Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02236183 2008-02-20
PROCESS AND APPARATUS FOR
CONVERTING OIL SHALE OR TAR SANDS TO OIL
FIELD OF THE INVENTION
The present invention relates to a continuous process for
producing synthetic crude oil (SCO) from oil shale or tar sand
and an apparatus for its practice. More specifically, the
present invention provides a process for treating dry tar sand
or shale without prior beneficiation, in a reactor operating
at elevated pressure and temperature conditions, in the
presence of substantially only hydrogen gas. The spent shale
or tar sand can then be used to prepare soil and construction
compositions.
BACKGROUND OF THE INVENTION
There are some tar sand systems that are successful in
making SCO, such as those in the Canadian Athabasca tar sand
area that surface mine and process the tar sands, where they
first separate sand (85 wt.%) from bitumen (15 wt.%) to avoid
processing the sand in the reaction systems. The separated
bitumen is converted to sweet, light crude oil by conventional
refinery type operation. Separation of the sand from the
bitumen requires beneficiating operations such as floatation
cells and secondary separation equipment and processing and
equipment to prepare the tar sand for flotation. Tailing oil
recovery is necessary to clear the sand for disposal, however
40214606.3 -1-
54222 vl
CA 02236183 2005-01-05
the sand is not completely cleared of bitumen.
Existing technology uses a large number of physical and
chemical processing units for the treatment of wet tar sands,
e.g., fluid cokers, LC finer, tumblers (being phased out by
hydro-pumping), beneficiation including: primary separation
vessels with floatation cells and secondary separation systems
necessary to recover the bitumen from the tar sand; tailing
oil recovery systems which result from the sand not being
completely cleared of bitumen; tailing settling ponds which
are necessary to settle and separate fine clays and other
undesirable solids from the water required for floatation
since the water must be reused to maximize clean-up to reduce
environmental problems. These systems require large facilities
along with the maintenance and reclamation required.
For example, U.S. Patent Nos. 5,340,467 and 5,316,467 to
Gregoli, et al. relate to the recovery of hydrocarbons
(bitumen) from tar sands. In the Gregoli, et al. patent
process, tar sand is slurried with water and a chemical
additive and then sent to a separation system. The bitumen
recovery from tar sand processes described in U.S. Patent Nos.
5,143,598 to Graham et al. and 4,474,616 to Smith, et al. also
involve the formation of aqueous slurries. Other processes
involving slurries, digestion, or extraction processes are
taught in U.S. Patent Nos. 4,098,674 to Rammler, et al.,
4,036,732 to Irani, et al., 4,409,090 to Hanson, et al.,
-2-
54222 vl
CA 02236183 2005-01-05
4,456,536 to Lorenz, et al. and Miller, et al.
In situ processing of tar sand is also known as seen
from the teachings of U.S. Patent Nos. 4,140,179, 4,301,865
and 4,457,365 to Kasevich, et al. and 3,680,634 to Peacock,
et al.
U.S. Patent No. 4,094,767 to Gifford relates to
fluidized bed retorting of tar sands. In the process
disclosed by the Gifford patent, raw tar sand is treated in a
fluidized bed reactor in the presence of a reducing
environment, steam, recycle gases and combustion gases. The
conversion of the bitumen, according to the Gifford patent,
is through vaporization and cracking, thereby leaving a coked
sand product. The steam and oxygen, according to Gifford are
"injected into the fluidized bed in the decoking area above
the spent sand cooling zone, and below the input area in the
cracking zone for fresh tar sand."
The process and apparatus of the present invention avoid
the use of the large number of physical and chemical
processing units used in the processing of wet tar sand by
using a single continuous reactor system to hydrocrack and
hydrogenate the dry tar sand. Moreover, because the present
invention directly hydrogenates dry tar sand, larger
quantities of valuable sweet, light crude oil is obtained.
Moreover, with the present invention, less gas and
substantially no coke is produced.
-3-
54222 vl
CA 02236183 2005-01-05
BRIEF SUMMARY OF THE INVENTION
The present invention relates to a continuous process for
converting oil bearing material, e.g., oil shale or tar sand,
and an apparatus for its practice.
Accordingly, one aspect of the present invention is to
provide a continuous process and an apparatus for its practice
where oil bearing material such as the kerogen in oil shale or
the bitumen in tar sand is continuously treated.
Another aspect of the present invention relates to the
treatment of dry tar sand.
An object of the present invention is providing a
process for converting tar sand to oil through the use of
substantially only hydrogen.
Another object of the present invention is providing a
heat recovery process whereby hydrogen provides the heat
necessary to bring the raw tar sand up to reactor temperature.
A still further object of the present invention is
providing a process where hydrogen is used for
hydrocracking and hydrogenating the bitumen in the tar sand
or oil shale.
A further objective of the present invention is providing
a process for using recycle and make-up hydrogen as a heat
transfer vehicle.
