Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 022~3872 1998-11-06
W O 98/38940 PCT~US98/03936
-- 1
Method and Apparatus for Altering the Osmotic Pressure
of Cryopreserved White Stem Cells
Technical Field
The following invention relates generally to instrumentalities which take
cryopreserved white stem cells from their frozen condition to a constituency which
is compatible with transfusion without compromising the vitality of the white stem
cells.
Background Art
Transfusions inv~lving white stem cells can provide profound therapeutic
benefits in certain situations. Ongoing research by the present inventors has
continued to shed light on phenomena which can appreciably alter the vitality ofthe white stem cells, thereby improving efficacy.
One issue involves the white stem cells themselves, their procurement,
l 5 concentration and preservation for subsequent use. Details appurtenant thereto are
reflected in the present inventors' co-pending application Serial No. 08/349,747,
filed December 5, 1994.
The following citations reflect activity by third parties known to applicants
and of record in the above-referenced pending application. These citations are
included to discharge applicants' acknowledged duty to disclose relevant prior art. It
is submitted, however, that none of these citations teach signally nor render
obvious when considered in any conceivable combination the next of instant
invention set forth hereinafter.
PATENT NO. ISSUE DATE INVENTOR
2,702,034 February 15, 1955 Walter
3,187,750 June 8, 1965 Tenczar, Jr,
4,004,975 January 25, 1977 Lionetti, et al.
4,098,456 July 4, 1978 Bayham
4,332,122 June 1, 1982 Williams
4,343,793 August 10, 1982 Wissler
4,744,907 May 17, 1988 Klimchak
4,887,411 December 19, 1989 Rondeau, et al.
4,902,287 February 20, 1990 Carmen, et al.
4,937,194 June 26, 1990 Pattillo, et al.
4,969,882 November 13, 1990 Carmen, et al.
5,004,681 April 2, 1991 Boyse, et al.
5,023,043 June 11, 1991Kotzlowski, et al.
5,101,017 March 31, 1992Rubinstein, et al.
. . .. _ .
CA 022~3872 1998-11-06
: W 0 98/38940 PCTrUS98/03936
-2-
5,104,788April 14, 1992 Carmen, et al.
5,154,716October 13, 1992Bauman, et al.
5,192,553March 9, 1993 Boyse
5,316,681May31, 1994 Serres
5,397,479March 14, 1995 Kass, et al.
FOREIGN PATENT DOCUMENTS
PATENT NO.ISSUE DATE INVENTOR
WO 91/02202February 21, 1991 Richard
WO 92/16800October 1, 1992 Richard
WO93/03891 March 4, 1993 Knippscheer
OTHER PRIOR ART (Including Author Title Date, Pertinent Pa~es, Etc.)
Korbling, et al., Transfusion "Description of a Closed Plastic Bag System for the
Collection and Cryopreservation of Leukaphersis-Derived Blood Mononuclear
Leukocytes and CFUc from Human Donors", Volume 20, Number 3, pages 293 - 300,
l 5 May-June 1960.
Rubinstein, et al., Stored Placental Blood ~or Unrelated Bone Marrow
Reconstitution, May 1993, 27 Pages.
Rubinstein, et al., Processing And Cryopreservation Of Placental/Umbilical Cord
Blood For Unrelated Bone Marrow Reconstitution, October 1995, 4 Pages.
The other prior art listed above, catalog the prior art of which the applicants
are aware and are tendered to discharge applicants' acknowledged duty to disclose
prior art. These references diverge even starkly from the instant invention
specifically distinguished hereafter.
