Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02283672 1999-09-15
0050/47873
Novel herbicidal hydroximic acid derivatives
Description
The present invention relates to novel substituted hydroximic
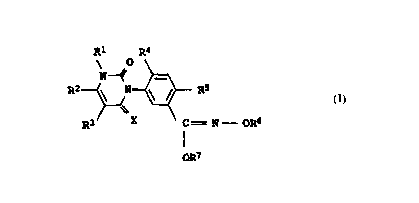
acid derivatives of the formula I
Ri Ra
\ 0
N--~
RZ \ N ~ ~ R5
\\
R3 X C = N = OR6
YR7
where X and the substituents R1 to R7 have the following meanings:
X is oxygen or sulfur;
Y is oxygen or sulfur;
R1 is hydrogen, C1-C4--alkyl, amino, C1-CQ-alkylamino or
di(C1-C4-alkyl)amino;
RZ is C1-C3-haloalkyl;
R3 is hydrogen, halogen or C1-C4-alkyl;
R4 is hydrogen or halogen;
R5 is cyano or halogen;
R6 is C1-C6-alkyl, C2-C6-alkenyl, C3-C6-alkynyl or
phenyl-C1-C6-alkyl, it being possible for the phenyl ring to
be unsubstituted or to have attached to it one to three
substituents each selected from the group consisting of
cyano, nitro, halogen, C1-C4-alkyl, C1-C6-haloalkyl,
C1-C6-alkoxy, CZ-C6-alkenyloxy, C3-C6-alkynyloxy and
(C1-C6-alkoxy)carbonyl;
CA 02283672 1999-09-15
,' 0050/47873
2
R7 is a C1-C6-alkyl, C2-C6-alkenyl, C3-C6-alkynyl or
phenyl-C1-C6-alkyl group, it being possible for these 4 groups
to be unsubstituted or to have attached to them one or two
substituents each selected from the group consisting of
halogen, C1-C3-alkoxy, C1-C3-alkoxyimino,
(C1-C3-alkoxy)carbonyl, (C1-C3-alkylamino)carbonyl,
di(C1-C3-alkyl)aminocarbonyl and CO-N(~C1-C3-alkyl)-
(C1-C3-alkoxy), it being possible for the 4 last-mentioned
radicals, in turn, to have attached to them a
(C1-C3-alkoxy)carbonyl or C1-C4-alkoxy group;
and the agriculturally useful salts of the compounds I.
Furthermore, the invention relates to
- the use of the compounds I as herbicides and/or .for the
desiccation/defoliation of plants,
- herbicidal compositions and compositions for the
desiccation/defoliation of plants which comprise compounds I
as active substances,
- processes for preparing the compounds I and herbicidal
compositions and compositions for the desiccation/defoliation
of plants using the compounds I,
- methods for controlling undesirable vegetation and for the
desiccation/defoliation of plants using the compounds I, and
- novel intermediates of the formula VII.
EP-A 408 382 disclosess herbicidally active hydroximic acid
derivatives of the formula
Rn X 1 R2
~ N-~ ~
(C1 C6-haloalkyl) ~ N ~ ~ Rp
(II)
/ OCA2-COORr
Ro O C-N
Rq
_
where Rn is hydrogen, hydroxymethyl or C1-C3-[halo]alkyl, Ro is
hydrogen, nitro, halogen, C1-C6-[halo]alkyl or hydroxymethyl, Rp
is nitro, cyano or halogen, Rq is hydrogen, C1-C3-alkyl, -alkoxy
or -alkoxy-C1-C2-alkyl and Rr is hydrogen, C1-C4-alkyl,
CZ-C3-haloalkyl, C3-C6-cycloalkyl, phenyl or benzyl.
CA 02283672 1999-09-15
0050/47873
3
- DE-A 44 24 791 discloses certain hydroximic acids of cinnamic
acid.
It is an object of the present invention to provide novel
hydroximic acid derivatives which have a good herbicidal action.
The object also extends to providing novel compounds which act as
desiccants/defoliants.
We have found that this object is achieved in accordance with the
invention by the hydroximic acid derivatives of the formula I.
Preferred compounds of the formula I can be seen from the
sub-claims and from the description which follows.
Depending on the substitution pattern, the compounds of the
formula I can contain one or more chiral centers, in which case
they exist in the form of enantiomer or diastereomer mixtures.
This invention provides both the pure enantiomers or diasteromers
and mixtures thereof.
Agriculturally useful salts are in particular the salts of those
cations and the acid addition salts of those acids whose cations
and anions, respectively, do not adversely affect the herbicidal
activity of the compounds I. Suitable cations are therefore in
particular the ions of the alkali metals, preferably sodium and
potassium, of the alkaline earth metals, preferably calcium,
magnesium and barium, and of the transition metals, preferably
manganese, copper, zinc and iron, and the ammonium ion,~which may
carry one to four C1-Cq-alkyl substituents, and/or one phenyl or
benzyl substituent, preferably diisopropylammoniurn,
tetramethylammonium, tetrabutylammonium, trimethylbenzylammonium,
and furthermore phosphonium ions, sulfonium ions, preferably
tri(C1-Cq-alkyl)sulfonium and sulfoxonium ions, preferably
tri(C1-Cq-alkyl)sulfoxonium.
Anions of useful acid addition salts are primarily chloride,
bromide, fluoride, hydrogensulfate, sulfate, dihydrogenphosphate,
hydrogenphosphate, phosphate, nitrate, hydrogencarbonate,
carbonate, hexafluorosilicate, hexafluorophosphate, benzoate, and
the anions of C1-Cq-alkanoic acids, preferably formate, acetate,
propionate and butyrate.
The organic moieties mentioned for the substituents R1 to R3, R6,
R~ or as radicals on phenyl rings are collective terms for
individual enumerations of each of the group members, as is the
meaning halogen. All carbon chains, ie. all alkyl, haloalkyl,
CA 02283672 1999-09-15
' .' 0050/47873
4
alkenyl, alkynyl, alkoxy, alkenyloxy, alkynyloxy, alkylamino and
phenylalkyl moieties can be straight-chain or branched.
Halogenated substituents preferably have attached to them one to
five identical or different halogen atoms.
Examples of individual meanings are:
- halogen: fluorine, chlorine, bromine or iodine, preferably
fluorine or chlorine;
- C1-C4-alkyl; CH3, C2H5, CHZ-CZHS, CH(CH3)Z, n-CqH9r
CH(CH3)-CZHS, CH2-CH(CH3)Z or C(CH3)3:
- C1-C6-alkyl and the alkyl moiety of C1-C6-alkoxy-C1-C6-alkyl:
methyl, ethyl, n-propyl, 1-methylethyl, n-butyl,
1-methylpropyl, 2-methylpropyl, 1,1-dimethylethyl, n-pentyl,
