Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02284139 1999-09-28
1. Field of the Invention
The present invention relates to an iron powder, to a
method for making the iron powder, and to a powder magnetic
core using the iron powder, which core has high permeability
over a high-frequency range and which may be used as a reactor
core, or a noise filter core or a substitute for a ferrite
compact.
2. Desc_rip ~~n of the R la d Ar
Miniaturization of electronic components is rapidly
progressing with reduction in size of electronic devices.
Although many electronic components use ferrite compacts
having excellent high-frequency characteristics and low iron
loss (core loss) , inexpensive powder magnetic cores having high
thermal stability of magnetic characteristics have been
examined as substitutes for ferrite compacts. The powder
magnetic cores are formed by mixing iron powder with a resin
binder, compaction-molding the mixture and then curing the
resin binder, and are used for reactor cores and noise filter
cores. The powder magnetic cores not requiring a sintering
step do not have cracks and chips due to shrinkage during
sintering and enable the formation of articles having thin
articles or complicated shapes. Thus, it is anticipated that
electronic components will be miniaturized and novel
2
CA 02284139 1999-09-28
electronic components, which cannot be produced with
conventional materials, will be produced to satisfy design
requirements.
Characteristics required for powder magnetic cores are
high permeability and low iron loss in the high-frequency
region. When the dependence of the initial permeability, at
room temperature and 50 Hz to 1 MHz , on the frequency is measured
and the frequency at which the initial permeability is 80~ of
the direct-current initial permeability is defined as a
criticalfrequency, a high direct-currentinitialpermeability
and a high critical frequency are required. Alternatively,
high effective permeability and a low iron loss are required.
The density of the magnetic core and the presence of an
effective demagnetizing field affect the permeability, and
thus, a higher density of the magnetic core and a lower effective
demagnetizing field will result in a higher permeability. The
critical frequency increases as the eddy current loss decreases,
and thus , a higher insulating capability between particles as
constituents of the magnetic core, and a lower iron loss, will
resultin a higher critical frequency. Theinsulating property
between particles is evaluated by the direct-current
resistivity of the magnetic core, and the direct-current
resistivity increases as the insulating property becomes
higher.
3
CA 02284139 1999-09-28
A proposed method for decreasing the effective
demagnetizing field is to flatten the iron powder particles
used as a raw material for powder iron cores, as disclosed in,
for example, Japanese Laid-Open Patent Nos. 62-72102, 63-
233508 and 61-223101. Although the direct-current initial
permeability is improved by this method, a flattening results
in increasing of contact areas between powder particles.
Thus, the insulating property between the particles tends to
decrease, and the iron loss tends to increase.
Techniques for producing magnetic cores having a high
direct-current initial permeability and a high critical
frequency are examined by considering improved insulating
layers, as disclosed in, for example, Japanese Laid-Open Patent
No. 8-260114. Since most insulating layers form hard coatings
on the surfaces of particles, the compactness of the powder
magnetic core and thus the density are decreased during
compaction. Improvement of permeability due to such
flattening is, therefore, decreased.
Since the powder magnetic cores using conventional iron
powder have low densities, the iron powder is readily corroded
not only at the surface of the magnetic core but also in the
interstices. Thus, this powder iron core has less reliability
when used as an electronic component, as compared with ferrite
compacts.
4
CA 02284139 1999-09-28
Accordingly, it is an obj ect of the present invention to
provide an iron powder for a powder magnetic core which has
high corrosion resistance, can be used as a substitute for
ferrite compacts, and has a high initial permeability over a
high-frequency region; to provide a method for making the iron
powder; and to provide a powder magnetic core using the iron
powder.
We have discovered that flat reduced iron powder, obtained
by flattening porous reduced iron powder and then annealing
the flattened powder, improves the magnetic characteristics
of the iron powder.
An important feature of the present invention is to
provide a flat-particle iron powder comprising reduced iron
powder obtained by reducing iron oxide, wherein the flat-
particle iron powder has an average aspect ratio of at least
about 5 and an average ferrite grain size of about 2 to 20 Vin.
Preferably, the flat-particle iron powder is covered with
an insulating agent, and the insulating agent comprises an
amine-quinone compound having an amine-quinone repeating unit.
It is further important in the present invention to
provide a powder magnetic core, prepared by compaction molding
of a mixture of a ferromagnetic material, comprising a
flat-particle iron powder and a binder, wherein the flat-
5
CA 02284139 1999-09-28
particle iron powder comprises reduced iron powder obtained
by reducing iron oxide, and has an average aspect ratio of at
least about 5 and an average ferrite grain size of about 2 to
20 Wn.
Preferably, the flat-particle iron powder is covered with
an insulating agent, and the insulating agent comprises an
amine-quinone compound having an amine-quinone repeating unit.
