Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02286909 1999-10-12
WO 98/46605 PGT/US98/07207
5,7-DISUBSTITUTED 4-AMINOPYRIDO[2,3-D]PYRIMIDINE COMPOUNDS AND THEIR USE AS
ADENOSINE KINASE INHIBITORS
Technical Field
The present invention relates to a method of inhibiting adenosine kinase by
administering 5,7-disubstituted-4-aminopyrido[2,3-d]pyrimidine compounds, to
pharmaceutical compositions containing such compounds, as well as to certain
5,7-
disubstituted-4-aminopyrido[2,3-d)pyrimidine compounds.
Background Of The Invention
Adenosine kinase (ATP:adenosine 5'-phosphotransferase, EC 2.7.1.20) is a
ubiquitous enzyme which catalyzes the phosphorylation of adenosine to AMP,
using ATP,
preferentially, as the phosphate source. Adenosine kinase has broad tissue and
species
distribution, and has been isolated from yeast, a variety of mammalian sources
and certain
microorganisms. It has been found to be present in virtually every human
tissue assayed
including kidney, liver, brain, spleen, placenta and pancreas. Adenosine
kinase is a key
enzyme in the control of the cellular concentrations of adenosine.
Adenosine is a purine nucleoside that is an intermediate in the pathways of
purine
nucleotide degradation and salvage. Adenosine also has many important
physiologic
2o effects, many of which are mediated through the activation of specific
ectocellular receptors,
termed P 1 receptors (Burnstock, in Cell Membrane Receptors for Drugs and
Hormones,
1978, (Bobs and Straub, eds.) Raven, New York, pp. 107-118; Fredholm, et al.,
Pharmacol. Rev. 1994, 46: 143-156).
In the central nervous system, adenosine inhibits the release of certain
neurotransmitters (Corradetti, et al., Eur. J. Pharmacol. 1984,104: 19-26),
stabilizes
membrane potential (Rudolphi, et al., Cerebrovasc. Brain Metab. Rev. 1992, 4:
346-360),
functions as an endogenous anticonvulsant (Dragunow, Trends Pharmacol. Sci.
1986, 7:
128-130) and may have a role as an-endogenous neuroprotective agent (Rudolphi,
et al.,
Trends Pharmacol. Sci., 1992, 13: 439-445). Adenosine may play a role in
several
3o disorders of the central nervous system such as schizophrenia, anxiety,
depression and
Parkinson's disease. (Williams, M., in Psychopharmacology: The Fourth
Generation of
Progress; Bloom, Kupfer (eds.), Raven Press, New York, 1995, pp 643-655.
Adenosine has also been implicated in modulating transmission in pain pathways
in
the spinal cord (Sawynok, et al., Br. J. Pharmacol., 1986, 88: 923-930), and
in mediating
the analgesic effects of morphine (Sweeney, et al., J. Pharmacol. Exp. Ther.
1987, 243:
CA 02286909 1999-10-12
WO 98146605 PCT/US98/07207
657-665). In the immune system, adenosine inhibits certain neutrophil
functions and
exhibits and-inflammatory effects (Cronstein, J. Appl. Physiol. 1994, 76: 5-
13). An AK
inhibitor has been reported to decrease paw swelling in a model of adjuvant
arthritis in rats
(Firestein, et.al., Arthritis and Rheumatism, 1993, 36, S48.
Adenosine also exerts a variety of effects on the cardiovascular system,
including
vasodilation, impairment of atrioventricular conduction and endogenous
cardioprotection in
myocardial ischemia and reperfusion (Mullane and Williams, in Adenosine and
Adenosine
Receptors, 1990 (Williams, ed.) Humana Press, New Jersey, pp. 289-334). The
widespread actions of adenosine also include effects on the renal,
respiratory,
gastrointestinal and reproductive systems, as well as on blood cells and
adipocytes.
Adenosine, via its A1 receptor activation on adipocytes, plays a role in
diabetes by inhibiting
Iipolysis [Londos, et al., Proc. Natl. Acad. Sci. USA, 1980, 77, 2551.
Endogenous adenosine release appears to have a role as a natural defense
mechanism
in various pathophysiologic conditions, including cerebral and myocardial
ischemia,
seizures, pain, inflammation and sepsis. While adenosine is normally present
at low levels
in the extracellular space, its release is locally enhanced at the sites) of
excessive cellular
activity, trauma or metabolic stress. Once in the extracellular space,
adenosine activates
specific extracellular receptors to elicit a variety of responses which tend
to restore cellular
function towards normal (Bruns, Nucleosides Nucleotides, 1991, 10: 931-943;
Miller and
Hsu, J. Neurotrauma, 1992, 9: S563-S577). Adenosine has a half life measured
in
seconds in extracellular fluids (Moser, et al., Am. J. Physiol. 1989, 25: C799-
C806), and
its endogenous actions are therefore highly localized.
The inhibition of adenosine kinase can result in augmentation of the local
adenosine
concentrations at foci of tissue injury, further enhancing cytoprotection.
This effect is likely
to be most pronounced at tissue sites where trauma results in increased
adenosine
production. thereby minimizing systemic toxicities.
Pharmacologic compounds directed towards adenosine kinase inhibition provide
potentially effective new therapies for disorders benefited by the site- and
event-specific
potentiation of adenosine. Disorders where such compounds may be useful
include
ischemic conditions such as cerebral ischemia, myocardial ischemia, angina,
coronary artery
bypass graft surgery (CABG), percutaneous transluminal angioplasty (PTCA),
stroke, other
thrombotic and embolic conditions, and neurological disorders such as
epilepsy, anxiety,
schizophrenia, nociperception including pain perception, neuropathic pain,
visceral pain, as
well as inflammation, arthritis, immunosuppression, sepsis, diabetes and
gastrointestinal
disfunctions such as abnormal gastrointestinal motility.
A number of compounds have been reported to inhibit adenosine kinase. The most
potent of these include 5'-amino-5'-deoxyadenosine (Miller, et al., J. Biol.
Chem. 1979,
-2-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
254: 2339-2345), 5-iodotubercidin (Wotring arid Townsend, Cancer Res. 1979,
39: 3018-
3023) and 5'-deoxy-5-iodotubercidin (Davies, et al., Biochem. Pharmacol. 1984,
33: 347-
355).
Adenosine kinase is also responsible for the activation of many
pharmacologically
active nucleosides (Miller, et al., J. Biol. Chem. 1979, 254: 2339-2345),
including
tubercidin, formycin, ribavirin, pyrazofurin and 6-(methylmercapto)purine
riboside. These
purine nucleoside analogs represent an important group of antimetabolites
which possess
eytotoxic, anticancer and antivirai properties. They serve as substrates for
adenosine kinase
and are phosphorylated by the enzyme to generate the active form. The loss of
adenosine
kinase activity has been implicated as a mechanism of cellular resistance to
the
pharmacological effects of these nucleoside analogs (e.g. Bennett, et al.,
Mol. Pharmacol.,
1966, 2: 432-443; Caldwell, et al., Can. J. Biochem., 1967, 45: 735-744;
Suttle, et al.,
Europ. J. Cancer, 1981, 17: 43-51). Decreased cellular levels of adenosine
kinase have
also been associated with resistance to the toxic effects of 2'-deoxyadenosine
(Hershfield
and Kredich, Proc. Natl. Acad. Sci. USA, 1980, 77: 4292-4296). The
accumulation of
deoxyadenosine triphosphate (dATP), derived from the phosphorylation of 2'-
deoxyadenosine, has been suggested as a toxic mechanism in the immune defect
associated
with inheritable adenosine deaminase deficiency (Kredich and Hershfield, in
The Metabolic
Basis of Inherited Diseases, 1989 (Scriver, et al., eds.}, McGraw-Hill, New
York, pp.
1045-1075).
B.S. Hurlbert et al. (J. Med. Chem., 1 l: 711-717 (1968)) disclose various 2,4-
diaminopyrido[2,3-d]pyrimidine compounds having use as antibacterial agents.
R. K.
Robins et al. (J. Amer. Chem. Soc., 80:3449-3457 (1958)) disclose methods for
preparing
a number of 2,4-dihydroxy-, 2,4-diamino-, 2-amino-4-hydroxy- and 2-mercapto-4-
2S hydroxypyrido[2,3-d]pyrimidines having antifolic acid activity. R. Sharma
et al., (Indian J.
Chem., 31B: 719-720 (1992)) disclose 4-amino-5-(4-chlorophenyl)-7-(4-
nitrophenyl)pyrido(2,3-d]pyrimidine and 4-amino-5-(4-methoxyphenyl)-7-(4-
nitrophenyl)pyrido[2,3-d]pyrimidine compounds having antibacterial activity.
A. Gupta et
al., (J. Indian Chem. Soc., 71: 635-636 (1994)) disclose 4-amino-5-(4-
fluorophenyl)-7-(4-
3o fluorophenyl)pyrido[2,3-d]pyrimidine and 4-amino-5-(4-chlorophenyl)-7-(4-
fluorophenyl)pyrido[2,3-d]pyrimidine compounds having antibacterial activity.
L. Prakash
et al., Pharmazie, 48: 221-222 (1993)) disclose 4-amino-5-phenyl-7-(4-
aminophenyl)pyrido[2,3-d]pyrimidine, 4-amino-5-phenyl-7-(4-
bromophenyl)pyrido[2,3-
d]pyrinudine, 4-amino-5-{4-methoxyphenyl}-7-(4-aminophenyl)pyrido[2,3-
d]pyrimidine,
- 35 and 4-amino-5-(4-methoxyphenyl)-7-(4-bromophenyl)pyrido[2,3-d]pyrimidine
compounds
having antifungal activity. P. Victory et al., Tetrahedron, 51: 10253-10258
(1995))
discloses the synthesis of 4-amino-5,7-diphenylpyrido[2,3-d]pyrimidine
compounds from
-3-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
acyclic precursors. Bridges et al.(PCT application WO 95/19774, published July
27, 1995)
disclose various bicyclic heteroaromatic compounds as having utility for
inhibiting tyrosine
kinase of epidermal growth factors.
Summarv Of The Invention
The present invention provides for 5,7-disubstituted-4-aminopyrido[2,3-
d)pyrimidine compounds having utility as adenosine ldnase inhibitors.
In one aspect, the present invention provides a method of inhibiting adenosine
kinase
by administering a compound of formula (n
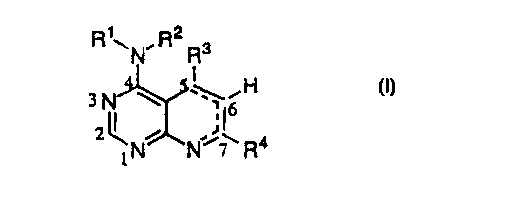
RyN,R2Rs
I
H
io ~N N~~ R4
(n
wherein
R 1 and R2 are independently selected from H, loweralkyl, C 1-C6alkoxyC 1-
C6alkyl, arylCl-C6alkyl, -C(O)C1-C6alkyl, -C(O)aryl, -C(O)heterocyclic or may
join
together with the nitrogen to which they are attached to form a 5-7 membered
ring optionally
containing 1-2 additional heteroatoms selected from O, N or S;
R3 is selected from the group consisting of loweralkyl, loweralkenyl,
loweralkynyl,
cycloallcyl, aryl, arylalkyl, heteroaryl, heterocyclic group, heteroarylalkyl
or
heterocycloalkyl wherein the heteroaryl and heterocyclic groups are linked
directly or
indirectly by a ring carbon;
R4 is selected from the group consisting of loweralkyl, loweralkenyl,
loweralkynyl,
cycloallcyl, aryl, arylalkyl, heteroaryl, heterocyclic group heteroarylalkyl
or
heterocycloalkyl; and a dashed line --- indicates that a double bond is
optionally present
provided that proper valencies are maintained.
In particular, the method of inhibiting adenosine kinase comprises exposing an
adenosine kinase to an effective inhibiting amount of a compound of Formula I
of the
present invention. Where the adenosine kinase is located in vivo, the compound
is
administered to the organism.
In still another aspect, the present invention provides a method of treating
ischemia,
neurological disorders, nociperception , inflammation, immunosuppression,
gastrointestinal
disfunctions, diabetes and sepsis in a mammal in need of such treatment,
comprising
administering to the mammal a therapeutically effective amount of a compound
of Formula I
of the present invention.
In a preferred aspect, the present invention provides a method of treating
cerebral
-4-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
ischemia, myocardial ischemia, angina, coronary artery bypass graft surgery,
percutaneous
transluminal angioplasty, stroke, thrombotic and embolic conditions, epilepsy,
anxiety, .
schizophrenia, pain perception, neuropathic pain, visceral pain, arthritis,
sepsis, diabetes
and abnormal gastrointestinal motility in a mammal in need of such treatment,
comprising
administering to the mammal a therapeutically effective amount of a compound
of Formula I
of the present invention.
The present invention also contemplates the use of pharmaceutically acceptable
salts
and amides of compounds having Formula I.
In another aspect, the present invention.provides a compound of formula (I)
RvN~R2 Rs
H
,6
2N i
to ~N N'~~ R4
(I)
wherein
R 1 and R2 are independently selected from H, loweralkyl, C 1-C6alkoxyC 1-
C6aikyl, arylC I -C6alkyl, -C(O)C 1-C6alkyl, -C{O)aryl; -C(O)heterocyclic or
may join
together with the nitrogen to which they are attached to form a 5-7 membered
ring optionally
15 containing 1-2 additional heteroatoms selected from O, N or S;
R3 is selected from the group consisting of loweralkyl, loweralkenyl,
loweralkynyl,
cycloalkyl, aryl, arylalkyl, heteroaryl, heterocyclic group, heteroarylalkyl
or
heterocycloalkyi wherein the heteroaryl and heterocyclic groups are linked
directly or
indirectly by a ring carbon;
20 R4 is selected from the group consisting of loweralkyl, loweralkenyl,
loweralkynyl,
cycloalkyl, aryl, arylalkyl, heteroaryl, heterocyclic group heteroarylalkyl or
heterocycloalkyl; and a dashed line --- indicates that a double bond is
optionally present
provided that proper valencies are maintained;
with the proviso that the compound may not be selected from the group
consisting
25 of:
(a) 4-amino-5-(4-chlarophenyl}-7-{4-nitrophenyl)pyrido[2,3-d]pyrimidine;
(b) 4-amino-5-(4-methoxyphenyl)-7-(4-nitrophenyl)pyrido[2,3-d]pyrimidine;
(c) 4-amino-5-(4-fluorophenyl)-7-(4-fluorophenyl)pyrido[2,3-d]pyrimidine;
(d) 4-amino-5-(4-chlorophenyl)-7-(4-fluorophenyl)pyrido[2,3-d]pyrinudine;
30 (e) 4-amino-5-phenyl-7-(4-aminophenyl)pyrido[2,3-d]pyrimidine;
(f) 4-amino-5-phenyl-7-(4-bromophenyl)pyrido[2,3-d]pyrimidine;
(g) 4-amino-5-(4-methoxyphenyl)-7-(4-aminophenyl)pyrido[2,3-d]pyrimidine;
(h) 4-amino-5-(4-methoxyphenyl)-7-(4-bromophenyl)pyrido[2,3-d]pyrimidine; and
-5-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
(i) 4-amino-5,7-diphenylpyrido[2,3-d]pyrimidine.
In another aspect, the present invention provides a pharmaceutical composition
comprising a therapeutically effective amount of a compound of Formula I above
in
combination with a pharmaceutically acceptable carrier.
In another aspect, the present invention provides a process for the
preparation of
adenosine kinase inhibiting compounds having the formula
R~~N, R2 R3
I
3N 4~ \6H
i~ 4
1 N N ~ R , wherein R 1 and RZ are hydrogen,
the method comprising
(a) contacting a ketone having the formula R4-CO-CH3, wherein R4 is as defined
above,
with an aldehyde having the formula R3-CHO, wherein R3 is as defined above and
malononitrile in the presence of an ammonium salt under anhydrous conditions
and isolating
a first intermediate compound having the structure
R3
NC
4
H2N N R
(b) contacting the first intermediate compound with formamide at reflux for
from about 1 to
about 8 hours, and isolating the compound of formula I with double bonds
between the 7
and 8 position and the 5 and 6 position and optionally reducing to form a
partially saturated
right side or a fully saturated right side to form a compound of formula I.
Detailed De, cription of the Invention
The present invention relates to 5,7-disubstituted-4-aminopyrido[2,3-
d]pyrimidine
compounds that are useful in inhibiting adenosine kinase, to pharmaceutical
compositions
containing such compounds, to a method of using such compounds for inhibiting
adenosine
kinase, and to novel 5,7-disubstituted-4-aminopyrido[2,3-dJpyrimidine
compounds.
In one aspect, the present invention provides 5,7-disubstituted-4-
aminopyrido[2,3-
d]pyrimidine compounds that are adenosine kinase inhibitors. An adenosine
kinase
inhibitor of the present invention is a compound of the Formula I, shown
above.
