Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02266725 1999-03-23
WO 98/30995 PCT/US97/00813
M1::THOD AND APPARATUS FOR TESTING
THE EFFICACY OF PATIENT SUPPORT SYSTEMS
1 BACRGROilNI) OF THE INVENTION
2
3 A decubitus ulcer, or pressure ulcer as it is
4 more commonly known, is a localized wound of
variable depth caused by prolonged pressure in a
6 patient allowed to lie too still in bed over an
7 extended period of time. Sustained compression of
8 the cutaneous and subcutaneous tissue between the
9 bony prominences of the patient's body and the
support structure, e.a, the mattress, has been cited
11 as a primary cause of pressure ulcer formation.
12 Thus, the: sites most often affected in bed-ridden
13 patients include the sacrum, greater trochanter,
14 heel and scapula - these are the sites which usually
experience higher pressures or loads due to body
16 weight distribution.
17
18 From the treatment of patients in acute care
19 facilities to their care in the home setting, the
incidence of pressure ulcer development, and the
21 degeneration of tissues associated with such ulcers,
22 once formed, present a significant health care
23 problem Moth in terms of the amount of financial
24 resources expended in treatment and, more
CA 02266725 1999-03-23
WO 98/30995 PCT/US97/00813
1 importantly, in the morbidity and mortality
2 associated with the complications which often arise.
3 Depending on the severity of the pressure ulcer and
4 the medical condition of the patient, it has been
estimated that the cost of treatment can be as high
6 as $40,000 (Brandeis et.al., JAMA 264:2905-2908
7 (1990)). In one study it was reported that the rate
8 of occurrence of bacteremia associated with pressure
9 ulcers was 3.5 events per 10,000 hospital
discharges. The hospital mortality rate from this
11 complication alone was estimated to be 50% (Allman,
12 Decubitis 2:30-33 (1989)).
13
14 Although current understanding of pressure
ulcer etiology is incomplete, it is known that the
16 development of such ulcers is the result of a myriad
17 of factors which often interact with one another in
18 a complex manner. It has been recognized that
19 purely conservative measures can be used to control
one or more of these factors and that these measures
21 alone can result in the prevention of pressure ulcer
22 development and in more effective treatment of those
23 which have developed. One conservative measure
24 which has been identified to be of critical
importance in this regard, is the choice of
26 effective support surfaces, such as wheelchair
27 cushions as well as other seating devices and, more
28 importantly, mattresses, which are utilized in the
29 day-to-day patient care. In the following
2
CA 02266725 1999-03-23
WO 98/30995 PCT/US97I00813
1 discussion, the general term "support structure"
2 will encompass such varied products as beds,
3 mattresses, cushions, mattress overlays and covers,
4 and sheets, in addition to operating room tables and
other t~rpes transitional structures i.e. all types
6 of products with which a patient might have contact.
A1.'. of the products mentioned above impinge
8 upon defined areas of a patient's body and so
9 present their own unique set of problems and
concerns for healthcare workers and researchers in
11 the field of pressure ulcer prevention. For
12 example, the areas at risk of pressure ulcer
13 development as a result of inadequately designed
14 seating devices center primarily around the ischium
but can involve the posterior regions of the knee
16 joints a,nd lower thigh. More generalized areas are
17 at risk of pressure ulcer development however, when
18 the design of operating room tables and other
19 transitional structures is examined.
21
22 FACTOR8 TO HE CONSIDERED IN THE DESIGN OF A SUPPORT
2 3 BTRUCTUP,E
24 There is no universal support surface which can
be effectively used with every patient who might be
26 at risk of developing a pressure ulcer. Indeed, the
27 criteria for developing any type of support
28 structure transcend the purely practical constraints
29 of economy, durability and ease of use. The factors
3
CA 02266725 1999-03-23
WO 98/30995 PCT/C1S97/00813
1 which prevent the design of the universal support
2 structure are those associated with the individual
3 patient such as diagnosis; tissue history (previous
4 incidence of tissue breakdown, surgical repair or
stress); and body build (percentage of body fat and
6 its distribution or locali2ation) . In the design of
7 any type of support structure for the prevention, or
8 treatment, of pressure ulcers, the interaction
9 between these and other patient-related variables
together with the three mechanical forces of
11 pressure, shear and friction, all of which have been
12 implicated in the cause or exacerbation of pressure
13 ulcers, is of great importance. A review of the
14 three mechanical forces and their interaction with
the support structure, as well as a discussion of
16 the means used to measure them, is informative at
17 this juncture.
