Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02240993 1998-06-19
W O 97/23687 PCTAJS96119091
Black Liquor Gasification Process And
Regeneration Of Pulping Liquor
Background of the Invention
This invention relates to a system and process for the processing
5 of the spent pulping liquor from a kraft pulping process, known as black
liquor, to recover the chemicals and produce fresh pulping liquor. More
specifically, the system and process involve black liquor gasification in
a fluidized bed reactor to produce a product gas and a dry solids
product, without the formation of any smelt, and then the recovery of
10 the chemicals to produce fresh pulping liquor streams with controlled
compositions.
The kraft pulping process employs an alkaline pulping liquor,
known as whits liquor, to react with the lignins in the wood and free
the fibrous portions. Following a series of filtering and washing steps,
15 the fibrous portion is separated as raw pulp and the remaining spent
cooking liquor, which is dark in color, is known as weak black liquor.
This liquor, which is approximately 85% water, is then subjected to a
series of various types of evaporation to produce strong black liquor
with solids content greater than 50%. The strong black liquor is then
20 ready for the chemical recover phase.
The typical prior art process for treating black liquor to recover
chemicals employs what is commonly referred to as a chemical recovery
furnace. In these furnaces, which are operated as boilers for the
generation of steam, the strong black liquor is fired to burn the organic
25 content and to form a smelt composed primarily of sodium sulfide and
sodium carbonate. This smelt is drained from the smelt bed in the
bottom of the furnace, dissolved in water to form green liquor and then
causticized to form the white pulping liquor containing sodium sulfide
and sodium hydroxide.
CA 02240993 1998-06-19
W O 97n36~7 PCT~US96/19091
U.S. Patent5,284,550entitled "BlackLiquorGasificationProcess
Operating At Low Pressure Using A Circulating Fluidized Bed," which
issued February 8,1 g94 and U.S. Patent 5,425,850 ent}tled "CFB Black
Liquor Gasification System Operating At Low Pressures," which issued
June 20, 1995 and which are both assigned to the same assignee as
the present application, describe and claim one such system and
process for replacing a chemical recovery furnace. Referring to the
subject matter of U.S. Patents 5,284,550 and 5,425,850, they
basically involve the replacement of the chemical recovery furnace with
a black liquor gasification system using a circulating fluidized bed
reactor arrangement including the arrangement for processing the gases
and solids which are produced to generate fresh cooking liquor. In the
processes disclosed in these prior patents, kraft black liquor is gasified
under substoichiometric conditions to form a product gas rich in sulfid~,
primarily tlzS with some COS, and a solid bottoms product containing
primarily Na2CO3 along with some unreacted Na2SO4 and some Na2S.
The bottoms product is dissolved to form what is referred to as green
liquor which is then reacted to convert the Na2CO3 to NaOH. This is
done by a causticizing process where slaked lime, Ca~OH)z, is added to
convert the Na2CO3 to NaOH and CaCO3. The solid CaC03 is then
calcined in a kiln to convert it to CaO which is then slaked and recycled
to the causticizer. The resulting liquor is referred to as white liquor with
a high sodium content and is recycled to the digester. The sulfur-rich
product gas is separately processed in a reactor or scrubber to recover
the sulfur compounds usually as Na2S. This sulfur recovery from the
product gas may be carried out by wet scrubbing with NaOH and/or Na2
CO3 or it may be by dry scrubbing w}th calcium compounds ~CaO,
Ca~OH)2). In any case, the sulfur in the product gas (H2S, COS) is
converted to high sulfide white liquor containing NazS and/or NaHS.
