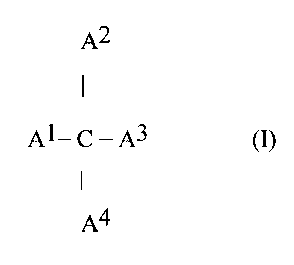Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
- CA 02274110 1999-06-04
wo rcrrn~rr
HAIR CQNDITIONING COMPOSITIONS C~IIPRIS~NG
WATER-INSOLUBLE HIGH MOLECULAR WEIGHT OILY COMPOUND
S
TECHNICAL FIELD
The present invention relates to hair conditioning compositions
comprising a water: insoluble high molecular weight oily compound.
BACKGROUND
Human hair becomes soiled due to its contact with the surrounding
environment and from sebum secreted by the scalp. The soiling of the hair
causes it to have a dirty or greasy feel, and an unattractive appearance. The
soiling of the hair necessitates shampooing with regulari#y.
Shampooing cleans the hair by removing excess soil and sebum.
However, shampooing can leave the hair in a wet, tanghad, and generally
unmanageable state. Once the hair dries, it is often left in a dry, rough,
lusterless, or frizzy condition due to removal of the hair's natural oils and
other natural conditioning and moisturizing components. The hair can further
.be left with increased levels of static upon drying which can interfere with
combing and result in a condition commonly referred to as "fly-away hair", or
- contribute to an undesirable phenomena of "split ends", particularly for
long
hair.
A variety of approaches have been developed to alleviate these after
shampoo problems. These approaches range from post shampoo
application of hair conditioner such as leave-on and rinse-off products, to
hair
condi~oning shampoos which attempt to both cleanse and conditron the hair
from a single product. Although some ~u~m p~,~ ~,e ease and
convenience of a shampoo which includes conditior~rrs, a substantial
proportion of consumers prefer the more conventional conditioner
formulations which are applied to the hair as a separate step from
shampooing, usually subsequent to shampooing. Conditioning formulation
can be in the form of rinse-off products or leave-on products, and can be in
the form of an emulsion, cream, gel, spray, and mousse. Such consumers
who prefer the conventional conditioner formulations value the relatively
higher conditioning effect, or convenience of changing the amount of
CA 02274110 1999-06-04
wo - PCTHJS96I19397
2
conditioning depending on the condition of hair or portion of hair. Further,
consumers prefer conditioners which provide smoothness and softness to the
hair and wet combing benefits.
A common method of providing conditioning benefit to the hair is
through the use of hair conditioning agents such cationic surfactants and
polymers, silicone conditioning agents, and hydrocarbon and other organic
oils, and solid aliphatics such as fatty alcohols. Cationic surfactants and
polymers, as well as oils and aliphatics are known to enhance hair shine and
provide moistness, softness, and static control to the hair, however, are also
ZO known to provide stickiness or greasy or waxy feeling.
High molecular weight substances, for example, silicone polymers are
also known to provide condfiioning benefits such as smoothness and
combing ease. Without being bound by theory, it is believed that, due to the
low surface tension of silicone polymers, silicone polymer provides favorable
conditioning benefits to the hair. However, a large amount of silicone
polymer compound causes dry feel or frizzy condition.
Japan Laid open S55-124711 discloses a-hair composition comprising
a branched ester such as pentaerythrittol rnonoisostearate, methylphenyl-
silicone compound, polyoxyethylene fatty acid ester and water. Japan Laid
open H5-124921 discloses a cosmetic composition comprising a nonionic
amph'rphiiic compound having at least one long chain branched alkyl or
alkenyi group and structure of a lamellar liquid crystal. The nonionic
amphiphilic indudes m~hyl branched fatty acid ester of pentaerythritol.
Ther_e~temains a desire to provide hair conditioning compositions with
improved conditioning benefits such as smoothness, softness, and ease of
combing when the hair is wet.
In the present invention, a hair conditioning composition comprising a -
water insoluble high molecular weight oily compound which is in a liquid form
and has a molecular weight of at least about 800 has been developed. This
hair condfioning -composition provides improved conditioning benefits such
__ as moistness, softness, free flowing, decreased stickiness; and static
control,
and which known conditioning agents can be incorporated in the
composifion.
CA 02274110 1999-06-04 _
WO l ~./US9~9397
3
_ SUMMARY
The present invention relates to a hair conditioning composition
comprising:
(a) a water insoluble high molecular weight oily compound having a
molecular weight of at least about 800, spedfic gravity of at least about
0.9, is in a liquid form at 25°C, and has the following formuta-(1):
A2
AL C - A3 (I)
A4
wherein A1, A2, A3 and A4 are independently alkyl, alkenyl, aryl, alkylaryl,
hydroxyalkyl, alkoxyl, alkoxyalkyl, acyl, acylalkyl, and alkylacyloxyi group
having C1 to about C3p or the formula -(CH2)n-O-OCR wherein R is from
C1 to about C30 of branched or straight chain alkyl or alkenyl and n is an -
integer from 1 to about 30; and
(b) a carrier.
Such compositions satisfy the need for a hair conditioning composition
which has improved conditioning benefits such as moistness, softness, free
flowing, decreased stickiness, and static control, and which known
conditioning agents can be incorporated in the composition.
DETAILED DESCRIPTION
All percentages herein are by weight of the compositions unless
otherwise indicated. AH ratios are weight ratios unless otherwise indicated:
All percentages, ratios, and levels of ingredients referred to herein are by
weight and are based on the actual amount of the ingredient, and do not
indude solvents, fillers, or other materials with which the ingredient may be
combined as commercially available products, unless othervvise-indicated.
The invention_hereof can comprise, consist of, or consist essentially of
the essential elements described herein as well as any of the preferred or
optional ingrediectts also described herein.
All publications, patent applications, and issued patents mentioned
herein are hereby incorporated in their entirety by reference.
- CA 02274110 1999-06-04
WO 98n4401 _ PCT/1JS96/19397 -
4
WATER-INSOLUBLE h+IGH MOLECULAR WEIGHT OILY COMPOUND
The hair conditioning composition of the present invention comprises a
water-insoluble high molecular weight oily compound and a carrier. The
water-insoluble high molecular weight oily compounds of the present -
invention are those which provide excellent conditioning benefrts such as
smoothness to the hair-~d ease of combing. Without being bound by .
theory, it is believed that, the water insoluble high molecular weight oily
compound of this invention is capable of being deposited on and conditions
the hair. It is also believed that this water insoluble high molecular weight
oily compound covers the surface of the hair and as a result reduces hair
friction to deliver smoothness to the hair. The water-insoluble high molecular
weight oily compound is chemically stable under normal use and storage
conditions.
As used herein, the term "water-insoluble oily compound" is meant that
the compound is not sufficiently soluble in water at room temperature. When
the compound is mixed with water at above 1.0% concentration, more
preferably at above 0.5% concentration, the compound is temporarily
dispersed to form unstable colloid in water, then is quickly separated from
water into two phases in a short period.
The water-insoluble high molecular weight oily compound useful in the
present invention has a molecular weight of at least about 800, preferably at
least about 1000, more preferably at least about 1200 as long as the
compound has a specific gravity of at least about 0.9, and is in a liquid form
at 25°C.
The water-insoluble high molecular weight oily compound useful
herein include those of the following formula (I):
A2
Al- C - A3 ( I
A4
wherein A1, A2, A3 and A4 are independently alkyl, alkenyl, aryl, alkytaryl,
hydroxyatkyl, alkoxyl, alkoxyalkyl, acyl, acylatkyl, and alkylacyloxyl group
having C1 to about C30 or the formula -(CH2)n-O-OCR wherein R is from C1
to about C30 of branched or straight chain alkyl or alkenyl and n is an
integer
from 1 to about 30, and preferably equals 1.
CA 02274110 1999-06-04
wo PCT~9~I1939'1
Preferably, the water insoluble high molecular weight oily compound
of
this invention is an ester wherein all of A1, A2, A3 and A4 are ester
groups or
three of A1, A2, A3 and A4 are ester groups and the remainder is
an alkyl
group. More preferably, the water insolub~ high molecular weight
oily
5 compounds of this invention are esters of a fatty acid of from
about
C12 to
about C22 with pentaerythritol, esters of a fatty acid of from about
C12 to
about C~ with trimethylolalkane, and mixtures thereof.
Preferable water-insoluble high molecular weight oily compounds are
those which have the same substituent for A1, A2, A3 and A4 and wherein
these substituents are ester groups.
The preferred water insoluble high nurlecular weight oily compounds
of this invention are pentaerythrkol tetraisostearate, trimethylotpropane
triisostearate, pentaerythritol tetraoleate, trimethylolpropane trioleate,
and
mixtures thereof.
