Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02267978 1999-04-07
WO 98/16107 PCT/EP97/05639
_y
Method for the prevention of the reinfestation of warm-blooded animals
b~ectoparasites
The present invention relates to the use of compounds of formula I in the
systemic control
of ectoparasites in warm-blooded animals, especially dogs and cats.
Blood-sucking ectoparasites of the order Insecta, which include, for example,
Cteno-
cephalides fells and C. canis (cat and dog fleas)) lice, mosquitos, tabanids,
tsetse-flies and
other biting flies, and those of the order Acarina, such as, for example,
Boophilus,
Amblyomma, Anocentor, Dermacentor, Haemaphysalis, Hyalomma) Ixodes,
Rhipicentor,
Margaropus, Rhipicephalus, Argas, Otobius and Ornithodorus (ticks) and the
like) infest
many warm-blooded animals, including farm animals, such as cows, pigs, sheep
and
goats) fowl, such as hens, turkeys and geese) animals bred for their fur, such
as mink,
foxes, chinchillas, rabbits and the like, and domestic animals, such as cats
and dogs.
Ticks can be subdivided into hard and soft ticks and are characterised by the
fact that they
infest one, two or three host animals. They attach themselves to a suitable
host animal and
suck up blood or body fluid. Replete female ticks drop from the host animal
and lay large
numbers of eggs {2000 to 3000) in a suitable niche in the ground or in any
other protected
site, where the larvae hatch. The larvae in turn seek a host animal, in order
to suck its
blood. Larvae of ticks that infest only one host animal moult twice and, in so
doing,
become nymphs and, finally, adult ticks without leaving the host that was
first chosen.
Larvae of ticks that infest two or three host animals drop from the host
animal after inges-
ting the blood) moult in the surrounding area and, as nymphs or adult ticks,
seek a second
or third host in order to suck its blood.
Ticks are responsible for transmitting and passing on many human and animal
diseases
throughout the world. The most important ticks, on account of their economic
conse-
quences, are Boophilus) Rhipicephalus, Ixodes, Hyalomma, Amblyomma and Derma-
centor. They are carriers of bacterial, viral) rickettsial and protozoal
diseases and cause
tick-paralysis and tick-toxicosis. Even a single tick can cause paralysis as
its saliva passes
into the host animal as the tick takes in food. Diseases caused by ticks are
usually trans-
mitted by ticks that infest several host animals. Such diseases, for example
babesiosis,
anaplasmosis, theiieriasis and heartwater, are responsible for death or injury
in a large
number of domestic and farm animals all over the world. In many countries
having a
temperate climate, ixodid ticks pass the agent of the chronically destructive
lyme disease
A 11
CA 02267978 1999-04-07
WO 98116107 PCTIEP97105639
_2_
from wild animals to humans. in addition to transmitting diseases, ticks are
also respon-
sible for high economic losses in cattle production. The losses are
attributable not only to
the death of the host animals, but also to damage to the skins, loss of
growth, reduction in
milk production and reduced value of the meat. Although the harmful effects of
tick infesta-
tion in animals have been known for many years and enormous progress has been
made
in tick-control programmes, no totally satisfactory methods of controlling or
eliminating
those parasites have yet been found and, in addition, ticks have often
developed a resis-
tance to chemical active ingredients.
Likewise, flea infestation in domestic animals and pets is a problem for the
animal owner to
which there have as yet been found only unsatisfactory solutions. Owing to the
complica-
ted life cycle of the flea, none of the known methods of controlling fleas is
totally satis-
factory, especially since most of the known methods are aimed principally at
controlling the
fully grown fleas in the fur and take no account at all of the various
juvenile stages of the
fleas, which live not only in the fur of the animal) but also on the floor, on
carpets, on the
animal's sleeping place, on chairs, in the garden and in all the other places
with which the
infested animal comes into contact. Adult cat and dog fleas (Ctenocephalides
fells and
C. canis) normally live in the fur of the host cat or host dog. They live on
the blood of the
host animal and lay their eggs in its fur. Since those eggs are not self-
adhering, however,
they generally soon fall off and can be found on the floor, on carpets, in the
dog's or cat's
basket, on the chairs used by the animal, in the garden, in the yard, etc..
That means that the whole of the pets' living area is contaminated with flea
eggs, from
which the larvae develop within two days. The larvae have three distinct
stages of develop-
ment, each of which lasts three days. In the final stage the lama spins its
cocoon and
becomes a pupa. Under favourable conditions, i.e. at 33~C and a relative
humidity of 65 %,
the metamorphosis from egg to pupa takes place in about 8 to 10 days. After
about
another 8 days the young, fully-formed fleas develop in the cocoons that are
still lying on
the floor, the carpets, the sleeping places, the chairs, etc.. The young adult
fleas remain
there until they sense the presence of an acceptable host animal, then they
hatch from
their cocoons and attempt to jump onto the host animal. Thus it takes at least
three weeks
for an egg to develop into a young adult flea that is capable of reinfesting
the host animal.
CA 02267978 1999-04-07
WO 98I16107 PCT/EP97/05639
-3-
The young flea may, however) remain in its cocoon for months, possibly for up
to a year.
On the other hand, under less favourable conditions the development from egg
to young
adult flea may take 4 to 5 months. To reach sexual maturity, fleas require
blood as food in
order to be able to reproduce, and that blood must be from the appropriate
host animal.
That long life cycle, which proceeds separately from the host animal, has a
significant
influence on the successful control of fleas on the host animal.
Even when the fleas in the fur of the host animal can be successfully
controlled, i.e. when
all the adult fleas are killed with a suitable active ingredient, the cat or
the dog is neverthe-
less exposed for weeks or even months to the risk of reinfestation by newly
hatched young
fleas from its living space.
Flea infestation of dogs and cats has unpleasant consequences not only for the
animal to
be treated but also for the animal's owner. Such drawbacks lead, for example,
to local irri-
tation or troublesome itching. A large number of animals become allergic to
the excreta of
the fleas, which leads to very itchy and crusty skin changes around the sites
of the bites on
the animal's body. Those skin changes normally have a diameter of
approximately 3 mm or
more and often make the animal prone to biting and cause it to scratch,
leading to loss of
fur in places.