A still further object of the present invention is to
produce dry, relatively clean sand as waste that will not
-4-
54222 vl
CA 02236183 2005-01-05
pollute and can be used as excellent landfill for permanently
improved and desirable land.
Objects and advantages of the invention are set forth in
part herein and in part will be apparent herefrom, or may be
learned by practice with the invention, the same being
realized and attained by means of the flow charts, process
steps, structures, instrumentalities and combinations pointed
out in the appended claims. Accordingly, the invention resides
in the novel steps, parts, structures, arrangements,
combinations and improvements herein shown and described.
BRIEF DESCRIPTION OF THE DRAWINGS
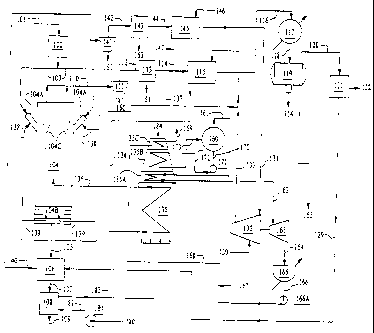
FIG. 1 shows the flow diagram of one embodiment
according to the present invention.
FIG. 2 shows a fluidized bed reactor for converting
bitumen in tar sand to viable products in accordance with the
present invention.
FIG. 3 shows a stand-alone fired heater used in the
process according to the present invention.
FIG. 4 shows a compressor for supplying the hydrogen for
use in the present invention.
FIG. 5 shows the flow chart of an acid gas recovery
system for use in the present invention.
FIG. 6 shows the mass balance for one embodiment of the
present invention.
-5-
54222 vl
CA 02236183 2005-01-05
DETAILED DESCRIPTION OF THE INVENTION
In the present invention the hydrocarbon content of the
hydrocarbon bearing solids, e.g., dry tar sand or oil shale is
reacted in a fluidized bed reactor with hydrogen and the
process is operated to avoid decompression of the hydrogen. In
the present invention, the hydrocarbon bearing solid does not
include bituminous or anthracite coals or similar type
material. A first portion of a substantially only hydrogen
stream is used to feed the oil shale or tar sand, which has
been comminuted and reduced in size to form particles that are
capable of being fluidized, e.g., fluidizable, into the
reactor. A second portion of the hydrogen stream is used as
the fluidizing medium. The hydrogen stream that is used in the
present invention is formed from fresh make-up hydrogen and
recycle hydrogen generated during the process, or obtained
from other hydrogen producing processes. A mixed fresh-make-up
and recycle hydrogen stream is discharged from a compressor at
a first temperature and pressure, and a portion is diverted
for admixture with the fluidizable particles of tar sand or
oil shale which are injected into the fluidized bed reactor in
a fan like flow, at an acute angle relative to the vertical
axis of the reactor or a horizontal plane. The remainder of
the hydrogen stream at said first temperature is indirectly
heated to a second higher temperature by indirect heat
-6-
54222 vl
CA 02236183 2005-01-05
exchange with overhead products from the fluidized bed
reactor. The hydrogen stream at said second temperature is
conveyed to a direct fired heater where the hydrogen stream is
heated to a third temperature higher than said second
temperature and then used as the fluidizing medium in the
reactor to fluidize the tar sand or oil shale fluidizable
particles that have been injected with the first portion of
the hydrogen stream.
In the fluidized bed reactor the bitumen in the tar
sand or the kerogen in the oil shale and hydrogen are
reacted via endothermic and exothermic reactions to produce
spent tar sand or oil shale and an overhead product stream
that contains hydrogen, hydrogen sulfide, sulfur gases, C1 +
C2 hydrocarbons, ammonia, fines (sand particles and clay)
and vaporous products. The overhead product stream is first
separated in cyclone separators within the reactor which
help maintain the bed level and separate solids. The first
separated overhead product is conveyed to a series of
additional separators to provide a particle free clean
product stream. The cleaned product stream at a first
temperature is conveyed to a first heat exchange unit where
heat is transferred to a second portion of the hydrogen
stream and results in a product stream at a second
temperature lower than said first product stream
temperature. The product stream at said second temperature
-7-
54222 vi
CA 02236183 2005-01-05
is conveyed to a condenser to further reduce its temperature
to a third temperature lower than the second product stream
temperature. The product stream at said third temperature
contains liquid and gas fractions and is conveyed to a
separator where the gas fraction is removed, sent to an
amine scrubber, and recycled as a scrubbed recycle hydrogen
stream, and the liquid fraction is removed as oil product
(SCO). The cooled, absorbed overhead hydrogen stream is
conveyed to a heat exchanger where it contacts spent tar
sand or spent shale and its temperature is elevated due to
heat transferred from the spent discharge. The hydrogen
stream at the elevated temperature is conveyed to a cyclone
separator, or other suitable separating devices to remove
particles. It then flows to the amine system to regenerate
the amine solution. It is eventually conveyed to a
compressor where it is combined with fresh make-up hydrogen
for use in the fluidized bed reactor as the first and second
portions of the hydrogen stream.
