Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02284826 1999-12-23
1
..
PREPARATION OF
COPPER-INDIUM-GALLIUM-DISELENIDE
PRECURSOR FILMS BY ELECTRODEPOSITION FOR
FABRICATING HIGH EFFICIENCY SOLAR CELLS
s
The United States government has rights in this invention pursuant to
National Renewable Energy Laboratory (NREL) contract No. 1326.
15
BACKGROUND OF THE INVENTION
1. Field of the Invention
The field of the present invention relates to the preparation of thin film
semiconductor devices. More particularly, the present invention relates to
electrodepositionof copper-indium-gallium-diselenidefiims for solar cells.
2. Description of the Related Art
Chalcopyrite ternary thin films of copper-indium-diselenide (CuInSe.Z) and
copper-indium-gallium-diselenide(Culnl_,,Ga,Se2), both of which are
generically
referred to as Cu(In,Ga)Se2, CIGS, or simply CIS, have become the subject of
considerable interest and study for semiconductor devices in recent years.
Sulphur can also be, and sometimes is, substituted for selenium, so the
compound
is sometimes also referred to even more generically as Cu(In,Ga)(Se,S)i so as
to
encompass all of these possible combinations. These devices are also referred
to
as I-III-VIZ devices according to their constituent elemental groups.
CA 02284826 1999-09-22
WO 98/48079 PCT/US98/06212
2
These devices are of particular interest for photovoltaic device or solar
cell absorber applications. For photovoltaic applications, the p-type CIGS
layer
is combined with an n-type CdS layer to form a p-n heterojunction CdS/CIGS
device. The direct energy gap of CIGS results in a large optical absorption
coefficient, which in turn permits the use of thin layers on the order of 1-2
m.
An additional advantage of CIGS devices is their long-term stability.
Various methods have been reported for fabricating CIGS thin fihns.
Some of the earliest techniques involved heating copper and indium on a
substrate
in the presence of a selenium-containing gas, including H2Se. The heating of
copper and indium films in the presence of a selenium-containing gas is known
as
selenization. One drawback to selenizing with H2Se is that H2Se gas is highly
toxic, thus presenting serious hazards to humans in large scale production
environments.
In U.S. Patent No. 5,045,409, Eberspacher et al. disclose depositing
copper and indium films by magnetron sputtering, and depositing a selenium
film
by thermal evaporation, followed by heating in the presence of various gases.
Other methods for producing CIS films have included Molecular Beam Epitaxy,
electrodeposition either in single or multiple steps, and vapor deposition of
single
crystal and polycrystalline films.
Although vapor deposition techniques have been used to yield solar cells
with efficiencies as high as seventeen percent (17%), vapor deposition is
costly.
Accordingly, solar cells made by vapor deposition have generally been limited
to
devices for laboratory experimentation, and are not suitable for large scale
production. On the other hand, thin film solar cells made by electrodeposition
techniques are generally much less expensive. However, solar cells produced by
electrodeposition generally suffer from low efficiencies. For example, in
Solar
Cells with Improved Efficiency Based on Electrodeposited Copper Indium
Diselenide Thin Films, ADVANCED MATERIALS, Vol. 6 No. 5 (1994),
Guillemoles et al. report solar cells prepared by electrodeposition with
efficiencies on the order of 5.6
%.
~ _ r Y. _
CA 02284826 1999-12-23
3
SUMMARY OF THE INVENTION
Accordingly, it is a general object of this invention to provide an
improved process for fabricating high quality thin film Cu(In,Ga)Se2 solar
cells.
It is also an object of this invention to provide low cost, high-quality thin
film solar cells having high conversion efficiencies.
It is a further object of this invention to provide a process for producing
Cu-In, Cu-Se, Cu-In-Se, and Cu-In-Ga-Se thin films that have applications in
solar and non-solar cells.
It is a still further object of this invention to provide a process for
electrodepositing a gallium-containing thin-film solar cell precursor.
