Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 022~3480 1998-10-30
W O97142140 PCTrUS97/08342
EXPLOSIVE FO ~ ULATIONS
BACKG~OUND OF I~v~Nl~loN
For over a decade, the military has been devoting a
large amount of research and development funding to
research projects directed to reducing the impact and
shock sensitivity of the main explosive charge in
munitions. A main challenge is to reduce sensitivity o~
the main explosive charge without decreasing performance
while also not significantly increasing cost. one of
the main charge explosives in munitions ~ormulations is
cyclotetramethylene-tetranitramine (HMX). The only
known practical way to reduce the sensitivity of these
formulations is to increase the amount of inerts and
less sensitive components therein and thus decrease the
sensitivity of the formulation but this also reduces the
performance of the formulation. Further, extensive
discussion of this problem is set forth in U. S. Patent
No. 4,842,659. In this patent it is stated that
insensitive munitions must be developed to improve the
combat survivability of an armament vehicle. It has
been found that munitions utilized in some weapon
systems are vulnerable to sympathetic detonation. For
2~ instance, the cannon caliber ammunition stored aboard
these vehicles is vulnerable to initiation via shape
charge jet and then propagation of the reaction due to
sympathetic detonation.
This sympathetic detonation and propagation
scenario can be summarized as follows: If a round is hit
by a shape charge jet, it is initiated. As a result,
the fragments that are generated by the blast then
~ strike the other rounds that are adjacent to it. The
latter rounds then initiate, contributing to the overall
reaction and damage sustained by the vehicle, crew, and
CA 022~3480 1998-10-30
WO 97/42140 PCTrUS97108342
other munitions. The m~ch~isms of reaction for the
initiation of the surrounding rounds are due to the
blast and fragments imping-ng on the aforesaid adjacent
round. The probability of sympathetic detonation can be
reduced in several ways. This can be done by
reconfiguring the ammunition compartments within the
vehicle. It can also be accomplished by packaging the
ammunition with anti-fratricide materials. However,
each of the aforesaid solutions will reduce the amount
of space available for the storage of ammunition. The
most acceptable solution to the problem is to reduce the
sensitivity of the energetic material to sympathetic
detonation. Incorporating less sensitive energetic
material will reduce the vulnerability of initiation
from the cited threats without reducing the number of
rounds stored in the vehicle. It has been found that by
reducing the vulnerability to sympathetic detonation of
the energetic materials used in these munitions, the
pro~ability of catastrophic reaction can be minimized.
The m~h~nis~ generally accepted within the
explosives community for detonating or deflagrating
explosives is the creation of very localized regions of
high temperature, i.e., hot spots. The application of
impact or shock on the explosive can generate hot spots
in the following ways: (1) by adiabaticly compressing
air (or explosive vapor) bubbles trapped in or purposely
introduced into the explosive, (2) ~y intercrystalline
friction, (3) by friction of the impacting surfaces,
(4) by plastic deformation of a sharply-pointed
impacting surface, and (5) by viscous heating of the
impacted material as it flows past the periphery of the
impacting surfaces.
In the compression and movement of explosive
crystals due to impact or shock, explosives like HMX
rapidly evolve into simpler products like H2O, CO, N2,
CA 02253480 1998-10-30
WO 97142140 PCTrUS97/08342
H2, CH20, HCN, and C2H2 as well as free radicals and
unstable intermediates. This mixture of products is
unstable and subject to detonation when exposed to a low
intensity shock induced spark o~ static electricity.
The creation and build-up of static electricity may be
an additional source of energy which contributes to the
detonation of the explosive and its decomposition
products.
BRIEF SUMMARY AND OBJECTS OF INVENTION
The present invention is directed to HMX formula-
tions in which the HMX is coated with shock sensitivity
reducing agents to reduce the shoc~ sensitivity of HMX.
Agents which were found to be useful in this inven-
tion were from four primary classes of compounds. The
classes are: 1) Quaternary Ammonium Salts; 2) Anionic
Aliphatic and Aromatic Compounds; 3) Fatty Acid Esters;
and 4) Amine Derivatives;
"Quaternary ammonium salts" are cationic nitrogen
containing compounds with four various aliphatic or
aromatic groups as discussed above for the amine
derivatives. The selected anion is generally a halogen,
acetate, phosphate, nitrate, or methosulfate radical.
Inclusive in this category are quaternary imidazolinium
salts where two of the ~;rh~tiC group bonds are
contained within the imidazole ring.
"Anionic aliphatic and aromatic compounds" are
compounds normally containing a water insolu~le
aliphatic group with an attached hydrophilic group.
They are often used as surfactants. The hydrophilic
portion of these anionic compounds is a phosphate,
sulfate, sulfonate, or carboxylate; sulfates and
sulfonates predominate.
CA 022~3480 1998-10-30
W 097142140 PCTrUS97/08342
"Fatty acid esters" is a term used broadly that
covers a wide variety of nonionic materials including
fatty esters, fatty alcohols and their derivatives.
Although once limited to compounds obtained from natural
fats and oils, the term "fatty" has come to mean those
compounds which correspond to materials obtainable from
fats and oils, even if obtained by synthetic processes.
