Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02275049 1999-06-17
Title
DRUG DELIVERY COMPOSITION FOR DENTAL APPLICATIONS
FIELD OF THE INVENTION
The present invention pertains to a drug delivery composition, suitable for
sustained release of one or more biologically active agents.
BACKGROUND OF THE INVENTION
A. Endodontic Disease and Treatment
Endodontic disease is a process in which the dental pulp tissue and the
tissues supporting the tooth become affected by infection from oral
microorganisms
invading the tooth. Endodontic disease is a frequent occurrence that affects
approximately
70% of the populations in countries with well developed dental care modes, and
by
estimate, over 95% of the populations in other parts of the world. Endodontic
disease is
one of the major causes of tooth loss in these populations.
Endodontic disease is an all-inclusive term for a variety of clinical
conditions, that are forms of either pulpitis or periradicular periodontitis.
Pulpitis is an
inflammation of the dental pulp that can be caused by mechanical, physical and
microbial
stimuli. If the stimulus is not eliminated in the early stages of pulpitis,
the disease process
escalates beyond potential repair, and leads to pulpal necrosis. Frequently at
this stage,
oral microorganisms infect the necrotic pulp and the pulp space inside the
tooth,
consisting of the pulp chamber and root canals. If allowed to persist, this
root canal
infection causes the onset of periradicular periodontitis, an infective
disease of the tissues
that support the tooth, the periodontal ligament and alveolar bone. Symptoms
that may be
encountered in the various stages of the disease process frequently lead to
the loss of the
affected tooth. Untreated or intractable root canal infection ultimately leads
to tooth loss.
CA 02275049 1999-06-17
Periradicular periodontitis can be treated by debridement of the infected
pulp tissue and disinfection of the root canal. Disinfection of the root canal
is achieved by
a combination of three modalities: (i) instruments (files and reamers) are
used to debride
the pulpal tissue and enlarge the root canal; (ii) antimicrobial irrigating
solutions, mainly
sodium hypochlorite, are used to flush debris, digest tissue remnants, and
kill
microorganisms; and (iii) antimicrobial medicaments are applied to the root
canal
between consecutive treatments to kill surviving microorganisms. Such
treatment is
effective and it results in complete healing of the diseased sites in 46% to
93% of the
cases (Friedman S, in: Essential Endodontology (Q~rstavik D & Pitt Ford TR,
eds.)
Blackwell Science, Oxford (1998)). Absence of healing, or treatment failure,
is mainly
attributed to residual root canal infection (Sjogren U, et al., Int. Endodon.
J. 30: 297-306
(1997)).
Root canal microorganisms can persist in spite of the aforementioned
procedures, mainly when harbored in root canal irregularities and dentinal
tubules, where
they may be inaccessible to the instruments, irrigants and medicaments. Thus
the ability
of the instruments to disinfect the root canal is minimal (Bystrom A &
Sundqvist G,
Scand. J. Dent. Res. 89:321-328 (1981); Dalton BC, et al., J. Endodon. 24:763-
768
(1998)), and even the irrigants are insufficient to do so predictably (Sjogren
U, et al., Int.
Endodon. J. 30: 297-306 (1997)). Most of the available root canal medicaments,
such as
camphorated paramonochlorphenol or iodine potassium iodide are strongly
antimicrobial;
however, they are effective for only hours, and after they expire,
microorganisms can
repopulate the root canal (Bystrom A, et al., Endod. Dent. Traumatol. 1:170-
175 (1985)).
Another commonly used root canal medicament, calcium hydroxide, can be
effective for
weeks (Bystrom A, et al., Endod. Dent. Traumatol. 1:170-175 (1985)); however,
it is not
very effective against specific enteric bacteria (f~rstavik D & Haapasalo M,
Endod. Dent.
Traumatol. 6:142-149 (1990)), that are frequently associated with failures of
root canal
treatment (Sundqvist G, et al., Oral Surg. 85:86-93 (1998)). The ability of
calcium
hydroxide to kill microorganisms harbored in the dentinal tubules also is
questionable
(Heling I, et al., Int. Endod. .I. 25:15-19 (1992)).
2
CA 02275049 1999-06-17
The disadvantage exists, therefore, that the current root canal treatment
modalities, including instruments, irrigants and medicaments, are insufficient
to
predictably disinfect the root canal.