CA 02240993 1998-06-19
Wo ~7/23687 PCT/US96/19091
S(lmmary of the Invention
The invention involves the gasification of black liquor in a
fluidized bed reactor to recover both sodium and sulfur for use in the
kraf~ pulping process. The system will produce soiid sodium and solid
sulfur compounds in the gasifier suitable for conversion into kraft white
liquor. Specifically, the invention employs a calcium reactant which is
added to the gasifier to react with the sulfur compounds (I f2S and COS)
directly in the gasifier rather than in a separate scrubberfreactor. The
calcium reactant may be CaO, CaC03 or Ca(OH)2 including materials
which contain these reactants such as limestone and dolomite. The
presence of the calcium compounds allows sulfur to be captured in the
gasifier at the same time pyrolysis of black liquor occurs. Sulfur reacts
with calcium (CaO, CaC03 or Ca(OH)2) to form solid calcium sulfide.
The solids from the gasifier containing Na2CO3, Na2S and CaS, as wen
1 5 as some unreacted calcium compounds, are drained from the gasifier for
conversion into kraft pulping liquor. The sodium compounds ~Na2CO3
and Na2S) are dissolved to form green liquor containing undissolved
calcium compounds. The sodium carbonate is converted to NaOH
through standard causticization. Calcium sulfide is converted to sodium
hydrosulfide (NaHS) by reaction with sodium hydroxide (NaOH~. These
processes produce a white liquor suitable for use in Icraft pulping.
Brief Description of the Drawings
Figure 1 is a process flow diagram of a black liquor gasification
system according to the prior art.
Figure 2 is a process flow diagram illustrating a black liquor
gasification system incorporating the present invention.
Figure 3 is a process flow diagram similar to Figure 2 but
modified for the combined conversion of CaS and Na2CO3.
CA 02240993 1998-06-19
PCT/US96/19091
W O 97/23687
Figure 4 is a process flow diagram illustrating a black liquor
gasification system according to the present invention modified to
provide multiple white liquor streams with variable sulfidity levels.
Description of the Preferred Embodiment
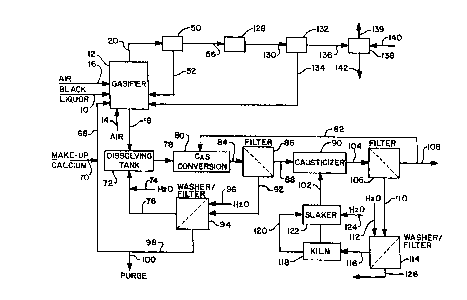
Figure 1 is a representation of the process flow diagram for a
black liquor gasification system as described in the previously
mentioned prior U.S. Patents 5,284,550 and ~,4Z5,850. Strong black
liquor 10 derived from the pulp digestion process is fed to the
circulating fluidized bed gasifier 12. Fluidizing air 14 and reaction air 16
are also fed into the gasifier 12 as explained by the two prior patents
previously identified. The gasification process is carried out with
substoichiomet-ic oxygen levels and the primary net reactions with
respect to sodium and sulfur which occur in the gasifier are as follows:
Na2S04 + 4C--Na2S + 4C0
Na2S04 + 4C0 ~ Na2S + 4C02
Na2S + ~2~ + C~2 - Na2CO3 + H2S
Na2S04 + 4H2 ~ NazS + 4H20
S + H2 H2S
2Na + C + 3/202 ~ Na2C03
2Na + S ~ Na2S
The total air to the gasifier is generally in the range of 20% to
50% of stoichiometric which results in the gasification of more than
60% and up to 99% of the sulfur contained in the black liquor. The
remaining sulfur reacts with sodium to form Na2S which remains a solid
and is discharged out the bottom along with the Na2C03 and any
unreacted Na2S04. The solids which are formed, primarily Na2C03, are
collected and drained from the bottom of the gasifier as bottoms solids
stream 18 while the gas product 20 is removed from the top of the
gasifier 12. The gas stream 20 contains sulfur, primarily as H2S, in
CA 02240993 1998-06-19
W O 97/23687 PCTAUS96/19091
addit;on to the other products of the substoichiometric oxidation
process, namely CO2, CO, H2, H20 and N2.
