Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02268478 1999-04-12
WO 99/07326 PCT/CA98/00754
Title:
"ANTIN>ZCROBIAL CEMENT COMPOSITIONS"
Related Applications
This application claims the benefit of U.S. Provisional Application No.
60/055361) filed August 1 l, l997.
Field of the Invention
This invention relates to cement compositions having antimicrobial properties)
and particularly to cement compositions containing antimicrobial zeolites.
Background of the Invention
Numerous types of cements have been developed for use in medicine and
dentistry. Generally, medical and dental cements comprise a powder component
comprising finely divided metal oxides, metal hydroxides, calcium
hydroxyapatite
bioglasses) silicate-cement glazes, or glass ionomer particles, which is
induced to react
with a liquid medium containing phosphoric acid, polyelectrolytes such as
polycarboxylic acid, or salicylic acid derivatives. These cements set through
ionic
interactions between the various components.
These cements are typically used in medicine as bone cements, implant
components and bone substitutes, and in dentistry as adhesives, sealants and
restorative
(filling) materials.
One cement type used in dentistry and medicine is glass ionomer cement (GIC).
glass ionomer cements are preferred for use as dental adhesives and
restoratives) for
example to restore cavities in dental tissue, and to seal the interfacial area
between a
filling and surrounding dental tissue, for example as sealers in root
fillings. However,
due to their limited physical strength, glass inomer cements are less
frequently used as
restorative materials for surfaces that undergo considerable physical stress.
SUBSTITUTE SHEET (RULE 26)
CA 02268478 1999-04-12
WO 99/07326 PCT/CA98/00754
Glass ionomer cements typically comprise at least two components; firstly, a
glass ionomer cement powder comprising an acid-soluble calcium
fluoroafuminosilicate
glass powder; and secondly, a polyanion) also referred to as a
"polyelectrolyte", in a
water base. When the solid and liquid components are combined to form a paste,
the
surfaces of the glass particles are attacked by the polyanion. Calcium)
aluminum,
sodium and fluorine ions are leached into the aqueous medium, and the
polyanion chains
are cross-linked by the calcium ions to form a solid mass. The aluminum ions
also
become sonically bound within the cement mix. The unreacted portions of the
glass
particles become sheathed by silica gel, so that the set cement consists of an
agglomeration of unreacted powder particles surrounded by silica gel in an
amorphous
matrix of hydrated calcium and aluminum polysalts. The poiyanion also reacts
with
calcium ions in the substrate, for example a tooth, forming a chemical bond
between the
cement and the substrate. Other monomer components may be added in order to
carry
out a dual cure process, for example where polymer chains are generated by
light-curing
the monomer components.
Therefore, glass ionomer cement cures primarily by means of ionic reactions in
which "ion bridges" are formed between the polyanion, the ions originating
from the
glass ionomer particles) and the substrate to which the cement is being
adhered. As a
result of this ion bridging, glass ionomer cements have satisfactory adhesion
to hard
dental tissue, such as teeth and bones) which are comprised of calcium
hydroxyapatite. It
is desirable to provide dental cements having antimicrobial properties. One
particularly
important potential application of antimicrobial cements is in endodontic
treatment of
root canal systems. The goals of endodontic therapy include disinfection of
the root
canal system and subsequent bacteria-tight sealing of the root canal system.
Disinfection
is accomplished during the root canal treatment by instruments) irrigants and
medicaments. Sealing is accomplished by root filling materials, such as gutta
percha
(rubber) and sealer cements. However, the sealing properties of known root
filling
materials are insufficient to prevent leakage and bacterial ingress into the
filled root
canal. The interfacial area between the endodontic filling materials and the
dentin wall
of the root canal is particularly susceptible to bacterial ingress,
particularly where the
- z-
SUBSTITUTE SHEET (RULE 26)
CA 02268478 1999-04-12
WO 99/07326 PCT/CA98/00754
technical quality of the root filling is poor. Consequently) bacteria that
challenge the
endodontically treated tooth may proliferate through the filled root canal
system and
cause treatment failure.
Glass ionomer cements have shown some promise as antimicrobial endodontic
fillers. These cements release fluoride ions over a period of time and are
believed to
provide an antimicrobial effect. However) fluoride-releasing glass ionomer
cements
have several disadvantages. Firstly) fluoride ions may participate in ionic
bridging
between dental tissue and the glass ionomer cement, and thus form part of the
cement
matrix. When the fluoride is released from the cement into the oral cavity and
is
replaced by other anions, structural changes occur in the cement which may
compromise
its mechanical strength. Secondly, the quick release rate of fluoride from the
cement
provides a high initial dose, which rapidly decreases within a few days or
weeks. The
fluoride ions must be regenerated regularly in order to provide a continuous
effect
against bacteria.
Therefore, the disadvantage exists that no cements have yet been developed for
medicine and dentistry which provide a long term antimicrobial effect.
A further, more specific, disadvantage exists in that no cements have been
developed for use as endodontic filling materials which provide a long term
antimicrobial effect.
Other types of materials having antimicrobial properties have been developed.
For example, U.S. Patent No. 4,938,958) issued on July 3, 1990 to Niira et
al., which is
incorporated herein by reference) discloses zeolites having antimicrobial
properties.
