Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02269720 1999-04-23
i ,. ~ -::._.--~~ .. , . ~
SPECIFICATION
NOVFd. HETEROCYCLIC AMIDE COMPOUND AND PHARMAeCEUTiCAL USE THEREOF
Technical Field
The present invention relates to a novel heterocyclic amide compound, a
pharmacologically acceptable salt thereof, a pharmaceutical composition
thereof,
and a pharmaceutical use thereof. More particularly, the present invention
relates to pyridoneacetamide and pyrimidoneacetamide derivatives useful
pharmacologically, diagnostically and for the prophylaxis and treatment of
diseases, and a pharmacologically acceptable salt thereof. Moreover, the
present
invention relates to an intermediate necessary for the synthesis of the above-
mentioned heterocyclic amide compound.
Backg~rouad Ant
Angiotensin II shows physiological activities such as vasopression by
strong contraction of blood vessel, stimulation of aldosterone secretion from
adrenal cortex (aldosterone retains sodium), and the like, and is considered
to be a
causative substance or risk factor of diseases such as hypertension,
hypercardia,
myocardial infarction, arteriosclerosis, diabetic and non-diabetic renal
diseases,
vascular restenosis after PTCA (percutaneous transluminal coronary
angioplasty)
and the like.
It is known that this angiotensin II is generated by cleavage of two amino
acid residues from angiotensin I, which is a peptide consisting of ten amino
acids
present in a living body, and that angiotensin converting enzyme (ACE) is
involved
in said cleavage. Thus, numerous ACE inhibitors have been developed for the
prophylaxis and treatment of the above-mentioned diseases.
Meanwhile, actions of a chymase group including human heart chymase,
human mast cell chymase and human cubs chymase, which is one of the
subfamilies of serine protease, have been drawing attention in recent years.
It has been clarified that chymase is involved in the course of generation,
which is independent from ACE, of angiotensin II in the conversion of the
above-
mentioned angiotensin I to angiotensin II (Okunishi et al., Jpn. J. Pharmacol.
1993, 62, p. 207 etc. and others). Also, chymase is known to use, as
substrates,
numerous physiologically active substances such as extracellular matrix,
cytokine,
substance P, VIP (vasoactive intestinal polypeptide), apoprotein B and the
like, and
known to be responsible for the activation of other proteases such as
collagenase
1
CA 02269720 1999-04-23
(Igakuno ayumi, Miyazaki et al., 1995, 172, p. 5S9).
Therefore, chymase inhibitors are expected to become inhibitors of
angiotensin II action, as well as agents for the prophylaxis and treatment of
various diseases caused by chymase, since it inhibits generation of ACE non-
dependent angiotensin II. A patent application drawn to a chymase inhibitor
based on these ideas has been already filed (W093/25574).
The above-mentioned patent application W093/25574 in the name of
PFIZER Inc. discloses a series of peptide compounds which are chymase
(inclusive
of human heart chyrrrase) inhibitors. However, these compounds are peptide
compounds which are unsatisfactory in terms of oral absorption, and no
pharmacological test data are available.
Patent applications filed by ZENECA Inc. (Japanese Patent Unexamined
Publication Nos. 286946/ 1993, 56785/ 1994 and W093/21210), J. Med. Chem.
1994, 37, p. 1259, J. Med. Chem. 1994, 37, p. 3090, J. Med. Chem. 1994, 37, p.
3303, J. Med. Chem. 1994, 37, p 3313, J. Med. Chem. 1995, 38, p 98, J. Med.
Chem. 1995, 38, p 212 and others disclose or report heterocyclic compounds
which are human leukocyte elastase inhibitors, and they compounds are known
to selectively inhibit human leukocyte elastase.
Patent applications filed by ICI Americans Inc. (now ZENECA Inc.)
(Japanese Patent Unexamined Publication Nos. 45395/ 1989), J. Am. Chem. Soc.,
1992, 114, p 1854, J. Med. Chem. 1995, 38, p. 76, J. Med. Chem. 1995, 38, p.
3972 and others disclose or report peptide compound having heterocycle. These
compounds are also known to selectively inhibit human leukocyte elastase.
It is therefore an object of the present invention to provide novel
compounds having superior chymase inhibitory activity, pharmaceutical
compositions thereof and chymase inhibitors.
Disclosure of the Iav~entioa
The present inventors have conducted intensive studies in an attempt to
achieve the above-mentioned objects, and found that, by modifying or
converting a
part of the structure of the compound disclosed by ZENECA Inc. and the like,
compounds can be obtained that inhibit chymase group, inclusive of human heart
chymase, with high selectivity, without inhibiting other enzymes such as human
leukocyte elastase, which exhibit superior characteristics in absorption,
safety and
stability in blood, which resulted in the completion of the present invention.
2
CA 02269720 1999-04-23
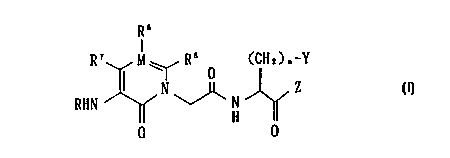
Accordingly, the present invention realtes to a heterocyclic amide
compound of the formula (I)
wherein
R6
R~ M~ R5
O (CH2)n-Y
RHN N~N Z I
H ~ ()
O O
R is a hydrogen atom, alkyl, -CHO, -CONHZ, -CORi, -COORi,
-CONHORl, -CONHRi, -CONR1R1', -CONHSOzRI, -COSRI,
-COCOR2, -COCOOR2, -CONHCOOR2, -COCONR3R4, -CSXRl,
-S02WR1, -S02NR1R1' or -S02E
wherein Rl and Rl' may be the same or different and each is independently
alkyl, cycloalkyl, cycloalkylalkyl, aryl, arylalkyl, heteroaryl,
heter~oarylalkyl,
heterocycle or heterocyclic alkyl, Rz, R3 and R4 may be the same or
different and each is independently a hydrogen atom, alkyl or arylalkyl,
or -NR3R4 may in combination form a heterocycle, X is a single bond,
-NH-, -O- or -S-, W is a single bond, -NH-, -NHCO-,
-NHCOO- or -NHCONH-, and E is a hydroxyl group or amino;
R5, R6 and R'
may be the same or different and each is independently hydrogen atom or
alkyl, or one of R5, R6 and R' is aryl, arylalkyl, arylalkenyl, heteroaryl,
heteroarylalkyl or heteroarylalkenyl and the rest are hydrogen atom;
M is a carbon atom or nitrogen atom, provided that when M is a nitrogen
atom, R6 is void;
Y is cycloalkyl, aryl or heteroaryl;
Z is a group of the formula (i)
formula (ii)
N ~a~b
i i R8 (i)
~ ~c
d
R
N R$
(ii)
A w R9
3
CA 02269720 1999-04-23
or formula (iii)
N R$
(iii)
w R9
wherein R8 and R9 may be the same or ditl'erent and each is independently
hydrogen atom, alkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, halogen,
trifluoromethyl, cyano, nitro, -NRl~Rl~', -NHS02R1~, -ORi~, -COORl~,
-CONHS02R1~ or -CONRI~Rlo' wherein Rl~ and Rl~' may be the same or
ditl'erent and each is independently hydrogen atom, alkyl, cycloalkyl,
cycloalkylalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl or
trifluoromethyl,
or -NR'~R'~' may in combination form heterocycle, A is -O-, -S- or
-NR12- wherein R12 is a hydrogen atom, alkyl, cycloalkyl or cycloalkylalkyl,
and a, b, c and d are each a carbon atom or one of them is a nitrogen atom
and the rest are carbon atom; and
n is 0 or 1,
wherein, of the above-mentioned groups, alkyl, cycloalkyl, cycloalkylalkyl,
aryl,
arylalkyl, arylalkenyl, heteroaryl, heteroarylalkyl, heteroarylalkenyl,
heterocycle
and heterocyclic alkyl each optionally has a substituent, (hereinafter to be
also
referred to as compound (I))
or a pharmacologically acceptable salt thereof.
The present invention also relates to the above-mentioned heterocyclic
amide compound of the formula (I), wherein Y is an optionally substituted
aryl,
and a pharmacologically acceptable salt thereof ; the above-mentioned
heterocyciic
amide compound of the formula (I), wherein Z is a group of the formula (i),
and a
pharmacologically acceptable salt thereof ; and the above-mentioned
heterocyclic
amide compound of the formula. (I), wherein one of R5, R6 and R' is optionally
substituted aryl and the rest is hydrogen atom, provided that when M is a
nitrogen
atom, R6 is void, and a pharmacologically acceptable salt thereof.
The present invention further relates to a compound of the formula (II)
R6
I
R~ M\ R5
O ~CH2)n-Y
N~N
RHN ~ ,~l H
IO OH
4
CA 02269720 1999-04-23
wherein each symbol is as defined above (hereinafter to be also referred to as
compound (II)), which is useful for the synthesis of compound (I). In
addition, the
present invention relates to a compound of the formula (~~XVI)
Rs
R~ M~ R5
O ~CH2)n-Y
(
N~N H
RHN ~ H
O O
wherein each symbol is as defined above (hereinafter to be also referred to as
compound (XXVI)), which is useful for the synthesis of compound (I) and which
lias superior chymase inhibitory activity, and a pharmacologically acceptable
salt
thereof.
The present invention moreover relates to a pharmaceutical composition
containing compound (I), compound (XX~II) or a pharmacologically acceptable
salt
thereof, and a pharmacologically acceptable carrier, a pharmaceutical use
thereof,
particularly, to a chymase inhibitor.
The symbols used in the present specification are explained in the
following.
Alkyl at R, Rl, Rl', Rz - Rx~, Rx~' and Rlz preferably has 1 to 6 carbon atoms
and may be linear or branched. Examples thereof include methyl, ethyl, n-
propyl,
isopropyl, n-butyl, isobutyl, sec-butyl, tent-butyl, n-pentyl, isopentyl, n-
hexyl and
the like.
- C.~cloalkyl at Rx, Rl', Rl~, Rx~', Rxz ~d Y preferably has 3 to '~ carbon
atoms
and is exemplified by cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
cycloheptyl
and the like.
G~cloalkylalkyl at R', Rl', Rl~, Rlo' and Rxz has the same cycloalkyl moiety
as mentioned above and the alkyl moiety which preferably has 1 to 3 carbon
atoms and which may be linear or branched. Examples thereof include
cyclopropylmethyl, 2-cyclobutylethyl, 3-cyclopentylpropyl, cyclohexylmethyl, 2-
cyclohexylethyl, cycloheptylmethyl and the like.
Aryl at Rx, Rl', R5 - Rx~, Rlo' and y is preferably phenyl, naphthyl or an
ortho-fused bicyclic group having 8 to 10 cyclic atoms wherein at least one
ring is
an aromatic ring (e.g., indenyl and the like), and the like.
Arylalkyl at Rx, Rx', Rz - Rl~ and Rx~' has the same aryl moiety as mentioned
CA 02269720 1999-04-23
above and the alkyl moiety which preferably has 1 to 3 carbon atoms and which
may be linear or branched. Examples thereof include benzyl, phenethyl, 3-
phenylpropyl, 1-naphthylinethyl, 2-naphthylmethyl, 2-( 1-naphthyl)ethyl, 2-(2-
naphthyl)ethyl, 3-( 1-naphthyl)propyl, 3-(2-naphthyl)propyl and the like.
Arylalkenyl at R5 - R' has the same aryl moiety as mentioned above and
the alkenyl moiety which preferably has 2 to 6 carbon atoms and which may be
linear or branched. Examples thereof include styryl, 3-phenyl-2-propenyl, 4-
phenyl-3-butenyl, 5-phenyl-4-pentenyl, 6-phenyl-5-hexenyl, 3-(1-naphthyl)-2-
propenyl, 4-(2-naphthyl)-3-butenyl and the like.
Heteroaryl at Rl, Rl', R5 - Rl~, Rl~' and Y is preferably a 5 or 6-membered
cyclic group having a carbon atom and 1 to 4 hetero atoril(s) (oxygen atom,
sulfur
atom and nitrogen atom), an ortho-fused bicyclic hetero aryl having 8 to 10
cyclic
atoms derived threrefrom, which is particularly exemplified by benzo
derivatives
and derivatives obtained by fusing propenylene, trimethylene or tetramethylene
therewith, a stable N-oxide thereof and the like. Examples thereof include
pyrnolyl, furyl, thienyl, oxazolyl, isoxawlyl, imidazolyl, thiazolyl,
isothiawlyl,
pyrazolyl, triazolyl, tethazolyl, 1,3,5-oxadiazolyl, 1,2,4-oxadiazolyl, 1,2,4-
thiadiazolyl, pyridyl, pyrranyl, pyrazinyl, pyrimidinyl, pyridazinyl, 1,2,4-
triazinyl,
1,2,3-triazinyl, 1,3,5-triazinyl, benzoxazolyl, ben2othiazolyl,
benzoimidazolyl,
thianaphtenyl, isothianaphtenyl, benzofuranyl, isobenzofuranyl, chromenyl,
isoindolyl, indolyl, indawlyl, isoquinolyl, quinolyl, phthalazinyl,
quinoxalinyl,
quinazolinyl, cinnolinyl, benzoxazinyl and the like.
Heteroarylalkyl at Rl, Rl', R5 - Rl~ and Rl~' has the same heteroaryl moiety
as mentioned above and the alkyl moiety which preferably has 1 to 3 carbon
atoms and which may be linear or branched. Examples thereof include 2-
pyrrolylmethyl, 2-pyridylmethyl, 3-pyridylinethyl, 4-pyridylinethyl, 2-
thienylinethyl, 2-(2-pyridyl)ethyl, 2-(3-pyridyl)ethyl, 2-(4-pyrinyl)ethyl, 3-
(2-
pyrrolyl)propyl and the like.
Heteroarylalkenyl at RS - R' has the same heteroaryl moiety as mentioned
above and the alkenyl moiety which preferably has 2 to 6 carbon atoms and
which
may be linear or branched. Examples thereof include 2-(2-pyridyl)ethenyl, 3-(2-
pyridyl)-2-propenyl, 4-(3-pyridyl)-3-butenyl, 5-(2-pyrrolyl)-4-pentenyl, 6-(2-
thienyl)-5-hexenyl and the like.
Heterocycle represented by Rl and Rl' is a 4 to 6-membered ring having a
6
CA 02269720 1999-04-23
carbon atom and 1 to 4 hetero atoms) (oxygen atom, sulfur atom and nitrogen
atom) . Examples thereof include azetidinyl, pyrrolidinyl, piperidinyl,
piperidino,
piperazinyl, morpholinyl, morpholino, thiomorpholinyl, oxothiomorpholinyl,
dioxothiomorpholinyl, tetrahydropyranyl, dioxacyclohexyl and the like.
Heterocycle represented by -NR3R4 and -NRl~Rl~' is a 4 to 6-membered
ring having a carbon atom, at least one nitrogen atom and optionally other
hetero
atom (oxygen atom or sulfur atom). Examples thereof include azetidinyl,
pyrrolidinyl, piperidino, piperazinyl, morpholino, thiomorpholino,
oxothiomorpholino, dioxothiomorpholino and the like.
Heterocyclic alkyl at Rl and Rl' has the same heterocyclic moiety as
mentioned above for Rl and Rl', and linear or branched alkyl moiety preferably
having 1 to 3 carbon atoms. Examples thereof include azetidinylethyl,
pyrrolidinylpropyl, piperidinylmethyl, piperidinoethyl, piperazinylethyl,
morpholinylpropyl, morpholinomethyl, thiomorpholinylethyl,
oxothiomorpholinylethyl, dioxothiomorpholinylethyl, tetrahydropyranylpropyl,
dioxacyclohexylmethyl and the like.
Halogen at R$ and R9 is exemplifeid by fluorine, chlorine, bromine and
iodine.
Of the above-mentioned substituents, alkyl, cycloalkyl, cycloalkylalkyl,
aryl, arylalkyl, arylalkenyl, heteroaryl, heteroarylalkyl, heteroarylalkenyl,
heterocycle and heterocyclic alkyl are optionally substituted by one or more
substituents shown below.
The substituents for these substituents are exemplified by halogen,
hydroxyl group, nitro, cyano, trifluoromethyl, alkyl, alkoxy, alkylthio,
formyl,
acyloxy, oxo, phenyl, arylalkyl, -COORa, -CH2COORa, -OCH2COORa,
-CONRbRc, -CH2CONRbRc, -OCHZCONRbRc, -COO(CHZ)2NReRf, -S02T1,
-CONRdSOZTI, -NReRf, -NRgCHO, -NRgCOT2, -NRgCOOT2,
-NRhCQNRiRj, -NRkS02T3, -SOzNRIRm, -S02NRnCOT4 and the like.
Of the exemplified substituents for the above-mentioned substituents,
halogen, alkyl and arylalkyl may be those mentioned above. Alkoxy preferably
has 1 to 6 carbon atoms and may be linear or branched. Examples thereof
include methoxy, ethoxy, propoxy, butoxy, pentyloxy, hexyloxy and the like.
All~ylthio preferably has 1 to 6 carbon atoms and may be linear or branched.
Examples thereof include methylthio, ethylthio, propylthio, butylthio,
pentylthio,
7
CA 02269720 1999-04-23
hexylthio and the like. Acyloxy preferably has 1 to 6 carbon atoms and may be
linear or branched. F~xamples thereof include formyloxy, acetyloxy,
propionyloxy,
butyryloxy, valeryloxy, pivaloyloxy, hexanoyloxy and the like.
Ra - Rn show hydrogen atom, alkyl (same as above) or arylalkyl (same as
above). -NRbRc ,-NReRf ,-NRiRj and -NRlRm in combination may form a
heterocycle (same as those exemplified for the above-mentioned -NR3R4 and
-NRl~Rl~', which is optionally substituted by the above-mentioned
substituents).
-NRe Rf can also show a heterocycle having =O (e.g., 2-pyrlrolidinon-1-yl,
succinimide, oxazolidin-2-on-3 yl, 2-benzoxazolinon-3-yl, phthalimide, cis-
hexahydrophthalimide and the like). Tl - T4 are the same as those exemplified
above for Rl and are optionally substituted by the above-mentioned
substituents.
Q is =O or =S.
The compound (I),compound (II) and compound (XXVI) can exist as
optically active compounds or racemates due to the asymmetric carbon atom
bound with -(CH,jn-Y group. The racemates can be resolved into each
optically active compound by a method known per se. When these compounds
have additional asymmetric carbon atom, the compound can exist as a mixture of
diastereomers or a single diastereomer, which can be also resolved into each
optically active compound by a method known per se.
The compound (I), compound (11) and compound (~~XVi) can show
polymorphism and exist as two or more tautomers or solvates (e.g., ketone
solvate,
hydrate and the like) .
Therefore, the present invention encompasses any of the above-mentioned
stereoisomers, optical isomers, polymorphs, tautomers, solvates and optional
mixtures thereof, and the like.
When the compound (I) and compound (XXVI) are acidic compounds, the
pharmacologically acceptable salts thereof include, for example, alkali metal
salt
(e.g., salt with lithium, sodium, potassium and the like), alkaline earth
metal salt
(e.g., salt with calcium, magnesium and the like), aluminum salt, ammonium
salt,
salt with organic base (e.g., triethylamine, morpholine, piperidine,
triethanolamine
and the like) and the like.
When the compound (I) and compound (~~XVI) are basic compounds, the
pharmacologically acceptable salts thereof include inorganic acid addition
salt (e.g.,
salt with hydrochloric acid, hydrobromic acid, hydriodic acid, sulfuric acid,
8
CA 02269720 1999-04-23
phosphoric acid and the like), organic acid addition salt (e.g.,
methanesulfonic acid,
benzenesulfonic acid, p-toluenesulfonic acid, formic acid, acetic acid,
trifluoroacetic acid, oxalic acid, citric acid, malonic acid, fumaric acid,
glutaric acid,
adipic acid, maleinic acid, tartaric acid, succinic acid, mandelic acid, malic
acid
and the like), salt with amino acid (e.g., salt with g~utamic acid, aspartic
acid and
the like) and the like.
Of the compounds (I) of the present invention, preferred are a compound
of the formula (I) wherein Y is optionally substituted aryl ; compound of the
formula
(I) wherein Z is a group of the formula (i) ; a compound of the formula (I)
wherein
one of R5, R6 and R' is optionally substituted aryl and the rest are hydrogen
atom,
provided that when M is a nitrogen atom, R5 is void, and the like.
More preferred are the compounds of the following Examples 2, 4, 5, 8, 14,
15, 17, 19, 23, 26, 28, 30, 31, 32, 34, 36, 38, 40, 45, 46, 50, 52, 53, 54,
55, 56, 57,
58, 59, 60, 62, 64, 71, 72, 73, 74, 76, 78, 80, 82, 84, 86, 88, 90, 92, 93, 94
and the
like.
The production method of the inventive compound (I) is shown in the
following Scheme I.
9
CA 02269720 1999-04-23
(C~n Y
R6
H2N~Z R~ '
(CH~n~
cb2HN N
O H OH
CBI) ~ t~)
(CH~~Y
H2N~Z
A' oEI" I (R ~ cbz)
R~ ~
Rs
tC~_Y I (R ~ ~
cbzNN N Z
O H OR''
CIY) I~$H)
Rb
R~ ( R,~ .
o tC~J~ Y
H2N ~N~Z
O H'~ OrR'~
CV)
R~ i Rs
0 (CH~"Y
N ---~ B ._,~. I _
RHN
O ~ OR''
(VI : R ~ H)
l0
CA 02269720 1999-04-23
wherein R11 is a hydroxy protecting group, cbz is benzyloxycarbonyl and other
symbols are as defined above.
