Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02241258 1998-06-22
WO 97123779 PCT/GB96/03213
1
1 has Inlet System
2
3 The present invention relates to a gas inlet system
4 which has been designed to introduce reference gases of
any type, inert or combustible, into isotope ratio mass
6 spectrometer systems.
7
8 In particular, the system permits the introduction of a
9 reference gas in such a way that this gas is subjected
to the same physical conditions as the analytes thus
21 meeting the conditions of an internal standard. Hence,
12 it combines the advantages of a reference compound
13 added into a sample with the advantage of allowing the
14 positioning of reference gas pulses as desired within a
given chromatogram.
16
3.7 The commercial availability of gas isotope ratio mass
18 spectrometers (GIRMS) directly coupled to a gas-
19 chromatography (GC) via an on-line combustion interface
(C), based on the design by Matthews and Hayes [1], GC-
22 C-IRMS has become a powerful tool in all areas of
22 applied analytical chemistry [2,3].
23
24 Whatever the specific application, the user is
confronted with the dilemma of how to refer measured
26 stable isotope ratios of the anaiytes to a calibrated
CA 02241258 1998-06-22
WO 97/23779 PCTlG1396/03213
2
1 isotope standard. The best results are obtained when
2 an internal standard compound of known isotopic
3 composition is added to the sample mixture [4]. The
4 major advantage is that standard and analytes are
subjected to identical physical conditions during
6 analysis. However, this advantage may be lost in cases
7 in which the standard or analytes elute late in the
8 chromatogram where increasing peak distortion
9 negatively influences accuracy and precision of
l0 measured isotope ratios. Adding an internal standard
11 mixture representative of the whole range of analytes,
12 on the other hand, is of little help since it will
13 create problems caused by co-elution or at least
14 overlap of analytes and individual compounds of the
internal standard mixture.
16
17 It is standard practice to introduce pulses of an
18 isotopically calibrated gas, such as COZ from a gas
19 cylinder via an independent second capillary directly
into the ion source of the IRMS [3,5,6]. Although it
21 is commonly accepted that this mode of calibration does
22 not take account of the physical conditions to which
23 analytes are subjected, because of its user-
24 friendliness and practicability its use is common.
Recent observations of isotopic fractionation occurring
26 during gas-liquid chromatography (GLC) followed by on-
27 line combustion have been reported [7].
28
29 The present invention aims to optimise isotopic
calibration and provide an approach that maintains the
31 free positioning of reference peaks while at the same
32 time subjecting the reference compound to the same
33 physical conditions as the analytes.
34
It is an object of the present invention to provide an
36 improved method and apparatus for introducing a
CA 02241258 2006-03-13
-3-
reference gas in isotope ratio mass spectrometer systems.
The present invention provides a reference gas inlet for an isotope ratio mass
spectrometer system comprising three pipes which intersect at an intersection
point,
wherein a secondary flow of carrier gas and a reference gas can enter the
inlet via a first
and second pipe respectively, and can exit the inlet together via a third
pipe, wherein
the inlet is switchable between an open and closed mode, wherein when the
inlet is in
the closed mode, the reference gas leaves the system via an outlet and, when
the inlet is
in the open mode, at least a portion of the reference gas exits with the
secondary flow
of carrier gas through the third pipe characterised in that, in use at least a
portion of the
reference gas is at all times able to leave the system via the outlet, and
when the inlet is
in the open mode at least a portion of the secondary carrier gas is able to
leave the
system via the outlet.
In another aspect of the invention, there is provided an isotope ratio mass
spectrometer
system comprising a reference gas inlet of the invention.
In another aspect of the invention, there is provided a system for internal
standardization comprising a reference gas inlet of the invention, wherein the
reference
gas can be introduced into the secondary flow of carrier gas that joins a
primary flow of
carrier gas transporting a sample containing an elemental analyte before both
the
primary and secondary flows pass into a module that converts the sample into a
gaseous
form for mass spectrometry analysis.
Therefore in IRMS systems fitted with a combustion interface, combustible
gases can
be used as a reference gas.
Preferably the inlet comprises three pipes which intersect at an intersection
point,
wherein a flow of carrier gas and reference gas can enter the inlet via
separate pipes and
can exit the inlet together via a third pipe at a point before a secondary
flow enters the
combustion interface.
