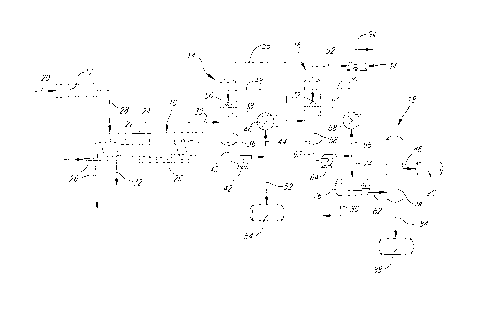Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02260570 1999-O1-29
PROCESS FOR THE PRODUCTION OF
PHENOLIC-RICH PYROLYSIS OILS FOR USE IN MAKING
PHENOL-FORMALDEHYDE RESOL RESINS
s The present invention relates to a process for
the production of phenolic-rich pyrolysis oils suitable
for use in making phenol-formaldehyde resol resins. More
particularly, the invention is directed to the production
of phenolic-rich pyrolysis oils from lignocellulosic
~o materials.
Phenol-formaldehyde or phenolic resins are
typically cross-linkable polymeric resins. There are two
types of phenolic resins; both types are made from phenol
and aldehydes, usually pure phenol and formaldehyde. One
15 type of phenolic resin, novolak, is made under acidic
conditions using excess phenol; the acid catalyzes the
reaction of phenol and formaldehyde to form the cross-
linkable polymeric resin. Novolak resins are used for the
formation of molded pieces and articles. The other type of
zo phenolic resin, resol, is made under basic conditions
using excess formaldehyde; a small amount of a base is
added to the phenol to catalyze the reaction thereof with
formaldehyde and form the cross-linkable polymeric resin.
Resol resins are used as adhesives for gluing together the
z5 veneer plies of exterior-grade plywood panels and the
flakes of oriented strand board panels. The cured adhesive
is resistant to moisture, preventing delamination of the
panels.
Because phenol is produced primarily from
3o petroleum, its price and availability are linked to that
of petroleum. Consequently, phenolic resins are relatively
expensive. A number of attempts have thus been made in
recent years to at least partially substitute the
petroleum-based phenol in phenolic resins with inexpensive
35 phenols derived from lignocellulosic wastes such as bark,
sawdust, wood chips and the like.
The pyrolysis of lignocellulosic materials is
known to produce a complex mixture of phenolic compounds
- 1 -
CA 02260570 1999-O1-29
which are derived primarily from the lignin contained in
the feedstock. Such a complex mixture of phenolic
compounds requires expensive fractionation in order to
provide a phenolic fraction which can be used as a
substitute for the petroleum-based phenol in the synthesis
of phenolic resins. For example, in an attempt to
formulate new adhesives for wood, Chum and Black have
proposed in US Patent No. 4,962,269 a process for
fractionating fast-pyrolysis oils derived from
to lignocellulosic materials to produce a phenolic
compounds/neutrals fraction. The process involves a series
of liquid-liquid extraction steps, wherein the phenolic
compounds partitioned in an organic phase from the
pyrolysis oil phase and the organic phase is then treated
5 with an aqueous alkali metal bicarbonate solution. The
complexity and lengthy solvent extraction associated with
a relatively low yield of the phenolic compounds/neutrals
fraction limit the industrial applications of such a
process. The same disadvantages are encountered in the
zo fractionation process described in US Patent No.
4,233,465.
It is therefore an object of the present
invention to overcome the above drawbacks and to provide a
process for the production of phenolic-rich pyrolysis oils
z5 which can be directly used for making phenol-formaldehyde
resol resins.
According to one aspect of the invention, there
is provided a process for the production of a phenolic-
rich pyrolysis oil, which comprises the steps of:
3o a) pyrolysing lignocellulosic material at a
temperature of no more than about 550°C under an absolute
pressure of no more than about 50 kPa to produce
pyrolysis vapors; and
b) condensing the pyrolysis vapors to obtain a
35 condensate consisting of a phenolic-rich pyrolysis oil
having a dew point of about 65 to about 75°C under an
absolute pressure of about 15 to about 20 kPa.
