Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02254357 1998-11-17
Docket No. 337462001200
DIAZONIUM ION ASSAY REAGENTS AND
METHODS FOR THEIR USE
TECHNICAL FIELD
This invention relates to stable diazonium ion compounds, and methods for
their use in conducting assays for the detection and quantitative analysis of
bilirubin
in plasma, blood or other samples.
BACKGROUND ART
Bilirubin is a principal component of bile pigment in body fluid. Bilirubin
present in serum is a product of the decomposition of heme originating from
hemoglobin in red blood cells. Two fractions of bilirubin are present in blood
serum,
unconjugated and conjugated bilirubin. Conjugated bilirubin is bilirubin which
is
conjugated with glucuronic acid in the liver and rendered water soluble. The
conjugated bilirubin also is referred to as "direct" bilirubin, and the
bilirubin not
conjugated with glucuronic acid is referred to as "indirect" bilirubin or
unconjugated
bilirubin. Normally, only small amounts of bilirubin are found in the blood,
the
normal concentration being for direct bilirubin up to about 0.25 mg/100 mi
serum
(_< 4.3 mol/L); and for indirect bilirubin up to about 0.75 mg/100 ml serum
pa-187428 v5
CA 02254357 2007-06-27
(S 12.7 mol/L). The content of bilirubin in blood increases with an increase
in
decomposition of hemoglobin and a decrease in liver function.
The determination of both bilirubin fractions is of importance in medical
diagno4is. Normally, bilirubin is excreted from the gall bladder with the
biliary fluid
into the intestine. However, this mechanism is disturbed in various disease
states.
Thus, for example, in the case of increased hemoglobin breakdown, the
bilirubin
conjugation system can be overloaded so that the ratio of direct/indirect
bilirubin is
changed. In the case of liver cell damage, disturbances of the outflow in the
biliary
capillary or bile duct obstructions, the excretion of the bilirubin via the
gall bladder
into the intestine is reduced or completely blocked. This leads to increased
bilirubin
concentrations in the blood. The absolute concentration of the bilirubin and
the ratio
of direct/indirect bilirubin can thereby be influenced. Thus, from the
measurement of
both values, important diagnostic conclusions can be made regarding the nature
and
localization of certain diseases of the liver, gall bladder and intestinal
tract.
Generally, the total bilirubin is usually determined first, and then the
direct bilirubin
content is measured. The indirect bilirubin portion is obtained from the
difference
between the two values. Methods for measuring serum bilirubin are reviewed in
Doumas and Wu, Critical Reviews in Clinical Laboratory Sciences, 28:415-445
(1991); and Lott and Doumas, Clin Chem., 39:641-647 (1993).
Analytical tests which pernut the quantitative analysis of bilirubin are very
useful clinically. The most widely used assay for bilirubin has been the so
called
diazo method. In the diazo method, a sample suspected of containing bilirubin
is
contacted with a reagent composition which includes a diazonium salt. The
diazonium salt reacts with bilirubin to form two azobilirubin fiagments. The
2
CA 02254357 2007-06-27
azobilirubin has an extinction coefficient which is higher than that of
bilirubin itself
and is easily detectable.
Many diazonium salts have been used in the diazo method for determining
bilirubin. For example, diazotized sulfanilic acid couples with bilirubin to
give a
yellow diazobilirubin pigment. Details of the diazo method for quantitative
analysis
of bilirubin are described in Doumas et al., Clin Chem., 31:1779-1789 (1985);
M. Michaelsson, Scand J. Clin. Lab. Invest., 13 (Suppl.), 1-80 (1961); H.
Malloy,.T.
Biol, Chem., 119, 481(1939); and Z.K. Shihabi, et al., American Journal
ofMedical
Technology, 43(10), 1004-1007 (1977).
Other diazonium salts, such as 2,4- and 2,5-dichlorophenyldiazonium salts,
have been used for the detection of bilirubin in serum and urine. However,
methods
using these diazonium salts are known to be relatively insensitive, and some
of these
diazonium salts, when dry, are explosively unstable, i.e., subject to shock
induced
decomposition. U.S. Patent No. 4,468,467 to Babb et al. Another diazonium
compound which has been used for the determination of bilirubin is diazotized
sulfanilamide. Chin-Chung Chen et al., Clfn Chem., 26:990 (1980). SynermedT"
(Synermed, Inc., Quebec, Canada) total bilirubin reagent is commercially
available
which includes a stabilized diazonium salt of 3,5-dichloroaniline, 3,5-
dichlorophenyl
diazonium tetrafluoroborate, which reacts with bilirubin to form azobilirubin
which
absorbs maximally at 540 nm. The red azobilirubin formed can be shifted to a
blue
color which absorbs at 600 nm upon the addition of alkali. Caffeine and
surfactants
are used as reaction accelerators.
U.S. Patent No. 4,468,467 to Babb et al. describes certain substituted
sulfanilamide and carbonamide diazonium salts for bilirubin assays. U.S.