CA 02241258 2006-03-13
- 3a
The reference gas can be introduced into a flow of carrier gas (e.g. Helium)
that joins or
is equal to the primary flow of carrier gas transporting a sample containing
the
elemental analyte at a point before both flows pass into a module that
converts the
sample into a gaseous form for mass spectrometric analysis.
Preferably the reference gas inlet comprises a hollow T-piece, two ends of
which are
positioned in the secondary carrier gas line wherein reference gas can be
carried into
th~ "~."~~~,, ft",~ o~fo,.;r,~ tt,o ;rfo,.~~,.o
CA 02241258 1998-06-22
WO 97/23779 PCT/GB96/03213
4
1 In one particular embodiment the reference gas inlet
2 pipe comprises a sheathing pipe and an inner pipe
3 moveable within the sheathing pipe towards and away
4 from the intersection of the inlet.
6 The invention will now be further described without
7 limitation with reference to the accompanying drawings
8 wherein:
9
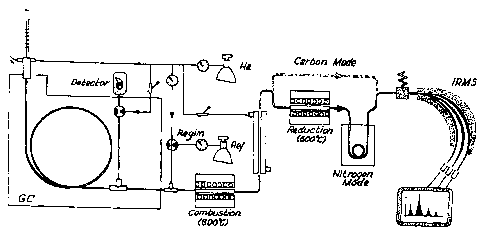
to Fig. li~Illustrates a gas chromatography on-line
11 combustion interface isotope ratio mass spectrometry
12 system.
13
14 Fig. 2~ Illustrates the position of a reference gas
inlet module in a gas chromatography on-line combustion
16 interface isotope ratio mass spectrometry system.
17
'8 Fig. 3A i Schematic of the T-piece connecting the second
i9 carrier gas stream and the GC effluent to the
combustion furnace.
21
22 Fig. 3B / Schematic of the reference gas inlet scheme
23 using a moveable capillary.
24
Fig. 4/ Illustrates an arrangement whereby the
26 reference gas may be introduced by means of a moveable
27 piston arrangement.
28
29 Fig. 5 'Illustrates the combination of two reference
gas inlet modules.
31
32 Fig. 6 ~ Ion current of mJz of COZ pulses introduced into
33 the combustion furnace via REGIS (numbers over each
34 peak indicate pulse width time).
'
36 Fig. 7A Effect of GC parameter and choice of reference
. . ~ ,~ :"
CA 02241258 1998-06-22
WO 97/23779 PCT/GB96/032I3
1 peak - methylcaproate (C6) was chosen as internal as
2 internal standard and its 6'3C-value was arbitrarily set
3 to 0.000~/u.
4
5 Fig. 7B Effect of GC parameter and choice of reference
6 peak - COz was chosen as an internal standard and its
7 6'3C-value was arbitrarily set to -11.84 to match the
8 dl3C-value chosen for C6. The set point was based upon
9 the FAME analyses carried out at 4°C/min.
11 Fig. 8A Effect of GC parameter and choice of reference
12 peak - methylundecanoate (CIA) was chosen as internal
13 standard and its a'3C-values was arbitrarily set to
14 O.OOd~oo.
16 Fig. 8B Effect of GC parameter and choice of reference
17 peak - COZ was chosen as internal standard and its 613C-
18 value was set to -10. 67!~lo to match the 8I3C-value of
19 O.OOt~loo chosen for C". This set point was based on the
FAME analyses carried out at 8°C/min.
21
22 Fig. 9 Ion current chromatogram of m/z 44 of CO~ pulses
23 (R) and n-O-propyl, N-trifluoroacetyl derivatives of 5
24 amino acids (S) recorded using an ORCHID system fitted
with a REGIS module.
26
27 Fig. 10 /Ion current chromatogram of m/z 28 of NZ pulses
28 (R) and n-O-propyl, N-trifluoroacetyl derivatives of 5
29 amino acids (S) recorded using an ORCHID system fitted
with a REGIS module.
