Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02251850 1998-10-15
Water-soluble Tetrazolium Salt Compounds
Technical Field
The present invention relates to novel water-soluble tetrazolium salt
compounds. More specifically, the present invention relates to water-soluble
tetrazolium salt compounds which can be suitably used for, for example,
quantitative
measurement of dehydrogenases and other, and to methods for measurement by
using
the compounds.
Background Art
Quantitative measurement of various dehydrogenases, such as lactate
dehydrogenase (abbreviated occasionally as "LDH" hereinafter in the
specification),
alcohol dehydrogenase, and glutamate dehydrogenase, have conventionally been
conducted by using tetrazolium salt compounds. A property of the tetrazolium
salt
compound is its ability to receive a hydrogen released by the action of a
dehydrogenase,
among variety types as mentioned above, via an intermediate electron
transporter
such as reduced nicotinamide-adenine dinucleotide (abbreviated occasionally as
"NADH" hereinafter in the specification) to give a corresponding formazan
compound.
Accordingly, dehydrogenases can be quantitatively determined by measuring the
absorbance of the resulting formazan compound.
Among these dehydrogenases, lactate dehydrogenase distributes all over
somatic cells, and in particular, abundantly exists in myocardia, livers,
skeletal
muscles, and kidneys. It is known that serum LDH activity markedly increases
in
patients suffered from diseases such as myocardial infarct, malignant tumor,
hepatic
failure, progressive muscular atrophy, intravascular hemolysis, and
megaloblastic
anemia. Accordingly, by measuring serum LDH activity, highly useful clinical
information for diagnosis can be obtained.
In recent years, in order to detect trace substances in blood, e.g., uric acid
and
bile acid, with high sensitivity, it has been desired to develop a method for
measuring a
dehydrogenase which is less susceptible to biogenous substances. For this
purpose,
3, 3'- [3, 3'-dimethoxy-( 1,1'-biphe nyl)-4, 4'-diyl]-bis [2-(4-nitrophenyl)-5-
phenyl]-2H-
tetrazolium chloride (abbreviated occasionally as "nitro-TB" hereinafter in
the
1
CA 02251850 1998-10-15
specification) and other have generally been used as hydrogen acceptors.
However, the formazan compound formed by vitro-TB after the acceptance of a
hydrogen has low water solubility, which causes a practical problem. In
particular, in
automatic analysis, the resulting formazan compound precipitates inside a
measuring
system including tubes and cells, and may add positive errors on measured
values. In
order to solve the problem, it has been desired to develop a method utilizing
a
tetrazolium salt which produces a water-soluble formazan compound.
As water-soluble tetrazolium salt compounds which form formazan compounds
having sufficient solubility, Japanese Patent LJnexamined Publication
No.(Hei)7-
70092/1995 discloses compounds of the following general formula (2):
R2 -O3S
N
N~ ~ ~ / S03-
~+
N=N M+
(2)
wherein R1 and R~ independently represent hydrogen atom or vitro group, and M
represents an alkali metal or an ammonium.
Since these compounds produce formazan compounds having extremely high
detection sensitivity, measurement with higher sensitivity can be achieved by
using
these compound compared to the conventionally used vitro-TB. In addition,
because
of the water-solubility of the formazan compounds, they are free from adhesion
to a
measuring apparatus, and accordingly, useful for clinical diagnostics. In
particular,
the compounds of the above general formula wherein R' and R'-' are vitro
groups have a
feature of efficient reactivity with NADH to give a formazan compound
exhibiting
extremely high absorbance.
However, researches by the inventors of the present invention revealed that
these compounds have a problem of low storage stability when stored in the
state of an
aqueous solution. Because aqueous solutions are often stored, after their
preparation,
for a long period of time such as for 3 to 6 months in usual clinical
analyses, it is
desired that tetrazolium compounds used as hydrogen acceptors have high
storage
stability in the state of an aqueous solution.
