Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02278747 1999-07-21
WO 98/35946 PCT/US98/00623 -
4-AMINOALKOXY-1,3-DIHYDROBENZOIMIDAZOL-2-0NE DERIVATIVES) THEIR
PREPARATION AND THEIR USE AS DOPAMINE AUTORECEPTOR (D2) AGONISTS
Field of Invention
This invention relates to a series of 4-aminoalkoxy-1,3-dihydrobenzoimidazol-2-
ones having dopaminergic properties and thus have utility in treating
Parkinson's disease,
to Tourette's syndrome, schizophrenia, and alcohol and drug addiction.
Background of the Invention
The compounds of this invention are dopamine agonists having various degrees
of
intrinsic activity and are essentially free from extrapyramidal side effects.
Some of the
compounds are selective autoreceptor agonists, and therefore partial agonists
(i.e, activate
only autoreceptors versus postsynaptic D2 dopamine receptors). Efforts to
induce
antipsychotic activity with dopamine autoreceptor agonists have been
successful (Dorsini et
al., Adv. Biochem. Psychopharmacol.) 16, 645-648, 1977; Tamminga et al.,
Science,
200, 567-568; and Tamminga et al., Psychiatry) 398-402, 1986).
2o As selective autoreceptor agonists, the invention compounds provide
functional
modulation of the dopamine systems of the brain without the excessive blockade
of the
postsynaptic dopamine receptors which have been observed to be responsible for
the
serious side effects frequently exhibited by agents found otherwise clinically
effective for
the treatment of schizophrenia. Activation of the dopamine autoreceptors
results in reduced
2s neuronal firing a well as inhibition of dopamine synthesis and release and
therefore
provides a means of controlling hyperactivity of the dopaminergic systems.
The compounds of this invention were also found to have high intrinsic
activity and
therefore they can behave as the natural neurotransmitter, i.e., as full
agonists. As such,
they are useful in the treatment of diseases having abnormal concentrations of
dopamine
3o could be used as dopamine surrogates such as schizophrenia, Parkinson's
disease and
Tourette's syndrome. Such agents are partial agonists at the postsynaptic
dopamine D2
receptor and are thereby useful in the treatment of alcohol and drug
addiction.
In the Belgian patent 850,166, Ciba-Geigy discloses compounds represented by
the
compound of the formula below which have both a and (3-adrenergic properties
and are
35 useful as cardiovascular and antihypertensive agents.
1
CA 02278747 1999-07-21
WO 98/35946 PG"f/US98/00623 -
HN \ I O~~t Bu
a- NH OH
O
CGP-12177
Summary of the Invention
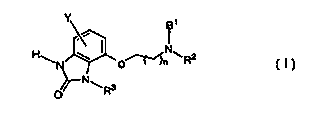
Compounds of this invention are 4-aminoalkoxy-1,3-dihydro-benzoimidazol-2-
s ones which are illustrated by Formula I below
HEN \ I o~N~R2
~N~
O
to I
wherein:
R' is hydrogen or C1-C6 alkyl;
RZ is selected from hydrogen, straight-chain and branched C1-Cto alkyl,
cyclohexylmethyl or -(CH2)mAr where Ar is phenyl, naphthyl, thienyl,
1 s furanyl or pyridinyl, each optionally substituted by one or two
substituents
selected independently from C,-C6 alkyl, halogen, C,-C6 alkoxide and
trifluoromethyl;
or NR'RZ is 1, 2, 3, 4-tetrahydroquinolin-1-yl or 1, 2, 3, 4-
tetrahydroisoquinolin-
2-yl;
2o m is 1-5;
nis 1 or2;
R3 is hydrogen or C,-C6 alkyl;
Y is halogen, C,-C6 alkyl, and C,-C6 alkoxy;
and the pharmaceutically acceptable salts thereof.
25 Acid addition salts can be formed with an invention compound and a
pharmaceutically acceptable acid including, but not limited to, the
hydrochloride,
hydrobromide, hydroiodide, sulfate, phosphate, nitrate, acetate, fumarate,
succinate,
citrate, maleate, lactate, and benzoate salts.
The compounds of this invention are dopamine autoreceptor agonists, that is,
they
3o serve to modulate the synthesis and release of the neurotranmitter
dopamine. They are thus
2
CA 02278747 1999-07-21
WO 98/35946 PCT/US98/00623 -
useful for treatment of disorders of the dopaminergic system, such as
schizophrenia,
Parkinson's disease and Tourette's syndrome. Such agents are partial agonists
at the
postsynaptic dopamine DZ receptor and are thereby useful in the treatment of
alcohol and
drug addiction.
s
Detailed Description of the Invention
The compounds of Formula I are generally prepared by the overall sequence
depicted in Schemes I -N. When one or both of R' and R2 is hydrogen, it is
desirable to
protect the basic nitrogen with a suitable protecting group such as the
trifluoroacetyl group
t o or t-butyloxycarbonyl group. Scheme I outlines a procedure to prepare an
invention
compound where R3 is H.
Scheme I
is
R2
H O~C~
/ NH2 / NH2 "O~N~Ri
-~ I ~ / NH2 trifluorwnW c
N02 \ N02 ~ ~ NO anhydndc
1 fn=1or21
O'\ /CF3 O~CF3
O~n~~R~ HZNNHz O~/~N'~R1 cm
/ NH2 lo% Pa/c / NH2
or
NOp Raney Nickel ~ NH2
ethanol
O'\'CF3
H
O~'~IN~R1 O
n
H 1. IC2C03
N O MeOH/H20 / N
HCI salt
N~ 2. Hct ~ ( O
H
3
CA 02278747 1999-07-21
- WO 98/35946 PCT/US98/00623 -
Scheme II shows a synthetic route for invention compounds where R3 is not H.
Scheme II
s
O\ /CF3 O \ /CF3
~N NN' 1
O~n ~RZ trifluoroacetic O~n ~R
NH2 anhydride / NHCOCF3 DMSO
\ N02 \ ~ NO
2
3
O'\ /CF3
H
O~N~Ri O~N~R1
NR3COCFg KzCOs / NHR3 BoczO
\ , NO Me o \ ~ N02
2
Z $
~oc ~oc
O~nN~Rt O~N~R1
NHR3 HZNNHZ NHR3 CDI
--~ ~ -.-
\ I N02 Pd/C \ ~ NH
2
L
to
~oc ~oc
R~ N~O R3 Rte ~,~0 R3
N 1. ~A N
O 2. Hct ~ ~ ~O
a H H
Scheme III shows a synthetic route for invention compounds where neither of R'
and RZ is H.
4
CA 02278747 1999-07-21
WO 98/35946 PCT/US98/00623 -
Scheme III
\ \
' N I / N
O~n O~n
/ NH2 H2NNH2 / NH2 CDI
NOZ lo~ ~ I NH2 Hct
ethanol
2k a
N
O~n
H
/ N
HCI salt
N
H
Scheme IV shows the procedure used to prepare an intermediate where Y is Cl.
Scheme IV
Io
O~C~ O~CI
/ NH2 NCS ~ / NH2
\ ~ N02 CH3CN C \ ~ NOp
ZZ
The following specific examples illustrate the synthetic procedures for the
~ 5 preparation of intermediates and invention compounds and should not be
construed as
limiting the scope of this disclosure. Those skilled in the art of organic
synthesis may be
aware of still other routes to prepare invention compounds. Reactants and
intermediates are
either commercially available or can be prepared according to standard
literature
procedures.
5
CA 02278747 1999-07-21
WO 98/35946 PCT/US98/00623 -
Intermediate 1 a
2-(2-Chloro-ethoxy)-6-vitro-phenylamine
s Method 1.
To a solution of 2-amino-3-nitrophenol (5.0 g, 32.4 mmol)) triphenylphosphine
(12.8 g, 48.7 mmol) and 2-chloroethanol (3.9 g, 48.7 mmol) in tetrahydrofuran
(120 mL)
at 0 - 5° C was added over 30 min a solution of diethyl
azodicarboxylate (8.5 g) 48.7
1 o rnlnol) in tetrahydrofuran (75 mL). The mixture was warmed to 2'~°
C and stirred for 18
hr. The solvent was removed under vacuum to give a dark brown oil.
