Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
-~ CA 02244136 1998-07-23
~.
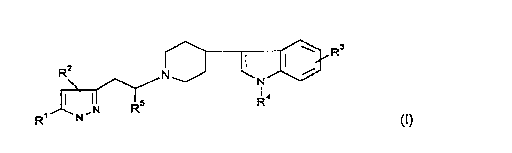
1-Pyrazol-3-ylethyl-4-indol-3-ylpiperidines
The invention relates to novel l-pyrazol-
3-ylethyl-4-indol-3-ylpiperidine derivatives o~ the
formula I
R~ ~ R-
in which
R1 is H or A
R2 is H, phenyl which is mono- to trisubstituted by
Hal, NO2, CoN(R4)2, So2N(R4)2, cyano, A or R4-o
R3 is H, Hal, A, A-O-, amino, cyano, carboxamide, NO2,
S~2N(R )2
R4 is H or A,
R5 is H or A,
A is alkyl having 1-6 C atoms or an alkyl having 1-6
C atoms, which is mono- to trisubstituted by
fluorine
Hal is F, Cl, Br or I
and their salts, which have proved to be substances
having pharmaceutically advantageous actions.
A large number of medicaments for the treatment
of diseases which are caused by malfunctions or
disorders of the central nervous system are known from
the technical and patent literature. The majority of
these medicaments, however, either have serious side
effects or a relatively non-specific spectrum of
action.
The invention was therefore based on the object
of making available novel compounds which as
medicaments act sel -tively on the central nervous
system, but at the same time are low in side effects
and have no dependence potential or only a very low
dependence potential.
~, CA 02244136 1998-07-23
-- 2
It was also an object of the invention to make
available a process whereby the appropriate compounds
can be prepared in the highest possible yields and high
purities.
These objects were achieved by the present
invention.
It has now been found that compounds of the
formula I in which the radicals Rl - R5, A and Hal have
the meanings given, and their physiologically
acceptable salts have a broad spectrum of useful
pharmacological properties They thus show, in
particular, actions on the central nervous system,
especially dopamine-stimulating (anti-Parkinson) and
serotonin-agonistic and -antagonistic actions.
Specifically, the compounds of the formula I induce
contralateral pivoting behaviour in hemiparkinson rats
(detectable by the method o~ Ungerstedt et al., Brain
Res. 24, (1970), 485-493). They inhibit the binding of
tritiated dopamine agonists and antagonists to striatal
receptors (detectable by the method of Schwarcz et al.,
J. Neurochemistry 34, (1980), 772-778 and Creese et
al., European J Pharmacol. 46, (1977), 377-381) and
the binding of tritiated serotonin ligands to
hippocampal receptors (Cossery et al., European J.
Pharmacol 140, (1987), 143-155). Moreover, changes in
DOPA accumulation in the striatum and 5-HTP
accumulation in the n. raphe occur (Seyfried et al.,
European J. Pharmacol. 160, (1989), 31-41).
Additionally, the compounds inhibit the glossomaxillary
reflex in the anaesthetized rat (detectable following
the method of Barnett et al., European J. Pharmacol.
21, 81973), 178-182, and of Ilhan et al., European J.
Pharmacol. 33 (1975), 61-64). Analgesic and hypotensive
ef~ects also occur; thus in catheterized conscious,
spontaneously hypertensive rats (strain
SHR/Okamoto/NIH-MO-CHB-Kisslegg; method cf. Weeks and
Jones, Proc. Soc. Exptl. Biol. Med. 104, (1960), 646-
648) the directly measured blood pressure after oral
administration of the compounds is lowered.
. CA 02244136 1998-07-23
., t
The invention further relates to a process for
the preparation of compounds of the given formula I and
o~ their salts, characterized in that a compound o~ the
formula II
Rl ~ (11 )
in which
Rl R~ and R5 have the abovementioned meanings and
Z is Hal, O-SO2CH3, O-SO2CF3, OSO2-C6H4 or O-
S~2 ~ C6Hs ~
is reacted with a compound of the formula III
HN~ ~NJ3'
R4 (
in which
R3 and R4 have the abovementioned meanings,
or in that
a compound of the formula IIa
~OH
1~N 1 o (lla)
in which
Rl and R2 have the meanings indicated above, is
converted into an activated form, then reacted wit~ a
compound of the formula III under conditions such as
are known for the formation of peptide bonds, and the
desired compound of the formula I is formed from the
compound thus obtained by a reduction reaction,
, ~ CA 02244136 1998-07-23
L
- 4 -
and/or in that by treating with a strong base compounds
of the formula I are liberated as free bases,
and/or in that a base of the formula I is converted
into the associated acid addition salt using an acid.
Compounds of the formula I can have a chiral
centre Appropriate compounds of the formula I can
occur in several enantiomeric forms. All these forms
(e.g. D- and L-forms) and their mixtures (e.g. the DL-
forms) are included in the formula I.
Above and below, the radicals or parameters Rl
to Rs, A and Hal have the meanings indicated in the
formulae I to III, i~ not expressly stated otherwise.
If several marked identically groups are present in the
molecule, they can assume different definitions
independently of one another.