- 2 -
CA 02260570 1999-O1-29
Applicant has found quite unexpectedly that by
condensing pyrolysis vapors derived from lignocellulosic
material at a temperature ranging from about 65 to about
75°C and an absolute pressure ranging from about 15 to
s about 20 kPa, or at equivalent temperature/pressure
thermodynamic conditions, one obtains a condensate
consisting of a phenolic-rich pyrolysis oil which is
capable of functioning as efficiently as petroleum-based
phenols in the production of phenol-formaldehyde resol
to resins. Such a phenolic-rich pyrolysis oil can thus be
directly used in making phenol-formaldehyde resol resins.
The present invention therefore provides, in
another aspect thereof, a phenolic-rich pyrolysis oil
derived from lignocellulosic material and having a dew
15 point of about 65 to about 75°C under an absolute pressure
of about 15 to about 20 kPa.
According to a further aspect of the invention,
there is also provided a method of preparing phenol-
formaldehyde resol resins, which comprises substituting a
zo phenolic-rich pyrolysis oil as defined above for a portion
of phenol in a phenol-formaldehyde resol composition.
According to still another aspect of the
invention, there is provided a phenol-formaldehyde resol
resin containing a phenolic-rich pyrolysis oil as defined
z5 above .
The lignocellulosic material used as feedstock
advantageously comprises bark waste, preferably softwood
bark waste, since such a feedstock enables one to obtain
pyrolysis oils having high contents of phenolic compounds.
3o Examples of suitable softwoods include fir, pine, spruce,
larch and mixtures thereof. The bark waste is preferably
in the form of particles having a particle size of about
15 to about 25 mm in order to ensure optimum heat
transfer.
35 According to a preferred embodiment, step (a) is
carried out at a temperature of about 400 to about 550°C
and an absolute pressure of about 10 to about 50 kPa. Care
- 3 -
CA 02260570 1999-O1-29
should be taken to not exceed a temperature of about 550°C
and a pressure of about 50 kPa, since extensive secondary
cracking of the pyrolysis vapors occurs at temperatures
and pressures above 550°C and 50 kPa, respectively.
s Preferably, the pyrolysis is carried out at a temperature
of about 475°C and a pressure of about 15 to about 20 kPa
to provide the desired phenolic-rich pyrolysis oil in an
optimum yield while reducing extensive secondary cracking
of the pyrolysis vapors.
~o As previously indicated, step (b) is carried out
at a temperature ranging from about 65 to about 75°C and
an absolute pressure ranging from about 15 to about 20
kPa, or at equivalent temperature/pressure thermodynamic
conditions. Operating within such temperature and pressure
~s ranges enables one to avoid condensing water vapor,
carboxylic compounds of low molecular weight such as
acetic and formic acids and undesirable odorous compounds
of low molecular weight, which occurs at temperatures
below 65°C, and also to avoid polymerization reactions and
zo the formation of highly viscous liquids, which occur at
temperatures above 75°C. Preferably, step (b) is carried
out to obtain a phenolic-rich pyrolysis oil having a dew
point of about 68-70°C under an absolute pressure of about
15 to about 20 kPa. The phenolic-rich pyrolysis oil which
z5 is obtained in step (b) has been found to contain about 70
wt.% of phenolic compounds such as monophenols,
polyphenols, flavanoids, low-molecular-weight lignins and
tannins, about 19 wt.% of neutral compounds such as
ketones, aldehydes, steroids and furfural derivatives, and
3o about 11 wt.% of sugars such as levoglucosane and high
molecular weight carboxylic acids such as fatty acids.
According to another preferred embodiment, non-
condensed pyrolysis vapors obtained in step (b) are
further condensed at a temperature of no more than about
35 45°C, preferably at about 15-30°C, and an absolute
pressure of about 15 to about 20 kPa, or at equivalent
temperature/pressure thermodynamic conditions, to obtain a
- 4 -
CA 02260570 1999-O1-29
further condensate comprising an organic phase in
admixture with an aqueous phase. Since the organic phase
contains desirable phenolic compounds which were not
condensed in step (b), it is preferably separated from the
s aqueous phase and the separated organic phase is subjected
to an evaporation so as to recover a residue comprising a
phenolic fraction boiling above 125°C under atmospheric
pressure. Such a phenolic fraction can be mixed with the
aforesaid phenolic-rich pyrolysis oil for use in making
~o phenol-formaldehyde resol resins.
