Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02238710 1998-OS-27
BOEHRINGER MANNHEIM GMBH
4273/00/kJG
New amino alcohol derivatives, processes for their
production and pharmaceutical agents containing these
compounds
The present invention concerns new amino alcohol
derivatives, processes for their production as well as
pharmaceutical agents which contain these substances.
In healthy persons the anabolic and catabolic processes
in the bone are almost in equilibrium i.e. the activity
of the osteoblasts and osteoclasts is balanced. However,
if this equilibrium is disturbed in favour of the
osteoclasts and/or in favour of the osteoblasts the bone
mass is reduced and there is a negative change in bone
structure and function.
Inhibitors of bone resorption such as oestrogens,
calcitonin and bisphosphonates have previously been used
to treat disturbances of bone metabolism. However, the
use of these substances is limited and also does not
exhibit the desired effect in all cases. Compounds which
have a stimulating effect on the formation of bone and
in addition contribute to increasing an already reduced
bone mass are therefore extremely important for the
treatment of disturbances of bone metabolism. Substances
with an osteoanabolic action for the therapy of
osteoporosis were described in the European Patent
Applications EP-A-625522 and EP-A-524023.
CA 02238710 1998-OS-27
- 2 -
Surprisingly it was now found that amino alcohol
derivatives of the present invention have a stimulating
effect on the formation of bone and are therefore
suitable for the broad treatment of disturbances of bone
metabolism. They can be used particularly well for cases
in which the formation of bone is disturbed i.e. they
are suitable for the treatment of osteopenic diseases of
the skeletal system such as e.g. osteoporosis including
osteogenesis imperfecta but also to support bone
regeneration and osteoinduction such as e.g. in
orthopaedic and orthodontic indications, in the healing
of fractures, osteosyntheses, pseudoarthroses and the
settling of bone implants.
Based on these properties they can also be used in the
prevention of osteoporosis.
As a result of their influence on bone metabolism they
additionally form a basis for the treatment of
rheumatoid arthritis, osteoarthritis and degenerative
arthrosis.
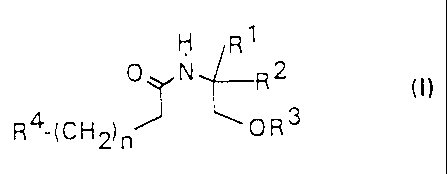
The present invention concerns new compounds of the
general formula (I)
R1
0 N R2
(I)
R4- CH ~ OR3
( 2) n
in which
R1 - hydrogen or methyl
R2 - lower straight-chained or branched alkyl with 1 to
CA 02238710 1998-OS-27
- 3 -
carbon atoms
R3 - hydrogen or lower alkyl
n - 0 - 12
R4 - alkyl, alkenyl or alkinyl with 6 to 24 carbon
atoms,
wherein in the case that
R4 denotes alkyl, -(CH2)n-R4 may not be an unbranched
alkyl chain with 8, 10, 12, 14 or 16 carbon atoms
and in the case that
R2 denotes methyl or isobutyl, -(CH2)n-R4 may not be
(all-cis-4,7,10,13)-octadecatetraene
as well as pharmacologically acceptable salts and
optical isomers thereof.
In EP-A-208961 amino alcohol derivatives of formula (I)
are described in which R1 denotes hydrogen or methyl,
R3 denotes hydrogen, R2 denotes methyl or isopropyl and
in which -(CH2)n-R4 denotes an unbranched alkyl chain
with 14 carbon atoms. In J. Med. Chem. 35, 2939-51
(1995) amino alcohol derivatives of formula (I) are
described in which R1, R3 denote hydrogen, R2 denotes
methyl or isobutyl and in which -(CH2)n-R4 denotes an
unbranched alkyl chain with 14 carbon atoms. A compound
of formula (I) is described in Biochem. J. 288, 167-73
CA 02238710 1998-OS-27
- 4 -
(1992) in which R1, R3 denote hydrogen, R2 denotes
isobutyl and in which -(CH2)n-R4 denotes an unbranched
alkyl chain with 10 carbon atoms. In J. Lipid Res. 13
(1), 86-91 (1972) and in DE-A-3418525 compounds of
formula (I) are described in which R1, R3 denote
hydrogen, R2 denotes ethyl and in which -(CH2)n-R4
denotes an unbranched alkyl chain with 8, 10, 12, 14 and
16 carbon atoms. All compounds are described as
intermediate products without information on a possible
use as pharmaceutical agents. Compounds of formula (I)
in which R1, R3 are hydrogen, R2 is methyl or isobutyl
and in which -(CH2)n-R4 is (all-cis-4,7,10,13)-
octadecatetraene have been described in Life Sci. 56
(23/24), 2041-8 (1995) as cannabinoid receptor ligands.
Therefore pharmaceutical agents are also a subject
matter of the invention which contain compounds of
formula I
R1
0 N R2
(I)
R4- CH ~ OR3
( 2) n
in which
R1 - hydrogen or methyl
R2 - lower straight-chained or branched alkyl with 1 to
carbon atoms
R3 - hydrogen or lower alkyl
n - 0 - 12
CA 02238710 1998-OS-27
- 5 -
R4 - alkyl, alkenyl or alkinyl with 6 to 24 carbon
atoms
as well as pharmacologically acceptable salts and
optical isomers thereof.
Lower alkyl is intended in all cases to represent a
straight-chained or branched C1-C6 alkyl group such as
e.g. methyl, ethyl, propyl, isopropyl, butyl, isobutyl,
pentyl or hexyl in particular methyl, ethyl, propyl and
butyl.