The binder referred to above is preferably a thermosetting
resin, and the thermosetting resin is preferably at least one
resin selected from the group consisting of polymeric resins
having a quinone-amine repeating unit, epoxy resins, phenolic
resins, and polyamide resins.
Another feature of the present invention is to provide
a method of making a flat-particle iron powder for a powder
magnetic core, which method comprises the steps of flattening
a reduced ion powder obtained by reduction of iron oxide,
annealing and disintegrating the flat-particle iron powder,
classifying the size of disintegrated flat-particle iron
powder particles by screening, and treating the surfaces of
the resulting flat-particle iron powders with an insulating
material. The reduced iron powder is mixed with metallic soap
or wax, and then flattened by using a mill.
Preferably, the step of treating the surface of the
6
CA 02284139 1999-09-28
insulating material comprises dissolving a compound having an
amine-quinone repeating unit in a solvent, adding the solution
dropwise into the flat-particle iron powder, mixing the
flat-particle iron powder, and then removing the solvent.
Preferably, the mill is selected from the group consisting of
a vibrating ball mill, a vibrating rod mill, a disk mill and
a rotating ball mill.
These and other important features are shown by way of
illustration in the drawings, which are not intended to define
or to limit the scope of the invention, which is defined in
the appended claims.
BRTEF DE~GRTPmrON OF THE DRAWrurS
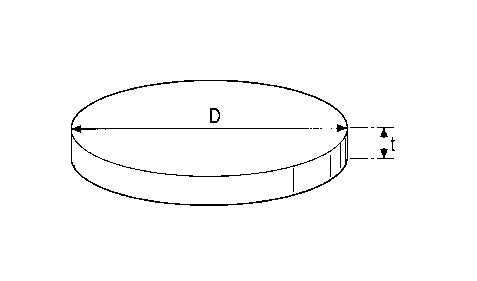
Fig. 1 is a schematic view of a typical flat-particle iron
powder in accordance with this invention; and
Fig. 2 is a graph showing the relationship between the
effective permeability and the average aspect ratio of a
flat-particle iron powder in accordance with this invention.
Flat-particle iron powders in accordance with the present
invention are made of reduced iron powder prepared by reduction
of iron oxide. Pure iron powder is generally classified into
two groups, reduced iron powder and atomized iron powder,
depending upon the manufacturing processes. The reduced iron
7
CA 02284139 1999-09-28
powder has the general format of a sponge or net, and comprises
a multiplicity of long entangled arms . Thus , the reduced iron
powder has a larger effective aspect ratio than that of atomized
iron powder, even when they have the same average aspect ratio.
As a result, the reduced iron powder has a greatly decreased
demagnetizing field and is useful for forming magnetic
materials.
The average aspect ratio D/t of a flat-particle iron
powder in accordance with the present invention is at least
about 5. The expression "average aspect ratio D/t" means the
average particle diameter D, based upon sieve classification,
divided by the average thickness t of the particle.
Fig. 1 is a schematic view of a typical flat-particle iron
powder. The average particle diameter D is determined by sieve
classification so that the total weight of the particles having
diameters larger than the average particle diameter D is equal
to the total weight of the particles having diameters smaller
than the average particle diameter D. The average thickness
t is determined by visual observation of 50 particles using
a scanning electron microscope (SEM) . Preferably, the average
aspect ratio D/t is at least about 10. When the flat-particle
iron powder is mixed with a predetermined amount of binder and
the mixture is molded at a given pressure, a large average aspect
ratio creates a decreased effective demagnetization field and
8
CA 02284139 1999-09-28
thus an increased direct-current initial permeability. On the
other hand, an average aspect ratio of less than about 5 does
not substantially contribute to a decrease in the
demagnetization field.
Since a flat-particle iron powder has a large contact area
between particles when it is molded, insulation between
particles decreases even when the type and amount of the binder
is optimized. As a result, eddy currents flowing between
particles increase and iron loss decreases.
Particles have defects on their surfaces, such as
distortion and cracks, which are formed during pulverization
and working. Such defects cause a decrease in permeability.
Since the effects of these defects are suppressed as the
particle size increases, the permeability increases as the
particle size increases. A large particle size, however,
causes an increase in passage of the eddy currents on the
particle surface, and thus an increase in eddy current loss.
As a result, the dependence of permeability on frequency is
deteriorated, that is, the critical frequency decreases.
Accordingly, iron powder having a larger diameter is
advantageous for making components which require high
permeability in a low-frequency region, in addition to a
high-frequency region. When the iron powder is used in a
high-frequency region, a smaller particle size is preferred.
9
CA 02284139 1999-09-28
That is, the particle size is preferably about 2 mm or less
and more preferably about 500 dim or less . The average particle
size is preferably about 50 to 180 Nm, and more preferably about
75 to 120 Nm.