Patent No.
4,902,477 to Katsuyama et al. discloses an analytical element for quantitative
analysis of bilirubin by a diazo method, which includes certain aryldiazonium
salts
3
CA 02254357 1998-11-17
Docket No. 337462001200
having in the aryl group a substituent which is an alkoxycarbonyl group, an
alkylaminosulfonyl group or an alkylaminocarbonyl group. U.S. Patent No.
4,892,833 to Weiss et al. discloses aryldiazonium salts wherein the aryl group
is
substituted with halogen groups and lower alkoxy groups for use in the
determination
of bilirubin.
Many of the assay reagents used in current assays have problems associated
with their use. For example, in the case of automated bilirubin assays using a
commercially available dichloroaniline diazonium tetrafluoroborate salt, the
diazonium form of the chloroaniline derivatives is known to interact strongly
with
indican, a compound found in the serum of renal dialysis patients making it
unsuitable for this sample type. Hemolyzed samples also cannot be used in many
of
the assays due to extensive interference by hemoglobin. Some of the reagents
have a
relatively low solubility in aqueous systems, thus reducing their usefulness
in these
systems. Additionally, many of the reagents are unstable in the liquid form,
cannot
be readily transported, and do not have a useful shelf-life.
It is an object of the invention to provide diazonium ion compounds which
can be used as assay reagents for the detection of bilirubin in body fluid
samples. It
is a further object of the invention to provide diazonium ion compounds which
are
thermally stable making long distance shipping more feasible. It is yet
another object
of the invention to provide diazonium ion compounds which are thermally
stable,
have a long term shelf-life, and may be stored while retaining activity, for
example,
for periods of one year or more.
4
pa-187428 v5
CA 02254357 1998-11-17
Docket No. 337462001200
DISCLOSURE OF THE INVENTION
Diazonium ions which are useful as reagents for the assay of bilirubin content
in a sample, such as a body fluid sample, are provided. Also provided are
methods of
assaying to detect or quantitate bilirubin in a sample, wherein a sample
suspected to
contain bilirubin is contacted with a diazonium ion compound as disclosed
herein,
and then the product of the reaction of the diazonium ion and the bilirubin is
detected,
for example, spectrophotometrically.
In one embodiment, the diazonium ion compounds of Formula I are provided:
+N N
R5 / Ri
I
~
R4 R2
R3
Formula I
wherein at least one of R,, R2, R3, R4 or R5 is H, alkyl, sulfonate or nitro,
and
wherein the compound is capable of reacting with bilirubin in a sample to
produce a
detectable product.
Preferred are compounds of Formula I wherein:
one of R,, R2, R3, R4 or RS is C1-C3 alkyl, preferably methyl;
one of R,, R2, R3, R4 or R5 is sulfonate or nitro, preferably nitro; and
the remainder of R,, R2, R3, R4 and R5 are H.
In one preferred embodiment, the diazonium ion is 2-methyl-3-nitroaniline
diazonium ion (2-methyl-3-nitro-l-benzenediazonium ion), having the structure:
5
pa-187428 v5
CA 02254357 1998-11-17
Docket No. 33 7462001200
+N N
CH3
N02
2-methyl-3-nitroaniline diazonium ion
Other preferred compounds include 4-aminotoluene-3-sulfonic acid
diazonium ion.
Preferred compounds are diazonium ion compounds which are formed from
precursor compounds including an amine group, wherein the precursor amine
compounds have a solubility of at least about 0.038 mg/ml in acidic aqueous
solutions, e.g., in 100 mM HCl at a pH of about 1Ø
Also provided are reagent compositions including the diazonium ions. The
reagent compositions may be in liquid or solid form, and further may include
other
components, such as buffers, carriers and/or solubilizers. Salts including the
diazonium ion and a counteranion also are provided. Preferred salts include
tetrafluoroborate, hexafluorophosphate, and metal double salts such as the
zinc
double chlorides.
Using the assay methods disclosed herein, the total amount of bilirubin in a
sample may be quantitated. In another embodiment, the sample includes direct
and
indirect bilirubin, and the method may further include detecting the
concentration of
direct and indirect bilirubin in the sample. Thus, the level of direct,
indirect, and total
bilirubin in a sample may be measured and correlated with the presence or
absence of
a disease or disorder, for example, of the liver, gall bladder or intestines.
6
pa-187428 v5
CA 02254357 2007-06-27
In accordance with one aspect of the present invention, there is provided a
method of
assaying for bilirubin in a sample, the method comprising: a) contacting a
sample suspected
to contain bilirubin with a diazonium ion compound of Formula I:
+N N
R5 R,
R4 R2
R3
wherein one of RI, R2, R3, R4 or R5 is methyl; one of Rl, R2, R3, R4 or R5 is
sulfonate
or nitro; and the remainder of RI, R2, R3, R4 and R5 are H; b) permitting the
compound to
react with bilirubin in the sample to produce a detectable product; and c)
detecting the
product.