The hydroxy-protecting group at R11 is, for example, acetyl, trimethylsilyl,
tert-butyl dimethylsilyl, triisopropylsilyl, tent-butyl diphenylsilyl and the
like.
As shown in the above scheme I, compound (III) is first condensed with
amine A to give compound (VII), or compound (III) is first condensed with
amine A'
to give compound (I~.
The compound (III) is described in publications (Japanese Patent
Unexamined Publication Nos. 56785/ 1994 and 286946/ 1993, Warner et al., J.
Med. Chem. 1994, 37, p 3090, Damewood et ai., J. Med. Chem. 1994, 37, p 3303,
Veale et al., J. Med. Chem. 1995, 38, p 98, W093/21210 and the like). It can
be
prepared by a conventional method based on these publications. The methods
for prepareing amine A and amine A' are described later.
Preferable condensing agent used for this condensation to activate the
carboxylic acid of compound (III) is exemplified by dicyclohexylcarbodiimide
(DCC)/hydroxybenztrzazole (HOBT), N-(3-dimethylarninopropyl)-N'-
ethylcarbodiimide (WSCI) or hydrochloride thereof/HOBT, WSCI or hydrochloride
thereof/4-dimethylaminopyridine (DMAP), 2-ethoxy-1-ethoxycarbonyl- I,2-
dihydroquinoline (EEDQ), carbonyldiimidazol (CDIJ / HOBT, diethylphosphoryl
cyanide and the like.
This reaction is generally carried out in an inert solvent, wherein the inert
solvent to be used may be any as long as it is aprotic. Preferred are
acetonitrile,
dichloromethane, chloroform, N,N-dimethylforlnamide and the like. This
condensation is carried out at a temperature of -30 - 80~C, preferably 0 -
25~C.
The hydroxyl group of the obtained compound (VII) may be protected to
give compound (I~. Conversely, the hydroxy-protecting group (R11) of compound
(I~ may be eliminated to give compound (VII).
The benzyloxycarbonyl group of compound (I~ can be removed by a
conventional method such as hydrogenation decomposition and the like to
convert
the compound to compound (V).
The amino group bound to the carbon atom on the heterocyclic ring
(pyridone ring or pyrimidone ring) of compound (~ can be acylated or
sulfonylated
by a conventional method to give compound (VI) wherein R is a substituent
other
than a hydrogen atom.
11
CA 02269720 1999-04-23
The compound (VI) wherein R is -CHO, -CONH2, -COR1, -COOR1,
-CONHORI, -CONHRI, -CONR1R1', -CONHSOZRI, -COSRI, -COCOR2,
-COCOORZ, -CONHCOORz or -COCONR3R4 can be synthesized using an
activated carboxylic derivative such as acid halide, or using carboxylic acid
and a
coupling agent, or other method.
When compound (VI) wherein R is -CONHZ, -CONHRi, -CONHS02R1
or -CONHCOOR2 is synthesized, a method using isocyanate and the like can be
used. For example, a method using carbonyldiimidazole, phosgene, diphosgen
(trichloromethylchloroformate), triphosgene [bis(trichloromethyl)carbonate)
and
the like together with alcohol of the formula R10H, thiol of the formula R1SH
or
amine of the formula R1NH2, (Rl)2NH, R10NH2, and a base such as triethylamine
and the like, is employed.
When a compound ('VI) wherein R is -CSXRI is synthesized, a method
using an activated thiocarboxylic derivative (e.g., thioylchloride, lower
alkyl ester of
dithioic acid and the like, and the like), a method using thioic acid and a
coupling
agent, and the like are employed. In addition, for example, a method using
dimethyl trithiocarbonate and the like together with alcohol of the formula
R10H,
thiol of the formula R1SH or amine of the formula R1NHZ and the like, is
employed.
When a compound (Vn wherein X is -NH- is synthesized, a method using
isothiocyanate and the like can be employed.
When a compound (VI) wherein R is -SOZWRI, -SOZNR1R1' or -SOZE is
synthesized, the following method and the like is suitably used for
sulfonylation.
For example, a method using sulfonic acid of the formula HO-SOZWRI,
HO-SOZNR1R1' or HO-S02E, or the corresponding halogenated acid, particularly
sulfonyl (or sulfamoyl) chloride of the formula Cl-SOZWRI, Cl-SOZNR1R1' or
Cl-S02E and an organic base (e.g., triethylamine, pyridine and the like) or
inorganic base (e.g., sodium carbonate, potassium carbonate and the like) in
an
inert solvent (e.g., dichloromethane, tetrahydrofuran, toluene and the like),
can be
employed.
When compound (VI) has a group of the formula -COORa (carboxyl)
wherein Ra is hydrogen atom as the substituent for each substituent at R or Z,
for
example, the corresponding ester synthesized using an acid-protecting group
that
can be preferably removed [compound (VI) having -COORa wherein Ra is not
hydrogen atom as the substituent for the substituent) is decomposed to gave
this
12
CA 02269720 1999-04-23
compound. This decomposition is conducted by an optional method from
various methods well Down in organic chemistry, such as basic hydrolysis
using,
for example, lithium hydroxide, sodium hydroxide and the like, or
hydrogenation
decomposition of benzyl ester and the like.
When compound (VI) has a gxoup of the formula -COORa, -CONRbRc,
-COO(CH~2NReRf or -CONRdS02T1 as the substituent for each substituent at
R or Z, for example, compound of the formula HORa, HNRbRc, HO(CH2)ZNReRf or
HNRdSOZTI (Ra - Rf are not hydrogen atom) and compound (Vn having a group of
the formula -COORa (carboxyl), wherein Ra is hydrogen atom, as the substituent
of the substituent, or an activated derivative thereof, are reacted to give
this
compound.
When compound (VI) has a group of the formula -OCH2COORa or
-OCH2CONRbRc as the substituent for each substituent at R5 - R', R or Z, for
example, a compound of the formula BrCH2COORa, ICH2COORa,
BrCH2CONRbRc or ICH2CONRbRc (Ra - Rc are not hydrogen atom) and
compound (V17 having hydroxyl group as the substituent of the substituent, are
reacted in the presence of a base such as sodium hydride and the like to give
this
compound.
When the compound (VI) has a group of the formula -NRgCOT2,
-NRgCOOT2, -NRhCQNRiRj, -NRkSO2T3 or acyloxy as the substituent for each
substituent at R5 - R', R or Z, for example, the corresponding compound (VI)
having hydroxyl group, or amino group of the formula -NHRg, -NHRh or
-NHRk as the substituent of the substituent is reacted with an activated
derivative of an acid, which is represented by the formula HOCOT2, HOCOOTz,
HOCQNRiRj, HOSOZT3 and the like, to give this compound.
When compound (VI) contains heteroaryl N-oxide in R5 - R', R or Z, the
corresponding compound (VI) containing heteroaryl in the group at R5 - R', R
or Z
is oxidized with a conventional oxidizing agent such as dioxirane and the like
in
acetone to give this compound.
While conversion of the substituent of each substituent at R, Z and the
like, and the like has been explained by referring to the case of compound
(VI), it
does not mean that such conversion and the like are possible only with regard
to
compound (VI), but are possible with regard to various other compounds as long
as it does not affect other functional gxoups contained in the chemical
structure.
13
CA 02269720 1999-04-23
For example, when the substituent of each substituent at R, Z and the like is
amino, hydroxyl group and the like, conversion in the form of compound (I)
rather
than compound (VI) is preferable.
The hydroxy-protecting group (R11) of compound (~ and compound (VI) is
removed to give compound (II). This compound (II) is useful as the synthetic
intermediate of compound (I).
This hydroxy-protecting group is removed in an inert solvent such as
tetrahydrofuran and the like, a sing tetrabutyl ammonium fluoride and the
like,
wherein the a se of acid such as acetic acid and the like for buffering the
reaction
mixture is preferable.
Then, the hydroxyl group of compound (II) is oxidized to give compound (I) .
The oxidation is preferably carried out, for example, by a method using an
excess of dimethyl sulfoxide, water soluble carbodiimide and dichloroacetic
acid as
a catalyst in an inert solvent such as toluene and the like at approximately
room
temperature. Useful other methods include a method wherein an aqueous alkali
solution of potassium permanganate is used ; a method wherein oxalyl chloride,
dimethyl sulfoxide and tertiary amine are used ; a method wherein acetic
anhydride and dimethyl sulfoxide are used ; a method wherein pyridine-sulfur
trioxide complex and dimethyl sulfoxide are used ; a method wherein a
chromium(VI) oxide - pyridine complex is used in methylene chloride ; a method
wherein a hypervalent iodine reagent such a.s periodinane (e.g., 1,1,1-
triacetoxy-
l,l-dihydro-1,2-benziodoxol-3(1H)-one and the like) is used in dichloromethane
or
dimethylformamide ; and the like.
In the compound (VII) obtained by condensation of compound (III) and
amine A or eliminating hydroxy-protecting group of compound (I~, the hydroxyl
group is oxidized according to the above-mentioned method to give a compound
(I)
having amino protected by benzyloxycarbonyl.
Then, benzyloxycarbonyl of this compound is deprotected under mild
reaction conditions to give compound (I) wherein R is hydrogen atom. For
example, acid decomposition by reaction with trifluoromethanesulfonic acid or
triffuoroacetic acid in the presence of anisole or thioanisole, or
hydrogenation
decomposition using palladium carbon and the like as a catalyst, is applied
for
deprotection.
When the above-mentioned acylation reaction and the like is additionally
14
CA 02269720 1999-04-23
applied, compound (I) wherein R is other than hydrogen atom can be obtained.
Scheme II shows a different method for producing compound (I~. This
method is applied solely when M is a carbon atom.
R~
R~ MYR.s
NH
c~zHN
O
CVIIi~
cc~,-Y
ORu
trv)
wherein each symbol is as defined above.
As shown in the above Scheme II, compound (IV) can be obtained by
reacting compound (VIII) (compound disclosed in publication (see Japanese
Patent
Unexamined Publication No. 56785/ 1994, Warner et al., J. Med. Chem. 1994, 37,
p 3090, Damewood et al., J. Med. Chem. 1994, 37, p 3303) or a compound
obtained by a conventional method based on these publications] and compound B.
The method for producing compound B is stated later.
As disclosed in, for example, Japanese Patent Unexamined Publication No.
5678S/ 1994 and J. Med. Chem. 1994, 37, p 3303, this reaction is carried out
by
treating compound (VIII) with a base such as sodium hydride, potassium hydride
and the like in an inert solvent such as aprotic solvent, particularly N,N-
dimethylformamide, tetrahydrofuran and the like at -30 - 80~C, preferably 0 -
30~C,
and reacting the obtained compound with compound B at -30 - 80~C, preferably 0
- 30~C. The thus obtained compound (IVY is introduced into compound (I)
according to the method discussed under Scheme I.
Scheme III shows methods of synthesizing amine A and amine A' using
w CA 02269720 1999-04-23
cyanohydrin compound (XIII) as a key intermediate.
Scheme III
(~ 2)n: Y (~ ~"-Y (C~)" Y
~- --~- ~OH
RpHN C02H RPHN C02Rq RPHN
(IX) (X) lXl)
(CH~"Y
RpHN~CHO
( Xlt )
. 1
(~2)~ Y . (CH2)~ Y
RpHN~CN -~ RpHN~CN
OH ORi ~
( X111 ) ( XVf )
1 1
(CHI"-Y (CHI"-Y
H
RPHN ~ORr RPHN NH2
OH OR"
( XiV ) ( XW )
1 1
( H~~ Y (Chh) ~ Y
RPHN Z ~ RPHN~Z
OH OR' ~
( XV ) ( XVIII )
1 1
A A'
16
CA 02269720 1999-04-23
wherein Rp is amino-protecting group, Rq and Rr are each alkyl and other
symbols are as defined above.
The amino-protecting group at Rp is exemplified by benzyloxycarbonyl
(cbz), tert-butoxycarbonyl (BOC) and the like.
Alkyl at Rq and Rr has 1 to 6 carbon atoms and is exemplified by those
mentioned above.
First, compound (IX) is esterified to give compound (~.
The esterification is carried out by reacting compound (IX) with alkyl
halide corresponding to Rq in the presence of a base such as potassium
hydrogencarbonate and the like, or reacting compound (I~ with diazoalkane, and
the like.
While the a-amino acid, wherein amino group is protected, shown by the
formula (I~, is mostly marketed, but those that are not can be synthesized by
obtaining an amino acid from aldehyde Y-(CHZ)nCHO by a method for Strecker
synthesis or other well known method and protecting amino group of the
resultant
amino acid.
Then, by reducing compound (X) using, for example, aluminum
diisobutylhydride, compound (XII) can be obtained easily. Alternatively, as
shown in the report of Fehrentz et al. (Synthesis, l983, p 676), compound (IX)
may be condensed with N,O-dimethylhydroxylamine to give amide derivative, and
this derivative is reduced with lithium aluminum hydride to give compound
(XII) .
As a different method, compound (~ may be reduced with, for example,
sodium borohydride/ lithium chloride to give compound (XI), and compound (XI)
is
oxidized by the oxidation method discussed for conversion of compound (II) to
compound (I) to ultimately give compound (XII).
Then, compound (?~I) is treated with cyanide salt, preferably potassium
cyanide or sodium cyanide, in the presence of auxiliary solvent such as
tetrahydrofuran, ethyl acetate and dioxane, in an aqueous solution to give
compound (X1II) . Alternativelly, the compound (XII) may be treated with
acetone
cyanohydrin to give compound (XIII) . It is also possible to obtain compound
(XIII)
by the use of trimethylsilyl cyanide. In this case, subsequent treatment with
an
acid removes trimethylsilyl.
When compound (XIII) is treated with hydrogen chloride in the presence of
alcohol represented by Rr OH (e.g., methanol, ethanol, propanol, butanol and
the
17
CA 02269720 1999-04-23
like), hydrochloride salt of imidate compound (XI~ can be obtained. Generally,
compound (XI~ is not isolated before proceeding to the next step, but the
isolation is possible on demand. Method for synthesizing and handling imidate
compound and the like are explained in the book of 1'atai ("The Chemistry of
Amidines and Imidates", Wiley-Interscience, 1975) and publication of Nielson
et al.
CChem. Rev. 1961, 61, p 1979).
Then, based on the report of Edwards et al. (J. Med. Chem. 1995, 38, p
76J, Tsutsumi et al. (J. Med. Chem. 1994, 37, p 3492) or Costanzo et al. (J.
Med.
Chem. 1996, 39, p 3039), compound (X~ having a hetero ring represented by Z
can be obtained from compound (XIV).
Amine A wherein Z is thiazole can be produced by a different method
mentioned below using thioamide.
First, the hydroxyl group of compound (XIII) is protected by a hydroxy-
protecting group (R11) to give compound (XVI).
This compound (XVI) is treated with hydrogen sulfide by a conventional
method in the presence of a basic catalyst (e.g., triethylamine and the like)
as
necessary to give compound (XVII).
Then, based on the report of Schmidt et al. (Synthesis, l986, p 992) or
Wiley et al. (Org. Reactions 1957, 6, p 367), compound (XVIII) wherein Z is
thiazole
can be obtained from compound (XVII).
The hydroxyl group of the compound (X~ obtained above may be
protected with a hydroxy-protecting group (R11) to convert the compound to
compound (XVIII) . Conversely, the hydroxy-protecting group (R11) of compound
(XVIII) may be eliminated to give compound (X~.
Lastly, the amino-protecting group of compound (X~ is removed to give
amine A and the amino-protecting group of compound (XVIII) is removed to give
amine A'.
Scheme IV shows methods of synthesizing amine A by reacting
compound (XII) using heterocyclic reagent G-Z previously formed.
~C~i2~'Y G-Z ~~e'Y
RPHN~CHO ~ RpHN~Z ------~- A
CXII) t~~H
18
CA 02269720 1999-04-23
wherein G is a group imparting nucleophilicity to the 2-position carbon atom
of the
group represented by Z (e.g., lithium, trimethylsilyl and the like) and other
symbols
are as defined above.
As is shown in the report of Tsutsumi et al. (J. Med. Chem. 1994, 37, p
3492), the heterocyclic reagent G-Z, wherein G is lithium, is directly reacted
with
compound (XII) to give compound (X~.
The above-mentioned reagent G-Z, wherein G is lithium, can be
produced and used as described in, for example, Wasserman et al. (Tetrahedron
Lett. 1981, 22, p 1737), Schroeder et al. (Liebigs Ann. Chem. 1975, p 533),
Beraud
et al. (Bull. Soc. Chem. France 1962, p 2072j, Shirlet et ~al. (J. Am: Chem.
Soc.
1957, 79, p 4922), Ogura et al. (J. Org. Chem. 1974, 39, p 1374) or Jason et
al.
CChem. Ber. 1973, 106, p 594 and p 2815).
As shown in the report of Dondoni et al. (J. Org. Chem. 1988, 53, p 1748),
Edwards et al. (J. Med. Chem. 1995, 38, p 76), Tsutsumi et al. (J. Med. Chem.
1994, 37, p 3492) or Costanzo et al. (J. Med. Chem. 1996, 39, p 3039), the
heterocyclic reagent G-Z, wherein G is trimethylsilyl, is first reacted with
compound (XII) to introduce the compound into O-trimethylsilyl derivative of
the
corresponding alcohol.
Then, by a conventional method, trimethylsilyl is removed to give
compound (X~ . Preferably, the reaction proceeds in an inert solvent such as
tetrahydrofuran, dichloromethane and the like in the presence or absence of
cesium fluoride or tetrabutyl ammonium fluoride at 0 - 60~C.
As shown in the report of, for example, Dondi et al. (Tetrahedron Lett.
198S, 26, p 5477, J. Chem. Soc. Chem. Commun. 1984, p 258J, Medici et al.
(Tetrahedron Lett. 1983, 24, p 2901) or Edwards et al. (J. Med. Chem. 1995,
38, p
76), the above-mentioned reagent G-Z, wherein G is trimethylsilyl, can be
produced and used in a manner similar to the case with 2-
trimethylsilyloxazole,
2-trimethylsilylbenzothioazole and 2-trimethylsilylthiazole.
Then, the amino-protecting group of compound (XV) is removed to give
amine A.
Scheme V shows methods of synthesizing amine A using compound (XiX)
having N-methoxy-N-methylamido group and heterocyclic reagent G-Z via
heterocyclic ketone.
19
CA 02269720 1999-04-23
Scheme V
(~~n'Y
(C~ri Y ~ ~ G-Z (CH 2)~ Y
~CO H Rpt~'I OMe
2 O Rah
C IX ) ( Xl7C ) O
( X7C )
1
(~ 2)n'Y (CH~Jn-Y
~Z ~Z
H2N
OH O
( XY ) ( )0(1 )
~llt )
i (R = cbz)
wherein each symbol is as defined above.
As shown in the report of Weinreb et al. (Tetrahedron Lett. 1981, 22, p
3815) and Dufore et al. (J. Chem. Soc. Perkin Trans. I 1986, p 1895), compound
(IX) is condensed with N,O-dimethylhydroxylamine to give compound (XIX~, and
the compound is reacted with the heterocyclic reagent G-Z, wherein G is
lithium,
to give compound (X~. The heterocyclic reagent G-Z, wherein G is lithium, can
be produced as mentioned above.
Then, compound (XX) is reduced with, for example, sodium borohydride
and the like to give compound (XV) with ease and the amino-protecting group is
removed to give amine A.
The compound (XXI) obtained by removing the amino-protecting group of
coumpound (XX) is condensed with compound (III) by the method described with
respect to the condensation using amine A under Scheme I to directly give
compound (I) wherein R is benzyloxycarbonyl.
While amine A can be obtained as mentioned above, the hydroxyl group
of amine A is protected with hydroxy-protecting group (R11) to give amine A'.
It is
preferable that hydroxy-protecting group be introduced while amino is
protected
with protecting group Rp, and the amino-protecting group be removed
thereafter.
Scheme VI shows methods of synthesizing compound B.
CA 02269720 1999-04-23
(C~n-Y p (~2)n'Y 0 (CH~n-Y
H2N Z -._,~. Ct ~N Z - > t~ ~ Z
H N
OH OH H Ru
(XXII)
(XXIII)
wherein each symbol is as defined above.
For example, as shown in the report of Damewood et al. (J. Med. Chem.
1994, 37, p 3303), amine A is first reacted with chloroacetyl chloride in an
inert
solvent such as tetrahydrofuran and the like in the presence of an organic
base
such as N-methylmorpholine and the like at -20 - 60~C, preferably 0 - 30~C, to
give
compound (XXIt).
The hydroxyl group of this compound (XXII) is protected with the
aforementioned protecting group (R11), preferably silyl, such as tert-
butyldimethylsilyl and the like, to give compound (XXIII).
Then, in an inert solvent such as acetone, compound ~ is reacted
with sodium iodide or potassium iodide at -20 - 60~C, preferably at 0 - 30~C,
to give
a desired compound B.
Scheme VII shows a different method of synthesizing compound (VII).
21
CA 02269720 1999-04-23
(CH~~-Y
R
R~ (~ Rs H2N ~Oli~ R7 Rg Rs
C X11 ) ") ~~~ O (CHI"-Y
cbzHN vCO~H cbzHN ~ N~OH
H
C ili ) O( 7t7CV )
~ (R~cbz)
Rg
R~ -'I, Rs
O (CH?J~-Y
cbzHN ~N~CtiO
O H
( 7CX111' )
Rg
R7 M ~O (~n Y
( "" ) -- ~~ ~
cbzHN ~H~CN
O Oti
( XXW ).
wherein each symbol is as defined above.