The flat-particle iron powder in accordance with the
present invention is composed of substantially pure iron of
an a-Fe or ferritic single phase, and the ferritic phase has
an average crystal grain size of about 2 to 20 Nm. Impurities
are concentrated in the grain boundary of the ferritic phase,
and are often precipitated as inclusions. The precipitate
inhibits migration of the magnetic wall and causes pinning of
the magnetic wall. The pinning causes a decrease in
permeability and an increase in hysteresis loss. Thus, it is
preferable that the crystal grain size in the ferritic phase
of the iron powder used as a magnetic material favor being larger .
When the average grain size of the ferritic phase is less than
about 2 Nm, the number of the crystal grain boundaries increases
in the iron powder. Thus, the permeability decreases
significantly and the hysteresis loss increases. Since the
reduced iron powder is a sponge or net composed of long entangled
arms, the grain size of the crystal is limited to the width
of the arms, that is, about 20 ~Im. Preferably, the average
grain size is about 5 to 10 Nm.
CA 02284139 1999-09-28
The average ferrite grain size is determined as follows .
The iron powder is embedded in a resin, and the hardened resin
is polished and etched. An optical photograph at a
magnification of 400 is taken from the etched cross section.
The photographic image is analyzed using a computer. The
grain sizes of 50 ferrite crystals are determined, and the
average grain size is calculated.
The flat-particle iron powder in accordance with the
present invention is prepared by mixing reduced iron with a
metallic soap or a lubricant such as wax and then shaping the
reduced iron to a flat shape, as herein defined, in a vibrating
ball mill, a vibrating rod mill, a disk mill, or a rotating
ball mill.
Reduced iron powder used in the present invention is
prepared by conventional reduction of iron oxide. The iron
oxide used as a raw material is mill-scale powder and/or iron
ore powder.
The flat-particle iron powder in accordance with the
present invention is suitable for a powder magnetic material
using a thermosetting resin as a binder. The flat-particle
iron powder is mixed with the thermosetting resin and the
mixture is shaped by compaction molding. The thermosetting
resin is then hardened to form a powder magnetic core. The
compaction molding is performed by a typical powder
11
CA 02284139 1999-09-28
metallurgical process.
The thermosetting resin used as the binder is at least
one resin selected from the group consisting of polymeric
resins having an amine-quinone-repeating unit, epoxy resins,
phenolic resins, and polyamide resins. Among these, the epoxy
resins are preferably used. The binder is preferably added in
an amount of about 0.1 to 10 percent by weight and more
preferably about 0.5 to 5 percent by weight with respect to
the flat-particle iron powder (100 percent by weight).
Prior to compression molding of the flat-particle iron
powder in accordance with the present invention, the flat-
particle iron powder is preferably covered with an amine-
quinone compound having an amine-quinone-repeating unit. In
the present invention, pores in the porous structure of the
flat-particle iron powder may be filled with the amine-quinone
compound. Thus, the covering in the present invention also
includes such an embodiment. The thickness of the covering or
coating layer is about 5 nm to 2 Nm. The thickness is determined
by the carbon and nitrogen contents in depth profile analysis
by Auger-electron spectroscopy. The insulating layer on the
iron powder increases insulation between the iron powder
particles in the powder magnetic core and thus significantly
decreases the iron loss of the magnetic core. Furthermore, the
12
CA 02284139 1999-09-28
insulating layer improves corrosion resistance of the iron
particles. It is believed that hydrogen bonds formed between
oxygen atoms of quinone groups in the amine-quinone compound
and the iron powder surfaces formed by oxidation facilitate
adsorption and bonding of the coating layer on the iron powder.
Preferable amine-quinone compounds are amine-quinone
monomers having at least one hydroxyl group in each molecule .
An amine-quinone-polyurethane resin prepared by
polymerization of such a compound is particularly preferred.
The amine-quinone monomer having at least one hydroxyl group
has high affinity for iron powder and facilitates the formation
of a uniform insulating layer. The amine-quinone-
polyurethane resin has high affinity for iron powder and high
thermal resistance, and is suitable for use in a high-
temperature atmosphere.
Preferably, the content of the amine-quinone compound
added to the iron powder is in the range of about 0. O1 to 0.3
percent by weight and more preferably about 0 . 05 to 0 . 2 percent
by weight. A content of less than about 0. O1 percent by weight
does not produce insulating effects, whereas a content
exceeding about 0.3 percent by weight causes agglomeration of
the iron powder due to crosslinking of the resin lying over
the adj acent iron particles during the removal of the organic
solvent. The agglomerated iron powder inhibits homogeneous
13
CA 02284139 1999-09-28
mixing with a binder. The coating is performed as follows . A
solution of an amine-quinone compound in an organic solvent
is added dropwise to the flat-particle iron powder while the
flat-particle iron powder is mixed, and then the organic
solvent is removed. The concentration of the amine-quinone
compound in the solution is preferably about 5 to 80 percent
by weight, and more preferably about 20 to 60 percent by weight.