6a
CA 02254357 1998-11-17
Docket No. 337462001200
BRIEF DESCRIPTION OF THE FIGURES
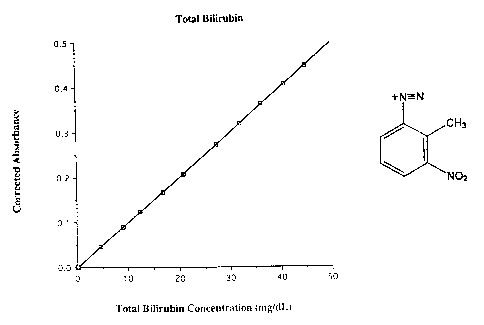
Figure 1 is a graph of absorbance vs. total bilirubin concentration obtained
in
a bilirubin assay using 2-methyl-3-nitroaniline diazonium ion.
BEST MODE FOR CARRYING OUT THE INVENTION
Diazonium compounds are provided which are useful as assay reagents. In
particular, the diazonium compounds are useful in assays for detecting or
quantitating
bilirubin in a body fluid sample. In one embodiment, diazonium compounds of
the
general Formula I below are provided:
+N=N
R5 ~ R,
I
~
R4 R2
R3
Formula I
wherein at least one of R,, R2, R3, R4 or RS is H, alkyl, sulfonate or nitro,
and wherein
the compound is capable of reacting with bilirubin in a sample to produce a
detectable
product. Preferred alkyl groups include C 1-C3 alkyl groups including methyl,
ethyl
and propyl. A particularly preferred alkyl group is methyl.
In a preferred embodiment:
one of R,, R2, R3, R4 or RS is C 1-C3 alkyl, preferably methyl;
7
pa-187428 v5
CA 02254357 1998-11-17
Docket No. 337462001200
one of R,, R2, R3, R4 or R5 is sulfonate or nitro, preferably nitro; and
the remainder of R,, R2, R3, R4 and RS are H.
Exemplary compounds include 4-aminotoluene-3-sulfonic acid diazonium
ion. Other exemplary compounds include the diazonium ions of 2-methyl-4-
nitroaniline, 2-methyl-5-nitroaniline, 2-methyl-5-nitroaniline hydrate, 2-
methyl-6-
nitroaniline, and 5-methyl-2-nitroaniline.
In one preferred embodiment, the compound is the diazonium ion of 2-
methyl-3-nitroaniline shown below:
+N=N
CH3
X
\ NO2
2-methyl-3-nitroaniline diazonium ion
The compounds advantageously may be used in assays to detect and
quantitate bilirubin in a body fluid sample, such as a serum sample. Preferred
compounds of Formula I are those which are thermally stable and have a long
shelf
life. The compounds may be used to identify disease states such as impaired
function
of the liver, gall bladder or intestinal tract.
Preferred compounds are diazonium ion compounds which are formed from
precursor compounds including an amine group, wherein the precursor compounds
have a solubility of at least about 0.038 mg/mi, or optionally at least about
0.5 mg/ml,
or preferably at least about 2.0 mg/ml in acidic aqueous solutions, e.g., 100
mM HCI,
at a pH of about 1Ø For example, 2-methyl-3-nitroaniline, the precursor of 2-
8
pa-187428 v5
CA 02254357 1998-11-17
Docket No. 337462001200
methyl-3-nitroaniline diazonium ion, has a solubility of about 2.3 mg/ml in
100 mM
HC1, at a pH of about 1Ø
Synthesis of Diazonium Compounds
The diazonium compounds may be synthesized using methods for generating
diazonium cations known in the art. Heinrich Zollinger, "Diazo Chemistry I,
Aromatic and Heteroaromatic Compounds," VCH Publishers, New York, NY, 1994,
Chapter 2. In general, the diazonium ions may be prepared by diazotization of
the
free arylamine using sodium nitrite and an acid such as hydrochloric acid to
produce
the desired diazonium salt. The desired anion for the diazonium salt may be
provided
by including a salt of the anion in the diazotization reaction mixture. For
example, if
sodium hexafluorophosphate is included in the reaction mixture, the
hexafluorophosphate diazonium salt is produced. Alternatively, the
tetrafluoroborate
salt may be produced. Other salts include the metal double salts, in
particular the zinc
double chlorides, ZnCl4z-. Thus, compositions within the scope of the
invention
include salts of the diazonium cations.
An exemplary synthesis is the synthesis of 2-methyl-3-nitroaniline diazonium
ion (2). In this embodiment, 2-methyl-3-nitroaniline (1) is converted to the
diazonium ion (2) by reaction with sodium nitrite in an acidic aqueous medium.