The bottoms stream 18 from the gasifier 12, which is a solids
stream containing primarily Na2CO3 but with some small amount of
5 Na2S, is fed to the dissolving tank 22. The sodium solids are dissolved
in a liquid stream 24 which may be water or a weak liquor or scrubber
liquor stream to form green liquor. In any event, the sodium sulfide
content of the selected dissolving liquid 24 is low. The resulting green
liquor stream 26 contains more than 70% and up to 95% sodium as
10 sodium carbonate on a mole basis.
The green liquor stream 26 is fed to the causticizer 28 where
siaked lime, Ca(OH)2, is added from line 30 to convert the Na2CO3 to
NaOH and CaC03. The slurry 32 from the causticizer 28 is fed to the
settling tank 34 where the solids, primariiy CaCO3, are separated out as
a sludge 36 leaving the low sulfide white liquor stream 38. The CaCO3
sludge 36 is washed with water in the mud washer 40 leaving a weak
wash stream 42 which can be used in the plant, as needed. The
washed CaC03 44 is fed to the kiln 46 for calcining to CaO and then to
the slaker 48 for conversion back to CalOH~2. The white liquor stream
20 38 is composed mainly of NaOH with small amounts of Na2S and is
recycled to the digester.
The gas product 20 from the gasifier 12 would first be cleaned
of entrained particulate material at 50 by some form of mechanical
separator such as a cyclone with the removed solids being recycled at
25 52 back to the gasifier. The remaining gas stream from the solids
separator means 50 may be cooled at 54 down to the saturation
temperature via recovery of heat. If any additional fine dust removal is
needed, the gas would then be sent through an electrostatic
precipitator, bag filter or some other form of dust removal equipment
30 (not shown). For further details of the mechanical separation, cooling
CA 02240993 1998-06-19
W O 97~3687 PCT/US96/19091
and dust removal, see the previously mentioned prior patents
5,284,550 and 5,425,850.
The cleaned and cooled gas product stream 56 is fed to the sulfur
recovery scrubber 58. The scrubber 58, which operates in a known
5 manner, employs a liquor stream 60 containing sodium values ~Na2CO3
and NaOH) to react with the sulfur compounds, primarily H2S with some
COS, to form a liquor stream 62. Regarding the scrubbing liquor stream
60, it may in fact be several different liquor streams from various
sources in the plant. The primary reactions which take place in the
10 scrubber 58 are as follows:
AbsorDtion Reactions
H2S ~ Na2CO3 --NaHS + NaHCO3
COS + Na2CO3 + H20 ~ NaOH + NaHS + 2CO2
COS + H20--C02 + H2S
CO2+ Na2CO3 + H20--2NaHCO3
Neutralization Reaction
NaHCO3 + NaOH--Na2CO3 +H20
The clean overhead gas 64 from the scrubber 58 now contains
primarily CO, CO2, H2, H20, CH4 and N~. There is sufFicient heating
20 value in this gas stream 64 so it is typically burned in combustion
equipment such as a steam generator or lime kiln. The liquor stream
62 from the scrubber 58 contains primarily Na2S with smaller
amounts of Na2CO3. This green liquor stream 62 is fed to a holding
tank 66 from which it is used to prepare a high sulfide white liquor
25 stream which will typically involve another causticizing operation for
the Na2CO3.
The present invention improves upon the prior art system of
Figure 1 which has just been described in that it provides for the
capture of the sulfur in the gasifier and eliminates the requirement for
30 a scrubberlreactor to remove the sulfur from the gases. In the present
invention, the gasifier is operated with a bed of particles consisting of
CA 02240993 1998-06-19
W O 9~/23687 PCTfUS9~/19091
those described in the prior art plus one or more calcium compounds,
CaO, Ca(OH)2 and/or CaC03, which react with the sulfur compounds to
form CaS.
