Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
' CA 02237133 1998-OS-07
NITROGEN DIOXIDE PASSIVE SAMPLING SYSTEM
TI~CHNICAL ;FIELD
'The invention relates to passive samplers and sampling media for nitrogen
dioxide.
B,~CKGROUlfD OF THE INVENTION
Nitrogen dioxide (NOZ ) is a criteria air pollutant. It is required to be
monitored by law in many countries because it is a major source of acid rain
and is the only
gaseous air pollutant which contributes to visibility reduction.
Many sampling methods for monitoring NOZ in the ambient air have been
developed. These methods are generally categorized as either active methods or
passive
methods. Examples of active methods are described i n the following
publications:
1. Federal Register, "Sodium Arsenate method for the determination of Nitrogen
Dioxide in the Atmosphf;re", Vol. 42, p62971, 12/14/1977.
2. Federal Register, "TGS-ANSA Method for the Determination of Nitrogen
Dioxide in the Atmosphere", Vol. 42, p62971, 12/14/1977.
3 . Ellis, E.C.; Margeson, J. H. "Evaluation of TEA Procedure for
Determination of
Nitrogen Dioxide in Ambient Air", EP.A report NO. 650/4.74-031 July 1974.
4. Lipari, L. "New Solid - sorbent: Method for Ambient Nitrogen Dioxide
monitoring", Anal. Chern. 1984, 56, 1920-1826.
5. Blacker J.H. "Triethanolalnine :for Collecting NOZ in the TLV Range", Am.
Ind.
Hyg. Assoc. J., 1973, 34, 390-396.
CA 02237133 1998-OS-07
Active methods use devices to pump air through collection devices to collect
the NOZ .
,A passive or diffusive sampler is a device which samples an atmospheric gas
at
a rate controlled by the physical process of diffusion through a static air
layer or permeation
through a membrane. Passive samplers rely upon a concentration gradient across
a diffusion
barrier to produce a mass transfer of gaseous molecules. The principle of
operation is based
on Fick's first law of diffusion:
41=-DA do
dx
(1)
where J = diffusion transfer rate,
D= diffusion coefficient
A= effective cross-sectional area
x= distance along the diffusion path
c= analyte concentration at distance x
The negative siign in equation 1 indicates that the concentration of the
analyte decreases in the
direction of diffusion.
:Equation 1 may be simplified as follows:
Q=R.,.Cat ~2)
'where Q= mass uptake
RS = sampling rate
Ce = concentration of the analyte
t = sampling time
-2-
CA 02237133 1998-OS-07
From equation 2 it can be seen that the key parameter related to the correct
measurement of
NO Z in the atmosphere using a passive sampler is its sampling rate. Active
samplers have a
known sampling rate, which is the pump's flow rate. A passive sampler's
sampling rate
depends on many factors including NOZ concentration. ambient temperature,
relative humidity,
wiind direction, wind speed, the exposure and structure of the sampler and the
collection
media. Examples of passive sampling vmethods may be found in the following
publications:
1. 1?almes, E.D.,; Gunnison.) A.F.; DiMatti:o, J.; Tomczyk, C. "Personal
Sampler
~:or Nitrogen Dioxide", Am. Ind. Hyg. fl.ssoc. .1., 1976, 37, 570-577.
2. perm, M. "Further Development of a Diffusion Sampler for NOZ", IVL-
T 86/180, Swedish Environmental Research Institute 1986.
3. Mulik, J.D.; Williams, D. "Passive Sampling Devices for NOZ", Proceedings
of
~'he 1986 EPAlAPCA symposium of mecxsurement of Toxic Air Pollutants,
l~aleigh, NC, April 1986, pp61-79.
4. hair, A.J.; Penkett, S.A.; Ovola, P. "Development of a Simple Passive
'technology for the Determination of N itrogen Dioxide in Remote Continental
l:,ocations", Atmos. Environ., 1991, 25(9), 1927-1939.
5. Ogawa & Company US~~ Inc. "NO-NOz Simultaneous Sampling Protocol", June
1994.
6. 1=IIOSH Method 6700, 1984.
