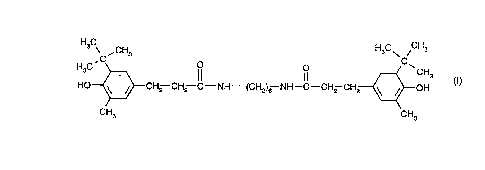Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 022~172 1998-12-03
A-21465/A
Stabilisation of polyamide, Polyester and Polvacetal
The present invention relates to compositions comprising a polyamide, polyester or polyke-
tone, to a specific phenolic antioxidant, to the use thereof for stabilising polyamides, poly-
esters or polyacetals against oxidative, thermal and/or light-induced degradation as well as
to a process for stabilising these plastic materials.
It is known from US patent specifications 3,691,131 and 3,860,558 that polyamides may be
stabilised with phenolic antioxidants in the presence of metal hypophosphites, such as
potassium hypophosphite or sodium hypophosphite, and of copper salts of organic acids.
In polyamides, polyesters or polyacetals, these known stabiliser mixtures cannot meet all the
demands made on them. It is known that such stabiliser mixtures reduce the starting colour
as well as the colour development of the polyamide during oven ageing and that they further-
more reduce the impairment of the mechanical properties of the polyamide to during oven
ageing and exposure to light.
It has now been found that a specific phenolic antioxidant, which is selected from US patent
specifications 3,691,131 and 3,860,558, is particularly suitable as ~ ser for polyamides,
polyesters or polyacetals in the absence of metal hypophosphi~es and copper salts of orga-
nic acids. The polyamides, polyesters or polyacetals stabilised in this manner have improved
properties with regard to oxidative, thermal or/und light-induced degradation.
Accordingly, this invention relates to compositions comprising
a) a polyamide, polyester or polyacetal which is subject to oxidative, thermal or light-in-
duced degradation, and
b) the compound of formula I
C 3 o C
H3C~CH2--CH2--C--NH--(CH2)6 NH--C--CH2--CH2~ CH3 (1)
CH3 CH3
CA 022~172 1998-12-03
with the proviso that, if component (a) is a polyamide, then the composition contains no me-
tal hypophosphite and no copper salt of an organic acid.
Component (b) of the novel composition, or the compound of formula 1, is known and bears
the Chemical Abstracts registry number [37042-77-6]. The preparation of the compound of
formula I is disclosed in GB-A-1 251 840, Example 1, page 5.
Polyamides will be understood to mean aliphatic and aromatic polyamides or copolyamides
which are derived from diamines and dicarboxylic acids and/or from aminocarboxylic acids or
their corresponding lactams. Suitable polyamides are for example: PA 6, PA 11, PA 12, PA
46, PA 6.6, PA 4.6, PA 6.9, PA 6.10 or PA 6.12, PA 10.12, PA 12.12 and also amorphous
polyamides of the type Trogamid PA 6-3-T and Grilamid TR 55. Polyamides of the cited type
are commonly known and are commercially available.
Interesting compositions are those wherein component (a) is polyamide 6, polyamide 6.6,
polyamide 4.6, polyamide 11 or polyamide 12 or copolymers thereof, in particular polyamide
6 or polyamide 6.6 or elastomer-modified polyamide 6 or polyamide 6.6 blended with poly-
propylene.
The polyesters used may be homo- or copolyesters which are composed of aliphatic, cyclo-
aliphatic or aromatic dicarboxylic acids and diols or hydroxycarboxylic acids.
The aliphatic dicarboxylic acids may contain 2 to 40 carbon atoms, the cycloaliphatic dicar-
boxylic acids 6 to 10 carbon atoms, the aromatic dicarboxylic acids 8 to 14 carbon atoms,
the aliphatic hydroxycarboxylic acids 2 to 12 carbon atoms and the aromatic, e.g. cycloali-
phatic, hydroxycarboxylic acids 7 to 14 carbon atoms.
The aliphatic diols may contain 2 to 12 carbon atoms, the cycloaliphatic diols 5 to 8 carbon
atoms and the aromatic diols 6 to 16 carbon atoms.
Aromatic diols are those wherein two hydroxyl groups are bound to one or to different aro-
matic hydrocarbon radicals.
CA 022~172 1998-12-03
The polyesters may also be branched with small amounts, e.g. 0.1 to 3 mol%, based on the
dicarboxylic acids present, of more than difunctional monomers (e.g. pentaerythritol, trimelli-
tic acid, 1,3,5-tri(hydroxyphenyl)benzene, 2,4-dihydroxybenzoic acid or 2-(4-hydroxyphenyl)-
2-(2,4-dihydroxyphenyl)propane) .
Polyesters consisting of at least 2 monomers may be randomly distributed or they may be
block copolymers.
Suitable dicarboxylic acids are linear and branched saturated aliphatic dicarboxylic acids,
aromatic dicarboxylic acids and cycloaliphatic dicarboxylic acids.
Suitable aliphatic dicarboxylic acids are those containing 2 to 40 carbon atoms, for example
oxalic acid, malonic acid, dimethylmalonic acid, succinic acid, pimelic acid, adipic acid, tri-
methyladipic acid, sebacic acid, azelaic acid and dimeric acids (dimerisation products of
unsaturated aliphatic carboxylic acids such as oleic acid), alkylated malonic and succinic
acids such as octadecylsuccinic acid.
Suitable cycloaliphatic dicarboxylic acids are: 1,3-cyclobutanedicarboxylic acid, 1,3-cyclo-
pentanedicarboxylic acid, 1,3- and 1,4-cyclohexanedicarboxylic acid, 1,3- and 1,4-(dicarbo-
xylmethyl)cyclohexane, 4,4'-dicyclohexyldicarboxylic acid.
Suitable aromatic dicarboxylic acids are: in particular terephthalic acid, isophthalic acid, o-
phthalic acid, and also 1,3-, 1,4-, 2,6- or 2,7-naphthalenedicarboxylic acid, 4,4'-diphenyldi-
carboxylic acid, 4,4'-diphenylsulfonedicarboxylic acid, 4,4'-benzophenonedicarboxylic acid,
1,1,3-trimethyl-5-carboxyl-3-(p-carboxylphenyl)indane, 4,4'-diphenyl ether dicarboxylic acid,
bis-p-(carboxylphenyl)methane or bis-p-(carboxylphenyl)ethane.
The aromatic dicarboxylic acids are preferred, and among those in particular terephthalic
acid, isophthalic acid and 2,6-naphthalenedicarboxylic acid.
Other suitable dicarboxylic acids are those containing -CO-NH groups; they are described in
DE-A-2 414 349. Dicarboxylic acids containing N-heterocyclic rings are also suitable, for
example those which are derived from carboxylalkylated, carboxylphenylated or carboxyben-
zylated monoamine-s-triazinedicarboxylic acids (cf. DE-A-2 121 184 and 2 533 675), mono-
CA 022~172 1998-12-03
or bishydantoins, optionally haiogenated benzimidazoles or parabanic acid. In this context,
the carboxyalkyl groups may contain 3 to 20 carbon atoms.
Suitable aliphatic diols are the linear and branched aliphatic glycols, in particular those con-
taining 2 to 12, preferably 2 to 6 carbon atoms in the molecule, for example: ethylene glycol,
1,2- and 1,3-propylene glycol, 1,2-, 1,3-, 2,3- or 1,4-butanediol, pentyl glycol, neopentyl gly-
col, 1,6-hexanediol, 1,12-dodecanediol. A suitable cycloaliphatic diol is e.g. 1,4-dihydroxy-
cyclohexane. Other suitable aliphatic diols are, for example, 1,4-bis(hydroxymethyl)cyclo-
hexane, aromatic-aliphatic diols such as p-xylylene glycol or 2,5-dichloro-p-xylylene glycol,
2,2-(~-hydroxyethoxyphenyl)propane and polyoxyalkylene glycols, such as ethylene glycol,
triethylene glycol, polyethylene glycol or polypropylene glycol. The alkylenediols are prefer-
ably linear and preferably contain 2 to 4 carbon atoms.
Preferred diols are the alkylenediols, 1,4-dihydroxycyclohexane and 1,4-bis(hydroxymethyl)-
cyclohexane. Ethylene glycol, 1,4-butanediol and 1,2- and 1,3-propylene glycol are particu-
larly preferred.
Other suitable aliphatic diols are the ,B-hydroxyalkylated, in particular ~-hydroxyethylated bis-
phenols, such as 2,2-bis[4'-(~-hydroxyethoxy)phenyl]propane. Other bisphenols are cited
hereinafter.