The compound (III) is condensed with compound (~ by the method
described with respect to the condensation with amine A under Scheme I to
convert the compound to compound (). This compound is oxidized by the
method described with respect to the conversion of compound (II) to compound
(I)
to give compound (XXVI' ).
While the alcohol compound of the formula (XXIV) may be marketed,
those that are not can be obtained by removing the amino-protecting group of
compound (XI).
Then, compound (XXVI' ) is subjected to cyanohydrination reaction by the
method described with respect to Scheme III, i.e., the method for conversion
of
compound (XII) to compound (XIn), to give compound (XXVII) . After converting
nitrite to imidate or thioamide, the heterocycle is formed by the
aforementioned
method to give compound (VII).
It is also possible to directly synthesize compound (VII) by the method
22
CA 02269720 1999-04-23
described with respect to Scheme IV and using compound (XXVI' ).
The inventive compound (~~XVI) is obtained as compound (XXVI' ) when R
is benzyloxycarbonyl. When R is not benzyloxycarbonyl, it can be obtained from
compound (XX~ or (XXVI' ).
The compound (XXVI) is derived from compound (XXVj by protecting the
hydroxyl group of compound (XXV) with a protecting group (R11) and following
the
method described with respect to the conversion of compound (I~ to compound
(I)
under Scheme I. ~l~lhen it is derived from compound (XXVI' ), aldehyde group
of
compound (~~XVI' ) is protected as acetal using methanol, ethylene glycol and
the
like, the compound is subjected to the method described with respect to the
conversion of compound (I~ to compound (VI) under Scheme I, and acetal of the
aldehyde gxoup is deprotected.
Scheme VIII shows method of directly synthesizing compound (I) via
compound (XXXI) having N-methoxy-N-methylamido group.
(~ 2)n-Y
t Rs H2N ~2Ra p7 i~ Rs
C XXVnI ) ~ Or (CH~n-Y
cbzH vC~H cbzHN ~H~CO2Rq
O 0(7007t)
(m)
R' M R5 C -Y
~n
cbzHN H~C~H
O
(XXX)
a
R' M R' (CH2)n-Y
Me
cbzHN - _v -N OMe
O H O
~~)
G-2
~ (R = c~z)
23
CA 02269720 1999-04-23
wherein each symbol is as defined above.
The compound (III) is condensed with compound (XXVIII) by the method
described with respect to the condensation with amine A under Scheme I to
convert the compound to compound (XXI~. Ester of this compound is
hydrolyzed or subjected to suitable deprotection to give compound (~~. Using
this compound according to the method described under Scheme V, compound (I)
wherein R is benzyloxycarbonyl via compound (XXXI) can be obtained.
While the ester of the formula (XXVIII) may be marketed, those that are
not can be obtained by removing the amino-protecting group of compound (X).
The compound (I), compound (II) and compound (XXVI) of the present
invention thus obtained can be subjected to known separation and purification
methods, such as concentration, extraction, chromatography, reprecipitation,
recrystallization and the like, to give a compound having an optional purity.
The pharmacologically acceptable salts of this compound (I) and
compound (XXVI) can be produced by a known method. Farther, various
isomers of these compounds and the like can be produced by a known method.
The compound (I) and compound (XXVI) and pharmacologically
acceptable salts thereof of the present invention have superior inhibitory
action on
chyrnase group in mammals such as human, dog, cat and the like.
Thus, the compound (I), compound (XXVI) and pharmacologically
acceptable salts thereof of the present invention are useful as inhibitors of
chymase group, inclusive of human heart chymase, and useful for the
prophylaxis
and treatment of various diseases caused by chymase, inclusive of the diseases
considered to be caused by angiotensin II, such as hypertension, hypercardia,
myocardial infarction, arteriosclerosis, diabetic and non-diabetic
retinopathy,
vascular restenosis after PTCA and the like. In addition, they show superior
characteristics in absorption, safety, stability in blood and the like.
When the compound (I), compound (XXVI) and pharmacologically
acceptable salts thereof of the present invention are used as pharmaceutical
products, a pharmacologically acceptable carrier and the like are used to give
a
pharmaceutical composition in the form of granule, tablet, capsule, injection,
ointment, cream, aerosol and the like, which is administered orally or
parenterally.
The above-mentioned preparation contains compound (I), compound (XXVI) or a
pharmacologically acceptable salt thereof in an effective amount.
24
CA 02269720 1999-04-23
The dose of the compound (I), compound (~~XVI) and a pharmacologically
acceptable salt thereof varies depending on the administration route,
condition,
body weight, age and the like of patients, and is appropriately set according
to the
administration object. In general terms, for oral administration to an adult,
the
dose of 0.01- 1000 mg/kg body weight/day, preferably 0.05 - 500 mg/kg body
weight/day, which is given in a single dose or several doses a day.
The present invention is explained in detail in the following by way of
Reference Examples and Examples, to which the present invention is not
limited.
1H -NMR was determined at 300 or 500 MHz. The chemical shift of 1H -
NMR was based on tetramethylsilane (TMS) as an internal standard and relative
delta ( ~ ) value was shown in parts per million (ppm) . The coupling constant
was
shown using s (sing~et), d (doublet), t (triplet), q (quartet), m (multiplet),
dd (doublet
of doublet), brs (broad sing~et), ABq (AB quartet) and the like, and obvious
multiplicity by Hz. The thin layer chromatography (TLC) and column
chromatography were performed using silica gel (Merck). Condensation was
performed using a rotary evaporator manufactured by Tokyo Rikakikai Co., Ltd.
Re~ereace E~mple 1
Synthesis of [5-benzyloxycarbonylamino-2-(4-fluorophenyl)-6-oxo-1,6-dihydro-1-
pyrimidinylJacetic acid
( 1) Into a solution of 4-fluorobenznitrile (50.9 g, 0.420 mol) in ethanol
(500 mL)
was blown hydrogen chloride to saturation under ice-cooling and the mixture
was
stirred at room temperature for 21 hours. The solvent was evaporated under
reduced pressure and the obtained crystals were washed with ether and dried in
vacuo to give ethyl 4-fluorobenzimidate hydrochloride as white crystals (78.8
g,
92%).
(2) To a solution of the objective compound (78.8 g, 0.387 mol) of Step ( 1)
in
ethanol (350 mL) was added dropwise aminoacetaldehyde diethyl acetal (62 mL,
0.43 mol) under ice-cooling, and the mixture was stirred at 5~C for 16 hours.
Eethanol was evaporated under reduced pressure and the obtained concentrate
was added to 1N aqueous sodium hydroxide solution (750 mL) and extracted with
chloroform. The extract was dried over magnesium sulfate and the solvent was
evaporated under reduced pressure to give a colorless oil containing N-(2,2-
diethoxyethyl)-4-fluorobenzamidine.
(3) To a solution of the objective compound (crude product obtained by the
CA 02269720 1999-04-23
above-mentioned reaction) of Step (2) in ethanol ( 150 mL) was added dropwise
at
room temperature diethylethoxymethylene malonate (86 mL, 0.43 mol) . After the
dropwise addition, the mixture was heated to 100~C and stirred for 3 hours.
The
solvent was evaporated under reduced pressure and the obtained residue was
separated and purified by silica gel column chromatography ( 1:1 ethyl acetate
hexane) to give ethyl 1-(2,2-diethoxyethyl)-2-(4-fluorophenyl)pyrimidin-6( 1H)-
one-
5-carboxylate as a pale-yellow oil ( 135 (yield from the objective compound of
Step
( 1 ) 92%).
(4) To a solution of the objective compound ( 135 g, 0.3S8 mol) of Step (3) in
pyridine (480 mL) was added lithium iodide ( 120 g, 0.895 mol) and the mixture
was heated to 100~C and stirred for 16 hours. The organic solvent was
evaporated under reduced pressure and toluene ( 100 mL) was added. The
residual trace amount of pyridine was evaporated under reduced pressure. The
residue was added to a saturated aqueous sodium hydrogencarbonate solution
(500 mL) and organic material other than carboxylic acid was extracted with
ethyl
acetate. After removing insoluble material by filtration, the aqueous layer
was
separated. The aqueous layer and the insoluble material were combined and 2N
hydrochloric acid (ca. 1 L) was added to adjust the pH to 3 and the mixture
was
extracted with ethyl acetate. The extract was washed with saturated brine and
dried over magnesium sulfate. The solvent was evaporated under reduced
pressure to give a brown oil containing 1-(2,2-diethoxyethyl)-2-(4-
fluorophenyl)pyrimidin-6(1H)-one-5-carboxylic acid.
(5) To a solution of the objective compound (crude product obtained by the
above-mentioned reaction) of Step (4) and triethylamine (87.5 mL, 0.63 mol) in
1,4-dioxane (900 mL) was added dropwise at room temperature
diphenylphosphoryl azide (84 mL, 0.37 mol). After the dropwise addition, the
mixture was heated to 110~C and stirred for 2 hours. The mixture was cooled to
room temperature and benzyl alcohol (44 mL, 0.43 mol) was added. The reaction
mixture was heated again to 110~C and the mixture was stirred for 4 hours. The
reaction mixture was cooled to room temperature and 1,4-dioxane was evaporated
under reduced pressure. The residue was added to a saturated aqueous
ammonium chloride solution ( 1 L) and extracted with ethyl acetate. The
extract
was washed successively with 1N aqueous sodium hydroxide solution and
saturated brine, dried over magnesium sulfate and concentrated under reduced
26
CA 02269720 1999-04-23
pressure. The residue was separated by silica gel column chromatography ( 1:2
ethyl acetate:hexane) to give a mixture of [5-benzyloxycarbonylamino-2-(4-
fluorophenyl)-1,6-dihydro-6-oxo-1-pyrimidinyl]acetaldehyde diethyl acetal and
benzyl alcohol as a pale-yellow oil ( 126 ~ (69% as objective compound).
(6) To a solution of objective compound of Step (5) ( 126 g, mixture with
benzyl
alcohol, 0.247mo1 as objective compound of Step (5)) in tetrahydrofuran (THF)
(650 mL) was added 1 N hydrochloric acid (500 mL) and the mixture was stirred
at
70~C for 14 hours. The reaction mixture was cooled to room temperature and
THF was evaporated under reduced pressure. To the obtained concentrate was
added a saturated aqueous sodium hydrogencarbonate solution to adjust its pH
to
7 and the mixEure was extracted with ethyl acetate. The extract was dried over
magnesium sulfate and the solvent was evaporated under reduced pressure to
give a white solid containing [5-benzyloxycarbonylamino-2-(4-ffuorophenyl)-1,6-
dihydro-6-oxo-1-pyrimidinyl]acetaldehyde.
(7) To a mixture of the objective compound (crude product obtained by the
above-mentioned reaction) of Step (6), 2-methyl-2-propanol (900 mL) and 2-
methyl-2-butene ( 106 mL, 1.00 moll was added a solution of sodium
dihydrogenphosphate dihydrate ( 180 g, 1.15 moll and sodium chlorite (80%
content, 136 g, 1.20 moll in water (400 mL) and the mixture was stirred at
room
temperature for 2 hours. The insoluble material was removed by filtration and
the organic solvent was evaporated under reduced pressure. The obtained
concentrate was added to 2N hydrochloric acid (650 mL) and the mixture was
extracted with ethyl acetate. The extract was washed with saturated brine,
dried
over magnesium sulfate and concentrated under reduced pressure. Ethyl
acetate-hexane ( 1 : 1) was added to the residue for crystallization to give
the title
compound as a white solid ( 10.6 g). The insoluble material obtained earlier
was
added to 1N hydrochloric acid (500 mL) and the mixture was extracted with
ethyl
acetate. The extract was washed with saturated brine and dried over magnesium
sulfate: The solvent was evaporated under reduced pressure to give the title
compound as a white solid (67.7 g, total yield 80%).
iH-NMR (500MHz, DMSO-ds) 8 13.3 (brs, 1H), 8.99 (s, 1H),
8.46 (s, 1H), 7.56 (dd, J = 5.4, 8.9 Hz, 2H), 7.44 (d, J = 7.2 Hz, 2H), 7.30-
7.42 (m,
5H), 5.19 (s, 2H), 4.53 (s, 2H)
IR (KBr) 3650-2300, 1720, 1660, 1600 cm 1
27
CA 02269720 1999-04-23
Reference Example 2
Synthesis of 2-amino-1-(2-benzoxazolyl)-1-hydroxy-3-phenylpropane
( 1) To a mixture of L-phenylalaninol (20.2 g, 0.134 mol), sodium carbonate
(21.2 g,
0.200 mol) and 1,4-dioxane ( 150 mL) was added a solution of benzyloxycarbonyl
chloride (19.1 mL, 0.134 mol) in 1,4-dioxane (50 mL) and the mixture was
stirred
at room temperature for 3 hours. Water (300 mL) was added to the reaction
mixture and the obtained mixture was added to ice-cooled 0.5N hydrochloric
acid
(500 mL) . The precipitated crystals were collected by filtration and washed
with
hexane and dried to give N-benzyloxycarbonyl-I~phenylalaninol as white
crystals
(28.8 g, 76%).
(2) To a solution of the objective compound ( 10.7 g, 37.5 mmol) of Step( 1)
and
triethylamine (21.3 mL, 153 mmol) in dichloromethane ( 100 mL) was added a
solution of sulfur trioxide-pyridine complex (23.9 g, 150 mmol) in dimethyl
sulfoxide (DMSO) ( 100 mL) at -10~C. The obtained solution was stirred at 10-
20~C for 45 min and added to the saturated brine (400 mL). The mixture was
extracted with ether. The extract was washed successively with 1N hydrochloric
acid, a saturated aqueous sodium hydrogencarbonate solution and saturated
brine, dried over magnesium sulfate and concentrated under reduced pressure to
give N-benzyloxycarbonyl-If-phenylalaninal as a white solid (0.6 g,
quantitatively).
(3) To a solution of the objective compound (5.00 g, 17.6 mmol) of Step (2)
and
acetone cyanohydrin (4.8 mL, 53 mmol) in dichloromethane (50 mL) was added
triethylamine ( 1.5 mL, 11 mmol) and the mixture was stirred at room
temperature
for 4 hours. The solvent was evaporated under reduced pressure and the
obtained concentrate was added to water ( 100 mL). The mixture was extracted
with ethyl acetate. The extiract was washed with saturated brine, dried over
magnesium sulfate and concentrated under reduced pressure. The residue was
separated and purified by silica gel column chromatography (2:1 hexane:ethyl
acetate) to give N-benzyloxycarbonyl-DL-phenyh~rar~inal cyanohydrin as a pale-
yellow solid (5.15 g, 94%).
(4) To a mixture of chloroform (30 mL) and ethanol (28 mL, 0.48 mol) was added
dropwise acetyl chloride (31 mL, 0.44 mol) under ice-cooling over 20 min. The
mixture was stirred at 0~C for 10 min and a solution of the objective compound
(4.50 g, 14.5 mmol) of Step (3) in chloroform (30 mL) was added. The mixture
was
stirred at 0~C for 3 hours. The solvent was evaporated under reduced pressure
to
28
CA 02269720 1999-04-23
give a pale yellow solid. Thereto were added ethanol ( 100 mL) and o-
aminophenol ( l .90 g, 17.4 mmol) and the mixture was heated to 90~C and
stirred
for 6 hours. The solvent was evaporated under reduced pressure and the
obtained concentrate was added to 0.5N aqueous sodium hydroxide solution ( 100
mL) and the mixture was extracted with ethyl acetate. The extract was washed
successively with 0.5N hydrochloric acid, a saturated aqueous sodium
hydrogencarbonate solution and saturated brine, dried over magnesium sulfate
and concentrated under reduced pressure. The residue was separated and
purified by silica gel column chromatography (60:1 chloroform:methanol) to
glue
1-(2-benzoxazolyl)-2-benzyloxycarbonylamino-1-hydroxy-3-phenylpropane as a
pale-brown solid (5.12 g, 88%).
(5) To a solution of the objective compound (3.63 g, 9.02 mmol) of Step (4) in
methanol (50 mL) was added under nitrogen atmosphere 10% palladium carbon
(480 m~ and the mixture was stirred at room temperature for 18 hours under
hydrogen atmosphere. The catalyst was filtered off' and washed with methanol.
The filtrate was concentrated under reduced pressure to give the title
compound
as a brown solid (2.43 g, 100%).
1H-NMR (500 MHz, DMSO-d6) S 7.74-7.68 (m, 2H), 7.41-7.15 (m, 7H),
6.17 (m, 0.3H), 6.08 (brs, 0.7H), 4.61 (m, 0.7H), 4.54 (m, 0.3H),
3.34 (m, 1H), 3.05 (dd, J = 13.4, 3.8 Hz, 0.3H),
2.78 (dd, J = 13.4, 5.9 Hz, 0.7H), 2.60 (dd, J = 13.4, 7.9 Hz, 0.7H),
2.S3 (dd, J = 13.4, 8.9 Hz, 0.3H), 1.47 (brs, 2H)
IR (KBr) 3400, 3020, 1585, 1555 crri 1
Refereace L~ample 3
Synthesis of 2-amino-1-hydroxy-1-[5-(methoxycarbonyl)benzoxazol-2-yl]-3-
phenylpropane
( 1) To a solution of 4-hydroxy-3-nitrobenzoic acid ( 15.8 g, 86.3 mmol) in
1,2-
dichloroethane ( 150 mL) were added methanol ( 14 mL) and conc. sulfuric acid
(0.5
mL) and the mixture was heated to 80~C and stirred. Methanol (9 mL) was added
on the way and the mixture was stirred for 21 hours. The reaction mixture was
added to a saturated aqueous sodium hydrogencarbonate solution (400 mL) and
extracted with chloroform. The extract was washed with saturated brine, dried
over magnesium sulfate and concentrated under reduced pressure to give methyl
4-hydroxy 3-nitrobenzoate as a yellow solid ( 11.5 g, 68%).
29
CA 02269720 1999-04-23
(2) To a solution of the objective compound ( 11.4 g, 57.8 mmol) of Step( 1)
in ethyl
acetate (300 mL) was added under nitrogen atmosphere 10% palladium carbon
( 1.80 g) and the mixture was stirred at room temperature for 18 hours under
hydrogen atmosphere. The catalyst was filtered off and washed with ethyl
acetate.
The filtrate was concentrated under reduced pressure. The obtained solid was
washed with ether - hexane ( l : l) and dried in vacuo to give methyl 3-amino-
4-
hydroxybenzoate as a pale-brown solid (9.34 g, 97%).
(3) Using the objective compound of Step (2) and by a reaction similar to that
in
Reference Example 2-(4), 2-benzyloxycarbonylamino-1-hydroxy-1-[5-
(methoxycarbonyl)benzoxazol-2 yl]-3-phenylpropane was obtained as a pale-
brown solid ( 1.80 g, 81%).
(4) Using the objective compound of Step (3) and by a reaction similar to that
in
Reference Example 2-(5), the title compound was obtained as a pale-brown solid
( 1.14 g, 98%).
1H-NMR (500 MHz, DMSO-d fi) ~ 8.27 (d, J = 1.3 Hz, 0.4H),
8.25 (d, J = 1.3 Hz, 0.6H), 8.03 (dd, J = 8.6, 1.3 Hz, 0.4H),
8.02 (dd, J = 8.6, 1.3 Hz, 0.6H), 7.84 (d, J = 8.6 Hz, 0.4H),
7.81 (d, J = 8.6 Hz, 0.6H), 7.28-7.23 (m, 4H), 7.18-7.13 (m, 1H),
4.77-4.73 (m, 1H), 3.89 (s, 3H), 3.58 (m) 0.6H), 3.50 (m, 0.4H),
3.06 (dd, J = 13.6, 4.8 Hz, 0.4H), 2.88 (dd, J = 13.6, 7.3 Hz, 0.6H),
2.81 (dd, J = 13.6, 6.8 Hz, 0.6H), 2.65 (dd, J = 13.6, 8.2 Hz, 0.4H)
IR (KBr) 3300, l710, 1615 cm 1
Refereaoe E~mple 4
Synthesis of 2-amino-1-hydroxy-1-[6-(methoxycarbonyl)benzoxazol-2 yl]-3-
phenylpropane
( 1 ) Using 3-hydroxy-4-nitrobenzoic acid and by a reaction similar to that in
Reference Example 3-( 1), methyl 3-hydroxy-4-nitrobenzoate was obtained as a
yellow solid ( 13.8 g, 85%).
(2) Using the objective compound of Step (1) and by a reaction similar to that
in
Reference Example 3-(2), methyl 4-amino-3-hydroxybenzoate was obtained as a
white solid ( 11.0 g, 95%).
(3) Using the objective compound of Step (2) and by a reaction similar to that
in
Reference Example 2-(4), 2-benzyloxycarbonylamino-1-hydroxy-1-[6-
(methoxycarbonyl)benzoxazol-2 yl]-3-phenylpropane as a brown solid (4.02 g,
CA 02269720 1999-04-23
68%).
(4) Using the objective compound of Step (3) and by a reaction similar to that
in
Reference Example 2-(5), the title compound was obtained as a pale-brown solid
(688 mg, 83%).
