Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02261218 1999-02-22
1
CATALYST FOR REMOVAL OF NITROGEN OXIDES AND METHOD FOR
REMOVAL OF NITROGEN OXIDE BY USE OF THE CATALYST
This invention relates to catalyst for the removal of
nitrogen oxides and to a method for the removal of
nitrogen oxides by the use of the catalyst. More
particularly, it relates to a honeycomb catalyst to be
used for the removal of nitrogen oxides contained in waste
gases discharged from boilers, heating furnaces, gas
turbines, diesel engines, and various industrial processes
and to a method for efficient removal of such nitrogen
oxides by the use of the catalyst.
This application is a divisional of co-pending
Canadian Patent Application Serial No.2,022,935-7, filed
August 8, 1990.
In the methods currently available for the removal of
nitrogen oxides from waste gases, the method of selective
catalytic reduction which attains selective removal of
nitrogen oxides even from a waste gas of a high oxygen
content, operates effectively with only a small amount of
a reducing agent, and allows use of ammonia as a reducing
agent by reason of economy, has been predominating.
As respects the shape of the catalyst to be used in
this method of selective catalytic reduction, the catalyst
in the honeycomb construction proves to be effective for
the reason that the dust in the waste gas is not readily
deposited on the catalyst bed and the pressure loss
suffered by the catalyst bed is small. At present,
therefore, the honeycomb catalyst is in extensive utility.
Concerning the method for effective removal of nitrogen
oxides by the use of a honeycomb catalyst, it is disclosed
in Japanese Patent Publication SHO 54(1979)-29,419, for
example, that when the through holes in the honeycomb
catalyst are so defined as to possess an equivalent
diameter in the range of 2 to 30 mm and an opening ratio
in the range of 50 to 80% and produces a gas flow rate in
the range of 0.5 to 60 m/sec, the honeycomb catalyst
CA 02261218 1999-07-07
2
experiences no clogging of tree through holes with the
dust, suffers only from a smal7_ pressure loss, and enjoys
a high ratio of removal of nitrogen oxides (hereinafter
referred to as "nitrogen removal ratio").
In recent years, tree environmental pollution
with nitrogen oxides represented by the acidic rain has
been worsening on the global scale. In the circumstances,
an earnest desire has been expressed to perfect as a
measure for decreasing the emanation of nitrogen oxides a
technique for nitrogen removal waste gases with high
efficiency at a low cost. For the sake of this technique,
it is extremely important to promote compaction of a
nitrogen removal apparatus by decreasing the amount of
catalyst required therefor and, at the same time, lower
the pressure loss of the catalyst bed to the fullest
possible extent and decrease the power consumption
required for the operation of a fan.
The method disclosed in Japanese Patent
Publication SHO 54(1979)-29,419 is not enough at all to
fulfil the requirement described above. The desirability
of developing a catalyst for the removal of nitrogen
oxides which possesses a still higher capacity for
denitrification and suffers o~aly from a small pressure
loss or a method for efficient removal of nitrogen oxides
has been finding enthusiastic recognition.
Incidentally, it i;s widely known that in
proportion as the opening ratio in the cross section of a
given honeycomb catalyst is increased, the thickness of
partition walls separating the through holes is inevitably
decreased (hereinafter referred to occasionally as "cell
wall thickness") and, as the result, the pressure loss of
the catalyst bed is lowered and. the geometric surface area
CA 02261218 1999-07-07
3
of the catalyst is increased, with the result that the
nitrogen removal activity is enhanced. For the pressure
loss of the catalyst bed to be' suppressed to the fullest
possible extent, it is necessary that the opening ratio of
the catalyst should be increased as much as possible.
The present invention, therefore, is directed
towards the provision of a novel honeycomb catalyst for
the removal of nitrogen oxides and a method for the
efficient removal of nitrogen oxides by the use of the
catalyst.
This invention furthE~r is directed towards the
provision of a catalyst for the removal of nitrogen oxides
which allows ample suppression of pressure loss, excels in
the ability to nitrify waste' gas, the enjoys highly
satisfactory durability and a method for efficient removal
of nitrogen oxides by the use o:E this catalyst.