Alkyl is intended in all cases to represent a straight-
chained or branched C6 - Clg alkyl group such as e.g.
hexyl, isohexyl, 2,2-dimethylhexyl, 5-methylhexyl,
heptyl, isoheptyl, 6-methylheptyl, octyl, isooctyl,
nonyl, isononyl, decyl, isodecyl, undecyl, isoundecyl,
dodecyl, isododecyl, tridecyl, isotridecyl, tetradecyl,
isotetradecyl, pentadecyl, isopentadecyl, hexadecyl,
heptadecyl, isoheptadecyl or octadecyl in particular
heptyl, decyl and dodecyl.
Alkenyl denotes in all cases a monounsaturated or
polyunsaturated, optionally substituted, residue with 6
- 20 carbon atoms such as e.g. O1-hexenyl, ~1-octenyl,
O9-nonenyl, O1-decenyl, Olo-decenyl, 01.4-decadienyl,
O1, 4, 7-decatrienyl, 01.4, 7, lo-hexadecatetraenyl, O1-
dodecenyl, ~5-dodecenyl, X1,4-undecadienyl, 014-tetra-
decenyl, in particular ~1-decenyl, 01~4-decadienyl,
01,4,7-decatrienyl in which the double bonds can be cis
or trans and in the case of polyunsaturated compounds
all combinations are possible.
Alkinyl denotes in all cases a monounsaturated or
CA 02238710 1998-OS-27
- 6 -
polyunsaturated optionally substituted, residue with 6 -
20 carbon atoms such as e.g. O1-decinyl, O1-noninyl,
01,3_tetradecadiinyl, 01~3-hexadecadiinyl, 01~3-octa-
decadiinyl, in particular O1-decinyl.
Compounds of the general formula (I) contain at least
one asymmetric carbon atom and therefore optically
active compounds of the general formula (I) are also a
subject matter of the present application.
Compounds of the general formula (I) are obtained by
known processes for the formation of carboxylic acid
amides from the amino alcohols of the general formula
(II)
R1
HzN R2
(II)
OR3
in which Rl, R2 and R3 have the above-mentioned meanings
and carboxylic acid derivatives of the general formula
(III) ,
O X
4- ~ (III)
R (CH2)n
in which R4 and n have the meanings stated above and X
can be a hydroxy or an activation group whereby if X
denotes hydroxy, the carboxyl group can be activated by
the carbodiimide process and if X denotes an activating
group, mixed anhydrides come into consideration and
CA 02238710 1998-OS-27
- 7 -
especially with lower alkyl esters of carbonic acid such
as ethyl or isobutyl esters or active esters in
particular p-nitrophenyl, 2,4,5-trichlorophenyl,
N-hydroxysuccinimide or 1-hydroxybenzotriazole esters,
or by condensation with nitriles of the general formula
(IV)
R4-(CH2)n+1-CN (IV)
in which R4 and n have the meanings stated above (cf.
Liebigs Ann. Chem. 986-96 (1979)).
Compounds of the general formula (II) are produced by
known processes preferably by the reduction of amino
acids or they are commercially available.
Compounds of the general formula (III) are produced by
known processes from compounds of the general formula
(V)
R4-(CH2)n+1-COOH (V)
in which R4 and n have the meanings stated above.
Compounds of the general formula (IV) are produced by
known processes for the synthesis of nitriles or they
are commercially available.
Compounds of the general formula (V) are produced by
known processes for chain elongation or synthesis of
carboxylic acids or they are commercially available.
CA 02238710 1998-OS-27
g
Pure enantiomers of the compounds of formula (I) can be
obtained by using optically active amino alcohols which
can be produced by known processes e.g. by classical
racemate resolution via salt formation with optically
active acids or by reduction of optically active amino
acids.
Compounds of the formula (I) can be administered orally,
enterally, parenterally, topically, nasally, pulmonary
or rectally in a liquid, solid or aerosol form in all
usual non-toxic pharmaceutically acceptable carrier
materials, adjuvants and additives. The compounds of
formula (I) can be also applied locally on/or in the
bones (possibly in a surgical operation). In this
connection the term parenterally encompasses
subcutaneous, intravenous and intramuscular
administration or infusions. Oral forms of application
can be for example tablets, capsules, coated tablets,
syrups, solutions, suspensions, emulsions, elixirs etc.
which can contain one or several additives from the
following groups such as e.g. flavourings, sweeteners,
dyes and preservatives. Oral forms of administration
contain the active component together with non-toxic
pharmaceutically acceptable carrier materials which are
suitable for the production of tablets, capsules, coated
tablets etc. such as e.g. calcium carbonate, sodium
carbonate, lactose, calcium phosphate or sodium
phosphate; starch, mannitol, methylcellulose, talcum,
highly dispersed silicic acids, higher molecular fatty
acids (such as stearic acid), peanut oil, olive oil,
paraffin, miglyol, gelatin, agar-agar, magnesium
stearate, bee wax, cetyl alcohol, lecithin, glycerol,
animal and vegetable fats, solid high molecular polymers
(such as polyethylene glycols). Tablets, capsules,
coated tablets etc. can be provided with an appropriate
CA 02238710 1998-OS-27
_ g _
coating such as e.g. glyceryl monostearate or glyceryl
distearate to prevent undesired side-effects in the
stomach or to increase the duration of action by a
delayed absorption in the gastro-intestinal tract. As an
injection medium sterile injectable aqueous or oily
solutions or suspensions are preferably used which
contain the usual additives such as stabilizers and
solubilizers. Such additives can for example be water,
isotonic saline solution, 1,3-butanediol, fatty acids
(such as oleic acid), monoglycerides and diglycerides or
miglyol. All suitable non-irritating additives can be
used for the rectal application which are solid at
normal temperatures and liquid at rectal temperature
such as e.g. cacao butter and polyethylene glycol. For
aerosol application the pharmaceutically common carrier
media are used. Creams, tinctures, gels, solutions or
suspensions etc. containing pharmaceutically common
additives are used for external applications.