Zeolites are aluminosilicates having a three dimensional skeletal structure
and
represented by the following formula:
XM2/n0-A120,-YS i0z-ZH20
In the general formula, M represents an ion-exchangeable ion and in general a
monovalent or divalent metal ion, n represents atomic valency of the (metal)
ion) X and
Y represent coefficients of metal oxide and silica respectively) and Z
represents the
number of water of crystallization.
_ :,_
SUBSTITUTE SHEET (RULE 26)
CA 02268478 1999-04-12
WO 99/07326 PCT/CA98/00754
In antimicrobial zeolites) such as those taught by Niira et al., ion-
exchangeable
ions present in the zeolite are completely or partially replaced by ammonium
and
antimicrobial metal ions such as silver, copper, zinc, mercury, tin, lead,
bismuth)
cadmium, chromium and thallium,
Numerous materials have been prepared containing antimicrobial zeoiites. For
example, U.S. Patent No. 4,9I I,898, issued March 27, 1990 to Hagiwara et al.;
U.S.
Patent No. 5,556,699) issued September 17, 1996 to Niira et al.; and U. S.
Patent No.
4,937,273, issued June 26) 1990 to Okuyama et al. disclose blending
antimicrobial
zeolites with polymers to form antimicrobial fibres for fabrics) films for
packaging or
laminating) and flexible foams.
Composite materials are known comprising a polymerizable binder reinforced
with inert organic or inorganic filler particles. Such materials are commonly
used as
dental restorative materials such as fillings. It would be desirable to
provide such
materials having antimicrobial properties) and antimicrobial zeolites are
known to be
incorporated into polymeric materials. However, the inventors are not aware of
any
commercially available composite materials incorporating antimicrobial
zeolites for use
as dental restorative materials. Several disadvantages exist with respect to
incorporation
of antimicrobial zeolites in polymeric materials, which may explain their lack
of use.
Firstly, the exchangeable ions contained in the zeolite diffuse poorly through
polymeric
materials. Therefore, a high concentration of antimicrobial zeolite is
required to produce
an anti microbial composite material. Secondly, antimicrobial zeolites have
been known
to cause staining of the polymeric materials in which they are incorporated.
This is
particularly disadvantageous in composites for dental restorative materials,
which must
be colour-stable. The staining problem is aggravated by the need to
incorporate large
amounts of zeolites in the polymeric material. Thirdly, it is believed by some
that
zeolites do not incorporate antimicrobial metal ions in amounts sufficient to
provide an
antimicrobial effect, for example in U.S. Patent No. 5,009,898, issued April
23, 199I to
Sakuma et al., at column 2, lines 29 to 31.
Therefore, the disadvantage exists that antimicrobial zeolites have not
successfully been incorporated in dental restorative materials.
_ .t_
SUBSTITUTE SHEET (RULE 26)
. _, . _ ~_ ....u_.~.~ _ .__ _ ..~. -...._r..._._..
CA 02268478 1999-04-12
WO 99/07326 PCT/CA98/00754
Although the above discussion has focussed on composite polymer systems and
glass ionomer cements used in dentistry) similar problems exist with cements
in general.
Specifically, there is a need for antimicrobial cements in other fields, such
as bone
cements for use in medicine, as well as in building materials in industrial,
institutional
and residential applications. For example, tiles are frequently used as a
floor or wall
covering in wet or humid areas. Grouts which are used to fill joints between
tiles are
frequently subject to deterioration and discolouration by molds and mildew, as
well as
contamination by bacteria. To date, there has been no completely satisfactory
solution to
this problem.
Summary of the Invention
To at least partially overcome the above disadvantages, the inventors have
developed antimicrobial cement compositions containing antimicrobial zeolites.
The
cement compositions of the invention generally comprise a cement containing an
antimicrobial zeolite in an amount sufficient to prevent growth of bacteria in
the
composition and over the hardened cement formed when the composition is cured.
The cement compositions of the invention have a wide variety of possible
applications, including dentistry, medicine, as well as in building materials
for
institutional, industrial and domestic use. Preferably, the antimicrobial
cement
compositions according to the present invention are used in dentistry or
medicine. For
these applications, the antimicrobial dental cement compositions according to
the present
invention preferably comprise an anionic component, an inorganic particulate
component) and an antimicrobial zeolite. The anionic component preferably
comprises a
liquid medium containing an acid such as phosphoric acid, a polyelectrolyte
such as a
polycarboxylic acid) or a salicylic acid derivative. The inorganic particulate
component
preferably comprises finely divided metal oxides, metal hydroxides, calcium
hydroxyapatite bioglasses) silicate-cement glazes, or glass ionomer particles.
The
antimicrobial zeolite is preferably as described in Niira et al. '958,
discussed above, and
most preferably contains silver ions.
_ ;_
SUBSTITUTE SHEET (RULE 26)
CA 02268478 1999-04-12
WO 99/07326 PCT/CA98/00754
Most preferably, the antimicrobial cement composition according to the
invention
is an antimicrobial glass ionomer cement composition comprising a
polyelectrolyte, glass
ionomer particles and antimicrobial zeolite particles.
The antimicrobial zeolite is preferably contained in the cement composition of
the invention in an amount of from about 0.1 to about 30 percent by weight of
the
powder component, more preferably from about 0.2 to about 20 percent by weight
of the
powder component.
