Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02236171 1998-04-28
W O 97/16438 PCTAJS96/17481-
TITLE OF THE INVENTION
TRITERPENE DERIVATIVES VVITH IMMUNOSUPPRESSANT
ACTIVITY
5 BACKGROUND OF THE ~VENTION
Immunoregulatory abnormalities have been shown to exist
in ~ wicle v~riety of "autoimmune" and chr-nic inflammatcry diseases~
including systemic lupus erythematosis, chronic rheumatoid arthritis,
type I and II diabetes mellitus, infl~mm~tory bowel disease, biliary
10 cirrhosis, uveitis, multiple sclerosis and other disorders such as Crohn's
disease, ulcerative colitis, bullous pemphigoid, sarcoidosis, psoriasis,
ichthyosis, Graves ophth~lmopathy and asthma.
Although the underlying pathogenesis of each of these
conditions may be quite different, they have in common the appearance
15 of a variety of autoantibodies and self-reactive Iymphocytes. Such self-
reactivity may be due, in part, to a loss of the homeostatic controls
under which the normal immune system operates.
Similarly, following a bone-marrow or an organ
transplantation, the host lymphocytes recognize the foreign tissue
20 antigens and begin to produce antibodies which lead to graft rejection.
One end result of an autoimmllne or a rejection process is
tissue destruction caused by infl:~mmz~tory cells and the mediators they
release. Anti-infl~mm~tory agents such as NSAlD's act principally by
blocking the effect or secretion of these mediators but do nothing to
25 modify the immunologic basis of the disease. On the other hand,
cytotoxic agents, .such as cyclophosphamide, act in such a nonspecific
fashion that both the normal and autoimmune responses are shut off.
Indeed, patients treated with such nonspecific immunosuppressive agents
are as likely to succumb from infection as they are from their
30 autoimmune disease.
Cyclosporin A (CsA), which was approved by the US FDA
in 19~3 is currently the leading drug used to prevent rejection of
transplanted organs. In 1993, FK-506 (Prograf) was approved by the
US FDA for the prevention of rejection in liver transplantation. CsA
CA 02236171 1998-04-28
W O 97/16438 PCT~US96/17481 '
and FK-506 act by inhibiting the body's immlm~ system from
mobilizing its vast arsenal of natural protecting agents to reject the
transplant's foreign protein. In 1994, CsA was approved by the US
FDA for the treatment of severe psoriasis and has been approved by
s European regulatory agencies for the treatment of atopic dermatitis.
Though they are effective in fighting transplant rejection, CsA and FK-
are kn-lwn to cause sev*r~ Lndesir~hle side effects inclllding
nephrotoxicity, neurotoxicity, and gastrointestinal discomfort.
Newer, safer drugs exhibiting less side effects are
10 constantly being searched for in the field.
Four active components of Spachea correa were recently
identified which inhibit thymidine uptake of T cells and are useful as
immnnosuppressive agents in ~nim~ls, including man.
H H Formula 1 (a) b is a single
H ~¦' bond and R is OAc
H""~ ~
b~ C H31~0 Formula 1 (b) b is a double
H ~ ', bond and R is OAc
,~ H 1 OH COOCH3
0~( CH~CH~OAc Formula l(c) b is a single
--~Ac OAC bond and R is OH
CH3~\ OAc Formula 1 (d) b is a double
~ bond and R is OH
These compounds are useful as immllnosuppressive agents
in ~nim~l~, including man. The present invention describes newly
developed immllnosuppressive compounds derived from the compounds
described in Formulae l(a) through l(d) and which have the relative
20 stereochemistry depicted above.
CA 02236171 1998-04-28
W O 97/16438 PCTAJS96/17481 -
SUMMARY OF THE INVENTION
A class of triterpene derivatives of the general structural
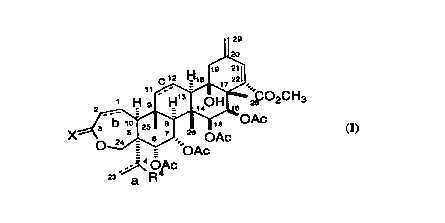
Formula I
,~ O~c
are useful as immllnosuppressants.
As immunosuppressants, the compounds of this invention
are useful in the treatment of autoimmune diseases, the prevention of
rejection of foreign organ transplants and/or related afflictions, diseases
10 and illnesses. Also within the scope of this invention are
pharmaceutical formulations comprising a compound of Formula I and
a pharmaceutical carrier, as well as, pharmaceutical formulations
comprising a compound of Formula I, a second immllnosuppressant
compound and a pharmaceutical carrier.
DETAILED DESCRIPTION OF THE ~VENTION
A. Scope of the Invention
The present invention is related to compounds of formula I,
20 including but not limited to those specified in the examples, which are
- useful in a m~mm~ tn subject for the treatment and prevention of the
resistance by transplantation of organs or tissue, graft-versus-host
diseases brought about by medulla ossium transplantation; rheumatoid
arthritis, systemic lupus erythematosus, Hashimoto's thyroiditis,
25 multiple sclerosis, myasthenia gravis, type I diabetes uveitis, juvenile-
onset or recent-onset diabetes mellitus, posterior uveitis, allergic
CA 02236171 1998-04-28
W O 97/16438 PCTAUS96/17481-
encephalomyelitis, glomerulonephritis, infectious diseases caused by
pathogenic microorg~ni~m.~, infl~mm~tory and hyperproliferative skin
diseases, psoriasis, atopical derm~titi.~, contact dermatitis, eczematous
dermatitises, seborrhoeis dermatitis, Lichen planus, Pemphigus, bullous
s pemphigoid, Epidermolysis bullosa, urticaria, angioedemas, vasculitides,
erythemas, cutaneous eosinophilias, Lupus erythematosus, acne,
Alopecia areata, keratoconjunctivitis. vernal conjunctivitis, llveitis
associated with Behcet's disease, keratitis, herpetic keratitis, conical
cornea, dystrophia epithelialis comeae, corneal leukoma, ocular
10 pemphigus, Mooren's ulcer, Scleritis, Graves' opthalmopathy, Vogt-
Koyanagi-Harada syndrome, sarcoidosis, etc.; pollen allergies,
reversible obstructive airway disease, bronchial asthma, allergic asthma,
intrinsic asthma, extrinsic asthma and dust asthma, chronic or inveterate
asthma, late asthma and airway hyper-responsiveness, bronchitis, gastric
15 ulcers, vascular damage caused by ischemic diseases and thrombosis,
ischemic bowel diseases, infl~mm~tory bowel diseases, necrotizing
enterocolitis, intestinal lesions associated with thermal burns and
leukotriene B4-mediated diseases, Coeliac diseases, proctitis,
eosinophilic gastroenteritis, mastocytosis, Crohn's disease, ulcerative
20 colitis, migraine, rhinitis, eczema, interstitial nephritis, Good-pasture's
syndrome, hemolytic-uremic syndrome, diabetic nephropathy, multiple
myositis, Guillain-Barre syndrome, Meniere's disease, polyneuritis,
multiple neuritis, mononeuritis, radiculopathy, hyperthyroidism,
Basedow's disease, pure red cell aplasia, aplastic anemia, hypoplastic
2s anemia, idiopathic thrombocytopenic purpura, autoimmune hemolytic
anemia, agranulocytosis, pernicious anemia, megaloblastic anemia,
anerythroplasia, osteoporosis, sarcoidosis, fibroid lung, idiopathic
interstitial pneumonia, dermatomyositis, leukoderma vulgaris, ichthyosis
vulgaris, photoallergic sensitivity, cutaneous T cell Iymphoma,
30 arteriosclerosis, atherosclerosis, aortitis syndrome, polyarteritis nodosa,
myocardosis, scleroderma, Wegener's granuloma, Sjogren's syndrome,
adiposis, eosinophilic fascitis, lesions of gingiva, periodontium, alveolar
bone, substantia ossea dentis, glomerulonephritis, male pattern alopecia
or alopecia senilis by preventing epilation or providing hair germination
CA 02236171 1998-04-28
W O 97/16438 PCTAJS96/17481 -
and/or promoting hair generation and hair growth; muscular dystrophy;
Pyoderma and Sezary's syndrome, Addison's disease, ischemia-
reperfusion injury of organs which occurs upon preservation,
transplantation or ischemic disease, for example, thrombosis and cardiac
s infraction, endotoxin-shock, pseudomembranous colitis, colitis caused
by drug or radiation, ischemic acute renal insufficiency, chronic renal
insllfficiencv~ toxinosis caused by hlng-oxvgen or drll~ for ex~mple,
paracort and bleomycins), lung cancer, pulmonary emphysema,
cataracta, siderosis, retinitis, pigmentosa, senile macular degeneration,
10 vitreal scarring, corneal alkali bum; dermatitis erythema multiforrne,
linear IgA ballous dermatitis and cement dermatitis, gingivitis,
periodontitis, sepsis, pancreatitis, diseases caused by environmental
pollution, aging, carcinogenis, metastasis of carcinoma and
hypobaropathy; disease caused by histamine or leukotriene-C4 release;
15 Behcet's disease, autoimmune hepatitis, primary biliary cirrhosis
sclerosing cholangitis), partial liver resection, acute liver necrosis,
necrosis caused by toxin, viral hepatitis, shock, or anoxia, B-virus
hepatitis, non-A/non-B hepatitis, cirrhosis, alcoholic cirrhosis, hepatic
failure, fillmin~nt hepatic failure, late-onset hepatic failure, "acute-on-
20 chronic" liver failure, augmention of chemotherapeutic effect,preventing or treating activity of cytomegalovirus infection, HCMV
infection, and antiinfl~mm~tory activity; and treatment of
immunodepression or a disorder involving immunodepression,
including AIDS, cancer, senile dementia, trauma, chronic bacterial
25 infection, and certain central nervous system disorders.
More particularly this invention relates to compounds of
the general structural formula I:
CA 02236171 1998-04-28
W O 97/16438 PCTnJS96tl7481-
~2CH3
~4 O~C
I
or a pharmaceutically acceptable salt, crystal form or hydrate, wherein:
5 Xis: O;S,NH,HandRl;
a is: a single bond, or a double bond when R4 is absent;
b and c are independently: a single bond, or a double bond;
n is: 1 to 4;
m is: 1 to 4;
1S r is: 0 or 1;
s is: 0 or 1 ;
R l and R2 are independently:
a) H, or
b) (Cl-C6)-alkyl, wherein alkyl is unsubstituted or substituted
with one, two or three substituents selected from the group
consisting of: Br, Cl, F, I, (cl-c6)-alkoxy~ vinyl, cyano,
oxo, nitro, hydroxy, CHO, CO2H, COCl-C6-alkyl,
CO2C 1 -C6-aLkyl, CONR 1 R2, NR 1 R2, NR 1 COC 1 -C6-alkyl,
aryl, wherein aryl is defined as phenyl or naphthyl,
CA 02236171 1998-04-28
W O 97/16438 PCTrUS96/17481 -
unsubstituted or substituted with one, two or three
substituents selected from the group consisting of: Br, Cl,
F, I, (Cl-C6)-alkoxy, phenyl, phenoxy, cyano, nitro,
hydroxy, CHO, CO2H, COC1-C6-alkyl, CO2Cl-C6-alkyl,
CONR 1 R2, NR 1 R2, NR 1 COC 1 -C6-alkyl and any two of
adjacent substituents can be joined to form a 5-, 6- or 7-
memhered fuse(l ring .said ring containing I or 2 o~ygt.n
atoms and the remainder carbon atoms, heteroaryl, wherein
heteroaryl is defined as a 5 or 6-membered ring substituted
with one and two heteroatoms selected from O, S, N,
unsubstituted or substituted with one, two or three
substituents selected from the group consisting of: Br, Cl,
F, I, (Cl-C6)-alkoxy, cyano, nitro, hydroxy, CHO, CO2H,
COC 1 -C6-alkyl, CO2C 1 -C6-alkyl, CONR 1 R2, NR 1 R2,
NRlCOCl-C6-aLkyl, any two adjacent substituents can be
joined to form a 5-, 6- or 7-membered fused ring said ring
cont~ining 1 or 2 oxygen atoms and the remainder carbon
atoms, or any two adjacent substituents can be joined
together to form a benzo-fused ring;
R3 is:
a) -(Cl-C6)-alkyl, alkyl as defined above;
b) -(Cl-C6)-alkenyl, wherein alkenyl is unsubstituted or
substituted with one, two or three substituents selected from
from the group consisting of: Br, Cl, F, I, (Cl-C6)-alkoxy,
cyano, oxo, nitro, hydroxy, CHO, CO2H, COCl-C6-alkyl,
CO2C 1 -C6-alkyl, CONR 1 R2, NR 1 R2, NR 1 COC 1 -C6-alkyl,
aryl as defined above, and heteroaryl as defined above;
c) -(Cl-C6)-alkynyl, wherein alkynyl is unsubstituted or
substituted with one, two or three substituents selected from
the group consisting of: Br, Cl, F, I, (Cl-C6)-alkoxy,
- cyano, oxo, nitro, hydroxy, CHO, CO2H~ COCl-C6-alkyl,
CO2C 1 -C6-alkyl, CONR 1 R2, NR 1 R2, NR 1 COC 1 -C6-alkyl,
aryl as defined above, and heteroaryl as defined above,
CA 02236171 1998-04-28
WO 97/16438 PCTAJS96/17481 -
d) -aryl, aryl as defined above, or
e) -heteroaryl, heteroaryl as defined above;
R4 is:
a) absent and a is a double bond,
b) -H,
c) -OH,
d) =O,
e) -o[(c=o)or]scl-clo-alkyl~ alkyl as defined above,
f) -o[(c=o)or]sc2-clo-alkenyl~ as defined above,
g) -O[(C=O)Or]sC2-C6-alkynyl, alkynyl as dei~ined above,
h) -o[(c=o)or]s(c3-c7)-cycloalkyl~
i) -o[(c=o)or]saryL aryl as defined above,
j) -O[(C=O)Or]sheteroaryl~ heteroaryl as defined above,
k) -O(CH2)nO(CH2)mheteroaryl, heteroaryl as defined above,
1) -O(CH2)nO(CH2)maryl, aryl as defined above,
m) -OC(-O)NRl R2,
n) -oso2R3~ or
o) -NRlR2.