Suitable ester compounds include, for example, pentserythritol
tetraisostearate and trimethylolpropane triisostearate which are
available
from Kokyo Alcohol with tradenames KAKPTI and KAKTTI, and
pentaerythritol tetraoleate and trimethylolpropane trioleate which
are
available from Shinnihon Rika with tradenames PTO and --ENUJERUBU
TP3S0, respectively.
The water insoluble high molecular weight oily compound is used at
a
level of from about 0.1 % to about 20.0%, preferably from about 0.1
% to
about 10.0%, more preferably from about 0.29~o to about 5.0% by weight
of
the composition.
CA_~ R
The hair cxanditioning composition of the present invention comprises
a
carrier. The level and species of the carrier are set accorcling
to the
compatibility with other components, and desired characteristic of
the
product.
The carrier useful in the present invention include water, lower
alkyl
aloohols, polyhydric alcohols, and mixtures thereof. The lower alkyl
alcohol
useful herein are C1-Cg alkyl monohydric alcohols, preferably C2-C3
alkyl
aicohols. The preferred lower alkyl alcohol is ethyl alcohol, isopropyl
alcohol,
and mixtures thereof. The polyhydric alcohols useful herein include,
for
example, propylene glycol, hexylene glycol, glycerin, and propane
diol, and
mixture ther~f.
CA 02274110 1999-06-04
wo rcrmsxm93m
s
NONVOLATILE WATE~t-INSOLUBLE LOW MOLECULAR WEIGHT OILY
COMPOUND
The hair conditioning composition of the present invention may further
comprise a nonvolatile water-insoluble low molecular weight oily compound.
The nonvolatile water-insoluble low molecular weight oily compounds of the
present invention are those which provide excellent conditioning benefits to
the hair. Without being bound by theory, it is believed that, the nonvolatile
water-insoluble low molecular weight oily compounds may penetrate to the
hair easily and result in providing softness to the hair and increasing hair
flexibility.
As used herein, the term "nonvolatile" is meant that the water-
insoluble low molecular weight oily compounds exhibit very low or no
significant vapor pressure at ambient conditions, e.g., 1 atmosphere at
25°C.
The nonvolatile water-insoluble low molecular weight oily compound
i 5 useful in the present invention has a molecular weight of less than about
800,
preferably less than about 500, and more preferably less than about 300 as
long as the compound has a speciftc gravity of at least about 0.8 and is in a
liquid form at 25°C.
Preferably, the nonvolatile water insoluble low molecular weight oily
compound useful herein is selected from the group consisting of hydrocarbon
having from 10 to about 40 carbon atoms, fatty aicohols having from about
10 to about 30 carbon atoms, fatty acids having from about 10 to about 30
carbon atoms, fatty acid derivatives, fatty alcohol derivatives, and mixtures
thereof.
Useful hydrocarbons include straight chain, cyclic, and branched chain
hydrocarbons which can be either saturated or unsaturated. Suitable
hydrocarbons having from 10 to about 40 carbon atoms include, for example
isoparaffln and mineral oil. These hydrocarbons are available, for example,
from Exxon Chemical Co. with tradenames Isopar and Witco Corp. with
tradenames Benol.
The fatty alcohols useful herein include those having from about 10 to
_ about 30 carbon atoms, preferably from about 12 to about'22 carbon atoms,
and more preferably from about 16 to about 22 carbon atoms. These fatty
ahhols can be straight or branched chain alcohols and can be saturated or
unsaturated alcohols, preferably unsaturated alcohois. Suitable fatty
alcohols include, for example, oleyl alcohol, isostearyl alcohol,
CA 02274110 1999-06-04 _
wo ~n~oi rc~ritrsm93m
tridecylalcohol, decyl tetradecyl ak~hol, and ociyl dodecyl alcohol. These
alcohols are available, for example, from Shinnihon Rika.
The fatty acids useful herein include those having from about 10 to
about 30 carbon atoms, preferably from about 12 to about 22 carbon atoms,
and more preferably from about 16 to about 22 carbon atoms. These fatty
acids can be straight or branched chain acids and can be saturated or
unsaturated. Suitable fatty acids include, for example, oleic acid, linoleic
acid, isostearic acid, linolenic acid, and ethyl linolenic acid.
The fatty acid derivatives and fatty alcohol derivatives are defined
herein to include, for example, esters of fatty alcohols, alkoxylated fatty
alcohols, alkyl ethers of fatty alcohols, alkyl ethers of alkoxyiated fatty
alcohols, and mixtures then~f. Nonlimiting examples of fatty acid derivatives
and fatty alcohol derivatives, include, for example, methyl linoleate, ethyl
linoleate, isopropyl linoleate, isodecyl oleate, isopropyl oleate, ethyl
oleate,
octyldodecyl oleate, oleyl oleate, decyl cleats, butyl oleate, methyl oleate,
_
octyldodecyl stearate, octykiodecyl isostearate, octyldodecyl isopalmitate,
octyl isopeiargonate, octyl pelargonate, hexyl isostearate, isopropyl
isostearate, isodecyl isononanoate, and Oleth-2.
Highly preferred nonvolatile water-insolt~le tow molecular weight oily
compound is selected ftom the group consisting of octyl dodecyl isostearate,
methyl myristate, oleyl alcohol, hydrocarbons having from about 14 to about
40 of carbon atoms, and mixtures thereof.
Nonlimiting examples of the above compounds are found in
International Cosmetic Ingredient Dictionary, Fifth EdiSon, 1993, and CTFA
Cosmetic Ingredient Handbook, Second Edition, 1992, both of which are
incorporated by reference herein in their entirety.
The nonvolatile k~nr molecular weight oily compound is comprised at a
level of from about 0.1 % to about 10.0%, preferably from about 0.2°r6
to
about 5.0%, more prefierably from about 0.2°~ to about 2.0% by weight
of the
composition. -
SOLID_WATIaR IMS~ ~ ~ IA!.IPHAT~,C COMPOU(~I'D _
The hair conditioning composition of the present invention may further
comprise a solid water-insoluble aliphatic compound. Without being bound
by theory, it is believed that, the solid water-insoluble aliphatic compound
of
. 35 this invention covers the hair surtace, and provides smooth feel on the
hair
and ease of combing by reducing friction coefficient.
CA 02274110 1999-06-04
WO 98/24401 - 8 PCT/US96/19397
As used herein, the term "solid water-insoluble" aliphatic compound is
meant that the compound is not sufficiently soluble in water at room
temperature: lNhen the compound is mixed with water at above 1.0%
concentration, more preferably at above 0.5% concentration, the compound
remains in the original state and does not dissolve. By the term "solid" what
is meant is that the cocapound has a resistance to pressure, does not easily'
change in shape, and is distinguished from a gum, paste, liquid or gaseous
property at 25°C.
The solid water insoluble aliphatic compound useful in the present
invention include those selected from the group consisting of hydrocarbons
having at least about 20 carbon atoms, saturated fatty aicohols having at
least about 14 carbon atoms, fatty acids having at least about 10 carbon
atoms, fatty acid derivatives, fatty alcohol derivatives, steroids, and
mixtures
thereof. The solid water insoluble aliphatic compounds such as
hydrocarbons, saturated fatty aicohols, fatty acids, fatty acid derivatives or
fatty alcohol derivatives are distinguished from the compounds exemplified in
the part of "nonvolatile water-insoluble low molecular wea,~ht oily compound"
in view of its different characteristics.
It is recognized that some of these compounds can have properties as
nonionic surfactants and can alternatively be classif~d as such. However, a
given classification is not intended to be a limitation on that particular
compound, but is done so for convenience of classification and
nomenclature.
The saturated fatty alc~hols useful herein are those having at least
about 14 carbon atoms, preferably from about 14 to about 22 carbon atoms.
These saturated fatty alcohots can be straight or branched chain alcohols.
Suitable saturated fatty alcohols include, for example, cetyl alcohol, stearyl
alcohol, and behenyl alcohol. These are commercially available, for
example, from Shinnihon Rika with trader amen Konol series, or from Nippon
Oil with tradenames NAA series.
The fatty acids ~eful herein are thane having at feast about 10 carbon
atoms. These fatty acids can be straight or branched chain acids and can be
saturated or unsaturatied. Also included are diaads, triacids, and other
multiple aads which meet the carbon number requirement herein. Suitable
fatty acids include, for example, stearic acid, behenic acid, palmitic acid,
archidonic acid and sebacic Aad. These are available, for example, from
CA 02274110 1999-06-04
WO 98J24401 _ P~~g96119397
9
Akzo with tradenames Noe-Fat, from Witco Corp. with tradenames
Hystrene,
or from Vevy with tradenarnes derma.