1-methylbutyl, 2-methylbutyl, 3-methylbutyl,
2,2-dimethylpropyl, 1-ethylpropyl, n-hexyl,
1,1-dimethylpropyl, 1,2-dimethylpropyl, 1-methylpentyl,
2-methylpentyl, 3-methylpentyl, 4-methylpentyl,
1,1-dimethylbutyl, 1,2-dimethylbutyl, 1,3-dimethylbutyl,
2,2-dimethylbutyl, 2,3-dimethylbutyl, 3,3-dimethylbutyl,
1-ethylbutyl, 2-ethylbutyl, 1,1,2-trimethylpropyl,
1,2,2-trimethylpropyl, 1-ethyl-1-methylpropyl or
1-ethyl-2-methylpropyl, preferably C1-C4-alkyl, in particular
methyl or ethyl;
_ C1-C6_haloalkyl: C1-C6-alkyl as mentioned above which is
partially or fully substituted by fluorine, chlorine, bromine
and/or iodine, eg. CHZF, CHF2, CF3, CH2C1, CH(C1)2, C(C1)3,
chlorofluoromethyl, dichlorofluoromethyl,
chlorodifluoromethyl, 1-fluoroethyl, 2-fluoroethyl,
2,2-difluoroethyl, 2,2,2-trifluoroethyl,
2-chloro-2-fluoroethyl, 2-chloro-2,2-difluoroethyl,
2,2-dichloro-2-fluoroethyl, 2,2,2-trichloroethyl, C2F5,
3-fluoropropyl, 3-chloropropyl or CF2-C2F5, preferably
C1-C4-haloalkyl, in particular trifluoromethyl or
1,2-dichloroethyl; -
- CZ-C6-alkenyl: ethenyl, prop-1-en-1-yl, prop-2-en-1-yl,
1-methylethenyl, n-buten-1-yl, n-buten-2-yl, n-buten-3-yl,
1-methylprop-1-en-1-yl, 2-methylprop-1-en-1-yl,
1-methylprop-2-en-1-yl, 2-methylprop-2-en-1-yl,
n-penten-1-yl, n-penten-2-yl, n-penten-3-yl, n-penten-4-yl,
1-methylbut-1-en-1-yl, 2-methylbut-1-en-1-yl,
CA 02283672 1999-09-15
~' 0050/47873
3-methylbut-1-en-1-yl, 1-methybut-2-en-1-yl,
2-methylbut-2-en-1-yl, 3-methylbut-2-en-1-yl,
1-methylbut-3-en-1-yl, 2-methylbut-3-en-1-yl,
3-methylbut-3-en-1-yl, 1,1-dimethylprop-2-en-1-yl,
5 1,2-dimethylprop-1-en-1-yl, 1,2-dimethylprop-2-en-1-yl,
1-ethylprop-1-en-2-yl, 1-ethylprop-2-en-1-yl,
n-hex-1-en-1-yl, n-hex-2-en-1-yl, n-hex-3-en-1-yl,
n-hex-4-en-1-yl, n-hex-5-en-1-yl, 1-methylpent-1-en-1-yl,
2-methylpent-1-en-1-yl, 3-methylpent-1-en-1-yl,
4-methylpent-1-en-1-yl, 1-methylpent-2-en-1-yl,
2-methylpent-2-en-1-yl, 3-methylpent-2-en-1-yl,
4-methylpent-2-en-1-yl, 1-methylpent-3-en-1-yl,
2-methylpent-3-en-1-yl, 3-methylpent-3-en-1-yl,
4-methylpent-3-en-1-yl, 1-methylpent-4-en-1-yl,
2-methylpent-4-en-1-yl, 3-methylpent-4-en-1-yl,
4-methylpent-4-en-1-yl, 1,1-dimethylbut-2-en-1-yl,
1,1-dimethylbut-3-en-1-yl, 1,2-dimethylbut-1-en-1-yl,
1,2-dimethylbut-2-en-1-yl, 1,2-dimethylbut-3-en-1-yl,
1,3-dimethylbut-1-en-1-yl, 1,3-dimethylbut-2-en-1-yl,
1,3-dimethylbut-3-en-1-yl, 2,2-dimethylbut-3-en-1-yl,
2,3-dimethylbut-1-en-1-yl, 2,3-dimethylbut-2-en-1-yl,
2,3-dimethylbut-3-en-1-yl, 3,3-dimethylbut-1-en-1-yl,
3,3-dimethylbut-2-en-1-yl, 1-ethylbut-1-en-1-yl,
1-ethylbut-2-en-1-yl, 1-ethylbut-3-en-1-yl,
2-ethylbut-1-en-1-yl, 2-ethylbut-2-en-1-yl,
2-ethylbut-3-en-1-yl, 1,1,2-trimethylprop-2-en-1-yl,
1-ethyl-1-methylprop-2-en-1-yl,
1-ethyl-2-methylprop-1-en-1-yl or
1-ethyl-2-methylprop-2-en-1-yl, preferably C3- or CQ-alkenyl;
- C3-C6-alkynyl: prop-1-yn-1-yl, prop-2-yn-3-yl,
n-but-1-yn-1-yl, n-but-1-yn-4-yl, n-but-2-yn-1-yl,
n-pent-1-yn-1-yl, n-pent-1-yn-3-yl, n-pent-1-yn-4-yl,
n-pent-1-yn-5-yl, n-pent-2-yn-1-yl, n-pent-2-yn-4-yl,
n-pent-2-yn-5-yl, 3-methylbut-1-yn-1-yl,
3-methylbut-1-yn-3-yl, 3-methylbut-1-yn-4-yl,
n-hex-1-yn-1-yl, n-hex-1-yn-3-yl, n-hex-1-yn-4-yl,
n-hex-1-yn-5-yl, n-hex-1-yn-6-yl, n-hex-2-yn-1-yl,
n-hex-2-yn-4-yl, n-hex-2-yn-5-yl, n-hex-2-yn-6-yl,
n-hex-3-yn-1-yl, n-hex-3-yn-2-y1, 3-methylpent-1-yn-1-yl,
3-methylpent-1-yn-3-yl, 3-methylpent-1-yn-4-yl,
3-methylpent-1-yn-5-yl, 4-methylpent-1-yn-1-yl,
4-methylpent-2-yn-4-yl or 4-methylpent-2-yn-5-yl, preferably
C3- or C4-alkynyl, in particular prop-2-yn-3-yl;
CA 02283672 1999-09-15
0050/47873
6
- phenyl-C1-C6-alkyl: for example benzyl, 1-phenyleth-1-yl,
2-phenyleth-1-yl, 1-phenylprop-1-yl, 2-phenylprop-1-yl,
3-phenylprop-1-yl, 1-phenylprop-2-yl, 2-phenylprop-2-yl,
1-phenylbut-1-yl, 2-phenylbut-1-yl, 3-phenylbut-1-yl,
4-phenylbut-1-yl, 1-phenylbut-2-yl, 2-phenylbut-2-yl,
1-phenylbut-3-yl, 2-phenylbut-3-yl,
1-phenyl-2-methylprop-3-yl, 2-phenyl-2-methylprop-3-yl,
3-phenyl-2-methylprop-3-yl or 2-benzylprop-2-yl, preferably
phenyl-C1-C4-alkyl, in particular 2-phenyleth-1-yl;
- C1-C3-alkoxy and the alkoxy moieties of (C1-C3-alkoxy)carbonyl
and CO-N(C1-C3-alkyl)-(C1-C3-alkoxy): OCH3, OC2H5, OCHz-C2H5 or
OCH(CH3)z;
- C1-C6-alkoxy and the alkoxy moiety of
C1-C6-Alkoxy-C1-C6-alkyl: methoxy, ethoxy, n-propoxy,
1-methylethoxy, n-butoxy, 1-methylpropoxy, 2-methylpropoxy,
1,1-dimethylethoxy, n-pentoxy, 1-methylbutoxy,
2-methylbutoxy, 3-methylbutoxy, 1,1-dimethylpropoxy,
1,2-dimethylpropoxy, 2,2-dimethylpropoxy, 1-ethylpropoxy,
n-hexoxy, 1-methylpentoxy, 2-methylpentoxy, 3-methylpentoxy,
4-methylpentoxy, 1,1-dimethylbutoxy, 1,2-dimethylbutoxy,
1,3-dimethylbutoxy, 2,2-dimethylbutoxy, 2,3-dimethylbutoxy,
3,3-dimethylbutoxy, 1-ethylbutoxy, 2-ethylbutoxy,
1,1,2-trimethylpropoxy, 1,2,2-trimethylpropoxy,
1-ethyl-1-methylpropoxy or 1-ethyl-2-methylpropoxy,
preferably C1-C4-alkoxy, in particular OCH3, OC2H5 or
OCH(CH3)2:
- (C1-C6-alkoxy)carbonyl: COOCH3, COOCZH5, COOCHz-C2H5,
COOCH(CH3)z, COO(n-C4Hg), COOCH(CH3)-CzHs, COOCHZ-CH(CH3)z,
COOC(CH3)3, COO(n-CSH11), 1-methylbutoxycarbonyl,
2-methylbutoxycarbonyl, 3-methylbutoxycarbonyl,
2.2-dimethylpropoxycarbonyl, 1-ethylpropoxycarbonyl,
n-hexoxycarbonyl, 1,1-dimethylpropoxycarbonyl,
1,2-dimethylpropoxycarbonyl, 1-methylpentoxycarbonyl,
2-methylpentoxycarbonyl, 3-methylpentoxycarbonyl,
4-methylpentoxycarbonyl, 1,1-dimethylbutoxycarbonyl,
1~2-dimethylbutoxycarbonyl, 1,3-dimethylbutoxycarbonyl,
2,2-dimethylbutoxycarbonyl, 2,3-dimethylbutoxycarbonyl,
3,3-dimethylbutoxycarbonyl, 1-ethylbutoxycarbonyl,
2-ethylbutoxycarbonyl, 1,1,2-trimethylpropoxycarbonyl,