Another group of suitable aliphatic diols is that of the heterocyclic diols described in
DE-A-1 812 003, DE-A-2 342 432, DE-A-2 342 372 and DE-A-2 453 326. Examples are:N,N'-bis(~-hydroxyethyl)-5,5-dimethylhydantoin, N,N'-bis(~-hydroxypropyl)-5,5-dimethylhy-
dantoin, methylene-bis-[N-(~-hydroxyethyl)-5-methyl-5-ethylhydantoin], methylene-bis-[N-(~-
hydroxyethyl)-5,5-dimethylhydantoin], N,N'-bis(~-hydroxyethyl)benzimidazolone, N,N'-bis(~-
hydroxyethyl)-(tetrachloro)benzimidazolone or N,N'-bis(,~-hydroxyethyl)-(tetrabromo)benzi",i-
dazolone.
SUit~hlE aromatic diols are mononuclear diphenols and, in particular, dinuclear diphenols
carrying a hydroxyl group at each aromatic nucleus. The term aromatic is understood as re-
ferring preferably to hydrocarbon-aromatic radicals, for example phenylene or naphthylene.
In addition to e.g. hydroquinone, resorcinol or 1,5-, 2,6- and 2,7-dihydroxynaphthalene, men-
CA 02255172 1998-12-03
tion is to be made in particular of the bisphenols which may be represented by the following
formulae:
R' R' R' R'
H~>~h A~,OH ~AJ~\
HO ~ \~
R" R" R" R"
R' R'
R" R"
R' R'
~3A~oH
R" R"
~A ~A ~3A~
R" R"
R' R'
~OH .
R" R"
The hydroxyl groups may be in m-position and, in particular, in p-position. R' and R" in these
formulae may mean alkyl containing 1 to 6 carbon atoms, halogen, such as chloro or bromo
and, preferably, hydrogen atoms. A may be a direct bond, or oxygen, sulfur, -SO-, -SO2-,
CA 022~172 1998-12-03
'c=o . -P(O)(C,-C20alkyl)-, unsubstituted or substituted alkylidene, cycloalkylidene or
alkylene.
Examples of unsubstituted or substituted alkylidene are: ethylidene, 1,1- or 2,2-propylidene,
2,2-butylidene, 1,1-isobutylidene, pentylidene, hexylidene, heptylidene, octylidene, dichloro-
ethylidene, trichloroethylidene.
Examples of unsubstituted or substituted alkylene are methylene, ethylene, phenylmethy-
lene, diphenylmethylene, methylphenylmethylene. Examples of cycloalkylidene are cyclo-
pentylidene, cyclohexylidene, cycloheptylidene and cyclooctylidene.
Examples of bisphenols are: bis(p-hydroxyphenyl) ether or bis(p-hydroxyphenyl) thioether,
bis(p-hydroxyphenyl)sulfone, bis(p-hydroxyphenyl)methane, bis(4-hydroxyphenyl)-2,2'-bi-
phenyl, phenylhydroquinone, 1,2-bis(p-hydroxyphenyl)ethane, 1-phenyl-bis(p-hydroxyphe-
nyl)methane, diphenyl-bis(p-hydroxyphenyl)methane, diphenyl-bis(p-hydroxyphenyl)ethane,
bis(3,5-dimethyl-4-hydroxyphenyl)sulfone, bis(3,5-dimethyl-4-hydroxyphenyl)-p-diisopropyl-
benzene, bis(3,5-dimethyl-4-hydroxyphenyl)-m-diisopropylbenzene, 2,2-bis(3',5'-dimethyl-4'-
hydroxyphenyl)propane, 1,1- or 2,2-bis(p-hydroxyphenyl)butane, 2,2-bis(p-hydroxyphenyl)-
hexafluoropropane, 1,1-dichloro- or 1,1,1-trichloro-2,2-bis(p-hydroxyphenyl)ethane, 1,1-bis-
(p-hydroxyphenyl)cyclopentane and, in particular, 2,2-bis(p-hydroxyphenyl)propane (bisphe-
nol A) and 1,1-bis(p-hydroxyphenyl)cyclohexane (bisphenol C).
Suitable polyesters of hydroxycarboxylic acids are, for example, polycaprolactone, polypiva-
lolactone or the polyesters of 4-hydroxycyclohexanecarboxylic acid or 4-hydroxybenzoic
acid.
Also suitable are polymers which may predominantly contain ester bonds but also other
bonds, for example polyester amides or polyester imides.
Polyester containing aromatic dicarboxylic acids have become most important, in particular
the polyalkylene terephthalates. Accordingly, those inventive moulding compositions are
preferred in which the polyester is composed of at least 30 mol%, preferably of at least
40 mol%, of aromatic dicarboxylic acids and of at least 30 mol%, preferably of at least
CA 022~172 1998-12-03
40 mol%, of alkylenediols containing preferably 2 to 12 carbon atoms, based on the poly-
ester.
In this case the alkylenediol is preferably linear and contains 2 to 6 carbon atoms, such as
ethylene-, tri-, tetra- or hexamethylene glycol, and aromatic dicarboxylic acid denotes tereph-
thalic and/or isophthalic acid.
Particularly suitable polyesters are PET, PETG (glycol-modified polyethylene terephtl,alate)
or PBT (polybutylene terephthalate) and corresponding copolymers, PET and its copolymers
being especially preferred.
Polyacetals are, for example, homopolymers or copolymers of paraformaldehyde, such as in
particular polyoxymethylene (POM), and those polyoxymethylenes which contain comono-
mers, such as ethylene oxide, and polyacetals which are modified by thermoplastic polyure-
thanes, acrylates or MBS.
Useful compositions are those, as described above, wherein component (b) is present in an
amount of 0.01 to 1 %, preferably of 0.02 to 0.8 %, for example of 0.03 to 0.6 %, based on
the weight of component (a).
~esides the components (a) and (b), the novel compositions can contain additional additives
or cost~hilisers, such as the following:
1. Antioxidants
1.1. Alkylated monoPhenols, for example 2,6-di-tert-butyl-4-methylphenol, 2-tert-butyl-4,6-di-
methylphenol, 2,6-di-tert-butyl-4-ethylphenol, 2,6-di-tert-butyl-4-n-butylphenol, 2,6-di-tert-bu-
tyl-4-isobutylphenol, 2,6-dicyclopentyl-4-methylphenol, 2-(a-methylcyclohexyl)-4,6-dimethyl-
phenol, 2,6-dioctadecyl-4-methylphenol, 2,4,6-tricyclohexylphenol, 2,6-di-tert-butyl-4-metho-
xymethylphenol, nonylphenols which are linear or branched in the side chains, for example,
2,6-di-nonyl-4-methylphenol, 2,4-dimethyl-6-(1'-methylundec-1'-yl)phenol, 2,4-dimethyl-6-(1'-
methylheptadec-1'-yl)phenol, 2,4-dimethyl-6-(1'-methyltridec-1'-yl)phenol and mixtures there-
of.
CA 022~172 1998-12-03
-- 8 --
1.2. Alkylthiomethylphenols. for example 2,4-dioctylthiomethyl-6-tert-butylphenol, 2,4-dioc-
tylthiomethyl-6-methylphenol, 2,4-dioctylthiomethyl-6-ethylphenol, 2,6-di-dodecylthiomethyl-
4-nonylphenol.
1.3. Hvdroquinones and alkylated hvdro~uinones. for example 2,6-di-tert-butyl-4-methoxy-
phenol, 2,5-di-tert-butylhydroquinone, 2,5-di-tert-amylhydroquinone, 2,6-diphenyl-4-octade-
cyloxyphenol, 2,6-di-tert-butylhydroquinone, 2,5-di-tert-butyl-4-hydroxyanisole, 3,5-di-tert-
butyl-4-hydroxyanisole, 3,5-di-tert-butyl-4-hydroxyphenyl stearate, bis(3,5-di-tert-butyl-4-
hydroxyphenyl) adipate.
1.4. Toco~herols. for example a-tocopherol, ,B-tocopherol, ~-tocopherol, ~-tocopherol and
mixtures thereof (Vitamin E).
1.5. Hydroxvlated thiodiPhenvl ethers. for example 2,2'-thiobis(6-tert-butyl-4-methylphenol),
2,2'-thiobis(4-octylphenol), 4,4'-thiobis(6-tert-butyl-3-methylphenol), 4,4'-thiobis(6-tert-butyl-
2-methylphenol), 4,4'-thiobis(3,6-di-sec-amylphenol), 4,4'-bis(2,6-dimethyl-4-hydroxyphe-
nyl)disulfide.