As summarized above, the present invention relates to a method of inhibiting
3U adenosine kinase comprising administering a compound of formula l
-6-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
R ~~N- R2 R3
H
3 N ~,
2~ i u6
1 N N ~~ R a (I)
wherein
RI and R2 are independently selected from H, loweralkyl, arylC1-C6alkyl, -
C(O)C1-C6allcyl, -C(O)aryl, -C(O)heterocyclic or may join together with the
nitrogen to
which they are attached to from a 5-7 membered ring optionally containing 1-2
additional
heteroatoms selected from O, N or S;
R3 and R4 are independently selected from the group consisting of:
C1-C6~Yl~
C2-C6alkenyl,
C2-C~alkynyl,
C3-Cgcycloalkyl,
heteroarylCp-C6alkyl or substituted heteroarylCp-C6alkyl,
optionally substituted cycloalkyl,
arylCp-C6alkyl or substituted arylCp-C6alkyl,
heteroarylC2-C6alkenyl or substituted heteroarylC2-C6alkenyl,
arylC2-C6alkenyl or substituted arylC2-C6alkenyl,
heteroarylC2-C6alkynyl or substituted heteroarylC2-C6alkynyl,
aryIC2-C6alkynyl or substituted arylC2-C6alkynyl wherein the 1-4 heteroaryl or
aryl
substituents are independently selected from
halogen, oxo, C02R5, cyanoCl-C6alkyl, heteroarylCp-C6alkyl,
heterocyclicCp-C6alkyl, Cl-C6alkyloxy, C1-C6alkyloxyCl-C6alkyl,
arylCp-C6allcyl, arylCl-C6alkyloxy, RSR6NC(O), cyano, C2-C6alkenyl,
C2-C6alkynyl, C 1-C6alkyl, C2-C6allcenyldialkylmalonyl, CF3, HO-, C I-
C6alkyloxyCl-C6alkyloxy, CI-C6aIkylSOn wherein n is 1-2, C1-
C6alkylthio, Cl-C6alkylacryl, CF30, CF3, C1-C4alkylenedioxy, C1-
C~alkylacryl, RSR6N(CO)NRS, N-formyl(heterocyclic), NO2, NRSR6Cp-
C6alkyl, (R50)(R60)-P(O)- Cp-C6alkyl,
wherein RS and R6 are independently selected from H, C1-C6alkyl,
HC(O), Cl-C6alkyloxyCl-C6alkyl, CI-C6allcyloxy, CI-
3o C6alkylC(O), CF3C(O), NR~RgCI-C6alkyl, phthalimidoCl-
C(C(O), Cl-C6alkylSOn where n is 1-2, CNC1-C6alkyl,
R~R8NC(O)NR~-, heteroaryl, NR~RgCI-C6a1ky1C(O), C1-
C6allcyloxycarbamidoC 1-C6allcyl,
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
wherein R~ and Rg are independently selected from those
variables identified for RS and R6 or
RS and R6 or R~ and Rg may join together with the nitrogen atom to
which they are attached to form a 5-7 membered unsubstituted or
substituted ring optionally containing 1-3 additional heteroatoms
selected from O, N or S wherein the substituents are selected from
Cl-C6alkyl and
a dashed line --- indicates a double bond is optionally present.
The preferred compounds utilized in the above method of inhibiting adenosine
kinase are
selected from a compound of formula II
RvN,R2 R3
H
\~
2~N N' 'R4
1 (In
wherein Rl-Rg and n are as defined above. The present invention also relates
to
compounds of formula II with Rl-Rg and n as defined above with the proviso
that the
specific known compounds identified above for a compound of formula I are
excluded.
In a preferred embodiment, an adenosine kinase inhibitor of the present
invention is
a compound of Formula (II) above, wherein R4 is aryl or heteroaryl and
substituted versions
thereof.
In a more preferred embodiment, an adenosine kinase inhibitor of the present
invention is a compound of Formula (II) above, wherein R4 is aryl or
heteroaryl and
substituted versions thereof and R3 is loweralkyl, aryl, arylalkyl or
heteroaryl and
substituted versions thereof wherein the substituents are as identified above.
In another preferred embodiment, an adenosine kinase inhibitor of the present
invention is a compound of Formula (I) above, wherein R4 is selected from the
group
consisting of: phenyl; thiophene-2-yl; 3-methyl-2-oxobenzoxazolin-6-yl; 2-
(dimethyiamino)-
5-pyrimidinyl; 2-(N-formyl-N-methyl amino)-5-pyrimidinyl; 2-(N-methoxyethyl-N-
methyl
amino)-5-pyrimidinyi; 2-(N-methylamino)-5-pyrimidinyl; 2-(1-morpholinyl)-5-
pyrimidinyl;
2-(1-pyrrolidinyl)-5-pyrimidinyl; 2-dimethylamino-5-pyrimidinyl; 2-furanyl; 2-
oxobenzoxazolin-6-yl; 2-pyridyl; 3-(dimethylamino)phenyl; 3-amino-4-
methoxyphenyl; 3-
bromo-4-(dimethylamino)phenyl; 3-methoxyphenyl; 3-methyl-4-(N-acetyl-N-
methylamino)phenyl; 3-methyl-4-(N-formyl-N-methylamino)phenyl; 3-methyl-4-(N-
methyl-
N-(trifluoroacetyl)amino)phenyl; 3-methyl-4-{N-methylamino)phenyl; 3-methyl-4-
pyrrolidinylphenyl; 3-pyridyl; 3,4-dichlorophenyl; 3,4-methylenedioxyphenyl;
3,4,5-
trimethoxyphenyl; 4-(acetylamino)phenyl; 4-(dimethylamino)-3-fluorophenyl; 4-
_g_
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
(dimethylamino)phenyl; 4-(imidazol-1-yl)phenyl; 4-(methylthio)phenyl; 4-
(morpholinyl)phenyl; 4-(N-(2-(dimethylamino)ethyl)amino)phenyl; 4-(N-(2-
methoxyethyl)amino)phenyl; 4-(N-acetyl-N-methylamino)phenyl; 4-(N-ethyl-N-
formylamino)phenyl; 4-(N-ethylamino}phenyl; 4-(N-formyl-N-{2-
methoxyethyl}amino)phenyl; 4-(N-isopropylamino)phenyl; 4-(N-methyl-N-((2-
dimethylamino)ethyl)amino)phenyl; 4-(N-methyl-N-(2-(N-
phthalimidyl)acetyl}amino)phenyl; 4-(N-methyl-N-(2-cyano)ethylamino)phenyl; 4-
(N-
methyl-N-(2-methoxyethyl)amino)phenyl; 4-(N-methyl-N-(3-
methoxy)propionylamino)phenyl; 4-(N-methyl-N-acetylamino)phenyl; 4-(N-methyl-N-
formylamino)phenyl; 4-(N-methyl-N-trifluoroacetylamino)phenyl; 4-(N-
morpholinyl)phenyl; 4-(thiophene-2-yl)phenyl; 4-(ureido)phenyl; 4-(2-
(dimethylamino)acetylamino)phenyl; 4-(2-{2-
methoxy)acetylamino)ethyl)amino)phenyl; 4-
(2-methoxy)ethoxyphenyl; 4-(2-oxo-3-oxazolidinyl)phenyl; 4-(4-methoxy-2-
butyl)phenyl;
4-(4-methylpiperidinyl)phenyi; 4-(5-pyrimidinyl)phenyl; 4-aminophenyl; 4-
bromophenyl;
4-butoxyphenyl; 4-carboxamidophenyl; 4-chlorophenyl; 4-cyanophenyl; 4-
diethylaminophenyl; 4-diethylmalonylallylphenyl); 4-dimethylaminophenyl; 4-
ethoxyphenyl; 4-ethylphenyl; 4-fluorophenyl; 4-hydroxyphenyl; 4-
imidazolylphenyl; 4-
iodophenyl; 4-isopropylphenyl; 4-methoxyphenyl; 4-methylaminophenyl; 4-
methylsulfonylphenyl; 4-morpholinylphenyl; 4-N-(2-(dimethylamino)ethyl)-N-
formylamino)phenyl; 4-N-(3-methoxypropionyl)-N-isopropyl-amino)phenyl; 4-N-
ethyl-N-
(2-methoxyethyl)amino)phenyl; 4-N-formylpiperazinylphenyl; 4-nitrophenyl; 4-
piperidinylphenyl; 4-(3-pyridyl)phenyl; 4-pyrrolidinylphenyl; 4-t-
butylacrylphenyl; 5-
(dimethylamino)thiophene-2-yl; 5-amino-2-pyridyl; 5-dimethylamino-2-pyrazinyl;
3-
dimethylaminopyridazin-6-yl; 5-dimethylamino-2-pyridyl; 5-pyrimidinylphenyl; 6-
(N-
methyl-N-formylamino)-3-pyridinyl; 6-(N-methyl-N-methoxyethylamino)-3-
pyridinyl; 6-(2-
oxo-3-oxazolidinyl)-3-pyridinyl; 6-dimethylamino-3-pyridinyl; 6-imidazolyl-3-
pyridinyl; 6-
morpholinyl-3-pyridinyl; 6-pyrrolidinyl-3-pyridinyl; 6-(2-propyl)-3-pyridinyl;
and (4-
formylamino)phenyl.
In another preferred embodiment, an adenosine ldnase inhibitor of the present
invention is a compound of Formula (I) above, wherein R3 is selected from the
group
consisting of: (thiophene-2-yl)methyl; (thiophene-3-yl)methyl; butyl;
cycloheptyl; pentyl;
thiophene-2-y1; 1-(3-bromophenyl)ethyl; 2-(N-
phenylmethoxycarbonyl)aminophenyl; 2-(3-
bromophenyl)ethyl; 2-(3-cyanophenyl)methyl; 2-(4-bromophenyl)ethyl; 2-(S-
chloro-2-
(thiophen-3-yl)phenyl; 2-bromophenyl; 2-furanyl; 2-methylpropyl; 2-
phenylethyl;
3s phenylmethyl; 2,3-dimethoxyphenyl; 2,3-methylenedioxyphenyl; 3-(furan-2-
yl)phenyl; 3-
(thiophen-2-yl)phenyI; 3-(2-pyridyl)phenyl; 3-(3-methoxybenzyl)phenyl; 2-(3-
aminopropynyl)phenylmethyl; 3-benzyloxyphenyl; 3-bromo-4-fluorophenyl; 3-bromo-
5-
-9-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
iodophenyl; 3-bromo-5-methoxyphenyl; 3-bromophenyl; 3-bromophenylmethyl; 3-
carboxamidophenyl; 3-chlorophenyl;.3-cyanophenyl; 3-diethylmalonylallylphenyl;
3-
dimethylaminophenyl; 3-ethoxyphenyl; 3-fluoro-5-trifluoromethylphenyl; 3-
fluoiophenyl; 3-
hydroxyphenyl; 3-iodophenyl; 3-methoxyethyoxyphenyl; 3-methoxyphenyl; 3-
methylphenyl; 3-methylsulfonylphenyl; 3-methylthiophenyl; 3-t-
butylacrylphenyl; 3-
trifloromethyoxyphenyl; 3-trifluoromethylphenyl; 3-vinylpyridinylphenyl; 3,4-
dichlorophenyl; 3,4-dimethoxyphenyl; 3,4-methylenedioxyphenyl; 3,4,5-
trimethoxyphenyl;
3,5-di(trifluoromethyl)phenyl; 3,5-dibromophenyl; 3,5-dichlorophenyl; 3,5-
dimethoxyphenyl; 3,5-dimethylphenyl; 4-(2-propyl)phenyl; 4-(2-
propyl)oxyphenyl; 4-
i0 benzyloxyphenyl; 4-bromophenyl; 4-bromothiophene-2-yl; 4-butoxyphenyl; 4-
dimethylaminophenyl; 4-fluoro-3-trifluoromethylphenyl; 4-methoxyphenyl; 4-
neopentylphenyl; 4-phenoxyphenyl; 5-bromothiophene-2-yl; 5-cyclohexyl; 5-
cyclopropyl;
5-hexyl; 5-methyl; 5-phenyl; (2-bromo-5-chlorophenyl)methyl; (2-
bromophenyl)methyl; and
(5-chloro-2-(3-methoxyphenyl)phenyl)methyl.
Exemplary and preferred adenosine ldnase inhibitor compounds of the invention
utilized in the method recited herein include the compounds listed below
wherein R1 and R2
in a compound of formula II are selected from H, the groups identified at the
5-position are
included within R3 and the groups identified at the 7-position are included
within R4, RS-
Rg are as described in the specific compound:
4-amino-5-(4-dimethylaminophenyl)-7-(4-bromophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(4-dimethylaminophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(4-methoxyphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-S-(4-dimethylaminophenyl)-7-(4-methoxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(4-(2-propyl)phenyl)-7-(4-methoxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(4-neopentylphenyl)-7-(4-methoxyphenyl)pylzdo[2,3-d]pyrimidine;
4-amino-5-(4-butyloxyphenyl)-7-(4-methoxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(4-methoxyphenyl)-7-(4-bromophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(4-(2-propyl)oxyphenyl)-7-(4-methoxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(4-butoxyphenyl)-7-(4-N-formylpiperazinylphenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(4-benzyloxyphenyl)-7-(4-methoxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(4-phenoxyphenyI)-7-(4-methoxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(4-(2-propyl)phenyl)-7-(4-diethylmalonylallylphenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(4-(2-propyl)phenyl)-7-(4-t-butylacrylphenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
-10-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
4-amino-5-(3,4-dimethoxyphenyl)-7-(4-dimethylaminophenyl)pyrido [2,3-
d]pyrimidine;
4-amino-5-(3-t-butylacrylphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-methoxyphenyl-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3,5-dimethoxyphenyl-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-diethylmalonylallylphenyl)-7-{4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-vinylpyridinylphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-trifluoromethylphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]PY~dine;
4-amino-5-(3-carboxamidophenyl)-7-{4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-cyanophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-benzyloxyphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-methoxyphenyl)-7-(4-methoxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-butoxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-(2-pyridyl)phenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-methylphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-chlorophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-fluorophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-methoxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-methoxyphenyl)-7-(4-bromophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-phenylpyrido [2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-ethylphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-bromophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-cyanophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-hydroxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-iodophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-ethoxyphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-trifloromethyoxyphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3,5-dichlorophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrirnidine;
-11-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
4-amino-5-(3-bromo-4-fluorophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-hydroxyphenyl}-7-(4-dimethylaminophenyl)pyrido [2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-morpholinylphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-{4-piperidinylphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(imidazol-1-yl)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-chlorophenyl)pyrido[2,3 ~d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-isopropylphenyl)pyrido[2,3-d]pyrimidine;
4-amino-S-(3-bromophenyl)-7-(4-trifluorophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-diethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-S-(3-bromophenyl)-7-(3,4,5-trimethoxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-{3-methoxybenzyl)phenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
'4-amino-5-(3-methoxyethyoxyphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3,4-methylenedioxyphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]PY~dine;
4-amino-5-(3-bromophenyl)-7-(4-ethoxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(2'-thiophene)pyrido[2,3-d]pyrimidine;
4-amino-S-(3-bromophenyl)-7-{4-fluorophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-dimethylaminophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-phenyl-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3,4,5-trimethoxyphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-nitrophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-{4-iodophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-{3-bromophenyl)-7-(3,4-methylenedioxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(thiophen-2-yl)-7-(4-morpholinylphenyl)pyrido [2,3-d]pyrimidine;
4-amino-5-(3,5-dimethoxyphenyl)-7-(thiophen-2-yle)pyrido [2,3-d]pyrimidine;
4-amino-5-{3-bromophenyl)-7-(4-carboxamidophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-{4-(2-methoxy)ethoxyphenyl)pyrido[2,3-
d]PY~dine;
4-amino-5-(3,5-dimethoxyphenyl)-7-(4-morpholinylphenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-trifluoromethylphenyl)-7-(thiophene-2-yl)pyrido [2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-aminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromo-4-fluorophenyl)-7-(thiophene-2-yl)pyrido [2,3-d]pyrimidine;
-12-
CA 02286909 1999-10-12
WO 98!46605 PCT/ITS98/07207
4-amino-5-(3-bromo-4-fluorophenyl)-7-(2-furanyl)pyrido [2,3-d]pyrimidine;
4-amino-5-(3,5-dimethoxyphenyl)-7-(4-iodophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3,5-dimethoxyphenyl)-7-(4-imidazolylphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3,5-dimethoxyphenyl)-7-(4-(thiophene-2-yl)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3,5-dimethoxyphenyl)-7-(4-(3-pyridyl)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(4-methylpiperidinyl)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-S-(3-bromophenyl)-7-(4-pyrrolidinylphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(4-bromothiophene-)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(4-bromothiophene-2-yl)-7-(4-morpholinylphenyl)pyrido[2,3
d]pyrimidine;
4-morpholinyl-5-(3-bromophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(5-bromothiophene-2-yl)-7-(4-morpholinylphenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(4-bromophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(acetylamino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3,S-dimethoxyphenyl)-7-(5-pyrimidinylphenyl)pyrido[2,3-
d]pyrimidine;
4-(4-fluorophenyl)amino)-5-(3-bromophenyl)-7-(4-
dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(4-bromothiophene-2-yl)-7-(4-pyrrolidinylphenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(4-bromothiophene-2-yl)-7-(thiophene-2-yl)pyrido[2,3-d]pyrimidine;
4-amino-~-(3-bromophenyl)-7-(5-(dirnethylamino)thiophene-2-yl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromo-5-iodophenyl)-7-(4-(dimethylamino)phenyl)pyrido [2,3-
d]pyrimidine;
4-amino-5-(3,5-di(trifluorornethyl)phenyl)-7-(4-
(dimethylamino)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-S-(3,5-di(trifluoromethyl)phenyl)-7-(4-morpholinylphenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3,5-dibromophenyl)-7-(4-(dimethylamino)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3,5-dibromophenyl)-7-(4-morpholinylphenyl)pyrido[2,3-d]pyrimidine;
-13-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
4-amino-5-(4-bromothiophene-2-yl)-?-(4-(4-methylpiperidinyi)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3,5-dibromophenyl)-?-(4-(dimethylamino)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-?-(3-(dimethylamino)phenyl}pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-?-(4-methylsulfonylphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-?-(3-methoxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-?-(4-(methylthio)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-?-(3,4-dichlorophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-?-(4-{N-methyl-N-formylamino)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-?-(4-methylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromo-4-fluorophenyl)-?-(4-methylsulfonylphenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-?-(3-amino-4-methoxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-?-(3-bromo-4-(dimethylamino)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-?-(3-methyl-4-(dimethylamino)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-S-(3-bromophenyl)-?-(4-(N-methyl-N-
trifluoroacetylamino)phenyl)pyrido [2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-?-(4-(dimethylamino)-3-fluorophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-?-(4-(N-ethyl-N-formylamino)phenyl)pyrido(2,3-
d]pyrimidine;
4,4-bis(acetylamino)-5-(3-bromophenyl)-?-(4-(N-methyl-N-