1,2,~2-trimethylpropoxycarbonyl,
1-ethyl-1-methylpropoxycarbonyl or
1-ethyl-2-methylpropoxycarbonyl, preferably
CA 02283672 1999-09-15
0050/47873
7
(C1-C4-alkoxy)carbonyl, in particular COOCH3, COOCZHS or
COOCH(CH3)2~
- C2-C6-alkenyloxy: ethenyloxy, prop-1-en-1-yloxy,
prop-2-en-1-yloxy, 1-methylethenyloxy, n-buten-1-yloxy,
n-buten-2-yloxy, n-buten-3-yloxy, 1-methylprop-1-en-1-yloxy,
2-methylprop-1-en-1-yloxy, 1-methylprop-2-en-1-yloxy,
2-methylprop-2-en-1-yloxy, n-penten-1-yloxy,
n-penten-2-yloxy, n-penten-3-yloxy, n-penten-4-yloxy,
1-methylbut-1-en-1-yloxy, 2-methylbut-1-en-1-yloxy,
3-methylbut-1-en-1-yloxy, 1-methylbut-2-en-1-yloxy,
2-methylbut-2-en-1-yloxy, 3-methylbut-2-en-1-yloxy,
1-methylbut-3-en-1-yloxy, 2-methylbut-3-en-1-yloxy,
3-methylbut-3-en-1-yloxy, 1,1-dimethylprop-2-en-1-yloxy,
1,2-dimethylprop-1-en-1-yloxy, 1,2-dimethylprop-2-en-1-yloxy,
1-ethylprop-1-en-2-yloxy, 1-ethylprop-2-en-1-yloxy,
n-hex-1-en-1-yloxy, n-hex-2-
en-1-yloxy, n-hex-3-en-1-yloxy, n-Hex-4-en-1-yloxy, n-hex-5-
en-1-yloxy, 1-methylpent-1-en-1-yloxy, 2-methylpent-1-en-
1-yloxy, 3-methylpent-1-en-1-yloxy,
4-methylpent-1-en-1-yloxy, 1-methylpent-2-en-1-yloxy,
2-methylpent-2-en-1-yloxy, 3-methylpent-2-en-1-yloxy,
4-methylpent-2-en-1-yloxy, 1-methylpent-3-en-1-yloxy,
2-methylpent-3-en-1-yloxy, 3-methylpent-3-en-1-yloxy,
4-methylpent-3-en-1-yloxy, 1-methylpent-4-en-1-yloxy,
2-methylpent-4-en-1-yloxy, 3-methylpent-4-en-1-yloxy,
4-methylpent-4-en-1-yloxy, 1,1-dimethylbut-2-en-1-yloxy,
1,1-dimethylbut-3-en-1-yloxy, 1,2-dimethylbut-1-en-1-yloxy,
1,2-dimethylbut-2-en-1-yloxy, 1,2-dimethylbut-3-en-1-yloxy,
1,3-dimethylbut-1-en-1-yloxy, 1,3-dimethylbut-2-en-1-yloxy,
1,3-dimethylbut-3-en-1-yloxy, 2,2-dimethylbut-3-en-1-yloxy,
2,3-dimethylbut-1-en-1-yloxy, 2,3-dimethylbut-2-en-1-yloxy,
2,3-dimethylbut-3-en-1-yloxy, 3,3-dimethylbut-1-en-1-yloxy,
3,3-dimethylbut-2-en-1-yloxy, 1-ethylbut-1-en-1-yloxy,
1-ethylbut-2-en-1-yloxy, 1-ethylbut-3-en-1-yloxy,
2-ethylbut-1-en-1-yloxy, 2-ethylbut-2-
en-1-yloxy, 2-ethylbut-3-en-1-yloxy, 1,1,2-trimethylprop-2-
en-1-yloxy, 1-ethyl-1-methylprop-2-en-1-yloxy, 1-ethyl-2-
methylprop-1-en-1-yloxy or 1-ethyl-2-methylprop-2-en-1-
yloxy, in particular prop=2-en-1-yloxy;
C3-C6-alkynyloxy: prop-1-yn-1-yloxy, prop-2-yn-1-yloxy,
n-but-1-yn-1-yloxy, n-but-1-yn-3-yloxy, n-but-1-yn-4-yloxy,
n-but-2-yn-1-yloxy, n-pent-1-yn-1-yloxy, n-pent-1-yn-3-yloxy,
n-pent-1-yn-4-yloxy, n-pent-1-yn-5-yloxy,
n-pent-2-yn-1-yloxy, n-pent-2-yn-4-yloxy,
n-pent-2-yn-5-yloxy, 3-methyl-but-1-yn-3-yloxy,
CA 02283672 1999-09-15
0050/47873
8
3-methylbut-1-yn-4-yloxy, n-hex-1-yn-1-yloxy,
n-hex-1-yn-3-yloxy, n-hex-1-yn-4-yloxy, n-hex-1-yn-5-yloxy,
n-hex-1-yn-6-yloxy, n-hex-2-yn-1-yloxy, n-hex-2-yn-4-yloxy,
n-hex-2-yn-5-yloxy, n-hex-2-yn-6-yloxy, n-hex-3-yn-1-yloxy,
n-hex-3-yn-2-yloxy, 3-mathylpent-1-yn-1-yloxy,
3-methylpent-1-yn-3-yloxy, 3-methylpent-1-yn-4-yloxy,
3-methylpent-1-yn-5-yloxy, 4-methylpent-1-yn-1-yloxy,
4-methylpent-2-yn-4-yloxy or 4-methylpent-2-yn-5-yloxy, in
particular prop-2-yn-1-yloxy;
- C1-C4-alkylamino: NH-CH3, NH-C2H5, NH-CH2-C2H5, NH-CH(CH3)2.
NH-(n-CQHg), NH-CH(CH3)-C2H5, NH-CH2-CH(CH3)2 or NH-C(CH3)3:
- (C1-C3-alkylamino)carbonyl: CO-NH-CH3, CO-NH-C2H5,
CO-NH-CH2-C2H5 or CO-NH-CH(CH3)2, in particular CO-NH-CH3 or
CO-NH-C2H5;
- di(C1-C3-alkyl)amino: N(CH3)2, N(C2H5)2, N(n-C3H7)2,
N[CH(CH3)2]2. N(n-C4Hg)2, N[CH(CH3)-C2H5]2, N[CH2-CH(CH3)2]2.
N[C(CH3)3]2, N(CH3)-C2H5, N(CH3)-CH2-C2H5, N(CH3)-CH(CH3)2,
N(CH3)-(n-C4Hg), N(CH3)-CH(CH3)-C2H5, N(CH3)-CH2-CH(CH3)2,
N(CH3)-C(CH3)3i N(C2H5)-CH2-C2H5i N(C2H5)-CH(CH3)2r
N(C2H5)-(n-C4Hg). N(C2H5)-CH(CH3)-C2H5, N(C2H5)-CH2-CH(CH3)2,
N(C2H5)-C(CH3)3. N(n-C3H7)-CH(CH3)2. N(N-C3H7)-(n-C4Hg).
N(n-C3H7)-CH(CH3)-2H5, N(n-C3H7)-CH2-CH(CH3)2,
N(n-C3H7)-C(CH3)3, N[CH(CH3)21-(n-CaHg).
N[CH(CH3)2]-CH(CH3)-C2H5, N[CH(CH3)2-CH2-CH(CH3)2.
N[CH(CH3)2]-C(CH3)3, N(n-C4Hg)-CH(CH3)-C2H5,
N(n-C4Hg)-CH2-CH(CH3)2, N(n-C4Hg)-C(CH3)3,
N[CH(CH3)-C2H5]-CH2-CH(CHg)2, N[C(CH3)3]-CH(CH3)-C2H5 or
N[C(CH3)3]-CH2-CH(CH3)2, in particular N(CH3)2 or N(C2H5)2:
di(C1-C3-alkyl)aminocarbonyl: CO-N(CH3)2, CO-N(C2H5)2.
CO-N(n-CgH~)2, CO-N[CH(CH3)z12. CO-N(CH3)-C2H5,
CO-N(CH3)-CH2-C2H5, CO-N(CH3)-CH(CH3)2, CO- N(C2H5)-CH2-C2H5.
CO-N(C2H5)-CH(CH3)2 or CO-N(n-C3H~)-CH(CH3)2, in particular
CO-N(CH3)2 or CO-N(C2H5)2:
- C1-C3-alkoxyimino: methoxyimino, ethoxyimino, n-propyloxyimino
or i-propyloxyimino. -
Preferred compounds I are those where the substituents have the
following meanings:
X is oxygen;
CA 02283672 1999-09-15
v 0050/47873
R1 is methyl or amino;
9
R2 is trifluoromethyl or trifluoroethyl;
R3 is hydrogen;
R4 is halogen, in particular fluorine;
R5 is cyano or chlorine;
R6 is C1-C6-alkyl, C3-C6-alkenyl, C3-C6-alkynyl or
phenyl-C1-C6-alkyl, it being possible for the phenyl ring to
have attached to it one to three substituents each selected
from the group consisting of cyano, vitro, halogen,
C1-C4-alkyl, C1-C4-haloalkyl, C1-C4-alkoxy, C3-C5-alkenyloxy .
and C3-CS-alkynyloxy;
R6 is, in particular, C1-C4-alkyl or phenyl-C1-C4-alkyl, it
being possible for the phenyl ring to have attached to it one
to three substituents each selected from the group consisting
of cyano, vitro, halogen, C1-C4-alkyl, C1-C4-haloalkyl,
C1-C4-alkoxy, C3-CS-alkenyloxy and C3-CS-alkynyloxy;
R~ is C1-C4-alkyl having one or two substituents each selected
from the group consisting of C1-C3-alkoxyimino,
(C1-C3-alkoxy)carbonyl, (C1-C3-alkylamino)carbonyl,
di(C1-C3-alkyl)aminocarbonyl and
CO-N(C1-C3-alkyl)-(C1-C3-alkoxy), it being additionally
Possible for the last four radicals, in turn, to have
attached to them a (C1-C3-alkoxy)carbonyl group.