An application directly on/or in the bones (possibly in
a surgical operation) can either be achieved in a
solution or suspension or bound to a carrier
advantageously by infusion or injection (preferably
locally) Carrier-bound compounds of formula (I) can for
example be administered as gels, pastes or as a coating
on implants.
Biocompatible and preferably biodegradable materials are
used as a carrier. The materials preferably themselves
additionally induce wound healing or osteogenesis.
For the local application it is preferable to embed the
compounds of formula (I) in polymeric gels or films, to
immobilize them in this manner and to apply this
CA 02238710 1998-OS-27
- 10 -
preparation directly to the site on the bones to be
treated. Such polymeric base gels or films are composed
for example of glycerol, methylcellulose, hyaluronic
acid, polyethylene oxides and/or polyoxamers. Collagen,
gelatine and alginate are also suitable and described
for example in WO 93/00050 and WO 93/20859. Further
polymers are polylactic acid (PLA) and copolymers of
lactic acid and glycollic acid (PLPG) (Hollinger et al.,
J. Biodem. Mater. Res. 17 71-82 (1983)) as well as the
bone derivative "demineralized bone matrix" (DBM)
(Guterman et al. Kollagen Rel. Res. 8 419-4319 (1988).
Polymers which are used for example to adsorb TGFf3 are
also suitable and described in EP-A-0 616 814 and in EP-
A-0 567 391 and synthetic bone matrices according to WO
91/18558.
Other suitable carriers for compounds of formula (I) are
materials which are usually used in the implantation of
bone substitutes or of other therapeutically active
substances. Such carriers are based for example also on
calcium sulfate, tricalcium phosphate, hydroxyapatite
and polyanhydrides. Apart from these biodegradable
carriers, carriers are also suitable which are not
biodegradable but biocompatible. Such carriers are for
example sintered hydroxylapatite, bioglass, aluminates
or other ceramic materials (e. g. calcium aluminate
phosphate). These materials are preferably used in
combination with the biodegradable materials such as in
particular polylactic acid, hydroxylapatite, collagen or
tricalcium phosphate. Further non-degradable polymers
are described for example in the US patent 4,164,560.
It is particularly preferable to use a carrier which
continuously releases the compounds of formula (I) at
the site of action. Slow release pellets from Lnnovative
CA 02238710 1998-OS-27
- 11 -
Research of America, Toledo, Ohio, USA are for example
particularly suitable for this. Pellets are particularly
preferably used which release the compounds of formula
(I) over several days preferably up to 100 days at a
daily dose of 1-l0 mg/kg per day.
The dosage can depend on various factors such as the
mode of administration, species, age and/or individual
state. The daily dose that has to be administered of the
active substance is 0.01 mg to approximately 100 mg/kg
body weight, preferably 0.1 to 10 mg/kg body weight and
can be administered singly or divided into several
doses.
Apart from the compounds mentioned in the examples and
compounds derived by combining all meanings of the
substituents stated in the claims the following amino
alcohol derivatives are preferred within the sense of
the present invention:
Preferred compounds (PC):
(1) [1-(Hydroxymethyl)-ethyl]-octanamide
(2) [1-(Hydroxymethyl)-propyl]-octanamide
(3) [1-(Hydroxymethyl)-pentyl]-octanamide
(4) [1-(Hydroxymethyl)-ethyl]-7-methyloctanamide
(5) [1-(Hydroxymethyl)-butyl]-7-methyloctanamide
(6) [1-(Hydroxymethyl)-propyl]-7,7-dimethyloctanamide
(7) [1-(Hydroxymethyl)-pentyl]-7,7-dimethyloctanamide
(8) [1-(Hydroxymethyl)-ethyl]-nonanamide
(9) [1-(Hydroxymethyl)-butyl]-nonanamide
(10) [1-(Hydroxymethyl)-ethyl]-4-methylnonanamide
(11) [1-(Hydroxymethyl)-propyl]-8-methylnonanamide
(12) [1-(Hydroxymethyl)-ethyl]-decanamide
(13) [1-(Hydroxymethyl)-butyl]-decanamide
CA 02238710 1998-OS-27
- 12 -
(14) [1-(Hydroxymethyl)-propyl]-undecanamide
(15) [1-(Hydroxymethyl)-pentyl]-undecanamide
(16] [1-(Hydroxymethyl)-ethyl]-10-methylundecanamide
(17) [1-(Hydroxymethyl)-butyl]-10-methylundecanamide
(18] [1-(Hydroxymethyl)-propyl]-dodecanamide
(19) [1-(Hydroxymethyl)-pentyl]-dodecanamide
(20] [1-(Hydroxymethyl)-pentyl]-11-methyldodecanamide.