1.6. Alkvlidenebis~henols. for example 2,2'-methylenebis(6-tert-butyl-4-methylphenol), 2,2'-
methylenebis(6-tert-butyl-4-ethylphenol), 2,2'-methylenebis[4-methyl-6-(a-methylcyclohexyl)-
phenol], 2,2'-methylenebis(4-methyl-6-cyclohexylphenol), 2,2'-methylenebis(6-nonyl-4-me-
thylphenol), 2,2'-methylenebis(4,6-di-tert-butylphenol), 2,2'-ethylidenebis(4,6-di-tert-butylphe-
nol), 2,2'-ethylidenebis(6-tert-butyl-4-isobutylphenol), 2,2'-methylenebis[6-(a-methylbenzyl)-
4-nonylphenol], 2,2'-methylenebis[6-(a,a-dimethylbenzyl)-4-nonylphenol], 4,4'-methylenebis-
(2,6-di-tert-butylphenol), 4,4'-methylenebis(6-tert-butyl-2-methylphenol), 1,1-bis(5-tert-butyl-
4-hydroxy-2-methylphenyl)butane, 2,6-bis(3-tert-butyl-5-methyl-2-hydroxybenzyl)-4-methyl-
phenol, 1,1,3-tris(5-tert-butyl-4-hydroxy-2-methylphenyl)butane, 1,1-bis(5-tert-butyl-4-hy-
droxy-2-methyl-phenyl)-3-n-dodecylmercaptobutane, ethylene glycol bis[3,3-bis(3'-tert-butyl-
4'-hydroxyphenyl)butyrate], bis(3-tert-butyl-4-hydroxy-5-methyl-phenyl)dicyclopentadiene,
bis[2-(3'-tert-butyl-2'-hydroxy-5'-methylbenzyl)-6-tert-butyl-4-methylphenyl]terephthalate, 1,1-
bis(3,5-dimethyl-2-hydroxyphenyl)butane, 2,2-bis(3,5-di-tert-butyl-4-hydroxyphenyl)propane,
2,2-bis(5-tert-butyl-4-hydroxy2-methylphenyl)-4-n-dodecylmercaptobutane, 1,1,5,5-tetra-(5-
tert-butyl-4-hydroxy-2-methylphenyl)pentane .
CA 022~172 1998-12-03
g
1.7. O-, N- and S-benzvl compounds. for example 3,5,3',5'-tetra-tert-butyl-4,4'-dihydroxydi-
benzyl ether, octadecyl-4-hydroxy-3,5-dimethylbenzylmercaptoacetate, tridecyl-4-hydroxy-
3,5-di-tert-butylbenzylmercaptoacetate, tris(3,5-di-tert-butyl-4-hydroxybenzyl)amine, bis(4-
tert-butyl-3-hydroxy-2,6-dimethylbenzyl)dithioterephthalate, bis(3,5-di-tert-butyl-4-hydroxy-
benzyl)sulfide, isooctyl-3,5-di-tert-butyl-4-hydroxybenzylmercaptoacetate.
1.8. HYdroxybenzylated malonates. for example dioctadecyl-2,2-bis(3,5-di-tert-butyl-2-hydro-
xybenzyl)malonate, di-octadecyl-2-(3-tert-butyl-4-hydroxy-5-methylbenzyl)malonate, di-dode-
cylmercaptoethyl-2,2-bis(3,5-di-tert-butyl-4-hydroxybenzyl)malonate, bis[4-(1,1,3,3-tetrame-
thylbutyl)phenyl]-2,2-bis(3,5-di-tert-butyl-4-hydroxybenzyl)malonate.
1.9. Aromatic hvdroxybenzyl comPounds. for example 1,3,5-tris-(3,5-di-tert-butyl-4-hydroxy-
benzyl)-2,4,6-trimethylbenzene, 1,4-bis(3,5-di-tert-butyl-4-hydroxybenzyl)-2,3,5,6-tetrame-
thylbenzene, 2,4,6-tris(3,5-di-tert-butyl-4-hydroxybenzyl)phenol.
1.10. Triazine com~ounds. for example 2,4-bis(octylmercapto)-6-(3,5-di-tert-butyl-4-hydro-
xyanilino)-1,3,5-triazine, 2-octylmercapto-4,6-bis(3,5-di-tert-butyl-4-hydroxyanilino)-1,3,5-tri-
azine, 2-octylmercapto-4,6-bis(3,5-di-tert-butyl-4-hydroxyphenoxy)-1,3,5-triazine, 2,4,6-tris-
(3,5-di-tert-butyl-4-hydroxyphenoxy)-1,2,3-triazine, 1,3,5-tris-(3,5-di-tert-butyl-4-hydroxyben-
zyl)isocyanurate, 1,3,5-tris(4-tert-butyl-3-hydroxy-2,6-dimethylbenzyl)isocyanurate, 2,4,6-tris-
(3,5-di-tert-butyl-4-hydroxyphenylethyl)-1,3,5-triazine, 1,3,5-tris(3,5-di-tert-butyl-4-hydroxy-
phenyl,~,roFionyl)-hexahydro-1,3,5-triazine, 1,3,5-tris(3,5-dicyclohexyl-4-hydroxybenzyl)iso-
cyanurate.
1.11. Benzyl~hosphonates, for example dimethyl-2,5-di-tert-butyl-4-hydroxybenzylphospho-
nate, diethyl-3,5-di-tert-butyl-4-hydroxybenzylphosphonate, dioctadecyl3,5-di-tert-butyl-4-hy-
droxybenzylphosphonate, dioctadecyl-5-tert-butyl-4-hydroxy-3-methylbenzylphosphonate,
the calcium salt of the monoethyl ester of 3,5-di-tert-butyl-4-hydroxybenzylphosphonic acid.
1.12. Acvlaminophenols. for example 4-hydroxylauranilide, 4-hydroxystearanilide, octyl N-
(3,5-di-tert-butyl-4-hydroxyphenyl)carbamate.
CA 022~l72 l998-l2-03
- 10- -
1.13. Esters of ~-(3,5-di-tert-butvl-4-hydroxyphenyl)ProPionic acid with mono- or polyhydric
alcohols, e.g. with methanol, ethanol, n-octanol, i-octanol, octadecanol, 1,6-hexanediol, 1,9-
nonanediol, ethylene glycol, 1,2-propanediol, neopentyl glycol, thiodiethylene glycol, diethy-
lene glycol, triethylene glycol, pentaerythritol, tris(hydroxyethyl) isocyanurate, N,N'-bis(hydro-
xyethyl)oxamide, 3-thiaundecanol, 3-thiapentadecanol, trimethylhexanediol, trimethylolpro-
pane, 4-hydroxymethyl-1-phospha-2,6,7-trioxabicyclo[2.2.2]octane.
1.14. Esters of B-(5-tert-butyl-4-hydroxv-3-methylPhenvl)ProPionic acid with mono- or poly-
hydric alcohols, e.g. with methanol, ethanol, n-octanol, i-octanol, octadecanol, 1,6-hexane-
diol, 1,9-nonanediol, ethylene glycol, 1,2-propanediol, neopentyl glycol, thiodiethylene glycol,
diethylene glycol, triethylene glycol, pentaerythritol, tris(hydroxyethyl) isocyanurate, N,N'-
bis(hydroxyethyl)oxamide, 3-thiaundecanol, 3-thiapentadecanol, trimethylhexanediol, trime-
thylolpropane, 4-hydroxymethyl-1-phospha-2,6,7-trioxabicyclo[2.2.2]octane.
1.15. Esters of ~-(3.5-dicvclohexvl-4-hydroxyPhenyl)ProPionic acid with mono- or polyhydric
alcohols, e.g. with methanol, ethanol, octanol, octadecanol, 1,6-hexanediol, 1,9-nonanediol,
ethylene glycol, 1,2-propanediol, neopentyl glycol, thiodiethylene glycol, diethylene glycol,
triethylene glycol, pentaerythritol, tris(hydroxyethyl)isocyanurate, N,N'-bis(hydroxyethyl)ox-
amide, 3-thiaundecanol, 3-thiapentadecanol, trimethylhexanediol, trimethylolpropane, 4-hyd-
roxymethyl-1 -phospha-2,6,7-trioxabicyclo[2.2.2]octane.