2
CA 02251850 1998-10-15
Disclosure of the Invention
The inventors of the present invention conducted various studies to provide
compounds which have high storage stability as an aqueous solution, and in
addition,
maintains the characteristic excellent detection sensitivity of the compounds
of the
general formula (2). As a result, they found that the compounds of the general
formula (1) set out below have the desired features. The present invention was
achieved on the basis of these findings.
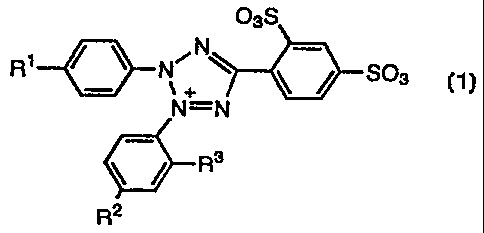
The present invention thus provides tetrazolium salt compounds represented
by the following general formula (1):
03S
N
N~ ~ ~ / S03_
~+
N=N M+
R3
2 (1)
R
wherein R' and R'-' independently represent hydrogen atom, vitro group, cyano
group,
carboxyl group, or a halogen atom; R3 represents an alkyl group or an alkoxyl
group,
and M represents an alkali metal or an ammonium.
According to preferred embodiments of the present invention, there are
provided the aforementioned tetrazolium salt compounds wherein both R' and R''
are
vitro groups, and Rv is a C1_~ alkyl group or a C,_~ alkoxy group; and the
aforementioned
tetrazolium salt compounds wherein both R' and R~ are vitro groups, R~j is
methyl
group or methoxy group, and M is sodium.
According to another aspect of the present invention, there is provided a
reagent for the measurement of a dehydrogenase which comprises the
aforementioned
tetrazolium salt compound. As a preferred embodiment of the present invention,
there is provided the aforementioned reagent which is used for a measurement
utilizing reduced nicotinamide adenine dinucleotide as an intermediate
electron
transporter.
According to further aspect of the present invention, there are provided
3
CA 02251850 2005-03-16
formazan compounds represented by the following general formula (G):
Na(~3S
N
R9 ~ ' H' ~ ' / S~~Na
N=N
/ R~
(6)
R~
«therein R' and R'= independently represent hydrogen atom, nitro group, cyano
group,
carboxyl group or a halogen atom, and R3 represents an alkyl group or an
alkoxyl
group.
According to still further aspect of the present invention, there is provided
a
method for the measurement of a dehydrogenase «therein the compound of the
above
general formula (1) is used as a hydrogen acceptor. According to preferred
embodiments of the aforementioned methods, there are provided the method which
comprises the step of measuring the absorbance of the formazan compound
represented by the above general formula (G); and the method wherein reduced
nicotinamide adenine dinucleotide is used as an intermediate electronic
transporter.
Brief Description of the Drawings
Figure 1 depicts the changes of absorbance of the compound of the present
invention (Compound a) and a compound disclosed in Japanese Patent Unexamined
Publication No.(Hei) 7-70092/1995 (Compound b) when stored at 4°C. In
the figure, o
represents results obtained by using the compound of the present invention
(Compound a), and a represents results obtained by using the compound b.
Figure 2 depicts calibration curves of NADH obtained by absorption spectrum
measurements. In the figure, o represents results obtained by using the
compound of
the present invention (Compound a), and a represents results obtained by using
the
compound disclosed in Japanese Patent Unexamined Publication No.{Hei) 7-
70092/1995 (Compound b).
Figure 3 depicts calibration curves of NADH obtained by absorption spectrum
measurements. In the figure, O represents results obtained by using the
compound of
the present invention (Compound a), and o represents results obtained by using
nitro-
TB.
4
CA 02251850 1998-10-15
Best Mode for Carrying Out the Invention
In the above general formula (1), R' and R'-' independently represent hydrogen
atom, nitro group, cyano group, carboxyl group or a halogen atom. As the
halogen
atom, any of fluorine atom, chlorine atom, bromine atom, and iodine atom can
be used.