31
~ 32 The primary flow of carrier gas transporting the gas-
33 chromatographically separated sample constituents and
34 the secondary flow of carrier gas (which, as
appropriate, transports the reference gas) both enter
36 the combustion interface i.e. at the bottom of the
CA 02241258 1998-06-22
WO 97/23779 PCT/GB96/03213
6
1 oxidation furnace (Fig. 3A). The secondary flow of
2 carrier gas continuously enters the combustion
3 interface at a given gas pressure and flow rate.
4
A low dead volume T-piece situated in the second
6 carrier gas line accommodates three pipes, an inlet
7 pipe I, an outlet pipe o, and a sheathing pipe S (Fig.
8 3B). The second carrier gas enters the T-piece via
9 pipe I and leaves it via pipe O. The sheathing pipe S
opposite pipe O houses another pipe (pipe R) through
11 which the reference gas can flow.
12
13 In "switch-off" position A, the end of pipe R lies
14 before the intersection of the T-piece. Gas pressure
and flow rates of the second carrier gas and of the
16 reference gas are pre-set in such a way that reference
17 gas flowing out of pipe R is forced by the second
18 carrier gas to leave the system through pipe S. In
19 "switch-on" position B, pipe R is moved forwards by
means of a suitable remote controllable device (e. g.
21 pull/thrust action miniature solenoid) in such a way
22 that pipe R now lies in the intersection of the T-
23 piece. Reference gas flowing from pipe R is thereby
24 carried with by the second carrier gas flow, passing
through the combustion interface and eventually
26 entering the IRMS.
27
28 The described method and apparatus allow analytes in
29 the primary gas flow to be measured against an internal
reference gas.
31
32 Owing to the layout described above, the reference gas
33 is subject to all the same processes in the combustion
34 interface so that any influences of these on the ,
measurement characteristics affect both the standards
36 and the analytes. Both the analyte peak and the
CA 02241258 1998-06-22
WO 97/23779 PCT/GB96/03213
7
1 reference gas peak are of Gaussian shapes. Peak
2 detection and integration parameters are common for
3 both the analyte gas peak and the reference gas peak
4 which increases accuracy and precision.
6 At a given reference gas pressure, peak height and peak
7 area can be changed by setting the period of the
8 switch-on time accordingly. For a fixed period of
9 switch-on time, peak height and peak area can be
changed by setting~the reference gas pressure
11 appropriately. Since there is always a constant flow
12 of reference gas emanating from pipe R, no pressure
13 shock-waves are created and the reference gas is always
L4 equilibrated with the primary gas flow.
16 Additionally, as there is always a certain amount of
17 second carrier gas emanating from pipe S, no incoming
18 leak is created, i.e. no atmospheric gases can enter
19 the system. This is particularly important for
analysis of nitrogen containing compounds.
21
22 Twa principles guided the new approach: 1) the desire
23 to combine the advantage of adding an internal standard
24 to the sample at will anywhere in a given chromatogram
without interfering with the gas-chromatographic
25 separation; 2) recent observations of isotopic
27 fractionation of saturated FAMEs during GLC-C-IRMS that
28 prompted the hypotheses of the combustion interface
29 being a major contributing factor to this thermodynamic
isotope effect [7].
31
32 Since gases of low molecular weight and high volatility
33 such as CO2, N2, CHQ etc are hardly if at all retained
by
34 common GLC stationary phases, i.e. are not subject to
solute-stationary phase interaction, any design aiming
36 to introduce such gases at injector or column head
CA 02241258 1998-06-22
WO 97/23779 PCT/GB96/03213
8
1 level could be ruled out from the start. The obvious
2 place to position a gas inlet was between column end
3 and combustion furnace.
4
To do this, a second continuous helium stream was
6 introduced into the combustion interface via a low deal
7 volume T-piece positioned between column end and
8 combustion furnace (Fig. 3A). This T-piece was
9 connected to the combustion furnace in such a way that
its temperature was well above 360'C thus preventing
11 condensation of analytes due to cold spots.
12
13 A remote switchable device was positioned in the
14 capillary carrying the second helium stream. This
device was designed in such a way that the reference
16 gas was always flowing and thus equilibrated with
17 ambient pressure and, hence, not subject to isotopic
18 fractionation. When switched on, the reference gas was
19 introduced pressure pulse free into the second helium
2o stream (Fig. 3B). The flow rate of the reference gas
21 could be freely adjusted but was typically set to
22 <0.3m1/min.