Since the phenolic-rich pyrolysis oil and/or the
phenolic fraction boiling above 125°C under atmospheric
pressure may contain high molecular weight carboxylic
acids in quantities which inhibit the reaction of such oil
~s and/or fraction with formaldehyde in the production of
phenol-formaldehyde resol resins, the phenolic-rich
pyrolysis oil and/or phenolic fraction can be purified by
extraction with an organic solvent selected from the group
consisting of C5-Cg saturated hydrocarbons and petroleum
zo ether to obtain a first fraction comprising phenolic oil
with a minor portion of the high molecular weight
carboxylic acids and a second fraction comprising the
organic solvent and a major portion of the high molecular
weight carboxylic acids dissolved therein, the first and
z5 second fractions being immiscible with one another,
separating the first and second fractions from one another
and recovering the first fraction. Preferably, the organic
solvent utilized is hexane.
The above purification method is also useful for
3o removing hydrocarbons which may be present in the
phenolic-rich pyrolysis oil and/or phenolic fraction and
which are inert in the reaction of such oil and/or
fraction with formaldehyde, but which have a diluting
effect.
35 The present invention therefore provides, in
another aspect thereof, a method of purifying a phenolic-
_ 5 -
CA 02260570 1999-O1-29
rich pyrolysis oil containing high molecular weight
carboxylic acids, which comprises the steps of:
a) extracting the oil with an organic solvent
selected from the group consisting of C5-Cg saturated
s hydrocarbons and petroleum ether to obtain a first
fraction comprising a phenolic oil with a minor portion of
the high molecular weight carboxylic acids and a second
fraction comprising the organic solvent and a major
portion of the high molecular weight carboxylic acids
~o dissolved therein, the first and second fractions being
immiscible with one another;
b) separating the first and second fractions
from one another; and
c) recovering the first fraction.
15 Further features and advantages of the invention
will become more readily apparent from the following
description of preferred embodiments, with reference to
the accompanying drawings in which the single figure is a
schematic illustration of a vacuum pyrolysis plant for
zo treating lignocellulosic material to produce a phenolic-
rich pyrolysis oil, according to the invention.
The vacuum pyrolysis plant illustrated comprises
a double-tray reactor 10 for pyrolysing under vacuum
softwood bark waste, a pre-treatment unit 12 for pre-
z5 treating the bark waste prior to being fed to the reactor
10, primary and secondary condenser units 14 and 16 for
condensing the pyrolysis vapors generated in the reactor
and a fractionation unit 18 for recovering a phenolic
fraction from the condensate discharged from the secondary
3o condenser unit 16. The unit 12 includes a grinder for
reducing coarse bark waste fed via line 20 into particles
having a particle size of about 15-25 mm and a dryer for
drying the particles to a moisture content of about 10-
wt.%.
35 The reactor 10 is a horizontal moving bed
reactor of the type described in copending US patent
application Serial No. 08/811,172 filed March 4, 1997,
- 6 -
CA 02260570 1999-O1-29
which includes two trays 22,24 arranged one above the
other and heated by means of molten salt circulating
through conduit 26 in contact with the trays 22 and 24.
The bark particles which are fed via line 28 to the
reactor 10 fall onto the upper tray 22 on which they are
conveyed along one direction by a conveyor system (not
shown) and then fall onto the lower tray 24 on which they
are conveyed in the opposite direction, while being heated
at a temperature of about 400-550°C by the molten salt
~o circulating in the conduit 26. The reactor 10 is provided
with a discharge outlet 30 for discharging the non-
condensable gases and condensable vapors generated in the
reactor and a discharge outlet 32 for discharging the
solid carbonaceous material formed therein. The discharge
5 outlet 30 is connected via the primary and secondary
condenser units 14 and 16 to a vacuum pump 34 for
maintaining an absolute pressure of about 15 to about 20
kPa in the reactor 10.
The gases and vapors discharged from the reactor
zo 10 through the outlet 30 are sent to the primary condenser
unit 14. The condenser unit 14 comprises a packed bed
tower 36 containing a packing 38 of Raschigs rings onto
which are sprayed fine oil droplets having a temperature
of about 65 to about 75°C, obtained by recirculating a
zs portion of the oil condensed in the tower 36 via line 40,
pump 42, line 44, heat exchanger 46 where the oil is
cooled to a temperature of 65-75°C and then via line 48 to
spray nozzle 50. As the gases and vapors ascend the tower
36 and pass through the packing 38 of Raschigs rings, they
3o encounter the cooled oil droplets sprayed by the nozzle
50, resulting in the condensation of the vapors having a
dew point of about 65-75°C under the operating
subatmospheric pressure conditions, thereby obtaining a
substantially water-free phenolic-rich pyrolysis oil which
35 accumulates at the bottom of the tower 36. The portion of
the phenolic-rich pyrolysis oil which is not recirculated
is sent via line 52 to the storage tank 54.