In the above formulae, the group A has 1 to 6,
preferably 1, 2, 3 or 4, C atoms. Speci~ically, A is
preferably methyl, ethyl, propyl, isopropyl, butyl,
isobutyl, sec-butyl or tert-butyl, furthermore also
pentyl, 1-, 2- or 3-methylbutyl, 1,1-, 1,2- or
2,2-dimethylpropyl, 1-ethylpropyl, hexyl, 1-, 2-, 3- or
4-methylpentyl. The group A is furthermore a straight-
chain or branched alkylene group having 1 to 6, in
particular having 1 to 4 C atoms, preferably methylene
or ethylene, but also, for example, ethylidene,
trimethylene, -CH(CH3)CHz-~ -CHzCH(CH3)-~ -c(cH3)z-
~
propylidene, tetramethylene, -CH(CH3)-(CH2) 2-
-CH2-CH(CH3)-CHz-, -(CHz)2-CH(CH3)-, -C(CH3)z-CHz-
~-CHz~C(CH3) 2 - / - CH(CH2CH3)-CH2-/ -CHzCH(CH2CH3)-,
-CH(CH2CH2CH3)-, -C(CH3)(CH2CH3)-, -CH(CH3)CH(CH3)- or
-CH[CH(CH3) 2] - . This group, however, can also be the
corresponding alkyl group having 1 to 6 C atoms, which
is mono- to trisubstituted by fluorine.
Accordingly, the group R4-o-, if R4 is A, is in
particular a straight-chain or branched alkylene group
having 1 to 6, in particular having 1 to 4, C atoms
., CA 02244136 1998-07-23
- 5 -
bonded via an oxygen atom. A-O is pre,erably methoxy,
ethoxy, 1~ or 2-propoxy, l-butoxy, isobutoxy, sec-
butoxy or tert-butoxy.
A group designated by phenyl is preferably an
unsubstituted phenyl. A substituted phenyl group is
preferably monosubstituted. Such a phenyl group,
however, can also be di- or trisubstituted, it being
possible for the substituents to be identical or
different. Preferred substituents are F, Cl, methoxy
and OH. Possible substituents, however, are also NO2,
cyano, A or A-O, it being possible ~or A and A-O- to
have the abovementioned meanings.
Speci~ically, a phenyl group is pre~erably o-,
m- or p-fluorophenyl, o-, m- or p-chlorophenyl, o-, m-
or p-methoxyphenyl, o-, m- or p-ethoxyphenyl, 2, 3-,
2,4-, 2,5-, 2,6-, 3,4- or 3,5-dimethoxyphenyl,
3-hydroxy-4-methoxyphenyl, 3-methoxy-4-hydroxyphenyl,
2,3-, 2,4-, 2,5-, 2,6-, 3,4- or 3,5-dihydroxyphenyl,
2,3- or 3,4-methylenedioxyphenyl.
Hal corresponds to a halogen radical and can be
fluorine, chlorine, bromine or iodine. It is
particularly pre~erably ~luorine or chlorine.
Rl is preferably a hydrogen or has the meanings of A.
R2 is preferably hydrogen or a phenyl which is mono-,
di- or trisubstituted by a Hal, A or R4-o-, R4
pre~erably having the meaning o~ A, but in
particular also a phenyl mono-, di- or
trisubstituted by CoN(R4) 2 or So2N(R4) 2 ~
R3 is preferably a hydrogen, a radical having the
meaning of A, in particular methyl or ethyl, or
having the meaning of R4-o, i.e. preferably methoxy
~r ethoxy. Particularly preferably, it has the
meaning of a cyano, carboxamido or nitro group. In
particular, it can also be an optionally
substituted sulfonyl group. Possible substituents
, CA 02244136 1998-07-23
of this sulfonyl group are Hal, A, or A which is
mono- to trisubstituted by Hal.
R4 is preferably hydrogen, but can also optionally
have the meanings of A, in particular it is a
methyl or ethyl radical.
Rs is, like R4, preferably hydrogen, but can also
optionally have the meanings of A, in particular
it is a methyl or ethyl radical.
Among the compounds of the formula I, those are
preferred in which at least one o~ the radicals
indicated has one of the preferred meanings indicated.
Some groups of preferred compounds are those of the
formulae Ia to Id, which correspond to the formula I,
but in which
in Ia R1 is hydrogen or methyl;
in Ib R1 is hydrogen or methyl and
R2 is hydrogen or a phenyl which is mono- to
trisubstituted by Hal,
in Ic R1 is methyl and
R3 is hydrogen, fluorine, hydroxyl, cyano,
carboxamide, ethyl, methoxy or a
trifluoromethylsulfonyloxy,
in Id R1 is methyl and
R4 is hydrogen or methyl.
The compounds of the formula I and also their
starting compounds are prepared by methods known per se
to the person skille' in the art, such as are described
in the literature (e.g. in the standard works such as
Houben-Weyl, Methoden der organischen Chemie [Methods
of Organic Chemistry], Georg Thieme Verlag Stuttgart),
namely under reaction conditions which are known and
'j CA 02244136 1998-07-23
-- 7
suitable for the reactions mentioned. Use can also be
made in this case o~ variants which are known per se,
but not mentioned here in greater detail.
The starting substances can, if desired, also
be ~ormed in situ, such that they are not isolated ~rom
the reaction mixture, but immediately reacted further
to give the compounds of the formula I
Starting compounds ~or the preparation o~
compounds of the formula I can be obtained by
liberating them from their functional derivatives by
solvolysis, in particular hydrolysis, or by
hydrogenolysis. Compounds of the formula I are
preferably obtained by coupling reactions known to the
person skilled in the art of the compounds o~ the
formulae II or IIa with those of the formula III, if
appropriate a~ter selective hydrogenation. If possible,
the synthesis o~ compounds o~ the ~ormula I is carried
out such that a solvolysis is unnecessary for the
liberation o~ the desired compound o~ the ~ormula I,
especially as compounds of this structure are
frequently unstable under such conditions.