The
reaction is illustrated in Scheme I below. The 2-methyl-3-nitroaniline
diazonium ion
(2) is capable of reacting with bilirubin to form a detectable product, and is
thus
useful in assays for detecting and quantitating bilirubin in a sample. The 2-
methyl-3-
nitroaniline diazonium ion precursor, 2-methyl-3-nitroaniline, also
advantageously
has good solubility in aqueous systems. 2-methyl-3-nitroaniline has a
solubility of at
least about 2.3 mg/ml in 100 mM HCl at a pH of about 1Ø
9
pa-187428 v5
CA 02254357 1998-11-17
Docket No. 337462001200
NH2 +N=N
CH3 CH3
NaNO2
HCl
N02 H2O N~2
(1) 4 C (2)
Scheme I
Reagent Compositions
Reagent compositions including the diazonium ion compounds for use in
assays may be provided in a variety of forms including solutions and solid
forms.
In one embodiment, the reagent composition is in the form of a stable liquid
reagent solution. In a preferred embodiment, the diazonium ion compound is
provided in an aqueous acidic solution, at a preferred concentration of about
0.25 mM
to 15 mM, at pH less than about 7, preferably about pH 0.5 to 2. The solid or
liquid
reagent composition further optionally may include other added materials, such
as
buffers or solubilizers.
Buffer systems which may be used include citric acid/tris-(hydroxymethyl)-
aminomethane, citric acid/aqueous sodium hydroxide solution, acetic
acid/aqueous
sodium hydroxide solution, acetic acid/sodium acetate, potassium hydrogen
phthalate/aqueous sodium hydroxide solution or phosphate buffers. Preferred
buffer
systems include an acetate, sodium acetate/acetic acid system.
Solutions used in the bilirubin assays can include solubilizers and
detergents.
Exemplary solubilizers include dimethylformamide, dimethyl sulphoxide,
tetrahydrofuran, dioxan and various glycols such as polyethylene glycol. Other
exemplary solubilizers include a non-ionic detergent, such as polyoxyethylated
octyl
pa-187428 v5
CA 02254357 1998-11-17
Docket No. 337462001200
phenol (e.g., Triton(& X 100, Rohm & Haas, Philadelphia, PA), which can be
added at
a concentration of, for example, from about 0.1 to 1.0% (w/v). Further
examples of
non-ionic detergents which can be used include polyoxyethylene sorbitan
monolaurate (e.g., Tween 20, ICI, Wilmington, DE), polyoxyethylene sorbitan
monopalmitate (Tween(V 40, ICI, Wilmington, DE), and polyglycol ether
surfactants
such as Tergitol 15-S-30 (Union Carbide Corp., Houston, TX). Ionic detergents
also may be used, such as alkyl betaine (Empigen BB, Albright and Wilson,
Ashland,
VA). In one embodiment, aqueous test solutions including a solubilizer are
provided,
including about 1 to 13% (w/v) solubilizer, wherein the pH is less than 7,
preferably
about pH 0.5 to 7.
The diazonium ion compound may be provided in a formulation in
combination with any of a variety of materials such as a carrier, buffer
and/or
solubilizer. Suitable carriers include water, preferably an acidic aqueous
solution.
Salts including the diazonium ion compound and a counteranion also are
provided.
Preferred salts include tetrafluoroborate, hexafluorophosphate and the metal
double
salts such as the zinc double chlorides.
Reagent compositions also may include the diazonium ion compound or salt
thereof and an acid. The diazonium ion compound or salt thereof may be stored
at an
acid pH. Where the reagent composition is in the form of an aqueous solution,
useful
acids include mineral acids, such as hydrochloric acid and sulfuric acid. For
a dry
reagent composition, acids which are solid when anhydrous may be used, such as
malic, sulfosalicylic, tartaric, succinic, cyclohexanesulfamic, p-
toluenesulfonic and
citric acid. If the reagent composition includes a carrier, an acid may be
included
which does not degrade the carrier matrix. Alternatively, a combination of
materials
which are capable of generating the acid in situ on contact with water may be
included, such as a solid adduct of a Friedel Crafts salt and an organic Lewis
base
11
pa-187428 v5
CA 02254357 1998-11-17
Docket No. 33746200 L 200
with a weak acid such as an organic acid as described in U.S. Pat. No.
3,814,586.
The presence of the acid can promote the coupling of the diazonium ion salt
with
bilirubin. The amount of acid in the reagent may vary. For example, acids may
be
added to an aqueous solution of the diazonium ion compound in an effective
amount
to provide a reagent solution having a pH less than about 7, preferably about
pH 0.5
to 2. The acid also may be present in a dry analytical element used in the
assay.
The reagent composition including the diazonium ion compound may be
prepared in a variety of solid forms, or combination of forms. The reagent
composition may be prepared as a powder or tablets which are reconstituted
with
water or suitable diluent to produce a reagent solution. Techniques for making
solid
forms of reagent compositions and materials such as fillers and binders, known
in the
art, may be used.