18
19 (1) Contact Pressure
Contact pressure is that force exerted on the
21 cutaneous and subcutaneous tissues by the patient's
22 body weight and bony prominences on one side and the
23 support structure on the other. The incorporation
24 of material into a support structure which has the
ability to reduce, redistribute or modify the
26 pressure forces generated by a patient's body weight
27 and bony prominences is of obvious importance in the
28 design of an effective support structure for the
29 prevention or treatment of pressure ulcers.
4
CA 02266725 1999-03-23
WO 98/30995 PCT/US97100813
1 At present there are a number of devices which
2 are routinely utilized by designers of support
3 structures to measure the ability of their products
4 to reduce contact pressure. These devices range
from simple pneumatic types to the more complex,
6 which utilize electro-pneumatic and electro-
7 resistive means. Such devices may employ algorithms
8 to sense: pressure change. The pressure change, so
9 detected,, is translated by the device and displayed
in a standardized form.. Some measuring devices use
11 fluids instead of air to sense changes in pressure.
12
13 Pneumatic and electro-pneumatic measuring
14 devices consist essentially of a pressure-reading
instrument connected to a probe. The probe consists
16 of an inflatable bladder in the pneumatic devices
17 or, in the electro-pneumatic devices, an inflatable
18 bladder containing a wire grid on each of its two
19 opposing walls (electrical connection is broken when
the grids are separated). The uninflated pneumatic-
21 type probes are placed beneath the body site to be
22 measured and air is supplied until, in the electro-
23 pneumatic devices, the two grids are separated or,
24 in the pneumatic devices, until internal pressure is
equal to external loading pressure. The contact
26 pressure is calculated as that pressure which
27 corresponds to the pressure between the body site
28 and the underlying support structure as measured by
29 the attached pressure-reading instrument.
5
CA 02266725 1999-03-23
WO 98/30995 PCT/US97/00813
1 Electro-resistive devices for measuring contact
2 pressure consist of a probe containing sensors
3 composed of materials whose electrical resistance
4 properties vary with the pressure which is applied
to their surface. Such electro-resistive devices
6 for measuring contact pressure can contain single-,
7 or multiple-sensor-probes. Strain gages or strain
8 gage assemblies are usually included as component
9 parts of such pressure measuring instruments. Just
as in the pneumatic-type devices, the change in
il resistance of the sensors) is measured by
1?, appropriate instrumentation and, by virtue of
13 calibration methods, the contact pressure between
14 the body site and underlying support structure can
be estimated from the change in resistance as
16 recorded on the attached instrumentation.
17
18 (2) Shear
19 Shear is defined as a mechanical stress which
is applied parallel to a plane of interest. Shear
21 is proportional to the pressure at any given site.
22 Like pressure, it exerts a degree of trauma on
23 cutaneous and subcutaneous tissues, thereby
24 compromising circulation and, as such, it is likely
to be an important factor in so-called "pressure
26 ulcer" formation.
27
28 The majority of support structures are
29 contained in an external covering material to
6
CA 02266725 1999-03-23
WO 98/30995 PCT/US97/00813
1 protect the interior from patient discharges. The
2 external covering material can produce shear stress
3 and one manifestation of such shear stress is the
4 so-called "hammock effect." The hammock effect
occurs when the support structure external covering
6 material supports the bulk of the patient's body
7 weight in a manner which is independent of the
8 interior of the support structure. In this
9 situation, the external covering material has a
tendenc~,r to cause relative movement of the cutaneous
11 and sur~cutaneous tissues along the sides of the
12 contact area between the external covering material
13 and the patient's body. Shear forces and stress are
14 also generated when the head region of a patient's
hospita:L-type bed is raised relative to the lower
16 portions resulting in slippage of the patient's
17 lower body regions.
18
19 Although clinical literature discusses the
significance of shear forces in the development and
21 progression of pressure ulcers in bed-ridden
22 patient:, it does not define specific means for
23 measuring the shear forces which cause the observed
24 clinical- effects. Indeed, to date, no procedures
have been described which will accurately measure
26 the total shear forces experienced by various sites
27 on a patient's body. Nor are there any means
28 presently available which are capable of determining
29 how much of what is presently recorded as a
7
CA 02266725 1999-03-23
WO 98/30995 PCT/US97/00813
1 "pressure" effect is, in actual fact, a shear
2 effect.