To achieve the foregoing and other objects and advantages in accordance
with the purpose of the present invention, as embodied and broadly described
herein, the process of this invention includes electrodepositing a layer of
CuxlnyGaZSeõ (x=0-2, y=0-2, z=0-2, n=0-3), preferably using direct current in
combination with high frequency alternating current. Electrodeposition baths
containing 0.1-0.2 molar (M) copper ions, 0.05-0.15 M indium ions obtained
from indium chloride, 0.05-0.15 M gallium ions obtained from gallium chloride,
0.01-0.03 M selenium ions, and at least 0.3 M lithium chloride were found to
produce simultaneous co-electrodeposition of copper, indium, selenium, and
2 0 appreciable amounts of gallium with a good morphology, when an
electrodeposition potential having a high frequency alternating current
superimposed upon a DC current was applied. Following simultaneous co-
electrodeposition, additional material was vapor deposited to adjust the final
composition of the deposited film very close to stoichiometric
Cu(Inl_xGa,,)SeZ.
This unique two-step film deposition process allows precursor metal films
to be deposited by inexpensive electrodeposition, and then adjusted using the
more expensive but more precise technique of physical vapor deposition (PVD)
to
bring the final film into the desired stoichiometric range. Solar cells may
then be
completed as for example by chemical bath deposition (CBD) of CdS followed by
sputtering of ZnO, and addition of bi-layer metal contacts as well as optional
anti-
reflective coating.
CA 02284826 1999-12-23
4
A solar cell made according to the process disclosed herein achieved a
device conversion efficiency of 13.6%. This represents a significant
improvement over the 9.4% conversion efficiency device disclosed in U.S.
Patent
application serial number 08/571,150, now U.S. Patent No. 5,730,852, of which
this application is a continuation-in-part.
The present invention also includes electrodeposition solutions and
process parameters whereby gallium may be co-electrodeposited in appreciable
amounts along with copper, indium, and selenium, while still obtaining a
densely
packed, uniform morphology film suitable for processing into a photovoltaic
cell.
This co-electrodeposition of gallium further decreases the amount of
stoichiometry adjustment that must be made by the later PVD step.
Additional objects, advantages, and novel features of the present invention
will be set forth in part in the description that follows, and in part will
become
apparent to those skilled in the art upon examination of the following
description
and drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
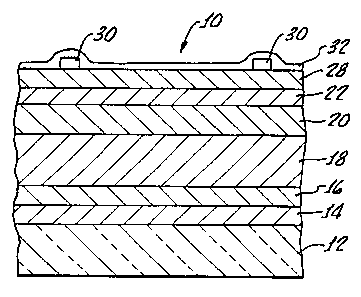
FIG. 1 is a cross sectional view of a CIGS photovoltaic device prepared
according to the present invention.
FIG. 2 is a cross sectional view of the conducting zinc oxide layer 28
shown in FIG. 1.
FIG. 3 is a scanning electron microscope photograph of the
electrodeposited precursor film of Example 1 of the present invention.
FIG. 4 is a graph of the Auger electron spectroscopy analysis for the cell
of Example 1.
FIG. 5 is a graph of the Auger electron spectroscopy analysis for the cell
of Example 2.
FIG. 6 is a graph of the Auger electron spectroscopy analysis for the cell
of Example 3.
FIG. 7 is the x-ray analysis results for the electrodeposited film, and the
finished films of Examples 1-3.
CA 02284826 1999-09-22
~ 98/48079 PCT/US98/06212
FIG. 8 is a graph of the relative quantum efficiency verses wavelength for
the cells of Examples 1-3.
FIG. 9 is a graph showing the Current versus Voltage characteristics of
the cells of Examples 1-3.
5
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The present invention includes an essentially two-step process for
fabricating high quality, low cost thin film CIGS semiconductor devices that
exhibit photovoltaic characteristics and are especially adaptable for solar
cell
applications. In the first step, a precursor film of Cu,,InyGaZSen (x=0-2, y=0-
2,
z=0-2, n= 0-3) is electrodeposited on a substrate such as glass coated with
molybdenum. This first step may include a unique process and electrodeposition
bath for electrodepositing gallium concurrently with other elements, as well
as the
unique use of an alternating current in conjunction with a direct current.