Compounds of the formula I, where R1 to R5 have the meanings
mentioned in Table 1 and R6 and R7 represent in each case one row
of Tables 2 and 3, respectively, are particularly preferred.
Table 1:
No. R1 R2 ~ R3 R4 R5
1,1 CH3 CF3 H F CI
1.2 CH3 CF3 H F CN
1.3 CH3 CF3 H H C1
1.4 CH3 CF3 H H CN
1.5 NHZ CF3 H F C1
1.6 NH2 CF3 H F CN
CA 02283672 1999-09-15
0050/47873
No . R1 R2 R3 R4 R5
1.7 NH2 CF3 ~I~H ~~ H C1
1.8 NHz CF3 H H CN
5
Table 2:
No R6
.
10 2 CH3
1
2.2 C2H5
2.3 CH(CH3)CH3
2.4 CH2CH=CH2
2.5 CH2C~CH
2.6 CH(CH3)C~CH
2.7 CH2C6H5
Table 3:
No R7
.
3.1 CH3
3.2 CH2CH=CH2
3,3 CH2C~CH
3.4 CHzCOOCH3
3.5 CHZCOOC2H5
3.6 CH2COOCH2CH2CH3
3.7 CHZCOOCHyCOOCH3
3.8 CH2CON(OCH3)CH3
3.9 CH2-C6H5
3.10 CH2C(NOCH3)COOCH3
3lI CH(CH3)CH3
3.12 CH(CH3)C~CH
3.13 CH(CH3)COOCH3
3.14 CH(CH3)COOCH2CH3
3.15 CH(CH3)CON(OCH3)CH3
_
Compound 1.1. of Table 1 where R6 has the meaning of 2.1. and R7
has the meaning of 3.3. is cited hereinbelow as 1.1./2.1./3.3.
CA 02283672 1999-09-15
0050/47873
11
The hydroximic acid derivatives I according to the invention are
obtainable by various routes, preferably by one of the processes
described hereinbelow.
a) Alkylation of a hydroxamic acid derivative of the formula III:
R1 R4
\ 0
N--
R2 ~ N ~ ~ R5 (III)
\\
R3 X C- N - OR6
II
H
As a rule, the process is carried out in an inert solvent or
diluent, preferably in the presence of a base.
Examples of suitable solvents are protic solvents, such as
the lower alcohols, preferably ethanol, if desired, as a
mixture with water, or aprotic solvents such as aliphatic or
cyclic ethers, preferably 1,2-dimethoxyethane,
tetrahydrofuran and dioxane, aliphatic ketones, preferably
acetone, amides, preferably dimethylformamide, sulfoxides,
preferably dimethylsulfoxide, ureas such as tetramethylurea
and 1,3-dimethyltetrahydro-2(1H)pyrimidinone, carboxylic
esters such as ethyl acetate, or halogenated aliphatic or
aromatic hydrocarbons such as dichloromethane and
chlorobenzene.
Alkylation is normally carried out with the halide,
preferably chloride or bromide, the sulfate, a sulfonate,
preferably a methanesulfonate (mesylate) such as
trifluoromethanesulfonate (triflate) or a benzenesulfonate
such as p-toluenesulfonate (tosylate) and
p-bromobenzenesulfonate (brosylate), or with a diazo
compound, eg. diazomethane.
Suitable bases are inorganic bases, eg. carbonates, such as
potassium carbonate and sodium carbonate, hydrogen carbonates
such as potassium hydrogen carbonate and sodium hydrogen
carbonate, alkali metal hydrides such as sodium hydride and
potassium hydride, and organic bases, eg. amines, such as
triethylamine, pyridine and N,N-diethylaniline, or alkali
metal alkoxides such as sodium methoxide, sodium ethoxide and
potassium tert-butoxide.
CA 02283672 1999-09-15
0050/47873
12
It is preferred to use 0.5 to 2 times the molar amount of
both base and alkylating agent, based on the amount of III.
In general, a reaction temperature of from (-78~C) to the
boiling point of the reaction mixture, in particular from
(-60) to 60~C, is suitable.
Alkylation of the compounds of the formula III normally gives
not only substituted hydroximic acid derivatives I according
to the invention, but also the corresponding amide-nitrogen-
substituted cinnamohydroxamide derivatives. The ratio in
which the two products are formed depends on the reaction
temperature, on the alkylating agent, the base used and also
on the particular starting compound III. The desired compound
I can normally be separated from the by-products in a manner
known per se, eg. by crystallization or chromatography.
b) Alkylation of a substituted hydroximic acid IV which
corresponds to the formula I with hydrogen on place of R6:
R1 R4
\ 0
N-
R2 ~ N ~ ~ R5 (IV)
\\
R3 X ~=N-OH
YR7
As regards the reaction conditions, what has been said above
for process variant a) also applies here.
c) Reaction of a hydroximino halide of the formula V with an
alcohol or thiol derivative
Ri R4
\ O
N-
R2 ~ N- ~ - ~ RS + R~YH
\\ H
R3 X ~-N-0~ R6
(V) Hal
CA 02283672 1999-09-15
0050/47873
13
It is advantageous to use, as alcohol/thiol derivatives, the
alcohols/thiols R7YH or their salts, in particular those of
the alkali metals or alkaline earth metals.
Examples of suitable solvents or diluents are aliphatic or
cyclic ethers such as diethyl ether and tetrahydrofuran,
aliphatic ketones such as acetone, hydrocarbons such as
n-pentane, cyclohexane and petroleum ether, aromatic
hydrocarbons such as benzene and toluene, halogenated
aliphatic or aromatic hydrocarbons such as dichloromethane
and chlorobenzene, esters such as ethyl acetate, amides such
as dimethylformamide and N-methylpyrrolidone, sulfoxides such
as dimethyl sulfoxide, and mixtures of these. The alcohols
and alcohol derivatives themselves are also suitable as
solvents or diluents.
The ratio of V to alcohol/thiol or alcohol/thiol derivative
R~YH is not critical. Approximately equimolar amounts are
normally employed. However, it may also be expedient to
employ an excess of the alcohol/thiol or the alcohol/thiol
derivative, so that it acts simultaneously as solvent or
diluent.
In general, a reaction temperature of from (-78)~C to the
boiling point of the particular reaction mixture is suitable,
in particular from 0 to SO~C.
The reaction of V with an alcohol/thiol R7YH or a derivative
thereof is particularly advantageously carried out in the
presence of a base, suitable bases being not only inorganic
bases, eg. carbonates, hydrogen carbonates or alkali metal
hydrides, but also organic bases, eg. amines such as
triethylamine, pyridine and N,N-dimethylaniline, or alkali
metal alkoxides. An alkoxide/thiolate of the alcohol/thiol
R7YH is expediently used as the base.
The base can be employed in catalytic, substoichiometric or
stoichiometric amounts or in excess of up to five times the
molar amount, based on V.
The hydroximino halides of the formula V are obtainable for
example by halogenating the corresponding carbonyl compounds
of the formula III.
CA 02283672 1999-09-15
0050/47873
14
This halogenation reaction is normally carried out in an
inert solvent or diluent, suitable substances being, in
particular, aprotic, organic liquids, for example aliphatic
or aromatic hydrocarbons, such as n-hexane benzene, toluene
and o-, m- and p-xylene, halogenated aliphatic hydrocarbons
such as methylene chloride, chloroform and
1,2-dichloroethane, halogenated aromatic hydrocarbons, such
as chlorobenzene, tertiary amines such as
N,N-dimethylaniline, or nitriles such as acetonitrile.
Suitable halogenating agents are, above all, thionyl
chloride, phosphorus pentachloride, phosphorus oxychloride,
phosphorus pentabromide or phosphorus oxybromide. The use of
a mixture of phosphorus pentachloride and phosphorus
oxychloride or of phosphorus pentabromide and phosphorus
oxybromide may also be particularly advantageous, in which
case the process can be carried out without diluent in an
excess of phosphorus oxychloride or phosphorus oxybromide.
When using thionyl chloride as the halogenating agent, it is
recommended to add a catalytic amount of dimethylformamide.
A mixture of a tetrahalomethane, such as carbon tetrachloride
and carbon tetrabromide, and an unsubstituted or substituted
triphenylphosphane, eg. triphenylphosphane or
tri-(o-tolyl)phosphane has proved particularly suitable.