The fatty acid derirratives and the fatty alcohol derivatives
are deftned
herein to include ester of the fatty acids, including the fatty
alcohol esters of
the acid and the ethoxytated alcohol esters of the acid. Nonlimiting
examples
of fatty acid derivatives and fatty alcohol derivatives include,
for example
,
glyceryl monostearate, stearyl stearate, ethyl stearate, cetyl
stearate, cetyl
palmitate and myristyl myristate.
Suitable steroids include, for example, cholesterol, from Nikko
with
tradenames Nikkol Aguasome !.A.
A highly preferred solid water insoluble aliphatic compound is
saturated fatty alcohol having from about C14 to about C22.
Nonlimiting examples of the above compounds are found in
Intemationai Cosmetic Ingn3d~nt Dictionary, Fifth Edition, 1993,
and CTFA
Cosmetic Ingredient Handbook, Second Edition, 1992, both of which
are
incorporated by reference herein in their entirety.
The solid water insoluble aliphatic compound is comprised at
the level
of from about 0.1 % to about 20%, prefierabty from about 1 %
to about 15%,
more preferably from about 4% to about 10r6 of the composition
ADDITIONAL CONDITIONING AGENTS -
In addition, other conditioning agents known in the industry
may be
comprised in the present invention. Suitable conditioning agents
include
cationic surfactartts, cationic polymers, silicone compounds,
and mixtures
thereof. These conditioning agents are comprised at a level of
from about
0.01 % to about 20% of the conditioning composkion of the present
invention.
Cationic Surfactant
The hair conditioning compositions of the present invention can
_
additionally comprise one or more cationic surfactants as a conditioning
agent.
The cationic surfactants useful herein are any known to the artisan.
Among the cat~nic surfactants usefut herein are those corresponding
to the general formula (t): -
Rl
~
R2_ N+_ R3 X-
R4
CA 02274110 1999-06-04
WO 98/24401 PCT/U8%/19397
wherein at least one of -R1, R2, R3, and R4 is selected from an aliphatic
group of from 8 to 30 carbon atoms or an aromatic, alkoxy, polyoxyalkylene,
alkylamido, hydroxyalkyl, aryl or alkylaryl group having up to about 22 carbon
atoms, the remainder of R1, R2, R3, and R4 are independently selected from
5 an aliphatic group of from 1 to about 22 carbon atoms or an aromatic,
alkoxy,
polyoxyalkylene, alkylamido, hydroxyalkyi, aryl or alkylaryl group having up
to
about 22 carbon atoms; and K is a salt-forming anion such as those selected
from halogen, (e.g. chloride, bromide), acetate, citrate, lactate, glycolate,
phosphate, nitrate, sulfonate, sulfate, alkylsulfate, and alkyl sulfonate
10 radicals. _ The aliphatic groups can contain, in addition to carbon and
hydrogen atoms, ether linkages, and other groups such as amino groups.
The longer chain aliphatic groups, e.g., those of about 12 carbons, or higher,
can be saturated or unsaturated. Preferred is when R1, R2, R3, and R4 are
independently selected from C1 to about C22 alkyl. Nonlimiting examples of
1~ cationic surfactants useful in the present invention include the materials
having the following CTFA designations: quatemium-8, quatemium-24,
quaternium-26, quaternium-27, quatemium-30, quatemium-33, quatemium-
43, quatemium-52, quaternium-53, quatemium-56, quatemium-80,
quatemium-62, quatemium-70, quatemium-72, quatemium-75, quatemium-
77, quatemium-78, quaternium-80, quatemium-81, quatemium-82,
quaternium-83, quatemium-84, and mixtures thereof.
Also preferred are hydrophilically substituted cationic surfactants in
which at least one- of the substituents contain one or more aromatic, ether,
ester, amido, or amino moieties present as substituents or as linkages in the
radical chain, wherein at toast one of the R1 - R4 radicals contain one or
more hydrophilic moieties selected from alkoxy (preferably C1 - Cg alkoxy),
pofyoxyalkylene (preferably C1 - C3 polyoxyalkylene), alkylamido,
.hydroxyalkyl, alkylester, and combinations thereof. Preferably, the
hydrophiiicatly substituted cationic conditioning surfactant contains from 2
to
about 10 nonionic hydrophile moieties locate! within the above stated
ranges. Preferred hydrophilically substituted cationic surfactants include
those of the formula (II) through (VII) below:
z1
_
~3 ( ~2 ) ri ~2- N+- ( CH2 CH20 ) XH X' ( I I
I
(CH2CH20) ~,H
CA 02274110 1999-06-04
wo poi PCT//Z1189~6I19347
_ 11
wherein n is ftom 8 to about 28, x+y is from 2 to about 40, Z1 is a short
chain
alkyl, preferably a C1 - C3 alkyl, more preferably methyl, or - (CH2CH2O)ZH
wherein x+y+z is up to 60, and X is a salt forming anion as defined above;
' S R6 R8
RS- N+_ ( CIi2 ) m =~+_ R9 2X' ( I I I )
R10
wherein m is 1 to 5, one or more of R5, R6, and R7 are independently an C 1
- C30 alkyl, the remainder are - CH2CH20H, one or two of R8, R9, and R10
are independently an C1 - C30 alkyl, and remainder are - CH2CH20H, and
X is a salt forming anion as mentioned above;
O Z2 O
()
R11_ C~ _ ( CH2 ) p -N+_ ( CH2 ) q NFiCRl2 X' ( IV )
Z3
wherein Z2 is an alkyl, preferably a C~ - Cg alkyl, more preferably methyl,
and Z3 is a short chain hydroxyalkyl, preferably hydroxymethyl or
hydroxyethyl, p and q independently are integers from 2 to 4, inclusive,
preferably from 2 to 3, inclusive, more prefienably 2, R11 and R12 ,
independently, are substituted or unsubstituted hydrocarbyls, preferably C12
- C20 alkyl or alkenyl, and X is a salt forming anion as defined above;
Z4
R13_ N+. (CH2CH0)aH X' (V)
I
- ZS ~3
wherein R'! 3 is a hydrocarbyl, preferably a C 1 - C3 alkyl, more preferably
methyl, Z4 and Z5 are, independently, short chain hydrocarbyls, preferably _
C2 - C4 aNryl or alkenyl, more preferably ethyl, a is from 2 to about 40,
preferably from about 7 to about 30, and X is a sad forming anion as defined
ate;
R14 . _
CA 02274110 1999-06-04
WO 98/Z4401 - PCT/U89(~19397 -
12
Z6- N+- CH2CCH3- A X- (VI)
R15 OH
wherein R14 and R15, independently, are C1 - C3 alkyl, preferably methyl,
Z6 is a C12 - C22 hydrocarbyl, alkyl carboxy or alkylamido, and A is a
protein, preferably a coNagen, keratin, milk protein, silk, soy protein, wheat
_
protein, or hydrolyzed forms thereof; and X is a salt forming anion as defined
above;
O R16
HOCH2- (CHOH) 4-CNH ( CH2 ) b-N+-CH2CH20H X- (VII)
Rl7
wherein b is 2 or 3, R16 and R17, independently are C1 - C3 hydrocarbyls
preferably methyl, and X is a salt forming anion as defined above.
Nonlimiting examples of hydrophilically substituted cationic surfactants
useful
in the present invention include the materials having the following CTFA
designations: quaternium-16, quatemium-61, quatemium-71, quaternium-79
hydrolyzed collagen, quatemiurn-79 hydrolyzed keratin, quatemium-79
hydrolyzed milk protein, quatemium-79 hydrolyzed silk, quatemium-79
hydrolyzed soy protein, and quatemium-79 hydrolyzed wheat protein. Highly
preferred compounds include commercially available materials; VARIQUAT
K1215 and 838 from Witco Chemical, MACKPRO KLP, MACKPRO WLW,
MACKPRO-It~LP, MACKPRO NSP, MACKPRO NLW, MACKPRO WIMP,
MACKPRO NLP, MACKPRO SLP from Mclntyre, ETHOQUAD 18/25,
ETHOQUAD OI12PG, ETHOQUAD C/25, ETHOQUAD S/25, and
ETHODUOQUAD from Akzo, DEHYQUAT SP from Henkel, and ATLAS
6265 from ICI Americas.
Salts of primary, secondary, and tertiary fatty amines are also suitable
cationic surfactants. The alkyl groups of such amines preferably have from
_ about 12 to about 22 carbon atoms, and can be substituted or unsubstituted.