In accordance with the present invention, there
is provided a honeycomb catalyst for effecting catalytic
reduction and removal of nitrogen oxides in a waste gas in
the presence of ammonia, whi~~h honeycomb catalyst (I)
contains a catalytically active substance comprising 60 to
99.5% by weight of a binary oxide containing titanium and
silicon and 40 to 0.5% by weight of the oxide of at least
one metal selected from the group consisting of vanadium,
tungsten, molybdenum, copper, manganese, cerium and tin,
and (II) possesses pores comprising substantially two
independent pore groups each of a uniform pore diameter,
such that the pore volume occupied by one pore group
having a pore diameter in the range of 0.01 to 0.03 ~m
accounts for a proportion in the range of 50 to 80% of the
total pore volume and the pore volume occupied by the
other pore group having a pore' diameter in the range of
CA 02261218 1999-07-07
4
0.8 to 4 ~,m accounts for a proportion in the range of 10
to 30% of the total pore volume', wherein said binary oxide
containing titanium and silicon comprises 40 to 95 atomic
of titanium and 60 to 5 atomic % of silicon.
In accordance with another aspect of the
invention, there is provided a method for the removal of
nitrogen oxides in waste gars, which method comprises
causing the waste gas to contact the aforementioned
catalyst in the presence of ammonia.
The main effects of the present invention are
as follows:
(1) By the use of the honeycomb catalyst of the
present invention, the pressur~= loss of catalyst bed can
be lowered conspicuously. As a result, the power
consumption required for the ~~peration of a fan can be
reduced and the denitrifying process can be appreciably
economized.
(2) The honeycomb cataly~>t of the present invention
continues to exhibit high denitrifying activity for a long
time.
(3) The honeycomb catalyst of this invention
possesses durability enough to satisfy commercial
applications.
(4) Since the honeycomb catalyst of this
invention continues to possess a high nitrogen removal
activity for a long time, suffers only from a small
pressure loss of catalyst bed, and enjoys thorough
durability, it can effect efficient and economic removal
of nitrogen oxides in waste gas.
In the description which follows, reference is
made to the accompanying drawings, wherein:
CA 02261218 1999-02-22
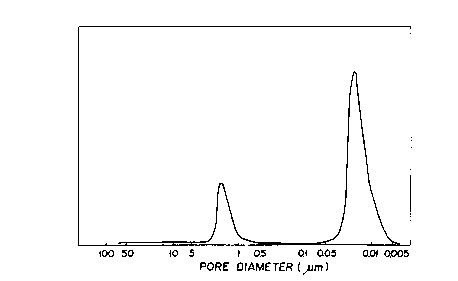
Fig. 1 is a diagram showing the pore diameter
distribution of a honeycomb obtained in Example 1;
Fig. 2 is a diagram showing the pore diameter
5 distribution of a catalyst obtained in Control 1; and
Fig. 3 is a diagram showing the pore diameter
distribution of a catalyst obtained in Control 2.
Now, the present invention will be described in
detail below with respect to the components (I) to (IV)
sequentially in the order mentioned.
The catalytically active substance for the catalyst
of a honeycomb construction of this invention to be used
for the removal of nitrogen oxides (hereinafter referred
to as "honeycomb catalyst of this invention") comprises
(A) 60 to 99.5% by weight of a binary oxide containing
titanium and silicon (hereinafter referred to briefly as
"Ti02-Si02" ) and (B) 40 to 0 . 5 % by weight of the oxide of
at least one metal selected from the group consisting of
vanadium, tungsten, molybdenum, copper, manganese, cerium
and tin.
In the composition of Ti02-Si02 as the component (A) ,
titanium is preferable to account for a proportion in the
range of 40 to 95% and silicon for a proportion in the
range of 60 to 5%, respectively in the atomic percentage.
If the proportion of titanium is less than 40%, the
nitrogen removal activity of the catalyst is unduly low.
If this proportion exceeds 95%, the oxidizing activity of
SO2 is unduly high.
The component (B) is the oxide of at least one metal
selected from the group consisting of vanadium, tungsten,
molybdenum, copper, manganese, cerium, and tin.
The proportion of the component (A) is in the range
of 60 to 99.5% by weight, preferably 80 to 99% by weight,
and the proportion of (B) is in the range of 40 to 0.5% by
weight, preferably 20 to 1% by weight. If the proportion
of the component (A) is less than 60% by weight, the cost
of raw materials for the catalyst is high and the
denitrifying activity cannot be expected to be
CA 02261218 1999-02-22
6
proportionately increased. Conversely, if this proportion
exceeds 99.5% by weight, the undesirability arises that
the denitrifying activity is unduly low.
The method for the preparation of the honeycomb
catalyst of this invention is not specifically defined.
This honeycomb catalyst can be prepared by various
methods. Now, a typical method of preparation will be
described below. It should be noted, however, that this
invention is not limited to this method.