At least equimolar amounts of halogenating agent and starting
compound III are required for complete reaction. In general,
an excess of halogenating agent of up to approximately eight
times the molar amount, based on III, has a favorable effect
on the course of the reaction.
In general, the reaction temperature is from O~C to the
reflux temperature of the reaction mixture, preferably at
from 20 to 120~C.
d) Conversion of a nitrile of the formula VI into a compound of
the formula I in two steps:
CA 02283672 1999-09-15
0050/47873
R1 R4
\ O R7~ H2~ I
N-
RZ ~ N ~ ~ RS
5
R3 X CN
(VI)
The reaction is normally carried out in two steps by first
10 reacting the nitrile VI with an alcohol/thiol R7YH and then
reacting the resulting imido ester with an hydroxylamine
H2N-OR6, if desired without isolating the imido ester from the
reaction mixture.
VI can be reacted with R7YH in an inert solvent or diluent or
in the absence of a solvent in an excess of the
alcohol/thiol. An acidic or "Lewis"-acidic catalyst is
frequently beneficial, preferably in an approximately
catalytic amount or in an amount of up to approximately 200
mold, based on the amount of VI.
Particularly suitable inert solvents or diluents are organic
solvents, eg. aliphatic or cyclic ethers such as diethyl
ether, tetrahydrofuran and dimethoxyethane, aliphatic, cyclic
or aromatic hydrocarbons such as n-pentane, petroleum ether,
cyclohexane, toluene and the xylenes, amides such as.
dimethylformamide and N-methylpyrrolidone, halogenated
hydrocarbons such as dichloromethane, chlorobenzene and
1,2-dichloromethane, or mixtures of these.
Suitable acidic catalysts are inorganic, preferably
anhydrous, acids, eg. hydrogen chloride, hydrogen bromide,
nitric acid, sulfuric acid, also oleum, or perchloric acid,
and organic acids such as acetic acid, propionic acid,
p-toluenesulfonic acid and trifluoroacetic acid. Examples of
"Lewis"-acidic catalysts are titanium tetrachloride, tin(II)
chloride, iron(III) chloride, aluminum trichloride,
ethylaluminum dichloride, titanium tetraisopropoxide and
boron trifluoroetherate. -
The amount of R~YH is not critical. Normally, 1 to 10 mol of
alcohol/thio per mole of VI suffice for an optimum reaction
of VI. If the process is carried out in the alcohol/thiol in
question in the absence of a solvent, this alcohol may also
be present in a larger excess.
CA 02283672 1999-09-15
0050/47873
16
If the imido ester is obtained in salt form in the first
step, it is recommended to liberate the neutral compound
before carrying out the reaction of hydroxylamine H2N-OR6.
Hydroxylamines which are obtainable in the form of their
salts, in particular as hydrochlorides, hydrobromides or
sulfates, or which are obtained in salt form during the
preparation can be liberated by adding a suitable base before
they are reacted, especially suitable bases being those
mentioned for method a).
The resulting imido ester with H2N-OR6 is generally reacted in
an inert solvent or diluent. Suitable substances are also
alcohols such as methanol, ethanol and isopropanol, nitriles
such as acetonitrile, amines such as triethylamine, pyridine
and N,N-dimethylaniline, or else water besides the
abovementioned solvents.
It is expedient to react imido ester and hydroxylamine with
each other in approximately equimolar amounts. To react the
imido ester as completely as possible, however, it may be
advantageous to employ the hydroxylamine H2N-OR6 in an excess,
of up to approximately 10 mold.
For both steps, the reaction temperature is generally at from
(-20) to 120~C, in particular at from O~C to the boiling point
of the reaction mixture.
e) Conversion of an oxime of the formula VII
R4
R8- R5
\ / (VII)
C=NOR6
YR~
where R8 is nitro, amino,_isocyanato, isothiocyanato,
(C1-C6-alkyl)carbamato or phenylcarbamato
into the hydroximic acid derivatives of the formula I
according to the invention by a method described in
WO 93/06090.
CA 02283672 1999-09-15
' 0050/47873
17
The compounds of the formula VLI are novel. They themselves are
obtainable by one of the processes described above for the
preparation of compounds I.
f) Sulfurization of a hydroximic acid derivative of the formula
I where X = oxygen:
I (X=O) sulfurizati~ I (X=S)
The sulfurization is generally carried out in an inert solvent or
diluent, for example in an aromatic hydrocarbon such as toluene
or a xylene, in an ether such as diethyl ether,
1,2-dimethoxyethane and tetrahydrofuran, or in an organic amine
such as pyridine.
Particularly suitable sulfurizing agents are phosphorus (V)
sulfide and 2,4-bis(4-methoxyphenyl)-1,3,2,4-
dithiadiphosphetane-2,4-dithione ("Lawesson's Reagent").
Usually 1 to 5 times the molar amount, based on the starting
material to be sulfurized, is sufficient for virtually complete
conversion.
The reaction temperature is generally from 20 to 200°C, preferably
from 40°C to the boiling point of the reaction mixture.
The hydroxamic acid derivatives of the formula III where Y =
oxygen are accessible for example from benzoic acids of the
formula VIII:
R4
R1 0
~N~ + H2N-OR6
R2 ~ N ~ ~ R5 --~ III
R3 X COOH
VIII
The reaction is normally carried out in an inert solvent or
diluent in the presence of a condensation auxiliary or without
solvent in an excess of the condensation auxiliary.
CA 02283672 1999-09-15
' 0050/47873
18
Especially suitable solvents or diluents are organic solvents,
eg. aliphatic or cyclic ethers such as diethyl ether,
tetrahydrofuran and dimethoxyethane, aliphatic, cyclic or
aromatic hydrocarbons such as n-pentane, petroleum ether,
cyclohexane, toluene and the xylenes, alcohols such as methanol,
ethanol and i-propanol, amides such as dimethylformamide and
N-methylpyrrolidone, nitriles such as acetonitrile, amines such
as triethylamine, pyridine and N,N-dimethylaniline, halogenated
hydrocarbons such as dichloromethane, chlorobenzene and
1,2-dichloromethane, or water. Mixtures of these are also
suitable.
Suitable condensation auxiliaries are, for example, oxalyl
chloride, carbonyl diimidazole, carbodiimides such as
dicyclohexylcarbodiimide, halogenating agents such as thionyl
chloride, phoshorus oxychloride, phosgene, phosphorus trichloride
and phoshorus pentachloride, or methyl or ethyl chloroformate.
preferred is the use of an halogenating agent, first giving an
acid halide "in situ", which then further reacts with the
hydroxylamine H2N-OR6 to give the product III.
However, it is also possible to prepare specifically the acid
halide in a separate process step and, if desired, subsequently
to react it in purified form with the hydroxylamine H2N-OR6.
Hydroxylamines which are obtainable in the form of their salts,
in particular as hydrochlorides, hydrobromides or sulfates, or
which are obtained during preparation in salt form, can be
liberated - prior to their reaction with VIII, if desired also in
the reaction mixture with the condensation auxiliary and VIII -
by adding a suitable base.
gases which are especially suitable for this purpose are those
mentioned for method a).
The amounts of condensation auxiliary, VIII and hydroxylamine
HZN-OR6 are not critical. They are expediently used in
approximately equimolar amounts of the starting materials. If
desired, the condensation auxiliary may also be employed in an
excess, in which case the process may even be carried out without
inert solvent.
CA 02283672 1999-09-15
0050/47873
19
Hydroxamic acid derivatives III where Y = sulfur are
advantageously obtainable by sulfurization of the corresponding
derivatives III where Y = oxygen, similar to process f).
Here, the amount of sulfurizing agent can be 0.5 to 5 mol, based
on 1 mole of the compound III (Y=0) to be sulfurized.
All the above-described processes are expediently carried out
under atmospheric pressure or under the inherent pressure of the
reaction mixture in question.
As a rule, the reaction mixtures are worked up by methods known
per se, for example by removing the solvent, partitioning the
residue in a mixture of water and a~suitable organic solvent and
working up the organic phase to obtain the product.
The substituted hydroximic acid derivatives of the formula I
according to the invention can be obtained from the preparation
as isomer mixtures which, if desired, can be separated into the
pure isomers by the methods conventionally used for this purpose,
eg. by means of crystallization or chromatography on an optically
active adsorbate. Pure optically active isomers can, for example,
also be prepared from suitable optically active starting
materials.
Substituted hydroximic acid derivatives I with C-H acidic
substituents can be converted into their alkali metal salts in a
manner known per se by reaction with a base of the corresponding
cation.
Salts of I whose metal ion is not an alkali metal ion can
normally be prepared by double decomposition of the corresponding
alkali metal salt in aqueous solution.
Other metal salts, such as manganese, copper, zinc, iron,
calcium, magnesium and barium salts, can be prepared from the
sodium salts in the customary manner, and also ammonium and
phosphonium salts by means of ammonia, phosphonium, sulfonium or
sulfoxonium hydroxides.