An embodiment of the invention are the compounds of
structural Formula I,
ll29
,~0
~H~
~O2CH3
~ gAc
I
25 or a pharmaceutically acceptable salt, crystal form or hydrate, wherein:
CA 02236171 1998-04-28
W O 97/16438 PCTAUS96/17481-
X is: O, S, or NH;
a is: a single bond;
b and c are independently: a single bond or a double bond;
nis: 1 to4;
mis: 1 to 4;
r is: O or 1;
sis: Oorl;
Rl and R2 are independently:
a) H, or
b) (Cl-C6)-aLkyl, wherein alkyl is unsubstituted or substituted
with one, two or three substituents selected from the group
consisting of: Br, Cl, F, I, (Cl-C6)-alkoxy, vinyl, cyano,
oxo, nitro, hydroxy, CHO, CO2H, COCl-C6-alkyl,
CO2C 1 -C6-alkyl, CONR 1 R2, NR 1 R2, NR 1 COC 1 -C6-alkyl,
aryl, wherein aryl is defined as phenyl or naphthyl,
unsubstituted or substituted with one, two or three
substituents selected from the group consisting of: Br, Cl,
F, I, (Cl-C6)-alkoxy, phenyl, phenoxy, cyano, nitro,
hydroxy, CHO, CO2H, COCl-C6-alkyl, Co2cl-c6-alk
CONR 1 R2, NR 1 R2, NR 1 COC 1 -C6-alkyl and any two of
adjacent substituents can be joined to form a 5-, 6- or 7-
membered fused ring said ring cont~ining 1 or 2 oxygen
atoms and the remainder carbon atoms, heteroaryl, wherein
- heteroaryl is defined as a 5 or 6-membered ring substituted
with one and two heteroatoms selected from O, S, N,
unsubstituted or substituted with one, two or three
CA 02236171 1998-04-28
W O 97/16438 PCT~US96/17481 -
- 10 -
substituents selected from the group consisting of: Br, Cl, .
F, I, (Cl-C6)-alkoxy, cyano, nitro, hydroxy, CHO, CO2H,
COCl-C6-alkyl, CO2Cl-C6-alkyl, CONR1R2, NRlR2,
NRlCOCl-C6-alkyl, any two adjacent substituents can be
joined to form a 5-, 6- or 7-membered fused ring said ring
cont~ining 1 or 2 oxygen atoms and the remz~in~ler carbon
atoms, or any two adjacent ,substituents c~n be joine-l
together to forrn a benzo-fused ring;
R3 is:
a) -((~1-C6)-alkyl, alkyl as defined above;
b) -(C1-C6)-aLkenyl, wherein alkenyl is unsubstituted or
substituted with one, two or three substituents selected from
from the group consisting of: Br, Cl, F, I, (Cl-C6)-alkoxy,
cyano, oxo, nitro, hydroxy, CHO, CO2H~ COCl-C6-alkyl,
CO2C 1 -C6-alkyl, CONR 1 R2, NR 1 R2, NR 1 COC 1 -C6-alkyl,
aryl as defined above, and heteroaryl as defined above;
c) -(Cl-C6)-alkynyl, wherein alkynyl is unsubstituted or
substituted with one, two or three substituents selected from
the group consisting of: Br, Cl, F, I, (Cl-C6)-alkoxy,
cyano, oxo, nitro, hydroxy, CHO, CO2H, COCl-C6-alkyl,
C02C 1 -C6-aL~cyl, CONR 1 R2, NR 1 R2, NR 1 COC 1 -C6-alkyl,
aryl as defined above, and heteroaryl as defined above,
d) -aryl, aryl as defined above, or
2~ e) -heteroaryl, heteroaryl as defined above;
R4 is:
a) absent and a is a double bond;
b) -H,
c) -OH,
d) =O,
e) -O[(C=O)Or]sCl-clo-alkyl~ alkyl as defined above,
f) -o[(c=o)or]sc2-clo-alkenyl~ as defined above,
g) -O[(C=O)Or]sC2-C6-alkynyl, alkynyl as defined above,
CA 02236171 1998-04-28
W O 97/16438 PCT~US96/17481 -
- 11 -
h) -o[(c=o)or]s(c3-c7)-cycloalkyl~
i) -o[(c=o)or]saryl~ aryl as defined above,
j) -O[(C=O)Or]sheteroaryl~ heteroaryl as defined above,k) -o(cH2)no(cH2)mheteroaryl~ heteroaryl as defined above,
1) -o(cH2)no(cH2)maryL aryl as defined above,
m) -OC(=O)NRl R2,
n~ -oso2R3~ or
o) -NRlR2.
An embodiment of this embodiment of the invention are the
compounds of structural Formula I,
L2209
~H
2 1 H g H 14OH~2~02CH3
X~OAc
OAc
~4 OAc
I
or a pharmaceutically acceptable salt, crystal form or hydrate, wherein:
X is: O;
a is: a single bond;
20 b and c are independently: a single bond or a double bond;
n is: I to 4;
-
mis: I to4;
2s
CA 02236171 1998-04-28
W O 97/16438 PCTAUS96/17481
- 12 -
r is: O or l;
sis: Oorl;
R 1 and R2 are independently:
a) H, or
b) (Cl-C~)-aLkvl, wherein alkyl is unsubstituted or~suhstitllted
with one, two or three substituents selected from the group
consisting of: Br, Cl, F, I, (C1-C6)-alkoxy, vinyl, cyano,
oxo, nitro, hydroxy, CHO, C02H, COCl-C6-alkyl,
C02Cl-C6-aLkyl, CONRlR2, NRlR2, NRlCOCl-C6-aLkyl,
aryl, wherein aryl is defined as phenyl or naphthyl,
unsubstituted or substituted with one, two or three
substituents selected from the group consisting of: Br, Cl,
F, I, (Cl-C6)-alkoxy, phenyl, phenoxy, cyano, nitro,
hydroxy, CHO, C02H, COCl-C6-alkyl, C02Cl-C6-alkyl,
CONR 1 R2, NR 1 R2, NR 1 COC 1 -C6-aLkyl and any two of
adjacent substituents can be joined to form a S-, 6- or 7-
membered fused ring said ring cont~ining 1 or 2 oxygen
atoms and the remainder carbon atoms, heteroaryl, wherein
heteroaryl is defined as a 5 or 6-membered ring substituted
with one and two heteroatoms selected from 0, S, N,
unsubstituted or substituted with one, two or three
substituents selected from the group consisting of: Br, Cl,
F, I, (Cl-C6)-alkoxy, cyano, nitro, hydroxy, CHO, C02H,
COCl-C6-alkyl, C02Cl-C6-aL~yl, CONRlR2, NRlR2,
NR 1 COCl -c6-alkyl~ any two adjacent substituents can be
joined to form a 5-, 6- or 7-membered fused ring said ring
cont~ining 1 or 2 oxygen atoms and the remainder carbon
atoms, or any two adjacent substituents can be joined
together to form a benzo-fused ring,
CA 02236171 1998-04-28
W O 97/16438 PCTAUS96/17481-
- 13 -
R3 is:
a) -(Cl-C6)-alkyl, alkyl as defined above,
b) -aryl, aryl as defined above, or
c) -heteroaryl, heteroaryl as defined above;
R4 is:
~) -O[(C=O!O~SCl-Clo-~lkyl~ ~lkyl a.c (1efined ab(lve.
b) -o[(c=o)or]s(c3-c7)-cycloalkyl~
c) -o[(c=o)or]saryl~ aryl as defined above,
lo d) -O[(C=O)Or]sheteroaryl~ heteroaryl as defined above,
e) -O(CH2)nO(CH2)mheteroaryl, heteroaryl as defined above,
f) -O(CH2)nO(CH2)maryl, aryl as defined above,
g) -OC(=O)NRl R2, or
h) -oso2R3
A preferred embodiment of this embodiment are the
compounds of structural Formula I, as recited in above,or a
pharmaceutically acceptable salt, crystal form or hydrate, wherein:
20 R4 is:
a) -o[(c=o)or]saryl~ wherein aryl is defined as phenyl or
naphthyl, unsubstituted or substituted with one, two or
three substituents selected from the group consisting of: Br,
Cl, F, I, (Cl-C6)-alkoxy, phenyl, phenoxy, cyano, nitro,
hydroxy, CHO, CO2H, COCl-C6-alkyl, Co2cl-c6-alk
CONR 1 R2, NR 1 R2, NR 1 COC 1 -C6-alkyl and any two of
adjacent substituents can be joined to form a 5-, 6- or 7-
membered fused ring said ring cont~ining 1 or 2 oxygen
atoms and the remainder carbon atoms, or
b) -o[(c=o)or]sheteroaryl~ wherein heteroaryl is defined as a
S or 6-membered ring substituted with one and two
heteroatoms selected from O, S, N, unsubstituted or
substituted with one, two or three substituents selected from
CA 02236171 1998-04-28
W O 97/16438 PCTrUS96/17481-
- 14 -
the group consisting of: Br, Cl, F, I, (Cl-C6)-alkoxy,
cyano, nitro, hydroxy, CHO, CO2H, COC1-C6-alkyl,
C02Cl -C6-aLkyl, CONR lR2, NR 1 R2, NR 1 COCl -C6-alkyl,
any two adjacent substituents can be joined to form a 5-, 6-
s or 7-membered fused ring said ring cont~inin~ 1 or 2
oxygen atoms and the remainder carbon atoms, or any two
~diacent sllb,stit~lellts c~n be ioine(l t~ gether tC! ~n a
benzo-fused ring.
Another embodiment of this invention are the compounds
of structural Formula I,
~22o9
~H~3
X~ 1 s~OAc
,~ OAc
I
or a pharmaceutically acceptable salt, crystal form or hydrate, wherein:
Xis: HandRl;
a is: a single bond;
20 b and c are independently: a single bond or a double bond;
n is: 1 to 4;
mis: 1 to4;
CA 02236171 1998-04-28
W O 97/16438 PCTrUS96/17481-
r is: 0 or 1;
s is: O or l;
s R l and R2 are independently:
a) H, or
b~ (C I -C~)-alkyl. wherein alkyl is unsubstituted or substituted
with one, two or three substituents selected from the group
consisting of: Br, Cl, F, I, (cl-c6)-alkoxy~ vinyl, cyano,
oxo, nitro, hydroxy, CHO, CO2H, COCl-C6-alkyl,
CO2C 1 -C6-alkyl, CONR 1 R2, NR 1 R2, NR 1 COC 1 -C6-alkyl,
aryl, wherein aryl is defined as phenyl or naphthyl,
unsubstituted or substituted with one, two or three
substituents selected from the group consisting of: Br, Cl,
lS F, I, (Cl-C6)-alkoxy, phenyl, phenoxy, cyano, nitro,
hydroxy, CHO, CO2H~ COCl-C6-alkyl, Co2cl-c6-alk
CONRlR2, NRlR2, NRlCOCl-C6-alkyl and any two of
adjacent substituents can be joined to form a 5-, 6- or 7-
membered fused ring said ring cont~ining 1 or 2 oxygen
atoms and the remainder carbon atoms, heteroaryl, wherein
heteroaryl is defined as a 5 or 6-membered ring substituted
with one and two heteroatoms selected from O, S, N,
unsubstituted or substituted with one, two or three
substituents selected from the group consisting of: Br, Cl,
2s F, I, (cl-c6)-alkoxy~ cyano, nitro, hydroxy, CHO, C02H,
COC 1 -C6-aL~yl, CO2C 1 -c6-alkyl~ CONR 1 R2, NR 1 R2,
NRlCOC1-C6-aLkyl, any two adjacent substituents can be
joined to form a S-, 6- or 7-membered fused ring said ring
cont:~ining 1 or 2 oxygen atoms and the rem~in~ler carbon
~ 30 atoms, or any two adjacent substituents can be joined
together to form a benzo-fused ring;
R3 is:
a) -(Cl-C6)-alkyl, alkyl as defined above;
CA 02236171 1998-04-28
W O 97/16438 PCTAUS96/17481-
- 16 -
b) -(cl-c6)-alkenyl~ wherein aL~enyl is unsubstituted or
substituted with one, two or three substituents selected from
from the group consisting of: Br, Cl, F, I, (CI-C6)-alkoxy,
cyano, oxo, nitro, hydroxy, CHO, CO2H, COCl-C6-alkyl,
CO2C 1 -C6-aLkyl, CONR 1 R2, NR 1 R2, NR 1 COC 1 -C6-alkyl,
aryl as defined above, and heteroaryl as defined above;
c) -(Cl-C~)-alkvnvl. wherein aL~vnvl is un~sub~stituted or
substituted with one, two or three substituents selected from
the group consisting of: Br, Cl, F, I, (Cl-C6)-alkoxy,
cyano, oxo, nitro, hydroxy, CHO, CO2H, COCl-C6-alkyl,
CO2C 1 -C6-alkyl, CONR 1 R2, NR 1 R2, NR 1 COC 1 -C6-alkyl,
aryl as defined above, and heteroaryl as defined above,
d) -aryl, aryl as defined above, or
e) -heteroaryl, heteroaryl as defined above;
R4 is:
a) absent and a is a double bond;
b) -H,
c) -OH,
20d) =O,
e) -o[(c=o)or]scl-clo-aL~cyl~ aL~yl as defined above,
f) -o[(c=o)or]sc2-clo-alkenyl~ as defined above,
g) -O[(C=O)Or]sC2-C6-aL~cynyl, alkynyl as defined above,
h) -O[(C=O)Or]s(C3-C7)-cycloalkyl,
2si) -o[(c=o)or]saryl~ aryl as defined above,
j) -O[(C=O)Or]sheteroaryl, heteroaryl as defined above,
k) -O(CH2)nO(CH2)mheteroaryl, heteroaryl as defined above,
I) -O(CH2)nO(CH2)maryl, aryl as defined above,
m) -OC(=O)NRl R2,
30n) -0So2R3~ or
o) NRIR2.
