Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02244098 1998-07-23
W O 97/27316 PCT~US97/01291
POLYM~RIC FILM, ASSAY AND METHO3
FOR DIRECT COLO~IM~TRIC D~TECTION OF ANALYTES
BACKGROUND OF THE INV~NTION
Field of Invention
The present invention relates to a polymeric film useful
in an assay and method for direct detection of small molecules,
biomolecules and detectable analytes. The method and assay
utilize observable spectral changes in monomolecular polymeric
films, which changes occur upon the selective binding of a
molecule, biomolecule or analyte to the polymeric film. The
polymeric film comprises a lipid bilayer with an affinity
ligand specific to the analyte, which layer responds to the
binding of the analyte to the ligand by changes in light
absorption spectra. The change is qualitatively and
quantitatively detectable.
~ackarQund and Related Arts
lS Analytical chemistry methods for detection of most of
chemical and biological molecules and/or analytes are virtually
unavailable due to the destruction of the analyte
characteristics during preparation and analysis steps and also
becau~e, typically, of the small amount of the analyte present
in the test sample.
While useful in their own right, analytical chemistry
methods are of limited or no practical applicability to many
biological materials in which assessment would be valuable.
Unless expensive and cumbersome gas chromatography methods are
2~ used, large quantities of analytes are generally required to
accomplish detection. Often, quantitative results are either
limited or not available.
Medical-biologiCal systems analysis including direct
microscopic observation using various cell-staining and classic
pathology techniques also have limitations. Well developed
microbiological techniques, such as culturing, colony
characterization, and observation of metabolic and nutrient
limitations are used to augment these techni~ues. While
culturing and direct tissue observation techniques have served
CA 02244098 l998-07-23
W O 97/27316 PCTrUS97/01291
as the bulwark of medical detection processes for many years,
they also have considerable limitations. Pathological analysis
o~ patient tissues to determine the stage of development o~ a
disease and the identi~ication of the causative pathogens
generally requires an invasive procedure. On the other hand,
culturing the pathogen ~rom various body ~luid or other samples
is time consuming and expensive.
A breakthrough in medicine occurred with the development
of immunoassay methods. In these methods, an antibody is
developed which specifically binds to a target of interest.
While costly in both their development and production,
antibodies from animals typically allow a very accurate
analysis of a number o~ analytes which had previously been
virtually unassessable in both research and clinical
situations
An important technical advancement in immunoassay was the
development o~ monoclonal antibodies. Because the antibody
itself is a small molecule, it is preferably labeled in some
way so that the binding event can be detected. This can be
done with a dye, fluorescent, radioactive or other label.
~onversely, if binding inhibition occurs between a known amount
of introduced, labeled analyte and the material to be analyzed,
the diminution of the signal indicates the presence of the test
analyte. If the agglutination of the antibody particles is o~
suf~icient volume and density, the ~ormation o~ a precipitant
can also serve to signal the presence of an analyte.
In recent years, the research and medical communities have
come to rely heavily on immunoassay techni~ues to detect and
~uantify biological materials. While successful in many
respects, the indirect nature of immunoassay methods as well
a~ their dependence on antibody materials results in a variety
of complications, problems, and assay limitations. The
development and production of antibodies r~;n~ expensive, and
these molecules are sensitive to environmental changes. Also,
-only those materials to which antibodies can be produced can
be detected by these systems.
Many small biological molecules are notoriously difficult
CA 02244098 1998-07-23
W O 97/27316 PC~rUS97/01291
to assay in a direct manner due to the severe limitation of
environmental ranges which they can tolerate without losing
their specific characteristics. For these molecules,
immunoassays have been heavily relied upon. The requirement
that an antibody be developed and produced for each possible
target limits the efficacy o~ immunoassay methods in such
applications as designer drug development and screening.
A disadvantage of immunoassay systems is readily apparent
in rapidly evolving pathogens such as the influenza virus,
where the external coat of the pathogen which is normally
available for immune reactions constantly shifts its antibody
recognition elements and, therefore, becomes immunologically
unrecognizable.
Certain types of analytical chemistry techniques were
optimized by the immobilization of one or more of the
components of a reaction. For instance, if the material to be
tested is present in only a small quantity in a test sample,
the detectable analyte may be at so small a concentration that
it is beyond the detection capabilities of any normal assay
system. In these instances, immobilization systems have proven
to be advantageous.
Many immobilizing materials such as Sephadex columns are
available. Re~uirementS for these materials are their specific
binding properties, their relatively inert reaction to other
materials so that they themselves do not interact ln the test
reaction or otherwise inter~ere with the assay for
immobilization, and their structure regularity providing
predictability in the testing situation.
Classically, immobilization has been accomplished on
columns, liposomes or other surfaces. The use o~ such
materials provides many advantages for an assay system. For
instance, these materials allow easy segregation of reactants
from the other sample components.
In a typical immobilization scheme, the analyte is
concentrated by adhesion to the immobilizing material for which
it has a specific af~inity. The testing then takes place on
an area sur~ace limited to immobilized sur~ace, rather than in
CA 02244098 1998-07-23
WO 97127316 PCTr~S97/01291
the defused three-dimensional array of the original sample
~luid. The results are concentrated in a smaller area, and are
more likely to be detected.
Bilayer films emplaced on surfaces have been used to
provide the immobilization matrixes. Chemical modifications
of these surfaces by organic monomolecular films have recently
been used in an e~fort to develop new materials. The
techniques o~ molecular self-assembly, such as that described
in Lanqmuir, 3, 932, (1987) are used for coating sur~aces with
a well-de~ined, ~uasi two-~im~n.qional array o~ molecules.
These bilayer ~ilms became useful as immobilizing supports ~or
analytic reactions. Bio-sensors based on these films can
detect molecules o~ diagnostic signi~icance such as glucose and
urea, as described for example in Thin Solid Films, 180:65,
~lg89) and 210: 443 (1992). In these cases, classic analytical
chemistry systems are immobilized on the films in order to
improve the readout o~ the test results and otherwise simpli~y
and improve the detection capabilities of the test procedure.
Similarly, the detection of receptor-ligand interaction
is accomplished by indirect assays such as the enzyme-linked
immunosorbent assay. Although biotechnological ~unctionalized
~ilms have led to molecular recognition at an interface, the
problem of translating the molecule recognition event into a
measurable signal has remained problematic until the advent of
~he subject invention.
Detection o~ the immobilized reaction products is
generally carried out by coupling the immobilized matrix to a
~econdary device such as an optical fiber (Colloid Inter~ace
~ci., 124: 146 (1988)), quartz oscillator (Thin Solid Films,
21Q: 471 (1992)), or electrode surfaces (Chemical Letters, 627
(1990) ) .
Some o~ the analytes bound to the immobilized matrixes are
detectable by the fluorescent label, where the fluorescence or
its quenched state indicate the occurrence of a binding event.
Immobilized matrixes may ~urther be made o~ a bi-lipid layer
where the detection materials are embedded in the sur~ace of
the supporting bi-lipid layer (Advanced Materials, 3: 532
CA 02244098 1998-07-23
W O 97/27316 PCTrUS97/~1291
~1991) ) .
Polydiacetylene films are known to change color from blue
to red with increasing temperature or changing pH due to
~con~ormational changes in the conjugated backbone ~Lanamuir,
8: 594 (1992)). While it has been a goal of the research
community to exploit this characteristic in the detection of
b~r.dir~g event~, researchers have yet to develop a method using
this phenomenon in practical applications.
It would be, therefore, highly desirable if the direct
detection method could be provided for detection of very small
chemical and biological molecules present in minute amounts.
It would be ideally advantageous to develop technology of
monomolecular film supports in a simple and unique way so that
the binding would event cause a readily observable change in
the support material that could be directly detected.
It i6 therefore, a primary obiective to provide a simple
and reliable assay ~or detection of minute amounts of various
chemical and biological analytes using a novel polymeric film
for direct colorimetric detection o~ analytes.
20SUMMARY
It is one object of the present invention to provide a
polymeric film, use~ul in an assay for detection of minute
amounts of chemical or biological analytes, a method and assay
for direct colorimetric detection of small molecules of various
analytes using observable color changes in a monomolecular
polymeric ~ilm occurring when the molecule binds to a ligand
of the film polymer bilayer.
It is another object of the present invention to provide
an assay suitable for detection o~ the presence of minute
amounts of chemical or biological molecules by directly
detecting the binding event when the analyte specifically binds
to a ligand of the polymer bilayer.
It is a further object of the present inventi~n to provide
a method and assay ~or a direct detection o~ viruses, bacteria,
parasites, and other pathogens, or drugs, hormones, cell wall
~ragments, enzymes and their interactions, as well as other
biologically relevant materials.
CA 02244098 1998-07-23
W O 97/27316 PCTAUS97101291
It is another object o~ the present invention to provide
a method and assay for the development o~ drugs and improvement
o~ drug activity by observing competitive binding or
inhibition o~ natural binding events between some or all
sur~aces o~ the polymeric ~ilm and the natural bioactive ligand
of the tested biomolecule.