Pre~erred starting substances ~or the
solvolysis or hydrogenolysis are those which otherwise
correspond to the abovementioned formulae, but instead
o~ ~ree amino and/or hydroxyl groups contain
corresponding protected amino and/or hydroxyl groups,
pre~erably those which, instead of an H atom which is
bonded to an N atom, carry an amino protective group,
in particular those which, instead of an HN group,
carry an R'-N group in which R' is an amino protective
group, and/or those which, instead of the H atom o~ a
hydroxyl group, carry a hydroxyl protective group, e.g.
those which, instead o:E a group -COOH, carry a group
-COOR", in which R~ is a hydroxyl protective group.
Several - identical or dif~erent - protected
amino and/or hydroxyl groups can also be present in the
molecule of the starting substance. If the protective
groups present are different from one another, in many
cases they can be removed selectively.
CA 02244136 1998-07-23
_ 8 -
The expression ~amino protective group" is
generally known and relates to groups which are
suitable for protecting (for blocking) an amino group
~rom chemical reactions, but which are easily removable
after the desired chemical reaction has been carried
out at another position in the molecule. Typical of
such groups are, in particular, unsubstituted or
substituted acyl, aryl (e.g. dinitrophenyl (DNP),
aralkoxymethyl (e.g. benzoxymethyl (BOM)) or aralkyl
groups (e.g. benzyl, 4-nitrobenzyl, triphenylmethyl).
As the amino protective groups are removed a~ter the
desired reaction (or reaction se~uence), their nature
and size is otherwise uncritical; however, those having
1-20, in particular 1-8, C atoms are preferred. The
expression "acyl group" is to be interpreted in the
widest sense in connection with the present process. It
includes acyl groups derived from aliphatic,
araliphatic, aromatic or heterocyclic carboxylic acids
or sul~onic acids, and in particular alkoxycarbonyl,
aryloxycarbonyl and especially aralkoxycarbonyl groups.
Examples o~ acyl groups of this type are alkanoyl such
as acetyl, propionyl, butyryl; aralkanoyl such as
phenacetyl; aroyl such as benzoyl or tolyl;
aryloxyalkanoyl such as phenoxyacetyl; alkoxycarbonyl
such as methyoxycarbonyl, ethoxycarbonyl; 2,2,2-tri-
chloroethoxycarbonyl, isopropoxycarbonyl, tert-
butoxycarbonyl (BOC), 2-iodoethoxycarbonyl; aralkyloxy-
carbonyl such as benzyloxycarbonyl (CBZ), 4-methoxy-
benzyloxycarbonyl, 9-fluorenylmethoxycarbonyl (FMOC).
Preferred amino protective groups are BOC, DNP and BOM,
furthermore CBZ, benzyl and acetyl.
The expression ~'hydroxyl protective group is
likewise generally known and relates to groups which
are suitable for protecting a hydroxyl group from
chemical reactions, but which are easily remov~ble
after the desired chemical reaction has been carried
out at another position in the molecule. Typical of
such groups are unsubstituted or substituted aryl,
aralkyl or acyl groups, ~urthermore also alkyl groups.
CA 02244136 1998-07-23
The nature and size of the hydroxyl protective groups
is not critical, as after the desired chemical reaction
or reaction sequence they are removed again; groups
having 1-20, in particular 1-10, C atoms are preferred.
Examples of hydroxyl protective groups are, inter alia,
tert-butyl, benzyl, p-nitrobenzyl, p-toluenesulfonyl
protective groups and acetyl, benzyl and acetyl being
particularly preferred.
The functional derivatives of the compounds of
the ~ormula I to be used as starting substances can be
prepared by the customary methods, such as are
described, for example, in the appropriate standard
works and the relevant patent literature, ~or example
by reaction of compounds which correspond to the
formulae II and III, but in which at least one of these
compounds carries a protective group instead of an H
atom.
The liberation both of the compounds of the
~ormula II, IIa or III and, i~ appropriate, of the
compounds of the formula I from their functional
derivatives takes place - depending on the protective
group used - e.g. with strong acids, expediently with
trifluoroacetic acid or perchloroacetic acid, but also
with other strong inorganic acids, such as hydrochloric
acid or sulfuric acid, strong organic carboxylic acids,
such as trichloroacetic acid or sulfonic acids, such as
benzene- or p-toluenesulfonic acid, the presence of an
additional solvent is possible, but not always
necessary.
Suitable inert solvents are preferably organic,
for example carboxylic acids such as acetic acid,
ethers such as tetrahydro~uran (THF) or dioxane, amides
such as dimethylformamide (DMF), furthermore also
alcohols such as methanol, ethanol or isopropanol and
also water. Mixtures of the abovementioned solvents are
furthermore possible. Trifluoroacetic acid is
preferably used in excess without addition of a further
solvent, perchloric acid in the form of a mixture o~
acetic acid and 70 ~ perchloric acid in the ratio 9:1.
, CA 02244136 1998-07-23
I~
-- 10
The reaction temperatures for the removal o~ protective
groups are expediently between approximately 0 and
50~C; the reaction is pre~erably carried out between 15
and 30~C (room temperature).
The BOC group can be removed, ~or example,
preferably using 40 ~ trifluoroacetic acid in
dichloromethane or using approximately 3 to 5 N HCL in
dioxane at 15 to 60~C, the FMOC group using an
approximately 5 to 20 ~ solution of dimethylamine,
diethylamine or piperidine in DMF at 15 to 50~C.