The compounds I and their agriculturally useful salts are
suitable as herbicides, both in the form of isomer mixtures and
in the form of the pure isomers. The herbicidal compositions
comprising I effect very good control of vegetation on non-crop
areas, especially at high rates of application. In crops such as
CA 02283672 1999-09-15
0050/47873
wheat, rice, maize, soybeans and cotton they act against
broad-leaved weeds and grass weeds without damaging the crop
plants substantially. This effect is observed especially at low
rates of application.
5
Depending on the application method in question, the compounds I,
or compositions comprising them, can additionally be employed in
a further number of crop plants for eliminating undesirable
plants. Examples of suitable crops are the following:
10 Allium cepa, Ananas comosus, Arachis hypogaea, Asparagus
officinalis, Beta vulgaris spec. altissima, Beta vulgaris spec.
raps, Brassica napus var. napus, Brassica napus var.
napobrassica, Brassica rapa var. silvestris, Camellia sinensis,
Carthamus tinctorius, Carya illinoinensis, Citrus limon, Citrus
15 sinensis, Coffea arabica (Coffea canephora, Coffea liberica),
Cucumis sativus, Cynodon dactylon, Daucus carota, Elaeis
guineensis, Fragaria vesca, Glycine max, Gossypium hirsutum,
(Gossypium arboreum, Gossypium herbaceum, Gossypium vitifolium),
Helianthus annuus, Hevea brasiliensis, Hordeum vulgare, Humulus
20 lupulus, Ipomoea batatas, Juglans regia, Lens culinaris, Linum
usitatissimum, Lycopersicon lycopersicum, Malus spec., Manihot
esculenta, Medicago sativa, Musa spec., Nicotiana tabacum
(N.rustica), Olea europaea, Oryza sativa, Phaseolus lunatus,
Phaseolus vulgaris, Picea abies, Pinus spec., Pisum sativum,
prunus avium, Prunus persica, Pyrus communis, Ribes sylvestre,
Ricinus communis, Saccharum officinarum, Secale cereale, Solanum
tuberosum, Sorghum bicolor (s. vulgare), Theobroma cacao,
Trifolium pratense, Triticum aestivum, Triticum durum, Vicia
faba, Vitis vinifera and Zea mays.
Moreover, the compounds I may also be used in crops which have
been made fully or partially tolerant to the action of herbicides
due to breeding including genetic engineering methods.
Furthermore, the substituted hydroximic acid derivatives I are
also suitable for the desiccation and/or defoliation of plants.
As desiccants, they are especially suitable for desiccating the
aerial parts of crop plants such as potatoes, oilseed rape,
sunflowers and soybeans. This allows completely mechanical
harvesting of these important crop plants.
Also of economic interest is facilitated harvesting, which is
made possible by concentrating, over a period of time,
dehiscence, or reduced adhesion to the tree, in the case of
citrus fruit, olives or other species and varieties of pomaceous
CA 02283672 1999-09-15
0050/47873
21
fruit, stone fruit and nuts. The same mechanism, ie. promotion of
the formation of abscission tissue between fruit or leaf and
shoot of the plants, is also essential for readily controllable
defoliation of useful plants, in particular cotton.
Moreover, a shortened period of time within which the individual
cotton plants ripen results in an increased fiber quality after
harvesting.
The compounds I, or the compositions comprising them, can be
employed, for example, in the form of directly sprayable aqueous
solutions, powders, suspensions, also highly-concentrated
aqueous, oily or other suspensions or dispersions, emulsions, oil
dispersions, pastes, dusts, materials for spreading or granules,
by means of spraying, atomizing, dusting, spreading or pouring.
The use forms depend on the intended purposes; in any case, they
should guarantee the finest possible distribution of the active
ingredients according to the invention.
Suitable inert additives are essentially: mineral oil fractions
of medium to high boiling point such as kerosene and diesel oil,
furthermore coal tar oils and oils of vegetable or animal origin,
aliphatic, cyclic and aromatic hydrocarbons, eg. paraffins,
tetrahydronaphthalene, alkylated naphthalenes and their
derivatives, alkylated benzenes and their derivatives, alcohols
such as methanol, ethanol, propanol, butanol and cyclohe~anol,
ketones such as cyclohexanone or strongly polar solvents, eg.
amines such as N-methylpyrrolidone or water.
Aqueous use forms can be prepared from emulsion concentrates,
suspensions, pastes, wettable powders or water-dispersible
granules by adding water. To prepare emulsions, pastes or oil
dispersions, the substituted hydroximic acid derivatives as such
or dissolved in an oil or solvent, can be homogenized in water by
means of wetting agent, tackifier, dispersant or emulsifier.
However, it is also possible to prepare concentrates composed of
active substance, wetting agent, tackifier, dispersant or
emulsifier and, if appropriate, solvent or oil, and these
concentrates are suitable for-dilution with water.
Suitable surfactants are the alkali metal, alkaline earth metal
and ammonium salts of aromatic sulfonic acids, eg. ligno-,
phenol-, naphthalene- and dibutylnaphthalenesulfonic acid, and of
fatty acids, of alkyl- and alkylaryl sulfonates, of alkyl
sulfates, lauryl ether sulfates and fatty alcohol sulfates, and
salts of sulfated hexa-, hepta- and octadecanols, and of fatty
CA 02283672 1999-09-15
0050/47873
22
alcohol glycol ether, condensates of sulfonated naphthalene and
its derivatives with formaldehyde, condensates of naphthalene, or
of the naphthalenesulfonic acids, with phenol and formaldehyde,
polyoxyethylene octylphenyl ether, ethoxylated isooctyl-, octyl-
or nonylphenol, alkyiphenyl and tributylphenyl polyglycol ether,
alkylaryl polyether alcohols, isotridecyl alcohol, fatty
alcohol/ethylene oxide condensates, ethoxylated castor oil,
polyoxyethylene alkyl ethers or polyoxypropylene alkyl ethers,
lauryl alcohol polyglycol ether acetate, sorbitol esters,
lignin-sulfite waste liquors or methylcellulose.
Powders, materials for spreading and dusts can be prepared by
mixing or concommitantly grinding the active substances with a
solid carrier.
Granules, eg. coated granules, impregnated granules and .
homogeneous granules, can be prepared by binding the active
ingredients to solid carriers. Solid carriers are mineral earths
such as silicas, silica gels, silicates, talc, kaolin, limestone,
lime, chalk, bole, loess, clay, dolomite, diatomaceous earth,
calcium sulfate, magnesium sulfate, magnesium oxide, ground
synthetic materials, fertilizers such as ammonium sulfate,
ammonium phosphate, ammonium nitrate, ureas and products of
vegetable origin such as cereal meal, tree bark meal, wood meal
and nutshell meal, cellulose powders or other solid carriers.
The concentrations of the active ingredients I in the
ready-to-use products can be varied within wide ranges. In
general, the formulations comprise approximately from 0.001 to
98~ by weight, preferably 0.01 to 95~ by weight, of at least one
active ingredient. The active ingredients are normally employed
in a purity of from 90~ to 100, preferably 95~ to 100
(according to NMR spectrum).
The formulation examples below illustrate the preparation of such
formulations:
I. 20 parts by weight of the compound No. 4.01 are dissolved
in a mixture composed of 80 parts by weight of alkylated
benzene, 10 parts by weight of the adduct of 8 to 10 mol of
ethylene oxide and 1 mol of oleic acid N-monoethanolamide,
5 parts by weight of calcium dodecylbenzenesulfonate and
5 parts by weight of the adduct of 40 mol of ethylene oxide
and 1 mol of castor oil. Pouring the solution into
100,000 parts by weight of water and finely distributing it
therein gives an aqueous dispersion which comprises 0.02
CA 02283672 1999-09-15
0050/47873
23
by weight of the active ingredient.
II. 20 parts by weight of the compound No. 4.02. are dissolved
in a mixture composed of 40 parts by weight of
cyclohexanone, 30 parts by weight of isobutanol, 20 parts
by weight of the adduct of 7 mol of ethylene oxide and
1 mol of isooctylphenol and 10 parts by weight of the
adduct of 40 mol of ethylene oxide and 1 mol of castor oil.
Pouring the solution into 100,000 parts by weight of water
and finely distributing it therein gives an aqueous
dispersion which comprises 0.02% by weight of the active
ingredient.
III' 20 parts by weight of the active ingredient No. 4.03. are
dissolved in a mixture composed of 25 parts by weight of
cyclohexanone, 65 parts by weight of a mineral oil fraction
of boiling point 210 to 280~C and 10 parts by weight of the
adduct of 40 mol of ethylene oxide and 1 mol of castor oil.