acetylamino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-?-(4-(N-acetyl-N-methylanvno)phenyl)pyrido[2,3-
d]pyrinudine;
4-amino-5-(3-bromophenyl)-?-(4-(N-ethylamino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-?-(4-(N-methyl-N-(2-
methoxyethyl)amino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-?-(4-(N-isopropylamino)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-?-(4-N-ethyl-N-(2-
methoxyethyl)amino)phenyl)pyrido[2,3-d]pyrimidine;
-14-
CA 02286909 1999-10-12
WO 98!46605 PCT/US98/07207
4-amino-5-(3-bromophenyl)-7-(4-N-(3-methoxypropionyl)-N-isopropyl-
amino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-N-(2-(dimethylamino)ethyl)-N-
formylamino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(N-(2-
(dimethylamino)ethyl)amino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(N-methyl-N-(2-
cyano)ethylamino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(N-methyl-N-(3-
methoxy)propionylamino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(3-methyl-4-(N-formyl-N-
methylamino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(3-methyl-4-(N-methylamino)phenyl)pyrido[2,3-
d]pyrimidine;
i5 4-amino-5-(3-bromophenyl)-7-(4-(4-methoxy-2-butyl)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(N-methyl-N-(2-(N-
phthalimidyl)acetyl)amino}phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(3-methyl-4-(N-methyl-N-
2o (trifluoroacetyl)amino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(3-methyl-4-(N-acetyl-N-
methylamino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-dimethy!amino-3-pyridinyl)pyrido[2,3-
d]pyrimidine;
25 4-amino-5-(3-cyanophenyl)-7-(4-methylsulfonylphenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-cyanophenyl)-7-(4-(N-methyl-N-formylamino)-phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-(N-methyl-N-formylamino)-3-
pyridinyl)pyrido[2,3-d]pyrimidine;
30 4-amino-5-(3-bromophenyl)-7-(6-morpholinyl-3-pyridinyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-(N-methyl-N-methoxyethylamino)-3
pyridinyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-pyrolidinyl-3-pyridinyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(2-(dimethylamino)-5-pyrimidinyl)pyrido[2,3-
35 d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(2-(N-methoxyethyl-N-methyl amino)-5-
pyrimidinyl)pyrido[2,3-d]pyrimidine;
-15-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
4-amino-5-(3-bromophenyl)-7-(2-(N-formyl-N-methyl amino)-5-
pyrimidinyl)pyrido[2,3-d)pyrimidine;
4-amino-5-(3-bromophenyl)-7-(2-(N-methylamino)5-pyrimidinyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(2-( 1-pyrrolidinyl)-5-pyrimidinyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(2-( 1-morpholinyl)-5-pyrimidinyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-(2-oxo-3-oxazolidinyl)-3-pyridinyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(2-pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(3-pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-S-(3-(thiophen-2-yl)phenyl)-7-(4-dimethylaminophenyl)pyrido(2,3-
d]pyrimidine;
4-amino-5-(3-(furan-2-yl)phenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-(3-methoxyphenyl)phenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pylimidine;
4-amino-5-phenyl-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-chlorophenyl)-7-(4-(morpholinyl)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromo-4-fluorophenyl)-7-(4-(morpholinyl)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-chlorophenyl)-7-(4-iodophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-chlorophenyl)-7-(4-(thiophen-2-yl)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-chlorophenyl)-7-(4-(5-pyrimidinyl)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromo-4-fluorophenyl)-7-(4-iodophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(4-bromothiophene-2-yl)-7-(4-methoxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)methyl-7-(4-(dimethylamino)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(2-phenylethyl)-7-(4-diethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(2-methylpropyl)-7-(4-diethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(butyl)-7-(4-diethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(2-(4-bromophenyl)ethyl)-7-(4-diethylaminophenyl)pyrido[2,3-
d]pylimidine;
4-amino-5-(butyl)-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(2-(3-cyanophenyl}methyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
-lb-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
4-amino-5-(2-(N-phenylmethoxycarbonyl)aminoethyl)-7-(4-
dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(cycloheptyl)-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(2-{5-chloro-2-(thiophen-3-yl)phenylmethyl)-7-(4-
dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-{pentyl)-7-(4-diethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-hexyl-7-(4-diethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(2-{3-bromophenyl)ethyl)-7-(4-diethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-((2-bromophenyl)methyl)-7-(4-diethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-cyclopropyl-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-cyclohexyl-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-((2-bromo-5-chlorophenyl)methyl)-7-(4-diethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-methyl-7-(4-diethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(2,3-methylenedioxyphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidirie;
4-amino-5-(3-fluoro-5-trifluoromethylphenyl)-7-(4-
dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(2-bromophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3,5-dimethylphenyl)-7-{4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-{3,4-dichlorophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(4-fluoro-3-trifluoromethylphenyl)-7-(4-
dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromo-5-methoxyphenyl)-7-(4-morpholinylphenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromo-5-methoxyphenyl)-7-(4-pyrrolidinylphenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromo-5-methoxyphenyl)-7-(4-piperidinylphenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromo-5-methoxyphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-methylthiophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromo-5-methoxyphenyl)-7-(thiophene-2-yl)pyrido[2,3-d]pyrimidine;
-17-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
4-amino-5-(2,3-dimethoxyphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-methylsulfonylphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pvrimidine;
4-acetylamino-5-(3-bromophenyl)-7-(4-dimethylaminophenyl)pvrido[2,3-
d]pyrimidine;
4-formylamino-5-(3-bromophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-(methoxyacetyl)amino-5-(3-bromophenyl)-7-(4-diethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-trifluoroacetylamino-5-(3-bromophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-pentanoylamino-5-(3-bromophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]PY~dine;
4-benzoylamino-5-(3-bromophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]PY~dine:
4-(N-BOC-glycyl)amino-5-(3-bromophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-(N-phthalimidylglycyl)amino-5-(3-bromophenyl)-7-{4-
2o dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-(ethoxycarbonyl)amino-5-(3-bromophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-(ethylaminocarbonyl)amino-5-(3-bromophenyl)-7-(4-
dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-allylamino-S-(3-bromophenyl)-7-(4-dimethylaminophenyl) pyrido[2,3-
d]pyrimidine;
4-(2-(N,N-dimethylamino)ethylamino)-5-(4-bromophenyl)-7-(4-
dimethylaminophenyl) pyrido[2,3-d]pyrimidine;
4-(4-(N,N-dimethylamino)butylamino)-5-(3-bromophenyl)-7-(4-
dimethylaminophenyl) pyrido[2,3-d]pyrimidine;
4-(N-allyl-N-formylamino)-5-(4-dimethylaminophenyl)-7-(4-
bromophenyl)pyrido[2,3-d]pyrimidine;
4-diacetylamino-5-(p-dimethylaminophenyl)-7-(4-
bromophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(5-amino-2-pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(5-dimethylamino-2-pyridyl)pyrido[2,3-
d]pyrimidine;
-18-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
4-amino-5-(3-bromophenyl)-7-(5-dimethylamino-2-
pyrazinyl)pyrido [2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(2-oxobenzoxazolin-6-yl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-( 1-methyl-2-oxobenzoxazolin-6-
yl)pyrido[2,3-d]pyrimidine;
4-amino-5-((5-chloro-2-(3-methoxyphenyl)phenyl)methyl)-7-(4-
dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-{(thiophene-2-yl)methyl)-7-(4-diethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-((thiophene-3-yl)methyl)-7-(4-diethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-((2-bromophenyl)methyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]PY~dine;
4-amino-5-(3-bromophenyl)-7-(4-(N-formyl-N-(2-
methoxyethyl)amino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(N-(2-methoxyethyl)amino)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-{N-methyl-N-((2-
dimethylamino)ethyl)amino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(2-
methoxy)acetylamino)ethyl)amino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-((4-formylamino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(2-(dimethylamino)acetylamino)phenyl)pyrido
[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-{2-oxo-3-oxazolidinyl)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-{6-(2-propyl)-3-pyridinyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(3-methyl-4-pyrrolidinylphenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-imidazolyl-3-pyridinyl)pyrido(2,3-d]pyrimidine;
4-amino-5-phenylmethyl-7-(4-diethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(2-(3-aminopropynyl)phenylmethyl)-7-(4-diethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-~-( 1-(3-bromophenyl)ethyl)-7-(4-diethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-~-(4-dimethylaminophenyl)-7-(4-bromophenyl)pyrido[2,3-d]pyrimidine;
4-amino-~-(2-furanyl)-7-(4-(N-morpholinyl)phenyl)pyrido[2,3-d]pyrimidine;
-19-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
4-amino-5-(3-bromophenyl)-7-(2-dimethylamino-5-pyrimidinyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(ureido)phenyl)pyrido [2,3-d]pyrimidine;
4-amino-5-( 1-phenylmethyl-3-piperidinyl)-7-(4-diethylaminophenyl)pyrido[2,3-
d]pyrimidsne;
4-amino-5-(3-bromophenyl)-7-(6-(3-methyl-5-isoxazolyl))-3-
pyridinyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-chloro-3-pyridinyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-methoxy-3-pyridinyl)pyrido[2,3-d]pyrimidine;
l0 4-amino-5-(3-bromophenyl)-7-(6-(1,2,4-triazol-4-yl)-3-pyridinyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(2-morpholinyl-5-pyrimidinyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(2-thiazolyl}-7-(4-pyrrolidinylphenyl)-pyrido[2,3-d]pyrimidine;
15 4-amino-5-(3-bromophenyl)-7-(6-pyrazolyl-3-pyridinyl))-pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-( 1-methyl-ureido)phenyl)-pyridoj2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(N-methyl-N-(2-pyrimidinyl)amino)phenyl)-
20 pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(3-fluoro-4-(N-formyl-N-methylamino)phenyl)-
pyrido[2,3-d]pyrimidine;
4-formylamino-5-(3-bromophenyl)-7-(3-fluoro-4-(N-formyl-N-
methylamino)phenyl)-pyrido[2,3-d]pyrimidine;
25 4-amino-5-(3-bromophenyl)-7-(4-(N-methyl-N-methylsulfonylamino)-
phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-(N-methyl-N-methylsulfonylamino)-3-
pyridinyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-( 1-methyl-5-indolinyl)pyrido[2,3-d]pyrimidine;
30 4-amino-5-(3-bromophenyl)-7-( 1-methyl-5-benzimidazolyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-dimethylamino-3-pyridazinyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3bromophenyl)-7-(6-morphoiinyl-3-pyridazinyl)pyrido[2,3-
35 d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-pyrrolidinyl-3-pyridazinyl)pyrido[2,3-
d]pyrimidine;
-20-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
4-amino-5-(3-bromophenyl)-7-(5-morpholinyl-2-pyrazinyl)pyrido(2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(5-(N-(2-methoxyethyl)-N-methylamino)-2-
pyrazinyl)pyrido [2,3-d]pvrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(morpholinylmethyl)-phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-{5-(N,N-bis(2-methoxyethyl)amino)-2-
pyridinyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(imidazolylmethyl)-phenyl)pyrido[2,3-
1o d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(S-( 1-morpholinyl)-2-pyridinyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-((dimethylamino)methyl)-phenyl)pyrido[2,3-
d]PY~dine:
15 4-amino-5-(3-bromophenyl)-7-(5-(4-hydroxy-1-piperidinyl)-2-
pyridinyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(S-(N-formyl-N-methylamino)-2-
pyridinyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(5-(2-propenyl)-2-pyridinyl)pyrido[2,3-
2o d]pyrimidine;
4-amino-S-(3-bromophenyl)-7-(3-(2-methoxyethyl)-2-oxo-6-
benzoxazolyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-( 1-(N-formylamino)-ethyl)phenyl)pyrido [2,3-
d]pyrimidine;
25 4-(methylamino)-5-(3-bromophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine hydrochloride;
4-(2-methoxyethylamino)-5-(3-bromophenyl)-7-(4-
dimethylaminophenyl)pyrido[2,3-d]pyrimidine hydrochloride;
4-amino-5-(3-bromophenyl)-7-(4-( 1-methyl-2-imidazolyl)phenyl)pyrido[2,3-
3o d]pyrimidine trihydrochloride;
4-amino-5-(3-bromophenyl)-7-(4-(aminomethyl)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(2-bromo-4-(dimethylamino)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(dimethylaminoethyl)phenyl)pyrido[2,3-
35 d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(3-(dimethylamino)propynyl)phenyl)pyrido[2,3-
d)pyrimidine;
-21-
CA 02286909 1999-10-12
WO 98/46605 PCT/fJS98/07207
4-amino-5-(3-bromophenyl)-7-(4-(3-amino-3-methylbutynyl)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-dimethylphosphonatophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(3-(methoxypropynyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-carboxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-methyl-3-oxo-2H-4H-pyrido[3,2-b]-1,4-oxazin-
7-yl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(2-(dimethylamino)ethyl)-3-oxo-2H-4H-
to pyrido[3,2-b]-1,4-oxazin-7-yl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(2,3-dihydro-3-(dimethylaminoethyl)-2-
oxobenzoxazol-6-yl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-methyl-3-oxo-2H-4H-benzo-1,4-oxazin-7-
yl)pyrido[2,3-d]pyrimidine;
15 4-amino-5-(3-bromophenyl)-7-(2,2,4-trimethyl-3-oxo-ZH-4H-benzo-1,4-oxazin-7-
yl)pyrido[2,3-d]pyrimidine;
4-amino-5-cyclohexyl-7-(4-(2-dimethylamino)ethyl)-2H-4H-benzo-3-oxo-1,4-
oxazin-7-yl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(5-( 1-methylethyl)-2-pyridyl)pyrido[2,3-
20 d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-{S-piperidin-1-ylpyrid-2-yl)pyrido[2,3-
d]pyrimidine;
4-amino-5-( 1-(4-bromophenyl)ethyl)-7-(6-morpholinylpyrid-3-yl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-{4-((N-formylamino)methyl)phenyl)pyrido[2,3-
25 d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-( 1-methyl-1-(N-methylamino)ethyl)phenyl)-
pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-( 1-(dimethylamino)-1-
methylethyl)phenyl)pyrido[2,3-d]pyrimidine;
30 4-amino-5-(3-bromophenyl)-7-(N-acetyl-5-indolinyl)pyrido[2,3-d]pyrimidine;
4-amino-5-cyclohexyl-7-(6-chloro-3-pyridyl)pyrido[2,3-d]pyrimidine:
4-amino-5-( 1-(2-bromophenyl}ethyl)-7-(6-diethylamino-3-pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-( 1-(2-bromophenyl)ethyl)-7-(6-morpholinyl-3-pyridyl)pyrido[2,3-
35 d]pyrimidine;
4-amino-5-( 1-(2-bromophenyl)ethyl)-7-(4-(N-methyl-N-formyl)amino)-
phenyl)pyrido[2,3-d]pyrimidine;
-22-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
4-amino-5-cyclohexyl-7-(6-morpholinyl-3-pyridyl)pyrido [2,3-d]pyrimidine;
4-amino-5-((2-bromophenyl)methyl)-7-(6-morpholinyl-3-pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(4-tetrahydropyranyl)-7-(6-morphoiinyl-3-pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-cyclohexyl-7-(6-dimethylamino-3-pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-5-( 1-ethylpropyl)-7-(6-dimethylamino-3-pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-cyclopentyl-7-(6-morpholinyl-3-pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-5-cyclohexyl-7-(2-chloro-3-pyridyl)pyrido[2,3-d]pyrimidine;
l0 4-amino-5-(3,5-dimethylcyclohexyl)-7-(6-dimethylamino-3-pyridyl)pyrido.[2,3-
d]pyrimidine;
4-amino-5-((N-(benzyloxycarbonyl)-4-piperidinyl)methyl)-7-(6-morpholinyl-3-
pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-5-cyclohexyl-7-(6-bromo-3-pyridyl)pyrido[2,3-d]pyrimidine;
15 4-amino-5-cyclohexyl-7-(3-cyanophenyl)pyrido[2,3-d]pylimidine;
4-amino-5-{ t -(2-bromophenyl)ethyl)-7-(6-dimethylamino-3-pyridazinyl)pyrido
[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-imidazolyl-3-pyridazinyl)pyrido(2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-{6-(azacycloheptanyl)-3-pyridazinyi)pyrido[2,3-
20 d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-(N-methyl-N-( 1-methylethyl))amino)-3-
pyridazinyl)pyrido[2,3-d]pyrimidine;
4-amino-5-{ 1-(2-bromophenyl~thyl)-7-(6-morpholinyl-3-pyridazinyl)pyrido[2,3-
d]pyrimidine;
25 4-amino-5-cyclohexyl-7-(6-(4-acetylpiperazinyl)-3-pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-cyclohexyl-7-(6-(4-acetyl-1,4-diazacycloheptanyl)-3-
pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-cyclohexyl-7-(b-(4-methyl-1,4-diazacycloheptanyl)-3-
pyridyl)pyrido[2,3-d]pyrimidine;
30 4-amino-5-cyclohexyl-7-(6-(N-methyl-N-(2-(2-pyridyl)ethyl)amino)-3-
pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-5-cyclohexyl-7-(6-2-(N-(N',N'-dimethylaminoethyl)-N-methylamino)-3-
pylidyl)pyrido[2,3-d]pyrimidine;