(21) [1-(Hydroxymethyl)-butyl]-11-methyldodecanamide
(22] [1-(Hydroxymethyl)-pentyl]-tridecanamide
(23) [1-(Hydroxymethyl)-pentyl]-12-methyltridecanamide
(24] [1-(Hydroxymethyl)-ethyl]-tetradecanamide
(25) [1-(Hydroxymethyl)-pentyl]-tetradecanamide
(26] [1-(Hydroxymethyl)-butyl]-13-methyltetradecanamide
(27) [1-(Hydroxymethyl)-pentyl]-13-methyltetra-
decanamide
(28] [1-(Hydroxymethyl)-ethyl]-pentadecanamide
(29) [1-(Hydroxymethyl)-propyl]-pentadecanamide
(30] [1-(Hydroxymethyl)-butyl]-pentadecanamide
(31) [1-(Hydroxymethyl)-butyl]-14-methylpentadecanamide
(32] [1-(Hydroxymethyl)-pentyl]-14-methylpenta-
decanamide
(33) [1-(Hydroxymethyl)-ethyl]-hexadecanamide
(34] [1-(Hydroxymethyl)-ethyl]-15-methylhexadecanamide
(35) [1-(Hydroxymethyl)-propyl]-15-methylhexadecanamide
(36] [1-(Hydroxymethyl)-pentyl]-15-methylhexadecanamide
(37) [1-(Hydroxymethyl)-ethyl]-heptadecanamide
(38] [1-(Hydroxymethyl)-pentyl]-heptadecanamide
(39) [1-(Hydroxymethyl)-propyl]-16-methylhepta
decanamide
(40] [1-(Hydroxymethyl)-pentyl]-16-methylhepta-
decanamide
(41) [1-(Hydroxymethyl)-pentyl]-octadecanamide
(42] [1-(Hydroxymethyl)-butyl]-17-methyloctadecanamide
(43) [1-(Hydroxymethyl)-pentyl]-17-methyloctadecanamide
(44] [1-(Hydroxymethyl)-ethyl]-nonadecanamide
CA 02238710 1998-OS-27
- 13 -
(45) [1-(Hydroxymethyl)-pentyl]-nonadecanamide
(46] [1-(Hydroxymethyl)-ethyl]-18-methylnonadecanamide
(47) [1-(Hydroxymethyl)-ethyl]-eicosanamide
(48] [1-(Hydroxymethyl)-butyl]-eicosanamide
(49) [1-(Hydroxymethyl)-butyl]-19-methyleicosanamide
(50] [1-(Hydroxymethyl)-ethyl]-heneicosanamide
(51) [1-(Hydroxymethyl)-propyl]-docosanamide
(52) [1-(Hydroxymethyl)-pentyl]-tricosanamide
(53) [1-(Hydroxymethyl)-pentyl]-tetracosanamide
(54) [1-(Hydroxymethyl)-butyl]-heptacosanamide
(55) [1-(Hydroxymethyl)-pentyl]-heptacosanamide
(56) [1-(Hydroxymethyl)-pentyl]-hexacosanamide
(57) [1-(Hydroxymethyl)-ethyl]-heptacosanamide
(58) [1-(Hydroxymethyl)-propyl]-octacosanamide
(59) [1-(Hydroxymethyl)-butyl]-triacontanamide
(60) [1-(Hydroxymethyl)-pentyl]-heptenamide
(61) [1-(Hydroxymethyl)-ethyl]-trans-9-hexadecenamide
(62) [1-(Hydroxymethyl)-propyl]-trans-9-hexadecenamide
(63) [1-(Hydroxymethyl)-pentyl]-trans-9-hexadecenamide
(64) [1-(Hydroxymethyl)-propyl]-(all-cis-11,14,17)-
eicosatrienamide
(65) [1-(Hydroxymethyl)-butyl]-(all-cis-11,14,17)-
eicosatrienamide
(66) [1-(Hydroxymethyl)-pentyl]-(all-cis-11,14,17)-
eicosatrienamide
(67) [1-(Hydroxymethyl)-propyl]-cis-10-heptadecenamide
(68) [1-(Hydroxymethyl)-pentyl]-cis-10-heptadecenamide
(69) [1-(Hydroxymethyl)-pentyl]-cis-10-nonadecenamide
(70) [1-(Hydroxymethyl)-ethyl]-cis-3,cis-6-nona-
dienamide
(71) [1-(Hydroxymethyl)-butyl]-cis-3,cis-6-nona-
dienamide
(72) [1-(Hydroxymethyl)-ethyl]-cis-10-pentadecenamide
(73) [1-(Hydroxymethyl)-pentyl]-cis-10-pentadecenamide
(74) [1-(Hydroxymethyl)-butyl]-cis-12-octadecenamide
CA 02238710 1998-OS-27
- 14 -
(75) [1-(Hydroxymethyl)-propyl]-cis-13-octadecenamide
(76) [1-(Hydroxymethyl)-pentyl]-cis-13-octadecenamide
(77) [1-(Hydroxymethyl)-ethyl]-cis-7-octadecenamide
(78) [1-(Hydroxymethyl)-ethyl]-cis-8-eicosenamide
(79) [1-(Hydroxymethyl)-butyl]-cis-8-eicosenamide
(80) [1-(Hydroxymethyl)-ethyl]-traps-9-tetradecenamide
(81) [1-(Hydroxymethyl)-propyl]-traps-9-tetradecenamide
(82) [1-(Hydroxymethyl)-pentyl]-traps-9-tetradecenamide
(83) [1-(Hydroxymethyl)-pentyl]-cis-9,cis-11-octadeca-
dienamide
(84) [1-(Hydroxymethyl)-ethyl]-cis-9,cis-12-octadeca-
dienamide
(85) [1-(Hydroxymethyl)-butyl]-cis-9,cis-12-octadeca-
dienamide
(86) [1-(Hydroxymethyl)-propyl]-traps-9-octadecenamide