1H-NMR (500 MHz, DMSO-d6) ~ 8.25 (d, J = 1.0 Hz, 0.4H),
8.23 (d, J = 1.0 Hz, 0.6H), 8.03-7.97 (m, 1H),
7.85 (d, J = 8.4 Hz, 0.4H), 7.83 (d, J = 8.3 Hz, 0.6H),
7.31-7.14 (m, 5H), 6.26 (d, J = 5.9 Hz, 0.4H), 6.15 (brs, 0.6H),
4.66 (m, 0.6H), 4.S8 (m, 0.4H), 3.89 (s, 3H), 3.3S (m, 1H),
3.05 (dd, J = 13.S, 3.8 Hz, 0.4H), 2.80 (dd, J = l3.4, 6.1 Hz, 0.6H),
2.62 (dd, J = 13.4, 7.7 Hz, 0.6H), 2.54 (dd, J = 13.5, 8.8 Hz, 0.4H),
1.52 (brs, 2H)
IR (KBr) 3330, 1705, 1600 cm 1
Reference Example S
Synthesis of benzyl 3-amino-4-hydroxybenzoate
( 1 ) Using 4-hydroxy-3-nitrobenzoic acid and benzyl alcohol and by a reaction
similar to that in Reference Example 3-(1), a mixture of benzyl 4-hydroxy-3-
nitrobenzoate and benzyl alcohol was obtained as a yellow oil (9.01 ~.
(2) To a solution of the objective compound (mixture with benzyl alcohol, 9.01
g) of
Step ( 1 ) in a mixture of THF ( 130 mL) and water (50 mL) were added iron
powder
(9.15 g, 164 mmol) and 1 N hydrochloric acid (7 mL) and the mixture was
stirred at
room temperature for 2.5 hours. The insoluble material was filtered off
through
celite and washed with methanol. The filtrate and washing solution were
combined and the organic solvent was evaporated under reduced pressure. The
concentrate was added to a saturated aqueous sodium hydrogencarbonate
solution ( 150 mL) and the mixture was extracted with ethyl acetate. The
extract
was washed with saturated brine, dried over magnesium sulfate and concentrated
under reduced pressure. The residue was separated and purified by silica gel
column chromatography ( 1:1 hexane:ethyl acetate) to give the title compound
as a
pale-brown solid (3.02 g) (yield from 4-hydroxy-3-nitrobenzoic acid, 45%).
1H-NMR (500 MHz, DMSO-d6) ~ 9.8 (brs, 1H), 7.44-7.37 (m, 4H),
7.34 (t, J = 6.9 Hz, 1H), 7.27 (d, J = 2.0 Hz, 1H),
7.14 (dd, J = 8.2, 2.0 Hz, 1H), 6.72 (d, J = 8.2 Hz, 1H), 5.25 (s, 2H), 4.8
(brs, 2H)
IR (KBr) 3250, 1665, 1585, 1510 ciri 1
31
CA 02269720 1999-04-23
Refereace Example 6
Synthesis of 2-amino-1-hydroxy-1-(5-nitrobenzoxazol-2-yl)-3-phenylpropane
( 1) Using 2-amino-4-nitrophenol and by a reaction similar to that in
Reference
Example 2-(4), 2-benzyloxycarbonylamino-1-hydroxy-1-(5-nitrobenzoxazol-2 yl)-
3-phenylpropane was obtained as a brown solid (2.77 g, 35%).
(2) To a solution of the objective compound (2.01 g, 4.50 mmol) of Step ( 1)
and
anisole ( 1.60 mL, 14.7 mmol) in dichloromethane (50 mL) was added under ice-
cooling trifluoromethanesulfonic acid (2.39 mL, 27.0 mmol) and the mixture was
stirred at 0~C to room temperature for 1.5 hours. A saturated aqueous sodium
hydrogencarbonate solution (45 mL) was added under ice-cooling and the mixture
was stirred for 15 min and the mixture was extracted with ethyl acetate. The
extract was washed with saturated brine, dried over magnesium sulfate and
concentrated under reduced pressure. The residue was separated and purified
by silica gel column chromatography (93:7 dichloromethane:methanol) to give
the
title compound as a yellow solid ( 1.19 g, 85%).
iH-NMR (300 MHz, DMSO-d6) ~ 8.60 (d, J = 2.3 Hz, 1H),
8.31 (dd, J = 8.9, 2.3 Hz, 1H), 7.96 (d, J = 8.9 Hz, 1H),
7.30-7.12 (m, 5H), 6.23 (brs, 1H), 4.68 (brs, 1H), 3.33 (m, 1H),
2.82 (dd, J = 13.3, 6.4 Hz, 1H), 2.64 (dd, J = 13.3, 7.6 Hz, 1H),
1.59 (brs, 2H)
IR (KBt~ 3325, 3050, 2940, 1620, 1570, 1520 crn 1
Refereace ample ?
Synthesis of 2-amino- 1-hydroxy-1-(5-methoxybenzoxazol-2-yl)-3-phenylpropane
( 1 ) Using 2-amino-4-methoxyphenol and by a reaction similar to that in
Reference
Example 2-(4), 2-benzyloxycarbonylarnino-1-hydroxy-1-(5-methoxybenzoxazol-2-
yl)-3-phenylpropane was obtained as a dark brown solid (3.09 g, 85%).
(2) Using the above-mentioned compound and by a reaction similar to that in
Reference Example 2-(5), the title compound was obtained as a brown solid (
1.92 g,
92%).
iH-NMR (500 MHz, DMSO-d6) Q7.60 (d, J = 8.8 Hz, 0.4H),
7.59 (d, J=8.8 Hz, 0.6H), 7.32-7.15 (m, 6H), 6.98-6.93 (m, 1H),
6.14 (d, J = 5.6 Hz, 0.4H), 6.0S (brs, 0.6H), 4.56 (m, 0.6H),
4.51 (m, 0.4H), 3.80 (s, 3H), 3.3 (rn, 1H),
3.02 (dd, J = 13.4, 3.7 Hz, 0.4H), 2.75 (dd, J = 13.4, 5.0 Hz, 0.6H),
32
CA 02269720 1999-04-23
2.57 (dd, J = 13.4, 8.0 Hz, 0.6H), 2.51 (m, 0.4H), 1.46 (brs, 2H)
IR (KBr) 3320, 3000, 2900, 2810, 2630, 1600, 1555 crri 1
Refereace Earample 8
Synthesis of 2-amino- 1-hydroxy-1-(2-oxazolinyl)-3-phenylpropane
( 1 ) To a mixture of chloroform (9 mL) and ethanol (8.3 mL, 0.14 mol) was
added
dropwise acetyl chloride (9.1 mL, 0.13 mol) over 20 minutes under ice-cooling.
The mixture was stirred at 0~C for 10 min and a solution of the objective
compound ( 1.33 g, 4.29 mmol) of Reference Example 2-(3) in chloroform (9 mL)
was added. The mixture was stirred at 0~C for 3 hours and the solvent was
evaporated under reduced pressure to give a pale-brown solid. Thereto were
added dichloromethane ( 18 mL), monoethanolamine (0.51 mL, 8.5 mmol) and
triethylamine ( 1.2 mL, 8.6 mmol), and the mixture was stirred at room
temperature for 18 hours. The reaction mixture was added to 1N aqueous
sodium hydroxide solution (50 mL) and the mixture was extracted with ethyl
acetate. The extract was washed with saturated brine, dried over magnesium
sulfate and concentrated under reduced pressure. The residue was separated
and purified by silica gel column chromatography (30:1 chloroform:methanol) to
give 2-benzyloxycarbonylamino-1-hydroxy-1-(2-oxazolinyl)-3-phenylpropane as a
white solid (509 mg, 33%).
(2) Using the above-mentioned compound and by a reaction similar to that in
Reference Example 2-(5), the title compound was obtained as a white solid (284
mg, 91%).
1H-NMR (300 MHz, DMSO-ds) Q7.33-7.12 (m, 5H), 4.20 (m, 2H),
3.90(dd, J = 8.4, 4.6 Hz, 1H), 3.72 (m, 2H), 3.04 (m, 1H),
2.90 (dd, J = 13.4, 4.1 Hz, 0.4H), 2.80-2.65 (m, 1.2H),
2.42 (dd, J = 13.4, 8.8 Hz, 0.4H)
ample 1
Synthesis of 2-[5-benzyloxycarbonylamino-2-(4-fluorophenyl)-6-oxo- 1,6-dihydro-
1-pyrimidinyl]-N-( 1-[(2-benzoxazolyl)carbonyl]-2-phenylethyl]acetamide
( 1 ) To a solution of the title compound ( 1.90 g, 4.48 mmol) of Reference
Example 1
and the title compound ( 1.37 g, 5.11 mmol) of Reference Example 2 in DMF ( 15
mL) were added HOBT ( 1.21 g, 8.95 mmol) and WSCI hydrochloride ( 1.03 g, 5.37
mmol) and the mixture was stirred at room temperature for 4 hours. The
reaction mixture was added to 0.5N hydrochloric acid ( 100 mL) and the mixture
33
CA 02269720 1999-04-23
was extracted with ethyl acetate. The extt act was washed successively with a
saturated aqueous sodium hydrogencarbonate solution and saturated brine,
dried over magnesium sulfate and concentrated under reduced pressure. The
obtained solid was washed with ether, and dried in vacuo to give 2-[5-
benzyloxycarbonylamino-2-(4-fluorophenyl)-6-oxo-1,6-dihydro-1-pyrimidinyl]-N-
[ 1-[(2-benzoxazolyl) hydroxymethyl]-2-phenylethyl]acetamide as a white solid
(2.43
g, 84%).
(2) To a solution of the objective compound (2.12 g, 3.27 mmol) of Step ( 1)
in a
mixture of DMSO (20 mL) and toluene (20 mL) were added WSCI hydrochloride
(3.13 g, 16.3 mmol) and dichloroacetic acid (0.54 mL, 6.5 mmol) and the
mixture
was stirred at room temperature for 3.5 hours. The reaction mixture was added
to 1N hydrochloric acid ( 100 mL) and extracted with ethyl acetate. The
extract
was washed with a saturated aqueous sodium hydrogencarbonate solution and
the precipitated white solid was collected by filtration. The filtrate was
further
washed with saturated brine, dried over magnesium sulfate and concentrated
under reduced pressure. The residue and the precipitate collected earlier were
combined and separated and purified by silica gel column chromatography (S:1
dichloromethane : ethyl acetate) to give the title compound as white crystals
( 1.69
g, 80%). The crystals were recrystal)ized from ethyl acetate to give white
crystals
(1.43 g).
mp 222-225~C
1H-NMR (S00 MHz, DMSO-d6) ~ 8.98 (d, J = 6.9 Hz, 1H), 8.86 (s, 1H),
8.40 (s, 1H), 8.00 (d, J = 8.0 Hz, 1H), 7.91 (d; J = 8.0 Hz, 1H),
7.66 (t, J = 8.0 Hz, 1H), 7.S5 (t, J = 8.0 Hz, 1H), 7.46-7.40 (m, 4H),
7.38 (t, J = 7.1 Hz, 2H), 7.33 (t, J = 7.1 Hz, 1H),
7.25 (t, J = 6.7 Hz, 2H), 7.22-7.13 (m, 5H), 5.56 (m, 1H), S.17 (s, 2H), 4.S3
(d, J =
16.6 Hz, 1H), 4.4S (d, J = 16.6 Hz, 1H),
3.31 (dd, J = 14.2, 4.8 Hz, 1H), 2.96 (dd, J = 14.2, 9.0 Hz, 1H)
IR (KBr) 3360, 3270, 3040, 1705, 1655, 1600, 1520 cmm
MS (SIMS, positive) m/z 646 (MH+)
Example 2
Synthesis of 2-[S-amino-2-(4-fluorophenyl)-6-oxo-1,6-dihydro-1-pyrimidinyl]-N-
[ 1-[(2-benzoxazolyl)carbonyl]-2-phenylethyl]acetamide
To a solution of the tide compound ( 1.14 g, 1.77 mmol) of Example 1 in a
34
CA 02269720 1999-04-23
mixture of methanol ( 15 mL) and THF (25 mL) was added 10% palladium carbon
( 188 m~ under a nitrogen atmosphere and the mixture was stirred at room
temperature for 4 hours under a hydrogen atmosphere. The catalyst was
removed by filtration and washed with chloroform-methanol ( 10:1 ) and the
filtrate
was concentrated under reduced pressure. The residue was separated and
purified by silica gel column chromatography (50:1 chloroform:methanol) to
give
the title compound as white crystals (704 mg, 78%).
mp 233-236~C
1H-NMR (500 MHz, DMSO-d6) ~ 8.92 (d, J = 6.8 Hz, 1H),
8.01 (d, J = 8.0 Hz, 1 H), 7.91 (d, J = 8.0 Hz, 1 H),
7.66 (t, J = 8.0 Hz, 1 H), 7.56 (t, J = 8.0 Hz, 1 H),
7.35 (dd, J = 8.6, 5.5 Hz, 2H), 7.28 (s, 1H), 7.26 (t, J = 8.0 Hz, 2H), 7.23-
7.17 (m,
3H), 7.09 (t, J = 8.0 Hz, 2H), 5.55 (m, 1H), S.13 (s, 2H),4.48 (d, J = 16.7
Hz, 1H),
4.4l (d, J = 16.7 Hz, 1H),
3.30 (dd, J = 14.1, 4.9 Hz, 1H), 2.97 (dd, J = 14.l, 8.9 Hz, 1H)
IR (KBr) 3400, 3330, 3250, 3040, 1705, 1655, 1600, 1525, 1500 crri 1
MS (SIMS, positive) m/z 512 (MH+)
E~mple 3
S~mthesis of 2-[5-benzyloxycarbonylamino-2-(4-fluorophenyl)-6-oxo- 1,6-dihydro-
1-pyrimidinyl]-N-( 1-[[5-(methoxycarbonyl)benzoxazol-2-yl]carbonyl]-2-
phenylethyl]acetamide
( 1) Using the title compound of Reference Example 1 and the title compound of
Reference Example 3 and by a reaction similar to that in Example 1-( 1 ), 2-[5-
benzyloxycarbonylamino-2-(4-fluorophenyl)-6-oxo-1,6-dihydro-1-pyrimidinyl]-N-
[ 1-[[5-(methoxycarbonyl)benzoxazol-2-yl]hydroxymethyl]-2-
phenylethyl]acetarnide
was obtained as a pale-brown solid ( 1.66 g, 72%).
(2) Using the objective compound of Step ( 1) and by a reaction similar to
that in
Example 1-(2), the title compound was obtained as white crystals ( 1.28 g,
82%).
mp 218-222~C
1H-NMR (500 MHz, DMSO-d6) ~ 9.04 (d, J = 6.8 Hz, 1H), 8.84 (s, 1H),
8.51 (d, J = 1.5 Hz, 1 H), 8.40 (s, 1 H), 8.23 (dd, J = 8.7, 1. 5 Hz, 1 H),
8.04 (d, J = 8.7
Hz, 1H), 7.45-7.40 (m, 4H), 7.38 (t, J = 7.1 Hz, 2H),
7.33 (t, J = 7.1 Hz, 1H), 7.26 (t, J = 7.3 Hz, 2H), 7.22-7.13 (m, 5H),
5.51 (m, 1H), 5.17 (s, 2H), 4.53 (d, J = 16.8 Hz, 1H),
CA 02269720 1999-04-23
4.45 (d, J = 16.8 Hz, 1H), 3.92 (s, 3H), 3.31 (m, 1H),
2.97 (dd, J = 14.2, 8.9 Hz, 1 H)
IR (KBr) 3350, 3260, 3050, 1710, 1670, 1655, I600, 1520 crri 1
MS (APCI, positive) m/z 704 (MH+)
E~mple 4
Synthesis of 2-[5-amino-2-(4-fluorophenyl)-6-oxo-1,6-dihydro-1-pyrimidinyl]-N-
[ 1-[[5-(methoxycarbonyl)benzoxazol-2-yl]carbonyl]-2-phenylethyl]acetamide
To a solution of the title compound (462 mg, 0.657 mmol) of Example 3
and anisole (0.21 mL, 1.9 mmol) in dichloromethane ( 13 mL) was addded under
ice-cooling trifluoromethanesulfonic acid (0.35 mL, 4.0 mmol) and the mixture
was stirred at 0~C to room temperature for 1 hour. A saturated aqueous sodium
hydrogencarbonate solution ( 13 mL) was added under ice-cooling and the
mixture
was stirred for 30 min. The the reaction mixture was added to a saturated
aqueous sodium hydrogencarbonate solution (50 mL) and extracted with ethyl
acetate. The extract was washed with saturated brine, dried over magnesium
sulfate and concentrated under reduced pressure. The residue was separated
and purified by silica gel column chromatography (30:1 chloroform:methanol) to
give the title compound as pale yellow crystals (368 mg, 98%).
mp 208-213~C
1H-NMR (500 MHz, DMSO-d6) ~ 8.97 (d, J = 6.7 Hz, 1H),
8.51 (d, J = 1.6 Hz, 1H), 8.24 (dd, J = 8.7, 1.6 Hz, 1H),
8.05 (d, J = 8.7 Hz, 1H), 7.35 (dd, J = 8.8, 5.6 Hz, 2H),
7.28-7.17 (m, 6H), 7.08 (t, J = 8.8 Hz, 2H), 5.50 (m, 1H), 5.12 (s, 2H), 4.48
(d, J =
16.8 Hz, 1H), 4.41 (d, J = 16.8 Hz, 1H), 3.93 (s, 3H),
3.31 (m, 1 H), 2.97 (dd, J = 14.1, 8.9 Hz, 1 H)
IR (KBr) 3370, 170S, 1655, 1600 crri 1
MS (SIMS, positive) m/z 570 (MH+)
L~rample 5
Synthesis of 2-[5-amino-2-(4-fluorophenyl)-6-oxo-1,6-dihydro-1-pyrimidinyl]-N-
[ 1-[(5-carboxybenzoxazol-2-yl)carbonyl]-2-phenylethyl]acetamide
To a solution of the title compound ( 180 mg, 0.316 mmol) of Example 4 in
a nvxture of dimethylsulfide (5 mL) and dichloromethane (5 mL) was added under
ice-cooling aluminum bromide (680 mg, 2.55 mmol) and the mixture was stirred
at 0~C to room temperature for 4 hours. Water ( 10 mL) and 1 N hydrochloric
acid
36
CA 02269720 1999-04-23
( 1 mL) were added and the mixture was stirred at room temperature for 1 hour.
The precipitate was collected by filtration and washed with water and
chloroform.
The obtained solid was separated and purified by silica gel column
chromatography (2:1 chloroform:methanol) to give the title compound as yellow
crystals (146 mg, 83%).
mp 207-214~C
1H-NMR (500 MHz, DMSO-d6) ~ 8.94 (d, J = 6.8 Hz, 1H), 8.46 (s, 1H),
8.24 (d, J = 8.6 Hz, 1H), 7.87 (d, J = 8.6 Hz, 1H),
7.35 (dd, J = 8.7, 5.5 Hz, 2H), 7.29-7.18 (m, 6H),
7.11 (t, J = 8.7 Hz, 2H), 5.S5 (m, 1H), 5.13 (s, 2H),
4.48 (d, J = 16.6 Hz, 1H), 4.42 (d, J = 16.6 Hz, 1H), 3.30 (m, 1H),
2.97 (dd, J = 14.1, 8.8 Hz, 1H)
IR (KBr) 3300, 1700, 1655, 1600, 1520, 1500 crri '
MS (SIMS, negative) m/z 554 (MH-)
sample 6
Synthesis of 2-[5-benzyloxycarbonylamino-2-(4-fluorophenyl)-6-oxo- 1,6-dihydro-
1-pyrimidinyl]-N-[ 1-[[6-(methoxycarbonyl)benzo~cazol-2-yl]carbonyl]-2-
phenylethyl]acetamide
( 1) Using the title compound of Reference Example 1 and the title compound of
Reference Example 4 and by a reaction similar to that in Example 1-( 1), 2-[5-
benzyloxycarbonylamino-2-(4-fluorophenyl)-6-oxo-1,6-dihydro-1-pyrimidinyl]-N-
[ 1-[[6-(methoxycarbonyl)benzoxazol-2-yl]hydroxymethyl]-2-
phenylethyl]acetamide
was obtained as a white solid ( 1.09 g, 75%).