4-amino-5-cyclohexyl-7-(6-azetidinyl-3-pyridyl)pyrido[2,3-d]pyrimidine;
35 4-amino-5-cyclohexyl-7-(6-{3-(N-
methylacetamido)pyrrolidinyl)pyridyl)pyrido[2,3-
d]pyrimidine;
-23-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
4-amino-5-cyclohexyl-?-(6-(3-(formamido)pyrrolidinyl)pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-cyclohexyl-?-(4-oxo-1-phenyl-1,3,8-triazaspiro[4.5[decan-8-
yl)pyrido[2,3-d]pyrimidine;
4-amino-S-cyclohexyl-?-(6-(2-methoxymethyl)pyrrolidin-1-yl)pvridyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-cyclohexyl-?-(6-(N-methoxyethyl-N-propylamino)pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-cyclohexyl-?-(N-methyl-N-(2,2-dimethoxyethyl)amino)pyrido[2,3-
d]pyrimidine;
4-amino-5-cyclohexyl-?-(6-(4-(dimethylamino)piperidinyl)pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-cyclohexyl-?-(6-(4-(aminocarbonyl))piperidinyl)pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-cyclohexyl-?-(N-methyl-N-(3-(diethylamino)propyi)aminopyrid-3-
yl)pyrido[2,3-d]pyrimidine;
4-amino-5-cyclohexyl-?-(6-(N-methyl-N-(4-pyridyl)ethylamino)pyrid-3-
yl)pyrido[2,3-d]pyrimidine;
4-amino-5-cyclohexyl-?-(6-(N-methyl-N-(3-pyridylmethylamino)pyrid-3-
yl)pyrido[2,3-d]pyrimidine;
4-amino-5-( 1-{2-bromophenyl)ethyl)-?-( 1-methyl-5-indolyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-( 1-(2-bromophenyl)ethyl)-?-{ 1-methyl-2,3-dioxo-5-indolyl)pyrido
[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-?-(3-fluoro-4-(1-morpholinyl)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-~-(3-bromophenyl)-?-(4-hydroxy-3-nitrophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-?-(6-(4,4-ethylenedioxypiperidinyl)-3-
pyridyl)pyrido[2>3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-?-(6-(4-oxopiperidinyl)-3-pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-S-(3-bromophenyl)-?-(6-(4-formylpiperazinyl)-3-pyridyl)pyrido[2,3-
d]pyrimidine:
4-amino-5-(3-bromophenyl)-?-(6-(4-methylpiperazinyl)-3-pyridyl)pyrido[2,3-
d]pyrimidine:
4-amino-5-(3-bromophenyl)-?-(6-thiomorpholinyl-3-pyridyl)pyrido[2,3-
d]pyrimidin;
-24-
CA 02286909 1999-10-12
WO 98/46605 PCTIUS98/07207
4-amino-5-(3-bromophenyl)-7-(6-(4,4-dioxothiomorpholinyl)-3-pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-{2-bromophenyl)-7-(6-morpholinyl-3-pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromo-4-methoxyphenyl)-7-(6-morpholinyl-3-pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(4-bromophenyl)-7-(6-morpholinyl-3-pyridyl)pyrido(2,3-d]pyrimidine;
4-amino-5-(3-chiorophenyl)-7-(6-morpholinyl-3-pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(5-chloro-6-morpholinyl-3-pyridyl)pyrido[2,3-
d]pyrimidine;
l0 4-amino-5-(3-bromophenyl)-7-(6-(N-oxidomorpholinyl)-3-pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-(N-(2-hydroxyethoxyethyl)amino)-3-
pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-(N-(2-hydroxyethoxyethyl)-N-formylamino)-3-
15 pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-(N-{2-hydroxyethoxyethyl)-3-pyridyl-N-
oxide)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-(3-hydroxy)morpholinyl)-3-pyridyl)pyrido[2,3-
d]pyrimidine;
20 1-(5-(4-amino-5-(3-bromophenyl)pyrido[2,3-d]pyrimidin-7-yl)-2-pyridyl)-
piperidine-4-phosphate, disodium salt;
4-amino-5-(3-bromophenyl)-7-(4-methylenylpiperidinyl)-3-pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-hydroxy-4-(hydroxymethyl)piperidinyl)-3-
25 pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-(4,4-ethylenedioxypiperidinyl)-3-
pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-5-cyclohexyl-7-(6-(4-oxo-piperidinyl)-3-pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-cyclohexyl-7-(6-(4-methylenylpiperidinyl)-3-pyridyl)pyrido[2,3-
3o d]pyrimidine;
4-N-(iminomethyl)amino-5-cyclohexyl-7-(6-dimethylamino-3-pyridyl)pyrido[2,3-
d]pyrimidine;
and pharmaceutically acceptable salts and amides thereof. In addition, the
and pharmaceutically acceptable salts and amides thereof. The partially
saturated and fully
35 saturated versions of the above compounds are also included within the
scope of the method
of inhibiting adenosine kinase in a patient in need of treatment thereof. The
above
compounds may be treated with hydrogen and a catalyst to form a compound of
formula I
-25-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
wherein the double bonds on the right side are absent or there is a double
bond between the
5,6 carbons; the 6,7 carbons or the 7 carbon, 8 nitrogen.
Exemplary and preferred compounds of the invention, again with the variables
R1-
Rg as specifically shown below, include:
4-amino-5-(4-dimethylaminophenyl)-7-(4-bromophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(4-dimethylaminophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(4-methoxyphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(4-dimethylaminophenyl)-7-(4-methoxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(4-(2-propyl)phenyl)-7-(4-methoxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(4-neopentylphenyl)-7-(4-methoxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(4-butyloxyphenyl)-7-(4-methoxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(4-methoxyphenyl)-7-(4-bromophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(4-(2-propyl)oxyphenyl)-7-(4-methoxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(4-butoxyphenyl)-7-(4-N-formylpiperazinylphenyl)pyrido[2,3-
d]PY~dine;
4-amino-5-(4-benzyloxyphenyl)-7-(4-methoxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(4-phenoxyphenyl)-7-(4-methoxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(4-(2-propyl)phenyl)-7-(4-diethylmalonylallylphenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(4-(2-propyl)phenyl)-7-(4-t-butylacrylphenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3,4-dimethoxyphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-t-butylacrylphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3- methoxyphenyl-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3.5-dimethoxyphenyl-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-diethylmaionylallylphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-vinylpyridinylphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]PY~dine:
4-amino-5-(3-trifluoromethylphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
-26-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
4-amino-5-(3-carboxamidophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-cyanophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-benzyloxyphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-methoxyphenyl)-7-(4-methoxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-butoxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-(2-pyridyl)phenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-methylphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-chlorophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-fluorophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-methoxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-methoxyphenyl)-7-(4-bromophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-phenylpyrido [2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-ethylphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-bromophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-cyanophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-hydroxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-iodophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-ethoxyphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-trifloromethyoxyphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3,5-dichlorophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromo-4-fluorophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-hydroxyphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-morpholinylphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-piperidinylphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(imidazol-1-yl)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-chlorophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-isopropylphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-trifluorophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-diethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(3,4,5-trimethoxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-(3-methoxybenzyl)phenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
-27-
CA 02286909 1999-10-12
WO 98/46605 PCTNS98/07207
4-amino-5-(3-methoxyethyoxyphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3,4-methylenedioxyphenyl)-7-(4-dimethylaminophenyl)pyrido [2,3-
d)pyrimidine;
4-amino-5-(3-bromophenyl)-7-(~-ethoxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(2'-thiophene)pyrido[2,3-d)pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-fluorophenyl)pyrido[2,3-d)pyrimidine;
4-amino-5-(3-dimethylaminophenyi)-7-(4-dimethylaminophenyl)pyrido[2,3-
d)pyrimidine;
4-amino-5-phenyl-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3,4,5-trimethoxyphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-nitrophenyl)pyrido[2,3-d)pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-iodophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(3,4-methylenedioxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(thiophen-2-yI)-7-(4-morpholinylphenyl)pyrido [2,3-d]pyrimidine;
4-amino-5-(3,5-dimethoxyphenyl)-7-(thiophen-2-yle)pyrido [2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-carboxamidophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(2-methoxy)ethoxyphenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3,5-dimethoxyphenyl)-7-(4-morpholinylphenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-trifluoromethylphenyl)-7-(thiophene-2-yl)pyrido [2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-aminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromo-4-fluorophenyl)-7-(thiophene-2-yl)pyrido [2,3-d)pyrimidine;
4-amino-5-(3-bromo-4-fluorophenyl)-7-(2-furanyl)pyrido [2,3-d]pylvrudine;
4-amino-5-(3,5-dimethoxyphenyl)-7-(4-iodophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3,5-dimethoxyphenyl)-7-(4-imidazolylphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3,5-dimethoxyphenyl)-7-(4-(thiophene-2-yl)phenyl)pyrido [2,3-
d]pyrimidine;
4-amino-5-(3,5-dimethoxyphenyl)-7-(4-(3-pyridyl)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(4-methylpiperidinyl)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-pyrrolidinylphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(4-bromothiophene-)-7-(4-dimethylanunophenyl)pyrido[2,3-
d]pyrimidirre;
4-amino-5-(4-bromothiophene-2-yl)-7-(4-morpholinylphenyl)pyrido[2,3-
d]pyrimidine;
-28-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98707207
4-morpholinyl-5-(3-bromophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino=5-(5-bromothiophene-2-yl)-7-{4-morpholinylphenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(4-bromophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-{4-(acetylamino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-S-(3-bromophenyl}-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3,5-dimethoxyphenyl)-7-(5-pyrimidinylphenyl)pyrido[2,3-
d]pyrimidine;
4-(4-fluorophenyl)amino)-5-(3-bromophenyl)-7-(4-
1o dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(4-bromothiophene-2-yl)-7-(4-pyrrolidinylphenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(4-bromothiophene-2-yl)-7-(thiophene-2-yl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(5-(dimethylamino)thiophene-2-yl)pyrido[2,3-
15 d]pyrimidine;
4-amino-5-(3-bromo-5-iodophenyl)-7-(4-(dimethylamino)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3,5-di(trifluoromethyl)phenyl)-7-(4-
(dimethylamino)phenyl)pyrido[2,3-
d]pyrimidine;
20 4-amino-5-(3,5-di(trifluoromethyl)phenyl)-7-(4-morpholinylphenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3,5-dibromophenyl)-7-(4-(dimethylamino)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3,5-dibromophenyl)-7-(4-morpholinylphenyl)pyrido[2,3-d]pyrimidine;
25 4-amino-5-(4-bromothiophene-2-yl)-7-(4-(4-
methylpiperidinyl)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3,5-dibromophenyl)-7-(4-(dimethylamino)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(3-{dimethylamino)phenyl)pyrido [2,3-d]pyrimidine;
30 4-amino-5-(3-bromophenyl)-7-(4-methylsulfonylphenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(3-methoxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(methylthio)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(3,4-dichlorophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(N-methyl-N-formylamino)phenyl)pyrido[2,3-
35 d]pyrimidine:
4-amino-5-(3-bromophenyl)-7-(4-methylaminophenyl)pyrido[2,3-d]pyrimidine;
-29-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
4-amino-5-(3-bromo-4-fluorophenyl)-7-(4-methylsulfonylphenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(3-amino-4-methoxyphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(3-bromo-4-(dimethylamino)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(3-methyl-4-(dimethylamino)phenyl)pyrido[2,3-
d]pylimidine;
4-amino-5-(3-bromophenyl)-7-(4~(N-methyl-N-
trifluoroacetylamino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(dimethylamino)-3-fluorophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(N-ethyl-N-formylamino)phenyl)pyrido[2,3-
d]pyrimidine;
4,4-bis(acetylamino)-5-(3-bromophenyl)-7-(4-(N-methyl-N-
acetylamino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(N-acetyl-N-methylamino)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(N-ethylamino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(N-methyl-N-(2-
methoxyethyl)amino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(N-isopropylamino)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-S-(3-bromophenyl)-7-(4-N-ethyl-N-(2-
methoxyethyl)amino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-N-(3-methoxypropionyl)-N-isopropyl-
amino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-{4-N-(2-(dimethylamino)ethyl)-N-
formylamino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-{N-(2-
(dimethylamino)ethyl)amino)phenyl)pyrido[2.3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(N-methyl-N-(2-
cyano)ethylamino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(N-methyl-N-(3-
methoxy)propionylamino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(3-methyl-4-(N-formyl-N-
methylamino)phenyl)pyrido[2,3-d]pyrimidine;
-30-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
4-amino-S-(3-bromophenyl)-7-(3-methyl-4-(N-methylamino)phenyl)pyrido(2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(4-methoxy-2-butyl)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-S-(3-bromophenyl)-7-(4-(N-methyl-N-(2-(N-
phthalimidyl)acetyl)amino)phenyl)pyrido(2,3-d]pyrimidine;
4-amino-S-(3-bromophenyl)-7-(3-methyl-4-(N-methyl-N-
(trifluoroacetyl)amino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-S-(3-bromophenyl)-7-(3-methyl-4-(N-acetyl-N-
methylamino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-S-(3-bromophenyl)-7-(6-dimethylamino-3-pyridinyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-cyanophenyl)-7-(4-methylsulfonylphenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-cyanophenyl)-7-(4-{N-methyl-N-formylamino)-phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-S-(3-bromophenyl)-7-(6-(N-methyl-N-formylamino)-3-
pyridinyl)pyrido[2,3-d]pyrimidine;
4-amino-S-(3-bromophenyl)-7-(6-morpholinyl-3-pyridinyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-(N-methyl-N-methoxyethylamino)-3-
pyridinyl)pyrido[2,3-d]pyrimidine;
4-amino-S-(3-bromophenyl)-7-(6-pyrrolidinyl-3-pyridinyl)pyrido[2,3-
d]pyrimidine;
4-amino-S-(3-bromophenyl)-7-(2-(dimethylamino)-S-pyrimidinyl)pyrido[2,3-
d]pyrimidine;
4-amino-S-(3-bromophenyl)-7-(2-(N-methoxyethyl-N-methyl amino)-S-
pyrimidinyl)pyrido(2,3-d]pyrimidine;
4-amino-S-(3-bromophenyl)-7-(2-(N-formyl-N-methyl amino)-S-
pyrimidinyl)pyrido[2,3-d]pyrimidine;
4-amino-S-(3-bromophenyl)-7-(2-(N-methylamino)S-pyrimidinyl)pyrido[2,3-
d]pyrimidine;
4-amino-S-(3-bromophenyl)-7-(2-(1-pyrrolidinyl)-S-pyrimidinyl)pyrido[2,3-
d]pyrimidine;
4-amino-S-(3-bromophenyl)-7-(2-( 1-morpholinyl)-S-pyrimidinyl)pyrido(2,3-
d]pyrimidine;
4-amino-S-(3-bromophenyl)-7-(6-(2-oxo-3-oxazolidinyl)-3-pyridinyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(2-pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-S-(3-bromophenyl)-7-(3-pyridyl)pyrido(2,3-d]pyrimidine;
-31-
CA 02286909 1999-10-12
WO 98/46605 PCT/LTS98/07207
4-amino-5-(3-(thiophen-2-yl)phenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
dIPY~~e~
4--amino-5-(3-(furan-2-yl)phenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]PY~dine;
4-amino-5-(3-(3-methoxyphenyl jphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-S-phenyl-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-chlorophenyl)-7-(4-(morpholinyl)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromo-4-fluorophenyl)-7-(4-(morpholinyl)phenyl)pyrido[2,3-
l0 d]pyrimidine;
4-amino-5-(3-chlorophenyl)-7-(4-iodophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-chlorophenyl)-7-(4-(thiophen-2-yl)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-chlorophenyl)-7-(4-(5-pyrimidinyl)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromo-4-fluorophenyl)-7-(4-iodophenyl)pyrido[2,3-d]pyrimidine;
15 4-amino-S-(4-bromothiophene-2-yl)-7-(4-methoxyphenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)methyl-7-(4-(dimethylamino}phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(2-phenylethyl)-7-(4-diethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(2-methylpropyl)-7-(4-diethylaminophenyl)pyrido[2,3-d]pyrimidine;
20 4-amino-5-(butyl)-7-(4-diethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(2-(4-bromophenyl)ethyl)-7-(4-diethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(butyl)-7-(4-dimethylaminophenyl)pyrido [2,3-d]pyrimidine;
