Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02270469 1999-04-30
WO 98/22101 PCT/US97/21489
1
METHOD OF USING CYCLOOXYGENASE-2 INHIBITORS
AS ANTI-ANGIOGENIC AGENTS
Field of the Invention
This invention is in the field of the prevention
and treatment of angiogenesis. More specifically, this
invention relates to the use of cyclooxygenase-2
inhibitors or derivatives thereof in preventing and
treating angiogenesis-related disorders.
Background of the Invention
Prostaglandins play a major role in the
inflammation process and the inhibition of prostaglandin
production, especially production of PGG2, PGH2 and
PGE2, has been a common target of anti-inflammatory drug
discovery. However, common non-steroidal anti-
inflammatory drugs (NSAID's) that are active in reducing
the prostaglandin-induced pain and swelling associated
with the inflammation process are also active in
affecting other prostaglandin-regulated processes not
associated with the inflammation process. Thus, use of
high doses of most common NSAID's can produce severe
side effects, including life threatening ulcers, that
limit their therapeutic potential. An alternative to
NSAID's is the use of corticosteroids, which also
produce severe adverse effects, especially when long
term therapy is involved.
NSAIDs have been found to prevent the
production of prostaglandins by inhibiting
. enzymes in the human arachidonic
acid/prostaglandin pathway, including the enzyme
cyclooxygenase (COX). The recent discovery of an
inducible enzyme associated with inflammation
CA 02270469 1999-04-30
WO 98/22101 PCT/US97/21489
2
(named "cyclooxygenase-2 (COX-2)" or
"prostaglandin G/H synthase II") provides a
viable target of inhibition which more
effectively reduces inflammation and produces
fewer and less drastic side effects.
Angiogenesis is the development of new blood
vessels into a tissue or organ. Under normal
conditions, angiogenesis is observed in wound
healing and embryonal development. Uncontrolled
angiogenesis is associated with neoplastic
disease, tumor metastasis and other angiogenesis-
related diseases.
Although originally developed for their anti-
inflammatory properties, glucocorticoids are now
recognized to have a wide variety of therapeutic uses.
For example, many steroids with anti-inflammatory
activity inhibit angiogenesis (U. S. Pat. No. 5,646,136).
Compounds which selectively inhibit
cyclooxygenase-2 have been described in U.S.
patents 5,380,738, 5,344,991, 5,393,790,
5,434,178, 5,474,995, 5,475,018, 5,510,368 and WO
documents W096/06840, W095/21817, W096/03388,
W096/03387, W096/03392, W096/25405, W096/24584,
W096/03385, W096/16934, W095/15316, W094/15932,
w094/27980, wo95/00501, W094/13635, wo94/20480,
and W094/26731.
[Pyrazol-1-yl]benzenesulfonamides have been
described as inhibitors of cyclooxygenase-2 and have
shown promise in the treatment of inflammation,
arthritis, and pain, with minimal side effects in pre-
clinical and clinical trials. Their use for preventing
colon cancer has been described in U.S. Patent No.
5,466,823. However, their use for treating or preventing
CA 02270469 1999-04-30
WO 98/22101 PCT/US97/21489
3
angiogenesis-related diseases has not been previously
described.
There have been several publications describing the
benefits of inhibiting angiogenesis. !n10 patent
publication No. 96/19469 describes that COX-2 inhibitors
would be useful to prevent and/or treat tumor
angiogenesis and diabetic retinopathy.
The present invention is directed to the use of
inhibitors of cyclooxygenase-2 for the treatment and
prevention of tumor growth and metastasis that are
dependent on the angiogenic process. In addition, the
treatment and prevention of non-neoplastic angiogenesis-
related disorders, such as retinopathies, and
endometriosis is also included.
Detailed Description of the Invention
The present invention provides a method for
treating or preventing angiogenesis-related disorders in
a subject in need of such treatment or prevention, the
method comprises treating the subject with a
therapeutically effective amount of a cyclooxygenase-2
inhibitor or derivative or pharmaceutically-acceptable
salt thereof.
The method above would be useful for, but not
limited to, the treatment of angiogenesis-related
disorders in a subject. According to the present
invention, the compounds of Formula I are administered
to a subject in need of angiogenesis inhibition. The
method would be useful for treatment of neoplasia,
including metastasis; ophthalmological conditions such
as corneal graft rejection, ocular neovascularization,
retinal neovascularization including neovascularization
following injury or infection, diabetic retinopathy,
CA 02270469 1999-04-30
WO 98/22101 PCT/US97/21489
4
retrolental fibroplasia and neovascular glaucoma;
ulcerative diseases such as gastric ulcer; pathological,
but non-malignant, conditions such as hemangiomas,
including invantile hemaginomas, angiofibroma of the
nasopharynx and avascular necrosis of bone; and
disorders of the female reproductive system such as
endometriosis.
The term "treatment" includes partial or total
inhibition of angiogenesis, including neoplastic growth,
spreading or metastasis, as well as partial or total
destruction of the neoplastic cells.
The term "prevention" includes either preventing
the onset of clinically evident angiogenesis altogether
or preventing the onset of a preclinically evident stage
of angiogenesis in individuals at risk. Also intended to
be encompassed by this definition is the prevention of
metastasis of malignant cells or to arrest or reverse
the progression of malignant cells. This includes
prophylactic treatment of those at risk of developing
angiogenesis.
The phrase "therapeutically-effective" is intended
to qualify the amount of each agent which will achieve
the goal of improvement in disease severity and the
frequency of incidence over treatment of each agent by
itself, while avoiding adverse side effects typically
associated with alternative therapies.
The term "subject" for purposes of treatment
includes any human or animal subject who has any
one of the known angiogenesis-related disorders.
For methods of prevention, the subject is any human
or animal subject, and preferably is a human
subject who is at risk for obtaining an
angiogenesis-related disorder, such as metastasis.
CA 02270469 1999-04-30
WO 98/22101 PCT/US97/21489
The subject may be at risk due to exposure to
carcinogenic agents, being genetically predisposed
. to have the angiogenesis, and the like. Besides
being useful for human treatment, these compounds
5 are also useful for veterinary treatment of
mammals, including companion animals and farm
animals, such as, but not limited to, horses, dogs,
cats, cows, sheep and pigs. Preferably, subject
means a human.
Inhibitors of the cyclooxygenase pathway in
the metabolism of arachidonic acid used in the
prevention and treatment of angiogenesis may
inhibit enzyme activity through a variety of
mechanisms. By the way of example, the inhibitors
used in the methods described herein may block
the enzyme activity directly by acting as a
substrate for the enzyme. The use of
cyclooxygenasse-2 selective inhibitors is highly
advantageous in that it minimize the gastric side
effects that can occur with non-selective
NSAID's, especially where prolonged prophylactic
treatment is expected.
The term "cyclooxygenase-2 inhibitor"
denotes a compound able to inhibit
cyclooxygenase-2 without significant inhibition
of cyclooxygenase-1. Preferably, it includes
compounds which have a cyclooxygenase-2 ICSp of
less than about 0.2 uM, and also have a
selectivity ratio of cyclooxygenase-2 inhibition
over cyclooxygenase-1 inhibition of at least 50,
and more preferably of at least 100. Even more
preferably, the compounds have a cyclooxygenase-1
ICSp of greater than about 1 uM, and more
preferably of greater than 10 uM.
CA 02270469 1999-04-30
WO 98/22101 PCT/US97/21489
6
The method provided herein relates to the use
of cyclooxygenase-2 inhibitors or derivatives
thereof in the prevention and treatment of
angiogenesis. In the preferred embodiments, the
cyclooxygenase-2 inhibitor is selected from
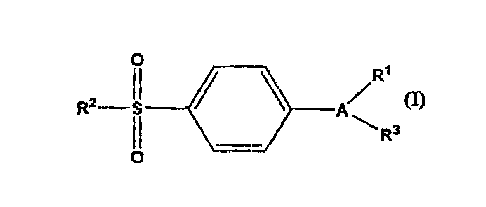
compounds of Formula I
R1
~S
O A
~ R3
wherein A is a substituent selected from
oxazolyl, isoxazolyl, thienyl, dihydrofuryl, furyl,
pyrrolyl, pyrazolyl, thiazolyl, imidazolyl,
isothiazolyl, cyclopentenyl, phenyl and pyridyl;
wherein R1 is at least one substituent
selected from heterocyclyl, cycloalkyl,
cycloalkenyl and aryl, wherein R1 is optionally
substituted at a substitutable position with one or
more radicals selected from alkyl, haloalkyl,
cyano, carboxyl, alkoxycarbonyl, hydroxyl,
hydroxyalkyl, haloalkoxy, amino, alkylamino,
arylamino, nitro, alkoxyalkyl, alkylsulfinyl, halo,
alkoxy and alkylthio;
wherein R2 is methyl or amino; and
wherein R3 is a radical selected from hydrido,
halo, alkyl, alkenyl, alkynyl, oxo, cyano,
carboxyl, cyanoalkyl, heterocyclyloxy, alkyloxy,
alkylthio, alkylcarbonyl, cycloalkyl, aryl,
haloalkyl, heterocyclyl, cycloalkenyl, aralkyl,
heterocyclylalkyl, acyl, alkylthioalkyl,
hydroxyalkyl, alkoxycarbonyl, arylcarbonyl,
aralkylcarbonyl, aralkenyl, alkoxyalkyl,
arylthioalkyl, aryloxyalkyl, aralkylthioalkyl,
aralkoxyalkyl, alkoxyaralkoxyalkyl,
alkoxycarbonylalkyl, aminocarbonyl,
CA 02270469 1999-04-30
WO 98/22101 PCT/US97/21489
7
aminocarbonylalkyl, alkylaminocarbonyl, N-
arylaminocarbonyl, N-alkyl-N-arylaminocarbonyl,
alkylaminocarbonylalkyl, carboxyalkyl, alkylamino,
N-arylamino, N-aralkylamino, N-alkyl-N-
aralkylamino, N-alkyl-N-arylamino, aminoalkyl,
alkylaminoalkyl, N-arylaminoalkyl, N-
aralkylaminoalkyl, N-alkyl-N-aralkylaminoalkyl, N-
alkyl-N-arylaminoalkyl, aryloxy, aralkoxy,
arylthio, aralkylthio, alkylsulfinyl,
alkylsulfonyl, aminosulfonyl, alkylaminosulfonyl,
N-arylaminosulfonyl, arylsulfonyl, N-alkyl-N-
arylaminosulfonyl; or a pharmaceutically-
acceptable salt thereof.