They can generally be subclassified as: (l) fatty
esters (e.g., sorbitan esters (e.g., mono- and di-
glycerides)), (2) fatty alcohols, and (3) polyhydric
ester-alcohols. The exact classification of these
compounds can become quite confused due to the presence
of multiple functional groups. For example, ethers
containing at least one free -OH group fall within the
definition of alcohols, (e.g., glycerol-1,3-distearyl
ether). Synthetic compounds such as polyethylene glycol
esters can also be included in this category.
"Amine derivatives" describes a wide variety of
aliphatic nitrogen bases and their salts. ~mines and
their derivatives may be considered as derivatives of
ammonia in which one or more of the hydrogens have been
replaced by aliphatic groups. Preferred amine salts are
formed by reaction with a carboxylic acid to form the
corresponding salt. The amine and the carboxylic
aliphatic groups can be unsubstituted alkyl, alkenyl,
aryl, alkaryl, and aralkyl or substituted alkyl,
alkenyl, aryl, alkaryl and aralkyl where the
substituents are groups consisting of halogen, carboxyl,
or hydroxyl.
Agents evaluated are presented in Table 1 of the
example. The focus in obtaining these materials was
availability and toxicity. Secondarily, water
insolubility was highly desired due to ease of
incorporation into existing explosives manufacturing
processes.
CA 02253480 1998-10-30
W O 97/42140 PCTrUS97/08342
-- 5 --
The agents listed in Table 1 were classified in
accordance with the four primary classifications listed
above. Classification of some of the agen~s were
- assumed based upon MSDS information since the exact
chemical structure was proprietary. Agents were
o~tained representing all four categories. Compounds
from all three subclassification referenced above for
the fatty acid esters are also represented. The list of
possible compounds that can be employed within these
categories is almost infinite due to the a7iphatic group
size, structure (branched or straight), additional
functional groups, quantity, combination, and arrange-
ment. Since the evaluation cou~d become endless, agents
were chosen to represent the widest variety practical
within each chosen category.
It is an object of this invention to reduce the
impact and shock sensitivity of HMX formulations without
significantly reducing the performance of the main
charge explosive.
It is another object of this invention to reduce
the sensitivity of HMX formulations without
significantly increasing the cost of manufacturing the
HMX formulations.
other objects and variations of this invention will
become obvious to the skilled artisan from a reading of
the following detailed specification.
BRIEF DESCRIPTION OF THE DRAWINGS
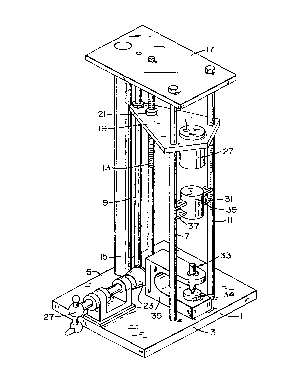
Figure 1 is a pictorial view of the HDC Impact
Machine.
CA 02253480 1998-10-30
W O97/42140 PCTrUS97/08342
DET~TT~n DESCRIPTION OF THE INVENTION
The invention is a high energy explosive
formulation characterized by reduced susceptibility to
impact and sympathetic detonation due to shock forces,
the formulation comprising ~MX and a shock sensitivity
reducing agent, the shock sensitivity reducing agent
being present in an amount effective to impart an
increase in HDC Impact Value to the formulation which is
statistically significant. A HDC Impact Value of 36.06
centimeters has been found to be statistically
significant for HMX. The shock sensitivity reducing
agent may be a quaternary ammonium compound; an anionic
aliphatic or aromatic compound; a fatty acid ester; or a
long chain amine.
Preferred quaternary ammonium compounds have the
formula
R2 +
R1 ~ R3 X
~4
wherein Rl is hydrogen, alkyl having 8-22 carbon atoms,
aryl having 6-30 carbon atoms, al~aryl having 7-30
35 carbon atoms, aralkyl having 7-30 carbon atoms, or
H(OCH2CH2)n wherein n is 1 to 50,
H(~CHCH2)n
C~3
wherein n is 1 to 50, alkaryl having 8-20 carbon atoms,
or hydroxyethyl. R2 is the same as Rl, R3 is hydrogen,
alkyl having 1-Z2 carbon atoms, aryl having 6-30 carbon
atoms, H(OCH2CH2~n - wherein n is 1 to 150, or hydroxy-
CA 02253480 1998-10-30
W O97142140 PCTrUS97/08342
ethyl, R4 is hydrogen or alkyl having ~-4 carbon atoms,
and X- is halogen, carboxylate having 2-22 car~on atoms,
nitrate, sulfate, methosulfate or phosphate.
