Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02247397 2002-02-25
WO 9713174'l PCT/LJS97/02872
TITLE OF THE INVENTION
LASER DRILLING PROCESS FOR PRODUCING A PLURALITY OF HOLES IN CHEMICAL
nOSAGE FORMS USING ACOUSTO-OPTIC DEFLECTOR
BACK~~ROUND OF' THE INVENTION
There is a need within the pharmaceutical industry to
produc~° an opening in the surface of many types of dosage folzns. For
example, certain controlled release devices rely on an opening through
an outer coating or housing and into the core of the device, as a means
of releasing material stored within the core to the environment of use.
Often these controlled release devices rely on osmotic
pressure, diffusion or surface hydratian to deliver the contents of the
core through the opening.
U.S. Patent 4,088,864 reported the use of a laser to
produce outlet passage-way in the walls of tablets which dispense their
contents osmotically. This technique comprised moving the pills in
succession along a predetermined path at a predetermined velocity;
tracking the moving pills seriatim with a laser of a wavelength which
is absorbable by the walls. The laser beam dimensions at the wall, the
Iaser power and the firing duration were such as to cause the laser beam
to heat and pierce the wall and produce an outlet passageway 4 to 2000
microns in diameter through the wall and into the device core.
There is further a need to produce dosage forms containing
multiple holes through the outer coating and into the core. The holes
expose; multiple portions of the dosage form core to the environment
of use., allowing for delivery of the drug stored within the core.
Jain, N.K. and Naik S.U., J. Pharm Sci., 73, 1806-1811
~( 1984), have reported on the use of a laser to drill holes in capsules.
To valy the number of pores, the capsule was mounted on a linear drive
and moved at a speed of 2 mm/sec. :By changing the laser frequency
CA 02247397 1998-08-26
WO 97/31747 PCTIUS97102872
-2-
and keeping the power and pulse width constant 25 to I00 pores were
drilled on the body of the capsule shell.
Technology required to produce multiple patterns of
openings through the dosage form shell or coating without repositioning
of the dosage form has previously not been available. A process which
provides for rapid though-put of dosage forms, capable of providing
such a pattern of openings, without such manipulation is desirable.
Recently, laser systems which employ a linear array of
individual laser tubes have been developed. These systems allow the
user to pulse only those lasers needed so as to produce a linear array
of laser beams. In U.S. Patent 5,049,721, such a system was used to
provide markings in an outer jacket of repetitively spaced sections along
the length of a moving cable. As the cable was moved along, the lasers
were pulsed, via a computer program, to produce letters and symbols.
In U.S. 5,376,771, this technology was applied to create multiple arrays
of holes in dosage form devices rapidly and precisely.
SUMMARY OF THE INVENTION
A process for producing a plurality of apertures in
dosage forms using a laser whose beam is deflected by an acousto-
optic deflector is presented. Using this process, the apertures may be
produced by individual pulses of laser energy, wherein the laser beam
is redirected by a synchronized mirror or mirrors to reproduce similar
pluralities of apertures at further faces or areas of the same dosage
form. The resultant apertures may be arranged in the form of an m
by n matrix to generate a desired pattern of apertures, where m and
n range from I to about 1000, and more preferably from about 10
to about 200. This technology is particularly effective in producing
apertures in pharmaceutical dosage forms such as tablets, capsules,
lozenges, boluses, pills, wafers, disks, expandable devices, patches,
suppositories, collars, pellets, controlled release devices, slow release
devices and other medicament delivery devices, and particularly when
the dosage form is film coated. When the film coating is water insol-
CA 02247397 1998-08-26
WO 97/31747 PCT/CTS97/02872
-3-
uble and water impermeable, this process offers a rapid and effective
means of producing apertures for contact of the dosage form core with
the environment of use since the aperture may be drilled through the
coating and into the core of the dosage form to insure exposure of the
core when in use. While the apertures may be of any size and shape,
one preferred embodiment includes apertures which are circular where
the diameter ranges from about 100 microns to about 2000 microns.