3
4 As stated previously, strain gages are often
used as the primary means of detection in devices
6 which are used to measure different types of force.
7 The principle upon which the operation of the strain
8 gage is based is, in essence, a simple one: the load
9 placed on the gage or housing which contains the
gage produces a force;.the force causes the gage to
il strain or stretch in response to its application;
12 the force alters the physical properties of the gage
13 such that there is a change in its electrical
14 properties such as resistance; this resistance
change can be detected and converted into an
16 accurate measurement of force.
17
18 For the above reasons, strain gages are
19 particularly suited for use in instrumentation for
the direct measurement of shear forces. The
21 tendency of tissue to deform due to shear can be
22 detected by the change in such properties as
23 electrical resistance in the attached gage. In this
24 regard, the Y series of encapsulated foil strain
gages and G series of foil strain gage manufactured
26 by Omega Engineering, Inc. of Stamford, Connecticut;
27 and the semiconductor strain gages such as types C,
28 D, E, F, G, H, and L supplied by Kulite
8
CA 02266725 1999-03-23
WO 98/30995 PCT/US97/00813
1 5emiconoluctor Products, Inc. of Leonia, New Jersey
2 are useful.
3
4 (3) Friction
Friction is defined as the force generated
6 between two surfaces as they move across one
7 another. As such, it is a factor which is
8 considered to be of some importance in not only the
9 formation of pressure ulcers, but also in the
progressive deterioration of tissues which occurs as
11 a result: of their development. When, for example,
12 the external covering of the support structure,
13 described earlier, moves relative to the skin of a
14 patient, frictional forces are generated. When such
frictional forces are exerted on the patient's skin,
16 the skin is exposed to frictional drag which causes
17 abrasion. of its outermost layers.
18
19 When examining the forces of friction, two
factors must be considered - the actual force with
21 which tlae patient's body is pushing against the
22 external covering material and the relative
23 smoothness, softness or lubricity of the external
24 covering material which contacts the skin.
26 The coefficient of friction is the product of
27 such support structure properties as external
28 covering material smoothness, softness and lubricity
29 and the clinical characteristics of the opposing
9
CA 02266725 1999-03-23
WO 98/30995 PCT/US97f00813
1 external skin. Current methods for measuring
2 friction usually involve dragging a weighted sled,
3 with the material of interest on its contact side,
4 across the surface of the skin.
6 Frictional drag produces a strain on tissue and
7 so an alternative means of measuring its magnitude
8 would be by the use of localized force indicators
9 such as strain gages.
11 CONCL08ION
12 From the foregoing discussion of the importance
13 of contact pressure, shear and friction in "pressure
14 ulcer" development and progression, as well as that
regarding the methods available for their
16 measurement, it is apparent that, at the present
17 time, a support structure's ability to reduce the
18 incidence and severity of pressure ulcers cannot be
19 accurately determined prior to it being marketed.
The current pre-marketing test procedures used to
21 determine contact pressure, shear and friction are
22 inadequate or nonexistent - there are no universally
23 accepted means of, or procedures for, measuring
24 contact pressure in this context. Reproducibility
of results from both within and between testing
26 centers is impractical; the various pressure
27 measuring devices, discussed earlier, produce
28 different readings under the same test conditions.
29 Likewise, the determination of frictional drag,
CA 02266725 1999-03-23
WO 98/30995 PCT/US97/00813
1 mentioned above, is not universally applicable. In
2 addition, there are no methods currently available
3 to measure shear in this context.
4
As a result of the current inadequacies in pre-
6 market testing, patients are exposed to an unknown
7 risk of developing "pressure" ulcers while being
8 treated in healthcare facilities and precious
9 healthcare resources are being potentially wasted on
equipment with no measure of efficacy in the area of
11 pressure ulcer prevention or amelioration.
12
13 TOWARDS A UNIVERBAL TESTING BCHEME
14 As discussed previously, the choice of a
suitabl~a support structure for the patient at risk
16 of developing so-called "pressure ulcers" is vital
17 in the prevention of this serious and potentially
18 life-threatening condition. As an initial
19 preventative measure, it not only is the most
effective means of controlling the problem, but also
21 the most economical. Unfortunately, there are no
22 universally accepted means currently available to
23 test the various types of commercially available
24 support structures before they are introduced into
hospita7_s and other healthcare institutions.