An embodiment of thi.s embodiment are the compounds of
structural Formula I,
CA 02236171 1998-04-28
W O 97/16438 PCTrUS96/17481 -
S~ ~CH3
",~ 04~C
a
I
or a pharmaceutically acceptable salt, crystal form or hydrate, wherein:
S Xis: HandRl;
a is: a single bond;
b and c are independently: a single bond or a double bond;
nis: 1 to4;
m is: 1 to 4;
1S r is: 0 or 1;
s is: 0 or 1;
R 1 and R2 are independently:
a) H, or
b) (Cl-C6)-alkyl, wherein alkyl is unsubstituted or substituted
with one, two or three substituents selected from the group
consisting of: Br, Cl, F, I, (Cl-C6)-alkoxy, vinyl, cyano,
oxo, nitro, hydroxy, CHO, CO2H, Cocl-c6-alkyl~
2s CO2C l -c6-alkyl~ CONR 1 R2, NR 1 R2, NR I COC I -C6-alkyl,
aryl, wherein aryl is defined as phenyl or naphthyl,
CA 02236171 1998-04-28
W O 97/16438 PCTAUS96/17481 -
- 18 -
unsubstituted or substituted with one, two or three
substituents selected from the group consisting of: Br, Cl,
F, I, (Cl-C6)-alkoxy, phenyl, phenoxy, cyano, nitro,
hydroxy, CHO, CO2H, COC1-C6-alkyl, CO2C1-C6-alkyl,
CONR 1 R2, NR. 1 R2, NR 1 COC 1 -C6-alkyl and any two of
adjacent substituents can be joined to form a 5-, 6- or 7-
membered fused ring said rirlg containing 1 or 2 oxy~;en
atoms and the remainder carbon atoms, heteroaryl, wherein
heteroaryl is defined as a 5 or 6-membered ring substituted
with one and two heteroatoms selected from O, S, N,
unsubstituted or substituted with one, two or three
substituents selected from the group consisting of: Br, Cl,
F, I, (C1-C6)-alkoxy, cyano, nitro, hydroxy, CHO, CO2H,
COC 1 -C6-aLkyl, C02C 1 -C6-aLkyl, CONR 1 R2, NR 1 R2,
NRlCOCl-C6-alkyl, any two adjacent substituents can be
joined to form a 5-, 6- or 7-membered fused ring said ring
cont~ining 1 or 2 oxygen atoms and the rem~in~ler carbon
atoms, or any two adjacent substituents can be joined
together to form a benzo-fused ring;
R3 is:
a) -(Cl-C6)-alkyl, alkyl as defined above;
b) -(Cl-C6)-alkenyl, wherein alkenyl is unsubstituted or
substituted with one, two or three substituents selected from
from the group consisting of: Br, Cl, F, I, (Cl-C6)-alkoxy,
cyano, oxo, nitro, hydroxy, CHO, CO2H, COCl-C6-alkyl,
CO2C 1 -C6-aL~yl, CONR 1 R2, NR 1 R2, NR 1 COC I -C6-alkyl,
aryl as defined above, and heteroaryl as defined above;
c) -(C1-C6)-alkynyl, wherein alkynyl is unsubstituted or
substituted with one, two or three substituents selected from
the group consisting of: Br, Cl, F, I, (Cl-C6)-alkoxy,
cyano, oxo, nitro, hydroxy, CHO, CO2H, COC1-C6-alkyl,
CO2C 1 -C6-alkyl, CONR 1 R2, NR 1 R2, NR I COC 1 -C6-alkyl,
aryl as defined above, and heteroaryl as defined above,
CA 02236171 1998-04-28
W O 97/16438 PCT~US96/17481-
- 19 -
d) -aryl, aryl as defined above, or
e) -heteroaryl, heteroaryl as defined above;
R4 is:
a) -OH,
b) -o[(c=o)or]scl-clo-aLkyl~ aL~cyl as defined above,
c) -o~(c=o)orl~(c~-c7)-cycloalkyl~
d) -O[(C=O)Or]saryl, aryl as defined above,
e) -O[(C=O)Or]sheteroaryl~ heteroaryl as defined above,
f) -o(cH2)no(cH2)mheteroaryl~ heteroaryl as defined above,
g) -O(CH2)nO(CH2)maryl, aryl as defined above,
h) -OC(=O)NRl R2, or
i) -oso2R3.
An embodiment of the invention is a compound selected
from the group consisting of:
4,6,7,15,16-pentakis(acetyloxy)- 18-hydroxy-22-methoxycarbonyl-
[6OC,7OC,15~ 1613]D:A-Friedo-A-homo-27,30-dinor-24-oxaoleana-
1,20(29),21 -trien-3-one;
4-(2-bromobenzoyl)oxy-6,7,15,16-tetrakis(acetyloxy)- 1 ~S-hydroxy-22-
methoxycarbonyl [6a,7a,1513,16,13]D :A-Friedo-A-homo-27,30-dinor-24-
oxaoleana- 1,20(29),21 -trien-3-one;
4,6,7,15,16-pentakis(acetyloxy)- 18-hydroxy-22-methoxycarbonyl-
[6a,70c,15,13,16,1~]D:A-Friedo-A-homo-27,30-dinor-24-oxaoleana-
1,20(29),21 -triene;
4,6,7,15,16-pentakis(acetyloxy)-21,22-epoxy- 18-hydroxy-22-
methoxycarbonyl[6O~,7Oc,15,B,16,B,21~13,22,13]D:A-Friedo-A-homo-27,30-
- dinor-24-oxaoleana-20(29)-en-3-one;
CA 02236171 1998-04-28
W O 97/16438 PCT~US96/17481 -
- 20 -
4,6,7,15,16-pentakis(acetyloxy)- 18-hydroxy-22-methoxycarbonyl-
[60c,7cc,15,B,16,13]D:A-Friedo-A-homo-27,30-dinor-24-oxaoleana-
20(29),21 -dien-3-one;
4-(2-bromobenzoyl)oxy-6,7,15,16-tetrakis(acetyloxy)- 18-hydroxy-22-
methoxycarbonyl[6cc,70~,15,13,16~1~]D:A-Friedo-A-homo-27,30-dinor-24-
oxaoleana-20(29) 21-dien-3-one:
6,7,15,16-tetrakis(acetyloxy)-21,22-epoxy-4,18-dihydroxy-22-
0 methoxycarbonyl[6a,7~c,15,~,16~,21 (3,2213]D: A-Friedo-A-homo-27,30-
dinor-24-oxaoleana-20(29)-en-3-one; and
4-(2-bromobenzoyl)oxy-6,7,15,16-tetrakis(acetyloxy)-21,22-epoxy- 18-
hydroxy-22-methoxycarbonyl[60~,70~,15l3,16,13,21,13,22~3]D:A-Friedo-A-
homo-27,30-dinor-24-oxaoleana-20(29)-en-3 -one .
The compounds of the present invention have asymmetric
centers and this invention includes all of the optical isomers and
mixtures thereof.
In addition compounds with carbon-carbon double bonds
may occur in Z- and E- forms with all isomeric forms of the
compounds being included in the present invention.
As used herein, the term "aL~yl" includes those alkyl groups
of a designated number of carbon atoms of either a straight, branched,
or cyclic configuration. Examples of "alkyl" include methyl, ethyl,
propyl, isopropyl, butyl, sec-and tert-butyl, pentyl, hexyl, heptyl,
cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl,
norbornyl, and the like. "Alkoxy" represents an alkyl group of
indicated number of carbon atoms attached through an oxygen bridge,
such as methoxy, ethoxy, propoxy, butoxy and pentoxy.
"Alkenyl" is intended to include hydrocarbon chains of a
specified number of carbon atoms of either a straight- or branched-
configuration and at least one unsaturation, which may occur at any
point along the chain, such as ethenyl, propenyl, butenyl, pentenyl,
CA 02236171 1998-04-28
W O 97/16438 PCTAJS96/17481 -
dimethyl pentenyl, and the like, and includes E and Z forms, where
applicable. "Halogen", as used herein, means fluoro, chloro, bromo and
iodo.
The term "aryl" is defined as a phenyl or naphthyl ring
s which is optionally substituted at any available carbon atoms with one,
two or three substituents selected from the group consisting of: Br, Cl,
F, I, (Cl-C6)-alkoxy. phenyl, phenoxy~ cyano~ oxo~ nitro. hydroxy,
CHO~ CO2H~ COC 1 -C6-alkyl~ CO2C 1 -C6-aLkyl~ CONR 1 R2~ NR 1 R2,
NR1COC1-C6-alkyl. The aryl may also be substituted with a fused
10 5-, 6-, or 7-membered ring cont~ining one or two oxygens and the
rem~ining ring atoms being carbon, the fused 5-, 6-, or 7-ring being
selected from the group consisting of: dioxolanyl, dihydrofuranyl,
dihydropyranyl, and dioxanyl.
The term "heteroaryl" as utilized herein is intended to
15 include the following a 5 or 6-membered ring substituted with one or
two heteroatoms selected from O, S, N, and is unsubstituted or
substituted with one, two or three substituents selected from the group
consisting of: Br, Cl, F, I, (Cl-C6)-alkoxy, cyano, nitro, hydroxy, CHO
C02H, COCl-C6-alkyl~ C02Cl-C6-alkyL CONRlR2, NRlR2
20 NR 1COCl-C6-aL~yl, any two adjacent substituents can be joined to form
a 5-, 6- or 7-membered fused ring said ring cont~ining 1 or 2 oxygen
atoms and the remainder carbon atoms, or any two adjacent substituents
can be joined together to form a benzo-fused ring. Heteroaryl groups
within the scope of this definition include but are not limited to:
2s acridinyl, carbazolyl, cinnolinyl, quinoxalinyl, pyrrazolyl, indolyl,
benzotriazolyl, furanyl, thienyl, benzothienyl, benzofuranyl, quinolinyl,
isoquinolinyl, pyrazinyl, pyridazinyl, pyridinyl, pyrimidinyl, and
pyrrolyl which are substituted or unsubstituted as defined above.
In the compounds of Formula I, the heteroaryl group may
30 be optionally substituted with the substituents listed above at any
available carbon atom or nitrogen atom (if present), but compounds
bearing certain substitutents, directly substituted to a nitrogen may be
relatively unstable and are not preferred. The heteroaryl may also be
fused to a second 5-, 6-, or 7-membered ring containing one or two
CA 02236171 1998-04-28
W O 97/16438 PCTAJS96/17481-
- 22 -
oxygens selected from the, the rem~ining ring atoms being carbon,
selected from the group consisting of: dioxolanyl, dihydrofuranyl,
dihydropyranyl, and dioxanyl.
Phs~ reutically acceptable salts include both the metallic
s (inorganic) salts and organic salts; a list of which is given in
Remingfon 's Pharmaceutical Sciences, l 7th Edition, pg. l 4 l 8 ( l 985). It
is well known to one skilled in the art that an appro~riate salt form is
chosen based on physical and chemical stability, flowability, hydro-
scopicity and solubility. As will be understood by those skilled in the
0 art, pharmaceutically acceptable salts include, but are not limited to salts
of inorganic acids such as hydrochloride, sulfate, phosphate,
diphosphate, hydrobromide, and nitrate or salts of an organic acid such
as m~1~te, maleate, fumarate, tartrate, succinate, citrate, acetate, lactate,
methanesulfonate, p-toluenesulfonate or palmoate, salicylate and
15 stearate. Similarly pharmaceutically acceptable cations include, but are
not limited to sodium, potassium, calcium, aluminum, lithium and
ammonium (especially ammonium salts with secondary amines).
Preferred salts of this invention for the reasons cited above include
potassium, sodium, calcium and ammonium salts. Also included within
20 the scope of this invention are crystal forrns, hydrates and solvates of
the compounds of Formula I.
-
CA 02236171 1998-04-28
W O 97116438 PCT~US96/17481-
- 23 -
RI3ACTION SCHEME A
~29
2 H~8 ~ O
CH3
2~\ OAc
Li, NH3
THF
,~2CH3
28~ OAc
OAc 11
s As seen in Scheme A, compound I, 4,5,6,15,16-pentakis
(acetyloxy)-21,22-epoxy- 18-hydroxy-22-methoxycarbonyl[6a, 7a,
15~, 16~, 21,13, 2213]D:A-Freido-A-homo-27,30-dinor-24-oxaoleana-
1,20(29)-diene-3-one, isolated from Spachea correa in liquid ammonia
with lithium metal will result in the reduction of the Cl olefin group to
produce the saturated lactone. Alternative methods for reducing the Cl
olefin group and/or the C20(29) olefin that are known in the art may
also be employed. US Serial Number 08/476,806 filed on June 7, 1995
describes the isolation of compound I and is hereby incorporated by
CA 02236l7l l998-04-28
WO 97/16438 PCT~US96/17481 -
- 24 -
reference. The resultant lactone can then be converted to the oxepin
analog by procedures described in Reaction Scheme B.
It should also be noted that compounds of Formula I having
the 11,12-double bond can be prepared using the starting material,
s 4,6,7,15,16-pentakisacetoxy-21,22-epoxy- 18-hydroxy-22-
methoxycarbonyl[60~,7Oc, l 5,B,16~B,21,(3,22,B]D:A-Freido-A-homo-27,30-
dinor-24-oxaleana-1 11.20(29)-trien-3-one. isolated fro~ Snaehea
c~rrea and following the procedures described herein. However, there
may be reactions where it will not be possible to selectively operate on
one of the double bonds, for example, ozonolysis.
REACTION SCHEME B
Il 29
12 H18~C~
CH3
2~4 OAc
I (b is a double bond)
Il (b is a single bond)
1. LiAlH(OtBu)3
2. Et3SiH, BF30Et2
,a~
CH3
O Ac
25/~4 OAc
CA 02236l7l l998-04-28
W O 97/16438 PCTrUS96/17481-
As seen in Scheme B, compound I [(4,6,7,15,16-
pentakis(acetyloxy)-21,22-epoxy- 18-hydroxy-22-methyoxycarbonyl-
[6O~,70~,15,13,1613,21,13,22~]D:A-Freido-A-homo-27,30-dinor-24-
oxaoleana-l, 20(29)-dien-3-one], isolated from Spachea correa can be
5 converted to its oxepin analog in a two step process. US Serial Number
08/476,806 filed June 7, 1995, describing the isolation of compound I
and is hereby incorporated bv reference. Lactone T is first redluced to
the lactol. This can be accomplished by using a variety of reducing
agents including di-isobutylal7-minnm hydride (DIBAL-H) and sodium
lo bis(2-methoxyethoxy)aluminum hydride (Red-AI). A more optimal
reducing agent is the use of lithium tri-t-butoxyal~lminllm hydride in an
inert solvent such as dichloromethane at reduced temperatures,
preferably 0~C. The purified lactol intermediate is then reacted with
triethylsilane and a Lewis acid such as borontrifluoride diethyl etherate
15 to give the ether (oxepin) analog of I.