(87) [1-(Hydroxymethyl)-butyl]-traps-9-octadecenamide
(88) [1-(Hydroxymethyl)-ethyl]-cis-9-octadecenamide
(89) [1-(Hydroxymethyl)-propyl]-cis-9-octadecenamide
(90) [1-(Hydroxymethyl)-ethyl]-(all-traps-9,11,13,15)-
octadecatetraenamide
(91) [1-(Hydroxymethyl)-pentyl]-(all-traps-9,11,13,15)-
octadecatetraenamide
(92) [1-(Hydroxymethyl)-butyl]-(all-cis-9,11,13,15)-
octadecatetraenamide
(93) [1-(Hydroxymethyl)-pentyl]-(all-cis-9,11,13,15)-
octadecatetraenamide
(94) [1-(Hydroxymethyl)-ethyl]-cis-11-octadecenamide
(95) [1-(Hydroxymethyl)-pentyl]-cis-11-octadecenamide
(96) [1-(Hydroxymethyl)-ethyl]-cis-13-docosenamide
(97) [1-(Hydroxymethyl)-propyl]-(all-cis-13,16,19)-
docosatrienamide
(98) [1-(Hydroxymethyl)-pentyl]-(all-cis-13,16,19)-
docosatrienamide
(99) [1-(Hydroxymethyl)-ethyl]-(all-cis-9,12,15)-
octadecatrienamide
CA 02238710 1998-OS-27
- 15 -
(100) [1-(Hydroxymethyl)-ethyl]-(all-cis-8,11,14)-
eicosatrienamide
(101) [1-(Hydroxymethyl)-propyl]-(all-cis-8,11,14)-
eicosatrienamide
(102) [1-(Hydroxymethyl)-butyl]-(all-cis-8,11,14)-
eicosatrienamide
(103) [1-(Hydroxymethyl)-pentyl]-(all-cis-8,11,14)-
eicosatrienamide
(104) [1-(Hydroxymethyl)-ethyl]-trans-11-octadecenamide
(105) [1-(Hydroxymethyl)-pentyl)-trans-13-docosenamide
(106) [1-(Hydroxymethyl)-propyl]-traps-9,trans-12-
octadecadienamide
(107) [1-(Hydroxymethyl)-ethyl]-cis-9-tetradecenamide
(108) [1-(Hydroxymethyl)-propyl]-cis-9-tetradecenamide
(109) [1-(Hydroxymethyl)-butyl]-cis-9-tetradecenamide
(110) [1-(Hydroxymethyl)-ethyl]-cis-9-hexadecenamide
(111) [1-(Hydroxymethyl)-methylbutyl]-cis-9-hexa-
decenamide
(112) [1-(Hydroxymethyl)-butyl]-cis-9-hexadecenamide
(113) [1-(Hydroxymethyl)-ethyl]-10-undecenamide
(114) [1-(Hydroxymethyl)-pentyl]-(all-cis-8,11,14)-
eicosatrienamide
(115) [1-(Hydroxymethyl)-pentyl]-cis-ll,cis-14-
eicosadienamide
(116) [1-(Hydroxymethyl)-ethyl]-cis-11-eicosenamide
(117) [1-(Hydroxymethyl)-pentyl]-cis-~11-eicosenamide
(118) [1-(Hydroxymethyl)-ethyl)-cis-15-tetracosenamide
(119) [1-(Hydroxymethyl)-propyl]-cis-15-tetracosenamide
(120) [1-(Hydroxymethyl)-pentyl]-11-dodecenamide
(121) [1-(Hydroxymethyl)-ethyl]-9-decenamide
(122) [1-(Hydroxymethyl)-butyl]-16-heptadecenamide
(123) [1-(Hydroxymethyl)-ethyl]-(all-cis-11,14,17)-
eicosatrienamide
(124) [1-(Hydroxymethyl)-butyl)-(all-cis-11,14,17)-
eicosatrienamide
CA 02238710 1998-OS-27
- 16 -
(125) [1-(Hydroxymethyl)-pentyl]-(all-cis-11,14,17)-
eicosatrienamide
(126) [1-(Hydroxymethyl)-methylbutyl]-cis-13-
eicosenamide
(127) [1-(Hydroxymethyl)-ethyl]-cis-l3,cis-13-
docosadienamide
(128) [1-(Hydroxymethyl)-propyl]-(all-cis-7,10,13,16)-
docosatetraenamide
(129) [1-(Hydroxymethyl)-ethyl]-22-tricosenamide
(130) [1-(Hydroxymethyl)-ethyl]-9-tetradecynamide
(131) [1-(Hydroxymethyl)-butyl]-9-tetradecynamide
(132) [1-(Hydroxymethyl)-ethyl]-13-eicosynamide
(133) [1-(Hydroxymethyl)-ethyl]-10,12-nonacosadiinamide
(134) [1-(Hydroxymethyl)-ethyl]-10,12-octadecadiinamide
(135) [1-(Hydroxymethyl)-pentyl]-10,12-octadecadiinamide
(136) [1-(Hydroxymethyl)-butyl]-9-octadecynamide
(137) [1-(Hydroxymethyl)-propyl]-9-octadecynamide
(138) [1-(Hydroxymethyl)-methylbutyl]-9-octadecynamide
(139) [1-(Hydroxymethyl)-ethyl]-10-undecynamide
(140) [1-(Hydroxymethyl)-butyl]-10,12-tricosadiynamide
(141) [1-(Hydroxymethyl)-ethyl]-10,12-pentacosadiynamide
(142) [1-(Hydroxymethyl)-ethyl]-10,12-heptacosadiynamide
The following examples exhibit some of the process
variants that can be used to synthesize the compounds
according to the invention. However, they are not
intended to limit the subject matter of the invention.