Particularly useful are amido substituted tertiary fatty amines. Such amines,
useful herein, include stearamidopropyldimethylamine,
stearamidopropyldiethylamine, stearamidoethyldiethylamine,
stearamidoethyldimethylamine, palmitamidopropyldimethylamine,
palmitamidopropyidiethylamine, palrnitamidoethyldiethylamine,
palmitamidoethyldimethylamine, behenamidopropyldimethylamine,
CA 02274110 2002-02-28
13
behenamidopropyldiethylamine, behenamidoethyldiethytamine,
behenamidoethyldimethylamine, arachidamidopropyldimethylamine,
arachidamidopropyldiethylamine, arachidamidoethyldiethylamine,
arachidamidoethyfdimethylamine, diethylaminoethylstearamide. Also useful
are dimethylstearamine, dimethylsoyamine, soyamine, myristylamine,
tridecyiamine, ethylstearylamine, N-tallowpropane diamine, ethoxylated (with
5 moles of ethylene oxide) stearylamine, dihydroxyethylstearylamine, and
arachidylbehenylamine. These amines can also be used in combination with
acids such as L-glutamic acid, lactic acid, hydrochloric acid, malic acid,
succinic acid, acetic acid, fumaric acid, tartaric acid, citric acid, L-
glutamic
hydrochloride, and mixtures thereof; more preferably L-glutamic acid, lactic
acid, citric acid. Cationic amine surfactants inciud-ed among those useful in
the present invention are disclosed in U.S. Patent 4,275,055, Nachtigal, et
al., issued June 23, 1981.
The cationic surfactants for use herein may also include a plurality of
ammonium quaternary moieties or amino moieties, or a mixture thereof.
The cationic surfactants for use herein may be used as emulsifying
agents in this composition.
Cationic Polymers
The hair conditioning compositions of the present invention can further
comprise one or more cationic polymer as a conditioning agent. As used
herein, the term "polymer" shall include materials whether made by
polymerization of one type of monomer or made by two (i.e., copolymers) or
more types of monomers.
Preferably, the cationic polymer is a water soluble cationic polymer.
By 'water soluble" cationic polymer, what is meant is a polymer which is
suffiaentty soluble in water to form a substa~tialiy clear solution to the
naked
eye at a concentration of 0.1 % in water (distilled or equivalent) at
25°C. The
preferred polymer will be suffiaently soluble to form a substantially clear
solution at 0.5% concentration, more preferably at 1.0% conosntration.
The cationic~polymers hereof will generally have a weight average
molecular weight which is at feast about 5,000, typically at least about
10,000, and is less than about 10 million. Preferably, the molecular weight is
from about 100,000 to about 2 million. The cationic polymers will generally
have cationic nitrogen-containing moieties such as quaternary ammonium or
cationic amino moieties, and mixtures thereof.
CA 02274110 1999-06-04
wo poi PCTIU996I19397
14
The cationic charge density is preferably at least about 0.1 meqlgram,
more preferably at least about 1.5 meq/gram, even more preferably at least
about 1.1 meq/gram, still more preferably at least about 1.2 meqlgram.
Cationic charge density of the cationic polymer can be determined according
to the Kjekiahl Method. Those skilled in the art will recognize that the
charge
density of amino-containing polymers may vary depending upon pH and the
isoelectric point of the amino groups. The charge density should be within
the above limits at the pH of intended use.
Any anionic counterions can be utilized for the cationic polymers so
long as the water solubility criteria is met. Suitable counterions include
halides (e.g., CI, Br, 1, or F, preferably CI, Br, or I), sulfate, and
methylsulfate.
Others can also be used, as this list is not exclusive.
The cationic nitrogen-containing moiety will be present generally as a
substituent, on a fraction of the total monomer units of the cationic hair
conditioning polymers. Thus, the cationic polymer can comprise copolymers,
terpolymers, etc. of quaternary ammonium or cationic amine-substituted
monomer units and other non-cationic units referred to herein as spacer
monomer units. Such polymers are known in the art, and a variety can be
found in the CTFA Cosmetic Ingredient Dictionary, 3rd edition, edited by
Estrin, Crosley, and Haynes, (The Cosmetic, Toiletry, and Fragrance
Association, Inc., Washington, D.C., 1982).
Suitable cationic polymers include, for example, copolymers of vinyl
monomers having cationic amine or quaternary ammonium functionalities
with water soluble spacer monomers such as acrylamide, methacryiamide,
alkyl and dialkyl acrylamides, alkyl and dialkyl methacrylamides, alkyl
acrylate, alkyl methacrylate, vinyl caprolactone, and vinyl pyrrolidone. The
. alkyl and dialkyl substituted monomers preferably have C1 - C7 alkyl groups,
more preferably C1 - C3 alkyl groups. Other suitable spacer monomers
include vinyl esters, vinyl alcohol (made by hydrolysis of polyvinyl acetate),
malefic anhydride, propylene glycol, and ethylene glycol.
The cationic amines can be primary, secondary, or tertiary amines,
depending upon the particular species and the pH of the composition. In
ger~ral, secondary and tertiary amines, especially tertiary amines, are
prefierred. _
Amine-substituted vinyl rrronomers can be polymerized in the amine
form, and then optionally can be converted to ammonium by a quaternization
reaction. Amines can also be similarly quaternized subsequent to formation
- CA 02274110 1999-06-04
WO 9&/2440! PCT/US9~/19397
of the polymer. For example, tertiary amine functionalities can -be
quaternized by reaction with a salt of the formula R'X wherein R' is a short
chain alkyl, preferably a C1 - C7 alkyl, more preferably a C1 - C3 alkyl, and
X
is an anion which forms a water soluble salt with the quaternized ammonium.
5 Suitable cationic amino and quaternary ammonium monomers include,
for example, vinyl connpounds substituted with dialkylaminoalkyl acryiate,
dialkylaminoalkyl methacrylate, monoalkylaminoaikyl acrylate,
monoalkylaminoalkyl methacryiate, trialkyl methacryloxyalkyl ammonium salt,
trialkyl acryloxyalkyl ammonium salt, diallyl quaternary ammonium salts, and
10 vinyl quaternary ammonium monomers having cyclic cationic nitrogen-
containing rings such as pyridinium, imidazolium, and quatemized
pyrrolidone, e.g., alkyl vinyl imidazolium, alkyl vinyl pyridinium, alkyl
vinyl
pyrrolidone salts. The alkyl portions of these monomers are preferably lower
alkyls such as the C1 - C3 alkyls, more preferably C1 and C2 alkyls. Suitable
15 amine-substituted vinyl monomers for use herein include dialkylaminoalkyl
acrylate, dialkylaminoalkyl methacrylate, diaikyiaminoalkyl acrylamide, and
dialkylaminoalkyl methacrylamide, wherein the alkyl groups are preferably C1
- C7 hydrocarbyls, more preferably C1 - Cg, alkyls.
The cationic polymers hereof can comprise mixtures of monomer units
derived from amine- and/or quaternary ammonium-substituted monomer
and/or compatible spacer monomers.
Suitable cationic hair conditioning polymers include, for example:
copolymers - of 1-vinyl-2-pyrroiidone and 1-vinyl-3-methylimidazolium salt
(e.g., chloride satt) (referred to in the industry by the Cosmetic, Toiletry,
and
Fragrance Association, "CTFA", as Polyquatemium-16), such as those
cornmeraally available from BASF Wyandotte Corp. (Parsippany, NJ, USA)
under the LUVIQUAT tradename (e.g., LUVIQUAT FC 370); copolymers of 1-
vinyl-2-pymolidone and dimethylaminoethyl methacrylate (referred to in the
industry by CTFA as Polyquatemium-11) such as those commercially
available from Gaf Corporation (Wayne, NJ, USA) under the GAFQUAT
tradename (e.g.; GAFQUAT 755N); cationic diallyl quaternary ammonium-
containing polymers, including, for example, dimethyldiallylammonium -
' chlorkle homopolymer and copolymers of acryiamide and
dimethyidiallylammonium chloride, referred to in the industry (CTFA) as
Polyquatemium 8 and Polyquatemium 7, respectively; and mineral acid salts
of amino-alkyl esters of homo- and co-polymers of unsaturated carboxylic
CA 02274110 2002-02-28
acids having from 3 to 5 carbon atoms, as described in U.S. Patent
4,009,256.
Other cationic polymers that can be used include polysaccharide
polymers, such as cationic cellulose derivatives and cationic starch
derivatives.