A preferred class of compounds which inhibit
cyclooxygenase-2 consists of compounds of Formula I
wherein A is selected from oxazolyl, isoxazolyl,
pyrazolyl, imidazolyl, cyclopentenyl, phenyl, and
pyridyl; wherein R1 is selected from 5- and 6-
membered heterocyclyl, lower cycloalkyl, lower
cycloalkenyl and aryl selected from phenyl,
biphenyl and naphthyl, wherein R1 is optionally
substituted at a substitutable position with one or
more radicals selected from lower alkyl, lower
haloalkyl, cyano, carboxyl, lower alkoxycarbonyl,
hydroxyl, lower hydroxyalkyl, lower haloalkoxy,
amino, lower alkylamino, phenylamino, lower
alkoxyalkyl, lower alkylsulfinyl, halo, lower
alkoxy and lower alkylthio; wherein R2 is methyl or
amino; and wherein R3 is a radical selected from
hydrido, oxo, cyano, carboxyl, lower
alkoxycarbonyl, lower carboxyalkyl, lower
cyanoalkyl, halo, lower alkyl, lower alkyloxy,
lower cycloalkyl, phenyl, lower haloalkyl, 5- or 6-
membered heterocyclyl, lower hydroxylalkyl, lower
aralkyl, acyl, phenylcarbonyl, lower alkoxyalkyl,
5- or 6-membered heteroaryloxy, aminocarbonyl,
CA 02270469 1999-04-30
WO 98/22101 PCT/US97/21489
8
lower alkylaminocarbonyl, lower alkylamino, lower
aminoalkyl, lower alkylaminoalkyl, phenyloxy, and
lower aralkoxy; or a pharmaceutically-acceptable
salt thereof.
A more preferred class of compounds which
inhibit cyclooxygenase-2 consists of compounds of
Formula I wherein A is selected from oxazolyl,
isoxazolyl, pyrazolyl, imidazolyl and
cyclopentenyl; wherein R1 is selected from pyridyl
optionally substituted at a substitutable position
with one or more methyl radicals, and phenyl
optionally substituted at a substitutable position
with one or more radicals selected from methyl,
ethyl, isopropyl, butyl, tert-butyl, isobutyl,
pentyl, hexyl, fluoromethyl, difluoromethyl,
trifluoromethyl, cyano, carboxyl, methoxycarbonyl,
ethoxycarbonyl, hydroxyl, hydroxymethyl,
trifluoromethoxy, amino, N-methylamino, N,N-
dimethylamino, N-ethylamino, N,N-dipropylamino, N-
butylamino, N-methyl-N-ethylamino, phenylamino,
methoxymethyl, methylsulfinyl, fluoro, chloro,
bromo, methoxy, ethoxy, propoxy, n-butoxy, pentoxy,
and methylthio; wherein R2 is methyl or amino; and
wherein R3 is a radical selected from hydrido, oxo,
cyano, carboxyl, methoxycarbonyl, ethoxycarbonyl,
carboxypropyl, carboxymethyl, carboxyethyl,
cyanomethyl, fluoro, chloro, bromo, methyl, ethyl,
isopropyl, butyl, tert-butyl, isobutyl, pentyl,
hexyl, difluoromethyl, trifluoromethyl,
pentafluoroethyl, heptafluoropropyl, difluoroethyl,
difluoropropyl, methoxy, ethoxy, propoxy, n-butoxy,
pentoxy, cyclohexyl, phenyl, pyridyl, thienyl,
thiazolyl, oxazolyl, furyl, pyrazinyl,
hydroxylmethyl, hydroxylpropyl, benzyl, formyl,
phenylcarbonyl, methoxymethyl, furylmethyloxy,
aminocarbonyl, N-methylaminocarbonyl, N,N-
CA 02270469 1999-04-30
WO 98/22101 PCT/iTS97/21489
9
dimethylaminocarbonyl, N,N-dimethylamino, N-
ethylamino, N,N-dipropylamino, N-butylamino, N-
methyl-N-ethylamino, aminomethyl, N,N-
dimethylaminomethyl, N-methyl-N-ethylaminomethyl,
benzyloxy, and phenyloxy; or a pharmaceutically-
acceptable salt thereof.
A family of specific compounds of particular
interest within Formula I consists of compounds
and pharmaceutically-acceptable salts thereof as
follows:
5-(4-fluorophenyl)-1-[4-(methylsulfonyl)phenyl)-3-
(trifluoromethyl)pyrazole;
4-(4-fluorophenyl)-5-[4-(methylsulfonyl)phenyl]-1-
phenyl-3-(trifluoromethyl)pyrazole;
4-(5-(4-chlorophenyl)-3-(4-methoxyphenyl)-1H-
pyrazol-1-yl)benzenesulfonamide
4-(3,5-bis(4-methylphenyl)-1H-pyrazol-1-
yl)benzenesulfonamide;
4-(5-(4-chlorophenyl)-3-phenyl-1H-pyrazol-1-
yl)benzenesulfonamide;
4-(3,5-bis(4-methoxyphenyl)-1H-pyrazol-1-
yl)benzenesulfonamide;
4-(5-(4-chlorophenyl)-3-(4-methylphenyl)-1H-pyrazol-
1-yl)benzenesulfonamide;
4-(5-(4-chlorophenyl)-3-(4-nitrophenyl)-1H-pyrazol-
1-yl)benzenesulfonamide;
4-(5-(4-chlorophenyl)-3-(5-chloro-2-thienyl)-1H-
pyrazol-1-yl)benzenesulfonamide;
4-(4-chloro-3,5-diphenyl-1H-pyrazol-1-
yl)benzenesulfonamide
4-[5-(4-chlorophenyl)-3-(trifluoromethyl)-1H-
pyrazol-1-yl]benzenesulfonamide;
4-[5-phenyl-3-(trifluoromethyl)-1H-pyrazol-1-
yl]benzenesulfonamide;
CA 02270469 1999-04-30
WO 98/22101 PCT/US97/21489
4-[5-(4-fluorophenyl)-3-(trifluoromethyl)-1H-
pyrazol-1-yl]benzenesulfonamide;
4-[5-(4-methoxyphenyl)-3-(trifluoromethyl)-1H-
pyrazol-1-yl]benzenesulfonamide;
5 4-[5-(4-chlorophenyl)-3-(difluoromethyl)-1H-pyrazol-
1-yl]benzenesulfonamide;
4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-
pyrazol-1-yl]benzenesulfonamide;
4-[4-chloro-5-(4-chlorophenyl)-3-(trifluoromethyl)-
10 1H-pyrazol-1-yl]benzenesulfonamide;
4-[3-(difluoromethyl)-5-(4-methylphenyl)-1H-pyrazol-
1-yI]benzenesulfonamide;
4-[3-(difluoromethyl)-5-phenyl-1H-pyrazol-1-
yl]benzenesulfonamide;
15 4-[3-(difluoromethyl)-5-(4-methoxyphenyl)-1H-
pyrazol-1-yl]benzenesulfonamide;
4-[3-cyano-5-(4-fluorophenyl)-1H-pyrazol-1-
yl]benzenesulfonamide;
4-[3-(difluoromethyl)-5-(3-fluoro-4-methoxyphenyl)-
20 1H-pyrazol-1-yl]benzenesulfonamide;
4-[5-(3-fluoro-4-methoxyphenyl)-3-(trifluoromethyl)-
1H-pyrazol-1-yl]benzenesulfonamide;
4-[4-chloro-5-phenyl-1H-pyrazol-1-
yl]benzenesulfonamide;
4-[5-(4-chlorophenyl)-3-(hydroxymethyl)-1H-pyrazol-
1-yl]benzenesulfonamide;
4-[5-(4-(N,N-dimethylamino)phenyl)-3-
(trifluoromethyl)-1H-pyrazol-1-
yl]benzenesulfonamide;
2-methyl-4-[1-[4-(methylsulfonyl)phenyl-4-
(trifluoromethyl)-1H-imidazol-2-yl]pyridine;
2-methyl-6-[1-[4-(methylsulfonyl)phenyl-4-
(trifluoromethyl)-1H-imidazol-2-yl]pyridine;
4-[2-(6-methylpyridin-3-yl)-4-(trifluoromethyl)-1H-
imidazol-1-yl]benzenesulfonamide;
2-(3,4-difluorophenyl)-1-[4-(methylsulfonyl)phenyl]-
4-(trifluoromethyl)-1H-imidazole;
CA 02270469 1999-04-30
WO 98/22101 PCT/US97/21489
11
4-[2-(4-methylphenyl)-4-(trifluoromethyl)-1H-
imidazol-1-yl]benzenesulfonamide;
2-(4-chlorophenyl)-1-[4-(methylsulfonyl)phenyl]-4-
methyl-1H-imidazole;
2-(4-chlorophenyl)-1-[4-(methylsulfonyl)phenyl]-4-
phenyl-1H-imidazole;
2-(4-chlorophenyl)-4-(4-fluorophenyl)-1-[4
(methylsulfonyl)phenyl]-1H-imidazole;
2-(3-fluoro-4-methoxyphenyl)-1-[4-
(methylsulfonyl)phenyl-4-(trifluoromethyl)-1H-
imidazole;
1-[4-(methylsulfonyl)phenyl]-2-phenyl-4-
trifluoromethyl-1H-imidazole;
2-(4-methylphenyl)-1-[4-(methylsulfonyl)phenyl]-4-
trifluoromethyl-1H-imidazole;
4-[2-(3-chloro-4-methylphenyl)-4-(trifluoromethyl)-
1H-imidazol-1-yl]benzenesulfonamide;
2-(3-fluoro-5-methylphenyl)-1-[4-
(methylsulfonyl)phenyl]-4-(trifluoromethyl)-1H-
imidazole;
4-[2-(3-fluoro-5-methylphenyl)-4-(trifluoromethyl)-
1H-imidazol-1-yl]benzenesulfonamide;
2-(3-methylphenyl)-1-[4-(methylsulfonyl)phenyl]-4-
trifluoromethyl-1H-imidazole;
4-[2-(3-methylphenyl)-4-trifluoromethyl-1H-imidazol-
1-yl]benzenesulfonamide;
1-[4-(methylsulfonyl)phenyl]-2-(3-chlorophenyl)-4-
trifluoromethyl-1H-imidazole;
4-[2-(3-chlorophenyl)-4-trifluoromethyl-1H-imidazol-
1-yl]benzenesulfonamide;
4-[2-phenyl-4-trifluoromethyl-1H-imidazol-1-
yl]benzenesulfonamide;
4-[2-(4-methoxy-3-chlorophenyl)-4-trifluoromethyl-
1H-imidazol-1-yl]benzenesulfonamide;
1-allyl-4-(4-fluorophenyl)-3-[4-
(methylsulfonyl)phenyl]-5-(trifluoromethyl)-1H-
pyrazole;
CA 02270469 1999-04-30
WO 98/22101 PCT/US97/21489
12