The structure of the compounds was ensured by 1H and
optionally by 13C-NMR spectroscopy. The purity of the
substances was determined by means of C, H, N elemental
analysis as well as by thin layer chromatography.
CA 02238710 1998-OS-27
- 17 -
General working instructions
General working instruction A'
25 mmol carboxylic acid of formula III is dissolved in
125 ml THF. After addition of 30 mmol 1,1'-carbonyl-
diimidazole it is boiled for 10 min under reflux.
50 mmol amino alcohol of formula II is added at room
temperature. After a further 3 hours under reflux it is
concentrated in a vacuum. The residue is taken up in
diethyl ether, washed with water, 0.5 N NaOH and water.
The organic phase is dried over magnesium sulfate and
concentrated in a vacuum.
General working instruction B~
A solution of 25 mmol chloroformic acid isobutyl ester
dissolved in 25 ml absolute dichloromethane is added
dropwise at -10°C to a solution of 25 mmol carboxylic
acid of formula III and 25 mmol triethylamine in 100 ml
absolute dichloromethane. After 15 min a solution of
30 mmol amino alcohol of formula II and 30 mmol
triethylamine in 75 ml absolute dichloromethane is added
dropwise. It is stirred for a further 30 min at -10°C
before it is slowly heated to room temperature. The
reaction mixture is concentrated in a vacuum, taken up
in diethyl ether and w4shed with water, 0.5 N NaOH and
water. The organic phase is dried over magnesium sulfate
and concentrated in a vacuum.
General working instruction C:
A solution of 25 mmol carboxylic acid chloride of
CA 02238710 2001-11-21
- 18 -
formula III in 30 ml absolute dichloromethane is added
dropwise at 10°C to a solution of 25 mmol amino alcohol
of formula II and 25 mmol triethylamine in 100 absolute
dichloromethane. After stirring for 48 hours at room
temperature it is concentrated in a vacuum, the residue
is taken up in diethyl ether and washed with water, 0.5
N HC1 and saturated NaCl. The organic phase is dried
over magnesium sulfate and concentrated in a vacuum.
Examale 1
R-N-[1-(Hydroxymethyl)-propyl]-eicosanamide
carboxylic acid: eicosanoic acid
alcohol: R-2-amino-1-butanol
It is prepared according to the general working
instruction A. Yield 84 % colourless crystals; Fp 90-
92°C
Example 2
R-N-[1-(Hydroxymethyl)-propyl]-(all-cis-9,12,15)-
octadecatrienamide
carboxylic acid: linolenic acid
alcohol: R-2-amino-1-butanol
It is prepared according to the general working
instruction B. It was purified by chromatography on
silica gel with ethyl acetate/isohexane (1:1). Yield
61 % colourless oil.
CA 02238710 1998-OS-27
- 19 -
Example 3
N-[1-(Hydroxymethyl)-pentyl]-eicosanamide
carboxylic acid: eicosanoic acid
alcohol: 2-amino-1-hexanol
It is prepared according to the general working
instruction C: yield 65 % colourless crystals.
Example 4
N-[1-(Hydroxymethyl)-pentyl]-cis-3,cis-6-nonadienamide
carboxylic acid: cis-3,cis-6-nonadienoic acid
alcohol: 2-amino-1-hexanol
It is prepared according to the general working
instruction B. It was purified by chromatography on
silica gel with ethyl acetate/isohexane (1:1). Yield
35 % colourless oil.
Example 5
N-[1-(Hydroxymethyl)-pentyl]-nonanamide
carboxylic acid: nonanoic acid
alcohol: 2-amino-1-hexanol
It is prepared according to the general working
instruction A. Yield 95 % colourless oil.
CA 02238710 2001-11-21
- 20 -
Example 6
R-N-[1-(Hydroxymethyl)-3-methylbutyl]-(all-cis-9,12,15)-
octadecatrienamide
carboxylic acid: linolenic acid
alcohol: R-2-amino-4-methyl-1-pentanol
It is prepared according to the general working
instruction B. It was purified by chromatography on
silica gel with ethyl acetate/isohexane (1:1). Yield
94 % colourless oil.
Example 7
R-N-[1-(Hydroxymethyl)-butyl]-(all-cis-9,12,15)-
octadecatrienamide
carboxylic acid: linolenic acid
alcohol: R-2-amino-1-pentanol
It is prepared according to the general working
instruction B. It was purified by chromatography on
silica gel with ethyl acetate/isohexane (I:1). Yield
86 % colourless oil.
Example 8
R-N-[1-(Hydroxymethyl)-propyl]-cis-3,cis-6-nonadienamide
carboxylic acid: cis-3,cis-6-nonadienoic acid
alcohol: R-2-amino-1-butanol
CA 02238710 2001-11-21
- 21 -
Synthesis of cis-3 cis-6-nonadienoic acid
cis-3,cis-6-Nonadien-1-of (150 mmol) is dissolved in
140 ml acetone and admixed with a mixture of 14 g
chromiumtrioxide in 42 ml water and 12.6 ml concentrated
sulphuric acid.,After 6 h at room temperature it is
diluted with 70 ml water and the mixture is extracted
with diethyl ether. The combined organic phases are
extracted with soda solution, the soda solution is
acidified and extracted with dichloromethane. After
drying over magnesium sulfate it is concentrated and a
colourless oil is obtained. (yield: 54 %)
It is prepared according to the general working
instruction A. Yield 95 % colourless oil.