7. perm, M.; Rodhe, H. "Measurement of Air Concentration of SOZ, NOZ and NH3
at Rural and Remote Sites in Asia", J. .Armos. Chem., 1997, 27, 17-29.
-3-
CA 02237133 1998-OS-07
'rriethanolamine (TEA) is a commonly used agent in passive samplers because
it captures NO-~ very efficiently and may be analyzed easily by ion
chromatography to a very
low detection limit. TEA is typically coated on cellulose or glass fibre
filters or on metallic
screens. However, numerous difficulties exist with the use of such TEA
sampling media. It
ha.s been found that prior art TEA-coated cellulose or glass fibre filters or
metallic screens
demonstrated v~ridely varying sampling rates as ambient temperature is
decreased from 27°C to
6°C. Similarly, a reduction in relative humidity from 79% to 10% is
known to increase NOz
sampling rates by about 50%. When I~-OZ concentration increases 10-fold from
20 parts per
billion (ppb) to about 200 ppb, the NOz sampling rate has been known to
increase about
2~~0%.
la is therefore desirable t,o have a passive sampling media for NOz which
exhibits a more; stable sampling rate across a wide range of ambient
temperature and other
variables which affect prior art passive sampling media.
SiIMMARY OF THE INVENTION
l:n one aspect of the invention, the invention is a nitrogen dioxide sampling
medium which has a sampling rate which is relatively insensitive to
atmospheric fluctuations
in temperature, relative humidity and other ambient conditions. In general
terms, the
sampling media comprises silica gel combined with triethanolamine (TEA) or
molecular sieve
combined with TEA.
l:n another aspect of the iinvention, the invention is an all-season sampler
for
collecting atmospheric nitrogen dioxide comprising:
(a) a sampler body having a downwardly opening cavity;
(b) sampling media disposed within the cavity comprising silica gel combined
with
~:riethanolamine (TEA) o:r molecular sieve combined with TEA; and
-4-
CA 02237133 1998-OS-07
(c) means for retaining the sampling media within the cavity.
The sampler may further comprise an a.ir diffusion barrier and a support ring
disposed
between the retainer means and the diffusion barrier thereby creating an air
gap between the
diffusion barrio;r and the sampling media.
B1ZIEF DESCF;IPTION OF THE DF,A'WINGS
lEmbodiments of the invention will now be described with reference to the
accompanying drawings in which:
1~ figure 1 is a cross-sectional depiction of the preferred embodiment of the
sampler of the present invention.
Figure 2 is a cross-sectional depiction of the sampler mounted within a rain
shelter.
DIETAILED DIESCRIPTION (BEST MODE) OF THE INVENTION
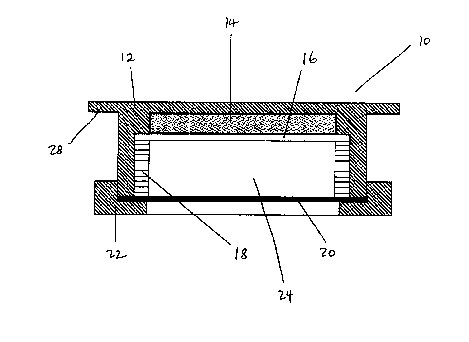
In one aspect of the invention, there is disclosed a passive NOZ sampler ( 10)
as
shown in Figure 1. In general terms, the preferred embodiment of the sampler (
10) comprises
a lbody ( 12), sampling media ( 14), a screen ( I 6), a support ring ( 18), a
diffusion barrier (20)
and a cover (2~?).
'Che body ( 12) defines a cavity (24) which is preferably cylindrical and
closed
at one end. TI ~e sampling media ( 14) i s retained within the cavity (24) by
the screen ( 16). In
the preferred embodiment, two polyester screens are used, having mesh sizes of
110 and 20
respectively.
_5_
CA 02237133 1998-OS-07
'the support ring ( 18) fits snugly in the cavity (24) and retains the screens
( 16)
in place. The support ring ( 18) also creates an air gap between the sampling
media ( 14) and
the diffusion barrier (20). The diffusion barrier (20) is preferably a Teflon~
film which acts
both as a gas diffusion controller and prevents contamination of the sampling
media by air-
borne particles. A ring-shaped cover (22) may be friction fit over the body (
12) and the
diffusion barrier (20) to hold the assembly of the sampler ( 10) together.
