Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02246~28 1998-08-14
W O 97129g92 PCTIAU97/00073
-- 1 --
RED MUD PROCESSING
FIELD OF THE INVENTION
The present invention relates to a method for
recovering soda and/or alumina values from red mud
produced in the Bayer process for extracting alumina from
bauxite.
The Bayer process was first developed in 1888 and
presently accounls for over 90~ of the world's alumina
production. The process utilises a digestion solution at
elevated temperat:ure to digest alumina :in bauxite. The
digestion solution is typically caustic soda but other
solutions in which alumina can be dissolved may be used.
For example, the digestion solution may be potassium
hydroxide or ammonium hydroxide.
Throughout this specification the expression "a
Bayer process" i'3 to be understood to mean a process as
described in the preceding paragraph.
To digest the alumina, the ~austic soda solution is
typically at a temperature in the range of 150-280~C,
with the temperature used being largely dependent upon
the nature of the bauxite. The alumina rich liquor phase
is separated from undissolved impurities by settling and
alumina is recovered from the li~uor phase by
precipitation of aluminium hydrate crystals. The
aluminium hydrate crystals are calcined to produce
anhydrous aluminium oxide. The slurry of undissolved
impurities resulting from digestion of the bauxite with
caustic soda at elevated temperature i9 commonly referred
to as red mud and typically comprises inert iron oxides,
titanium oxides and silica compounds. Prior to discharge
from the process, the red mud is typically washed with
water to reco~er ,-ntrained caustic soda in Rolution.
During digestion, in addition to reacting with
alumina in bauxite, the caustic soda also reacts with
silica which is typically present in bauxite. The
consumption of caustic soda resulting from reaction with
silica minerals :Ls a world-wide problem in the alumina
refining industry. In bauxite deposits which
CA 02246~28 1998-08-14
W O 97t29992 PCT/AU97/00073 -- 2
contain high concentrations of silica minerals, the
caustic soda lo~:s associated with reaction with silica
can represent a significant fraction of overall alumina
production costs. Silica may be present in bauxite
deposits in various forms inclu.ding kaolinite
(Al203.2SiO2.2H20'l and quartz. In ge:neral, kaolinite
accounts for the majority of the reactive silica found in
bauxite. Durinq processing, di.ssolved silica, alumina
and sodium combine to precipitate out of solution as a
sodium-aluminium-silicate desilication product (DSP).
Each tonne of silica that dissolves from bauxite consumes
approximately l.1.8 tonnes of caustic soda in forming DSP.
The DSP is discharged from the process as a significant
component (up to 40~ by weight) of the red mud waste
product and hence soda and alumina values are lost. It
would therefore be desirable to recover soda and/or
alumina values from red mud.
BACKGROUND ART
Various methods have been proposed. for recovering
DSP soda from red mud and the majority of such processes
are based on the reaction of .Lime (CaO~ with DSP at
elevated temperatures. Such pr-ocesses have not found
wide commercial u.se principally because several tonnes of
lime are required per tonne of soda. recovered and
reaction kinetics are such that a significant thermal
energy input is required.
US patent no. 4044095 teaches a process in which red
mud is treated with concentrated caustic soda at high
temperatures (in the order of 300~C~ in the presence of
lime. The DSP in the red mud is converl-ed to a sodium-
calcium-silicate and, in a second stage of the process,
the sodium-calcium-silicate is converted to calcium-
silicate releasing soda for recovery. In addition to
soda, alumina is recovered from DSP in t:he process. US
patent no. 934270 also teaches the digestion of red mud
with lime at elevated temperature to recover soda from
DSP. The processes of US patent nos. 4044095 and g38270
are elevated t.emperature processes in which the amount of
CA 02246~28 1998-08-14
W O 97/29992 PCT/AU97/00073
- 3
lime consumed exceeds the amount of soda recovered.
US 2992893 teaches a process similar to that
described above which uses finely divided slaked lime and
employs vigorous stirring. The thenmal energy
requirement of the process is demonstrated by the
reaction condit_ons taught by the patent, namely 30
minutes at 255~C,, 6 hours at 90~C and 8 hours at 75~C.