Furthermore, flea-infested animals are constantly exposed to the risk of
infestation by
Dipyiidium caninum, a type of tape worm, which is transmitted by fleas.
Flea infestation is not only extremely troublesome for the affected animal,
but also has
unpleasant consequences for the animal's owner, until it finally becomes
evident to him
from the unusual behaviour of his pet that it is ill and is suffering and that
he must help it.
What is more) it can become unpleasant for the animal's owner if he has to
give up keep-
ing his infested animal, or if it dies or is removed temporarily from its
usual environment,
since in the event of the prolonged absence of a suitable host animal, the
newly hatched
fleas on the floor will be forced to infest the human, although they are
unable to reproduce
with human blood as their only source of food. Even when the dog or the cat is
present,
the animal's owner can be bitten by the fleas.
CA 02267978 1999-04-07
WO 98l16107 PCT/EP97/05639
-4-
In addition, dog and cat fleas) or their excreta, can lead to allergy-like
skin disorders in
some people, which in many cases means that the pet must be destroyed. The
desire for
effective control of fleas in dogs and cats has therefore always existed.
A number of conventional methods of control are known, but they have various
disadvan-
tages. 1f, for example, flea combs are used, the animal's owner has no
alternative but to
comb the animal intensively and often which, depending on the size of the
animal) may
take from a few minutes to an hour and will not be accepted patiently by every
animal.
However, not every animal owner is prepared to devote the time to this. The
use of corres-
ponding anti-flea shampoos is often unsuccessful, since most cats, and also
many dogs)
can be bathed, if at all, only by force, with the result that water and
medicament are spilt
and have to be cleared up. In addition) the effect of such a bath treatment
lasts about a
week at most, and the laborious procedure has to be repeated. The same or very
similar
problems can -be expected with the use of dips or rinses. The use of dusting
powders is
generally also not accepted by the animal without resistance, since it takes
several minutes
to treat the whole surface of the fur uniformly, and some of the dust will
inevitably get into
the mouth, nose and eyes of the animal. Even with careful application, it
cannot be
ensured that the animal and the human will not inhale any powder. It is
virtually inevitable
that the human will also come into contact with the composition to a greater
or lesser
extent.
When using sprays, many people may be unpleasantly surprised to find that most
animals,
especially cats) run away or react aggressively at the mere sound of the
spray. In addition,
sprays also have all the disadvantages listed for dusting powders, added to
which they
become even more finely dispersed in the atmosphere and are therefore inhaled
by human
and animal. Fleas are frequently controlled by means of so-called flea
collars, which
ensure good effectiveness temporarily. This treatment has a certain weakness,
owing
especially to its locally very limited area of application. Although the
killing action in the
region of the neck and chest is generally 7 00 %, more remote parts of the
body are
scarcely affected. In addition, those collars are active for a limited time.
Furthermore) many
of the collars are unattractive and may annoy the animal. It is also possible
nowadays to
buy medallions) which can be hung from conventional collars and are said to be
effective.
Although they are attractive in appearance, the action of those medallions is
unsatis-
factory, since they have inadequate contact with the fur. Some anti-flea
organophosphorus
compounds are also available as spot-on formulations and are thus applied to a
locally
CA 02267978 1999-04-07
WO 98I16107 PCT/EP97I05639
-5-
limited area of the fur. They generally have good short-term activity against
adult fleas, but
the compositions used often have toxic properties that present problems. Some
organophosphorus compounds are also administered orally, but they are subject
to strict
safety restrictions and must on no account be administered simultaneously with
other
organophosphorus compounds.
Overall it may be said that most of the conventional methods seek only to kill
the adult pest
and, in some cases, they control that pest completely satisfactorily in the
short term.
However) what has hitherto not adequately been taken into account in the case
of most of
the known pest control methods is the fact that, owing to the particular life
cycle of those
ectoparasites, dogs and cats are repeatedly reinfested, partly because contact
with the
eggs, larvae and young adult fleas and ticks on the floor or in the immediate
vicinity of the
animal is unavoidable, and partly because many pet animals constantly come
into contact
with infested members of their own species. Constantly recurring reinfestation
is not
prevented by most conventional compositions.
In addition to numerous insecticidal compositions for controlling
ectoparasites, systemically
active agents have, therefore, also been proposed for some time, for example
pyrethroids
(US 3 962 458 and US 4 031 239), octahydrophenanthridines (US 4 006 236), a-
cyano-m-
phenoxybenzyl-a-alkylnaphthalene-2-acetates (US 4 053 631 ), alkanolamines
(US 4 323 582) and triazine and benzoylurea derivatives (US 4 973 589). Some
juvenile-
hormone-like classes of nitrogen heterocycles have also been described as
having
systemic activity (US 5 439 924).