The second step is physical vapor deposition of copper, indium, gallium,
and/or selenium. In this second step the composition of the overall film is
carefully controlled so that the resulting thin film is very close to
stoichiometric
Cu(Ini_xGaX)Se2. Both of these steps may be performed on substrates having
large surface areas. Accordingly, the process of the present invention allows
large area, high efficiency solar cells to be economically produced.
Referring now to FIG. 1, CdS/CIGS photovoltaic device 10 includes a
substrate 12 which may be, for example, soda-lime silica glass or amorphous
7059 glass. Substrate 12 further includes a back contact layer 14 of
molybdenum, about 1-2 m thick. The molybdenum may be deposited using DC
sputtering from a rotating cylindrical magnetron target (CMAG). To improve
adhesion between the Mo layer 14 and the precursor film to be deposited, an
additional adhesion layer 16 of copper may also be deposited as by
electrodeposition. After Mo layer 14 and optional copper adhesion layer 16
have
been deposited, the substrate should be degreased as for example with propanol
and dried in flowing nitrogen gas.
CA 02284826 1999-09-22
V~O 98/48079 PCT/US98/06212
6
A metallic precursor film 18 is then deposited by electrodeposition. The
precursor film contains one or more of the elements copper, indium, gallium,
and
selenium. Electrodeposition is generally a less expensive method of depositing
these metals than vapor deposition. However, it is not possible to control the
ratios of metals deposited during electrodeposition as precisely as desired.
Consequently, prior CIS layers deposited entirely by electrodeposition
produced
low conversion efficiencies. In the present invention, the electrodeposition
step is
integrated with the vapor deposition step that follows. This allows precursor
metal to be deposited in bulk using an economical electrodeposition step,
followed by a vapor deposition step to carefully control the final metal
ratios.
This results in economical production yet high efficiencies of the resulting
cell.
The composition of metal precursor film 18 is generally denoted as
CuXInYGaZSen
(x = 0-2, y= 0-2, z=0-2, n=0-3). The metal precursor film 18 should be
deposited to about 1-3 ,um thick, with thickness being controlled by
coulometric
measurements.
It has been found that electrodepositing the films using an AC voltage in
addition to a DC voltage produces improved results. An AC voltage improves
the morphology of the film. It is also believed that the AC voltage improves
nucleation (growth) of the thin film by allowing additional nucleation centers
to
be created. For an entirely aqueous plating solution, the applicable DC
voltage
range is approximately 1-5 VDC, with a preferred voltage of approximately 3
VDC. Improved results may be obtained by superimposing an AC voltage of
0.2-5.0 VAC at 1-100 Khz, with preferred values of approximately 3.5 VAC at
10-30 KHz. The plating solution is adjusted to have a pH of approximately 1.0
to 4.0, and more preferably to about 1.4 to 2.4. The plating solution should
preferably be at about 10 C to 80 C, and more preferably at about 24 C.
Adding a supporting electrolyte to the plating bath can additionally increase
the
conductivity of the plating solution, allowing for a further increase in the
electrodeposition rate. Salts such as NaCl, LiCl, or Na2SO4 have been found to
be suitable supporting electrolytes for use with certain embodiments of the
present invention.
CA 02284826 1999-12-23
7
..
In completely aqueous solutions, electrolysis of water molecules begins to
occur to an undesirable extent at voltage levels that are too high. The
resulting
02- and OH- ions combine with deposition metal ions or deposited metal to form
unwanted metal oxides and hydroxides on the precursor film 18. To overcome
this disadvantage, the water in the plating solution may be either partially
or
completely replaced by one or more organic solvents such as dimethyl sulfoxide
(DMSO). Increasing the organic solvent content of the electrodeposition
solution
allows the cathodic potential to be increased without unacceptable increases
in
metal oxide and hydroxide formation rates. The increased cathodic potential
increases the deposition rate of the precursor films. An additional advantage
is
that increasing the cathodic potential increases the deposition rate of
gallium
relative to the deposition rates of other deposited metals. Therefore, using a
solution containing one or more organic solvents allows the cathodic potential
to
be selected from a wider range so as to achieve a more desired stoichiometry
of
the as-deposited precursor film 18. When an organic solvent is used, the
preferred cathodic potential is approximately 3-10 VDC and 0.2-5.0 VAC at
approximately 1-100 KHz. Value of approximately 5 VDC and 0.45 VAC at
approximately 18.1 KHz were found to yield good results.