As the final step in root canal treatment, the canal is filled and the tooth
crown rerstored, to prevent future ingress of microorganisms and recurrent
infection
subsequent to treatment. The goal of resisting microbial ingress is
particularly
challenging, considering that the root canal treated teeth are expected to
function for the
patientis lifetime, while being constantly at risk of microbial ingress
through leakage in
the margins of the restorations, and invasion of dentinal tubules from
infected sites in the
gums and periodontal defects. Currently used root filling materials do not
effectively
resist microbial ingress (Friedman S, et al., .l. Endodon. 23:557-561 (1997);
Friedman S,
et al. J. Dent. Res. abstract 78:206 (1999)), and therefore, they do not
provide sufficient
protection against recurrent root canal infection. Consequently, infection of
the filled root
canal, and resulting treatment failure, can occur even if a previous root
canal infection
was effectively eliminated, and also in teeth that were not infected prior to
root filling.
Such recurrent infection, or treatment failure, leads to tooth loss in about
23% of the
cases (Petersson K, et al., Endod. Dent. Traumatol. 5:153-158 (1989)).
The further disadvantage exists, therefore, that the currently used root
filling materials do not prevent treatment failure from microbial ingress and
propagation
in the filled root canal.
In response to the shortcomings of the aforementioned conventional
modalities for root canal disinfection, researchers have explored
possibilities to employ
chlorhexidine and its salts as a root canal medicament. Chlorhexidine
effectively kills
oral microorganisms when applied to treat gum disease (Oosterwaal PJM, et al.,
J. Clin.
Periodontol. 18:97-100 (1991); Schaeken MJM, et al., J. Dent. Res. 70:150-153
(1991)),
and it is effective against microorganisms that cause periradicular
periodntitis, including
enteric bacteria (Henessey T.D., J. Periodont. Res. 8 suppl. 12:61-67 (1973);
Emilson
C.G., Scand. J. Dent. Res. 85:255-265 (1977)) that can resist the action of
other
3
CA 02275049 1999-06-17
medicaments (Q~rstavik D & Haapasalo M, Endod. Dent. Traumatol. 6:142-149
(1990)).
The antimicrobial efficacy of chlorhexidine equals that of the conventional
root canal
irrigants and medicaments (Delany G.M. et al., Oral Surg. 53:518-523 (1982);
Ohara
P.K. et al., Endod. Dent. Traumatol. 9:95-100 (1993); Vahdaty A. et al.,
Endod. Dent.
Traumatol. 9:243-248 (1993); Jeansonne M.J. & White R.R. ,J. Endodon. 20:276-
278
(1994); Siqueira J.F. & de Uzeda M. J. Endodon. 23:167-169 (1997); Barbosa
C.A.M. et
al., J. Endodon. 23:297-300 (1997); Heling I. & Chandler M.P. Int Endod J 31:8-
14
(1998); Kuruvilla J.R. & Kamath M.P. J. Endodon. 24:472-476 (1998); Ayhan H.
et al.,
Int. Endod. J. 32:99-102 (1999); D'Arcangelo C. et al., J. Endodon. 25:351-353
(1999)).
Unlike the other medicaments, however, chlorhexidine binds to the oral tissues
including
dentin (Emilson C.G. et al., J. Periodont. Res. 8 suppl. 12:17-21 (1973)). The
bound
chlorhexidine may kill microorganisms that approach the dentin surface, and it
may alter
the physical and chemical properties of the dentin surface to prevent
microbial
colonization (Emilson C.G. et al., J. Periodont. Res. 8 suppl. 12:17-21
(1973)). and
affects residual antimicrobial activity of the dentin surface (Parsons GJ, et
al., Oral Surg.
49:455-459 (1980)). Root canal dentin demonstrates a short-term residual
antimicrobial
activity after exposure to chlorhexidine for several minutes (White RR, et
al., J. Endodon.
23:229-231 ( 1997); Jung S, et al., J. Endodon. 25:288 abstract ( 1999));
however, to
predictably resist microbial ingress and prevent recurrent infection in the
long term, a
substantive antimicrobial activity must be induced. To predictably affect
substantive
antimicrobial activity, the canal has to be exposed to chlorhexidine for a
longer time than
afforded by irrigation alone (Heling I. et al., Int. Endod. ,I. 25:20-24
(1992)), preferably
for one week (Komorowski R & Friedman S, J. Endodon. 23:263 abstract (1997)).
