Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02258305 2005-06-29
29276-747
_1_
Decomposina polymers usina NOR-HALS compounds
The invention relates to a method of reducing the molecular weight of
polymers,
for example waste plastics and used plastics, in which at least one of the so-
called
"NOR-HALS" compounds described below is added and heating is carried out at
temperatures of 280°C or more.
In connection with the disposal of plastics, chemical recycling, i.e. the
decomposing of polymers to give oligomers and low molecular mass products, is
increasing in importance in comparison to the conventional thermomechanical
reforming processes.
Chemical recycling can take place in a variety of ways, for example by
hydrogenation, visbreaking, gasification or pyrolysis (U. Hofmann, M. Gebauer,
Kunststoffe 83, 259 (1993); D.E.Vesper, U.Guhr Kunststoffe 83, 905 (1993),
H. Wanjek, U. Stabel, Kunststoffe 84, 109 (1994); G. Menges, J. Bandrup, Kunst-
stoffe 84, 114 (1994); P.Mapleston, Mod. Plast. Int. 1993, 32), or else by an
extrusion process. Oligomers in particular are produced in this case [W.
Hasberg,
D. Vesper, M. Gebauer, Kunststoffe 84, 103 (1994), W. Micheli, V. Lackner,
paper
given at Int: Conf. on Advances in the Stabilization and Degradation of
Polymers,
Lucern, CH, 1994,177). The use of catalysts has also been proposed
(DE 4 224 990).
To adapt the molecular weight distribution in the case of plastics such as
polypropylene use is made, during the preparation of compound formulations,
primarily of peroxides, which bring about the decomposition of excessively
long
chains. These peroxide compounds break down at comparatively low temperatures
and are therefore of only limited suitability .for the targeted decomposing of
polymers
at higher temperatures (i.e. above customary processing temperatures). The use
of
these compounds in relatively high concentrations also carries with it a
safety risk,
which necessitates appropriate protective measures.
1t is therefore advantageous to provide additives which accelerate
decomposition
and become active only at high temperatures. For these purposes, suitable
additives
CA 02258305 1998-12-15
-2-
have now been found within the class of the "NOR-HALS" compounds described
below.
The invention accordingly relates to a method of reducing molecular weight in
polymers at temperatures of 280°C or more, in particular from 280 to
400°C, for
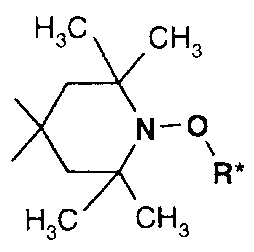
which NOR-HALS compounds comprising at least one group
G-CHCH3 G' G2
o-N (I), in which G is hydrogen or methyl and G1 and G2 are
G-CHZ
CM3
hydrogen, methyl or together are =O, are added as decomposition catalysts. It
is
judicious to operate by an extrusion process.
It is self evident that the polymers must be exposed to the elevated
temperature
for a sufficient period of time for the desired decomposition to occur. This
period of
time is normally longer than the time during which elevated temperatures are
employed in the processing of the polymers. The decomposition times may vary
greatly depending on the temperature, the amount of material to be decomposed
and the nature of the extruder if used. They are usually from about 2 to 120
minutes,
in particular from 5 to 90 minutes. As is common knowledge, so-called
sterically
hindered amines (Hindered Amine Light Stabilizers, HALS) are frequently added
as
light stabilizers to the polymers at the processing stage.
The NOR-HALS compounds described are, however, also suitable for limiting the
molecular weight and/or controlling the molecular weight distribution of
polymers,
especially polypropylene, in the course of compounding, and in this context,
as
described earlier for peroxides, they bring about decomposition of the chains.
In the method of the invention the NOR-HALS compounds are present judiciously
to the extent of from 0.01 to 10.0 for example to the extent of from 0.05 to
5.0
preferably to the extent of from 0.05 to 3.0, but in particular to the extent
of from 0.1
to 2.0% by weight. One or more of these compounds may be at hand in connection
with this method. The weight percentages are based on the total amount of
these
CA 02258305 1998-12-15
-3-
compounds. The basis for calculation in this context is the total weight of
the polymer
excluding the NOR-HALS compounds.
The NOR-HALS compounds in accordance with the method are derivatives of
polyalkylpiperidines which comprise at least one group of the formula
G-CHCH3 G' G2
-o-N 1 (or II or III, as described below)
G-CH2
CH3
in which G is hydrogen or methyl and G~ and G2 are hydrogen, methyl or
together
are =O; the polyalkylpiperidine groups of the formula 1 or II are preferably
substituted
in position 4 by one or two polar substituents or a polar spiro ring system.
Examples of such compounds can be found in the US Patents No. 4 590 231,
300 647, 4 831 134, 5 204 473, 5 004 770, 5 096 950, 5 021 478, 5 118 736,
5 021 480, 5 015 683, 5 021 481, 5 019 613, 5 021 486, 5 021 483, 5 145 893,
5 286 865, 5 359 069, 4 983 737, 5 047 489, 5 077 340, 5 021 577, 5 189 086,
5 015 682, 5 015 678, 5 051 511, 5 140 081, 5 204 422, 5 026 750, 5 185 448,
5 180 829, 5 262 538, 5 371 125, 5 216 156, 5 300 544.
Preference within the method is given to the use of compounds which comprise a
group of the formula
CH3 G~
G-CH2 G2
G'-'' O- N
G-CH2
CH3
in which G is hydrogen, G, and G2 are as defined above and G11 is hydrogen,
C1-ClBalkyl, C2-C,salkenyl, C3-C~Balkynyl, C5-C~2cycloalkyl, C6-
C,obicycloalkyl,
C5-CBCycloalkenyl, phenyl, naphthyl, C~-C~2phenylalkyl, phenyl or phenylalkyl
substituted by alkyl or phenyl having 7 to 14 carbon atoms, or is a group of
the
formula -CO-D' in which D' has the definitions C,-C,Balkyl, C1-C~Balkoxy,
phenyl, or
CA 02258305 1998-12-15
-4-
phenyl substituted by hydroxy, C~-CBalkyl, C1-Csalkoxy, amino or amino mono-
or
disubstituted by C,-CBalkyl or phenyl.
