Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02276185 1999-06-25
LIQUID ELECTROLYTE BATTERY
The invention relates to a liquid electrolyte or electrolytic battery,
which is preferably used in moving vehicles, such as e.g. cars, boats or
aircraft.
The efforts more particularly made by the vehicle industry for lightweight
construction also concerns the economizing of battery weight. However,
there is at the same time an increasing demand for a higher battery power,
because in addition to conventional energy for starting the vehicle,
energy is also required for additional units such as electric window
regulators, actuators or servomotors for adjusting seats and for the elec-
tric heating of the seats. It is desirable to keep the battery power at a
constant high level over the entire battery life.
The prior art discloses various measures for increasing the power of a
conventional lead acid battery. The term power is here understood to mean
the capacity of the battery and the current output and consumption capa-
bility of the battery.
A particular problem arising with lead acid batteries is the optimum com-
plete use of the electrode surface. Figs. 1 to 3 are intended to illus-
trate the problem known from the prior art. Fig. 1 is a sectional repre-
sentation of a car battery 1 along its electrodes 2, which have a grid form
in the present construction. The level of the battery acid 3 is designated
3a. Research has revealed that the chemical characteristics of the battery
acid differ significantly in the areas designated a, b and c. Thus, in
area a the acid concentration is too high, which leads to corrosion and
ultimately to the disintegration of the plates. In area c the acid concen-
tration is too low, i.e. the electrolytic characteristics necessary for the
operation of the battery do not exist.
Only in the central area b does the acid have the optimum stoichiometric
ratio. Thus, the existing electrode surface is not utilized in an optimum
manner due to the inadequate acid characteristics in areas a and b. It is
clear to the expert that the areas are not sharply defined in the manner
shown.
CA 02276185 1999-06-25
- 2 -
In order to also improve the stoichiometric ratio in areas a and b, it is
known from the prior art to circulate the battery acid, i.e. the electro-
lyte, in order to obtain a better intermixing. This simultaneously pre-
vents the formation of deposits, which impair the function and life of the
battery.
DE U1 9114909 discloses a storage battery, in which by means of the intro-
duction of gas from a pressurized gas source, an electrolyte circulation is
brought about. Due to their complicated construction such devices are
unsuitable for vehicle batteries, particularly as additionally a pressur-
ized gas source is needed.
The prior art also discloses electrolyte intermixing devices, which can be
called hydrostatic pumps. Figs. 29a, 29b and 29c show the basic operation
of such a device. Fig. 29a is a sectional representation of an electrolyte-
filled battery box, which has a double bent plate 21, a portion of the
angle projecting beyond the electrolyte surface. To facilitate under-
standing the electrode plates are not shown. If the battery box installed
in a vehicle moves at a uniform speed v, i.e. the vehicle neither acceler-
ates nor decelerates, the electrolyte surface is flat and horizontal. Fig.
29b shows that during a braking process, due to the mass moment of inertia,
the electrolyte builds up to a wave in the travel direction and the electro-
lyte splashes over the upper portion of the plate edge. As now the liquid
level between the angle and the casing wall is higher, according to fig.
29c the electrolyte flows downwards until the two levels have evened out.
The arrows show the electrolyte flow direction.
This principle is inter alia described in US 4,963,444 US 5,096,787 and US
5,032,476. However, the inventors of the present invention have found that
with the devices known from this prior art it is not possible to achieve an
optimum electrolyte intermixing.
Therefore the problem of the invention is the provision of a liquid electro-
lyte battery for vehicles, in which the necessary higher battery capacity
and life are to be achieved mainly through an improved electrolyte inter-
mixing.
CA 02276185 1999-06-25
- 3 -
This problem is solved by the batteries according to claims 1, 10 and 15.