It is yet another object of the invention to detect the
presence o~ biomolecules, using visible color changes or
colorimetric detection in the lipid bilayer o~ the polymeric
~ilm o~ the invention which color changes occur as a result o~
the speci~ic binding of the biomolecules to the bilayer.
It is an additional obiect of the present invention to
provide a simple to use, inexpensive and reliable assay and a
test ~it ~or qualitative and quantitative detection o~ minute
amounts o~ small molecules, which assay is stable in a wide
range o~ environmental and laboratory conditions when the
analyte is mixed with a number o~ other materials.
It is still another object o~ the present invention to
provide a polymerized bilayer film ~or direct detection o~ the
presence o~ analyte comprising:
a) a ligand having direct a~inity ~or the analyte or
which ~unctions as a competitive binder to the analyte;
b) a linear structural linker having two terminal ends,
wherein said linker is attached at its ~irst terminal end to
said ligand;
c) a conjugated polymer backbone to which said structural
linker is bound at its second terminal end;
d) orienting head groups bound to the sur~ace o~ the
conjugated polymer backbone in positions not occupied by the
~0 structural linker; and
e~ a support structure;
wherein said ~ilm undergoes detectable spectral
modi~ication upon binding o~ a target analyte to the ligand
moiety.
35BRIEF DESCRIPTION OF THE DRAWINGS
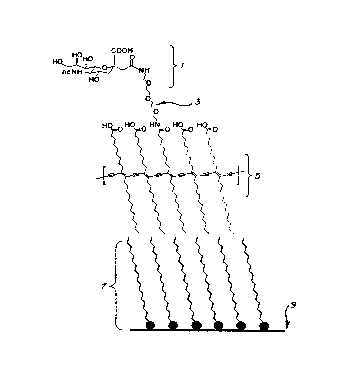
Figure 1 provides a schematic view o~ one embodiment o~
the subject invention.
CA 02244098 1998-07-23
W O 97/27316 PCTrUS971~1291
Figure 2 is an optical micrograph of one of the inventive
polymeric ~ilms (Figure 2A) and the optical micrograph
illustrating the colorimetric response to the influenza virus
(Figure 2B).
Figure 3 shows the structures of the compounds used in
film formation tested in competitive inhibition experiments.
Figure 4 illustrates ~uantification of the visible
absorption spectrum before and a~ter incubation with virus.
Figure 5 is a plot of the colorimetric response of a
sialoside bilayer film to increasing concentrations of an
influenza virus.
Figure 6 illustrates usefulness of the invention for
detection of the influenza virus by detecting colorimetric
response in the presence or absence of the binding inhibitor.
DEFINITIONS
As used herein:
"Analyte" means a detectable chemical molecule,
biomolecule or a portion thereof which is detectable by a
specific binding to a ligand of the polymeric film of the
invention, resulting in changes in the spectral characteristics
of the polymeric film.
"Ligand" means a hydrophilic lipid monomer or its
derivative to which the tested analyte binds at a speci~ic
recognition and detection site, which can be rendered polymeric
by linking the ligand through a linking arm to a polymerized
thin bi-layer film. The ligand is typically specific either
to the individual analyte or to a group thereof. The ligand
forms a part of detecting heads of the polymeric film. The
ligand is attached to one terminal end of spacer or linker
molecules and is polymerizied in admixture with a hydrophobic
matrix lipid monomer. The ligand can be monovalent or
multivalent.
"Linker" or "spacer" means a linear structure molecule
linked through one terminal end to the ligand and through the
second terminal end to the base ~ilm. Specifically, the linker
i9 attached to one of several monomers which have been
polymerized into a chromatic detection element. A structural
CA 02244098 1998-07-23
W O 97/27316 PCT~US97/01291
linker has a sufficient length and conformability to allow
binding of multiple sites on the analytes. The linker may be,
for example, tetraethylene glycol.
"Conjugated polymer'l, 'Ipolymer backbonell, "conjugated
polymer bac~bone" means a layer of a polymerized ligand and
matrix monomer bi-layer assembly able to signal binding
occurring at the surface of the film by a chromatic transition.
The polymer backbone may be, for example, polydiacetylene. The
polymer backbone is bound to a structural linker.
IlPolymer bi-layer" is made of polymerizable ligand and
matrix lipid monomers, polymerized into a chromatic detection
element. Binding of the analyte to the ligand linked to the
polymer backbone induces stress within the bi-layer, changing
the effective conjugation length o~ the polymer backbone. The
matrix lipid monomers suitable for polymerization are lipid
monomers. Such moieties include: acetylenes, diacetylenes,
alkenes, thiophenes, imides, acrylamides, methacrylates,
vinylether, malic anhydrides, urethanes, allylamines, siloxanes
or vinylpyridinium etc. Lipids containing these groups can be
made into homopolymers or mixed polymers. The preferred group
for use in this invention is diacetylene, due to its uni~ue
optical properties in the polydiacetylene polymerized ~orm.
However, other polymerizing groups could be used when they
provide an observable change in properties upon a binding
event.
~ Ligand lipid monomers" are carbohydrates, amino acids and
such other molecules having carboxylic groups.
"Lipid monomers" are readily polymerized into polymeric
films by ultraviolet irradiation or other means for polymer
backbone formation. Most pre~erred monomers are diacetylene
monomers such as polydiacetylene and octadicyltrichlorosilane.
"Lipid orienting head groups" means lipids forming,
stabilizing and structurally orienting the polymer bi-layer to
facilitate color stability until the binding of the analyte
disturbs it.
'ILipid tail(s)" means a moiety serving to anchor the
polymerized film to the support surface.
.
CA 02244098 1998-07-23
W O 97/27316 P~T~US97/01291
DETAILED DESCRIPTION OF THE INrVENTION
The present invention concerns an assay and method for the
direct colorimetric detection of small molecules, biomolecules
and analytes binding in a receptor-ligand-like interaction
using a no~el polymeric thin ~ilm construct. The invention
further concerns the thin poly~eric ~ilm as well as the method
for producing the polymeric films ~or speci~ic detection o~
various analytes through their binding to a ligand, where the
ligand or its derivative are rendered polymeric by polymeric
linking o~ the ligand through a linking arm to a polymerized
thin bi-layer ~ilm.
The presence o~ the investigated analyte detected by
binding of the analyte to the ligand is observed through
changes in the spectral characteristics o~ the polymeric ~ilm.
The polymer-ligand assembly encompasses speci~ic molecular
recognition and detection sites, all contained within a single
molecular assembly.
The present invention allows, ~or the ~irst time, direct
detection o~ small chemical and biological molecules, such as
pathogens and drugs, using observable spectral changes in
monomolecular ~ilms. The present invention thus represents an
entirely new approach to the direct detection o~ small
molecules, biomolecules and analytes using color changes in a
monomolecular film which changes occur when these materials
speci~ically bind to the target molecule.
The subject invention represents a dramatic advancement
o~er both chemical and immunoassay systems as it enables both
~ualitative and guantitative detection o~ small, otherwise
undetectable, chemical molecules, biomolecules and/or
identi~iable analytes in a simple, ~ast and practical way. The
present invention combines the advantages o~ both immunoassay
and chemical analysis in a single system.
Additionally, the assay o~ the invention is use~ul for
environmental testing by detection o~ various analytes in their
3~ most advantageous environmental conditions by the allowing
rigorous, direct analysis to occur even in very narrow
environmental ranges. The speed and simplicity o~ the color
CA 02244098 1998-07-23
W O 97/27316 PCTAUS97/01291
change indicator of the subject invention are its hallmark
advantages.
In general, the present invention requires no pre-analysis
purification step. The results are easily read by an untrained
observer, and the test can be conducted in ambient conditions.
Very mild testing conditions allow the detection o~ small
biomolecules in a near natural state, providing information as
to their interactions and avoiding the risk o~ modification or
degradation of the analyte. This feature o~ the subject
lo invention is due to the high specificity of the ligands
incorporated into the detecting ~ilm. Additionally, the
inventive direct assay system avoids the expense,
complications, and increased inaccuracies inherent in the
indirect systems currently available.
I. The Polvmerized Bilayer Film
The polymeric film according to the invention is a multi-
layer assemblage which allows ~or the direct detection of the
presence of a wide range of analytes by changes in color
spectral criteria.
A. PreParation of PolYmer Film
Polymeric detection ~ilms o~ the invention are simple and
easy to prepare.
Briefly, monomeric lipids having hydrophilic groups, such
as carbohydrates, amino acids, etc. which groups are able to
bind to biological materials, are used as the ligands ~or
detection of these materials. Monomeric matrix lipids, having
hydrophobic groups, are used to assemble and orient the film.