G" is in particular C,-Ciaalkyl, C3-CBalkenyl, C3-CBalkynyl, C5-C$cycloalkyl,
C~-C9phenylalkyl, C2-C~Balkanoyl, C3-Csalkenoyl, e.g. C1-C~ealkyl, C5-
CBCycloalkyl or
C~-C9phenylalkyl.
Particular preference is given to the use of NOR-HALS compounds comprising
HsC CHs
the group ~N-o (III).
~-~( R*
Ha / 'CHs
R* in this formula is C~-C2oalkyl, OH-substituted C1-C2oalkyl, optionally C1-
C4alkyl-
substituted C5-C~2cycloalkyl, C~-C9phenylalkyl or O- or S-interrupted C2-
C2oalkyl,
preferably C1-Cl2alkyl, benzyl or CS-Cacycloalkyl especially C6-Cloalkyl or
cyclohexyl.
The use of the classes of so-called sterically hindered amine derivatives
described below under (a) to (h) and carrying at least one group of the
formula I as
indicated above is of particular interest:
(a) Compounds of the formula IV
GCHz CHs G'
11 12
G-O-N O G
GCHZ CH3
IV
in which n is a number from 1 to 4, G and G' are each independently of one
another hydrogen or methyl, G" is as defined above and
G'2, if n is = 1, is hydrogen, C,-C~aalkyl which can be interrupted by one or
more
than one oxygen atom, 2-cyanoethyl, benzyl, glycidyl, a monovalent radical of
an
aliphatic, cycloaliphatic, araliphatic, unsaturated or aromatic carboxylic
acid,
carbarmic [sic] acid or phosphorus-containing acid, or a monovalent silyl
radical,
preferably the acyl radical of an aliphatic carboxylic acid having 2 to 18
carbon
atoms, of a cycloaliphatic carboxylic acid having 7 to 15 carbon atoms, of an
a,f3-
CA 02258305 1998-12-15
-5-
unsaturated carboxylic acid having 3 to 5 carbon atoms or of an aromatic
carboxylic
acid having 7 to 15 carbon atoms, it being possible for the carboxylic acid to
be
substituted in the aliphatic, cycloaliphatic or aromatic moiety by from 1 to 3
groups
-COOZ'2, in which Z'2 is hydrogen, C,-C2oalkyl, C3-Cl2alkenyl, C5-
C~cycloalkyl,
phenyl or benzyl;
if n is = 2, C2-C,2alkylene, C4-Cl2alkenylene, xylylene, a divalent acid
radical of an
aliphatic, cycloaliphatic, araliphatic or aromatic dicarboxylic acid,
dicarbamic acid or
phosphorus-containing acid, or a divalent silyl radical, preferably the acyl
radical of
an aliphatic dicarboxylic acid having 2 to 36 carbon atoms, of a
cycloaliphatic or
aromatic dicarboxylic acid having 8 to 14 carbon atoms or of an aliphatic,
cycloaliphatic or aromatic dicarbamic acid having 8 to 14 carbon atoms, and
the
dicarboxylic acid can be substituted in the aliphatic, cycloaliphatic or
aromatic moiety
by 1 or 2 groups -COOZ'2;
if n is = 3, a trivalent acid radical of an aliphatic, cycloaliphatic or
aromatic
tricarboxylic acid, it being possible for the radical to be substituted in the
aliphatic,
cycloaliphatic or aromatic moiety by -COOZ'2, or a trivalent acid radical of
an
aromatic tricarbamic acid or of a phosphorus-containing acid, or a trivalent
silyl
radical; or,
if n is = 4, a tetravalent acid radical of an aliphatic, cycloaliphatic or
aromatic
tetracarboxylic acid.
The stated acid radicals comprise in each case radicals of the formula (-CO)~R
in
which the definition of n is as defined above and the definition of R conforms
to the
given definitions.
C~-Cl2alkyl substituents are for example methyl, ethyl, n-propyl, isopropyl, n-
butyl,
sec-butyl, tert-butyl, n-hexyl, n-octyl, 2-ethylhexyl, n-nonyl, n-decyl, n-
undecyl or
n-dodecyl.
G" or G'2 defined as C~-ClBalkyl can for example be the abovementioned groups
and in addition for example n-tridecyl, n-tetradecyl, n-hexadecyl or n-
octadecyl.
G" defined as C3-Csalkenyl can for example be 1-propenyl, allyl, methallyl,
2-butenyl, 2-pentenyl, 2-hexenyl, 2-octenyl or 4-tert-butyl-2-butenyl.
CA 02258305 1998-12-15
-6-
G" defined as C3-CBalkynyl is preferably propargyl. G" defined as
C~-C~2phenylalkyl is preferably 2-phenethyl or benzyl.
G" defined as C2-CiBalkanoyl is for example propionyl, butyryl, octanoyl and
preferably acetyl. As C3-Csalkenoyl it is preferably acryloyl or methacryloyl.
G'2 defined as the monovalent acyl radical of a carboxylic acid is for example
the
acyl radical of acetic acid, hexanoic acid, stearic acid, acrylic acid,
methacrylic acid,
benzoic acid or f3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionic acid;
preferably it is the
acyl radical of stearic acid, acrylic acid or methacrylic acid.
G'2 defined as a monovalent silyl radical is for example a radical of the
formula
-(C~H2~)-Si(Z')2Z", in which j is an integer from 2 to 5 and Z' and Z" are
each
independently one another C1-C4alkyl or C1-C4alkoxy.
G'2 defined as the divalent acid radical of a dicarboxylic acid is for example
the
acid radical of malonic acid, succinic acid, glutaric acid, adipic acid,
subaric [sic] acid,
sebacic acid, malefic acid, itaconic acid, phthalic acid, dibutylmalonic acid,
dibenzylmalonic acid, butyl(3,5-di-tert-butyl-4-hydroxybenzyl)malonic acid or
bicycloheptenedicarboxylic acid.