Cationic polysaccharide polymer materials suitable for use herein
include those of the formula:
R1
~
A - O - ( R - N+- R3 ) g-
I
2
R
wherein: A is an anhydroglucose residual group, such as a
starch or cellulose
anhydroglucose residual, R is an alkylene oxyalkylene, poiyoxyalkyiene,
or
hydroxyalkylene group, or combination thereof, R1, R2; and
R3
independently are alkyl, aryl, _ alkylaryl, arylalkyl, alkoxyalkyl,
or alkoxyaryl
groups, each group containing up to about 18 carbon atoms,
and the total
number of carbon atoms for each cationic moiety (i.e., the
sum of carbon
atoms in R1, R2 and R3) preferably being about 20 or less,
and X is an
anionic counterion, as previously described:
Cationic cellulose is available from Amerchol Corp. (Edison,
NJ, USA)
in their Polymer JR~ and LR~ series of polymers, as salts
of hydroxyethy!
cellulose reacted with trimethyl ammonium substituted epoxide,
referred to in
the industry (CTFA) as Polyquatemium 10. Another type of cationic
cellulose
includes the polymeric quaternary ammonium salts of hydroxyethyl
cellulose
reacted with aauryl dimethyi ammonium-substituted epoxide,
referred to in the
industry (CI-FA) as Potyquatemium 24. These materials ana
available from
Amerchol Corp. (Edison, NJ, USA) under the tradename Polymer
LM-200~.
Other cationic polymers that can be used include cationic
guar gum
derivatives, such as guar hydroxypropyltrimonium chloride
(commercially
available from Celanese Corp. in their Jaguar R series). Other
materials
include quaternary nitrogen-containing cellulose ethers (e.g.,
as described in
U.S. Patent 3,962,418), and aopoiymen3 of
ether~ed cellulose and starch (e.g., as described in U.S.
Patent 3,958,581).
Silicone corn unds
CA 02274110 1999-06-04
WO 98124401 PCTIUS96/19397
17
Other conditioning agents useful herein include silicone compounds.
The hair conditioning compositions hereof can include volatile soluble or
insoluble, or nonvolatile soluble or insoluble silicone conditioning agents.
By_
soluble what is meant is that the silicone conditioning agent is miscible with
the carrier of the composition so as to form part of the same phase. By
insoluble what is meant is that the silicone forms a separate, discontinuous
phase from the carrier, such as in the form of an emulsion or a suspension of
droplets of the silicone.
The nonvolatile dispersed silicones for use herein will preferably have
a viscosity of from about 1,000 to about 2,000,000 centistokes at 25oC, more
preferably from about 10,000 to about 1,800,000, and even more preferably
from about 100,000 to about 1,500,000. The viscosity can be measured by
means of a glass capillary viscometer as set forth in Dow Coming Corporate
Test Method CTM0004, July 20, 1970, which is incorporated by reference
I S herein in its entirety. Suitable silicone fluids include polyalkyl
siloxanes, .
polyaryl siloxanes, polyalkylaryl siloxanes, polyether siloxane copolymers,
and mixtures thereof. Other nonvolatile silicone compounds having hair
conditioning properties can also be used.
The nonvolatile dispersed silicone compounds herein -also include
polyalkyl or polyaryi siloxanes with the following structure (1)
R R R
A - Si- O - ( Si- O ] x - Si- A ( I )
_ _1_ I
R R R
when3in R is alkyl or aryl, and x is an integer from about 7 to about 8,000.
"A" represents groups which block the ends of the silicone chains. The alkyl
. _ 30 or aryl groups substituted on the siloxane chain (R) or at the ends of
the
siloxane chains (A) can have any structure as long as the resumng silicone
remains fluid at room temperature, is dispersible, is neither irritating;
toxic nor
- otherwise harmful when applied to the hair, is compatible with the other
components of the composition, is chemically stable under normal use and
storage conditieons, and is capable of being deposited on and conditions the
hair. Suitable A groups include hydroxy, methyl, methoxy, ethoxy, propoxy,
and aryloxy. The two R groups on the silicon atom may represent the same
group or different groups. Preferably, the two R groups represent the same
group. Suitable R groups include methyl, ethyl, propyl, phenyl, methylphenyl
CA 02274110 1999-06-04
WO 98124401 PCT/U896/19397
18
and phenylmethyl. - The preferred silicone compounds are
polydimethylsiloxane, polydiethylsiioxane, and polymethylphenylsiloxane.
Polydimethylsiloxane, which is also known as dimethicone, is especially
preferred. The polyalkylsiloxanes that can be used include, for example, -
polydimethylsiloxanes. These silicone compounds are available, for
example, from the General Electric Company in their ViscasilR and SF 96
series, and from Dow Corning in their Dow Corning 200 series.
Polyalkylaryl siloxane fluids can also be used and include, for
example, polymethylphenylsiloxanes. These siloxanes are available, for
example, from the General Electric Company as SF 1075 methyl phenyl fluid
or frorri Dow Corning as 556 Cosmetic Grade Fluid.
Especially preferred, for enhancing the shine characteristics of hair,
are highly arylated silicone compounds, such as highly phenylated polyethyl
silicone having refractive index of about 1.46 or higher, especially about
1.52
or higher. When these high refractive index silicone compounds are used,
they should be mixed with a spreading agent, such as a surfactant or a
silicone resin, as described below to decrease the surface tens'ron and
enhance the film forming ability of the material.
The nonvolatile dispersed silicone compounds that can be used
include, for example, a polypropylene oxide modified polydimethylsiloxane
although ethylene oxide or mixtures of ethylene oxide and propylene oxide
can also be used. The ethylene oxide and polypropylene oxide level should
be sufficiently low so as not to interfere with the dispersibility
characteristics
of the silicone. These material are also known as dimethicone copolyols.
Other nonvolatile dispersed silicone compounds include amino
substituted materials. Suitable alkylamino substituted silicone compounds
iri~lude those represenfied by the following structure (II)
~3 R
3o I I
HO - [Si- O ] X - [Si - O] Y - H ( II)
I I
~3 [ ~2 ) 3
_ I
_ (
«2 ) 2
I
~2
- CA 02274110 1999-06-04
WO 98124401 PCTIUS96I19397 _
19
wherein R is CHg or OH, x and y are integers which depend on the molecular
weight, the average molecular weight being approximately between 5,000
and 10,000. This polymer is also known as "amodimethicone".
Suitable amino substituted silicone fluids include those represented by
the formula (III)
(R1 )aG~a-Si-(-OSiG2)~~-0SiGb(R1 )a_b)m-O-SiGg-a(R1 )a (III)
in which G is chosen from the group consisting of hydrogen, phenyl, OH,
C1-Cg alkyl and preferably methyl; a denotes 0 or an integer from 1 to 3, and
preferably equals 0; b denotes 0 or 1 and preferably equals 1; the sum n+m
is a number from 1 to 2,000 and preferably from 50 to 150, n being able to
denote a number from 0 to 1,999 and preferably from 49 to 149 and m being
able to denote an integer from 1 to 2,000 and preferably from 1 to 10; R1 is
a monovalent radical of formula CqH2qL in which q is an integer from 2 to 8
and L is chosen from the groups
-N(R2)CH2-CH2-N(R2)2
-N(R2)2 _
-N(R2)3A
-N(R2)CH2-CH2-NR2H2A-
in which R2 is chosen from the group consisting of hydrogen, phenyl, benzyl,
a saturated hydrocarbon radical, preferably an alkyl radical containing from 1
_ 25 to 20 carbon atoms, and A- denotes a halide ion.
An especially preferred amino substituted silicone corresponding to
formula (III) is the polymer known as "trimethylsilylamodimethicone", of
formula (1~:
cx3 ox
I I
(~3) 3Si- O - [Si- O) n- [Si- OJ m- Si (CH3 ) 3 (IV)
I I
~3 ( CHZ ) 3
I
NH _
- I
( ~2 ) 2
I
~2
CA 02274110 1999-06-04
wo 9snm PCT/US96119397
In this formula n and m are selected depending on the exact molecular
weight of the compound desired.
Other amino subst~uted silicone polymers which can be used are
represented by the formula (~:
5
CA 02274110 2002-02-28
21
R4CH2-CHOH-CH2-N+(R3)3(~
R3
(CH3) 3Si- 0 - [Si- O] r- LSi- O] S- Si (CH3) 3 (V)
R3 R3
where R3 denotes a monovalent hydrocarbon radical having
from 1 to 18
carbon atoms, preferably an alkyl or alkenyl radical such
as methyl; R4
denotes a hydrocarbon radical, preferably a C1 - C1g alkylene
radical or a
C1 - Clg, and more preferably C1 - Cg, alkyleneoxy radical;
Q- is a halide
ion, preferably chloride; r denotes an average statistical
value from 2 to 20,
preferably from 2 to 8; s denotes an average statistical
value from 20 to 200,
1 S and preferably from 20 to 50. A preferred polymer of this
class is available
from Union Carbide under the name "UCAR SILICONE ALE 56."