- 7
CA 02260570 1999-O1-29
The non-condensable gases and non-condensed
vapors comprising water vapor, carboxylic compounds of
relatively low molecular weight, phenolic compounds of
relatively low molecular weight and undesirable odorous
s compounds of low molecular weight leaving the tower 36 are
sent via line 56 to the secondary condenser unit 16. The
condenser unit 16 is similar to the unit 14, but operates
at a lower temperature, e.g. about 15 to about 30°C. The
unit 16 comprises a packed bed tower 58 containing a
~o packing 60 of Raschigs rings onto which are sprayed fine
droplets of condensate having a temperature of 15-30°C,
obtained by recirculating a portion of the condensate
formed in the tower 58 via line 62, pump 64, line 66, heat
exchanger 68 where the condensate is cooled to a
15 temperature of 15-30°C and then via line 70 to spray
nozzle 72. The condensate formed in the tower 16 comprises
an organic phase in admixture with an aqueous phase, the
organic phase containing the phenolic compounds of low
molecular weight. The portion of the condensate which is
zo not recirculated is sent via line 74 to the fractionation
unit 18 comprising a decanter 76 and an evaporation tower
78 for recovering the phenolic compounds contained in the
organic phase.
In the decanter 76, the organic phase and
z5 aqueous phase are separated from one another by
decantation. The separated aqueous phase is sent via line
80 to a water treatment unit (not shown), whereas the
organic phase obtained is sent via line 82 to the
evaporation tower 78, where it is subjected to an
3o evaporation. The tower 78 includes a bottom outlet 84 for
discharging a residue comprising a phenolic fraction
boiling above 125°C under atmospheric pressure, and a
lateral outlet 86 for discharging the fraction boiling at
a temperature less than 125°C. The phenolic fraction which
35 contains phenolic compounds of relatively low molecular
weight is sent to the storage tank 88 and is
advantageously mixed with the phenolic-rich pyrolysis oil
_ g -
CA 02260570 1999-O1-29
contained in the tank 54 for use in making phenol-
formaldehyde resol resins. The other fraction having a
boiling point below 125°C is sent to the storage tank 90.
The non-condensable gases leaving the tower 58
s are sent via line 92, vacuum pump 34 and line 94 to
combustion utilities (not shown).
The following non-limiting examples further
illustrate the invention.
EXAMPLE 1
~o A feedstock comprising 194.5 kg of softwood bark
waste having a moisture content of 6.5 wt.% and consisting
of 70 vol.o fir, 28 vol.% spruce and 2 vol.% larch was
pyrolyzed in reactor 10 at a temperature of 468°C under an
absolute pressure of 18.9 kPa. The pyrolysis vapors were
~s condensed in tower 36 at a temperature of 71°C under the
same pressure. 53.27 kg of a phenolic-rich pyrolysis oil
were obtained, representing a yield of 29.37 % on
anhydrous wood basis.
Non-condensed vapors leaving the tower 36 were
zo condensed in tower 58 at a temperature of 27°C under an
absolute pressure of 18.9 kPa, to obtain 38.07 kg of a
condensate comprising an organic phase in admixture with
an aqueous phase. The organic and aqueous phases were
separated from one another in decanter 76 and the
z5 separated organic phase (7.82 kg) was subjected to an
evaporation in tower 78. 5.44 kg of a phenolic fraction
boiling above 125°C under atmospheric pressure were
recovered, representing a yield of 2.96 % on anhydrous
wood basis.
3o The phenolic fraction boiling above 125°C and
discharged from the tower 78 was combined with the
phenolic-rich pyrolysis oil discharged from the tower 36.