It is preferred that both R' and R' are nitro groups.
As the alkyl group represented by R~', for example, a C,_a alkyl group can be
used. More specifically, methyl group, ethyl group, n-propyl group, isopropyl
group,
n-butyl group, sec-butyl group, tert-butyl group and other can be used. As the
alkoxy
group represented by R~, for example, a C,_~ alkoxy group can be used. More
specifically, methoxy group, ethoxy group, n-propoxy group, isopropoxy group,
n-
butoxy group, sec-butoxy group, tert-butoxy group and other can be used. R3
may
preferably be a C,_~ alkyl group or a C,_., alkoxy group, and more preferably
methyl
group or methoxy group.
As the alkali metal represented by M, for example, sodium, potassium and
other can be used, and sodium is preferred.
The compounds of the present invention represented by the general formula
(1) can be prepared by a conventional method. For example, a hydrazine
compound
represented by the following general formula (3):
R~ ~ ~ NHNH2 (3)
wherein R' represents hydrogen atom, nitro group, cyano group, carboxyl group
or a
halogen atom can be reacted with an aldehyde compound represented by the
general
formula (4):
Na03S
OHC ~ ~ S03Na
in an alcoholic solution to obtain a hydrazone compound represented by the
general
formula (5):
CA 02251850 1998-10-15
Na03S
R~ / ~ H-N=H ~ / S~sNa
and then the resulting compound can be reacted with a corresponding diazonium
salt
in an organic solvent or water under basic condition to obtain a formazan
compound
represented by the general formula (6).
Na03S
N
R~ ~ ~ H~ ~ ~ / S~3Na
N=N
Rs
(6)
R2
In the above general formula (6), R' and R' independently represent hydrogen
atom,
nitro group, cyano group, carboxyl group, or a halogen atom, and R3 represents
an
alkyl group or an alkoxyl group, and they may preferably be the substituents
specifically explained as to R', R'-' and R~3 of the above general formula
(1).
In the reactions mentioned above, sodium hydroxide, potassium hydroxide or
other may be used as a basifying agent. Then, the resulting formazan compound
of
the general formula (G) can be oxidized by using an oxidizing agent such as
butyl
nitrite in an alcoholic solvent to obtain the tetrazolium salt compound of the
general
formula (1).
The compounds of the present invention have a characteristic feature that
they receive a hydrogen produced by the action of a dehydrogenase via NADH or
other,
and they, per se, are reduced to form the formazan compounds represented by
the
above general formula (6). Accordingly, the concentration of a dehydrogenase
such as
lactate dehydrogenase, alcohol dehydrogenase, and glutamate dehydrogenase can
be
quantitatively measured by using the compounds of the present invention as
hydrogen
acceptors. They can also be used for quantitative measurement of reducing
substances such as NADH as described in the following examples. In general,
the
absorbance of the formazan compound of the general formula (6) formed in a
reaction
system may be measured to perform the quantitative measurement of these
substances,
6
CA 02251850 1998-10-15
or alternatively, the measurement can also be performed by means of
fluorescent
measurement or other spectrometric means. Methods for the measurement of a
dehydrogenase utilizing an intermediate electronic transporter such as NADH
are well
known in the art, per se, and reaction conditions, measurement means and other
can
be appropriately chosen by one of ordinary skill in the art.
Examples
The present invention will be further explained more specifically by referring
to the following examples. However, the scope of the present invention is not
limited
to the following examples.
Example 1: Synthesis of the compound of the general formula (1) wherein R' and
R2 are
nitro groups and Rv is methoxy group (Compound a)
p-Nitrophenylhydrazine (18.4 g) and sodium 4-formyl-1,3-benzenedisulfonate
(37.2 g) were mixed with methanol, and the mixture was heated under reflux for
two
hours. The resulting precipitates were collected by filtration to obtain a
hydrazone in
a 85%yield. The hydrazone obtained (6.7 g) was dissolved in water (200 ml),
and
cooled to 0'C. Separately, 5-nitro-o-anisidine (2.7 g) was diazotized in a
conventional
manner, and the product was added to the hydrazone solution obtained above.