'
References disclosing suitable nonvolatile dispersed silicone
compounds include U.S. Patent No. 2,826,551, to Geen; U.S.
Patent No.
3,964,500, to Drakoff, issued June 22, 1976; U.S. Patent
No. 4,364,837, to
Pader; and British Patent No. 849,433, to Woolston, - - '
and "Silicon Compounds" distributed by - - petrarch
Systems, Inc., 1984. This reference provides an extensive,
though not
. exclusive, listing of suitable silicone compounds.
Another nonvolatile dispersed silicone that can be especially
useful is
a silicone gum. The term "silicone gum", as used herein,
means a
polyorganosiloxane material having a viscosity at 25C of
greater than or
equal to 1,000,000 centistolces. It is recognized that the
silicone gums
described herein can also have some overlap with the above-disclosed
silicone compounds. This overlap is not intended as a limitation
on any of
these materials. Silicone gums are described by Petrarch,
and others
including U.S. Patent No. 4,152,416, to Spitzer et al., issued
May 1, 1979
and Noll; Waiter, Chemistry and Technology. of Silicones,
New York:
Academic Press 1968. Also describing silicone gums are General
Electric
Silicone Rubber Product Data Sheets SE 30, SE 33, SE 54 and
SE 76.
The "silicone gums" will typically knave a mass molecular weight in
excess of about 200,000, generally between about 200,000 and about
1,000,000. - Speafic examples include polydimethylsiloxane,
CA 02274110 1999-06-04
W4 98/?,4401 PCTI(J896/19317
22
poly(dimethylsiloxane rnethyivinylsiloxane) copolymer, poly(dimethylsiloxane
diphenylsiloxane methylvinylsiloxane) copolymer and mixtures thereof.
Also useful are silicone resins, which are highly crosslinked polymeric
siloxane systems. The crosslinking is introduced through the incorporation of
tri-functional and tetra-functional silanes with mono-functional or di-
functional,
or both, silanes during manufacture of the silicone resin. As is well _
understood in the art, the degree of crosslinking that is required in order to
result in a silicone resin will vary according to the specific silane units
incorporated into the silicone resin. In general, silicone materials which
have
a sufficient level of trifunctional and tetrafunctional siloxane monomer
units,
and hence, a sufficient level of crosslinkingt such that they dry down to a
rigid, or hard, film are considered to be silicone resins. The ratio of oxygen
atoms to silicon atoms is indicative of the level of crosslinking in a
particular
silicone material. Silicone materials which have at least about 1.1 oxygen
atoms per silicon atom will generally be silicone resins herein. Preferably,
the ratio of oxygenailicon atoms is at least about 1.2:1Ø Silanes used in
the
manufacture of silicone resins include monomethyl-, dimethyl-, trimethyl-,
monophenyl-, Biphenyl-, methylphenyl-, monovinyl-, and
methylvinylchlorosilanes, and tetrachlorosilane, with the methyl substituted
silanes being most commonly utilized. Preferred resins are offered by
General Electric as GE SS4230 and SS4267. Commercially available -
silicone resins will generally be supplied in a dissolved form in a low
viscosity
volatile or nonvolatile silicone fluid. The silicone resins for use herein
should _
be supplied and incorporated into the present compositions in such dissolved
form, as will be readily apparent to those skilled in the art. Without being
bound by theory, it is believed that the silicone resins can enhance
deposition
of other silicone compounds on the hair and can enhance the glossiness of
hair with higirrefractive index volumes.
Other useful silicone resins are silicone resin powders such as the
- material given the CTFA designation polymethylsilsequioxane, which is
commercially available as TospearlTM from Toshiba Silicones.
Background material on silicone compounds, including sections
discussing silicone fluids, gums, and resins, as well as the manufacture of
silicone compounds, can be found in Encyclopedia of Polymer Science and
Engineering, Volume 15, Second Edition, pp 204-308, John alley & Sons,
Inc.,-1989, which is incorporated herein by reference in its entirety.
CA 02274110 1999-06-04
WO 924401 PCT/U896119397 -
23
Silicone materials and silicone resins in particular, can conveniently
be
_
identified according to a shorthand nomenclature system well known
to those
skilled in the art as the "MDTQ" nomenclature. Under this system,
the
silicone is described according to the presence of various siloxane
monomer
units which make up the silicone. Briefly, the symbol M denotes
the mono-
functional unit (CH3)3SIE3}~; D denotes the difunctional unit (CH3)2Si0;
T
denotes the trifunctional unit (CH3)Si01,5; and Q denotes the quadri-
or
tetra-functional unit Si02. Primes of the unit symbols, e.g., M',
D', T, and Q'
denote substituents other than methyl, and must be specifically
defined for
each occurrence. Typical alternate substituents include groups such
as vinyl,
phenyl, amino, hydroxyl, etc. The molar ratios of the various units,
either in
terms of subscripts to the symbols.indicating the total number of
each type of
unit in the silicone, or an average thereof, or as specifically
indicated ratios in
combination with molecular weight, complete the description of the
silicone
material under the MDTQ system. Higher relative molar amounts of
T, Q, T
and/or Q' to D, D', M andlor or M' in a silicone resin is indicative
of higher
levels of crosslinking. As discussed before, however, the overall
Level of
crosslinking can also be indicated by the oxygen to silicon ratio.
The silicone resins for use herein which are preferred are MQ, MT,
MTQ, MQ and MDTQ resins. Thus, the preferred silicone substituent
is
methyl. Especially preferred are MQ resins wherein the M:Q ratio
is from
about 0.5:1.0 to about 1.5:'1.0 and the average molecular weight
of the resin
is from about 1000 to about 10,000.
ADDITIONAL SURFACTAN S
Hair conditioning compositions of the present invention may further
comprise additional surfactant. Such addi~onal surfactants comprise
amphoteric surfactants, zwitterionic surfactants, nonionic surtactants,
anionic
surfactants, and mixtures thereof which do not affect the oondirioning
composition of the present invention.
The additional surfactant is particularly useful for oomposihons
in
form of spray or mousse, wherein the additional surfactant is used
to
suspend the conditioning agents and other components which are insoluble
_
in the carrier. Additional surfactants are typically inducted at
a level by weight
of from about 0.1 % to about 15%, preferably from about 0.3!o to
about 10%
of the composition. The level and species are selected adding to
the
compatibility with other components, and desired characteristic
of the
pr~uct. _ __
CA 02274110 1999-06-04
wo 9sr~oi . PCT/US9f119397
24
Amnhoteric and Zwitterionic Surfactants.
The hair conditioning compositions of the present invention can
comprise amphoteric andlor zwitterionic surfactants.
Amphoteric surfactants for use herein include the derivatives of
aliphatic secondary and tertiary amines in which the aliphatic radical is
straight or branched and one of the aliphatic substituents contains from about
8 to about 18 carbon atoms and one contains an anionic water soiubilizing
group; e.g., carboxy, sulfonate, sulfate, phosphate, or phosphonate.
Zwitterionic surfactants for use herein include the derivatives of
aliphatic quaternary ammonium, phosphonium, and sulfonium compounds, in _
which the aliphatic radicals are straight or branched, and wherein one of the
aliphatic substituents contains from about 8 to about 18 carbon atoms and
one contains an anionic group, e.g., carboxy, sutfonate, sulfate, phosphate,
or phosphonate. A general formula for these compounds is:
(R3)x
R2_1,+_CH2_R4_Z-
where R2 contains an alkyl, elkenyl, or hydroxy alkyl radical of from about 8
to about 18 carbon atoms, from 0 to about 10 ethylene oxide moieties and
from 0 to about 1 glyceryl moiety; Y is selected from the group consisting of
nitrogen, phosphorus, and sulfur atoms; R3 is an alkyl or monohydroxyalkyl
group containing 1 to about 3 carbon atoms; X is 1 when Y is a sulfur atom,
and 2 when Y is a nitrogen or phosphorus atom; R4 is an alkylene or
hydroxyalkylerje of from 1 to about 4 carbon atoms and Z is a radical
selected from the group consisting of carboxylate, sulfonate, sulfate,
phosphonate, and phosphate groups.