The
substantive antimicrobial activity cannot be induced by any other
conventionally used
root canal medicament, nor can it be induced by a short term irrigation of the
root canal
with chlorhexidine.
The further disadvantages exist, therefore, that the commonly used root
canal medicaments, and even chlorhexidine used as an irrigant, cannot induce
substantive
antimicrobial activity in the root canal.
4
CA 02275049 1999-06-17
Hence, a major therapeutic goal is the development of a vehicle to deliver
chlorhexidine to root canals for extended periods of time, to (i) kill
residual root canal
microorganisms; and (ii) induce substantive antimicrobial activity that can
prevent
microbial ingress and recurrent infection subsequent to treatment, and
consequent
treatment failure.
B. Use of Sustained-release Pharmaceutical Compositions in the Treatment of
Endodontic and Other Diseases
To allow long-term delivery of medicaments to specific sites for medical
and dental treatment applications, researchers have developed sustained-
release
pharmaceutical compositions that can be inserted into the target site and
slowly release
the medicament contained within them. The most investigated devices for
sustained
release comprise incorporation of a medicament into a polymeric matrix, which
is then
shaped into a convenient form for insertion in the target site. Specifically,
Friedman M
(U.S. Pat. No. 5,002,769) discloses a sustained-release device containing
chlorhexidine
for use in treatment of periodontal disease. This sustained-release device
incorporates a
biodegradable polymer to be used as a periodontal implant. This device is more
effective
than calcium hydroxide in disinfection of root canal dentin in bovine teeth
(Heling I, et
al., Int. Endod. J. 25:15-19 (1992)). Bovine root canals medicated with this
device show
resistance to microbial colonization in vitro, in contrast to canals medicated
with calcium
hydroxide (Heling I, et al., Int. Endod. J. 25:20-24 (1992)). Such substantive
antimicrobial activity may inhibit the recurrence of infection subsequent to
treatment, and
lower the rate of treatment failures.
The biodegredable sustained-release device is not well suited for a root
canal application inside the tooth. The minimal amount of fluids present in
the root canal,
if any, is insufficient to degrade the polymer carrier and release the
chlorhexidine.
Furthermore, the device may not be completely degraded when it is time to
permanently
fill the root canal, and the clinician cannot verify whether fragments of the
polymer
device still remain in the root canal or not.
CA 02275049 1999-06-17
Loesche WJ (U.S. Pat. No. 4,568,535) discloses an ethylcellulose based,
non-biodegradable periodontal implant; however, this non-biodegradable
sustained-
release composition contains metronidazole for periodontal use, and it is not
suitable for
endodontic use.
Due to the strong, positive charge of chlorhexidine and the consequent
binding capability, many polymers used for sustained drug delivery become
unsuitable
for chlorhexidine. For example, cellulose acetate phthalate, used for
sustained release of
metronidazole, strongly binds with chlorhexidine resulting in undetectable
release of
chlorhexidine (Wu XY & Lee PI, unpublished data). Chlorhexidine is also
incompatible
with many pharmaceutical excipients that are used to plasticize the polymers,
such as
dibutyl phthalate, diamyl phthalate, diisobutyl carbonyl phthalate, butyl
diglycol
carbonate, and tricresyl phosphate. These characteristics of chlorhexidine
make the
formulation of the delivery composition difficult.
Moreover, preparing a device with a shape and size suitable for insertion
into root canals, while maintaining considerable mechanical strength, is
another
challenge.
The disadvantage exists, therefore, that the aforementioned sustained-
release compositions are unsuitable for delivery of chlorhexidine for
endodontic use, to
disinfect infected root canals and render them resistant to recurrent
infection in the long
term.
Hence, a major therapeutic goal is the development of a sustained-release
composition that (i) releases the drug by a mechanism rather than by
biodegradation; (ii)
is sized in conformity with the small dimensions of root canals; (iii) is
capable of
delivering chlorhexidine for weeks to disinfect infected root canals and
render them
resistant to recurrent infection; and (iv) can be retrieved at the end of the
medication
period without residual debris remaining in the root canal.