Removal o~ the DNP group is carried out, for example,
also using an approximately 3 to 10 ~ solution of
2-mercaptoethanol in DMF/water at 15 to 30~C.
Hydrogenolytically removable protective groups
(e.g. BOM, CBZ or benzyl) can be removed, for example,
by treating with hydrogen in the presence of a catalyst
(e.g. of a noble metal catalyst such as palladium,
expediently on a support such as carbon). Suitable
solvents in this case are those indicated above, in
particular, ~or example, alcohols such as methanol or
ethanol or amides such as DMF. The hydrogenolysis is
generally carried out at temperatures between 0 and
approximately 100~C and pressures between approximately
1 and 200 bar, preferably at 20 to 30~C and 1 to 10
bar. Hydrogenolysis o~ the CBZ group is readily carried
out, for example, on 5 to 10 ~ Pd-C in methanol at 20
to 30~C.
Compounds of the formula I can pre~erably be
obtained by reaction of a pyrazole derivative of the
~ormula II with a compound of the formula III. Use is
expediently made here of the methods known per se for
the N-alkylation of amines.
The leaving group Z of the formula II is
preferably Cl, Br, I, C1- to C6-alkylsulfonyloxy, such
as ethane- or ethanesulfonyloxy, Cl- to C6-fluoro-
alkylsulfonyloxy, such as trifluoromethanesul~onyloxy
or C6-C10-arylsulfonyloxy such as benzene-, p-toluene-
or 1- or 2-naphthalenesul~onyloxy.
,~ CA 02244136 1998-07-23
The reaction is carried out in an inert
solvent, e.g. a halogenated hydrocarbon, such as
dichloromethane, trichloromethane or carbon
tetrachloride, an ether, such as THF or dioxane, an
amide such as DMF or dimethylacetamide, or a nitrile
such as acetonitrile. Suitable solvents are also
dimethyl sulfoxide, toluene or benzene It is also
possible, however, to use mixtures of these solvents.
This reaction can be carried out at temperatures
between approximately -10 and 200~C, preferably between
O and 120~C. Preferably, the reaction is carried out in
the presence of an additional base, e.g. of an alkali
metal or alkaline earth metal hydroxide or carbonate
such as sodium, potassium or calcium hydroxide, or
sodium, potassium or calcium carbonate. I~ the leaving
group Z is different from I, an addition o~ an iodide
such as potassium iodide is recommended.
The starting substances of the formula II can
be prepared by methods known from the literature.
The starting substances of the formula III can
be prepared by methods generally known to the person
skilled in the art, such as are described in handbooks
on indole chemistry. As protective groups of the
piperidine nitrogen, benzyl and BOC are preferred.
These protective groups are particularly preferred in
order to introduce a substituent designated by R4, which
is unequal to hydrogen, on the indole nitrogen. The
latter reaction takes place in particular in the
presence of a strong base, to be precise preferably
from the group NaH, KH, KOC(CH3)3 or n-, sec- or tert-
butyllithium. The removal of the "amino protective
group" is then carried out according to one of the
methods described above.
Starting substances of the formula IIa can be
prepared by method known from the literature, in
particular analogously to the following Example 5.
Coupling reactions of compounds of the general
formula IIa with compounds of the general formula III
can be carried out under conditions such as are known
_
CA 02244136 1998-07-23
- 12 -
~or the ~ormation o~ peptide bonds. Corresponding
methods are described, for example, in "Aminosauren,
Peptide, Proteine" [Amino Acids, Peptides, Proteins],
Jakubke, Hans-Dieter; Jeschkeit, Hans; Verlag Chemie,
Weinheim (1982), but also in Wunsch E. (1974),
"Synthese von Peptiden" [Synthesis of Peptides] in:
Houben-Weyl, ~Methoden der organischen Chemie" [Methods
of Organic Chemistry], Vol. 15, 1/2 (Ed., Muller, E.)
Georg Thieme Verlag, Stuttgart. These methods include,
inter alia, the azide method, the mixed anhydride
method using chlorocarbonic acid monoesters as
anhydride-forming agents, various activated ester
methods and the carbodiimide method, as well as its
modified form, the DCC additive process. From this
carbonyl compound obtained by the linkage reaction, the
desired compound of the ~ormula I can be liberated by
reduction under suitable conditions.
In particular, this is carried out in the
presence o~ a catalyst of the complex hydride group.
Preferably, this reaction is carried out in a solvent
from the ether group. Particularly preferably,
tetrahydro~uran is used as a solvent. In general, this
reduction is carried out under mild conditions at
temperatures from -78 to +66~C, preferably at room
temperature. In particular, this reduction can also be
carried out, however, using sodium bis(2-methoxy-
ethoxy)aluminium hydride (e.g. Vitride~). For this
purpose, the latter is employed in excess. As solvent,
in this case suitable ethers are preferred.