The most preferred dental uses of the antimicrobial cement compositions of the
invention include endodontic filling materials, dental cements) dental lining
and
restorative materials, dental sealers, and dental luting adhesives, for
example to adhere
metal brackets to dental tissue. The most preferred medical uses include bone
cements,
implant components and bone substitutes.
Most preferably) antimicrobial glass ionomer cement compositions of the
invention are used in endodontic therapy to provide a bacteria-tight seal in a
root canal
system. The antimicrobial glass ionomer cement composition of the invention
may
preferably be used to fill the root canal or may preferably be used as a
sealer in
combination with a core root canal filling material such as gutta percha
(rubber).
The cement compositions of the present invention overcome many of the
difficulties experienced with previously known antimicrobial cement
compositions. It is
believed that when zeolites are incorporated into a cement composition
according to the
invention, for example a Mass ionomer cement composition, it is the zeolites,
and not the
exchangeable metal ions contained therein, which form ionic bridjes with the
polyacrylic acid) the glass particles and the substrate. Therefore, when
antimicrobial
ions are released from within the zeolites, the structures of the zeolites and
the cement
matrix as a whole are not significantly altered.
Furthermore) the inventors have found that antimicrobial zeolites contained in
cement compositions of the invention release antimicrobial metal ions in a
highly
controlled fashion, providing a substantially constant release of such ions
over an
extended period of time. Therefore, the inventors expect that antimicrobial
cement
- G-
SUBSTITUTE SHEET (RULE 26)
~ ....._~_..~._"-....~.......__.... ....._.__ ....._.. .__.._._.._._......-
~........... ...._..,..__...... ..._.. . ..__.......
CA 02268478 1999-04-12
WO 99/07326 PCT/CA98/00754
compositions according to the invention will retain their mechanical stability
as well as
their antimicrobial activity over a relatively long period of time.
Because the structure of the antimicrobial cement matrix of the invention
differs
significantly from that of a polymeric composite material, the cement
compositions of
the invention overcome many of the problems experienced with polymer resins
containing antimicrobial zeolites. For example, the inventors have found that
antimicrobial ions contained in the zeolites of the cement compositions of the
invention
may more easily diffuse through the cement matrix to be released in the oral
cavity or at
the interface between the root filling and the dental tissue. This permits the
amount of
zeolite in the cement to be relatively low, yet sustain the antimicrobial
activity of the
cement. The use of smaller amounts of zeolites permits cements of the
invention to be
produced economically) and also avoids problems such as staining which becomes
more
severe when zeolite content is increased. Furthermore) because antimicrobial
metal ions
may diffuse to the surface from the interior of the cement, more antimicrobial
metal ions
are available for release, and therefore the cement composition of the present
invention is
expected to maintain its antimicrobial activity for a longer period of time.
Accordingly, it is one object of the present invention to provide an
antimicrobial
cement composition containing antimicrobial zeolites.
It is another object of the present invention to provide an antimicrobial
cement
composition containing antimicrobial zeolites which is useful in dentistry and
medicine.
It is yet another object of the present invention to provide an antimicrobial
cement composition containing antimicrobial zeolites for use as a dental
sealer, adhesive
and filler, which provides controlled, long-term release of antimicrobial
metal ions.
It is yet another object of the present invention to provide an antimicrobial
glass
ionomer cement composition containing antimicrobial zeofites for use as a
dental sealer,
adhesive and filler, the cement composition providing controlled, long-term
release of
antimicrobial metal ions.
It is yet another object of the present invention to provide an antimicrobial
glass
ionomer cement composition for use as an endodontic filling and sealing
material which
provides long term protection against ingress of bacteria into a filled root
canal.
_ 7_
SUBSTITUTE SHEET (RULE 26)
._ . _.... ~.. ...~......_...,........ ... ........,~~._..._ .._. -,......_.
_.. ......._..
CA 02268478 1999-04-12
WO 99/07326 PCT/CA98/00754
Brief Description of the Drawings
Further aspects and advantages of the present invention will become apparent
from the following description, taken together with the accompanying drawings,
in
which:
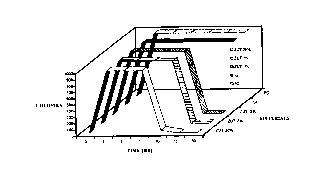
Figure 1 is a plot of optical density versus incubation times, comparing the
antimicrobial activity of an antimicrobial GIC composition according to
Example 1 of
the invention with a conventional GIC composition;
Figure 2 is a flow chart description of the microbiology study of Example 2;
Figure 3 shows bacterial colony counts from substrates of Example 2 following
exposure to bacteria for 1, 3, 7, 10, 15, 30 hours. Prior to inoculation the
substrates were
incubated in a bacteria free medium for 84 days. Averages of three
measurements were taken
for bacterial colony counts. ( 1000 colonies were labelled as having complete
jrowth);
Figure 4 shows the change in the average optical density values measured for
each
group from Example 2 at 84 days as a function of bacteria culture time.
Figure S shows the statistical significance of interactions between the
different ZUT
materials of Example 2 as measured by the lumped optical density values (over
the entire 84
day experiment) versus the bacteria culture time. Statistical differences were
found between
the ZUT materials at 7 and 10 hours. (p < 0.000l).