Other preferred quaternary ammonium chloride
formulations are bis(hydrogenated tallow alkyl) dimethyl
quaternary ammonium chloride; trimethyl tallow alkyl
quaternary ammonium chloride; (CH3)3N+R Cl , wherein R
is a mixture of long chain aliphatic and unsaturated
aliphatic alkyl groups containing 14 to 18 carbon atoms;
hydrogenated tallow alkyl (2-ethylhexyl) dimethyl
quaternary ammonium methosulfate, N,N,N-tris(2-hydroxy-
ethyl) tallow alkyl ammonium acetate;
C~O
(HOCH2CH2)3N R oC 3
wherein R is a mixture of aliphatic and unsaturated
aliphatic alkyl groups containing 14 to 18 carbon
atoms;
dimethyl di(cocoalkyl) quaternary ammonium chloride;
R2N+(CH3)2 Cl , wherein R is C6 - C18 alkyl and
unsaturated alkyl groups; methyl bis(2-hydroxyethyl)
cocoalkyl quaternary ammonium chloride; trialkyl
polyalkoxyalkylene quaternary ammonium chloride; and
R3N+CH2CH2(OCH2CH2)nOH Cl , wherein R is methyl and n is
1-250.
A preferred anionic aliphatic shock sensitivity
reducing compound is sodium alkane sulfonate where the
~ alkane group has 6-18 carbon atoms.
A preferred anionic compound is a soap or detergent
based on the lithium, potassium or sodium salts of
carboxylic acids containing about 8-26 carbon atoms or
similar salts based on alkylbenzene sulfonates. Also
CA 02253480 1998-10-30
W O 97/42140 PCTnUS97/08342
-- 8 --
the salt may be a triethanolamine salt of a carboxylic
acid having about 8 to about 26 carbon atoms or
triethanolamine salts based on alkylbenzene sulfonates
wherein the alkyl groups contains 8--18 carbon atoms.
Preferred long chain amines are bis(2-hydroxyethyl)
tallow alkyl amine, (HOCH2CH2)2NR wherein R is C12-Cl8.
/R
2 2
\ R1
wherein R1 is Cl2--C18;
[H(OCH2CH2)nOCH2CH2]2N
2~
wherein R is C12 to C18 and n is 1-150, and
~Rl
tH (~CH2CH2 ) n~CH2CH2
wherein Rl is C12 to c18 and n is 1 to about 150. The
long chain amine may be ethoxylated cocoalkyl amine
where cocoalkyl is C8-C18 saturated or unsaturated
group.
Preferred fatty acid esters are glycerol esters
having the formula
H2O ~ H2OH
~HOH ~HO HO
CH2~H CH2~H HOH
wherein R is about C8 to C18,
CA 02253480 1998-10-30
WO97/42140 PCT~US97108342
Other shock sensitivity reducing compounds useful
in this invention are water soluble or water dispersible
quaternary ammonium salts which include: Arquad 2HT-75
from Akzo Chemicals Inc. (bis(hydrogenated tallow alkyl)
dimethyl quaternary ammonium chloride);
Arquad T50 from Akzo Chemical Inc. (trimethyl
tallow alkyl quaternary ammonium chloride)
(CH3)3 N+R cI where R is a mixture of long chain
aliphatic and unsaturated aliphatic groups containing 14
to 18 carbon atoms;
Arquad HTL8-MS from Akzo Chemicals Inc.
(hydrogenated tallow alkyl (2-ethylhexyl) dimethyl
quaternary ammonium methosulfate);
~ thoquad T~13-50 from Akzo Chemicals Inc. (~ J
tris (2-hydroxyethyl) tallow alkyl ammonium acetate),
(HocH2cH2)3 N R O C CH3 ,
wherein R is a mixture of aliphatic and unsaturated
aliphatic alkyl groups containing 14 to 18 carbon atoms;
Arquad 2C-75 from Akzo Chemicals Inc., dimethyl
di(cocoalkyl) quaternary ammonium chloride
R2N+(CH3)2 Cl wherein R = C6-C1~ alkyl and unsaturated
alkyl ~ r GU~a;
Ethoquad C~12-75 from Akzo Chemicals Inc. (methyl
bis~2-hydroxyethyl~ cocoalkyl quaternary ammonium
chloride);
Markstat AL-12 from Witco Chemical Corp. (trialkyl
polyalkoxyalkylene quaternary ammonium chloride); and
Staticide 30006 from ACL Inc. (a quaternary
ammonium c4mpound) (Structure proprietary.)
other useful quaternary ammonium salts are derived
from diamines, triamines or polyamines.
For example quaternary ammonium salts derived from
-
CA 022~3480 1998-10-30
W O97/42140 PCTrUS97/08342
-- 10 --
ethylenediamine; diethylenetriamine; hexamethylene-
diamine; 1-4 cyclohexane-bis-methylamine (can use cis,
trans or cis~trans mixture); phenylenediamine. Typical
salts would be hexamethyl ethylene diammonium chloride;
hexamethylene phenylene diammonium sulfate; and dimethyl
tetrahydroxyethyl 1-4 cyclohexylenedimethylene
diammonium chloride.
Water soluble anionic aliphatic compounds and
aromatic compounds which are useful include: Dehydat
93P from Henkel Corp. which is a sodium alkane sulfonate
(alkane not specified but probably C8-C18).
Soaps or detergents based on the lithium,
potassium, sodium on triethanolamine salts of carboxylic
acids containing 8 to 26 carbon atoms or similar salts
based on alkylbenzene sulfonates.
Other useful salts include: sodium octanoate,
sodium decanoate, sodium laurate, sodium myristate,
sodium palmitate, sodium stearate, sodium oleate, sodium
linoleate.