In the preparation Ti02-Si02 as the component (A),
at least one compound selected from the group consisting
of inorganic titanium compounds such as titanium chloride
and titanium sulfate and organic titanium compounds such
as titanium oxalate and tetraisopropyl titanate can be
used as a titanium source and at least one compound
selected from the group consisting of inorganic silicon
compounds as colloidal silica, finely powered silicic
acid, water glass, and silicon tetrachloride and organic
silicon compounds such as tetraethyl silicate as a silicon
source.
The aforementioned compounds as the titanium source
and the silicon source are weighed out in amounts such
that the atomic percentages of titanium and silicon fall
respectively in the range of 40 to 95% and 60 to 5% and
they are retained in the state of an acidic aqueous
solution or a sol in a concentration in the range of 1 to
100 g/liter, preferably 5 to 80 g/liter, as oxides at a
temperature in the range 10° to 100°C, preferably 10° to
50°C. The mixture of the compounds is stirred and aqua
ammonia is added dropwise thereto as a neutralizing agent
to give rise to a coprecipitated compound. The
coprecipitated compound is separated by filtration,
thoroughly washed, then dried by heating at a temperature
in the range of 80 to 140°C, preferably 100° to 120°C,
for
a period in the range of 1 to 10 hours, preferably 5 to 10
hours, and further calcined at a temperature in the range
of 450° to 700°C, preferably 500° to 650°C, for a
period in
CA 02261218 1999-02-22
7
the range of 1 to 10 hours, preferably 3 to 10 hours, to
obtain TiOz-Si02.
In the preparation of the oxide of at least one metal
selected from the group consisting of vanadium, tungsten,
molybdenum, copper, manganese, cerium, and tin as the
component (B), the starting material may be suitably
selected from among oxides, hydroxides, ammonium salts,
oxalates, and halides of the metals mentioned above.
Specifically, the vanadium sources which are usable herein
include ammonium metavanadate, vanadyl sulfate, vanadyl
oxalate, and vanadium oxide and the tungsten sources which
are usable herein include tungsten oxide, ammonium
paratungstate, and tungstic acid, for example.
The aforementioned component (A) and the aqueous
solution of a starting material for the component (B)
added thereto in conjunction with a molding auxiliary are
mixed, kneaded, and molded in the form of a honeycomb with
an extrusion molding device. From the molded product, a
honeycomb catalyst of the present invention is obtained by
drying the molded product at a temperature in the range of
50° to 120°C, preferably 50° to 100°C, and then
calcining
the dried product in the air at a temperature in the range
of 450° to 700°C, preferably 500° to 650°C, for a
period in
the range of 1 to 10 hours, preferably 2 to 6 hours.
The specific surface area (BET surface area) of the
honeycomb catalyst of the present invention is preferable
to be not less than 80 mz/g, preferably in the range of 80
to 250 mz/g.
The opening ratio in the cross section (cross section
perpendicular to the through holes) of the honeycomb
catalyst of the present invention is preferable to be in
the range of 80 to 90%, preferably 80 to 88%. In the
present invention, an economic effect is obtained as in
lowering the pressure loss of the catalyst bed as
described above and decreasing the power consumption
required for the operation of a fan by allowing the
catalyst to have an opening ratio of not less than 80% in
CA 02261218 1999-02-22
8
the cross section thereof. If the opening ratio in the
cross section exceeds 90%, the catalyst no longer has
practicability because the cell walls have a very small
thickness and the strength of the catalyst is unduly low.
The thickness of the partition walls separating the
through holes in the honeycomb catalyst of this invention
is in the range of 0.2 to 0.8 mm, preferably 0.2 to 0.7
mm. If this thickness is less than 0.2 mm, the strength
is unduly low. Conversely, if the thickness exceeds 0.8
mm, the undesirability arises that the pressure loss is
unduly large.
The through holes in the honeycomb catalyst of this
invention have diameters in the range of 2 to 8 mm,
preferably 3 to 7 mm.
The honeycomb catalyst of this invention possesses
pores comprising substantially two independent pore groups
each of a uniform pore diameter, such that the pore volume
occupied by one pore group having a pore diameter in the
range of 0.01 to 0.03 ~m accounts for a proportion in the
range of 50 to 80%, preferably 50 to 70% of the total pore
volume and the pore volume occupied by the other pore
group having a pore diameter in the range of 0.8 to 4~m
accounts for a proportion in the range of 10 to 30%,
preferably 15 to 30% of the total pore volume.