F~carnples of amphoteric and zwitterionic surfactants also include -
sultaines and amidosultaines. Sultaines, including amidosultaines, include
for example, cocodimethylpropylsultaine, stea~yklimethylpropylsultaine,
lauryl-bis-(2-hydroxyethyi)propyisultaine and the like; and the amidosultaines
__ such as cocamidodimethylpropylsultaine,
stearylamidododimethylpropylsultaine, laurylamido-bis-(2
hydroxyethyl)propytsultaine, and the like. Preferred are
amidohydroxysultaines such as the - C12 - C18
hydrocarbylamidopropyihydroxysultaines, especially C12 - C14
hydrocarbytamidopropylhydroxysultaines, e,g.,
laurylamidopropylhydroxysultaine and cocamidopropylhydroxysultaine. Other
CA 02274110 2002-02-28
sultaines are described in U.S. Patent 3,950,417.
Other suitable amphoteric surfactants are the aminoalkanoates of the
formula RNH(CH2)nCOOM, the iminodialkanoates of the formula
5 RN[(CH2)mCOOM]2 and mixtures thereof; wherein n and m are numbers
from 1 to about 4, R is Cg - C22 alkyl or alkenyl, and M is hydrogen, alkali
metal, alkaline earth metal, ammonium or alkanolammonium.
Examples of suitable aminoalkanoates include n-alkylamino
propionates and n-alkyliminodipropionates, specific examples of which
10 include N-lauryi-beta-amino propionic acid or salts thereof, and N-lauryl-
beta
imino-dipropionic acid or salts thereof, and mixtures thereof.
Other suitable amphoteric surfactants include those represented by
the formula
15 R3
I
R~CON - (CH2)~ - N+ - CH2Z
- R2
wherein R1 is Cg - C~ alkyl or alkenyl, preferably C12 - Cog, R2 and R3 is
independently selected from the group consisting of hydrogen, -CH2C02M, -
CH2CH20H, -CH2CH20CH2CH2COOM, or -(CH2CH20)mH wherein m is
an integer from 1 to about 25, and R4 is hydrogen, -CH2CHZOH, or
CH2CH20CH2CH2COOM, Z is C02M or CH2C02M, n is 2 or 3, preferably
2, M is hydrogen or a ration, such as alkali metal (e.g., lithium, sodium,
potassium), alkaline earth metal (beryllium, magra3sium, calcium, strontium,
barium), or ammonium. This type of surfactant is sometimes classfied as an
imidazoiine-type amphoteric surfactant, although it should be recbgnized that
it does not necessarily have to be derimred, directly or indirectly, through
an
imidazoline intermediate. Suitable rnateriais of this type are marketed under
the tradename MIRANOL and are understood to comprise a complex mixture
of species, and can exist in protonated and non-protonated species
depending upon pki with respect to species that can have a hydrogen at R2.
All such variations and speaes are meant to be encompassed by the above
fomnula.
Examples of surfactants of the above formula are monocarboxylates
and di-carboxytates. Examples of these materials include
cocoamphocarboxypropionate, cocoamphocarboxypropionic acid,
CA 02274110 1999-06-04
wo rcTnls9sri9~~
Zs
cocoamphocarboxyglycinate (alternately referred to as cocoamphodiacetate),
and cocoamphoacetate.
Commercial amphoteric surfactants include those sold under the trade
names MIRANOL C2M CONC. N.P., MIRANOL C2M CONC. 0.P., MIRANOL
C2M SF, MIRANOL CM SPECIAL (Miranol, Inc.); ALKATERIC 2CIB (Alkaril
Chemicals); AMPHOTERGE W 2 (Lonza, Inc.); MONATERIC CDX-38,
MONATERIC CSH-32 (Mona Industries); REWOTERIC AM-2C (Rewo
Chemical Group); and SCHERCOTERIC MS-2 (Scher Chemicals).
Betaine surfactants, I.e. zwitterionic surfactants, suitable for use in the
I O conditioning compositions are those represented by the formula:
O R4 R2
RS- IC - N - (CH2) m1 n N+- Y- R1
R3
wherein: R~ is a member selected from the group consisting of
COOM and CH(OH)CH2S03M
R2 is lower alkyl or hydroxyalkyl; R3 is lower alkyl or hydroxyalkyl; R4 is a
member selected from the group consisting of hydrogen and lower alkyl; R~
is higher alkyl or alkenyl; Y is lower alkyl, preferably methyl; m is an
integer
from 2 to 7, preferably from 2 to 3; n is the integer 1 or 0; M is hydrogen or
a
ration, as previously described, such as an alkali metal, alkaline earth
metal,
or ammonium. The term "lower alkyl" or "hydroxyalkyl" means straight or
branch chained, saturated, aliphatic hydrocarbon radicals and substituted
hydrocarbon radicals having from one to about three carbon atoms such as,
for example, rra~thyl, ethyl, propyl, isopropyl, hydroxypropyl, hydroxyethyl,
and the like. The term "higher alkyl or alkenyl" means straight or branch
chained saturated (I.e., "higher alkyf~ and unsaturated (I.e., "higher
atkenyl")
aliphatic hydrocarbon radicals having from about eight to about 20 carbon
atoms such as, for example, lauryl, cetyl, stearyl, oleyl, and the like. tt
should
be understood that the term "higher alkyl or alkenyl" includes mixtures of
radicals which may contain one or more intem>ediate linkages such as ether
or poiyether linkages or non-functional substitutents such as hydroxyl or
halogen radicals wherein the radical remains of hydrophobic character.
Examples of surfactant betaines of the above formula wherein n is
zero which are useful herein include the alkylbetaihes such as
cocodimethylcarboxymethylbetaine, lauryldimethylcarboxymethylbetaine,
CA 02274110 1999-06-04
wo PGT/US96119397
27
lauryldimethyl-alpha-carboxyethyfbetaine,
cetykiimethylCarboxymethylbetaine, lauryl-bis-(2-hydroxyethyl)-
carboxymethylbetaine, stearyl-bis-(2-hydroxypropyl)carboxymethylbetaine,
ofeykiimethyi-gamma-carboxypropylbetaine, lauryl-bis-(2-hydroxypropyl)-
alpha-carboxyethylbe#aine, etc. The sulfobetaines may be represented by
cocodimethylsulfopropylbetaine, stearyldirr~ethylsulfopropylbetaine, lauryi-
bis-
(2-hydroxyethyl)-sulfopropylbetaine, and the like.
Speck examples of amido betaines and amidosulfobetaines useful in
the conditioning compositions include the amidocarboxybetaines, such as
cocamidodimethylcarboxymethylbetaine,
laurylamidodimethylcarboxymethylbetaine,
cetylamidodimethylcarboxymethylbetaine, iaurylamido-bis-(2-hydroxyethyi)-
carboxymethylbetaine, cocamido-bis-(2-hydroxyethyl)-carboxymethylbetaine,
etc. The amidosulfobetaines may be represented by
cocamidodimethylsulfopropylbetaine,
stearylamidodimethylsulfopropylbetaine, laurylamido-bis-(2-hydroxyethyl)-
su~opropylbetaine, and the like.
Nonionic Surfactan"~
The hair conditioning compositions of the present inven~on can
20. comprise a nonionic surfactant. Nonionic surfactants include those
compounds produced by condensation of alkylene oxide groups, hydrophilic
in nature, with an organic hydrophobic compound, which may be aliphatic or
alkyl aromatic in nature.