23
24 In an alternative embodiment of the present invention
the reference gas inlet system is of a T-shaped
26 configuration which incorporates a piston arrangement
27 as shown in Fig. 4. The piston arrangement must be in
28 an 'open' mode (as shown in Fig. 4) for a reference gas
29 to flow into the gas isotope analysis system. To
prevent a reference gas from entering the analysis
31 system, the piston arrangement must be in a 'closed'
32 mode wherein the piston cooperates with the valve seat
33 such that the reference gas is vented to the atmosphere
34 via a restriction outlet.
36 Figure 5 illustrates one way in which two of the T-
CA 02241258 1998-06-22
WO 97/23779 PCT/GB96/03213
9
1 shaped gas inlet systems can be linked to allow more
2 than one reference gas to be used.
3
4 Since any gas serving as a reference was introduced
into the combustion interface in front of the
' 6 combustion furnace, it is possible to use a combustible
7 gas as reference compound. Any such gas would be
8 subject to the physical conditions of both the
9 combustion process and the interface.
11 Introducing reference gas pulses via this module
12 resulted in virtually Gaussian shaped peaks that could
~.3, hardly be distinguished from peaks caused by combusted
14 analytes. Peak area of the reference gas was linear
dependent on pulse width time from 1 to 10 s (Table 1).
15 Pulse width longer than 15 s, i.e. pulse width times of
17 the same order as peak width, resulted in almost
18 rectangular peak shapes (Fig. 6).
19
First tests with the Reference Gas Inlet System for
21 internal Standardisation (REGIS] were carried out using
22 CO,, as reference gas. Because only relative changes
23 were to be monitored its s'3C-value was arbitrarily set
24 to zero. During all tests the column head pressure of
the second helium stream, and the COz pressure were kept
26 constant.
27
28 All relative a~3C-values in %° are given as mean of three
29 repetitions and the error bars are ~2Q. Identical
aliquots of a FAME mixture were injected in splitless
31 mode. The split was kept closed for 6 seconds and
32 opened thereafter for the rest of the run. The split
33 flow was set to 30 ml/min. Column temperature was held
34 at 70°C for 5 minutes and then programmed to 270°C at
n°C/min, where n = 4, 6 or 8. Column head pressure was
36 set to flow rate of 1.20 ml/min corresponding to a
CA 02241258 1998-06-22
WO 97/23779 PCT/GB96/032I3
1 linear velocity of 28.4 ml/min at a column temperature
2 of 70°C.
3
4 The results of these experiments are presented in Table
5 2. Under isothermal conditions excellent precision was .
6 observed, independent of whether the GC effluent was
7 diverted from or directed into the combustion
8 interface. The average si3C-value of -O.OI3 ~ 0.048
9 (95~ confidence limit based on ten degrees of freedom)
10 obtained from these isothermal runs served as the
11 target value for subsequent studies when temperature
12 gradients were run on the GC in constant pressure mode.
13
14 Selecting only a single active reference peak, a
noticeable shift towards more negative 8'3C-values was
16 observed with steeper temperature gradients (Table 2).
17 Here, average 8~3C-values were -0.063 _+ 0.103 °/~ (95~
18 confidence limit based on ten degrees of freedom)
19 obtained from these isothermal run served as a target
value for subsequent studies when temperature gradients
21 were run on the GC in constant pressure mode.
22
23 Selecting only a single active reference peak, a
24 noticeable shift towards more negative 8~3C-values was
observed with steeper temperature gradients (Table 2).
26 Here, average d'3C-values were -0.063 -1 0.087°/~, and -
27 0.166 ~ 0.103°/~, and -0.417 ~ 0.193°/~ for 4'C/min,
28 6'C/min, and 8'C/min, respectively. Clearly, these
29 shifts were attributable to the change, i.e. decrease,
in carrier gas flow caused by the rise in column
31 temperature since these were the only dependent
32 variables. By selecting three active reference peaks,
33 however, average 8~3C-values became virtually identical
34 with the target value obtained under isothermal
conditions.