6
CA 02275049 1999-06-17
SUMMARY OF THE INVENTION
The present invention relates to pharmaceutical compositions suitable for
dental therapy, preferably for placement in the root canals and pulp chambers
of teeth,
and capable of treating root canal infection and preventing recurrent
infection subsequent
to completion of treatment.
Broadly stated, the present invention involves a drug delivery composition
comprising a cylindrical device containing at least one biologically active
agent wherein
the device has a polymer matrix core which can be coated or not coated by
another
polymer or by the same polymer. The polymer matrix core may contain other
pharmaceutical excipients, such as fillers like starches and binders like
polyvinyl
pyrrolidone. The device may be manufactured with one end thicker or wider than
the
other end to conform with the shape of the dental root canal and pulp chamber.
In detail, the invention provides a sustained-release composition that
permits the sustained release of biologically active agents such as
antimicrobial agents or
anti-inflammatory agents in a root canal and pulp chamber of a tooth, and that
comprises
an essentially needle-like device specifically adapted for implementation in a
root canal
of a patientis tooth. The device contains an effective amount of at least one
biologically
active agent such as antimicrobial agents like chlorhexidine, triclosan,
clindamycin,
tetracyclines, doxycycline, metronidazole, and penicillins, or anti-
inflammatory agents
such as corticosteroids, flurbiprofen, piroxicam, indomethacin, and other non-
steroidal
anti-inflammatory drugs, a polymer matrix and a polymer film coating being
optional.
The invention also provides a method of preparing the device that contains
at least one biologically active agent, the method comprising:
(1) obtaining a mixture of a suitable polymer and other pharmaceutical
excipients;
(2) mixing the polymer mixture with at least one biologically active agent;
(3) molding the mixture to form a cylindrical or needle-like shape; and
7
CA 02275049 1999-06-17
(4) coating the core matrix with a polymer film; with step (4) being optional.
The invention further provides a method of administering at least one
biologically active agent to a root canal and pulp chamber of a patientis
tooth in need of
such an agent, which comprises administering to the patient an essentially
needle-like
device specifically adapted for implementation in a dental root canal, wherein
the device
contains an effective amount of at least one biologically active agent such as
antimicrobial agents like chlorhexidine, triclosan, clindamycin,
tetracyclines,
doxycycline, metronidazole, and penicillins, or anti-inflammatory agents such
as
corticosteroids, flurbiprofen, piroxicam, indomethacin, and other non-
steroidal anti-
inflammatory drugs.
Brief Description of the Drawings
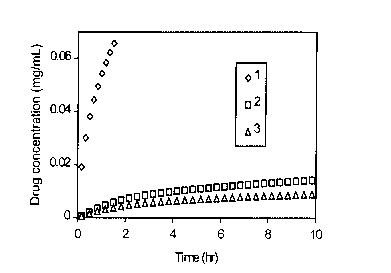
Figure 1 shows the short-term concentrations of chlorhexidine released
from the delivery composition using three different formulations.
Figure 2 shows the cumulative amount of chlorhexidine released from the
delivery composition of formulation 2, that was removed from the solution and
placed in
a fresh buffer solution after 17 hours.
Figure 3 shows the long-term release rates of chlorhexidine for delivery
compositions using four different formulations.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The sustained-release drug delivery composition of the invention is a
water-permeable polymeric solid that is cast in a cylindrical or needle-like
form. Such a
sustained-release composition is formed from solid polymers or solidification
of gel-like
polymers.
The sustained-release drug delivery composition of the invention is
formed to contain biologically active agents, including antimicrobial agents
like
8
CA 02275049 1999-06-17
chlorhexidine, triclosan, clindamycin, tetracyclines, doxycycline,
metronidazole, and
penicillins, or anti-inflammatory agents such as corticosteroids,
flurbiprofen, piroxicam,
indomethacin, and other non-steroidal anti-inflammatory drugs, preferably
chlorhexidine
salts or tetracyclines, most preferably chlorhexidine salts. Such sustained-
release drug
delivery composition is cylindrical or needle-like, preferably needle-like and
specially
adapted to allow its insertion into the root canals and pulp chamber of the
teeth of
patients.
The nature of the preferred components of the sustained-release drug
delivery composition of the invention is described in greater detail below.