A dry analytical element also may be used including the reagent composition
on a suitable support. Contact of the support with a sample can dissolve the
reagent
composition and then the presence of bilirubin in the sample is detected. Dry
analytical elements may be formulated which comprise a carrier matrix
impregnated
with the reagent composition. Useful carrier materials are insoluble and
maintain
structural integrity when exposed to water or physiological fluids such as
serum or
urine. Exemplary matrixes include paper, cellulose, wood, glass fiber, and
woven
and nonwoven fabrics. For example, a dry analytical element may be made by
applying a solution containing the reagent composition to the matrix and then
drying.
Assays
In one embodiment, reagent compositions including stable diazonium ion
compounds are provided in solution which may be used in an assay to detect or
quantitate bilirubin in a body fluid sample. The bilirubin in the sample
reacts with
the diazonium ion compound to produce azobilirubin in an acidic solution which
12
pa-187428 v5
CA 02254357 1998-11-17
Docket No. 337462001200
absorbs maximally at about 540 mn. In alkaline media the chromophore shifts
to, for
example, about 600 nm. The reaction product can be detected using a
spectrophotometer or other analyzer capable of measuring absorbance at the
desired
wavelength. For example, the Hitachi line of analyzers may be used (Boehringer
Mannheim Diagnostics, Inc., Indianapolis, IN). The levels of bilirubin in a
blood,
plasma or serum sample thus can be accurately measured using the diazonium
compounds.
For many diagnostic applications, it is important to quantitate direct
bilirubin
in addition to total bilirubin. Methods for quantitating direct, indirect and
total
bilirubin using diazo reagents are developed in the art. See for example, Lott
and
Doumas, Clin. Chem., 39:641-647 (1993); and Doumas and Wu, Critical Reviews in
Clinical Laboratory Sciences, 28:415-445 (1991).
To quantitate total bilirubin, the diazonium compound is reacted with a
sample suspected to contain bilirubin in the presence of an accelerator such
as
caffeine. After the reaction is complete (about 10 minutes) the azobilirubin
product is
detected spectrophotometrically by absorbance at about 540 nm. Alternatively,
the
solution may be changed to alkaline by, for example, the addition of alkaline
tartrate
reagent, and the absorbance at about 598 nm may be measured. Other
accelerating
reagents (or promoters) which can be used, which promote the formation of
diazobilirubin, include dyphylline, sodium acetate, sodium benzoate and gum
arabic.
The absorbance is detected using a sample blank containing the assay
components
without the bilirubin sample. Bilirubin from the National Institute for
Standards and
Technology (NIST), SRM 916a, may be used for preparing standard solutions for
calibration of the bilirubin assay.
In some diagnostic applications, it is important to be able to detect direct
bilirubin without reaction of the diazonium compound with the unconjugated
13
pa-187428 v5
CA 02254357 1998-11-17
Docket No. 337462001200
bilirubin. In general, the direct reacting bilirubins (bilirubin mono and
diglucuronide
and delta bilirubin) are reacted with diazonium ion in the absence of an
accelerator.
In the assay, the diazonium ion compound is combined with the bilirubin
sample,
such as a serum sample. Other components of the assay solution may include
water,
buffers, stabilizers and HCI. At the end of the coupling (about 10 minutes),
the
absorbance of the azopigment is measured near 540 nm, or after adding a base
such as
alkaline tartrate, near 598 nm. The pH is preferably as low as possible to
prevent the
reaction of the unconjugated bilirubin. To keep unconjugated bilirubin from
reacting,
the serum sample may be diluted with HC1(for example 100 mmol/L) and incubated
for at least about 5 minutes before adding diazo reagent.
Preferably, the assay is conducted in aqueous systems. In an exemplary assay
for total bilirubin in a sample, such as a plasma or serum sample, two assay
formulations are provided, an acidic solubilizer formulation (referred to
herein as a
Total Bilirubin Rl Formulation), and a reagent formulation (referred to herein
as a
Total Bilirubin R2 Formulation), which includes the diazonium ion compound
(See
Tables 1 and 2). The assay described herein may be modified, by for example,
modification of components of the R1 and R2 Formulation, and incubation times
or
temperatures. The following assay conditions and formulations are provided by
way of example.
In the assay, 4-6 l of the sample is added to 250 l of Total Bilirubin Rl
Formulation, and mixed (a ratio of about 1:62 to about 1:42 sample:R1
Formulation). If the concentration of bilirubin in the sample is found to be
greater
than 35 mg/dL, the sample can be diluted 1 + 1 with physiological saline and
reassayed. The mixture is incubated, for example, at 25, 30 or 37 C for about
3 to
5 minutes. A sample blank is included for each patient sample, standard and
control. After about 5 minutes, 65 l of the acidic Total Bilirubin R2
Formulation
14
pa-187428 v5
CA 02254357 1998-11-17
Docket No. 337462001200
is added (a volume equal to about 13/50 of the volume of the Rl Formulation).
The solution is mixed, and the absorbance at about 540 nm is detected using a
spectrophotometer, preferably before about 10 minutes after the end of the
incubation reaction. The absorbance is compared with a standard of known total
bilirubin concentration, and the concentration of the sample thereby
determined.