CA 02236171 1998-04-28
WO 97/16438 PCT~US96/17481 -
- 26 -
REACTION SCHEME C
~20
~ ~2CH3
4 -
1. LiAlH(OtBu)3
2. Et3AI
29
12 H1~ ~~
Et~2c H3
4 -
In a variation of Scheme B, ether (oxepin) derivatives
5 substituted at C3 can also be prepared. Thus in Reaction Scheme C,
lactone I is first reduced to the lactol as described in Reaction Scheme B.
The purified lactol intermediate is then reacted with a trialkylalllminllm
reagent, as exemplified in this scheme by triethylaluminum (Et3AI) to
give the ethyl derivative. The allyl derivative can be prepared with
10 allyltrimethylsilane and a Lewis acid, such as borontrifluoride diethyl
etherate.
CA 02236171 1998-04-28
W O 97/16438 PCTrUS96/17481 -
REACTION SCHEME D
ll29
12 1--1B 22 C~
C H3
8~ OAc
2 OAc
WCI6, nBuLi
THF, 50 ~C
~22o9
X~OACC02C H3
2~ OAc
The C21-C22 epoxide of lactone or ether derivatives can be
s converted to the olefin by use of a WC16/BuLi complex (1:2) in
tetrahydrofuran (THF) by procedures developed by Sharpless et al. (J.
Am. Chem. Soc., 94, 653~-6540, 1972). This conversion can be
achieved before or after any of the reaction schemes described.
CA 02236171 1998-04-28
W O 97/16438 PCT~US96/17481 -
- 28 -
RE~CTION SCHEME E
~ M~
H ~ CO2CH3
o~OAc
CH3 4 OAc
HCI/THF
or
CH3(CI)AI[N(OCH3)CH3]
=~CO2C H3
~OAc
CH3 4 OH
Lactone or ether derivatives can be selectively de-acetylated
s at C4 to give the corresponding alcohol by reacting it with an aqueous
solution of HCI (preferably 2M to 3M concentration) in THF. It can
also be prepared by reacting I with CH3(CI)Al[N(OCH3)CH3] (Weinreb
reagent) in inert solvents such as THF, toluene or methylene chloride.
If a product from this reaction contains the epoxide, it can
1~) be removed by the method described in Reaction Scheme D.
CA 02236171 1998-04-28
W O 97/16438 PCT~US96/17481-
- 29 -
REACTION SCHEME F
Il
H ~OH CO2CH3
X~ ~M~OAc
CH3~4 OH
[~] ~
I Me¦¦
H ~/~CO2CH3
~\ /~OAc
b Me Me
~OAc 'OAc
CH3 4 O
The C4 hydroxy group can be oxidized to the
corresponding ketone by a variety of oxidizing agents. The Jones
reagent (chromic acid and sulfuric acid in H20), pyridinium
chlorochromate, and oxalyl chloride plus DMSO all will achieve this
conversion.
CA 02236171 1998-04-28
W O 97/16438 PCTAUS96/17481-
- 30 -
REACTION SCHEME G
CO~CH~
~\ ~--OAc
X \~", OAc
CH3 4 OH
(PhO)3MePI
HMPT, 75 ~C
J~
H ~ ~--CO2CH3
=~ ~OAc
s The C4 hydroxy group can be dehydrated to give the
olefin. Reaction of the alcohol with tris-phenoxymethylphosphonium
iodide in hexamethylphosphorous triamide (HMPT) at 75~C will achieve
this conversion.
CA 02236171 1998-04-28
W O 97/16438 PCTAJS96/17481 -
- 31 -
REACTION SCHEME H
R4 COCI ~M~
H ~i co2CH3
~OAc
H ~CO2CH3
~OAc
CH3 4 OH
~'
CDI, R1R2NHH ~ CO2CH3
o(~OAc
C H3~4 O
O~NR1R2
CA 02236171 1998-04-28
W O 97/16438 PCT~US96/17481-
REACTION SCHEME H (CONT'D~
Il
CDI, R40H
co2CH3
~OAc
~-- OAc OAC
CH3 4 0
o~oR4
Me¦
~)H CO2CH3
~OAc
X~o ~ ~ 0
CH3~4 OH
R40H, Tf20, base H I~OH~--CO2CH3
~ OAc
~"'OAOAC
CH3 ~\oR4
As depicted in Reaction Scheme H, esters at C4 can be
s prepared by reaction of a preformed carboxylic acid chloride with the
C4 alcohol derivative (Reaction Scheme E) in a basic solvent such as
pyridine. It should be understood that R4 is used to represent a portion
of the R4 definition, e.g. R4 can be an alkyl carbonate which is depicted
in the scheme as oC(=o)oR4, R4 representing the alkyl substituent.
10 The acid chlorides, when not purchased, are prepared by stirring the
carboxylic acids in reagents such as oxalyl chloride or thionyl chloride.
Esters may also be prepared by reaction of the acid chloride and C4
.
CA 02236171 1998-04-28
W O 97/16438 PCTrUS96/17481-
alcohol with silver cyanide (AgCN) in an aprotic solvent such as HMPA.
C4 sulfonate derivatives are prepared in a similar manner by reaction
with sulfonyl chlorides.
C4 carbonate and carbamate derivatives are prepared by
5 first reacting the C4 alcohol derivative with carbonyldiimidazole (CDI)
to obtain the imidazolecarbonyl intermediate which is then reacted with
an alcohol or amine (R lR2N~! to give the correspollding c~rhon~te or
carbamate derivatives.
C4 ether derivatives can also be prepared. The best
10 procedure involves reacting an alcohol with trifluoromethanesulfonic
anhydride (Tf20, triflic anhydride) to obtain the preformed triflate in
dichloromethane at reduced temperature, preferably -7~s~C. To this
solution is added the triterpene alcohol, the reaction mixture is warmed
to room temperature and stirring is continued until reaction is complete.
5 Ethers may also be prepared by heating a mixture of triterpene C4
alcohol, the appropriate aL~ylhalide and an excess of silver oxide
(Ag20) in an aprotic invert solvent such as THF.
CA 02236171 1998-04-28
W O 97/16438 PCTAJS96/17481 -
- 34 -
l~EACTION SCHEME I
H~CO2C H3
c~\ r~e ~OAc
X~ o OAC
CH3 4 0
NHR1 R2
NaCNBH3
H ~ CO2CH3
--OAc
O----A 'OAC
R1 R2N ~C H
Amines at C4 can be prepared from the C4 ketone
described in Reaction Scheme F by reaction with an amine NHR 1 R2 in a
5 variety of solvents with a reducing agent such as sodium
cyanoborohydride .
UTILITY
The present invention is related to compounds of
formula I, including but not limited to those specified in the examples,
which are useful in a m~mm~lian subject for the treatment and
prevention of immunemediated diseases such as the resistance by
transplantation of organs or tissue such as heart, kidney, liver, medulla
1~ ossium, skin, cornea, lung, pancreas, intestinum tenue, limb, muscle,
CA 02236171 1998-04-28
W O 97/16438 PCT~US96/17481 -
- 35 -
nervus, duodenum, small-bowel, pancreatic-islet-cell, including xeno
transplants, etc.; graft-versus-host diseases brought about by medulla
ossium transplantation; autoimmlme diseases such as rheumatoid
arthritis, systemic lupus erythematosus, I~himoto's thyroiditis,
s multiple sclerosis, myasthenia gravis, type I diabetes uveitis, juvenile-
onset or recent-onset diabetes mellitus, posterior uveitis, allergic
encephalomyelitis. glomel~llonephritis, alld the lil~e~ and further
infectious diseases caused by pathogenic microorg~ni~m.~. Further uses
may include the treatment and prophylaxis of infl~mm~tory and
hyperproliferative skin diseases and cutaneous manifestations of
immunologically mediated illnesses, such as psoriasis, atopical
dermatitis, contact dermatitis and further eczematous dermatitises and
further eczematous dermatitises, seborrhoeis dermatitis, Lichen planus,
Pemphigus, bullous pemphigoid, Epidermolysis bullosa, urticaria,
angioedemas, vasculitides, erythemas, cutaneous eosinophilias, Lupus
erythematosus, acne and Alopecia areata; various eye diseases
(autoimmune and otherwise) such as keratoconjunctivitis, vernal
conjunctivitis, uveitis associated with Behcet's disease, keratitis, herpetic
keratitis, conical cornea, dystrophia epithelialis corneae, corneal
leukoma, ocular pemphigus, Mooren's ulcer, Scleritis, Graves'
opthalmopathy, Vogt-Koyanagi-Harada syndrome, sarcoidosis, etc.;
pollen allergies, reversible obstructive airway disease, which includes
condition such as asthma (for example, bronchial asthma, allergic
asthma, intrinsic asthma, extrinsic asthma and dust asthma), particularly
2s chronic or inveterate asthma (for example, late asthma and airway
hyper-responsiveness), bronchitis and the like; infl~mm~tion of mucous
and blood vessels such as gastric ulcers, vascular ~l~m~ge caused by
ischemic diseases and thrombosis, ischemic bowel diseases,
infl~mm~tory bowel diseases, necrotizing enterocolitis, intestinal lesions
~ 30 associated with thermal burns and leukotriene B4-mediated diseases;
intestinal infl~mm~tions/allergies such as Coeliac diseases, proctitis,
- eosinophilic gastroenteritis, mastocytosis, Crohn's disease and ulcerative
colitis; food-related allergic diseases which have symptomatic
manifestation remote from the gastrointestinal tract (e.g. migraine,
CA 02236171 1998-04-28
W O 97/16438 PCT~US96/17481 -
- 36 -
rhinitis and eczema); renal diseases such as interstitial nephritis, Good-
pasture's syndrome, hemolytic-uremic syndrome and diabetic
nephropathy; nervous diseases such as multiple myositis, Guillain-Barre
syndrome, Meniere's disease, polyneuritis, multiple neuritis,
5 mononeuritis and radiculopathy; endocrine diseases such as
hyperthyroidism and Basedow's disease; hematic diseases such as pure
red cell aplasia. aplastic anemia. hvpoplastic anemia. idiopathic
thrombocytopenic purpura, autoimmune hemolytic anemia,
agranulocytosis, pernicious anemia, megaloblastic anemia and
10 anerythroplasia; bone diseases such as osteoporosis; respiratory diseases
such as sarcoidosis, fibroid lung and idiopathic interstitial pneumonia;
skin disease such as dermatomyositis, leukoderma vulgaris, ichthyosis
vulgaris, photoallergic sensitivity and cutaneous T cell Iymphoma;
circulatory diseases such as arteriosclerosis, atherosclerosis, aortitis
15 syndrome, polyarteritis nodosa and myocardosis; collagen diseases such
as scleroderma, Wegener's granuloma and Sjogren's syndrome;
adiposis; eosinophilic fascitis; periodontal disease such as lesions of
gingiva, periodontium, alveolar bone and substantia ossea dentis;
nephrotic syndrome such as glomerulonephritis; male pattern alopecia
20 or alopecia senilis by preventing epilation or providing hair germination
and/or promoting hair generation and hair growth; muscular dystrophy;
Pyoderma and Sezary's syndrome; Addison's disease; active oxygen-
mediated diseases, as for example organ injury such as ischemia-
reperfusion injury of organs (such as heart, liver, kidney and digestive
25 tract) which occurs upon preservation, transplantation or ischemic
disease (for example, thrombosis and cardiac infraction): intestinal
diseases such as endotoxin-shock, pseudomembranous colitis and colitis
caused by drug or radiation; renal diseases such as ischemic acute renal
insufficiency and chronic renal insufficiency; pulmonary diseases such
30 as toxinosis caused by lung-oxygen or drug (for example, paracort and
bleomycins), lung cancer and pulmonary emphysema; ocular diseases
such as cataracta, siderosis, retinitis, pigmentosa, senile macular
degeneration, vitreal scarring and corneal alkali bum; dermatitis such as
erythema multiforme, linear IgA ballous dermatitis and cement
CA 02236171 1998-04-28
W O 97/16438 PCTAUS96/17481-
dermatitis; and others such as gingivitis, periodontitis, sepsis,
pancreatitis, diseases caused by environmental pollution (for example,
air pollution), aging, carcinogenis, metastasis of carcinoma and
hypobaropathy; disease caused by histamine or leukotriene-C4 release;
s Behcet's disease such as intestinal-, vasculo- or neuro-Behcet's disease,
and also Behcet's which affects the oral cavity, skin, eye, vulva,
articulation. epididymis lung, kidney and so on. Furthermo~e~ the
compounds of the invention are useful for the treatment and prevention
of hepatic disease such as immllnogenic diseases (for example, chronic
10 autoimmune liver diseases such as the group consisting of autoimmune
hepatitis, primary biliary cirrhosis and sclerosing cholangitis), partial
liver resection, acute liver necrosis (e.g. necrosis caused by toxin, viral
hepatitis, shock, or anoxia), B-virus hepatitis, non-A/non-B hepatitis,
cirrhosis (such as alcoholic cirrhosis) and hepatic failure such as
5 fillmin~nt hepatic failure, late-onset hepatic failure and "acute-on-
chronic" liver failure (acute liver failure on chronic liver diseases), and
moreover are useful for various diseases because of their useful activity
such as augmention of chemotherapeutic effect, preventing or treating
activity of cytomegalovirus infection, particularly HCMV infection, and
20 antiinfl~mm~tory activity; and
The compounds of the present invention may also be used
in the treatment of immunodepression or a disorder involving
immllnodepression, such as AIDS, cancer, senile dementia, trauma
(including wound healing, surgery and shock) chronic bacterial
2s infection, and certain central nervous system disorders.
