 12 over the socket 16 and presses it firmly
into
place on the surrounding bone to complete its adaptation to the site. The
surgeon
s then inserts the implant member 14 in the socket 16 through the barrier 12.
The gum
20 is sutured in place over the barrier 12 and around the implant cover screw
15, the
repositioned gum 20 holding the integrated guided-tissue-regeneration barrier
12
firmly in place on the bone and the barrier 12 in turn helping to stabilize
the implant
member 14 by virtue of its tight fit to the implant member 14. As a result of
the barrier
io 12 preventing migration of fibroblasts and epithelial cells into the
extraction socket
16, osteoblasts populate the healing blood clot around the implant member 14,
achieving osteointegration.
In O~~eration with the Implant Member inserted in the Prior to Packa~inct
is In operation when the guided-tissue-regeneration barrier is combined
with the root-form dental implant in sterile packaging, the surgeon evaluates
the site
clinically before tooth extraction and views its radiograph image to determine
whether
the tooth is replaceable with an implant-barrier combination 10, and if so,
what size
implant member 14 the implant-barrier combination 10 should have and how to
cut
2o and shape the integrated guided-tissue-regeneration barrier 12. The surgeon
then
elevates the gum 20 about the tooth, remove the tooth, mechanically debrides
the
socket 16, confirms or modifies implant member 14 choice and cuts and contours
the
CA 02235705 1998-04-23
-19-
integrated guided-tissue-regeneration barrier 12, taking care to hold the
combination
by the attached handle 30 so as not to touch or contaminate the implant member
14.
The surgeon places the implant-barrier combination 10 so that the implant
member
14 is inserted in the socket 16 and the integrated guided-tissue-regeneration
barrier
s 12 is in position over the bone 20 around the socket 16. The surgeon then
completes
the adaptation of the integrated guided-tissue-regeneration barrier 12 to the
alveolar
bone by pressing it firmly against the bone 20. The gum 18 is sutured in place
over
the barrier 12 and around the implant cover screw 15, the repositioned gum 18
holding the integrated guided-tissue-regeneration barrier 12 firmly in place
on the
io bone 20 and the barrier 12 in turn helping to stabilize the implant member
14 by
virtue of its tight fit to the implant member 14.
The barrier membrane 13 composed of titanium mesh or resorbable
membrane material is biocompatible and causes little risk of infection, as
noted
above. The titanium mesh or resorbable membrane material bonded to titanium
is reinforcement ribbing will retain the desired shape and circulation can be
established
through them. Consequently, it will not penetrate the overlying gum 18 as
polytetrafluoroethylene and titanium foil tend to do. As a result of its
biocompatibiliy,
small risk of infection, shape retention and possibility of establishment of
circulation,
the integrated guided-tissue-regeneration barrier 12 does not have to be
removed in
2o a subsequent surgical procedure.
Once osteointegration has occurred, an abutment 17 and a prosthetic
tooth 19 can be attached to the implant member 14.
CA 02235705 1998-04-23
-20-
Since various modifications can be made in my invention as herein
described, and many apparently widely different embodiments of the same made
within
the spirit and scope of the claims without departure from such spirit and
scope, it is
intended that all matter contained in the accompanying specification shall be
interpreted
as illustrative only and not in a limiting sense.