While there is no theoretical limit to the number of apertures which
may be drilled in a dosage form using this process, in a preferred
embodiment of the process, from 5 to about 1000 apertures are drilled
in each face of each dosage form. In a more preferred embodiment of
this invention from about 10 to about 200 apertures are drilled in each
face of each dosage form.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1, is a side view of the process wherein dosage
forms are continuously moved through the optical enclosure on a chain
feeder mechanism.
Figure 2, is a diagrammatic view of the optical enclosure of
the system.
Figure 3, is a plan view of an apertured dosage form
containing 22 apertures on each face.
Figure 4, is the laser drilling timing sequence.
DESCRIPTION OF THE INVENTION
The present invention is directed to a novel process for
producing a plurality of apertures in chemical dosage forms at high
speed using a laser whose beam is deflected by an acousto-optic
deflector.
This novel process provides for the production of dosage
forms which are able to deliver their contents once in an environment
of use. For example, pharmaceutically active product may be dispensed
to an animal, including man, in need thereof; flavoring, sugar, or sugar
substitutes may be dispensed from confections; chemicals useful in the
CA 02247397 1998-08-26
WO 97J31747 PCT/US97J02872
-4-
treatment of water may be added to water reservoirs using dosage
forms which are prepared using this novel process. By "dosage form"
is meant any device capable of delivering a chemical which requires a
plurality of apertures through which the chemical may move into the
environment of use. The term "pharmaceutical dosage form", as used
herein, refers to a dosage form useful in the delivery of a pharma-
ceutically active agent to a patient, in need thereof, the dosage form
having been prepared using the process of this invention.
The environment of use is not limited. It may be of a
biological nature, for example, when the device is used pharmaceutical
drug delivery, or for the preparation of a confection. This novel
process may also be used in industrial environments such as water
or air treatment, or any other area in need of delivery of a chemical
through a plurality of apertures.
The term "dosage form" further includes, but is not limited
to items such as coated or uncoated tablets, capsules, lozenges, boluses,
pills, wafers, disks, expandable devices, patches, suppositories, collars,
pellets, controlled release devices, slow release devices, room freshener
devices, water treatment delivery devices, confections, candies, and
other chemical delivery devices. This process is particularly useful
when used to produce a pharmaceutical dosage form which is film
coated, since the apertures can then be used to expose portions of the
core of the pharmaceutical dosage form to the environment where the
dosage form will ultimately be used, such as the stomach and intestine.
This process is particularly useful in the production of a
pharmaceutical dosage form when the film coating applied to the dosage
form is insoluble in an aqueous environment or impermeable to aqueous
solutions or where the coating is both impermeable and insoluble in an
aqueous environment. By an "aqueous environment" is meant, an
environment which is, at least in part, water. Examples of the type of
environment where such a dosage form would be used includes, but is
not limited to the mouth, bucal cavity, stomach, Large and small
intestine, vagina, and nasal passages.
CA 02247397 1998-08-26
WO 97/31747 PCT/US97/02872
-
Since release rate of the contents of the pharmaceutical
dosage form contemplated for use in this process is a function of the
number and size of the apertures, it is critical that the method produce
apertures which are uniform in both size and number. The method
5 presented herein provides for both rapid and accurate drilling of dosage
forms where the aperture diameters are consistent and the number of
apertures in the dosage form are constant.
In a preferred embodiment of this invention, the apertures
are drilled in the face of the dosage form. By "face" is meant the most
significant or prominent surface or surfaces of a dosage form. For
example, when the faces of a pharmaceutical dosage form such as a
tablet or capsule are discussed, the word "face" or "faces" is used to
describe the opposed surfaces with the greatest area.
While the apertures may be of any size and shape, one
preferred embodiment includes apertures which are circular where the
diameter ranges from about 100 microns to about 2000 microns. While
there is no theoretical limit to the number of apertures which may be
drilled in a dosage form using this process, in a preferred embodiment
of the process, from about 5 to about 1000 apertures are drilled in each
face of each dosage form. In a more preferred embodiment of this
invention from about 10 to about 200 apertures are drilled in each face
of each dosage form.
The dosage form to be processed is passed at constant
velocity through an optical enclosure during which it is bombarded a
number of times by pulses of laser energy, thereby producing a plural-
ity of apertures. The desired spatial separation of the apertures is
achieved by delivering the pulses at predetermined beam deflection
angles as the dosage form passes through specific positions in its
direction of travel. In such a way, a pattern, such as a rectangular
matrix or array, can be produced on a face or-in one general area of
the dosage form.