26 Moreover, the majority of testing procedures which
27 are presently utilized, only take into account the
28 contribution of contact pressure in the development
11
CA 02266725 1999-03-23
WO 98/30995 PCT/US97/00813
1 of pressure ulcers and so, in light of the foregoing
2 discussion, are deficient.
3
4 Most initial evaluations of support structures
are performed using individual volunteer subjects of
6 varying physical characteristics of weight, height,
7 anatomical frame, gender and age. The results of
8 such evaluations cannot be duplicated or
9 extrapolated to different physiognomies.
Furthermore, such evaluations are performed merely
11 to provide the documentation required to introduce
12 the support structures into the healthcare facility.
13
14 In reality, at the present time, the true
efficacy parameters of the product can only be
16 determined from its,actual performance once in-use
17 in the facility. Not only are such means of
18 assessing efficacy undesirable, exposing, as they
19 do, patients to an unknown risk of developing
pressure ulcers, but they also provide a potentially
21 meaningless assessment. Even in well-designed,
22 randomized clinical trials, great care must be
23 exercised to ensure strict adherance to the study
24 protocol. Such studies are usually conducted over
extended periods of months or even years and involve
26 a large expenditure of financial resources. Failure
27 to comply with the study protocol can result in
28 invalidation of the results and so negate the value
12
CA 02266725 1999-03-23
WO 98/30995 PCT/US97/00813
1 of the study for use in justifying clinical
2 outcomes.
3
4 Pressure sore development, as discussed
previously, occurs as a result of the interaction of
6 a variety of factors. The presence of these various
7 factors, or their interaction, cannot be assessed in
8 the scientifically uncontrolled environment of the
9 healthcare facility.
11 As a result of the inability of current
12 methodologies and procedures to accurately assess
13 the efficacy of support structures used in the
14 prevention and treatment of pressure ulcers,
valuable healthcare resources are being drained from
16 an already overburdened system. Resources are being
17 wasted not only in the purchase of products whose
18 efficacy is largely unknown, but also in the
19 treatment of pressure ulcers which develop as a
result of exposing patients to products which are
21 not efficacious.
22
23 A ~~referred methodology for testing support
24 structure=s to determine their ability to prevent
pressure ulcer formation would be one which is both
26 uncomplicated in its utilization and universal in
27 nature i..e., one which would give reproducible
28 results no matter where in the world testing was
29 conducted. In addition, given the earlier
13
CA 02266725 1999-03-23
WO 98130995 PCT/US97I00813
1 discussion of the present inability to accurately
2 measure the three mechanical forces which have been
3 cited as being of importance in pressure ulcer
4 formation, such testing methodology would also
include, at a minimum, some means of accurately
6 measuring contact pressure, shear and friction.
7
8 The present invention contemplates provision of
9 a standardized testing system and method for
l0 evaluation of the efficacy of support structure
11 products in the prevention and treatment of pressure
12 ulcers. The manner in which this has been achieved
13 is by the incorporation of sensors of contact
14 pressure, shear and friction into the design of an
anthropomorphic model which is representative of the
16 human body in respect to such features as anatomical
17 contours; height, weight and weight distribution;
18 and compliance, flexibility and tissue thickness.
19
As a result of the flexible and adaptable
21 nature of its design, the present invention can also
22 be adapted to include an assessment of other factors
23 which might be considered important in the
24 development of pressure ulcers. The effect of these
factors on pressure ulcer development can be
26 assessed alone or in conjunction with other
27 variables such as those of contact pressure, shear
28 and friction already mentioned. Examples of such
29 other factors are temperature and moisture
14
CA 02266725 1999-03-23
WO 98/30995 PCT/US97J00813
1 accumulation within the skin due to sweating, normal
2 moistures loss from the body and moisture
3 accumulation due to uncontrolled factors such as
4 incontinence. In this regard, the physical
properties of the material or materials used to
6 simulate human skin and subcutaneous tissues in the
7 anthropomorphic model combined with the ability to
8 incorporate means of simulating normal moisture loss
9 and sweating within the model e-g, fluid reservoirs
and heating filaments, would enable clinical
11 investigators or researchers to examine and monitor
12 the role= of these factors in the formation and
13 progression of pressure ulcers.