G'2 defined as the trivalent radical of a tricarboxylic acid is for example
the acid
radical of trimellitic acid, citric acid or nitrilotriacetic acid.
G'2 defined as the tetravalent radical of a tetracarboxylic acid is for
example the
tetravalent acid radical of butane-1,2,3,4-tetracarboxylic acid or of
pyromellitic acid.
G'2 defined as the divalent radical of a dicarbarmic [sic] acid is for example
the
hexamethylenedicarbamic acid radical or the 2,4-tolylenedicarbamic acid
radical.
Preferred compounds are those of the formula IV in which n is 1 or 2, G and G'
are hydrogen, G" is C6-C~oalkyl or cyclohexyl and G'2 is the acyl radical of
an
aliphatic monocarboxylic acid having 12 to 18 carbon atoms or the diacyl
radical of
an aliphatic dicarboxylic acid having 4 to 12 carbon atoms. Important examples
of
alkylpiperidine compounds from this class are:
1) 1-octyloxy-4-hydroxy-2,2,6,6-tetramethylpiperidine
2) 1-cyclohexyloxy-4-hydroxy-2,2,6,6-tetramethylpiperidine
CA 02258305 1998-12-15
_7_
3) 1-cyclohexyloxy-4-stearoyloxy-2,2,6,6-tetramethylpiperidine
4) bis(1-cyclohexyloxy-2,2,6,6-tetramethylpiperidin-4-yl) succinate
5) bis(1-cyclohexyloxy-2,2,6,6-tetramethylpiperidin-4-yl) adipate
6) bis(1-octyloxy-2,2,6,6-tetramethylpiperidin-4-yl) sebacate.
13
G
CH, 1
GCHz G
(b) Compounds of the formula ,1 is (V),
G-O-N N G
GCHZ
CH,
n
in which n has the meaning 1 or 2 and G, G' and G" have the definitions given
in
(a), G'3 is hydrogen, C1-Cl2alkyl, C2-CShydroxyalkyl, C5-C~cycloalkyl, C~-
Caaralkyl,
C2-C~8alkanoyl, C3-CSalkenoyl, benzoyl or a group of the formula
cH, 1
GCH, G
G'-O-N (Va), in which
GCH,
CH,
G'4, if n is = 1, is hydrogen, C1-Clsalkyl, C3-CBalkenyl, C5-C~cycloalkyl; C1-
C4alkyl
which is substituted by a hydroxy, cyano, alkoxycarbonyl or carbamide group;
glycidyl; a group of the formula -CH2-CH(OH)-Z or of the formula -CONH-Z, in
which
Z is hydrogen, methyl or phenyl;
if n is = 2, is C2-C~2alkylene, C6-C,2arylene, xylylene, a -CH2CH(OH)-CH2-
group
or a group -CH2-CH(OH)-CH2-O-D-O- in which D has the definition C2-
Cioalkylene,
C6-C,Sarylene, C6-C,2cycloalkylene; or, provided that G'3 is not alkanoyl,
alkenoyl or
benzoyl, G'4 can also be 1-oxo-C2-C,2alkylene, a divalent radical of an
aliphatic,
cycloaliphatic or aromatic dicarboxylic acid or dicarbamic acid or the group -
CO-; or,
if n is = 1, G'3 and G'4 taken together are the divalent radical of an
aliphatic,
cycloaliphatic or aromatic 1,2-dicarboxylic acid or 1,3-dicarboxylic acid.
The C1-C,2alkyl substituents or C,-C,salkyl substituents have the definition
indicated under (a).
CA 02258305 1998-12-15
-g_
The CS-C~cycloalkyl substituents are preferably cyclohexyl.
G'3 defined as C~-Caaralkyl is preferably 2-phenethyl or benzyl. G'3 defined
as
C2-CShydroxyalkyl is preferably 2-hydroxyethyl or 2- or 3-hydroxypropyl.
G'3 defined as C2-ClBalkanoyl is for example propionyl, butyryl, octanoyl,
dodecanoyl, hexadecanoyl, octadecanoyl, preferably acetyl. As C3-CSalkenoyl it
is
preferably acryloyl.
G'4 defined as C2-CBalkenyl is for example allyl, meth-allyl, 2-butenyl, 2-
pentenyl,
2-hexenyl or 2-octenyl.
G'4 defined as C,-C4alkyl which is substituted by a hydroxy, cyano,
alkoxycarbonyl or carbamide group can for example be: 2-hydoxyethyl [sic],
2-hydroxypropyl, 2-cyanoethyl, methoxycarbonylmethyl, 2-ethoxycarbonylethyl,
2-aminocarbonylpropyl or 2-(dimethylaminocarbonyl)ethyl.
C2-Cl2AIkylene substituents are for example ethylene, propylene, 2,2-
dimethylpropylene, tetramethylene, hexamethylene, octamethylene, decamethylene
or dodecamethylene.
C6-C~SArylene substituents are for example o-, m- or p-phenylene, 1,4-
naphthylene or 4,4'-diphenylene.
C6-C,2Cycloalkylene is preferably cyclohexylene.
Preferred compounds are those of the formula V in which n is = 1 or 2, G is
hydrogen, G" is Cs-C~oalkyl or cyclohexyl, G'3 is hydrogen, C1-Cl2alkyl or a
group of
the formula Va and, if n is = 1, G'4 is hydrogen or C~-C~2alkyl and, if n is =
2, is
C2-CBalkylene or 1-oxo-C2-Caalkylene.
(c) Compounds of the formula
GCHz CHI G1
O
11 15
G O-N G
O
GCHz CH3
n
CA 02258305 1998-12-15
_g_
in which n is 1 or 2 and G, G' and G" have the definitions given under (a) and
G'S, if n is = 1, is C2-Caalkylene, C2-CBhydroxyalkylene or C4-
C22acyloxyalkylene or, if
n is = 2, is the group (-CH2)2C(CH2-)2.