Specifically, the honeycomb catalyst of this invention is
characterized by the fact that, as illustrated in Fig. 1,
the two pore groups are present independently of each
other and exhibit very sharp pore diameter distributions.
If the pores form one pore group as illustrated in
Fig. 2 or if the pores form two independent pore groups,
partly overlap one another, and possess no uniform pore
diameter as illustrated in Fig. 3, the objects of this
invention are not accomplished.
The fact that the pore groups exhibit very sharp pore
diameter distributions in the honeycomb catalyst of this
invention means that the two pore groups are each formed
of pores of a highly uniform diameter and this very fact
CA 02261218 1999-02-22
9
is believed to explain why the honeycomb catalyst of this
invention exhibits high satisfactory denitrifying
activity. Specifically, the true cause remains yet to be
elucidated definitely. When a waste gas is to be diffused
within pores of a catalyst, the diffusion of the waste gas
proceeds easily in the pore groups each having a uniform
diameter as in the honeycomb catalyst of this invention.
As the result, it is believed that even when the cell
walls have a small thickness, the honeycomb catalyst is
improved in denitrifying activity without reference to the
cell wall thickness.
The total pore volume of the honeycomb catalyst of
this invention is preferable to be in the range of 0.3 to
0.55 cc/g.
The pore diameter, pore diameter distribution, and
pore volume of the honeycomb catalyst of this invention
have been determined by the use of a mercury injection
type porosimeter.
The honeycomb catalyst possessing pores comprising
two independent pore groups each having a uniform diameter
according with the present invention can be produced by
(1) a method which comprises adding during the course of
molding an organic polymeric compound such as resin or
cellulose or an inorganic salt such as ammonium nitrate
which is volatilized and decomposed in the step of
calcination, (2) a method which comprises adding a powder
such as silica sand, a,-alumina, cordierite, or zirconia
and mixing the powder with the raw materials, or (3) a
method which comprises suitably adjusting the particle
diameter of the raw material powders, for example.
Typically, the organic polymeric compounds which are
usable in the method of (1) include polyethylene resin,
acrylic resin, and crystalline cellulose, for example.
Typically, the inorganic salts which are similarly usable
herein include ammonium nitrate, ammonium oxalate, and
ammonium carbonate, for example. The amount of such a
polymeric compound to be added is in the range of 5 to 30%
CA 02261218 1999-02-22
by weight, preferably 10 to 30% by weight.
The average particle diameter and the amount of
addition of the powder in the method of (2) are desired to
5 be respectively in the range of 1 to 20~m, preferably 5 to
20~m, and in the range of 5 to 30%, preferably 10 to 30%.
In the case of the method of (3), the average
particle diameter of the powder as a raw material is
generally in the range of 2 to 30~m, preferably 5 to 20~m.
10 If the particle diameter is unduly small, the honeycomb
catalyst possessing pore distributions aimed at by this
invention cannot be prepared.
The kind of the waste gas to be treated with the
honeycomb catalyst of this invention is not specifically
defined. The honeycomb catalyst of the present invention
can be used for the removal of nitrogen oxides contained
in waste gases discharged from boilers, heating furnaces,
gas turbines, diesel engines, and various industrial
processes.
Specifically, it can be effectively used for a waste
gas approximately containing 0 to 3,000 ppm of sulfur
oxides (SOX), 1 to 20% by volume of oxygen, 1 to 15% by
volume of carbon dioxide, 5 to 15% by volume of steam, 0
to 30 g/Nm3 of soot, and 100 to 1,000 ppm of nitrogen
oxides (NOX, mainly NO) . The waste gas from the ordinary
boiler has a gas composition falling in the aforementioned
ranges. The honeycomb catalyst of the present invention
can be also used for the treatment of such special gases
as nitrogen oxides-containing waste gases containing no
sulfur oxide and nitrogen oxides-containing waste gases
containing halogen compounds.
Though the conditions of the treatment are variable
with the kind, behavior, etc. of a given waste gas,
ammonia (NH3) is generally used in an amount the range of
0.5 to 3 parts by volume, preferably 0.5 to 1.1. parts by
volume, based on 1 part by volume of nitrogen oxide (NoX).
In the case of a waste gas from a boiler, for example,
since the NOX is formed mostly of NO, the molar ratio of NO
CA 02261218 1999-02-22
11
to NH3 is desired to be approximately in the range of 1 .