The combined oils were subjected to chemical analysis. The
results are shown in Table I:
_ g -
CA 02260570 1999-O1-29
TABLE 1
Compound type Wt.% Molecular weight
distribution
Hydrocarbons 3 200 < Mw < 300
Sugars 9 Mw < 300
Acids 1.5 M < 100
Fatty acids 10 150 < Mw < 300
Alcohols (linear) 1 Mw < 250
Esters 1 Mw < 250
Phenols 10 100 < Mw < 250
Ketones 2 Mw < 250
Hydroxy ketones 1 Mw < 250
Cyclic alcohols 1.5 Mw < 250
Steroids 2 350 < Mw < 450
Triterpenoids 2 350 <Mw < 450
Flavonoids 18 280 < Mw < 400
Tannins and 25 380 < Mw < 500
Derivatives
Polyphenols and 5 Mw > 500
Polyflavonoids
Labile compounds 8 N.D. (2)
Total 100
(1) approximate values (moisture and ash-free basis)
(2) N.D. - non determined.
As it is apparent from the above Table, the
phenols, flavonoids, tannins, polyphenols and
polyflavonoids which constitute phenolic compounds
represent 58 wt.% of the total weight of the oil
~o composition.
EXAMPLE 2
A feedstock comprising 205.25 kg of softwood
bark waste having a moisture content of 6.5 wt.% and
consisting of 70 vol.o fir, 28 vol.o spruce and 2 vol.%
larch was pyrolyzed in reactor 10 at a temperature of
400°C under an absolute pressure of 18.7 kPa. The
pyrolysis vapors were condensed in tower 36 at a
temperature of 70°C under the same pressure. 50.71 kg of a
phenolic-rich pyrolysis oil were obtained, representing a
zo yield of 26.49 % on anhydrous wood basis.
- 10 -
CA 02260570 1999-O1-29
Non-condensed vapors leaving the tower 36 were
condensed in tower 58 at a temperature of 35°C under an
absolute pressure of 18.7 kPa, to obtain 45.44 kg of a
condensate comprising an organic phase in admixture with
an aqueous phase. The organic and aqueous phases were
separated from one another in decanter 76 and the
separated organic phase (10.19 kg) was subjected to an
evaporation in tower 78. 7.09 kg of a phenolic fraction
boiling above 125°C under atmospheric pressure were
~o recovered, representing a yield of 3.69 0 on anhydrous
wood basis.
EXAMPLE 3
A feedstock comprising 205.32 kg of softwood
bark waste having a moisture content of 11.7 wt.o and
5 consisting of 70 vol.o fir, 28 vol.% spruce and 2 vol.o
larch was pyrolyzed in reactor 10 at a temperature of
483°C under an absolute pressure of 16.7 kPa. The
pyrolysis vapors were condensed in tower 36 at a
temperature of 68°C under the same pressure. 39.85 kg of a
zo phenolic-rich pyrolysis oil were obtained, representing a
yield of 21.98 % on anhydrous wood basis.
Non-condensed vapors leaving the tower 36 were
condensed in tower 58 at a temperature of 43°C under an
absolute pressure of 16.7 kPa, to obtain 45.59 kg of a
z5 condensate comprising an organic phase in admixture with
an aqueous phase. The organic and aqueous phases were
separated from one another in decanter 76 and the
separated organic phase (13.63 kg) was subjected to an
evaporation in tower 78. 9.81 kg of a phenolic fraction
3o boiling above 125°C under atmospheric pressure were
recovered, representing a yield of 5.41% on anhydrous wood
basis.
- 11 -
CA 02260570 1999-O1-29
EXAMPLE 4
A phenol-formaldehyde resol resin having code
No. E-7-7-A was prepared from the phenolic-rich pyrolysis
oil obtained in Example 1, by substituting such an oil for
40 wt.% of the phenol in an industrial formulation, and
was used for the manufacture and evaluation of oriented
strand board (OSB) panels. An industrial phenol-
formaldehyde resol resin without phenol replacement and
having code No. E-7-7 was also prepared for comparison.
~o The panel test results are shown in Table 2:
TABLE 2
RESIN OSB Panel
Code Phenol pH 215C Density Internal Torsion
Replace- Press (kg/m3) Bonding Shear
ment Cycle (MPa) (in. lb.)
(wt.s) (Sec.)
E-7-7-A 40 10.2 150 638 0.396 9.7
210 642 0.515 23.1
E-7-7 0 - 150 639 0.048 1.4
210 644 0.446 14.5
As shown in the Table above, the mechanical
~s properties of the panel prepared using the resin having
code E-7-7-A (40% phenol replacement) are superior to
those of the panel prepared using the resin having code
E-7-7 (without phenol replacement).
- 12 -