14
In 'the past, anthropomorphic devices have found
16 extensive use in studies of various aspects of motor
17 vehicle safety and, in a related context, as parts
18 of model systems designed to assess the effects of
19 motor vehicle accidents on the vehicle occupants.
As such, the idea of placing sensors of various
21 types on the surface of, and within, the
22 anthropomorphic device, is not of itself new.
23 However, one novel aspect of the
present invention,
24 and one= which distinguishes it from the
anthropomorphic systems proposed to date, is its
26 placement of particular sensing means at various
27 experimentally-predetermined positions of
28 physiolo~~ical importance to the development and
29 clinical progression of pressure The
ulcers.
CA 02266725 1999-03-23
WO 98130995 PCT/US97/00813
1 placement of the sensing means is of such sensitive
2 nature that their output as regards contact
3 pressure, shear and friction can be correlated to
4 the actual forces acting on the cutaneous and
subcutaneous tissues in vivo. In this way, the
6 anthropomorphic model of the present invention can
7 be used to accurately assess, in a standardized
8 manner, the external factors which will predispose
9 an individual to pressure ulcer formation and their
pathological progression.
11
12 This ability to accurately assess the role of
13 external factors in pressure ulcer development and
14 pathological progression imparted by the
anthropomorphic model of the present invention is
16 especially valuable when it is remembered that many
17 pressure ulcers are initiated beneath the surface of
18 the skin, usually in the deeper tissue regions. By
19 the time these pressure ulcers are visible on the
surface of the skin, they have usually already
21 severely undermined large areas of tissue between
22 the bone and skin surface, often forming channels,
23 sinus tracts and large areas of dead or missing
24 tissue. An understanding of the way in which
external factors translate into internal forces
26 beneath the surface of the skin is critical to not
27 only the assessment of the efficacy of new support
28 structures, but also to an understanding of the
16
CA 02266725 1999-03-23
WO 98/30995 PCT/LTS97/008I3
1 pathology of pressure ulcer development and
2 progression.
3
4 In addition, the positioning of the various
sensors within the anthropomorphic model combined
6 with the: ability to manufacture the model to a
7 variety of specifications which are representative
8 of the varied forms of the human body allows the
9 contribution of the various forces to be accurately
assessed for an infinite varietry of body types. The
11 system is thus capable of separating the various
12 forces in a manner which has not been possible up
13 until the present time. Such an ability is an
14 invaluable component of not only a testing system,
but also any system designed to investigate the
16 individual and combined effect of such variables as
17 contact pressure, shear, friction, moisture
18 accumulation and temperature in pressure ulcer
19 developmeant and progression.
21 The design of the anthropomorphic model system
22 also lends itself to testing and study programs
23 involving actual human tissue. Sections of human
24 tissue may be introduced into the compartmentalized
structurE: of the anthropomorphic model and tissue
26 viability assessed over time relative to the
27 application of various support structures. The
28 viabilit~~ of the sections of human tissue will be
29 proportional to the forces acting upon them and so
17
CA 02266725 2004-10-18
78041-1
indicative of the efficacy of the support structure being
tested.
S'ITMMARY OF THE INVENTION
A broad aspect of the invention provides an
anthropomorphic model system comprising: an anthropomorphic
model simulating the major dynamic characteristics of a
human, said anthropomorphic model being adaptably
representative of specific classes of human body form as
regards body build and including flexible human skin and
subcutaneous tissue simulating materials comprising a
cutaneous region and a subcutaneous region covering at least
parts of the model; sensing means located at predetermined
positions on, and in the vicinity of, the surface of the
skin defining the cutaneous region and within the
subcutaneous region, within the interior of said
anthropomorphic model, said sensing means for measuring
physical parameters acting on said anthropomorphic model
when arranged in life-like positions resting on a support
structure, such physical parameters including pressure,
shear and friction forces; and means for detecting and
displaying signals from said sensing means whereby decreased
or increased signals from said sensing means are indicative
of the forces existing at and within the cutaneous and
subcutaneous regions.