The advantage of the invention according to claim 1 is that the liquid
electrolyte circulating device brings about a high degree of intermixing
and consequently the battery capacity is significantly increased and simul-
taneously the battery life is lengthened. The liquid electrolyte circulat-
ing device has no free moving parts, whose movement could be impeded by
electrolyte deposits. Therefore this device operates very reliably. In
addition, the liquid electrolyte circulating device is very inexpensively
manufacturable and can be readily integrated into the battery manufacturing
technology. Unlike in the case of the prior art, hydrostatic electrolyte
pumps, this device pumps the electrolyte from bottom to top. The inventors
have proved that in this way intermixing can be significantly improved.
Obviously it is advantage for intermixing purposes if the thicker bottom
acid is forced upwards and runs out over the horizontal part of the inter-
mixing device, in order to mix with the thinner surface acid.
In the case of a liquid electrolyte battery further developed in accordance
with claim 2, parallel to the vertical edges is provided a second, plate-
like element, in order to form a flow channel. Thus, the flow conditions
can be set in a more clearly defined manner and optimized.
In a liquid electrolyte battery further developed according to claim 3,
the first plate-like element and the second plate-like element are con-
structed in one piece as angles, so that in certain cases an easier
assembly is possible.
In a liquid electrolyte battery further developed according to claim 4, in
the vicinity of the upper edge of the first plate-like element is provided
a first return flow preventer for preventing the return flow of a first
electrolyte wave, which improves intermixing.
In a liquid electrolyte battery further developed according to claim 5,
the return flow preventer is constructed as a web-like material extension
of the first plate-like element, which is particularly cost-effective.
CA 02276185 1999-06-25
- 4 -
In a liquid electrolyte battery further developed according to claim 6, the
return flow preventer is constructed as a flap valve, which particularly
effectively prevents a return flow.
In a liquid electrolyte battery further developed according to claim 7, the
liquid electrolyte circulating device is placed on both casing sides,
which brings about an improved intermixing.
The advantage of the invention according to claim 10 is that through heat
convection there is even a thorough mixing or intermixing when the battery
is only slightly moved or not moved at all, the heating elements being so
positioned that a powerful electrolyte flow can be produced.
According to claim 11 use is made of panel heaters, which are placed on or
in the casing wall. If the battery is constructed from two cell groups,
which are interconnected by a common partition, the heating means according
to claim 12 can be placed on said partition located in the centre of the
battery. Virtually no heat losses occur in this embodiment.
In claim 13 for the protection of the electrode plates a heat shield is
provided, so that the electrolyte heated by the heating means does not pass
directly to the electrode plates. In a particularly preferred embodiment
according to claim 14, part of the mechanical circulating device is simul-
taneously used as a heat shield, so that both a mechanically caused and a
thermally caused circulation of the electrolyte takes place.
The advantage of the invention according to claim 15 is that in the same
way as for producing a convection by means of heating elements, an inter-
mixing still occurs if the battery moves only slightly or is stationary.
A Peltier element is highly suitable as the cooling element in accordance
with claim 16.
The cooling involves the same effect, but it is brought about with differ-
ent means. Thus, according to claim 17, there can be a combination with the
mechanical circulating device.
CA 02276185 1999-06-25
- 5 -
Further measures and advantages of the invention can be gathered from the
following description of embodiments in conjunction with the attached
diagrammatic drawings.
Fig. 1 shows a side longitudinal section of a liquid electrolyte battery
according to the prior art.
Fig. 2 shows the plan view of an open liquid electrolyte battery according
to the prior art.
Fig. 3 shows the view of fig. 1, the liquid electrolyte battery undergoing
an acceleration and the electrolyte level is inclined.
Fig. 4 shows a first embodiment of the invention.
Fig. 5 shows the first movement phase of the electrolyte surface during
an acceleration.
Fig. 6 shows the second movement phase of the electrolyte surface after
acceleration.
Fig. 7 shows the third movement phase of the electrolyte surface after
the acceleration.
Fig. 8 shows a second embodiment of the invention.
Fig. 9 is a plan view of an open, inventive liquid electrolyte battery
with a one-sided circulating device.
Fig. 10 shows a plan view of an open, inventive liquid electrolyte battery
with a two-sided circulating device.