I. The Components of the Drug Delivery Composition of the Invention
As herein aforementioned, the present invention pertains to a sustained-
release drug delivery composition comprising a suitable device containing at
least one
biologically active agent. The contained agents include antimicrobial agents
like
chlorhexidine, triclosan, clindamycin, tetracyclines, doxycycline,
metronidazole, and
penicillins, or anti-inflammatory agents such as corticosteroids,
flurbiprofen, piroxicam,
indomethacin, and other non-steroidal anti-inflammatory drugs. Any one of
these agents
may be incorporated into the drug delivery composition alone or in combination
with one
or more of the other agents.
A. The Polymeric Matrix Core of the Composition
The core in the drug delivery composition of the invention comprises a
polymer matrix. In order to provide a non-degradable polymeric matrix for
sustained-
release of biologically active agents, it is preferable to employ a polymeric
matrix
composed of water-permeable polymer. Such polymer matrices do not undergo
significant degradation when the device is inserted into the root canal of a
tooth or any
other target site and thus are retrievable at the end of the medication period
or whenever
desired. Such polymer matrices are preferably comprised of cellulose
derivatives such as
ethylcellulose, methylcellulose, methylpropyl cellulose; polyurethanes;
silicone rubbers;
9
CA 02275049 1999-06-17
polyesters; polyamides; gutta percha; and polysaccharides, most preferably
ethylcellulose. Such matrix core also may be comprised of pharmaceutical
excipients
such as fillers, binders, pigments, flavours, preservatives, and colours.
B. The Polymeric Coating of the Composition
The device of the drug delivery composition of the invention also may
comprise a coating. In order to provide a non-degradable polymeric coating for
sustained-
release of biologically active agents, it is preferable to employ a polymeric
coating
composed of water-permeable polymer. Such polymer coating does not undergo
significant degradation when the device is inserted into the root canal of a
tooth or any
other target site and thus is retrievable at the end of the medication period
or whenever
desired. Such polymer coating is preferably comprised of cellulose derivatives
such as
ethylcellulose, methylcellulose, methylpropyl cellulose; polyurethanes;
silicone rubbers;
polyesters; polyamides; gutta percha; polysaccharides, polyethylene;
polypropylene;
acrylic polymers; and waxes, most preferably ethylcellulose .Such coating also
may be
comprised of pharmaceutical excipients such as fillers, binders, pigments,
flavours,
preservatives, or colours.
The sustained-release drug delivery composition of the invention may
contain inert excipients to improve the ease of insertion of the device or to
wet the device.
For example, inert excipients may include water, saline, glycerol, propyl
glycol, ethanol,
and buffer solutions.
C. Biologically Active Agents of the Composition
The biologically active agents of the sustained-release drug delivery
composition of the invention include antimicrobial agents like chlorhexidine,
triclosan,
clindamycin, tetracyclines, doxycycline, metronidazole, and penicillins, or
anti-
inflammatory agents such as corticosteroids, flurbiprofen, piroxicam,
indomethacin, and
other non-steroidal anti-inflammatory drugs, preferably chlorhexidine salts or
tetracyclines, most preferably chlorhexidine salts. Chlorhexidine in liquid or
solid state
CA 02275049 1999-06-17
can be incorporated in the sustained-release drug delivery composition,
provided as a free
base or salt of chlorhexidine, preferably as a salt of chlorhexidine, most
preferably as
chlorhexidine acetate or gluconate. Any one of the aformentioned biologically
active
agents may be incorporated into the drug delivery composition alone or in
combination
with one or more of the other agents.
The sustained-release drug delivery composition of the invention also may
contain biologically active agents outside of the device for immediate action
of therapy.
Such biologically active agents may include antimicrobial agents like
chlorhexidine,
triclosan, clindamycin, tetracyclines, doxycycline, metronidazole, and
penicillins, or anti-
inflammatory agents such as corticosteroids, flurbiprofen, piroxicam,
indomethacin, and
other non-steroidal anti-inflammatory drugs, preferably chlorhexidine salts or
tetracyclines, most preferably chlorhexidine salts.
II. Process for Formulation of the Drug Delivery Composition
A method for the formulation of the sustained-release drug delivery
composition of the invention containing at least one biologically active agent
is provided
below.