As the number of elements to be simultaneously electrodeposited
increases, the difficulties increase. Obtaining simultaneous electrodeposition
of
four elements in pre-defined ratios with good morphology, for example, can be
an extremely difficult task. The parameters that must be simultaneously
adjusted
include but are not limited to: total solution molarity, relative molarities
of
constituents, from which compounds to obtain the desired constituent elements,
pH, temperature, voltage, waveform characteristics, and electrolytic fluid.
Because of the complexities involved in simultaneous co-electrodeposition, it
is
believed that gallium had never before been successfully co-electrodeposited
along with copper, indium, and selenium to produce a photovoltaic device. The
present invention includes solutions and process parameters whereby gallium
may
be co-electrodeposited in appreciable amounts along with the other three
constituent elements of CIGS.
CA 02284826 2006-05-31
8
If desired, a second electroplating solution may be employed to adjust the
stoichiometry of the electrodeposited film prior to the vapor deposition
phase.
For example, a first electrodeposition step may produce a CIGS precursor film
with less gallium than optimally desired. Although the gallium content can be
increased during the vapor deposition phase, it may be less expensive to
deposit a
certain amount of gallium using a second electrodeposition solution to niake a
coarse stoichiometric adjustment prior to proceeding to fine stoichiometric
adjustment at the vapor deposition step. Another potential motivation for
using a
second electrodeposition solution is to achieve a composition gradient in the
deposited film, as suggested by U.S. Patent No. 4,335,266 issued to Michelsen
et al.,which t e a c h e s composition-
graded CIGS thin films for solar cell and other applications. Yet another way
of
achieving composition grading during electrodeposition is to vary process
parameters such as cathodic potential, ionic concentrations, pH, or
temperature,
as electrodeposition proceeds.
Several examples of electrodeposited precursor fihns fabricated according
to the present invention are given. These examples include Cu-In-Ga-Se, In-Se,
Cu-Se, and Cu-In-Se, precursor films. The solution for co-depositing all four
elements includes ions of each of the elements of copper, indium, gallium, and
selenium. The metal ions may be supplied in the form of dissolved metal salts.
For precursor films that do not contain gallium, gallium should be added to
raise
the energy gap.
In the discussion and claims that follow, electrodeposition potential is
expressed in terms of a voltage without specifying positive or negative
voltage. It
is to be understood that the substrate or working electrode on which the thin
film
is to be deposited is to be connected as the electrodeposition cathode, with
the
counter electrode being connected as the anode. Accordingly, the
electrodeposition voltages discussed herein are to be understood as negative
voltages. In accordance with this convention, where electrodeposition voltages
are expressed as, e.g., "at least 1.0 volt", this indicates that an
electrodeposition
voltage that is at least as negative as -1.0 volt with respect to the counter
CA 02284826 1999-12-23
= ~ ~
9
..
electrode is to be applied to the substrate. Discussing the electrodeposition
voltages as unsigned voltages is to be understood as merely a shorthand way of
referring to the absolute potential difference between the electrodes.
After the precursor film 18 has been electrodeposited it should-be cleaned.
A suitable method is to rinse precursor film 18 with deionized water and dry
it in
flowing nitrogen gas. After precursor film 18 has been cleaned, an additional
layer 20 of copper, indium, gallium; and/or selenium, is deposited by physical
vapor deposition to adjust the final film composition to the ratios of
approximately Cu= 1-1.2: (In,Ga)= 1-1.2: Se= 2-2.5, and most preferably to
approximately 1:1:2. That is, the final film composition is adjusted to
approximately Cul(In,Ga),Se2, which is to say Cui(In1_xGa_x)Se2 where x is
within
the range of 0 to 1, inclusive. By controlling the ratio of In/Ga the energy
gap
between the CdS and the CIGS layers can be adjusted to the optimal or nearly
optimal value. An energy gap of approximately 1.45 eV is considered optimal
for terrestrial solar energy conversion, and is achieved by an In/Ga ratio of
approximately 3:1. For cells prepared according to the method disclosed
herein,
a Ga/(In+Ga) atomic ratio of 0.34 - 0.50 is preferred, with a ratio of 0.39
producing the highest observed efficiency. The substrate (precursor film)
temperature should be 300 C to 600 C during PVD, and preferably about
2 0 550 C.