.As shown in Figure 2, tb~e samplers ( 1 CI) may be mounted in a rain shelter
(26).
The shoulder (;? 8) built into the body ( 12) facilitates mounting of the
samplers ( 10) so that the
cavity (24) faces downward.
In another aspect of the invention, there is disclosed a new sampling media
for
NO 2 comprising a combination of silica. gel as a carrier and triethanolamine
(TEA) as the NOZ
binding agent. Silica gel has a very hil;h internal surface area and
apparently bonds well with
TEA. The silica gel surface presents many acidic hydroxyl groups which, it is
surmised, may
be bonded to by TEA. Other materials which may be suitable for combining with
TEA in
accordance with this aspect of the invention include molecular sieve
(silicaceous clay) which
also has a very high internal surface area and a high affinity for TEA,
similar to silica gel.
In the preferred embodiment, the silica gel used has a mesh size of about
60/100 and an average pore size of about 22A and has internal surface area of
approximately
66.9 square meters per gram of gel. Silica gel with an average pore size of
60A and 150A
will also yield satisfactory results. In an alternative embodiment, molecular
sieve 13X, with
an internal surface area of approximately 600 square meters per gram and an
average pore
size of approximately 13A may be used.
(reparation of the sampling media is exemplified by the method described
herein. This rr.~ethod or any other method producing t:he media is not
intended to be limiting
of the scope of the invention claimed herein.
-6-
CA 02237133 1998-OS-07
EXAMPLE 1
Prepar;~tion of the Sampling Media
a. preparation of TEA coating solution
Ten grams of TEA (BDH, Toronto, Canada) and one gram of glycerol were
dissolved
in 100 ml of methanol and de-ionized {DI) water (Fisher Scientific, Nepean
Canada)
solution (50:50 volume). The coating solution was kept in a plastic bottle
with a cap
sealed with Teflon tape, and sto red at 4°C.
b. :silica gel cleaning
Two hundred grams of silica ge:l (W.R. Grace & Co., Baltimore, Maryland, 22 A,
60/100 :mesh) was washed with DI water to remove fine particles. After
washing, the
silica gesl was transferred to several glass petri dishes and dried in a
vacuum oven at
60°C and 15-mm Hg pressure for 3 hours. During drying, a small stream
of room air
purified by active carbon was directed at the gel in order to speed the drying
process.
After drying, the silica gel was transferred to a glass column with frit at
the column
bottom. The column was installed in a gas chromatograph (GC) oven with 20
ml/min
of helium as carrier gas. The silica gel was heated at 220°C for 24
hours, then cooled
down to room temperature. The heat-treated silica gel was transferred in a
glass bottle
with a c:ap sealed with Teflon tape. The glass bottle was stored at
4°C.
CA 02237133 1998-OS-07
c. Coating procedure
50 ml of each the heat-treated siilica gel and the TEA coating solution was
added into
a 20 crr~ (diameter) glass petri dish. After 5 minutes, the excess solution
was decanted
and the petri dish was placed into a vacuum oven and dried for 2 hours. The
vacuum
drying conditions were the same: as described above in section b. After
drying, the
coated silica gel was transferred to a glass bottle with a cap sealed with
Teflon tape,
Then, the glass bottle was put into a resealable plastic bag, sealed and kept
at 4°C.
d. ~4nalytical procedure
The spectrophotometric method, the flow injection analysis method and the
continuous
flow analysis method, are used for the nitrite analysis. These methods are
well known
in the art of nitrite analysis.
_g_
CA 02237133 1998-OS-07
The novel sampling media of the present invention was tested in the laboratory
in
10
accordance with the analytical procedures referred to above.
1. Temperature study
Cellulo:;e filters (Millipore, Bed:Ford, MA) coated with the same TEA coating
solution
and dried in th~~ vacuum oven under thc: same conditions for the sampling
media prepared in
accordance with Example 1 above (hereinafter referred to as "CHEMIXTM") were
compared
wiah CHEMIX'~M. Table 1 lists the comparison results. The comparison was
conducted at -
2 7 °C and 20°C., the relative humidity was about 4%, the cross
diffusion barrier surface
velocity was 0.5 cm/sec, and the NOZ concentrations were 30 ppb and 150 ppb.