The
reaction mixture was kept at -5 to 0°C and added dropwise with an
aqueous solution
obtained by dissolving NaOH (2.6 g) in water (40 ml). After the dropwise
addition,
the mixture was stirred overnight at room temperature. The reaction mixture
was
added with hydrochloric acid and concentrated. The precipitates formed by the
addition of isopropanol were collected by filtration to obtain a formazan in a
63% yield.
The resulting formazan (5 g) was suspended in methanol (250 ml), added with
concentrated hydrochloric acid (6.7 ml) and butyl nitrite (4.1 g), and then
the mixture
was stirred at room temperature overnight. The reaction mixture was
concentrated,
and added with isopropanol. The deposited precipitates were collected by
filtration,
and the crude product was recrystallized from ethanol to obtain the captioned
tetrazolium salt (Compound a) in a 45% yield.
Elemental analysis: Calculated. (C.,oH,LN601,S~Na) C: 40.00%, H: 2.18%, N:
14.00 %;
Found. C: 39.78%, H: 2.15%, N: 13.76%
7
CA 02251850 1998-10-15
Example 2: Synthesis of the compound of the general formula (1) wherein R' and
R'' are
nitro groups and R3 is methyl group
The captioned compound was prepared in the same manner as in Example 1.
Elemental analysis: Calculated. (C,oH13N60",S.,Na) C: 41.10%, H: 2.24%, N:
14.38%;
Found. C: 40.86%, H: 2.44%, N: 14.17%
Example 3: Storage stability test
50 mM Tris buffer (pH 8.0) containing 1 mM of Compound a was stored at 4'C
for 0, l, 7, 14, 31 and 90 days, and absorbances at 460 nm were measured. At
the
same time, the compound of the above general formula (2) described in Japanese
Patent Unexamined Publication No.(Hei) 7-70092/1995 wherein both R' and R'-'
are
nitro groups (Compound b: see, the section of "Background Art") was stored in
the
same manner, and changes of absorbance were measured. Fig. 1 shows the
relationships between the days of storage and absorbances deriving from
Compound a
and Compound b. Compound b exhibited remarkable increases of absorbance,
whilst
Compound a of the present invention gave no increase of absorbance for 90
days, which
revealed its excellent stability.
Example 4: Measurement of NADH concentration
50 mM Tris buffer (pH 8.0, 5 ml) containing 0.1 mM of Compound a and 5 a
M of 1-methoxy-5-methylphenazinium methosulfate was added with 0, 10, 20, 30,
40 or
50 a 1 of 5 mM NADH, and allowed to react at room temperature for 5 minutes,
and
then absorbances were measured. Figure 2 shows the relationship between NADH
concentrations and absorbances. Measurement was performed in the same manner
by using Compound b. Each of the compounds gave a linear calibration curve
between
NADH concentration and absorbance starting from the origin, and exhibited
almost
the same detection sensitivity. On the other hand, when the relationship
between
NADH concentration and absorbance was measured by using nitro-TB in the same
manner, it was found that detection sensitivity was apparently inferior to
that of the
compound of the present invention as shown in Figure 3.
8
CA 02251850 1998-10-15
Industrial Applicability
The tetrazolium salt compounds of the present invention give water-soluble
formazans when used as hydrogen acceptors. Accordingly, no deposition is
occurred
in a measuring apparatus, and measurements by using automatic analyzers are
facilitated. Furthermore, the compounds of the present invention have
characteristics that they exhibit higher sensitivity compared to nitro-TB,
which is
generally used as a hydrogen acceptor in clinical diagnostics, and can easily
detect
NADH. In addition, they have the feature of excellent storage stability in the
state of
an aqueous solution. Accordingly, the compounds of the present invention are
extremely useful as, for example, reagents for clinical diagnostics.
9