Also useful are sodium, lithium or potassium salts
of mixed acids such as those obtained from tallow and
coconut oil. A typical one would ~e a sodium salt of
mixed acids containing 12, 14, 16 and 18 carbon atoms.
Some typical useful alkylbenzene sulfonates
include: dodecylbenzenesulfonic acid, dodecylbenzene-
sulfonic acid sodium salt, dodecylbenzenesulfonic acid
triethylamine salt, nonylbenzenesulfonic acid, nonyl-
benzenesulfonic acid sodium salt, and mixed C10 to C13
alkylbenzenesulfonic acid salts. Useful sodium alkane-
sulfonates include sodium dodecanesulfonate, sodium
stearylsulfonate, and sodium myristylsulfonate. Useful
alkylnaphthalenesulfonate salts include sodium
isopropylnaphthalenesulfonate, sodium nonylnaphthalene-
sulfonate. A useful ~-olefin sulfonate is mixed
l-octene, l-decenesulfonic acid sodium salt. A useful
CA 02253480 1998-10-30
WO 97/42140 PCTrUS97/Q8342
dialkyl sulfosuccinate is di Z-ethylhexyl sulfosuccinic
acid sodium salt. A useful amidosulfonate is sodium N-
oleoyl-N-methyl taurate. A useful sulfoethyl ester of
fatty acid is sodium sulfoethyl oleate.
A useful alcohol sulfate is sodium lauryl sulfate.
Ethoxylated alcohol sulfates such as sodium poly-
ethoxyethylene sulfate; ethoxylated alkyl phenol
sul~ates; phosphate esters - usually used as a mixture
of mono, di, and triester are useful in this invention.
lo Useful fatty acid esters are glycerol esters such
as ~lycerol monostearate, glycerol distearate, and
glycerol dilaurate which are usually a mixture of mono
and diesters. Many products are derived from naturally
occurring fats such as tallow, lard, cottonseed,
safflower oil and the like and will be mixtures of fatty
acids cont~;n;ng about 12 to about 18 carbon atoms.
Also useful are polyoxyethylene esters; amine
derivatives, and bis(2-hydroxyethyl) tallow alkyl amine.
Other operable amines include dialkylethanolamines in
which the alkyl groups contain 12 to 18 carbon atoms;
ethoxylated amines such as alkyl polyethoxyethylamines
in which the alkyl group is about 12 to 18 carbon atoms,
and ethoxylated cocoamine.
Shock sensitivity reducing agents useful in this
invention exhibit anti-static properties.
DESCRIPTION OF HDC IMPACT MACHINE
The impact sensitivity of HMX explosives is
determined on a drop weight test machine comprising a
me~-hA~;sm for dropping a 5 kilogram weight from a chosen
height on a selected sample of explosive. The sample
weight is normally 0.025 or .035 grams. The sensitivity
value is expressed as the height in cm from which the
weight is dropped for the probability of an explosion to
CA 022~3480 1998-10-30
WO 97/42140 PCTrUS97/08342
- 12 -
be 50 percent.
The HDC impact machine is shown in Figure 1. The
machine comprises metal bas3 plate 1 which is generally
square, about 16 inches per side, and is about one and
one-half inches thick. On the base plate there are
located three tapped holes to receive guide rods 7, 9
& 11. Two of the holes are located about four (4)
inches from the front edge 3 of the base plate and three
(3) inches on either side of a center line ext~n~;ng
from the front edge 3 to the back on opposite edge 5 of
the generally s~uare base plate. The third hole is
located on said center line about ten and one-half
inches from the front edge 3. In the three holes are
mounted two guide rods 7 and 9 and a graduated guide
rod 11. The graduated guide rod 11 has centimeter
graduations formed thereon and are used to indicate the
height of a five kilogram weight used with the apparatus
~ cll~fied later herein). A guide rod 7 is mounted in a
hole spaced about 4 inches from the front edge 3 of the
mounting block 1. A guide rod 9 is mounted in the third
hole formed in the base plate as described above. A
fourth hole is formed in the ~ase plate 1 to receive a
lift rod 13. The hole is located eight and one-half
inches from the front edge of said bace plate. The lift
rod 13 is threaded its full length and is mounted for
rotation in a bearing (not shown) located in said fourth
hole. A fifth hole is formed in the base plate centered
and is three inches from the back edge of the base
plate 1. In this hole is mounted a support rod 15.
A top plate 17 having the dimension of ten by
thirteen inches is provided with holes positioned in the
same configuration as the holes in the base plate for
receiving the upper ends of the guide rods 7, 9 and 11,
the lift rod 13 and the support rod lS to space and hold
all five rods parallel to each other.
CA 022~3480 1998-10-30
W O97142140 PCTrUS97/Q8342
- 13 -
A magnet retainer plate 19 is provided and has
holes matching the pat~ern of those in the top plate 17
and the base plate l, with the exception of the support
rod receiving hole. The magnet retainer plate 19 is
positioned ~etween the base plate 1 and the top
plate 17. Guide rod 7 and graduated guide rod 11 pass
through the holes located on the front portion o~ the
magnet retainer plate 19 and guide rod 9 passes through
the hole located at the back of the magnet retainer
plate. The lift rod 13 is threaded through a lift rod
nut 21 which is attached to the magnet retainer pla~e
over the corresponding hole in the plate. The lift rod
is mounted in bushings for rotational movement to move
the magnet retainer plate up and down between the base
plate 1 and the top plate 17. The lift rod has a 45~
miter gear 23 attached to its lower end adjacent the
base plate 1 to cooperate with a second miter gear
mounted on a ball crank shaft and handle 27 which will,
when turned, rotate the lift rod 13 for moving the
magnet retainer plate up and down as required.