Figure 6 shows the cumulative silver ion release (ppm) for ZUT .2% and ZUT 2%
of
Example 2 over a period of 84 days.
Detailed Description of Preferred Embodiments
Preferred antimicrobial cement compositions according to the present invention
comprise a cement and an antimicrobial zeolite.
The type of cement used in the preferred antimicrobial cement compositions of
the
invention is at least partially dependent on the intended use of the cement
composition. For
example, antimicrobial cements for building materials used in industrial,
institutional and
domestic applications preferably comprise a hydraulic cement and an
antimicrobial zeolite.
The term "hydraulic cement" is to be understood as including any mixture of
fine-
ground lime, alumna and silica that will set to a hard product by admixture of
water which
_ R_
SUBSTITUTE SHEET (RULE 26)
.. T .. _ . _._.._.._ __..._..~.~ _ _ ..._.. ~.~..,.~_.~.__~..,. . _ ___
..._.._..._ _~ .
CA 02268478 1999-04-12
WO 99/07326 PCT/CA98/00754
combines chemically with the other ingredients to form hydrate. A preferred
hydraulic
cement is Portland cement.
Preferred types of building materials into which antimicrobial cement
compositions
according to the present invention may be incorporated include grout, mortar
and concrete,
which are preferably formed by admixture of the antimicrobial cement
composition with an
aggregate selected from the group comprising sand, gravel and crushed stone,
and optionally
with other conventional ingredients. For example, a cement composition of the
invention
may be used as a component in a mildew and bacterial resistant tile grout for
use in
bathrooms, hospitals or other areas where cleanliness is important.
The present invention also includes within its scope tiles and similar
building
materials incorporating antimicrobial zeolites, whether or not such tiles or
other
materials are made from or contain cement.
More preferably, the antimicrobial cement composition of the invention
contains
a cement for medical or dental use. Preferred medical cements include bone
cements,
implant components and bone substitutes, and preferred dental cements include
cements
for use as dental adhesives, sealers, fillers and restorative materials. Most
preferably, the
cement is an endodontic filler or an endodontic sealer to be used in
combination with a
core filling material of different composition, for endodontic treatment of
root canal
systems.
Preferred types of cements for medical and dental use preferably comprise an
anionic component, an inorganic particulate component, and an antimicrobial
zeolite.
The anionic component preferably comprises a liquid medium containing an acid
such as
phosphoric acid) a polyelectroiyte such as a polycarboxylic acid, or a
salicylic acid
derivative. The inorganic particulate component preferably comprises a finely
divided
metal oxides, metal hydroxides, calcium hydroxyapatite bioglasses, silicate-
cement
glazes) or glass ionomer particles.
Most preferably, the antimicrobial cement composition according to the
invention
is an antimicrobial glass ionomer cement composition comprising a
polyelectrolyte, glass
ionomer particles and antimicrobial zeolite particles. This antimicrobial
cement
_o_
SUBSTITUTE SHEET (RULE 26)
....~~ . ...__~._ _ _.__....~___....._ _ _.__~._..._
CA 02268478 1999-04-12
WO 99/07326 PCT/CA98/00754
composition is particularly preferred as an endodontic filling and sealing
material for use
in endodontic treatment of root canal systems.
The preferred antimicrobial glass ionomer cement compositions according to the
present invention include resin modified glass ionomer cements which include a
small
amount of a resin monomer to provide the cement with improved compliance, as
welt as
metal-modified glass ionomer cements.
Zeolitic structures are comprised primarily of an aluminosilicate framework of
alkali or alkali earth metals, with a regular three-dimensional skeleton
consisting of a
methane-type tetrahedron of linked Si04 and A104, the oxygen atoms being
shared. This
framework can house exchangeable ions) typically cations.
Antimicrobiai zeolites which are preferably used in the antimicrobial cement
compositions of the present invention include any zeolites in which the
exchangeable
ions have antimicrobial activity. Preferred ions include ammonium ion and
antimicrobial metal ions. Preferably) the antimicrobial metal ions are
selected from one
or more members of the group comprising silver) copper) zinc, mercury) tin,
lead,
bismuth) cadmium, chromium and thallium ions; more preferably silver, copper
and zinc
ions; and most preferably silver ions. The content of the antimicrobial metal
ions in the
zeolite is preferably from about 0.1 % to about I S% by weight of the zeolite.
Particularly preferred zeolites used in the cement compositions of the
invention
contain silver ions and ammonium ions, and may preferably also contain copper
and/or
zinc ions. Silver ions are preferably contained in the zeolite in an amount of
from about
0.1% to about I S% by weight of the zeolite. In zeolites which contain copper
and/or
zinc ions) the preferred total content of copper and/or zinc ions is from
about 0. I % to
about 8% by weight of the zeolite. Ammonium ion is preferably contained in the
zeolite
in an amount of less than about 20% by weight of the zeolite, more preferably
from
about 0.5% to about I _S% of the zeolite) and most preferably from about I .5%
to about
S% by weight of the zeolite.
The most preferred zeolites for use in the cement composition according to the
invention are zeolites sold under the trade mark ZeomicT"" by Shinagawa Fuel
Co., Ltd.
The particle size of the zeolite particles is preferably from about 20 to
about 50 microns.