AU 88102/8,' teaches a process similar to that
described in relation to US 4044095 which utilises a
single digestion stage at lower caustic concentrations
but with a higher rate of lime addition. DSP is
converted to a,n iron substituted calcium-aluminium-
silicate of the hydrogarnet type with high soda and
alumina recovery.
In the Mud Caustication Process (~. Solmar and J.
Zoldi, "Lime in the Bayer Process, Present State and
Future Trends~, TMS Light Metal (1993)) lime is reacted
with DSP under atmospheric conditions to liberate soda
and form a hydrogarnet. The Mud Caustication Process
also rec~ires high levels of lime consumption and
relatively long reaction times. As much as 2-3 tonnes of
CaO can be consumed per tonne of soda recovered. No
alumina is recovered in the Mud Caustication Process and
the degree of soda recovery is adversely affected where
soluble caustic ]evels in the red mud slurry exceed about
30 grams per litre.
In the "Lime-Soda-Sinter" process red mud is mixed
with lime and soda ash prior to being sintered at
temperatures in t:he order of 1000~C. DSP is converted to
an insoluble calcium silicate and soluble sodium
aluminate which is recovered by leaching the sinter
products. US patent no. 4045537 teaches a variation on
this process involving the addition of a carbonaceous
substance for red muds having a high iron content because
iron consumes lime and soda ash. Although Lime-Soda-
Sinter processes result in high recoveries of soda and
alumina, their industrial application has been limited
because of the cost of high temperature operation and the
CA 02246~28 1998-08-14
W O 97~9992 PCT/AU97~073
-- 4
technical compIexity of sintering so as to cause
frittering or sintering of the particles without
substantial fusion or melting.
Mechanica~l activation is a process in which
mechanical energy is utilised to increase the chemical
reactivity of a system. Mechanochemical reactions are
induced which result in changes in chemical composition
and structure as a consequence of the input of mechanical
energy. US patent no. 532850~ teaches a mechanical
activation process in which chemica:L reduction of
reducible metal compounds with a reductant is
mechanically activated during milling in a high energy
ball mill to refine and manufacture metals and alloys.
During milling, the energy imparted to the reactants
through ball-reactant collision event:s enables the
starting materials to react re~ulting i.n the reduction
reaction proceeding without the need for high
temperatures or melting to increase reaction rates.
SUMMARY OF THE INVENTION
In a first aspect, the present invention provides a
method for recovering soda and/or alumina values from DSP
formed in a Bayer process, the method comprising
mechanically activating the DSP to induce a
mechanochemical reaction whereby soda and/or alumina
values are solubilised.
In a second aspect, the present invention provides a
method for recovering alumina values from red mud formed
in a Bayer process wherein the alumina values are alumina
values other than alumina values derivab:Le from DSP, the
method compri~ing mechanically activating the red mud and
recovering the alumina values.
DESCRIPTION OF PREFERRED EMBODIMENT~
The method according to the rst aspect of the
present invention is applicable to either the mechanical
activation treatment of red mud or a DSP concentrate of
red mud. A DSP concentrate may be formed by subjecting
red mud to a separation technique, for example, a gravity
separation technique or a hydrocycloning technique.
CA 02246~28 1998-08-14
W O 971~9992 PCT/AU97/0~073
- 5
During the caustic digestion o~ bauxite in a Bayer
process it is not uncommon for red mud to contain alumina
values that have neither been dissolved and separated in
the liquor phase nor have been reacted in the formation
of DSP. Typically, such alumina values are derived from
boehmite and, where red mud is mechanic~lly activated in
accordance with the second aspect of the present
invention, such alumina values are believed to be
recoverable. Red mud may also contain alumina values
derived from alumina which was dissolved during digestion
but which precipitated from the alumina rich liquor phase
prior to separation of the alumina rich liquor phase.
Again, such alumina values are believed to be recoverable
in accordance ~ith the second aspect of the present
invention.
The particle size of the red mud/DSP may be reduced
by grinding or the like prior to mechanical activation.