4-[1-ethyl-4-(4-fluorophenyl)-5-(trifluoromethyl)-
1H-pyrazol-3-yl]benzenesulfonamide;
N-phenyl-[4-{4-luorophenyl)-3-[4-
(methylsulfonyl)phenyl]-5-(trifluoromethyl)-1H-
pyrazol-1-yl]acetamide;
ethyl [4-(4-fluorophenyl)-3-[4-
(methylsulfonyl}phenyl]-5-(trifluoromethyl)-1H-
pyrazol-1-yl]acetate;
4-{4-fluorophenyl)-3-[4-(methylsulfonyl)phenyl]-1-
20 (2-phenylethyl)-1H-pyrazole;
4-(4-fluorophenyl)-3-[4-(methylsulfonyl)phenyl]-1-
(2-phenylethyl)-5-(trifluoromethyl)pyrazole;
1-ethyl-4-(4-fluorophenyl)-3-[4-
(methylsulfonyl}phenyl]-5-(trifluoromethyl)-1H-
pyrazole;
5-(4-fluorophenyl)-4-(4-methylsulfonylphenyl)-2-
trifluoromethyl-1H-imidazole;
4-[4-(methylsulfonyl)phenyl]-5-(2-thiophenyl)-2-
(trifluoromethyl)-1H-imidazole;
5-difluoromethyl-4-(4-methylsulfonylphenyl)-3-
phenylisoxazole;
4-[3-ethyl-5-phenylisoxazol-4-yl]benzenesulfonamide;
4-[5-difluoromethyl-3-phenylisoxazol-4-
yl]benzenesulfonamide;
4-[5-hydroxymethyl-3-phenylisoxazol-4-
yl]benzenesulfonamide;
4-[5-methyl-3-phenyl-isoxazol-4-
yl]benzenesulfonamide;
1-[2-(4-fluorophenyl)cyclopenten-1-yl]-4-
(methylsulfonyl)benzene;
1-[2-(4-fluoro-2-methylphenyl)cyclopenten-1-yl]-4-
(methylsulfonyl)benzene;
1-[2-(4-chlorophenyl)cyclopenten-1-yl]-4-
(methylsulfonyl)benzene;
1-[2-(2,4-dichlorophenyl)cyclopenten-1-yl]-4-
(methylsulfonyl)benzene;
CA 02270469 1999-04-30
WO 98/22101 PCT/US97/21489
13
1-[2-(4-trifluoromethylphenyl)cyclopenten-1-yl]-4-
(methylsulfonyl)benzene;
1-[2-(4-rnethylthiophenyl)cyclopenten-1-yl]-4-
(methylsulfonyl)benzene;
1-[2-(4-fluorophenyl)-4,4-dimethylcyclopenten-1-yl]-
4-(methylsulfonyl)benzene;
4-[2-(4-fluorophenyl)-4,4-dimethylcyclopenten-1-
yl]benzenesulfonamide;
1-[2-(4-chlorophenyl)-4,4-dimethylcyclopenten-1-yl]-
4-(methylsulfonyl)benzene;
4-[2-(4-chlorophenyl)-4,4-dimethylcyclopenten-1-
yl]benzenesulfonamide;
4-[2-(4-fluorophenyl)cyclopenten-1-
yl]benzenesulfonamide;
4-[2-(4-chlorophenyl)cyclopenten-1-
yl]benzenesulfonamide;
1-[2-(4-methoxyphenyl)cyclopenten-1-yl]-4-
{methylsulfonyl)benzene;
1-[2-(2,3-difluorophenyl)cyclopenten-1-yl]-4-
(methylsulfonyl)benzene;
4-[2-{3-fluoro-4-methoxyphenyl)cyclopenten-1-
yl]benzenesulfonamide;
1-[2-(3-chloro-4-methoxyphenyl)cyclopenten-1-yl]-4-
(methylsulfonyl)benzene;
4-[2-(3-chloro-4-fluorophenyl)cyclopenten-1-
yl]benzenesulfonamide;
4-[2-(2-methylpyridin-5-yl)cyclopenten-1-
yl]benzenesulfonamide;
ethyl 2-[4-(4-fluorophenyl)-5-[4-(methylsulfonyl)
phenyl]oxazol-2-yl]-2-benzyl-acetate;
2-[4-(4-fluorophenyl)-5-[4-
(methylsulfonyl)phenyl]oxazol-2-yl]acetic acid;
2-(tert-butyl)-4-(4-fluorophenyl)-5-[4-
(methylsulfonyl)phenyl]oxazole;
4-(4-fluorophenyl)-5-[4-(methylsulfonyl)phenyl]-2-
phenyloxazole;
4-(4-fluorophenyl)-2-methyl-5-[4-
CA 02270469 1999-04-30
WO 98/22101 PCT/L1S97/21489
14
(methylsulfonyl)phenyl]oxazole; and
4-[5-(3-fluoro-4-methoxyphenyl)-2-trifluoromethyl-4-
oxazolyl]benzenesulfonamide.
A family of specific compounds of more
particular interest within Formula I consists of
compounds and pharmaceutically-acceptable salts
thereof as follows:
4-[5-(4-chlorophenyl)-3-(trifluoromethyl)-1H-
pyrazol-1-yl]benzenesulfonamide;
4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-
pyrazol-1-yl]benzenesulfonamide;
4-[5-(3-fluoro-4-methoxyphenyl)-3-(difluoromethyl)-
1H-pyrazol-1-yl]benzenesulfonamide;
3-[1-[4-(methylsulfonyl)phenyl]-4-trifluoromethyl-
1H-imidazol-2-yl]pyridine;
2-methyl-5-[1-[4-(methylsulfonyl)phenyl]-4
trifluoromethyl-1H-imidazol-2-yl]pyridine;
4-[2-(5-methylpyridin-3-yl)-4-(trifluoromethyl)-1H
imidazol-1-yl]benzenesulfonamide;
4-[5-methyl-3-phenylisoxazol-4-
yl]benzenesulfonamide;
4-[5-hydroxymethyl-3-phenylisoxazol-4-
yl]benzenesulfonamide;
[2-trifluoromethyl-5-(3,4-difluorophenyl)-4-
oxazolyl]benzenesulfonamide;
4-[2-methyl-4-phenyl-5-oxazolyl]benzenesulfonamide;
and
4-[5-(3-fluoro-4-methoxyphenyl-2-trifluoromethyl)-
4-oxazolyl]benzenesulfonamide.
A subclass of cyclooxygenase-2 inhibitors is
selected from compounds of Formula II
CA 02270469 2005-08-22
R~
g5
H2 1
o'r5 \ / -N I I
O
R~
wherein R4 is selected from hydrido, alkyl,
haloalkyl, alkaxycarbonyl., ayano, cyanoalkyl, carboxyl.
5 aminocarbonyl, alkylaminacarbonyl,
cycloalkylami.nocarbonyl, arylaminocarbonyl,
carboxyalkylaminocarbc~ny7., carbo~cyalkyl,
aralkoxycarbony7.alkylamimocarbonyl, aminocarborxylalkyl,
alkoxycarbonylcyanaall~:Eny1 anc~ hydroxyalkyl;
10 wherein R5 is selected from hydrido, alkyl, cyano,
hydraxyalkyl, cycloalkyl, alkylsulfonyl and hale; and
wherein R~ is selected from aralkenyl, aryl.
cycloalkyl, cycloalkenyl and heterocyclic; wherein R~ is
optionally substituted ate a Substitutahle position with
15 one or more radicals selE~cted from halo, alkylthio,
alkylsulfonyl, cyano, nit:ro, haloalkyl, alkyl, hydroxyl.
alkenyl, hydroxyalkyl; carboxyl, cycloalkyl, alkylamino,
dialkylamino, alkoxycarbonyl, ami.nvcarbQnyl, alkoxy,
halaalkoxy, sulfa~nyl, he~teracyclic and amino;
or a pharmaceutical:Ly-acceptable salt or
derivative thereof.
A class of compound; of particular interest
consists of those compowzds of Formula rr wherein R~ is
selected from lower halo~31ky1; wherein R5 is hydridQ;
and wherein R6 is selected from phenyl optionally
substituted at a substitutable position with one or
more radicals selected f;ram halo, lower alkyl and lower
a~.ko~cy; or a p~.ax~naceut~.wally-acceptable salt or
derivative thereof.
CA 02270469 1999-04-30
WO 98/22101 PCT/US97/21489
16
A family of specific compounds of particular
interest within Formula I consists of compounds,
derivatives and pharmaceutically-acceptable salts
thereof as follows:
4-[5-(4-chlorophenyl)-3-(trifluoromethyl)-1H-pyrazol-1-
yl]benzenesulfonamide;
4-[5-phenyl-3-(trifluoromethyl)-1H-pyrazol-1-
yl]benzenesulfonamide;
4-[5-(4-fluorophenyl)-3-(trifluoromethyl)-1H-pyrazol-1-
yl]benzenesulfonamide;
4-[5-(4-methoxyphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-
yl]benzenesulfonamide;
4-[5-(4-chlorophenyl)-3-(difluoromethyl)-1H-pyrazol-1-
yl]benzenesulfonamide;
4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-
yl]benzenesulfonamide;
4-[3-(difluoromethyl)-5-(4-methylphenyl)-1H-pyrazol-1-
yl]benzenesulfonamide;
4-[3-(difluoromethyl)-5-phenyl-1H-pyrazol-1-
yl]benzenesulfonamide;
4-[3-(difluoromethyl)-5-(4-methoxyphenyl)-1H-pyrazol-1-
yl]benzenesulfonamide;
4-[3-(difluoromethyl)-5-(3-fluoro-4-methoxyphenyl)-1H-
pyrazol-1-yl]benzenesulfonamide; and
4-[5-(3-fluoro-4-methoxyphenyl)-3-(trifluoromethyl)-1H-
pyrazol-1-yl]benzenesulfonamide.
A family of specific compounds of more particular
interest within Formula I consists of compounds and
pharmaceutically-acceptable salts or derivatives thereof
as follows:
4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-
yl]benzenesulfonamide;
4-[5-(4-chlorophenyl)-3-(difluoromethyl)-1H-pyrazol-1-
yl]benzenesulfonamide; and
CA 02270469 1999-04-30
WO 98/22101 PCT/ITS97/21489
17
4-[5-(3-fluoro-4-methoxyphenyl)-3-(difluoromethyl)-1H-
pyrazol-1-yl]benzenesulfonamide.
Derivatives are intended to encompass any compounds
which are structurally related to the cyclooxygenase-2
inhibitors or which possess the substantially equivalent
biologic activity. By way of example, such inhibitors
may include, but are not limited to, prodrugs thereof.
Such compounds can be formed in vivo, such as by
metabolic mechanisms.