Both lipids are mixed in proportion from about 5-40~ o~
ligand/matrix lipids in an organic solvent, such as chloroform,
benzene, hexane, xylene, and others, or in a mixture of the
organic solvent with an aqueous solvent, such as a mixture of
chloro~orm/methanol, preferably about 9:1. The solution of
ligand/matrix lipids in the solvent is slowly added, typically
in small drops, onto the surface of the water, where it orients
ltsel~ by attaching the hydrophilic groups to the water and by
projecting the hydrophobic groups out o~ the water. During
this step, the organic solvent either evaporates or is removed
CA 02244098 1998-07-23
W O 97127316 PCT~U~97/0129 11
leaving behind the lipids as a ~luid film positioned in the
water sur~ace. For better ~ilm ~ormation and per~ormance, the
lipids are either applied in high concentration on small water
area or, typically, are compressed in 9andwich-like manner by
placing barriers on both sides o~ the lipid ~ilm. Then, the
lipid monomers are polymerized by, ~or example, exposure to the
W light forming thereby a ~loating polymer which is, to a
naked eye, visibly blue. This polymer is then mounted on, or
attached to, the separately prepared support structure, such
1~ as, for example, glass, microscopic glass, celluloid, etc., or
any other ~irm supporting structure which can be coated with
an anchoring hydrophobic sur~actant such as octadicylsiloxane,
or hexamethyldisiloxane, and others, wherein the siloxane group
provides anchoring. The final assembly has blue color.
The inventive diacetylenic lipid monomers such as compound
1 ~Fig. 1) are readily polymerized in monolayers by ultraviolet
irradiation to ~orm a conjugated polydiacetylene backbone of
alternating eneyne groups using standard art methods (Colloid
PolYmer Science, 255: 36 (1977), and J. o~ PolYmer Science,
~etters to the Editor, 16: 205 (1978)). In one embodiment
o~ the invention, a thermo-chromatic polydiacetylene bilayer
is assembled on a support, and then used ~or the detection
procedure. The polydiacetylene layer is ~unctionalized with
a receptor speci~ic ligand ~or the target molecule which is to
be detected. Both qualitative and quantitative ~indings as to
the presence o~ the target material can be obtained using
various embodiments o~ the subject invention.
In one embodiment of the present invention, the bilayer
is composed o~ a sel~-assembled monolayer of
octadecyltrichlorosilane and a Langmuir-Blodgett monolayer of
polydiacetylene (Lanqmuir-Blodqett Films, Roberts., Ed., Wiley
New York, (1966)). The polydiacetylene layer in this case is
~unctionalized with an analog o~ sialic acid. Sialic acid is
the receptor-specific ligand ~or the in~luenza virus
hemagglutinin, as well as ~or other pathogens. The sialic acid
ligand serves as a molecular recognition element.
This colorimetric technology is coupled to materials whose
CA 02244098 1998-07-23
W O 97/~7316 PCT~US97/01291
12
chemical properties can be tailored to bind a variety o~ small
organic molecules. Many organic hosts ~orm inclusion complexes
with dipolar protic and aprotic compounds. Certain inclusion
compounds, or clathrates, such as compounds 1 and 2 seen in
Reaction Scheme 1 have been shown to be highly selective
sorbents for organic solvent vapors (Anqew, Chem. Int. Ed.
Enql., 32: 110 (1993)~. For example, compound 1 has a
pronounced affinity for dioxane and little affinity for
butanol, acetone, methanol, 2-propanol, cyclohexane, toluene
and water. The lack o~ af~inity to cycloh~ne is particularly
remarkable given the similarity in chemical structure.
~ompound 2 on the other hand, shows a pronounced affinity for
1-butanol over the same group o~ solvents. This breakthrough,
combined with colorimetric detection led to a new class o~
chemically sensitive materials immobilized on sur~aces.
~urfaces which have both the clathration element and the
detection element built into a single supra-molecular assembly
represent a novelty of the method for direct detection of a
wide variety of environmental cont~m;n~nts.
Clathrate-formingcompounds coupledto the polydiacetylene
polymer ~orm a new class o~ materials which are chemically
sensitive, robust, and have unique optical properties. These
materials of~er a novel, yet simple method o~ detecting the
presence of organic solvents by monitoring the color changes
which occur in the ~ilm upon binding of the offending compound.
No technical expertise is re~uired to use such a detector; thus
it is suitable for on-site analysis by persons with little or
no technical experience. The molecular level understanding of
why clathrate-forming compounds of a given structure complex
with a given test molecule leads to a wide variety of
clathrate-~orming polymeric thin films. All these are within
the scope of the invention.
B. The Com~osition o~ the PolYmeric Film
The inventive assay film is composed of the base polymeric
~i-layer ~ilm, whose sur~ace contains both orienting and
detecting head groups. The detecting head groups are composed
of a ligand specific to the analyte in question, which is bound
CA 02244098 1998-07-23
W O 97/27316 PCTrUS97/01291
13
to one terminal end o~ a linear structural linker. This
linker, in turn, is bound to the base film by its second
terminal end. The base film surface is also provided with
lipid orienting head groups.
A schematic depiction of one embodiment of the present
invention is seen in Figure 1.
Receptor-bindlng lipid ligand (1? which is hydrophilic in
nature is shown attached through the terminal end of its linker
or spacer molecule (3) to the second lipid matrix monomer of
the polymeric film which is hydrophobic in nature. Both the
ligand and matrix lipid are polymerized into a chromatic
detection element (5). The chromatic detection element (5)
which is, through its hydrophobic side, connected to a
monolayer support layer (7) which is also hydrophobic and is,
in turn, attached to a support structure, such as a microscopic
slide (9). Alternatively, any surface which will accommodate
the hydrophobic detection element (5) can be substituted for
the elements (7) and (9). For example, a plastic surface could
serve in their place.
The polymeric film comprises a ligand lipid monomer,
optionally a compound used as a linker or spacer, and a matrix
lipid, both polymerized into the detection polymeric film
having detection and orienting head groups, said film mounted
on the support system.
1. Liaand ~rou~
A ligand or its derivative is a hydrophilic lipid monomer
to which the tested analyte binds at a specific recognition and
detection site, which can be rendered polymeric by linking o~
the ligand through a linking arm of the linker or spacer to a
polymerized thin bi-layer film. The ligand is typically
specific either to the individual analyte or to a group
thereof. The ligand forms a part of the detecting heads of the
polymeric film and it can be monovalent or multivalent.
The ligand group of the present invention, seen in Figure
1 ~1) is selected from a wide variety of materials. The main
criteria ~or such selection are that the ligand has an a~finity
and specificity for the analyte of choice. The ligand may be
CA 02244098 1998-07-23
W O 97/27316 PCT~US97/01291
14
directed to a single entity or to a broad range o~ materials,
such as when a class of materials is to be assayed.
Appropriate ligands include peptides, carbohydrates, nucleic
acids or any organic molecules which bind to receptors. For
instance, all in~luenza strains use the same binding sites when
binding to a host receptor molecule. Thus, the host receptor
molecule is advantageously employed ~or screening ~or all
in~luenza strains, including those which have not yet been
characterized.
Ligands are also advantageously used in the present
invention when they ~unction as competitive binders to the
analyte, ~or instance, when a pathogen is introduced with a
test material which is to be tested ~or the presence o~ a
receptor molecule. In absence o~ the receptor molecule, the
pathogen binds to the assay bilayer and produces a color. To
the degree that the pathogen sur~ace binds to the receptor
molecule introduced in the test material, the binding is
diminished. In this way, the presence o~ a receptor molecule
is detected and quanti~ied.
2. Linker
A linker or spacer may be a separate li n~ structure
molecule linked through one terminal end to the ligand and
through the second terminal end to the base ~ilm or a part of
the ligand or matrix. Speci~ically, the linker is attached to
one o~ several monomers which have been polymerized into a
chromatic detection element. A structural linker has a
su~icient length and con~ormability to allow binding of
multiple sites on the analytes. The linker may be, ~or
example, tetraethylene glycol.
3. Polymer backbone
Polymer backbone is a layer o~ a polymerized bi-layer
assembly able to signal binding occurring at the sur~ace o~ the
~ilm by a chromatic transition visible as a change o~ color
~rom blue to red. The polymer backbone may be, ~or example,
polydiacetylene. The polymer backbone is bound to polymer bi-
layers.
Polymer bi-layer is made o~ polymerizable monomers,
~ CA 02244098 1998-07-23
W O 97/27316 PCTAUS97/01291
preferably ligand/matrix lipids polymerized into a chromatic
detection element. Binding of the analyte to the ligand linked
to the polymer backbone induces stress within the bi-layer
changing the effective conjugation length of the polymer
backbone.
The monomers suitable for polymerization are lipid
m.onom.ers. The matriX lipid monomers are such moieties as:
acetylenes, diacetylenes, alkenes, thiophenes, imides,
acrylamides, methacrylates, vinylether, malic anhydrides,
urethanes, allylamines, siloxanes or vinylpyridinium etc.
Lipids containing these groups can be made into homopolymers
or mixed polymers. The preferred group for use in this
invention is the diacetylene group due to its unique optical
properties in the polydiacetylene, its polymerized form. The
most preferred matrix monomers are diacetylene monomers such
as polydiacetylene and octadicyltrichlorosilane, however, other
polymerizing groups could be used when they provide an
observable change in properties upon a binding event. The
second component of the bi-layer are the ligands described
above.
Lipid monomers are readily polymerized into monolayers by
ultraviolet irradiation or by other means.