The methods of the present invention are suitable
for either batch processing or continuous processing of
red mud/DSP concentrate.
According l_o the first aspect of the present
invention, DSP/red mud can be mechanically activated
without any externally applied heat with soda and/or
alumina values recoverable by conventional washing
techniques following conversion into soluble or partially
soluble forms. The solubilisation of soda and/or alumina
values from DS~' is believed to be thermodynamically
favoured at a~ient temperature but in prior art
processes input of thermal energy is believed to have
been required due to kinetic limltations. In the absence
of mechanical activation it is believed not to be
possible to solubilise soda and/or alumina values from
DSP at ambient temperature within a commercially feasible
period of time. Without wishing to be bound by theory,
the mechanical activation of DSP is believed to increase
the reaction kinetics or increase the chemical reactivity
of the DSP with the result that a che~ical reaction or
phase change oc-urs which produces a new compound or
CA 02246~28 1998-08-14
W O 97n9992 PCT/AU97/00073
-- 6
phase that can ke more readily processed ~or extraction
of soda and/or a]umina values. l'he mechanical activation
of ambient temperature DSP is believed to generate
localised regions of high temperature and pressure.
Localised temperatures are believed to be as high as
400~C even though average temperature may only be 40~C-
60~C.
The recovery of soda and/or alumina values can be
enhanced by mechanically activating the DSP in the
presence of a reagent. Any reagent which is
thermodynamically capable of reacting with DSP to
solubilise soda and/or alumina values may be used.
Suitable reagents include oxides and hy(1roxides such as
CaO, NaOH and Ca~OH) 2. Without wishing to be bound by
theory, one class of reagents are believed to undergo a
cation exchan~e mechanism with DSP in which sodium is
released and replaced with a reagent cation. For
example, the reagent cation may be selected from Ca2+, K+,
Ba2+ and Mg2~
Preferably, the red mu~/DSP concentrate is
mechanically activated in a mechanical mill. The
expression mechanical mill is to ~e understood to include
ball mills, nutating mills, tower mills, planetary mills,
vibratory mills, attritor mills, gravity-dependent-type
ball mills, jet mills, rod mills, high pressure roller
mills and the like. By way of example, a ball mill is a
vessel which cont:ains grinding media which is kept in a
state of continuous relative motion by input of
mechanical energy. The grinding media is typically steel
or ceramic balls. Energy is imparted to the DSP within a
ball mill by ball-DSP-ball and ball-DSP-mill collisions
with the energy being sufficient to cause mechanical
activation of the DSP. In a preferred embodiment of the
present invention, mechanical activation and thermal
treatment can be combined by the use of a thermally
insulated high energy mill such as an attritor. With
such high intensity mills power inputs of the order of
100kW/m3 can be achieved. The thermal energy generated
CA 02246~28 1998-08-14
W O 97129992 PCTIAU97100073
during milling can result in temperature elevation.
Without wishing to be bound by theory, utilisation of the
generated heat during milling i9 believed to
substantially increase reaction kinetics through the
combined effects of mechanical and thermal activation
with the resul~: that milling time and cost may be
reduced. Accordingly, process efficiency is increased by
utilising the generated thermal energy which would
otherwise tend to be lost.
At least preferred embodiments of the first aspect
of the present invention are advantageous when compared
with prior art processes because
(1) DSP can be mechanically activated at ambient
temperat~re;
(2) where lime is utilised as a reagent, the level of
lime consumption is low;
(3) processing t:imes are relatively short; and
(4) the presence of caustic soda in sol~tion in DSP does
not adversely affect recovery of soda/alumina
values.
EXAMPLES
The ensuing examples are set forth for the purposes
of illustration only and are not to be construed as
limiting the scope of the present invention in any way.
Example 1
A red mud slurry containing 470 grams of solids per
litre was loaded together with 3kg of 6mm grinding balls
into a 1 litre capacity horizontal attritor mill operated
with a rotor speed of 600rpm. In some t:ests the red mud
was mechanically activated in the presence of either CaO
or NaOH with the mass ratio of red mud:reagent being
varied in different tests. The charge ratio (grinding
balls:red mud solids g/g) and milling time were varied in
different tests.