G'5 defined as C2-Caalkylene or C2-CBhydroxyalkylene is for example ethylene,
1-methylethylene, propylene, 2-ethylpropylene or 2-ethyl-2-
hydroxymethylpropylene.
G'S defined as C4-C22acyloxyalkylene is for example 2-ethyl-2-
acetoxymethylpropylene.
(d) Compounds of the formulae VIIA, VIIB and VIIC, preferably compounds of the
formula VIIC:
1 G16
GCHZ CH3 G
11 N-c-o
G O-N 17
C N G
GCHZ CHI
O
n
(VIIA),
1 r,
GCH2 CH3 G
11 o c-r2
G O-N
N C - O
GCHz CH3
VIIB ,
1 r,
GCHz CH3 G
11 o c-rZ
G O-N 17
C N G
GCH2 CH3
O
(VIIC),
in which n is 1 or 2 and G, G' and G" have the definitions given in (a),
CA 02258305 1998-12-15
-10-
G'6 is hydrogen, C,-C~2alkyl, allyl, benzyl, glycidyl or C2-Csalkoxyalkyl, and
G", if
n is = 1, is hydrogen, C,-C~2alkyl, C3-CSalkenyl, C~-C9aralkyl, C5-
C~cycloalkyl,
C2-C4hydroxyalkyl, C2-Cfialkoxyalkyl, C6-Cioaryl, glycidyl or a group of the
formula
-(CH2)p-COO-Q or of the formula -(CH2)P O-CO-Q, in which p is 1 or 2 and Q is
C,-C4alkyl or phenyl; if n is = 2, is C2-Cl2alkylene, C4-C,2alkenylene, C6-
C,2arylene, a
group - CH2-CH(OH)-CH2-O-D-O-CH2-CH(OH)-CH2-, in which D is [sic] the
definition
C2-Cloalkylene, C6-C,Sarylene, C6-C,2cycloalkylene, or a group -CH2CH(OZ')CH2-
(OCH2-CH(OZ')CH2)2- , in which Z' is hydrogen, C~-ClBalkyl, allyl, benzyl, C2-
l2alkanoyl or benzoyl.
T' and T2 are each independently of one another hydrogen, C1-CiBalkyl or
C6-C~oaryl or C~-C9aralkyl each of which can be substituted by halogen or C~-
C4alkyl,
or T' and T2 form, together with the linking carbon atom, a C5-C,4cycloalkane
ring.
The substituents C,-C~2alkyl are for example methyl, ethyl, n-propyl,
isopropyl,
n-butyl, sec-butyl, tert-butyl, n-hexyl, n-octyl, 2-ethylhexyl, n-nonyl, n-
decyl, n-undecyl
or n-dodecyl.
Substituents defined as C1-ClBalkyl can for example be the groups indicated
above or for example n-tridecyl, n-tetradecyl, n-hexadecyl or n-octadecyl.
The substituents C2-Csalkoxyalkyl are for example methoxymethyl, ethoxymethyl,
propoxymethyl, tert-butoxymethyl, ethoxyethyl, ethoxypropyl, n-butoxyethyl,
tert-
butoxyethyl, isopropoxyethyl or propoxypropyl.
G" defined as C3-C5-alkenyl is for example 1-propenyl, allyl, methallyl, 2-
butenyl
or 2-pentenyl.
G", T' and T2 defined as C~-C9aralkyl are preferably 2-phenethyl or benzyl. If
T'
and T2 together with the carbon atom form a cycloalkane ring then this ring
can for
example be a cyclopentane, cyclohexane, cyclooctane or cyclododecane ring.
G" defined as C2-C4hydroxyalkyl is for example 2-hydroxyethyl, 2- or
3-hydroxypropyl or 2-, 3- or 4-hydroxybutyl.
G", T' and T2 defined as C6-C,oaryl is [sic] preferably phenyl or a- or B-
naphthyl
each of which can be substituted by halogen or C~-C4alkyl.
CA 02258305 1998-12-15
-11-
G" defined as C2-Cl2alkylene is for example ethylene, propylene, 2,2-dimethyl-
propylene, tetramethylene, hexamethylene, octamethylene, decamethylene or
dodecamethylene.
G" defined as C4-C~2alkenylene is preferably 2-butenylene, 2-pentenylene or
3-hexenylene.
G" defined as Cs-C,2arylene is for example o-, m- or p-phenylene, 1,4-
naphthylene or 4,4'-diphenylene.
Z' defined as CZ-,2alkanoyl is for example propionyl, butyryl, octanoyl,
dodecanoyl, but is preferably acetyl.
D defined as C2-C,oalkylene, C6-C,Sarylene or C6-Cl2cycloalkylene is as
defined
under (b).
(e) Compounds of the formula VIII, which are optionally preferred,
is
G
N~N
1~ / G20
G N
(VIII),
in which n is = 1 or 2 and G'8 is one of the groups of the formula
G1 CH3
CHzG
H
E-(A)X CH N-O G1'
CH3 CH2G
or
2 G1 CHs
G CH2G
11
E-(A)-N N-O G ,
X
CH G
CH3 z
CA 02258305 1998-12-15
-12-
in which G and G" are as defined in (a), where G is preferably hydrogen and G"
is preferably C,-C~°alkyl or cyclohexyl, and G' and G2 is [sic]
hydrogen or methyl or
taken together is [sic] the substituent =O,
E is -O- or -NG'3-, A is C2-Csalkylene or -(CH2)3-O-, and x is either 0 or 1,
G'3 is hydrogen, C,-C,2alkyl, C2-CShydroxyalkyl or C5-C~cycloalkyl,
G9 is identical with G'$ or is one of the groups -NG2'G22, -OG23, -NHCH20G23
or
-N(CH20G23)2,
G2°, if n is = 1, is identical with G'8 or G'9 and, if n is = 2, is a
group -E-B-E- in
which B is C2-Cgalkylene or C2-CBalkylene which is interrupted by 1 or 2
groups
N(G2')-, G2' is C,-Cl2alkyl, cyclohexyl, benzyl or C~-C4hydroxyalkyl or a
group of the
formula (Va),
G22 is C1-C,2alkyl, cyclohexyl, benzyl or C1-C4hydroxyalkyl, and
G23 is hydrogen, C,-Cl2alkyl or phenyl, or G2' and G22 taken together
-CH2CH2
are C4_5alkylene or C4_Soxaalkylene, e.g. O or a group of the
-CH2CH
-CH2CH2
formula: N-G~ 1 , or G2' is a group of the
-CH2CH
HsC CHa
C4H9 N
G~1 O-N N
N\/ N
HsC CH ~3
formula: C4H9 N
H3C 1/CH3
H3C N CH3
O
CA 02258305 1998-12-15
-13-
C1-C~2AIkyl substituents are for example methyl, ethyl, n-propyl, n-butyl, sec-
butyl, tert-butyl, n-hexyl, n-octyl, 2-ethylhexyl, n-nonyl, n-decyl, n-undecyl
or
n-dodecyl.