Fig. 11 shows a third embodiment of the invention in detail.
Fig. 12 shows a fourth embodiment of the invention in detail.
CA 02276185 1999-06-25
- 6 -
Fig. 13 shows a fifth embodiment of the invention.
Fig. 14 shows a sixth embodiment of the invention.
Fig. 15 shows another embodiment of the invention, where circulation takes
place by heating.
Fig. 16 shows another embodiment of the invention, in which circulation
takes place by cooling.
Fig. 17 shows a combination of mechanical and thermal circulation.
Fig. 18 shows a further combination of mechanical and thermal circulation.
Fig. 19 shows a combination of mechanical and thermal circulation by
cooling.
Fig. 20 shows an angular mixing device with flow slots.
Fig. 21 shows an angular mixing device in conjunction with specially
constructed flow channels.
Fig. 22 shows another form of a mixing device.
Fig. 23 shows another form of a mixing device.
Fig. 24 shows another form of a mixing device.
Fig. 25 shows another form of a mixing device.
Fig. 26 shows another form of a mixing device.
Fig. 27 shows another form of a mixing device.
Fig. 28 shows a further form of a mixing device, which automatically
adapts to different acid levels.
CA 02276185 1999-06-25
- 7 -
Fig. 29 shows the closest prior art.
Figs. 1 and 2 serve for illustration purposes and show a liquid electrolyte
battery according to the prior art with a casing 1 having side walls la, lb,
lc, ld, a casing bottom le and a lid lf. Electrodes 2 are placed in vert-
ically standing manner in the individual cells lg, each of which contains a
liquid electrolyte 3, which is approximately 1 cm above the upper edge of
the electrodes 2.
Fig. 3 shows the representation of fig. 1, the liquid electrolyte battery
undergoing an acceleration and the electrolyte level is inclined. This
situation arises if the battery is e.g. installed in a car in such a way
that the electrode plates extend in the direction of travel, which in the
present example passes in the image plane from left to right. If the mov-
ing vehicle is decelerated, as a result of the mass moment of inertia of
the electrolyte, it splashes in the direction of travel, which is only
diagrammatically intimated by the sloping level. During travel there is
only a slight electrolyte movement between the plates, without any signif-
icant intermixing occurring.
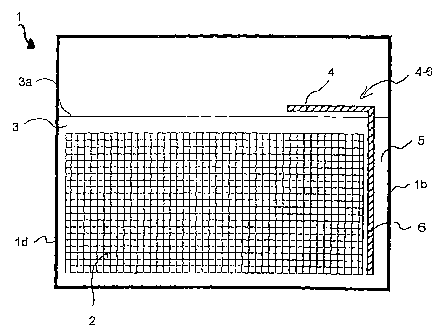
Fig. 4 shows a first embodiment of the invention. An angle 4-6 is inserted
in such a way that a vertical side is parallel to the vertical plate edges.
Its horizontal side is parallel to the upper edge 2a of the plates 2 in
the vicinity of the level 3a of electrolyte 3.
If the above-described braking situation arises, the angle portion 4 pre-
vents the described splashing movement of the electrolyte 3. Fig 5 diagram-
matically shows the movements forced on the electrolyte by angle 4-6.
Particular mention is made of the fact that the electrolyte in the vertical
flow channel 5, which forms between the vertical side of the angle and the
cell wall, is forced strongly upwards, which is diagrammatically repre-
sented by the upwardly directed arrows.
Fig. 6 shows the next time period, in which an electrolyte crest 3b has
formed on the horizontal angle side and subsequently flows away as a wave
in the arrow direction and runs out again at wall ld and can again form an
electrolyte crest (fig. 7).
CA 02276185 1999-06-25
- 8 -
The above statements make it clear that the angle and its spatial arrange-
ment in the battery casing forces a cycle on the electrolyte, circulation
taking place from bottom to top, so that a very intense intermixing occurs.