Another broad aspect of the invention provides a
method for measuring the efficacy parameters of support
structures to be used in the prevention and treatment of
decubitus or pressure ulcer formation comprising: resting an
anthropomorphic model simulating the major dynamic
characteristics of a human and having flexible human skin
and subcutaneous tissue simulating materials including a
cutaneous region and a subcutaneous region; placing sensing
18
CA 02266725 2004-10-18
78041-1
means at specific locations on and within said cutaneous and
subcutaneous regions of said model on a support structure to
be tested for efficacy, said sensing means capable of
measuring physical parameters comprising at least one of
temperature, moisture accumulation, pressure, shear and
friction; and means for collecting and interpreting data
obtained from said sensing means to determine loading at
sensing locations to thereby determine likelihood of injury
to a human resting on such support.
A further broad aspect of the invention provides
an anthropomorphic model simulating the major dynamic
characteristics of a human, said anthropomorphic model being
adaptably representative of specific classes of human body
form as regards body build and including flexible human skin
simulating materials including a cutaneous region and a
subcutaneous region covering at least parts of the model;
sensing means located within the flexible human skin
simulating materials of said anthropomorphic model for
measuring physical parameters such as pressure, shear and
friction forces acting on said anthropomorphic model; and
means for detecting signals from said sensing means for
ascertaining at least one of the pressure, shear and
friction forces existing within the flexible skin simulating
materials.
A still further broad aspect of the invention
provides a method for measuring the external and internal
pressures and forces sensed by parts of an anthropomorphic
model simulating the major dynamic characteristics of a
human, said anthropomorphic model including flexible human
skin simulating materials with a cutaneous region and a
subcutaneous region comprising: placing sensing means at
specific locations on, and within, said flexible human skin
of said model; said sensing means capable of measuring
18a
CA 02266725 2004-10-18
78041-1
physical parameters comprising at least one of temperature,
moisture accumulation, pressure, shear and friction; resting
said anthropomorphic model on a support structure; and
sensing and interpreting data obtained from said sensing
means to determine at least one of temperature, moisture
accumulation, pressure, shear and friction at a sensing
location on, and within, said flexible human skin.
The present invention achieves its objectives in a
simple, straightforward yet elegant manner. The
anthropomorphic model is of stable construction and composed
of durable materials so that it will retain over an extended
period of time, its characteristics of human-like contour;
weight and weight distribution; tissue compliance,
flexibility and thickness. Its specifications can be so
rigidly delineated that it is capable of being manufactured
in a reproducible manner in different shapes, sizes and
weights which are representative of various classes of male
and female body types.
The sensor means capable of measuring contact
pressure and shear are placed at discrete, predetermined
locations on the surface of the anthropomorphic model and at
experimentally predetermined depths of physiological
importance within the portions of the model which correspond
to the inner tissues. Sensor means capable of measuring
friction are also located on or near the surface of the
model at experimentally predetermined positions of
physiological importance. Although, the location of the
sensor means correspond to those areas of a patient's body
where pressure ulcers are
18b
CA 02266725 1999-03-23
WO 98/30995 PCT/LTS97/00813
1 known to develop, the flexibility of the testing
2 system is such that sensor means may also be easily
3 located at positions which normally experience lower
4 loadinc~s. This permits the actual measurement of
forces at an almost infinite variety of sites both
6 before and after the anthropomorphic model has been
7 placed on a support structure. Placement of sensor
8 means at discrete positions on the surface of the
9 anthro~~omorphic model as well as at predetermined
positions of clinical. importance within the model
11 enable the three forces of contact pressure, shear
12 and friction to be measured in as close to the real
13 life situation as is possible.
14
The sensor means are placed so that they are
16 easily accessible and so can be removed to check
17 accuracy or replaced when no longer functional by
18 relatively unskilled individuals. The output of
19 each sensor is measured, processed and recorded by
suitable instrumentation equipped with programs to
21 collate the data in a form that can be easily
22 interpreted. If desirable, the means for
23 transmitting the data obtained from the sensing
24 means c:an be located entirely within the model,
thereby obviating the need for the attachment of any
26 external means for signal transduction.
27
28 It is therefore an object o~ the present
29 invention to provide an anthropomorphic model which
19
CA 02266725 1999-03-23
WO 98/30995 PCT/US97100813
1 embodies a standardized testing system which
2 produces quantifiable measurements of contact
3 pressure, shear and friction for the evaluation of
4 the efficacy of support structures designed to
prevent pressure ulcers or reduce the trauma
6 associated with existing pressure ulcers, so that
7 manufacturers and healthcare facilities alike can
8 determine the efficacy of support structures in a
9 reproducible manner for all types of patient.