Referring to Figure 2, the present invention has the same basic
arrangement of a circulating fluidized bed gasifier 12 fed with black
liquor 10 and fluidizing and combustion air 14 and 16. A similar
overhead gas stream 20 is produced and the solids are separated at 50
and recycled back to the gasifier in line 52. This line 52 would actually
comprise a conventional solids return system consisting of a discharge
duct from the bottom of the separator ~0 and a fluidization seal system,
known as a G valve or a seal pot. This is to assure one way flow of
solids from the separator 50 back to the gasifier. The remaining gas
stream 56 will be discussed hereinafter.
The operation of the gasifier in the present invention, as shown
in Figure 2, differs from the prior art gasifiers, such as shown in Figure
1, in that calcium compounds are introduced into the gasifier through
line 68. The calcium compounds in line 68 comprise recycled calcium
compounds and make-up calcium compounds added as needed at 70.
The source of the recycle calcium compounds will be apparent from the
further description. In the gasifier, the relevant reactions involving the
calcium, sodium and sulfur at the substoichiometric, reducing conditions
include the gasifier reactions previously indicated plus the following
reactions involving the added calcium compounds:
CaO + H2S ~ CaS + H20
Ca(OH)2 + H2S CaS + 2H20
CaC03 + H2S CaS + H20 + CO2
The CO2 and water vapor which are formed exit with the gas from
the top of the gasifier while almost all of the solid compounds which
are circulated in the gas stream 20 are separated at 50 and returned
to the gasifier 12. Ultimately, the solid compounds exit out of the
bottom of the gasifier at 18. The solid product from the gasifier
CA 02240993 1998-06-19
W 097123687 PCT~US96/19091
contains primarily Na2CO3, Na2S, CaS and any unreacted Na2SO4, CaO
and CaC03.
The gasifier must be operated in a temperature range where the
solids do not melt and agglomerate. Some solid compounds formed in
the normal course of black fiquor pyrolysis, such as certain sodium and
potassium salts, tend to melt at temperatures as low as 500~C to
600~C. The sodium and potassium salts also react with higher melting
sodium compounds, Na2CO3 ~851 ~C) and Na2S (1180~C), to form
eutectics which lower the bed melting temperature. Such reactions
tend to reduce the allowable operating temperature of the gasifier.
Generally the gasifier will operate between 650~C and B~0~C and most
likely between 700~C - 750~C. However, with the circulating solids
that includes calcium compounds, the gasifier may be operated at
higher temperatures. The presence of the dry, high melting point
calcium compounds will counter the agglomerating effect that the
melting of other, lower melting solids would have on the fluidization
characteristics. First, the dry calcium compounds will bind to and coat
any melted compounds so that they will not agglomerate. Second,
there will be a dilution of any melted compounds by the dry calcium
compounds, so that any agglomeration will be insignificant and will not
cause the collapse of the fluidized bed. Third, there may be reactions
~unknown~ which mitigate the formation of eutectics and raise the bed
melting temperature. The advantage of being able to operate the
gasifier at a higher temperature is that the reactions involved will
proceed at a higher rate. Also, the high temperatures result in high
absorption rates of sulfur. Sulfur absorption up to 9~% or more may be
achieved in the range of 700 to 900~C.
The solids stream 18 from the gasifier is fed to the dissolving
tank 72. Water, either as make-up water 74 or the weak wash return
stream 76, is added to the tank 72 where the Na2CO3 and Na2S are
dissolved to form green liquor containing solid CaS, CaO, CaC03 and
CA 02240993 1998-06-19
W O 97/236N7 PCT~US96/19091
inert compounds originating from the black liquor. These inert
compounds are referred to as ash or non-process elements (NPEs). The
CaO hydrates with the water to form Ca~OH)2. Sodium sulfide
hydrolyzes to form two compounds consisting of sodium hydrosulfide
5 ~NaHS) and sodium hydroxide lNaOHJ. The reactions are as follows:
Na2S ~ H2O - NaHS + NaOH
CaO + H2O ~ Ca(OH)2
The liquor stream 78 is fed to a reaction tank 80 to convert the
sulfur in CaS into NaHS. White liquor 82 is fed to the tank where the
10 active compound, sodium hydroxide (NaOHJ, reacts with CaS to form
NaHS and calcium hydroxide Ca(OH~2. The main reactions occurring
during CaS conversion are as follows:
CaS + NaOH + H20 Ca(OH)2 + NaHS
Na2CO3 + CalOH)2--CaCO3 (~) + 2NaOH
15 These reactions are time and temperature sensitive. The greatest
conversion occurs at temperatures above 80~C. NaOH is required to
convert CaS to NaHS and the CalOH)2 is required for the conversion
of Na2CO3 to NaOH. These reactions complement each other and
help minirnize the addition of NaOH. Since Na2CO3 is far more
20 abundant than CaS, the balance of the Na2CO I is converted in a
conventional causticizer.