Purification by
chromatography (1.3 kg silica gel, 30% hexane - ethyl acetate) afforded 3.1 g
(44.2%) of
an orange solid, mp 71-73 °C; MS (+)PBEI mle 216/218 (M').
t s Elemental analysis for C8H9C1N203:
Calc'd: C, 44.36; H, 4.19; N, 12.93
Found: C, 44.45; H, 4.02; N, 12.97
Method 2.
A slurry containing 2-amino-3-nitrophenol (32.0 g, 0.208 mol), 1,2-
dichloroethane
(260.0 g, 2.65 mol), potassium carbonate (35.0 g, 0.252 mol) and 2-butanone
(750 mL)
was refluxed for 24 hr. The mixture was cooled, filtered and the solids were
washed with
ethyl acetate. The filtrate was concentrated to an oily residue that was
dissolved in ethyl
2s acetate (500 mL). The organic layer was washed with 1 N sodium hydroxide
{250 mL),
water (500 mL), and brine (2X 500 mL), dried over anhydrous magnesium sulfate.
Concentration of the filtered solution and trituration of the residue with
hexane afforded
37.8 g (84.6%) of product as an orange solid, ~mp 71-73 °C; MS (+)PBEI
mle 216/218
(M+).
Intermediate 1 b
2-(3-Bromo-propoxy)-6-vitro-phenylamine
Following the procedure of method 2 above, and using 1,3-dibromopropane, the
title compound was as a yellow solid, (78.7%) mp 88-89 °C; MS EI mle
274/276 (M+).
3s
Elemental analysis for C9H11BrN203:
Calc'd: C, 39.29; H, 4.03; N, 10.18
Found: C, 39.71; H, 3.91; N, 10.27
6
CA 02278747 1999-07-21
WO 98/35946 PCT/US98/00623 -
Intermediate 2a
2-(2-Benzylamino-ethoxy)-6-vitro-phenylamine
s A mixture of 2-(2-chloro-ethoxy)-6-vitro-phenylamine (1~, 3.0 g, 13.8 mmol)
and
benzylamine (9.0 g, 84.0 mmol) was heated neat at 100-110° C for 6 hr.
The excess
benzylamine was removed by distillation under vacuum (70-75 °C / 0.1
mm) . The residue
was poured into 1 N sodium hydroxide (300 mL) and extracted with ethyl acetate
(2X) 300
mL). The combined organic layer was washed with water (2X, 300 mL) and brine
(300
t o mL). The ethyl acetate layer was dried over anhydrous magnesium sulfate,
filtered, and the
solvent removed under vacuum to give 5.1 g of crude red oil. Purification by
chromatography (500 g silica gel, ethyl acetate : 2 M NH3 in methanol, 20 : 1
) afforded
3.54 g (89.3%) of a red semi-solid, mp 33-60 °C; MS EI mle 287 (M').
1 s Elemental analysis for C 1 SH 17N303:
Calc'd: C, 62.71; H, 5.96; N) 14.62
Found: C, 62.64; H, 6.04; N, 14.23
'DMSO can be used as a solvent in this reaction
2o Using this general procedure and utilizing 2-(2-chloro-ethoxy)-6-vitro-
phenylamine
or 2-(3-bromo-propoxy)-6-vitro-phenylamine or 4-chloro-2-(2-chloro-ethoxy)-6-
nitro
phenylamine and 4-methyl-benzylamine, 1-naphthalene-methylamine, 4-tert-butyl
benzylamine, thiophene-2-methyl-amine, 4-chloro-bcnzylamine, thiophene-3-
methyl
amine, 1,2,3,4-tetrahydroisoquinoline or 3-phenyl-1-propylamine produced the
following
2s intermediates Zb-21, respectively:
2h 2-[2-(4-Methyl-benzylamino)-ethoxy]-6-vitro-phenylamine as a yellow
solid (89 %), mp 55-57 °C; EI mle 301 (M').
Elemental analysis for C16H19N303~
Calc'd: C, 62.71; H, 5.96; N, 14.62
Found: C, 62.64; H, 6.04; N, 14.23
2c 2-(3-Benzylamino-propoxy)-6-vitro-phenylamine as a viscous orange oil
ss (85.5 %); MS EI mle 301 (M').
Elemental analysis for C16H19N303:
Calc'd: C, 63.77; H, 6.36; N, 13.94
7
CA 02278747 1999-07-21
WO 98/35946 PCT/US98/00623
Found: C, 63.66; H, 6.28; N, 13.89
s
2~i 2-{2-[(Naphthalen-1-ylmethyl)-amino]-ethoxy}-6-vitro-phenylamine
as a yellow solid (76.3 %), mp 66-67 °C; MS EI mle 337 (M').
Elemental analysis for C19H19N3~3:
Calc'd: C, 67.64; H, 5.68; N, 12.45
Found: C, 67.20; H, 5.66; N, 12.26
l0 2~ 2-[2-(4-tent-Butylbenzylamino)-ethoxy]-6-vitro-phenylamine
as an orange viscous oil (83.3 %); MS EI mle 343 (M').
Elemental analysis for C19H25N3~3 ' 0.25 H20:
Calc'd: C, 65.59; H, 7.39; N, 12.07
1 s Found: C, 65.89; H, 7.20; N, 11.94
2f 2-Nitro-6-{2--[(thiophen-2-ylmethyl)-amino]-ethoxy}-phenylamine as
a red semi-solid material (88.5%); MS EI mle 389 (M').
2o Elemental analysis for C13H15N3~3S~
Calc'd: C, 53.23; H, 5.15; N, 14.32
Found: C, 52.86; H, 4.93; N, 14.1 S
2-[2-(4-Chloro-benzylamino)-ethoxy]-6-vitro-phenylamine as an orange
2s solid (87.8 %), mp 61-62 °C; MS EI mle 322/324 (M~).
Elemental analysis for C15H16N3~3 ' 0.25 H20:
Calc'd: C, 55.22; H, 5.10; N, 12.88
Found: C, 55.27; H, 4.96; N, 12.88
2h 2-(2-Benzylamino-ethoxy)-4-chloro-6-vitro-phenylamine as a orange-
brown colored solid (54.0 %), mp 87-88 °C; MS EI mle 321/323 (M+).
Elemental analysis for C15H16CIN303:
3s Calc'd: C, 55.99; H, 5.01; N, 13.06
Found: C, 55.85; H, 4.90; N, 13.13
8
CA 02278747 1999-07-21
WO 98/35946 PCT/US98/00623 -
2i 4-Chloro-2-nitro-6- f 2-[(thiophen-2-ylmethyl)-amino]-ethoxy}-
phenylamine as a yellow solid (44.0 %), mp 74-75 °C; MS EI mle 327/329
(M'")
Elemental analysis for C13H14C1N302S:
s Calc'd: C, 47.67; H, 4.33; N, 12.75
Found: C, 47.54; H, 4.11; N, 13.06
io
2i 4-Chloro-2-vitro-6-{2-[(thiophen-3-ylmethyl)-amino]-ethoxy}-
phenylamine as a yellow solid (33.3 %), mp 77-78 °C; MS EI mle 327/329
(M').
Elemental analysis for C13H14C1N302S:
Calc'd: C) 47.67; H, 4.33; N) 12.75
Found: C, 47.54; H, 4.18; N) 12.80
is ~k_ 2-[2-(3,4-Dihydro-1H-isoquinolin-2-yl)-ethoxy]-6-vitro-phenylamine
as a yellow solid (87.1 %), mp 95-97 °C; MS EI mle 313 (M+).
Elemental analysis for C17H19N302:
Calc'd: C, 65.16; H, 6.11; N, 13.41
2o Found: C, 64.87; H, 6.11; N, 13.40
21 2-Nitro-6--[2-(-phenyl-propylamino)-ethoxy]-phenylamine as a viscous
orange oil (83.9%); MS EI mle 315 (M').
2s Elemental analysis for C,~HZ1N303~0.25 H20:
Calc'd: C, 63.83; H, 6.77; N, 13.14
Found: C, 63.90; H, 6.56; N, 13.07
Intermediate 3a
N-[2-(2-Amino-3-vitro-phenoxy)-ethyl]-N-benzyl-2,2,2-trifluoro-acetamide
To a solution of 2-(2-benzylamino-ethoxy)-6-vitro-phenylamine (2,~, 0.5 g,
1.74
mmol) and triethylamine (0.32 mL) 3.48 mmol) in anhydrous methylene chloride
(10 mL)
3s at 23° C was added trifluoroacetic anhydride (0.32 mL, 2.26 mmol).