Examp 1 a 9
R-N-[1-Hydroxymethyl)-pentyl]-(all-cis-9,12,15)octadeca-
trienamide
carboxylic acid: linolenic acid
alcohol: R-2-amino-1-hexanol
It is prepared according to the general working
instruction B. It was purified by chromatography on
silica gel with ethyl acetate/isohexane (1:1). Yield
48 % colourless oil.
CA 02238710 1998-OS-27
- 22 -
Example 10
R-N-[1-Hydroxymethyl)-pentyl]-cis-9,cis-12-octadeca-
dienamide
carboxylic acid: linoleic acid
alcohol: R-2-amino-1-hexanol
It is prepared according to the general working
instruction C. It was purified by chromatography on
silica gel with ethyl acetate/heptane (2:1). Yield 55
colourless oil.
Example 11
N-[1-Hydroxymethyl)-pentyl]-cis-9-hexadecenamide
carboxylic acid: palmitoleinic acid
alcohol: 2-amino-1-hexanol
It is prepared according to the general working
instruction C. It was purified by chromatography on
silica gel with ethyl acetate/heptane (1:1). Yield 75
colourless oil.
Example 12
S-N-[1-Hydroxymethyl)-pentyl]-cis-9-octadecenamide
carboxylic acid: oleic acid
alcohol: S-2-amino-1-hexanol
CA 02238710 1998-OS-27
- 23 -
It is prepared according to the general working
instruction C. It was purified by chromatography on
silica gel with ethyl acetate/heptane (2:1). Yield 40
colourless amorphous powder.
Example 13
8-N-[1-Hydroxymethyl)-pentyl]-9-octadecynamide
carboxylic acid: octadecynoic acid
alcohol: S-2-amino-1-hexanol
It is prepared according to the general working
instruction C. It was purified by chromatography on
silica gel with ethyl acetate/heptane (2:1). Yield 51
colourless crystals.
Example 14
R-N-[1-hydroxymethyl)-pentyl]-cis-9-octadecenamide
carboxylic acid: oleic acid
alcohol: R-2-amino-1-hexanol
It is prepared according to the general working
instruction C. It was purified by chromatography on
silica gel with ethyl acetate/heptane (2:1). Yield 65
colourless crystals.
CA 02238710 2001-11-21
- 24 -
Example 15
S-N-[1-Hydroxymethyl)-pentyl]-trans-9-octadecenamide
carboxylic acid: elaidic acid
alcohol: S-2-amino-1-hexanol
It is prepared according to the general working
instruction C. It was purified by chromatography on
silica gel with ethyl acetate/heptane (2:1). Yield 55 0
colourless crystals.
Example 16
R-N-[1-Hydroxymethyl)-pentyl]-9-octadecynamide
carboxylic acid: octadecynoic acid
alcohol: R-2-amino-1-hexanol
It is prepared according to the general working
instruction C. It was purified by chromatography on
silica gel with ethyl acetate/heptane (2:1). Yield 61 0
colourless crystals.
Example 17
R-N-[1-Hydroxymethyl)-pentyl]-trans-9-octadecenamide
carboxylic acid: elaidic acid
alcohol: R-2-amino-1-hexanol
It is prepared according to the general working
CA 02238710 1998-OS-27
- 25 -
instruction C. It was purified by chromatography on
silica gel with ethyl acetate/heptane (2:1). Yield 54 0
colourless amorphous powder.
Example 18
R-N-[1-Hydroxymethyl)-pentyl]-eicosanamide
carboxylic acid: eicosanoic acid
alcohol: R-2-amino-1-hexanol
It is prepared according to the general working
instruction C. It was purified by chromatography on
silica gel with ethyl acetate/heptane (2:1). Yield 49
colourless crystals.
Example 19
N-[1-Hydroxymethyl)-pentyl]-cis-9-tetradecenamide
carboxylic acid: myristeoleic acid
alcohol: 2-amino-1-hexanol
It is prepared according to the general working
instruction C. It was purified by chromatography on
silica gel with ethyl acetate/heptane (2:1). Yield 84
colourless oil.
CA 02238710 1998-OS-27
- 26 -
Example 20
N-[1-Hydroxymethyl)-pentyl]-9-octadecynamide
carboxylic acid: octadecynoic acid
alcohol: 2-amino-1-hexanol
It is prepared according to the general working
instruction C. It was purified by chromatography on
silica gel with ethyl acetate/heptane (1:1). Yield 65
colourless oil.
Example 21
N-[1-Hydroxymethyl)-pentyl]-trans-9-octadecenamide
carboxylic acid: elaidic acid
alcohol: 2-amino-1-hexanol
It is prepared according to the general working
instruction C. It was purified by chromatography on
silica gel with ethyl acetate/heptane (1:1). Yield 80
colourless oil.
Example 22
N-[1-Hydroxymethyl)-pentyl]-9-tetradecynamide
carboxylic acid: tetradecynoic acid
alcohol: 2-amino-1-hexanol
It is prepared according to the general working
CA 02238710 1998-OS-27
- 27 -
instruction C. It was purified by chromatography on
silica gel with ethyl acetate/heptane (2:1). Yield 92
colourless oil.
Example 23
8-N-[1-Hydroxymethyl)-pentyl]-cis-9,cis-12-octadeca-
dienamide
carboxylic acid: linoleic acid
alcohol: S-2-amino-1-hexanol
It is prepared according to the general working
instruction C. It was purified by chromatography on
silica gel with ethyl acetate/heptane (2:1). Yield 47
colourless oil.
Example 24
N-[1-Hydroxymethyl)-pentyl]-cis-9,cis-12-octadeca-
dienamide
carboxylic acid: linoleic acid
alcohol: 2-amino-1-hexanol
It was prepared according to the general working
instruction B. Yield 98 % colourless oil.