Water-permeable polymers including cellulose and derivatives such as
ethylcellulose, methylcellulose, methylpropyl cellulose; polyurethanes;
silicone rubbers;
polyesters; polyamides; gutta percha; and polysaccharides, most preferably
ethylcellulose
are dissolved in a suitable solvent or solvent mixture preferably alcohols or
ethyl acetate,
most preferably ethanol. One or more pharmaceutical excipients such as fillers
(e.g.,
starches, lactose, cellulose, bicalcium phosphate) and binders (e.g.,
polyvinyl pyrrolidone,
starches, sucrose, lactose, glucose, Acacia) are introduced into the polymer
solution.
Biologically active agents including antimicrobial agents and/or anti-
inflammatory agents, preferably chlorhexidine salts or tetracyclines, most
preferably
chlorhexidine salts, are dissolved or dispersed in the polymer solution
forming a
homogeneous solution or suspension.
11
CA 02275049 1999-06-17
In general, the ratio of the chlorhexidine in the polymer solution will vary
from 1 g chlorhexidine to 2 to 5 g polymer. Most of the solvent is evaporated
in a
fumehood at room temperature, and the remaining gel-like mixture is
transferred to a
mold to form the needle-like device and allowed to dry completely at room
temperature.
The prototype device was manufactured as described above with a length of 17
mm and a
diameter of 0.6 to 0.8 mm. The weight of the device was approximately 8 mg
including 2
to 3 mg of chlorhexidine. Four different formulations were used in this work,
to
manufacture compositions with different drug loading and drug release
mechanisms.
In order to be inserted into a root canal of a patientis tooth to treat or
prevent periradicular endodontic disease, the device of the sustained-release
drug delivery
composition of the invention is preferably needle-like having a diameter in
the range
from 0.1 to 4.0 mm, and preferably having a diameter in the range from 0.2 to
1.0 mm,
most preferably approximately 0.25 mm. It is desirable to employ devices
having a length
in the range from 1 to 18 mm, and is preferably in the range from 6 to 15 mm,
most
preferably approximately 12 mm. Such device may be manufactured with a variety
of
diameters and lengths to suit different tooth dimensions. Such device may be
tapered with
one end thicker or wider than the other end to conform with the shape of the
dental root
canal and pulp chamber, the preferred taper not exceeding 4% so that if the
device is 12
mm long and the tip at one end is 0.25 mm then the tip at the other end is
0.73 mm.
Devices having such dimensions and, therefore, suitable for insertion into the
root canal
of a patientis tooth may be employed to treat or prevent periradicular
endodontic disease.
Having now generally described this invention, the same will be better
understood by reference to certain specific examples which are included herein
for
purposes of illustration only and are not intended to limit the scope of the
invention,
unless specified.
EXAMPLE 1
Preparation and Evaluation of the Sustained-release Drug Delivery Composition
12
CA 02275049 1999-06-17
Various grades of ethylcellulose (Ethocel, Dow Chemical) of different
molecular weights, namely V 100, V45, and V 10 were dissolved in absolute
ethanol to
form a 10 wt % solution with different weight ratio of V100/V45N10. A typical
ratio
was 5/10/1. The pharmaceutical excipient starch (Aldrich) was introduced to
the polymer
solution. The weight of starch used was 40% to 50% of the dry ethylcellulose
used. The
biologically active agent chlorhexidine acetate was dissolved or dispersed in
the polymer
solution to form a mixture containing 20% to 50% chlorhexidine in the total
solid mass.
After most of the solvent was evaporated at room temperature in a fumehood,
the
remaining gel-like mixture was transferred into a mold and dried at room
temperature to
form the needle-like device having a length of 17 mm and a diameter of 0.6 to
0.8 mm.
The weight of the device was about 8 mg including 2 to 3 mg of chlorhexidine
acetate. A
coating was applied over the core by dipping the core into a solution
comprising 8%
ethylcellulose in ethanol and drying at room temperature.
Four different formulations were prepared all having different drug
loading and drug release mechanisms, as follows: ( 1 ) Formulation 1 - the
chlorhexidine
concentration was 30%, no filler was added and no coating was applied; (2)
Formulation
2 - the chlorhexidine constituted 45 wt % of the solid core composition, no
filler was
added but the core was coated with 8% ethylcellulose; (3) Formulation 3 - it
was similar
to formulation 2 except for the addition of starch as the filler; and (4)
Formulation 4 - it
was also similar to formulation 2 except for being coated twice.