4-amino-5-(2-(3-cyanophenyl)methyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
25 d]pyrimidine;
4-amino-5-(2-(N-phenylmethoxycarbonyl)aminoethyl)-7-(4-
dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(cycloheptyl)-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(2-(5-chloro-2-(thiophen-3-yl)phenylmethyl)-7-(4-
30 dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(pentyl)-7-(4-diethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-hexyl-7-(4-diethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(2-(3-bromophenyl)ethyl)-7-(4-diethylaminophenyl)pyrido[2,3-
d]pyrimidine;
35 4-amino-5-((2-bromophenyl)methyl)-7-(4-diethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-cyclopropyl-7-(4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
-32-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
4-amino-5-cyclohexyl-7-(4-dimethylaminophenyl)pyrido [2,3-d]pyrimidine;
4-amino-5-((2-bromo-5-chlorophenyl)methyl)-7-(4-diethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-methyl-7-(4-diethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(2,3-methylenedioxyphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-fluoro-5-trifluoromethylphenyl)-7-(4-
dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(2-bromophenyl)-7-{4-dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3,5-dimethylphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3,4-dichlorophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(4-fluoro-3-trifluoromethylphenyl)-7-(4-
d:methylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromo-5-methoxyphenyl)-7-(4-morpholinylphenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromo-5-methoxyphenyl)-7-(4-pyrrolidinylphenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromo-5-methoxyphenyl)-7-(4-piperidinylphenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromo-5-methoxyphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrirnidine;
4-amino-5-(3-methylthiophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromo-5-methoxyphenyl)-7-(thiophene-2-yl)pyrido[2,3-d]pyrimidine;
4-amino-5-{2,3-dimethoxyphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-methylsulfonylphenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-acetylamino-5-(3-bromophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-formylamino-5-(3-bromophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-(methoxyacetyl)amino-5-(3-bromophenyl)-7-(4-diethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-trifluoroacetylamino-5-(3-bromophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
-33-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
4-pentanoylamino-5-(3-bromophenyl)-7-(4-dimethylaminophenyl)pyrido(2,3-
d]pyrimidine;
4-benzoylamino-5-(3-bromophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-(N-BOC-glycyl)amino-5-(3-bromophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-(N-phthalimidylglycyl)amino-5-(3-bromophenyl)-7-(4-
dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-(ethoxycarbonyl)amino-5-(3-bromophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
1o d]pyrimidine;
4-(ethylaminocarbonyl)amino-5-(3-bromophenyl)-7-(4-
dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-allylamino-5-(3-bromophenyl)-7-(4-dimethylaminophenyl) pyrido[2,3-
d]pyrimidine;
15 4-(2-(N,N-dimethylamino)ethylamino)-5-(4-bromophenyl)-7-(4-
dimethylaminophenyl) pyrido[2,3-d]pyrimidine;
4-(4-(N,N-dimethylamino)butylamino)-5-(3-bromophenyl)-7-(4-
dimethylaminophenyl) pyrido(2,3-d]pyrimidine;
4-(N-allyl-N-formylamino)-5-(4-dimethylaminophenyl)-7-(4-
2o bromophenyl)pyrido[2,3-d]pyrimidine;
4-diacetylamino-5-(p-dimethylaminophenyl)-7-(4-
bromophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(5-amino-2-pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(5-dimethylamino-2-pyridyl)pyrido[2,3-
d]pyrimidine;
25 4-amino-5-{3-bromophenyl)-7-(5-dimethylamino-2-
pyrazinyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(2-oxobenzoxazolin-6-yl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-( 1-methyl-2-oxobenzoxazolin-6-
yl)pyrido[2,3-d]pyrimidine;
30 4-amino-5-((5-chloro-2-(3-methoxyphenyl)phenyl)methyl)-7-(4-
dimethylaminophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-((thiophene-2-yl)methyl)-7-(4-diethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-((thiophene-3-yl)methyl)-7-(4-diethylaminophenyl)pyrido[2,3-
35 d]PYi'~dine;
4-amino-5-((2-brornophenyl)methyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine;
-34-
CA 02286909 1999-10-12
WO 98/46605 PCTlUS98/07207
4-amino-5-{3-bromophenyl)-7-(4-(N-formyl-N-(2-
methoxyethyl)amino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(N-(2-methoxyethyl)amino)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(N-methyl-N-((2-
dimethylamino)ethyl)amino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(2-
methoxy)acetylamino)ethyl)amino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-({4-formylamino)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(2-(dimethylamino)acetylamino)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(2-oxo-3-oxazolidinyl)phenyl)pyrido [2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-(2-propyl)-3-pyridinyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(3-methyl-4-pyrrolidinylphenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-imidazolyl-3-pyridinyl)pyrido[2,3-d]pyrimidine;
4-amino-5-phenylmethyl-7-(4-diethylaminophenyl)pyrido [2,3-d]pyrimidine;
4-amino-5-(2-(3-aminopropynyl)phenylmethyl)-7-(4-diethylaminophenyl)pyrido[2,3-
2o d]pyrimidine;
4-amino-5-( 1-(3-bromophenyl)ethyl)-7-(4-diethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(4-dimethylaminophenyl)-7-(4-bromophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(2-furanyl)-7-(4-(N-morpholinyl)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(2-dimethylamino-5-pyrimidinyl)pyrido[2,3-
d]pyrimidine;
4-amino-S-(3-bromophenyl)-7-(4-(ureido)phenyl)pyrido [2,3-d]pyrimidine;
4-amino-5-( 1-phenylmethyl-3-piperidinyl)-7-(4-diethylaminophenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-brornophenyl)-7-(6-(3-methyl-5-isoxazolyl))-3-
pyridinyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-chloro-3-pyridinyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-methoxy-3-pyridinyl)pyrido[2,3-d]pyrimidine; --
4-amino-5-(3-bromophenyl)-7-(6-( 1,2,4-triazol-4-yl)-3-pyridinyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(2-moipholinyl-5-pyrimidinyl)pyrido[2,3-
d]pyrimidine;
-35-
CA 02286909 1999-10-12
WO 98146605 PCT/US98/07207
4-amino-5-(2-thiazolyl)-7-(4-pyrrolidinylphenyl)-pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-pyrazolyl-3-pyridinyl))-pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-( 1-methyl-ureido)phenyl)-pyrido [2,3-
d]pyrimidine:
4-amino-5-(3-bromophenyl)-7-(4-(N-methyl-N-(2-pyrimidinyl)amino)phenyl)-
pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(3-fluoro-4-(N-formyl-N-methylamino)phenyl)-
pyrido[2,3-d]pyrimidine;
4-formylamino-5-(3-bromophenyl)-7-(3-fluoro-4-{N-formyl-N-
methylamino)phenyl)-pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(N-methyl-N-methylsulfonylamino)-
phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-(N-methyl-N-methylsulfonylaminol-3-
pyridinyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-( 1-methyl-5-indolinyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-( 1-methyl-5-benzimidazolyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-dimethylamino-3-pyridazinyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3bromophenyl}-7-(6-morpholinyl-3-pyridazinyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-pyrrolidinyl-3-pyridazinyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(5-morphoiinyl-2-pyrazinyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(5-(N-(2-methoxyethyl)-N-methylamino)-2-
pyrazinyl)pyrido [2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(morpholinylmethyl}-phenyl}pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(5-(N,N-bis(2-methoxyethyl)amino)-2-
pyridinyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(imidazolylmethyl)-phenyl)pyrido[2,3-
d]PY~dine;
4-amino-~-(3-bromophenyl)-7-(5-(1-morpholinyl)-2-pyridinyl)pyrido[2,3-
d]pyrimidine;
-36-
CA 02286909 1999-10-12
WO 98/46605 PCT/LTS98/07207
4-amino-5-(3-bromophenyl)-7-(4-((dimethylamino)methyl)-phenyl)pyrido [2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(5-(4-hydroxy-1-piperidinyl)-2-
pyridinyl)pyrido[2,3-dJpyrimidine;
4-amino-5-(3-bromophenyl)-7-(5-(N-formyl-N-methylamino)-2-
pyridinyl)pyrido(2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(5-(2-propenyl)-2-pyridinyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(3-(2-methoxyethyl)-2-oxo-6-
benzoxazolyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-( 1-(N-formylamino)-ethyl)phenyl)pyrido[2,3-
d]pyrimidine;
4-(methylamino)-5-(3-bromophenyl)-7-(4-dimethylaminophenyl)pyrido[2,3-
d]pyrimidine hydrochloride;
:~-(2-methoxyethylamino)-5-(3-bromophenyl)-7-(4-
dimethylaminophenyl)pyrido[2,3-d]pyrimidine hydrochloride;
~-amino-5-(3-bromophenyl)-7-(4-( 1-methyl-2-imidazolyl)phenyl)pyrido[2,3-
dJpyrimidine trihydrochloride;
4-amino-5-(3-bromophenyl)-7-(4-(aminomethyl)phenyl)pyrido[2,3-d]pyrimidine;
:~-amino-5-(3-bromophenyl)-7-(2-bromo-4-(dimethylamino)phenyl)pyrido[2,3-
d]pyrimidine;
-1-amino-5-(3-bromophenyl)-7-(4-(dimethylaminoethyl)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-{3-bromophenyl)-7-(4-(3-(dimethylamino)propynyl)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-(3-amino-3-methylbutynyl)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-dimethylphosphonatophenyl)pyrido[2,3-
d]pyrimidine;
:l-amino-5-(3-bromophenyl)-7-(4-(3-(methoxypropynyl)pyrido[2,3-d]pyrimidine;
-t-amino-5-(3-bromophenyl)-7-(4-carboxyphenyl)pyrido[2;3-d]pyrimidine;
-1-amino-5-(3-bromophenyl)-7-(4-methyl-3-oxo-2H-4H-pyrido[3,2-b]-1,4-oxazin-
7-yl)pyrido[2,3-d]pyrimidine;
4-amino-5-{3-bromophenyl)-7-(4-(2-(dimethylamino)ethyl)-3-oxo-2H-4H-
pyrido[3,2-b]-1,4-oxazin-7-yl)pyrido[2,3-d]pyrimidine;
~-amino-5-(3-bromophenyl)-7-(2,3-dihydro-3-(dimethylaminoethyl)-2-
oxobenzoxazol-6-yl)pyrido[2,3-d]pyrimidine;
-37-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
4-amino-5-(3-bromophenyl)-7-(4-methyl-3-oxo-2H-4H-benzo-1,4-oxazin-7-
yl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(2,2,4-trimethyl-3-oxo-2H-4H-benzo-1,4-oxazin- 7-
yl)pyrido[2,3-d]pyrimidine;
4-amino-5-cyclohexyl-7-(4-(2-dimethylamino)ethyl)-2H-4H-benzo-3-oxo-1,4-
oxazin-7-yl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(5-( 1-methylethyl)-2-pyridyl)pyrido[2,3-
d]PY~dine;
4-amino-5-(3-bromophenyl)-7-(5-piperidin-1-ylpyrid-2-yl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(1-(4-bromophenyl)ethyl)-7-(6-morpholinylpyrid-3-yl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-((N-formylamino)methyl)phenyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-( 1-methyl-1-(N-methylamino)ethyl)phenyl)-
pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-( 1-(dimethylamino)-1-
methylethyl)phenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(N-acetyl-5-indolinyl)pyrido[2,3-d]pyrimidine;
4-amino-5-cyclohexyl-7-(6-chloro-3-pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-5-( 1-(2-bromophenyl)ethyl)-7-(6-diethylamino-3-pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-( 1-{2-bromophenyl)ethyl}-7-(6-morpholinyl-3-pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-( 1-(2-bromophenyl)ethyl)-7-(4-(N-methyl-N-formyl}amino)-
phenyl)pyrido[2,3-djpyrimidine;
4-amino-5-cyclohexyl-7-(6-morpholinyl-3-pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-5-((2-bromophenyl)methyl)-7-(6-morpholinyl-3-pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(4-tetrahydropyranyl)-7-(6-morpholinyl-3-pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-S-cyclohexyl-7-(6-dimethylamino-3-pyridyl)pyrido[2,3-d]pyrimidine:
4-amino-5-( 1-ethylpropyl)-7-(6-dimethylamino-3-pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-cyclopentyl-7-(6-morpholinyl-3-pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-5-cyclohexyl-7-(2-chloro-3-pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3,5-dimethylcyclohexyl)-7-(6-dimethylamino-3-pyridyl)pyrido[2,3-
d]pyrimidine:
-38-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
4-amino-5-((N-{benzyloxycarbonyl)-4-piperidinyl)methyl)-7-(6-morpholinyl-3-
pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-5-cyclohexyl-7-(6-bromo-3-pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-5-cyclohexyl-7-(3-cyanophenyl)pyrido[2,3-d]pyrirnidine;
4-amino-5-(1-(2-bromophenyl)ethyl)-7-(6-dimethyIamino-3-pyridazinyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyi)-7-(6-imidazolyl-3-pyridazinyl)pyrido[2,3-
d]pyrimidine;
4-amino-S-(3-bromophenyl)-7-(6-(azacycloheptanyl)-3-pyridazinyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl}-7-(6-(N-methyl-N-( 1-methylethyl))amino)-3-
pyridazinyl)pyrido[2,3-d]pyrimidine;
4-amino-5-( 1-(2-bromophenyl)ethyl)-7-(b-morpholinyl-3-pyridazinyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-cyclohexyl-7-(6-(4-acetylpiperazinyl)-3-pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-cyclohexyl-7-(6-(4-acetyl-1,4-diazacycloheptanyl)-3-
pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-cyclohexyl-7-{6-(4-methyl-1,4-diazacycloheptanyl)-3-
pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-5-cyclohexyl-7-{6-(N-methyl-N-(2-(2-pyridyl)ethyl)amino)-3-
2o pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-S-cyclohexyl-7-(6-2-(N-(N',N'-dimethylaminoethyl)-N-methylamino)-3-
pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-5-cyclohexyl-7-(6-azetidinyl-3-pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-5-cyclohexyl-7-(6-(3-(N-
methylacetamido)pyrrolidinyl)pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-cyclohexyl-7-(6-(3-(formamido)pyrrolidinyl)pyridyl)pyrido[2,3-
d]PY~dine;
4-amino-5-cyclohexyl-7-(4-oxo-1-phenyl-1,3,8-triazaspiro[4.5 [decan-8-
yl)pyrido[2,3-d]pyrimidine;
4-amino-5-cyclohexyl-7-(6-(2-methoxymethyl)pyrrolidin-1-yl)pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-cyclohexyl-7-{6-(N-methoxyethyl-N-propylamino)pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-cyclohexyl-7-(N-methyl-N-(2,2-dimethoxyethyl)amino)pyrido[2,3-
d]pyrimidine;
4-amino-5-cyclohexyl-7-(6-(4-(dimethylamino)piperidinyl)pyridyl)pyrido[2,3-
d]pyrimidine;
-39-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
4-amino-5-cyclohexyl-7-(6-(4-(aminocarbonyl))piperidinyl)pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-cyclohexyl-7-(N-methyl-N-(3-(diethylamino)propyl)aminopyrid-3-
yl)pyrido[2,3-d]pylimidine;
4-amino-5-cyclohexyl-7-(6-(N-methyl-N-(4-pyridyl)ethylamino)pyrid-3-
yl)pyrido[2,3-d]pyrimidine;
4-amino-5-cyclohexyl-7-(6-(N-methyl-N-(3-pyridylmethylamino)pyrid-3-
yl)pyrido[2,3-d]pyrimidine;
4-amino-5-( 1-(2-bromophenyl)ethyl)-7-( 1-methyl-5-indolyl}pyrido[2,3-
d]pyrimidine;
4-amino-S-( 1-(2-bromophenyl)ethyl)-7-( 1-methyl-2,3-dioxo-5-
indolyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(3-fluoro-4-( 1-morpholinyl)phenyl)pyrido(2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(4-hydroxy-3-nitrophenyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-(4,4-ethylenedioxypiperidinyl)-3-
pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-(4-oxopiperidinyl)-3-pyridyl)pyrido[2,3-
d)pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-(4-formylpiperazinyl)-3-pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-(4-methylpiperazinyl)-3-pyridyl}pyrido[2,3-
d]pyrimidine;
4-amino-S-(3-bromophenyl)-7-(6-thiomorpholinyl-3-pyridyl)pyrido[2,3-
d]pyrimidin;
4-amino-5-{3-bromophenyl)-7-(6-(4,4-dioxothiomorpholinyl)-3-pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(2-bromophenyl)-7-(6-morpholinyl-3-pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromo-4-methoxyphenyl)-7-(6-morpholinyl-3-pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-(4-bromophenyl)-7-(6-morpholinyl-3-pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-chlorophenyl)-7-(6-morpholinyl-3-pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(5-chloro-6-morpholinyl-3-pyridyl)pyrido(2,3-
d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-(N-oxidomorpholinyl)-3-pyridyl)pyrido[2,3-
d]pyrimidine;
-40-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98l07207
4-amino-5-(3-bromophenyl)-7-(6-(N-(2-hydroxyethoxyethyl)amino)-3-
pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-(N-(2-hydroxyethoxyethyl)-N-formylamino)-3-
pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-(N-(2-hydroxyethoxyethyl)-3-pyridyl-N-
oxide)pyrido[2,3-d]pyrimidine;
4-amino-5-(3-bromophenyl)-7-(6-(3-hydroxy)morpholinyl)-3-pyridyl)pyrido[2,3-
d]pyrimidine;
1-(5-(4-amino-5-(3-bromophenyl)pyrido[2,3-d]pyrimidin-7-yl)-2-pyridyl)-
1o piperidine-4-phosphate, disodium salt;
4-amino-5-(3-bromophenyl)-7-(4-methylenylpiperidinyl)-3-pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-S-(3-bromophenyl)-7-(4-hydroxy-4-(hydroxymethyl)piperidinyl)-3-
pyridyl)pyrido[2,3-d]pyrimidine;
1S 4-amino-S-(3-bromophenyl)-7-(6-(4,4-ethylenedioxypiperidinyl)-3-
pyridyl)pyrido[2,3-d]pyrimidine;
4-amino-5-cyclohexyl-7-(6-(4-oxo-piperidinyl)-3-pyridyl)pyrido[2,3-
d]pyrimidine;
4-amino-5-cyclohexyl-7-(6-(4-methylenylpiperidinyl)-3-pyridyl)pyrido[2,3-
d]pyrimidine;
2o 4-N-(iminomethyl)amino-5-cyclohexyl-7-(6-dimethylamino-3-pyridyl)pyrido[2,3-
d]pyrimidine;
and pharmaceutically acceptable salts and amides thereof. In addition, the
partially
hydrogenated or fully hydrogenated versions wherein the 5,6 and/or the 7,8
double bonds
are hydrogenated of the compounds identified above are also included within
the scope of
25 the invention. The preferred substitution pattern on the R3 group when it
is selected from,
for example, a substituted aryl group, is having at least one substituent at
the meta position.