Following milling the slurry was washed from the
mill in a manner to ensure 100% recovery of the slurry
and the solids were separated from the initial wash
liquor by centrifuging. The separated solids were dried
CA 02246528 1998-08-14
W O 97/29992 PCT/AU97/00073
- 8
ln an oven at 10l3~C, ground with a mortar and pestle and
washed with a cold 10~ ammonia solution for 10 minutes
followed by vacuum filtering and drying at 100~C. The
washing procedure was repeated.
The milled, washed and dried solids were analysed by
X-ray diffraction (XRD) to identify crystalline phases
present and by X-ray fluorescence (XRF) to provide a
standard chemical analysis to determine the chemical
composition cf the solids. Chemical analyses of the
initial wash liquor were carried out on selected samples.
Soda recovery was calculated from the total soda
content of the feed and product (milled'~ samples. Soda
values in the product were normalised to the feed sample
by the Tie element method using TiO2 which is unaffected
by the milling proce~s and remains constant in the red
mud. The corrected soda value was calculated from:
N O N o ( TiO2)(~o~a~
The soda removed was then expressed as a percentage
of the soda in the feed sample:
%Soda Recove~y = (N~2o~ Na2O(~)x100
Na20(f~,~
Similar equations were used to calculate ~ alumina
recovery.
Table 1 sets out the results of a series of ten
tests which were carried out on two samples of red mud.
The first sample was red mud obtained from Queensland
Alumina Limited's processing plant at Gladstone,
Australia and the second sample was a portion of the
first sample which had been washed with a 10~ ammonia
solution and dried to remove any entrained liquor from
the Bayer process. The dried ~econd sample was mixed
with distilled water to form a 470 c~rams per litre
slurry.
CA 02246528 1998-08-14
W O 97~9992 PCT/AU97/00073
-- 9 _
t~ >1
-,l V
> a~ 0 ~D ~ ao ~r
U
>~
V
O > ~ CO ~ ~DL' O W
o\P ~V
~ C ~ ~ ~ ' ' ~ N ~
-~ 1 o 'V ~ o ~V o o o o
j~ ~ V ~ ~ ~~ ~ V C~ ~~
P p:
" c C ~ a
~; v z; a ~ u ~ ~ ~; u z ~
~V o ~ ~ ~ ~1 ~ ~ ~1 ~ ~ ~1
o c)' o o o o o ~ ~ ~
~ IV VJ
'~. Ei 1~ o cl o o o o o o o o
Z ~ t' a~ o~ ~
V~
~V
o
SUBSTITUTE SHEET (RULE 26)
CA 02246~28 1998-08-14
W O 97/29992 PCT/AU97100073
- 10
The following observations can be made from the
results of the ten tests.
* The milling of red mud with CaO results in high
levels of soda recovery. The recovery of 66~ of soda
originally contained in the red mud was achieved (see
Test Nos. 4 and 7). In addition to soda, up to 18~ of
the alumina contained in the red mud was also recovered.
* No significant differences in soda recovery were
observed between the arnmonia washed and unwashed red mud
samples. For ammonia washed red mud, a rnass ratio of red
mud to CaO ol~ 24:1 gave 66~ recovery (see Test No. 7).
For unwashed red mud, 66~ recovery was obtained in a
sample with a mass ratio of 13.4:L (see Test No. 4).
* Milling with CaO achieved higher levels of soda
recovery. In ~'est No. 4, milling red mud with CaO
resulted in 66~ soda recovery as compared with 47~
recovery obtainecl in Test No. 5 where the red mud was
milled under identical conditions but for the absence of
CaO. Similarly, in Test No. 8, 58~ soda recovery was
achieved as compared with 46~ soda recovery in Test No.
10. Alumina recovery was also enhanced when red mud was
milled with CaO.