The effluent green liquor 84 from the CaS conversion at 80 now
contains NaOH and NaHS as dissolved compounds plus CaCO3,
unreacted Ca(OH)2 and the non-process elements as suspended solids.
25 The green liquor is fed to a solids separation device 86 to clarify the
green liquor. Clarified green liquor 88 is discharged and sent to
causticizer 90 for final conversion into white liquor.
Solids 92 from filter 86 are washed at 94 with water 96 to
remove residual sodium compounds and the solids 98 are returned to
30 the gasifier. ~he liquid 76 separated from the solids is returned to the
dissolving tank 72. The solids 94 returned to the gasifier will contain
CA 02240993 1998-06-19
W O 97/23687 PCT/US96/19091
non-process elements that flow through the chemical recovery process
as inert material. The non-process elements which are contained in the
btack liquor will build up to high levels if not removed. In order to
maintain a low level of non-process elements, the solids stream can be
purged at 100 or filtered to maintain the desired level. Make-up calcium
70 will be introduced as required to achieve the required calcium
content to the gasifier 12.
The final conversion process to form white liquor occurs in the
causticizer 90. Slaked lime (Ca~OH)2~ 102 is fed into the causticizer
which converts Na2CO3 to NaOH by the following equation:
Na2CO3 + CalOH)2 > CaCO~ + 2NaOH
Unclarified white liquor 104 is discharged from the causticizer 90
and sent to a filter 106 to remove the calcium solids. Clarified white
liquor 108 is then sent to the digester for pulping of wood. Some white
liquor 82 is diverted to the CaS conversion process as previously
explained.
Calcium carbonate, which is the primary solid 110 removed from
white 1i4uor, is processed through conventional calcining and slaking
processes. The CaC03 is first washed with water 1 12 and dewatered
in the filter 1 14. The dewatered CaCO3 1 16 is calcined in a lime kiln
1 18 to produce lime (CaO~ 120 which is slaked at 1~2 with water t 24
to form the caicium hydroxide ~CalOH)2) 102 for use in the causticizer
90. Liquid 126 taken from the washer 114 may be returned to the
dissolving tank 72 or the causticizer 90 as desired.
The clean overhead gas 56 from the separator 50 now contains
primarily CO, CO2, H2, CH4, H20, CH4 and N2 and probably some small
quantity of unreacted H2S and COS. There is sufficient heating value
in this gas stream 56 so it is burned in the combustion equipment 128
which, for example, could be a steam generator or the lime kiln 1 18.
The flue gas stream 130 from the combustion equipment 128 is cleaned
at 132 to remove any remaining entrained particulate solids which are
CA 02240993 1998-06-19
WO 97123687 PCT/US96/19091
1 1
recycled at 134 to the gasifier 12 or sent to disposal. The cleaner 132
may be a hot gas filter, a baghouse, an electrostatic precipitation or a
wet scrubber. Because there is not 100% H2S removal in the gasifier
12 and the gas stream 56 contains low concentrations of H2S which
5 will be oxidized to SO2 in the combustion equipment 128, the flue gas
stream 136 from the cleaner 132 conta;ns some SO2 which can be
vented to the atmosphere if the SO2 level is low enough or the flue gss
can be scrubbed at 138. A scrubbing solution 140, such as a NaOH
solution, will convert the SO2 to Na2SO3 and Na2SO4. The resulting
10 scrubbin~ effluent 142 containing the sulfite and sulfate compounds,
both of which can be used in pulping, is then returned to the digestion
cycle. Production of sodium sulfite can be controlled with the black
liquor gasification process by controlling the proportion of sulfur which
is gasified as H2S and other reduced sulfur compounds. This is
15 accomplished by controlling the mole ratio of calcium to sulfur in the
reactor. The inclusion of this scrub~er 138 reduces the total sulfur
emissions from the plant and conserves sulfur for use in the process.