Disclosure Of Invention
2 5 The instant in~ention takes the cryoprotected white blood cells of the
previous pending application and conditions the unfrozen white stem cells for
subsequent transfusion. In the above-referenced patent application, the white stem
cells will have been modified with a starch, such as HES, and cryopreservatives
including DMSO and Dextran. The DMSO is understood to pass through the walls
of the white stem cells and displace water therein, raising the osmotic pressure of
the white stem cells. Concurrently, the Dextran further insulates the white stemcells by their affinity to the outer periphery of the cell, surrounding the cell and
further displacing the water. It is the sequestration of the water from the white stem
cells which protect the cells from the sharp crystalline nature of the water as it
freezes and protects the white stem cells by minimizing the crystalline water's
affinity to puncture the cells. However, prior to transfusion, the osmotic pressure
within the white stem cells must be returned to a lower pressure compatible withambient conditions within the recipient of the white stem cells in order to enhance
CA 022~3872 1998-11-06
WO 98/38940 PCT/US98/03936
~ -- 3 --
the vitality of the cells. Otherwise, the white stem cell's vitality would be
compromised by the pressure differential upon transfusion to the recipient. In
addition the DMSO should be diluted and a majority of the DMSO removed prior to
cell transfusion.
In order to achieve pressure normalization, the white stem cells are first
thawed and then transferred to an aseptic transfusion bag having air and a
volumetric capacity approximately eight times greater than the capacity of the
storage container originally housing the frozen white stem cells. Assume that the
white stem cell freezing bag initially held 25 milliliters of product. Once transferred
10 to the transfusion bag, a volume of sterile saline preferably six (6) times greater (e.~$.,
in the present case, 150 milliliters of sterile saline) is admitted into the bag from a
diluent bag. Preferably, this dose of sterile saline is admitted at a slow rate, i.~. a drop
at a time, ~ia a drip reducer preferably o~er a four to ten minute span. This slow
drip rate allows the osmotic pressure to be reduced, gently, asymptotically
15 approaching the recipient's osmotic pressure. Thus, after the volume of sterile
saline has been mixed into the transfusion bag, the white stem cells will have been
pressure normalized. Further, the DMSO is caused to go into solution with excesssaline. The white stem cells are thereafter sequestered from the DMSO/saline
solution .
The transfusion bag can now administer the white stem cells by a transfusion
IV coupling. The effect of the dilution by using sterile saline involves the gentle
reduction of osmotic pressure in the white stem cells from an elevated pressure
(compared to ambient osmotic pressure of a transfusion recipient) to one which is
compatible with the recipient's osmotic pressure. This assures that the transfusion
25 will not initiate white stem cell fracture upon transfusion which would alter the
treatment s efficacy. Also dilution of the DMSO, suspension of the DMSO and
removal of the DMSO from the white stem cells reduces the likelihood of DMSO
induced side effects.
Industrial Applicabilitv
The industrial applicabillity of this invention shall be demonstrated through
discussion of the following objects of the invention.
Accordingly, it is the primary object of the present invention to provide a
new and novel method and apparatus for preserving the vitality of cryopreserved
white stem cells.
It is a further object of the present invention to provide an instrumentality
which delivers the white stem cell with an osmotic pressure compatible with the
osmotic pressure of an intended recipient while simultaneously diluting the
.
CA 022~3872 1998-11-06
W 098/38940 PCT~US98103936
~ -4-
cryopreservative with a diluent, such as saline. The DMSO is sent into solution
with the saline.
It is a further object of the present invention to provide an instrumentality asset forth above which can achieve reliability and repeatability.
It is a further object of the present invention to provide an instrumentality asset forth above which is extremely safe to use.
Viewed from a first vantage point it is an object of the present invention to
provide a method for normalizing the osmotic pressure of frozen white stem cellshaving a cryopreservative for administration to a patient, the steps including:
thawing the white stem cells, diluting the cryopreservative, and reducing the
osmotic pressure of the white stem cells prior to administration to the patient.Viewed from a second vantage point it is an object of the present invention to
provide an aseptic apparatus for taking a white stem cell bag having frozen
cyroprotected white stem cells and conditioning the white stem cells prior to
therapeutic administration to a patient, comprising, in combination: a first aseptic
bag having means for accessing an interior of the white stem cell bag to receivewhite stem cells therefrom, means for reducing the osmotic pressure of the
cryoprotected white stem cells, and means for transfusing the pressure correctedwhite stem cells.