(2) Using the objective compound of Step ( 1) and by a reaction similar to
that in
Example 1-(2), the title compound was obtained as pale-yellow crystals (521
mg,
50%).
mp 247-250~C
iH-NMR (500 MHz, DMSO-d6) ~ 9.02 (d, J = 6.7 Hz, 1H), 8.84 (s, 1H),
8.45 (s, 1H), 8.40 (s, 1H), 8.12 (s, 2H), 7.46-7.41 (m, 4H),
7.38 (t, J = 7.1 Hz, 2H), 7.33 (t, J = 7.1 Hz, 1H),
7.25 (t, J = 7.2 Hz, 2H), 7.22-7.13 (m, 5H), 5.53 (m, 1H), 5.17 (s, 2H),4.53
(d, J =
16.7 Hz, 1H), 4.45 (d, J = 16.7 Hz, 1H), 3.93 (s, 3H),
3.30 (m, 1H), 2.97 (dd, J = 14.2, 8.9 Hz, 1H)
IR (KBr) 3370, 3240, 3020, 2920, 1715, 1655, 1600, 1520 crri 1
37
CA 02269720 1999-04-23
MS (SIMS, positive) m/z 704 (MH+)
Example 7
Synthesis of 2-[5-amino-2-(4-fluorophenyl)-6-oxo-1,6-dihydro-1-pyrimidinyl]-N-
[ 1-[[6-(methoxycarbonyl)benzoxazol-2-yl]carbonyl]-2-phenylethyl]acetamide
Using the title compound of Example 6 and by a reaction similar to that in
Example 4, the title compound was obtained as yellow crystals (284 mg, 88%).
mp 197-200~C
iH-NMR (500 MHz, DMSO-ds)~ 8.97 (d, J = 6.7 H2, 1H), 8.46 (s, 1H),
8.12 (s, 2H), 7.35 (dd, J = 8.7, 5.5 Hz, 2H), 7.28-7.17 (m, 6H),
7.10 (t, J = 8.7 Hz, 2H), 5.52 (m, 1H), 5.12 (s, 2H),
4.48 (d, J = 16.6 Hz, 1H), 4.40 (d, J = 16.6 Hz, 1H), 3.93 (s, 3H),
3.31 (m, 1H), 2.97 (dd, J = 14.1; 8.9 Hz, 1H)
IR (KBr) 3350, 3000, 1705, 1655, 1600, 1500 crri 1
MS (SIMS, positive) m/z 570 (MH+)
ample 8
Synthesis of 2-[5-amino-2-(4-ffuorophenyl)-6-oxo-1,6-dihydro-1-pyrimidinyl]-N-
[ 1-[(6-carboxybenzoxazol-2-yl)carbonyl]-2-phenylethyl]acetamide
Using the title compound of Example 7 and by a reaction similar to that in
Example 5, the title compound was obtained as pale-yellow crystals (91 mg,
52%).
mp 235-241~C
iH-NMR (500 MHz, DMSO-d6) ~ 8.93 (d, J = 6.8 Hz, 1H), 8.29 (s, 1H),
8.14(d,J=8.4 Hz, 1H),7.91(d,J=8.4 Hz, 1H),
7.35 (dd, J = 8.7, 5.6 Hz, 2H), 7.28-7.17 (m, 6H),
7.11 (t, J = 8.7 Hz, 2H), 5.56 (m, lI~, 5.13 (s, 2H),
4.49 (d, J = 16.7 Hz, 1H), 4.42 (d, J = 16.7 Hz, 1H), 3.33 (m, 1H),
2.96 (dd, J = 14.0, 9.0 Hz, 1 H)
IR (KBr) 3330, 1700, 1650, 1600, 1555 crri 1
MS (SIMS, negative) m/z 554 (MH-)
ample 9
Synthesis of 2-[5-benzyloxycarbonylamino-2-(4-fluorophenyl)-6-oxo- 1,6-dihydro-
1-pyrimidinyl]-N-( 1-formyl-2-phenylethyl)acetamide
( 1) Using the title compound of Reference Example 1 and lrphenylalaninol and
by
a reaction similar to that in Example 1-( 1), 2-[5-benzyloxycarbonylamino-2-(4-
fluorophenyl)-6-oxo-1,6-dihydro-1-pyrimidinyl]-N-( 1-hydroxymethyl-2-
38
CA 02269720 1999-04-23
phenylethyl)acetamide was obtained as white crystals (6.51 g, 81%).
(2) Using the objective compound of Step ( 1) and by a reaction similar to
that in
Example 1-(2), the title compound was obtained as white crystals (5.45 g,
93%).
mp 138-141 ~C
1H-NMR (500 MHz, CDC13 ) ~ 9.62 (s, 1H), 8.77 (brs, 1H),
7.53 (dd, J = 8.7, 5.2 Hz, 2H), 7.48 (s, 1H), 7.43-7.33 (m, 5H),
7.28-7.08 (m, 7H), 6.52 (d, J = 6.8 Hz, 1H), 5.24 (s, 2H),
4.77 (q, J = 6.6 Hz, 1H), 4.52 (ABq, J = l5.4 Hz, 2H), 3.17 (m, 2H)
IR (KBr) 3270, 3010, 1725, 1645, 1600, 1520 crri 1
MS (SIMS, positive) m/z 529 (MH+)
L~ample 10
Synthesis of 2-[5-benzyloxycarbonylamino-2-(4-ffuorophenyl)-6-oxo- 1,6-dihydro-
1-pyrimidinyl]-N-[ 1-[[5-(benzyloxycarbonyl)benzoxazol-2-yl]carbonyl]-2-
phenylethyl]acetamide
( 1) To a solution of the title compound (4.06 g, 7.68 mmol) of Example 9 and
acetone cyanohydrin (2.1 mL, 23 mmol) in dichloromethane (50 mL) was added
triethylamine (0.64 mL, 4.6 mmol) and the mixture was stirred at room
temperature for 2 hours. The precipitate was collected by filtration and
washed
with ethyl acetate. The filtrate and washing solution were combined and the
organic solvent was evaporated under reduced pressure. The obtained
concentrate was added to water ( 100 mL) and extracted with ethyl acetate. The
extract was washed with saturated brine, dried over magnesium sulfate and
concentrated under reduced pressure. The obtained solid was washed with ether,
combined with the precipitate obtained earlier and dried in vacuo to give 2-[5-
benzyloxycarbonylamino-2-(4-fluorophenyl)-6-oxo-1,6-dihydro-1-pyrimidinyl]-N-
[ 1-(cyanohydroxymethyl)-2-phenylethyl]acetamide as a white solid (3.68 g,
86%).
(2) To a mixture of chloroform ( 10 mL) and ethanol (4.5 mL, 77 mmol) was
added
dropwise under ice-cooling acetyl chloride (5.0 mL, 70 mmol) over 15 min. The
mixture was stirred at 0~C for 10 min and the objective compound ( 1.30 g,
2.34
mmol) of Step (1) was added. The mixture was stirred at 0~C to room
temperature for 3 hours. The solvent was evaporated under reduced pressure to
give white solid. Thereto were added ethanol ( 13 mL) and the title compound
(735 mg, 3.02 mmol) of Reference Example 5 and the mixture was heated to 65~C
and stirred for 6 hours. The solvent was evaporated under reduced pressure and
39
CA 02269720 1999-04-23
the obtained concentrate was added to a 0.5N aqueous sodium hydroxide solution
(50 mL) and extracted with ethyl acetate. The extract was washed with
saturated
brine and concentrated under reduced pressure. The residue was separated and
purified by silica gel column chromatography (50:1 chloroform:methanol) to
give a
mixture containing 2-[5-benzyloxycarbonylamino-2-(4-fluorophenyl)-6-oxo-1,6-
dihydro-1-pyrimidinyl]-N-( 1-[[5-(benzyloxycarbonyl)benzoxazol-2-
yl]hydroxymethyl]-2-phenylethyl]acetamide as a pale-yellow solid (395 m~.
(3) Using a mixture containing the objective compound of Step (2) and by a
reaction similar to that in Example 1-(2), the title compound was obtained as
pale-yellow crystals ( 179 mg, yield from 2-[5-benzyloxycarbonylamino-2-(4-
fluorophenyl)-6-oxo-1,6-dihydro-1-pyrimidinyl]-N-[ 1-(cyanohydroxymethyl)-2-
phenylethyl]acetamide, 10%).
mp 221-225~C
1H-NMR (500 MHz, DMSO-d6) ~ 9.02 (d, J = 6.8 Hz, 1H), 8.83 (s, 1H),
8.55 (d, J = 1.4 Hz, 1 H), 8.40 (s, 1 H) , 8.26 (dd, J = 8.8, 1.4 Hz, 1 H),
8.0S (d, J = 8.8
Hz, 1H), 7.S2 (d, J = 7.2 Hz, 2H), 7.46-7.35 (m, 9H),
7.32 (t, J = 7.1 Hz, 1H), 7.28-7.12 (m, 7H), 5.51 (m, 1H), 5.42 (s, 2H),5.17
(s, 2H),
4.53 (d, J = 16.6 Hz, 1H), 4.45 (d, J = 16.6 Hz, 1H),
3.32 (m, 1H), 2.96 (dd, J = 14.1, 9:0 Hz, 1H)
IR (KBr) 3370, 1715, 1670, 1655, l600, 1520 crri 1
MS (SIMS, positive) m/z 780 (MH+)
Example 11
Synthesis of 2-[5-benzyloxycarbonyl,amino-2-(4-ffuorophenyl)-6-oxo- 1,6-
dihydro-
1-pyrimidinyl]-N-[ 1-[[5-(ethylaminocarbonyl)benzoxazol-2 yl]carbonyl]-2-
phenylethyl]acetamide
( 1) To a solution of the objective compound (2.00 g, 2.83 mmol) of Example 3-
( 1) in
DMSO (250 mL) was added a 1N aqueous sodium hydroxide solution (30 mL) and
the mixture was stirred at mom temperature for 1 hour. The reaction mixture
was added to 0.1N hydrochloric acid (1000 mL) and extracted with ethyl
acetate.
The extract was washed with saturated brine, dried over magnesium sulfate and
concentrated under reduced pressure. The obtained solid was washed with ether
and dried in vacuo to give 2-[5-benzyloxycarbonylamino-2-(4-ffuorophenyl)-6-
oxo-
1,6-dihydro-1-pyrimidinyl]-N-[ 1-[(5-carboxybenzoxazol-2-yl) hydroxymethyl]-2-
phenylethyl]acetamide as a white solid ( 1.58 g, 81%).
CA 02269720 1999-04-23
(2) To a solution of the objective compound (450 mg, 0.651 mmol) of Step (1),
ethylamine hydrochloride (67 mg, 0.82 mmol) and H013T ( 176 mg, 1.30 mmol) in
DMF ( 10 mL) were added N-ethylirlorpholine (0.10 mL, 0.79 mmol) and WSCI
hydrochloride ( 148 mg, 0.772 mmol) and the mixture was stirred at room
temperature for 3.5 hours. The reaction mixture was added to 1N hydrochloric
acid (80 mL) and extracted with ethyl acetate. The extract was washed
successively with a saturated aqueous sodium hydrogencarbonate solution and
saturated brine, dried over magnesium sulfate and concentrated under reduced
pressure. The obtained solid was washed with ether and dried in vacuo to give
2-(5-benzyloxycarbonylamino-2-(4-fluorophenyl)-6-oxo- 1,6-dihydro-1-
pyrimidinyl]-N-[ 1-[[5-(ethylaminocarbonyl)benzoxazol-2-yl]hydroxymethyl]-2-
phenylethyl]acetamide as a white solid (397 mg, 85%).
(3) To a solution of the objective compound (448 mg, 0.623 mmol) of Step (2)
in
DMSO (5 mL) was added Dess-Martin periodinane (446 mg, 1.05 mmol) and the
mixture was stirred at room temperature for 1.5 hours. To the reaction mixture
was added a saturated aqueous sodium hydrogencarbonate solution (5 mL)
containing sodium thiosulfate in a concentration of 0.22 g/mL, and the mixture
was stirred at room temperature and extracted with ethyl acetate. The
insoluble
material in the extract was collected by filtration and the filtrate was
washed
successively with a saturated aqueous sodium hydrogencarbonate solution and
saturated brine, dried over magnesium sulfate and concentrated under reduced
pressure. The residue and the insoluble material obtained earlier were
combined,
and separated and purified by silica gel column chromatography (50:1
chloroform:methanol) to give the title compound as pale-yellow crystals (268
mg,
60%).
mp 256-261~C
1H-NMR (500 MHz, DMSO-ds) ~ 9.00 (d, J = 6.9 Hz, 1H), 8.86 (s, 1H),
8.65 (t, J = 5.5 Hz, 1H), 8.46 (d, J = 1.6 Hz, 1H), 8.40 (s, 1H),
8.14 (dd, J = 8.7, 1.6 Hz, 1H), 7.97 (d, J = 8.7 Hz, 1H),
7.46-7.40 (m, 4H), 7.38 (t, J = 7.1 Hz, 2H), 7.33 (t, J = 7.1 Hz, 1H),
7.26 (t, J = 7.2 Hz, 2H), 7.22-7.13 (m, 5H), 5.54 (m, 1H), 5.17 (s, 2H), 4.53
(d, J =
16.4 Hz, 1H), 4.46 (d, J = 16.4 Hz, 1H), 3.3 (m, 3H),
2.95 (dd, J = 14.1, 9.1 Hz, 1H), 1.16 (t, J = 7.2 Hz, 3H)
IR (KBr) 3350, 32S0, 3020, 17l5, 1655, 1600, 1520 crri 1
41
CA 02269720 1999-04-23
Example 12
Synthesis of 2-[5-amino-2-(4-fluorophenyl)-6-oxo-1,6-dihydro-1-pyrimidinylJ-N-
[ 1-[(5-(ethylaminocarbonyl)benzoxazol-2 yl]carbonyl]-2-phenylethylJacetamide
Using the title compound of Example 11 and by a reaction similar to that
in Example 4, the title compound was obtained as pale yellow crystals ( 111
mg,
68%).
mp 233-239~C
1H-NMR (S00 MHz, DMSO-d6) ~ 8.94 (d, J = 6.8 Hz, 1H),
8.65 (t, J = 5.5 Hz, 1H), 8.46 (d, J = 1.6 Hz, 1H),
8.l4 (dd, J = 8.7, 1.6 Hz, 1H), 7.98 (d, J = 8.7 Hz, 1H),
7.35 (dd, J = 8.7, 5.5 Hz, 2H), 7.29-7.18 (m, 6H),
7.10 (t, J = 8.7 Hz, 2H), S.53 (m, 1H), 5.12 (s, 2H),
4.48 (d, J = 16.7 Hz, 1H), 4.42 (d, J = 16.7 Hz, 1H), 3.3 (m, 3H),
2.96 (dd, J = 14.1, 9.0 Hz, 1 H), 1.16 (t, J = 7.2 Hz, 3H)
IR (KBr) 3300, 1705, 1640, 1600, 1525, 1500 crri 1
Example 13
Synthesis of 2-[5-benzyloxycarbonylamino-2-(4-ffuorophenyl)-6-oxo-1,6-dihydro-
1-pyrimidinylJ-N-[1-[(5-nitrobenzoxazol-2 yl)carbonyl]-2-phenylethylJacetamide
( 1) Using the tide compound of Reference Example 1 and the title compound of
Reference Example 6 and by a reaction similar to that in Example 1-(1), 2-[5-
benzyloxycarbonylamino-2-(4-fluorophenyl)-6-oxo-1,6-dihydro-1-pyrimidinylJ-N-
[ 1-[(5- nitrobenzoxazol-2 yl)hydroxymethyl]-2-phenylethyl]acetamide was
obtained as a pale-yellow solid (2.54 g, 99%).
(2) Using the objective compound of Step ( 1) and by a reaction similar to
that in
Example 1-(2), the title compound was obtained as a pale-brown solid ( 1.47 g,
59%).
1H-NMR (300 MHz, DMSO-d6) ~ 9.07 (d, J = 6.8 Hz, 1H),
8.90 (d, J = 2.2 Hz, 1H), 8.85 (s, 1H), 8.52 (dd, J = 9.1, 2.2 Hz, 1H), 8.40
(s, 1H),
8.18 (d, J = 9.1 Hz, 1H), 7.53-7.10 (m, 14H),
5.49 (m, 1H), 5.17 (s, 2H), 4.58 (d, J = 16.8 Hz, 1H),
4.44 (d, J = 16.8 Hz, 1H), 3.4-3.25 (m, 1H),
2.97 (dd, J = 14.2, 8.9 Hz, 1 H)
IR (KBr) 3350, 3050, 1715, 1655, 1600, 1520 crnl
~ple 14
42
CA 02269720 1999-04-23
Synthesis of 2-[5-benzyloxycarbonylarnino-2-(4-fluorophenyl)-6-oxo- 1,6-
dihydro-
1-pyrimidinyl]-N-[1-[(5-aminobenzoxazol-2-yl)carbonyl]-2-phenylethyl]acetamide
To a solution of the title compound ( 1.340 g, 1.940 mmol) of Example 13
in a mixture of THF (40 mL), water (7 mL) and methanol (7 mL) were added iron
powder (2.734 g, 48.95 mmol) and 1 N hydrochloric acid ( 1.64 mL), and the
mixture was stirred at room temperature for 18 hours. The insoluble material
was removed through celite and washed with chloroform. The filtrate and
washing solution were combined and washed with a saturated aqueous sodium
hydrvgencarbonate solution and saturated brine, dried over sodium sulfate and
concentrated under reduced pressure. The residue was separated and purified
by silica gel column chromatography (96:4 chloroform:methanol) and (97:3
chloroform:methanol) to give the title compound as an organge solid ( 1.004 g,
78%).
1H-NMR (300 MHz, DMSO-dfi)~ 8.93 (s, 1H), 8.92 (d, J = 6.6 Hz, 1H),
8.41 (s, 1H), 7.54 (d, J = 8.9 Hz, 1H), 7.48-7.13 (m, 14H),
6.95 (d, J = 2.0 Hz, 1 H), 6.92 (dd, J = 8.9, 2.0 Hz, 1 H) , 5.56 (m, 1 H),
S.37 (s, 2H),
5.17 (s, 2H), 4.S3 (d, J = 16.7 Hz, 1H),
4.44 {d, J = 16.7 Hz; 1H), 3.25 (dd, J = l4.0, 4.4 Hz, 1H),
2.92 (dd, J = 14.0, 8.9 Hz, 1 H)
IR (KBr) 3325, 3000, 1695, 1650, 1600, 1510 crri 1
P~ample 15
S~mthesis of 2-[5-amino-2-(4-fluorophenyl)-6-oxo-1,6-dihydro-1-pyrimidinyl]-N-
[ 1-[(5-aminobenzoxazol-2-yl)carbonyl]-2-phenylethyl]acetamide
Using the title compound of Example 14 and by a reaction similar to that
in Example 4, the title compound was obtained as a yellow solid (228 rng,
83%).
1H-NMR (S00 MHz, DMSO-d6) ~ 8.83 (d, J = 7.0 Hz, 1H),
7.53 (d, J = 8.8 Hz, 1H), 7.36 (m, 2H), 7.28 (s, 1H),
7.26 (t, J = 7.3 Hz, 2H), 7.23-7.17 (m, 3H), 7.13 (t, J = 8.8 Hz, 2H),
6.96 (d, J = 2.2 Hz, 1 H), 6.92 (dd, J = 8.8, 2.2 Hz, 1 H), 5.56 (m, 1 H),
5.35 (s, 2H),
5.14 (s, 2H), 4.48 (d, J = 16.6 Hz, 1H),
4.41 (d, J = 16.6 Hz, 1H), 3.2S (dd, J = 14.1, 4.8 Hz, 1H),
2.93 (dd, J = 14.l, 8.8 Hz, 1H)
IR (KBr) 3250, 3000, 1700, 1650, 1600, 1520, 1500 crri 1
MS (SIMS, positive) m/z 527 (MH+)
43
CA 02269720 1999-04-23
sample 16
Synthesis of 2-[5-benzyloxycarbonylamino-2-(4-ffuorophenyl)-6-oxo-1,6-dihydro-
1- pyrimidinyl)-N-[1-([5-(trifluoromethanesulfonylamino)benzoxazol-2-
yl]carbonyl]-2-phenylethyl]acetamide
To a solution of the title compound ( 149 mg, 0.226 mmol) of Example 14
in THF (3 mL) was added triethylamine (0.038 mL, 0.273 mmol) and the mixture
was cooled to -78~C. Thereto was added dropwise anhydrous
triffuoromethanesulfonic acid (0.046 mL, 0.273 mmol). The obtained mixture
was stirred at the same temperature for 1 hour. Water ( 10 mL) was added and
the mixture was allowed to warm to room temperature and extracted with ethyl
acetate and chloroform. The extract was washed with saturated brine, dried
over
magnesium sulfate and concentrated under reduced pressure. The residue was
separated and purified by silica gel column chromatography (95:5
chloroform:methanol - 90:10 chloroform:methanol) to give the title compound as
a
pale yellow solid ( 145 mg, 81%).
1H-NMR (300 MHz, DMSO-d6) ~ 8.99 (d, J = 6.9 Hz, 1H), 8.90 (s, 1H),
8.41 (s, 1H), 7.96 (d, J = 8.9 Hz, 1H), 7.83 (d, J = 2.0 Hz, 1H),
7.50 (dd, J = 8.9, 2.0 Hz, 1H), 7.44-7.14 (m, 14H), 5.54 (m, 1H),
5.17 (s, 2H), 4.53 (d, J = 16.8 Hz, 1H), 4.45 (d, J = 16.8 Hz, lI-~,
3.4-3.1 (m, 1H), 2.94 (dd, J = 14.1, 9.0 Hz, 1H)
IR (KBr) 3325, 3025, 1705, 1650, 1600, 1520 crri 1
MS (SIMS, positive) m/z 793 (MH+)
Example 17
Synthesis of 2-(5-amino-2-(4-fluorophenyl)-6-oxo-1,6-dihydro-1-pyrimidinyl]-N-
[ 1-[[5-(triffuoromethanesulfonylamino)benzoxazol-2 yl]carbonyl]-2-
phenylethyl]acetamide
Using the title compound of Example 16 and by a reaction similar to that
in Example 4, the title compound was obtained as a pale-yellow solid (67 mg,
58%).
mp 165-170~C
1H-NMR (500 MHz, DMSO-ds) ~ 8.91 (d, J = 6.9 Hz, 1H),
7.91 (d, J = 8.9 Hz, 1 H), 7.79 (d, J = 2.0 Hz, 1 H),
7.48 (dd, J = 8.9, 2.0 Hz, 1H), 7.36 (m, 2H), 7.29 (s, 1H),
7.26 (t, J = 7.3 Hz, 2H), 7.23-7.17 (m, 3H), 7.12 (t, J = 8.8 Hz, 2H),
44
CA 02269720 1999-04-23
5.53 (m, 1H), 4.48 (d, J = 16.6 Hz, 1H), 4.42 (d, J = 16.6 Hz, 1H),
3.31 (dd, J = 14.1, 4.7 Hz, 1H), 2.95 (dd, J = 14.1, 8.9 Hz, 1H)
IR (KBr) 3300, 3000, 1645, 1600, 1520, 1500 crri 1
MS (SIMS, positive) m/z 659 (MH+)
F~ample 18
Synthesis of 2-[5-benzyloxycarbonylamino-2-(4-ffuorophenyl)-6-oxo-1,6-dihydro-
1-pyrimidinyl]-N-[ 1-[(5-methoxybenzoxazol-2-yl)carbonyl]-2-
phenylethyl]acetamide
( 1) Using the title compound of Reference Example 1 and the title compound of
Reference Example 7 and by a reaction similar to that in Example 1-(1), 2-[5-
benzyloxycarbonylamino-2-(4-fluorophenyl)-6-oxo-1,6-dihydro-1-pyrimidinyl]-N-
[ 1-[(5-methoxybenzoxazol-2 yl) hydroxymethyl]-2-phenylethyl]acetamide was
obtained as a pale-red solid (810 mg, 93%).