Having generated the first pattern on a particular dosage
form, the laser beam can be redirected by means of a mirror or mirrors
CA 02247397 1998-08-26
WO 97/31747 PCTIUS97/02872
-6-
synchronized with the travel velocity, to reproduce similar patterns at
further faces or areas of the same dosage form.
The pattern is defined by a computer file containing the
delivery timings of laser pulses together with the corresponding values
of beam deflection angle. It is thereby possible to define different
patterns for individual dosage forms, or more commonly, for different
batches or types of dosage forms.
By "optical enclosure" is meant the area in which the
laser beam is contained, and through which the dosage forms pass in
order to be drilled or processed.
By "apertures" is meant holes or openings starting at the
surface of the dosage form and extending into the dosage form to a
predetermined depth. Alternatively, the apertures may go completely
through the dosage form. The apertures may pierce the coating of a
dosage form thus exposing the interior of the dosage form to the
environment of use. Additionally, the apertures may provide an exit
means for the chemical stored inside a dosage form to be expelled under
osmotic pressure, diffusion, surface hydration, erosion, or mechanical
force.
The apertures may be arranged closely so as to produce
perforations which define an area of the dosage form which is to be
discarded prior to use or expelled during use. Further, the apertures
may be arranged in a manner which produces a pattern which identifies
the dosage form prior to or during use. Additionally the pattern may
be used to produce a design, spell out a code, trademark or other
symbol.
The pattern may constitute an array containing any number
of apertures. When boluses and other large dosage forms are prepared
for example, m x n arrays containing from 1 to 1000 or more apertures
may be needed. Thus, it would not be outside this invention for a
dosage farm to contain 1000 columns of apertures each containing
50 to 100 apertures (i.e. m = 1000 and n = SO to 100).
When other smaller dosage forms are prepared, m x n
arrays containing from 10 to 50 apertures may be required. Thus, it
CA 02247397 2005-10-31
would be within this invention for a dosage form to contain 5 columns of 10
apertures each (i.e. m = 5 and n = 10).
The laser drilling system may be used either alone or in conjunction with a
printing means to inscribe alpha-numeric characters or other symbols on the
dosage form using technology such as that described in U. S. Patent 5,049,721,
in
such a manner that the characters mask or hide the apertures.
The number and size of the apertures is determined by the end use of the
dosage form. For example, such apertures could be used to limit or enhance the
delivery rate of the chemical to the environment of use.
i o In the pharmaceutical field, the dosage form may consist of a tablet or
other
drug delivery device. The drug delivery device may be coated or uncoated.
Uncoated tablets may contain apertures in order to assure rapid disintegration
of
the tablet or to produce incursions which help in breaking the tablet. Coated
tablets may contain apertures to assist in entry of fluid from the environment
of
i5 use, allow for passage of drug from the core of the tablet to the
environment or to
define the amount of core area exposed to the environment.
The dosage form may be a core which comprises a polymer which forms
gelatinous microscopic particles upon hydration and a medicament, the core
being
completely coated with a water insoluble and water impermeable coating. This
2 o process for producing a plurality of apertures may then be used to drill a
predetermined number of apertures into the surface of the dosage form. If the
dosage form has distinct faces, apertures may be drilled in all of the faces,
either
sequentially or simultaneously. In a system of this type, the apertures
provide
access to the solution which makes up the environment of use. The solution
2 5 hydrates the polymer at the exposed surfaces. The polymer forms gelatinous
microscopic particles which move from the tablet into the environment of use,
carrying with them the active ingredient.
CA 02247397 1998-08-26
WO 97/31747 PCTIUS97/02872
_ 8 -
DESCRfPTION OF PREFERRED EMBODIMENT
An example of the present invention will now be described
with reference to the accompanying drawings. This example is not
designed to limit the scope of this invention in any way.