Nonlimiting examples of Formulations for the Total Bilirubin assay are
shown below in Tables 1 and 2:
Table 1: Total Bilirubin R1 Formulation
Component Concentration
alkyl betaine (Empigen BB) 4-13% (w/v), e.g., 11% (w/v)
Potassium Iodide 0-12.5 mM, e.g., 12.5 mM
Antifoam FG- 10 Emulsion 1500 ppm
Sodium Acetate = 3H20 50-150 mM, e.g., 85 mM
Sulfamic Acid 50-200 mM, e.g., 110 mM
Table 2: Total Bilirubin R2 Formulation
Component Concentration
HC1 50-500 mM, e.g., 100 mM
2-methyl-3-nitroaniline 1-15 mM, e.g., 3 mM
Sodium nitrite 1-20 mM, e. g. , 8 mM
Sulfamic Acid 1-25 mM, e.g., 5 mM
The components of the Total Bilirubin Rl and R2 Formulations may be
obtained from commercial suppliers. For example, alkyl betaine (C 12-C 14
alkyl
betaine, Empigen BB) may be obtained from Albright & Wilson, (Ashland, VA);
potassium iodide, sulfamic acid, and HCl are available from J.T. Baker
(Phillipsburgh, NJ); Antifoam FG-10 emulsion (an emulsion including
polydimethylsiloxane) is available from Dow Corning (Midland, MI); sodium
acetate = 3H20 is available from Fisher Scientific (Itaska, IL); 2-methyl-3-
nitroaniline
pa-187428 0
CA 02254357 1998-11-17
Docket No. 337462001200
is available from Aldrich Chemical Co., St. Louis, MO; and sodium nitrite is
available from Sigma Chemical Company, St. Louis, MO.
The above formulations all are made up in water. Upon combining the
components to form the R2 Formulation, the nitrite reacts with the acid to
form
nitrous acid which subsequently reacts with the 2-methyl-3-nitroaniline, to
form the
2-methyl-3-nitroaniline diazonium ion. Thus, after the reaction, nitrite and 2-
methyl-
3-nitroaniline substantially are no longer present in the R2 Formulation.
Direct Bilirubin also may be quantitated in a sample. In an exemplary assay
for direct bilirubin in a sample, such as a plasma or serum sample, two assay
formulations again are provided, an acidic formulation (referred to herein as
a
Direct Bilirubin R1 Formulation), and a reagent formulation (referred to
herein as a
Direct Bilirubin R2 Formulation), which includes the diazonium ion compound
(see
Tables 3 and 4 below). While the components of the R1 and R2 Formulation and
incubation times may be designed and optimized using knowledge of bilirubin
assays developed in the art, the following assay conditions and formulations
are
provided by way of example.
In an exemplary assay for direct bilirubin concentration in a serum sample,
6 1 of the sample is added to 250 l of Direct Bilirubin R1 Formulation, and
mixed (a ratio of about 1:42 sample:R1 Formulation). If the concentration of
bilirubin in the sample is greater than 20 mg/dL, the sample can be diluted 1
+ 1
with physiological saline and reassayed. The mixture is incubated at 25, 30 or
37 C for about 30 seconds to 5 minutes. A sample blank is included for each
patient sample, standard and control. After 5 minutes, 65 l of the Direct
Bilirubin
R2 Formulation is added (a volume equal to 13/50 of the volume of the Direct
Bilirubin Rl formulation). The solution is mixed, and the absorbance at about
540 nm is detected using a spectrophotometer, preferably before about 10
minutes
16
pa-187428 v5
CA 02254357 1998-11-17
Docket No. 337462001200
after the end of the incubation reaction. The absorbance is correlated with a
standard curve prepared using samples of known bilirubin concentration,
thereby
permitting the determination of direct bilirubin concentration in the sample.
Nonlimiting examples of Formulations for the Direct Bilirubin assay are
shown below in Tables 3 and 4:
Table 3: Direct Bilirubin R1 Formulation
Component Concentration
Betaine monohydrate 1-8% (w/v), e.g., 2 % (w/v)
Thesit 0.05-0.3% (w/v), e.g., 0.2 % (w/v)
Potassium Iodide 0.1-12 mM, e.g., 2 mM
Sodium Acetate - 3H20 50-150 mM, e.g., 85 mM
Sulfamic Acid 50-300 mM, e.g., 200 mM
Table 4: Direct Bilirubin R2 Formulation
Component Concentration
HCl 100 mM
2-methyl-3-nitroaniline 0.25-12 mM, e.g., 3 mM
Sodium Nitrite 8-11 mM, e.g., 8
Sulfamic Acid 5 mM
The components of the Direct Bilirubin Formulations can be obtained from
commercial suppliers. For example, betaine monohydrate and sodium nitrite may
be
obtained from Sigma Chemical Co. (St. Louis, MO). Thesitt
(dodecylpoly(ethyleneglycolether)õ) can be obtained from Boehringer Mannheim
Corporation (Indianapolis, IN); potassium iodide, sulfamic acid and HCI may be
obtained from J.T. Baker (Phillipsburgh, NJ); sodium acetate - 3HZ0 is
available from
Fisher Scientific (Itaska, IL); and 2-methyl-3-nitroaniline is available from
Aldrich
Chemical Co. (St. Louis, MO).