Viewed from a third vantage point it is an object of the present invention to
provide a previously frozen white stem cell product having a viable white stem cell
concentration of at least 80% and an osmotic pressure compatible with a recipient.
These and other objects will be made manifest when considering the
following detailed specification when taken in conjunction with the appended
drawing figures.
Brief Description Of Drawings
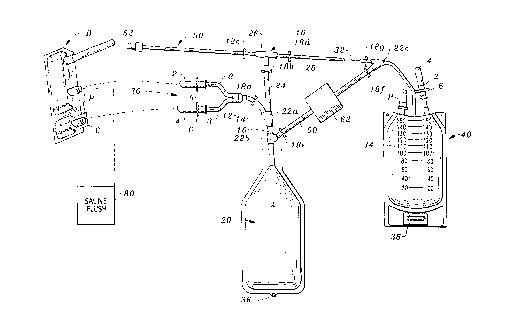
Figure 1 is a perspective view of the apparatus of the present invention.
Figure 2 is a flow chart of the methodology associated with the bags of figure
1.
3 0 Figure 3 is a schematic depiction of the white stem cell being diluted.
Best Mode(s) For Carrying Out The Invention
Referring to the drawings, wherein like reference numerals denote like parts
throughout the various drawing figures, reference numeral 10 is directed to the bag
set according to the present invention.
In its essence, the bag set 10 includes a transfusion bag 20 which receives the
white stem cells including cryopreservative from a white stem cell bag B via spikes
CA 022~3872 1998-11-06
W 098t38940 rCT~US98/03936
-5-
30. The transfusion bag 20 receives dilution, preferably in the form of sterile saline
from a diluent bag 40. After the white stem cells have been diluted with saline, the
solution containing the cryopreservative is moved from the transfusion bag 20 tothe diluent bag 40 and the white stem cells remaining in the transfusion bag 20 are
5 transfused via transfusion tube 50.
More specifically, the white stem cell bag B includes a pair of spaced parallel
portals P. These portals P are accessed by a pair of spaced parallel spikes 2 spaced
from each other a distance comparable to the portals P, once the caps C from theportals P have been removed. Similarly, these spikes 2 are provided with covers 4
l0 which must be removed in order to access the spikes which are protected
therewithin. Slightly upstream from the spikes 2 are spacers 6, which provide a
positive stop abutment to limit the degree of incursion of the spikes 2 within the bag
B. Each of the spikes has a hollow interior allowing the white stem cells and
cryoprotectant, when thawed, to pass within tubes 8 that in turn communicate ~ia a
l 5 manifold 12 to a conduit 14 leading into a channel 16 and thence into the
transfusion bag 20. Because the bag B is substantially full, it is preferred that the
transfusion bag 20 include aseptic air A therewithin to provide ease of transfereither by squeezing air from the transfusion bag 20 into bag B and/or squeezing the
contents from bag B to thwart vapor lock. In other words, air can be allowed into
20 the bag B whereupon the cryoprotected white stem cells can be released from the bag
B into the transfusion bag 20.
Once the cryoprotected white stem cells have been introduced into the
transfusion bag 20, the conduit 14 is occluded with a clamp 18a. The conduit 14
communicates with the channel 16 via a Y-adapter 22a. The Y-adapter 22a also
25 allows access to the interior of the transfusion bag 20 via a passageway 24. The
passageway 24 is protected by another clamp 18b. The passageway 24 communicates
with a T-adapter 26 which is protected on a left side by a clamp 18c and on the right
side by clamp 18d. Clamp 18d controls access between the transfusion bag 20 and the
diluent bag 40 via an access 28. Interposed along access 28 is a drip reducer 32. This
3 0 drip reducer 32 controls the rate at which fluid proceeds through access 28 from the
diluent bag 40 to the transfusion bag 20 once the appropriate clamps 18 have been
manipulated.