Mounted on the magnet retainer plate 79 is an
electromagnet 29 where~y the height of the magnet may be
ad~usted by the operator by turning the ball crank
handle to move the magnet retainer plate 19 up or down
as necessary.
A five kilogram weight 31 is provided and is
adapted to be held by the electromagnet. The weight is
provided with opposed flanges 37 which cooperate with
guide rod 7 and graduated guide rod 11 whereby when the
weight 31 is released from the electromagnet 29 the
weight will freely fall to contact a plunger assembly 33
which strikes an anvil 34. Mounted on the base plate 1
is an anvil and plunger holder 35. The holder is
attached to the base plate in a position to hold the
anvil and plunger directly below the five kilogram
CA 022~3480 1998-10-30
W O 97/42140 PCT~US97/08342
- 14 -
weight so that the falling weight will strike the
plunger which in turn will strike a sample located on
the anvil. Also, a second anvil surface ~not shown) is
mounted in the bottom center of the five kilogram
weight. The anvils are made from tool steel heat
treated to 56 to 60 points Rockwell Hardness. The
plunger 33 is made from tool steel heat treated to 56 to
60 points Rockwell Hardness. The plunger may be two
inches in length overall, 0.50 inches in diameter and is
tapered at near one end from 0.50 to 0.303 inches which
extends for about 3~16 of an inch to form the striker
portion of the plunger. Both ends of the plunger are
ground to be perpendicular to the center line of the
plunger. The anvils are cylinders which are one and one
half inches tall and one and one quarter inches in
diameter. The plunger is slidingly mounted in a bushing
mounted in the plunger holder 35 which is centered
directly over the second or bottom anvil 34.
In use the lift rod 13 is rotated to raise the
electro magnet to preselected heights. The five
kilogram weight will freely fall the preselected
distance to strike the upper end of the plunger which in
turn will strike a sample placed in a sample cup which
is located directly below the small end of the plunger.
The sample cup is made from brass and is 0.008 inches
thick, 0.303 inches in diameter and 0.20 inches in
height.
A detailed procedure for using the HDC Impact
machine follows:
Interferences in the test may be: 1) a machine
loosely assembled or not in proper alignment may produce
incorrect values; 2) a rough surface or cracks on the
anvil or plunger may produce low sensitivity values; 3)
insufficient or unevenly distributed sample may produce
incorrect values; 4) a sample containing glass, metal,
CA 02253480 1998-10-30
W O97142140 PCT~US97/08342
- 15 -
or other gritty matter foreign to the product may
produce low sensitivity values; and 5) wet samples or
samples containing oil, grease, and or soft plastics may
produce high sensitivity values.
Equipment needed is: 1) a sample splitter or glazed
paper; 2) caps, percussion, 0.303 in diameter, 0.~00 in
height, and 0.008 inches thick; 3) spoon, loading, 0.025
and 0.035 gm; 4) spatula, wood; 5) tong, laboratory;
6) brush, approximately 2 inches wide; 7) oven, steam
heated; and 8) a HDC Impact machine. The machine shall
be tested with a sample having a known sensitivity
range. The results are plotted on a control chart and
corrections taken if the first point fails to plot
within control limits or if 5 successive points all plot
on one side of the center line.
Position 25 brass percussion caps, with open end
up, on a flat surface. Fill the 0.025 gram loading
spoon with the dry explosive and smooth off the excess
by drawing a wooden spatula over the flat surface of the
spoon. Dump the remaining portion into one of the
prepared caps. Repeat Step 2 until each percussion cap
is loaded. Ascertain explosives to be evenly
distributed in each cap. Remove fumes and dust from the
area of the impact machine. Using the laboratory tongs,
place a loaded percussion cap on the anvil of the impact
machine. While holding the cap with the tong, insert
the plunger through the guide hole above the anvil and
into the percussion cap. Turn the electromagnet switch
to the ~ON" position. Adjust the height of the
electromagnet by turning the ball crank handle until the
base of the lower magnet arm coincides with the 35 cm
mark on the guide rod 11. Lower the safety shield (not
shown in drawing) and lift the weight vertically until
it is held in place by the electromagnet. (The weight
normally re5ts upon a safety shield while the machine is
CA 022~3480 1998-10-30
W Og7/42140 PCTrUS97/08342
being charged). Face the opposite direction from the
impact machine, turn the electromagnet switch to the
~OFF" position, allowing the weight to fall and strike
the top of the plunger. Lift the weight. ~m; ne the
percussion cap to determine if an explosion has
occurred. An exploded cap is usually disintegrated;
however, partial explosions may be determined by
inspecting the cap for parts of the rim blown away. An
explosion may also be recognized by a sharp report or ~y
smoke in the area of the plunger. Clean all unexploded
material and parts of the percussion cap from the anvil,
plunger, and base plate with a brush or cloth. Repeat
Steps 5 thru 12 raising the electromagnet 5 cm after
each non-explosion and lowering the electromagnet 5 cm
after each explosion. The first non-explosion after an
explosion is considered as the starting point of the 20
tests. Record this height in cm. Raise the
electromagnet 5 cm and repeat Steps 5 thru 12. Raise or
lower the electromagnet as required and repeat the steps
until 20 tests have been completed. Record each test
result. Assume each test exploding at a recorded height
would have exploded at greater heights. Assume each
non-explosion at a recorded height would fail to explode
at heights less than the recorded height. Perform
calculations for impact value.