4. Li~id Detectin~ and Orientinq Groups
The lipids are important in forming and structurally
orienting the film's bi-layer so that binding of the analyte
results a detectable color change. The detection is based on
a structuring effect of the orienting groups appropriately
stabilizing the physical structure of the bi-layer to
facilitate color stability until the binding of the analyte to
the molecular recognition ligand groups happens. The binding
causes sufficient steric perturbation or stress of the
structure to result in a color change. The stability and
relative rigidity engendered by the orienting lipids unites the
bi-layer, so that a steric change in one area triggers a larger
ef~ect in the surface as a whole which can be readily observed.
The observed spectral changes are due to stresses induced
by binding which change the e~fective con~ugation length of the
CA 02244098 1998-07-23
W O 97/27316 PCTrUS97/01291
16
polymer backbone.
Materials, suitable as ligand detecting groups in the
present invention are hydrophilic lipids including -CH20H, -
CH20CONHPh, -CH20CONHEt, -CH2CH(Et)OCONHPh, -~CH2)90H,
CH20COPh, -CH20CONHMe, -CH20Ts, -CH(OH)Me;
-CH20COR2, wherein R2 is n-C5Hl1, n-C7Hl5, n-CgHlg, n-C1lH23, n-
Cl3H27, n-Cl5H3l, n-Cl7H35, Ph, PhO, or o-(HO2C)C6H4;
-OSo2R2, wherein R2 is Ph, p-MeC6H4, p-FC6H~, P-ClC6H4, p-
BrC6H4, p-MeOC6H4, m-CF3C6H4, 2-CloH7, or Me; and
-CO2M, wherein M is K,HNA, or Ba/2.
The pre~erred materials which can be employed as head
groups in the present invention are:
- CH20CONHR2 or -CH2CONHR2 where R2 is Et, n-Bu, n-C6Hl3,
n-C8Hl7, n-Cl2H25, cyclo-C6Hll, Ph, p-MeC6H4, m-MeC6H4, o-ClC6H4,
m-ClC6H4, P-ClC6H4, o-MeOC6H4, 3-Thienyl, Me, Et, Ph, 1-CloH7, Et,
Ph, EtOCOCH2, BuOCOCH2, Me, Et, i-Pr, n-C6Hl3, EtOCOCH2,
BuOCOCH2, Ph, or 2,4(NO2)2C6H30CH2, CH2CH20H.
The most preferred detection groups are taken from -
CH2COX, where X is OH, MeO or any salt thereof.
Materials suitable for use as matrix orienting groups are
hydrophobic lipids used to assemble and orient the film. The
groups comprising the tails of the lipids are of a wide
variety. Serving to anchor the polymerized film to the support
surface these moieties can be any of the following: CH3-' CH30-
, neo-C5HllO-, cyclo-C6HllO-, PhCH20-, p-AcC6H40-, p-BzC6H40-, p-
BrC6H4COCH20-, p-(PhCH=CHCO)C6H40-, p-(PhCOCH=CH)C6H~O-, o-
BZC6H4NH-, p-BZC6H4NH-, MeOCH2CH2NH-, n-C6Hl3NH-, EtO-. The
preferred group in this invention is the methyl group.
5. Film Su~ort
The supporting structure (9) to which the film is attached
can be a variety of materials. The material used in certain
embodiments of the invention, such as Example 1 is a
microscopic glass slide which has been made hydrophobic by
treatment with an appropriate surfactant such as
=octadecyltrichlorosilane. Any material which is somewhat
hydrophobic such as plastic, mica metal, ceramic or other
relatively uniform polymeric sur~ace can be used. Glass is the
CA 02244098 1998-07-23
W O 97/27316 PCTAUS97/~1291
17
preferred transferrant support in this invention due to its
transparency for ease in reading the color changes. However,
non transparent materials can be employed using a reflectance
type measurement.
~. Rece~tor-Bindinq Molecules Used as Liqands
Receptor-bindins molecules are materials on the surface
of a host cell to which a pathogen attaches itself as a prelude
to the infective event. Selecting such molecules as the ligand
group o~ the present invention provides a specific recognition
site for these pathogens as these molecules tend to be highly
genetically conserved in the pathogen having obvious
criticality to the pathogen survival. Different strains of the
same pathogen will generally not produce a false negative
result when molecules suCh as the ligand group in the subject
invention are selected. Receptor molecules tend to be smaller
and less complex, and often less hydrophobic.
Receptor-binding molecules such as those described above
are detectable by the current invention as an increasing number
of receptor molecules have been recognized, identified,
isolated, and synthesized for a large number of pathogens.
Many of these receptors have been improved for use in various
analytic and treatment sy~tems.
A good example of the usefulness of the invention is the
sialic acid derivative used ~or detection of influenza or
malaria. Examples of the receptors for a number of pathogens
are provided in the application as Table l. All o~ these, as
well as any other ligands falling within the s~ope of the
invention are within the scOpe of the subject invention.
Example 1 describes one exemplary use of sialic acid
derivatives as one preferred embodiment for the use o~
receptor-binding molecules.
II. AssaY Testin~ Conditions
The as~ay of the invention is performed under gentle
testing conditions sensitive to tested analytes. The inventive
polymeric thin film construct employs ligands and analytes
which are stable or possess appropriate binding characteristics
specifically within a limited in vitro or narrow environmental
CA 02244098 1998-07-23
W O 97127316 PCTrUS97/01291
18
range o~ conditions. The present invention meets stringent
limitations'even within this narrow range of such conditions.
This allows, ~or instance, maintaining three dimensional
conformations of sensitive biochemicals and biomolecules
throughout the testing procedure.
The test is extremely simple in that the tested analyte
or molecule is contacted with the polymeric film of the
invention and the color change and its intensity are observed
and measured for quantitation. Typically, this process lasts
only about 30 minutes.
The present invention ~unctions well even in extremely
limited conditions. The assay conditions, such as pH,
salinity, and temperature can be carefully controlled by
~eedback controls, titration and other techniques without
interfering with the accuracy or sensitivity of the analysis.
Because of this wide experimental range advantage of the
present invention, intact cells or sensitive subcellular
inclusions are assayed without disturbing their structural
integrity. Subtle cellular development stages, such as the
various stages o~ malaria in~ection can be monitored.
Additionally, the association between various ~actors can be
tested or monitored even during the interaction process using
the method of the subject invention.
The inventive film and method are suitable for assaying
25 ~of very small biological or other molecules for which
antibodies cannot be developed. These target materials include
organic solvents or pollutants present at extremely low levels.
There are special opportunities made available by the advances
achieved by the subject inventors for drug screening in both
forensic and clinical applications. Inhibition techniques
applied to the subject invention allow the testings of
materials which are of a minute size or have a small number or
single valiancy.
The unexpected spectral signal achieved ~y the present
invention is due to a physical perturbation of the bilayer
which occurs as a result of the binding event of for example,
multivalent materials, such as viruses and cell membrane
CA 02244098 1998-07-23
W O 97/27316 PCTrUS97/01291
19
~ragments, which perturbation then is detected using the
subject inventive method. Thus, multivalent materials
generally elicit a particularly strong response in the assay
system because o~ conformational changes introduced into the
bi-lipid layer as a result o~ binding, causing physical
recon~iguration o~ structure.
Pre-binding o~ smaller, single valent analyte materials
to a carrier may also prove advantageous to increasing the
e~icacy of the subject invention. For instance, the analyte
may be bound to a polymer or the sur~ace o~ a liposome. This
would concentrate the binding event on the inventive bi-lipid
sur~ace to speci~ic points, increasing the spectral
modi~ication at each point of contact. Additionally, the
curved sur~ace o~ the liposome to which the analyte is attached
would serve to tug the peripherally bound analytes away ~rom
the bi-lipid surface and ~orce analytes centrally located on
the liposome into the bi-lipid sur~ace. This pre-binding step
then results in increased torsion, perturbation and signal
generation on the bi-layer sur~ace.
The assay of the invention is suitable ~or detection of
weak binding analytes as well as ~or multivalent analytes. ~he
multivalent ~eature o~ the polymer-linked ligands of the
subject invention provides a heightened binding capacity in the
case o~ naturally multivalent analytes. Multivalency can also
be provided ~or limited valency analytes prior to the test
procedure to imbue them with this advantage o~ the subject
invention. The inventive exploitation o~ multivalency allows
a speci~ic but weak interaction to be ampli~ied many ~old.
A structural linker of su~icient length and
con~ormability aids the binding o~ multiple sites on the
analyte even when they are conformationally separated on a
curved sur~ace. As a result o~ these special ~eatures, the
present invention can detect many ligands previously unsuitable
for assay evaluation.
The main criteria ~or e~ective indication o~ the presence
o~ an analyte is that the sur~ace o~ the indicating bi-layer
be su~iciently perturbed to produce the re~uisite spectral
CA 02244098 1998-07-23
W O 97/27316 PCTrUS97/01291
. 20
change. Binding the analyte to an immobilizing particle serves
this purpose, as it concentrates the analyte in a small area,
and further provides a three-dimensional aspect over a
relatively large area to even a small analyte.