(2) Using the above-mentioned compound and by a reaction similar to that in
Example 1-(2), the title compound was obtained the as pale-yellow crystals
(906
mg, 57%).
mp 233-235~C
1H-NMR (500 MHz, DMSO-d6 Q8.98 (d, J = 6.9 Hz, 1H),
8.92 (s, 1H), 8.41 (s, 1H), 7.81 (d, J = 9.1 Hz, 1H),
7.52 (d, J = 2.4 Hz, 1H), 7.46-7.41 (m, 4H), 7.38 (t, J = 7.1 Hz, 2H), 7.33
(t, J = 7.1
Hz, 1H), 7.28-7.14 (m, 8H), 5.55 (m, 1H), 5.17 (s, 2H),4.53 (d, J = 16.7 Hz,
1H),
4.44 (d, J = 16.7 Hz, 1H), 3.86 (s, 3H),
3.30 (dd, J = 14.1, 4.5 Hz, 1 H), 2.94 (dd, J = 14.1, 9.0 Hz, 1 H)
IR (KBr) 3390, 3300, 1705, 1660, 1605, 1520 crri 1
MS (SIMS, positive) m/z 676 (MH+)
Ea~mple 19
Synthesis of 2-[5-amino-2-(4-fluor~ophenyl)-6-oxo- 1,6-dihydro- 1-pyrimidinyl]-
N-
[ 1-[(5-methoxybenzoxazol-2-yl)carbonyl]-2-phenylethyl]acetamide
Using the title compound of Example 18 and by a reaction similar to that
in Example 4, the title compound was obtained as pale-yellow crystals (586 mg,
92%).
mp 163-169~C
iH-NMR (500 MHz, DMSO-d6) Q8.92 (d, J = 6.9 Hz, 1H),
7.82 (d, J = 9.1 Hz, 1H), 7.S3 (d, J = 2.4 Hz, 1H),
CA 02269720 1999-04-23
7.35 (dd, J = 8.6, 5.6 Hz, 2H), 7.29-7.18 (m, 7H),
7.11 (t, J = 8.6 Hz, 2H), 5.54 (m, 1H), 5.16 (s, 2H),
4.48 (d, J = 16.5 Hz, 1H), 4.41 (d, J = 16.5 Hz, 1H), 3.86 (s, 3H),
3.30 (dd, J = 14.1, 4.4 Hz, 1H), 2.95 (dd, J = 14.l, 9.1 Hz, 1H)
IR (KBr) 3390, 3270, 170S, 1660, l605, 1505 crri 1
MS (SIMS, positive) m/z 542 (MH+)
Synthesis of 2-[5-amino-2-(4-fluorophenyl)-6-oxo- 1,6-dihydro- 1-pyrimidinyl]-
N-
[ 1-[(5-hydroxybenzoxazol-2-yl)carbonyl]-2-phenylethyl]acetamide
To a solution of the title compound (452 mg, 0.835 mmol) of Example 19
in dichloromethane ( 10 mL) was added under ice-cooling a solution ( 1.0 M,
8.4
mL, 8.4 mmol) of boron tribromide in dichloromethane and the mixture was
stirred at 0~C to room temperature for 4 hours. Methanol ( 1.5 mL) was added
and the mixture was stirred for 10 min. The reaction mixture was added to a
saturated aqueous sodium hydrogencarbonate solution (SO mL) and extracted
with ethyl acetate. The extract was washed with saturated brine, dried over
magnesium sulfate and was concentrated under reduced pressure. The residue
was separated and purified by silica gel column chromatography ( 10:1
chloroform:methanol) to give the title compound as a yellow solid (340 g,
77%).
1H-NMR (500 MHz, DMSO-d6) 89.95 (brs, 1H),
8.89 (d, J = 6.9 Hz, 1H), 7.69 (d, J = 8.9 Hz, 1H),
7.35 (dd, J = 8.5, 5.6 Hz, 2H), 7.29-7.07 (m, 10H), 5.S4 (m, 1H),
5.15 (s, 2H), 4.48 (d, J = 16.5 Hz, 1H), 4.41 (d, J = 16.5 Hz, 1H),
3.27 (dd, J = 14.1, 4.7 Hz, 1H), 2.94 (dd, J = 14.1, 9.0 Hz, 1H)
IR (KBr) 3410, 3290, 170S, 1660, l605, 1520, 1505 crri 1
MS (SIMS, positive) m/z 528 (MH+)
Example 21
Synthesis of 2-[5-benzyloxycarbonylamino-2-(4-fluorophenyl)-6-oxo-1,6-dihydro-
1-pyrimidinyl]-N-(2-phenyl-1-[(2-thiazolyl)carbonyl]ethyl]acetamide
( 1) To a solution of the title compound (724 mg, 1.37 mmol) of Example 9 in
dichloromethane ( 15 mL) was added 2-trimethylsilylthiazole (0.23 mL, 1.4
mmol)
and the mixture was stirred at room temperature for one day. A solution ( 1M,
2.5
mL, 2.5 mmol) of tetrabutyl ammonium fluoride in THF was added and the
mixture was stirred for 30 min. The reaction mixture was added to a saturated
46
CA 02269720 1999-04-23
aqueous sodium hydrogencarbonate solution (50 mL) and extracted with
chloroform. The extract was dried over anhydrous magnesium sulfate and
concentrated under reduced pressure. The residue was separated and purified
by silica gel column chromatography (50: lchloroform:methanol) to give 2-[5-
benzyloxycarbonylamino-2-(4-fluorophenyl)-6-oxo-1,6-dihydro-1-pyrimidinyl]-N-
[2-phenyl-1-[(2-thiazolyl)hydroxymethyl] ethyl]acetamide as a white solid (665
mg,
79%).
(2) Using the above-mentioned compound and by a reaction similar to that in
Example I-(2), the title compound was obtained as white crystals (554 mg,
86%).
mp 209-211~C
1H-NMR (500 MHz, DMSO-d6) 88.92 (s, 1H), 8.85 (d, J = 7.5 Hz, 1H), 8.41 (s,
1H),
8.30 (d, J = 3.0 Hz, 1H), 8.21 (d, J = 3.0 Hz, 1H),
7.47-7.41 (m, 4H), 7.38 (t, J = 7.1 Hz, 2H), 7.33 (t, J = 7.1 Hz, 1H), 7.27-
7.18 (m,
SHj, 7.15 (d, J = 7.0 Hz, 2H), 5.67 (m, 1H), 5.17 (s, 2H),4.55 {d, J = 16.6
Hz, 1H),
4.42 (d, J = 16.6 Hz, 1H),
3.19 (dd, J = 14.0, 4.2 Hz, 1H), 2.88 (dd, J = 14.0, 9.2 Hz, 1H)
IR (KBr) 3360, 1725, 1655, 1605, 1525 cm 1
MS (SIMS, positive) m/z 612 (MH+)
Example 22
Synthesis of 2-[5-amino-2-(4-ffuorophenyl)-6-oxo-1,6-dihydro-1-pyrimidinyl]-N-
[2-phenyl-1-[(2-thiazolyl)carbonyl]ethyl]acetamide
Using the title compound of Example 21 and by a reaction similar to that
in Example 4, the title compound was obtained as white crystals (370 mg,
100%).
mp 199-203~C
1H-NMR (500 MHz, DMSO-dfi) Q8.79 (d, J = 7.5 Hz, 1H),
8.30 (d, J = 2.9 Hz, 1H), 8.21 (d, J = 2.9 Hz, 1H),
7.36 (dd, J = 8.5, 5.6 Hz, 2H), 7.29-7.14 (m, 8H), S.67 (m, 1H),
S.15 (s, 2H), 4.50 (d, J = 16.6 Hz, 1H), 4.39 (d, J = 16.6 Hz, 1H),
3.19 (dd, J = 14.0, 4.3 Hz, 1 H), 2.89 (dd, J = 14.0, 9.2 Hz, 1 H)
IR (KBr) 3380, 1650, 160S, 1500 crri 1
MS (SIMS, positive) m/z 478 (MH+)
Example 23
Synthesis of 2-[5-benzyloxycarbonylamino-2-(4-ffuorophenyl)-6-oxo-1,6-dihydro-
1-pyrimidinyl]-N-[ 1-[(2-oxazolinyl)carbonyl]-2-phenylethyl]acetamide
47
CA 02269720 1999-04-23
( 1 ) Using the title compound of Reference Example 1 and the title compound
of
Reference Example 8 and by a reaction similar to that in Example 1-( 1 ), a
mixture
containing 2-[5-benzyloxycarbonylamino-2-(4-ffuorophenyl)-6-oxo-1,6-dihydro-1-
pyrimidinylJ-N-[ 1-[(2-oxazolinyl)hydroxymethyl]-2-phenylethyl]acetamide was
obtained as a white solid (521 mg).
(2) Using the above-mentioned compound and by a reaction similar to that in
Example 1-(2), the title compound was obtained as white crystals ( 173 mg,
yield
from the title compound of Reference Example 8, 23%).
mp 215-220~C
1H-NMR (300 MHz, DMSO-dfi) Q8.92 (t, J = 5.7 Hz, 1H), 8.90 (s, 1H), 8.68 (d, J
=
7.2 Hz, 1H), 8.42 (s, 1H), 7.5l-7.08 (m, 14H),
5.2S (m, 1H), 5.18 (s, 2H), 4.50 (d, J = 16.8 Hz, 1H),
4.42 (d, J = 16.8 Hz, 1H), 3.68 (t, J = 6.1 Hz, 2H), 3.48 (m, 2H),
3.10 (dd, J = 14.0, 4.2 Hz, 1H), 2.77 (dd, J = 14.0, 8.9 Hz, 1H)
IR (KBr) 3380, 3270, 1725, 1655, 1600, 1520 crri 1
~ple 24
Synthesis of 2-[5-amino-2-(4-fluorophenyl)-6-oxo- 1,6-dihydro-1-pyrimidinyl]-N-
[ 1-[(2-oxazolinyl)carbonyl]-2-phenylethyl]acetamide
Using the title compound of Example 23 and by a reaction similar to that
in Example 4, the title compound was obtained as white crystals (48 mg, 49%).
mp 180-18S~C
1H-NMR (300 MHz, DMSO-ds) Q8.92 (t, J = 5.8 Hz, 1H),
8.62 (d, J = 7.2 Hz, 1H), 7.40 (dd, J = 8.7, 5.5 Hz, 2H),
7.30-7.09 (m, 8H), 5.24 (m, 1H), 5.15 (s, 2H),
4.46 (d, J = 16.7 Hz, 1 H), 4.38 (d, J = 16.7 Hz, 1 H),
3.68 (t, J = 6.2 Hz, 2H), 3.48 (m, 2H), 3.10 (dd, J = 14.0, 4.2 Hz, 1H), 2.78
(dd, J =
14.0, 8.8 Hz, 1H)
IR (KBr) 3350, 1655, 1600, 1530 crri 1
The compounds obtained in the above-mentioned Examples are shown in
Tables 1- 4, wherein Me is methyl, Et is ethyl and Bn is benzyl.
48
CA 02269720 1999-04-23
O cu
O
U ~ U
Z-( Z~ O O O O
Z~. Z~ Z~ Z
N
~~ ~
N
0 LL lL LL U_ lL LL
_ I
I
O~ O~ O
O O O
O
z
A., t- N C'0 d' nt7 cfl
49
Table 2
s
N~R H2C~Y
N Z
RHN ~ ~ ~'
O " O
Example No. R . . RS ~ Y ~ Z
,F
? H
o Co~ute
N o
H ~OW C~ o
9 0~ F . -CH=O.
O F N.
'10 0~
0
O F N CONHEt
11 0~
o
F CONHEt
12 H
<IMG>
51
CA 02269720 1999-04-23
O
0 0 ~ ~ ~o , ~o
w ~ ~ Z~ Z=~
N
O
C7N
LL tL lL 4:. !L Lt-
u~
~Z O ~.
O
z
O~ O T N
N N N N N
52
CA 02269720 1999-04-23
Example 25 - Example 94
The compounds shown in Tables 5 to 9 were synthesized according to the
above-mentioned Examples. In the Tables, Me is methyl and Et is ethyl.
53
CA 02269720 1999-04-23
Table 5
NYR50 H2C~Y
RHN I N~H~Z
O O
Example No. R R5 Y Z
F
25
F - F
26 ~ ~ ~ J'o
p F C02Et
27 ~ J~' ~ I~ ~o
F G02Et
28 H ~ J'o
F N CONHp
29 ~'
F N CONH2
30 H
p F N CONMe2
31 0~' J!
32 H ~F
CONMe2
p F CONEi2
33 ~ ~ ~ ~'o
F N CONEt2
34 H ~ ~ ~' o
O F N N
35 ~ ~ ~ J'o
F N N
36 H ~ ~ J'o
0
37 ~ F
38
3g o
54
CA 02269720 1999-04-23
Table 6
N~ R O H2C~Y
RHN N~ N~
H
O O
Example No. R RS Y Z
F F
40 H
0
41 ~~~ ~ ~ ~o
42 H
0
~ COZEt
43
C02Et
44 H
O N
45 ~~~
N
46 H
0
0
47
Me O
48 H
Me ~
~ C02Me
49
Me O
COpMe
50 H
Me O
~ C02Et
51
Me
C02Et
52 H ~Me
O CONHZ
53
Me
CONH2
54 H
Me O
CA 02269720 1999-04-23
Table 7
N~ R O H2C~Y
RHN N''' N~
H
O O
Example R R5 Y Z
No.
O CONHEt
55 ~~ Ms ~o
H M ~OCONHEt
56
O CONEtz
57 ~o~ Me
H ~CONEtp
58
0 Me
59
Me
60 H ~M8
0
61 ~co2Me
~o
~ 0
N C02Me
62 H
O N - C02Et
63
64 H . ~ 2Et
0
0
65 I O~ ~OCONH2
66 H ~CONH2
/' j~ III .~I'O
O N CONHEt
67
N CONHEi
68 H ~ ~ ~o
O N CONEt2
69
56
, CA 02269720 1999-04-23
Table 8
N~ R O H2C~Y
RHN N~ N~ Z
H "
O O
Example No. R R5 Y Z
~CONEtp
7~ ~'ul~~[~ (~''[o
O
71 ~
72 H
0
O COpMe
73 ~~ ~1 ~ ~o
74 co2Me
H
C02Me
75
COpMe
76
O C02Me
77 CI
C02Me
78 H ,l
o
O cl C02Me
79
N02
COpMe
80 H
O N02 C02Et
81 ~~ CI J'o
CI ~~C02Et
82 H
0
O C02E1
83 ~~ ~I ,l
co2Et
84 H I~ J:
o
cl
57
CA 02269720 1999-04-23
Table 9
N~ R 0 H2C~Y
RHN N v N~
H
O O
Example No. R RS Y Z
0
85 ~~~ ~oMe
86 H
OMe
~ C02Me
OMe
~OMe ~ ~C02Me
88 H I I /'i I
~ C02Et
89 ~ ~ OMe
H ~OMe ~ ~C02Et
~ C02Me
91
~C02Me
92 H I I ~'',.'i I
H ~NH2 ~O~C02Me
93
94 Met
58
CA 02269720 1999-04-23
The spectrum data of the representative compounds from the
compounds obtained in the above-mentioned Examples are shown in the
following.