A base o~ the ~ormula I can be converted into
the associated acid addition salt using an acid. For
this reaction, possible acids are in particular those
which give physiologically acceptable salts. Thus
inorganic acids can be used, e.g. sulfuric acid, nitric
acid, hydrohalic acids, such as ~ ~drochloric acid or
hydrobromic acid, phosphoric acids, such as
orthophosphoric acid, sulfamic acid, furthermore
organic acids, in particular aliphatic, alicyclic,
araliphatic, aromatic or heterocyclic mono- or
~, . CA 02244136 1998-07-23
polybasic carboxylic, sulfonic or sulfuric acids, e.g.
formic acid, acetic acid, trifluoroacetic acid,
propionic acid, pivalic acid, diethylacetic acid,
malonic acid, succinic acid, pimelic acid, fumaric
acid, maleic acid, lactic acid, tartaric acid, malic
acid, citric acid, gluconic acid, ascorbic acid,
nicotinic acid, isonicotinic acid, methane- or
ethanesulfonic acid, ethanedisulfonic acid,
2-hydroxyethanesulfonic acid, benzenesulfonic acid, p-
toluenesulfonic acid, naphthalenemono- and disulfonic
acids, and laurylsulfuric acid. Salts with
physiologically unacceptable acids, e.g. picrates, can
be used for the isolation and/or purification of the
compounds of the formulae I.
If desired, the free bases of the formula I can
be liberated from their salts by treatment with strong
bases, such as sodium or potassium hydroxide, or sodium
or potassium carbonate.
As already pointed out above, the compounds of
the ~ormula I can contain one or more chiral centres
and can therefore be present in racemic or in optically
active form. Racemates which are obtained can be
separated into the enantiomers mechanically or
chemically by methods known per se. Preferably,
diastereomers are formed from the racemic mixture by
reaction with an optically active resolving agent.
Suitable resolving agents are, for example, optically
active acids, such as the D- and L-forms of tartaric
acid, diacetyltartaric acid, dibenzoyltartaric acid,
mandelic acid, malic acid, lactic acid or the various
optically active camphorsulfonic acids such as
~-camphorsulfonic acid.
Also advantageous is resolution of enantiomers
with the aid of a column packed with an optically
active resolving agent (e.g. dinitrobenzoylph~yl-
glycine). A suitable eluent in this connection is, for
example, a mixture of hexane/isopropanol/acetonitrile.
Of course, it is also possible to obtain
optically active compounds of the formula I according
CA 02244136 1998-07-23
to the methods described above by using starting
substances (e.g. those of the formula II) which are
already optically active.
The novel compounds o~ the ~ormula I and their
physiologically acceptable salts can therefore be used
as pharmaceutical active compounds for axiolytics,
antidepressants, neuroleptics, anti-Parkinson agents
and/or antihypertensives. They are of use for the
treatment and prophylaxis of anxiety states, for the
treatment of panic attacks, schizophrenia, delusional
obsessions, Alzheimer's disease, migraine, anorexia,
bulimia, sleep disorders and drug abuse or suitable ~or
the control of sequelae of cerebral infarcts but also
for the treatment of extrapyramidal motor side effects
of neuroleptics. However, they can also be used as
intermediates for the preparation of other
pharmaceutical active compounds
The compounds of the general formula I and
their physiologically acceptable salts can therefore be
used for the production of pharmaceutical preparations
by bringing them into suitable dose form together with
at least one excipient or auxiliary and, if desired,
with one or more other active compounds. The
preparations thus obtained can be employed as
pharmaceuticals in human or veterinary medicine.
Possible excipients are organic or inorganic substances
which are suitable for enteral (e.g. oral or rectal) or
parenteral administration and do not react with the
novel compounds, for example water, vegetable oils,
benzyl alcohols, polyethylene glycols, glycerol
triacetate and other fatty acid glycerides, gelatin,
soya lecithin, carbohydrates such as lactose or starch,
magnesium stearate, talc or cellulose.
Tablets, coated tablets, capsules, syrups,
juices or drops are used in particular for oral
administration. Especially of interest are coated
tablets and capsules having enteric coatings or capsule
shells. Suppositories are used for rectal
administration and solutions, preferably oily or
CA 02244136 1998-07-23
- 15 -
aqueous solutions, ~urthermore suspensions, emulsions
or implants, for parenteral administration.
The active compounds claimed according to the
invention can also be lyophilized and the lyophilizates
obtained used, for example, for the production of
injection preparations.
The preparations indicated can be sterilized
and/or contain auxiliaries such as preservatives,
stabilizers and/or wetting agents, emulsifiers, salts
for affecting the osmotic pressure, bu~er substances,
colourants and/or flavourings. If desired, they can
also contain one or more other active co~pounds, e.g.
one or more vitamins, diuretics or anti-inflammatories.
The compounds of formula I according to the
invention are generally administered in analogy to
other known commercially available preparations ~or the
indications claimed, preferably in doses between
approximately 1 mg and 50 mg, in particular between S
and 30 mg, per dose unit. The daily dose is pre~erably
between approximately 0.02 and 20 mg/kg, in particular
0.2 and 0.4 mg/kg, of body weight.
The specific dose for each individual patient
depends, however, on all sorts of factors, for example
on the efficacy of the specific compound employed, on
the age, body-weight, general state of health, sex, on
the diet, on the time and route of administration, on
the excretion rate, pharmaceutical substance
combination and severity of the particular disorder to
which the therapy relates. Oral administration is
preferred.
In the following, examples are given which are
used to illustrate the invention, but which do not
restrict the invention to the examples given.
All temperatures below are indicated in ~C.