Preferred nonlimiting examples of nonionic surfactants for use herein
include the following:
(1) polyethylene oxide condenses of alkyl pherwls, e.g., the
condensation products of alkyl phenols having an alkyl group containing from
about 8 to about 20 carbon atoms in either a straight chain or branched chain
configuration, with ethylene oxide, the said ethylene oxide beir~ present in
amounts equal to from about 10 to about 60 moles of ethylene oxide per
_ mole of alkyl phenol;
(2) those derived from- he cxrndensa#ion of ethylene oxide with the
' product resulting from the reaction of propylene oxide and ethylene diamine
products;
(3) condensation products of aliphatic a~ohols having from about 8 to
about 18 carbon atoms, in either straight chain or branched chain
configura~ons, with ethylene oxide, e.g., a coconut alcohol ethylene oxide
CA 02274110 2002-02-28
28
condense having from about 10 to about 30 moles of ethylene oxide per mole
of coconut alcohol, the coconut alcohol fraction having from about 10 to
about 14 carbon atoms;
(4) long chain tertiary amine oxides of the formula [R1R2R3N-~ O]
where R1 contains an alkyl, alkenyl or monohydroxy alkyl radical of from
about 8 to about 18 carbon atoms, from 0 to about 10 ethylene oxide
moieties, and from 0 to about 1 glyceryl moiety, and R2 and R3 contain from
about 1 to about 3 carbon atoms and from 0 to about 1 hydroxy group, e.g.,
methyl, ethyl, propyl, hydroxyethyl, or hydroxypropyl radicals;
(5) long chain tertiary phosphine oxides of the formula (RR'R"P-~ O]
where R contains an aHcyl, alkenyl or monohydroxyalkyl radical ranging from
about 8 to about 18 carbon atoms in chain length, from 0 to about 10
ethylene oxide moieties and from 0 to 1 glyceryl moieties and R' and R" are
each alkyl or monohydroxyalkyl groups containing from about 1 to about 3
carbon atoms;
(6) long chain dialkyl sulfoxides containing one short chain alkyl or
hydroxy alkyl radical of from 1 to about 3 carbon atoms (usually methyl) and
one long hydrophobic chain which include alkyl, alkenyl, hydroxy alkyl, or
keto alkyl radicals containing from about 8 to about 20 carbon atoms, from 0
to about 10 ethylene oxide moieties and from 0 to 1 gtyceryl moieties;
(7) alkyl polysaccharide (APS) surfactants (e.g. alkyl polyglycosides),
examples of which ane described in U.S. Patent 4,565,647,
and which discloses APS
surfactants having a hydrophobic group with about 6 to about 30 carbon
atoms and a polysaccharide (e.g., polyglycoside) as the hydrophilic group;
optionally, there can be a polyalkylene-oxide group joining the hydrophobic
and hydrophilic moieties; and the alkyl group (i.e., the hydrophobic moiety)
can be saturated or unsaturated, branched or unbranc~d, and unsubstihrted
or substituted (e.g., with hydroxy or cyclic rings); a preferred material is
alkyl
polyglucoside which is commercially available from Henkel, ICI Americas,
and Seppic; and
(8) poiyoxyethyiene alkyl ethers such as those of the formula
RO(CH2CH2)nH and polyethylene glycol (PEG) giyceryl fatty esters, such as
those of the formula R(O)OCH2CH(OH)CH2(OCH2CH2)nOH, wherein n is
from 1 to about 200, preferably from about 20 to about 100, and R is an alkyl
having from about 8 to about 22 carbon atoms.
OTHER O~'TIONAL COMPONENTS
CA 02274110 1999-06-04
WO 9$I?A401 PCTIUS96I19397
28
A wide variety of other components can optionally be formulated into
the present composition. These include: other conditioning agents such as
hydrolysed collagen, hydrolysed keratin, proteins, plant extracts, and
nutrients; hair-hold polymers; other surfactants such as anionic surfactants;
additional thickening agents and suspending agents such as xanthan gum,
= guar gum, hydroxyethylcellulose, methylGellulose, starch and starch
derivatives; viscosity modifiers such as methanolamides of long chain fatty
acids such as cocomonoethanolamide; pearlescent aids such as ethylene
. glycol distearate; preservatives ~ such as benzyl alcohol, methyl paraben,
propyl paraben and imidazolidinyl urea; solvents such as polyvinyl alcohol,
ethyl alcohol and volatile and non-volatile silicone fluids of kwv molecular
weight; pH adjusting agents, such as citric acid, sodium citrate, succinic
acid,
phosphoric acid, sodium hydroxide, sodium carbonate; salts, in general, such
as potassium acetate and sodium chloride; coloring,agents, such as any of
the FD&C or DEC dyes; hair oxidizing (bleaching) agents, such as hydrogen
peroxide, perborate and persulfate salts; hair reducing agents such as the
thioglycolates; perfumes; sequestering agents, such as dis~ium
ethylenediamine tetra-acetate; and polymer plasticizing agents, such as
glycerin, disobutyl adipate, butyl stearate, and propylene glycol; and
ultraviolet and infrared screening and absorbing agents such as octyl
salicylate, and propellants such as fluorohydrocarbons, dimethyl ether,
carbon dioxide, nitrogen, and LPG gas. Such optional ingredients generally
are used individually at levels from about 0.019~o to about 10.0%, preferably
from about 0.05% to about 5.0% by weight of the composfion.
METHOD OF USE
The hair conditioning compositions of the present invention are used in
conventional ways to provide the conditioning benefits such as moistness,
softness, free flowing, decreased stickiness, and static control. Such method
of use depends on the type of composition employed but generally involves
application of an efFecfrve amount of the product to the hair, which may then
be rinsed from the hair (as in the case of hair rinses) or allowed to remain
on
' the hair (as in the case of gets, lotions, sprays, mousses, and creams).
"Effective amount'-' means an amount sufficient enough to provide a
conditioning benefit. In general, from about 1g to about 50g of the hair
conditioning composition is applied to the hair and/or the scalp. If the
compositions are not in uniform condition, for example, separated into two
CA 02274110 1999-06-04
WO 98I~4401 PCT/U5961i9397
phases, the compositions need to be mixed before use. The composition is
distributed throughout the hair, typically by rubbing or massaging the hair
and
scalp. Preferably, the composition is applied to wet or damp hair prior to
drying of hair. After such compositions are applied to the hair, the hair is
5 dried and styled in acxx~rdance with the preference of the user. In the
alternative, the composition is applied to dry hair, and the hair is then
combed
or styled in accordance with the preference of the user.
EXAMPLES
10 The following examples further describe and demonstrate
embodiments within the scope of the present invention. The examples are
given solely for the purpose of iliustrai>on and are not to be construed as
limitations of the present invention, as many variations thereof are possible
without departing from the spirit and s~pe of the invention. Ingredients are
15 identified by chemical or CTFA name, or otherwise defined below.
The hair conditioning ingredients herein are expressed by weight
percentage of the total compositions, unless otherwise specified.
The components shown bek~nr can be prepared by any conventional
method well known in the art. A suitable method and formulation are as
20 follows:
CA 02274110 1999-06-04
wo ~sn~oi rc~rrtrs~sng3~ -
31
I- I
For Example I through VI, the combination of
stearyltrimethylammonium chloride and L-glutamic acid, or MATMAC are
. added into water under agitation at above 70°C. Then, stearyl alcohol
and
cetyl alcohol are added with agitation. After cooled down below 60°C,
other
ingredients except for denatured ethyl alcohol and perfume are added to the
above and agitated. After the obtained mixture is cooled down under
50°C,
denatured ethyl alcohol and perfumes are added. The obtained composition
provides smoothness and soft feel to the hair.
Example No. I II III IV V V_I
Pentaerythrito) tetraisostearate 0.50 - 0.25 - 0.25
Trimethylolpropane triisostearate - 0.5 - 0.25 - 0.5
Methyl myristate 0.5 - 0.4 - - -
1 S Oleyl alcohol - - - - 0.4 -
Mineral oil _ _ _ - 0.3 -
Octyl dodecyl isostearate - 0.3 - 0.25 - 0.3
Stearyl ahrhol 4.0 2.6 2.0 2.8 4.0 -
Cetyl alcohol 6.0 4.2 3.0 4.2 3.0 7.0
Sold paraffin - 1.0 -- 1.0 - -
Stearamidopropykiimethylamine -2.0 2.0 3.0 2.0 - 2.0
L-Glutamic aad 0.64 0.64 1.0 0.64 - 0.64
MATMAC*~ _ _ - - 0.25 -
Silicone mixture*2 5.0 1.0 - 1.0 2.0 4.0
Preservative 0.2 0.3 0.2 0.25 0.1 0.5
Perfume 0.2 0.25 0:1 0.2 0.4 0.2
- Denatured ethyl alcohol - - - - 10.0 - _
r up to 100%
'1 MATMAC; monoalkyl trimethyl ammonium chloride having C20 - C22
alkyls .
*2 Silicone mixture; 85%/15% cwt. basis) mixture of D5 cyciomethicone and
dirricone gum (weight average molecular weight of about 400,000 to
about 600,000).
CA 02274110 1999-06-04
WO 98124481 PCTIUS96/l9397
32
For Examples VII through X, All ingredients are added into a
denatured ethyl alcohol under agitation. The obtained composition is packed
into packages equipped with a spraying device to make a conditioning spray.
The obtained composition provides smoothness and soft feel to the hair.
Example No. VII VIII IX X
Pentaerythritol isostearate 1.50 - - 0.5
Trimethylolpropane triisostearate- 1.0 1.5 -
Methyl myristate 1.50 1.0 -
Oleyl alcohol - - - 0.5
Mineral oil . - - 1.5 -
Cetyl alcohol - 1:0 -
MATMAC* 1 - 0.2 - 0.2
Preservative 0.1 0.1 0.1 0.1
Perfume 0.1 0.1 0.1 0.1
Denatured ethyl alcohol up
to
100%
*1 MATAAAC; monoatkyl trimethyl ammonium chloride having C20 - C22
alkyls