Any organic solvent which can dissolve the amine-quinone
compound may be used. Examples of such organic solvents
include cyclohexanone, tetrahydrofuran (THF),
dimethylformamide (DMF), and mixtures thereof with ketonic
solvents . Mixing is preferably performed using, for example,
an attritor or an explosion-proof high-speed mixer until the
iron powder agglomerates formed by segregation of the resin
solution in the powder disappear. The solvent is removed and
then the iron powder covered with the resin is dried, for example,
in a vacuum drier.
The preferable amine-quinone-polyurethane resins in the
present invention are polyurethane resins modified with diols
having amino and quinone groups represented by the chemical
formula (1)
14
CA 02284139 1999-09-28
O
I
N~R~OH ( 1 )
HO' R2~
N
I
Rt. O
wherein R1 is hydrogen, branched, linear or cyclic C1-C6 alkyl,
aralkyl or phenyl which may be substituted by linear, branched
or cyclic alkyl; and R2 is a linear, branched or cyclic C1-
Cl6alkylene chain, phenylene, aralkylene, alkarylene, or a
polyethylene oxide represented by the chemical formula (2):
- ( CHZCHzO ) nCH2CH2- ( 2 )
wherein n is an integer of 0 to 50. For example, Rl may be an
ethyl , n-propyl , i so-propyl , benzyl or phenyl group , and Rz may
be a methylene, ethylene, propylene or iso-propylene.
The amine-quinone-polyurethane resin is prepared by
reaction of a diol having amine-quinone represented by the
chemical formula (1) , a linear diol not having amine-quinone ,
and a diisocyanate. Preferred diols have a molecular weight
of approximately 500 to 5, 000 . Examples of such diols include
polycaprolactone diol(PCL), poly(hexamethylene carbonate)
diol (PHC) , poly (butylene adipate) diol (PBA) ,
poly(hexamethylene adipate)diol(PHA), and 1,4-butane diol
(BD). Examples of preferable diisocyanates include tolylene
diisocyanate (TDI) and 4,4'-diphenylmethane diisocyanate or
methylene di(4-phenylisocyanate) (MDI). Predetermined
CA 02284139 1999-09-28
amounts of raw materials are mixed to form a desired
amine-quinone-polyurethane resin. For example, 5 to 40
percent by weight of diol oligomer containing amine-quinone
groups is added with respect to the total weight of the PCL
and the TDI and the mixture is heated at approximately 60°C for
1 hour for polymerization.
Terminal groups of the urethane molecules in the
amine-quinone-polyurethane resin, coated on the flat-particle
iron powder and epoxy groups in the epoxy resin as a binder,
form crosslinks by condensation. The epoxy resin is tightly
bonded to the iron powder, resulting in increased mechanical
strength of the powder magnetic core. In compression molding,
an amine-quinone-polyurethane resin may be compounded in the
binding resin, if necessary. Also, in this case, crosslinks
between the epoxy groups and the amine-quinone-polyurethane
molecules cause increased mechanical strength of the hardened
powder magnetic core. At least about 1 percent by weight of
amine-quinone-polyurethane resin must be added to 100 percent
by weight of epoxy resin to ensure satisfactory mechanical
strength, and such a content of amine-quinone-polyurethane
resin is preferable. The upper limit of the content depends
on the type of the binder used together. When an excessive
amount of amine-quinone-polyurethane resin is added, the
16
CA 02284139 1999-09-28
mechanical strength decreases. The resin component of the
binder may be composed of only the amine-quinone-polyurethane
resin. When iron powder not covered with a binder containing
an amine-quinone-polyurethane resin is mixed with a binder
containing an amine-quinone-polyurethane resin and is
subj ected to compaction molding, the resulting powder magnetic
core also has substantially the same advantages as those of
the above-described powder magnetic core.
Mill-scale reduced iron powders A and atomized iron
powders B shown in Table 1 were dry-pulverized in a vibrating
ball mill and sieved using a sieve having openings of 106 Eim
or 2 mm. Each powder was placed into a tube furnace and annealed
at 800°C for 1 hour in a hydrogen atmosphere of a dew point of
60°C to prepare a sintered cake. The sintered cake was
disintegrated and sieved by a sieve having openings of 106 Nm
or 2 mm to prepare a flat-particle iron powder. The average
aspect ratio, the average particle size, and the average
ferritic grain size of the resulting powder are shown in Table
2. The flat-particle iron powder was mixed with 1 percent by
weight of epoxy resin and the mixture was molded at a molding
pressure of 686 MPa to form a ring with an outer diameter of
38 mm, an inner diameter of 25 mm and a thickness of 6.5 mm.
