Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02281231 1999-0~8-31
-1-
Method for Producing Ele~ro- or El~ctro~esi-deposited hiim
With A Cont:oged 1 Orlmtation
1. Field of the Invention
This invention relates to a method of producing electro- or
electrolcss-deposited film with a controlled crystal orientation and, in
particular, to a method for controlling the c.,rystal orientation in order to
provide improved product properties.
2. Description of Related Art
A thin film is conventionally deposited and developed on a
substrate by various deposition method, including a wet method such as
io electro- or electroless-deposition method, .and a dry method such as
sputtering
method, PVD method, CVD method and the like. Among other things, in the
wet method, a material (ag., metal) in electrolytic solution assumes an sonic
state and is deposed on a substrate to form a thin film by electro-deposition
method or electroless-deposition method. In this instance, it is desirable to
control the crystal orientation of the deposited film to improve the product
property of the film. The crystal orientation of the deposited film is
generally
controlled by:
(1) orienting crystals of the d~-posited film into conformity with
the crystal orientation of the substrate, or
(2) subjecting the deposited film to stresses through the substrate,
(3) controlling an overvoltage applied to the olectro-deposition
environment.
The method (1) above is generally known as epitaxial method, and
requires no particular explanations. The method (2) about is usually seen
2s when stresses are added to the substrate during the deposition process due
to a
99060 (11-40,804)
- CA 02281231 1999-08-31
-2-
difference in terms of the coefficient of thermal expansion between the
substrate and the deposited film. Furtherr~nore, the method (3) above utilizes
a phenomenon wherein an easy axis of the crystals in the deposited material,
in which clectro-deposition tends to readily occur, changes depending upon
s the applied overvoltage. For example, in the case of an electro-deposited Zn
film, the crystals of the deposited Zn film are oriented into the c-aus whop
the
overvoltage is low, and into the a axis or b-axis under an increased
overvoltage.
Beside the above-mentioned methods (1), (2) and (3), there may be
io instances wherein the crystal orientation of the deposited film is
controlled by
controlling temperature of the substrate or the temperature or flow of the
electrolytic solution, etc.
However, these methods suffer from a serious problem that they
can be applied only to specific substances. Moreover, while the lauown
15 method for developing the deposited film allows the development of a
deposited film comprising crystals oriented in a thermodynamically stable
direction, it is still difficult, if not impossible, to developla deposited
film
comprising crystals which are oriented in other direction.
2o It is therefore an object of the present invention to provide a
method of producing electro- or electroless-deposited film with a controlled
crystal orientation, which can be widely applied without being limited to
specific materials or to specific cxystal orientation of the deposited film.
In general, a substance has magnetism and is classified into
25 magnetic material and non-magnetic material. The magnetic material refers
to ferromagnetic body, while the non-magnetic material refers to paramagnetic
body or diamagnetic body. Moreover, a crystal of the substance has different
magnetic susceptibility accofding to the crystal orientation. Therefore, when
99060 (11-40.804)
CA 02281231 1999-08-31
-3-
the material having different magnetic susceptibility according to the crystal
orientation is electro- or electroless-deposited on a substrate while applying
a
magnetic field, the deposited crystals of the material on the substrate are
oriented so that the direction of the crystal orientation having a higher
s magnetic susceptibility is in parallel with the direction in which the
magnetic
field is applied. Such a phenomenon is utilized in the present invention.
According to the invention, a material to be electro- or electroless-
deposited is made to have an ionic state in a conventional manner and is then
aggregated, dectro-deposited or electroless-deposited on a substrate.
1o A magnetic field is applied to the elcctro-deposition or clcctroless-
deposition
environment, i.e., an environment which surrounds the substrate and the
material in electrolytic state.
Since the crystals of the substance have magnetic anisotropy, when
the substance is electro- or electroless-deposited while being applied with a
15 magnetic field, the crystals of the deposited substance are oriented on the
substrata with the crystal orientation having a higher magnetic susceptibility
being in parallel with the direction in which the magnetic field is applied.
Therefore, by applying the magnetic field so that the crystals of the
deposited
substance on the substrate are oriented to have the desired crystal
orientation
zo according to the invention, it is possible to obtain a deposition film
having a
desired crystal orientation.