- ~o-
SUBSTITUTE SHEET (RULE 26)
CA 02268478 1999-04-12
WO 99/07326 PCT/CA98/00754
Cement compositions according to the present invention preferably contain from
about 10% to about 95% filler, with the antimicrobial zeolite being present in
amounts of
up to about 30% by weight of the filler. More preferred cements according to
the present
invention are antimicrobial glass ionomer cement compositions containing
antimicrobial
zeolite particles in amounts of up to about 20% by weight of the glass ionomer
particles,
and more preferably from about 0.2% to about 20% by weight of the glass
ionomer
particles.
In particularly preferred antimicrobial cement compositions of the invention)
the
cement compositions contain antimicrobial zeolites in amounts which
substantially do
not cause staining of the cement composition) but which provide the cement
composition
with acceptable antimicrobial activity. The preferred amount of antimicrobial
zeolite to
accomplish these objects is from about 0.2% to about 2% by weight of the
powder
component of the antimicrobial cement composition.
The present invention also includes within its scope the incorporation of
antimicrobial zeolites in dental cements selected from the group comprising
zinc
phosphate, zinc oxide eugenol (ZOE), silicophosphate and polycarboxylate
dental
cements.
The present invention also includes within its scope the use of antimicrobial
zeolite cements in combination with conventional) non-cementitious dental
restorative
materials including polymeric materials such as gutta percha, polymeric
composite
materials, calcium hydroxide, and pulp canal sealers, as well as in
combination with
porcelain for crowns.
The inventors have found that antimicrobial cement compositions according to
the present invention are capable of preventing) or reducing by at least SO%,
the ability
of bacteria to penetrate through filled root canals for periods of at least 90
days. The
ions leach out of the antimicrobiai zeolite to the surface of the root canal
filler or sealer
of the invention, and are believed to disrupt bacterial activities such as
cellular
respiration) enzyme activation and active transport from the cell wall, with
subsequent
inhibition of the bacterial cell function and proliferation.
SUBSTITUTE SHEET (RULE 26)
CA 02268478 1999-04-12
WO 99/07326 PCT/CA98/00754
The antimicrobial cement compositions of the present invention are also
expected
to have efficacy against a wide range of bacteria, including the at least
fifty strains of
bacteria which have been isolated from root canals, such as
Enterococcrrsyfac~calis,
Actinomyces, Lactobacillus, black-pigmented Bact~roides,
Peptortrc,~ptococc~rs, non-
pigmented Bacteroidc.~a~) Veillo~etia, Fusobacterirrnr ~ucleatum, and
ftr~e~ptococcrrs
muta~s~. The cement compositions of the invention preferably also have
activity against
molds, fungi and algae.
Examples
Example 1
A variation of the direct contact test (DCT) was used to compare the
bactericidal
ability of a preferred antimicrobial glass ionomer cement composition of the
invention
with that of a conventional glass ionomer cement. The DCT test is suitable to
test
insoluble materials such as cements since it relies on direct and close
contact between the
test microorganism and the tested material and is virtually independent of the
diffusion
properties of both the tested material and the media.
The results of Example t are shown in Figure 1. The antimicrobial cement
composition tested comprised a glass ionomer cement sold under the trade mark
ChemFill IIT"', containing 2% by weight of a silver-containing zeolite sold
under the trade
mark ZEOM1C by Shinagawa Fuel Co., Ltd.) which is identified in figure 1 as
ZUT2. The
antimicrobial glass ionomer cement ZUT2 was placed in a vial containing brain
heart infusion
(BHI) culture media and was inoculated with Errt~rococcn.s,faecali.s. The
optical density of
the vial was observed over a period of 30 hours, turbidity in the culture
media being
indicative of bacterial proliferation. The optical density of antimicrobial
glass ionomer
cement sample ZUT2 was compared with samples containing inoculated media,
inoculated
ChemFill II without zeolite (CF), and a paper disc control (PD).
Optical density is a means by which to measure the clarity of a solution
containing particulate) in this case bacterial, growth. Optical density is
measured by
determining the degree of light that can be transmitted through the test
specimen. A low
-12-
SUBSTITUTE SHEET (RULE 26)
_ T _~.~.......-.._.__. __W_ _ _ ...~_._ .~__. .
CA 02268478 1999-04-12
WO 99/07326 PCT/CA98/00754
value indicates low bacterial growth activity, while a high value indicates
the presence of
elevated bacterial growth activity.
The results of this test showed that the antimicrobial glass ionomer cement
composition ZUT2 effectively prevented all growth of Enterococcn.s_ faecalis
from its
surface within 10 hours of inoculation. On the other hand, the samples of the
unmodified
glass ionomer cement CF and the paper disc control PD showed elevated growth
of
bacteria as indicated by high turbidity in the culture vessels.
Example 2
A study was undertaken to characterize the anti-bacterial properties of
various
antimicrobial glass ionomer cement compositions of the present invention (ZUT
formulations) and to compare them to a standard glass ionomer formulation.
Materials
were assessed for their physical characteristics and their ability to suppress
adherent E.
faecalis and sustain anti-bacterial function over a period of 3 months.