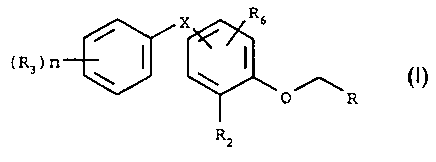
Surprisingly, it has now been found that the compounds of formula
Rs
(R3)n
(I),
O R
Rz
wherein
n is 0, 1, 2 or 3, and when n is greater than 1 the radicals R3 are identical
or different;
R is C(CH3)=CHCOOR,, wherein R~ is C1-Csalkyl; C(CH3)=CHC(=O)Re, wherein Re is
C,-Csalkyl, NH-C,-Csalkyl or N(C,-Csalkyl)2; CH2C(CH3)(Rg)-OR,o, wherein R9
and R,o
are each independently of the other H or C,-Csalkyl; phenyl that is
substituted from one
to five times, the substituents being selected from the group consisting of
hydrogen,
CA 02267978 1999-04-07
WO 98116I07 PCT/EP97/05639
-6-
C,-Csalkyl, C,-Csalkoxy and halogen; pyridyl that is unsubstituted or
substituted from
one to four times, the substituents being selected from the group consisting
of
hydrogen, C,-Csalkyl and halogen; CH(R")OC(=O)NHR,2) wherein R" is hydrogen or
methyl and R,2 is C,-Csalkyl; CH20C(=O)N(R,3)R,4, wherein R,3 and R,4 are each
independently of the other hydrogen or C,-Csalkyl; CH2NHCOOR,S, wherein R,5 is
C,-Csatkyl; CH2CH2C=CH; C---CH; CH(R,6)ZR,~, wherein R16 is hydrogen or C,-
Csalkyl,
Z is oxygen or sulfur and R,~ is a five- or six-membered, aromatic or non-
aromatic ring
containing from one to three nitrogen atoms;
0
Rla , wherein R,e is hydrogen or C,-Csalkyl;
0
1,4-dioxane that is unsubstituted or substituted from one to seven times by C,-
Csalkyl;
or
Rs R1
~~ , wherein either R, is C,-Csalkyl, halo-C,-C3alkyl, C2-C4alkenyl,
R4
Y
C2-C4alkynyl) C,-C3alkoxy or C3-Cficycioalkyl and R4 is hydrogen or C,-
C3alkyl; or R,
and R4, together with the carbon atom to which R) and R4 are bonded, form a
ring
having 4, 5 or 6 ring members, the ring structure, which may contain a carbon-
carbon
double bond, being composed either only of carbon atoms or of 1 oxygen atom
and 3,
4 or 5 carbon atoms and the ring being unsubstituted or mono- or di-
substituted by
identical or different C,-C3alkyl radicals; R5 is hydrogen or C,-C3alkyl; and
Y is nitrogen,
oxygen or sulfur;
R2 is hydrogen, C,-C3alkyl, halo-C,-C3alkyl, C2-C3alkenyl, C,-C3alkoxy, halo-
C,-C3alkoxy,
fluorine, chlorine or bromine;
R3 is C,-C3alkyl, halo-C,-C3alkyl, C,-C3alkoxy, halo-C,-C3alkoxy, fluorine,
chlorine or
bromine;
R6 is hydrogen, halogen or C,-C3alkyi; and
X is methylene, O, S or C(=O),
have systemic activity in the control of ectoparasites in warm-blooded
animals. When
administered in very small doses to test animals) the compounds exhibit high
ovicidal
activity against ectoparasites. The term "ectoparasite", as used here, has its
normal
CA 02267978 1999-04-07
WO 98I16107 PCTIEP97105639
-7_
meaning according to the prior art and includes fleas, ticks, lice, mosquitos,
tabanids,
tsetse-flies and other biting flies and, especially, the above-mentioned
species.
Unless defined otherwise) the general terms used hereinbefore and hereinafter
have the
meanings given below.
The halogen atoms that come into consideration as substituents of haloalkyl
and halo-
alkoxy are fluorine and chlorine as well as bromine and iodine, with fluorine,
chlorine and
bromine being preferred.
Unless defined otherwise, carbon-containing groups and compounds preferably
each
contain from 1 up to and including 4, especially 1 or 2, carbon atoms.
C3-C6Cycloalkyl is cyclopropyl, cyclobutyl, cyclopentyl or cyclohexyl.
Alkyl - as a group per se and as a structural element of other groups and
compounds, such
as alkoxy, haloalkyl and haloalkoxy - is, in each case giving due
consideration to the
number of carbon atoms contained in the group or compound in question, either
straight-
chained or branched and is methyl) ethyl, propyl, isopropyl, butyl, isobutyl,
sec-butyl or tert-
butyl, or pentyl or hexyl or in each case an isomer thereof. Preferred alkyl
groups R, are
C,-C3alkyl groups, especially C2-C3alkyl groups.
Alkenyi and afkynyl contain one or more, preferably not more than two,
unsaturated
carbon-carbon bonds. Examples that may be mentioned are vinyl, allyl,
methallyl, prop-1-
en-1-yl, 2-methyl-prop-1-en-1-yt, b ut-2-en-1-yl, ethynyt, propargyl, prop-1-
yn-1-yl an d but-1-
yn-1-yl.
Halo-substituted groups, i.e. haloalkyl and haloalkoxy) may be partially
halogenated or
perhatogenated. Examples of haloalkyl - as a group per se and as a structural
element of
other groups and compounds, such as haloalkoxy - are methyl substituted from
one to
three times by fluorine, chlorine and/or by bromine, such as CHF2 or CF3;
ethyl substituted
from one to five times by fluorine, chlorine and/or by bromine, such as
CH2CH2F, CHZCF3,
CF2CF3, CF2CCl3, CF2CHCI2, CF2CHF2, CF2CFCt2, CF2CNBr2, CF2CHCIF, CF2CHBrF or
CC1FCHC1F; and propyl or isopropyl each substituted from one to seven times by
fluorine,
chlorine and/or by bromine, such as CH2CHBrCH2Br, CF2CHFCF3) CH2CF2CF3 or
CH(CF3)2.