After PVD, the films should then be annealed. Annealing improves the
homogeneity and quality of the films. A high quality CIGS film is one that
does
not exhibit an excessive amount of copper nodules, voids, or vacancies in the
film
which would reduce conversion efficiencies. Annealing the films at 250 C to
500 C in a vacuum, followed by slow cooling at a rate of approximately
3 C/min to avoid thermal shock was found to yield good results. Because
selenium has a much higher vapor pressure than either copper, indium, or
gallium, selenium may be lost from the film during the high temperature steps
of
vapor deposition and annealing. To compensate, the atmosphere during these
steps may contain a moderate overpressure of selenium. In the preferred
embodiment, the film is selenized at a rate of 5-100 A/s during cool-down from
PVD temperature to annealing temperature.
CA 02284826 1999-09-22
Wfl 98/48079 PCT/US98/06212
Once the CIGS layers 18 and 20 collectively are deposited and annealed, a
thin layer 22 of n-type semiconductor comprising cadmium sulfide is deposited
next. CdS layer 22 is preferably deposited by chemical bath deposition (CBD)
to
a thickness of approximately 200-1000 A. The CBD bath may be prepared from
5 0.08 gm CdSO4, 2.5 gm thiourea, and 27.5 gm NH4OH dissolved in 200 ml
water. The deposition temperature should be approximately 40-80 C.
A layer 28 of conducting wide bandgap n-type semiconductor materials is
deposited next. In the preferred embodiment, layer 28 comprises two zinc oxide
layers 24 and 26 as shown in FIG. 2. First zinc oxide layer 24 is deposited
with
10 RF sputtering at approximately 0.62 watts/cm2 in an argon plasma at 10
millitorrs pressure. Second zinc oxide layer 26, comprising approximately 1-5
%
A1203-doped zinc oxide, is also prepared using RF sputtering at approximately
1.45 watts/cm2 in an argon plasma at 10 millitorrs pressure. In an exemplary
embodiment the resistivity of the first layer was 50-200 ohm/cm2, and
resistivity
of the second layer was 15-20 ohm/cm2. The transmissivity of the overall ZnO
layer was 80-85 %.
Bi-layer metal contacts 30 may then be prepared with an e-beam system
or other techniques. In an exemplary ernbodiment a first metal contact layer
was
500-1000 A thick Ni and the second metal contact layer was 1-3 m thick Al.
Metal contacts 30 will generally be laid out in fine grid lines across the
collecting
surface of the device and connected to a suitable current collecting electrode
(not
shown). The efficiency of the resulting device can be further increased by
adding
an antireflection coating 32, such as a 600-1000 A layer of MgF2 by electron
beam. A device prepared according to Example 3 below exhibited a conversion
efficiency of 13.6%.
Example 1
A thin film containing copper, indium, gallium, and selenium was
deposited onto a glass substrate coated with approximately 500 A Mo, and
processed into a photovoltaic cell. The thin film was obtained by preparing a
solution containing ions of copper, indium, and selenium, and further
including
_ 7 .r _,
CA 02284826 1999-12-23
11
..
ions of gallium in a concentration of at least 0.05 molar, and at least 0.3
molar
LiC1. More particularly, the electrodeposition bath comprised 2.1286 gm
Cu(N03)2=H20, 7.9625 gm InCl3, 1.3929 gm H2SeO3, and 9.2063 gm Ga(No3)3,
and 14.08 gm LiCl dissolved in 450 ml of water. The resulting bath comprised
approximately 0.014 M copper, 0.08 M indium, 0.08 M gallium, and 0.023 M
selenium ions. The pH was adjusted to 1-2. Deposition proceeded at 24 C.