C,-C4Hydroxyalkyl substituents are for example 2-hydroxyethyl, 2- or
3-hydroxypropyl or 2-, 3- or 4-hydroxybutyl.
A defined as C2-Csalkylene is for example ethylene, propylene, 2,2-
dimethylpropylene, tetramethylene or hexamethylene.
GZ' and G22 together defined as CQ Csalkylene or -oxaalkylene are for example
tetramethylene, pentamethylene or 3-oxapentamethylene.
Important examples of polyalkylpiperidine compounds from this class are
compounds of the following formulae
R R
I I
~~ R-NH-(CH2)3-N-(CH2)2-N-(CH2)3-NH-R , in which R =
H3C CHs
N Calls
-~ ~N N-O
N~N
C4H9 N H3C CH3
H3C ~CH3
H3C N CH3
O
CA 02258305 1998-12-15
-14-
8)
i
H3C N CH3
H3C CH3
N_CaHs
H C CH N~N H C
3 3 ~ ~ 3 CH3
O-N N N N \N-O
H3C CH3 Calls Calls H3C CH3
HsCO
H3C N CH3
H3C CH3
9) N-Calls
H C CH N- \ N H C
3 3 ~ ~ 3 CH3
H3C0-N N N N \N-OCH3
H3C CH3 Calls Calls H3C CH3
H~N~CH2-CH2-OH
H C CH N \_N H C CH
3 3 I 3 3
O-N N N N N-O
H3C CH3 Calls Calls H3C CH3
CA 02258305 1998-12-15
-15-
(f) oligomeric or polymeric compounds whose structural repeating unit
comprises
a N-substituted 2,2,6,6-tetraalkylpiperidine radical of the formula I,
especially of the
formula III, preferably polyesters, polyethers, polyamides, polyamines,
polyurethanes, polyureas, polyaminotriazines, poly(meth)acrylates,
poly(meth)acryl-
amides and their copolymers comprise [sic] such radicals.
Radicals referred to as alkyl without further specification are preferably n-
alkyl; for
example, octyl (the radical CBH~~) is preferably n-octyl (the radical (CH2]~-
CH3).
Preferred NOR-HALS compounds in the method of the invention are sterically
hindered amine derivatives of the formula IV or VIII (groups (a) and (e)) and
also
oligomeric or polymeric compounds of group (f).
The synthetic organic polymer to be decomposed in accordance with the
invention normally comprises from 0.01 to 10% by weight of the sterically
hindered
amine derivative. Advantageous ranges lie from 0.05 to 5%, especially from 0.1
to
2% by weight of the sterically hindered amine derivative.
In the method of the invention it is possible to employ individual NOR-HALS
compounds or mixtures thereof. In the case of a mixture of compounds the
stated
amounts are based in each case on the total amount of sterically hindered
amine
derivatives used.
With particular preference, NOR-HALS compounds of the following structures are
used:
H3C CH3
R* O-N O (CH2)
n
H3C CH3
2
;n=1 to8;
H3C CH3
R* O-N O (CH2)a CH3
H3C CH3 ; a = 0 to 24;
CA 02258305 1998-12-15
-16-
R*
~O
I
H3C N CHs
HsC CHs
N-RS
H C CH N~N H C
3 3 ~ 3 CH3
R*O-N N N N N-OR*
I I
HsC CHs R5 R5 H3C CHs ~ R5 = E..I~ C1_C~oalkyl or
C5-C8cycloalkyl;
in which R* is C1-C2oalkyl or optionally C~-C4alkyl-substituted C5-
C,2cycloalkyl,
the compounds
H3C
~O
I
H3C N CHs
H3C CHs
N-C4H9
H C CH N~N H C
3 3 ~ 3 CH3
H3C0-N N N N N-OCHs
H3C CHs C4H9 C'H9 H3C CHs
R R
R-NH-(CH2)3-N-(CH2)2-N-(CH2)3-NH-R
where R =
CA 02258305 1998-12-15
-17-
HsC CHs
N Calls
~N 'N-OR*
N \ /N
H3C CH3
C4H9 N
HsC CH3
HsC N
CH3
OR*
H3C CH3
H,~CB-O-N O (CH2)
H3C CH3
R-NH-CH2CH2CH2-NR-CHZCH2-NR-CH2CH2-NH-R where R =
HsC CHs
N Calls
~N 'N-O
N\\ 'N
H3C CH3
C4H9 N
H3C I /CH3
H3C i CH3
O
CA 02258305 1998-12-15
_18_
\O
I
H3C N CH3
H3C CH3
N -C4H9
HC CH N \_N HC
3 3 ~ 3 C'H3
O-N N N N N-O
I I
H3C CH3 C4H9 C4H9 H3C CH3
H3C
~O
I
H3C N CH3
H3C CH3
N-C4H9
HC CH N \_N HC
3 3 ~ 3 CH3
H3C0-N N N N N-OCH3
H3C CH3 C4H9 C4H9 H3C CH3
O
C
N
N~N
N ~ N ~CH2)s N m
H3C CH3 H3C I ' CH3
H3C N CH3 H3C N ~ CH3
OCH3 pCH3
CA 02258305 1998-12-15
-19-
CH3 CH3
NH-C-CH2 C-CH3
CH3 CH3
N ~ ~ N (CH2)6 N
~N
CH ~ CH3 CH3 CH3 m
3
CH3 N CH3 CH3 N CH3
OR OR
~ ~ Jn
O' _N_ 'O
I
O NH
O NH
NOR*
= Polymer chain, typically ethylene copolymer or
styrene copolymer with malefic anhydride
-;~~CH2 i H-
n
O N O (CH2)"_2~
ICH3
N
I
OR
The specified amines are known compounds; many of them are obtainable
commercially.