1.16. Esters of 3.5-di-tert-butvl-4-hvdroxvPhenyl acetic acid with mono- or polyhydric alco-
hols, e.g. with methanol, ethanol, octanol, octadecanol, 1,6-hexanediol, 1,9-nonanediol,
ethylene glycol, 1,2-propanediol, neopentyl glycol, thiodiethylene glycol, diethylene glycol,
triethylene glycol, pentaerythritol, tris(hydroxyethyl)isocyanurate, N,N'-bis(hydroxyethyl)ox-
amide, 3-thiaundecanol, 3-thiapentadecanol, trimethylhexanediol, trimethylolpropane, 4-hy-
droxymethyl-1 -phospha-2,6,7-trioxabicyclo[2.2.2]octane.
1.17. Amides of ,B-(3.5-di-tert-butyl-4-hydroxyphenyl)Propionic acid e.g. N,N'-bis(3,5-di-tert-
butyl-4-hydroxyphenylpropionyl)hexamethylenediamide, N,N'-bis(3,5-di-tert-butyl-4-hydroxy-
phenylpropionyl)trimethylenediamide, N,N'-bis(3,5-di-tert-butyl-4-hydroxyphenylpropionyl)-
hydrazide, N,N'-bis[2-(3-[3,5-di-tert-butyl-4-hydroxyphenyl]propionyloxy)ethyl]oxamide (Nau-
gard~XL-1 supplied by Uniroyal).
CA 022~172 1998-12-03
1.18. Ascorbic acid (vitamin C)
1.19. Aminic antioxidants. for example N,N'-di-isopropyl-p-phenylenediamine, N,N'-di-sec-
butyl-p-phenylenediamine, N,N'-bis(1,4-dimethylpentyl)-p-phenylenediamine, N,N'-bis(1-
ethyl-3-methylpentyl)-p-phenylenediamine, N,N'-bis(1-methylheptyl)-p-phenylenediamine,
N,N'-dicyclohexyl-p-phenylenediamine, N,N'-diphenyl-p-phenylenediamine, N,N'-bis(2-naph-
thyl)-p-phenylenediamine, N-isopropyl-N'-phenyl-p-phenylenediamine, N-(1,3-dimethylbutyl)-
N'-phenyl-p-phenylenediamine, N-(1-methylheptyl)-N'-phenyl-p-phenylenediamine, N-cyclo-
hexyl-N'-phenyl-p-phenlenediamine, 4-(p-toluenesulfamoyl)diphenylamine, N,N'-dimethyl-
N,N'-di-sec-butyl-p-phenylenediamine, diphenylamine, N-allyldiphenylamine, 4-isopropoxydi-
phenylamine, N-phenyl-1-naphthylamine, N-(4-tert-octylphenyl)-1-naphthylamine, N-phenyl-
2-naphthylamine, octylated diphenylamine, for example p,p'-di-tert-octyldiphenylamine, 4-n-
butylaminophenol, 4-butyrylaminophenol, 4-nonanoylaminophenol, 4-dodecanoylaminophe-
nol, 4-octadecanoylaminophenol, bis(4-methoxyphenyl)amine, 2,6-di-tert-butyl-4-dimethyl-
aminomethylphenol, 2,4'-diaminodiphenylmethane, 4,4'-diaminodiphenyimethane, N,N,N',N'-
tetramethyl-4,4'-diaminodiphenylmethane, 1,2-bis[(2-methylphenyl)amino]ethane, 1,2-bis-
(phenylamino)propane, (o-tolyl)biguanide, bis[4-(1',3'-dimethylbutyl)phenyl]amine, tert-octy-
lated N-phenyl-1-naphthylamine, a mixture of mono- and dialkylated tert-butyl/tert-octyldiphe-
nylamines, a mixture of mono- and dialkylated nonyldiphenylamines, a mixture of mono- and
dialkylated dodecyldiphenylamines, a mixture of mono- and dialkylated isopropyUisohexyldi-
phenylamines, a mixture of mono- und dialkylated tert-butyldiphenylamines, 2,3-dihydro-3,3-
dimethyl-4H-1,4-benzothiazine, phenothiazine, a mixture of mono- und dialkylated tert-butyU-
tert-octylphenothiazines, a mixture of mono- und dialkylated tert-octyl-phenothiazines, N-
allylphenothiazin, N,N,N',N'-tetraphenyl-1,4-diaminobut-2-ene, N,N-bis(2,2,6,6-tetramethyl-
piperid-4-yl-hexamethylenediamine, bis(2,2,6,6-tetramethylpiperid-4-yl)sebacate, 2,2,6,6-
tetramethylpiperidin-4-one, 2,2,6,6-tetramethylpiperidin-4-ol.
2. UV absorbers and liqht stabilisers
2.1. 2-(2'-Hydroxyphenyl)benzotriazoles, for example 2-(2'-hydroxy-5'-methylphenyl)benzo-
triazole, 2-(3',5'-di-tert-butyl-2'-hydroxyphenyl)benzotriazole, 2-(5'-tert-butyl-2'-hydroxyphe-
nyl)benzotriazole, 2-(2'-hydroxy-5'-(1,1,3,3-tetramethylbutyl)phenyl)benzotriazole, 2-(3',5'-di-
tert-butyl-2'-hydroxyphenyl)-5-chloro-benzotriazole, 2-(3'-tert-butyl- 2'-hydroxy-5'-methylphe-
CA 022~l72 l998-l2-03
12
nyl)-5-chloro-benzotriazole, 2-(3'-sec-butyl-5'-tert-butyl-2'-hydroxyphenyl)benzotriazole, 2-(2'-
hydroxy-4'-octyloxyphenyl)benzotriazole, 2-(3',5'-di-tert-amyl-2'-hydroxyphenyl)benzotriazole,
2-(3',5'-bis(a,a-dimethylbenzyl)-2'-hydroxyphenyl)benzotriazole, 2-(3'-tert-butyl-2'-hydroxy-5'-
(2-octyloxycarbonylethyl)phenyl)-5-chloro-benzotriazole, 2-(3'-tert-butyl-5'-[2-(2-ethylhexyl-
oxy)carbonylethyl]-2'-hydroxyphenyl)-5-chloro-benzotriazole, 2-(3'-tert-butyl-2'-hydroxy-5'-(2-
methoxycarbonylethyl)phenyl)-5-chloro-benzotriazole, 2-(3'-tert-butyl-2'-hydroxy-5'-(2-metho-
xycarbonylethyl)phenyl)benzotriazole, 2-(3'-tert-butyl-2'-hydroxy-5'-(2-octyloxycarbonylethyl)-
phenyl)benzotriazole, 2-(3'-tert-butyl-5'-[2-(2-ethylhexyloxy)carbonylethyl]-2'-hydroxyphenyl)-
benzotriazole, 2-(3'-dodecyl-2'-hydroxy-5'-methylphenyl)benzotriazole, 2-(3'-tert-butyl-2'-hy-
droxy-5'-(2-isooctyloxycarbonylethyl)phenylbenzotriazole, 2,2'-methylene-bis[4-(1,1,3,3-tetra-
methylbutyl)-6-benzotriazole-2-ylphenol]; the transesterification product of 2-[3'-tert-butyl-5'-
(2-methoxycarbonylethyl)-2'-hydroxyphenyl]-2H-benzotriazole with polyethylene glycol 300;
[R--CH2CH2--COO - CH2CH2~ where R = 3'-tert-butyl-4'-hydroxy-5'-2H-benzotriazol-2-
ylphenyl, 2-[2'-hydroxy-3'-(a,a-dimethylbenzyl)-5'-(1,1,3,3-tetramethylbutyl)phenyl]benzotri-
azole; 2-[2'-hydroxy-3'-(1,1,3,3-tetramethylbutyl)-5'-(a,a-dimethylbenzyl)phenyl]benzotri-
azole.
2.2. 2-HydroxybenzoDhenones. for example the 4-hydroxy, 4-methoxy, 4-octyloxy, 4-decyl-
oxy, 4-dodecyloxy, 4-benzyloxy, 4,2',4'-trihydroxy and 2'-hydroxy-4,4'-dimethoxy derivatives.
2.3. Esters of suhstituted and unsubstituted benzoic acids, as for example 4-tertbutyl-phenyl
s~'icylate, phenyl s~l ylate, octylphenyl salicylate, dibenzoyl resorcinol, bis(4-tert-butylben-
zoyl) resorcinol, benzoyl resorcinol, 2,4-di-tert-butylphenyl 3,5-di-tert-butyl-4-hydroxybenzo-
ate, hexadecyl 3,5-di-tert-butyl-4-hydroxybenzoate, octadecyl 3,5-di-tert-butyl-4-hydroxyben-
zoate, 2-methyl-4,6-di-tert-butylphenyl 3,5-di-tert-butyl-4-hydroxybenzoate.