A method of treating a condition in a m~mm~l, the
treatment of which is effected or facilitated by Kv 1.3 inhibition,
comprising the ~lmini~tration, in an amount that is effective at
inhibiting KV1 3~ of a compound of Formula I. The method of treating
30 a condition in a m~mm~l, the treatment of which is effected or
facilitated by Kvl.3 inhibition, wherein the condition is selected from
- the group consisting of: immlmemediated diseases such as the resistance
by transplantation of organs or tissue such as heart, kidney, liver,
medulla ossium, skin, cornea, lung, pancreas, intestinum tenue, limb,
CA 02236171 1998-04-28
W O 97/16438 PCT~US96/17481 -
- 38 -
muscle, nervus, duodenurn, small-bowel, pancreatic-islet-cell, including
xeno transplants, etc.; graft-versus-host diseases brought about by
medulla ossium transplantation; autoimmune diseases such as rheumatoid
arthritis, systemic lupus erythematosus, Hashimoto's thyroiditis,
s multiple sclerosis, myasthenia gravis, type I diabetes uveitis, juvenile-
onset or recent-onset diabetes mellitus, posterior uveitis, allergic
encephalomvelitis. glomerulonephritis~ and the like; and further
infectious diseases caused by pathogenic microorg~ni.cmc. Fur~er uses
may include the treatment and prophylaxis of infl~mm~tory and
10 hyperproliferative skin diseases and cutaneous manifestations of
immllnologically mediated illnesses, such as psoriasis, atopical
dermatitis, contact dermatitis and further eczematous dermatitises and
further eczematous derrnatitises, seborrhoeis derm~ti~i~, Lichen planus,
Pemphigus, bullous pemphigoid, Epidermolysis bullosa, urticaria,
angioedemas, vasculitides, erythemas, cutaneous eosinophilias, Lupus
erythematosus, acne and Alopecia areata; various eye diseases
(autoimmllne and otherwise) such as keratoconjunctivitis, vernal
conjunctivitis, uveitis associated with Behcet's disease, keratitis, herpetic
keratitis, conical cornea, dystrophia epithelialis corneae, corneal
20 leukoma, ocular pemphigus, Mooren's ulcer, Scleritis, Graves'
opthalmopathy, Vogt-Koyanagi-Harada syndrome, sarcoidosis, etc.;
reversible obstructive airway disease, which includes condition such as
asthma (for example, bronchial asthma, allergic asthma, intrinsic
asthma, extrinsic asthma and dust asthrna), particularly chronic or
25 inveterate asthma (for example, late asthma and airway hyper-
responsiveness), bronchitis and the like; infl~mm~tion of mucous and
blood vessels such as gastric ulcers, vascular damage caused by ischemic
diseases and thrombosis, ischemic bowel diseases, infl~mm~tory bowel
diseases, necrotizing enterocolitis, intestinal lesions associated with
30 thermal burns and leukotriene B4-mediated diseases; intestinal
infl~mm~tions/allergies such as Coeliac diseases, proctitis, eosinophilic
gastroenteritis, mastocytosis, Crohn's disease and ulcerative colitis;
food-related allergic diseases which have symptomatic manifestation
remote from the gastrointestinal tract (e.g. migraine, rhinitis and
-
CA 02236171 1998-04-28
W O 97/16438 PCTrUS96/17481 -
- 39 -
eczema); renal diseases such as interstitial nephritis, Good-pasture's
syndrome, hemolytic-uremic syndrome and diabetic nephropathy;
nervous diseases such as multiple myositis, Guillain-Barre syndrome,
Meniere's disease, polyneuritis, multiple neuritis, mononeuritis and
5 radiculopathy; endocrine diseases such as hyperthyroidism and
Basedow's disease; hematic diseases such as pure red cell aplasia, aplastic
anemia, hypoplastic anemia~ idiopathic thrombocvtopenic puIpura
autoimmune hemolytic anemia, agranulocytosis, pernicious anemia,
megaloblastic anemia and anerythroplasia; bone diseases such as
10 osteoporosis; respiratory diseases such as sarcoidosis, fibroid lung and
idiopathic interstitial pneumonia; skin disease such as dermatomyositis,
leukoderma vulgaris, ichthyosis vulgaris, photoallergic sensitivity and
cutaneous T cell lymphoma; circulatory diseases such as arteriosclerosis,
atherosclerosis, aortitis syndrome, polyarteritis nodosa and
15 myocardosis; collagen diseases such as scleroderma, Wegener's
granuloma and Sjogren's syndrome; adiposis; eosinophilic fascitis;
periodontal disease such as lesions of gingiva, periodontium, alveolar
bone and substantia ossea dentis; nephrotic syndrome such as
glomerulonephritis; male pattern aleopreia or alopecia senilis by
20 preventing epilation or providing hair germination and/or promoting
hair generation and hair growth; muscular dystrophy; Pyoderma and
Sezary's syndrome; Addison's disease; active oxygen-mediated diseases,
as for example organ injury such as ischemia-reperfusion injury of
organs (such as heart, liver, kidney and digestive tract) which occurs
2s upon preservation, transplantation or ischemic disease (for example,
thrombosis and cardiac infraction): intestinal diseases such as endotoxin-
shock, pseudomembranous colitis and colitis caused by drug or
radiation; renal diseases such as ischemic acute renal insufficiency and
chronic renal insufficiency; pulmonary diseases such as toxinosis caused
30 by lung-oxygen or drug (for example, paracort and bleomycins), lung
cancer and pulmonaly emphysema; ocular diseases such as cataracta,
- siderosis, retinitis, pigmentosa, senile macular degeneration, vitreal
scarring and corneal alkali bum; dermatitis such as erythema
multiforme, linear IgA ballous dermatitis and cement dermatitis; and
CA 02236171 1998-04-28
W O 97/16438 PCTAUS96/17481-
- 40 -
others such as gingivitis, periodontitis, sepsis, pancreatitis, diseases
caused by environmental pollution (for example, air pollution), aging,
carcinogenis, metastasis of carcinoma and hypobaropathy; disease caused
by histamine or leukotriene-C4 release; Behcet's disease such as
s intestinal-, vasculo- or neuro-Behcet's disease, and also Behcet's which
affects the oral cavity, skin, eye, vulva, articulation, epididymis, lung,
kidney and so on. Furthermore, the compounds of the invention are
useful for the treatment and prevention of hepatic disease such as
immllnogenic diseases (for example, chronic autoimmune liver diseases
10 such as the group consisting of autoimml-ne hepatitis, primary biliary
cirrhosis and sclerosing cholangitis), partial liver resection, acute liver
necrosis (e.g. necrosis caused by toxin, viral hepatitis, shock, or anoxia),
B-virus hepatitis, non-A/non-B hepatitis, cirrhosis (such as alcoholic
cirrhosis) and hepatic failure such as fillmin~nt hepatic failure, late-
15 onset hepatic failure and "acute-on-chronic" liver failure (acute liver
failure on chronic liver diseases), and moreover are useful for various
diseases because of their useful activity such as augmention of
chemotherapeutic effect, preventing or treating activity of
cytomegalovirus infection, particularly HCMV infection, and
20 antiinfl~mm~tory activity; and immunodepression or a disorder
involving immunodepression, such as AIDS, cancer, senile dementia,
trauma (including wound healing, surgery and shock), chronic bacterial
infection, and certain central nervous system disorders.
An embodiment of the invention is a method for the
25 treatment of autoimmune diseases. Another embodiment of the invention
is a method for the prevention of rejection of foreign organ transplants
comprising ~lmini~tering to a patient in need of such treatment a
therapeutically effective amount of a compound of formula I.
One end result of an autoimml-ne or a rejection process is
30 tissue destruction caused by infl~mm~tory cells and the mediators they
release. Anti-infl:~mm~tory agents such as NSAID's and corticosteroids
act principally by blocking the effect or secretion of these mediators,
CA 02236171 1998-04-28
W O 97/16438 PCTrUS96/17481 -
- 41 -
but do nothing to modify the immlmologic basis of the disease. On the
other hand, cytotoxic agents, such as cyclophosphamide, act in such a
nonspecific fashion that both the normal and autoimmllne responses
are shut off. Indeed, patients treated with such nonspecific immllno-
suppressive agents are as likely to succumb from infection as they are
from their autoimmlme disease.
Cyclospnrin ~ which was ~I)pr~ved hv ~e ~T~ A
in 1983, is currently the le~lling drug used to prevent rejection of
transplanted organs. The drug acts by inhibiting the body's immllne
system from mobilizing its vast arsenal of natural protecting agents to
reject the transplant's foreign protein. Though cyclosporin A is
effective in fighting transplant rejection, it is nephrotoxic and is known
to cause several undesirable side effects including kidney failure,
abnormal liver function and gastrointestinal discomfort.
Newer, safer drugs exhibiting fewer side effects are
constantly being searched for in the field. The present invention
provides for immlmosuppressant agents which are inhibitors of a
voltage dependent potassium channel, Kv 1.3, that is found on human
T-lymphocytes.
Potassium channels modulate a number of cellular events
such as muscle contraction, neuro-endocrine secretion, frequency and
duration of action potentials, electrolyte homeostasis, and resting
membrane potential. These channels comprise a family of proteins that
have been classified according to their biophysical and pharmacological
2s characteristics. Inhibition of K+ channels, in their role as modulators
of the plasma membrane potential in hllm~n T -lymphocytes, has been
postulated to play a role in eliciting immunosuppressive responses. In
regulating membrane potential, K+ channels play a role in the
regulation of intracellular Ca++ homeostasis, which has been found to
be important in T-cell activation. The biochemical characterization of
K+ channels is underdeveloped, due to the paucity of selective high
affinity probes.
Functional voltage-gated K+ channels can exist as
multimeric structures formed by the association of either identical or
CA 02236171 1998-04-28
W O 97/16438 PCTAUS96/17481
- 42 -
dissimilar subunits. This phenomena is thought to account for the wide
diversity of K+ channels. However, subunit compositions of native K+
channels and ~e physiologic role that particular channels play are, in
rnost cases, still unclear.
s The KV1.3 channel is a voltage-gated potassium channel
that is found in neurons, blood cells, osteoclasts and T-lymphocytes.
The ~handy ~nd (~ahal7m lahor~ories propose~ a hypotl~e~is that
blocking the Kvl.3 channel would elicit an immllnosuppressant
response. (Chandy et al., J. Exp. Med. 160, 369, 1984; Decoursey et al
0 Nature, 307, 465, 19~S4). However, the K+ channel blockers employed
in their studies were non-selective. Until research with the peptide
margatoxin, a peptide found in scorpion venom, no specific inhibitor of
the Kv 1.3 channel existed to test this hypothesis. Although a laboratory
(Price et al., Proc. Natl. Acad. Sci. USA, 86, 10171, l9g9) showed that
1S charybdotoxin would block KV1.3 in hllm~n T cells, charybdotoxin was
subsequently shown to inhibit four different K+ channels (KV1.3 and
three distinct small conductance Ca l l activated K+ channels) in hllm~n
T-lymphocytes, limiting the use of this toxin as a probe for the
physiological role of Kv 1.3 (Leonard et al., Proc. Natl. Acad. Sci. USA,
89, 10094, 1992). Margatoxin, on the otherhand, blocks only Kvl.3 in
T-cells, and has immunosuppressant activity in both in vitro and in vivo
models. (Lin et al., J. Exp. Med, 177, 637, 1993). Since the
compounds of the embodiments of this invention produce blockade of
KV1~3~ they will also inhibit T-cell activation.
Also within the scope of this invention is a method of
treating a condition in a m~mm~l, the treatment of which is effected or
facilitated by KV1.3 inhibition, comprising the ~mini~tration of a
pharmaceutical composition comprising a suitable pharmaceutical
carrier and a compound of Formula (I), in an amount that is effective at
3~ inhibiting Kv 1.3.
Also within the scope of this invention is a combination
therapy comprising a compound of formula I and one or more
immllnosuppressant agents. These imm7mosuppressant agents within the
scope of this invention include, but are not limited to, IMUREK(~)
CA 02236171 1998-04-28
W O 97/16438 PCTAUS96tl7481-
- 43 -
~ azathioprine sodium, brequinar sodium, SPANIDIN(~) gusperimus
trihydrochloride (also known as deoxyspergualin), mizoribine (also
known as bredinin), CELLCEPT(g) mycophenolate mofetil, NEORAL(~)
Cyclosporin A (also marketed as different formulation of Cyclosporin A
5 under the trademark SANDIMMUNE(~)), PROGRAF(~) tacrolimus (also
known as FK-506) and RAPIMMUNE(~' sirolimus (also known as
rap~myrin)~ lefllunQmide ~also kno~n as ~Y~A-486)~ ,,lucocortcoids,
such as prednisolone and its derivatives, antibody therapies such as
orthoclone (OKT3) and Zenapax and antithymyocyte globulins, such as
10 thymoglobulins.
Using the methodologies described below, representative
compounds of the invention were evaluated and found to exhibit IC50
values of at least <10 ,uM in any of the assays thereby demonstrating
and confirming the utility of the compounds of the invention as Kv 1.3
5 inhibitors and immunosuppressants.
T CELL IL-2 ASSAY
Peripheral blood mononuclear (MNC) cells from healthy
20 donors were separated by density centrifugation with ficoll-hypaque
(LSM, Organon Teknika, Durham, NC), followed by rosetted with
neuraminidase treated sheep red blood cells (SRBC). After another
centrifugation with leucocyte separation medium (LSM), the SRBC of
the rosetted T cells were then lysed with ammonium chloride lysing
25 buffer (GIBCO, Grand Island, NY). Such purified T cells were
resuspended at 3 X 106/ ml in RPMI 1640 culture medium (GIBCO)
supplemented with 10% fetal calf serum (Sigma, St. Louis, MO), 100
mM glutamine, 1 mM sodium pyruvate, 0.1 mM non-essential amino
acids, and 1 % penn-strep (GIBCO). The cell suspension was
30 immediately distributed into 96 well round-bottom microculture plates
(Costar) at 200 ~l/well. The various dilutions of test compound were
- then added in triplicate wells at 25 ~l/well, incubated for 30 min at
37~C. Ionomycin (125 ng/ml), and PMA (1 or 5 ng/ml), were added to
the appropriate wells. The culture plates were then incubated at 37~C in
a humidified atmosphere of 5% CO2 - 95% air for 18-24 hours. The
CA 02236171 1998-04-28
W O 97/16438 PCT~US96/17481 - -
- 44 -
supernzlt~ntc were removed, and assayed for IL-2 with an IL-2 capture
ELISA, using monoclonal anti-IL-2, and biotinylated goat anti-IL-2
antibodies (unconjugated antibodies purchased from R&D System,
Minneapolis, MN). The ELISA was developed with streptavidin
s conjugated peroxidase (Zymed, San Francisco, CA) and substrate for
peroxidase (Sigma). Mean OD and units of IL-2 of the replicate wells
were calculated from standard curve~ created with recomhin~nt TT,-2
(Collaborative Biomedical Products, Bedford, ~A) and the results
were expressed as concentration of compound required to inhibit IL-2
production of T cells by 50%.