36
CA 02241258 1998-06-22
WO 97/23779 PCT/GB96/03213
I1
1 Similar observations were made when the combustible gas
2 isobutane (10 vol% in He) was used. In contrast to COZ,
3 for which precision was usually better than 0.1°/~, the
4 additional combustion process Was reflected in a
precision of about 0.2°/~, only (Table 3). When
6 selecting three active reference peaks, average 813C-
7 values obtained from temperature gradient analyses
8 became identical with the calculated target value of
9 +0.007 ~ 0.156°/~ (95% confidence limit based on ten
degrees of freedom).
11
12 Although the observed precision of ~0.156°/~ was very
23 similar to that of ~0.11°/~ reported by S A Baileys et
14 a1. [8] it was likely that by using Cu0/Pt at 820'C for
combustion, complete combustion of isobutane.could not
16 be achieved. For compete combustion of natural gases
17 such as methane, propane and isobutane, higher
18 temperatures, i.e. --1000'C, are reported to be more
19 suitable [9]. The furnace used in this study was
unable to deliver such high temperatures.
21
22 First experiments with a FAME mixture and CO,_ as
23 reference compound clearly showed the merits of this
24 alternative way of standardisation. As reported
previously [7], a significant difference in ~13C-values
26 of > +2.00°/~ on average was observed when comparing
27 8130-values obtained at 4°C/min with those obtained at
28 8'C/min and when only one analyte was chosen as
29 internal reference compound (Fig. 8A). It must be
emphasized that these differences could only be
31 detected because no time shift correction [3, 10, 11]
32 was employed by the data evaluation software.
33
34 Evaluating data based on 95% confidence limits (two
degrees of freedom) as well as within ~ 2Q, sl3C-values
36 for G6, CII, C12, Cla, C~6, C,s, and CZ° became virtually
CA 02241258 1998-06-22
WO 97/23779 PCT/GB96/03213
12
1 identical when four COZ pulses introduced via the REGTS
2 were chosen as internal reference peaks (Fig. 7B and
3 8B) .
4
This method of internal isotopic calibration cannot
6 account for those isotope effects which occur in the GC
7 column due to different solute-stationary phase
8 interaction dominated by Van der Waals dispersion
9 forces, leading to an earlier elution of the heavier
isotope [12]. This is clearly reflected in the fact
11 that only a 95% agreement between Si3C-values measured
12 with different temperature gradients could be achieved
13 when expressing FAME 813C-values versus CO~ after its
14 isotope ratio has been calibrated against Cti with 8"C
C11 = 0.000°/~ (Fig. 8B) . It may therefore be argued
16 that this method is not a true internal calibration.
17 But, as pointed out earlier, permanent gases are not
18 subject to solute-stationary phase interaction on
19 stationary phases used in GLC. For the time being,
this method of isotopic calibration constitutes the
2I best possible compromise of the two existing methods.
22
23 The finding of an agreement of as high a value as 95~
24 supports the hypothesis that the combustion interface,
because of its numerous connections and changes in
26 capillary diameter between the GC and the IRMS,
27 contributes significantly to the process of peak shapes
28 becoming asymmetric and, hence, amplifying the
2~ chromatographic isotope effect in GLC [13,7].
31 With the inlet pipe of the invention reference gas is
32 introduced in a continuously flowing second stream of
33 carrier gas before the interface and, hence, subject to
34 same influences by this interface as the analyte(s) .
therefore acting as an internal standard. The design
36 of the REGIM ensures no interference of the reference
CA 02241258 1998-06-22
WO 97/23779 PCT/GB96/03213
13
1 gas with the analyte. The principal design of the
2 REGIM module allows for any conceivable combination of
3 modules and therefore provides the means of introducing
4 e.g. two reference gases or one reference gas and an
oxidation agent for use with a combustion interface
6 alternatively and/or simultaneously). Owing to the
principal design of the REGIM, flow and pressure of the
8 reference gas(s) are always equilibrated and are,
9 therefore, not subject to isotope fractionation by the
switching process. Flow and pressure of the reference
11 gas{es) are set up in such a way that no pressure
12 pulses are created by the switching process. Reference
13. gas introduced. into the system results in a signal as
34 the same appearance as an analyte signal and is
processed in the same way as such.