The substrate was employed as the working electrode, and platinum gauze
was used as the counter electrode in a two electrode system. The
electrodeposition voltage comprised a DC component of at least 0.5 volt. More
particularly, the electrodeposition voltage comprised a DC voltage of at least
1.0
volt and an AC voltage of at least 0.5 V at a frequency of at least 1.0 KHz
superimposed thereon. Still more particularly, the electrodeposition voltage
comprised a DC component of 3.0 volts, and an AC component of 3.5 volts
pulsed at 20 KHz superimposed thereon. The voltage was supplied by a power
i5 source obtained from Team Specialty Products Corporation of Albuquerque,
New
Mexico. The AC component is nominally a square wave. However, due to the
complex impedances of the power supply and the remainder of the
electrodeposition equipment operating at 20 KHz, it will be understood that
the
voltage as measured at the substrate will not be a perfect square wave. Thus,
the
applied voltage is more properly described using the broader term of AC
"pulsed" rather than the narrow term of a "square wave". This convention will
be maintained throughout this disclosure and appended claims. The resulting as-
deposited precursor layer had a composition of Cu1.OOIn0.34Gao.oZSeo.91. FIG.
3 is
a scanning electron microscope photograph of the as-deposited film. The
photograph shows the film to be tight, densely packed, and uniform.
After electrodeposition, additional In, Cu, Ga, and/or Se were added to
the film by physical evaporation to adjust the final composition to
Culn,.xGaSe2,
with the ratio of Ga/(In+Ga) being 0.16. The films were allowed to crystallize
at 550 C for five minutes. The substrate (precursor film) temperature during
the
physical evaporation step was also 550 C. The film was then selenized by
CA 02284826 1999-09-22
WO 98/48079 PCTIUS98/06212
12
exposure to selenium vapor during the cool-down time, with cooling at
approximately 20 C per minute.
FIG. 7 is the X-ray analysis results for the electrodeposited film, and the
finished films of Examples 1-3. The X-ray analysis of the as-deposited
precursor
film indicates the presence of both the CIGS phase and the Cu2Se phase. The X-
ray analysis of the film after final film composition adjustment shows only
the
CIGS phase. The shifts in 2-theta values are due to different Ga
concentrations in
the absorber layers.
Photovoltaic devices were completed by chemical bath deposition of
approximately 500 A CdS followed by radio frequency sputtering of 500 A
intrinsic ZnO and 3500 A of A1203-doped ZnO. Bilayer Ni/Al top contacts were
deposited using an e-beam system. An anti-reflection coating of 100 nm of MgF2
was applied as the final step.
The device was evaluated at AM1.5 illumination (1000 W/m2, 25 C,
ASTM E892 global). The device was also characterized by Auger electron
spectroscopy (AES). FIG. 4 is the AES analysis results for the fmished
photovoltaic cell showing the atomic distribution of the film at varying
depths
within the film. FIG. 8 shows the relative quantum efficiency of the cell as a
function of wavelength. FIG. 9 shows the Current versus Voltage
characteristics
of the finished cell. The cell exhibited an overall efficiency of 12.4%. Other
performance parameters for this cell are listed in Table 1 below.
Example 2
A cell was prepared according to Example 1, but the PVD step was
conducted to adjust the final Ga/(In+Ga) ratio to 0.26 rather than 0.16. The
device efficiency improved from 12.4 % to 13.2 %. The AES analysis is shown in
FIG. 5. The relative quantum efficiency is shown in FIG. 8. The Current vs.
Voltage performance is shown in FIG. 9.
..... . .T. . . . 1... .... . __ .._. ...... ..... .
CA 02284826 1999-12-23
13
..
Example 3
A cell was prepared according to Example 1, but the PVD step was
conducted to adjust the final Ga/(In+Ga) ratio to 0.39. The overall device
efficiency improved to 13.6%. The AES analysis is shown in FIG. 6. The
relative quantum efficiency is shown in FIG. 8. The Current vs. Voltage
performance is shown in FIG. 9. Performance parameters for the cells of
Examples 1-3 are given in Table 1 below.