Long-term testing has revealed that the electrolyte concentration in areas
a, b and c is virtually the same and also has the correct stoichiometric
ratio. Thus, also electrode portions a and c, which could only be partly
utilized in conventional batteries, are now completely utilized.
Besides increasing the battery capacity, the invention has numerous further
advantages. The improved intermixing ensures that there is no solid elec-
trolyte deposit formation, which in conventional batteries reduces both
power and service life. Particular mention is also made of the greatly
improved cold starting characteristics of the inventive battery.
It is clear to the expert that the battery according to the invention has a
preferred installation direction and this is chosen in such a way that
there is an optimum circulation of the electrolyte. Thus, in a car the
battery must be so installed that the electrode plates are oriented in the
direction of travel.
Figs. 8 and 10 to 14 show further embodiments of the invention. Thus, fig.
8 shows on the angle 4-6 a web-like extension 4b, which can be vertical or
inclined. This extension 4b prevents the rapid flow back of the electro-
lyte and consequently improves its intermixing.
Fig. 9 is a plan view of the opened battery with six cells, in which is in
each case placed an angle 4-6.
As the battery, particularly in cars, is accelerated in both directions by
braking and accelerating actions, intermixing is improved if two angles per
cell are installed in oppositely directed manner, as shown in fig. 10 and
as a result of the similarity of the action no further explanation is
required by the expert.
Fig. 11 shows a further and/or additional possibility for preventing return
electrolyte flow. As shown in fig. 11, on angle 4-6 can be placed a
CA 02276185 1999-06-25
- 9 -
flexible plate in such a way that a valve action occurs. If the electro-
lyte rises in the flow channel, the flexible plate engages on the wall to
which it is fixed, i.e. the valve is opened. If the electrolyte flows back,
the valve closes. The constructional details and the opening and closing
phase are shown in figs. lla to llc and should require no additional
explanations for the expert.
Fig. 12 shows a modification of the principle according to fig. 11. The
structure and action of this flap valve are apparent from the drawings.
Fig 13 shows a second return flow preventer 9, which can be used in oppo-
sition to the direction of travel in car batteries. In place of a horiz-
ontal side, said device has a rearwardly open volume 10. If, according to
fig. 7, a back-flowing wave is formed, it passes over the slope l0a and is
held back by the open volume 10, so that the electrolyte sinks downwards
along the vertical side and consequently an intermixing takes place.
It must be stressed that the return flow preventers shown can be further
modified. Thus, at e.g. specific points in the angles openings can be pro-
vided, in order to prevent the formation of dead areas, i.e. areas where
an inadequate intermixing takes place. The dimensioning of the return flow
preventer for a specific battery type requires no inventive activity on the
part of the expert when knowing the teaching involved. The expert will
also take further intermixing-aiding measures, which are not expressly
mentioned in the present invention. Thus, it is e.g. advantageous to so
design the electrolyte flow paths that within the flows forced by the
battery movement a limited flow resistance occurs, which can inter alia be
achieved by very smooth walls and by the avoiding of projections where
eddies can form.
Fig. 14 shows a double-sided embodiment according to fig. 10, where the two
angles are linked by a perforated plate 11. This embodiment is advantage-
ous from the assembly standpoint, because the electrode plates are held
together in clamp or clip-like manner and can be easily automatically
fitted. The action of the perforation is made clear to the expert on
referring to fig. 5.
CA 02276185 1999-06-25
- 10 -
Fig. 15 shows another embodiment of the invention, where electrolyte cir-
culation takes place by heating. For this purpose electric heating elem-
ents are located in the lower area of the battery box. On heating, the
neighbouring electrolyte also heats and rises upwards and consequently
brings about an intermixing. The heating elements are preferably of a very
flat type, such as film or foil heaters. These heaters can be gathered
from the prior art. As a result of the simple action, no further operat-
ional explanations are needed. It is stressed that the heaters 12 are not
mainly used for heating the electrolyte, but instead for producing a
convective flow, which brings about an intermixing. Therefore the heaters
are not uniformly distributed over the entire bottom surface and are
instead located at predetermined points, so that a very strong flow is
produced.