The term "hydrido" denotes a single hydrogen
atom (H). This hydrido radical may be attached,
for example, to an oxygen atom to form a hydroxyl
radical or two hydrido radicals may be attached
to a carbon atom to form a methylene (-CH2-)
radical. Where used, either alone or within
other terms such as "haloalkyl", "alkylsulfonyl",
"alkoxyalkyl" and "hydroxyalkyl", the term
"alkyl" embraces linear or branched radicals
having one to about twenty carbon atoms or,
preferably, one to about twelve carbon atoms.
More preferred alkyl radicals are "lower alkyl"
radicals having one to about ten carbon atoms.
Most preferred are lower alkyl radicals having
one to about six carbon atoms. Examples of such
radicals include methyl, ethyl, n-propyl,
isopropyl, n-butyl, isobutyl, sec-butyl, tert-
butyl, pentyl, iso-amyl, hexyl and the like. The
term "alkenyl" embraces linear or branched
radicals having at least one carbon-carbon double
bond of two to about twenty carbon atoms or,
preferably, two to about twelve carbon atoms.
More preferred alkenyl radicals are "lower
alkenyl" radicals having two to about six carbon
atoms. Examples of alkenyl radicals include
ethenyl, propenyl, allyl, propenyl, butenyl and
CA 02270469 1999-04-30
WO 98/22101 PCT/US97/21489
18
4-methylbutenyl. The term "alkynyl" denotes
linear or branched radicals having two to about
twenty carbon atoms or, preferably, two to about
twelve carbon atoms. More preferred alkynyl
radicals are "lower alkynyl" radicals having two
to about ten carbon atoms. Most preferred are
lower alkynyl radicals having two to about six
carbon atoms. Examples of such radicals include
propargyl, butynyl, and the like. The terms
"alkenyl", "lower alkenyl", embrace radicals
having "cis" and "trans" orientations, or
alternatively, "E" and "Z" orientations. The
term "cycloalkyl" embraces saturated carbocyclic
radicals having three to twelve carbon atoms.
More preferred cycloalkyl radicals are "lower
cycloalkyl" radicals having three to about eight
carbon atoms. Examples of such radicals include
cyclopropyl, cyclobutyl, cyclopentyl and
cyclohexyl. The term "cycloalkenyl" embraces
partially unsaturated carbocyclic radicals having
three to twelve carbon atoms. More preferred
cycloalkenyl radicals are "lower cycloalkenyl"
radicals having four to about eight carbon atoms.
Examples of such radicals include cyclobutenyl,
cyclopentenyl, cyclopentadienyl, and
cyclohexenyl. The term "halo" means halogens
such as fluorine, chlorine, bromine or iodine.
The term "haloalkyl" embraces radicals wherein
any one or more of the alkyl carbon atoms is
substituted with halo as defined above.
Specifically embraced are monohaloalkyl,
dihaloalkyl and polyhaloalkyl radicals. A
monohaloalkyl radical, for one example, may have
either an iodo, bromo, chloro or fluoro atom
within the radical. Dihalo and polyhaloalkyl
radicals may have two or more of the same halo
atoms or a combination of different halo
CA 02270469 1999-04-30
WO 98/22101 PCT/US97/21489
19
radicals. "Lower haloalkyl" embraces radicals
having 1-6 carbon atoms. Examples of haloalkyl
radicals include fluoromethyl, difluoromethyl,
trifluoromethyl, chloromethyl, dichloromethyl,
trichloromethyl, trichloromethyl,
pentafluoroethyl, heptafluoropropyl,
difluorochloromethyl, dichlorofluoromethyl,
difluoroethyl, difluoropropyl, dichloroethyl and
dichloropropyl. The term "hydroxyalkyl" embraces
linear or branched alkyl radicals having one to
about ten carbon atoms any one of which may be
substituted with one or more hydroxyl radicals.
More preferred hydroxyalkyl radicals are "lower
hydroxyalkyl" radicals having one to six carbon
atoms and one or more hydroxyl radicals.
Examples of such radicals include hydroxymethyl,
hydroxyethyl, hydroxypropyl, hydroxybutyl and
hydroxyhexyl. The terms "alkoxy" and "alkyloxy"
embrace linear or branched oxy-containing
radicals each having alkyl portions of one to
about ten carbon atoms. More preferred alko~cy
radicals are "lower alkoxy" radicals having one
to six carbon atoms. Examples of such radicals
include methoxy, ethoxy, propoxy, butoxy and
tert-butoxy. The term "alkoxyalkyl" embraces
alkyl radicals having one or more alkoxy radicals
attached to the alkyl radical, that is, to form
monoalkoxyalkyl and dialkoxyalkyl radicals. The
"alkoxy" radicals may be further substituted with
one or more halo atoms, such as fluoro, chloro or
bromo, to provide haloalkoxy radicals. More
preferred haloalkoxy radicals are "lower
haloalkoxy" radicals having one to six carbon
atoms and one or more halo radicals. Examples of
such radicals include fluoromethoxy,
chloromethoxy, trifluoromethoxy, trifluoroethoxy,
fluoroethoxy and fluoropropoxy. The term "aryl",
CA 02270469 1999-04-30
WO 98/22101 PCT/US97/21489
alone or in combination, means a carbocyclic
aromatic system containing one, two or three
rings wherein such rings may be attached together
in a pendent manner or may be fused. The term
5 "aryl" embraces aromatic radicals such as phenyl,
naphthyl, tetrahydronaphthyl, indane and
biphenyl. Aryl moieties may also be substituted
at a substitutable position with one or more
substituents selected independently from alkyl,
10 alkoxyalkyl, alkylaminoalkyl, carboxyalkyl,
alkoxycarbonylalkyl, aminocarbonylalkyl, alkoxy,
aralkoxy, hydroxyl, amino, halo, vitro,
alkylamino, acyl, cyano, carboxy, aminocarbonyl,
alkoxycarbonyl and aralkoxycarbonyl. The term
15 "heterocyclyl" embraces saturated, partially
unsaturated and unsaturated heteroatom-containing
ring-shaped radicals, where the heteroatoms may
be selected from nitrogen, sulfur and oxygen.
Examples of saturated heterocyclyl radicals
20 include saturated 3 to 6-membered heteromonocylic
group containing 1 to 4 nitrogen atoms (e. g.
pyrrolidinyl, imidazolidinyl, piperidino,
piperazinyl, etc.); saturated 3 to 6-membered
heteromonocyclic group containing 1 to 2 oxygen
atoms and 1 to 3 nitrogen atoms (e. g.
morpholinyl, etc.); saturated 3 to 6-membered
heteromonocyclic group containing 1 to 2 sulfur
atoms and 1 to 3 nitrogen atoms (e. g.,
thiazolidinyl, etc.). Examples of partially
unsaturated heterocyclyl radicals include
dihydrothiophene, dihydropyran, dihydrofuran and
dihydrothiazole. The term "heteroaryl" embraces
unsaturated heterocyclyl radicals. Examples of
unsaturated heterocyclyl radicals, also termed
"heteroaryl" radicals include unsaturated 3 to 6
membered heteromonocyclic group containing 1 to 4
nitrogen atoms, for example, pyrrolyl,
CA 02270469 2005-08-22
21
pyrx-olinyl, im~dazolyl, pyrazolyl, pyxidyl,
pyrimidyl, pyrazinyl, pyridazinyl, triazolyl
(e.g_, 4H-1,2,4--triazolyl, 1H-1,2,3-triazoly3.,
2H-2,2,3-txiazolyl, etc.) tetxazolyl (e.g. 1H-
tetrazolyl, 2H-tetrazolyl, etc.), etc.;
wnsaturated condensed heterocyclyl group
containing l to 5 nitrogen atoms, for example,
indolyl, isoindolyl, indolizinyl, benzimidazolyl,
quinolyl, isoquinolyl, zndazolyl, bezrzotriazolyl,
1o tetrazolopyridazir~yl (e.g.., tetrazolo[1,5-
b]pyridazinyl, etc.), etc:.; unsaturated 3 to 6-
membered heteromonocyclic: group containing an
oxygen atom, for example, pyxanyl, furyl, etc.;
unsaturated 3 to 6-membered heteromvnocyclic
group containing a sulfur- atom, for example,
thienyl, etc.; unsatuxat~:d 3- to 5-membered
heteromonocyclic group acsz~taining 7. to 2 oxygen
atoms and 1 to 3 nitrogen atoms, for example,
oxazolyl, isoxazolyl, Qx<<diazolyl (e. g., 1,2,4-
2Q oxadiazolyl, x.,3,4~oxadieizolyl, 1,2,5-
oxadiazolys, etc.) etr.; unsaturated condensed
heterocyclyl group containing 1 to 2 oxygen atoms
and 1 to 3 nitrogen atom~a (e_g_ benzoxazolyl,
benzOxadiazolyl, etc.): unsaturated 3 to 6-
membered heteromonocyclic: group containing 1 to 2
sulfur atoms and 1, to 3 nitrogen atoms, for
example, thiazolyl, thiac~iazolyl (e. g., 1,2,4-
thiadi.azolyl, 1,3,4--thiadiazolyl, 1,2,5-
thiadiazolyl, etc.) etc.; unsaturated coxadensed
heterocyclyl group containing 1 to Z sulfur atoms
and 1 to 3 nitrogen atom; (e_g., henzothiazolyl,
benzothiadiazolyl, etc..) and the like. The term
also embraces radical: m:nere heterocyclyl
rad~.cals are fused with aryl radicals. Examples
of such fused bicyalic radicals include
benzofuran, benzothiophewe, and the like_ Said
°heterocyclyl. gxoup° may have 1 to 3 substituents
CA 02270469 1999-04-30
WO 98/22101 PCT/~JS97/21489
22
such as alkyl, hydroxyl, halo, alkoxy, oxo, amino
and alkylamino. The term "alkylthio" embraces
radicals containing a linear or branched alkyl
radical, of one to about ten carbon atoms
attached to a divalent sulfur atom. More
preferred alkylthio radicals are "lower
alkylthio" radicals having alkyl radicals of one
to six carbon atoms. Examples of such lower
alkylthio radicals are methylthio, ethylthio,
propylthio, butylthio and hexylthio. The term
"alkylthioalkyl" embraces radicals containing an
alkylthio radical attached through the divalent
sulfur atom to an alkyl radical of one to about
ten carbon atoms. More preferred alkylthioalkyl
radicals are "lower alkylthioalkyl" radicals
having alkyl radicals of one to six carbon atoms.
Examples of such lower alkylthioalkyl radicals
include methylthiomethyl. The term
"alkylsulfinyl" embraces radicals containing a
linear or branched alkyl radical, of one to ten
carbon atoms, attached to a divalent -S(=O)-
radical. More preferred alkylsulfinyl radicals
are "lower alkylsulfinyl" radicals having alkyl
radicals of one to six carbon atoms. Examples of
such lower alkylsulfinyl radicals include
methylsulfinyl, ethylsulfinyl, butylsulfinyl and
hexylsulfinyl. The term "sulfonyl", whether used
alone or linked to other terms such as
alkylsulfonyl, denotes respectively divalent
radicals -S02-. "Alkylsulfonyl" embraces alkyl
radicals attached to a sulfonyl radical, where
alkyl is defined as above. More preferred
alkylsulfonyl radicals are "lower alkylsulfonyl"
radicals having one to six carbon atoms.