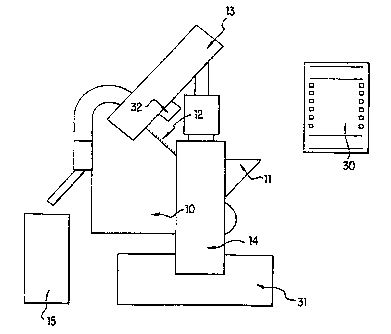
In Figure 1, dosage forms (12) are delivered to a moving
conveyer system ( 10) from a storage container ( 11 ). The storage
container ( 11 ) has a capacity of up to 200,000 dosage forms. The
moving conveyer system (10), preferably a chain or belt transport
mechanism, transports the dosage form into and out of the optical
enclosure (13) of the laser (14). The apertured dosage forms are
collected in a final storage container (15).
1'he laser ( 14) is mounted vertically on one side of the
apparatus with the beam directed vertically upwards through a
polarization rotator, onto a mirror. The mirror directs the beam into
an optical enclosure (13) consisting of a number of optical components
mounted at approximately 90° to the chain transport mechanism.
The components in the optical enclosure are shown in
Figure 2 and consist primarily of the following:
An acousto-optic deflector {16) which is a solid-state
beam steering device. This is used to steer the beam,
which ultimately scans across the tablet. It consists of
a germanium crystal with 10 {typical) lithium niobate
acoustic transducers in a phased array on one side. These
launch an acoustic wave across the crystal that interacts
with the optical beam.
Three cylindrical lenses (33,34,35), which are used to focus
the beam into the aperture of the acousto-optic deflector
( 16), and subsequently recollimate. These are five inch
focal length antireflection coated zinc selenide planoconvex
lenses.
CA 02247397 1998-08-26
WO 97/31747 PCT/US97/02872
_9_
A special aluminum rotating mirror (17) with a 180° lobe.
Machined from aluminum, nickel electroplated and gold
coated. The mirror rotates once for every tablet that passes
the lasing point and diverts the beam, first to one side of
each tablet, and then the other, as the lobe passes in and out
of the beam path.
Two deflecting mirrors (I8,19) to direct the beam to the
lasing point. These are gold coated silicon flat mirrors.
Two lenses (22,23) (one each side), of 5" focal length to
focus the beam onto the tablet.
Under certain conditions, two special sensor units (24,25)
IS (one of each on each side) which monitor the beam energy
in each pulse and the presence of the corresponding visible
flashes to confirm that drilling has taken place. The power
sensor, comprises a 45° zinc selenide component having a
90° residual reflection of typically 1 %, a secondary focus
lens and a pyrodetector is included. The sensor analyses
the change in power loading on the pyrodetector and
thereby infers the laser power during the pulse.
Two small mirrors (26,27) and beam dumps (28,29)
( 1 ° dia gold coated silicon beam dumps water cooled black
anodized fitting with conical beam absorbing aperture).
These absorb the beam energy when it is not being
deflected to the target.
The whole optical cavity is maintained at positive pressure
by a small fan drawing in air from outside the machine to prevent
ingress of any dust generated by the drilling process.
CA 02247397 1998-08-26
WO 97/31747 PCT/US97/02872
-10-
One possible array of 22 is found in Figure 3. Alternative
designs may be generated by controlling the timing and deflection of
beam pulses as the tablet passes through the optical enclosure.
Referring back to Figure 1, the machine interface (30)
allows the operator to exercise control of the machine. The laser power
supply unit (31) is located in the base of the machine, and the acousto-
optic deflector driver (32) is located in close proximity to the deflector,
on one of the outer faces of the optical enclosure.
Referring to Figure 3, the apertures (40) are a plurality of
drilled openings which pierce the outer surface coating of the dosage
form.
The diameter and depth of the apertures in the dosage form
is a function of the duration of each pulse, the laser power, the optical
resolution, and to some extent the composition of the dosage form.
In alternative embodiments of the present invention, other
laser systems may be used to produce the apertures in the dosage form.
The laser drill of the preferred embodiment includes a carbon dioxide
laser (150W, 225W, or greater). Other lasers, including, but not being
limited to, an argon laser, another carbon dioxide laser, a neodymium:
YAG laser, an erbium:YAG laser, and an excimer Iaser, may be used so
long as the laser is able to produce apertures in the dosage form (12).