The preferred substitution pattern on the R4 position when it is selected
from, for example, a
substituted heteroaryl group, is having at least one substituent at the para
position. The
present invention is therefore directed to compounds of formula I or II with
the variables
3o recited as above wherein, in the case of R3 selected from substituted aryl
or heteroaryl
groups and R4 selected from substituted aryl or heteroaryl groups, the
substiuents on the R3
group are meta and the substituents on the R4 group are para. In addition, the
present
invention encompasses pro-drugs of the above compounds which may be active in
their own
right or are metabolized or convened to the non pro-drug form as exemplified
above. The
35 invention is not limited to synthetic versions of the claimed compounds and
includes the
compounds-per-se or pro-drugs or metabolites thereof regardless of how or
where they are
manufactured or made.
-41-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
The term "acyl", as used herein, refers to a moiety attached by a carbonyl
linkage, as
for example, loweralkyl-carbonyl or aryl-carbonyl, wherein loweralkyl and aryl
are as
defined herein. Examples of acyl include, for example, acetyl, propionyl,
hexanoyl,
trifluoroacetyl, benzoyl, 4-methylbenzoyl, methoxyacetyl, pentanoyl, N-
Bocglycylimidazoyl, N-phthalimidylglycyl and the like or others as specified
herein.
The term "aryl" or "substituted aryl" as used herein, refers to a carbocyclic
aromatic
radical, including, for example, phenyl and 1- or 2-naphthyl, which may be
unsubstituted or
substituted respectively by independent replacement of one, two or three of
the hydrogen
atoms thereon with Cl, Br, F, I, cyano, carboxamido, hydroxy, loweralkoxy,
loweralkyl,
loweralkenyl, loweralkynyl, amino, loweralkylamino, di(loweralkylamino), N-
loweralkyl-
N-loweralkoxyamino, trifluoromethyl or methoxymethyl groups. In addition, the
term
"aryl" refers to a phenyl group substituted with one ureido, methylsulfonyl,
pyrimidinyl,
pyridinyl, pyridazinyl, morpholinyl, phenyl-loweralkoxy, phenyl-loweralkenyl
or
cycloalkyl-loweralkyl group. Examples of aryl radicals include, but are not
limited to, 3-
bromophenyl, 3-chlorophenyl, 4-chlorophenyl, 3-methoxyphenyl, 3-(2-
propyl)phenyl, 3,4-
dimethoxyphenyl, 3-trifluromethylphenyl, 3-trifluoro-4-fluorophenyl, 4-(N-
methyl-N-
methoxyl)ethylaminophenyl, 4-dimethylaminophenyl, 3-fluoro-4-methylphenyl, 4-
methylphenyl, 4-cyanophenyl, 4-propylmethyl, 3,5-dichlorophenyl, 3,4-
methylenedioxyphenyl, 3-cyanopropylphenyl, 4-ureidophenyl, 3-
methylsulfonylphenyl, 3-
carboxamidopropylphenyl.
The term "arylalkyl" refers to a ioweralkyl radical having appended thereto an
aryl
group, as defined above, as for example benzyl and phenylethyl.
The term "aryloxy" refers to a aryl radical which is appended to the molecule
via an
ether linkage (i.e., through an oxygen atom), as for example phenoxy,
naphthyloxy, 4-
chlorophenoxy, 4-methylphenoxy, 3,5-dimethoxypehenoxy, and the like.
The term "cycloalkyl" refers to a cyclic saturated hydrocarbon radical having
from 3
to 7 ring atoms. Examples of cycloalkyl include cyclopropyl, cyclobutyl,
cyclopentyl,
cyclohexyl and cycloheptyl. Cycloalkyl is also described as C3-CgcycloalkyL
The term "cycloalkyl-loweralkyl" refers to a loweralkyl radical as defined
below
substituted with a cycloalkyl group as defined above by replacement of one
hydrogen atom.
Examples of cycloalkyl-loweralkyl include cyclopropylmethyl, cycloburylethyl,
cyclopentylmethyl, cyclohexylmethyl and cycloheptylbutyl, and the like.
The term "heteroaryl" or "substituted heteroaryl" refers to a monocyclic
aromatic
radical having from five to seven ring atoms of which one ring atom is
nitrogen, oxygen or
sulfur; zero, one or two ring atoms are additional heteroatoms independently
selected from
S, O and N; and the remaining ring atoms are carbon, the radical being joined
to the rest of
the molecule via any of the ring atoms. A heteroaryl group may be
unsubstituted or
-42-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
substituted by independent replacement of one, two or three of the hydrogen
atoms thereon
with Cl, Br, F, I, cyano, carboxamido, hydroxy, loweralkoxy, lowerallryl,
loweralkenyl,
loweralkynyl, amino, loweralkylamino, di(loweralkylamino), N-loweralkyl-N-
loweralkoxyamino, trifluoromethyl or methoxymethyl groups. In addition, the
term
"heteroaryl " refers to a heteroaryl group substituted with one ureido,
methylsulfonyl,
pyrimidinyl, pyridinyl, pyridazinyl, morpholinyl, phenyl-loweralkoxy, phenyl-
loweralkenyl
or cycloallcyl-loweralkyl group. In addition a heteroaryl group may be
substituted by
replacement of any two adjacent hydrogen atoms with a grouping of atoms to
form a fused
benzene ring, e.g., benz derivatives such as indole, benzoxazole and the like.
Examples of
heteroaryl include pyridinyl, pyrazinyl, pyrimidinyl, pyrrolyl, pyrazolyl,
imidazolyl,
thiazolyl, oxazolyl, isoxazolyl, thiadiazolyl, oxadiazolyl, furanyl,
thiophenyl, 5-
methylthiophene-2-yl, 5-nitrothiophene-2-yl, 5-methylfuranyl, benzofuranyl,
benzothiophenyl, and the like and those additionally described herein.
The term "heterocyclic" refers to a saturated or unsaturated monocyclic ring
system
radical having from four to seven ring atoms of which one is nitrogen or
oxygen; zero, one
or two ring atoms are additional heteroatoms independently selected from S, O
and N; and
the remainder are carbon, the radical being joined to the rest of the molecule
via any of the
ring carbon atoms and being optionally substituted, either on a nitrogen or a
carbon atom, by
an additional radical selected from among aryl(loweralkyl), alkoxycarbonyl,
loweralkyl,
halo(loweralkyl), amino(loweralkyl), hydroxy-substituted loweralkyl, hydroxy,
loweralkoxy, halogen, amino, loweralkylamino, and amino, (loweralkyl)amino or
alkanoylamino of from one to eight carbon atoms in which the amino group may
be further
substituted with alkanoyl of from one to eight carbons, an alpha-amino acid or
a
polypeptide. Examples of heterocyclic include pyrrolidine, tetrahydrofuran,
dihydropyrrole,
isoxazolidine, oxazolidine, tetrahydropyridine, piperidine, piperazine,
morpholine,
thiomorpholine, aziridine and azetidine or those additionally described
herein.
The term "heterocyclic-loweralkyl" refers to a loweralkyl radical as defined
below
substituted with a heterocyclic-group as defined above by replacement of one
hydrogen
atom. Examples of cycloallcyl-lowerallcyl include pyrrolidinylmethyl,
piperidinylethyl, and
the like.
The term "loweralkyl", as used herein, refers to saturated, straight- or
branched-
chain hydrocarbon radicals containing from one to six carbon atoms including,
which may
be unsubstituted or substituted by independent replacement of one, two or
three of the
hydrogen atoms thereon with Cl, Br, F, I, cyano, carboxamido, hydroxy,
loweralkoxy,
amino, loweralkylamino, iminoloweralkylamino,di(loweralkylamino) or N-
loweralkyl-N-
lowerallcoxyamino groups. Examples of loweralkyl include, but are not limited
to, methyl,
ethyl, propyl, isopropyl, ~a-butyl, tert-butyl, neopentyl, n-hexyl,
hydroxyethyl,
-43-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
methoxymethyl, trifluoromethyl, 3-cyanopropyl, 3-carboxamidopropyi, and the
like. In
certain cases, the group "Cl-C6alkyl" is described and has a similar meaning
as above for
loweralkyl but is more specifically recited. Likewise, the term "CO-C6alkyl"
indicates the
carbon atoms which may be present in the alkyl chain including zero. These
terms are also
provided adjacent to aryl or heteroaryl or other generic group and represent
or have the same
meaning as, for example, "arylalkyl" or "heteroarylalkyl".
The term "loweralkenyl", as used herein, refers to mono-unsaturated straight-
or
branched-chain hydrocarbon radicals containing from two to six carbon atoms
including, but
not limited to, vinyl, propenyl, n-butenyl, i-butenyl, n-pentenyl, and n-
hexenyl. These
variables are also recited as, for example, C2-C(alkenyl.
The term "loweralkoxy" refers to a loweralkyl radical which is appended to the
molecule via an ether linkage (i.e., through an oxygen atom), as for example
methoxy,
ethoxy, propoxy, 2-propoxy, 2-methyl-2-propoxy, tert-butoxy, pentyloxy,
hexyloxy,
isomeric forms thereof and the like. This term is also described as C1-
C(alkyloxy.
The term "loweralkynyl", as used herein, refers to straight- or branched-chain
hydrocarbon radicals possessing a single triple bond and containing from two
to six carbon
atoms including, but not limited to, ethynyl, propynyl, rc-butynyl, n-
pentynyl, and n-
hexynyl. This term is also described as C2-C6alkynyl.
The term "mammal" has its ordinary meaning and includes human beings.
In a further aspect of the present invention pharmaceutical compositions are
disclosed which comprise a compound of the present invention in combination
with a
pharmaceutically acceptable carrier.
The present invention includes one or more compounds, as set forth above,
formulated into compositions together with one or more non-toxic
physiologically tolerable
or acceptable diluents, carriers, adjuvants or vehicles that are collectively
referred to herein
as diluents, for parenteral injection, for oral administration in solid or
liquid form, for rectal
or topical administration, or the like. As is well known in the art, a
compound of the present
invention can exist in a variety of forms including pharmaceutically-
acceptable salts, amides
and the like.
Compositions may be prepared that will deliver the correct amount of a
compound or
compounds of the invention. The following dosages are thought to provide the
optimal
therapy: iv infusions: 0.1- 250 nmol/kg/minute> preferably from 1-50
nmol/kg/minute; oral:
0.01-250 ~.MoUkg/day, preferably from about 0.1-50 ~.Mol/kg/day; these oral
molar dosage
ranges correspond to 0.005-125 mg/kg/day, preferably 0.05-25 mg/kg/day. For
treatment
of acute disorders the preferred route of administration is intravenous; the
preferred method
of treating chronic disorders is orally by means of a tablet or sustained
release formulation.
_q.4_
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
"Pharmaceutically-acceptable amide" refers to the pharmaceutically-acceptable,
nontoxic amides of the compounds of the present invention which include amides
formed
with suitable organic acids or with amino acids, including short. peptides
consisting of from
1-to-6 amino acids joined by amide linkages which may be branched or linear,
wherein the
amino acids are selected independently from naturally-occurring amino acids,
such as for
example, glycine, alanine, leucine, valine, phenylalanine, proline,
methionine, tryptophan,
asparagine, aspartic acid, glutamic acid, glutamine, serine, threonine,
lysine, arginine,
tyrosine, histidine, ornithine, and the like.
"Pharmaceutically-acceptable salts" refers to the pharmaceutically-acceptable,
nontoxic, inorganic or organic acid addition salts of the compounds of the
present invention,
as described in greater detail below.
The compounds of the present invention can be used in the form of
pharmaceutically-acceptable salts derived from inorganic or organic acids.
These salts
include, but are not limited to, the following: acetate, adipate, alginate,
aspartate, benzoate,
benzenesulfonate, bisulfate, butyrate, camphorate, camphorsulfonate, citrate,
cyclopentanepropionate, digluconate, dodecylsulfate, ethanesulfonate,
flavianate, fumarate,
glucoheptonate, glycerophosphate, hemisulfate, heptonate, hexonoate,
hydrochloride,
hydrobromide, hydroiodide, 2-hydroxy-ethanesulfonate, lactate, maleate,
methanesulfonate,
nicotinate, 2-naphthalenesulfonate, oxalate, palmoate, pectinate, persulfate,
3-
2o phenylpropionate, phosphate, picrate, pivalate, propionate, succinate,
tartrate, thiocyanate,
tosylate, and undecanoate.
Appropriate cationic salts are also readily prepared by conventional
procedures such
as treating an acid of Formula I with an appropriate amount of base, such as
an alkali or
alkaline earth metal hydroxide, e.g., sodium, potassium, lithium, calcium, or
magnesium,
or an organic base such as an amine, e.g., dibenzylethylenediamine,
cyclohexylamine,
dicyclohexyiamine, triethylamine, piperidine, pyrrolidine, benzylamine, and
the like, or a
quaternary ammonium hydroxide such as tetramethylammonium hydroxide and the
like.
Also, the basic nitrogen-containing groups can be quaternized with such agents
as
loweralkyl halides, such as methyl, ethyl, propyl, and butyl chlorides,
bromides, and
iodides; dialkyl sulfates; long chain halides such as decyl, lauryl, myristyl,
and stearyl
chlorides, bromides and iodides; arylalkyl halides like benzyl and phenethyl
bromides, and
others. Water or oil-soluble or dispersible products are thereby obtained.
The salts of the present invention can be synthesized from the compounds of
Formula I which contain a basic or acidic moiety by conventional methods, such
as by
reacting the free base or acid with stoichiometric amounts or with an excess
of the desired
salt forming inorganic acid or base in a suitable solvent or various
combinations of solvents.
-45-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
Further included within the scope of the present invention are pharmaceutical
compositions comprising one or more of the compounds of formula (I) prepared
and
formulated in combination with one or more non-toxic pharmaceutically
acceptable carriers
compositions, in the manner described below.
Compositions suitable for parenteral injection may comprise pharmaceutically
acceptable sterile aqueous or nonaqueous solutions, dispersions, suspensions
or emulsions
and sterile powders for reconstitution into sterile injectable solutions or
dispersions.
Examples of suitable aqueous and nonaqueous carriers, diluents, solvents or
vehicles
include water, ethanol, polyols (propylene glycol, polyethylene glycol,
glycerol, and the
like), suitable mixtures thereof, vegetable oils {such as olive oil) and
injectable organic esters
such as ethyl oleate. Proper fluidity may be maintained, for example, by the
use of a coating
such as lecithin, by the maintenance of the required particle size in the case
of dispersions,
and by the use of surfactants.
These compositions may also contain adjuvants such as preserving, wetting,
emulsifying, and dispersing agents. Prevention of the action of microorganisms
may be
ensured by various antibacterial and antifungal agents, for example, parabens,
chlorobutanol, phenol, sorbic acid, and the like. It may also be desirable to
include isotonic
agents, for example, sugars, sodium chloride and the like. Prolonged
absorption of the
injectable pharmaceutical form may be brought about by the use of agents
delaying
2o absorption, for example, aluminum monostearate and gelatin.
If desired, and for more effective distribution, the compounds may be
incorporated
into slow-release or targeted-delivery systems, such as polymer matrices,
liposomes, and
microspheres. They may be sterilized, for example, by filtration through a
bacteria-retaining
filter, or by incorporating sterilizing agents in the form of sterile solid
compositions, which
may be dissolved in sterile water, or some other sterile injectable medium
immediately
before use.