Examples of such lower alkylsulfonyl radicals
include methylsulfonyl, ethylsulfonyl and
propylsulfonyl. The "alkylsulfonyl" radicals may
CA 02270469 1999-04-30
WO 98!22101 PCT/US97/21489
23
be further substituted with one or more halo
atoms, such as fluoro, chloro or bromo, to
provide haloalkylsulfonyl radicals. The terms
"sulfamyl", "aminosulfonyl" and "sulfonamidyl"
denote NH202S-. The term "acyl" denotes a radical
provided by the residue after removal of hydroxyl
from an organic acid. Examples of such acyl
radicals include alkanoyl and aroyl radicals.
Examples of such lower alkanoyl radicals include
formyl, acetyl, propionyl, butyryl, isobutyryl,
valeryl, isovaleryl, pivaloyl, hexanoyl,
trifluoroacetyl. The term "carbonyl", whether
used alone or with other terms, such as
"alkoxycarbonyl", denotes -(C=O)-. The term
"aroyl" embraces aryl radicals with a carbonyl
radical as defined above. Examples of aroyl
include benzoyl, naphthoyl, and the like and the
aryl in said aroyl may be additionally
substituted. The terms "carboxy" or "carboxyl",
whether used alone or with other terms, such as
"carboxyalkyl", denotes -C02H. The term
"carboxyalkyl" embraces alkyl radicals
substituted with a carboxy radical. More
preferred are "lower carboxyalkyl" which embrace
lower alkyl radicals as defined above, and may be
additionally substituted on the alkyl radical
with halo. Examples of such lower carboxyalkyl
radicals include carboxymethyl, carboxyethyl and
carboxypropyl. The term "alkoxycarbonyl" means a
radical containing an alkoxy radical, as defined
above, attached via an oxygen atom to a carbonyl
radical. More preferred are "lower
alkoxycarbonyl" radicals with alkyl porions
having 1 to 6 carbons. Examples of such lower
alkoxycarbonyl (ester) radicals include
substituted or unsubstituted methoxycarbonyl,
ethoxycarbonyl, propoxycarbonyl, butoxycarbonyl
CA 02270469 1999-04-30
WO 98/22101 PCT/US97/21489
24
and hexyloxycarbonyl. The terms "alkylcarbonyl",
"arylcarbonyl" and "aralkylcarbonyl" include
radicals having alkyl, aryl and aralkyl radicals,
as defined above, attached to a carbonyl radical.
Examples of such radicals include substituted or
unsubstituted methylcarbonyl, ethylcarbonyl,
phenylcarbonyl and benzylcarbonyl. The term
"aralkyl" embraces aryl-substituted alkyl
radicals such as benzyl, diphenylmethyl,
triphenylmethyl, phenylethyl, and diphenylethyl.
The aryl in said aralkyl may be additionally
substituted with halo, alkyl, alkoxy, halkoalkyl
and haloalkoxy. The terms benzyl and
phenylmethyl are interchangeable. The term
"heterocyclylalkyl" embraces saturated and
partially unsaturated heterocyclyl-substituted
alkyl radicals, such as pyrrolidinylmethyl, and
heteroaryl-substituted alkyl radicals, such as
pyridylmethyl, quinolylmethyl, thienylmethyl,
furylethyl, and quinolylethyl. The heteroaryl in
said heteroaralkyl may be additionally
substituted with halo, alkyl, alkoxy, halkoalkyl
and haloalkoxy. The term "aralkoxy" embraces
aralkyl radicals attached through an oxygen atom
to other radicals. The term "aralkoxyalkyl"
embraces aralkoxy radicals attached through an
oxygen atom to an alkyl radical. The term
"aralkylthio" embraces aralkyl radicals attached
to a sulfur atom. The term "aralkylthioalkyl"
embraces aralkylthio radicals attached through a
sulfur atom to an alkyl radical. The term
"aminoalkyl" embraces alkyl radicals substituted
with one or more amino radicals. More preferred
are "lower aminoalkyl" radicals. Examples of
such radicals include aminomethyl, aminoethyl,
and the like. The term "alkylamino" denotes
amino groups which have been substituted with one
CA 02270469 1999-04-30
WO 98/22101 PCT/US97/21489
or two alkyl radicals. Preferred are "lower N-
alkylamino" radicals having alkyl portions having
1 to 6 carbon atoms. Suitable lower alkylamino
may be mono or dialkylamino such as N-
5 methylamino, N-ethylamino, N,N-dimethylamino,
N,N-diethylamino or the like. The term
"arylamino" denotes amino groups which have been
substituted with one or two aryl radicals, such
as N-phenylamino. The "arylamino" radicals may
10 be further substituted on the aryl ring portion
of the radical. The term "aralkylamino" embraces
aralkyl radicals attached through an amino
nitrogen atom to other radicals. The terms "N-
arylaminoalkyl" and "N-aryl-N-alkyl-aminoalkyl"
15 denote amino groups which have been substituted
with one aryl radical or one aryl and one alkyl
radical, respectively, and having the amino group
attached to an alkyl radical. Examples of such
radicals include N-phenylaminomethyl and N-
20 phenyl-N-methylaminomethyl. The term
"aminocarbonyl" denotes an amide group of the
formula -C(=O)NH2. The term "alkylaminocarbonyl"
denotes an aminocarbonyl group which has been
substituted with one or two alkyl radicals on the
25 amino nitrogen atom. Preferred are "N-
alkylaminocarbonyl" "N,N-dialkylaminocarbonyl"
radicals. More preferred are "lower N-
alkylaminocarbonyl" "lower N,N-
dialkylaminocarbonyl" radicals with lower alkyl
portions as defined above. The term
"alkylaminoalkyl" embraces radicals having one or
more alkyl radicals attached to an aminoalkyl
radical. The term "aryloxyalkyl" embraces
radicals having an aryl radical attached to an
alkyl radical through a divalent oxygen atom.
The term "arylthioalkyl" embraces radicals having
an aryl radical attached to an alkyl radical
CA 02270469 1999-04-30
WO 98/22101 PCT/US97/21489
26
through a divalent sulfur atom.
The compounds utilized in the methods of the present
invention may be present in the form of free bases or
pharmaceutically acceptable acid addition salts thereof.
The term "pharmaceutically-acceptable salts" embraces
salts commonly used to form alkali metal salts and to
form addition salts of free acids or free bases. The
nature of the salt is not critical, provided that it is
pharmaceutically-acceptable. Suitable pharmaceutically-
acceptable acid addition salts of compounds of Formula I
may be prepared from an inorganic acid or from an
organic acid. Examples of such inorganic acids are
hydrochloric, hydrobromic, hydroiodic, nitric, carbonic,
sulfuric and phosphoric acid. Appropriate organic acids
may be selected from aliphatic, cycloaliphatic,
aromatic, araliphatic, heterocyclic, carboxylic and
sulfonic classes of organic acids, example of which are
formic, acetic, propionic, succinic, glycolic, gluconic,
lactic, malic, tartaric, citric, ascorbic, glucuronic,
malefic, fumaric, pyruvic, aspartic, glutamic, benzoic,
anthranilic, mesylic, 4-hydroxybenzoic, phenylacetic,
mandelic, embonic (pamoic), methanesulfonic,
ethanesulfonic, benzenesulfonic, pantothenic, 2-
hydroxyethanesulfonic, toluenesulfonic, sulfanilic,
cyclohexylaminosulfonic, stearic, algenic, (i-
hydroxybutyric, salicylic, galactaric and galacturonic
acid. Suitable pharmaceutically-acceptable base
addition salts of compounds of Formula I include
metallic salts made from aluminum, calcium, lithium,
magnesium, potassium, sodium and zinc or organic salts
made from N,N'-dibenzylethylenediamine, chloroprocaine,
choline, diethanolamine, ethylenediamine, meglumine (N-
methylglucamine) and procaine. All of these salts may
be prepared by conventional means from the corresponding
compound of Formula I by reacting, for example, the
appropriate acid or base with the compound of Formula I.
CA 02270469 2005-08-22
27
GENERAL SYNTHET7CC PROCEDURE.7~
The cyclooxygenase-~ inhibitor compounds df
the invention can be synthe:~ized according to the
following procedures of Schemes I-~, wherein the
RI-R~ substituents are a:~ de:fined for Formula T,
above, except where further noted.
I
I
R1
Base
3.,-O -~ ~3
R CCH3 xlcozcx3 F~ 0 0
a
°~ ~~° / \
EcoH, a s z
. Rz
O R2
Rz ~ O
NrN
Ri
Ri
3
4
Synthetic Scheme I shows the preparation of
cyclooxygenase-2 inhib~.tor compounds, as described
in tl.S. patent No. 5,521,207 a.nd WQ95t15316,
i5 embraced by Formula ~. Tn step ~., ketone 1
is treated with a base, hreferab,ix NaOMe
or NaH, and an ester, or ester
CA 02270469 2005-08-22
28
equivalent, to form the zxitcrmediate diketone 2 (in
the enol fox-~n) which is cased without further
purification. Tn step 2, d.iketone 2 in an
anhydrous protic solvent, such as absolute ethanol
or acetic acid, is treated with the hydrochloride
salt or the free base of a Substituted hydrazine at
reflex to afford a mixture ~af pyrazoles 3 and ~.
Recrystal~.ization or chromatography affords 3
usually as a solid_ Similar pyrazoles can be
prepared by methods descr~.bed in U.5_ Pat. Nos.
4,16,721, 5,051,51$, 5,:L34,1~2 and ~,9~.4,121.
Sc~~m~ T~
1.5
SRZ ~ ys~ O R~ SRS
R \ ~ 2 ) R CO-X Ri \ /
O O
5 5
RaN~F3Z
SO R2 8R2
x
\ /
R1 \ ! ~xid:i.2e
R3 ~ 'N R3 ~ N
~a
R~ R
$ 7
SchEme Iz shows the four step procedure for
forming cyclooxygenase-2 inhibitor pyrazales 8 as
described in U.s. patent No. 5,~8&,53~ (where Ra is
hydrido or alkyl) from ketoses 5. In step 1,
ketone 5 is reacted with a base, such as lithium
bis(trimethylsilyl)amide or lithium
diisopropylamide (bpA) to form the anion. In step
2, the anion is reacted with an acetylating reagent
to provide diketorie 6, rn :step 3, the reaction of
CA 02270469 2005-08-22
29
diketone 6 with hydrazine or a substituted
hydrazine, gives pyra2ole 7. In step 4, the
pyraaole 7 is oxidized with an oxzdlzing reagent,
such as Oxone~ (potassium pexoxyznonosuliate), 3-
chloroperbenzoic acid (MCPBA) or hydrogen peroxide,
to give a mixture of the desired 3-
(alkylsulfonyl)phenyl-pyrazole 8 ~.nd the 5-
(alkylsulfonyl)phenyl-pyrazole isomer. The desired
pyrazole 8, usually a white or pale yellow solid,
is obtained in pure form either by chromatography
or recrystallization.