16
17 The reference gas inlet system presented of this
18 invention is suggested as a valuable alternative to
19 existing methods of isotopic calibration. It yields
2o measurements of excellent precision and significantly
21 improves accuracy when multiple active reference peaks
22 are selected. Furthermore, it combines the user-
23 friendliness and practicability of the existing method
24 of external calibration with the advantage of standard
and analytes being subject to identical physical
26 conditions during passage through the combustion
2~ interface.
28
2g This system is especially useful when data are
processed without time shift correction as reference
31 compounds thus introduced pick up thermodynamic isotope
32 effects. Because of this property it can be
33 justifiably termed reference gas inlet system for
34 internal isotopic calibration.
36 Studies were carried out on an ORCHID GC-C-IRMS system
CA 02241258 1998-06-22
WO 97/23779 PCT/GB96/03213
14
1 (Europa Scientific, Crewe, CW1 1ZA, UK). The
2 combustion interface, comprising a Cu0/Pt combustion
3 furnace operated at 820'C, a Nafion tube acting as
4 water separator, and a Cu reduction furnace operated at
600'C transformed sample peaks eluted from the GC into -
6 dry COZ and N2. Tn 13C-studies, measured isotope ratios
7 are automatically corrected for contributions from
8 ISO[14] and expressed as 6-values in per mille[°/~)
9 units:
11 8 '3C= ( Raamptc-Rnmndara ) / Rsmndnrd X 10 0 0 Eqn . 1
12
13 where Rsample is the '3C/'ZC of the sample and Rstandard
14 is the '3C/'ZC ratio of the working standard. Since this
25 study was only concerned with changes rather than
16 absolute values, in all experiments one or more peaks
17 were chosen as internal reference peaks and their s"c-
18 values were arbitrarily set to zero.
19
The gas chromatograph was an HP 5890, Series II, fitted
2I with an electronic pressure control (EPC) thus
22 permitting the carrier gas management to be set either
23 to constant pressure or to constant flow mode.
24
Helium of 5.0 purity was used as carrier gas and was
26 passed through a high capacity gas purifier (Supeico
27 UK, Poole, BH17 7TG, UK) before entering the GC.
28 Column head pressure was controlled by an EPC. In
29 either mode, carrier gas was set to a linear velocity
of 28.4 cm/s at a column temperature of 70'C.
31 Experiments using, COZ and isobutane were carried out
32 either at a constant GC temperature of 70°C or using
33 one of three temperature gradients wherein the GC
34 temperature was held at 70°C for 2 minutes and
subsequently programmed to 270°C at rates of 4°C/min,
36 6'C/min, and 8'C/min, respectively.
CA 02241258 1998-06-22
WO 97!23779 PCT/GB96/03213
1 Separation of saturated FAMEs was achieved on a CP-Sil
2 19 CB capillary column (Chrompack, Middelburg, NL),
3 dimensions 25m x 0.25mm, 1.2 ~Cm film thickness. The
4 column was connected to a retention gap, lm x 0.25mm,
- 5 that was cyanopropyl/phenyl/methyl deactivated [15].
6
7 Data were analysed using the manufacturer provided
8 software (Europa Scientific ORCHID POST-PROCESSOR).
CA 02241258 1998-06-22
WO 97/23779 PCT/GB96/03213
16
Table 1
Dependence of peak area and peak height on pulse width of COZ pulses.''
Pulse WidthPeak Area [nA Peak Height Peak Width rel.8'3C[%]'',
[s] s] [nA] [s]
Pw Pab~ ph" at baseline
1 7.87 1.82 22 -0.100
2 16.45 3.69 25 -0.010
3 25.26 5.10 27 0.181
4 34.18 6.02 28 0.103
44.10 7.07 30 0.00*
6 54.00 7.77 31 -0.09 I
7 63.90 8.25 32 -0.083
8 72.78 8.47 33 0.071
9 83.09 8.76 34 0.250
93.15 8.96 35 -0.112
e' The COZ pulses were introduced under isothermal conditions (70°C)
while the GC eft7uent
was connected to the combustion interface.
''' Regression analysis yielded: pa=9.49pw - 2.61; r=0.99971; r==0.99942.