11 It is a further object of this invention to
12 provide an anthropomorphic model which will enable
13 clinical investigators and researchers to delineate
14 the role of, and assess the efficacy of support
structures in decreasing the effect of other factors
16 e.4. temperature and moisture accumulation, which
17 have been implicated in pressure ulcer development
18 and progression.
19
It is a further object of this invention to
21 provide an anthropomorphic model which simulates the
22 various forms of the human body in such a manner
23 that quantitative measurements of contact pressure,
24 shear and friction can be obtained at predetermined
positions corresponding to areas of the human body
26 at risk of pressure ulcer development in order to
27 facilitate research into the relationship of these
28 three mechanical forces to the development of
29 pressure ulcers.
CA 02266725 1999-03-23
WO 98/30995 PCT/US97/00813
1 It is another object of the present invention
2 to prov°ide an anthropomorphic model from which
3 quantif~.able measurements of contact pressure, shear
4 and friction can be obtained under conditions which
mimic t=hose which would be experienced by a
6 hospitalized patient such that various clinical
7 parameters of importance to the choice and useful
8 life of support structures can be measured in the
9 controlled environment of the laboratory.
11 BRIEF J1:8CRIPTION OF THE DRAWINGB
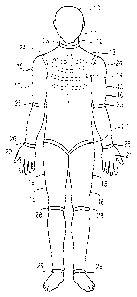
12 FIC~. 1 is a front elevational view of the
13 anthropomorphic model of this invention.
14
FI~~. 2 is a side elevational view of a typical
16 joint in the limbs.
17
18 FIC~. 3 is a side elevational view of the
19 anthropomorphic model of Fig. 1.
21 FIG.. 4 is an expanded schematic model of the
22 placement of sensors for detecting contact pressure,
23 shear a:nd friction forces in the human skin and
24 subcutaneous layers simulating material.
26 FIC~. 5(a) is a schematic representation of an
27 arrangement of sensors for detecting contact
28 pressure:, shear and friction forces; the data
29 receiving module, composed of signal processing
21
CA 02266725 1999-03-23
WO 98!30995 PCT/US97/00813
1 circuits and a signal transmitting terminus
2 assembly; data storage and retrieval\modules; and
3 computer processing assembly.
4
FIG. 5(b) is a schematic representation of one
6 embodiment of
7 the signal detection, transmitting and processing
8 pathway depicted in Fig. 5 (a) within a limb of the
9 anthropomorphic model.
11 Fig. 5(c) is a schematic representation of an
12 alternative embodiment of the signal detection,
13 transmitting and processing pathway depicted in Fig.
14 5(a) within a limb of the anthropomorphic model. In
this embodiment, the data receiving module is
16 attached by signal transmitting wires to the data
17 storage and retrieval module which is located at a
18 position outside the anthropomorphic model.
19
DESCRIPTION OF THE PREFERRED EMBODIMENT
21 A preferred embodiment of the invention
22 incorporates a number of features. The specific
23 form of those features presented in the preferred
24 embodiment of the invention is in accordance with
its use as a model system for testing various
26 support structures for their ability to prevent the
27 formation of pressure ulcers. This application has
28 been selected because of its importance. In other
22
CA 02266725 1999-03-23
WO 98/30995 PCT/US97/00813
1 applications, other specific forms may be
2 preferable.
3
4 Reference will now be made to the drawings,
whereby like parts are designated by like numerals.
6 The anthropomorphic model 10 includes head means 11,
7 neck means 12, and body means 13 which includes
8 chest/rib defining means 14. Limb means 15 include
9 a pair of arms 16, 17, a pair of legs 18, 19, and a
pair of hands 20, 21. Joint means 22 provide low
11 friction or frictionless articulated connections at
12 a neck joint 23, shoulder joints 24, elbow joints
13 25, wrist joints 26, hip joints 27, knee joints 28,
14 and ankle joints 29. Incorporation of the joint
means 22 into the design and structure of the
16 anthropomorphic model 10 enables the anthropomorphic
17 model :LO to be manipulated into a variety of
18 positions which have a direct relationship to the
19 human form and to changes in loading which result
from changes in relative positions of various body
21 parts. The anthropomorphic model 10 can be
22 manufacl~ured in a variety of size and weight classes
23 so that representatives of each class of human body
24 size and shape can be made available for testing.