2.4. Acrylates. for example ethyl a-cyano-~,~-diphenylacrylate, isooctyl a-cyano-,~,~-diphe-
nylacrylate, methyl a-carbomethoxycinnamate, methyl a-cyano-,B-methyl-p-methoxy-cinna-
mate, butyl a-cyano-~-methyl-p-methoxy-cinnamate, methyl a-carbomethoxy-p-methoxycin-
namate and N-(~-carbomethoxy-,~-cyanovinyl)-2-methylindoline.
CA 022~172 1998-12-03
- 13-
2.5. Nickel compounds, for example nickel complexes of 2,2'-thio-bis[4-(1,1,3,3-tetramethyl-
butyl)phenol], such as the 1:1 or 1:2 complex, with or without additional ligands such as n-
butylamine, triethanolamine or N-cyclohexyldiethanolamine, nickel dibutyldithiocarbamate,
nickel salts of the monoalkyl esters, e.g. the methyl or ethyl ester, of 4-hydroxy-3,5-di-tert-
butylbenzylphosphonic acid, nickel complexes of ketoximes, e.g. of 2-hydroxy-4-methylphe-
nyl undecylketoxime, nickel complexes of 1-phenyl-4-lauroyl-5-hydroxypyrazole, with or with-
out additional ligands.
2.6. Sterically hindered amines, for example bis(2,2,6,6-tetramethyl-4-piperidyl)sebacate,
bis(2,2,6,6-tetramethyl-4-piperidyl)succinate, bis(1,2,2,6,6-pentamethyl-4-piperidyl)sebacate,
bis(1-octyloxy-2,2,6,6-tetramethyl-4-piperidyl)sebacate, bis(1,2,2,6,6-pentamethyl-4-piperi-
dyl), n-butyl-3,5-di-tert-butyl-4-hydroxybenzylmalonate, the condensate of 1-(2-hydroxyethyl)-
2,2,6,6-tetramethyl-4-hydroxypiperidine and succinic acid, linear or cyclic condensates of
N,N'-bis(2,2,6,6-tetramethyl-4-piperidyl)hexamethylenediamine and 4-tert-octylamino-2,6-di-
chloro-1,3,5-triazine, tris(2,2,6,6-tetramethyl-4-piperidyl)nitrilotri~cet~te, tetrakis(2,2,6,6-tetra-
methyl-4-piperidyl)-1,2,3,4-butane-tetracarboxylate, 1,1'-(1,2-ethanediyl)-bis(3,3,5,5-tetra-
methylpiperazinone), 4-benzoyl-2,2,6,6-tetramethylpiperidine, 4-stearyloxy-2,2,6,6-tetrame-
thylpiperidine, bis(1,2,2,6,6-pentamethylpiperidyl)-2-n-butyl-2-(2-hydroxy-3,5-di-tert-butyl-
benzyl)malonate, 3-n-octyl-7,7,9,9-tetramethyl-1,3,8-triazaspiro[4.5]decan-2,4-dione, bis(1-
octyloxy-2,2,6,6-tetramethylpiperidyl)seb~c~te, bis(1-octyloxy-2,2,6,6-tetramethylpiperidyl)-
succinate, linear or cyclic condensates of N,N'-bis(2,2,6,6-tetramethyl-4-piperidyl)hexame-
thylenediamine and 4-morpholino-2,6-dichloro-1,3,5-triazine, the condensate of 2-chloro-4,6-
bis(4-n-butylamino-2,2,6,6-tetramethylpiperidyl )-1,3,5-triazine and 1,2-bis(3-aminopropyl-
amino)ethane, the condensate of 2-chloro-4,6-di-(4-n-butylamino-1,2,2,6,6-pentamethyl-
piperidyl)-1,3,5-triazine and 1,2-bis(3-aminopropylamino)ethane, 8-acetyl-3-dodecyl-7,7,9,9-
tetramethyl-1,3,8-triazaspiro[4.5]decane-2,4-dione, 3-dodecyl-1-(2,2,6,6-tetramethyl-4-pipe-
ridyl)pyrrolidin-2,5-dione, 3-dodecyl-1-(1,2,2,6,6-pentamethyl-4-piperidyl)pyrrolidine-2,5-di-
one, a mixture of 4-hexadecyloxy- and 4-stearyloxy-2,2,6,6-tetramethylpiperidine, a conden-
sation product of N,N'-bis(2,2,6,6-tetramethyl-4-piperidyl)hexamethylenediamine and 4-cyc-
lohexylamino-2,6-dichloro-1,3,5-triazine, a condensation product of 1,2-bis(3-aminopropyl-
amino)ethane and 2,4,6-trichloro-1,3,5-triazine as well as 4-butylamino-2,2,6,6-tetramethyl-
piperidine (CAS Reg. No. [136504-96-6]); N-(2,2,6,6-tetramethyl-4-piperidyl)-n- dodecylsuc-
cinimid, N-(1,2,2,6,6-pentamethyl-4-piperidyl)-n-dodecylsuccinimid, 2-undecyl-7,7,9,9-tetra-
methyl-1-oxa-3,8-diaza-4-oxo-spiro[4,5]decane, a reaction product of 7,7,9,9-tetramethyl-2-
CA 022~172 1998-12-03
- 14-
cycloundecyl-1-oxa-3,8-diaza-4-oxospiro [4,5]decane und epichlorohydrin, 1,1-bis(1,2,2,6,6-
pentamethyl-4-piperidyloxycarbonyl)-2-(4-methoxyphenyl)ethene, N,N'-bis-formyl-N,N'-bis-
(2,2,6,6-tetramethyl-4-piperidyl)hexamethylenediamine, diester of 4-methoxy-methylene-ma-
lonic acid with 1,2,2,6,6-pentamethyl-4-hydroxypiperidine, poly[methylpropyl-3-oxy-4-
(2,2,6,6-tetramethyl-4-piperidyl)]siloxane, reaction product of maleic acid anhydride-a-olefin-
copolymer with 2,2,6,6-tetramethyl-4-aminopiperidine or 1,2,2,6,6-pentamethyl-4-aminopipe-
ridine.
2.7. Oxamides. for example 4,4'-dioctyloxyoxanilide, 2,2'-diethoxyoxanilide, 2,2'-dioctyloxy-
5,5'-di-tert-butoxanilide, 2,2'-didodecyloxy-5,5'-di-tert-butoxanilide, 2-ethoxy-2'-ethyloxanilide,
N,N'-bis(3-dimethylaminopropyl)oxamide, 2-ethoxy-5-tert-butyl-2'-ethoxanilide and its mixture
with 2-ethoxy-2'-ethyl-5,4'-di-tert-butoxanilide, mixtures of o- and p-methoxy-disubstituted
oxanilides and mixtures of o- and p-ethoxy-disubstituted oxanilides.
2.8. 2-(2-HydroxvPhenyl)-1.3.5-triazines. for example 2,4,6-tris(2-hydroxy-4-octyloxyphenyl)-
1,3,5-triazine, 2-(2-hydroxy-4-octyloxyphenyl)-4,6-bis(2,4-dimethylphenyl)-1,3,5-triazine, 2-
(2,4-dihydroxyphenyl)-4,6-bis(2,4-dimethylphenyl)-1,3,5-triazine, 2,4-bis(2-hydroxy-4-pro-
pyloxyphenyl)-6-(2,4-dimethylphenyl)-1,3,5-triazine, 2-(2-hydroxy-4-octyloxyphenyl)-4,6-bis-
(4-methylphenyl)-1,3,5-triazine, 2-(2-hydroxy-4-dodecyloxyphenyl)-4,6-bis(2,4-dimethylphe-
nyl)-1,3,5-triazine, 2-(2-hydroxy-4-tridecyloxyphenyl)-4,6-bis(2,4-dimethylphenyl)-1,3,5-tri-
azine, 2-[2-hydroxy-4-(2-hydroxy-3-butyloxy-propoxy)phenyl]-4,6-bis(2,4-dimethyl)-1,3,5-tri-
azine, 2-[2-hydroxy-4-(2-hydroxy-3-octyloxy-propyloxy)phenyl]-4,6-bis(2,4-dimethyl)-1,3,5-
triazine, 2-[4-(dodecyloxy/tridecyloxy-2-hydroxypropoxy)-2-hydroxy-phenyl]-4,6-bis(2,4-di-
methylphenyl)-1,3,5-triazine, 2-[2-hydroxy-4-(2-hydroxy-3-dodecyloxy-propoxy)phenyl]-4,6-
bis(2,4-dimethylphenyl)-1,3,5-triazine, 2-(2-hydroxy-4-hexyloxy)phenyl-4,6-diphenyl-1,3,5-
triazine, 2-(2-hydroxy-4-methoxyphenyl)-4,6-diphenyl-1,3,5-triazine, 2,4,6-tris[2-hydroxy-4-(3-
butoxy-2-hydroxy-propoxy)phenyl]-1,3,5-triazine, 2-(2-hydroxyphenyl)-4-(4-methoxyphenyl)-
6-phenyl-1,3,5-triazine, 2-{2-hydroxy-4-[3-(2-ethylhexyl-1-oxy)-2-hydroxypropyloxy]phenyl}-
4,6-bis(2,4-dimethylphenyl)-1 ,3,5-triazine.