* Milling in a NaOH solution (see Test No. 3) resulted
in soda recovery similar to that achieved by milling red
mud with CaO which indicates that the caustic level in
the red mud slurry has no sig~ificant affect on soda
recovery. Removal of alumina was somewhat higher after
milling with NaOH.
* Reduction of the charge ratio had no significant
affect on the level of soda or alumina recovery (see Test
Nos. 1 and 8).
* Reduction of the milling time frorn l hour to 30
minutes using a charge ratio of 40:1 resulted in a
decrease in soda removal from 58~ to 48~ ~see Test Nos. 8
and 9).
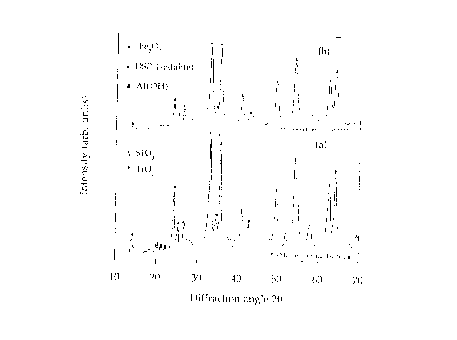
Typical XRD measurements of (a) the second sample
(ie. ammonia washed and dried slurry) and ~b) a sample of
the milled washecl and dried solids fro~ Test No. 7 are
CA 02246528 l998-08-l4
W O 97/29992 PCTIAU97/00073
illustrated in Figure 1. The XRD patterns show a
reduction in peak in~ensity and a broadening of the
diffraction peaks for the milled, washed and dried solids
from Test No. 7. Without wishing to be bound by theory,
this is believed to be a consequence of extreme reduction
in crystal size and the introduction of disorder and
crystal defects during milling. The intensities of the
DSP peaks relative to the Fe2O3 peaks are substantially
reduced during milling. Again without wishing to be
bound by theory, this is believed tO indicate that
amorphisation of the DSP, decomposition or a reaction
with other components of the red mud or CaO occurs during
milling, thereby enabling soda and alumina to be
recovered during subsequent washing.
Analysis of the initial wash liquor collected during
washing of selected samples is shown in Table 2. The
initial wash liquor contains only sodium and aluminium,
with the soda recovered as NaOH and Na2CO3.
TABLE 2
20 Te~tTotal Soda Caustic Alumina Alumina:Caustic
No. (g~L al3 (g/L as ~g~Ll
Na2C03) Na2C031
4 5.56 4.34 1.76 0.406
3.23 2.01 1.64 0.814
7 3.65 2.27 1.59 0.701
Example 2
A red mud slurry obtained from Queensland Alumina
Limited's processing plant at &ladstone, Australia
containing 580 grams of solids per litre was loaded into
a 5 litre capacit:y vertical attritor mill containing 15kg
of 6mm steel grinding balls which was operated with a
rotor speed of 600rpm. In some tests the red mud was
mechanically act vated in the presence of CaO with the
mass ratio cf red mud:CaO being varied in different
tests. The charge ratio ~grinding balls:red mud solids
g/g) and milling time were varied in different tests.
An identical procedure to that used in Example 1 was
used for wash-ng and drying the slurry recovered from the
CA 02246528 l998-08-l4
W 097/29992 PCTIAU97/00073
- 12 -
mill. Soda recovery and alumina recovery were calculated
in the same manner as used in Example 1 and Table 3 sets
out the results of a series of 10 tests. The results of
Example 2 indicate that different mechan:ical mills can be
used in the presen~ invention.
CA 02246528 1998-08-14
WO 97ngg92 PCT/AU97100073 - 13 -
a)
d~~ C~
~a J) ~ C' ~ ~ O~ O o o c~
o >
cn Oo ,~ c~CD a~ N 0~ ~ a~ d'
o\o ~
C
., ~
~ ~ ~ N ~I N
E~
O ~ O O O O ~ O ~
r~ ~ V ~ V V V S V ~~
v ~ ~ ~~ o o
o o o o o ~n o o o u~
~ ~ ~ ~ Ln ~D r ~D ~ o
E~
U~ o
SUBSTITUTE SHEET (RULE 26~