Pouring the solution into 100,000 parts by weight of water
and finely distributing it therein gives an aqueous
dispersion which comprises 0.02% by weight of the active
ingredient.
IV~ 20 parts by weight of the active ingredient No. 4.04. are
mixed thoroughly with 3 parts by weight of sodium
diisobutylnaphthalene-a-sulfonate, 17 parts by weight of
the sodium salt of a lignosulfonic acid from a sulfite
waste liquor and 60 parts by weight of pulverulent silica
gel and the mixture is ground in a hammer mill. Finely
distributing the mixture in 20,000 parts by weight of water
gives a spray mixture which comprises 0.1% by weight of the
active ingredient.
V. 3 parts by weight of the active ingredient No. 4.08. are
mixed with 97 parts by weight of finely divided kaolin.
This gives a dust which comprises 3% by weight of the
active ingredient.
VI. 20 parts by weight of the active ingredient No. 4.09. are
mixed intimately with 2 parts by weight of calcium
dodecylbenzenesulfonate, 8 parts by weight of fatty alcohol
polyglycol ether, 2 parts by weight of the sodium salt of a
phenol/urea/formaldehyde condensate and 68 parts by weight
of a paraffinic mineral oil. This gives a stable oily
dispersion.
CA 02283672 1999-09-15
0050/47873
24
VII. 1 part by weight of the compound No. 4.10 is dissolved in a
mixture composed of 70 parts by weight of cyclohexanone, 20
parts by weight of ethoxylated isooctylphenol and 10 parts
by weight of ethoxylated castor oil. This gives a stable
emulsion concentrate.
VIII. 1 part by weight of the compound No. 4.11 is dissolved in a
mixture composed of 80 parts by weight of cyclohexanone and
20 parts by weight of Wettol O EM 31 (non-ionic emulsifier
based on ethoxylated castor oil; BASF AG). This gives a
stable emulsion concentrate.
The active ingredients I, or the herbicidal compositions
comprising them, can be applied pre or post-emergence. If the
active ingredients are less well tolerated by certain crop
plants, application techniques may be used in which the
herbicidal compositions are sprayed, with the aid of the spray
apparatus, in such a way that they come into as little contact as
possible, if any, with the leaves of the sensitive crop plants
while reaching the leaves of undesirable plants which grow
underneath, or the bare soil (post-directed, lay-by).
Depending on the intended aim of the control measures, the
season, the target plants and the growth stage, the application
rates of active ingredient are from 0.001 to 3.0, preferably 0.01
to 1 kg/ha active substance (a.s.).
To widen the spectrum of action and to achieve synergistic
effects, the substituted hydroximic acid derivatives I can be
mixed and applied jointly with a large number of representatives
of other groups of herbicidally or growth-regulatory active
ingredients. Suitable components in mixtures are, for example,
1,2,4-thiadiazoles, 1,3,4-thiadiazoles, amides, aminophosphoric
acid and its derivatives, aminotriazoles, anilides,
(het)aryloxyalkanoic acids and their derivatives, benzoic acid
and its derivatives, benzothiadiazinones,
2-aroyl-1,3-cyclohexanediones, hetaryl aryl ketones,
benzylisoxazolidinones, meta-CF3-phenylderivatives, carbamates,
quinolinecarboxylic acid and ids derivatives, chloroacetanilides,
cyclohexane-1,3-dione derivatives, diazines, dichloropropionic
acid and its derivatives, dihydrobenzofurans,
dihydrofuran-3-ones, dinitroanilines, dinitrophenols, diphenyl
ethers, dipyridyls, halocarboxylic acids and their derivatives,
areas, 3-phenyluracils, imidazoles, imidazolinones,
N-phenyl-3,4,5,6-tetrahydrophthalimides, oxadiazoles, oxiranes,
phenols, aryloxy- or hetaryloxyphenoxypropionic esters,
CA 02283672 1999-09-15
0050/47873
phenylacetic acid and its derivatives, phenylpropionic acid and
its derivatives, pyrazoles, phenylpyrazoles, pyridazines,
pyridinecarboxylic acid and its derivatives, pyrimidyl ethers,
sulfonamides, sulfonylureas, triazines, triazinones,
5 triazolinones, triazolecarboxamides and uracils.
Moreover, it may be advantageous to apply the compounds I, alone
or in combination with other herbicides, in the form of a mixture
with additional other crop protection agents, for example with
10 pesticides or agents for controlling phytopathogenic fungi or
bacteria. Also of interest is the miscibility with mineral salt
solutions which are employed for treating nutritional and trace
element deficiencies. Non-phytotoxic oils and oil concentrates
can also be added.
Preparation examples
Example 1
3-[2-Fluoro-4-chloro-5-(0-allylhydroxylaminocarbonyl)-
phenyl]-2,4-dioxo-1-methyl-6-trifluoromethyl-1,2,3,4-tetrahydro-
pyrimidine
~H3 o F CH o F
\ 3
F3C N N ~ ~ C1 + H2N-Oallyl N-
F3C ~ N C1
O COC1
O C-NH-Oallyl
II
A g
0
3 g of allylhydroxylamine hydrochloride monohydrate were
Introduced in 100 ml of toluene, and 5.6 g of triethylamine were
added at room temperature (RT). To this there were added dropwise
9.6 g of A, dissolved in 80 ml of toluene, during which process
the temperature of the solution climbed to 35~C. The reaction
solution was subsequently stirred for 5 hours at room
temperature, 50 ml of water were then injected, the precipitate
which had separated out was removed and washed first with 50 ml
of water and then with 50 ml of petroleum ether. After the
precipitation had been dried at 50~C under reduced pressure, 5.5 g
of the desired product B of melting point 152-153~C were obtained.
CA 02283672 1999-09-15
~ 0050/4?8?3
26
Example 2
3-[2-Fluoro-4-chloro-5-(O-benzylhydroxylaminocarbonyl)-
phenyl]-2,4-dioxo-1-methyl-6-trifluoromethyl-1,2,3,4-tetrahydro-
pyrimidine
F
3
C N // + HZN-OCHZ- .CH3 /o F
F3 ~ ~N ~ ~ C1 phenyl N /~
~~ --~ F3C ~ N C1
O COC1
O C-NHOCHyphenyl
A ~ II
0
3.5 g of benzylhydroxylamine hydrochloride were introduced into
90 ml of toluene, and 4.5 g of triethylamine were added at room'
temperature (RT). To this there were added dropwise 7.7 g of A,
dissolved in 60 ml of toluene, during which process the
temperature of the solution climbed to 30~C. The reaction solution
was subsequently stirred for 5 hours at room temperature and then
washed 3 times with in each case 50 ml of water and dried over
sodium sulfate, and the solvent was distilled off under reduced
pressure. The resulting oil was purified by chromatography on
silica gel using methylene chloride as the eluent, subsequently
stirred with diisopropyl ether, and the precipitate which formed
was filtered off and washed with petroleum ether. After.the
precipitate had been dried at 50~C under reduced pressure, 5.5 g
of the desired product C of melting point 114-116~C were obtained.
Other hydroxamic acid derivatives can be prepared in a similar
manner.
Example 3
preparation of 3-[2-fluoro-4-chloro-5-methoxyimino-
(allyloxy)methylphenyl]-2,4-dioxo-1-methyl-6-trifluoromethyl-1,2,
3,4-tetrahydropyrimidine (Compound 4.02)
F
CH ~ - CH3 o F
N
F3C ~ N ~ ~ C1 F3C N N ~ ~ C1
O ~/C-NH-OCH3
O C=N-OCH3
O
D E Oallyl
CA 02283672 1999-09-15
0050/47873
27
2 g of D were introduced into 80 ml of acetone, and 0.8 g of
potassium carbonate were added. To this mixture there was added
dropwise 0.52 g of allyl bromide, and the reaction mixture was
stirred for 17 hours at room temperature (RT). The solvent was
subsequently distilled off under reduced pressure, and the
residue was taken up in 100 ml of methylene chloride, washed in
each case three times with 30 ml of water and dried over sodium
sulfate. The solid obtained after the solvent had been removed by
distillation was purified by chromatography on silica gel
(eluent: methylene chloride/ethyl acetate = 95:5). The resulting
oil was stirred with a 1:1 mixture of petroleum ether and
diisopropyl ether, and the precipitate which formed was filtered
off and washed with petroleum ether. After the precipitate had
been dried, 0.45 g of the desired product E of melting point
73-75~C was obtained.