A large variety o~ ligands may be employed in the subject
invention, allowing great flexibility in detecting a
multivalent test target. Ligand selection is based on the most
advantageous binding and steric characteristics, rather than
on accommodation of the test system. Thus, the most
advantageous ligands are selected based on such factors as
hydrophobicity and hydrophilicity, size, position of binding
site, and con~licting a~finities vis-a-vis the analyte to be
detected. Ligands which are advantageously employed in the
subject invention include carbohydrates, peptides, nucleotides,
heterocyclic compounds, and other organic molecules.
The inventive polymerized bi-layer assemblies film
structure and morphology are shown in Figure 1 as a ~chematic
diagram of the polymerized bi-layer assembly. The siloxane
linkages of the bottom monolayer are not shown. Fig. 2 (A)
shows an optical micrograph of the sialoside bilayer assembly
between crossed polarizers. Large domains up to 150 ~M are
visible. Scale: 1 cm = 20 ~M.
The initial investigations focused on the binding of the
influen~a virus to sialic acid as a model system for
colorimetric detection. The study is reported in Example 1.
A lipid monomer contains a carbon-linked sialic acid head group
that provides a molecular recognition site for the viral
lectin, hemagglutinin. Figure 3 shows the matrix lipid (11)
sialoside lipid ~13) and lactose lipid (15) used in LB film
formatting and compounds ~ - NeuAc (17), ~-0-NeuAc (19), and
~ glucose (21) used for competitive inhibition experiments as
described in Example 4. The syntheses of compound (13) is
reported in J. of The American Chemistrv Societv, 115: 1146
(1993). A carbon glycoside was used instead of the naturally
occurring oxygen glycoside to prevent hydrolysis by the
neuraminidase, which is also present on the surface of the
virus.
CA 02244098 l998-07-23
W O 97/27316 PCT~US97/01291
21
III. Tarqet Material 8
One of the advantages of the subject invention is its
usefulness for detection of a wide range of target materials,
binding events, and biochemical reactions amenable to analysis
using the current inventive techniques. Many of these
materials previously could not be detected using available
assays. The present invention utilizes specific binding
without the complications of immunoglobulin generation.
The rigor and outstanding advantages of the inventive
assay system allow the detection and quantitative evaluation
of materials which have been previously unachievable because
of the limitations of the prior art methods. The present
invention has already been tested in a unique assay method for
accurate detection of malaria parasitic infection as described
in Examp~e 9. Development of an effective assay for malaria
in transient stages has until now proven an intractable
challenge for either the immunological as6ay or analytical
chemical art methods.
IV. Oualitative and Ouantitative Evaluation
In the subject invention and the assay, various spectral
changes to the bi-layer are used to detect the presence or
absence o~ the target material. The conjugated polymer
bac~bone o~ the polymerized bilayer assembly signals binding
at the surface of the ~ilm by a chromatic transition. The
color or other spectral transition is readily visible to the
naked eye as a blue to red color change and can be quanti~ied
by visible absorption spectroscopy.
The film was designed to undergo color transition from
blue to red solely due to receptor-ligand interactions
occurring at the sur~ace of the bi-layer. The bi-layer
assembly incorporated both a molecular recognition site and a
detection element. This simple color-based sensor enables
~ rapid, qualitative detection of binding by visual inspection
o~ the ~ilm or quantitative detection by visible absorption
spectroscopy.
As shown above, the thin films of chemically
~unctionalized polydiacetylenes o~ the subject invention act
CA 02244098 1998-07-23
W O 97/27316 PCTrUS97/01291
22
as simple colorimetric biosensors. These films are derivatized
with a carbohydrate-based ligand which specifically binds bio-
organisms such as viruses. The conjugated polymeric film is
initially blue in color. Binding of a virus or other analyte
to the derivatized polymer causes a change in color of the film
from blue to red. The intensity of the resulting red color
corresponds roughly to the 9uantity of the virus. Means of
amplifying the spectral signal for quantification are well
known in the art, such as scintillators, may advantageously be
employed when low levels o~ analyte are present. Because of
the empirical nature of the signal, there are many
opportunities for automating the read out of the present
inventive assay system.
In one embodiment of the present invention, a blue color
shift is observed visually by the testing technician. Because
of the simplicity o~ the reaction, such observation may easily
be accomplished by an untrained person making the assay
suitable, for example, for at-home testing. Alternatively,
spectral test equipment, known in the art, may be employed to
determine a change in spectral color-shift beyond the limits
of simple visual observation, including optical density to a
particular illuminating light wavelength.
Spectral changes outside the human visual range can be
employed ef~ectively in the subject invention by use of various
spectral analyzers, such as light meters, or through technician
observation of the surface using various translating devices,
such as infrared and ultraviolet detectors.
Means of amplifying the spectral signal for quanti~ication
are well known in the art, such as scintillators, may
advantageously be employed when low levels of analyte are
present. Because of the empirical nature of the signal, there
are many opportunities for automating the read out of the
present inventive assay system.
For more precise quantitative measurement, the film is
scanned with a visible absorption spectrometer where the
relative change in the intensities at 620 nm (blue) and 550 nm
(red) i8 readily assessed as seen in Figure 4 (Table 2). The
CA 02244098 1998-07-23
W O 97/2731~ PCTrUS97/01291
23
extent o~ the color response is directly proportional to the
concentration o~ the analyte. The present example moves this
technology from the realm o~ biodiagnostics to the realm of
environmental diagnostics by exploring a new class of ligands
for which a precedent exists for binding small organic
molecules. These ligands are similarly tethered to the
polydiacetylene backbone which provides the colorimetric
detection.
UTILITY
The subject invention enjoys broad applications to the
detection of a very wide variety of analytes. These include
small biomolecules, the observation of binding and other
chemical events, and the detection of trace amounts of many
materials.
Because o~ the very broad applicability, important classes
of analytes are detectable by the present invention which have
previously proven difficult or impossible to detect by prior
art methods. Many viruses, bacteria and proteins related to
them, or infections caused by them can be detected. These
include such pathogens as influenza, HIV, and malaria, among
others. Direct colorimetric detection by the inventive
polymeric films o~ers new possibilities o~ diagnostic
application and screening for new drug candidates or binding
ligands.
The present invention i5 use:Eul for designer drug
development and screening. Currently, to assess competitive
inhibition o~ drug receptor molecules, radio-labeled materials
are typically used. ~owever, this process is time-consuming
and requires access to and handling o~ radio-labeled materials.
Other techniques, such as ~luorescence ~u~nch;ng, are limited
in that each test is self-contained, and there~ore a large
screening effort is prohibitively time consuming and expensive.
There are many advantages to-the genetically conserved
host recognition site being targeted by the embodiment o~ the
present invention. For example, a determination o~ a patient's
exposure to the flu will be definitive, and not limited to a
particular strain i~ the binding to the influenza pathogen
CA 02244098 1998-07-23
W O 97/27316 PCT~US97/01291
24
receptor ligand is detected. This advantage o~ the present
invention also avoids the need for a large number o~
immunological tests, as the clinician can rely on a single
assay. Addltionally, even newly evolved, uncharacterized flu
strains can be identified, ~urther avoiding false negative
tests.
An analogous limitation o~ immunoassays occurs in well
established pathogens sUch as malaria parasites. In these
organisms, phases of the life cycle which would allow for an
immune response have over time been 80 limited that the ~ lne
response is eliminated.
The present invention exploits the genetically
conservative host binding site to identify the pathogen. Even
in comparatively large parasites, the host binding site tends
to be held constant over time throughout the generations of
pathogens. Additionally, parasites are usually present in the
body in a large number of diverse life stages. In well
established parasites, the immune accessible sites often vary
considerably from stage to stage, the advantage being that the
host organism is unable to mount an ;m~llnological response with
sufficient rapidity to avoid the entrenchment o~ the parasite.
In this particular application o~ the subject invention,
various iterations of a drug can be ~uickly screened ~or
interference with infective binding by a pathogen. Table 1
provides a number o~ examples o~ the host receptor molecules
which provide the site o~ pathogen attachment required ~or
infectivity. All of these examples, along with many others,
can be exploited by the subject invention for drug development
and optimization. Multiple wells which can be made on a single
bi-layer sheet allow many subtle iterations of a candidate drug
to be tested, such as various levels of pH titering. By using
this invention for drug testing, the current chilling effect
on drug research of expensive, individual testing ~or each
sample would be eliminated.
The availability of high-volume inexpensive screening will
dramatically increase the speed of drug development. For this
purpose, a naturally occurrlng transmembrane receptor (TMR~ is
CA 02244098 1998-07-23
W O 97/27316 PCTAJS97/01291
reconstituted into a lipid bi-layer where the lipid layer is
constructed ~rom the polymerizable monomers. This is
particularly applicable to the inventive compounds that have
the two triple bonds in the chain. Once the receptor is
incorporated into the lipid, the lipid is irradiated and
~ polymerized to lock the TMR in place. Binding of small
molecules to the binding site in the TMR produces a
conformational change in the TMR which affects the lipids and
causes a color~change.