The objective of this study was to assess the efficacy of a modified
endodontic
filler (ZUT), consisting of an experimental glass ionomer (GI)) KT-308 (GC
Corp.,
Japan), and an anti-microbial agent (AA). Five experimental groups were
studied: Group
I- KT-308, Group 2- ZUT-.2%, Group 3- ZUT-2%, Group 4- ZUT-20%, Group 5- paper
disc (blank control)) where .2, 2, and 20% refer to the respective wt %
concentration of
AA in the ZUT materials. In order to evaluate the ability of each material to
eliminate
bacterial growth, the following experiment was carried out. Discs were
incubated in BHI
(Brain Heart Infusion) at 37~C for a period of 84 days. At 14-day intervals,
discs were
inoculated with a suspension of Ente~rncuccu.s faecali.c. The discs were
incubated at
37~C and removed at intervals of 1,3,7,10) 1 S and 30 hours. At each time
interval the
discs were washed in fresh BHI media to remove non-adherent bacteria, and then
transferred to fresh media. Following further incubation for 8 hours, 200 ~l
aliduots of
the medium were pippeted onto agar plates) which were then incubated for 8
hours and
assessed for colonies. The remaining medium was used to measure its optical
density in
a spectrophotometer. The three ZUT materials displayed bactericidal effects
throughout
the 84-day experiment) as expressed by complete elimination of bacteria within
l 5 hours
-m-
SUBSTITUTE SHEET (RULE 26)
CA 02268478 1999-04-12
WO 99/07326 PCT/CA98/00754
of inoculation. Groups 3, 4 and 5 displayed their bactericidal effects at 7,
10 and I S
hours respectively. Groups 1 and 2 were positive for growth during the entire
84 days.
It was concluded that ZUT provided a significant bactericidal effect relative
to glass
ionomer alone and that this effect was maintained for approximately 3 months
during
exposure to an aqueous environment.
Materials and Methods
The materials were arranged into five study groups, comprised of the
following:
Group 1 - KT-308 (a GIC); Group 2 - ZUT 0.2%; Group 3 - ZUT 2%; Group 4 - ZUT
20%; Group 5 - a paper disc (blank control). KT-308 is an experimental
cement/sealer
glass ionomer provided in kind by GC Corp., Japan. ZUT formulations were
prepared
with the GIC and Zeomic AJ 10D (Shinagawa Fuel Co., Nagoya, Japan), which is a
silver containing zeolitic agent. ZUT formulations were prepared by blending
KT with
Zeomic~ in percentages of 20%, 2%, and .2% with respect to the ceramic
content. Using
a custom made Teflon~ mold, the materials were prepared to form discs (n=I08
for each
group) with dimensions of 0.2S cm x 0.08 cm. A slight overfill of the mold was
introduced in order to compensate for material shrinkage. During setting, the
molds
were covered with Mylar sheets and glass slides to render the disc surfaces
optically
smooth. After being fully set, discs were placed into sterile 6-ml vials
containing Brain
Heart Infusion (BHI; Difco, Detroit, MI) medium, and incubated at 37~C for up
to 84
days. This is referred to as the incubation stock.
A modification of the direct contact test that was reported by Palenik et al
(Inhibition of microbial adherence and growth by various glass ionomers in-
vitro, Dental
Materials. I992; 8:16-20) was used to evaluate the antibacterial efficacy of
the materials.
A flowchart of the complete protocol is given in Figure 2 and is described as
follows. At
14-day intervals, eighteen discs from the experimental groups were removed
from the
incubated stock and inoculated with a 200A aliquot ofE.,faeccrli.c.
I:.,fcre~cali.c ATCC
292I2 was grown in BHI ( I 8~T SOOmI) from frozen stock culture (American Type
Culture Collection, Rockville) MD). The purity of the culture was maintained
by
-I:l_
SUBSTITUTE SHEET (RULE 26)
CA 02268478 1999-04-12
WO 99/07326 PCT/CA98/00754
quadrant streaking on a weekly basis. Discs were incubated in a humid
environment at
37~C to allow for bacterial adherence. At pre-defined incubation periods of 1,
3, 7) 10)
t 5 and 30 hours, three discs from each group were removed and gently rinsed
with 3 ml
of fresh BHI in sterile petridishes in order to discard non-adherent bacteria.
Each disc
was then transferred to a test tube containing 0.5 ml of fresh BHI) and
incubated for 8
hours. It was then washed twice for 1 S min with 3 ml of fresh BHI in a
sterile petridish
to again remove any non-adherent bacteria from its surface, transferred to a
sterile test
tube with 3 ml BHI, and incubated for a final 8 hours. An aliquot of 200 ~tl
from the
latter incubation medium was then pippeted onto an agar plate (BHI agar 26
g/500 ml)
and spread evenly on the surface. The agar plates) one for each disc, were
incubated for
8 hours and assessed for growth of bacterial colonies. The remaining
incubation solution
was pippeted into individual plastic curettes, and the optical density was
measured with a
spectrophotometer (LKB Biochrome) Cambridge) U.K.) at a wavelength of 560 nm.
The
absorbency of pure BHI was measured as a reference sample. Each disc that
showed a
significant reduction in growth, in the fore-mentioned procedures) was
subjected to a
direct sampling of its surface to assess for cell viability. The surface of
each disc was
gently scraped with a sterile scalpel blade (Lance, Sheffield) U.K.) and the
shavings
washed with 3 ml fresh BHI into a sterile petridish. The resulting medium was
pippeted
onto an agar plate, incubated for 8 hours, and assessed for growth of
bacterial colonies.