T CELL PROLIFERATION ASSAY
Peripheral blood mononuclear cells (MNC) from healthy
ls donors were separated by density centrifugation with ficoll-hypaque
(LSM, Organon Teknika, Durham, NC). After washing the MNC with
complete media (RPMI 1640 medium with 5% fetal calf serum, 100 mM
glllt~mine, 1 mM sodium pyruvate, 0.1 mM non-essential amino acid,
and 1% penn-strep, obtained from GIBCO, Grand Island, NY), they
were then irradiated at 7500 RADS, and resuspended at 4-4.5 x
106cells/ml in complete media. Another aliquot of MNC were rosetted
with neuraminidase treated SRBC. After another centrifugation with
LSM, the sheep red blood cells (SRBC) of these rosetted T cells were
then Iysed with ammonium chloride lysing buffer (GIBCO, Grand
Island, NY). After washing 2X with complete media, these purified T
cells were also resuspended at 2-2.5 x 106cells/ml in complete media.
The various dilutions of the compound were added in triplicates at 50
ul/well of a 96 well flat-bottom microculture plate (Costar, Cambridge,
MA). T cell suspension was then immediately distributed into the wells
3~ at 100 ul/well. After incubating the cells with compound for 30 min. at
37~C in a humidified atmosphere of 5% C02 - 95% air, 20 ,ul/well of
anti-CD3 (Ortho Diagnostic, NJ) at final conc. of 0.3 ng/ml was added,
followed by 50 ,ul of the irradiated MNC. The culture plates were then
incubated at 37~C in a humidified atmosphere of 5% CO2 - 95% air for
3s 72 hours. The proliferation of T Iymphocytes was assessed by
CA 02236171 1998-04-28
W O 97/16438 PCT~US96/17481-
- 45 -
measurement of tritiated thymidine incorporation. During the last 18-
24 hrs. of culturing, the cells were pulse-labeled with 2 ,uCi/well of
tritiated thymidine (NEN, Cambridge, MA). The cultures were
harvested on glass fiber filters using a multiple sample harvester
s (MACH-II, Wallac,Gaithersburg, MD). Radioactivity of filterdiscs
corresponding to individual wells was measured by standard liquid
scintillation countin~ methods (Betaplate Scint Counter~ Wallac). Mean
counts per minute of replicate wells were calculated and the results were
expressed as concentration of compound required to inhibit tritiated
10 thymidine uptake of T cells by 50%.
KVl.3-RUBIDIUM EFFLUX ASSAY
CHO cells transfected with Kvl.3 channels at site densities
15 of approximately 40,000 sites/cell are plated into 96 well culture plates
and m~int~ined in Iscove's Modified Dulbecco's Medium (IMDM, with
L-Glutarnine and HEPES, JRH 13iosciences). Cells are incubated
overnight with 86Rb+ (3 ,uCi/ml, Dupont-NEN) in the glllt~mine
supplemented IMDM. After aspiration of the media, 100 ,ul of Low K
20 Buffer (in mM, 6.5 KCl, 125 NaCI, 1 CaCl2, 2 MgCl2, 10 HEPES, pH
adjusted to 7.2 with NaOH) is added to each well followed by 100 ,ul test
samples in Low K Buffer also cont~ining 0.2% BSA and 2 mM ouabain.
Samples are tested at either 1 ,ug/ml for routine screening or at a variety
of concentrations encompassing at least 1/10 to 10 times the putative
25 ICso of test compound to determine potency. After a fixed
preincubation time, which is usually 10 min, the samples are aspirated.
The Kv 1.3 channels are opened by depolarization of the cells with High
K Buffer (final concentrations, in mM, 63.25 KCl, 68.25 NaCl, 1
CaCl2, 2 MgCl2, 10 HEPES, pH adjusted to 7.2 with NaOH) also
30 cont~ining test compounds. To measure 86Rb+ efflux through the
channels, aliquots of 100 ,ul are taken from each well after a given time
and added to plates cont~ining 100 ,ul MicroScint-40 (Packard) for
counting by liquid scintillation techniques. MicroScint-40 (100 ,ul) is
then added to each well of the cell plate to determine the rem~ining
35 86Rb+ activity. The efflux counts are norm~li7ed for the total amount of
CA 02236171 1998-04-28
W O 97/16438 PCTAJS96/17481 -
- 46 -
86Rb+ that was in the cells by ~d-ling the efflux counts to the cell plate
counts. Activity is determined by % inhibition of the efflux window that
is established using a saturating concentration of margatoxin (MgTX), a
39 amino acid peptide that is a potent blocker of Kv 1.3 channels (ICso =
S 100 p~).
D~)S~J~. Fl~ lS
As an immunosuppressive, these compounds are useful in
the treatment of autoimmune diseases, the prevention of rejection of
foreign organ transplants and/or related afflictions, diseases and
illnesses.
The compounds of this invention can be ~clmini~tered for
the treatment of autoimmune diseases, the prevention of rejection of
foreign organ transplants and/or related afflictions, diseases and illnesses
according to the invention by any means that effects contact of the active
ingredient compound with the site of action in the body of a warm-
blooded ~nim~l For example, ~lmini.~tration, can be oral, topical,
including transdermal, ocular, buccal, intranasal, inh~l~tion,
intravaginal, rectal, intracisternal and parenteral. The term
"parenteral" as used herein refers to modes of ~lministration which
include subcutaneous, intravenous, intramuscular, intraarticular
injection or infusion, intrasternal and intraperitoneal.
The compounds can be ~lmini~tered by any conventional
2s means available for use in conjunction with pharmaceuticals, either as
individual therapeutic agents or in a combination of therapeutic agents.
They can be ~lminictered alone, but are generally ~lmini.ctered with a
ph:~rm~ceutical carrier selected on the basis of the chosen route of
~(lmini~tration and standard pharmaceutical practice.
For the purpose of this disclosure, a warm-blooded ~nim~l
is a member of the ~nim~l kingdom possessed of a homeostatic
mechanism and includes m~mmals and birds.
The dosage ~lmini~tered will be dependent on the age,
health and weight of the recipient, the extent of disease, kind of
3s concurrent treatment, if any, fre~uency of treatment and the nature of
CA 02236171 1998-04-28
W O 97/16438 PCTAJS96/17481 -
- 47 -
the effect desired. Usually, a daily dosage of active ingredient
compound will be from about 1-500 milligrams per day. Ordinarily,
from 10 to 100 milligrams per day in one or more applications is
effective to obtain desired results. These dosages are the effective
s amounts for the treatment of autoimmllne diseases, the prevention of
rejection of foreign organ transplants and/or related afflictions, diseases
and illnesses.
The active ingredient can be a~lmini~tered orally in solid
dosage forms, such as capsules, tablets, troches, dragées, granules and
powders, or in liquid dosage forms, such as elixirs, syrups, emulsions,
dispersions, and suspensions. The active ingredient can also be
zl~lmini~tered parenterally, in sterile liquid dosage forms, such as
dispersions, suspensions or solutions. Other dosages forms that can also
be used to ~lmini~ter the active ingredient as an ointment, cream, drops,
transdermal patch or powder for topical ~lmini~tration, as an
ophth~lmic solution or suspension formation, i.e., eye drops, for ocular
~lmini~tration, as an aerosol spray or powder composition for
inh~l~tion or intranasal ~lmini~tration, or as a cream, ointment, spray
or suppository for rectal or vaginal ~lmini~tration.
Gelatin capsules contain the active ingredient and powdered
carriers, such as lactose, starch, cellulose derivatives, magnesium
stearate, stearic acid, and the like. Similar diluents can be used to make
compressed tablets. Both tablets and capsules can be manufactured as
sustained release products to provide for continuous release of
2s medication over a period of hours. Compressed tablets can be sugar
coated or film coated to mask any unpleasant taste and protect the tablet
from the atmosphere, or enteric coated for selective disintegration in the
gastrointestinal tract.
Liquid dosage forms for oral ~lmini~tration can contain
~ 30 coloring and flavoring to increase patient acceptance.
In general, water, a suitable oil, saline, aqueous dextrose
(glucose), and related sugar solutions and glycols such as propylene
glycol or polyethylene gycols are suitable carriers for parenteral
solutions. Solutions for parenteral administration preferably contain a
CA 02236171 1998-04-28
W O 97/16438 PCTAJS96/17481-
- 48 -
water soluble salt of the active ingredient, suitable stabilizing agents, and
if necessary, buffer substances. Antioxidizing agents such as sodium
bisulfite, sodium sulfite, or ascorbic acid, either alone or combined, are
suitable stabilizing agents. Also used are citric acid and its salts and
sodium EDTA. In addition, parenteral solutions can contain
preservatives, such as benzalkonium chloride, methyl- or
propylparaben, and chlorobutanol.
Suitable pharmaceutical carriers are described in
Remington's Pharmaceutical Sciences, A. Osol, a standard reference text
in this field.
For a~lmini~tration by inh~l~tion, the compounds of the
present invention may be conveniently delivered in the form of an
aerosol spray presentation from pressurized packs or nebulisers. The
compounds may also be delivered as powders which may be form~ ted
and the powder composition may be inhaled with the aid of an
insufflation powder inhaler device. The preferred delivery system for
inh~l~tion is a metered dose inhalation (MDI) aerosol, which may be
forml~ ed as a suspension or solution of a compound of Formula I in
suitable propellants, such as fluorocarbons or hydrocarbons.
For ocular aflministration, an ophthalrnic preparation may
be fo~nulated with an appropriate weight percent solution or suspension
of the compounds of Formula I in an appropriate ophth~lmic vehicle,
such that the compound is m~int~ined in contact with the ocular surface
for a sufficient time period to allow the compound to penetrate the
corneal and internal regions of the eye.
Useful pharmaceutical dosage-forms for ~lmini~tration of
the compounds of this invention can be illustrated as follows:
C~APSULES
A large number of unit capsules are prepared by filling
standard two-piece hard gelatin capsules each with l00 milligrams of
powdered active ingredient, 150 milligrams of lactose, 50 milligrams of
cellulose, and 6 milligrams magnesium stearate.
CA 02236171 1998-04-28
W O 97/16438 PCTrUS96/17481
- 49 -
SOFT GELATIN CAPSULES
A mixture of active ingredient in a digestible oil such as
soybean oil, cottonseed oil or olive oil is prepared and injected by means
5 of a positive displacement pump into gelatin to form soft gelatin
capsules cont~ining 100 milligrams of the active ingredient. The
capsules are washed and dried.
TABLETS
A large number of tablets are prepared by conventional
procedures so that the dosage unit is 100 milligrams of active
ingredient, 0.2 milligrams of colloidal silicon dioxide, 5 milligrams of
magnesium stearate, 275 milligrams of microcrystalline cellulose, 11
15 milligrams of starch and 98.8 milligrams of lactose. Appropriate
coatings may be applied to increase palatability or delay absorption.
INJECTABLE
A parenteral composition suitable for ~imini~tration by
injection is prepared by stirring 1.5% by weight of active ingredient in
10% by volume propylene glycol. The solution is made to volume with
water for injection and sterilized.
SUSPENSION
An aqueous suspension is prepared for oral administration
so that each 5 milliliters contain 100 milligrams of finely divided active
ingredient, 100 milligrams of sodium carboxymethyl cellulose, 5
30 milligrams of sodium benzoate, 1.0 grams of sorbitol solution, U.S.P.,
and 0.025 milliliters of vanillin.
The same dosage forms can generally be used when the
compounds of this invention are ~(lmini~tered stepwise or in conjunction
with another therapeutic agent. When drugs are atlmini~tered in
35 physical combination, the dosage form and aflmini~tration route should
be selected depending on the compatibility of the combined drugs. Thus
CA 02236171 1998-04-28
W O 97/16438 PCTAUS96/17481-
- 50 -
the term co~lmini~tration is understood to include ~e ~lmini~tration of
the two agents concomi~ntly or sequentially, or alternatively as a fixed
dose combination of the two active components.
The following examples illustrate the preparation of the
5 compounds of FoImula I and as such are not to be considered as
limiting the invention set forth in the claims appended hereto.
EXAMPLE 1
10 A Method Of Extracting The Compounds Of Formula 1 (a) and 1 (b)
From Spachea correa
H H
H~,.,H'3[~H
b~ C H3/~o Formula 1 (a) b is a single
H r H ~"~ bond and R is OAc
COOCH3
o~iCH3~ CH~OAc Formula l(b) b is a double
~Ac C bond and R is OAc
One gram of an ethanol extract of the roots of Spachea
correa was partitioned between 100 ml of hexane (twice) and 100 ml
of 90% aqueous methanol. After separation of the phases, the defatted
methanol was concentrated down under vacuum to give an aqueous
suspension. This was diluted out to 100 ml with water and extracted,
with 100 ml of methylene chloride.
The bioactive methylene chloride extract was dried down
to give 12 mg of residue. This was first fractionated by preparative thin
Iayer chromatography (TLC) on a 20 cm by 20 cm E. Merck silica gel
60F254 plate of lmm thickness using methylene chloride-ethyl acetate
1:1 (v/v) as solvent, then by high performance liquid chromatography
(HPLC) using a Zorbax RxC~ 4.6 mm x 25 cm column, operated at
50~C and eluted with a 50 minute gradient of acetonitrile:water (1:1,
CA 02236l7l l998-04-28
W O 97/16438 PCTrUS96/17481 -
v/v) to 100% acetonitrile, delivered at 1 ml/min, to afford 4 mg of
compound 1 (a) and 1 mg of 1 (b).
Homogeneity of the preparations was ascertained in several
TLC systems, such as E. Merck silica gel 60F254, methylene chloride-
ethyl acetate 1:1, Rf l(a) 0.4, Rf l(b) 0.3; V~ n KClg, methanol-
water 9:1, Rf l(a) 0.65, Rf l(b) 0.75 and by HPLC using a Zorbax
RxC~ column, acetonitrile-water 3:2, k' l(a) 4.15, k' l(b) 3.30; and by
NMR.
Mass spectra were recorded on JEOL SX-102A (electron
impact, EI,903V) and JEOL HX110 (Fast Atom Bombardment, FAB)
mass spectrometers. Exact mass measurements were performed at high
resolution (HR-EI) using perfluorokerosene (PFK) as the internal
standard. Trimethylsilyl derivatives were prepared with a 1:1 mixture
of BSTFA-pyridine at room temperature The FAB spectrum was run
in a matrix of dithiothreitol dithioerthritol (20/80).