Ga Area Voc Jsc Fill Total-Area
Example (In+Ga) (cm ) (mV) (mA/cm2) Factor Efficiency
(%)
1 0.16 0.413 521 34..9 68.2 12.4
2 0.26 0.420 602 31.7 69.4 13.2
3 0.39 0.419 689 27.6 71.6 13.6
Table 1
Performance Parameters for Photovoltaic Cells of Examples 1-3
Example 4
A bath containing approximately 0.016 M Cu(N03)2=H203, 0.08 M InCl3,
0.08 M H2SeO3, and 0.024 M Ga(No3)3 (relative ratios of approximately 1, 5, 5,
and 1.5, respectively) was prepared at a pH of 1.6. Electrodeposition
proceeded
at at least 2.0 volts DC and at least 2.0 volts AC at a frequency of at least
10
KHz superimposed thereon. More particularly, electrodeposition proceeded at
3.0 VDC with a pulsed AC voltage of 3.5 volts at a frequency of 20 KHz
superimposed thereon. ICP compositional-analysis revealed the following film
compositions before and after the precursor film was finished:
As-Deposited: Cu1.00In0.36Gao.o3Se1.00
After PVD adjustment: Cui.OJn1.04Gao.1gSe2.2z
Note that the as-deposited film contains the highest gallium content of any of
the
examples presented herein.
CA 02284826 1999-09-22
WO 98/48079 PCT/US98/06212
].4
A photovoltaic device was completed as before, with the fmal
Ga/(In+Ga) ratio adjusted to approximately 0.3. The fmal efficiency of the
device was 12.3 l .
Example 5
An electrodeposition bath was prepared by dissolving 1.9956 gm
Cu(N03)2=H20, 9.9531 gm InC13, 1.7411 gm H2SeO3, and 12.0832 gm Ga(No3)3,
and 15 gm LiCI in 450 ml of water (0.18 M copper ions, 0.10 Indium ions,
0.105 M gallium ions, and 0.29 M selenium ions). Electrodeposition proceeded
at a voltage of 3.00 VDC and 3.53 VAC superimposed thereon. The
composition of the as-deposited precursor layer, expressed as 1016 atoms/cm2,
was Cu1.OOIno.46Gao.o1Se1.16. The precursor layer was completed by PVD. The
finished device exhibited a conversion efficiency of 12.3 %.
Example 6
A metallic precursor film of In1-2Se1-3 was electrodeposited on glass
substrates coated with a Mo or Mo/Cu layer approximately 500 A thick. The
precursor film was deposited using an electroplating solution containing 2.25
gm
InC13 and 0.41 gm H2SeO3 dissolved in 200 ml of water. The pH of the solution
was adjusted between 1.4 and 2.4 using dilute HCI (10% by volume). The films
were deposited by applying a 2-5 V direct current voltage in combination with
an
alternating current voltage of 0.45 V at 18.1 KHz frequency. The films were 1-
3
m thick and adhered to the substrate.
Example 7
A metallic precursor film of Cu1-2Se1-3 was electrodeposited on a substrate
using an electroplating solution containing 6.21 gm Cu(N03)2=6H20 and 1.16 gm
H2SeO3 dissolved in 300 ml water. The pH was adjusted between 1.4 and 2.4
using dilute HC1 (10% by volume). The films were deposited by applying a 2-5
V direct current voltage in combination with an alternating current voltage of
T r,
CA 02284826 1999-09-22
Wfl 98/48079 PCT/US98/06212
0.45 V at 18.1 KHz frequency. As deposited layers were 1-3 m thick and
adhered to the substrate.