Within the scope of the invention preference is given to
CA 02267978 1999-04-07
WO 98I16107 PCT/EP97105639
-g_
(1 ) compounds of formula
x
i
(la)
\ \
O R
wherein R is C(CH3)=CHCOOR~, X is methylene or oxygen and R, is C,-Cfialkyl;
{2) compounds of formula la wherein R is C(CH3)=CHC(=O)R8, X is methylene or
oxygen
and R8 is C,-Csalkyl, NH-C,-Csalkyl or N(C,-Csalkyl}2;
(3) compounds of formula fa wherein R is CH2C(CH3)(R9)-OR,o, X is methylene or
oxygen
and R9 and R,o are each independently of the other H or C,-Csalkyl;
(4) compounds of formula la wherein R is phenyl that is substituted from one
to five times,
the substituents being selected from the group consisting of hydrogen, C,-
Cgalkyl,
C,-Csalkoxy and halogen, and X is methylene or oxygen;
(5) compounds of formula la wherein R is pyridyl that is unsubstituted or
substituted from
one to four times, the substituents being selected from the group consisting
of
hydrogen, C,-Csalkyl and halogen, and X is methylene or oxygen;
(6) compounds of formula la wherein R is CH(R")OC(=O)NHR,2, R" is hydrogen or
methyl, R,2 is C,-Csalkyl and X is methylene or oxygen;
(7) compounds of formula
X / Rz
tR3)n ~ ~ {Ib)
\ \
O R
wherein R is CH20C(=O)N(R,3)R,4) R2 is hydrogen, C,-C3alkyl, halo-C,-C3alkyl)
C,-C3-
alkoxy) halo-C,-C3alkoxy, fluorine, chlorine or bromine, R3 is C,-C3alkyl)
halo-C,-C3-
alkyl, C,-C3alkoxy) halo-C,-C3alkoxy, fluorine, chlorine or bromine, R,3 and
R,4 are
each independently of the other hydrogen or C,-Cgalkyl, n is 0, 1 or 2, and
when n is 2
the two radicals R3 are identical or different, and X is methylene or oxygen;
(8) compounds of formula Ib wherein R is CH2NHCOOR,5) R2 is hydrogen, C,-
C3alkyl,
halo-C,-C3alkyl) C,-C3alkoxy, halo-C,-C3alkoxy, fluorine, chlorine or bromine,
R3 is
C,-C3alkyl, halo-C,-Csaikyl, C,-C3alkoxy, halo-C,-C3alkoxy, fluorine, chlorine
or
_... t
CA 02267978 1999-04-07
WO 98I16107 PCT/EP97/05639
-9-
bromine) R,5 is C,-Csalkyl, n is 0, 1 or 2, and when n is 2 the two radicals
R3 are
identical or different, and X is methylene or oxygen;
(9) compounds of formula Ib wherein R is CH2CH2C=CH or C--_CH, R2 is hydrogen,
C,-C3-
alkyl, halo-C,-C3alkyl, C~-C3alkoxy, halo-C~-C3alkoxy, fluorine, chlorine or
bromine, R3
is C,-C3alkyl, halo-C,-C3alkyl, C,-C3alkoxy, halo-C,-C3alkoxy, fluorine,
chlorine or
bromine, n is 0, 1 or 2) and when n is 2 the two radicals R3 are identical or
different,
and X is methylene or oxygen;
(10) compounds of formula ib wherein R is CH(R,6)ZR,~, R2 and R,6 are
hydrogen, R3 is
C,-C3alkyl, halo-C,-C3alkyl, C,-C3alkoxy, halo-C,-C3alkoxy, fluorine) chlorine
or
bromine, n is 0, 7 or 2, and when n is 2 the two radicals R3 are identical or
different,
R,~ is a five- or six-membered, aromatic or non-aromatic ring containing from
one to
three nitrogen atoms) X is methylene or oxygen and Z is oxygen or sulfur;
0
(11 ) compounds of formula Ib wherein R is ~ R18 , R2 and R3 are
i
0
hydrogen, R,e is hydrogen or C,- CZ-C3alkenyl, Csalkyl and X is methylene or
oxygen;
(12) compounds of formula Ib wherein R is 1,4-dioxane that is unsubstituted or
substituted
from one to seven times by C,-Csalkyl, R2 is hydrogen, R3 is C,-C3alkyl, halo-
C~-C3-
alkyl, C,-C3alkoxy, halo-C,-C3alkoxy, fluorine, chlorine or bromine, n is 0, 1
or 2, and
when n is 2 the two radicals R3 are identical or different, and X is
rnethylene or
oxygen;
Rs R1
(13) compounds of formula Ib wherein R is < c~ , wherein either Ri is C1-C6-
\ Rq
Y
alkyl, halo-C,-C3alkyl, C2-C4alkenyl, C2-C4alkynyl, C,-C3alkoxy or C3-
Cscycloalkyl and
R4 is hydrogen or CI-C3alkyl; or R, and R4, together with the carbon atom to
which R,
and R4 are bonded) form a ring having 4, 5 or 6 ring members, the ring
structure,
which may contain a carbon-carbon double bond, being composed either only of
carbon atoms or of 1 oxygen atom and 3, 4 or 5 carbon atoms and the ring being
unsubstituted or mono- or di-substituted by identical or different C1-C3alkyl
radicals, R2
is hydrogen, C,-C3alkyl) halo-Cs-C3alkyl) C,-C3alkoxy) halo-C,-C3alkoxy,
fluorine,
CA 02267978 1999-04-07
WO 98I16107 PCTlEP97105639
-10-
chlorine or bromine) R3 is C,-C3aikyl, halo-C,-C3alkyl, C,-C3alkoxy, halo-C,-
C3alkoxy,
fluorine, chlorine or bromine) n is 0, 1 or 2, and when n is 2 the two
radicals R3 are
identical or different, X is methylene or oxygen, R5 is hydrogen or C,-C3alkyl
and Y is
nitrogen, oxygen or sulfur;
x , R2
(R~)n
(14) compounds of formula ~ ~ o o' .Rl (Ic) wherein
O/~\ Rq
R, is C,-Caalkyl, vinyl or cyclopropyl, preferably C,-C3alkyl;
(15) compounds of formula Ic wherein RZ is hydrogen) methyl, fluorine or
chlorine, prefer-
ably hydrogen or chlorine;
(t 6) compounds of formula is wherein R3 is fluorine or chlorine, preferably
fluorine;
(17) compounds of formula lc wherein R4 is hydrogen or methyl, preferably
hydrogen;
(18) compounds of formula Ic wherein X is oxygen or methylene, preferably
oxygen;
(19) compounds of formula Ic wherein n is 0, 1 or 2, and when n is 2 the two
radicals R3
are identical or different; R, is C,-C4alkyl, vinyl or cyclopropyl; RZ is
hydrogen, fluorine
or chlorine; R3 is fluorine or chlorine; R4 is hydrogen or methyl; and X is
oxygen or
methylene;
(20) compounds of formula Ic wherein n is 1 or 2) and when n is 2 the two
radicals R3 are
identical or different; R) is C,-C4alkyl; RZ is hydrogen or chlorine; R3 is
fluorine or
chlorine; Ra is hydrogen or methyl; and X is oxygen or methylene;
(21 ) compounds of formula Ic wherein n is 1 or 2, and when n is 2 the two
radicals R3 are
identical or different; R, is C,-C3alkyl; R2 is hydrogen; R3 is fluorine or
chlorine; R4 is
hydrogen; and X is oxygen.