Discs from groups 2 to 4 were characterized for changes in hardness using a
Knoop value hardness test (Leitz Wetzlar) Germany). A weight of 300 ponds was
used
for this test and ten disc specimens were measured from each group. Three
measurements were taken and averaged for each disc. Measurements were obtained
for
post incubation periods of 28 days and 84 days.
The incubation stock from 28) 56 and 84 days was analyzed for silver ions that
diffused from ZUT materials into the incubation stock. Ten samples each of ZUT
.2%
and ZUT 2% from each of the above time periods were diluted 1: l21 with 1%
Triton X-
100. A11 samples were measured by a graphite furnace atomic absorption
spectrophotometry (GFAAS) located in Dr Stanley Lugowski's laboratory at the
Centre
_ ti _
SUBSTITUTE SHEET (RULE 26)
CA 02268478 1999-04-12
WO 99l07326 PCTlCA98/00754
of Biomaterials, using Varian AA-87S spectrophotometer and Varian GTA-95
(Melbourne, Australia).
Colony counts and optical density data were statistically analyzed by a factor
analysis of variance (Statistical Analytical Systems, Cary, NC). Interactions
among the
three factors (materials) days and hours) were tested using the same
statistical method,
ANOVA. The least square means comparison was applied to examine interactions.
Correlation coefficients were calculated between optical density and colonies
for the data
combining interactions among the three factors.
Results
Bacterial colony counts for the 84-day samples are shown in Figure 3 and
typically represent the observed performance of all materials for the
different time points
over the 84-day experiment. The data reflect the measure of each material's
ability to
sustain adhesion and eventual growth of E. fcrc.~calis. While a11 materials
showed
evidence of growth after three hours of incubation with the bacteria, ZUT
materials
exhibited a complete elimination of bacteria within 15 hours of incubation.
The
strongest effect was exhibited by ZUT 20) which only had 43 colonies while ZUT
.2%,
ZUT 2% and the two controls had greater than l000 colonies at 10 hours. The
non-ZUT
materials exhibited no anti-microbial activity towards L _faccali.v throughout
the
complete incubation period. A plot of the optical density versus incubation
times
revealed a gradual drop in turbidity over 30 hours for media that contained
ZUT
materials (Figure 4). This gradual drop in turbidity corresponds to the drop
in colonies
observed for the different materials in Figure 3. In contrast, media
containing non-ZUT
materials permitted growth of bacteria, which was exhibited by the high
turbidity value.
A correlation coefFcient (r) calculated between optical density and colony
growth was
determined to be I" = 0.997. This correlation, obtained using a three-way
analysis
(ANOVA) was highly significant (p<0.0001). A three way ANOVA was also used to
assess the effects of media exposure time on the materials and their ability
to sustain
growth of bacteria as measured by optical density. The ZUT materials were
shown to
have a significant material/bacteria interaction (p<0.000 I ) for the 7 and 10
hour bacteria
_ t6 _
SUBSTITUTE SHEET (RULE 26)
CA 02268478 1999-04-12
WO 99/07326 PCT/CA98/00754
incubation periods (Figure 5), with a11 media exposure times (i.e. 14, 28....
and 84 days).
Based on this analysis) it was also shown that there was no prominent anti-
bacterial
activity demonstrated by Groups I and 5 for any time interval.
There were no viable cells found on the ZUT materials, which demonstrated an
absence of bacterial growth in the media (Table 1 ). Complete suppression of
bacterial
growth was observed at 15 hours for ZUT materials.
MATERIALS GROWTH GROWTH GROWTH
( 10 HR) ( 15 HR) (30 HR)
ZUT .2% + - -
ZUT 2 % + _ -
ZUT 20% + - -
Table 1: Viable E. faecalis cells removed from the surface ofZUT
materials is indicated by '+' at 10 hours. Absence of cells is
denoted by '-', indicating no cells were recovered after 15 hours.
Trace elemental analysis was conducted to study the amount of silver being
released from ZUT materials over the 84 period. Data in Figure 6 illustrate
that the
amount of silver ions released is in the order of parts per million. Note that
a ten fold
increase in zeolite content only translated to approximately a two fold
increase on silver
ion release over 28 days and an approximate S fold increase over 84 days
(Figure 6).
While there were slight variations in hardness values for ZUT materials
between the 28
and 84 day period, no significant difference was found between them (Table 2).
- 17-
SUBSTITUTE SHEET (RULE 26)
CA 02268478 1999-04-12
WO 99/07326 PCT/CA98/00754
IrITERVALS .2% ZUT 2% ZUT ZO% ZUT
28 DAYS 430.85 12.12387.15 16.37 3290.46 426.70
84 DAYS 477.92 10.29405.20 47.80 2501.66 211.85
Table 2: The Knoop values in the above table are of ZUT
materials after they were incubated for 28 and 84 days. A
weight of 300 ponds was used for all three materials. The
microhardness of each material is measured in kplmm2.
Discussion
The purpose of this study was to assess the ability of ZUT materials to
suppress
the growth of ~ fac~cali.s~. Materials were exposed to culture media for
different intervals
over a period of 84 days and then exposed to bacteria. Groups 2, 3, 4, which
contained
Zeomic~ in ratios of .2%) 2%, 20% proved to be effective against C.,
fcrc.~ccrli.s. E., facccrli.s
is one of the few facultative microorganisms and the most resistant to be
found in the
root canal. A common isolate found in infected root canals, it makes up a
small
percentage of the root canal flora) and may be favoured by ecological changes
that
establish infections, which are difficult to treat. E., faccalis has been used
previously in
studies of root canal dis-infection and is known to survive in environments
where the pI~
is as high as l 1.5.