Solid dosage forms for oral administration may include capsules, tablets,
pills,
powders, and granules. In such solid dosage forms, the active compound is
admixed with
at least one inert customary excipient (or carrier), such as sodium citrate or
dicalcium
phosphate, and additionally (a) fillers or extenders, as for example,
starches, lactose,
sucrose, glucose, mannitol and silicic acid; (b) binders, as for example,
carboxymethylcellulose, alginates, gelatin, polyvinylpyrrolidone, sucrose and
acacia; (c)
humectants, as for example, glycerol; (d) disintegrating agents, as for
example, agar-agar,
calcium carbonate, potato or tapioca starch, alginic acid, certain complex
silicates and
sodium carbonate; (e) solution retarders, as for example paraffin; (f)
absorption accelerators,
as for example, quaternary ammonium compounds; (g) wetting agents, as for
example, cetyl
alcohol and glycerol monostearate; (h) adsorbents, as for example, kaolin and
bentonite; and
-46-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
(i) lubricants, as for example, talc, calcium stearate, magnesium stearate,
solid polyethylene
glycols, sodium lauryl sulfate or mixtures thereof. In the case of capsules,
tablets and pills,
the dosage forms may also comprise buffering agents.
Solid compositions of a similar type may also be employed as fillers in soft
and
hard-filled gelatin capsules, using such excipients as lactose or milk sugar,
as well as high
molecular weight polyethylene glycols, and the like.
Solid dosage forms such as tablets, dragees, capsules, pills and granules may
be
prepared with coatings and shells, such as enteric coatings and others well
known in this art.
They may contain pacifying agents, and may also be of such composition that
they release
to the active compound or compounds in a certain part of the intestinal tract
in a delayed
manner. Examples of embedding compositions which may be used are polymeric
substances and waxes.
The active compounds may also be in micro-encapsulated form, if appropriate,
with
one or more of the above-mentioned excipients.
15 Liquid dosage forms for oral administration include pharmaceutically
acceptable
emulsions, solutions, suspensions, syrups and elixirs. In addition to the
active compounds,
the liquid dosage forms may contain inert diluents commonly used in the art,
such as water
or other solvents, solubilizing agents and emulsifiers, as for example, ethyl
alcohol,
isopropyl alcohol, ethyl carbonate, ethyl acetate, benzyl alcohol, benzyl
benzoate, propylene
20 glycol, 1,3-butylene glycol, dimethylformamide, oils, in particular,
cottonseed oil,
groundnut oil, corn germ oil, olive oil, castor oil and sesame oil, glycerol,
tetrahydrofurfuryl alcohol, polyethylene glycols and fatty acid esters of
sorbitan or mixtures
of these substances, and the like.
Besides such inert diluents, these liquid dosage forms may also include
adjuvants,
25 such as wetting agents, emulsifying and suspending agents, sweetening,
flavoring and
perfuming agents.
Suspensions, in addition to the active compounds, may contain suspending
agents,
as for example, ethoxylated isostearyl alcohols, polyoxyethylene sorbitol and
sorbitan
esters, microcrystalline cellulose, aluminum metahydroxide, bentonite, agar-
agar and
30 tragacanth, or mixtures of these substances, and the like.
Compositions for rectal or vaginal administrations are preferably
suppositories
which can be prepared by mixing the compounds of this invention with suitable
non-
irritating excipients or carriers such as cocoa butter, polyethylene glycol or
a suppository
wax, which are solid at ordinary temperatures but liquid at body temperature
and therefore,
35 melt in the rectum or vaginal cavity and release the active component.
Dosage forms for topical or transdermal administration of a compound of this
invention further include ointments, pastes, creams, lotions, gels, powders,
solutions,
-47-
CA 02286909 1999-10-12
WO 98/46605 PCT1US98/07207
sprays, inhalants or transdermal patches. Transdermal administration via a
transdermal
patch is a particularly effective and preferred dosage form of the present
invention. The
active component is admixed under sterile conditions with a pharmaceutically
acceptable
carrier and any needed preservative, buffers or propellants as may be
required. It is known
that some agents may require special handling in the preparation of
transdermal patch
formulations. For example, compounds that are volatile in nature may require
admixture
with special formulating agents or with special packaging materials to assure
proper dosage
delivery. In addition, compounds which are very rapidly absorbed through the
skin may
require formulation with absorption-retarding agents or barriers. Ophthalmic
formulations,
1o eye ointments, powders and solutions are also contemplated as being within
the scope of
this invention.
The present compounds may also be administered in the form of liposomes. As is
known in the art, liposomes are generally derived from phospholipids or other
lipid
substances. Liposomes are formed by mono- or multi-lamellar hydrated liquid
crystals that
I5 are dispersed in an aqueous medium. Any non-toxic, physiologically
acceptable and
metabolizable lipid capable of forming liposomes may be used. The present
compositions in
liposome form may contain, in addition to the compounds of the present
invention,
stabilizers, preservatives, excipients, and the like. The preferred lipids are
the
phospholipids and the phosphatidyl cholines (lecithins), both natural and
synthetic.
20 Methods to form liposomes are known in the art. See, for example, Prescott,
Ed., Methods
in Cell Bioloev. Volume XIV, Academic Press, New York, N. Y., (1976), p 33 et
seq.
Synthetic Methods
The compounds and processes of the present invention will be better understood
in
25 connection with the following synthetic schemes which illustrate the
methods by which the
compounds of the invention may be prepared. The R groups are as defined above
unless
otherwise noted below.
-48-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
he 1
R3
O CH3 O~,,H ~ NC
Rs + NC CN -
H2N N R4
1 2
3
R3 NH2 R3 .
NC O
H~NH N~
2
H2N N R4 ~ N- _R4
3 (I)
The compounds of the present invention may be synthesized by methods
illustrated
in Schemes 1 and 2. In accordance with Scheme 1, the 5,7-disubstituted
compounds
wherein R4 and R3 are aryl, heteroaryl, or a heterocyclic group may be
prepared by a
modification of a method of Kambe et al., Synthesis, 1980, 366-368. An
appropriately
substituted acetophenone (1, the "R4 Reagent"), wherein R4 is aryl,
heteroaryl, or a
heterocyclic group, an appropriately substituted aldehyde (2, the "R3
Reagent"), R3 is aryl,
heteroaryl, or a heterocyclic group, and malononitrile are heated in the
presence of
ammonium acetate, or another suitable ammonium salt, such as for example,
ammonium
propionate, ammonium iodide, or the like, in an aprotic solvent to produce
compound (3).
The water of the reaction may removed by use of a Dean Stark apparatus or by
another
suitable means, such as 4 ~ molecular sieves. Suitable aprotic solvents
include benzene,
toluene, methylene chloride, DMF, THF, dioxane, and the like. The reaction may
be
performed at from about 40 °C to about 200 °C, and preferably at
the reflux temperature of
the solvent, for from about 1 hour to about 24 hours, preferably about 4 hours
to 8 hours.
The product (3) is preferably purified by chromatography after isolation from
the reaction
mixture. The above reaction may also proceed by contacting the aldehyde (2)
with
malononitrile and isolating the resulting dicyano R3substituted alkene which
is then reacted
with the ketone ( 1 ) to form, upon addition of ammonium and cyclization,
compound (3).
Aliphatic aldehydes do not work effectively by this route. The ketone (1) may,
however,
include R4 as alkyl groups.
-49-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
The acetophenone starting materials (1) may be obtained commercially, or
prepared
easily by Friedel-Craft acylation of a suitable aromatic substrate, for
example. The
appropriate aldehyde starting materials (2) also may be obtained commercially,
or may be
prepared easily, for example by reductions of esters or acids with DIBAL or
another suitable
hydride reducing agent, or oxidation of alcohols under Swern conditions, for
example.
Compound (3) is then treated with excess formamide by heating at reflux. The
formation of product is monitored by TLC, and when the reaction is complete
(after about 1
to about 8 hours) the reaction mixture is cooled to room temperature. The 5,7-
disubstituted
pyrido[2,3-dJpyrimidine product (I) is then removed by filtration and purified
by column
chromatography. This compound may then be partially or fully reduced by
catalytic
hydrogenation to the partially saturated or fully saturated versions) (on the
right side of the
molecule) of the compounds shown in Scheme l or of Formula I. Stereoisomers
produced
during these reduction steps are included within the scope of the invention.
The present
invention also contemplates reductions which produce single bonds between the
5,6 and 7,8
positions and a double bond between the 6,7 carbons. The stereoisomers may be
isolated
and purified by conventional means.
In accordance with Scheme 2 are prepared compounds of Formula (I) wherein R4
is
preferrably an aryl, heteroaryl or heterocyclic group, and R3 is loweralkyl,
loweralkenyl,
loweralkynyl, or an arylalkyl group. In addition, R4 may be selected from
those additional
groups listed in R3.
Compound (4, the "R3 Reagent") may be obtained commercially or prepared from
the precursor ester (5) or alcohol (5) by suitable reactions. Compound (5) may
be reduced
with a suitable reducing agent, such as for example, diisobutylaiuminum
hydride or another
similar alkylaluminum hydride, under conditions well known to the art.
Compound (6) may
be oxidized to the aldehyde (4) Swern oxidation conditions, or other reactions
known to
those skilled in the art. The desired compound (4) is freshly prepared before
its use in the
reaction described below.
Compound (9), the "R4 Reagent")) may be prepared from the precursor alpha-
bromo
ketone (7) by a two-step procedure. Compound (7) is treated with
triphenylphosphine in the
3o presence of a base, such as for example, triethyl amine, to give compound
(8). Compound
(8) is then treated with an alkali metal base, such as NaOH or the like, to
give compound
(9). The procedure is normally accomplished by vigorous mixing of a solution
of (8) in an
organic solvent with an aqueous solution of base.
Compounds (4) and (9) are mixed and the mixture is held at ambient temperature
until the reaction is complete (monitoring by TLC), and the product ( 10) is
purified by
chromatography. A mixture of the cis and trans isomers is obtained and taken
to the next
step without further separation. Compound (10) is condensed with malononitrile
by
-50-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
heating in the presence of ammonium acetate as defined for Scheme 1 above to
produce
compound (11).
,S~heme 2
O red'n O oxid'n
R _!( '--~ R3-I( ~--- R3- CH2-OH
O- R6 H
..
Br P(Ph)3+ Bi P(Ph)3
O1 O1 O
R4 ---~ R4 -...,~ Ra
7 8 9
R3
P(Ph)3
~+
O H R4 ~ O R4
4 9 10
R3 R3
NC
+ NC~CN -
4
O R H2 IV R4
11
(I)
5
Compound (11 ) is then -treated with excess formamide by heating at reflux.
The
formation of product is monitored by TLC, and when the reaction is complete
(routinely,
after about 1 to about 8 hours) the reaction mixture is cooled to room
temperature. The 5,7-
disubstituted pyrido[2,3-d)pyrimidine product (I) is then removed by
filtration and purified
10 by column chromatography. In an alternate procedure, compound (11) is
treated by heating
with formamidine acetate in ethoxyethanol, followed by purification by flash
chromatography. In another alternate procedure, compound ( 11 ) and ammonium
sulfate are
-51-
CA 02286909 1999-10-12
WO 98/46605 PCT/LTS98/07207
heated at reflux in triethyl orthoformate for about 1 to about 8 hours, but
preferably about 2
hours. The reaction mixture is cooled and added to a mixture of ammonia in
ethanol. The
mixture is stirred for about 12 to 24 hours at 25 °C, then at reflux
for from one to 4 hours,
and the solvent is removed in vacuo. The residue is purified by trituration
with
chloroform/ethyl acetate, and the product may be converted to a hydrochloride
salt by
suspension in 3M HCI, followed by lyophilization.
Scheme 3
R3
R3
O~ CH3 I NC
4
R + NC CN ----~ ~ ~ a
{1) 12 H2 ~N R
(I)
Scheme 3 illustrates an alternate method for preparing the compounds (I) of
the
invention. Compounds (1), prepared as described above, are reacted with a
dicyanoalkene
compound (12} by heating with a suitable ammonium salt, such as for example,
ammonium
acetate, ammonium propionate, anunonium iodide, or the like, at reflux in an
alcoholic or
aprotic solvent to give the compound (I). Suitable solvents for the reaction
may be easily
determined by those skilled in the art, without undue trial and error, and may
include, for
example, ethanol, propanol, isopropanol, t-butanol, n-butanol, 1,2-
dichloroethane,
benzene, chloroform, carbon tetrachloride, toluene, dioxane, dimethoxyethane,
and the like.
A preferred solvent is 1,2-dichloroethane. The dicyano compounds (12} may be
prepared
2o from the precursor aldehyde (4) by treatment with malononitrile in 1:1
H20:EtOH in the
presence of a catalytic amount of glycine according to the method of Bastus
(Tetrahedron
Len. , 1963: 955), or alternately Mg0 in dichloromethane or a similar aprotic
solvent {cf.
Broekhuis, et al., Recl. J. R. Neth. Chem. Soc., ~9: 6-12 (1980); Moison, et
al.
Tetrahedron ( 1987), 43:537-542).
To prepare compounds of formula (I) wherein R1 and R2 are not both hydrogen
atoms, it is possible to prepared the desired derivative from the compound of
Formula (I)
wherein R1 and R2 are both hydrogen atoms. When R1 or R2 is loweralkyl this
may be
accomplished by reaction of the free amino group with the appropriate
allcylatin~ reagent,
such as an alkyl halide, an alkyl mesylate or an alkyl tosylate, for example,
in the presence
of a base such as triethylamine or potassium carbonate in a suitable solvent,
such as for
example, methylene chloride or THF. When R 1 or R2 is arylalkyl this may be
accomplished
by reaction of the free amino group with the appropriate arylalkyl halide, an
alkyl mesylate
-52-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98107207
or an alkyl tosylate, for example, in the presence of a base such as
triethylamine or
potassium carbonate in a suitable solvent, such as for example, methylene
chloride or THF.
When R1 or R2 is acyl this may be accomplished by reaction of the free amino
group with
the appropriate acid anhydride, acyl chloride or activated acyl group, in the
presence of a
base such as triethylamine or potassium carbonate in a suitable solvent, such
as for example,
methylene chloride or THF. When R1 and R2 are taken together with the nitrogen
atom to
which they are attached to form a 5-to-7 membered ring optionally containing
an additional
oxygen or nitrogen atom, the compound may be prepared by reacting a precursor
compound
having a halogen atom in place of the amino group at the 4-position with a S-7
membered
ring compound optionally containing an additional oxygen or nitrogen atom.
Examples of
such compounds include, but are not limited to, morpholine, piperidine,
pyrrolidine,
piperazine, thiomorpholine, and the like. Also, this alternate procedure may
be used to
prepare alkyl substituted amino compounds, for example by reacting the chloro
compound
with a mono- or disubstituted amine, such as for example, diethylamine, allyl
amine,
is dibutylamine. This reaction takes place readily in a solvent such as
methylene chloride, for
example, in the presence of a tertiary amine. The precursor compound having a
halogen
atom in place of the amino group at the 4-position may be prepared by
substitution of triethyl
orthofonmate for the formamide followed by chlorination of the ring by
treatment with
phosphorous oxychloride or thionyl chloride in the presence of DMF in Scheme 1
wherein
compound (3) is converted to compound (I).
Method of Inhibitin Kinase
In yet another aspect of the present invention a process of inhibiting
adenosine
kinase is disclosed. In accordance with that process, an adenosine kinase
enzyme is
exposed to an effective inhibiting amount of an adenosine kinase inhibitor
compound of the
present invention. Preferred such compounds for use in the process are the
same as set
forth above. Means for determining an effective inhibiting amount are well
known in the
art.
The adenosine kinase to be inhibited can be located in vitro, in situ or in
vivo.
Where the adenosine kinase is located i~: vitro, adenosine kinase is contacted
with the
inhibitor compound, typically by adding the compound to an aqueous solution
containing
the enzyme, radiolabeled substrate adenosine, magnesium chloride and ATP. The
enzyme
can exist in intact cells or in isolated subcellular fractions containing the
enzyme. The
enzyme is then maintained in the presence of the inhibitor for a period of
time and under
suitable physiological conditions. Means for determining maintenance times are
well known
in the art and depend inter alia on the concentrations of enzyme and the
physiological
conditions. Suitable physiological conditions are those necessary to maintain
adenosine
-53-
CA 02286909 1999-10-12
WO 98/46605 PCT1US98/07207
kinase viability and include temperature, acidity, tonicity and the like.
Inhibition of
adenosine kinase can be performed, by example, according to standard
procedures well
known in the art (Yamada, et al., Comp. Biochem. Physiol. 1982, 71B: 367-372).
Where the adenosine kinase is located in situ or in vivo, is typically
administered to a
fluid perfusing the tissue containing the enzyme. That fluid can be a
naturally occuring fluid
such as blood or plasma or an artificial fluid such as saline, Ringer's
solution and the like.
A method of inhibiting adenosine kinase in vivo is particularly useful in
mammals such as
humans. Administering an inhibitor compound is typically accomplished by the
parenteral
(e.g., intravenous injection or oral) administration of the compound. The
amount
1o administered is an effective inhibiting or therapeutic amount.