After 2 hr the reaction
was diluted with ether and washed with saturated sodium bicarbonate (3 x 80
mL) and the
9
CA 02278747 1999-07-21
WO 98/35946 PCT/US98/00623 -
organic layer dried over anhydrous magnesium sulfate. Filtration and
evaporation of the
solvent gave 0.55 g (81.7%) of yellow solid, mp 134-135 °C; MS EI mle
383 (M+).
Elemental analysis for C17H16F3N304:
s Calc'd: C, 53.27; H, 4.21; N, 10.96
Found: C, 53.09; H, 4.35; N, 10.93.
Following this general procedure and using 2-[2-(4-methyl-benzylamino)-ethoxy]-
6-vitro-phenylamine, 2-(3-benzylamino-propoxy)-6-vitro-phenylamine, 2- ( 2-
[(naphthalen-
l0 1-ylmethyl)-amino]-ethoxy}-6-vitro-phenylamine, 2-[2-(4-tert-
butylbenzylamino)-ethoxy]-
6-vitro-phenyl-amine, 2-vitro-6- { 2-[(thiophen-2-ylmethyl)-amino]-ethoxy } -
phenylamine,
2-[2-(4-chloro-benzylamino)-ethoxy]-6-vitro-phenylamine, 2-(2-benzylamino-
ethoxy)-4-
chloro-6-vitro-phenylamine) 4-chloro-2-vitro-6-{2-[(thiophen-2-ylmethyl)-
amino]-
ethoxy}-phenylamine, 4-chloro-2-vitro-6-{2-[(thiophen-3-ylmethyl)-amino}-
ethoxy}-
1 s phenylamine and 2-nitro-6-[ 2-(3-phenyl-propylamino)-ethoxy]-phenylamine
gave
respectively:
3b N-[2-(2-Amino-3-vitro-phenoxy)-ethyl]-2,2,2-trifluoro-N-(4-methyl-
benzyl) acetamide as a yellow solid (79 %), mp 172-173 °C; MS EI mlc
397 (M').
Elemental analysis for C1gH18F3N304;
Calc'd: C, 54.41; H, 4.57; N, 10.58
Found: C, 54.34; H, 4.33; N, 10.53
2s 3c N-[3-(2-Amino-3-vitro-phenoxy)-propyl]-N-benzyl-2,2,2-trifluoro-
acetamide as a yellow solid (67.8 %), mp 92-93 °C; MS EI mle 397 (M+).
Elemental analysis for C18H18F3N304;
Calc'd: C, 54.41; H, 4.57; N, 10.58
3o Found: C, 54.30; H, 4.50; N, 10.50
N-[2-(2-Amino-3-vitro-phenoxy)-ethyl]-2,2,2-trifluoro-N-
naphthalen-1-ylmethyl-acetamide as a yellow-orange colored solid (75.3 %), mp
133-135 °C; MS EI mle 433 (Mi).
Elemental analysis for C21H18F3N304:
Calc'd: C, 58.20; H, 4.19; N, 9.70
CA 02278747 1999-07-21
WO 98/35946 PCT/US98/00623 -
Found: C, 58.28; H, 4.07; N, 9.48
N-[2-(2-Amino-3-vitro-phenoxy)-ethyl]-N-(4-tert-butyl-benzyl)-
2,2,2-trifluoro-acetamide as a yellow solid (82.0 %), mp 80-82 °C; MS
EI mle 439
s (M+).
Elemental analysis for C21H24F3N3~4:
Calc'd: C) 57.40; H, 5.51; N, 9.56
Found: C, 57.09; H) 5.31; N, 9.40
to ~f N-[2-(2-Amino-3-vitro-phenoxy)-ethyl]-2,2,2-trifluoro-N-thiophen-
2-ylmethyl-acetamide as a yellow solid (77.4 %), mp 143-144 °C; MS EI
mle 389
(M').
Elemental analysis for C15H14F3N3~4S:
i s Calc'd: C, 46.27; H, 3.62; N, 10.79
Found: C, 46.19; H, 3.39; N, 10.64
N-[2-(2-Amino-3-vitro-phenoxy)-ethyl]-N-(4-chloro-benzyl)-2,2,2-
trifluoro-acetamide as a yellow solid (84.0 %), mp 138-139 °C; MS
(+)FAB mle
20 418/420 (M+H+)
Elemental analysis for C17H15C1F3N304:
Calc'd: C, 48.88; H, 3.62; N, 10.06
Found: C, 48.66; H, 3.47; N, 9.82
N-[2-(2-Amino-5-chloro-3-vitro-phenoxy)-ethyl]-N-benzyl-2,2,2-
trifluoro-acetamide as a yellow solid (67.9 %), mp 106-108 °C; MS
(+)FAB mle
418/420 (M+H+).
3o Elemental analysis for C17H15C1F3N304:
Calc'd: C, 48.88; H, 3.62; N, 10.06
Found: C, 48.96; H, 3.50; N, 10.03
N-[2-(2-Amino-5-chloro-3-vitro-phenoxy)-ethyl]-2,2,2-trifluoro-N-
ss thiophen-2-ylmethyl-acetamide as a yellow solid (59.6 %), mp 97-98
°C; MS EI mle
423/425 (M+).
11
CA 02278747 1999-07-21
WO 98/35946 PCT/US98/00623 -
Elemental analysis for C15H13C1F3N304S:
Calc'd: C, 42.51; H, 3.09; N, 9.92
Found: C, 42.37; H, 2.97; N, 9.84
s ~ N-[2-(2-Amino-5-chloro-3-vitro-phenoxy)-ethyl]-2,2,2-trifluoro-N-
thiophen-3-ylmethyl-acetamide as a yellow solid (80.0 %), mp 149-150
°C; MS EI
mle 423/425 (Ma).
Elemental analysis for C15H13C1F3N304S:
to Calc'd: C, 42.51; H, 3.09; N, 9.92
Found: C, 42.02; H, 2.95; N, 9.78
~c N-[2-(2-amino-3-vitro-phenoxy)-ethyl]-2,2,2-trifluoro-N-(3-phenyl-
propyl)-acetamide as a yellow solid (72.6%), mp 81-82 °C; MS EI mle 411
(M').
is
Elemental analysis for C19H20F3N3~4~
Calc'd: C, 55.47; H, 4.90; N, 10.21
Found: C, 55.57; H, 4.66; N, 10.23
2o Intermediate 4a
N-Benzyl-N-f2-12.3-diamino-~henoxvl-ethvll-2.2.2-trifluoro-acetamide
To a mixture of N-[2-(2-amino-3-vitro-phenoxy)-ethyl]-N-benzyl-2,2,2-trifluoro-
2s acetamide (,~, 2.4 g, 6.26 mmol) and 10 % palladium on carbon (0.40 g) in
ethanol (200
mL) at 50-55°C was added a solution of hydrazine hydrate (2.0 g) in
ethanol (25 mL). The
reaction was allowed to stir for 18 hr at 23°C, then the catalyst
filtered through solka floc
and the solvent removed under vacuum to afford 1.96 g (88.9 %) of an amber-
colored oil.
Crystallization from ethyl acetate - hexane gave a white solid, mp 118 -119
°C; MS
30 (+)FAB mle 354 (M+H+).