A wide variety of TMR's have been isolated. TMR's
including hormone, neurotransmitter, and other physiological
regulating receptors, are particularly useful in the
improvement and development of drugs using the present
invention. The use of naturally occurring TMR's in the subject
invention has particular applications to drug screening. The
subject invention has immediate pertinence in the development
of new drugs o~ which biological ef~ect depends on binding to
membrane bound receptors. For example, the dopamine receptor
binds the natural compound dopamine. In order to employ the
sub~ect invention to search for new compounds that behave like
dopamine, that is, bind to the dopamine receptor, the dopamine
receptor, used as a ligand, is exposed to the tested new
dopamine-like drugs. The approach for drug testing is set out
in Example 6 as one practical embodiment o~ the polymeric ~ilms
of the invention and their applications.
Because o~ the ease of screening available using the
subject invention, many small changes can be made in the
candidate drug structure and analyzed immediately, providing
great speed and flexibility in drug development and
optimization. By noting the area o~ modi~ication which
provides the greatest changes in ef~ectiveness, the critical
structures o~ the drug can be rapidly identi~ied. This allows
a critical ~ocusing of the drug modification effort which will
greatly increase the speed o~ drug development.
Basic research of drug interactions, optimization, and new
drug development is also made practical by the present
invention. Existing drugs can be analyzed to determine which
CA 02244098 l998-07-23
W O 97/27316 PCT~US97/01291
26
structures are of the greatest importance in their therapeutic
effect. These structures can then be optimized, and even
transpo~ed onto a more biologically acceptable, smaller, or
less expensive non-active structure. Such qualities as the
ability to traverse the blood-brain barrier can be conferred.
If two different drugs are available for the treatment of
a disease, their structure can be analyzed as to activity using
the technology of the subject invention. Then, their active
sites can be incorporated into a single drug. Additionally,
attendant structures which optimize activity can be
appropriately situated on the new hybrid drug. Any
interference in activity can be determined and ameliorated or
eliminated prior to expensive and lengthy animal or human
trials.
Another important application for the subject invention
and method is the inexpensive, accurate assaying of infective
states and other medical conditions. For instance, antibody
levels to a specific pathogen can be easily and inexpensively
monitored through competitive inhibition of a set amount of
pathogenic material placed in the analytic solution.
Additionally, certain antibodies can be detected through their
direct and specific binding to the inventive membrane.
A large variety of biologically related materials is
advantageously susceptible both to quantitative and qualitative
analysis using the subject invention. Infection by various
pathogens can be tested long before clinical manifestations are
observed. This is o~ a particularly critical advantage in
patients with depressed ;m~lln;ty, such as in newborns,
chemotherapy patients, donor organ recipients, and AIDS
victims.
In testing for pregnancy, human chorionic growth hormone
is assayed using the present invention. A rise in luteinizing
hormone heralds the onset of ovulation ~or both the achievement
o~ pregnancy and ~or use in natural birth control methods.
Because of the simplicity of readout, the subject
invention is highly suited for home use. For example, it
enables multiple testing at low cost needed in natural birth
=
CA 02244098 l998-07-23
W O 97/27316 PCTrUS97/01291
27
control methods or for assessing fertility to optimize the
chances o~ achieving pregnancy.
The inexpensive multiple te9ting capacity of the present
invention made possible through multiple wells on a single bi-
layer sheet provides an excellent incentive ~or extremely earlydetection of pregnancy. Detecting pregnancy prior to a missed
period is important in avoiding exposure to harmful factors,
which are critical to the final outcome, in the first ~ew days
of pregnancy. It is also important when a pregnant woman may
have been exposed to a disease that will have late or no
clinical manifestation ~or the mother, but could severely
damage the developing ~etus she carries. These diseases can
include rubella, toxoplasmosis, and other pathogens. The
present invention allows for simple and inexpensive screening
for such diseases.
Another important application ~or the present invention
is the monitoring of patients with chronic illnesses such as
diabetes. For instance, insulin blood levels can now be
regularly monitored at home using the subject invention. This
allows diabetics to tailor their insulin administration to more
accurately follow the insulin requirements. It also allows
them to quickly differentiate early symptoms o~ a transient
illness such as ~lu from undue variations in insulin levels.
The present invention also allows ~or the production of
a simple, at-home test for cholesterol levels allowing patients
to determine their cholesterol levels in the privacy o~ their
own home, encourages the more reticent to test their
cholesterol level and be aware of this o~ten critical
information. For patients with known hypercholesterolemia, the
present invention represents an ideal means to closely monitor
the palliative e~ects of treatment efforts. The multiple well
test kit made possible and practical by the present invention
is particularly useful for weekly or even daily monitoring of
these levels.
The monitoring of drugs and drug levels is a ~ertile area
of application for the present invention. Patients typically
display a wide range o~ metabolic levels and liver activity.
CA 02244098 1998-07-23
W O 97/27316 PCTrUS97/01291
28
This is particularly the case for those in a hospital
situation. Because blood drug levels cannot be easily
determined, the clinician is o~ten ~orced to under-medicate a
patient who could benefit from higher levels of administration.
Unfortunately, the doctor must err on the side of caution to
avoid the possibility of toxic levels being reached. The
present invention allows a more accurate titering of drug
administration, allowing better pain relief and other drug
benefits.
The present invention has important application in drug
abuse. When a patient suf~ers ~rom a possible overdose, the
actual blood levels of the drug and also its identity can be
very rapidly assessed by the treating physician using the
present invention. This information prevents potentially
harmful treatment for overdose by drugs which display the same
symptoms as those of the actual overdose substance.
Additionally, less draconian detoxi~ication measures can be
taken if lower than suspected drug levels are detected using
the subject invention. Conversely, toxic levels can be
detected even when the patient is not displaying symptoms which
would alert the clinician to the actual danger level.
The subject invention is further use~ul in a wide variety
of industrial applications. For instance, industrial enzymes
can be monitored as to their binding strength, as well as to
their presence in a media. Their loss can be monitored in an
effluent, and their appropriate dispersal can be monitored in
~eedstock and media.
The invention is very use~ul in determining optimal
conditions ~or enzyme activity on any particular substrate.
Additionally, the enzyme can be easily engineered for
optimization, including tailoring for specific uses or working
environments. This i8 done in a manner analogous to designer
drug evaluation as explained elsewhere. Thus, tolerance for
extreme pH environments, concentrated ~eedstock, cold and heat,
inter~ering additional materials, and other desirable tolerance
can be developed ~or industrial enzymes and other active
materials. The ability of the present inventive $ilms to
CA 02244098 1998-07-23
W O 97127316 PCTrUS97/01291
29
detect small molecules using TMR's as described in the drug
development section above also has excellent use in industrial
and environmental applications. Noteworthy among TMR's to be
used for this purpose are the olfactory TMR's. These can bind
small odorant molecules and have important applications as an
environmental sensor, among others.
The need for chemical sensors to measure analyte
concentrations for industrial process control applications, for
warning and sa~ety systems, in environmental analysis, etc. is
great. Classic chemical analysis, such as gas chromatography-
mass spectrometry are not conducive to on-site field analysis
because of the analytical turn around time, high cost,
impractical equipment for ~ield use and the need for
technically experienced personnel. The sensor which would be
useful in field work analysis therefore re~uires material which
is chemically sensitive and can specifically bind the analyte
in question, and a simple, user friendly method to detect when
binding of the analyte has occurred. In-line monitoring of
public water supplies (e.g. swimming pools, drinking water,~0 waste water streams, etc.) for contAm;nAnts can be developed.
EXAMPLE 1
Preparation of Polymer Film For Detection of Influenza Virus
This example illustrates the procedure used ~or
preparation of a polymeric film suitable for detection of
influenza virus.
A polymerized bilayer assembly shown in Figure 1 composed
of a self-assembled monolayer of octadecyltrichlorosilane (OTS)
and a monolayer of functionalized polydiacetylene was prepared.
The films were prepared by a modified LB technique in
which the carbohydrate ligand detection group is presented at
the surface of the bi-layer. Mixtures of 2~ to 5~ of
glycolipid monomer (13) seen in Figure 3 and matrix lipid
monomer (11) were spread on the water sur~ace o~ a standard LB
trough.
The matrix lipid uniformly dispersed the sialoside lipid,
which allowed optimum binding of the virus. Sialoside lipid
1~ to 5~ gave maximum binding of the virus to polymerized
CA 02244098 1998-07-23
W O 97/27316 PCTrUS97/01291
liposomes. Ideal m;~; ng of the two components was determined
by analysis of the Langmuir isotherms. Various ratios of
monomers (11) and ~13) give isotherms whose limiting areas and
collapse pressures change in direct proportion to the mole
fraction of (2) as expected for miscibility. The mixed
monolayer was compressed and polymerized on the water sur~ace.
The ~loating polymerized film was li~ted by the horizontal
touch method onto a glass slide previously coated with a self--
assembled monolayer of OTS. The resulting bi-layer assembly
lo presents an array of carbohydrate ligands at the sur~ace. The
tetraethylene glycol spacer in sialoside lipid (13) serves to
extend the carbohydrate ligand beyond the carboxylic acid head
groups o~ the matrix lipid (11).