An important reduirement of endodontic sealers is their insolubility. In this
context ZUT is not soluble and therefore its anti-microbial activity could not
be
measured using a routine afar diffusion test. An effective approach was to
measure its
ability to inhibit bacterial growth on its surface. A direct contact test
(DCT} is taught by
Weiss et al) Assessment of Antibacterial Activity of endodontic sealers by a
direct
- i8-
SUBSTITUTE SHEET (RULE 26)
CA 02268478 1999-04-12
WO 99/07326 PCT/CA98/00754
contact test, Endodontics and Dental Traumatology 1996; 32:179-184. The
modification
of the direct contact test (DCT) used in the current investigation was less
dependent on
the diffusion properties of the materials and was therefore advantageous to
this study.
During the 84 day period it was clearly demonstrated (Figure 6) that silver
ions
were diffusing into the surrounding media. The amount of silver ions detected
in media
varied from 319 to 3523 ppb (Figure 6) and was dependent on the percentage of
Zeomic~ present in the material. This release of silver ions showed a
continuous
accumulation through out the 84-day period) which indicates that the ion
exchange is
permitted over an extended period of time. Based on the quantity of zeolite
added to
each disc the highest possible amount of silver ions that could be released
into 2.5 ml of
incubation stock was calculated to be 7000 ppb for a single ZUT .2% disc.
Therefore
there would still be the potential for more silver ions to diffuse into the
media and
provide a sustained bactericidal effect beyond 84 days since ZUT .2% only
released
about one tenth of this amount (Figure 6). The levels of silver ions diffusing
through the
matrix were sufficient enough to reduce and ultimately resist adhesion
ofE..fcrc~cnli.s
(Figures 2 & 3). ZUT materials were able to suppress the adherence of bacteria
repeatedly for samples retrieved throughout the 84-day period. In a study
conducted by
Moroz et al. (Susceptibility of organisms which cause intestinal infections to
removal by
Silver, Khim Technol. (Kiebv), 1980' 2"275), it was stated that 50 to 200
parts per billion
of silver was sufficient enough to kill .S~rlnrnrrc.~l!u and E. cnli, as well
as bacteria highly
resistant to antibiotics (21 ). As indicated in Figure 6, over a period of 84
days an
average of more than 600 parts per billion of silver ions were released by ZUT
.2%. This
is three times the amount required to kill some bacteria) as reported by Moroz
et al.
The findings of this irr oitrw study suggest that ZUT materials that were in
contact
with the biological media sustain activity against adherence of L.,
fcrc.~ccrli.s for at least 84
days. It had been reported in U.S. Patent 5,5S6,699 to Niira et al, issued
September 17)
1996 that the silver can bind within the zeolitic structures and this most
likely reflects the
gradual and long lasting release of the ions, as well as the continued anti-
microbial
character of ZUT materials (Figure 2). Ag+, Zn2+, are a few of the cations
that can be
localized within the zeolitic structures, which are also potent anti-microbial
agents.
SUBSTITUTE SHEET (RULE 2fi)
CA 02268478 1999-04-12
WO 99/07326 PCT/CA98/00754
Cations, such as sliver are electrostaticaliy attracted to the negatively
charged
microorganism and may then undergo reactions on the surface. Once the canons
are in
the cell, they can act at sites of RNA, DNA, or enzymes, eventually disrupting
the entire
mechanism of the cell. The released silver is believed to exert a bactericidal
effect
through its' reducing action.
Since present day glass ionomers are thought to loose their structural
integrity
through a complex process of adsorption, disintegration and outward
transportation of
ions, there was interest in assessing changes in surface hardness as a
preliminary
determination of material stability. There were no signifcant changes in the
hardness
values of the ZUT materials between 1 to 3 months (Table 2) which indicates
that the
dif~'usion of silver ions on the structural nature of the ZUT materials
appears to minimal,
although further work will be required to assess compressive and tensile
strength
properties.
In summary this study indicates that ZUT materials have the potential to
suppress
microbial growth and metabolism in the local micro-environment of the root
canal. It
offers the clinician some information regarding the quality and properties of
ZUT-based
GICs. The ZUT materials were shown to inhibit and suppress growth of
E.,faeccrli.s. The
anti-bacterial effects were provided with low concentrations of Zeomic~ and
therefore
the handling properties of the materials still reflect those of traditional
GIC materials that
are close to an ideal filler, as required in endodontic practice.
Although the invention has been described in connection with certain preferred
embodiments, it is not intended to be limited thereto. Rather) it is intended
that the
invention cover all alternate embodiments as may be within the scope of the
following
claims. The invention also includes all embodiments which are functional
equivalents of
the specific embodiments and features which have been described herein.
It will be further understood that, although various features of the invention
have
been described with respect to one or another of the embodiments of the
invention, the
various features and embodiments of the invention may be combined or used in
conjunction with other fetures and embodiments of the invention as described
herein.
-20-
SUBSTITUTE SHEET (RULE 26)