Alternatively, diketone ~ can be formed from
ketone S by treatment with a base, such as sodium
hydride, in a solvent, such as dimethylformamide,
and further reacting with a nitrile to form an
aminoketone. Treatment of the aminoketone with
acid forms the diketone 6. Similar pyrazoles can
be prepared by methods described in U.S. Pat. No.
3,984,431.
CA 02270469 2005-08-22
3a
~ ch~m~~ z z z
O
.H R SOZRz
R O OR
0 0 $ase
0
g 10
11
\ \
, Cu, D
N
dp R2 deb. NaOFI.
a
Rz
TORb
la o°
13
i N Cu, 6
5Q.,R=
S02R3
R1
RI
1
R ~ ~ "~-'
T
14
5 Cycloo~cygenase-2 inlzibitor d~.ary7./heteroary3.
thiophenes (where ~ is S, and Rb is alkyl) can be
prepared by the methods desaxibed in LT. S. Patent
Nos. 4,427.93, 4,302,461, .x,381,311, 4,59x,205,
and 4,820,827, and PCT docmnents WO 95/00501 and
ZO W094/15932.
CA 02270469 2005-08-22
31
Similar pyrroles (where T is N), furanones a.nd
I ~urans (where T is Q) c~ be prepared by methods
j descrilaed a.n PCT documexits WO 95/00501 and
W09&/15932, and in EP799,823. .
S
S ch.~me IV
Ri Na~ ~ ~ '~ R
R2S~ p T$,SG1 Ra ~ OTSS
17
16
MC1>BA
OH T~SO
1
H+
Hz0 2
RZ~zg ~ O R 025 O
19
18
p3COC1
$a.Se
O
R3 Rl
O
NH"OAc
HOAc ~ R3
0
~ta025 / D
R20aS
20 21
Cyclooxygenase-2 a.nhibitor diaryl/hetexoaryl
vxazoles can be prepared by the methods described
in U_S_ Patent Nos_ 3,783,656. 3,644,499 and
3,647,$58, and PCT documents WO 95/00501 and
wo99/2?990. ~quzvalent oxa zole compounds can Ire
prepared aria W096/19963 an~i W096/19462_
CA 02270469 2005-08-22
32
i S ~~.~rr~~ V
NOH i O R~
Rz 1 ) 2 eq. ll~BtlL1 R1 OH
~ ~ .2) ~R3C0)20
23
1 ) C1S03H
2 ) NHQOH
N O R3
S02NF3z
24
Cycloo~ygenase-2 inhixfitor diaxyl/heteroaryl
isoxazoles can be prepared by the methods described
in TJnited States No. 5,533,272, PCT documents
wp92/05152, and Wo92/19504, arid European.
Publication EP 26928_ Su~.fonamides 24 can be formed
fQ from the hydrated isoxaz~~le 23 in a two step proce-
dure. First, hydrated is~axazo~.e 23 is treated at
about 0°C with two or three equivalents of chlorosul~
Tonic acid to form the corresponding sulfonyl chloride.
~ In step two, the sulfonyL chloride thus formed is
treated with concentrated ammonia to provide the
CA 02270469 1999-04-30
WO 98/ZZ101 PCT/US97/21489
33
sulfonamide derivative 24.
Scheme VI
0 O Alkylaluminuat NH
R1CN + ~5~~ ~ ~ ~ Reagwnt
s
Rz Solvent R
25 26
27 SOzR2
Rb
Ra
Alkylation;
p base
R3
R3
N~8
Rb Dehydration 1~ N Rb
R
R N
SOZRZ
SOzR2
2g 28
Scheme VI shows the three step preparation of
the cyclooxygenase-2 inhibitor imidazoles 29 of the
present invention. In step 1, the reaction of
substituted nitriles (R1CN) 25 with primary
phenylamines 26 in the presence of alkylaluminum
reagents such as trimethylaluminum,
triethylaluminum, dimethylaluminum chloride,
diethylaluminum chloride in the presence of inert
solvents such as toluene, benzene, and xylene,
gives amidines 27. In step 2, the reaction of
amidine 27 with 2-haloketones (where X is Br or C1)
CA 02270469 2005-08-22
34
in the presence of bases, such as sodium
bicarbonate, potassium carbonate, sodium carbonate,
potassium bicarbonate or hindered tertiary amines
such as N,N'-diisopropylethylamine, gixres'the 4,5-
S dihydroimidaaoles a8 (where Rb is alkyl). Some of
the suitable solvents for this reaction are
isapropanol, acetone and dimethylformamide. The
reaction, may be carried out at temperatures of
about 20°C t4 about 90°C. In step 3. the 4,5-
dihydrAimidazoles 28 may be dehydrated in the
presence 4f an acid catalyst such as 4-
toluenesulfot~,ic acid or mineral acids'to form the
1,2-diaubstituted imida~c~les ~9 of the invention.
Suitable solvents for th:LS dehydration step are
a _ g _ , toluene, .xylene and b~..nzene _ TrafluoroacEtic
acid cars be used as solvent and catalyst fpr this
dehydration step. .
rn some cases ( a . g . , w~~ere R3 ~ methyl. or
phenyl) the ixitermediate 28 may not he readily
isolable. The reaction, under the conditions
described above, proceeds: tm give the targeted
imidazoles directly.
Similarly, imidazolea c:an be prepared having
the sulfonylphenyl moiety ataached at positior~ 2
and Rl attached at the nitragen.atom at position 1.
Diaryl/heteroaryl im~.dazole~s can ?ae prepared by
the methods described in U_S. Patent Nos.
4,822,$05, and PCT document WO 93/14082.
Sc~.eme VII
CA 02270469 1999-04-30
WO 98/22101 PCT/US97/21489
O OTMS
R1~H TMSCN 1~CN
R H
catalyst
30 31 ~ 1 ) Base
0
2 ) H ~ ~ SR2
1 ) Base
32
2 ) x ~ \ sR2
O '~ ~ ~ sRz
0
Rl
OH
33
Oxidizing
agent
SRZ
0
R1
0
34
NH~OAc, HOAc
R CHO
SRZ S02R2
/ ~ /
R3~ ~ R3~
Oxidation
I Ri I Ri
H H
35 36
The subject imidazole cyclooxygenase-2
inhibitor compounds 36 of this invention may be
5 synthesized according to the sequence outlined in
Scheme VII. Aldehyde 30 may be converted to the
protected cyanohydrin 31 by reaction with a
trialkylsilyl cyanide, such as trimethylsilyl
cyanide (TMSCN) in the presence of a catalyst such
10 as zinc iodide (ZnI2) or potassium cyanide (KCN).
CA 02270469 1999-04-30
WO 98122101 PCT/US97/21489
36
Reaction of cyanohydrin 31 with a strong base
followed by treatment with benzaldehyde 32 (where
R2 is alkyl) and using both acid and base
treatments, in that order, on workup gives benzoin
33. Examples of strong bases suitable for this
reaction are lithium diisopropylamide (LDA) and
lithium hexamethyldisilazane. Benzoin 33 may be
converted to benzil 34 by reaction with a suitable
oxidizing agent, such as bismuth oxide or manganese
dioxide, or by a Swern oxidation using dimethyl
sulfoxide (DMSO) and trifluoroacetic anhydride.
Benzil 34 may be obtained directly by reaction of
the anion of cyanohydrin 31 with a substituted
benzoic acid halide. Any of compounds 33 and 34
25 may be used as intermediates for conversion to
imidazoles 35 (where R2 is alkyl) according to
chemical procedures known by those skilled in the
art and described by M. R. Grimmett, "Advances in
Imidazole Chemistry" in Advances in Heterocyclic
Chemistry, 12, 104 (1970). The conversion of 34 to
imidazoles 35 is carried out by reaction with
ammonium acetate and an appropriate aldehyde
(R3CH0) in acetic acid. Benzoin 36 may be
converted to imidazoles 38 by reaction with
formamide. In addition, benzoin 36 may be
converted to imidazoles by first acylating with an
appropriate acyl group (R3C0-) and then treating
with ammonium hydroxide. Those skilled in the art
will recognize that the oxidation of the sulfide
(where R2 is methyl) to the sulfone may be carried
out at any point along the way beginning with
compounds 35, and including oxidation of imidazoles
38, using, for examples, reagents such as hydrogen
peroxide in acetic acid, m-chloroperoxybenzoic acid
(MCPBA) and potassium peroxymonosulfate (OXONE~).
CA 02270469 2005-08-22
37
niaryl/heterc>ar~rl zmida~oles can be
prepared by the metk~.od~: dEacribed in U.S. Patent
Nos. 3,7~7,~75, 8,686,231, 4,503,()65, 9,~.72,42~,
4, 372, 969, 9, 576, 958, 3. 9C~1, 908, 5, 620, 999,
European publication EFf 372,945, and PCT
document WO 95/00501.
CA 02270469 2005-08-22
38
Sc~r.eme VIII
Rasoz \ / $_ _1.n-BuLi. THF, -~ g2soa \ / 2nc1
2 _ z~iClz
3y 39
9r R3
Pda
Bx 3H
SOaRa
l.n-8uL~, THF. ,7B°C 8r
2. znCI2
R3 ~3
41 a0
Pd° ~lBr
$02Ra
..
~1
R3 R3
42
Diaryl/heteroaryl cyclc~gentene cyclooxygenase~-
2 inhibitors can be preparecl by the methods
descr~.bed in U.S. Fatent No. 5,344,991, and PCT
document WO 95f00501.
CA 02270469 2005-08-22
39
S C~7.~I~'~e T~
SO2R2 Pd~ . PhCH3 .
C2H50H,
~r NazC03 , d
-!- Rl~I3 (C~H) 2
\ /
R3 R3
a4
a3
Similarly, Synthetic Scheme IX shows the
procedure far the preparation of 7.,2-diaxylbenzene
cyclooxygenase-2 inhibitor agents ~4~4 fzom 2-brom4-
biphenyl intErmediates 43 (prepared similar to that
described ~.n Synthetic Scheme VITI) and the
appropriate substituted phenyllaoronic acids. Using
a ooupling procedure similar to the one developed
by Suzuki et al. [Synth. Cammttn., 11, 513 (1987.)7.
intermediates ~3 are reac_te3 urith the bdronic acids
in toluene/ethanol at reflex in the presence of a
Pd° catalyst, e.g.,
tetrakis(triphenylphosphine)palladium(0), and 2M
sodium carbonate to give thca corresponding 1,2-
diaryZbenzene antiinflammatcary agents ~~ of this
inverition. Such terphenyl <:ompounds can be
prepared by the methods described in PCT patent
2Q document W095/15934,
CA 02270469 2005-08-22
SC1'l~nle X
0
u~a
rS
R2 ~ ( 4
a~i!