17
pa-187428 v5
CA 02254357 1998-11-17
Docket No. 337462001200
The above formulations are all made up in water. As noted above, upon
combining the components of the R2 Formulation, the nitrite reacts with the
acid to
form nitrous acid which subsequently reacts with the 2-methyl-3-nitroaniline,
to form
the 2-methyl-3-nitroaniline diazonium ion. Thus, after the reaction, nitrite
and 2-
methyl-3-nitroaniline are substantially no longer present in the Formulation.
Advantages and Properties of the Diazonium Ions
Preferred diazonium ion compounds within the scope of the invention are
compounds capable of reacting with bilirubin to form a product which can be
detected, for example spectrophotometrically. Compounds which are highly
reactive
with bilirubin are especially preferred.
Also preferred are diazonium ion compounds as defined herein which are
thermally stable. As used herein, "thermally stable" refers to compounds
and/or
reagent formulations containing the compounds, wherein about 60% of the
compound
remains undecomposed and active after being subjected to a temperature of
about
42 C for at least one day. The diazonium compounds preferably are stable
enough to
meet thermal requirements for international shipping. Subsequent to thermal
stressing of the reagent, it preferably also has sufficient levels, on the
order of at least
about 60% of initial, of active components to provide a shelf-life of at least
12
months at 4 C.
In a preferred embodiment, the compounds also substantially do not react with
potentially interfering compounds in a body fluid or other sample, such as
hemoglobin or indican. Preferably, the compound permits accurate measurement
of
bilirubin even in a sample containing up to 2000 mg/dL of hemoglobin. In
another
embodiment, the compound permits accurate measurement of bilirubin even in a
sample containing up to 10 mg/dL indican. Also preferred are compounds formed
18
pa-187428 v5
CA 02254357 1998-11-17
Docket No. 337462001200
from amine precursors which are soluble in an aqueous system. A problem with
prior
art compounds has been low solubility in aqueous systems.
One example of a preferred diazonium ion compound is 2-methyl-3-
nitroaniline diazonium ion. This compound does not substantially react with
interfering compounds in a serum or other body fluid sample. This compound
permits the accurate measurement of bilirubin in samples which are grossly
hemolyzed, which contain extremely high hemoglobin concentrations, for
example,
as high as 2000 mg dL-'. Other interfering compounds, such as indican, also do
not
react significantly with 2-methyl-3-nitroaniline diazonium ion. Bilirubin can
be
assayed even in samples with indican levels, for example, as high as 10 mg dL-
'. This
enables the assay of a wide range of samples.
The 2-methyl-3-nitroaniline diazonium ion also is highly reactive with
bilirubin, and is thermally stable. This compound is stable enough to meet
thermal
requirements for international shipping. The 2-methyl-3-nitroaniline diazonium
ion
withstands relatively high temperatures (42 C) for at least one day, with
decomposition of less than 40%. Subsequent to thermal stressing of the
compound,
for example in liquid reagent form as described in detail above, it has
sufficient levels
of active components to provide a shelf-life of at least 12 months at 4 C.
Arrhenius estimates of stability at 4 C indicate that the 2-methyl-3-
nitroaniline diazonium ion is about 2-2.5 times more stable than the
sulfanilamide
diazonium ion currently used in the quantitation of bilirubin. Thus, this
reagent
meets rigorous thermal stressing requirements and retains sufficient levels of
active
component to maintain a shelf-life of at least 12 months at 4 C. The 2-methyl-
3-
nitroaniline diazonium ion also is highly soluble in water or other aqueous
systems.
Thus, the 2-methyl-3-nitroaniline diazonium ion has many advantages as a
bilirubin assay reagent. The compound is thermally stable, is highly reactive
with
19
pa-187428 v5
CA 02254357 1998-11-17
Docket No. 337462001200
bilirubin, and permits accurate measurements even in the presence of high
levels of
hemoglobin, or other interfering compounds, such as indican. The use of this
compound permits the assay of a wide range of sample types, including body
fluids
such as serum, urine, and plasma. Assay of the levels of either total
bilirubin or direct
bilirubin in a body fluid sample thus permits the detection and diagnosis of a
variety
of metabolic disturbances, abnormal states, and diseases associated with
changes in
levels of either total, direct or indirect bilirubin including those affecting
the liver,
gall bladder and intestines.
The invention will be further understood by the following nonlimiting
examples.
pa-187428 v5
CA 02254357 1998-11-17
Docket No. 337462001200
EXAMPLES
Example 1: Preparation of 2-Methyl-3-Nitroaniline Diazonium ion and
Assay for Bilirubin.