The invention will now be described with reference to the
figures. FIG. 1 is a flow chart of one embodiment of the
present invention where tar sand is converted to oil. In
accordance with the present invention, tar sand from the run
of mine conveyor belt 101 is continuously fed to any suitable
sizing equipment 102 for classifying tar sand, at a
temperature of about 50 F. Tar sand is composed of bitumen and
-8-
54222 vl
CA 02236183 2005-08-16
sand.
The bitumen in the tar sand that is processed in the
present invention normally contains heavy metals which
catalytically help promote the endothermic and exothermic
reactions in reactor 104. However, it may be advantageous to
add additional catalyst. The tar sand processed in accordance
with the present invention is exemplified by the following,
non-limiting example:
TAR SAND FEED (WATER-FREE BASIS)
sand 84.6 wt. %
bitumen 15.4 wt. %
carbon 83.1 wt. %
hydrogen 10.6 wt. %
sulfur 4.8 wt. %
nitrogen 0.4 wt. %
oxygen 1.1 wt. %
nickel 75 PPM
vanadium 200 PPM
100 wt. % 100 wt. %
In the present invention dry tar sand having an average
particle size of that of sand is conveyed through conduit 103
as the feed for fluidized bed reactor 104, discussed in
greater detail in FIG. 2. Tar sand particles which are
oversized are either recycled to the sizing equipment 102, or
conveyed to any suitable equipment for reducing the size of
the oversized feed.
-9-
54222 vl
CA 02236183 2005-01-05
Tar sand is fed through pressure feeder rotary valves
104A which are circumferentially positioned adjacent and
around the upper end of the fluidized bed reactor 104, which
is described in detail greater in FIG. 2. The rotary feeders
104A are positioned at an angle of between 20 and 60 degrees
relative to the vertical reactor axis in order to "fan feed"
the fluidizable sized tar sand into the top of the reactor
104. More uniform dispersion of the tar sand in the fluidized
bed reactor can be obtained when three or more rotary feed
valves 104A are positioned equidistantly around the
circumference of the reactor. Although three feeders 104A are
preferred, the size of the reactor and the degree of fanning
desired will control the number of valve feeders. Thus, there
could be 4, 5, 6, 7 or more valve feeders used in the present
invention.
High pressure hydrogen is conveyed through lines 138 to
the feeders 104A, at a pressure of between 625 psi and 700
psi, preferably about 635 psi, to assist in injecting,
feeding and dispersing the tar sand into reactor 104.
Another portion of the hydrogen gas feed from line 137 for
fluidizing medium (hydrogen feed) is diverted through lines 139
and injected into the separator section 104B, at the bottom end of
the reactor.
The process performed in fluidized bed reaction 104
involves hydrocracking, which is an endothermic reaction, and
-10-
54222 vl
CA 02236183 2005-01-05
hydrogenation, which is an exothermic reaction, which
reactions are conducted to favor the production of liquid
fuels and minimize the production of gas yields. The reactor
operates at temperatures of between 800 F and 900 F,
preferably closer to 800 F to avoid cracking the large
fragments of hydrogenated bitumen in the tar sand.
It is advantageous to conduct the endothermic
hydrocracking and exothermic hydrogenating processing of tar
sand in reactor 104 in a predominantly hydrogen gas
environment. The hydrogen atmosphere in reactor 104 is
maintained at about 600 psi by fresh make-up hydrogen conveyed
through line 130 from a hydrogen plant and a hydrogen recycle
stream 129 which contains cleaned-up hydrogen. The volume of
recycle hydrogen to fresh make-up hydrogen is preferably at
least about 26 to 1.
Advantageously all the high pressure hydrogen for the
process of the present invention, for reaction in reactor 104
and the various heat exchange operations, is provided by the
steam powered compressor 132. Compressor 132 receives fresh
make-up hydrogen which is conveyed through line 130 and
recycle hydrogen which is conveyed through lines 129, 140,
142, 144 and 131. Compressor 132 is powered by steam conveyed
through line 162 from direct fired heater 135. 184 is the
fired heater stack gases line.
Reactor 104 operates in a highly agitated fashion
-t~-
54222 vl
CA 02236183 2005-08-16
insuring almost instant and complete reaction between the
bitumen components and hydrogen. The residence or retention
time of the tar sand in reactor 104 is about 15 minutes, but
could be between 10 and 20 minutes, depending on the
throughput and efficiency of the reactor process. The pressure
drop from the bottom to the top of the reactor 104 is about 35
psi.
Overhead products from reactor 104 are discharged from
reactor 104 through cyclone separators 104C, while solids are
discharged through the separator section located at the lower
end of reactor 104. The cyclone separators 104C discharge an
overhead stream, e.g., gas and vapor reaction components,
off-gas and product, through their upper ends into line 110,
while separated solids are discharged through the lower ends
of the dip legs. The cyclone separators 104C extend about 20
feet down into the reactor 104 and establish the bed height
in the reactor 104.