Compound of Example 26
2-[5-amino-2-(4-fluorophenyl)-6-oxo- 1,6-dihydro-1-pyrimidinyl]-N-[ 1-[(5-
fluorobenzoxazol-2-yl)carbonyl]-2-phenylethyl]acetamide
mp 222-227~C
1H-NMR (500 MHz, DMSO-d6) Q8.95 (d, J = 6.8 Hz, 1H),
7.98 (dd, J = 9.2, 4.3 Hz, 1H), 7.9l (dd, J = 8.4, 2.5 Hz, 1H),
7.S5 (dt, J = 2.5, 9.2 Hz, 1H), 7.35 (dd, J = 8.7, 5.6 Hz, 2H),
7.29-7.18 (m, 6H), 7.10 (t, J = 8.7 Hz, ~2H), 5.S0 (m, 1H), 5.15 (s, 2H), 4.48
(d, J =
16.7 Hz, 1H), 4.40 (d, J = 16.7 Hz, 1H), 3.30 (dd, J = l4.1, 4.7 Hz, 1H), 2.96
(dd, J
= 14.1, 8.9 Hz, 1H)
IR (KBr) 3330, 3020, 1710, 1655, 1605, 1525, 1505 crri 1
MS (SIMS, positive) m/z 530 (MH+)
Compound of Example 28
2-[5-amino-2-(4-fluorophenyl)-6-oxo- 1,6-dihydro-1-pyrimidinyl]-N-[ 1-[[5-
(ethoxycarbonyl)benzoxazol-2-yl)carbonyl]-2-phenylethyl]acetamide
mp 231-234~C
'H-NMR (300 MHz, DMSO-dfi) Q8.98 (d, J = 6.5 Hz, 1H),
8.51 (d, J = 1.4 Hz, 1H), 8.24 (dd, J = 8.7, 1.6 Hz, 1H),
8.05 (d, J = 8.7 Hz, 1H), 7.40-7.00 (m, 10H), 5.50 (m, 1H),
5.14 (s, 2H), 4.56-4.31 (m, 4H), 3.30 (m, 1H), 2.96 (m, 1H),
1.37(t,J=7.1Hz,3H)
IR (KBr) 3300, 1710, 1655, 1600, 1500 crri 1
MS (SIMS, positive) m/z 584 (MH+)
Compound of sample 30
2-[5-amino-2-(4-fluorophenyl)-6-oxo- 1,6-dihydro- 1-pyrimidinyl]-N-[ 1-[(5-
carbamoylbenzoxazol-2 yl)carbonyl]-2-phenylethyl]acetamide
mp 270~C
1H-NMR (300 MHz, DMSO-d6) Q8.96 (d, J = 6.8 Hz, 1H),
8.51 (s, 1H), 8.24-8.11 (m, 2H), 7.98 (d, J = 8.7 Hz, 1H),
7.56 (brs, 1H), 7.35 (dd, J = 8.4, 5.6 Hz, 2H), 7.30-7.15 (m, 7H),
7.10 (t, J = 8.7 Hz, 2H), 5.53 (m, 1H), 5.14 (s, 2H), 4.S8-4.30 (m, 2H), 3.30
(m, 1H),
59
CA 02269720 1999-04-23
2.96 (m, 1H)
IR (KBr) 3350, 1650, 1600 cm 1
Compound of Example 31
2-[5-benzyloxycarbonylamino-2-(4-fluorophenyl)-6-oxo-1,6-dihydro-1-
pyrimidinyl]-N-[1-[[5-(dimethylaminocarbonyl)benzoxazol-2-yl)carbonyl]-2-
phenylethyl]acetamide
mp 222-226~C
1H-NMR (300 MHz, DMSO-d6) Q9.00 (d, J = 6.8 Hz, 1H),
8.88 (s, 1H), 8.41 (s, 1H), 8.05 (s, 1H), 7.96 (d, J = 8.6 Hz, 1H),
7.67 (dd, J = 8.5, 1.5 Hz, 1H), 7.S5-7.02 (m, 14H), S.57 (m, 1H),
5.l7 (s, 2H), 4.54 (d, J = 16.4 Hz, 1H), 4.45 (d, J = 17.1 Hz, 1H),
3.16-2.65 (m, 8H)
IR (KBr) 3350, 1650, 1500 crri 1
Compound of sample 32
2-[5-amino-2-(4-fluorophenyl)-6-oxo-1,6-dihydro-1-pyrimidinyl]-N-[ 1-[[5-
(dimethylaminocarbonyl)benzoxazol-2 yl]carbonyl]-2-phenylethyl]acetamide
mp 129-133~C
1H-NMR (300 MHz, DMSO-d fi) ~ 8.94 (d, J = 6.8 Hz, 1 H),
8.05 (d, J = 1.2 Hz, 1H), 7.97 (d, J = 8.6 Hz, 1H),
7.68 (dd, J = 8.5, 1.5 Hz, 1H), 7.48-7.00 (m, 10H), 5.56 (m, 1H),
5.13 (d, J = 6.7 Hz, 2H), 4.49 (d, J = 16.8 Hz, 1H),
4.41 (d, J = 16.7 Hz, 1H), 3.12-2.72 (m, 8H)
IR (KBr) 33S0, 1650, 1610,-1500 crri 1
MS (SIMS, positive) m/z S83 (MH+)
Compound of Example 34
2-[5-amino-2-(4-fluorophenyl)-6-0~-1,6-dihydro-1-pyrimidinyl]-N-[ 1-[[5-
(diethylaminocarbonyl)benzoxazol-2-yl]carbonyl]-2-phenylethyl]acetamide
mp 120-124~C
1H-NMR (300 MHz, DMSO-d6) Q8.94 (d, J = 6.8 Hz, 1H),
7.98 (s, 1 H), 7.97 (d, J = 8.8 Hz, 1 H), 7.61 (dd, J = 8.5, 1.3 Hz, 1 H),
7.50-6.85 (m,
10H), 5.57 (m, 1H), 5.14 (s, 2H),
4.49 (d, J = 16.9 Hz, 1H), 4.42 (d, J = 17.2 Hz, 1H), 3.60-2.85 (m, 6H), 1.30-
0.90
(m, 6H)
IR (KBr) 3350, 1650, 1600 cm 1
CA 02269720 1999-04-23
MS (SIMS, positive) m/z 611 (MH+)
Compound of Example 36
2-[5-amino-2-(4-fluorophenyl)-6-oxo- 1,6-dihydro- 1-pyrimidinyl]-N-[ 1-
[(oxazolo[4,5-b]pyridin-2 yl)carbonyl]-2-phenylethyl]acetamide
mp 222-225~C
1H-NMR (500 MHz, DMSO-d6) 89.01 (d, J = 6.7 Hz, 1H),
8.76 (dd, J = 4.7, 1.1 Hz, 1H), 8.42 (dd, J = 8.3, 1.1 Hz, 1H),
7.70 (dd, J = 8.3, 4.7 Hz, 1H), 7.35 (dd, J = 8.7, 5.6 Hz, 2H),
7.29-7.19 (m, 6H), 7.09 (t, J = 8.7 Hz, 2H), 5.48 (m, 1H), 5.14 (s, 2H), 4.48
(d, J =
16.8 Hz, 1H), 4.40 (d, J = 16.8 Hz, 1H), 3.34 (m, 1H),
2.98 (dd, J = 14.1, 8.9 Hz, 1H)
IR (KBr) 3390, 1715, 1655, 1605, 1530, 1505 crri 1
MS (SIMS, positive) m/z 513 (MH+)
Compound of Example 38
2-[5-amino-2-(4-fluorophenyl)-6-oxo- 1,6-dihydro-1-pyrimidinyl]-N-[ 1-
[(benzothioazol-2-yl)carbonyl]-2-phenylethyl]acetamide
mp 196-200~C
1H-NMR (500 MHz, DMSO-ds) Q8.90 (d, J = 7.2 Hz, 1H),
8.28 (m, 2H), 7.68 (m, 2H), 7.35 (dd, J = 8.6, 5.5 Hz, 2H),
7.29-7.18 (m, 6H), 7.10 (t, J = 8.6 Hz, 2H), 5.7S (m, 1H), 5.16 (s, 2H), 4.52
(d, J =
16.5 Hz, 1H), 4.40 (d, J = 16.5 Hz, 1H),
3.27 (dd, J = 14.0, 4.5 Hz, 1 H), 2.97 (dd, J = 14.0, 9.0 Hz, 1 H)
IR (KBr) 3400, 3290, 305U, 1655, 1605, 1S55, 1505 crri 1
MS (SIMS, positive) m/z 528 (MH+)
Compouad of E~cample 40
2-[5-amino-2-(4-fluorophenyl)-6-oxo- 1,6-dihydro-1-pyrimidinyl]-N-[ 1-
[(benzoxazol-2-yl)carbonyl]-2-(4-fluorophenyl)ethyl]acetamide
mp 245-249~C
1H-NMR (300 MHz, DMSO-d6) Q8.94 (d, J = 7.0 Hz, 1H),
8.01 (d, J = 7.9 Hz, 1H), 7.92 (d, J = 8.2 Hz, 1H),
7.67 (t, J = 7.52 Hz, 1H), 7.56 (t, J = 7.4 Hz, 1H),
7.35 (dd, J = 8.5, 5.5 Hz, 2H), 7.28 (s, 1H),
7.25 (dd, J = 8.4, 5.6 Hz, 2H), 7.08 (t, J = 8.8 Hz, 4H), 5.51 (m, 1H), 5.15
(s, 2H),
4.48 (d, J = 16.9 Hz, 1H), 4.39 (d, J = 16.5 Hz, 1H),
61
CA 02269720 1999-04-23
3.29 (dd, J = 14.1, 4.8 Hz, 1H), 2.9S (dd, J = 14.0, 9.1 Hz, 1H)
IR (KBr) 3350, 1650, 1600, 1500 crri 1
MS (SIMS, positive) m/z 530 (MH+)
Compouad of Example 45
2-(5-benzyloxycarbonylamino-6-oxo-2-phenyl-1,6-dihydro-1-pyrimidinyl)-N-[ 1-
[(oxazolo[4,5-b]pyridin-2 yl)carbonyl]-2-phenylethyl]acetamide
mp 220-223~C
iH-NMR (300 MHz, DMSO-d6) Q9.04 (d, J = 6.7 Hz, 1H),
8.84 (s, 1H), 8.76 (dd, J = 4.7, 1.4 Hz, 1H), 8.44-8.38 (m, 2H),
7.69 (dd, J = 8.3, 4.7 Hz, 1H), 7.46-7.14 (m, 15H), 5.49 (m, 1H),
5.18 (s, 2H), 4.S1 (d, J = 16.6 Hz, 1H), 4.44 (d, J = 16.6 Hz, 1H),
3.34 (dd, J = 14.0, 5.0 Hz, 1H), 2.98 (dd, J = 14.0, 8.8 Hz, 1H)
IR (KBr) 3350, l720, 1655, 1600, 1510 crri 1
MS (SIMS, positive) m/z 629 (MH+)
Compouad of E,~mple 46
2-(5-amino-6-oxo-2-phenyl-1,6-dihydro-1-pyrimidinyl)-N-( 1-[(oxazolo[4,5-
b]pyridin-2 yl)carbonyl]-2-phenylethyl]acetamide
1H-NMR (300 MHz, DMSO-d6) d8.98 (d, J = 6.6 Hz, 1H),
8.76 (dd, J = 4.7, 1.2 Hz, 1H), 8.42 (dd, J = 8.3, 1.2 Hz, 1H),
7.70 (dd, J = 8.3, 4.7 Hz, 1H), 7.39-7.17 (m, 11H), 5.48 (m, 1H),
5.11 (s, 2H), 4.47 (d, J = 16.6 Hz, 1H), 4.40 (d, J = 16.6 Hz, 1H),
3.32 (m, 1H), 2.99 (dd, J = 14.0, 8.8 Hz, 1H)
IR (KBr) 3380, 1715, 1655, 1610, l530 cm 1
MS (SIMS, positive) m/z 495 (MH+)
Compouad of Example 50
2-[5-amino-6-oxo-2-(m-tolyl)-1,6-dihydro-1-pyrimidinyl]-N-[ 1-[[5-
(methoxycarbonyl)benzoxazol-2 yl]carbonyl]-2-phenylethyl]acetamide
mp 185-188~C
1H-NMR (500 MHz, DMSO-d6) Q8.95 (d, J = 6.5 Hz, 1H),
8.51 (d, J = 0.7 Hz, 1H), 8.23 (dd, J = 8.7, 0.8 Hz, 1H),
8.05 (d, J = 8.7 Hz, 1H), 7.30-7.06 (m, 10H), 5.53 (m, 1H),
5.10 (s, 2H), 4.46 (s, 2H), 3.93 (s, 3H),
3.30 (dd, J = 14.0, 5.0 Hz, 1H), 2.99 (dd, J = 14.0, 8.5 Hz, 1H),
2.28 (s, 3H)
62
CA 02269720 1999-04-23
IR (KBr) 3250, 3000, 2925, 1710, 1655, 1605, 1515 crri 1
MS (SIMS, positive) m/z 566 {MH')
Compound of Example 52
2-[5-amino-6-oxo-2-(m-tolyl)-1,6-dihydro-1-pyrimidinyl]-N-[ 1-((5-
(ethoxycarbonyl)benzoxazol-2-yl]carbonyl]-2-phenylethyl]acetamide
mp 197-199~C
1H-NMR (300 MHz, DMSO-d6) Q8.94 (d, J = 6.5 Hz, 1H),
8.51 (s, IH), 8.23 (dd, J = 8.7, 1.7 Hz, 1H), 8.04 (d, J = 8.7 Hz, 1H), 7.35-
7.00 (m,
10H), 5.51 (m, 1H), 5.09 (brs, 2H), 4.46 (s, 2H),
4.39 (q, J = 7.1 Hz, 2H), 3.30 (m, 1H), 2.98 (m, 1H), 2.27 (s, 3H),
1.37(t,J=7.1 Hz,3H)
IR (KBr) 3350, 1710, 1655, 1610 crri 1
MS (SIMS, positive) m/z 580 (MH+)
Compound of Example 53
2-[5-benzyloxycarbonylamino-6-oxo-2-(m-tolyl)-1,6-dihydro-1-pyrimidinyl]-N-
[ 1-((5-carbamoylbenzoxazol-2-yl)carbonyl]-2-phenylethyl]acetamide
mp 200-208~C
iH-NMR (300 MHz, DMSO-d~) 88.99 (d, J = 6.6 Hz, 1H),
8.86 (s, 1H), 8.50 (d, J = 1.3 Hz, 1H), 8.40 (s, 1H),
8.17 (dd, J = 8.8, 1.6 Hz, 1H), 7.97 (d, J = 8.7 Hz, 1H),
7.57 (brs, 1H), 7.50-7.05 (m, 15H), 5.53 (m, 1H), 5.17 (s, 2H),
4.50 (brs, 2H), 3.30 (m, 1H), 2.97 (m, 1H), 2.29 (s, 3H)
IR (KBr) 3250, 1655, l510 cm 1
Compound of Example 54
2-[5-amino-6-oxo-2-(m-tolyl)-1,6-dihydro-1-pyrimidinyl]-N-[ 1-[(5-
carbamoylbenzoxazol-2 yl) carbonyl]-2-phenylethyl]acetamide
mp 262-264~C
iH-NMR (300 MHz, DMSO-d6) Q8.93 (d, J = 6.7 Hz, 1H),
8.50 (d, J = 1.3 Hz, 1H), 8.17 (dd, J = 8.8, 1.6 Hz, 1H),
7.97 (d, J = 8.8 Hz, 1H), 7.57 (brs, 1H), 7.34-7.00 (m, 11H),
5.53 (m, 1H), 5.10 (brs, 2H), 4.46 (brs, 2H), 3.30 (m, 1H),
2.98 (m, 1H), 2.27 (s, 3H)
IR (KBr) 3350, 1650 cm 1
MS (SIMS, positive) m/z 551 (MH+)
63
CA 02269720 1999-04-23
Compound of Example 55
2-[5-benzyloxycarbonylamino-6-oxo-2-(m-tolyl)-1,6-dihydro-1-pyrimidinyl]-N-
[ 1-[[5-(ethylaminocarbonyl)benzoxazol-2 yl]carbonyl]-2-phenylethyl]acetamide
mp 207-2l4~C
iH-NMR (300 MHz, DMSO-d6) Q8.99 (d, J = 8.7 Hz, 1H),
8.86 (brs, 1 H), 8.67 (brs, 1 H), 8.45 (d, J = 1.3 Hz, 1 H), 8.40 (s, 1 H),
8.14 (dd, J = 8.7,
1.7 Hz, 1 H), 7.98 (d, J = 8.7 Hz, 1 H),
7.5S-7.08 (m, 14H), 5.54 (m, 1H), S.17 (s, 2H), 4.50 (brs, 2H),
3.30 (m, 1H), 2.97 (m, 1H), 2.29 (s, 3H), 1.16 (t, J = 7.2 Hz, 3H)
IR (KBr) 3350, 1655, 1510 crri 1
Compound of ample 56
2-[5-amino-6-oxo-2-(m-tolyl)-1,6-dihydro-1-pyrimidinyl]-N-[ 1-[[5-
(ethylaminocarbonyl)benzoxazol-2-yl]carbonylJ-2-phenylethyl]acetamide
mp 212-214~C
'H-NMR (300 MHz, DMSO-d6) Q8.93 (d, J = 6.8 Hz, 1H),
8.67 (brs, 1H), 8.46 (s, 1H), 8.14 (d, J = 8.8 Hz, 1H),
7.98 (d, J = 8.8 Hz, 1H), 7.48-7.02 (m, 10H), 5.53 (m, 1H),
5.10 (s, 2H), 4.46 (brs, 2H), 3.3S (q, J = 7.2 Hz, 2H), 3.30 (m, 1H),
2.97 (m, 1H), 2.27 (s, 3H), 1.16 (t, J = 7.1 Hz, 3H)
IR (KBr) 3350, 1640, 1605, 1510 crri 1
MS (SIMS, positive) m/z 579 (MH+)
Compound of E~mple 57
2-[5-benzyloxycarbonylamino-6-o~w-2-(m-tolyl)-1,6-dihydro-1-pyrimidinyl]-N
[ 1-[[5-(diethylaminocarbonyl)benzoxazol-2 yl]carbonyl]-2-
phenylethyl]acetamide
mp 95-100~C
iH-NMR (300 MHz, DMSO-d6) Q8.99 (d, J = 6.5 Hz, 1H),
8.86 (brs, 1H), 8.41 (s, 1H), 7.97 (s, 1H), 7.96 (d, J = 8.0 Hz, 1H),
7.61 (dd, J = 8.7, 1.4 Hz, 1H), 7.50-6.95 (m, 14H), S.55 (m, 1H),
5.17 (s, 2H), 4.50 (brs, 2H), 3.60-2.82 (m, 6H), 2.30 (s, 3H),
1.30-0.80 (m, 6H)
IR (KBr) 3350, 1720, 1650, 1505 crri 1
Compound of E~rampse 58
2-[5-amino-6-oxo-2-(m-tolyl)-1,6-dihydro-1-pyrimidinyl]-N-[ 1-[[5-
(diethylaminocarbonyl)benzoxazol-2-yl]carbonyl]-2-phenylethyl]acetamide
64
CA 02269720 1999-04-23
mp 117-120~C
1H-NMR (300 MHz, DMSO-d6) Q8.92 (d, J = 6.6 Hz, 1H),
7.97 (s, 1 H), 7.96 (d, J = 7.2 Hz, 1 H), 7.61 (dd, J = 8.6, 1.3 Hz, 1 H),
7.40-6.92 (m,
10H), 5.54 (m, 1H), 5.10 (s, 2H), 4.46 (brs, 2H),
3.60-2.90 (m, 6H), 2.28 (s, 3H), 1.30-0.90 (m, 6H)
IR (KBr) 3400, 16l0, 1520 cm 1
MS (SIMS, positive) m/z 607 (MH+)
Compound of Example 59
2-[5-benzyloxycarbonylamino-6-oxo-2-(m-tolyl)-1,6-dihydro-1-pyrimidinyl]-N-
[ 1-[(oxazolo[4,5-b]pyridin-2-yl)carbonyl]-2-phenylethyl]acetamide
mp 223-227~C
1H-NMR (300 MHz, DMSO-d6) Q9.03 (d, J = 6.6 Hz, 1H),
8.83 (s, 1H), 8.76 (dd, J = 4.7, 1.4 Hz, 1H), 8.43-8.38 (m, 2H),
7.69 (dd, J = 8.4, 4.7 Hz, 1H), 7.47-7.12 (m, 14H), 5.S1 (m, 1H),
5.17 (s, 2H), 4.50 (s, 2H), 3.33 (dd, J = 14.1, 4.9 Hz, 1H),
2.99 (dd, J = 14.1, 8.7 Hz, 1H), 2.29 (s, 3H)
IR (KBr) 3360, 3010, 1720, 1655, 1600, 1505 cm 1
MS (SIMS, positive) m/z 643 (MH+)
Compouad of E~mple 60
2-[5-amino-6-oxo-2-(m-tolyl)-1,6-dihydro-1-pyrimidinyl]-N-[1-[(oxazolo[4,5-
b]pyridin-2 yl)carbonyl]-2-phenylethyl]acetamide
1H-NMR (300 MHz, DMSO-d6) Q8.97 (d, J = 6.5 Hz, 1H),
8.76 (dd, J = 4.7, 1.4 Hz, 1H), 8.42 (dd, J = 8.4, 1.4 Hz, 1H),
7.70 (dd, J = 8.4, 4.7 Hz, 1H), 7.29-7.04 (m, 10H), 5.50 (m, 1H),
5.09 (s, 2H), 4.45 (s, 2H), 3.30 (m, 1H),
2.99 (dd, J = 14.1, 8.8 Hz, 1H), 2.27 (s, 3H)
IR (KBr) 3260, 1715, 1655, 1605, 1525 crrm 1
MS (SIMS, positive) m/z 509 (MH+)
Compound of Example 62
2-[5-amino-6-oxo-2-(3-pyridyl)-1,6-dihydro-1-pyrimidinyl]-N-[ 1-[[5
(methoxycarbonyl)benzoxazol-2 yl]carbonyl]-2-phenylethyl]acetamide
mp 141-147~C
1H-NMR (500 MHz, DMSO-d6) Q9.00 (d, J = 6.7 Hz, 1H),
8.58-8.53 (m, 2H), 8.53 (d, J = 1.5 Hz, 1H),
CA 02269720 1999-04-23
8.23 (dd, J = 8.7, 1.7 Hz, 1H), 8.04 (d, J = 8.7 Hz, 1H),
7.71 (dt, J = 7.9, 1.9 Hz, 1H), 7.35-7.15 (m, 7H), 5.52 (m, 1H),
5.24 (s, 2H), 4.52, 4.48 (ABq, J = 16.9 Hz, 2H), 3.93 (s, 3H),
3.32-3.26 (m, 1H), 2.97 (dd, J = l4.1, 8.6 Hz, 1H)
IR (KBr) 3275, 3000, 2925, 1710, 16S5, 1605, 1525 crri 1
MS (SIMS, positive) m/z 553 (MH+)
Compound of ample 64
2-[5-amino-6-oxo-2-(3-pyridyl)-1,6-dihydro-1-pyrimidinylJ-N-( 1-[[5-
(ethoxycarbonyl)benzoxazol-2-yl]carbonyl]-2-phenylethyl]acetamide
mp 148-152~C
1H-NMR (5~D MHz, DMSO-d6) Q9.00 (d, J = 6.8 Hz, 1H),
8.59-8.50 (m, 3H), 8.23 (dd, J = 8.7, 1.6 Hz, 1H),
8.04 (d, J = 8.7 Hz, 1H), 7.7l (dt, J = 8.0, 1.9 Hz, 1H),
7.35-7.16 (m, 7H), 5.52 (m, 1H), 5.24 (s, 2H),
4.S4 (d, J = 17.0 Hz, 1H), 4.47 (d, J = 17.0 Hz, 1H),
4.39 (q, J = 7.1 Hz, 2H), 3.30 (dd, J = 14.1, 4.8 Hz, 1H),
2.96 (dd, J = 14.1, 8.7 Hz, 1H), 1.38 (t, J = 7.1 Hz, 3H)
IR (KBr) 3400, 3300, 1710, 1655, 1605, 1525 crri 1
MS (SIMS, positive) m/z 567 (MH+)
Compound of Example 71
2-[5-benzyloxycarbonylamino-6-oxo-2-(3-pyridyl)-1,6-dihydro- 1-pyrimidinylJ-N-
[ 1-[(oxazolo[4,5-b]pyridin-2 yl)carbonylJ-2-phenylethylJacetamide
mp 190-195~C
1H-NMR (300 MHz, DMSO-d6) cS9.08 (d, J = 6.8 Hz, 1H),
8.93 (s, 1H), 8.75 (dd, J = 4.7, 1.4 Hz, 1H), 8.63-8.58 (m, 2H),
8.44 (s, 1H), 8.41 (dd, J = 8.4, 1.4 Hz, 1H),
7.77 (dt, J = 8.0, 1.9 Hz, 1H), 7.69 (dd, J = 8.4, 4.7 Hz, 1H),
7.47-7.17 (m, 11H), 5.49 (m, 1H), 5.18 (s, 2H),
4.56 (d, J = 16.9 Hz, 1 H), 4.49 (d, J = 16.9 Hz, 1 H), 3.33 (m, 1 H),
2.97 (dd, J = 14.0, 8.7 Hz, 1 H)
IR (KBr) 3380, 3280, 1720, 16S5, 1600, 1510 crri 1
MS (SIMS, positive) m/z 630 (MH+)
Compound of ample 72
2-[5-amino-6-oxo-2-(3-pyridyl)-1,6-dihydro-1-pyrimidinyl]-N-[ 1-[(oxazolo[4,5-
66
CA 02269720 1999-04-23
b]pyridin-2 yl)carbonyl]-2-phenylethyl]acetamide
1H-NMR (300 MHz, DMSO-d6) Q9.03 (d, J = 6.7 Hz, 1H),
8.76 (dd, J = 4.7, 1.4 Hz, 1H), 8.57-8.50 (m, 2H),
8.42 (dd, J = 8.4, 1.4 Hz, 1H), 7.72-7.65 (m, 2H), 7.32-7.17 (m, 7H), 5.48 (m,
1H),
5.23 (s, 2H), 4.53 (d, J = 16.8 Hz, 1H),
4.45 (d, J = 16.8 Hz, 1H), 3.33 (m, 1H),
2.98 (dd, J = 14.0, 8.6 Hz, 1 H)
IR (KBr) 33S0, 1710, 1655, 160S, 1525 crri 1
MS (SIMS, positive) m/z 496 (MH+)
Compouad of ample ?3
2-[5-benzyloxycarbonylamino-6-oxo-2-(4-pyridyl)-1,6-dihydro- 1-pyrimidinyl]-N-
[ 1-[[5-(methoxycarbonyl)benzoxazol-2-yl]carbonyl]-2-phenylethyl]acetamide
1H-NMR (300 MHz, DMSO-d6) Q9.05 (d, J = 6.8 Hz, 1H),
8.96 (s, 1 H), 8.61 (dd, J = 4.5, 1.5 Hz, 2H), 8.53 (d, J = 1.5 Hz, 1 H), 8.44
(s, 1 H),