If only a single heater or heaters with a high capacity are used, it may be
necessary for the protection of electrodes 2 to provide a heat protection
means between the latter and the heater. This heat protection ensures that
the strongly heated electrolyte does not come into contact with the elec-
trodes, because the latter could be damaged by the heat. In fig. 17 the
heat protection is constructed as a flat plate, which can.be dimensioned in
the same way as the first plate-like element 6. It must be ensured that
the heated electrolyte runs over the upper edge of the heat protection
means or can pass through an opening in the plate.
The particular advantage of convective mixing by heat infeed is that the
battery need not be moved. In the case of low external temperatures the
electrolyte is simultaneously heated, which is also desirable.
If, for operational reasons, battery heating is not desired, then according
to fig. 16 a convective mixing can also be brought about by a cooling
element. Due to the oppositely directed convection, the cooling element
is placed in the upper marginal area of the battery box, i.e. preferably
below the electrolyte level.
Fig. 18 shows a combination of mechanical and thermal circulation by heat-
ing. The vertical side of the angle for mechanical intermixing
CA 02276185 2006-03-01
- 11 -
simultaneously serves as a heat protection during thermal intermixing. To
permit the outflow of the heated, upwardly flowing electrolyte, the vert-
ical side has holes 4c.
As in the embodiment according to fig. 18, fig. 19 shows a combination of
mechanical and thermal circulation by cooling and the construction is
readily apparent from the drawing. Peltier elements are used for cooling
purposes. The function of this arrangement is apparent from the already
explained contexts and the drawing.
Fig. 20 shows intermixing angles with flow slots 14, which improve the flow
conditions for different electrolyte levels.
Fig. 21 shows an intermixing angle, which is suitable for forming different
flow channels 5a. Fig. 21a is a perspective view of the intermixing
angle and figs. 21b and 21c show the incorporated angle in plan view. In
the arrangement according to fig. 21c, there is a U-shaped flow channel
cross-section and in the arrangement according to fig. 21b two facing flow
channels.
Fig. 22 shows an intermixing plate 4, with fig. 22a showing a side cross-
section of a battery. The plate 4 is installed on the plate set and in
the same way as all the upper angle portions is preferably inclined
slightly towards the battery centre. In this embodiment the battery wall
takes over the function of the angle side 6.
Figs. 23 and 24 show intermixing devices, in which both angles are inter-
connected to form a U or box-like unit 15, 16. The slots 14 have an elec-
trolyte inflow/outflow function.
Fig. 25 shows an intermixing angle, which has on the lower portion of the
vertical angle side 6 a horizontal side 17, directed towards the battery
centre and having a length L. This constructional measure makes it poss-
ible to optimize the flow conditions on introducing the electrolyte into
the flow channel 5. The side 17 can also have holes or slots, if this
leads to a flow condition improvement.
CA 02276185 2006-03-01
, , .
- 12 -
Fig. 26 shows an intermixing angle, whose flow channel is formed by two
tubes 18.
Fig. 27 shows an intermixing angle similar to fig. 22. This angle has
portions 19, which permit an anchoring of the angle in the plate set and
simultaneously mechanically stabilize the set. This is merely a construc-
tional detail.
Fig. 28 shows an intermixing angle with a movable side 4a, which is pivot-
ably connected by means of a film hinge 20 to the vertical side 6. This
movable side 4a floats on the electrolyte surface 3a. In the case of a
suitable dimensioning, this arrangement improves the outflow of the elec-
trolyte flowing upwards through the flow channel 5, so that intermixing is
improved.
Taking account of the constructional and technological marginal conditions,
the expert will choose one of the indicated variants and will optionally
modify it without having to be involved in inventive activity.
By means of the embodiments described the expert can fully gather the
technical teaching of the invention. It is clear that these embodiments
can be further developed and modified or combined by an expert with the
aid of the inventive teaching. Thus, even the embodiments not expressly
mentioned or shown fall within the protective scope of the following claims.