The hot spent tar sand is continuously discharged at a
pressure of about 635 psi and a temperature of about 800 F
through lock hopper valving arrangement 104B in the lower end
of reactor 104 into line 105 which conveys the discharged
material to spent sand heat exchangers 106 and 108. Spent
sand is discharged through line 109.
The reactor overhead stream from the cyclone separators
104C is discharged into line 110, at a temperature of about
-12-
54222 vl
CA 02236183 2005-01-05
800 F and a pressure of about 600 psi. The overhead stream
discharged from the reactor 104 still contains dust and dry
waste particles, and is first conveyed through line 110 to
cyclone separator 111 where solids are separated and removed
through line 150. The gaseous effluent from separator 111 is
conveyed through line 112 to an electrostatic precipitator 113
for the final cleanup. The cleaned overhead stream from
precipitator 113 is removed and conveyed through line 114, and
separated solids are discharged through line 151. Cyclone
separator 111 and electrostatic precipitator 113 are of
conventional design and one of ordinary skill in the art
practicing the present invention can select suitable devices
for performing the described operation.
The cleaned stream from the precipitator 113, product,
vaporous components, and off gas, are conveyed to in-and-out
heat exchanger 115 through line 114. In the in-and-out
exchanger 115 the cleaned stream from line 114 is brought into
indirect heat exchange relationship with hydrogen being
conveyed through line 133, from compressor 132, i.e., recycle
and fresh make-up hydrogen, whereby heat is transferred from
the cleaned stream to the hydrogen in line 133 prior to the
hydrogen stream entering the fired heater 104. The cooled and
cleaned stream, products, vaporous components, off-gases, from
heat exchanger 115 is discharged into line 116 while hydrogen
is discharged into line 134 which conveys the hydrogen to the
-13-
54222 vi
CA 02236183 2005-01-05
direct fired heater 135.
The cooled stream being conveyed through line 116 is
introduced into condenser 117 and is discharged at a
temperature of about 100 F into line 118. The vapor and gas
stream from the condenser is conveyed through line 118 at a
temperature of 100 F and is introduced into separator 119
where vapors and liquid are separated and discharged.
Since the gas stream has been cooled down to about 100 F
and is still at a pressure of 480 psi, all carbon compounds C3
and above have been condensed are removed from the separator
119 through flow line 155 to storage. Sour water from the
separator is discharged through flow line 154. The crude oil
product stream in line 155 is a mixture of naphtha and gas
oils having an A.P.I. of approximately 33.5 and is a light
sweet crude. The gas stream in line 120 is conveyed to a
scrubbing system, e.g., at least one amine absorption column
121 where sulfur components, e.g., hydrogen sulfide and sulfur
dioxide gases, are absorbed and discharged through line 122
and conveyed to a suitable sulfur recovery plant. The amine
absorption system having amine absorber 121 is described in
greater detail in FIG. 5.
The only gases not absorbed and removed in the absorption
system having amine absorber 121 are unreacted recycle
hydrogen and C1 + C2 hydrocarbons which are conveyed through
line 129 to heat exchangers 106 so that the spent tar sand is
-14-
54222 vi
CA 02236183 2008-02-20
cooled and the recycle hydrogen and C1 + C2 hydrocarbons is
heated and discharged into line 140. The C. and C2
hydrocarbons in line 129 will not be absorbed nor condensed
but will be recycled with the unreacted hydrogen after
processing in units 141, 143 and 145 discussed hereinafter.
The C1 and CZ hydrocarbons will reach equilibrium within the
reactor 104 at about 2 vol.% and will then add to the
production of crude oil per ton of tar sand. A small offset
will be the increase in the recycle stream.
As discussed above, the spent sand from the reactor 104
is discharged into a succession of heat exchangers 106 and
108. The first heat exchanger 106 cools the sand from 792 F to
400 F using cool recycle hydrogen being conveyed through line
129. The cooled spent sand is conveyed in line 107 from heat
exchanger 106 and introduced into a second heat exchanger 108
so that the sand is cooled by cold air introduced through line
180 from blower 181 and through line 182, before discharging.
The air heated by the spent sand is discharged into line 183
which conveys the heated air to fired heater 135 for
combustion therein. Although two heat exchangers are shown,
the invention contemplates using more if necessary.
The heated and partial recycle hydrogen stream conveyed
through line 140 is introduced into cyclone 141, discharged
into line 142 which conveys the stream to precipitator 143,
and then through line 144 for introduction into reboiler 145.
40214606.3 -15-
54222 vl
CA 02236183 2005-01-05
FLUIDIZED BED REACTOR
FIG. 2 schematically shows the pressurized, continuously
operating fluid bed reactor 204 in accordance with the present
invention. Sized and screened tar sand or shale are conveyed
through lines 203 and fed through pressure feeder rotary
valves 204A into the top of the reactor 204. A portion of the
gases processed in compressor 132 (FIG. 1), and heated in
fired heater 135 (FIG. 1) are conveyed by line 236 and
introduced into fluidized bed reactor 204 in an upward
direction to fluidize the bed of the reactor 204. Another
portion of"the hydrogen gas from line 133 is conveyed through
line 237 to tar sand feed valves 204A through lines 238.