In another different formulation, the core of the drug delivery composition
containing chlorhexidine gluconate was prepared using PEG8000 and PEG400. A
coating
comprising 8% ethylcellulose in butanol/ethyl acetate (30:70 w/w) was applied
over the
core.
In vitro Drug Release
Short-term Drug Release Test
13
CA 02275049 1999-06-17
Each device of the drug delivery composition was immersed into 3.0 ml of
0.05 M buffer solution (pH = 7.4) in a cuvette at 37 °C with vigorous
stirring. The amount
of drug released into the solution was continuously monitored with a diode
array
spectrophotometer (Hewlett-Packard 8452A). The measurement lasted for more
than 20
hours, with a sampling interval of 10 minutes.
Long-term Drug Release Test
Each device of the drug delivery composition was immersed in 0.35 ml
deionized water in a small glass tube (SO mm long, internal diameter 5 mm)
without
stirring. The glass tubes were placed into a water bath at 37 °C. Every
24 hours, the drug
concentration in the medium was determined by spectrophotometry. The devices
were
immediately patted dry, and immersed into fresh deionized water in a separate
tube.
Subsequent release tests were then continued.
Results
As shown in Fig. 3, the drug was released quickly from the device of
formulation 1 with most of it consumed within 2 to 3 days; therefore,
formulation 1 is
ruled out because of too rapid release. For the device of formulation 4, the
drug release
rate was very low; therefore, formulation 4 may be suitable for an extended
application,
but its antimicrobial effectiveness needs to be verified in further
investigations. The drug
release profiles of the devices of formulations 2 and 3 are more desirable
because their
initial burst establishes an effective drug level in the solution, and the
following slow but
steady release is sufficient to sustain the drug concentration at a
therapeutic level. A
release rate greater than 0.02 mg/day can last 40 days or longer.
EXAMPLE 2
In vitro Substantive Antimicrobial Activity Tests
14
CA 02275049 1999-06-17
Objective
Two tests were carried out to asses the substantive antimicrobial activity
of root dentin after interaction with chlorhexidine as follows: (1) short-term
irngation of
root canals as conventionally performed in endodontic practice; and (2)
prolonged
immersion of root canals.
Methodology
The method of Q~rstavik D & Haapasalo M (Endod. Dent. Traumatol.
6:142-149 (1990)) was modified to prepare a bovine root model for the
antimicrobial
tests. The root was cut with a rotating diamond saw into 5 mm long segments,
or
specimens, each with an external diameter of approximately 7 mm. The root
canals of the
specimens were enlarged with an ISO 033 round bur, to standardize the internal
diameter
to 3.3 mm. The smear layer was removed from the root canal wall with 17% EDTA.
The
specimens were sterilized separately by autoclaving three times in test tubes
containing
brain heart infusion (BHI) broth for 30 minutes at 121 °C, coated with
nail varnish on the
outer surfaces, and mounted with sticky wax at the bottom of Petri dishes.
In the short-term irngation test the root canal of each specimen was
irrigated for 5 minutes with 10 ml of one of the following test solutions:
Group 1 - liquid
0.2% chlorhexidine gluconate; Group 2 - 5.25% sodium hypochlorite; Group 3 -
sterile
saline. Solutions were dispensed with sterile syringes, while the excess was
evacuated
with high power suction.
In the prolonged immersion test the root canal of each specimen was
completely immersed with one of the following test solutions: Group 4 - liquid
0.2%
chlorhexidine gluconate; Group 5 - 5.25% sodium hypochlorite; Group 6 -
sterile saline;
Group 7 - distilled water with the device of the drug release composition of
the invention
inserted into the canal. The specimens were then incubated at 37 °C for
7 days while
adding fresh solutions daily (in Group 7 distilled water was added).
CA 02275049 1999-06-17
After drying the canals, they were inoculated with a suspension of E.
faecalis (ATCC 29212) in BHI broth for a period of 3 weeks. A fresh inoculum
was
added every other day to maintain microbial viability.
After inoculation the specimens were rinsed with sterile water and dried.