Examples of materials to be decomposed catalytically are:
1. Polymers of monoolefins and diolefins, for example polypropylene,
polyisobutylene, polybut-1-ene, poly-4-methylpent-1-ene, polyisoprene or
polybutadiene, as well as polymers of cycloolefins, for instance of
cyclopentene,
norbornene or dicyclopentadiene; furthermore polyethylene (which optionally
can be
CA 02258305 1998-12-15
-20-
crosslinked or partially crosslinked), for example high density polyethylene
(HDPE),
low density polyethylene (LDPE), linear low density polyethylene (LLDPE),
branched
low density polyethylene (BLDPE).
The method of the invention is particularly suitable for polyolefins, i.e. the
polymers of monoolefins exemplified in the preceding paragraph, especially
polyethylene and polypropylene. They can be prepared by different, and
especially
by the following, methods:
a) radical polymerization (normally under high pressure and at elevated
temperature).
b) catalytic polymerization where the catalyst normally contains one or more
metals of group IVb, Vb, Vlb or VIII. These metals usually have one or more
ligands,
such as oxides, halides, alcoholates, esters, ethers, amines, alkyls, alkenyls
and/or
aryls that may be either p- or s-coordinated [sic]. These metal complexes may
be in
the free form or fixed on substrates, for example on activated magnesium
chloride,
titanium(III) chloride, alumina or silicon oxide. These catalysts may be
soluble or
insoluble in the polymerization medium. The catalysts as such may be active in
the
polymerization, or further activators may be used, for example metal alkyls,
metal
hydrides, metal alkyl halides, metal alkyl oxides or metal alkyloxanes, the
metals
being elements of groups la, Ila and/or Illa. The activators may be modified
for
example with further ester, ether, amine or silyl ether groups. These catalyst
systems
are usually termed Phillips, Standard Oil Indiana, Ziegler (-Natta), TNZ
(DuPont),
metallocene or single site catalysts (SSC).
2. Mixtures of the polymers mentioned under 1 ), for example mixtures of
polypropylene with polyisobutylene, polypropylene with polyethylene (for
example
PP/HDPE and/or LDPE, PP/EPDM) and mixtures of different types of polyethylene
(for example LDPE/HDPE, optionally with LLDPE).
3. Copolymers of monoolefins and diolefins with each other or with other vinyl
monomers, for example ethylene-propylene copolymers, linear low density
polyethylene (LLDPE) and its mixtures with low density polyethylene (LDPE),
propylene-but-1-ene copolymers, propylene-isobutylene copolymers, ethylene-but-
1-
ene copolymers, ethylene-hexene copolymers, ethylene-methylpentene copolymers,
CA 02258305 1998-12-15
-21 -
ethylene-heptene copolymers, ethylene-octene copolymers, propylene-butadiene
copolymers, isobutylene-isoprene copolymers, ethylene-alkyl acrylate
copolymers,
ethylene-alkyl methacrylate copolymers, ethylene-vinyl acetate copolymers and
their
copolymers with carbon monoxide, or ethylene-acrylic acid copolymers and their
salts (ionomers), as well as terpolymers of ethylene with propylene and a
diene, such
as hexadiene, dicyclopentadiene or ethylidene-norbonene; furthermore mixtures
of
such copolymers with one another and with polymers mentioned under 1 ), for
example polypropylene/ethylene-propylene-copolymers, LDPE/ethylene-vinyl
acetate
copolymers, LDPE/ethylene-acrylic acid copolymers, LLDPE/ethylene-vinyl
acetate
copolymers, LLDPE/ethylene-acrylic acid copolymers and alternating or random
polyalkylene/carbon monoxide copolymers and mixtures thereof with other
polymers,
for example polyamides.
4. Hydrocarbon resins (for example C5-Cs) including hydrogenated modifications
thereof (e.g. tackifiers) and mixtures of polyalkylenes and starch.
5. Polystyrene, polyp-methylstyrene), poly(alpha-methylstyrene).
6. Copolymers of styrene or alpha-methylstyrene with dienes or acrylic
derivatives, for examples styrene-butadiene, styrene-acrylonitrile, styrene-
alkyl
methacrylate, styrene-butadiene-alkyl acrylate and methacrylate, styrene-
malefic
anhydride, styrene-acrylonitrile-methyl acrylate; mixtures of high impact
strength of
styrene copolymers and another polymer, for example a polyacrylate, a diene
polymer or an ethylene-propylene-diene terpolymer; and block copolymers of
styrene
such as styrene-butadiene-styrene (SBS), styrene-isoprene-styrene, styrene-
ethylene/butylene-styrene or styrene-ethylene/propylene-styrene.
7. Graft copolymers of styrene or alpha-methylstyrene, for example styrene on
polybutadiene, styrene on polybutadiene-styrene or polybutadiene-acrylonitrile
copolymers, styrene and acrylonitrile (or methacrylonitrile) on polybutadiene;
styrene,
acrylonitrile and methyl methacrylate on polybutadiene; styrene and malefic
anhydride on polybutadiene; styrene, acrylonitrile and malefic anhydride or
maleimide
on polybutadiene; styrene and maleimide on polybutadiene, styrene and alkyl
acrylates or alkyl methacrylates on polybutadiene, styrene and acrylonitrile
on
ethylene-propylene-diene terpolymers, styrene and acrylonitrile on polyalkyl
acrylates
or polyalkyl methacrylates, styrene and acrylonitrile on acrylate-butadiene
CA 02258305 1998-12-15
-22-
copolymers, as well as mixtures thereof with the copolymers mentioned under
6), for
example those known as so-called ABS, MBS, ASA or AES polymers.