Another portion of the hydrogen gas feed from line 237 is
diverted through lines 239 and injected into the separator
section 204B, at the bottom end of reactor 204. Hydrogen
conveyed in lines 239 is injected into the separator section
204B of reactor 204 through injectors which are located at the
ends of flow lines.239 (not shown) and aid in heat retention
in the reactor system and spent sand discharge through line
205.
High temperature and high pressure hydrogen (make-up and
recycle) after passing through the direct fired heater 135, is
introduced into reactor 204 from line 236. Reaction products
and unreacted hydrogen exit the reactor through internal
-16-
54222 vl
CA 02236183 2005-01-05
cyclones 204C ensuring even flow out of the reactor. Although
two cyclone separators are shown, the invention contemplates
using as many as necessary to provide even flow of product
gases from reactor 204 and bed height maintenance. The hot
reactor effluent stream in line 210 is then conveyed to
physical and chemical units, described in FIG. 1 for cleanup
heat recovery and product separation.
DIRECT FIRED HEATER
As discussed above with reference to FIG. 1, a portion of
the fresh make-up and cleaned recycle hydrogen from the
compressor is conveyed to a direct fired heater. FIG. 3
schematically shows a fired heater 135 that is designed to
balance out the total energy required to operate the reactor
system. Preheated air conveyed through feed lines 183 is
combusted with fuel in the radiant section of fired heater 135
and elevates the temperature of the recycle and make-up
hydrogen that is conveyed through line 134. The fuel that is
combusted is obtained from the C3 fraction, e.g. propane, or
natural gas produced or purchased from the described process
or other sources. The hydrogen stream in lines 134 has been
preheated in the reactor in-out exchanger 115 to approximately
750 F. Since the hydrogen stream is circulated through the
radiant section of the heater 135 the temperature of the
hydrogen stream is elevated to a temperature of about 1200 F.
-17-
54222 vl
CA 02236183 2005-08-16
Circulation of the hydrogen stream through line 133, 134,
exchanger 115 and fired heater 135 is maintained by compressor
132 so that the 1200 F hydrogen stream can be introduced via
line 136 into reactor 104 (FIG. 1) or 204 (FIG. 2).
Waste heat from the radiant section of direct fired
heater 135 is recovered in convection section 135A, 135B and
135C. Steam separated in drum 160 is discharged into line 161
and introduced into convection section 135A where the steam
temperature is raised from about 596 F to about 800 F. After
passing through convection section 135A, the super heated,
high pressure steam is conveyed through line 162 to drive the
steam turbine 163 FIG. 4. Reduced temperature and pressure
steam from turbine 163 is conveyed to steam condenser 165 and
the condensate recirculated via line 166 and pump 166A. The
flow from pump 166A FIG. 1 is conveyed through line 168 and
combined with make-up water from line 167. The water being
conveyed in line 168 is introduced into convection section
135C, heated and discharged through line 169 for further
processing, e.g., deaeration.
Steam drum 160 separates steam which is conveyed to
radiant section 135A through line 161 to produce superheated
steam for the turbine compressor 163.
The steam circulation loop includes steam drum 160, line
170, recirculation pump 171 and lines 172-173 which conveys
boiler water through radiant section 135B and back into drum
-18-
54222 vl
CA 02236183 2005-08-16
160. Water for the boiler system is provided through feed line
167 which flows into line 168 which is in communication with
line 169 through convection section 135A to deaeration.
As discussed above, convection section 135A super heats
steam which is conveyed through line 162 to drive compressor
turbine 163, which drives compressor 132. Steam is generated
in convection section 135B and make-up water and turbine
condensate for boiler feed water are preheated in convection
section 135C.
COMPRESSOR SYSTEM
FIG. 4, schematically shows a compressor 132 driven by a
high pressure steam turbine 163 required to maintain
circulation of gases to operate the reactor system 104. Make-
up hydrogen 130 and recycle hydrogen 131, at approximately 450
psig and 100 F are pressurized by the compressor 132 to
approximately 670 psig and 122 F and discharged into line 133
which conveys and introduces the high pressure hydrogen into
the in-out exchanger 115 to be further heated by exchange with
reactor product gases.
High pressure steam in line 162, at 1500 psig and 800 F
drives the turbine 163. Exhaust steam 164 is condensed in
condenser 165, and along with make-up water 167 is fed to the
fired heater convection section 135C for preheating and reuse
as boiler feed water make-up.
19-
54222 vl
CA 02236183 2005-01-05
PRODUCT SEPARATION
The product separation of FIG. 1, components will be
described in greater detail with reference to FIG. 5, which
schematically shows the product separation from the
circulating gas stream and removal of,acid gasses in an amine
system. Partially cooled reactor effluent gases 116 from the
in-out exchanger 115 are further cooled in product condenser
117 and conveyed through line 118 to separator 119 where
condensed liquids are removed as product raw crude 155.