Elemental analysis for C17H18F3N302:
Calc'd: C, 56.58; H, 4.72; N, 12.38
Found: C, 57.49; H, 5.10; N, 11.86
3s
Following the above procedure and utilizing N-[2-(2-amino-3-vitro-phenoxy)-
ethyl)-2,2,2-
trifluoro-N-(4-methyl-benzyl) acetamide, N-[3-(2-amino-3-vitro-phenoxy)-
propyl]-N-
12
CA 02278747 1999-07-21
WO 98/35946 PCT/US98/00623 -
benzyl-2,2,2-trifluoro-acetamide, N-[2-(2-amino-3-nitro-phenoxy)-ethyl]-2,2,2-
trifluoro-
N-naphthalen-1-ylmethyl-acetamide, N-[2-(2-amino-3-nitro-phenoxy)-ethyl]-N-(4-
tert-
butyl-benzyl)-2,2,2-trifluoro-acetamide, N-[2-(2-amino-3-nitro-phenoxy)-ethyl]-
2,2,2-
trifluoro-N-thiophen-2-ylmethyl-acetamide, N-[2-(2-amino-3-nitro-phenoxy)-
ethyl]-N-(4-
s chloro-benzyl)-2,2,2-trifluoro-acetamide, N-[2-(2-amino-5-chloro-3-nitro-
phenoxy)-
ethyl]-N-benzyl-2,2,2-trifluoro-acetamide, N-[2-(2-amino-5-chloro-3-nitro-
phenoxy)-
ethyl]-2,2,2-trifluoro-N-thiophen-2-ylmethyl-acetamide, and N-[2-(2-amino-5-
chloro-3-
nitro-phenoxy)-ethyl]-2,2,2-trifluoro-N-thiophen-3-ylmethyl-acetamide afforded
respectively:
to
4~! N-[2-(2,3-Diamino-phenoxy)-ethyl]-2,2,2-trifluoro-N-(4-methyl-
benzyl)-acetamide as a white solid (85.0 %), mp 94-96 °C; MS EI mle 367
(M').
Elemental analysis for C 1 gH2pF3N302:
i s Calc'd: C) 58.85; H) 5.49; N, 11.44
Found: C, 58.91; H, 5.32; N, 11.45
4c N-Benzyl-N-[3-(2,3-diamino-phenoxy)-propyl]-2,2,2-trifluoro-
acetamide as a white solid (86.5 %), mp 56-58 °C; MS EI mle 367 (M').
Elemental analysis for C18H2pF3N302:
Calc'd: C, 58.85; H, 5.49; N, 11.44
Found: C, 59.00; H, 5.42; N, 11.48
2s 4~ N-[2-(2,3-Diamino-phenoxy)-ethyl]-2,2,2-trifluoro-N-naphthalen-1-
ylmethyl-acetamide as a viscous yellow oil (63.0 %); MS (+)FAB mle 404 (M+H').
Elemental analysis for C21H20F3N3~2:
Calc'd: C) 62.53; H, 5.00; N, 10.42
3o Found: C, 62.45; H, 4.98; N, 10.20
4~ N-(4-tert-Butyl-benzyl)-N-[2-(2,3-diamino-phenoxy)-ethyl]-2,2,2-
trifluoro-acetamide as a viscous brown oil (72.7 %); MS EI mle 409 (M+).
ss 4f N-[2-(2,3-Diamino-phenoxy)-ethyl]-2,2,2-trifluoro-N-thiophen-Z-
ylmethyl-acetamide as a white solid (41.0 %), mp 72-74 °C; MS (+)FAB
mle 404
(M+H+).
13
CA 02278747 1999-07-21
WO 98/35946 PCT/US98100623
Elemental analysis for C15H16F3N3~2S:
Calc'd: C, 50.13; H, 4.49; N, 11.69
Found: C, 50.09; H, 4.38; N, 11.59
s
4g N-(4-Chloro-benzyl)-N-[2-(2,3-diamino-phenoxy)-ethyl]-2,2,2-
trifluoro-acetarnide as a brown oil (80.9 %); MS EI mle 387/389 (M+).
Elemental analysis for C17H17C1F3N302:
t o Calc'd: C, 52.65; H, 4.42; N, 10.84
Found: C, 52.47; H, 4.51; N, 10.60
4h N-Benzyl-N-[2-(2,3-diamino-5-chloro-phenoxy)-ethyl]-2,2,2-
trifluoro-acetamide as a viscous brown oil (76.2 %); MS EI mle 387/389 (M+).
is
Elemental analysis for C17H17C1F3N302:
Calc'd: C, 52.65; H, 4.42; N, 10.84
Found: C, 52.47; H, 4.39; N, 10.90
20 4i N-[2-(2,3-Diamino-5-chloro-phenoxy)-ethyl]-2,2,2-trifluoro-N-
thiophen-2-ylmethyl-acetamide as a viscous brown oil (71.4 %); MS EI mle
393/395 (M+).
Elemental analysis for C15H15C1F3N302S:
2s Calc'd: C, 45.75; H, 3.84; N, 10.67
Found: C, 45.58; H, 3.93; N, 10.64
41 N-[2-(2,3-Diamino-5-chloro-phenoxy)-ethyl]-2,2,2-trifluoro-N-
thiophen-3-ylmethyl-acetamide as a viscous brown oil (75.0 %); MS EI mle
30 393/395 (M+).
Elemental analysis for C15H15C1F3N302S:
Calc'd: C, 45.75; H, 3.84; N, 10.67
Found: C, 45.39; H, 3.84; N, 10.56
Intermediate 5a
14
CA 02278747 1999-07-21
WO 98/35946 PCT/US98/00623
N-Benzyl-2,2,2-trifluoro-N-[2-(2-oxo-2,3-dihydro-1H-benzoimidazol-4
yloxy)-ethyl]-acetamide
A mixture of N-benzyl-N-[2-(2,3-diamino-phenoxy)-ethyl]-2.2,2-trifluoro-
acetamide (0.28 g, 0.804 mmol) and diimidazole carbonyl (0.326 g, 2.0 mrnol)
in
anhydrous tetrahydrofuran (10 mL) was stirred at 23° C for 2 hr. The
reaction was poured
into water and extracted with ethyl acetate (2 x 150 mL). The organic layer
dried over
anhydrous magnesium sulfate, filtered) and the solvent removed under vacuum.
to Purification by chromatography (60 g silica gel, ethyl acetate : hexane : 2
M NH3 in
methanol (15 : 5 : 1)) afforded 0.29 g (94.8%) of a colorless oil.
Crystallization from
hexane gave a white solid, mp 121-123 °C; MS EI mle 379 (M').
Elemental analysis for CI8H16F3N303;
1s Calc'd: C, 56.99; H, 4.25; N) 11.08
Found: C, 57.09; H, 4.U7; N, 11.10.
Utilizing N-[2-(2,3-diamino-phenoxy)-ethyl)-2,2,2-trifluoro-N-(4-methyl-
benzyl)-
acetamide, N-benzyl-N-[3-(2,3-diamino-phenoxy)-propyl]-2,2,2-trif7uoro-
acetamide, N-
20 [2-(2,3-diamino-phenoxy)-ethyl]-2,2,2-trifluoro-N-naphthalen-1-ylmethyl-
acetamide, N-
(4-tert-butyl-benzyl-N-[2-(2,3-diamino-phenoxy)-ethyl]-2,2,2-trifluoro-
acetamide, N-[2-
(2,3-diamino-phenoxy)-ethyl]-2,2,2-trifluoro-N-thiophen-2-ylmethyl-acetamide,
N-(4-
chloro-benzyl)-N-(2-(2,3-diamino-phenoxy)-ethyl]-2,2,2-trifluoro-acetamide, N-
benzyl-
N-[2-(2,3-diamino-5-chloro-phenoxy)-ethyl]-2,2,2-trifluoro-acetamide, N-[2-
(2,3-
2s diamino-5-chloro-phenoxy)-ethyl]-2,2,2-trifluoro-N-thiophen-2-ylmethyl-
acetamide and
N-[2-(2,3-diamino-5-chloro-phenoxy)-ethyl]-2,2,2-trifluoro-N-thiophen-3-
ylmethyl-
acetamide in the above general procedure afforded respectively:
5,~ 2,2,2-Trifluoro-N-(4-methyl-benzyl)-N-[2-(2-oxo-2,3-dihydro-1H-
3o benzoimidazol-4-yloxy)-ethyl]-acetamide ~ 0.1 ethyl acetate as a white
solid
(96.6 %)) mp 194-196 °C; MS (+)FAB mle 394 (M+H+).
Elemental analysis for C19H18F3N303 ~ 0.1 C4H802
Calc'd: C, 57.94; H, 4.71; N, 10.45
3s Found: C, 57.90; H, 4.60; N, 10.19
CA 02278747 1999-07-21
WO 98/35946 PCT/US98/00623
~c N-Benzyl-2,2,2-trifluoro-N-[3-(2-oxo-2,3-dihydro-1H-
benzoimidazol-4-yloxy)-propyl]-acetamide as a white solid (86.0 %), mp 114-116
°C; MS (+)FAB mle 394 (M+H+).