8x S ~Z.S
CIT~CN, EtOFI
-h HzN~R3 ° 1 R
R O
43 ~s 47
5
Diaryl/heteroaryl thiazole cyclooxygenase-2
inhibitors can be prepared by tb.e methods described
irr U.S. Patent No. 4,051,2_°.0, 4.632.930. European
10 Application EP 592,664, and PCT documents
W096/03392 and WO 95/00501. Isothiazoles can be
prepared as described in PC:I' document WO 95/00501.
15 Diaryl/heteroary7. ~~yra_d~.ne ayc~aoxygenase-2
inhibitors can be prepared by the methods described
in U_S. Patent Nos_ 5,1.6:9,f357, 4,01I,328, 4,,533,656,
and wp96/24584 and wp96/24°if35.
zo
Bi.ologzcal Evaluat~.ox~
Antiangiogenic Assay
To determir~e t'he ef=~ec~ts of C:OX-2 inhibitors on
angiogenesis in vivo, we: tasted selective campouzids in
the mouse and rat cornea.I znicropocket assay. The mouse
CA 02270469 1999-04-30
WO 98/22101 PCT/US97/21489
41
corneal neovascularization micropocket model was
performed with materials, reagents and procedures
essentially as described by Muthukkauppah et al., J.
Natl. Cancer Inst., 69, 699-708 (1982). In this assay,
a pellet containing basic fibroblast growth factor
(FGF) was implanted into the corneal stroma of the
mouse and the newly formed vessels were measured using
a slit lamp. In this model, we COX-2 is expressed in
the endothelial cells of the newly developed blood
20 vessels. A COX-2 inhibitor, 4-[5-(4-chlorophenyl)-3-
(difluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide
inhibited FGF-induced angiogenesis in the mouse (70-
90~) at a dose of 6 mg/kg/day.
In the rat micropocket assay, 4-[5-(4-chlorophenyl)-
3-(difluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide
given only once inhibited FGF-induced angiogenesis (~90~).
We also determined the effects of a COX-2
inhibitor (4-[5-(4-chlorophenyl)-3-(difluoromethyl)-1H-
pyrazol-1-yl]benzenesulfonamide) in the mouse cornea
using another angiogenic stimuli, vascular endothelial
growth factor (VEGF). In this model, angiogenesis was
inhibited (~50~) when the compound was given at a dose
of 6 mg/kg.
Metastasis Model
A Murine Lewis lung carcinoma assay is performed as
described by I. Anderson et al [Can. Res., 56, 715
(1996)]. A COX-2 inhibitor is effective in inhibiting
metastasis in this model.
The present invention comprises a pharmaceutical
CA 02270469 1999-04-30
WO 98/22101 PCT/US97/21489
42
composition for the treatment of angiogenic disorders,
comprising a therapeutically-effective amount of a
compound of Formula I in association with at least one
pharmaceutically-acceptable carrier, adjuvant or
diluent (collectively referred to herein as "carrier"
materials) and, if desired, other active ingredients.
The active compounds of the present invention may be
administered by any suitable route known to those
skilled in the art, preferably in the form of a
pharmaceutical composition adapted to such a route, and
in a dose effective for the treatment intended. The
active compounds and composition may, for example, be
administered orally, intra-vascularly,
intraperitoneally, intranasal, intrabronchial,
subcutaneously, intramuscularly or topically (including
aerosol). If the angiogenesis is localized, local
administration rather than system administration is
preferred. Formulation in a lipid vehicle may be used
to enhance bioavailability.
The administration of the present invention may be
for either prevention or treatment purposes. The methods
and compositions used herein may be used alone or in
conjunction with additional therapies known to those
skilled in the art in the prevention or treatment of
angiogenesis. Alternatively, the methods and compositions
described herein may be used as adjunct therapy. By way of
example, the cyclooxygenase-2 inhibitor may be
administered alone or in conjunction with other
antineoplastic agents or other growth inhibiting agents or
other drugs or nutrients.
There are large numbers of antineoplastic agents
available in commercial use, in clinical evaluation and
in pre-clinical development, which could be selected
for treatment of angiogenesis by combination drug
chemotherapy. Such antineoplastic agents fall into
CA 02270469 1999-04-30
WO 9$/22101 PCT/US97/214$9
43
several major categories, namely, antibiotic-type
agents, alkylating agents, antimetabolite agents,
hormonal agents, immunological agents, interferon-type
agents and a category of miscellaneous agents.
Alternatively, other anti-neoplastic agents , such as
metallomatrix proteases inhibitors (MMP), such as MMP-
13 inhibitors including batiastat, marimastat, Agouron
Pharmaceuticals AG-3340, and Roche RO-32-3555, or oc"j33
inhibitors may be used.
A first family of antineoplastic agents which may
be used in combination with a selective cyclooxygenase-
2 inhibitor consists of antimetabolite-type
antineoplastic agents. Suitable antimetabolite
antineoplastic agents may be selected from the group
consisting of 5-FU-fibrinogen, acanthifolic acid,
aminothiadiazole, brequinar sodium, carmofur, Ciba-
Geigy CGP-30694, cyclopentyl cytosine, cytarabine
phosphate stearate, cytarabine conjugates, Lilly DATHF,
Merrel Dow DDFC, dezaguanine, dideoxycytidine,
dideoxyguanosine, didox, Yoshitomi DMDC, doxifluridine,
Wellcome EHNA, Merck & Co. EX-015, fazarabine,
floxuridine, fludarabine phosphate, 5-fluorouracil, N-
(2'-furanidyl)-5-fluorouracil, Daiichi Seiyaku FO-152,
isopropyl pyrrolizine, Lilly LY-188011, Lilly LY-
264618, methobenzaprim, methotrexate, Wellcome MZPES,
norspermidine, NCI NSC-3.27?16, NCI NSC-264880, NCI NSC-
39661, NCI NSC-612567, Warner-Lambert PALA,
pentostatin, piritrexim, plicamycin, Asahi Chemical PL-
AC, Takeda TAC-788, thioguanine, tiazofurin, Erbamont
TIF, trimetrexate, tyrosine kinase inhibitors, tyrosine
protein kinase inhibitors, Taiho UFT and uricytin.
A second family of antineoplastic agents which may
be used in combination with a selective cyclooxygenase-
2 inhibitor consists of alkylating-type antineoplastic
CA 02270469 1999-04-30
WO 98/22101 PCT/US97/21489
44
agents. Suitable alkylating-type antineoplastic agents
may be selected from the group consisting of Shionogi
254-S, aldo-phosphamide analogues, altretamine,
anaxirone, Boehringer Mannheim BBR-2207, bestrabucil,
budotitane, Wakunaga CA-102, carboplatin, carmustine,
Chinoin-139, Chinoin-153, chlorambucil, cisplatin,
cyclophosphamide, American Cyanamid CL-286558, Sanofi
CY-233, cyplatate, Degussa D-19-384, Sumimoto
DACHP(Myr)2, diphenylspiromustine, diplatinum
cytostatic, Erba distamycin derivatives, Chugai DWA-
2114R, ITI E09, elmustine, Erbamont FCE-24517,
estramustine phosphate sodium, fotemustine, Unimed G-6-
M, Chinoin GYKI-17230, hepsul-fam, ifosfamide,
iproplatin, lomustine, mafosfamide, mitolactol, Nippon
Kayaku NK-121, NCI NSC-264395, NCI NSC-342215,
oxaliplatin, Upjohn PCNU, prednimustine, Proter PTT-
119, ranimustine, semustine, SmithKline SK&F-101772,
Yakult Honsha SN-22, spiromus-tine, Tanabe Seiyaku TA-
077, tauromustine, temozolomide, teroxirone,
tetraplatin and trimelamol.
A third family of antineoplastic agents which may
be used in combination with a selective cyclooxygenase-
2 inhibitor consists of antibiotic-type antineoplastic
agents. Suitable antibiotic-type antineoplastic agents
may be selected from the group consisting of Taiho
4181-A, aclarubicin, actinomycin D, actinoplanone,
Erbamont ADR-456, aeroplysinin derivative, Ajinomoto
AN-201-II, Ajinomoto AN-3, Nippon Soda anisomycins,
anthracycline, azino-mycin-A, bisucaberin, Bristol-
Myers BL-6859, Bristol-Myers BMY-25067, Bristol-Myers
BMY-25551, Bristol-Myers BMY-26605, Bristol-Myers BMY-
27557, Bristol-Myers BMY-28438, bleomycin sulfate,
bryostatin-1, Taiho C-1027, calichemycin,
chromoximycin, dactinomycin, daunorubicin, Kyowa Hakko
DC-102, Kyowa Hakko DC-79, Kyowa Hakko DC-88A, Kyowa
Hakko DC89-A1, Kyowa Hakko DC92-B, ditrisarubicin B,
CA 02270469 1999-04-30
WO 9$/22101 PCT/US97/21489
Shionogi DOB-41, doxorubicin, doxorubicin-fibrinogen,
elsamicin-A, epirubicin, erbstatin, esorubicin,
esperamicin-A1, esperamicin-Alb, Erbamont FCE-21954,
Fujisawa FK-973, fostriecin, Fujisawa FR-900482,
5 glidobactin, gregatin-A, grincamycin, herbimycin,
idarubicin, illudins, kazusamycin, kesarirhodins, Kyowa
Hakko KM-5539, Kirin Brewery KRN-8602, Kyowa Hakko KT-
5432, Kyowa Hakko KT-5594, Kyowa Hakko KT-6149,
American Cyanamid LL-D49194, Meiji Seika ME 2303,
10 menogaril, mitomycin, mitoxantrone, SmithKline M-TAG,
neoenactin, Nippon Kayaku NK-313, Nippon Kayaku NKT-01,
SRI International NSC-357704, oxalysine, oxaunomycin,
peplomycin, pilatin, pirarubicin, porothramycin,
pyrindamycin A, Tobishi RA-I, rapamycin, rhizoxin,
15 rodorubicin, sibanomicin, siwenmycin, Sumitomo SM-5887,
Snow Brand SN-706, Snow Brand SN-07, sorangicin-A,
sparsomycin, SS Pharmaceutical SS-21020, SS
Pharmaceutical SS-73138, SS Pharmaceutical SS-98168,
steffimycin B, Taiho 4181-2, talisomycin, Takeda TAN-
20 868A, terpentecin, thrazine, tricrozarin A, Upjohn U-
73975, Kyowa Hakko UCN-10028A, Fujisawa WF-3405,
Yoshitomi Y-25024 and zorubicin.