The compound of Formula l(a) runs underivatized by EI.
The molecular ion is observed a m/z 788 and three successive loses of
acetic acid are observed. The base peak is observed a m/z 334. The
compound does not silylate. Sc~nnin~ HR-EI indicated a molecular
formula of C40H52016. A table of the critical HR-EI data is given
below.
Observed m/z Formula Assignment
788.3220 C40H52016 M+
728.3040 C3gH4gO14 M-acetic acid
668.2834 C36H44012 M-2 x acetic acid
334.1417 C18H2206 base peak
13C NMR spectra were recorded for the compound of
Formula l(a) in CD2C12 at 100 MHz on a Varian Unity 400 NMR
spectrometer at 20~C. Chemical shifts are given in ppm relative to
tetramethylsilane (TMS) at zero ppm using the solvent peak at 53.8 ppm
as internal standard. The following data were observed: 15.0, 15..2,
16.8, 17.1, 20.7*, 20.9, 21.1, 21.6, 21.8, 22.2, 35.6, 40.8*, 42.1, 43.6,
CA 02236171 1998-04-28
W O 97/16438 PCT~US96/17481 -
- 52 -
45.1, 47.5, 49.3*, 53.5, 59.1, 62.6, 63.5, 66.1, 66.7*, 68.4*, 69.9, 73.9,
75.0, 75.6, 77.1*, 119.4, 123.7, 138.9, 143.0, 167.7, 169.2, 169.3*,
170.25, 170.31, 170.8, 171.3 ppm (where the * signifies the observation
as broad resonances). The carbon count of 40 is in agreement with the
5 molecular formula C4oH52ol6 derived by sc~nning HR EI-MS.
The lH NMR spectra of compound of Formula(a) is
provided as Fi~ure 1. The spectra was recorded at 400 MHz in CD~CI
on a Varian Unity 400 NMR spectrometer at 25~C. Chemical shifts are
in ppm relative to TMS at zero ppm using the solvent peak at ~5.32 as
10 the internal standard.
The mass spectra of the compound of Formula l(b) was
obtained as above. The following results were obtained.
Observed m/z Formula Assi~nment
786.3075 C40H50016 M+
726.2886 C3gH46014 M-acetic acid
666.2651 C36H42012 M-2 x acetic acid
606.2451 C34H38010 M-3 x acetic acid
489.2099 C26H33Og base peak
471.1992 C26H3 1 O8
1 3C NMR spectra were recorded for the compound of
Formulal(b) using the procedure described above. The following
results were observed: 14.8, 14.9, 17.3, 20.8, 20.9, 21.3, 21.7, 21.~,
2s 21.9, 27.1, 35.1, 40.6, 42.3, 45.4, 48.1, 50.4, 53.5, 54.1, 57.8, 63.7,
66.2, 67.8, 68.6, 71.4, 73.3, 73.8, 74.4, 1 19.5, 121.1, 124.3, 137.1,
138.9, 143.3, 167.6, 168.6, 169.3, 169.5, 169.9, 171.0, 171.7 ppm.
The carbon count of 40 is in agreement with the molecular
formula C40H50O16 derived by SC~nnin~ HR EI-MS.
CA 02236171 1998-04-28
W O 97/16438 PCT~US96/17481-
- 53 -
EXAMPLE 2
A Method Of Extracting The Compounds Of Formula l(c) And l(d)
From Spachea Correa
H H
~"" ~ H
b CH~o Formula l(c) b is a single
H ~" bond and R is OH
~HI~ OH COOCH3
O~ CH3rCH~OAc Formula l(d? b is a double
CH~AC OAc bond and R lS OH
OR
Analogs of the compounds of Formula l(a) and 1 (b) could
be detected in the crude extract and fractions thereof when the process
of Example 1 was carried out on a larger scale. Thus, 50 g of ethanol
extract were partitioned as described in Example 1 using 900 ml of each
solvent at each step.
Partial purification of the methylene chloride extract was
achieved by column chromatography on E. Merck silica gel 60 (120
ml), eluting with a step gradient of ethyl acetate in methylene chloride.
The step gradient was designed so that the column was washed first with
100 ~o methylene chloride and then with methylene chloride- ethyl
acetate mixtures of 9:1, 8:2, 3:2, 2:1, 1:1, 1:2, 2:8 and 1:9. Ultimately
the column was washed with 100% ethyl acetate. Fractions eluted with
methylene chloride-ethyl acetate 3:2 were enriched in compound of
Formula l(a) and l(b). These were resolved by HPLC using a Zorbax
RxC~$ 9 mm x 25 cm column, m~int~ined at 50~C and eluted at 4 ml/min
with acetonitrile-water 1:1 v/v. Three identical runs finally afforded
100 mg and 20 mg respectively of l(a) and l(b) after crystallization
from methanol. Later-eluting fractions from the silica gel column
above were found to contain at least two related compounds based on
UV spectra and color reactions on TLC plates. Material from the
CA 02236171 1998-04-28
W O 97/16438 PCTAUS96/17481 -
- 54 -
methylene chloride-ethyl actate 1:1 and 1:2 w~hin~ were combined
and evaporated down. Separation was achieved on the same HPLC
column as above, eluting with a 50 minute gradient of 30% to 50%
acetonitrile in water. Two identical runs gave 6 mg of purified
5 compound 1 (c). Fractions cont~ining the compound of Formula 1 (d)
were again processed by HPLC (same column) using acetonitrile-water
~:7 delivered i~ocratically to vield 2 mg of purifie~l Fc-rrrlllla ~
The mass spectra of these compounds were recorded on
a Finnigan TSQ700 mass spectrometer (electrospray ionization, ESI).
10 The samples were analyzed by LC/MS using a 2.1xlSOmrn C8 column
at 0.2ml/min. with a mobile phase of 45% acetonitrile/O.OlM aqueous
ammonium acetate at 50~C. Component 1 (d) had a retention time of
10.5 min. and a molecular weight of 744 which is observed a mlz: 745
(M+H), 762 (M+NH3), 786 (M + H + MeCN). Component 1 (c) has a
retention time of 11.8 and a molecular weight of 746 which is observed
at m/z: 747 (M+H), 764 (M+NH3) and 7~8 (M + H + MeCN).
The 13C NMR spectra obtained for ~e compound of
Formula l(c) using the conditions previously described is as follows:
15.1 (2x), 16.9, 19.8, 20.8, 20.91, 20.94, 21.9, 22.3, 35.6, 40.6, 42.2,
20 43.9, 45.0, 47.7, 50.8, 53.5, 55.6, 61.8, 63.5, 66.0, 67.6 (2x), 69.8,
70.0, 73.9, 75.0, 75.6, 119.3, 123.7, 139.0, 144.4, 167.8, 169.2, 169.5,
170.1, 170.4, 171.4 ppm.
The carbon count of 38 is in agreement with the molecular
formula C3gH50ol6 derived by sc~nning HR EI-MS.
EXAMPLE 3
Separation By HPLC
Compounds of this invention were characterized by the
following behavior during HPLC separation on a Zorbax RxCg 4.6 mm
x 25 cm column, m~int~ined at 50~C and eluted at 1 ml/min with
acetonitrile-water 3:2 v/v):
Compound l(a): k' = 4.15; l(b): k'=3.30; l(c): k'=2.30; l(d): k'=2.10.
CA 02236171 1998-04-28
W O 97/16438 PCTrUS96/17481-
- 55 -
Analyses using this HPLC system can be used to quantify
the compounds in the crude extract or other mixtures, by comparing the
absorbance of HPLC peaks at a wavelength of 220 nm with that
produced by injections of known (weighed) amounts of pure standards.
s
EXAMPLE 4
Additional Purification Procedure
A simplified purification process allows for rapid fraction-
ation of even larger amounts of crude extract and the preparation of
grarn amounts of the compounds of Formula l(a) and l(b).
The ethanol extract is first dissolved at 20 grams per
150 ml in methanol. This solution is diluted with 150 ml of water and
lS then extracted three times with methylene chloride using 150 ml of
methylene chloride each time. The pooled methylene chloride extracts
are evaporated down and fractionation proceeds by repeated column
chromatography on silica gel. One employs methylene chloride-
methanol 97:3 in a first step; the mixed compounds of Formula l(a) and
20 l(b) thus obtained are resolved by chromatographing on fresh silica gel
eluted with methylene chloride-ethyl acetate 3:1. Volume of elution for
the compound of Formula l(a) ranges from about 2 to about 3.5 column
volumes of solvent; that for the compound of Formula l(b) is about 3 to
about 4.5 column volumes. Finally, advantage is taken of the low
2s solubility of these compounds, and, after total resolution by
chromatography, the compounds of Formula 1 (a) and 1 (b) can be
precipitated and or crystallized from concentrated methanol solutions.
CA 02236171 1998-04-28
W O 97/16438 PCTnUS96/17481 -
- 56 -
EXAMPLE 5
4,6,7,15,16-Pentakis(acetyloxy)- 18-hydroxy-22-methoxycarbonyl-
[6O~,7OC,15~13,16,B]D:A-Friedo-A-homo-27,30-dinor-24-oxaoleana- 1,
20(29)~21 -trien-3 -one
Il
H ~¦CCO2C H3
o f \~ ~OAc
O ---'OAc
OAc
AcO ~
A solution of 233 mg (0.56 mmole) of tungsten
hexachloride in 8 mL of dry tetrahydrofuran was cooled to -78~C under
nitrogen. Then 0.70 mL (1.12 mmole) of 1.6 M butyllithium was added
and the solution was allowed to warm to room temperature over 30
min. Then a solution of 111 mg (0.141 mmole) of 4,6,7,15,16-
pentakis(acetyloxy)-21,22-epoxy- 18-hydroxy-22-methoxycarbonyl-
[6a,7Oc,15~3,1613,21,13,22~]D:A-Friedo-A-horno-27,30-dinor-24-
oxaoleana-1,20(29)-dien-3-one in 2 mL of dry tetrahydrofuran was
added and the solution was heated under nitrogen at 55~C for 14 h. The
mixture was applied to a 10 cm column of silica gel, which was washed
with 2: 1 ethyl acetate-hexane. The eluate was concentrated and purified
by silica gel chromatography with 2:1 ethyl acetate-hexane to afford 95
mg (88%) of the title compound as a white solid; lH NMR (CDC13)
7.10 (s, lH, C21-H); Mass Spectrum (APCI) 790 (M+NH4).
CA 02236171 1998-04-28
W O 97/16438 PCTrUS96/17481 -
- 57 -
EXAMPLE 6
4-(2-Bromobenzoyl)oxy-6,7,15,16-tetrakis(acetyloxy)- 18-hydroxy-22-
methoxycarbonyl[6a,70c,15~,16,13]D:A-Friedo-A-homo-27,30-dinor-24-
oxaoleana- 1.20(29) .21 -trien-3 -one
D~
11
H ~CO2C H3
o ~OAc
0 ~", OAc
Br o~\oAc
~0
Step A: 6,7,15,16-Tetrakis(acetyloxy)-21,22-epoxy-4, 18-
dihydroxy-22-methoxycarbonyl -
[6a,7Oc,15~,16,13,21 ~,22,13]D:A-Friedo-
A-homo-27.30-dinor-24-oxaoleana- 1.20(29)-dien-3-one
J~
~0
H ~ CO2C H3
o -- ~--OAc
O~ OAc
H O~ ~OAc
A solution of 102.1 mg (0.130 mmole) of 4,6,7,15,16-
pentakis(acetyloxy)-21,22-epoxy- 18-hydroxy-22-methoxycarbonyl-
CA 02236171 1998-04-28
W O 97/16438 PCT~US96/17481
- 58 -
[6a,7~c,15,B,16,13,2113,22,B]D:A-Friedo-A-homo-27,30-dinor-24-
oxaoleana-1,20(29)-dien-3-one in 4 mL of tetrahydrofuran and 2 mL of
3M aqueous HCI was heated at 40~C for 24h. The solution was diluted
with dichloromethane and the layers were separated. The organic layer
5 was washed with 0.1 M phosphate buffer (pH 7), then was dried over
MgSO4 and concentrated. The residue was purified by silica gel
chro~to~raphv with 2: 1 ethyl ~cetate-hexane to afford 44~9 mg ~f ~he
title compound as a white solid (46%); lH NMR (CDC13) ~ 4.20 (q, lH,
J = 4.3 Hz, C4-H); Mass Spectrum (APCI): m/e 764 (M+NH4).
Step B: 4-(2-Bromobenzoyl)oxy-6,7,15,16-tetrakis(acetyloxy)-
21,22-epoxy- 18-hydroxy-22-methoxycarbonyl-
[6a,7a,15~,16~,21,B,2213]D:A-Friedo-A-homo-27,30-
dinor-24-oxaoleana- 1 ~20(29)-dien-3-one
H ~C~~2c H3
0=,( ¦ 1 OAc
O ~", OAc
Br o~\OAC
¢~'~
To a solution of 17.5 mg (23.5 ,umole) of 6,7,15,16-
tetrakis(acetyloxy)-21,22-epoxy-4,1 g-dihydroxy-22-methoxycarbonyl-
2() [6a,7a,15,B,16,13,2113,22,B]D:A-Friedo-A-homo-27,30-dinor-24-
oxaoleana-1,20(29)-dien-3-one in 0.5 mL pyridine was added 27.~ mL
(237 ,umole) of benzoyl chloride. The solution was stirred at room
temperature for 4 h, then was concentrated under reduced pressure.
CA 02236171 1998-04-28
W O 97/16438 PCTrUS96/17481 -
- 59 -
The residue was first filtered through a plug of silica gel and then
purified by HPLC (Waters RCM, ~ Porosil, 10 mm X 10 cm) using a
mixture of 9.6:6 (5:4: 1 hexane-methyl tert-butyl ether-
acetonitrile:hexane) to afford 17.3 mg (88%) of the title compound as a
s white solid; lH NMR ~ 5.67 (lH, C4-H), 7.40-7.43 (m, 2H), 7.72 (dd,
lH, J = 2.2, 6.9 Hz), 7.78 (dd, lH, J = 2.3, 6.9 Hz); Mass Spectrum
(APCI): m/e 946~ 94~ (79Br-M+NH4, 81Br-M+NH4).