Elemental analysis for C19H18F3N3U3
Calc'd: C, 58.01; H, 4.61; N, 10.68
Found: C, 57.67; H, 4.37; N, 10.49
5~ 2,2,2-Trifluoro-N-naphthalen-1-ylmethyl-N-[2-(2-oxo-2,3-dihydro-
to 1 H-benzoimidazol-4-yloxy)-ethyl]-acetamide as a white solid (90.0 %), mp
88-90
°C; MS EI mle 429 (M+).
Elemental analysis for C22H18F3N303
Calc'd: C, 61.54; H, 4.23; N, 9.79
is Found: C, 61.34; H, 4.25; N, 9.52
Se N-(4-tert-Butyl-benzyl)-2,2,2-trifluoro-N-[2-(2-oxo-2,3-dihydro-
1H-benzoimidazol-4-yloxy)-ethyl]-acetamide as a white solid (84.9 %), mp 184-
185 °C; MS EI mle 435 (M+).
Elemental analysis for C22H24F3N3~3
Calc'd: C, 60.68; H, 5.55; N, 9.65
Found: C, 60.59; H, 5.55; N, 9.66
2s Sf 2,2,2-Trifluoro-N-[2-(2-oxo-2,3-dihydro-1H-benzoimidazol-4-
yloxy)-ethyl]-N-thiophen-2-ylmethyl-acetamide as a white solid (73.3 %), mp
49-50 °C; MS EI mle 385 (M+).
5g N-(4-Chloro-benzyl)-2,2,2-trifluoro-N-[2-(2-oxo-2,3-dihydro-1H-
so benzoimidazol-4-yloxy)-ethyl]-acetamide as a white solid (56.7 %), mp 190-
192
°C; MS (+)FAB mle 414/416 (M+H+).
Elemental analysis for C18H15C1F3N303
Calc'd: C, 52.25; H, 3.65; N, 10.16
ss Found: C, 52.28; H, 3.55; N, 10.20
16
CA 02278747 1999-07-21
WO 98/35946 PCTNS98/00623 -
~h_ N-Benzyl-N-[2-(6-chloro-2-oxo-2,3-dihydro-1H-benzoimidazol-4-
yloxy)-ethyl]-2,2,2-trifluoro-acetamide as a white solid (60.0 %), mp 171-173
°C;
MS (+)APCI mle 414.2/416.2 (M+H+).
s Elemental analysis for C18H15C1F3N303
Calc'd: C, 52.25; H, 3.65; N, 10.16
Found: C, 52.10; H, 3.56; N, 9.96
N-[2~(6-Chloro-2-oxo-2,3-dihydro-1H-benzoimidazol-4-yloxy)-
lo ethyl]- 2,2,2-trifluoro-N-thiophen-2-ylmethyl-acetamide as a white solid
(70.1
%), mp 153-154 °C; MS EI mle 419/421 (M+).
Elemental analysis for C16H13C1F3N303S:
Calc'd: C, 45.78; H, 3.12; N, 10.01
is Found: C, 45.85; H) 3.02; N) 9.73
5~' N-[2-(6-Chloro-2-oxo-2,3-dihydro-1 f1-benzoimidazol-4-yloxy)-
ethyl]- 2,2,2-trifluoro-N-thiophen-3-ylmethyl-acetamide as a white solid (77.8
%), mp 152-153 °C; MS EI mle 419/421 (M+).
Elemental analysis for C16H13C1F3N303S:
Calc'd: C, 45.78; H, 3.12; N, 10.01
Found: C, 45.86; H, 2.93; N, 9.76
Intermediate 6
N-[2-(2-{2,2,2-Trifluoroacetamidyl}-3-nitro-phenoxy)-ethyl]-N-benzyl
2,2,2-trifluoro-acetamide
To a suspension of N-[2-(2-amino-3-nitro-phenoxy)-ethyl]-N-benzyl-2,2,2-
trifluoro-acetamide (4.95 g, 12.9 mmol) in anhydrous methylene chloride (50
mL) at room
temperature was added trifluoroacetic anhydride (3.18 g, 15.1 mmol). After 15
min the
reaction was diluted with ether and washed with saturated sodium bicarbonate
(3x 80 mL)
3s and the organic layer dried over anhydrous magnesium sulfate. Upon
filtration and
evaporation of the solvent gave 5.84 g (94.4%) of yellowish white solid, mp
114-115 °C;
MS FAB mle 480 (M+H+).
17
CA 02278747 1999-07-21
WO 98/35946 PCT/US98/00623
Elemental analysis for C19H15F6N3~5
Calc'd: C, 47.61; H, 3.15; N, 8.77
Found: C, 47.35; H, 2.94; N, 8.69
s
Intermediate 7
N-[2-(1-Methyl-2-{2,2,2-Trifluoroacetamidyl}-3-nitro-phenoxy)-ethyl]-N-
lo benzyt-2,2,2-trifluoro-acetamide
A suspension of potassium carbonate (1.44g, 10.4 mmoll. h-[2-(2-{2,2,2-
trifluoroacetanudyl}-3-nitro-phenoxy)-ethyl]-N-benzyl-2,2,2-trifluoro-
aretamide ( 1.0 g)
2.09 mmol) and methyl iodide (2.96 g, 20.9 mmol, previously fihered through
basic
t s alumina) in anhydrous dimethylsulfoxide ( 11 mL) was allowed to stir at
room temperature
for 24 h. The reaction mixture was poured into methylene chloride (2()n mL)
and extracted
with water (2x 80 mL). The organic layer was dried over anhydrous magnesium
sulfate)
filtered, and the solvent removed under vacuum to afford a yellow thick oil.
Purification
by chromatography (30% ethyl acetate-hexanes) afforded 960 mg (93.3 %) of a
light
2o yellow solid, mp 90-92.5 °C; MS mle EI 493 (M+).
Elemental analysis for C2pH17F6N305
Calc'd: C, 48.70; H, 3.47; N, 8.57
Found: C, 48.50; H, 3.27; N, 8.39
Intermediate 8
N-Benzyl-2-(2-methylamino-3-nitro-phenoxy)-ethylamine
A suspension of potassium carbonate (2.52 g, 18.2 mmol) and N-[2-1-methyl-(2-
{ 2,2,2-trifluoroacetamidyl } -3-nitro-phenoxy)-ethyl]-N-benzyl-2,2,2-
trifluoro-acetamide
(900 mg, 1.82 mmol) in methanol-water (50 mL:3 mL) was heated to reflux for 2
h then
the solvent was evaporated and the residue dissolved in methylene chloride
(100 mL) and
3s extracted with water (80 mL). The organic layer was dried over anhydrous
magnesium
sulfate, filtered, and the solvent removed under vacuum. The residue was
further purified
18
CA 02278747 1999-07-21
WO 98/35946 PCT/US98/00623 -
by passing through a short pad of silica to afford 505 mg (92.1 %) of N-benzyl-
2-
(methylamino-3-vitro-phenoxy)ethylamine as a red oil; MS FAB mle 302 (M+H+).
Intermediate 9
s
N-Benzyl-[2-(2- methylamino-3-vitro-phenoxy)-ethyl]-carbamic acid tert
butyl ester
A solution of N-benzyl-2-(2-methylamino-3-nitro-phenoxy)-ethylamine
to (480 mg, 1.59 mmol) and di-tert-butyl Bicarbonate (348 mg, 1.59 mmol) in
anhydrous
tetrahydrofuran (6 mL) was allowed to stir for 3 hr. The reaction mixture was
poured into
methylene chloride (80 mL) and washed with water (50 mL). The organic layer
dried over
anhydrous magnesium sulfate, filtered, and the solvent evaporated to afford
593 mg (93 %)
of an orange solid, mp 91-93 °C; MS mle EI 401 (M').
is
Elemental analysis for C21H27N30$
Calc'd: C, 62.83; H, 6.78; N) 10.47
Found: C) 62.78; H) 6.53; N, 10.51
Intermediate 10
2s
N-Benzyl-[2-(2-methylamino-3-amino-phenoxy)-ethyl]-carbamic acid tert
butyl ester
To a mixture of N-benzyl-[2-(2-methylamino-3-vitro-phenoxy)-ethyl)-carbamic
acid tent-butyl ester (520 mg, 1.30 mmol) and ~ 10 % palladium on carbon ( 120
mg) in
ethanol (40 mL) at 50 °C was added a solution of hydrazine hydrate (
1.3 g) in ethanol ( 10
mL). The reaction was allowed to stir for 3 hr then the catalyst filtered
through celite and
so the solvent removed. Purification by chromatography (30 % ethyl acetate-
hexane) afforded
380 mg (78.9 %) of a clear oil; MS EI mle 371 (M'); IR (film) 3400, 3350, 1680
cm-1.