1. This is because the otherwise possible release of any
excess NH3 in its unaltered form into the ambient air must
be avoided. Where the occurrence of unaltered NH3 must be
curbed to the fullest possible extent, it is desirable to
lower the molar ratio of NH3/NOX below 1 . 1.
For the purpose of decreasing the pressure loss, the
flow speed of the waste gas is desired to be as low as
permissible. If the flow speed is less than 1 m/sec,
however, the undesirability arises that the catalyst bed
is clogged with soot or dust contained in the waste gas.
From the practical point of view, therefore, the flow
speed of the waste gas is suitably selected in the range
of 1 to 20 m/sec, preferably 2 to 10 m/sec. The flow
speed in this range is preferable because the low pressure
loss aimed at by the present invention can be obtained.
The reaction temperature is generally in the range of 200°
to 700°C, preferably 250° to 600°C. The special velocity
is generally in the range of 1000 to 100,000 hr-1,
preferably 3,000 to 20,000 hr-1. Though the pressure is
not specifically defined, it is desired to be in the range
of 0.01 to 10 kg/cm2, preferably 0.5 to 2 kg/cm2. The type
of the reaction vessel is not specifically defined.
Generally, there may be used reaction vessels of the
fixed-bed type, moving-bed type, fluidized-bed type, etc.
Now, the present invention will be described more
specifically below with reference to working examples.
Example 1
[Preparation of component A]
As a titanium source, an aqueous sulfuric acid
solution containing titanyl sulfate and having the
following composition was used.
TiOS04 250 g/1 (as TiOz)
Whole HzS04 1, 100 g/1
Separately, 286 liters of aqua ammonia (NH3,25%) way
added to 400 liters of water and 24 kg of silica sol
(containing about 30% by weight of Si02) (produced by
CA 02261218 1999-02-22
12
Nissan Chemical Co., Ltd. and marketed under trademark
designation of "Snowtex NCS-30)" was also added. Into the
resultant solution, a titanium-containing aqueous sulfuric
acid solution obtained by diluting 153 liters of the
aforementioned aqueous sulfuric acid solution of titanyl
sulfate with 300 liters of water was gradually added in a
stirred state, to form a coprecipitate. The reaction
mixture was left standing in the ensuant state for 15
hours, to obtain a Ti02-Si02 gel. This gel was separated
by filtration, washed with water, and dried at 200°C for 10
hours.
Then, the dried gel was calcined at 600°C for 6
hours, pulverized with a hammer mill, and classified with
a classifier, to obtain a powder having an average
particle diameter of 10~m.
The composition of the produced powder (hereinafter
referred to as "TS-1") was Ti . Si - 4 . I (atomic ratio)
and the BET surface area was 160 m2/g.
[Preparation of honeycomb catalyst]
Then, 0.45 liter monoethanolamine was mixed with 4.5
liters of water and, in the resultant solution, 0.907 kg
of ammonium paratungstate was dissolved and subsequently
0.444 kg of ammonium metavanadate was dissolved therein,
to produce a homogenous solution. This solution and 10 kg
of the aforementioned TS-1 were thoroughly mixed under
continued addition of a suitable amount of water, kneaded,
and then molded with an extrusion molding device to
produce a grating the square of 50 mm of visible surface
area, 84.6% of opening ratio, 6.9 mm of mesh, and 0.5 mm
of cell wall thickness. The grating was dried at 60°C and
then calcined under a current of air at 500°C for 5 hours.
The composition of the produced honeycomb
catalyst, expressed in the weight ratio of oxides, was TS-
1 . V205 4J03 - 90:3 . 7. The pore volume of the first pore
group and the second pore group having pore diameters
respectively in the range of 0.01 to 0.03 ~m and in the
range of 0.8 to 4 ~m accounted respectively for 68% and
CA 02261218 1999-02-22
13
18% of the total pore volume. The total pore volume was
0.44 cc/g.
The pore diameter distribution of the honeycomb
catalyst thus obtained was determined with a mercury
injection type porosimeter (produced by Shimadzu Ltd.).
The results are shown in Fig. 1.
It is noted from Fig. 1. that the honeycomb catalyst
possessed pores comprising two independent pore groups
each having a uniform pore diameter.
[Evaluation of quality of honeycomb catalyst]
A reaction vessel was packed with the honeycomb
catalyst and a model gas indicated hereinbelow was
supplied thereto under the conditions of 380°C of reaction
temperature, 25 Nm3/m2H (AV) of gas volume per gas contact
surface area of the catalyst, and 6 m/sec (380°C) of flow
speed of gas per cross section of the catalyst to
determine the denitrification ratio and the pressure loss
(per 1 m) .