The quantity of direct and total bilirubin in a serum or plasma sample is
detected. 2-methyl-3-nitroaniline diazonium ion is generated in a formulation
for the
total bilirubin assay (Total Bilirubin R2 Formulation) and in a formulation
for the
direct bilirubin assay (Direct Bilirubin R2 Formulation). Additionally, acidic
solutions are provided for the total bilirubin assay (Total Bilirubin R1
Formulation)
and for the direct bilirubin assay (Direct Bilirubin R1 Formulation). The
components
used to form the Formulations, which are made up in water, are shown below in
Tables 5-8.
Table 5: Total Bilirubin R1 Formulation
Component Concentration Supplier
Empigen BB 11.00% (w/v) Albright & Wilson
Antifoam FG-10 Emulsion 1500 ppm Dow Corning
Sodium Acetate - 3H2O 85 mM Fisher Scientific
Sulfamic Acid 110 mM J.T.Baker
Table 6: Total Bilirubin R2 Formulation
Component Concentration Supplier
HCl 100 mM J.T.Baker
2-methyl-3-nitroaniline 3 mM Aldrich
Sodium nitrite 8 mM Sigma
Sulfamic Acid 5 mM J.T. Baker
21
pa-187428 0
CA 02254357 1998-11-17
Docket No. 337462001200
Table 7: Direct Bilirubin R1 Formulation
Component Concentration Su lier
Betaine monohydrate 2.00% (w/v) Sigma
Thesit 0.20% (w/v) Boehringer Mannheim
Potassium Iodide 2 mM J.T. Baker
Sodium Acetate = 3HZ0 85 mM Fisher Scientific
Sulfamic Acid 200 mM J.T.Baker
Table 8: Direct Bilirubin R2 Formulation
Component Concentration Supplier
HCl 100 mM J.T.Baker
2-methyl-3-nitroaniline 3 mM Aldrich
Sodium Nitrite 8 mM Sigma
Sulfamic Acid 5 mM J.T.Baker
For the Total and Direct Bilirubin R2 Formulations, since the nitrite reacts
with the acid to form nitrous acid, which subsequently reacts with the 2-
methyl-3-
nitroaniline to form the 2-methyl-3-nitroaniline diazonium ion, nitrite as
such
substantially no longer exists in the reagent, nor does the 2-methyl-3-
nitroaniline.
The R2 Formulations after mixing of the components therein are stable for at
least
about 18 months at 4 C.
To form the R2 solution, 2-methyl-3-nitroaniline is dissolved in 100 mM HCl
at room temperature. The solution is cooled to - 4 C. The appropriate amount
of
solid sodium nitrite is slowly added, while keeping the solution cold, and
permitting
all the nitrite to completely dissolve. The appropriate amount of solid
sulfamic acid
is added to the solution, while keeping the solution cold, and ensuring
complete
dissolution of the sulfamic acid.
The assay is conducted using a clinical analyzer, such as the Boehringer
Mannheim Diagnostics, Inc., (Indianapolis, IN) Hitachi series of clinical
analyzers,
H717, H917, or H747. The calibration standard is Precical (Boehringer
Mannheim;
22
pa-187428 v5
CA 02254357 1998-11-17
Docket No. 337462001200
Indianapolis, IN), a commercially available calibrator having defmed level of
bilirubin. Serum based bilirubin sample control materials having known
concentrations of bilirubin which also may be used are Precitrol-A (PTA), an
abnormal control material, and Precitrol-N (PTN), a normal control material.
The
blank or zero calibrator used is saline.
In the assay, 6 L of the sample and 250 L R1 Formulation are incubated for
5 minutes at 37 C. Subsequently, 65 L R2 Formulation is added and the
solution
incubated for 5 minutes at 37 C. The absorbance at 546 nm 5 minutes after
addition
of R2 Formulation is measured and correlated with the standard to determine
bilirubin concentration. The Hitachi analyzer also subtracts absorbance at a
secondary wavelength, for example, of 660 or 700 nm.
Figure 1 illustrates a plot of absorbance vs. total bilirubin concentration
obtained using this method to analyze normal human serum based samples spiked
with different concentrations of pure unconjugated bilirubin (NIST-traceable).
The
Hitachi 717 Clinical Analyzer was used for analysis of the samples after
calibration
with Precical. The corrected absorbance on the y-axis corresponds to the
sample
absorbance minus the absorbance of the blank (saline). This plot illustrates
the
concentration obtained from the sample absorbance related to the calibrator
absorbance as well as the linearity of the method.
Although the foregoing invention has been described in some detail by way of
illustration and example for purposes of clarity and understanding, it will be
apparent
to those skilled in the art that certain changes and modifications may be
practiced.
Therefore, the description and examples should not be construed as limiting
the scope
of the invention.
23
pa-187428 v5