Table 1. ;3ampling rate and temperature
Nitrite Concentration
(~,g/ml)
at Different Temperatures
Collection Media
-27C 20C
CHEMIXTM 0.97 1.10
TEA-cellulose filter 0.19 0.96
Table 1 clearly shows that the sampling; rate of CHEMIXTM changed only
slightly when the
temperature reduction from 20°C to -27°C, but the sampling rate
for TEA coated filters
decreased about 80%.
2. Sampling rate comparison
3 0 Table 1 also indicates that the C HEMIXTM has higher sampling rates than
the TEA
coated filter. The CHEMIXTM collected about 5 times higher NOz than the TEA
coated filter
at -27°C; the ratio was about 1.5 times at room temperature.
-9-
CA 02237133 1998-OS-07
3. Relative humidity study
Table 2 lists the sampling rate comparison of the CHEMIXTM and the TEA coated
filter. The studies were conducted at 20°C, 150 ppb NOZ concentration,
0.5 cm/sec CDBS
velocity. The RHs ranged from 4% to 69%. It can be observed that the change of
the
relative humidity did not affect the CH:EMIXT'M sampling rate significantly,
but did affect the
TI~A coated filter's sampling rate.
Table 2. ;3ampling rate and relative humidity
Collection Nitrite
Media Concentration
(~,g/ml
j at Different
Temperatures
and
Different
Relative
Humidities
-27C 17C
4% 20% 40% 4% 50%
CHEMIXTM 0.95 0.96 0.99 1.08 1.12
TEA-filter 0.17 0.35 0.40 0.86 0.96
4. Stability study
After e~;posing the NOZ passive sampler at 150 ppb NOZ concentration, half of
the
passive sampleo~s were taken out off the chamber and sealed in a resealable
plastic bag which
w<~s stored at -:?0°C. The rest of the passive samplers in the chamber
were purged with zero
air at room temperature for 5 days. The test results are shown in Table 3. The
results show
nc~ significant difference between the samplers in a cooler and in the
chamber, which indicates
th;~t the CHEMIXTM NOZ complex is stable.
- 10-
CA 02237133 1998-OS-07
Table 3. Comparison of samplers in cooler and in chamber
Sampler Storing Condition Nitrite Concentration (~cg/ml)
Cooler (-20C) 0.14
Chambf;r Purged with Air (20C'.) 0.14
5. Capacity study
The N0, passive samplers were exposed at 16CI ppb NOz concentration, 3% RH and
20°C for one day, seven days and nine days separately. The results are
shown in Table 5. It
is concluded that the passive samplers c;an at least be used to monitoring 50
ppb NOZ
concentration in the atmosphere for one:-month exposure period.
Table 4. ~~apacity study
Collection Nitrite Collected
(~,g) at Different
Exposure Time
25.5 hours 170.5 hours 203 hours
Total 25.9 164.5 203.7
Per Hour 1.0 1.0 1.0
6. Practic,~l quantitative detection limit
From field blank results, it is found that the pooled standard deviation was
0.1 ug of
ni~.rite per blank. The practical quantitative detection limit, thus, can be
taken as 1 ug per
bl;~nk (10 times of the standard deviation). This is the equivalent to
exposure of the passive
sampler to 0.1 ppb for one month.
- 11 -
CA 02237133 1998-OS-07
7. Analytical recovery study
The standard nitrite solutions were spiked into the sampling media. After
drying in
the vacuum oven, the spiked media were extracted following the procedure
described above.
The recovery vas found to be 100%.
8. Precision study
The precision study is based on triplicate NOz passive sampler exposure. The
pooled
standard deviation was calculated. It was found the pooled standard deviation
is only about
5°.~°.
9. Interference
Based on other studies, a possible interference for sampling NOZ in air by
using
CI~EMEXTM rr~ight be peroxyacetyl nitrate ("PAN"). Other investigators have
designed an
experimental regime to test the potential interference of PAN and concluded
that there was no
sil;nificant interference from PAN when using TEA as collection media.
- 12-