A given model 10 may also be provided with means
26 (not sh~~wn), internal to the model, for attaching
27 discretsa concentrated weights, for example in the
28 vicinity of the shoulder blades, buttocks, hips,
23
CA 02266725 2004-10-18
78041-1
heels, etc. to simulate various weight classes using single
models.
Detachable portions 30 of the anthropomorphic
model 10 of this embodiment are composed of layers 100, 200,
300 of a flexible material 31 simulating human skin and
subcutaneous tissue. The flexible material 31 simulating
human skin and subcutaneous tissue may be uni- or multi-
layer depending upon the precise application and testing
procedures employed.
As shown in Fig. 3, the detachable portions 30 may
be placed at, and within, numerous selected portions of the
anthropomorphic model 10. These detachable portions may be
placed in any one of the models 10 manufactured in a variety
of (different) sizes.
The layers of flexible skin and subcutaneous
tissue simulating material 31 which make up the detachable
portions 30 of the anthropomorphic model 10 are especially
adapted to support or contain various types of sensing means
32, 33, 34. Mounted on the outer surface 100 of the skin
and subcutaneous tissue simulating material 31 are contact
pressure sensing means 32, shear force sensing means 33 and
friction sensing means 34. The middle layer 200 of skin and
subcutaneous tissue simulating material 31 contains contact
pressure sensing means 32 and shear force sensing means 33.
The innermost layer 300 of skin and subcutaneous tissue
simulating material 31 contains contact pressure sensing
means 32 and shear force sensing means 33.
24
CA 02266725 1999-03-23
WO 98/30995 PCT/US97/00813
1 Sensing means 32, 33, 34 are incorporated into
2 the anthropomorphic model 10 in such a way as to
3 facilitate ease of detachment and replacement even
4 by those unskilled in the art.
6 Each sensing means 32, 33, 34 is of modular
7 design and construction and is coupled to or is
8 integral with a data receiving module 35 composed of
9 a signal processing circuit 36 and a signal
transmitting terminus assembly 37, the latter being
11 connected by a plurality of leads 38 or a data bus
12 to data storage and retrieval modules 39 located in
13 predetermined parts of the anthropomorphic model l0
14 which do not interfere with the ability of the
system t:o take the necessary measurements. The data
16 storage and retrieval modules 39 are each enclosed
17 in a cwshioned and rugged protective housing 40.
18 The dat~j storage modules 39 receive and store the
19 output signals from the various sensors and transmit
them to a computer terminal 41. The computer 41 is
21 programmed to read the data supplied by each sensing
22 means :12, 33, 34, in a conventional manner.
23 Appropriate algorithms for performing mathematical
24 summing, averaging, statistical analysis or other
operations on the data generated by the various
26 sensors to be collated and displayed may be
27 conducted in a manner known to one of ordinary skill
28 in the a:rt.
CA 02266725 1999-03-23
WO 98/30995 PCTIUS97/00813
1 In an alternative embodiment of the invention,
2 each sensing means 32, 33, 34 is of modular design
3 and construction of a data receiving
and consists
4 module 35 composedof a varietyof signal processing
circuits 36 and a signal
transmitting
terminus
6 assembly 37 whichis connecte d by a plurality
of
7 leads 38 to data storage and retrieval modules
39
8 located at a position external to the
9 anthropom orphic
model
10.
11 Commonly available "off-the-shelf" items can be
12 used to sense and record contact pressure, shear and
13 friction. For example, suitable devices for sensing
14 shear forces are the RY21, RY61 and Y series of
strain gages manufactured by Omega Engineering, Inc.
16 of Stamford, Connecticut; suitable devices for
17 measuring contact pressure are those in the 170
18 series manufactured by Omega Engineering, Inc.; and
19 suitable devices for measuring friction are also
strain gages manufactured by Omega Engineering, Inc.
21 In addition, commonly available components can
22 be utilized to measure other variables,
23 quantificatation of which might be considered
24 desireable. For example, temperature measuring
instruments such as thermistors and thermocouples of
26 suitable dimensions for placement within the
27 anthropomorphic model are available from Cole-Parmer
28 of Niles, Illinois. Also, devices for measuring
29 skin moisture such as the Dermal Phase Meter are
26
CA 02266725 1999-03-23
WO 98130995 PCT/US97100813
1 manufactured by Nova Technology Corporation of
2 Gloucester, Massachusetts.
27