CALCULATION FOR IMPACT
1. Calculate the percentage explosions at a given
height.
CA 02253480 1998-10-30
W O 97142140 PCT~US97/083~2
Explosions, % = A x lO0
B
Where A - Number of explosions at a given height
B = Total number of explosions and non-
explosions at a given height
Record the percentage explosions.
2. Calculate the impact sensitivity as follows:
Impact sensitivity, cm = C - 5 (D-50)
D-E
Where c = The lowest height in cm at which more
than 50~ explosions occurred.
D = Percentage explosions greater than
50%.
E = Percentage explosions less than 50~.
5 = Difference in height in cm of each
test.
The invention will be further illustrated by
consideration of the following examples, which are
intended to be exemplary of the invention.
~MPLE
Compositions comprising HMX and a series of shock
sensitivity reducing agents were prepared according to
- the procedure set forth. The concentrations, the shock
sensitivity reducing agents and the HDC Impact Value
- 40 re~uired for detonation at different concentrations of
the agents in the HMX are shown in Table 1. Also there
is indicated in the Table the calculated concentration
CA 02253480 1998-10-30
W O97/42140 PCTrUS97/08342
- 18 -
required for the formulation to reach the statistically
significant increase in the HDC Impact Value.
DSC scans were run on HMX and each agent. Sample
size for the analysis was 4.5 to 5.5 mg. The analysis
was performed on a DSC (Differential ScAnn;ng
Calorimeter). Samples of HMX that were prepared for
impact testing with a 3% addition of an agent were also
~nalyzed by DSc to determine compatibility. None of the
mixtures showed abnormal exotherms.
The HMX was coated with the water soluble agents by
weighing 23.75 + 1.25 gms of the dry explosive with
varying amounts of the agents to produce an end
composition ranging from 0.05~ to 10.0%. Some of the
agents came from the manufacturer alcohol wet. For the
external coating, 5 ml of alcohol (isopropyl or methyl)
was added to the weighed agent. The agent, which was
mostly dissolved in the alcohol, was added to the dry
HMX and mixed in a 100 ml beaker for 5 minutes. The
beaker and contents were placed in a steam heated oven
(200~F) for 15 minutes. The heating and stirring
procedure was repeated until the explosive appeared dry
and the odor of the alcohol was gone. The standard HDC
impact test was run on each prepared sample. The lab
procedure is described herein.
Class 1 HMX was used in all the referenced aamples.
Class 1 has a median particle size diameter range of
45 - 300~ for HMX. The influence of particle size was
determined by externally coating Class 5 HMX with
several agents used with Class 1 HMX. Class 5 HMX has a
median particle size range of 25 - 4~. Particle size
does not have a major influence on impact response at a
given agent concentration.
External coating of HMX with the water insoluble
agents presented a unique problem. Some solvents that
would dissolve the agents would also dissolve the HMX.
CA 02253480 1998-10-30
W O97t42140 PCTrUS97/08342
-- 19 --
A coating procedure was developed which took advantage
of the low melting point (50-80~C) of the water
insoluble agents. The procedure consists of weighing
- 23.75 1.25 gms of the dried explosive into a 100 ml
beaker. The agent was added to the beaker along with
5 ml of water. The mixture was placed in a steam heated
oven at 200~E for about 15 minutes which was enough time
to melt the agent. The contents of the ~eaker were
stirred for 5 minutes. The beaker was placed in the
lo oven again. The heating and stirring procedure was
continued until all the water had evaporated. Impact
results indicate that this procedure produced homogenous
samples.
A limited number of conventional recrystallizations
were done in a 3 liter still using water soluble agents
to determine if the decrease in sensitivity that was
obtained from the external coatings would apply to the
recrystallization process.
The solu~le agent chosen for the evaluation with
HMX was bis(hydrogenated tallow alkyl~ dimethyl
quaternary ammonium chloride (2HT-75 - Akzo Chemicals).
HMX recrystallized with this agent (2% of the product)
had an impact of 60.7 cm which compares favorably to the
66.3 cm impact found with 2~ of the agent externally
coated as described above. It should be noted that the
filtered HMX from the recrystallization probably
contained less than 2% because of the loss of the agent
in the water filtrate. However, externally coated HMX
with only 0.1% agent had an impact of 65.0 cm.