The denitification ratio and the pressure loss were
determined as follows.
Nitrogen removal ratio: This property was found by
measuring the NOx concentrations at the inlet and outlet of
the catalyst bed with a NOX meter of the chemical light
emission type (produced by Yanagimoto Seisakujo) and
performing a calculation in accordance with the following
formula:
Nitrogen removal ration (%)
[(NOx concentration at inlet)-(NOX concentration at
outlet)]/(NOX concentration at inlet) x 100
Pressure loss: This property was found by measuring
the pressure difference between the inlet and outlet of
the catalyst bed and reducing the found pressure
difference to a value per m.
The results are shown in Table 1.
Model gas composition
NO 800ppm
CA 02261218 1999-02-22
14
O2 4%
SOz 1000ppm
H20 10%
NH3 8 0 Oppm
Nz Bal ance
Examples 2 to 5
Honeycomb catalyst possessing varied opening ratios
and cell wall thickness were obtained by following the
procedure of Example 1 and were tested for denitrification
ratio and pressure loss in the same manner as in Example
1. The results are shown in Table 1.
Control 1
The TS-1 powder obtained in Example 1 was further
pulverized with an air-current type pulverizing device to
obtain a powder having an average particle diameter of
lam. From this powder, a honeycomb catalyst having an
opening ratio of 62.4%, a cell wall thickness of 1.0 mm,
and a mesh of 3.95 mm was prepared by following the
procedure of Example 1.
This honeycomb catalyst possessed a total pore volume
of 0.40 cc/g and contained pores of only one pore group
having inner diameters in the range of 0.01 to 0.03 um as
illustrated in Fig. 2. The pore volume occupied by these
pores accounted for 90% of the total pore volume.
This honeycomb catalyst was tested for denitification
ratio and pressure loss in the same manner as in Example
1. The results are shown in Table 1.
Control 2
A honeycomb catalyst was prepared by following the
procedure of Control 1, excepting titanium oxide (Ti02)
having a specific surface area of 50 m2/g was added in an
amount to give a TS-1 . TiOZ = 10 . 2 (weight ratio) in the
molding of the catalyst.
The honeycomb catalyst thus obtained possessed a
total pore volume of 0.38 cc/g and contained two pore
groups partially overlapping and having no uniform
diameter as illustrated in Fig. 3.
CA 02261218 1999-02-22
15
This honeycomb catalyst was tested for
denitrification ratio and pressure loss in the same manner
as in Example 1. The results are shown in Table 1.
CA 02261218 1999-02-22
16
a~ a~
s
s'. ,-, ~r .-i ~ a ~S
~ ao c~ o o
rr M M N ~ ,p ~ j~
CO ep
b > b E
a ~, c.
a o a
o. v
o v
O r1 O Q
t~, O L~ c0 ~
,. ra O O
~ ~ O O
~ 0
N
~
0
0 ~ O
0 a
0 0
0 0
a0 0
0 0
a
0
>
o a~a~
y
v 8~
v
c ~ E
p,
'a~.~O p ~ ' O
O-~ N N N
'
. M
O O E
N ~ h' ""~ ' p ~'
. O .rl
A
b . ~d M
~
cd
O
O +~
CO
o p'
o~ 00 N O N O d~ O O
O
O O t0 N CO t0 M VI
CO C7
Ar ~ ~0 4 w
~ a y7p
O C
O
ra '~ Q. N G1N
N bD N h0
O ~ ~' ~ rr
~. p.cd
N ~ N ~r
i.
u7 N M ec~ a0 p N
~
N d: d' d! d~ M
d~ d!
O O O O O O
O
O '~
..1
i-. Vl fr
tr ..
C~ ~n tC r- ~ ~ ~
47 ~
O N
~
'
c ~"~~ a) E
~ p
d
co ~
do E s cd
.
~ n~o~
a~
v
._.., a ~ o
~ a
te
~n c~ o o " ~ .r
,
0 0 0 0 ~ ~ a~ ..~b
~
s, c no
U ~ i ho O C
4.a C U -I
o ~ ..aN >
t > a0cd
,y _ ,..,
p
do
co o ec cn ~ a~ o
r, rr
,
~' N a
N
n
~
'~
o c C o
C C
o o
o a
o
o o a
O w o s. E 40
~
a~
p
ra N Ch d' N
~ ~ a.
0 0 a
, r. r. r.
...
~
0 0 ~ o
W W W W W U i v
U
,
...,
w m