Overhead gases are conveyed through line 120 to an amine
absorber 121 where acid gasses H2S, COz and SO2 are absorbed by
a counter current circulating amine solution. The recycle
gases 121B flow from the top of the absorber 121 to recycle
hydrogen stream 129.
The rich amine solution 121C exits the bottom of the
absorber, flows through an amine exchanger 121D where it is
heated by exchange with hot lean amine solution 121L and
enters the top of an ainine stripper 121F through line 121E.
The lean amine solution passes through exchanger 121D to
exchanger 121N via line 121M and enters 121 via lean amine
feed line 121P. Absorbed acid gases are stripped from the
amine solution by the application of heat to the solution in
reboiler 145 (having reboiler feed line 146 and stripper
return line 147) and are conveyed through flow line 122 from
-20-
54222 vi
CA 02236183 2005-01-05
the stripper to sulfur recovery off-site. Hot recycle gases
are conveyed through line 144 from the spent sand cooler 106
via cyclone 141 and precipitator 143 to provide heat for
reboiler 145 and the partially cooled recycled gases 121G are
further cooled by cooler 121H and then flow through line 131
to the suction side of compressor 132. Solids removed by
cyclone 141 and precipitator 143 are discharged via lines 152
and 153 respectively.
Lean amine solution 121J is circulated by amine
circulation pumps 121K through the amine exchanger 121D and
amine cooler 121N to the top of the amine absorber 121 to
repeat the gas cleanup process.
EXAMPLE 1
The overall mass balance for the process according to
the present invention is shown in FIG. 6, where 1000
tons/hr of tar sand at 50 F are reacted with hydrogen to
produce 665 bbl/hr of synthetic crude oil. The following
Table provides the feed and product values for processing
1000 tons/hr. of tar sand.
RAW MATERIALS PRODUCTS
1000 TONS/HR. TAR SAND 665 BBL/HR SCO
1.6 MMSCF/HR HYDROGEN 5.2 MMSCF/HR STACK GAS
3.3 MMSCF/HR AIR 6600 LBS/HR SULFUR
0.5 MMSCF/HR NATURAL 850 TONS/HR SPENT SAND
GAS
-21-
54222 vl
CA 02236183 2008-02-20
REACTOR DIMENSIONS AND MASS AND ENERGY BALANCES
REACTOR 104
Column Diameter 20.00 ft
Cross Sectional Area 314.16 ft2
Void Fraction 0.85 (At Fluidization)
Cross Section of Sand 47.12 ft2
Cross Section of Gas 267.04 ft2
Reactor Volume 27394.26 ft3
Bed Diameter 20.00 ft
Bed Height 87.20 ft
Time-Space Constant 0.25 hr
Pressure Drop 35.00 psi
TAR SAND FEED
Sand Flow Rate 1000.00 tons/hr
Density of sand 121.68 lbs./ft3
Volumetric sand flow 16436.55 ft3/hr
Sand Velocity 5.81 ft/minute
Hold-up 15.00 minutes
HYDROGEN
Hydrogen Flow Rate 238661.44 lbs/hr
(45226343 SCF/hr)
Cp of H2 3.50 btu/lb- F (@900 F)
Hydrogen Recycle Ratio 26.52
Hydrogen Flow Rate 45.28 SCF/hr
Hydrogen Velocity 3.02 ft/s
OFF GAS
Gas Production 0.40 MMSCF/hr
MW 30.30 g/mole
Cp of flue gas 0.55 btu/lb- F
OFF GAS COMPOSITION wt. %
Co 0. 30 0
CO2 0.20%
H2S 31. 00 0
NH3 2 . 50 0
C3 66.00%
40214606.3 -22-
54222 vl
CA 02236183 2008-02-20
ENERGY BALANCE
OVER-ALL CONSIDERATIONS
Heat of Reaction 75.00 btu/lb. Bitumen
Cp Sand 0.19 btu/ton- F
Cp Bitumen 0.34 btu/lb- F
Cp Tarsand (sand+Bitumen) 426.70 btu/ton- F
Sand Feed Temperature 50.00 F
Sand temperature
at reactor inlet 50.00 F
Reaction temperature 800.00 F
Sand Feed 1,000.00 tons/hr
TAR SAND REACTOR
REACTOR CONDITIONS