8. Halogen-containing polymers, for example polychloroprene, chlorinated
rubber,
chlorinated or chlorosulfonated polyethylene, copolymers of ethylene and
chlorinated
ethylene, epichlorohydrin homo- and copolymers, especially polymers of halogen-
containing vinyl compounds, for example polyvinyl chloride, polyvinylidene
chloride,
polyvinyl fluoride, polyvinylidene fluoride; as well as copolymers thereof,
such as
vinyl chloride-vinylidene chloride, vinyl chloride-vinyl acetate or vinylidene
chloride-
vinyl acetate.
9. Polymers derived from alpha,beta-unsaturated acids and derivatives thereof,
such as polyacrylates and polymethacrylates, polymethyl methacrylates,
polyacrylamides and polyacrylonitriles impact-modified with butyl acrylate.
10. Copolymers of the monomers mentioned under 9) with each other or with
other unsaturated monomers, for example acrylonitrile-butadiene copolymers,
acrylonitrile-alkyl acrylate copolymers, acrylonitrile-alkoxyalkyl acrylate
copolymers,
acrylonitrile-vinyl halide copolymers or acrylonitrile-alkyl methacrylate-
butadiene
terpolymers.
11. Polymers derived from unsaturated alcohols and amines, or the acyl
derivatives or acetals thereof, such as polyvinyl alcohol, polyvinyl acetate,
polyvinyl
stearate, polyvinyl benzoate, polyvinyl maleate, polyvinyl butyral, polyallyl
phthalate
or polyallyl melamine; as well as their copolymers with olefins mentioned in
section 1.
12. Homo- and copolymers of cyclic ethers such as polyalkylene glycols,
polyethylene oxide, polypropylene oxide or copolymers thereof with bisglycidyl
ethers.
13. Polyacetals, such as polyoxymethylene and those polyoxymethylenes which
contain comonomers, for example, ethylene oxide; polyacetals modified with
thermoplastic polyurethanes, acrylates or MBS.
14. Polyphenylene oxides and sulfides, and mixtures thereof with styrene
polymers or polyamides.
15. Polyurethanes derived from polyethers, polyesters and polybutadienes with
terminal hydroxyl groups on the one hand and aliphatic or aromatic
polyisocyanates
on the other hand, as well as precursors thereof.
CA 02258305 1998-12-15
-23-
16. Polyamides and copolyamides derived from diamines and dicarboxylic acids
and/or from aminocarboxylic acids or the corresponding lactams, such as
polyamide
4, polyamide 6, polyamide 6/6, 6/10, 6/9, 6/12, 4/6, 12/12, polyamide 11,
polyamide
12, aromatic polyamides starting from m-xylene, diamine and adipic acid;
polyamides
prepared from hexamethylenediamine and isophthalic and/or terephthalic acid
and
with or without an elastomer as modifier, for example poly-2,4,4-
trimethylhexamethylene terephthalamide or poly-m-phenylene isophthalamide.
Block
copolymers of the aforementioned polyamides with polyolefins, olefin
copolymers,
ionomers or chemically bonded or grafted elastomers; or with polyethers, for
example with polyethylene glycol, polypropylene glycol or polytetramethylene
glycol.
Furthermore with polyamides or copolyamides modified with EPDM or ABS; and
polyamides condensed during processing (RIM polyamide systems).
17. Polyureas, polyimides, polyamide-imides and polybenzimidazoles.
18. Polyesters derived from dicarboxylic acids and dialcohols and/or from
hydroxycarboxylic acids or the corresponding lactones, such as polyethylene
terephthalate, polybutylene terephthalate, polyethylene naphthenate, poly-1,4-
dimethylolcyclohexane terephthalate, polyhydroxybenzoates, as well as block
polyether esters derived from polyethers with terminal hydroxyl groups;
furthermore
polyesters modified with polycarbonates or MBS.
19. Polycarbonates and polyester carbonates.
20. Polysulfones, polyether sulfones and polyether ketones.
21. Crosslinked polymers derived from aldehydes on the one hand and phenols,
urea or melamine on the other hand, such as phenol-formaldehyde resins, urea-
formaldehyde resins and melamine-formaldehyde resins.
22. Drying and non-drying alkyd resins.
23. Unsaturated polyester resins derived from copolyesters of saturated and
unsaturated dicarboxylic acids with polyhydric alcohols and vinyl compounds as
crosslinking agents, and also halogen-containing modifications thereof of low
flammability.
24. Crosslinkable acrylic resins derived from substituted acrylates, for
example
from epoxy acrylates, urethane acrylates or polyester acrylates.
CA 02258305 1998-12-15
-24-
25. Alkyd resins, polyester resins and acrylate resins crosslinked with
melamine
resins, urea resins, polyisocyanates or epoxy resins.
26. Crosslinked epoxy resins derived from polyepoxides, for example from
bisglycidyl ethers or cycloaliphatic diepoxides.
27. Natural polymers such as cellulose, natural rubber, gelatin and
derivatives
thereof modified chemically in a polymer-homologous manner, such as cellulose
acetates, cellulose propionates and cellulose butyrates, or the cellulose
ethers such
as methylcellulose; and also rosins and derivatives.
28. Mixtures (polyblends) of the aforementioned polymers, for example
PP/EPDM, polyamide/EPDM or ABS, PVC/EVA, PVC/ABS, PVC/MBS, PC/ABS,
PBTP/ABS, PC/ASA, PC/PBT, PVC/CPE, PVC/acrylates, POM/thermoplastic PUR,
PC/thermoplastic PUR, POM/acrylate, POM/MBS, PPO/HIPS, PPO/PA 6.6 and
copolymers, PA/HDPE, PA/PP, PA/PPO, PA/LDPE.