Films prepared in this manner exhibited a high degree of
order over a macroscopic range (50 to 150 ~M) as evidenced by
optical microscopy with the use of crossed polarizers as shown
in Figure 2. The films were further characterized by angle-
resolved X-ray photoelectron spectroscopy (XPS) and
ellipsometry. The XPS results indicate that the amide nitrogen
atoms and the carbonyl carbon atoms of the head groups are
localized at the sur~ace relative to the methylene carbons of
the lipid ~h~;n.c, demonstrating that the sialoside detection
group is presented at the surface of the film. Ellipsometric
analysis of the polydiacetylene monolayer coated on HF-treated
silicon indicated a film thickness o~ ~40 A, in agreement with
the expected value based on molecular modeling. The bi-layer
assembly had a visible absorption maximum of 620 nm and
appeared as a blue film.
EXAMPLE 2
Oualitative Detection of Influenza Viru~ Bindinqs
This example illustrates the detection of the binding of
influenza virus to the polymer film of Example 1.
When the film obtained in Example 1 was incubated with X31
in~luenza A virus in phosphate-buffered saline (PBS) buf~er,
at pH 7.41, the binding o~ the viral hemagglutinin to the
sialic acid residues on the surface resulted in a blue to red
color transition.
CA 02244098 1998-07-23
W O 97127316 PCTrUS97/01291
31
Figure (2)B shows the colorimetric response of the film,
supported on a glass microscope slide, readily visible to the
naked eye for qualitative evaluation of the presence of the
virus. The film on the left (blue) has been exposed to a blank
solution of PBS. The film on the right (red) has been exposed
to 100 HAUs of virus (CR = 77~). A colorimetric response of
~15~ was observed visually. No color change was observed when
the blue film was incubated with a blank solution of PBS
buffer. This result demonstrates a polydiacetylene color
transition arising from affinity binding (affinitychromism)
rather than thermal annealing (thermochromism).
Previous studies have shown that LB films composed of
lipid (1), Reaction Scheme 1, undergo a blue to red color
change when heated at 70~C, which corresponds to the
endothermal transition for lipid chain melting. Lipid chain
disorder and tangling decrease the effective conjugation length
of the polydiacetylene backbone. Similarly, Fourier transform
infrared and resonance Raman spectroscopy as well as X-ray data
~emonRtrate that lipid chain packing of the red form of the
polymer is different from that of the blue form. Thus,
conformational changes in the lipid c~nR af~ect the optical
properties of the polymer backbone. Binding of the viral
hemagglutinin to the sialoside bi-layer assembly appears to
affect the lipid chain conformations in a manner analogous to
thermal annealing.
EXAMPLE 3
Ouantitative Detection o~ Influenza Virus Binding
This example describes the quantitative detection of
binding of influenza virus to the polymeric film of the
invention.
In addition to qualitative evaluation by visual
inspection, the degree of color change is readily quantified
by standard visible absorption spectroscopy. The visible
absorption spectra of a bi-layer assembly prior to (solid line)
and a~ter (dashed line) viral incubation are shown in Figure
4. The bi-layer assembly was inserted into a quartz cuvette
containing P~3S buffer (pH 7.4), and the absorption spectrum was
CA 02244098 1998-07-23
WO 97/27316 PCT~US97/01291 32
obtained. Addition of influenza virus in PBS buf~er (pH 7.4)
resulted in a chromatic transition following a 30-min.
incubation period. Although the film color began to change
within seconds after exposure to virus, 30 min. was found to
be the average length of time required for the CR to reach a
plateau value in a nonstirred solution. These spectra
represent a CR of 50~.
The blue-colored film (solid line) before the exposure to
the virus, had a strong absorption m~; mum at 620 nm and a
weaker absorption at 550 nm. A~ter incubation with in~luenza
virus (dashed line), a dramatic change in the visible
absorption spectrum occurred. The m~; mllm at 550 nm increased
with a concurrent decrease in the m~;ml7m at 620 nm, resulting
in a red-colored film.
In order to quantify the response of a film to a given
amount of virus, the visible spectrum of the ~ilm before
exposure to virus was analyzed as equation
1. Bo = I62o/(~sso+I62o)
where Bo is defined as the intensity of absorption at 620 nm
divided by the sum of the absorption intensities at 550 nm and
620 nm. After exposure to influenza, the equation was
2. Bv = I620/(I55c+I620)
where Bv represents the new ratio of absorption intensities
after incubation with the virus. The colorimetric response
(CR) of a ~ilm is defined as the percent change in B upon
exposure to virus:
3. CR = [ (bo-Bv)/Bo)] 100~
The colorimetric response was directly proportional to the
quantity of influenza virus, measured in hemagglutinating units
(HAUs), where 1 HAU is de~ined as the highest dilution o~ stock
virus that completely agglutinates a standard erythrocyte
suspension.
Figure 5 shows a plot of the colorimetric response of a
sialoside bi-layer assembly versus successive additions of
influenza virus. A blue ~ilm containing 2~ of sialoside lipid
13 (Figure 3) and 98~ matrix lipid 11 (Figure 3) was
preincubated in PBS bu~er ~or 30 min., a~ter which successive
CA 02244098 1998-07-23
W O 97127316 PCT~US97/01291
33
aliquots of X31 influenza A virus were added. The film was
incubated for 30 min. following each addition of virus, and the
visible absorption spectrum was recorded. The CR was
calculated according to equation 3. Linear regression analysis
of the first six data points gives a slope of 0 53~ (r2 =
0.985).
Saturation of the colorimetric response occurs at ~80
HAUs. Incubating the red film with a buffer blank (no virus)
for 1 hour did not result in a return of the blue color. Thus,
the structural changes which result in the color change appear
to be irreversible under these conditions.
EXAMPLE 4
Com~etitive Inhibition Assays For In~luenza Virus
S~ecific Detection
This example illustrates utility of the invention in
competiti~e inhibition assays.
The specific nature of the interaction between the
influenza virus and the sialoside film surface was confirmed
by competitive inhibition assays. Figure 6 shows that the CR
of the film can be inhibited by compounds that bind to viral
hemagglutinin. Incubation of a sialoside bi-layer assembly
with 32 HAUs of influenza virus produced a colorimetric
response of 22.6~. However, the same concentration of virus
in the presence of 17.3 mM concentration of compound 17 (Kd =
2 mM) (Figure 6, column 4) completely suppressed the CR to less
than 0.5~ due to competitive inhibition. The CR was not
~;~;n;~h~d in the presence of 1~.3 mM (Figure 6, column 5)
concentration of compound 19 ~Kd ~ 50 mM) or compound 21
~Figure 6, column 6) that do not compete for binding to viral
hemagglutinin. The known inhibitor of influenza
hemagglutination, compound 17 see in Figure 3, has dissociation
constant X~ of 2 mM as determined by a standard
hemagglutination inhibition assay (HAI). Incubation of the
sialoside ~i-layer assembly with influenza virus in the
presence of the known binding inhibitor 17 resulted in no CR
~CR < 0.5~) and the ~ilm r~m~ blue. This result
demonstrates that the inhibitor effectively competed with the
CA 02244098 1998-07-23
W O 97127316 PCT~US97/0~291 34
sialoside surface for binding to the virus. When the blue film
was exposed to the same quantity of influenza in the presence
of a no~ nh; hitor (Figure 3, compound 19, Kd ~ 50 mM, or
glucose, compound 21), the color change was identical to a film
exposed to influenza alone.
In order to test the capability of the film to predict the
value of Kd for an inhibitor, the CR was measured for a series
of inhibitor concentrations. The CR increased in a linear
fashion (r2 = 0.995) with decreasing concentrations of
lo inhibitor 17. Bxtrapolation of this plot to CR = 0~ gives the
inhibitor concentration that completely prevents binding of the
virus to the surface. This value represents the m; n; m~lm
inhibitor concentration required to effectively compete with
the sialoside surface. The value obtained, 2.5 + 0.83 mM per
4 HAUs of virus, is in agreement with the value of 2 ~ 1.1 mM
o~tained by a standard HAI assay and 2.8 ~ O.30 mM as obtained
by nuclear magnetic resonance spectroscopy
The inventive inhibition assay described here is direct
and easy to perform. This approach avoids the need for red
blood cells, which are used in the standard HAI assay. In
addition, the subjectivity of reading microliter plates in the
standard HAI assay is replaced by a quantitative
spectrophotometric method. This methodology could be applied
to screening for new drug candidates or binding ligands.
EXAMPLE 5
Non-specific Adhesion
This example illustrates the non-existence of non-specific
adhesion interfering with the assay of the invention.
In order to assess the CR due to non-specific adhesion,
two experiments were performed. In the first experiment, films
incorporating lactose lipid (15) (Figure 3) were incubated with
the influenza virus. Lactose was not a ligand for the
hemagglutinin lectin. Incubation with loO HAUs of the virus,
which is a concentration corresponding to a m~ ml~m response
in the sialoside films, show only a small effect (CR of 2 to
4~). In the second experiment, films containing sialoside lipid
13 were exposed to concentrated solutions of bovine serum
CA 02244098 1998-07-23
W O 97/27316 PCT~US97/01291
albumin. Again, the same small CR was observed. These
results indicate that non-specific adhesion of virus or protein
to the film surface does not produce the dramatic color change
observed from specific receptor-ligand binding.