Heat required in reactor 356.03 MMbtu/hr
Heat generated in Reactor 22.50 MMbtu/hr
Additional Heat Required 335.24 MMbtu/hr
Minimum H2 for reaction 9000.00 lbs./hr
(1.71 MMSCF/hr)
Additional H2 Supplied 229736.15 lbs./hr
43.53 MMSCF/hr)
Total H2 Supplied 238736.15 lbs./hr
(45.24 MMSCF/hr)
C1-CZ Flow within H2 Stream
(at equilibrium -2 vol.%) 4594.72 lbs/hr
(0.08 MMSCF/hr)
Entering H2 Temperature 1200.00 F
Cp H2 3.50 btu/lb- F
Heat Supplied by C1-C2 1.01 MMbtu/hr
Heat Supplied by H2 334.23 MMbtu/hr
H2 Recycle ratio 26.53
REACTOR BOTTOMS COOLER:
Assures Efficient Removal of Exiting Solids
Cold Hydrogen Cooler Stream 1,148.68 lbs./hr
(0.22 MMSCF/hr)
Heat Removed 2.73 MMbtu/hr
Entering Hydrogen Temperature 121.64 F
Exiting Sand Temperature 791.60 F
SAND COOLER
SAND
Sand Flow Rate 850.00 tons/hr
Temperature of Entering Sand 791.60 F
Temperature of Spent Sand 180.00 F
Cp Sand 0.19 btu/lb- F
Heat Removed 198.59 MMbtu/hr
HYDROGEN COOLANT FLOW
Hydrogen Flow 238736.15 lbs/hr
(45.24 MMSCF/hr)
40214606.3 -23-
54222 vl
CA 02236183 1998-04-28
Docket No.: 3495-7000
Heat to Be Removed 182.96 MMbtu/hr
Entering Hydrogen Temperature 100.00 F
Exiting Hydrogen Temperature 318.96 F
AIR COOLANT
Air Required for Combustion 250000.00 lbs/hr
(3.27 MMSCF/hr)
Cp Air 0.25 btu/lb-OF
Entering Air Temperature 50.00 F
Exiting Air Temperature 300.00 F
Heat Removed 15.63 MMbtu/hr
AMINE REBOILER
HYDROGEN SUPPLY
Entering Hydrogen Temperature 318.96 F
Exiting Hydrogen Temperature 100.00 F
AMINE BOIL-OFF
Heat Available to the system 182.96 MMbtu/hr
IN-OUT HEAT EXCHANGER
HYDROGEN TO BE HEATED
Hydrogen Flow 238736.15 lbs/hr
(45.24 MMSCF/hr)
Inlet H2 Temperature 121.64 F
Exiting H2 Temperature 750.00 F
Total Heat Required 525.05 MMbtu/hr
OFF GAS HEAT SUPPLY
Off Gas flow rate 31978.89 lbs/hr
0.40 MMSCF/hr
Condensables in vapor phase 214941.75 lbs/hr
MW 30.30 lb/lb-mole
Cp Vapor 0.55 btu/lb-OF
Cp Liquid 0.45 btu/lb-OF @70 F
Cp Non-Condensables 3.00 btu/lb-OF
Heat of Vaporization 65.00 btu/lb
Hydrogen Recycle Flow
in Stream 229736.15 lbs/hr
(*43.53 MMSCF/hr)
Inlet Temperature 800.00 F
Exit Temperature 350.00 F
PRODUCT CONDENSER/COOLER
PRODUCT SIDE
Entering Temperature 350.00 F
Exiting Temperature 100.00 F
Condensate 214941.75 lbs/hr
665.29 bbl/hr
Heat Removal H2 201.02 MMbtu/hr
Off Gas 4.40 MMbtu/hr
Condensate 38.15 MMbtu/hr
Total 243.57 MMbtu/hr
24
CA 02236183 1998-04-28
Docket No.: 3495-7000
COOLER REQUIREMENT 243.57 MMbtu/hr
COMPRESSOR
HYDROGEN SIDE
Flow Rate 755412.69 SCF/min
45.32 MMSCF/hr
Pressure Out 670.00 psi
Pressure In 450.00 psi
DP 220.00 psi
gamma (Cp/Cv) 1.40
J Stages 3
Temperature Inlet 100.00 F
Mechanical Efficiency 0.80 *100%
Pb/Pa 1.14
Power Requirement per Stage 6366.67 hp
Total Power Required 19100.00 hp
Outlet Temperature 121.64 F
STEAM SUPPLY
Pressure 1500.00 psi
Temperature 800.00 F
Degree Superheat 200.00 F
Saturation Temperature 596.20 F
Steam Heat Value 1364.00 btu/lb
Flow Rate 10894.28 lbs/hr
FIRED HEATER
PRODUCTS TO BE HEATED
Hydrogen Flowrate 238736.15 lbs/hr
45.24 MMSCF/hr
Hydrogen Temperature 750.00 F
Water Flow Rate 10894.28 lbs/hr
Water Temperature 75.00 F
Heat Duty 517.83 MMbtu/hr
C3'S (FUEL PRODUCED BY THE PROCESS)
Flow Rate 4263.85 lbs/hr
(0.04 MMSCF/hr)
Heat of Combustion 20000.00 btu/lb
Cp 0.60 btu/lb-OF
Temperature in 75.00 F
Heat Supplied
(After temperature correction) 79.84 MMbtu/hr