CA 02244136 1998-07-23
- 16 -
Examples
Exam~le l
7-Ethyl-3-(1-benzyl-1,2,3,6-tetrahydro-4-pvridyl)indole
144 g (2.57 mol) of KOH are dissolved in 1.6 l
of methanol in a 2000 ml round-bottomed flask. 120 g
(approximately 0.86 mol) o~ 7-ethylindole and 161.4 g
(approximately 0.86 mol) of 1-benzylpiperidin-4-one are
then added to this solution. This reaction mixture is
stirred under reflux conditions ~or 5 hours. A ~urther
34.1 g (approximately 0.172 mol) of l-benzylpiperidin-
4-one are added and the mixture is stirred overnight
under reflux conditions. The reaction solution obtained
is concentrated in vacuo, the product being obtained in
crystalline ~orm. This crude product is recrystallized
from methanol.
Yield: 224 g of 7-ethyl-3-(1-benzyl-1,2,3,6-tetrahydro-
4-pyridyl)indole (~82 ~ of theory)
R~ 0.35 (ethyl acetate/petroleum ether 1:1)
Example 2
7-Ethyl-3-(4-piperidyl)indole
The 7-ethyl-3-(1-benzyl-1,2,3,6-tetrahydro-4-
pyridyl)indole obtained from Example 1 is dissolved in
a solvent mixture consisting of 2 l of methanol and
0.5 l of glacial acetic acid and hydrogenated at 22~C
in the course of 16 hours in the presence of a
palladium catalyst (Pd-C 5~). The catalyst is then
filtered o~f and the solvent is distilled off in vacuo.
The residue obtained is codistilled with toluene and
dissolved in 1 l of water. By the addition of sodium
hydroxide solution '32 ~), the pH of the solution is
rendered alkaline. The crystals precipitated in this
process are separated off and dried.
Yield: 153.6 g of 7-ethyl-3-(4-piperidyl)indole (- 95
of theory)
CA 02244136 1998-07-23
- 17 -
M.p. 189~C
Example 3
6-Methyl-2H-pyran-2,4-(3H)-dione
495 ml o~ sulfuric acid (90 ~) are heated to
130~C in a 2 l three-necked flask. 300 g of 3-acetyl-
3,4-dihydro-6-methylpyran-2,4-dione (dehydr~cet~c acid) are
added in small portions with stirring. The mixture is
then additionally stirred for some time After reaction
has taken place, the reaction mixture is poured onto
approximately 1500 g of ice. The crystals formed are
separated off.
Yield: 192 g of 3,4-dihydro-6-methylpyran-2,4-dione
(crude product) (85 ~ of theory)
Example 4
2-(5-MethYl-3-pyrazolyl)acethydrazide
191.6 g of 3,4-dihydro-6-methylpyran-2,4-dione
are dissolved in 800 ml of methanol. 190 g of NH2NH2 H2O
are added dropwise to this solution with stirring, the
temperature climbing to 70~C. Following the reaction,
the product formed is filtered off and further
processed as a crude product.
Yield: 193 g of 2-(5-methyl-3-pyrazolyl)acethydrazide
(82 % of theory)
M.p.: 153-154~C
Example 5
2-(5-Methyl-3-pyrazolyl)acetic acid
105 g of 2-(5-methyl-3-pyrazolyl)acethydrazide
and 720 ml of 2N sodium hydroxide solution are stirred
with one another and the mixture is heated under reflux
conditions for 4 hours. The reaction solution thus
~, CA 02244136 1998-07-23
L
- 18 -
obtained is then neutralized with hydrochloric acid.
After this, the reaction solution is concentrated in
vacuo. Crystals precipitating in the course of this are
separated o~ and ~urther processed directly as a crude
product.
Yield: 202 g of 2-(5-methyl-3-pyrazolyl)acetic acid
(crude product)
Example 6
EthYl 2-(5-methYl-3-pvrazolyl)acetate
95 g of 2-(5-methyl-3-pyrazolyl)acetic acid and
660 g of ethanol are treated with 74 ml of thionyl
chloride in a 2 l ~lask and stirred at room temperature
for approximately 72 hours and then allowed to stand
for a further 48 hours. The reaction solution is
concentrated under vacuum conditions. The residue
obtained is taken up in a solvent mixture consisting of
ethyl acetate/methanol in the ratio 2:1, and heated
under reflux conditions, the product going into
solution, but not by-products and sodium chloride. The
filtered mother liquor is concentrated in vacuo, the
product being obtained in crystalline form.
Yield: 141 g of ethyl 2-(5-methyl-3-pyrazolyl)acetate
(crude product)
ExamPle 7
2-(5-MethYl-3-Pyrazolyl)ethanol
100 g of ethyl 2-(5-methyl-3-pyrazolyl)acetate
(crude product) are suspended in 2 l of ethanol.
146.6 g of NaBH4 are added in portions to this
suspension with stirring. This reaction mixture is
stirred at room temperature for approximately
192 hours. Approximately 150 ml of water and 65 ml of
glacial acetic acid are added and the ethanol is
distilled off. The pasty residue obtained is taken up
CA 02244136 1998-07-23
in ethyl acetate and extracted several times with
water. The organic phase is dried, filtered and
concentrated under vacuum conditions. Further product
is separated o~ ~rom the aqueous phase by neutralizing
and extracting with ethyl acetate.
Yield: 49 g of 2-(5-methyl-3-pyrazolyl)ethanol
(- 75 ~ o~ theory)
Example 8
2-(5-MethYl-3-pyrazolyl)ethYl chloride
49 g o~ 2-(5-methyl-3-pyrazolyl)ethanol are
suspended in 78 ml of toluene and heated to reflux.