17
CA 02284139 1999-09-28
The ring was cured at 180 °C for 30 minutes in air to prepare
a test piece.
A coil was wound around the test piece and the dependence
of the initial permeability of the test piece on the frequency
was measured by an impedance analyzer Model 4824A made by the
Hewlett-Packard Company to determine the direct-current
initial permeability and the critical frequency. The results
are shown in Table 2. The average ferritic grain size was
determined as follows . The iron powder was embedded in a resin,
polished and etched to form a cross section including iron
powder. An optical photograph at a magnification of 400 of the
cross-section was taken and the images of 50 iron powder
particles were stored in a personal computer to measure
ferritic grain sizes in these powder particles and to calculate
the average of grain sizes in the 50 powder particles.
Comparison between EXAMPLE 1 and COMPARATIVE EXAMPLE 1
shows that a larger average aspect ratio causes a decreased
effective magnetic field and thus an increased direct-current
initial permeability.
Comparison between EXAMPLE 3 and COMPARATIVE EXAMPLE 3
and between EXAMPLE 4 and COMPARATIVE EXAMPLE 4 shows that the
powder magnetic core formed of reduced iron powder has a larger
direct-current initial permeability and a higher critical
18
CA 02284139 1999-09-28
frequency than those of the powder magnetic core formed of
atomized iron powder, even when the average aspect ratio is
the same.
Accordingly, the magnetic cores in accordance with the
present invention had superior magnetic characteristics.
E~~AM~LIiS 5 to 8 and GOMPA ATryF EXAMPLE 5 and 6
The mill-scale reduced iron powder A shown in Table 1 was
dry-pulverized in a vibrating ball mill and sieved using a sieve
having openings of 180 Eun. The powder was annealed as in
Example 1, but the annealing temperature was changed to 700
to 850°C. The sintered cake was disintegrated and sieved by
a sieve having openings of 180 Nm to prepare a flat-particle
iron powder. The flat-particle iron powder was molded and
cured as in EXAMPLE 1. A coil was wound around the test piece
and the dependence of the initial permeability of the test piece
on the frequency was measured to determine the direct-current
initial permeability and the critical frequency, as in EXAMPLE
1. The results are shown in Table 3. The average ferritic
grain size was determined as follows. The average particle
size of the crystal grains in the ferritic phase was determined
as in EXAMPLE 1.
Comparison between EXAMPLES 5 and 6 and COMPARATIVE
EXAMPLE 5 shows that a larger average aspect ratio caused a
19
CA 02284139 1999-09-28
decreased effective magnetic field and thus an increased
direct-current initial permeability. Comparison between
EXAMPLES 7 and 8 and COMPARATIVE EXAMPLE 6 shows that a larger
average diameter of the crystal grains in the ferritic phase
caused a larger direct-current initial permeability and a
higher critical frequency, even when the average aspect ratio
was the same. These results suggest that the pinning effect
of the magnetic wall due to the crystal grain boundaries was
suppressed.
ExAMPLE 9
The mill-scale reduced iron powder A shown in Table 1 was
dry-pulverized in a vibrating ball mill for a predetermined
time and sieved using a sieve having openings of 180 Nm. The
powder was annealed at 800°C for 1 hour in a hydrogen atmosphere
having a dew point of 60°C. The resulting sintered cake was
disintegrated and sieved by a sieve having openings of 180 ~,m.
The average aspect ratio of the resulting flat-particle iron
powder was measured. The flat-particle iron powder was mixed
with 1 percent by weight of epoxy resin, and the mixture was
molded at a pressure of 686 MPa to form a ring having an outer
diameter of 38 mm, an inner diameter of 25 mm and a thickness
of 6.5 mm. The ring was cured at 180°C for 30 minutes in air
to prepare a test piece.
CA 02284139 1999-09-28
A 40-turn primary coil and a 40-turn secondary coil were
wound around the test piece and the effective permeability of
the test piece was measured at 100 kHz and a maximum magnetic
flux density of 0.05 T using a BH analyzer Model E5060A made
by the Hewlett-Packard Company. The relationship between the
average aspect ratio and the effective permeability is shown
in Fig. 2, in which the error range is represented by error
bars.
The graph in Fig. 2 demonstrates that the effective
permeability increases as the average aspect ratio increased
for the same opening size of the sieve and significantly
increased when the average aspect ratio was 5 or more.
21
CA 02284139 1999-09-28
The mill-scale reduced iron powder A shown in Table 1 were
dry-pulverized as in EXAMPLES 1 and sieved using a sieve having
openings of 500 Nm. The powder was annealed as in Example 1.