Specific preference is given to the following compounds of formula I:
2-cyclopropyl-4-[4-(phenoxy)-phenoxymethyl]-1,3-dioxolane;
4-(2-chloro-4-phenoxy-phenoxymethyl)-2-ethyl-1,3-dioxolane;
4-(2-chloro-4-phenoxy-phenoxymethyl)-2-propyl-1,3-dioxolane;
4-(2-chloro-4-phenoxy-phenoxymethyl)-2-isopropyl-1,3-dioxolane;
4-(2-chioro-4-phenoxy-phenoxymethyl)-2-cyclopropyl-1,3-dioxolane;
r . . . ..... ......
CA 02267978 1999-04-07
WO 98/16107 PCT/EP97/05639
-11
4-[2-chioro-4-(3-fiuorophenoxy)-phenoxymethyl]-2-ethyl-1,3-dioxolane;
4-[2-chloro-4-(3-fluorophenoxy)-phenoxymethyl]-2-propyl-1,3-dioxolane;
4-[2-chloro-4-(3-fluorophenoxy)-phenoxymethyl]-2-isopropyl-1,3-dioxoiane;
4-[2-chloro-4-(3-fluorophenoxy)-phenoxymethyl]-2-cyclopropyl-1 (3-dioxolane;
4-[2-chioro-4-(3-chlorophenoxy}-phenoxymethyl]-2-ethyl-1,3-dioxolane;
4-[2-chloro-4-(3-chlorophenoxy)-phenoxymethyl]-2-propyl-1,3-dioxolane;
4-[2-chloro-4-(3-chlorophenoxy)-phenoxymethyl]-2-isopropyl-1,3-dioxoiane;
4-[2-chloro-4-(3,5-difluorophenoxy)-phenoxymethyl]-2-ethyl-1 (3-dioxolane;
4-[2-chioro-4-(3,5-difluorophenoxy)-phenoxymethyl]-2-propyl-1,3-dioxolane;
4-[2-chloro-4-(3,5-difluorophenoxy)-phenoxymethyl]-2-isopropyl-1,3-dioxolane;
4-[2-chioro-4-(3,4-dichlorophenoxy)-phenoxymethyl]-2-ethyl-1,3-dioxolane;
4-[2-chloro-4-(3-chloro-4-fiuorophenoxy)-phenoxymethyl]-2-ethyl-1,3-dioxolane;
4-(2-chloro-4-benzyl-phenoxymethyl)-2-ethyl-1,3-dioxolane;
4-(2-chloro-4-benzyl-phenoxymethyl)-2-propyl-1 (3-dioxolane;
4-(2-chloro-4-benzyl-phenoxymethyl)-2-isopropyl-1,3-dioxolane;
4-(2-chloro-4-benzyl-phenoxymethyl)-2-cyclopropyl-1 (3-dioxolane;
4-[2-chloro-4-(3-fluorobenzyi)-phenoxymethyl]-2-ethyl-1,3-dioxolane;
4-[2-chioro-4-(3-fluorobenzyl}-phenoxymethyl]-2-propyl-1,3-dioxoiane;
4-[2-chloro-4-(3,5-difluorobenzyl)-phenoxymethyl]-2-ethyl-1,3-dioxolane;
4-[2-chloro-4-(3,4-dichiorobenzyl)-phenoxymethyl]-2-ethyl-1,3-dioxoiane;
2-ethyl-4-[4-(3-fluorophenoxy)-phenoxymethyl]-4-methyl-1,3-dioxolane;
2-ethyl-4-[4-(3-fluorophenoxy)-phenoxymethyl]-1,3-dioxolane and
4-[4-(3,5-difluorophenoxy)-phenoxymethyl]-2-isopropyl-1,3-dioxoiane; but
especially to:
2-dimethyl-4-[4-(3-fluorophenoxy)-phenoxymethyl]-i,3-dioxolane and
2-dimethyl-4-[4-(3,5-difluorophenoxy)-phenoxymethyl]-1,3-dioxolane.
The compounds of formula I are known per se or can be prepared) for example,
according
to European Patent No. 559 612.
The compounds of formula I are known to be active against ectoparasites when
applied
topically (EP 559 612) in that they inhibit or completely suppress the further
development
of juvenile stages of development; however, that action has always been
determined on
direct application of the active ingredient to the parasites. What is
astonishing in connec-
tion with the present invention, however, is that full activity is still
achieved even when the
active ingredient is administered to the host animal in relatively low
concentrations and
CA 02267978 1999-04-07
WO 98/16107 PCT/EP97105639
-12-
reaches the adult parasite only by the circuitous route via the blood sucked
up by the
parasite.
The parasites to be controlled can be reached by the substances in two ways.
On the one
hand, chemicals can be taken up by the adult parasite via the ingested blood
and be
passed on from the adult parasite to the eggs, where the activity of the
chemicals then
manifests itself. Death may then occur in the egg, larval or pupal stage.
Tests on parasites
show reduced oviposition, high mortality of the larvae and, in the case of the
pupal stage,
an inhibition of hatching ability. On the other hand, the larvae are exposed
to the effect of
the excreta of the adult parasites, since they live on that excrement. The
parasites' excreta
still comprise large amounts of undigested blood from the host animal and
serve as a
source of protein for the developing parasites.