MAKE-UP METHANE
Combustion Temperature 2200.00 F
Heat Remaining to
be supplied by Methane 437.99 MMbtu/hr
Flow Rate 21653.89 lbs/hr
(0.51 MMSCF/hr)
Heat of Combustion
(After temperature correction)20227.00 btu/lb
Temperature in 75.00 F
CA 02236183 2008-02-20
COMBUSTION AIR
Air Required for Combustion 200000.00 lbs/hr
(2.61 MMSCF/hr)
Air Supplied 25 wt.% Excess 250000.00 lbs/hr
(3.27 MMSCF/hr)
COMPRESSOR SUCTION COOLER (5H)
OUTFLOWS
Hydrogen
Flowrate 200000.00 lbs/hr
Temperature 100.00 F
Required Coolant Supply 22.42 MMbtu/hr
MATERIAL BALANCE
TAR SAND REACTOR (104)
IN FLOWS
Sand
Flowrate 1000.00 tons/hr
Temperature 50.00 F
Pressure 14.70 psia (Force Fed)
Hydrogen
Flowrate 45.23 MMSCF/hr
Temperature 1200.00 F
Pressure 635.00 psi
C1-C2' s
Flowrate 0.08 MMSCF/hr
Temperature 1200.00 F
Pressure 635.00 psi
OUT FLOWS
Sand
Flowrate 850.00 tons/hr
Temperature 190.00 F
Pressure 600.00 psi
Off Gas
Flowrate 43.92 MMSCF/hr
Temperature 800.00 F
Pressure 600.00 psi
Composition wt%
H2 81.98
CO 0.05
COz 0.04
H2S 5.60
NH3 0.45
C3 11.92
40214606.3 -26-
54222 vl
CA 02236183 1998-04-28
Docket No.: 3495-7000
Product
Flowrate
(Vapor Phase) 214937.52 lbs./hr
Temperature 800.00 F
Pressure 600.00 psi
SAND COOLER (106, 108)
IN FLOWS
Sand
Flowrate 850.00 tons/hr
Temperature 791.92 F
Pressure 600.00 psi
Hydrogen
Flowrate 45.23 MMSCF/hr
Temperature 100.00 F
Pressure 500.00 psi
Air
Flowrate 3.27 MMSCF/hr
Temperature 50.00 F
Pressure 30.00 psi
OUT FLOWS
Sand
Flowrate 850.00 tons/hr
Temperature 200.00 F
Pressure 480.00 psi
Hydrogen
Flowrate 45.23 MMSCF/hr
Temperature 313.94 F
Pressure 480.00 psi
Air
Flowrate 3.27 MMSCF/hr
Temperature 300.00 F
Pressure 20.00 psi
IN-OUT HEAT EXCHANGER (115)
IN FLOWS
Hydrogen
Flowrate 45.23 MMSCF/hr
Temperature 147.60 F
Pressure 670.00 psi
Off Gas
Flowrate 43.92 MMSCF/hr
Temperature 800.00 F
Pressure 600.00 psi
Composition wt%
H2 81.94
CO 0.05
CO2 0.04
27
CA 02236183 1998-04-28
Docket No.: 3495-7000
H2S 5.60
NH3 0.45
C3 11.92
Product
Flowrate
(Vapor Phase) 214937.52 lbs./hr
Temperature 800.00 F
Pressure 600.00 psi
OUT FLOWS
Hydrogen Flowrate 45.23 MMSCF/hr
Temperature 750.00 F
Pressure 650.00 psi
Off Gas Flowrate 43.92 MMSCF/hr
Temperature 368.63 F
Pressure 580.00 psi
Off Gas Composition as Above
Product
Flowrate
(Vapor Phase) 214937.52 lbs./hr
Temperature 368.63 F
Pressure 580.00 psi
PRODUCT CONDENSERICOOLER (117)
IN FLOWS
Off Gas
Flowrate 43.92 MMSCF/hr
Temperature 368.63 F
Pressure 580.00 psi
Off Gas Composition as Above
Product
Flowrate
(Vapor Phase) 214937.52 lbs./hr
Temperature 368.63 F
Pressure 550.00 psi
OUT FLOWS
Off Gas
Flowrate 43.92 MMSCF/hr
Temperature 100.00 F
Pressure 540.00 psi
Off Gas Composition as Above
28
CA 02236183 1998-04-28
Docket No.: 3495-7000
Product
Flowrate
(as condensate) 214937.52 lbs./hr
Temperature 100.00 F
Pressure 540.00 psi
AMINE SYSTEM (121, FIG. 5)
IN FLOWS
Hydrogen
Flowrate 45.23 MMSCF/hr
Temperature 318.00 F
Pressure 470.00 psi
OUT FLOWS
Hydrogen
Flowrate 45.23 MMSCF/hr
Temperature 100.00 F
Pressure 450.00 psi
While particular embodiments of the present invention
have been illustrated and described herein, the present
invention is not limited to such illustrations and
descriptions. It is apparent that changes and
modifications may be incorporated and embodied as part of
the present invention within the scope of the following
claims.
29