3. Metal deactivators, for example N,N'-diphenyloxamide, N-salicylal-N'-salicyloyl hydrazine,
N,N'-bis(salicyloyl) hydrazine, N,N'-bis(3,5-di-tert-butyl-4-hydroxyphenylpropionyl) hydrazine,
3-salicyloylamino-1,2,4-triazole, bis(benzylidene)oxalyl dihydrazide, oxanilide, isophthaloyl
CA 022~l72 l998-l2-03
- 15-
dihydrazide, sebacoyl bisphenylhydrazide, N,N'-diacetyiadipoyl dihydrazide, N,N'-bis(salicyl-
oyl)oxalyl dihydrazide, N,N'-bis(salicyloyl)thiopropionyl dihydrazide.
4. Phosphites and Phos~honites. for example triphenyl phosphite, diphenyl alkyl phosphites,
phenyl dialkyl phosphites, tris(nonylphenyl) phosphite, trilauryl phosphite, trioctadecyl phos-
phite, distearyl pentaerythritol diphosphite, tris(2,4-di-tert-butylphenyl) phosphite, diisodecyl
pentaerythritol diphosphite, bis(2,4-di-tert-butylphenyl) pentaerythritol diphosphite, bis(2,6-di-
tert-butyl-4-methylphenyl)pentaerythritol diphosphite, diisodecyloxypentaerythritol diphos-
phite, bis(2,4-di-tert-butyl-6-methylphenyl)pentaerythritol diphosphite, bis(2,4,6-tris(tert-bu-
tylphenyl)pentaerythritol diphosphite, tristearyl sorbitol triphosphite, tetrakis(2,4-di-tert-butyl-
phenyl) 4,4'-biphenylene diphosphonite, 6-isooctyloxy-2,4,8, 1 0-tetra-tert-butyl-1 2H-dibenz-
[d,g]-1 ,3,2-dioxaphosphocin, 6-fluoro-2,4,8, 1 0-tetra-tert-butyl-1 2-methyl-dibenz[d,g]-1 ,3,2-di-
oxaphosphocin, bis(2,4-di-tert-butyl-6-methylphenyl)methyl phosphite, bis(2,4-di-tert-butyl-6-
methylphenyl)ethyl phosphite, 2,2',2"-nitrilo[triethyltris(3,3',5,5'-tetra-tert-butyl-1,1'-biphenyl-
2,2'-diyl)phosphite], 2-ethylhexyl(3,3',5,5'-tetra-tert-butyl-1,1'-biphenyl-2,2'-diyl)phosphite.
Especially preferred are the following phosphites:
Tris(2,4-di-tert-butylphenyl) phosphite (Irgafos~168, Ciba-Geigy), tris(nonylphenyl) phosphite,
(CH3)3C ~ C(CH3)3 (CH3)3C ~C(CH3)3
(A) H3C ~C (CHa)3 (CH3)3C--(~ p_O--CH CH--N (B)
(CH3)3C - - 3
CA 022~l72 l998-l2-03
- 16-
(CH3)3C ~C(CH3)3
P--O CH2CH(C4Hg)CH2CH3 (C)
~ ~0
(CH3)3C--~
C(CH3)3
(CH3)3C ~ o Xo ~C(CH3)3 (D
C(CH3)3 (CH3)3C
C(CH3)3 (CH3)3C
H3C~ oXo ~CH3 (E)
C(CH3)3 (CH3)3C
-- CH3
H3C--C--CH3
(F) H'7C-8 ~ P' X ,P--o--C18H37 ~[~0_p--OCH2CH3 (G)
_3 CH3 -- 2
5. Hydroxylamines, for example N,N-dibenzylhydroxylamine, N,N-diethylhydroxylamine, N,N-
dioctylhydroxylamine, N,N-dilaurylhydroxylamine, N,N-ditetradecylhydroxylamine, N,N-dihe-
xadecylhydroxylamine, N,N-dioctadecylhydroxylamine, N-hexadecyl-N-octadecylhydroxyl-
amine, N-heptadecyl-N-octadecylhydroxylamine, N,N-~ialkylhydroxylamine derived from hyd-
rogenated tallow amine.
6. Nitrones, for example N-benzyl-alpha-phenyl-nitrone, N-ethyl-alpha-methyl-nitrone, N-oc-
tyl-alpha-heptyl-nitrone, N-lauryl-alpha-undecyl-nitrone, N-tetradecyl-alpha-tridcyl-nitrone, N-
CA 022~172 1998-12-03
hexadecyl-alpha-pentadecyl-nitrone, N-octadecyl-alpha-heptadecyl-nitrone, N-hexadecyl-
alpha-heptadecyl-nitrone, N-ocatadecyl-alpha-pentadecyl-nitrone, N-heptadecyl-alpha-hep-
tadecyl-nitrone, N-octadecyl-alpha-hexadecyl-nitrone, nitrone derived from N,N-dialkylhydro-
xylamine derived from hydrogenated tallow amine.
7. Thiosvnergists, for example dilauryl thiodipropionate or distearyl thiodipropionate.
8. Peroxide scavengers. for example esters of ~-thiodipropionic acid, for example the lauryl,
stearyl, myristyl or tridecyl esters, mercaptobenzimidazole or the zinc salt of 2-mercapto-
benzimid~o'c, zinc dibutyldithiocarbamate, dioctadecyl disulfide, pentaerythritol tetrakis(~-
dodecylmercapto)propionate.
9. Basic co-stabilisers. for example melamine, polyvinylpyrrolidone, dicyandiamide, triallyl
cyanurate, urea derivatives, hydrazine derivatives, amines, polyamides, polyurethanes, alkali
metal salts and alkaline earth metal salts of higher fatty acids, for example, calcium stearate,
zinc stearate, magnesium behenate, magnesium stearate, sodium ricinoleate and potassium
palmitate, antimony pyrocatecholate or zinc pyrocatecholate.
10. Nucle~tina aqents. for example inorganic substances such as talcum, metal oxides such
as titanium dioxide or magnesium oxide, phosphates, carbonates or sulfates of, preferably,
alkaline earth metals; organic compounds such as mono- or polycarboxylic acids and the
salts thereof, e.g. 4-tert-butylbenzoic acid, adipic acid, diphenylacetic acid, sodium succinate
or sodium benzoate; polymeric compounds such as ionic copolymers (ionomers).
11. Fillers and reinforcinq agents, for example calcium carbonate, silicates, glass fibres,
glass beads, asbestos, talc, kaolin, mica, barium sulfate, metal oxides and hydroxides, car-
bon black, graphite, wood flour and flours or fibers of other natural products, synthetic fibers.
12. Other additives, for example plasticisers, lubricants, emulsifiers, pigments, rheology
additives, catalysts, flow-control agents, optical brighteners, flameproofing agents, antistatic
agents and blowing agents.
13. Benzofuranones and indolinones, for example those disclosed in U.S. 4,325,863;
U.S. 4,338,244; U.S. 5,175,312; U.S. 5,216,052; U.S. 5,252,643; DE-A-4316611;
CA 022~172 1998-12-03
DE-A-4316622; DE-A-4316876; EP-A-0589839 or EP-A-0591102 or 3-[4-(2-acetoxyethoxy)-
phenyl]-5,7-di-tert-butyl-benzofuran-2-one, 5,7-di-tert-butyl-3-[4-(2-stearoyloxyethoxy)phe-
nyl]benzofuran-2-one, 3,3'-bis[5,7-di-tert-butyl-3-(4-[2-hydroxyethoxy]phenyl)benzofuran-2-
one], 5,7-di-tert-butyl-3-(4-ethoxyphenyl)benzofuran-2-one, 3-(4-acetoxy-3,5-dimethylphe-
nyl)-5,7-di-tert-butyl-benzofuran-2-one, 3-(3,5-dimethyl-4-pivaloyloxyphenyl)-5,7-di-tert-butyl-
benzofuran-2-one, 3-(3,4-dimethylphenyl)-5,7-di-tert-butyl-benzofuran-2-one, 3-(2,3-dime-
thylphenyl)-5,7-di-tert-butyl-benzofuran-2-one.