29. Aqueous emulsions of natural or synthetic rubbers, e.g. natural rubber
latex
or latices of carboxylated styrene-butadiene copolymers.
30. Mixtures of the plastics specified under 1-29.
Suitable elastomers are, for example:
1.Polydienes, such as, for example, polybutadiene, polyisoprene or
polychloroprene; block polymers, such as, for example,
styrene/butadiene/styrene,
styrene/isoprene/styrene or acrylonitrile/butadiene copolymers.
2.Copolymers of mono- and diolefins with one another or with other vinyl
monomers, such as e.g. ethylene-alkyl acrylate copolymers, ethylene-alkyl
methacrylate copolymers, ethylene-vinyl acetate copolymers and also
terpolymers of
ethylene with propylene and a diene, such as hexadiene, dicyclopentadiene or
ethylidenenorbornene.
3.Halogen-containing polymers, such as e.g. polychloroprene, chlorinated
rubber,
chlorinated or chlorosulfonated polyethylene, epichlorohydrin homo- and
copolymers,
chlorotrifluoroethylene copolymers, polymers of halogen-containing vinyl
compounds,
such as e.g. polyvinylidene chloride, polyvinylidene fluoride; and copolymers
thereof,
such as vinyl chloride-vinylidene chloride, vinyl chloride-vinyl acetate or
vinylidene
chloride-vinyl acetate.
CA 02258305 2005-06-29
29276-747
-25-
4.Polyurethanes derived from polyethers, polyesters and polybutadiene with
terminal hydroxyl groups on the one hand and aliphatic or aromatic
polyisocyanates
on the other hand, and also their precursors.
S.Natural rubber.
6.Mixtures (polyblends) of the abovementioned polymers.
Z.Aqueous emulsions of natural or synthetic rubbers, such as e.g. natural
rubber
latex or latices of carboxylated styrene-butadiene copolymers.
Incorporation into the polymers can take place, for example, by mixing in the
NOR-HALS compounds or mixtures and, if desired, further additives by the
methods
customary in the art.
The NOR-HALS compounds or mixtures can also be added in the form of a
masterbatch comprising these compounds, for example, in a concentration of
from
2.5 to 25% by weight to the plastics that are to be decomposed.
According to another aspect of the invention, there is provided a use of NOR-
HALS
compounds for reducing molecular weight and, for example, for deoompo5ing
polymers.
The decomposition of waste plastics is particularly relevant. Here, the NOR-
HALS
compounds are suitable in particular for polypropylene and polyethylene,
especially
for polyolefin mixtures as are produced, for example, in the course of
collections and
separation processes.
Also suitable is the targeted decomposition of polymers or their mixtures, for
example that of polyethylene to give wax.
Further suitable decomposition catalysts and destabilizers are peroxides,
acidic
earths, zeolites, hydrocalcites or metal salts, e.g. of Fe, Zn or Cu.
The following examples illustrate the invention. The amounts used herein and
in
the description and the claims relate unless specified otherwise to the
weight.
Example 1: Decomposition of polypropylene/melt index measurements
CA 02258305 1998-12-15
-26-
Polypropylene powder is mixed with 0.5% of the additive indicated in Table I
[sic]
and melted at the stated temperature for 30 minutes. The product is drawn
through a
waterbath for cooling and then granulated. The melt index MFR [g/10 min] is
measured (at 190°C with 1.2 kg). A large increase in the melt index
denotes severe
chain degradation and hence good destabilization. The results are collated in
Table I
[sic].
Table 1
Example Additive Concentration Temperature MFR
1A (Comparative)Tinuvin770'' 0.5 % 280C 3.7
IB NOR-HALS-1'' 0.5 % 280C > 300
IC NOR-HALS-1 + 0.5 % 280C 220
IrganoxB 2153
CH3\ ,CH3
(CH2)e CO-O NH
i =O CH3 CH3
O
CH3 I 'CH3
CH3 -N/J~\CH3
H
2~ see Example 3
'' 1:2 Mixture of pentaerythrityl tetrakis-3-(3,5-di-tert-butyl-4-
hydroxyphenyl)propionate and tris(2,4-di-tert-butylphenyl) phosphite.
Example 2: An HDPE waste material is admixed with the stated additives in the
melt and the beginning of decomposition is determined by means of
CA 02258305 1998-12-15
-27-
thermogravimetric analysis (TGA). The lowest decomposition temperature T; is
possessed by the NOR-HALS-containing mixture 2C. The mixture 2B contains a
conventional decomposition catalyst.
Table 2: HDPE waste material, TGA measurement
Example Additive Concentration Temperature
2A (Comparative) none 0 % 491 C
2B zeolite ZSM 5 % 421
5
2C NOR-HALS-1 5 % 371
'' NOR-HALS-1: see Example 3
Example 3:
A PP/PE copolymer waste material from used battery casings is subjected to a
targeted decomposition by extruding it on a Rheocord° twin-screw
extruder (from
Haake) with the stated additives at 280°C and 70 rpm. Subsequently, the
MFR is
measured at 230°C and 2.16 kg in accordance with ISO 1133. In
comparison to a
material extruded without additive, a high MFR is obtained with the additives
of the
invention, which is evidence of corresponding decomposition. The decomposition
or
MFR can be adjusted by varying the state of the amount of additive.
CA 02258305 1998-12-15
-28-
Table 3
__ _ Additive MFR
230/2.16
3A PP/PE copolymer wastenone 6.8
material (comparative)
3B PP/PE copolymer waste0.5% NOR-HALS 1 32
material
3C PP/PE copolymer waste1.0% NOR-HALS 1 51
material
3D PP/PE copolymer waste1.0% NOR-HALS 2 51
material
3E PP/PE copolymer waste1.0% NOR-HALS 2 51
material
H3C CH3
R* O-N O (CH2)
n
H3C CH3
2
n;
n = 1, R* = cyclohexyl: NOR-HALS 1 (CA 260327)
n = 1, R*= methyl: NOR-HALS 2 (CA 270212)
n = 4, R*= cyclohexyl: NOR-HALS 3 (CA 260094).