In the method according to the present invention, it is preferred that
a porous plate is arranged adjacent to the substrate so as to suppress a flow
of
an electrolytic solution which occurs during the application of the magnetic
zs field. Such a porous plate serves to improve the property of the crystal
orientation, since the flow of the electrolytic solution is suppressed by the
porous plate during the electro- or electro:less-deposition.
The material to be subjected to the electro- or electroless-deposition
99060 (11-40,804)
CA 02281231 1999-08-31
-4-
may be a paramagnetic material or diamagnetic material. Even such
materials can be formed into a thin film having a desired crystal orientation,
by adequately controlling the direction of the magnetic field. This is because
a magnetic anisotropy is inherent not only to a ferromagnetic body, but also
to
a paramagnetic body or a diamagnetic body In this instance, it is preferred
that the magnetic field has an intensity which is at least on the order of 7T,
preferably on the order of 10 T.
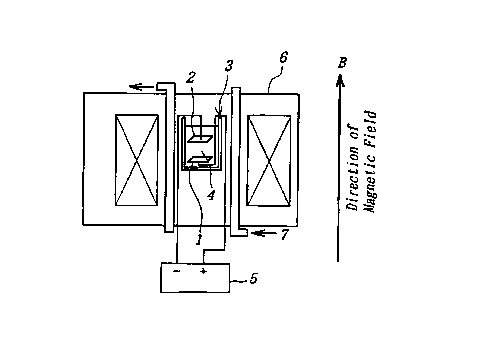
Fig. la and Fig. lb are schematic views showing a typical
1o arrangement of an clecrro-deposition apparatus which can be suitably used
for
carrying out the method according to the W vention;
Fig. 2a and Fig. 2b are explanatory views showing the principle of
controlling the orientation of metallic crystal by applying a magnetic field;
Fig. 3a is a chart showing the crystal orientation property of an
1s electro-deposited Zn film obtained while applying a magnetic field in
accordance with the invention; and
Fig. 3b is a similar chart showing the crystal orientation property of
another electro-deposited Zn film obtained. without applying a magnetic field;
Fig. 4 is a graph showing a difference in the crystal orientation
2o property of electro-deposited Zn films depending upon whether or not a
magnetic field is applied, and whether or not a flow-suppression porous plate
is arranged.
PRE ~MF.L~2
The present invention will be explained below in further detail,
2S with reference to some specific embodiments shown in the drawings.
There is shown in Fig. la and F:ig. lb a typical arrangement of an
electro-deposition apparatus which can be suitably used to carry out the
method according to the invention. As shown in these figures, a substrate 1
99060 (lI-40,804)
CA 02281231 1999-08-31
-5-
and a metal 2 for elcctro-deposition are accommodated within an electro-
deposition casing 3 which is filled with an electrolytic solution of the metal
4.
The apparatus further includes an electric power supply unit 5, a magnet 6
which surrounds the deposition casing 3. The magnet 6 is cooled and thereby
protected by a coolant 7. The magnet 6 may comprise a superconductive
magnet. As further shown in Fig. lb, a flow-suppression porous plato 8 may
be arranged adj scent to the substrate 1.
As shown in Fig. la and Fig. lb" according to the invention, the
electro-deposition of the metal 2 on the substrate 1 is carried out after the
1o metal 2 has been ionized within the casing 3 filled with the electrolytic
solution 4, by supplying an electric current from the power supply unit 6.
The clectro-deposition is typically carried while being applied with a
predetermined overvoltage and under a specific composition of the electrolytic
solution 4.
~5 According to the present invention, a magnetic field is applied in a
predetermined direction so that the electro- or eiectroless-deposition is
performed in an environment that is added with the magnetic field. In this
instance, the magnetic field may be applied in a direction which is
perpendicular to the surface of the metal 2 (Fig. 1 a), or in a direction
which is
2o parallel with the surface of the metal 2 (Fig;. 1 b).