The present invention thus has two objectives, on the one hand the afore-
described
method for the prevention of the reinfestation of dogs and cats by
ectoparasites) and
simultaneously of course the inhibition of the reproduction of ectoparasites
or, more
precisely: a method for the prevention of the reproduction of ectoparasites,
which
comprises administering to the ectoparasites as food blood that comprises an
effective
amount of at least one of the active ingredients defined above. This method
also includes
the objective of an effective amount of one of the said active ingredients
being admini-
stered to the host animal in the food and the ectoparasites ingesting the
active ingredient
by taking in the animal's blood on sucking blood.
It is material to the invention that the active ingredient selected from the
mentioned class of
compounds of formula I be administered in such a manner that it is taken up by
the adult,
sucking parasite with the blood of the host animal and can take effect in the
juvenile
stages. This is achieved according to the invention using various forms of
administration,
for example by administering a formulated active ingredient orally.
"Formulated" in this
case means, for example, in the form of a powder, a tablet, granules, a
capsule, an
emulsion, a foam, etc.; the animal need not necessarily be given the
composition directly,
rather it is advantageously mixed with its food. In addition to customary
formulation
ingredients, any composition that is to be administered orally may of course
comprise
further adjuvants that encourage the host animal to take the composition
voluntarily, for
example suitable odorants and flavourings. Oral administration, being easy to
carry out, is
one of the preferred subjects of this invention. A further form of
administration is parenteral
CA 02267978 1999-04-07
WO 98I16107 PCT/EP97/05639
-13-
administration, for example by subcutaneous injection or intravenous
injection, or with a
long-term composition (depot form) in the form of an implant.
Oral administration includes, for example, the administration of animal food
ready mixed
with the active ingredient, for example in the form of biscuits or treats,
chewable tablets,
water-soluble capsules or tablets, in a water-soluble form that can be added
to the food in
the form of drops or in other forms that can be mixed with the animal food.
The implants
also include any means that can be introduced into the body of the animal in
order to
release active ingredient.
Percutaneous forms of administration include) fvr example, subcutaneous,
dermal, intra-
muscular and even intravenous administration of injectable forms. In addition
to the
customary syringes with needles, needle-less high-pressure syringe devices, as
well as
pour-on and spot-on formulations, may be expedient.
By selection of a suitable formulation, it is possible to enhance the ability
of the active
ingredient to penetrate through the living tissue of the animal, and/or to
maintain its
availability. That is important when) for example, a very sparingly soluble
active ingredient
is used) the low solubility of which requires means for enhancing solubility)
since the
animal's body fluid is capable of dissolving only small amounts of active
ingredient at a
time.
The active ingredient may also be present in a matrix formulation which
physically prevents
the active ingredient from decomposing and maintains the constant availability
of active
ingredient. The matrix formulation is injected into the body and remains there
as a form of
depot from which active ingredient is released continuously. Such matrix
formulations are
known to a person skilled in the art. They are generally wax-like, semi-solid
substances, for
example vegetable waxes and polyethylene glycols having a high molecular
weight.
A high degree of active ingredient availability is also obtained by the
introduction of an
implant of the active ingredient into the animal. Such implants are widely
used in veterinary
medicine and often consist of silicone-containing rubber. The active
ingredient is dispersed
in the solid rubber or is located inside a hollow rubber body. Care must be
taken that the
active ingredient selected is soluble in the rubber implant, since it is first
dissolved in the
rubber and then seeps continuously out of the rubber material and into the
body fluid of the
animal to be treated.
CA 02267978 1999-04-07
WO 98I16107 PCT/EP97/05639
-14-
The rate of release of the active ingredient from the implant) and thus the
length of time
during which the implant exhibits activity, is generally determined by the
accuracy of the
calibration of the implant (amount of active ingredient in the implant}, the
environment of
the implant and the polymer formulation from which the implant has been
produced.
Administration of the active ingredient by means of an implant is a further
preferred
component of the present invention. Such administration is extremely
economical and
effective, because a correctly dimensioned implant ensures that the
concentration of active
ingredient in the tissue of the host animal is constant. It is possible
nowadays for implants
to be so made and implanted in a simple manner that they are capable of
delivering the
active ingredient over a period of several months. Once the implant has been
made, the
animal is not disturbed further, and there is no further need to be concerned
about the
dosage.
The administration of veterinary medicinal additives to animal food is well
known in the field
of animal health, It is usual first to prepare a so-called premix in which the
active ingredient
is dispersed in a liquid or is in finely divided form in solid carriers. That
premix can normally
comprise about 1 to 800 g of compound per kg of premix, depending on the
desired final
concentration in the food.
It is also known that active ingredients may be hydrolysed or weakened by the
constituents
of the food. Such active ingredients are routinely formulated in a protective
matrix, for
example in gelatin) before being added to the premix.
The present invention therefore relates also to a method for the systemic
prevention of the
reinfestation of host animals by ectoparasites, which comprises administering
to the said
host animal orally, parenterally or by means of an implant a lanricidally or
ovicidally effec-
tive amount of a compound that inhibits the growth of the ectoparasites)
preferably a
method in which a compound of formula I is used as a compound that inhibits
the growth
of the ectoparasites.
Accordingly, the present invention relates also to a method for the prevention
of the repro-
duction of ectoparasites, which comprises making available to the
ectoparasites as food
blood that comprises an effective amount of a parasite-growth-inhibiting
compound
selected from the compounds of formula 1. In other words, the present
invention relates
also to a method for the prevention of the reproduction of ectoparasites
living on host
CA 02267978 1999-04-07
WO 98I16107 PCTIEP97/05639
-15-
animals, which comprises administering an effective amount of one of the
parasite-growth-
inhibiting compounds of formula I to the host animal in the form of a fee
d additive and in that way enabling that compound to reach the parasites
living on the host
animal.
The compound of formula I is advantageously administered in a dose of from
0.01 to
800 mg/kg, preferably from 0.5 to 200 mg/kg, especially from 1 to 30 mg/kg of
body
weight, based on the host animal, with oral administration being preferred. A
good dose
that can be administered to the host animal regularly is from 1 to 100 mg/kg
of body
weight. Administration is advantageously effected daily, weekly, monthly or
half-yearly. For
the same active ingredient, the total dose may vary from one species of animal
to another
as well as within a species of animal, since it depends .infer alia on the
weight and constitu-
tion of the animal.