The costabilisers, with the exception of the benzofuranones listed under item 13, are added
in concenl,dtions of, for example, 0.01 to 10 %, based on the total weight of the polyamide,
polyester or polyacetal to be stabilised.
Other preferred compositions comprise further additives in addition to components (a) and
(b), in particular phenolic antioxidants, light stabilisers or/and processing stabilisers.
Particularly preferred additives are phenolic antioxidants (item 1 of the list), sterically hin-
dered amines (item 2.6 of the list), phosphites and phosphonites (item 4 of the list) and
peroxide scavengers (item 8 of the list).
Particularly interesting compositions are those comprising in addition to components (a) and
(b) as further additive at least one compound of the type of the organic phosphites or phos-
phonites (item 4 of the list).
Other preferred additional additives (stabilisers) are benzofuran-2-ones, such as those
described, inter alia, in U.S. 4,325,863; U.S. 4,338,244; U.S. 5,175,312; U.S. 5,216,052;
U.S. 5,252,643; DE-A-4 316 611, DE-A-4 316 622, DE-A-4 316 876, EP-A-0 589 839 or
EP-A-0591 102.
Examples of such benzofuran-2-ones are compounds of formula
CA 022~l72 l998-l2-03
R~15
"?~~
R12 H
wherein
R',1 is an unsubstituted or substituted carbocyclic or heterocyclic aromatic ring system;
R'-2 is hydrogen;
R'14 is hydrogen, alkyl of 1 to 12 carbon atoms, cyclopentyl, cyclohexyl or chloro;
R',3 has the meaning of R'12 or R',4 or is a radical of formula --(CH2)s C--OR',
O O O
Il 11 11
(CH2)s C--N(R 17)2 ~ --(CH2)s C--0--A--0--C--(CH2)s E
O O O O
--(CH2)s C--NR',8 A--NR',8 C--(CH2)s E, --(CH2)s a--NR',8 A--0--C--(CH2)s E,
O ~ o O
--(CH2)s C--N N--a--(CH2)s E, -CH2-S-R ,9, --CH(C6H5)--C--OR',6 or -D-E,
wherein
R'-6 is hydrogen, alkyl of 1 to 18 carbon atoms; alkyl of 2 to 18 carbon atoms which is inter-
rupted by oxygen or sulfur, dialkylaminoalkyl containing a total of 3 to 16 carbon atoms,
cyclopentyl, cyclohexyl, phenyl, or phenyl which is substituted by 1 to 3 alkyl radicals con-
taining together no more than 18 carbon atoms;
sisO, 1 or2;
the substituents R',7 are each independently of one another hydrogen,alkyl of 1 to 18 carbon
atoms, cyclopentyl, cyclohexyl, phenyl, phenyl which is substituted by 1 or 2 alkyl radicals
containing together no more than 16 carbon atoms, a radical of formula -C2H40H,
o
-C2H4-0-CtH2t+, or --C2H4 o--C--R'20 or, together with the linking nitrogen atom, form a
piperidine radical or morpholine radical;
t is 1 to 18;
CA 022~l72 l998-l2-03
- 20-
R'20 is hydrogen, alkyl of 1 to 22 carbon atoms or cycloalkyl of 5 to 12 carbon atoms;
A is alkylene of 2 to 22 carbon atoms which may be interrupted by nitrogen, oxygen or sulfur;
R'18 is hydrogen, alkyl of 1 to 18 carbon atoms, cyclopentyl, cyclohexyl, phenyl; phenyl or
benzyl which is substituted by 1 or 2 alkyl radical containing together no more than 16 car-
bon atoms;
R',g is alkyl of 1 to 18 carbon atoms;
D is -O-, -S-, -SO-, -sO2- or -C(R 21)2-;
the substituents R'21 are each independently of the other hydrogen or C,-C16alkyl, the two
R'2, containing together 1 to 16 carbon atoms, R'2, also being phenyl or a radical of formula
O o
Il 11
--(CH2)s C--OR',6 or --(CH2)s C--N(R~7)2 , wherein s, R',6 and R',7 have the
meanings cited above;
E is a radical of formula
C '
R,2 H
wherein R',1, R'12 and R'14 have the meanings cited above; and
R'15 is hydrogen, alkyl of 1 to 20 carbon atoms, cyclopentyl, cyclohexyl, chloro or a radical of
O O
ula CHz g OR 16 or --CH2--C--N(R',7)2 , wherein R',6 and R',7 have the
meanings cited above, or R'15, together with R',4, is a tetramethylene radical.
Those benzofuran-2-ones are preferred, wherein R',3 is hydrogen, alkyl of 1 to 12 carbon
atoms, cyclopentyl, cyclohexyl, chloro or a radical of formula --(CH2)s g--OR',
1~l
--(CH2)s C--N(R'~7)2 or -D-E, wherein s, R',6, R',7, D and E have the meanings given
CA 022~172 1998-12-03
above, R'16 preferably being hydrogen, alkyl of 1 to 18 carbon atoms, cyclopentyl or cyclo-
hexyl.
Other preferred benzofuran-2-ones are those, wherein R',1 is phenyl, or phenyl which is sub-
stituted by 1 or 2 alkyl radicals containing together no more than 12 carbon atoms; R'12 is
hydrogen; R'14 is hydrogen or alkyl of 1 to 12 carbon atoms;
o
R'13 is hydrogen, alkyl of 1 to 12 carbon atoms, --(CH2)s C--OR'16,
~l
--(CH2)s C--N(R',7)2 or -D-E; R'15 is hydrogen, alkyl of 1 to 20 carbon atoms,
O O
--CH2--C--OR'16 or --CH2 C--N(R'1,)2 , or R'1s, together with R'14, is a
tetramethylene radical, s, R'16, R'17, D and E having the meanings stated at the outset.
Particularly interesting benzofuran-2-ones are also those, wherein R'13 is hydrogen, alkyl of
1 to 12 carbon atoms or -D-E; R'12 and R'14 are each independently of the other hydrogen or
alkyl of 1 to 4 carbon atoms; and R',5 is alkyl of 1 to 20 carbon atoms, D and E having the
meanings stated at the outset.
Of special interest are, finally, also those benzofuran-2-ones, wherein R',3 is alkyl of 1 to 4
carbon atoms or -D-E; R',2 and R'14 are hydrogen; and R',5 is alkyl of 1 to 4 carbon atoms,
cyclopentyl or cyclohexyl, D being a group -C(R'2,)2- and E a radical of formula
~;[ IC~R~
R12 H 11
the substituents R'21 are identical or different and are each alkyl of 1 to 4 carbon atoms, and
R'11, R'12, R'14 and R'15 have the given meanings.
CA 022~172 1998-12-03
The amount of additionally added benzofuran-2-ones can vary within wide limits and they
may be present in the compositions of this invention in amounts of, for example, 0.0001 to
5 % by weight, preferably of 0.001 to 2 % by weight, more preferably of 0.01 to 2 % by
weight.
The incorporation of component (b) and optional further additives into component (a) [poly-
amide, polyester or polyacetal] is carried out by known methods, for example before or after
moulding or also by applying the dissolved or dispersed component (b) to component (a),
with or without subsequent evaporation of the solvent. Component (b) can also be added to
the materials to be stabilised [component (a)] in the form of a masterbatch which contains
these components in a concentration of, for example, 2.5 to 25 %.
Component (b) can also be added before or during polymerisation or before crosslinking.
Component (b) can be incorporated into the component (a) to be stabilised in pure form or
enc~psu~ted in waxes, oils, or polymers.
Component (b) can also be sprayed onto the component (a) to be stabilised. It is able to di-
lute other additives (e.g. the conventional additives indicated above) or their melts so that
they can be sprayed also together with these additives onto the component (a) to be stabi-
lised. Addition by spraying during the deactivation of the polymerisation catalysts is particu-
larly advantageous, it being possible to carry out spraying using, for example, the steam
used for deactivation.