Preferably, the diluent bag 40 is provided with gradations 34 indicating
volume. The diluent bag 40 includes a holder 36 as does the transfusion bag 20 to
3 5 allow each bag to be supported in an elevated position. The diluent bag 40 also is
preferably provided with a spike 2, spike cover 4 and spacer 6 as shown. In addition,
the diluent bag 40 is similarly provided with a port P comparable to the ports on the
white stem cell bag B.
CA 022~3872 1998-11-06
W O 98138940 PCT~US98/03936
-6
Saline is initially transferred from the diluent bag 40 to the transfusion bag 20
at a controlled rate via the drip reducer 32 by opening a clamp 18g (between reducer
32 and diluent bag 40), opening clamps 18d and 18b and closing all other clamps.Typically, the process can take four to ten minutes. Preferably, the dilution is equal
to the six times the volume of the contents of the white stem cell bag B and proceeds
at a slow rate. For example, assume 25 milliliters of cryoprotected white stem cells
have been moved into the transfusion bag 20. 150 milliliters of saline are
administered from the diluent bag 40 at a slow rate. The saline and the
cryoprotected white stem cells are mixed. This is done at a slow rate because this
l 0 reaction is exothermic and achieves the object to preserve the vitality of the white
stem cells.
After gentle mixing in the transfusion bag 20, the osmotic pressure of the
white stem cells has been normalized to approximately a pressure which the whitestem cells will experience when transfused into a patient. Further, the
l 5 cryoprotectant will have passed into solution with the excess diluent, saline. Next,
the cryoprotectant saline solution in the transfusion bag 20 is delivered to thediluent bag 40 through a pipeline 60. Pipeline 60 is accessed via another "Y" adapter
22b located on channel 16 and merges into access 28 between the drip reducer 32 and
diluent bag 40 via another Y-adapter 22c. A low micron filter 62, interposed in
pipeline 60, allows only the cryoprotectant saline solution therebeyond. Filter 62 is
optional and instead (or in combination therewith) the cryoprotectant solution can
be decanted or expressed off the white stem cells. Centrifuging the transfusion bag
20 can assist in driving the larger, heavier white stem cells to the bottom of bag 20.
The pipeline 60 is protected at both ends by clamps 18, one clamp 18e nearer
transfusion bag 20 and clamp 18f nearer diluent bag 40. The drip reducer 32 is also
cut off from pipeline 60 via a clamp 18g located on access 28 between the pipeline 60
and the reducer 32. Clamps 18a and 18b are also closed when removing fluid from
transfusion bag 20 to diluent bag 40 through filter 62.
A saline flush from source 80 may next wash out bag B and then be
3 0 introduced into transfusion bag 20 as was done with the initial contents of bag B (as
described above). Alternatively, the saline flush of bag B can be performed before
removing the cryoprotectant/saline solution from transfusion bag 20 to diluent bag
40 should it appear desireable to "wash" the residual cryoprotectant from the bag B
flush.
3 5 Next, the clamp 18b (controlling access to the passageway 24) is opened and all
other clamps (18a, 18e) along passageway 24 are closed. The clamp 18d (to the right
of T 26) is closed; the clamp 18c to the left of T 26 is opened (i.e. along transfusion
tube 50). The transfusion bag 20 is hung on an IV pole via its hook 36 and a
CA 022~3872 1998-11-06
W 098/38940 PCTrUS98/03936
transfusion connection 52 of transfusion tube 50 is connected to a conventional IV
for administration to the recipient.
Moreover, having thus described the invention, it should be apparent that
numerous structural modifications and adaptations may be resorted to without
5 departing from the scope and fair meaning of the instant invention as set forth
hereinabove and as described hereinbelow by the claims.