8.23 (dd, J = 8.7, 1.6 Hz, 1 H), 8.04 (d, J = 8.7 Hz, 1 H), 7.48-7.14 (m,
12H), 5.56 (m,
1 H), 5.18 (s, 2H), 4.50 (m, 2H),
3.92 (s, 3H), 3.38-3.23 (m, 1H), 2.96 (dd, J = 14.1, 8.8 Hz, 1H)
IR (KBr) 3275, 3025, 2925, 1720, 1660, 1595, 1510 crri 1
MS (SIMS, positive) m/z 687 (MH+)
Compound of sample ?4
2-[5-amino-6-oxo-2-(4-pyridyl)-1,6-dihydro-1-pyrimidinyl]-N-[ 1-[[5-
(methoxycarbonyl)benzoxazol-2 yl]carbonyl]-2-phenylethyl]acetamide
mp 238-240~C
1H-NMR (500 MHz, DMSO-ds) Q9.02 (d, J = 6.7 Hz, 1H),
8.57-8.S0 (m, 3H), 8.24 (dd, J = 8.7, 1.6 Hz, 1H),
8.0S (d, J = 8.7 Hz, 1H), 7.35-7.18 (m, 8H), 5.56 (m, 1H),
5.31 (brs, 2H), 4.48 (m, 2H), 3.93 (s, 3H), 3.38-3.29 (m, 1H),
2.97(dd,J=14.1,8.7 Hz,lH)
IR (KBr) 3275, 3025, 2950, 1710, 1655, 1610, 1590, 1530 crri 1
MS (SIMS, positive) m/z 553 (MH+)
Compound of ~anple ?6
2-[5-amino-2-(3-fluorophenyl)-6-oxo- 1,6-dihydro- 1-pyrimidinyl]-N-j 1-[[5-
(methoxycarbonyl)benzoxazol-2-yl]carbonyl]-2-phenylethyl]acetamide
mp 194.5-196~C
67
CA 02269720 1999-04-23
1H-NMR (300 MHz, DMSO-d6) 8 8.99 (d, J = 6.7 Hz, 1H),
8.51 (d, J = 1.6 Hz, 1H), 8.23 {dd, J = 8.7, 1.6 Hz, 1H),
8.04 (d, J = 8.7 Hz, 1H), 7.40-7.11 {m, lOH), 5.53 (m, 1H),
5.19 (brs, 2H), 4.52 (d, J = 17.2 Hz, 1H), 4.46 (d, J = 17.2 Hz, 1H),
3.93 (s, 3H), 3.31 (dd, J = 14.0, 4.7 Hz, 1H),
2.98 (dd, J = 14.0, 8.8 Hz, 1 H)
IR {KBr) 3450, 3350, 3080, 2960, 1720, 1675, l660, 1610, 1585,
1530 crri 1
MS (APCI, positive) m/z 570 (MH+)
Compouad of Example T8
2-[5-amino-2-(3-chlorophenyl)-6-oxo-1,6-dihydro-1-pyrimidinyl]-N-[ 1-[[5-
(methoxycarbonyl)benzoxazol-2 yl]carbonyl]-2-phenylethyl]acetamide
mp 197-199~C
1H-NMR (300 MHz, DMSO-dfi) ~ 9.00 (d, J = 6.8 Hz, 1H),
8.S1 (d, J = 1.6 Hz, 1H), 8.23 (dd, J = 8.7, 1.6 Hz, 1H),
8.04 (d, J = 8.7 Hz, 1H), 7.48-7.16 (m, 10H), 5.53 (m, 1H),
5.21 (brs, 2H), 4.53 (d, J = 16.8 Hz, 1 H), 4.45 (d, J = 16.8 Hz, 1 H),
3.93 (s, 3H), 3.30 (dd, J = 14.1, 4.9 Hz, 1 H),
2.99 (dd, J = 14.1, 8.7 Hz, 1H)
IR (KBr) 3420, 3280, 3080, 3020, 2950, 1720, 1675, 1660, 1610,
156S, 1530 crri 1
MS (APCI, positive) m/z 586 (MH+)
Compouad of Example 80
2-[5-amino-2-(3-nitrophenyl)-6-oxo- 1,6-dihydro-1-pyrimidinyl]-N-[ 1-[[5-
(methoxycarbonyl)benzoxazol-2 yl]carbonyl]-2-phenylethyl]acetamide
mp 202-203.5~C
1H-NMR (300 MHz, DMSO-d6) ~ 9.00 (d, J = 6.9 Hz, 1H),
8.50 (d, J = 1.4 Hz, 1H), 8.22 (dd, J = 8.7, 1.4 Hz, 1H),
8.21 (brs, 2H), 8.03 (d, J = 8.7 Hz, 1H), 7.78 (d, J = 7.8 Hz, 1H),
7.61 (t, J = 7.8 Hz, 1 H), 7.32 (s, 1 H), 7.28-7.13 (m, 5H),
5.53 (m 1H), 5.29 (brs, 2H), 4.57 (d, J = 16.5 Hz, 1H),
4.51 (d, J = 16.5 Hz, 1H), 3.93 (s, 3H),
3.29 (dd, J = 14.0, 4.8 Hz, 1H), 2.94 (dd, J = 14.0, 8.7 Hz, 1H)
IR (KBr) 3470, 3350, 3080, 2960, 1720, 1665, 1605, 1530, crri 1
68
CA 02269720 1999-04-23
MS (APCI, positive) m/z S97 (MH+)
Compound of sample 82
2-[5-amino-2-(4-chlorophenyl)-6-oxo-1,6-dihydro- 1-pyrimidinyl]-N-[1-[[5-
(ethoxycarbonyl)benzoxazol-2-yl]carbonyl]-2-phenylethyl]acetamide
1H-NMR (300 MHz, DMSO-dfi) ~ 9.02 (d, J = 6.7 Hz, 1H),
8.52 (d, J = 1.4 Hz, 1 H), 8.25 (d, J = 8.7, 1.4 Hz, 1 H),
8.06 (d, J = 8.7 Hz, 1H), 7.31-7.18 (m, 10H), 5.48 (m, 1H),
S.19 (brs, 2H), 4.48 (d, J = 16.6 Hz, 1H), 4.39 (d, J = 16.6 Hz, 1H),
4.37 (q, J = 7.1 Hz, 2H), 3.33 (dd, J = 14.0, 4.3 Hz, 1H),
2.97 (dd, J = 14.0, 8.9 Hz, 1 H), 1.37 (t, J = 7.1 Hz, 3H)
IR (KBr) 3380, 3280, 3040, 17l5, 1660, 1605, 1550, 1520, crri 1
MS (SIMS, positive) m/z 600 (MH+)
Compouad of sample 84
2-[5-amino-2-(3-chlorophenyl)-6-oxo-1,6-dihydro- 1-pyrimidinyl]-N-[ 1-[[5-
(ethoxycarbonyl)benzoxazol-2-yl]carbonylJ-2-phenylethyl]acetamide
mp 23S-237~C
1H-NMR (300 MHz, DMSO-ds) d' 8.99 (d, J = 6.4 Hz, 1H),
8.51 (d, J = 1.5 Hz, 1H), 8.23 (dd, J = 8.7, 1.5 Hz, 1H),
8.04 (d, J = 8.7 Hz, 1H), 7.46-7.16 (m, 10H), 5.S1 (m, 1H),
S.20 (brs, 2H), 4.52 (d, J = 16.7 Hz, 1H), 4.45 (d, J = 16.7 Hz, 1H),
4.39 (q, J = 7.1 Hz, 2H), 3.30 (dd, J = 14.0, 5.0 Hz, 1H),
2.98 (dd, J = 14.0, 8.6 Hz, 1H), 1.37 (t, J = 7.1 Hz, 3H),
IR (KBr) 3400, 3250, 3040, 2960, 1720, 17l0, 1675, 1650, 1610,
1585, 1560, 1530 cm 1
MS (APCI, positive) m/z 600 (MH+)
Compound of E'~umple 86
2-[5-amino-2-(3-methoxyphenyl)-6-oxo- 1,6-dihydro-1-pyrimidinyl]-N-[ 1-
[(benzoxazol-2-yl)carbonyl]-2-phenylethyl]acetamide
mp 167-17l~C
1H-NMR (300 MHz, DMSO-ds) ~ 8.92 (d, J = 6.8 Hz, 1H),
8.01 (d, J = 8.0 Hz, 1 H), 7.91 (d, J = 8.0 Hz, 1 H) ,
7.66 (t, J = 8.0 Hz, 1 H), 7.56 (t, J = 8.0 Hz, 1 H),
7.30-7.15 (m, 7H), 6.98-6.92 (m, 2H), 6.85 (d, J = 7.6 Hz, 1H),
5.57 (m, 1H), 5.13 (brs, 2H), 4.49 (d, J = 16.6 Hz, 1H),
69
CA 02269720 1999-04-23
4.43 (d, J = 16.6 Hz, 1H), 3.68 (s, 3H),
3.27 (dd, J = 14.0, 9.0 Hz, 1H), 2.99 (dd, 14.1, 8.6 Hz, 1H)
IR (KBr) 3450, 3300, 3080, 3030, 2970, 17l5, 1660, 1605, 1S80, 1530
cm 1
MS (APCI, positive) m/z 524 (MH+)
Compound of L~rample 88
2-[5-amino-2-(3-methoxyphenyl)-6-oxo- 1,6-dihydro- 1-pyrimidinyl]-N-( 1-[[5-
(methoxycarbonyl)benzoxazol-2-yl]carbonyl]-2-phenylethyl]acetamide
mp 184.5-186.0~C
1H-NMR (300 MHz, DMSO-d6) ~ 8.97 (d, J = 6.7 Hz, 1H),
8.51 (d, J = 1.6 Hz, 1H), 8.23 (dd, J = 8.?, 1.6 Hz, 1H),
8.04 (d, J = 8.7 Hz, 1H), 7.31-7.14 (m, 7H), 6.98-6.83 (m, 3H),
5.54 (m, 1H), 5.13 (brs, 2H), 4.50 (d, J = 16.6 Hz, 1H),
4.44 (d, J = 16.6 Hz, 1H), 3.93 (s, 3H), 3.69 (s, 3H),
3.30 (dd, J = 14.2, 5.3 Hz, 1H), 3.00 (dd, J = 14.2, 8.4 Hz, 1H)
IR (KBr) 3450, 3320, 3060, 3020, 2940, 1715, l660, 1605, 1530 crri 1
MS (APCI, positive) m/z 582 (MH+)
Compound of Example 90
2-[5-amino-2-(3-methoxyphenyl)-6-oxo- 1,6-dihydro- 1-pyrimidinyl]-N-[ 1-[[5-
(ethoxycarbonyl)benzoxazol-2 yl]carbonyl]-2-phenylethyl]acetamide
mp 1S3-156~C
1H-NMR (300 MHz, DMSO-ds) ~ 8.96 (d, J = 6.9 Hz, 1H),
8.51 (d, J = 1.6 Hz, 1H), 8.23 (dd, J = 8.7, 1.6 Hz, 1H),
8.04 (d, J = 8.7 Hz, 1H), 7.30-7.15 (m, 7H), 6.97-6.90 (m, 2H),
6.86 (d, J = 7.6 Hz, 1H), 5.53 (m, 1H), 5.12 (brs, 2H),
4.49 (d, J = 16.6 Hz, 1H), 4.44 (d, J = 16.6 Hz, 1H),
4.39 (q, J = 7.1 Hz, 2H), 3.69 (s, 3H), 3.30 (dd, J = 14.2, 4.8 Hz, 1H), 2.99
(dd, J =
14.1, 8.6 Hz, 1H), 1.37 (t, J = 7.0 Hz, 3H)
IR (KBr) 3450, 3250, 3050, 2950, 1720, 1660, 1600, 1580, 1530 crri 1
MS (APCI, positive) m/z 596 (MH+)
Compound of Example 92
2-(5-amino-6-oxo-2-phenyl-1,6-dihydro-1-pyrimidinyl)-N-[ 1-[[5-
(methoxycarbonyl)benzoxazol-2-yl]carbonyl]-2-phenylethyl]acetamide
mp 271-274~C
CA 02269720 1999-04-23
iH-NMR (300 MHz, DMSO-d6) S 8.95 (d, J = 6.6 Hz, 1H),
8.51 (d, J = 1.5 Hz, 1H), 8.24 (dd, J = 8.7, 1.5 Hz, 1H),
8.05 (d, J = 8.7 Hz, 1H), 7.41-7.16 (m, 11H), 5.53 (m, 1H),
5.11 (brs, 2H), 4.49 (d, J = 16.7 Hz, 1H), 4.42 (d, J = 16.7 Hz, 1H),
3.93 (s, 3H), 3.31 (dd, J = 14.0, 4.9 Hz, 1H),
2.98 (dd, J = 14.0, 8.7 Hz, 1 H)
IR (KBr) 3400, 3280, 3040, 2940, 1715, 1675, 1650, 1610, 1585, 1530 crri 1
Compouad of Example 93
2-[5-amino-2-(3-aminophenyl)-6-oxo- 1,6-dihydro-1-pyrimidinyl]-N-[ 1-[[5-
(methoxycarbonyl)benzoxazol-2-yl]carbonyl]-2-phenylethyl]acetamide
mp l25.0-127.5~C
1H-NMR (300 MHz, DMSO-d6) ~ 8.92 (d, J = 6.6 Hz, 1H),
8.52 (d, J = 1.6 Hz, 1H), 8.23 (dd, J = 8.7, 1.6 Hz, 1H),
8.04 (d, J = 8.7 Hz, 1H), ?.31-7.13 (m, 6H), 6.87(t, J = 7.6 Hz, 1H),
6.65-6.50 (m, 2H), 6.37 (d, J = 7.6 Hz, 1H), 5.56 (m, 1H),
5.25 (brs, 2H), 5.03 (brs, 2H), 4.S1 (d, J = 16.6 Hz, 1H),
4.44 (d, J = 16.6 Hz, 1H), 3.93 (s, 3H),
3.30 (dd, J = 14.1, 4.6 Hz, 1H), 3.03 (dd, J = 14.1, 8.4 Hz, 1H)
IR (KBr) 3400, 3340, 3050, 3010, 2940, 17l5, 1655, l600, 1530 crri 1
MS (APCI, positive) m/z 567 (MH+)
Compouad of Example 94
2-[5-acetamide-2-(4-fluoraphenyl)-6-oxo-1,6-dihydro-1-pyrimidinyl]-N-[1-
[(benzoxazol-2-yl)carbonyl]-2-phenylethyl]acetamide
mp 2S4-256~C
1H-NMR (300 MHz, DMSO-d6) ~ 9.50 (s, 1H), 9.02 (d, J = 6.9 Hz, 1H),
8.77 (s, 1H), 8.01 (d, J = 8.0 Hz, IH), 7.92 (d, J = 8.0 Hz, 1H),
7.67 (t, J = 8.0 Hz, 1 H), 7.56 (t, J = 8.0 Hz, 1 H),
7.43 (dd, J = 8.8, 5.5 Hz, 2H), 7.30-7.12 (m, 7H), 5.57 (m, 1H),
4.55 (d, J = 16.7 Hz, 1H), 4.45 (d, J = 16.7 Hz, 1H),
3.31 (dd, J = 14.1, 4.7 Hz, 1H), 2.96 (dd, J = 14.1, 8.9 Hz, 1H), 2.13 (s, 3H)
IR (KBr) 3470, 3360, 3080, 1695, 1655, 1605, 1S30, 1500 crri 1
Experimeatai Example 1: Inhibitory activity of the inventive compound on human
heart chymase
The effectiveness of the inhibitory activity of compound (I) and compound
71
CA 02269720 1999-04-23
(XXVI) of the present invention was evaluated based on the inhibitory activity
on
amidase activity of human heart chymase, which was determined as in the
following.
The inhibitory activity was quantitatively determined through variation in
fractional residual activity of the enzyme caused by the inventive compound in
defined serial concentration (< x 1, < x 10, < x 100-fold equivalents)
relative to 5 nM
chymase in the presence of synthetic substrate, succinyl-alanyl-alanyl-prolyl-
phenylalanine-p-nitroanilide (final concentration 2.5 mM). The inhibitory
effect
was analyzed by least square regression of Easson-Stedman plot {l?roc. Roy.
Soc. B.
1936, 121, p. 141) utilizing bimolecular equilibrium reaction linearization
formula.
The inhibitory activity was evaluated by the apparent inhibitory constant
(Kiapp)
obtained by this analysis and inhibitory constant (Ki) calculated from final
substrate concentration in the reaction mixture and Km values separately
determined. With regard to the quantlitative determination of initial rate of
the
enzyme reaction, the amount of p-nitroanivne produced by hydrolysis of the
substrate was spectrophotometrically detected in terms of an increased
absorbance which was obtained by subtracting absorbance at 650 nm wavelength
from the absorbance at 405 nm. The chymase inhibitory activity of the
compound of the present invention was calculated as ratio of residual activity
in
the presence of inhibitor relative to enzyme activity in the absence of
inhibitor, and
incorporation of the determination values was completed at a level less than
initial
rate guarantee absorbance at a concentration of the substrate used for the
enzyme,
after which analysis was performed.
The reaction mixture consisted of a buffer {pH 7.5, 140 ~,1) of Tris-HCl ( 100
mM) - KCl (2 M), the inventive compound dissolved in 20 ~,l of 10% dimethyl
sulfoxide {DMSO), substrate dissolved in 20 ~,1 of DMSO, and 20 ~.1 of
chymase,
thus amounting to 200 ~,1 in total.
Starting with the absorbance immediately after addition of the enzyme,
increases in absorbance were recorded as a progressive curve taken precisely
at
equal time internals.
From the above data, the di$'erence between absorbances at completion of
the reaction and immediately after addition was used to quantitatively
determine
the residual activity of the sample added with the inhibitor relative to a
control
wherein the inhibitor was not added. Alternatively, reaction rates of the
control
72
CA 02269720 1999-04-23
and the sample added with the inhibitor were calculated for certain time
period ( >__
20 min) with successive shift (every 10 to 30 minutes) of the period, and the
residual activity ratio was quantitatively determined and analyzed from the
respective reaction rates averaged through the entire reaction time.
The inhibitory activity against human leukocyte elastase was determined
using N-methoxysuccinyl-alanyl-alanyl-prolyl-valine-p-nitroanilide as a
substrate
and 0.1 M Tris-HCl (pH 8.0) containing 20 mM CaCl2 and 0.1% ~veen 80 as a
buffer, wherein other compositions and method were the same as above.
The results of human heart chymase inhibitory activity test of
representative compound (I) and compound (XXVI) of the present invention are
shown in Table 10.
Table 10
Example No. Ki M
3 1.30
0.076
7 0.056
9 1.84
0.183
17 0.130
21 0.111
23 0.048
26 0.093
31 0.016
40 0.990
44 0.002
45 0.024
53 0.066
55 0.059
58 0.012
64 0.045
72 0.074
73 0.059
80 0.042
88 0.005
93 0.006
94 o.os8
On the other hand, the inhibitory activity against human leukocyte
elastase was > 105 ~,M for every compound.
From the above results, it is evident that the compound (I) and compound
73
CA 02269720 1999-04-23
(XXVI) of the present invention does not inhibit human leukocyte elastase at
all,
but strongly inhibits human heart chymase.
Formulation ~mple 1 : tablet
( 1 ) Compound (I) of the present invention 10 mg
( 2 ) Fine particle No. 209 for direct pounding (Fuji Kagaku) 46.6 mg
Magnesium alumirlate metasilicate 20%
Corn starch 30%
Lactose 50%
( 3 ) Crystalline cellulose 24.0 mg
( 4 ) Calcium carboxylmethylcellulose 4.0 mg
( 5 ) Magnesium stearate 0.4 mg
( 1), (3) and (4) were respectively passed througk~ a 100 mesh sieve in
advance. The obtained (1), (3) and (4) and (2) were respectively dried to a
certain
water content and mixed at the above-mentioned weight ratios in a mixer. (5)
was
added to the homogeneous powder mixture and mixed for a short time (30
seconds) and the mixed powder was compressed (pounder : 6.3 mm ~ , 6.0 mmR)
to give tablets weighing 85 mg per tablet.
This tablet may be coated with a typical enteric film coating agent (e.g.,
polyvinyl acetal diethylamino acetate) or edible colorant, as necessary.
Formulation F~anple 2 : capsule
( 1 ) Compound (I) of the present invention 50 g
( 2 ) Lactose 93S g
( 3 ) Magnesium stearate 15 g
The above-mentioned ingredients were weigkled respectively and mixed
homogeneously. The mixed powder was packed in hard gelatin capsules by 200
mg per capsule.
Formulation Example 3 : injection
( 1 ) Hydrochloride of compound (I) of the present invention 5 mg
( 2 ) Sucrose 100 mg
( 3 ) Physiological saline 10 ml
A mixture of the above-mentioned ingredients was filtered through a
membrane filter, again sterilized by filtration and the filtrate was
aseptically
dispensed into vials. Nitrogen gas was filled and the vials were sealed to
give
intravenous injection.
74
CA 02269720 1999-04-23
The heterocyclic amide compound and pharmacologically acceptable salt
thereof of the present invention have superior inhibitory activity against
chymase
groups in mammals inclusive of human and can be administered orally or
parenterally. Therefore, they are useful as chymase inhibitors and can be used
for the prophylaxis and treatment of various diseases caused by chymase, such
as
those caused by angiotensin II.
The present invention is based on patent application Nos. 284471 / 1996
and 194106/ 199? filed in Japan, the contents of which are incorporated
hereinto
by reference.