A fourth family of antineoplastic agents which may
25 be used in combination with the selective
cyclooxygenase-2 inhibitor consists of a miscellaneous
family of antineoplastic agents selected from the group
consisting of alpha-carotene, alpha-difluoromethyl-
arginine, acitretin, Biotec AD-5, Kyorin AHC-52,
30 alstonine, amonafide, amphethinile, amsacrine,
Angiostat, ankinomycin, anti-neoplaston A10,
antineoplaston A2, antineoplaston A3, antineoplaston
A5, antineoplaston AS2-1, Henkel APD, aphidicolin
glycinate, asparaginase, Avarol, baccharin, batracylin,
35 benfluron, benzotript, Ipsen-Beaufour BIM-23015,
bisantrene, Bristo-Myers BMY-40481, Vestar boron-10,
bromofosfamide, Wellcome BW-502, Wellcome BW-773,
CA 02270469 1999-04-30
WO 98/22101 PCT/US97/21489
46
caracemide, carmethizole hydrochloride, Ajinomoto CDAF,
chlorsulfaquinoxalone, Chemes CHX-2053, Chemex CHX-100,
Warner-Lambert CI-921, Warner-Lambert CI-937, Warner-
Lambert CI-941, Warner-Lambert CI-958, clanfenur,
claviridenone, ICN compound 1259, ICN compound 4711,
Contracan, Yakult Honsha CPT-11, crisnatol, curaderm,
cytochalasin B, cytarabine, cytocytin, Merz D-609,
DABIS maleate, dacarbazine, datelliptinium, didemnin-B,
dihaematoporphyrin ether, dihydrolenperone, dinaline,
distamycin, Toyo Pharmar DM-341, Toyo Pharmar DM-75,
Daiichi Seiyaku DN-9693, elliprabin, elliptinium
acetate, Tsumura EPMTC, ergotamine, etoposide,
etretinate, fenretinide, Fujisawa FR-57704, gallium
nitrate, genkwadaphnin, Chugai GLA-43, Glaxo GR-63178,
grifolan NMF-5N, hexadecylphosphocholine, Green Cross
HO-221, homoharringtonine, hydroxyurea, BTG ICRF-187,
ilmofosine, isoglutamine, isotretinoin, Otsuka JI-36,
Ramot K-477, Otsuak K-76COONa, Kureha Chemical K-AM,
MELT Corp KI-8110, American Cyanamid L-623,
leukoregulin, lonidamine, Lundbeck LU-23-112, Lilly
LY-186641, NCI (US) MAP, marycin, Merrel Dow MDL-27048,
Medco MEDR-340, merbarone, merocyanine derivatives,
methylanilinoacridine, Molecular Genetics MGI-136,
minactivin, mitonafide, mitoquidone, mopidamol,
motretinide, Zenyaku Kogyo MST-16, N-(retinoyl)amino
acids, Nisshin Flour Milling N-021, N-acylated-
dehydroalanines, nafazatrom, Taisho NCU-190,
nocodazole derivative, Normosang, NCI NSC-145813, NCI
NSC-361456, NCI NSC-604782, NCI NSC-95580, octreotide,
Ono ONO-112, oquizanocine, Akzo Org-10172,
pancratistatin, pazelliptine, Warner-Lambert PD-111707,
Warner-Lambert PD-115934, Warner-Lambert PD-131141,
Pierre Fabre PE-1001, ICRT peptide D, piroxantrone,
polyhaematoporphyrin, polypreic acid, Efamol porphyrin,
probimane, procarbazine, proglumide, Invitron protease
nexin I, Tobishi RA-700, razoxane, Sapporo Breweries
RBS, restrictin-P, retelliptine, retinoic acid, Rhone-
CA 02270469 1999-04-30
WO 98/22101 PCT/US97/21489
47
Poulenc RP-49532, Rhone-Poulenc RP-56976, SmithKline
SK&F-104864, Sumitomo SM-108, Kuraray SMANCS, SeaPharm
SP-10094, spatol, spirocyclopropane derivatives,
spirogermanium, Unimed, SS Pharmaceutical SS-554,
strypoldinone, Stypoldione, Suntory SUN 0237, Suntory
SUN 2071, superoxide dismutase, Toyama T-506, Toyama T-
680, taxol, Teijin TEI-0303, teniposide, thaliblastine,
Eastman Kodak TJB-29, tocotrienol, Topostin, Teijin TT-
82, Kyowa Hakko UCN-O1, Kyowa Hakko UCN-1028, ukrain,
Eastman Kodak USB-006, vinblastine sulfate,
vincristine, vindesine, vinestramide, vinorelbine,
vintriptol, vinzolidine, withanolides and Yamanouchi
YM-534.
Examples of radioprotective agents which may be
used in the combination chemotherapy of this invention
are AD-5, adchnon, amifostine analogues, detox,
dimesna; 1-102, MM-159, N-acylated-dehydroalanines,
TGF- Genentech, tiprotimod, amifostine, WR-151327, FUT-
187, ketoprofen transdermal, nabumetone, superoxide
dismutase (Chiron) and superoxide dismutase Enzon.
Methods for preparation of the antineoplastic
agents described above may be found in the literature.
Methods for preparation of doxorubicin, for example,
are described in U.S. Patents No. 3,590,028 and No.
4,012,448. Methods for preparing metallomatrix
protease inhibitors are described in EP 780386,
W097/20824, W096/15096. Methods for preparing SOD
mimics are described in EP 524,101. Methods for
preparing Oc"~3, inhibitors are described in W097/08174.
In addition, the selective COX-2 inhibitor may be
administered in conjunction with other antiinflammatory
agents for maximum safety and efficacy, including
NSAID's, selective COX-1 inhibitors and inhibitors of
CA 02270469 1999-04-30
WO 98/22101 PCT/US97/21489
48
the leukotriene pathway, including 5-lipoxygenase
inhibitors. Examples of NSAID's include indomethacin,
naproxen, ibruprofen, salicylic acid derivatives such
as aspirin, diclofenac, ketorolac, piroxicam,
meloxicam, mefenamic acid, sulindac, tolmetin sodium,
zomepirac, fenoprofen, phenylbutazone, oxyphenbutazone,
nimesulide, zaltoprofen and etodolac.
The phrase "adjunct therapy" (or "combination
therapy"), in defining use of a cyclooxygenase-2
inhibitor agent and one or more other pharmaceutical
agent, is intended to embrace administration of each
agent in a sequential manner in a regimen that will
provide beneficial effects of the drug combination, and
is intended as well to embrace co-administration of
these agents in a substantially simultaneous manner,
such as in a single formulation having a fixed ratio of
these active agents, or in multiple, separate
formulations for each agent.
For oral administration, the pharmaceutical
composition may be in the form of, for example, a
tablet, capsule, suspension or liquid. The
pharmaceutical composition is preferably made in the
form of a dosage unit containing a particular amount of
the active ingredient. Examples of such dosage units
are capsules, tablets, powders, granules or a
suspension, with conventional additives such as
lactose, mannitol, corn starch or potato starch; with
binders such as crystalline cellulose, cellulose
derivatives, acacia, corn starch or gelatins; with
disintegrators such as corn starch, potato starch or
sodium carboxymethyl-cellulose; and with lubricants
such as talc or magnesium stearate.. The active
ingredient may also be administered by injection as a
composition wherein, for example, saline, dextrose or
water may be used as a suitable carrier.
CA 02270469 1999-04-30
WO 98/22101 PCT/US97/21489
49
For intravenous, intramuscular, subcutaneous, or
intraperitoneal administration, the compound may be
combined with a sterile aqueous solution which is
S preferably isotonic with the blood of the recipient.
Such formulations may be prepared by dissolving solid
active ingredient in water containing physiologically
compatible substances such as sodium chloride, glycine,
and the like, and having a buffered pH compatible with
physiological conditions to produce an aqueous
solution, and rendering said solution sterile. The
formulations may be present in unit or multi-dose
containers such as sealed ampoules or vials.
If the angiogenesis is localized in the G.I.
tract, the compound may be formulated with acid-stable,
base-labile coatings known in the art which begin to
dissolve in the high pH small intestine. Formulation to
enhance local pharmacologic effects and reduce systemic
uptake are preferred.
Formulations suitable for parenteral
administration conveniently comprise a sterile aqueous
preparation of the active compound which is preferably
made isotonic. Preparations for injections may also be
formulated by suspending or emulsifying the compounds
in non-aqueous solvent, such as vegetable oil,
synthetic aliphatic acid glycerides, esters of higher
aliphatic acids or propylene glycol.
Formulations for topical use include known gels,
creams, oils, and the like. For aerosol delivery, the
compounds may be formulated with known aerosol
exipients, such as saline, and administered using
commercially available nebulizers. Formulation in a
fatty acid source may be used to enhance
biocompatibility. Aerosol delivery is the preferred
CA 02270469 1999-04-30
WO 98/22101 PCT/US97121489
method of delivery for epithelial angiogenesis of the
lung for prevention application.
For rectal administration, the active ingredient may
5 be formulated into suppositories using bases which are
solid at room temperature and melt or dissolve at body
temperature. Commonly used bases include coca butter,
glycerinated gelatin, hydrogenated vegetable oil,
polyethylene glycols of various molecular weights, and
10 fatty esters of polyethylene stearate.
The dosage form and amount can be readily
established by reference to known treatment or
prophylactic regiments. The amount of therapeutically
15 active compound that is administered and the dosage
regimen for treating a disease condition with the
compounds and/or compositions of this invention depends
on a variety of factors, including the age, weight, sex
and medical condition of the subject, the severity of
20 the disease, the route and frequency of administration,
and the particular compound employed, the location of
the angiogenesis, as well as the pharmacokinetic
properties of the individual treated, and thus may vary
widely. The dosage will generally be lower if the
25 compounds are administered locally rather than
systemically, and for prevention rather than for
treatment. Such treatments may be administered as often
as necessary and for the period of time judged
necessary by the treating physician. One of skill in
30 the art will appreciate that the dosage regime or
therapeutically effective amount of the inhibitor to be
administrated may need to be optimized for each
individual. The pharmaceutical compositions may contain
active ingredient in the range of about 0.1 to 2000 mg,
35 preferably in the range of about 0.5 to 500 mg and most
preferably between about 1 and 200 mg. A daily dose of
about 0.01 to 100 mg/kg body weight, preferably between
CA 02270469 2005-08-22
51
about 0.1 and ab4ut 50 mg/kg body weight, may be
appropriate. The dai3y dose can be administered in one
to four doses per day.
Although this inventive has been described with
respect tv specific embodiments, the details of these
embodiments are not to be cv~strued as limitations.