I9
CA 02278747 1999-07-21
WO 98/35946 PCTNS98100623 -
Intermediate 11
N-Benzyl-[2-(2-oxo-1,3-dihydro-benzoimidazol-4-yloxy)-ethyl]-carbamic
acid tert-butyl ester
s
A mixture of N-benzyl-[2-(2-methylamino-3-amino-phenoxy)-ethyl]-carbamic acid
tent-butyl ester (330 mg, 0.89 mmol) and diimidazole carbonyl (577 mg, 3.56
mmol) in
anhydrous tetrahydrofuran (30 mL) was stirred a room temperature for 0.5 h and
then
heated to reflux for 3 h. The reaction was poured into water and extracted
with ethyl
1 o acetate (2 x 150 mL). The organic layer dried over anhydrous magnesium
sulfate, filtered,
and the solvent removed. Purification by chromatography (50 % ethyl acetate-
hexane)
afforded 268 mg (75.8 %) of a foam; MS FAB mle 398 (M+H+); IR (KBr) 3420)
3250)
1690 (bs) cm-1.
is
Intermediate 12
3-(2-(3,4-Dihydro-1H-isoquinolin-2-yl)-ethoxy]-benzene-1,2-diamine
2o The general procedure used in intermediate 4 using 2-[2-(3,4-dihydro-1H-
isoquinolin-2-yl)-ethoxy]-6-nitro-phenylamine (2k} afforded 3-[2-(3,4-dihydro-
1H-
isoquinolin-2-yl)-ethoxy]-benzene-1,2-diamine as a solid (95 %), mp 76-77
°C. This
material was characterized as the dihydrochloride 0.4 H20 salt; MS EI mle 283
(M+).
2s Elemental analysis for C17H21N30 ~ 2 HCl ~ 0.4 H20:
Calc'd: C, 56.17; H, 6.6U; N, 11.56
Found: C, 56.15; H, 6.68; N, 11.25
3o Intermediate 13
4-Chloro-2-(2-chloro-ethoxy)-6-vitro-phenylamine
A solution of 2-(2-chloro-ethoxy)-6-vitro-phenylamine ( la, 30.0 g, 0.14 mol),
N-
ss chlorosuccinamide and acetonitrile ( 1.3 L) was refluxed for 4 hr. The
mixture was
concentrated under vacuum and the residue was diluted with ethyl acetate (500
mL). The
organic layer was washed with water (2X, 250 mL) and brine (250 mL), dried
over
CA 02278747 1999-07-21
WO 98/35946 PCT/US98/00623
anhydrous magnesium sulfate) filtered) and the solvent removed under vacuum to
give an
orange solid residue. Crystallization from ethyl acetate-hexane gave 33.Sg
(95.3 %) as
orange solid, mp 109-110 °C; MS EI mle 250/252/254 (M+).
s Elemental analysis for C8H8C12N203:
Calc'd: C, 38.27; H, 3.21; N, 11.16
Found: C) 38.15; H, 3.10; N, 10.96
1 o Example 1
4-(2-Benzylamino-ethoxy)-1,3-dihydro-benzoimidazol-2-one
A suspension of potassium carbonate (1.15 g, 8.34 mmol) and N-benzyl-2,2,2-
ts trifluoro-N-[2-(2-oxo-1,3-dihydro-IH-benzoimidazol-4-yloxy)-ethylj-
acetamide (0.38g,
1.00 mmol) in methanol-water (30 mL:2 mL) was heated to reflux for 2 hr then
the solvent
was evaporated and the residue dissolved in ethyl acetate ( 100 mL) and
extracted with
water (80 mL). The organic layer was dried over anhydrous magnesium sulfate)
filtered,
and the solvent removed under vacuum to give the title compound as a white
solid, mp
20 132-135°C. Without further purification, this material was dissolved
in ethyl acetate-
methanol (1:1) and treated with an excess amount of 1 N HCl in ether to afford
0.30g
(75.0%) of the hydrochloride salt as a light tan-colored solid, mp 230-233
°C: MS EI mle
283 (M+).
2s Elemental analysis for C 16H 17N302~HC1
Calc'd: C, 60.09; H, 5.67; N, 13.14
Found: C, 59.84; H, 5.59; N, 12.92
3o Example 2
4-[2-(4-Methyl-benzylamino)-ethoxy]-I,3-dihydro-benzoimidazol-2-one
Following the general procedure used in example 1 and utilizing 2,2,2-
trifluoro-N-
3s (4-methyl-benzyl)-N-[2-(2-oxo-2,3-dihydro-1H-benzoimidazol-4-yloxy)-ethyl]-
acetamide
~ 0.1 ethyl acetate (Sb) afforded the title compound as a white solid (64.5 %
), mp 162-163
21
CA 02278747 1999-07-21
WO 98/35946 PCT/US98/00623
°C; MS (+)FAB mle 298 (M+H+). Treatment of the free base with ethereal
HCI gave a
white solid (90.0 %), mp 244-246 °C: MS (+)FAB mle 298 (M+H+).
Elemental analysis for C17H19N302~1.0 HCl~1.7 H20
s Calc'd: C, 56.17; H, 6.46; N, 11.56
Found: C, 55.94; H, 6.05; N, 11.42.
Example 3
to
4(7)-(2-Benzylamino-ethoxy)-1-(3)-methyl-1,3-dihydro-benzoimidazol-2-
one
To a solution of N-benzyl-[2-(2-oxo-1,3-dihydro-benzoimidazol-4-yloxy)-ethyl ]-
t s carbamic acid tert-butyl ester in anhydrous methylene chloride (7 mL) was
added
trifluoracetic acid (3 mL). After 15 min the reaction was poured into aqueous
saturated
sodium bicarbonate ( 150 mL) and extracted with methylene chloride (2 x 150
mL). The
organic layer dried and the solvent removed to afford I70 mg (87 %) a white
solid: mp
137-138°C; MS FAB 298 (M+H+). The fumarate salt was prepared by adding
a solution
20 of the free base ( 165 mg) in warm isopropanol ( 1 S mL) to an excess of
fumaric acid in
warm isopropanol (20 mL}. Upon completion of addition crystals began forming
and the
mixture was allowed to cool to room temperature and the crystals filtered to
afford 203 mg
of fumarate salt, mp 201.5-202.5 °C; MS ESI mle 298 (M+H').
2s Elemental analysis for C17HI9N302~C~04
Calc'd: C, 61.01; H, 5.61; N, 10.16
Found: C, 60.73; H, 5.36; N, 9.95
so Example 4
4-(3-Benzylamino-propoxy)-1,3-dihydro-benzoimidazol-2-one
Following the general procedure used in example 1 and using N-benzyl-2,2,2-
s5 trifluoro-N-[3-(2-oxo-2,3-dihydro- 1H-benzoimidazol-4-yloxy)-propyl]-
acetamide (Sc)
afforded the title compound as a light yellow solid foam (90.4 %}; MS EI mle
297 (M+).
Treatment of the free base with ethereal HCI gave the hydrochloride salt as a
white solid
(63.9 %), mp 243-244 °C: MS EI mle 297 (M+).