The polyamides, polyesters or polyacetals thus stabilised can be used in a very wide range
of forms, typically including films, fibres, filaments, moulded articles, profiles, or as binders
for paint systems, in particular powder coating compositions, adhesives or putties.
Component (b) is particularly suitable for use as processing stabiliser (heat stabiliser). For
this purpose, it is usefully added to component (a) before or during processing.
Accordingly, a preferred embodiment of this invention is the use of component (b) as stabi-
liser, in particular as processing stabiliser (heat st~ ' ser), for stabilising polyamides, poly-
esters or polyacetals against oxidative, thermal and/or light-induced degradation.
CA 022~l72 l998-l2-03
- 23 -
Component (b) is distinguished by its advantageous colour behaviour, i.e. Iittle discoloration
of the polyamides, polyesters and polyacetals, during processing.
Accordingly, this invention also relates to a process for stabilising a polyamide, polyester or
polyacetal against oxidative, thermal and/or light-induced degradation, which process com-
prises incorporating in, or applying to, said material at least one component (b).
The f~ wi.lg Examples illustrate the invention in greater detail. Parts and percentages are
by weight.
ExamPle 1: Stabilisation of polyamide 12.
100 parts of unstabilised polyamide 12 granules (Grilamid~L 20 G, of EMS, Switzerland) are
powdered by cryogenic grinding and charged with the stabilisers listed in Table 1. This mix-
ture is mixed in a Henschel mixer for 2 minutes at 70~C. The powder so obtained is then
dried at 80~C for 6 hours and is then extruded in a twin-screw extruder (type Berstorff) at a
maximum of 210~C and then granulated. The granules obtained are injection moulded in an
injection moulding apparatus (type Engel) at a maximum of 220~C to dumbbells 1.0 mm thick
and 67 mm long. The yellowness indes (Yl) of these dumbbells is determined in accordance
with ASTM D 1925-70. Low Yl values denote little discoloration, high Yl values strong disco-
loration of the samples. The less discoloration, the more effective the stabiliser or the stabi-
liser mixture. The results are compiled in Table 1.
CA 022~172 1998-12-03
- 24 -
Table 1:
. . Yellowness index
Example Stablllsers (dumbbells)
1 aa) 6.7
1ba) 0.25 % Irganox~245C) 8.9
1ca) 0.50 % Irganox~245C) 9.6
1da) 0.25 % Irganox~1098d) 9.2
1 ea) 0.50 % Irganox~1098d) 10.7
1fb) 0.25 % component (b)e)6.3
1 gb) 0.50 % component (b)e)6.4
1 ha) 0.25 % Irganox~245C) 6.8
0.25 % Irgafos~168fl
;a) 0-25 % IrganOx01o98d) 7.6
0.25 % Irgafos~168fl
1~b) 0.25 % cornponent (b)e) 5 7
J 0.25 % Irgafos0168~
1ka) 0-25 % Irganox~24sc)
0.25 % Irgafos~129)
0.25 % Irganox~1098d) 6.0
0.25 % Irgafos~129)
1 mb) 0-25 % component (b)e) 3 4
0.25 % Irgafos~129
a) Comparison Example.
b) Example of this invention.
c) Irganox~245 (Ciba Spezialitatenchemie AG) denotes a compound of formula AO-1
CA 022~172 1998-12-03
- 25 -
C H3C ~ ~CH3
H3C~ CH2--CH2--C--O--(CH2CH20)3--C--CH2--CH2= CH3 (AO1 )
CH3 CH3
d) Irganoxa1098 (Ciba Spezialitatenchemie AG) denotes a compound of formula AO2
C H3C ~ ~CH3
H3C >~ ~ ~ ~< CH3
HO~CH2--CH2 C--NH--(CH2)6 NH--C--CH2--CH2~,CH3
CH3 CH3 H C~ \
e) Component (b) denotes the compound of formula I
& ,CH3 H3C~ ~CH3
H3C~CH2--CH2--g--NH--(CH2)6 NH--C--CH2--CH2~oH ( )
CH3 CH3
f) Irgafos~168 (Ciba Spezialitatenchemie AG) denotes tris(2,4-di-tert-butylphenyl)phos-
phite.
g) Irgafos~12 (Ciba Spezialitatenchemie AG) denotes 2,2',2"-nitrilo[triethyl-tris(3,3'5,5'-
tetra-tert-butyl-1,1'-biphenyl-2,2'-diyl)phosphite]; Chemical Abstract registry number:
80410-33-9, and represents the compound of formula B.
CA 022~172 1998-12-03
(CH3)3C ~c(CH3)3
,P--O--CH2CH2 N (B)
(CH3)3C--(~
C(CH3)3
Example 2: Stabilisation of polyamide 6.
100 parts of unstabilised polyamide 6 granules (Ultramid~B3S, of BASF) are powdered by
cryogenic grinding and are charged with the stabilisers listed in Table 2. This mixture is then
mixed in a Henschel mixer for 2 minutes at 70~C. The powder to obtained is then dried at
80~C for 6 hours and extruded in a twin-screw extruder (type Berstorff) at a maximum of
240~C and then granulated. The granules obtained are injection moulded in an injection
moulding apparatus at a maximum of 240~C to little rods 4 x 6 mm thick and 50 mm long.
These rods are aged in a circulating air oven at 150~C. The time it takes for the impact
strength of the rods to fall to 80 KJ/m2 is measured in hours. The longer the time, the better
the stAhi~is~tion. The results are compiled in Table 2.
Table 2: Impact strength in oven agein test at 150~C
Time in hours at 150~C until
Example Stabiliserreaching an impact strength of
80 KJ/m2
2aa) 2
2ba) 0.25% Irganox~245C) 28
2ca) 0.25 % Irganox~1098d)30
2db) 0.25 % component (b)~) 38
See end of Table 1 for the explanation on footnotes a) to e).
CA 022~172 1998-12-03
ExamPle 3: Stabilisation of polyoxymethylene copolymer (POM)
100 parts of unstabilised POM copolymer (Hostaform~C, Hoechst) are mixed with 0.3 % of
calcium stearate and with the stabilisers listed in Tables 3, 4 and 5. The powder is then ex-
truded at a maximum of 190~C. One part of these granules is injection moulded in an injec-
tion moulding apparatus at a maximum of 200~C to platelets 2 mm thick, 40 mm wide and
60 mm long. The other part of the granules is injection moulded in an injection moulding
apparatus at a maximum of 200~C to little rods 4 x 6 mm thick and 50 mm long.
Thermogravimetrical measurement is carried out isothermally on part of these platelets un-
der a stream of air at 220~C. The time until the platelets lose 3 %, 6 % and 10 % of their
weight is measured in minutes. The longer the time, the better the stabilisation. The results
are compiled in Table 3.
Table 3: Thermogravimetrical measurement at 220~C
Time in minutes until
reaching a weight
Example Stabiliser loss of
3% 6% 10%
3aa) 0.30% Irganox~245C) 53 70 88
3ba) 0.30 % Irganox~1098d) 78 103 127
3cb) 0.30% component (b)e) 82 114 157
See end of Table 1 for the explanation on footnotes a) to e).
An oven ageing test is carried out on another part of the platelets at 1 40~C. The pl~teletc are
aged in a circulating air oven at 140~C, and the time until the platelets lose 2 % of their
weight is measured in hours. The longer the time, the better the stabilisation. The results are
compiled in Table 4.
CA 022~l72 l998-l2-03
- 28 -
Table 4: Weight loss in oven ageing test at 140~C
Time in hours until reaching
Example Stabilisera weight loss of 2 %
3da) 0.30 % Irganox~245C) 1300
3ea) 0.30 % Irganox~1098d) 2150
3fb) 0.30 % component (b)e)2700
See end of Table 1 for the explanation on footnotes a) to e).
The 4 x 6 mm thick and 50 mm long rods are also subjected to an oven ageing test in a cir-
culating air oven at 140~C, measuring the time in hours until the impact strength of the rods
has fallen from the original 110 KJ/m2 to 90 KJ/m2. The longer the time, the better the stabi-
lisation. The results are compiled in Table 5.
Table 5: Impact strength in oven agein- test at 1 40~C
Time in hours at 1 40~C until reaching
Example Stabiliseran impact strength of 90 KJ/m2
2ga) 0.30 % Irganox~245C) 770
2ha) 0.30 % Irganox~1098d) 800
2ib) 0 30 % component (b)e) 1250
See end of Table 1 for the explanation on footnotes a) to e).