When used according to the invention, the active ingredient will not normally
be admini-
stered in pure form, but preferably in the form of a composition that
comprises, in addition
to the active ingredient, constituents that assist administration) suitable
constituents being
those that are tolerated by the host animal. It is of course possible, as well
as controlling
the juvenile stages of development in accordance with the invention,
additionally to use
conventional methods to control the adult ectoparasites, although the latter
is not absolu-
tely essential.
Such compositions to be administered in accordance with the invention
generally comprise
from 0.1 to 99 % by weight) especially from 0.1 to 95 % by weight, of a
compound of
formula I and from 99.9 to 1 % by weight, especially from 99.9 to 5 % by
weight, of a solid
or liquid, non-toxic adjuvant) including from 0 to 25 % by weight, especially
from 0.1 to
25 % by weight, of a non-toxic surfactant.
Whereas commercial products will preferably be formulated as concentrates, the
end user
will normally employ dilute formulations. Such formulations may also comprise
further
auxiliaries, such as stabilisers, antifoams, viscosity regulators, binders and
tackifiers as
well as other active ingredients for obtaining special. effects. The materials
known from
veterinary medicinal practice for oral and parenteral administration and for
implants can be
used as formulation excipients. Some examples are given below.
Suitable carriers are especially fillers, such as sugars, for example lactose,
saccharose,
mannitol or sorbitol, cellulose preparations and/or calcium phosphates, for
example tri-
CA 02267978 1999-04-07
WO 98I16107 PCT/EP97105639
-16-
calcium phosphate or calcium hydrogen phosphate, and binders, such as starch
pastes
using, for example, corn, wheat, rice or potato starch, gelatin, tragacanth,
methylcellulose
andlor, if desired, disintegrators, such as the above-mentioned starches, also
carboxy-
methyl starch, cross-linked polyvinylpyrrolidone, agar, alginic acid or a salt
thereof, such as
sodium alginate. Adjuvants are especially flow conditioners and lubricants,
for example
silicic acid, talc, stearic acid or salts thereof, such as magnesium or
calcium stearate,
and/or polyethylene glycol. Drag~e cores can be provided with suitable,
optionally enteric,
coatings, there being used inter alia concentrated sugar solutions which may
comprise
gum arabic, talc, polyvinylpyrrolidone, polyethylene glycol and/or titanium
dioxide, or coat-
ing solutions in suitable organic solvents or solvent mixtures, or, for the
preparation of
enteric coatings, solutions of suitable cellulose preparations) such as
acetyicellulose
phthalate or hydroxypropylmethylcellulose phthalate. Dyes, flavourings or
pigments may be
added to the tablets or dragee coatings, for example for identification
purposes or to
indicate different doses of active ingredient.
Other orally administrable compositions are hard gelatin capsules, and also
soft sealed
capsules made of gelatin and a plasticiser, such as glycerol or sorbitol. The
hard gelatin
capsules may comprise the active ingredient in the form of granules, for
example in
admixture with fillers, such as lactose, binders, such as starches, and/or
glidants, such as
talc or magnesium stearate, and, if desired) stabilisers. In soft capsules,
the active ingre-
dient is preferably dissolved or suspended in suitable liquids) such as fatty
oils) paraffin oil
or liquid polyethylene glycols) to which stabilisers may also have been added.
Preference
is given inter alia to capsules that may easily be bitten through or swallowed
without being
chewed.
Suitable for parenteral administration are especially aqueous solutions of an
active ingre-
dient in water-soluble form) for example in the form of a water-soluble salt,
and also
suspensions of the active ingredient, such as corresponding oily injection
suspensions)
there being used suitable iipophilic solvents or vehicles, such as fatty oils,
for example
sesame oil, or synthetic fatty acid esters, for example ethyl oleate, or
triglycerides, or
aqueous injection suspensions that comprise viscosity-increasing substances,
for example
sodium carboxymethylcellulose, sorbitol andlor dextran, and, optionally)
stabilisers.
The compositions of the present invention can be prepared in a manner known
per se, for
example by means of conventional mixing) granulating, confectioning,
dissolving or lyophi-
lising processes. For example, compositions for oral administration can be
obtained by
CA 02267978 1999-04-07
WO 98I16107 PCT/EP97105639
-17-
combining the active ingredient with solid carriers, optionally granulating a
resulting
mixture, and processing the mixture or granules, if desired or necessary after
the addition
of suitable excipients, to form tablets or dragee cores.
The following Examples illustrate the invention described above, but do not
limit its scope
in any way. Temperatures are given in degrees Celsius.
Example 1: Tablets comprising 25 mg of active ingredient can be prepared as
follows:
Constituents (for 1000 tablets)
active ingredient 25.0 g
lactose 100.7 g
wheat starch 7.5 g
polyethylene glycol 6000 5.0 g
talcum 5.0 g
magnesium stearate 1.8 g
demineralised water q.s.
Preparation: All the solid ingredients are first forced through a sieve having
a mesh size of
0.6 mm. Then the active ingredient, the lactose, the talcum, the magnesium
stearate and
half the starch are mixed together. The other half of the starch is suspended
in 40 ml of
water and the suspension is added to a boiling solution of the polyethylene
glycol in 100 ml
of water. The resulting starch paste is added to the main batch and the
mixture is granula-
ted, if necessary with the addition of water. The granules are dried overnight
at 35~, forced
through a sieve having a mesh size of 1.2 mm and compressed to form tablets
which have
a diameter of about 6 mm and which are concave on both sides.
Example 2: Tablets comprising 0.02 g of active ingredient are prepared as
follows:
Composition (for 10 000 tablets)
active ingredient 200.00 g
lactose 290.80 g
potato starch 274.70 g
stearic acid 10.00 g
talc 200.00 g
magnesium stearate 2.50 g
colloidal silica 32.00 g
ethanol q.s.