Example 4
Preparation of 3-[2-fluoro-4-chloro-5-methoxyimino-
(methoxy)methylphenyl]-2,4-dioxo-1-methyl-6-trifluoromethyl-
1,2,3,4-tetrahydropyrimidine (Compound 4.01)
F
CH ~ CH3 o F
N N ~ ~ C 1 N-'\
F C
2 5 3 \\--(~~ ~ F3 C \ N ~ ~ C 1
O C-N-OCH3
// O C=N-OCH3
O I
D F OCH3
2 g of D were introduced into 100 ml of acetone, and 0.7 g of
potassium carbonate was added. To this mixture there was added
dropwise 0.83 ml of methyl tosylate and the reaction mixture was
refluxed for 5 hours. The solvent was subsequently distilled off
under reduced pressure, and the residue was taken up in 100 ml of
methylene chloride, washed in each case three times with 30 ml of
water and dried over sodium sulfate. The solid obtained after the
s°lvent had been removed by distillation was purified by
chromatography on silica gel (eluent: methylene chloride/ethyl
acetate = 90:10). The resulting oil was stirred with a 1:1
mixture of petroleum ether and diisopropyl ether, and the
resulting precipitate was filtered off and washed with petroleum
ether. After the precipitate had been dried, 0.65 g of the
desired product F of melting point 122-124~C was obtained.
CA 02283672 1999-09-15
~ 0050/47873
28
The compounds listed in Table 4 below were prepared following the
above-described processes. These compounds are exemplary,,of the
compounds of the formula I according to the invention, which can
be prepared by the processes described in the Examples or in the
above description.
Table 4
CH3 o F
N
F3C \ N / \ Cl I RZ ' Y CF O; R3 = H H3:
4 =
R F: R5 = C1}
O C=N-OR6
ORS
No. Rq R6 R7 m.p. (C)
4,01 F CH3 CH3 122-124
4.02 F CH3 CH2CH=CHZ 73-75
4.03 F CH3 CHZC6H5 oil
4.04 F CH3 CH2COOCH3 oil
4.05 F CH3 CH(CH3)COOCHg 011
4.06 F CH2CH=CH2 CHg oil
4.07 F CHZCH=CHZ CH2CH=CHZ ' oil
4.08 F CHZCH=CHy CHZCOOCHg 116-117
4.09 F CH2C6H5 CH3 127-129
4.10 F CHZC6H5 CHZCOOCH3 146-148
(cis)
4.11 F CH3 CH2C(NOCH3)COOCH3oil
4.12 F CH2(o-C1-C6Hq) CHyCO0CH3 o7.1
4.13 F CHZ-(m-C1-C6Hq) CH2COOCH3 oil
4.14 F CHZ-(p-C1-C6Hq) CHZCOOCHS oil
4.15 F CHZ-(o,m-C12-C6H3)CH2COOCH3 oil
4.16 F CHZ-(m,p-C12-C6H3)CHZCOOCH3 oil
4.17 F CHz-(m-CF3-C6Hq) CH2COOCH3 oil
4.18 F CHZ-C6H5 CH2COOCH3 oil
(trans)
419 I F I CH2-C6H5 ~ CH(CH3)COOCH3 oil
CA 02283672 1999-09-15
0050/47873
29
Example 5
Preparation of 3-[2-fluoro-4-chloro-5-[ethoxyimino(methylthio)-
methyl]phenyl]-2,4-dioxo-1-methyl-6-trifluoromethyl-1,2,3,4-
tetrahydropyrimidine
CH3 o F F
\ CH3 O
F3 N N ~ ~ C1 H3CJ \N~
F3C ~ N C1
O //C-NH-OCZHS O
~C=N-OCZHS
H3CS
G H
Initially 0.22 g of potassium carbonate and then 0.23 g of methyl
iodide were added dropwise to 0.69 g of G in 10 ml of absolute
ethanol. The mixture was stirred at room temperature (RT) for
3 hours and then the low-boiling proportions removed. The residue
was admixed with 50 ml of saturated aqueous sodium bicarbonate
solution. The resulting product of value was extracted from the
aqueous phase using 3 x 30 ml of ethyl acetate. The combined
organic phases were subsequently washed twice with water, dried
over sodium sulfate and finally concentrated. The crude product
was purified by medium pressure liquid chromatography (MPLC;
eluent mixture: cyclohexane/ethyl acetate = 3:1). Yield: 0.11 g
of the desired compound H as a yellow, very viscous oil.
Precursor
3-(2-Fluoro-4-chloro-5-(ethylhydroxylaminothiocarbonyl)phenyl-
2,4-dioxo-1-methyl-6-trifluoromethyl-1,2,3,4-tetrahydropyrimidine
CH3 o F
\ CH3 o F
F3C N N ~ ~ C1 Laraesson~s reagent \N='\
F3C ~ N ~ ~ C1
O
//C-NH-OCZHS O
C-NH-OCZHS
O
S
_
K G
A solution of 0.54 g of G and 0.27 g of Lawesson's reagent in
5 m1 of absolute tetrahydrofuran was heated to reflux temperature
for 12 hours. After cooling of the reaction mixture, the solvent
was distilled off. The residue was admixed with 50 ml of
saturated aqueous sodium bicarbonate solution. The mixture was
CA 02283672 1999-09-15
~' 0050/47873
then extracted with 3 x 30 ml of ethyl acetate. The combined
organic phases were washed twice with water. Yield: 0.69 g of
crude product which was methylated without any further
purification.
5
In a similar manner:
- 3-[2-fluoro-4-chloro-5-[(2-methylpropyl)hydroxylaminothio
carbonyl]phenyl]-2,4-dioxo-1-methyl-6-trifluoromethyl
10 1,2,3,4-tetrahydropyrimidine gave the corresponding compound
I where YR7 = SCH3;
- 3-[2-fluoro-4-chloro-5-(propyn-3-ylhydroxylaminothio-
carbonyl)phenyl]-2,4-dioxo-1-methyl-6-trifluoromethyl-
15 1,2,3,4-tetrahydropyrimidine gave the corresponding compound
I where YR7 = SCH3; yield: 23%; m.p.: 68-70°C;
- 3-[2-fluoro-4-chloro-5-(prop-2-ylhydroxylaminothiocarbonyl)-
phenyl]-2,4-dioxo-1-methyl-6-trifluoromethyl-1,2,3,4-tetra-
20 hydropyrimidine gave the corresponding compound I where
YR7 = SCH3; yield: 20%; colorless oil.
Use Examples for the herbicidal activity
The herbicidal action of the substituted hydroximic acid
derivatives I was demonstrated by the following greenhouse
experiments:
The culture containers used were plastic flowerpots containing
loamy sand with approximately 3.0% of humus as substrate. The
seeds of the test plants were sown separately for each species.
For the pre-emergence treatment, the active ingredients,
suspended or emulsified in water, were applied directly after
sowing by means of finely distributing nozzles. The containers
were irrigated gently to promote germination and growth and
subsequently covered with translucent plastic hoods until the
plants had rooted. This cover caused uniform germination of the
test plants unless this was adversely affected by the active
ingredients.
For the post-emergence treatment, the test plants were grown to a
plant height of from 3 to 15 cm, depending on the plant habit,
and only then treated with the active ingredients which had been
suspended or emulsified in water. To this end, the test plants
were either sown directly and grown in the same containers, or
CA 02283672 1999-09-15
0050/47873
35
31
they were first grown separately as seedlings and transplanted
into the test containers a few days prior to treatment. The rate
of application for the post-emergence treatment was 7.8 or
3.9 g/ha a.s.
Depending on the species, the plants were kept at from 10 - 25~C
and 20 - 35~C, respectively. The test period extended over 2 to
4 weeks. During this time, the plants were tended, and their
response to the individual treatments was evaluated.
Evaluation was carried out using a scale of from 0 to 100. 100
means no emergence of the plants, or complete destruction of at
least the aerial parts, and 0 means no damage or normal course of
growth. .
The plants used in the greenhouse experiments belonged to the
following species:
Scientific Name Common Name
~
Amaranthus retrofle Redroot pigweed
xus (AMARE)
Ipomoea spec. (IPOSS) morning glory
Setaria faberii (SETFA) giant foxtail
Setaria viridis (SETVI) green foxtail
Solanum nigrum (SOLNI) black nightshade
At a rate of application of 7.8 or 3.9 g/ha of a.s., compound No.
4,08 showed a very good herbicial action against undesired
broad-leaved plants and grass weeds when applied post-emergence.
Use Examples for the desiccant/defoliant activity
The test plants used were young cotton plants with 4 leaves
(without cotyledons) which had been grown under greenhouse
conditions (relative atmospheric humidity 50 to 70$; day/night
temperature 27/20~C}.
The young cotton plants were subjected to foliar treatment to
run-off point with aqueous preparations of the active ingredients
(with an addition of 0.15 by weight of the fatty alcohol
alkoxylate Plurafac ~ LF 7001), based on the spray mixture). The
amount of water applied was 1000 1/ha (converted). After 13 days,
CA 02283672 1999-09-15
0050/47873
32
the number of leaves shed and the degree of defoliation in % were
determined.
No leaves were shed in the untreated control plants.
11 a low-foam, nonionic surfactant from BASF AG
15
25
35
45