" Regression analysis yielded: ph=-0.279 + 2.335pw - 0.206pw' + 0.006pw';
r=0.99956;
r2=0.99913.
d' The COZ pulse with 5 s pulse width was chosen as active reference peak and
is marked with
an asterisk. Mean value is 0.023 °/~, median is -O.OI °% and
S.D. is 0.126.
~uasT~rur~ sr~E~-r hum 2s~
CA 02241258 1998-06-22
WO 97/23779 PCT/GB96/03213
17
0
0
V H
w N w
O 1F ~ N ... it U~'t(V y G
E N ~ ~ ~ O ~ ~ >
~
c~ O +I G
~" ~ O O' O ~ O + O~ 1 V
~
~ O v ~
00 c
v
O c P7
n E tE 4r
O
N
S C
M ~ V
N O n1
~ N
~D 00 tn M NO f~ O R E
E ~ ~ f~1 d' V1 ~O .~ _H
U G
o ' O ~ Q G O G O h E O C
~r.:
L U C a~
et
C '~ cif 7
NU
4. o
OO M
iF n
p
C O_ ~ et * ~ ~ o W ..
~ ~ ~ O O
~
E ~ O O O ~ O
G O '('(
Q o o o + > E
~
c ;
= G
E i.r~~ G v
N d C C d
a ~ ~ O
E ~ O c.~ ~ o N _ o
' ~ +t
~a E ~ 3 L
0 L O ~ O O O O O
N . O
O N
7 ca
'~ C
_
O O ~ ~ O
v n
~N
Q ~ p ~ ~ ~ r1 C 3 ~ ~n v
OD E ~ O ~ O ~ O ~ N ~ O ~ e... .'C
C .
O et t~ + O + O + O ~ _ 1 'O O C
~ O -
Ir a
O N
+ ~ c
3 .
c c
>'
E >
t~ C C N
'
~ E ~n
a a > '~ ~ o n r en ov p
~ o r~ ~O C ...
~
~, y
N R a c ~ N +I ca
w
,~ o ~ z a~ ~
: -/- O O O en .
O v ~ p ~
O
. + O O G V
- ~
a ~.=z ~
L :O
p a~ . y
N w vi E
C
C
n
.~6 n ~ ~Y N en ~O t/~ O ~ 7 >
S N
y p O ~ M O s R w ~ 00
O ~ ~ p ~ E
'~
= O O O ~ +f y
U a c
p
_ C ~ + O + + C O O ~ ~ ~ CW_ ~ R
$ ~
~
o .'~ ~ ~ ~ .. LZ. n~
' ' v
~"
_ o a 3 ~ s ~ +1
r
~ o
R
N ~ ~ O
C id
V ~ ~O N ~ ~ ~ d
_ N ~G .~ iF j ~ G
E ~ I~ N O V W C
d p ~ ~ o p
? ~ v
i. o
~ O O O y
> > ~ O ~ ~ [~ ~ =:
4: + '7 U
3
d Q 3 3
~ E
cv U a~
o
_
~ y
O ~' .C
~ O
U
V C O C O O ~ O .' cNe y ~ ~ a c
~ t~ ~O M N 0 ~' +( 3 U i y N t~..
N v
N p M O r O N y
c U H U U > ~.-.
U N C7 C7 Q
_'
ea o~ E
n
SUBST6TUTE SHEET RULE 26)
CA 02241258 1998-06-22
WO 97/23779 PCT/GB96/03213
18
0 0
i Q' ~ n '~ ~ w ~ ~ ~ 'v''a~
C > ~
. 0o c o $ o
O ~ ~ ~ O.
o
cf O' O O O O O O +I "
+ + + y
d e~ >
00 E b0
O ~ ~ CUC
C M
E b '.-
_ O ~ O
it O
~ ~ N N
R U
E U c ~ N N ~ o +I
n.
o "= -~ ~ C o C o C ~n 'd .C E
c.:
" v N v E ? C
E o N
Q '.' c s y
et
C O
~
N U e. >
~r _ .
O O
-C ? N i1 ~ at iF ~ O ~ N j
~ N ~
U
a ~ '-' ~ g ~ O .a-
~ e O O
_ O O
L ~O M O O + O + O O _
V d 'yn r
O ~ c 3 0
o
E +
t
C tQ
. ~ d .O V
~
V .-. a
x iF ~, O, ~ n ~ C L"
'c .!! y o0 ~o O C i a~
c ~
O O N t~1 th M ~~ C ~ G c':
" c o c o 0 0 0
cn d
c
o _ N R
,
H >
E
U
R
~' C _.