The steering system for the laser is an acousto-optic
deflector, driven by a module consisting of a voltage controlled
oscillator and broadband power amplifier. A preferred acousto-optic
deflector and drive module is available commercially, from lntraAction
Corp., Bellwood, Illinois, models AGD402A l and DE4020 respectively.
The latter is designed to provide a frequency sweep range from 30 to
50 MHz with a +/- 0.25% frequency linearity specification. The RF
power amplifier is capable of 20 watts output and can withstand any
mismatched load whether a short, open or complex impedance. The
system is also provided with an interlock circuit for thermal protection.
In addition to the optical components described above, the
main drive motor is also located in the optical enclosure. The latter is a
DC motor with integral tachometer to provide a speed control capability
CA 02247397 1998-08-26
WO 97/31747 PCT/LTS97/02872
-11-
and thereby prevent excursions into regimes which are beyond the
laser's capacity.
The rotating mirror is coupled directly to the motor shaft.
A toothed timing belt drive, also taken from the main
motor shaft, drives the tablet feeder mechanism via a harmonic drive
speed reducer. The latter is a compact gearbox of high quality, both in
terms of mechanical backlash and non-linearity.
An encoder is also coupled to the motor shaft. This
provides output signals which are used to regulate the timing of beam
pulse deliveries. In this way, if the machine speed varies slightly under
different operating conditions, the occurrence of beam pulses relative to
tablet position, and hence the pattern generated, is unaffected.
Since debris is produced by the lasing system, it is removed
by a dust extraction system, using nozzles and filtering air cleaner units.
The latter are commercially available, and can be set up to conform to
generally accepted international standards for the removal of
pharmaceutical dusts.
The laser drilling timing sequence can best be understood
by reference to Fig. 4, where the letters A through K indicate the
activity at any given point in the sequence. The following cycle shows
the drilling of holes in both faces of a dosage form:
(A) a process cycle enable bit is latched on by the start/stop key, this
allows the encoder pulses to now drive the laser;
(B) the leading edge of the next tablet to be drilled is detected as it sits
in its Garner;
(C) the index pulse is detected once per revolution of the motor shaft;
(D) on the trailing edge of the index pulse, the beam deflector is
position for the first hole;
(E) even pulses, of low to high transitions, are detected by a computer
and are used as a trigger to turn on the computer analog output and
fire the laser;
(F) the computer analog output command to fire the laser is turned on;
(G) the Laser remote control interface fires the laser;
(H) the laser is turned off;
CA 02247397 2005-10-31
-12-
(I) the energy plume detection sensor system is engaged; steps (E), (F), (G)
and (H) are repeated as necessary until all the apertures on the first face of
the tablet have been drilled, the mirror may then be rotated through
180°
and drilling on the reverse side, or second face, is initiated;
s (J) if drilling on a second face is desired, the laser beam is positioned to
drill
the first hole in the reverse side of the dosage form;
(K) the laser is fired and the process continues as above.
EXAMPLE
Tablets cores containing lovastatin, CARBOPOL 974 P~, trisodium citrate
to dihydrate and lactose in ratios of 5:2:4:2 were prepared by compression
using 1/4"
standard concave punches after wet granulation with 5% polyvinyl pyrollidone
and 90% ethanol 10% water. The tablets were coated to a thickness of 100
microns with a coating composition comprising cellulose acetate butyrate and
triethyl citrate, using a Glatt~ GPCG-3 column coater.
15 Twenty-two apertures were drilled in each face of the coated tablets, as
shown in Figure 3, using the laser drilling system described at full power and
at a
surface feed rate corresponding to approximately 48,000 tablets per hour. The
tablets were arranged in the carrier links at a spacing of two to an inch and
both
faces for the tablet were drilled serially, that is, one side and then the
other. The
a o approximate hole size, as measured by microscopic imaging using an
Analytical
Imaging Concepts~ IM4000, is 0.45 mm in diameter.
The in vitro release rate performance is determined at 37°C. in
isotonic,
phosphate buffer at pH 7.4 containing 0.4% w/w sodium dodecylsulfate using a
USP Apparatus 2 at 50 rpm. The cumulative percent lovastatin released was
z s measured against time. Approximately 80% of the contents was released in 8
hours. The last 20% of drug was released at a more constant rate and greater
than
>95% of the lovastatin content was released in less than 20 h.