Example 8
5 A metallic precursor film of Cu1_2In1_2Se1-3 was electrodeposited on a
substrate using an electroplating solution containing 4.47 gm CuC12, 5.67 gm
InC13 and 3.39 gm H2SeSO3 dissolved in 1050 ml water. The pH was adjusted
between 1.4 and 2.4 using dilute HCI (10% by volume). The films were
deposited by applying a 2-5 V direct current voltage in combination with an
10 alternating current voltage of 0.45 V at 18.1 KHz frequency. As deposited
layers
were 1-3 m thick and adhered to the substrate. The electrodeposited film was
slightly indium poor. Indium was then added by vapor deposition to adjust the
final content to approximately CuInSe2. CdS and ZnO were then added to
complete the solar cell. The resulting solar cell was exposed to ASTM E892-87
15 Global (1000 Wm 2) standard irradiance spectrum at 25 C. Performance
parameters for the finished solar cell, having an area of 0.4285 cm2, were
measured as:
-------------------------------------------------------------------------------
-----------------
Voc =0.4138V Vp.. =0.3121V
ISc = 15.40 mA Ip,,. = 12.96 mA
J5C = 35.94 mA cm 2 Pm,,, = 4.045 mW
Fill Factor = 63.47% Efficiency = 9.44%
-------------------------------------------------------------------------------
--------------
CA 02284826 1999-12-23
16
..
The device contained only Cu-In-Se, without any gallium. The device
exhibited an efficiency of 8.76 % without antireflective coating, and 9.44 %
after
an antireflective coating was added.
Example 9
A precursor film of Cu12In1-2Gao.01-1Se1-3 was electrodeposited using a
solution containing 1.12 gm Cu(N03)2=6H20, 12.0 gm InC13, 4.60 gm
Ga(NO3)3=xH2O and 1.80 gm H2SeO3 dissolved in 450 ml of water. This is
equivalent to approximately 2.49 gm/1 Cu(N03)2=6H20, 26.7 gnV1 InC13, 10.2
gm/1 Ga(NO3)3=xH2O and 4.0 gm/1 H2SeO3, and approximately 0.0084, 0.12,
0.28, and 0.31 molar of copper, indium, gallium, and selenium ions,
respectively. The pH was adjusted between 1.4 and 2.4 using dilute HC1 (10%
by volume). The films were deposited by applying a 2-5 V direct current
voltage
in combination with an alternating current voltage of 0.45 V at 18.1 KHz
frequency. As deposited layers were 1-3 m thick and adhered to the substrate.
Example 10
A precursor film of Cu1-21n1-2Ga0_01-1Se1.3 was electrodeposited using a
solution containing 1.496 gm Cu(No3)=5H20, 14.929 gm InC13, 1.523 gm
H2SeO3, and 7.192 gm Ga(NO3)3 dissolved in 450 ml of DMSO. The films were
deposited at 25 C and also at 50 C at an applied voltage of 5 VDC.
Example 11
A precursor film of Cu1-2In1_2GaQ 01-1Se1-3 was electrodeposited using a
solution containing 1.496 gm Cu(No3)=5H2O, 14.929 gm InC13, 1.523 gm
H2SeO3, and 7.192 gm Ga(NO3)3 dissolved in a mixture of 400 ml DMSO and 50
ml water. The films were deposited at 25 C and also at 50 C at an applied
voltage of 5 VDC.
CA 02284826 1999-09-22
WO 98/48079 PCT/US98/06212
17
Example 12
A precursor film of Cu1_2In1_2Ga0,01_1Se1_3 was electrodeposited using a
solution containing 1.496 gm Cu(No3)=5H2O, 14.929 gm InC13, 1.523 gm
H2SeO3, 7.192 gm Ga(N03)3, and 10 gm Na2SO4, and 20 gm LiCI dissolved in a
mixture of 400 ml DMSO and 50 ml water. The films were deposited at 25 C
and also at 50 C at an applied voltage of 5 VDC.
The present invention as described above may be incorporated in a variety
of applications, as for example the conversion of solar energy to electric
energy
for baseline power generation. Other applications include appliances such as
solar-powered calculators, battery charges such as those used with freeway
emergency call boxes, photoelectric eyes, night security light activators,
light
meters for photographic and other purposes, and the like.
Although the present invention has thus been described in detail with
regard to the preferred embodiments and drawings and examples thereof, it
should be apparent to those skilled in the art that various adaptations and
modifications of the present invention may be accomplished without departing
from the spirit and the scope of the invention. Accordingly, it is to be
understood that the detailed description and the accompanying drawings as set
forth hereinabove are not intended to limit the breadth of the present
invention,
which should be inferred only from the following claims and their
appropriately
construed legal equivalents.