CA 02238710 1998-OS-27
- 28 -
Example 25
N-[1-Hydroxymethyl)-pentyl]-6-heptenamide
carboxylic acid: 6-heptenoic acid
alcohol: 2-amino-1-hexanol
It was prepared according to the general working
instruction B. Yield 92 o colourless oil.
Example 26
N-[1-Hydroxymethyl)-pentyl]-cis-9-octadecenamide
carboxylic acid: oleic acid
alcohol: 2-amino-1-hexanol
It was prepared according to the general working
instruction A. Yield 78 % colourless oil.
Example 27
R-N-[1-Hydroxymethyl)-propyl]-cis-9,cis-12-octadeca-
dienamide
carboxylic acid: linoleic acid
alcohol: R-2-amino-1-butanol
It was prepared according to the general working
instruction B. It was purified by chromatography on
silica gel with ethyl acetate. Yield 54 o colourless
oil.
CA 02238710 1998-OS-27
- 29 -
Example 28
8-N-[1-Hydroxymethyl)-propyl]-cis-9,cis-12-octadeca-
dienamide
carboxylic acid: linoleic acid
alcohol: S-2-amino-1-butanol
It was prepared according to the general working
instruction B. It was purified by chromatography on
silica gel with ethyl acetate. Yield 48 % colourless
oil.
Example 29
N-[1-Hydroxymethyl)-ethyl]-traps-9-octadecenamide
carboxylic acid: elaidic acid
alcohol: 2-amino-1-propanol
It was prepared according to the general working
instruction B. It was purified by chromatography on
silica gel with ethyl acetate. Yield 72 % colourless
oil.
Example 30
N-[1-Hydroxymethyl)-butyl]-cis-9-octadecenamide
carboxylic acid: oleic acid
alcohol: 2-amino-1-pentanol
CA 02238710 1998-OS-27
- 30 -
It is prepared according to the general working
instruction C. It was purified by chromatography on
silica gel with ethyl acetate/heptane (3:1). Yield 88
colourless oil.
Example 31
N-[1-Iiydroxymethyl)-ethyl]-9-octadecynamide
carboxylic acid: octadecynoic acid
alcohol: 2-amino-1-propanol
It is prepared according to the general working
instruction C. It was purified by chromatography on
silica gel with ethyl acetate/heptane (1:1). Yield 68
colourless oil.
Example 32
Compounds of formula (I) were examined in a DNA
synthesis assay in primary cultures of osteoblasts from
fetal rat calvariae. The experiments were carried out in
accordance with Pfeilschifter et al., Endocrinology 126,
703 (1990).
Primary osteoblasts were isolated from foetal rat
calvariae by sequential digestion with collagenase. In
this process 5 cell fractions were obtained. A pool from
the cell fractions 3-5 were cultured in vitro. The cells
were cultured in an incubator at a relative air humidity
of 95 %, a C02 content of 5 % and a temperature of 37°C.
The test substances were examined in the cultures of the
first, second or third cell passage. For the
CA 02238710 1998-OS-27
- 31 -
examinations the cells were sown at a cell count of
7x103 cells (in 100 ~,1 culture medium) / well in
microtitre plates at least 76 hours before applying the
test substances. MEM Dulbecco (plus 4.5 g/1 glucose plus
3.7 g/1 NaHC03 without glutamine) was used as the
culture medium to which 5 % foetal calf serum (FCS) and
5000 U/ml penicillin/streptomycin had been added.
Immediately before addition of the test substances to
the cell culture the medium was replaced by 150 ~,1
medium which contained 1 mg/ml bovine serum albumin
(BSA) instead of FCS. Test substances were added at the
desired concentrations to the medium containing BSA.
TGFI31 (transforming growth factor !31) at concentrations
of 0.1 - 0.2 ng/ml were also examined as a positive
control. Three determinations were carried out per
(positive) control or substance concentration. The cell
cultures were incubated for 24 hours with test
substances, a thymidine probe (1 ~,Ci methyl-3H-thymidine
in 20 ~,1 PBS solution) being additionally present during
the last 3 hours. At the end of the incubation period
the cell cultures were washed three times with 0.9
saline solution and subsequently admixed with 100 ~,1
liquid scintillator each time (OptiPhase Supermix TM~).
Afterwards the radioactivity incorporated into the DNA
was measured in cpm in a liquid scintillation counter
(1450 MicroBeta~). Cell cultures which had only
received medium containing BSA served as controls
(100 %).
CA 02238710 1998-OS-27
- 32 -
Table I: Rate of DNA synthesis of primary osteoblastic
cells of foetal rat calvariae in percent
compared to the control (= 100 %)
Compound Example 1 ~g/ml
N-[1-hydroxymethyl)-pentyl]-(all- 9 367
cis-9,12,15)-octadecatrienamide
N-[1-hydroxymethyl)-pentyl]-cis- 10 475
9,cis-12-octadecadienamide
N-[1-hydroxymethyl)-pentyl]-cis-9- 11 191
hexadecenamide
N-[1-hydroxymethyl)-pentyl]-cis-9- 19 298
tetradecenamide
N-[1-hydroxymethyl)-pentyl]-9- 20 250
octadecynamide
N-[1-hydroxymethyl)-pentyl]-trans- 21 188
9-octadecenamide
N-[1-hydroxymethyl)-pentyl]-cis-9, 24 223
cis-12-octadecadienamide
N-[1-hydroxymethyl)-pentyl]-cis-9- 26 296
octadecenamide
N-[1-hydroxymethyl)-propyl]-cis-9, 27 252
cis-12-octadecadienamide