The sintered cake was disintegrated and sieved by a sieve
having openings of 500 dun to prepare a flat-particle iron powder .
The average ferrite particle diameter of the sieved flat-
particle iron powder was approximately 10 Nm. A predetermined
amount of solution containing 10 percent by weight of
amine-quinone-polyurethane resin in cyclohexanone was added
dropwise to the flat-particle iron powder, the mixture was
mixed in a high-speed mixer, and cyclohexanone was removed.
Flat-particle iron powders covered with different amounts of
amine-quinone-polyurethane resin were thereby prepared, as
shown in Table 4 . As in EXAMPLE 1, 1 percent by weight of epoxy
resin was mixed with 100 percent by weight of the covered
flat-particle iron powder and the mixture was molded at a
pressure of 686 MPa to form a ring having an outer diameter
of 38 mm, an inner diameter of 25 mm, and a thickness of 6.5
mm, and a rectangular parallelepiped having a width of 10 mm,
a length of 50 mm, and a thickness of 5 mm. These were cured
at 140°C for 30 minutes in air to form test pieces . A coil was
wound around the ring piece as in EXAMPLE 9, and the effective
22
CA 02284139 1999-09-28
permeability at 100 kHz and a maximum magnetic flux density
of 0.05 T and the iron loss at 100 kHz and maximum magnetic
flux density of 0.01 T were measured using the BH analyzer.
The direct-current resistivity in the longitudinal direction
of the rectangular parallelepiped was measured by a four-probe
method, and then the rectangular parallelepiped piece was
placed into a thermostat at 70°C and a relative humidity of 95~
for 48 hours to measure the corrosion area percentage formed
on the rectangular parallelepiped, as follows. A photograph
of the largest face of the surface on the rectangular
parallelepiped piece was taken and the area of the discolored
corroded region and the area of the uncorroded region were
determined by image analysis. The corrosion area ratio was
defined as the ratio of the corroded area to the overall area
of the observed face. The results are shown in Table 4.
The results of EXAMPLES 10 to 13 show that covering with
the amine-quinone-polyurethane resin caused a decreased iron
loss, an increased direct-current resistivity, improved
insulation between particles in the magnetic core, and a
decreased corrosion area ratio.
The mill-scale reduced iron powder A shown in Table 1 was
dry-pulverized as in EXAMPLE 10 so that the average aspect ratio
23
CA 02284139 1999-09-28
became 10, and was sieved using a sieve having openings of 500
elm. The flat-particle iron powder was annealed, as in EXAMPLE
1. The resulting sintered cake was disintegrated and sieved
by a sieve having openings of 500 E,Im. The average ferrite
particle diameter in the sieved flat-particle iron powder was
approximately 8 Nm. The flat-particle iron powder was mixed
with 1 percent by weight of epoxy resin containing an
amine-quinone-polyurethane resin in the amounts shown in Table
5, and the mixture was molded at a pressure of 686 MPa to form
a ring having an outer diameter of 38 mm, an inner diameter
of 25 mm and a thickness of 6.5 mm, and a rectangular
parallelepiped having a width of 10 mm, a length of 50 mm, and
a thickness of 5 mm. These were cured at 140°C for 30 minutes
in air to prepare test pieces. Magnetic characteristics were
measured as in EXAMPLE 10. The coil was unwound and the Radial
Crushing strength was measured according to ASTM B439-98. The
results are shown in Table 5.
The results of EXAMPLES 14 to 17 show that the binder
containing the amine-quinone-polyurethane resin caused a
decreased iron loss, an increased direct-current resistivity,
improved insulation between particles in the magnetic core,
a decreased corrosion area ratio, and an increased Radial
Crushing strength.
24
CA 02284139 1999-09-28
Polyols and diisocyanates shown in Table 6 were mixed with
2,5-bis(N-2-hydroxyethyl-N-methylamino)-1,4-benzoquinone
represented by the chemical formula (3) as an amine-quinone
monomer (AQM) according to the formulations shown in Table 6,
and were allowed to react at 60°C for 1 hour, in which the ratio
of the AQM in Table 6 represents percent by weight to the total
weight of all the raw materials. Amine-quinone-polyurethane
resins were thereby prepared. The molecular weights of the
resulting resins were approximately 5,000 to 50,000.
~2H4 OH
(3)
/ C2H4~
HO
These polyurethane resins were dissolved into 2-butanone
to prepare 10$-by-weight solutions. Each solution was added
dropwise to the flat-particle iron powder, prepared in EXAMPLE
14, having an average aspect ratio of 10 and an average ferrite
particle diameter of 8 dun, and the mixture was mixed in a
high-speed mixer. The solvent was removed.