The remaining gases 139 from the scrubber 138 may usually ~e
discharged to the atmosphere. The reactions which take place in the
20 SO2 scrubber 138 are as follows:
SO2 + Na2SO3 + H2O 2NaHSO3
NaHSO3 +NaOH ~ Na2SO3 + H20
2Na2SO3 + ~2 - 2Na2S~4
If desired, the cleaner 132 may precede the combustion equipment
128.
Figure 3 represents a modified version of the process described
in reference to Figure 2 in which both CaS and Na2CO3 are converted
together. Green liquor 78 containing calcium solids and non-process
elements are fed to a conversion process 144 in which the sulfur as
30 CaS is converted to NaHS and the sodium as Na2CO3 is converted to
NaOH by the following equations:
CA 02240993 1998-06-19
W O 97123687PCTAUS96/19091
12
CaS + NaOH + H20 r Ca(OH~2 + NaHS
Na2CO3 + Ca(OH)2 ~ CaCO3 (~) + 2NaOH
The resulting unclarified white liquor 146 containing calcium solids and
non-process elements are filtered at 148 to generate clarified white
liquor 150 and solids 152. The solids 152 are washed with water 154
in the washerlfilter 156 producing a solids stream 158 primarily of
CaCO3 and a liquid stream 160. The liquid wash stream 160 is returned
to the dissolving tank 72 along with whatever additional water may be
required .
A standard lime kiln 161 and slaker 162 system regenerates
Ca(OH)z 163 from the CaC03 in stream 158 for use in the causticizing
of sodium carbonate at 144. The sodium hydroxide generated during
this causticizing causes the conversion of CaS to NaltS. Non-process
elements contained in washed calcium carbonate stream 158 are purged
1 5 at 164 before entering the lime kiln 161 . Calcium carbonate 68
required to the gasifier is diverted upstream of the kiln 161. Make-up
calcium 70 as CaCO3, CaO or Ca~OH)2 is added as required to
compensate for calcium lost through purging non-process elements.
An alternative process for producing the white liquor for use in
the digestion process is illustrated in Fiyure 4. This involves the
production of split white liquor streams, one having a low sulfide
content and one having a high sulfide content. These split white liquor
streams can then be used for a multistage digestion process. A white
liquor stream with a high sulfide content is more desirable at the early
stages of delignification, while a white liquor with a lower sulfide
content and a higher NaOH concentration is more desirable later in the
delignification process to more effectively remove lignin without
effecting the pulp fiber strength.