A limited number of recrystallizations of KMX with
water insoluble agents were done. The insoluble agent
chosen for evaluation was distilled monoglyceride
(PA 208 - Eastman Chemical Company). The recrystallized
HMX containing 3% agent (3% of the product) had an HDC
Impact Value of 44.3 cm. Surface coating of 3% of the
CA 02253480 1998-10-30
W O 97/42140 PCTrUS97/08342
- 20 -
agent on HMX had an impact of 35.o cm. The
recrystallized HMX containing 3% of the agent had an
impact of 44.3 cm as compared to 51.3 cm when surface
coated. The close agreement between the impact results
obtained from recrystallizing or coating with the agent
in this case is due to the fact that little or no loss
occurred during filtration as occurs with the water
soluble agent.
The statistically significant impact values set
forth in the Table were determined as set forth.
A normal untreated HMX product has known average
and standard deviation values when tested on a st~n-l~rd
Holston impact machine. The impact value of a given
sample would not be expected to be more than 3 standard
deviation units larger than the average (the probability
of being less than 3 units above average from normal
distribution tables is 0.9987). Thus, if an agent is
added to a sample and the impact value of this sample is
more than 3 st~n~rd deviation units above the average,
it can be assumed that the additive has caused this
result and the result is said to be statistically
significant.
For the experiments, samples of a fixed product
with varying amounts of agent were prepared and the
impact value of each sample was determined. The impact
results were plotted against the %-additive in each
sample. From this graph, a %-additive above which the
impact value becomes more than 3 standard deviation
units greater than the average can be determined.
Observation of these graphs (covering a wide range
of products and %-additives) show that the curves, in
the region where the 3 standard deviation value
(critical value) is exceeded, are essentially linear
with some random variation. Based upon this, a linear
curve of the form
CA 02253480 1998-10-30
WO 97142140 ~lIU~97/08342
- 21 -
Y = mX + b
where Y = impact value
and x = %-additive
was fitted to the data by the method of least squares.
This formula was then used to calculate the ~-additive
at which the impact value ~ecomes greater than the
critical value.
This illustrative procedure describes using HMX as
the explosive component and bis(hydrogenated tallow
alkyl)dimethyl quaternary ammonium chloride (Arquad
2HT-5 from AKZO Chemical) as the shock sensitivity
reducing agent. This procedure illustrates the
preparation of a final mixture containing 99~ HMX and 1
Arquad 2HT-75. Other concentrations are prepared by
varying the proportions of the ingredients in the
mixture.
Compositions comprising HMX and a shock sensitivity
reducing agent (Ar~uad 2HT-75) are prepared following
the procedure set forth below:
A. Weigh 0.3333 grams of the Arquad 2HT-75 into a
100 ml beaker.
B. Add S ml H2O to provide a mixing media for
coating the HMX crystals with the Arquad
2HT-75. Other liquids such as isopropanol
will also work.
C. Stir the mixture of Arquad 2HT-75 and liquid
with a rubber tipped glass tipping rod until
the 2HT-75 is well dispersed.
CA 02253480 1998-10-30
WO 97/42140 PCTrUS97/08342
D. Weigh 24.7500 gms of HMX and pour into a
beaker containing the Arquad 2HT-75.
E. Stir the mixture with a rubber tipped stirring
rod for about 5 minutes.
. Place the beaker in a steam heated oven at
about 200~F for 15 minutes.
G. Remove the sample from the oven.
H. Stir the mixture with the rubber tipped glass
stirring rod for 5 minutes.
I. Place the beaker in the steam heated oven
(200~F) for another 15 minutes.
3. Remove the sample from the oven and stir for 5
minutes.
K. Weigh and record the weight of the beaker.
L. Return the beaker to the oven for 15 minutes.
M. Stir for S minutes and weigh the beaker.
N. Continue the heating and stirring procedure
until there is no weight loss after heating.
Table 1 also shows the test results using other
shock sensitivity reducing compounds, identified in the
Table, mixed with HMX in various concentrations. The
agents tested are representive of the large number of
compounds which are useful in this invention.
Table 1
o
Calculated Concentration
Required to Reach the
Conoentration % Statistically Significant ~
Shock Sensitivity in HMX HDC Impact HDC Impact Value
Reducinq Compound Formulation Value (cm) of 36.06 cm
Bis(hydrogenated tallow0.00 31.4 0.188%
alkyl)dimethyl quaternary 0.05 33.0
ammonium chloride - 0.10 33.6
Arquad 2HT-75 from 0.50 43.8
AKZO Chemicals Inc. 1.00 46.6
2.00 45.0
3.00 50.9
4.00 53.3 n
10.00 95.0
Trimethyl tallow alkyl 0.00 31.4 0.082%
quaternary ammonium 0~050 33.0
chloride - 0.100 37.7 ~ ~
Arquad T-S0 from 0.500 41.0 ~
AKZO Chemicals Inc. 1.000 37.9
2.Q0 49.3
3.00 47.3
4.00 48.4
10.00 89.0
Hydrogenated tallow 0.00 31.4 0.061%
alkyl(2-ethylhexyl) 0.10 39.0
dimethyl quaternary 1.00 46.7 ',
ammonium methosulfates 2.00 56.5
Arquad HTL8-MS from 3.00 58.5
AKZO Chemicals Inc. w
Table 1 (Cont'd.)