CA 02267978 1999-04-07
WO 98/Z6107 PCT/EP97I05639
_i8_
A mixture of the active ingredient, the lactose and 194.70 g of potato starch
is moistened
with an ethanolic solution of the stearic acid and granulated through a sieve.
After drying,
the remaining potato starch, the talc, the magnesium stearate and the
colloidal silica are
mixed in and the mixture is compressed to form tablets each weighing 0.1 g,
which may, if
desired, be provided with dividing notches for finer adaptation of the dose.
Example 3: Capsules comprising 0.025 g of active ingredient can be prepared as
follows:
Composition (for 1000 capsules)
active ingredient 50.00 g
lactose 249.80 g
gelatin 2.00 g
corn starch 10.00 g
talc 15.00 g
water q.s.
The active ingredient is mixed with the lactose, and the mixture is moistened
uniformly with
an aqueous solution of the gelatin and granulated through a sieve having a
mesh size of
1.2-1.5 mm. The granules are mixed with the dried corn starch and the talc and
introduced
in 300 mg portions into hard gelatin capsules (size 1).
Exam~ole 4: Premix I,feed additive)
0.25 part by weight of active ingredient and
4.75 parts by weight of secondary calcium phosphate, alumina) Aerosil)
carbonate or
chalk are mixed until homogeneous with
95 parts by weight of an animal food.
Example 5: Emulsifiable concentrate
20 parts by weight of active ingredient are mixed with
20 parts by weight of the emulsifier, e.g. a mixture of alkylarylpolyglycol
ether with
alkylarylpoiysulfonates, and with
60 parts by weight of a solvent
until the solution has been completely homogenised. Emulsions of the desired
concentra-
tion are obtained by dilution with water.
Example 6: Solutions le.q. for use as a drink additive)
15 percent by weight active ingredient in 2,2-dimethyl-4-hydroxymethyl-1,3-
dioxolane)
percent by weight active ingredient in diethylene glycol monoethyl ether,
. _...,._ . _ , ._
CA 02267978 1999-04-07
WO 98l16107 PCTIEP97l05639
-19-
percent by weight in polyethylene glycol 300, and
5 percent by weight in glycerol.
Example 7: Soluble powder
25 parts by weight of active ingredient,
1 part by weight of sodium lauryl sulfate,
3 parts by weight of colloidal silica gel, and
71 parts by weight of urea.
The constituents are mixed together and ground with one another until
homogeneous.
Further biologically active compounds or ingredients that are neutral towards
the active
ingredients and that have no adverse effect on the host animal to be treated,
as well as
mineral salts or vitamins, can be added to the compositions described.
Other compositions comprising compounds of formula t can also be prepared
analogously
to the described formulations of Examples i to 7.
Example 8: Flea control bvt oral administration of the active 'sngredient to
the host animal
cats with the proven ability permanently to maintain a flea population are
divided into
6 groups: 5 groups each being composed of 3 or 4 animals and a sixth group,
which
serves as the control group, being composed of 2 animals. Each of the cats is
infected with
100 cat fleas [Ctenocephaiides fells]. The cats of the 5 groups are treated
orally on day 0
with 50 mg of active ingredient per kg of body weight. The active ingredient
is administered
in the form of a capsule. The control group remains untreated.
After 3, 8 and 10 days, the flea eggs are collected from the paper beneath the
cats' boxes.
The eggs are counted and placed on a medium suitable for the growth of larvae
and
incubated. The number of pupae and fully grown fleas that develop is
determined.
Compounds of formula I exhibit good activity in this test. In particular, 4-[4-
(3-fluoro-
phenoxy)-phenoxymethyl]-2-ethyl-i ,3-dioxolane) 4-[4-(3,5-difluorophenoxy)-
phenoxy-
methyl]-2-isopropyl-1,3-dioxoiane, 2-dimethyl-4-[4-(3-fluorophenoxy)-
phenoxymethyl)-1,3-
dioxolane and 2-dimethyf-4-[4-(3,5-difluorophenoxy}-phenoxymethyl]-1,3-
dioxolane still
exhibit 100 % inhibition of the development of fleas after 45 days.
CA 02267978 1999-04-07
WO 98/16I07 PCT/EP9~/05639
-20-
Examale 9: Tick control by subcutaneous administration of the active
ingredient to the host
animal
13-day-old chicks of a fattened breed are infected with about 200 larvae of
the tick species
Amblyomma hebraeum and placed in pairs in cages. After three days) the chicks
are
injected subcutaneously with the active ingredient in an aqueous suspension.
Within 2 to 6
days of the treatment, i.e. 5 to 9 days after infection, the replete tick
larvae fall from the
chicks and can be collected and counted. The activity of the test compounds is
calculated
on the basis of the number of replete larvae on treated and untreated chicks
in accordance
with the formula %control = ~1- CuJ * 100 , where Ct is the average number of
larvae
from treated chicks and Cu is the average number of larvae from untreated
chicks.
Active ingredients that have a growth-inhibiting action on tick larvae do not
exhibit their
activity until the larvae have moulted to form nymphs. In order to determine
this type of
activity, the replete larvae are kept, separated into treatment groups, in an
incubator until
all the larvae collected from untreated chicks have moulted. The activity is
calculated using
the above-mentioned formula) with the average number of nymphs being used
instead of
the average number of larvae.
Table 1 shows the activity of a subcutaneousiy injected dose of 3 mg of the
active ingre-
dient per kg of body weight on the larvae and nymph formation of Amblyomma
hebraeum
in chicks. Reductions of more than 80 % can be regarded as systemic action.
Compound's % reduction larvae% reduction nymphs
1 83 83
2 82 83
3 78 80
'} compound 1: 2-ethyl-4-[4-(3-fluorophenoxy)-phenoxymethyl]-4-methyl-1,3-
dioxolane
compound 2: 2-cyclopropyl-4-[4-(phenoxy)-phenoxymethyl]-1,3-dioxolane
compound 3: 2-ethyl-4-[4-(3-fiuorophenoxy)-phenoxymethyl]-1,3-dioxolane