In this Figure 4 embodiment, solids 18 containing sodium and
calcium compounds plus the non-process elements are discharged from
CA 02240993 1998-06-19
W O 97/23687 PCT/US96/19091
the gasifier into a dissolving tank 72. The following reactions occur in
the dissolving tank 72:
Na2S + H20 ~ NaHS + NaOH
CaO + H2O--Ca(OH)2
Sodium compounds are dissolved to form a green liquor. In
order to generate two liquor streams of high and low sulfidity, the
calcium solids must be separated from the sodium compounds before
CaS is converted by reaction with NaOH. Since NaOH is formed in the
dissolving tank when NazS is hydrolvzed, some calcium sulfide may
begin conversion. If required to slow down the conversion process, the
green liquor 78 may be cooled at 165 before entering the solids
separation filter 166 where the solid CaS 167 is separated. The solids
167 are discharged into a CaS conversion tank 168 where CaS is
converted using NaOH. Most likely, the source of NaOH will be low
sulfidity white liquor 170. Water as needed is added at 172. The tank
168 is heated as necessary to a temperature between 80~C and 1 00~C
to promote the following reactions:
CaS + NaOtl + H20 ~ Ca(OH)2 + NaHS
Na2CO3 ~ Ca(OH)2 ~ Ca(C0)3 (~) 2NaOH
An unclarified high sulfidity white liquor 174is discharged from
the CaS conversion tank 168 to a filter 176 to separate the calcium
solids. Discharged from the filter 176 is a clarified high sulfidity white
liquor 178 and solids 180 containing mostly CaCO3, plus smaller
amounts of Ca(OH~2 and non-process elements. These solids 180, now
separated frorn the high sulfidity white liquor 178 are filtered (washed
and dewatered) at 182 with water 184. The liquid 186 which is similar
to weak wash is discharged from the filter 182 and recycled back to the
dissolving tank 72. Make-up water 74 is added if necessary to this
stream 186. The solids 68 discharged from the filter is recycled back
to the gasifier with whatever purge 188 may be required as mentioned
CA 02240993 l998-06-l9
W O 97/23687 PCT~US96/l9091
14
earlier. Make-up calcium 70 is again added to the level required in the
gasifier.
A second white liquor stream containing a low sulfidity ~low
NaHS) concentration is generated from the green liquor stream 190
5 discharged from first solids filter 166. This liquor is hi~h in Na2CO3 and
low in Na2S. The carbonate must be converted to NaOH. Th;s is done
in a conventional causticizing system using the lime kiln 192 and slaker
194 to produce the Ca~OH)2 stream 196 for the causticizer 198 as more
fully described in reference to Figure 2. The liquor 200 from the
causticizer 198 is filtered at 202 to give the low sulfidity white liquor
stream 204 and the solid CaCO3 stream 206. A portion 170 of the
white liquor 204 is recycled to a CaS conversion tank 168 while the
remainder is sent to the pulping process. The solid CaCO3 stream 2~6
is washed and filtered at 208 to yield the CaCO3 stream 210 for the
1 5 causticizing operation. The wash water 212 from the washerJfiller 208
is returned to the dissolving tank 72. As shown, water is supplied to
the washer/filter 208 at 214 while water 216 is supplied to the slaker
~94.
The invention can also produce a third pulping liquor in the form
20 of sodium sulfite (Na2SO3) Sulfite is produced from scrubbing SOz
contained in the flue gas 136 as described previously. The invention
not only produces sulfite as a byproduct of gasification, but can also
control the quantity of sulfite produced. Sulfur capture in the gasifier
is dependent on many factors including temperature, mixing
25 characteristics, gas and particle residence times and ratio of calcium to
sulfur ~molar basis). For the most part, all of the above factors will be
somewhat constant. However, the ratio of calcium to sulfur, also
known as the Ca to S mole ratio, can be easily controlled by controlling
the feed rate of calcium into the reactor. Reducing the CaJS mole ratio
30 will reduce sulfur capture as CaS and increase H2S production.
CA 02240993 1998-06-19
W O 97123687 PCTAJS96/19091
Increased H2S production and hence increased S02 production allows
increased production of sulfite in the S02 scrubber 138.
Since the recycled calcium compounds in line 68 are moist, some
of the heat in the gasifier is used merely to dry the calcium compounds.
5 Therefore, instead of injecting them directly back into the gasifier, they
may be injected through line 69 into the hot product gas from the
gasifier. For example, they may be injected into the separator 50 as
illustrated in Figure 4 such that the hot gases dry the calcium
compounds and then separates them from the gases along with other
10 solids for recycle to the gasifier. Alternatively, they could be injected
into a dedicated contact device located in line 56 and then separated
from the gases for recycle apart from the recycle 52 of the other solids.