When the electro-deposition is carried out under the application of
a magnetic field, according to the invention, the direction of the metallic
crystals can be controlled by the magnetic field, thereby allowing development
of a film on the substrate, of which the crystal orientation is aligned with
the
25 direction in which the magnetic field is applied. It is therefore possible
to
produce functional materials having excellent properties, e.g., thermoelectric
conversion efficiency, resistance to corrosion or abrasion, etc.
The intensity of the magnetic field to be applied during the electro-
99060 (11-40,804)
. CA 02281231 1999-08-31
-6-
or electroless-deposition is adjusted according to the magnetic property of
the
ioni2ed substance. For many substances, the magnetic field of not loss than
about 5 T is sufficient, though it is preferred in the case of a substance
having
especially paramagnetic properties to apply the magnetic field of not less
than
7 T, more preferably, not less than 10 T in order to control the crystal
orientation in a satisfactory manner.
The principle of the invention wall be explained blow with
reference to Fig. 2a and Fig. 2b. When a substance in an ionized state is
crystallized on a substrate, the crystal orientation of the dectro- or
electroless-
IO deposited film can be controlled by applying to the substrate a magnetic
field
in a predetermined direction. This is due to the fact that, as appreciated
from
Fig. 2a and Fig. 2b, previous to the electro- or electroless-deposition, the
crystals are rotated by anisotropy of the magnetization energy as a result of
anisotropy of magnetic susceptibility in the crystal axis. As indicated at
(1T)
in both Fig. 2a and Fig. 2b, it is possible to develop an electro-deposited
film
having a desired crystal orientation, by changing the direction in which the
magnetic field is applied.
For instance, Zn metal has a relative susceptibility x, i.e., the
magnetic susceptibility per permeability in vacuum, which is xa.b = -1.81x10'3
2o in directions along the a-axis and b-axis, and ~ _ -1.33x10-3 in the
direction
along the c-axis. The relative susceptibility x of Zu having a negative value
means that Zn is a diamagnetic substance. As used herein, the sign x
indicates the relative susceptibility, and the subscripts a, b and c indicate
the
axial direction of the crystal.
Whcn the magnetic field is applied upwards and in parallel with the
surface of the substrate, the c-axis of Zn crystals arc oriented in parallel
with
the direction of the magnetic field, i.e. parallel with the surface of the
substrate
since, as mentioned above, the values of xa and ~ are smaller than that of xC
99060 (I1-40,804)
CA 02281231 1999-08-31
Therefore, the crystals arc rotated and attached to the substrate as shown in
(II)
of Pig. 2a, so as to minimize the magnetization energy. In other words, the
crystals are oriented such that the direction of the crystal having higher
magnetic susceptibility is in parallel with the direction of the applied
magnetic
field. According to the invention, based on such principle, it is possible to
control the crystal orientation of Zn.
On the other hand, when the magnetic field is applied in parallel
with the surface of the metal 2 as shown in Fig. lb, it may become difficult
to
obtain a desired crystal orientation of the film, since the level of the
overvoltage fluctuates due to the occurrence of flow of the electrolytic
solution by Lorentz force. However, when a porous plate having a
permeability to ion is arranged adjacent to the substrate, it is possible to
advantageously suppress the flow of the electrolytic solution and to thereby
positively obtain a thin film having a desired orientation.
1s , The inventors carried out experiments wherein an ionized metal
was subjected to electro-deposition on a cropper substrate while applying a
magnetic field, and X-ray diffraction analysis was conducted with respect to
the electro-deposited film specimens thereby obtained. As a result, it has
been confirmed that the crystal orientation of the electro-deposited metal
films
2o could be controlled in advantageous manner. The following examples even
more clearly show the characteristic features of the invention.
The apparatus shown in Fig. la was used for the experiments. Zn
was ionized in the apparatus in which a copper substrate 1 and a Zn plate 2
are
2s immersed in the electrolytic solution within the casing 3. Subsequently, a
Zn
film having a thickness of about 20 pm was clectro-deposited on the substrate
1 while applying a magnetic field of 7 T in a direction which is perpendicular
to the surface of the substrate 1.
99060 (I1-40,804)
. . - CA 02281231 1999-08-31
-8-
With respect to the specimens of the electro-deposited Zn film,
their crystal orientations were analyzed by an X-ray diffractmeter. The result
of such analysis is shown in Fig. 3a. For the purpose of comparison, Fig. 3b
shows the result of analysis for comparison, obtained with respect to the
s specimens obtained by an electro-deposition carried out under the same
conditions except that the magnetic field v~~as not applied.