59.5 g o~ phosphorus oxychloride are added to this
suspension slowly in the course of 2 hours, a strongly
exothermic reaction taking place. After addition has
ended, the mixture is heated under reflux for a further
two hours. The reaction solution is then allowed to
stand at room temperature for at least 12 hours. The pH
o~ the solution is then adjusted to g using sodium
carbonate and it is extracted at least three times with
ethyl acetate. The combined ethyl acetate phases are
dried over magnesium sulfate, the latter is filtered
off and the solvent is distilled off down to dryness in
vacuo. As a residue, an oil is obtained which cannot be
completely crystallized. This crude product is puri~ied
by chromatography (silica gel 60; ethyl
acetate/petroleum ether 9:1).~0 Yield: 39.4 g of 2-(5-methyl-3-pyrazolyl)ethyl
chloride (~ 70 ~ of theory)
ExamPle 9
7-Ethyl-3-(1-(2-(5-methYl-3-pyrazolyl)ethyl)-4-
piPeridyl)indole
2.28 g (0.01 mol) of 7-ethyl-3-(4-piperidyl)-
indole (Example 2) and 1.44 g (0.01 mol) of 2(5-methyl-
., CA 02244136 1998-07-23
- 20 -
3-pyrazolyl)ethyl chloride (Example 8) are initially
introduced into 125 ml o~ acetonitrile in a 250 ml
round-bottomed flask and stirred under reflux
conditions for approximately 48 hours. The precipitate
~ormed during this time is separated of~. The
precipitate here is unreacted starting material. The
solvent of the reaction solution thus obtained is
distilled off and the residue obtained is separated by
chromatography (silica gel 60, ethyl acetate/methanol
3:2). A~ter concentrating the product-containing
fraction, the reaction product is precipitated as the
oxalate, separated off and dried.
Yield: 1.3 g o~ 7-ethyl-3-(1-(2-(5-methyl-
3-pyrazolyl)ethyl)-4-piperidyl)indole oxalate
(2 9 ~ of theory)
R~: 0.28 (ethyl acetate: methanol 2:1), amorphous
Example lQ
5-Fluoro-3-(1-(2-(3-(4-fluoroPhenyl)-5-methyl-
4-pyrazolyl)methylcarbonyl)-4-Piperidyl)indole
2.18 g (10 mmol) of 5-fluoro-3-(4-piperidyl)-
indole, 3.10 g (16 mmol) of 1-ethyl-3-(3'-dimethyl-
aminopropyl)carbodiimide hydrochloride and 2.70 g(20 mmol) of 1-hydroxybenzotriazole are taken up in
100 ml o~ dichloromethane and the mixture is stirred
for 1 hour. 2.34 g (10 mmol) of 2-(3-(6-fluorophenyl)-
5-methyl-4-pyrazolyl)acetic acid are then added and the
reaction solution thus obtained is stirred for
approximately 72 hours. The reaction mixture thus
obtained is extracted with sodium hydroxide solution
and then dried over magnesium sulfate. A~ter ~iltering
this solution, the solvent is distilled off in vacuo.
The -rude product thus obtained is not worked up but
directly used further in the next stage.
Yield: 6.5 g of 5-fluoro-3-(1-(2-(3-(4-fluorophenyl)-
5-methyl-4-pyrazolyl)methylcarbonyl)-
4-piperidyl)indole (crude product)
CA 02244136 1998-07-23
Example 11
5-Fluoro-3-(1-(2-(3-(4-fluorophenyl)-5-~ethyl-
4-~yrazolYl)ethYl)-4-piperidyl)indole
6.5 g (10 mmol) of 5-fluoro-3-(1-(2-(3-(4-
fluorophenyl)-5-methyl-4-pyrazolyl)methylcarbonyl)-4-
piperidyl)indole are dissolved in 100 ml of10 tetrahydrofuran and then treated with 5 ml (25 mmol) of
sodium bis(2-methoxyethoxy)aluminium hydride
(Vitride~). The reaction mixture thus obtained is
stirred at room temperature for two hours. After
reaction has ended, excess sodium bis(2-methoxyethoxy)-
aluminium hydride is destroyed by addition of water, acolourless gelatinous mass forming, which is filtered
off through kieselghur. The solvent is distilled off in
vacuo from the reaction solution thus obtained. By this
means 7.7 g of crude product are obtained, which is
purified by chromatography (silica gel 60; ethyl
acetate/petroleum ether 9:1).
Yield: 1.7 g o~ 5-fluoro-3-(1-(2-(3-(4-fluorophenyl)-
5-methyl-4-pyrazolyl)ethyl)-4-piperidyl)indole
(~ 40 ~ of theory)
4-~luoro-3(1-(2-(3-(4-fluorophenyl)-5-methyl-
4-pyrazolyl)ethyl)-4-piperidyl)indole was prepared
analogously.
ExamPle 12
5-Fluoro-1-methYl-3-(1-tert-butoxycarbonyl-
4-piPeridYl)indole
0.9 g (30 m,ol) of sodium hydride (80 ~) are
suspended in 300 ml of tetrahydrofuran. A solution
consisting of 9.55 g of 5-fluoro-3-(1-tert-butoxy-
carbonyl-4-piperidyl)indole and tetrahydrofuran is then
slowly added dropwise with cooling and additionally
. CA 02244136 1998-07-23
stirred ~or approximately one hour 1.9 ml (30 mmol) o~
methyl iodide are then added dropwise and the mixture
is additionally stirred ~or approximately two hours.
The solution is concentrated in vacuo and the residue
is taken up in ethyl acetate, extracted with water and
then dried over magnesium sul~ate. After filtering, it
is again concentrated down to a residue in vacuo The
crude product thus obtained is purified by
chromatography (silica gel 60; petroleum ether/ethyl
acetate).