22
CA 02278747 1999-07-21
WO 98/35946 PCT/US98/00623 -
Elemental analysis for C17H19N302 ~ HCI:
Calc'd: C, 61.17; H, 6.04; N, 12.59
Found: C, 60.92; H, 5.95; N, 12.41
s
Example 5
4-{Z-[(Naphthalen-1-ylmethyl)-amino]-ethoxy}-1,3-dihydro
benzoimidazol-2-one
to
Following the general procedure used in example 1 and using 2,2,2-trifluoro-N-
naphthalen-1-ylmethyl-N-[2-(2-oxo-2,3-dihydro-1H-benzoimidazol-4-yloxy)-ethyl]-
acetamide (Sd) gave the title compound as a white solid (67.4 %); MS EI mle
333 (M+).
is Elemental analysis for C2pH1gN302:
Calc'd: C, 72.05; H, 5.74; N) 12.60
Found: C, 71.72; H, 5.76; N, 12.22
Treatment of the free base with ethereal HCl gave the quarter hydrate of the
HCl as a white
2o solid (63.9 %), mp 223-225 °C: MS EI mle 333 (M+).
Elemental analysis for C 17H 19N302 ~ HCl ~ quarter hydrate:
Calc'd: C, 64.17; H, 5.52; N, 11.23
Found: C, 64.33; H, 5.42; N) 11.28
Example 6
4-[2-(4-tent-Butyl-benzylamino)-ethoxy]-1,3-dihydro-benzoimidazo!-2-one
Following the general procedure used in example 1 and using N-(4-tert-butyi-
benzyl)-2,2,2-trifluoro-N-[2-(2-oxo-2,3-dihydro-1H-benzoimidazol-4-yloxy)-
ethyl]-
acetamide (35g) gave the title as a white solid (84.5 %); MS EI mle 339 (M+).
3s Elemental analysis for C2pH25N3~2:
Calc'd: C, 70.77; H, 7.42; N, 12.38
Found: C, 70.59; H, 7.44; N, 12.28
23
CA 02278747 1999-07-21
WO 98/35946 PCT/US98/00623 -
Treatment of the title compound with ethereal HCI gave the hemihydrated HCl
salt as a
white solid, mp 224-226 °C: MS EI mle 339 (M+).
Elemental analysis for C2pH25N3~2 ' HCl ~ hemihydrate:
s Calc'd: C, 62.41; H, 7.07; N, 10.92
Found: C, 62.64; H, 6.93; N, 10.88
Example 7
to
4-{2-[(Thiophen-2-ylmethyl)-amino]-ethoxy}-1,3-dihydro-benzoimidazol
2-one
Following the general procedure used in example 1 and using 2,2,2-trifluoro-N-
[2-
ts (2-oxo-2,3-dihydro-1H-benzoimidazol-4-yloxy)-ethyl)-N-thiophen-2-ylmethyl-
acetamide
SUf , the title compound is obtained as a white solid (76.8 %); MS EI mle 289
(M+).
Elemental analysis for C14H15N3~2S:
Calc'd: C, 56.36; H, 5.41; N, 14.08
2o Found: C, 56.42; H, 5.04; N, 14.21
Conversion of the free base to the HCl salt with ethereal gave a white solid)
mp 240-241
°C: MS EI mle 289 (M+).
2s Elemental analysis for C1qH15N3~2S ~ HCI:
Calc'd: C, 51.61; H, 4.95; N, 12.90
Found: C, 51.22; H, 4.82; N, 12.70
Example 8
4-[2-(4-Chloro-benzylamino)-ethoxy]-1,3-dihydro-benzoimidazol-2-one
Following the general procedure used in example 1 and using N-(4-chloro-
benzyl}-
2,2,2-trifluoro-N-[2-(2-oxo-2,3-dihydro-1 H-benzoimidazol-4-yloxy)-ethyl]-
acetamide
3s (~}, the title compound is obtained as a white solid (77.6 %), mp 163-164
°C; MS
(+)FAB mle 318/320 (M+H+).
Elemental analysis for C16H16C1N302:
24
CA 02278747 1999-07-21
WO 98/35946 PCT/US98/00623 -
Calc'd: C, 60.48; H, 5.08; N, 13.22
Found: C, 60.17; H, 4.83; N, 13.20
Treatment of the free base with ethereal HCl yields the hydrochloride as a
white solid, mp
s >250 °C: MS EI mle 317/319 (M+).
Elemental analysis for C16H16C1N302 ~ HCI:
Calc'd: C, 54.25; H, 4.84; N) 11.86
Found: C, 54.18; H, 4.76; N, 11.87
to
Example 9
4-(2-Benzylamino-ethoxy)-6-chloro-1,3-dihydro-benzoimidazol-2-one
is
Following the general procedure used in example 1 and using N-(4-chloro-
benzyl)-
2,2,2-trifluoro-N-[2-(6-chloro-2-oxo-2,3-dihydro-1 H-benzoimidazol-4-yloxy)-
ethyl]-
acetamide {5h) afforded the title compound as a white solid (77.6 %), mp 192-
193 °C; MS
EI mle 317/319 (M+).
Elemental analysis for C16H16C1N302:
Calc'd: C, 60.48; H, 5.08; N, 13.22
Found: C, 60.24; H, 5.01; N, 13.09
2s Treatment of the free base with ethereal HCI gave the hydrochloride salt as
a white solid,
mp >250 °C: MS EI mle 317/319 (M+).
Elemental analysis for C16H16C1N302 ~ 1HC1:
Calc'd: C, 54.25; H, 4.84; N, 11.86
so Found: C, 54.23; H, 4.85; N, 11.69
CA 02278747 1999-07-21
WO 98/35946 PCT/US98/006Z3 -
Example 10
6-Chloro-4-{2-[(thiophen-2-ylmethyl)-amino]-ethoxy~-1,3-dihydro
benzoimidazol-2-one
s
to
Following the general procedure used in example 1 and using N-[2-(6-chloro-2-
oxo-2,3-dihydro-1H-benzoimidazol-4-yloxy)-ethyl]-2,2,2-trifluoro-N-thiophen-2-
yimethyl-acetamide ~) afforded the title compound as a white solid (89.0 %),
mp 179-180
°C; MS EI mle 323/325 (M+).
Elemental analysis for C14H14C1N302S:
Calc'd: C, 51.93; H, 4.36; N, 12.98
Found: C, 51.80; H, 4.23; N, 12.96
i s Treatment of the title compound with ethereal HCI gave the hydrochloride
as a white
solid(90.0 %), mp >250 °C: 1VIS EI mle 323/325 (M+).
Elemental analysis for C14H14C1N302S ~ HCI:
Calc'd: C, 46.68; H, 4.20; N, 11.66
2o Found: C, 46.52; H, 4.00; N, 11.57
Example 11
6-Chloro-4-{2-[(thiophen-3-ylmethyl)-amino]-ethoxy}-1,3-dihydro-
2s benzoimidazol-2-one
Following the general procedure used in. example 1 and using N-[2-(6-chloro-2-
oxo-2,3-dihydro-1H-benzoimidazol-4-yloxy)-ethyl]-2,2,2-trifluoro-N-thiophen-3-
ylinethyl-acetamide (~), the title compound is obtained as a white solid (89.0
%), mp 182-
30 183 °C; MS (+)FAB mle 324/326 (M+H+).
Elemental analysis for C14H14C1N302S:
Calc'd: C, 51.93; H, 4.36; N, 12.98
Found: C, 51.96; H, 4.30; N, 12.95
3s
'The title compound was treated with ethereal HC1 to obtain the hydrochloride
salt as a white
solid (90.0% ), mp, >250 °C: MS EI mle 323/325 (M+).
26
CA 02278747 1999-07-21
WO 98/35946 PCT/US98/00623 -
Elemental analysis~for C14H14C1N302S ~ HCI:
Calc'd: C, 46.68; H, 4.20; N) 11.66
Found: C, 46.29; H, 4.09; N, 11.51
Example 12
4-(2-(2,3-Dihydro-1H-isoquinolin-2y1)-ethoxy]-1,3-dihydro-
to benzoimidazol-2-one
Following the general procedure used in example 1 and using 2-[2-(3,4-dihydro-
1 H-isoquinolin-2-yl)-ethoxy)-6-vitro-phenylamine (~ afforded the title
compound as a
white solid (63.0 %)) mp 173-174 °C; MS EI mle 309 (M+).
is
Elemental analysis for C 18H 19N302:
Calc'd: C, 69.88; H, 6.19; N, 13.58
Found: C) 69.48; H, 6.01; N, 13.55
2o Treatment of the free base with ethereal HCl gave a quarter hydrate of the
hydrochloride
salt as a white solid (90.0% ), mp >250 °C: MS EI mle 323/325 (M+).