To 100 percent by weight of the flat-particle iron powder,
CA 02284139 1999-09-28
1 percent by weight of epoxy resin was added, and the mixture
was molded at a pressure of 686 MPa to form a ring having an
outer diameter of 38 mm, an inner diameter of 25 mm and a
thickness of 6 mm, and a rectangular parallelepiped having a
width of 10 mm, a length of 50 mm, and a thickness of 5 mm.
These were cured at 140°C for 30 minutes in air to prepare test
pieces. Magnetic characteristics were measured as in EXAMPLE
10. The coil was unwound and the Radial Crushing strength was
measured, as in EXAMPLE 14. The results are shown in Table 6.
The results of EXAMPLES 18 to 22 and COMPARATIVE EXAMPLE
7 show that the iron powder covered with the amine-
quinone-polyurethane resin had an increased direct-current
resistivity, a decreased iron loss, and an increased Radial
Crushing strength. These trends were noticeable when the
amine-quinone monomer (AQM) content increased.
The mill-scale reduced iron powder A shown in Table 1 was
dry-pulverized so that the average particle thickness became
approximately 2 Nm, and sieved using sieves having openings
of 106 Eun, 180 ~tm, 500 Nm, 1 mm, 2.0 mm, 2.5 mm, and 3.0 mm,
respectively. The sieved iron powders were annealed at 800°C
for 1 hour in a hydrogen gas atmosphere having a dew point of
60°C in a tube furnace. The resulting sintered cakes were
26
CA 02284139 1999-09-28
disintegrated and the product sieved using sieves having
openings of 106 Nm, 180 Nm, 500 EIm, 1 mm, 2.0 mm, 2.5 mm, and
3.0 mm respectively. The sieved iron powders were mixed with
1 percent by weight of epoxy resin, and the mixture was molded
at a pressure of 686 MPa to form rings having an outer diameter
of 38 mm, an inner diameter of 25 mm and a thickness of 6.5
mm. These were cured at 180°C for 30 minutes in air to prepare
test pieces. Magnetic characteristics of coiled ring pieces,
the dependence of the initial permeability on the frequency,
the direct-current initial permeability and the critical
frequency were measured using an impedance analyzer, as in
EXAMPLE 1. The results are shown in Table 7.
The results shown in Table 7 show that when the thickness
t of particles was constant, a larger opening of the sieve
resulted in an increased direct-current initial permeability
due to an increased average aspect ratio. When the maximum
particle size was larger than 2 mm, the critical frequency was
significantly decreased due to deterioration of insulation.
As described above , the powder magnetic core in accordance
with the present invention has high direct-current initial
permeability, high critical frequency, high effective
permeability, and low iron loss. Thus, this powder magnetic
core can well replace powder magnetic cores formed of
27
CA 02284139 1999-09-28
conventional ferrite sintered compacts. In addition, the
powder magnetic core in accordance with the present invention
has high mechanical strength and high corrosion resistance
compared to conventional powder magnetic cores.
28
CA 02284139 1999-09-28
M l0
O O
V1 O O
O O
rl O
P.i O O
'''~ O O
O
3
0
N O
O O
N
U
N N N
j1,r1 O O
O O
O
i~
N u7
O
M u7
O O
0 0
0 0 0
U
O
U O O
U o 0
E., 0 0
.f~
o ,-i
N rl
O O
N
S-1
N
-.-I o ~--i
N
~
..
O
rd
.rl
4-y
O
N ao .-I
~ o
~
~I
N 0
y
b
N M
A
S-i b f-I N t-I
N
U
_
3 ~ ~ 3
3
b ~ s~
H H O O H
O O
W ~ W ~ L~
N
b
2~
CA 02284139 1999-09-28
U
U ~ N .--IIn O O O rl
~ ,~, N O N O .-I00
-ri ~ v-~).-I ~ r-1 rlO
~'
U
w
rd -ri
f7.-I I~O Wit'M
01a0 0100 O 01
H N ~.
U !~
A N
W
4a
O
N
O M t~ u7
l!7Ln O O l0l0
.~ H ~i ~i
N
w
N
-rl O COl0 l0l0 l0l0
O O O O
O O
~
O ~ H H ~ ~ H
~
O O
V O
O M r-iri O O
O , y --1 .--I'-i ~t'd'
~ P.~'
W
O O
r~
U ~
-rl ~ M o0 l000 tnM
~
N ~ N ~ u7 u7cr l0
N
3
0
A w
0
N ~ 3 ~ ~C FCPq FCP4
~ H O
H
W W W
.--I,'~ M 'J d''J
rl M 'd'
H H H
a N ~
a
a ~ a a
a
w
x ~ x ~ x ~
x x x
w o w o w o
w w w
U U U
CA 02284139 1999-09-28
U
O
U ~ N ~f7~-1O oo,-It,c~
rl ,.~ r-I.-Ir-1 .-IN O ~ fd
,~,
~J
.-Ie-~~-~I r-I'-1.-1 ~ ~' ~7M r-100
ao
U w O
U
f +~ ri
+~ Wd -~
o
U N -ri ~oN .-i ~ o r1 -,
~.