Sterile round burs, ISO sizes 035, 037, 040, and 042 were used sequentially to
enlarge the
canals and thus recover dentin samples ranging in depth from 0.1 mm to 0.45
mm. The
dentin samples obtained with each bur were collected in a separate test tube
containing 3
ml of sterile BHI broth, and incubated for 24 hours at 37 °C. The
optical density of the
broth, proportional to the number of viable bacteria present in the dentin
sample, was
measured in a spectrophotometer at 540 nm. The mean and standard deviation
optical
density values were calculated for each group and statistically analyzed.
Results
Table 2. Optical density of the broth (mean ~ SD) as an indication of
microbial growth (n = 10 for Groups 1-6, n = 2 for Group 7).
Group Dentin depth
(mm)
0.1 0.2 0.35 0.45
1 0.720.12 0.760.07 0.760.10 0.840.06
2 0.640.13 0.660.10 0.650.09 0.730.10
3 0.650.16 0.620.07 0.690.08 0.710.08
4 0.0110.003 0.0150.005 0.0090.005 0.00960.005
0.530.09 0.690.14 0.770.16 0.670.21
6 0.690.25 0.590.17 0.660.21 1.060.21
7 / non detectablenon detectable/
Specimens from Group 1 demonstrated high optical density values that did
not differ from those observed for Groups 2, 3, 5 and 6. In contrast,
specimens from
Group 4 (prolonged immersion with liquid 0.2% chlorhexidine gluconate)
demonstrated
significantly lower optical density values than those observed for all other
groups. This
finding was consistent for all the dentin depths sampled. Specimens from Group
7
16
CA 02275049 1999-06-17
demonstrated significantly lower optical density values at dentin depths of
0.2 mm and
0.35 mm.
General Discussion
From the results of the studies chlorhexidine, such as contained in the drug
delivery compsition of the invention, appears to be an effective root canal
medicament
capable of reducing the potential for infection of the root dentin.
The drug delivery composition is suitable for sustained-release of
chlorhexidine over short and long periods of time, ranging from hours to
months.
The sustained-release drug delivery composition of the invention is very
suitable for insertion into root canals of teeth for the following reasons:
(i) the device of the composition of the invention does not become
degraded over time, therefore there is no concern about residual fragments of
it remaining
in the root canal and interfering with the root filling procedure;
(ii) insertion and removal of the device of the composition of the invention
is simple and does not require any instrumentation or procedures additional to
the
conventional ones used routinely in root canal treatment;
(iii) activation of the release of the chlorhexidine in the root canal does
not
require the presence of vital tissue, only an initial immersion of the root
canal with any
fluid, preferably chlorhexidine aqueous solution.
As will be realized from the above description and results, the sustained
release of chlorhexidine into the root canal and pulp chamber of the tooth is
a preferred
way of treating periradicular endodontic diseases and preventing root canal re-
infection
subsequent to treatment.
It will be evident to those skilled in the art that the invention is not
limited
to the details of the foregoing illustrative examples, and that the present
invention may be
17
CA 02275049 1999-06-17
embodied in other specific forms without departing from the essential
attributes thereof,
and it is therefore desired that the present embodiments and examples be
considered in all
respects as illustrative and not restrictive, reference being made to the
appended claims
rather than the foregoing description, and all changes which come within the
meaning and
range of equivalency of the claims are therefore intended to be embraced
therein.
18
CA 02275049 1999-06-17
Table 1. Comparison of the drug delivery composition of the invention with
relevant US patents.
Patent Materials Drugs) Shape/DeviceApplications Degradable
#
05,226,434 dental periodontics No
floss
05,002,769 implant periodontics Yes
05,438,076PMMA/MAA CPK film-formingperiodontics, No
oral or
(Eudragit), polymer dermatological
fungal
EC solution infections,
endodontics
coating
the
paper point
05,213,615 polymer cariology No
in
solution
05,614,223PHEMA/MAA fluoridetablets, attached to No
teeth
as core; enzyme capsules,
PHEMA/MMA inhibitor.globules,
half
as membrane football,
veneers
or thick
films
05,451,424polyurethaneCHX extrusion Yes
04,685,883 microspheres,periodontics Yes
slabs (adhesive)
04,568,535Ethylcellulosemetroni-implant periodontics No
dazole
05,019,096polyurethane,AgNO, coating film on catheters,No
silicones,CHX gloves. No
PLA Yes
Our devicecomposite CHX or needle-likeendodontics No
of
various other
polymers antibacte-
rial
agents
19