o
Calculated Concentration
Required to Reach the
Concentration % Statistically Significant ~
Shock Sensitivity in HMX HDC Impact HDC Impact Value
Reducinq Compound Formulation Value (cm) of 36.06 cm
Dimethyl di(cocoalkyl 0.00 31.4 0.654%
quaternary ammonium 0.10 34.2
chloride 1.00 37.9
Arquad 2C-75 from 2.00 44.0
AKZO Chemicals Inc. 3.00 54.2 D
N,N,N-Tris(2-hydroxy- 0.00 31.4 0.366~ ~
ethyl) tallow alkyl 0.10 33.1 n
ammonium acetate 1.00 43.7
(Ethoquad T~13-50 2.00 44.2
from AKZO Chemicals Inc.3.00 62.5 ~
Methyl bis(2-hydroxy- 0.00 31.4 0.088% ~ O
ethyl) cocoalkyl .10 36.7
quaternary ammonium 1.00 35.8 o
chloride 2.00 44.0
Ethoquad C/12-75 3.00 55.0
Trialkyl polyalkoxy- 0.00 31.4 0.482%
alkene quaternary 0.10 34.2
ammonium chloride 1.00 35.0
MARKSTAT AL-12 2.00 57.9
Witco Chemical Corp. 3.00 63.2 ù~
~w
Table 1 (Cont'd.)
Calculated Concentration
Required to Reach the
Concentration % Statistically Significant ~
Shock Sensitivity in HMX HDC Impact HDC Impact Value
Reducin~ Compound Formulation Value rcm) of 36.06 cm
Polyether (Trade 0.00 31.4 0.507%
Secret) 0.10 35.8
MARKSTAT AL-14 1.00 - 38.7
Witco Chemical Corp. 2.00 37.2
3.00 48.0 D
Quaternary Ammonium 0.00 31.4 0.299%
Compound~ (Proprietary)0.10 35.0 n
Staticide 3000G 1.00 44.3
~itco Chemical Corp. 2.00 52.1 ~ o
3.00 74.3
Sodium Alkane Sulfonate0.00 31.4 0.081% t
Dehydat 93P 0.10 37.1 o
Henkel Corporation 1.0 50.8
2.00 50,0
3.00 48.3
Distilled Monoglycerides0.00 31.4 0.314%
PA-208 0.05 31.2
Eastman Chemical Company0.10 30.0 t
0.50 40.0
1.00 35.0
1.50 37~5
2.0 39.1
3.0 51.3
4.0 53.5
10.0 95.0
Table 1 (Cont'd.)
~o
Calculated Concentration
Required to Reach the
Concentration % Statistically Significant ~
Shock Sensitivity in HMXHDC Impact HDC Impact Value
Reducing Compound Formulation Value (cm) of 36.06 cm
Distilled Monoglycerides 0.00 31.4 0.076%
Myverol 18-99 0.10 37.5
Eastman Chemical Company 1.00 41.0
2.00 42.5
3.00 49.2 D
Ethoxylated Tallow Amines 0.00 31.4 1.76%
Ethomeen T/120.05 28.0 n
AKZO Chemicals Inc. 0.10 33.3
0.50 31.4 ~ ~
1.00 3S.8 N
1.50 35.8
2.00 31.5 ~ O
3.00 43.3
4.00 40.8 o
10.00 65.0
Ethoxylated Cocoalkyl 0.00 31.4 0.072%
Amines 0.10 37.9
Kemamine AS-6501.00 45
Witco Chemical Company 2.00 46.7
3.00 47.1
Mono-diglycerides 0.00 31.4 0.31% ~'
Glycolube 1400.10 46.2
Glyco Inc. 1.00 41.7 x
2.00 55.0
3.00 55.0
Table 1 (Cont'd.)
Calculated Concentration
Required to Reach the
Concentration % Statistically Significant ~
Shock Sensitivity in HMX HDC Impact HDC Impact Value
Reducinq Compound Formulation Value (cm) of 36.06 cm
Dicarboxylic Acid Ester0.00 31.4 0.105%
of Saturated Aliphatic 0.10 35.8
Alcohols - Loxiol G60 1.00 41.3
Henkel Corporation 2.00 44.2
3.00 . 40.0
Fatty Acid Ester 0.00 31.4 0.041~ ~~
(Proprietary) 0.10 42.5 n
Dehydat 8312 1.00 44.2
Henkel Corporation 2.00 42.2 o
3.00 51.2
Quaternary Ammonium 0.00 31.4 0.071~ ~ ~
Compound - Cling Free 0.10 38.0 ,o
Extract - Proprietary 1.00 38.8 O
Beckner Inc. 2.00 56.1
3.00 48.2
Partial Polyglycerol 0.00 31.4 0.369
Fatty Acid Ester 0.10 34.2
(Proprietary) 1.00 42.5
Dehydat VAP 1726 2.00 41.2
Henkel Corporation 3.00 47.6
w
CA 02253480 1998-10-30
WO97t42140 PCT~US97/08342
- 28 -
The invention has been described in detail with
particular reference to preferred embodiments thereof,
but it will be understood that variations and
modifications will be effected within the spirit and
scope of the invention.