N fa +~ o~o ao 010 0~
~ ~ ~
H ''1 U -~- t7m n ao
1 W
t(7M ~'r-I
~1 U H y
~ v
uo00 ~ ~r
4) -,-I
W u1
N
G4
N
O
O -ri
S-I M 0001 r-IN lfl UI
~.
N S-1 SririW n o ~ t~o ~ ~
~ a "' n
~ S ~ .
.
O -rl ~ 3 N ~f7.-ICO
Ll 4-I Q ,x M N N t~
O ~ '.
M cr
E"~
b~
N D O
-ri ~ O O O O O O
a00000 0000 00
N ~ -I-I-1 -1--I--I -r~l
~-14 r r r . . . 1
r-I
o
O -.- t1~rl COM
-I _
O .1~
~
~
U ' l~I~ t~l~
~
~ ~-i.-Iri~-I
tT +~ O 4
a ~
U -
N ~ 00~ d~ N N N w
, ~ ~-~I rl~-Irl pa
N _
N S-I ~ I td
O
U +
.~ b
~
-~I N 3 ~ 00o u7,w o -'~
~ N
~ U
+ t~n ao t~~ n ~ 3
~ ~
>., m o 0
O r-1N
O Q O
,~
W O O O
~
~ I
~ o ~ 3 ~ ~ ~ ~ ~ FC
H H O -~
v1
a
w w
O .-IN M
N N
a a a a a a
LiJP.iW W
W W
w w U w w U
w w
CA 02284139 1999-09-28
O
N
(d -rl ~ Lf7I~Lf7 ~ ''~ l~~ ~-il0M o0
b~ ~ ~
ri ,~.,~
~.,
~
'b U1 N l~O r-I "Cj fl1 O M d~l0e-~N r-I
N ~ U U
N N N '-I ~ ~ ~ N N N N N .-1 r~
~
U m U ~ b w
N 'Li
O
~
.i-
O dw ~
_
''i
rti D cd
Q N ao m M ~ -~1 ~ ~ o ~ ~ g ~ +~
O U ~ a
o
A ~ ~ ~ oo m o u~
U R
O U
e N
P ..
r
~'
fn l0N d'M M N b
~
O
~I O 3 N O ~
t N r-i01
H a
,~ M M N N M 00
,-~
'
.rl
O ..
~"'., rl M M O1 ~ .~ ~ ~. u7~-1.-I01I~ u7 b '
u1 ~
O m ~ . . . . U w o00o ao~ co co td
U ro N
S-I O cr 00d'o N .-W-1 H rlrl ~-1 +~
3 '~ m
O ~
H a "~ M N N CO 4-I N (d
'
b
H H N
w
~ U
~ rd
~
.r +, ~
-I .
~
-r1 ~
9r +~ .r-I
-
i
U b ~ ~ ~ ~ ~ a ~ ~ O O O O O O r
~
~-Irl'-Iv--i ~, rlN M d'rl
~ -
O
~
W
U 3
'
w
N ~
+.r
+~ o
b
N m ~ O
_ ~ H H H H H H ~ H
_~ ~ O
~
A ~
~ ~ H
N N ~ O ~ ~ H H ~ ~
.rl
m O .
rl
W O rti u7 0 0 0 -~ O r-I
O
3
o ~ x ,~N -~ -~
O
.~ N d
> b
+~ ' .
r .~
~ a s~ ~ a.
p
a~
U Gf1P4U U
'O
c7
N
.
~ ~
H .-I.-Irl N N N H
a a a a a
a a a a a
w
~
x
w w w w w w w w w ~
w
32
CA 02284139 1999-09-28
U
U ~ N .--m~d~ oou'7ao to
N .--1O 01a0~ M
.-IW -1 O O O O
U
w
-ri r-I
~ ll~l0O N N d' d~
~
' O O .-IH .-I,-1 ,-1
\
r-i.--Ir-Ie-i~--I~-I r-I
U N
Ca N
W
O
~
U M I~.-101O a0 d~
-~ N O
n N ~ N u'7u7~r7~'u7u7 u~7
~
cd
~
ripr .
U
Ca
w
b
N
O
toO O
~ o o u~ o
O O H N N M
Q, -r-I
O V7
O
N
H
N
0 3 ~C~ ~ ~ ~ ~ r.C
O
N w
H
M W vo~ w W
~
N N N N N H H
a a a a a ~ ~
a a
~
a
w w w w w ~ o
w w
3~