Step C: 4-(2-Bromobenzoyl)oxy-6,7,15,16-tetrakis(acetyloxy)- 18-
hydroxy-22-methoxycarbonyl-[6a,7Oc,15,13,16,1~]D:A-
Friedo-A-homo-27,30-dinor-24-oxaoleana- 1,20(29),21 -
trien-3-one
H /--~CC~2CH3
of \~Ac
Br o~\oAc
~0
A solution of 218 mg (0.55 mmole) of tungsten
hexachloride in 8 mL of dry tetrahydrofuran was cooled to -78~C under
nitrogen. Then 0.69 mL (1.10 mmole) of 1.6M butyllithium was added
and the solution was allowed to warm to room temperature over 30
min. Then a solution of 50.2 mg (0.141 mmole) of 4-(2-
bromobenzoyloxy)-6,7,15,16-tetrakis(acetyloxy)-21,22-epoxy- 18-
hydroxy-22-methoxycarbonyl-[6(x,7cc,15,13,1613,21 ~13,22,(~]D:A-Friedo-A-
homo-27,30-dinor-24-oxaoleana-1,20(29)-dien-3-one in 2 mL of dry
tetrahydrofuran was added and the solution was heated under nitrogen at
CA 02236171 1998-04-28
W O 97/16438 PCTAJS96/17481-
- 60 -
55~C for 14 h. The mixture was washed with 2.5 MNaOH and brine,
then was dried over MgSO4. The filtrate was purified by HPLC
(Waters RCM, ,u Porosil, 25mm x 10cm) using a 8.9:4 mixture of
(5:4:1 hexane:methyl tert-butyl ether:acetonitrile):hexane to afford 31.3
5 mg (64%) of the title compound as a white solid; lH NMR (CDC13) ~
5.67 (lH, C4-H), 7.10 (s, lH, C21-H), 7.40-7.43 (m, 2H), 7.72 (dd, lH,
J = 2.2. 6.9 Hz), 7.78 (dd, lH, J = 2.3, 6.9 Hz); Mas.s ~pectn~m (APCJ)
m/e 930, 932 (79Br-M+NH4, 81Br-M+NH4).
EXAMPLE 7
4,6,7,15,16-Pentakis(acetyloxy)-21,22-epoxy- 18-hydroxy-22-
methoxycarbonyl[6a,7a,1513,1613]D:A-Friedo-A-homo-27,30-dinor-24-
oxaoleana- 1.20(29).21 -triene
H /~CO2CH3
~ OAc
o~J " OAc
,~OAc
AcO
Step A: 4,6,7,15,16-Pentakis(acetyloxy)-21,22-epoxy- 18-hydroxy-
22-methoxycarbonyl[6a,70c, l 5,B,16,B,21,(3,2213]D:A-Friedo-
A-homo-27.30-dinor-24-oxaoleana- 1.20(29)-dien-3-ol
CA 02236171 1998-04-28
W O 97/16438 PCTrUS96/17481 -
- 61 -
H ~'--~CO02CH3
H O
,~OAc
AcO
A solution of 3.0 g (3.8 mmole) of 4,6,7,15,16-
pentakis(acetyloxy)-21,22-epoxy- 18-hydroxy-22-methoxycarbonyl-
[60c,70c,15,13,16,(~,21,B,22l3]D:A-Friedo-A-homo-27,30-dinor-24-
s oxaoleana-1,20(29)-dien-3-one in 20 mL of dry dichloromethane was
cooled to 0~C under nitrogen. Then 9 mL of a lM solution of lithium
tri-(tert-butoxy)alllminllm hydride was added dropwise and the solution
was stirred at 0~C. After 18 h, the reaction was quenched by dropwise
addition of 20 mL of 2M aqueous H2SO4 and the mixture was diluted
with 200 mL of ether. The layers were separated and the aqueous layer
was washed with two 100 mL portions of ether. The organic layers
were sequentially washed with 20 mL of 2M aqueous H2SO4 and brine,
then were combined, dried over MgSO4, and concentrated to afford 2.9
g (99%) of the title compound, which was used directly in the next step.
CA 02236l7l l998-04-28
W O 97/16438 PCT~US96/17481-
- 62 -
Step B: 4,6,7,15,16-Pentakis(acetyloxy)-21,22-epoxy- 18-hydroxy-
22-methoxycarbonylL60~,7(x,15,13,16l3,21~,22l3]D:A-Friedo-
A-homo-2730-dinor-24-oxaoleana- 1 ~20(29)-diene
~0
H O~ CO2CH3
--OAc
O~ OAc
, ~OAc
AcO
A sample of 2.9 g of crude 4,6,7,15,16-pentakis(acetyloxy)-
21,22-epoxy- 18-hydroxy-22-methoxycarbonyl-
[60c,7a,15,13,16l3,21,B,22,B]D:A-Friedo-A-homo-27,30-dinor-24-
oxaoleana-1,20(29)-dien-3-ol was dissolved in 10 rnL of dry
dichloromethane under nitrogen. To this was added 10 mL of
triethylsilane, and the solution was stirred at room temperature for 10
min. Then 2 mL (20 mmole) of boron trifluoride etherate was added
and the mixture was stirred at room temperature for 15 min. The
15 reaction was quenched by addition of 10 mL of saturated aqueous
KHCO3 solution and the resulting mixture was partitioned between
ether and water. The water layer was washed with ether and the
organic extracts were washed with brine, then were combined, dried
over MgSO4, and concentrated. The residue was purified by
20 chromatography on silica gel using 30% ethyl acetate-hexane to afford
2.13 g (72%) of the title compound as a white solid; lH NMR (CDC13)
~ 4.14, 4.34 (dd, AB, 2H, J = 12 Hz, C3-H); Mass Spectrum (APCI) m/e
792 (M+NH4).
CA 02236171 1998-04-28
W O 97/16438 PCTAJS96/17481-
- 63 -
~ Step C: 4,6,7,15,16-Pentakis(acetyloxy)- 18-hydroxy-22-methoxy-
carbonyl[60~,70c,15,1~,16~]D:A-Friedo-A-homo-27,30-dinor-
24-oxaoleana- 1 ~20(29)~21 -triene
H ~--i~Co2cH3
f \~OAc
O~ OAc
,~OAc
AcO
A solution of 119 mg (0.300 mmole) of tungsten
hexachloride in 4 mL of dry tetrahydrofuran was cooled to -78~C under
nitrogen. Then 0.38 mL (0.61 mmole) of 1.6M butyllithium was added
10 and the solution was allowed to warm to room temperature over 30
min. A solution of 34.6 mg (0.045 mmole) of 4,6,7,15,16-
pentakis(acetyloxy)-21,22-epoxy- 18-hydroxy-22-methoxycarbonyl-
[60c,70~,15,1~,16,13,21,13,22~]D:A-Friedo-A-homo-27,30-dinor-24-
oxaoleana- l ,20(29)-diene 1n 2 mL of dry tetrahydrofuran was added
15 and the solution was heated under nitrogen at 50~C for 14 h. The
mixture was diluted with 20 mL ether, and the mixture was washed with
2.5 M NaOH and brine, dried over MgSO4, and concentrated. The
residue was purified by HPLC (Waters RCM, ,u Porosil, 25mm x 10cm)
using a 6.75:7.0 mixture of (5:4:1 hexane:methyl tert-butyl
20 ether:acetonitrile):hexane to to afford 27 mg (80%) of the title
- compound as a white solid; lH NMR (CDC13) o (s, lH, C21-H); Mass
Spectrum (APCI) 776 (M+NH4).
CA 02236171 1998-04-28
W O 97/16438 PCT~US96/17481-
- 64
EXAMPLE 8
4,6,7,15,16-Pentakis(acetyloxy)-21,22-epoxy- 18-hydroxy-22-
methoxycarbonyl[6a,7(x,1513,1613,21,13,2213]D:A-Friedo-A-homo-27,30-
s dinor-24-oxaoleana-20(29)-en-3-one
~2C H3
4-
As described in Scheme I, 4,5,6,15,16-pentakis(acetyloxy)-
21,22-epoxy-18-hydroxy-22-methoxycarbonyl[60c, 7(x, 15~(3, 16,13, 21~,
22,B]D:A-Freido-A-homo-27,30-dinor-24-oxaoleana-1,20(29)-diene-3-
one, isolated from Spachea correa in liquid ammonia with lithium metal
will result in the reduction of the Cl olefin group to produce the
saturated lactone.
EXAMPLE 9
4,6,7,15,16-Pentakis(acetyloxy)- 18-hydroxy-22-methoxycarbonyl-
[60c,70c, l 5,E~,1613]D:A-Friedo-A-homo-27,30-dinor-24-
oxaoleana-20(29)~21 -dien-3-one
-
CA 02236l7l l998-04-28
W O 97/16438 PCTrUS96/17481-
- 65 -
Jl
H ~CO2CH3
--OAe
O~ OAe
OAe
AeO ~
A solution of 233 mg (0.56 mmole) of tungsten
hexachloride in 8 mL of dry tetrahydrofuran is cooled to -78~C under
s nitrogen. Then 0.70 mL (1.12 mmole) of 1.6M butyllithium was added
and the solution is allowed to warm to room temperature over 30 min.
Then a solution of 111 mg (0.141 mmole) of 4,6,7,15,16-
pentakis(acetyloxy)-21,22-epoxy- 18-hydroxy-22-
methoxycarbonyl[6cc,7O~,15,13,16~,2113,22,1~]D:A-Friedo-A-homo-27,30-
dinor-24-oxaoleana-20(29)-en-3-one in 2 rnL of dry tetrahydrofuran is
added and the solution is heated under nitrogen at 55~C for 14 h. The
mixture is applied to a 10 cm column of silica gel, which is washed with
2: 1 ethyl acetate-hexane. The eluate is concentrated and purified by
silica gel chromatography with 2: 1 ethyl acetate-hexane to produce the
title compound.
EXAMPLE 10
4-(2-Bromobenzoyl)oxy-6,7,15,16-tetrakis(acetyloxy)- 18-hydroxy-22-
21) methoxycarbonyl[6a,7Oc,1513,16,(3]D:A-Friedo-A-homo-27,30-dinor-24-
oxaoleana-20(29).21 -dien-3-one
CA 02236171 1998-04-28
W O 97/16438 PCT~US96/17481 -
- 66 -
H ~CO2CH3
o~--~--OAc
~ ~"OAOAC
Br o~\oAc
~0
Step A: 6,7,15,16-Tetrakis(acetyloxy)-21,22-epoxy-4, 18-
dihydroxy-22-methoxycarbonyl[60c,7(x,15,B,16,13,21,13,22~3]-
s D:A-Friedo-A-homo-27,30-dinor-24-oxaoleana-20(29)-en-
3-one
~~
H ~ CO2C H3
0=~\~ ~OAc
O~ OAc
H o OAc
A solution of 102.1 mg (0.130 mmole) of 4,6,7,1 ~,16-
pentakis(acetyloxy)-21,22-epoxy- 18-hydroxy-22-methoxycarbonyl-
[6a,7a,15,13,16,3,21 ~,2213]D:A-Friedo-A-homo-27,30-dinor-24-
oxaoleana-20(29)-en-3-one in 4 mL of tetrahydrofuran and 2 mL of 3M
aqueous HCl is heated at 40~C for 24h. The solution is diluted with
dichloromethane and the layers are separated. The organic layer is
15 washed with 0.1M phosphate buffer (pH 7), then is dried over MgSO4
CA 02236171 1998-04-28
W O 97/16438 PCTAJS96/17481-
- 67 -
and concentrated. The residue is purified by silica gel chromatography
with 2:1 ethyl acetate-hexane to produce the title compound.
Step B: 4-(2-Bromobenzoyl)oxy-6,7,15,16-tetrakis(acetyloxy)-
s 21,22-epoxy- 18-hydroxy-22-methoxycarbonyl-
[60c,70c,15,13,16,13,2113,22,13]D :A-Friedo-A-homo-27,30-
clinor-24-ox~olean~ -20~29)-en-3-one
H /--~CO2CH3
~Ac
Br o~\OAC
~0
To a solution of 17.5 mg (23.5 ~mole) of 6, 7, lS, 16-
tetrakis(acetyloxy)-21,22-epoxy-4, 18-dihydroxy-22-methoxycarbonyl-
[6a,70c,15~,16l3,21~,22~]D:A-Friedo-A-homo-27,30-dinor-24-
oxaoleana-20(29)-en-3-one in 0.5 mL pyridine is added 27.5 mL (237
15 ,umole) of benzoyl chloride. The solution is stirred at room
temperature for 4 h, then is concentrated under reduced pressure. The
residue is first filtered through a plug of silica gel and then purified by
HPLC (Waters RCM, ,u Porosil, 10 mm X 10 cm) using a mixture of
9.6:6 (5:4:1 hexane-methyl tert-butyl ether-acetonitrile:hexane) to
20 produce the title compound.
CA 02236171 1998-04-28
W O 97/16438 PCTAUS96/17481 ~
- 68 -
Step C: 4-(2-Bromobenzoyl)oxy-6,7,15,16-tetrakis(acetyloxy)- 18-
hydroxy-22-methoxycarbonyl[6a,7Oc,151,~,16,~]D:A-Friedo-
A-homo-27~30-dinor-24-oxaoleana-20(29).21 -dien-3-one
H ~CO2C H3
O~ ~OAc
O \~ " OAc
Br o~\OAC
~
A solution of 218 mg (0.55 mrnole) of tungsten
hexachloride in 8 mL of dry tetrahydrofuran is cooled to -78~C under
nitrogen. Then 0.69 mL (1.10 mmole) of 1.6M butyllithium is added
0 and the solution is allowed to warm to room temperature over 30 min.
Then a solution of 50.2 mg (0.141 mmole) of 4-(2-bromobenzoyl)oxy-
6,7,15,16-pentakis(acetyloxy)-21,22-epoxy- 18-hydroxy-22-
methoxycarbonyl-[6a,70c,15,B,16,13,21l3,22,B~D:A-Friedo-A-homo-27,30-
dinor-24-oxaoleana-20(29)-en-3-one in 2 mL of dry tetrahydrofuran is
15 added and the solution is heated under nitrogen at 55~C for 14 h. The
mixture is applied to a 10 cm column of silica gel, which is washed with
2: 1 ethyl acetate-hexane. The eluate is concentrated and purified by
silica gel chromatography with 2: 1 ethyl acetate-hexane to produce the
title compound.