Yield: 6 7 g of 5-~luoro-1-methyl-3-(1-tert-butoxy-
carbonyl-4-piperidyl)indole (~ 67 ~ of theory);
oil
ExamPle 13
5-Fluoro-1-methYl-3-(4-piperidyl)indole
6.7 g (20 mmol) of 5-fluoro-1-methyl-3-(1-tert-
butoxycarbonyl-4-piperidyl)indole are stirred for one
hour in 150 ml o~ a hydrochloric acid/ether mixture
(considerable evolution of gas). This solution is then
concentrated in vacuo.
Yield: 5.0 g of 5-fluoro-1-methyl-3-(4-piperidyl)-
indole (~ 93 ~ of theory)
The following compounds were prepared
analogously to Example 9 or 11:
3-(1-(2-(5-methyl-3-pyrazolyl)ethyl)-4-piperidyl)-
indole-5-carbonitrile
M.p. 112-114~C
3-(1-(2-(5-methyl-3-pyrazolyl)ethyl -4-piperidyl)-
indole-5-carboxamide
M.p. 130-131.5~C
-
CA 02244136 1998-07-23
- 23 -
4-fluoro-3-(1-(2-(5-methyl-3-pyrazolyl)ethyl)-
4-piperidyl)indole, R~ 0.31, ethyl ether:methanol 2:1
5-fluoro-3-(1-(2-(5-methyl-3-pyrazolyl)ethyl)-
5 4-piperidyl)indole, R~ 0.38, ethyl ether:methanol 2:1
3-(1-(2-(5-methyl-3-pyrazolyl)ethyl)-4-piperidyl)-
indole, Rf 0.40, ethyl ether:methanol 2:1
4-fluoro-3-(1-(2-(3-(4-fluorophenyl)-5-methyl-
4-pyrazolyl)ethyl)-4-piperidyl)indole, R~ 0.52, ethyl
ether:methanol 2:1
5-~luoro-3-(1-(2-(3-(4-fluorophenyl)-5-methyl-
4-pyrazolyl)ethyl)-4-piperidyl)indole, Rf 0.53, ethyl
ether:methanol 2:1
5-fluoro-1-methyl-3-(1-(2-(5-methyl-3-pyrazolyl)ethyl)-
4-piperidyl)indole, m.p. 223~C
6-fluoro-3-(1-(2-(5-methyl-3-pyrazolyl)ethyl-
4-piperidyl)indole, R~ 0.62, ethyl ether:methanol 2:1
6-methoxy-3-(1-(2-(5-methyl-3-pyrazolyl)ethyl)-
4-piperidyl)indole, Rf 0.44, ethyl ether:methanol 2:1
7-methoxy-3-(1-(2-(5-methyl-3-pyrazolyl)ethyl)-
4-piperidyl)indole, Rf 0.30, ethyl ether:methanol 2:1
3-(1-(2-(5-methyl-3-pyrazolyl)ethyl)-1,2,3,6-tetra-
hydro-4-pyridyl)indole-5-carbonitrile
3-(1-(2-(5-methyl-3-pyrazolyl)ethyl)-1,2,3,6-tetra-
hydro-4-pyridyl)indole-5-carboxamide
The ~ollowing examples relate to pharmaceu~;cal
preparations:
CA 02244l36 l998-07-23
- 24 -
Example A: injection vials
A solution of 100 g of an active compound of
the ~ormula I and 5 g of disodium hydrogen phosphate is
adjusted to pH 6.5 in 3 l of double-distilled water
using 2N hydrochloric acid, sterile filtered, dispensed
into injection vials, lyophilized under sterile
conditions and aseptically sealed. Each injection vial
contains 5 mg of active compound.
Exam~le B: suppositories
A mixture of 20 g of an active compound o~ the
formula I is fused with 100 g of soya lecithin and
1400 g o:E cocoa butter, poured into moulds and allowed
to cool. Each suppository contains 20 mg of active
compound.
Example C: solution
A solution of 1 g of an active compound o~ the
formula I, 9.38 g of NaH2PO4 2H2O, 28.48 g of
Na2HP04-12H20 and 0.1 g of benzalkonium chloride in
940 ml of double-distilled water is prepared. The
solution is adjusted to pH 6.8, made up to 1 l and
sterilized by irradiation.
Example D: ointment
500 mg of an active compound of the formula I are
mixed with 99.5 g of petroleum jelly under aseptic
conditions.
Example E: tablets
A mixture of 1 kg of active compound of the
formula I, 4 kg of lactose, 1.2 kg of potato starch,
0.2 kg of talc and 0.1 kg of magnesium stearate is
CA 02244136 1998-07-23
- 25 -
compressed in a customary manner to give tablets such
that each tablet contains 10 mg of active compound.
Exam~le F: coated tablets
Analogously to Example E, tablets are pressed
which are then coated in a customary manner with a
coating o~ sucrose, potato starch, talc, tragacanth and
colourant.
Example G: capsules
2 kg of active compound o~ the ~ormula I are
~illed into hard gelatin capsules in a customary manner
such that each capsule contains 20 mg o~ the active
compound.
Example H: ampoules
A solution o~ 1 kg o~ active compound of the
~ormula I in 60 l o~ double-distilled water is sterile
~iltered, dispensed into ampoules, lyophilized under
sterile conditions and aseptically sealed. Each ampoule
contains 10 mg o~ active compound.