V c ..
~ O E H
p O
~ _p v N
>
G C ~ iF ~ x~ ~ iF G y v G
O ~ O~ g +I ~ ~ U
E '
V 0J
,r R ~ O O O O et ~ ~ a~
~ '~ <n + p ~- ~ + ~ ~ 6W
O ' p , o p
p R ~ _ ~ ,C
-~- O O O rdr;
C
E
N ~ C
>
~
i y0 ~ ~ O N _n
N c > oo 'w ~f' O O w'1 ~ ~
, ~ ~ cG d
_
E O ~ O O N N p +I LU. d ~' tC
U G
o R -f. O O O C O +. c~7 N y 4..
tr~ ..y". -_C
v ~ _
V O J U O
~.;z ~=
o '
c
~ v
E
a> V is
C > C m
. ,.- ~ G '-
b V O a) . C
1
~ ~ N ~ O N p01 ~ ~.~ .C W n .D
Op
O U G. O o O o O ~O _
.c R c ~ o c y
~
8 H O + C O, + O o -I- _ E a~ O' a 2
~ ~
... V
H
C v b
~
'y
+ 3 ~
+1
O O
V
> y ' O
f7 "
..
G
a
V1 H
.r
C ~ ~
U E '~ r'~ w n N in ~ _.. ,
~ ~e ~ ~ ._
a a'~
~O v U N ~ N t~ ~O U O .
.y R O C
y
d ~ N M ~ N 0 N
:)
~.
O ~ O ~- o, ~, + O ~ y ': '~ O
c
:9 ~ a~ C C _ R ~ N
.. ~
a~ O O C : i
L' O ca ~
C
o V
~
V s
N N
V
C 0 0 0 0 ,? ~ ~
.
v W o 0 0 0 -i-13U
o 0 o .~a''v
_
'
'
y w
h N V Q
V
E
SUBSTITUTE SHEET' (RULE 26)
CA 02241258 1998-06-22
WO 97/23779 PCT/GB96/03213
19
1 References
2
3 [1] D E Matthews and J M Hayes, Anal. Chem. 50, 1465-
4 1473 (1978)
[2] J T Brenna, Acc. Chem. Res., 27, 340-346 (1994)
6 and references cited therein
7 [3] W A Brand, J Mass Spectrom., 31, 225-235 (1996)
8 [4] R J Caimi, L H Houghton, and J T Brenna, Anal.
9 ~ Chem., 66, 2989-2991 (1994)
[5] European Patent, application number 88308156.4,
11 publication number 0 306 333, 08.03.1989, Bulletin
12 89/10
~.3 [6] D A Merrit, W A Brand, and J M Hayes, Org.
'
' 14 . Geochem., 21, 573-583 {1994)
[7] W Meier-Augenstein, P W Watt, and C D Langhans, J
16 Chromatogr, A, (1996) in press
17 [8] S A Baylis, K Hall, and E J Jumeau, Org. Geochem,
18 21, 777-785 (1994)
19 [9] B N Bopp, F J Sansone, T M Rust, and D A Merrit,
Anal. Chem., 67, 405-411 (1995)
21 [10] M Rautenschlein, K Habfast, and W A Brand in
22 Stable Isotopes in Paediatric, Nutritional, and
23 Metabolic Research, Intercept Ltd., Andover (UK),
24 (1990) pp 133-148
[11] K J Goodman and J T Brenna, Anal. Chem., 66,
26 12941301 (1994)
27 [12] M Matucha, W Jokisch, P Verner, and G Anders, J
28 Chromatogr., 588, 251-258 (1991)
29 [13] K J Goodman and J T Brenna, J Chromatogr. A, 698,
63-68 (1995)
31 [14] H Craig, Geochem. Cosmochim. Acta, 12, 133-I49
32 (1957)
33 [15] G R van der Hoff, P van Zoonen, and K Grob, J High
34 Resol. Chromatogr., 17, 37-42 {1994)