It can be appreciated that, when the elecixo-deposition is carried out
without applying a magnetic field, a su~ciently strong crystal orientation
property cannot be observed in the electro-deposited film, as shown in Fig.
3b.
1o On the other hand, when the electro-deposition is carried out while
applying a'
magnetic f eld according to the invention, there can be recognized a
significant
increase in the intensity of the X-ray at the (002) plane, which is the c-
plane,
and also a significant decrease in the intensity at the (110) plane, which
corresponds to the a- and b-planes, as shown in Fig. 3a. It can be thus
is understood that when an electro-deposition is carried out while applying a
magnetic field according to the invention, the crystal orientation can be
controlled such that the a- and b-planes are in parallel with the direction in
which the magnetic field is applied, and the c-plane is in parallel with the
surface of substrate.
20 Exam,
The apparatus shown in Fig. lb was used for the experiments, in
which Zn was ionized as in Example 1. Subsequently, a Zn film having a
thiclrness of about 20 p,m was electro-deposited on the substrate 1 while
applying a magnetic field of 7 T in a direction which is in parallel with the
2s surface of the substrate 1. The electro-deposition was carried out under
the
presence of a flow-suppression porous plate, and also carried out without
using the porous plate. With respect to the specimens of the electro-
deposited Zn film, their crystal aa~icntations were analyzed by an X-ray
99060 (11-40,804)
CA 02281231 1999-08-31
-9-
diffractmeter. The result of the analysis is shown in Fig. 4 which also shows
the result of comparative analysis for specimens of which the electro-
deposition was carried out under the same conditions except that the magnetic
field was not applied.
It can be appreciated that, when the electro-deposition is carried out
without a flow-suppression porous plate in the eleccro-deposition environment,
the orientation index at the (002) plane is :increased by application of the
magnetic field. This means that the c-plane orientation is enhanced with the
c-plane of the crystals oriented in parallel with the applied magnetic field.
On the other hand, when the electro-deposition is carried out with the flow-
suppression porous plate arranged in the e:lectro-deposition environment, the
orientation index of (110) plane is increased by application of the magnetic
field. This means that the a-plant and b-plant orientations arc enhanced with
the a- and b-planes of the crystals oriented in parallel with the applied
i5 magnetic field. In this way, the orientation of crystals can be effectively
controlled to the direction in which the magnetization energy is minimized due
to suppression of the flow of the electrolytic solution by the porous plate
adjacent to the substrate.
While the present invention has been described with reference to
20 some specific embodiments illustrated in the drawings, it is of course that
various changes or modifications may be made without departing from the
scope of the invention as defined by the appended claims.
For instance, when a metal having different corrosion resistance or
abrasion resistance according to its crystal direction is subjected to an
electro-
25 or electroless-deposition, it is possible to oricat the metal crystals into
a
selected plane exhibiting excellent corrosion resistance or abrasion
resistance,
or into a direction which is in parallel with the surface of the metal film on
a
substrate surface. Moreover, when the present invention is applied to a
99060(Ii-40,804)
. CA 02281231 1999-08-31
- lU-
thermoelectric material, it is possible to produce a material having a crystal
orientation that realizes an improved conversion efficiency between thermal
energy and electrical energy.
As above-mentioned, according to the invention, the crystal
orientation of an electro- or olcctroless-deposited film can be effectively
controlled to the desired direction due to the application of a magnetic field
to
a substance in its electrolytic state, for example, metal ion of various
substances. Therefore, according to the invention, it is possible not only to
selectively develop a metal place, which has an excellent resistance to
corrosion or abrasion, but also to produce a thermoelectric material having an
excellent energy conversion efficiency.
The invention can be widely applied even to non-magnetic
materials in contrast to the prior art wherein the material capable of
controlling
the crystal orientation of the electro-or electxoless-deposited film has been
~5 limited to a ferromagnetic body, such as cobalt-based alloy for magnetic
materials.
~oso (i i-ao,soa)