EXAMPLE 6
Druq Develo~ment
This example illustrates the utility of the invention for
drug development.
A receptor and its reciprocal binding partner (receptor-
binding molecule) known to be involved in the physiological
regulation of interest were selected. The binding partner was
incorporated into the inventive film according to Example 1.
In the case of neurotransmission and neurological drug
development, for instance, a dopamine receptor was employed.
In the case of drug development toward pathogens such as
influenza, for instance, the viruses hemagglutinin receptor
were employed. The binding partner incorporated into the film
was dopamine, or a dopamine analog or sialic acid or sialic
acid analog, respectively. The goal of the assay was to select
a drug which interacts with the binding site in a way which
effects physiological functions.
When the described receptor was present, it caused a color
change when allowed to bind to the inventive membrane
incorporating the binding partner.
A candidate drug was then introduced into the system. If
the drug bound to the receptor or modified the binding
partner's binding capacity, there was a concomitant decrease
in the color change observed in the subject inventive membrane
due to competitive inhibition. The ability of the candidate
drug to influence binding was quantitated by observing the
degree of decrease in signal as compared to the control.
Variations of the above approach are used to suit
different systems. In some cases it is necessary to organize
the receptors into large assemblages such as incorporation into
polymers, liposomes or membranes. This arrangement amplifies
the film changes when the receptors bind. However, when a
candidate drug is introduced which binds the receptor as above,
CA oi244098 1998-07-23
W O 97/27316 PCT~US97/01291
36
there is a concomitant decrease in the observed color change.
Another variation that is applicable to this invention
requires that the receptor portion be attached to the ~ilm.
A known receptor is covalently attached to the film at one or
more points. This can be accomplished ~y appending the
receptor to the monomer prior to film polymerization (as in the
foregoing example for binding partners) or a~ter the ~ilm is
polymerized through modification of the film's surface.
Binding partners then interact with the immobilized receptors
distorting the ~ilm, giving rise to the color change. In this
way, test candidates can be directly screened with receptors
giving a positive ~ilm response rather than absence of color
change, as in the previous variation.
EXAMPLE 7
Generalized A~roach
This example illustrates general utility o~ the invention.
Generic film is made where the binding partner is kept
constant and an intermediate linking moiety is varied to
accommodate an ancillary novel binding partner.
A film which has biotin on its surface binds the protein
streptavidin. This protein is tetravalent, therefore in its
bound ~tate it still has one or more sites available for biotin
binding. Novel binding partners are derivatized with biotin
to attach themselves to the ~ilm surface through the
intermediacy of the streptavidin protein. Only a single
biotinylated ~ilm is prepared, and built up in a sandwich
fashion with streptavidin, the biotinylated test binding
partner. Exposure of this assembly to the test receptor gives
the desired color change. As in the previous example
competitive assays are performed to identify new drug
candidates.
EXAMPLE 8
.ntra~ment and Detection of Small Orqanic Molecules
This example shows the development o~ a new class of
functional materials which specifically trap small organic
compounds and report the entrapment event by a colorimetric
change which can be detected visually. These materials act as
-
CA 02244098 l998-07-23
W O 97/273~6 PCTAUS97/01291
37
simple color-based sensor devices which detect the presence of
compounds such as solvents or other toxic pollutants in air or
water streams.
The first step involves the synthesis of lipid diacetylene
analogs of compounds 1 and 2 a seen in Reaction Scheme 1 by
elaborating the secondary methyl group into the orientation
group. The enantiometrically pure ester of pentacosadiynoic
acid 3 (PDA) is hydroxylated via molydenum peroxide oxidation
to alcohol 4. Diasteriomers are separated and the ester is
hydrolyzed to chiral lactate analogs 5 and 6. The ethyl esters
are formed and treated with Grignard reagents to give the
desired chiral lipid analogs 7 and 8. Variation in the R
groups result in a wide variety of new materials in which the
specific entrapment capabilities are reviewed.
The monomer-lipid clathrate is ordered and compressed on
the water surface using a Langmuir-Blodgett film apparatus.
Polymerization of-the monolayer by W irradiation yields the
blue colored material as previously described. The is lifted
onto a hydrophobized microscope slide. The ability of films
of 7 and 8 to entrap dioxane and l-butanol and to undergo the
expected color transition was tested. Because the technique
can be generalized, appropriate derivatives of 1 and 2 are
selected and tuning of the chemistry to specifically detect a
particular small molecule is determined. To date, little is
known about why the materials 1 and 2 are highly selective to
dioxane and l-butanol, respectively. By ~m; ni ng a series of
compounds, a variety of solvents was screened using the
colorimetric detection.technique to determine which solvent
forms the most suitable guest compound. By using computer
modeling, cavities were engineered to be of the specific size
and shape to bind to analyte molecules. The uncomplexed and
complexed film with a variety of standard surface techniques
were fully characterized. These include XPS, Auger, Leed,
ellipsometry, Raman spectroscopy and ST~. All of these enabled
3~ determination of the structural re~uirements of the clathrates
in order to rationally design new materials with specific
clathration properties.
CA 02244098 1998-07-23
W O 97/27316 PCTnUS97/01291
38
EXAMPLE 9
Petection of Malaria Merozoites
This example describes the ~ilms and conditions used for
detection of malaria merozoites.
The films contained sialic acid and were prepared
identically to those described in Example 1. The films were
exposed to erythrocyte cont~; n i ng solutions of malaria
merozoites. After overnight exposure to the pathogens the
~ilms became pink in color. The color response (CR) in each
lo case was nearly 100~.
CA 02244098 1998-07-23
W O 97/27316 PCTrUS97/01291
3g
t
~ ~ ,
u r c
a t
u
._
a ~ t,~l
rA. u '¢ ~ ~ t u
~eC r ~: ~ c
, -- t _ u ,, ~0 a
. o ~4 ~ ' a ~ ~ a 7 - ~ : u
C~ ~ U~11 rJ ~ _ ,A
~J A,~ rCS V _ r~ t
t ~ ~ c ~ o u r x ., u ~A~ a
~ A C C t A .
S.l U _ ~ ¢ . U 1~ ¢ U U O U U U U U U U
~, I 0-- r~.~ t; ' C mO ~ C C C C C C C C C ~ C
~_ V ~ _ U ~ _ _ _ _ _ _ _ ~ _
.~ 4. ~ . O O ~ -- ¢ >~ O ¢ ~ ; ¢ ¢ ¢
r~ ~~' I I t7'0 U t7l u'.'u~u u u u'ui u C u t7''u
m ~- u~ rO
m ~, ~- _ m m ~- m m t~ _
,A; 4 _ , ¢ A m ~ _ , , ~ ~ ~ . O e
c ,. ,_ " _ _ Q ~n~, ~ _ ~ . _ _ ~ ~ ;
-- O O ~-- O ~ _ m ~ ~ ~. Q~
L~ r ~1. ~ ~ r I I . : C ~ ~ 1~. r L ~ U ~r A_ C 7-- r ~ I
CA 02244098 l998-07-23
W O 97/27316 PCTnUS97/01291
1. Nature, 318: 663 (1985)
2. Science, 215: 182 (1982)
3. Proc. Natl. Acad. Sci. USA, 81: 4510 (1984)
4. ~. of Biol. Chem., 265: 12293 (1990)
5 5. Proc. Natl. Acad. Sci. USA, 82: 1494 (1985)
6. ~ature, 344: 70 (1990)
7. J. of Ne~roscience Research, 18: 102-107 (1987)
8. FEBS Letters, 211: 17-22 (1987)
9. Cell, 56: 855-865 (1989)
10 10. Cell, 56: 839-842 (1989)
11. Cell, 56: 849-853 (1989)
12. Nature, 333: 426-431 (1988)
13. Proc. Natl, Acad. Science, USA, 85: 7743-47 (1988)
14. Nature, 312: 763-770 (1985)
15 15. Cell, 56: 725-728 (1989)
16. J. Virol, 63: 3991 (1989)
17. Inas, 86: 10100 (1989)
18. Virol, 176: 337 (1990)
19. Med. Microbio. Imm., 179: 105 (1990)
20 20. In~ect. Imm., 24: 65 (1979)
21. Proc. Soc. EXP. Bio Med, 162: 299 (1979)
22. Virol, 172: 386 (198g)
23. J. Clin. Inv, 86: 2569 (1990)
24. J. Virol, 64: 2569 (1990)
25 25 Science, 248: 1410 (lsso)
26. Febs ~ett., 277: 253 (1990)
27. Infec. Imm., 57: 2378 (1989)
28. Microb. Lett., 57: 65 (1989)
29. In~ect. Imm., 40: 1060 (1990)
30 30. Infect. Imm., 25: 940 (1983)
31. Med. Vixol, 8: 213 (1989)
32. J. Virol, 64: 2582 (1990)
CA 02244098 1998-07-23
W O 97127316 PCT~US97/01291
41
~. CD l
_ ~ I
lll
~ I .
O 0 111
I T
~ ~ I
D ~ N
a~ -- _
~IllO ~ ~ I ~
D
T ~
I I
I I
,~ ,~, T :D
G11l1 1111 I /~I
D111 111 -- O
D~ o