Elemental analysis for C18H19N3O2 ~ HCl ~ 0.25 H20:
Calc'd: C, 61.71; H, 5.90; N, 11.99
2s Found: C, 61.90; H, 5.88; N, 11.97
Example 13
4-[2-(3-Phenyl-propylamine)-ethoxy]-1,3-dihydro-benzoimidazol-2-one
so Following the general procedures used in intermediates 4 and 5 and example
1, N-
[2-(2-amino-3-vitro-phenoxy)-ethyl]-2,2,2-trifluoro-N-(3-phenyl-propyl)-
acetamide (3k)
afforded the title comound as a white solid; MS (+)FAB mle 312 (M+H+).
Elemental analysis for C1gH21N3O2~O.S HZO:
ss Calc'd: C, 67.48; H, 6.92; N, 13.12
Found: C, 67.81; H, 6.76; N, 13.51
27
CA 02278747 1999-07-21
WO 98/35946 PCT/US98/00623 -
Treatment of the free base with ethereal HCl gave the hydrochloride salt as a
white solid
(90.9%), mp 243-245 °C; MS (+)FAB mle 312 (M+H+).
Elemental analysis for C18H2tN30z~HCI:
s Calc'd: C, 62.15; H, 6.38; N, 12.08
Found: C, 62.06; H, 6.21; N, 11.97
Pharmacology
A method for determining intrinsic activity at the dopamine D2 receptor was
t o recently reported [Lahti et al., Mol. Pharm., 42) 432-438, ( 1993)].
Intrinsic activity is
predicted using the ratio of the "low-affinity agonist" (LowAg) state of the
receptor and the
"high-affinity agonist" (HighAg) state of the receptor, i.e. LowAg/HighAg.
These ratios
correlate with the agonist) partial agonist, and antagonist activities of a
given compound,
which activities characterize a compounds ability to elicit an antipsychotic
effect.
1 s Affinity for the dopamine autoreceptor was established by a modification
of the
standard experimental test procedure of Seemen and Schaus) European Journal of
Pharmacology 203: 105-109, 1991, wherein homogenized rat striatal brain tissue
is
incubated with 3H-quinpiroie (Quin.) and various concentrations of test
compound, filtered
and washed and counted in a Betaplate scintillation counter.
2o High affinity for the dopamine D-2 receptor was established by the standard
experimental test procedure of Fields, et al., Brain Res., 1 ~6, 578 ( 1977)
and Yamamura et
al., eds., Neurotransmitter Receptor Binding, Raven Press, N.Y. (1978) wherein
homogenized limbic brain tissue is incubated with -~H-spiroperidol (Spiper.)
and various
concentrations of test compound, filtered and washed and shaken with
Hydrofluor
2s scintillation cocktail (National Diagnostics) and counted in a Packard 460
~ scintillation
counter.
The results of the tests with compounds representative of this invention are
given in
the immediately following table
28
CA 02278747 1999-07-21
WO 98/35946 PCTNS98/00623
Example ICSO (nM) ICgO
# D2 Quin. (nM)
D2 Spiper
1 0.51 60.6 118
2 0.29 28.5 98
3 2.92 1346 461
4 125.8 5979 47.5
0.60 38.7 64.5
6 0.81 47.8 59
7 0.51 254.6 499.2
8 0.30 99.5 331.7
9 0.48 34.6 70.6
0.47 58.0 123.4
11 0.31 67.0 216.1
12 12.0 657.5 55
13 0.30 30.0 100.(1
Pharmaceutical Composition
s Compounds of this invention may be administered neat or with a
pharmaceutical
carrier to a patient in need thereof. The pharmaceutical carrier may be solid
or liquid.
Applicable solid carriers can include one or more substances which may also
act as
flavoring agents, lubricants, solubilizers, suspending agents, fillers,
glidants, compression
aids, binders or tablet-disintegrating agents or an encapsulating material. In
powders, the
i o carrier is a finely divided solid which is in admixture with the finely
divided active
ingredient. In tablets, the active ingredient is mixed with a carrier having
the necessary
compression properties n suitable proportions and compacted in the shape and
size desired.
The powders and tablets preferably contain up to 99% of the active ingredient.
Suitable
solid carriers include, for example, calcium phosphate, magnesium stearate,
talc, sugars,
1 s lactose, dextrin, starch, gelatin, cellulose, methyl cellulose, sodium
carboxymethyl
cellulose, polyvinylpyrrolidine, low melting waxes and ion exchange resins.
Liquid carriers may be used in preparing solutions, suspensions, emulsions,
syrups
and elixirs. The active ingredient of this invention can be dissolved or
suspended in a
pharmaceutically acceptable liquid carrier such as water, an organic solvent)
a mixture of
2o both or pharmaceutically acceptable oils or fat. The liquid carrier can
contain other suitable
pharmaceutical additives such a solubilizers, emulsifiers, buffers)
preservatives,
sweeteners, flavoring agents, suspending agents, thickening agents, colors,
viscosity
29
CA 02278747 1999-07-21
WO 98/35946 PCT/US98/00623 -
regulators, stabilizers or osmo-regulators. Suitable examples of liquid
carriers for oral and
parenteral administration include water (particularly containing additives as
above, e.g.,
cellulose derivatives, preferable sodium carboxymethyl cellulose solution),
alcohols
(including monohydric alcohols and polyhydric alcohols, e.g., glycols) and
their
s derivatives, and oils (e.g., fractionated coconut oil and arachis oil). For
parenteral
administration the carrier can also be an oily ester such as ethyl oleate and
isopropyl
myristate. Sterile liquid carriers are used in sterile liquid form
compositions for parenteral
administration.
Liquid pharmaceutical compositions which are sterile solutions or suspensions
can
1 o be utilized by, for example, intramuscular, intraperitoneal or
subcutaneous injection.
Sterile solutions can also be administered intravenously. Oral administration
may be as
either a liquid or a solid dosage form.
The compounds of this invention may be administered rectally in the form of a
conventinal suppository. For administration by intranasal or intrabronchial
inhalation or
~ 5 insufflation) the compounds of this invention may be formulated into an
aqueous or
partrially aqueous solution, which can then be utilized in the form of an
aerosol. The
compounds of this invention may also be administered transdermally through the
use of a
rransdermal patch containing the active compound and a Garner that is inert to
the active
compound, is non-toxic to the skin, and allows delivery of the agent for
systemic
2o absorption into the blood stream via the skin. The carrier may take any
number of forms
such as creams and ointments, pastes, gels, and occlusive devices. The creams
and
ointments may be viscous liquid or semi-solid emulsions of either the oil in
water or water
in oil~type. Pastes comprised of absorptive powders dispersed in petroleum or
hydrophilic
petroleum containing the active ingredient may also be suitable. A variety of
occlusive
2s devices may be used to realease the active ingredient into the blood stream
such as a
semipernleable membrane covering a reservoir containing the active ingredient
with or
without a carrier, or a matrix containing the active ingredient. Other
occlusive devices are
known in the literature.
The dosage to be used in the treatment of a specific patient suffering a
dopamine
3o imbalance must be subjectively determined by the attending physician. The
variables
involved include the severity of the dysfunction, and the size, age, and
response pattern of
the patient.
Treatment will generally be initiated with small dosages less than the optimum
dose
of the compound. Thereafter the dosage is increased until the optimum effect
under the
ss circumstances is reached. Precise dosages for oral, parenteral, nasal, or
intrabronchial
administration will be determined by the administering physician based on
experience with
the individual subject treated and standerd madical principles.
CA 02278747 1999-07-21
WO 98/35946 PCT/US98/00623
Preferably the pharmaceutical composition is in unit dosage form, e.g., as
tablets or
capsules. In such form, the composition is sub-divided in unit dose containing
appropriate
quantities of the active ingredient; the unit dosage form can be packaged
compositions, for
example packed powders) vials) ampoules, prefilled syringes or sachets
containing liquids.
s The unit dosage form can be, for example, a capsule or tablet itself, or it
can be the
appropriate number of any such compositions in package form.
31