By a "therapeutically-effective amount" of the compound of the invention is
meant a
sufficient amount of the compound to treat adenosine kinase related disorders
or those
conditions or diseases which are ameliorated or modified by local inhibition
of the enzyme
which results in an increase in the concentration of adenosine. It will be
understood,
15 however, that the total daily usage of the compounds and compositions of
the present
invention is to be decided by the attending physician within the scope of
sound medical
judgment. The specific therapeutically-effective dose level for any particular
patient will
depend upon a variety of factors including the disorder being treated and the
severity of the
disorder; activity of the specific compound employed; the specific composition
employed;
2o the age, body weight, general health, gender and diet of the patient; the
time of
administration, route of administration, and rate of excretion of the specific
compound
employed; the duration of the treatment; drugs used in combination or
coincidental with
specific compound employed; and the like factors well known in the medical
arts and well
within the capabilities of attending physicians.
25 Compounds of the present invention inhibit adenosine kinase activity in
vitro and in
vivo. In vitro adenosine kinase activity can be measured using any of the
standard
procedures well known in the art. By way of example, cells containing
adenosine kinase,
such as IMR-32 human neuroblastoma cells, are cultured in the presence and
absence of an
inhibitor. Inhibition is measured as the ability to inhibit phosphorylation of
endogenous or
3o externally applied 14C-adenosine by these cells. The cells can be intact or
broken. The
specificity of adenosine kinase inhibitory activity is determined by studying
the effects of
inhibitors on adenosine A 1 and A2a receptor binding, adenosine deaminase
activity and
adenosine transport.
Compounds of the present invention are effective in inhibiting adenosine
kinase
35 activity in vivo. Numerous animal models for studying adenosine kinase
activity and the
affects of inhibiting such activity are well known in the art. By way of
example, adenosine
kinase inhibitors have been reported to protect rodents (e.g., mice and rats)
from seizures
-54-
CA 02286909 1999-10-12
WO 98/46605 ~'CT/US98/07207
induced by the subcutaneous administration of pentylenetetrazol (PTZ).
Typically the
rodents are injected with various doses of a given inhibitor followed at
various times by the
subcutaneous administration of from about 10 to about 500 milligrams per
kilogram of PTZ,
The injected animals are then observed for the onset of seizures.
The compounds of the invention were tested in vivo in the hot plate test of
analgesia
m in mammals such as mice. For example, the compounds of examples 6, 79, 104,
130, 133,
134, 137, 205, 246 and 256 in the procedure described directly below were
tested thirty
minutes after pretreatment with the drugs (30 ~.mol/kg i.p.) for latency to
10th jump (in
seconds). The longer the number of seconds, the more effective the drug at
masking the
pain felt from the hot plate. Compound 6 resulted in 152 seconds relative to
the vehicle
alone of 72.8~10.5 seconds (average~standard deviation); compound 79 resulted
in 143
seconds; compound 104 resulted in 180 seconds; compound 130 resulted in 158
seconds;
compound 133 resulted in 131 seconds; compound 134 resulted in 137 seconds;
compound
137 resulted in 159 seconds; compound 205 resulted in 158 seconds, compound
246
resulted in 160 seconds and compound 256 resulted in 143 seconds. Compounds of
the
invention are therefore potent pain relievers as demonstrated in this animal
model.
Mouse Hot Plate Assav
Male CF1 mice (Charles River) of approximately 25-30 g body weight are
pretreated
with 10 ml/kg of the test compounds, i.p. or p.o, in groups of 8 animals per
dose. At the
end of the pretreatment period, the mice are placed in an Omnitech Electronics
Automated 16
Animal Hot Plate Analgesia Monitor (Columbus, OH; Model AHP16AN) in
individual, 9.8
x 7.2 x 15.3 cm (1 x w x h) plastic enclosures on top of a copper plate warned
to 55oC.
Infared sensors located near the top of each enclosure record beam crossings
that occur as
the mice jump off of the heated surface. Latency times for each jump are
automatically
recorded, and latency to both the first and tenth jumps are used for data
analysis. Mice that
do not reach the criteria of 10 jumps by 180 seconds are immediately removed
from the
hotplate to avoid tissue damage, and they are assigned the maximum value of
180 seconds
as their latency to tenth jump.
Numerous other animal models of adenosine kinase activity have been described
[See, e.g., Davies" et al., Biochem. Pharmacol., 33:347-355 { 1984); Keil, et
al., Eur. J.
Pharmacol., 271:37-46 ( 1994); Murray, et al., Drug Development Res., 2$:410-
415
( 1993)].
Compounds of the present invention were also tested in vitro . The results of
some
representative studies are shown below in Tables 1 below. The Examples
provided before
the claims are all adenosine kinase inhibitors. The data indicate that the
compounds inhibit
adenosine kinase and are useful as adenosine kinase inhibitors. The compounds
of the
-55-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
invention including compounds of formula I and II with the variables recited
herein are also
useful as screening tools or as comparative indicators of adenosine kinase
inhibition activity
relative to unlrnown inhibitors or potential inhibitors.
Table 1
Inhibition of Adenosine Kinase by Representative Compounds of the Invention
Com ound of Exam le 1050 (nM)
No.
6 200
15 7
44 50
53 3
56 35
57 1
64 g
79
81 3
100 I 2
104 2
130 , 1
133 2
137 5
147 150
150 150
170 1
175 I 300
177 25
201 3
205 3
208 4
246 5
247 3
256 i
270 20
272 >100
274 2
283 8
288 0.3
29U I 1
291 I 0.6
292 i 10
303 1
304 ~ 1
306 ' 0.3
308 ~ 2
309 ~ 0.1
315 ; 0.3
319 j 1
-56-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
327 1
330 5
333 2
336 8
337 4
338 4.5
347 3
Method of Treating Cerebral Ischemia, Epilepsy,
Nociperception (Nociception) (Pain), Inflammation including
conditions such as Se tip c Sh?ck due to Sepsis Ln~fection
in yet another aspect of the present invention a method of treating cerebral
ischemia,
epilepsy, nociperception or nociception, inflammation including conditions
such as septic
shock due to sepsis infection in a human or lower mammal is disclosed,
comprising
administering to the mammal a therapeutically effective amount of a compound
of formula I
with Rl-R8 as defined herein. The preferred compounds are those of formula II
with the R
variables as defined previously. In particular, the present invention relates
to a method of
treating the above disorders comprising administering a compound of formula II
wherein R3
is a substituted aryl or heteroaryl moiety wherein the substituent
(preferrably halogen) is at
the meta position relative to the ring attachment and R4 is a substituted
heteroaryl or aryl
moiety wherein the substituent is at the para position relative to the ring
attachment. The
most preferred use is in the treatment of pain.
Alterations in cellular adenosine kinase activity have been observed in
certain
disorders. Adenosine kinase activity was found to be decreased, relative to
normal liver, in
a variety of rat hepatomas: activity of the enzyme giving a negative
correlation with tumor
growth rate (Jackson, et al., Br. J. Cancer, 1978, 37: 701-713). Adenosine
kinase activity
was also diminished in regenerating liver after partial hepatectomy in
experimental animals
(Jackson, et al., Br. J. Cancer, 1978, 37: 701-713). Erythrocyte Adenosine
kinase activity
was found to be diminished in patients with gout (Nishizawa, et al., Clin.
Chim. Acta 1976,
67: 1 ~-20). Lymphocyte adenosine kinase activity was decreased in patients
infected with
the human immunodeficiency virus (HIV) exhibiting symptoms of AIDS, and
increased in
asymptomatic HIV-seropositive and HIV-seronegative high-risk subjects,
compared to
normal healthy controls (Renouf, et al., Clin. Chem. 1989, 35: 1478-1481). It
has been
suggested that measurement of adenosine kinase activity may prove useful in
monitoring the
clinical progress of patients with HIV infection (Renouf, et al., Clin. Chem.
1989, 35:
1478-1481). Sepsis infection may lead to a systemic inflammatory syndrome
(SIRS),
characterized by an increase in cytokine production, neutrophil accumulation,
hemodynamic
effects, and tissue damage or death. The ability of adenosine kinase inhibitor
to elevate
adenosine levels in tissues has been demonstrated to ameliorate syndrome
symptoms, due to
-57-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
the known anti-inflammatory effects of adenosine. (Firestein, et al., J. of
Immunology,
1994: 5853-5859). The ability of adenosine kinase inhibitors to elevate
adenosine levels is
expected to alleviate pain states, since it has been demonstrated that
administration of
adenosine or its analogs results in antinociception or antinociperception.
(Swaynok, et al.,
Neuroscience, 1989, 32:557-569).
The following Examples illustrate preferred embodiments of the present
invention
and are not limiting of the specification and claims in any way.
Example 1
4-amino-5-(n-dimeth 1"~minophenvl)-7-(n-bromophen~pvridof2 3-dl~,vrimidine
A sample of 4-(4-bromophenyl)-3-cyano-6-(4-(dimethylamino)phenyl)pyridine-2-
amine ( 1 g), was suspended in formamide (20 mL), and the reaction was heated
to reflux.
After about 3 hours, the reaction was complete as monitored by TLC, and the
reaction
mixture was cooled to room temperature. The product was allowed to
precipitate, then
recovered by filtration and washed with water. Additional product was
recovered from the
filtrate. The product was purified by column chromatography eluting with 10%
MeOH/CH2CI2 to give the pure title compound. IR (KBr) 3503, 3398, 1731, 1658,
1510,
1467, 1278cm-1; MS m/z 421 (M+H)+.
The 4-(4-bromophenyl)-3-cyano-6-(4-(dimethylamino)phenyl)pyridine-2-amine
compound was prepared as follows:
The reagents, 4-bromoacetophenone ( 10 mmol, the "R4 reagent"), 4-
dimethylaminobenzaldehyde ( 10 mmol, the "R3 reagent"), malononitrile ( 10
mmol) and
ammonium acetate ( 1.4 g) were added to 25 mL of benzene. The reaction mixture
was
heated to reflux in a vessel fitted with a Dean-Stork apparatus. After 3.5
hours, the mixture
was cooled, and the solvent was removed. The residue was purified by flash
chromatography, eluting with methylene chloride, with optional addition of 5%
ethyl acetate
to the eluant. MS m/z 394 (M+H)+.
Examples 2-156
Following the procedures of Example 1, except substituting the appropriate
reagents
for R4 and R3 as indicated in Table 2 below, compounds of Examples 2-156 were
prepared
-58-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
Table 2
Examples 2-X56
~ Name R4 Reagent R3 Reagent yt~cal
I
N (for 7- {for 5- Data
o
.
osition) osition
~
2 4-amino-5-(4- 1-(4- 4- IR (KBr) 3440,
dimethylaminophenyl)-7-dimethylamino-dimethylamino-1615. 1760,
(
(4- phenyl)-ethanonebenzaldehyde1210cm-i:
MS m/z
dimethylaminophenyl)pyri 385 (M+H)+.
do[2,3-d] 'rrudine;
3 4-amino-5-(4- 1-(4- 4- IR (KBr) 3330,
methoxyphenyl)-7-(4-dimethylamino-methoxybenzalde1600, 1640,
1780.
dimethylaminophenyl)pyriphenyl)-ethanonehyde 1200cm-i;
MS m/z
do[2,3-d]pyrimidine; 3'72 M+ +
4 4-amino-5-(4- 1-(4- 4- IR (KBr) 3660,
dimethylaminophenyl)-7-methoxyphenyl)-dimethylamino-1600, 1620,
1510.
(4- ethanone benzaldehyde1360, 1240
cm-i;
methoxyphenyl)pyrido[2, MS miz 372
3-d]pyrimidine; M+H +
4-amino-5-(4- 1-(4- 4-isopropyl-IR (KBr) 3430,
isopropylphenyl)-7-(4-methoxyphenyl)-benzaldehyde3360, 1580,
1540
methoxyphenyl)pyrido[2,ethanone cm-i; MS m/z
3'71
3-d]pyrimidine; M+H +
6 4-amino-5-(4- 1-(4- 4-neopentyl-IR (KBr) 3480,
neopentylphenyl)-7-(4-methoxyphenyl)-benzaldehyde2960, 1580,
1510,
methoxyphenyl)pyrido[2,ethanone 1240 cm-i;
MS m/z
3-d]pyrimidine; 399 M+H +
7 4-amino-5-(4- 1-(4- 4- TR (KBr)
butoxyphenyl)-7-(4-methoxyphenyl)-butoxybenzaldeh3480,1600,
1580,
methoxyphenyl)pyrido[2,ethanone yde 1510, 1240,
1180
3-d]pyrimidine; cm-i; MS m/z
401
M+H +
8 4-amino-5-(4- 1-(4- 4- Iit (KBr)
3660,
methoxyphenyl)-7-(4-bromophenyl)-methoxybenzalde1600, 1680,
1520,
bromophenyl)pyrido[2,3-ethanone hyde 1240cm-i;
MS m/z
d]pyrimidine; 407 M+ +
9 4-amino-~-(4- 1-(4- 4-isopropoxy-IR (KBr) 3480,
isopropoxyphenyi)-7-(4-methoxyphenyl)-benzaldehyde2940, 1600,
1580,
methoxyphenyl)pyrido[2,ethanone 1504 cm-i;
MS m/z
3-d]pyrimidine; 386 M+H +
4-amino-5-(4- 1-(4-N- 4-butoxy- IR (KBr) 3480,
butoxyphenyl)-7-(4-N-fonmylpiperazinybenzaldehyde2940, 1660,
1600,
formylpiperazinylphenyl)pIphenyl)- 1580, 1510
cm-i:
yrido[2,3-d]pyrimidine;ethanone MS mi_ 483
(M+H +
11 4-amino-S-(4- 1-(4- 4-benzyloxy-IR (KBr) 3480,
benzyloxyphenyl)-7-(4-methoxyphenyl)-benzaldehyde3040, 1600,
1580,
methoxyphenyl)pyrido[2,ethanone 1560 cm-i;
MS m/z
3-d]pynmidine; 435 (M+H +
-59-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
12 4-amino-5-(4- 1-(4- 4-phenoxy- IR (KBr) 3456,
phenoxyphenyl)-7-(4-methoxyphenyl)-benzaldehyde3053, 1580,
1558,
methoxyphenyl)pyrido[2,ethanone 1247 cm-1;
MS m/z
3-d]pyrlmidine; 421 M+ +
13 4-amino-5-(4- 1-(4-(3- 4-isopropyl-IR (KBr) 3480,
isopropylphenyl)-7-(4-; (diethylmalonyl)benzaldehyde! 2980. 1735.
1580.
diethylinalonylallylphenyl)allyl) phenyl)- 1555 cm-I;
MS m~z
pvrido[2,3-d]pyrimidine;ethanone 539 M+H +
l4 4-amino-5-(4- 1-(4-t- 4-isopropyl-IR (KBr) 3471,
isopropylphenyl)-7-(4-t-butylacrylphenylbenzaldehyde2957, 1708,
1584,
butylacrylphenyl)pyrido[2)-ethanone 1556, 1149
cm'1~
,3-d]pyrimidine; MS m/z 467
M+H +
15 4-amino-5-(3- 1-(4- 3-bromo- IR (KBr) 3480,
bromophenyl)-7-(4- dimethylaminophbenzaldehyde1610, 1580,
1560,
dimethylaminophenyl)pyrienyl)-ethanone 1360, 1200
cm-1;
do[2,3-d]pyrimidine; MS m/z 421
M+H +
16 4-amino-5-(3,4- 1-(4- 3,4-dimethoxy-IR (KBr) 3450,
dimethoxyphenyl)-7-(4-dimethylaminophtxnzaldehyde1610.1580.1560,
dimethylaminophenyl)pyrienyl)-ethanone 1510 cm-1;
MS m/z
do[2,3-d]pyrirrudine; 402 M+H +
17 4-amino-5-(3-t- 1-(4- 3-(3- IR (KBr) 3480,
butylacryiphenyl)-7-(4-dimethylaminophformylphenyl)acr3400, 1700,
1610,
dimethylaminophenyl)pyrienyl)-ethanoneylic acid 1580, 1560
t-butyl cm-I;
do[2,3-d]pyrimldine; ester MS m/z 468
M+H +
18 4-amino-5-(3- 1-(4- 3-methoxy- 1It (KBr) 3475,
methoxyphenyl-7-(4-dimethylaminophbenzaldehyde1610, 1580,
1560,
dimethylaminophenyl)pyrienyl)-ethanone 1200 cm-1;
MS m/z
do[2,3-d]pyrimidine; 372 M+H +
19 4-amino-5-(3,5- 1-(4- 3,5-dimethoxy-IR (KBr) 3419,
dimethoxyphenyl-7-(4-dimethylaminophbenzaldehyde1637, 1600,
1572,
dimethylaminophenyl)pyrienyl)-ethanone 1371, 1202
cm-1;
do[2,3-d]pyrimidine; MS m/z 402
M+H +
20 4-amino-5-(3- 1-(4- 2-[2-(3- IR (KBr) 3480.
diethyhnalonylallylphenyl)dimethylaminophformylphenyl)vi1720, 1610;
1580,
-7-(4- enyl)-ethanonenyl]malonic 1558, 1524,1360
acid
dimethylaminophenyl)pyri diethyl estercm-1; MS m/z
540
do[2,3-d]pyrimldine; M+H +
21 4-amino-S-(3- 1-(4- 3-vinylpyridinyl-IR (KBr) 3480,
vinylpyridinylphenyl)-7-dimethylaminophbenzaldehyde1610, 1580,
1560,
(4- enyl)-ethanone 1513, 1360
cm-i;
dimethylaminophenyl)pyri MS m~~ 385
do[2,3-d]pyrimidine; M+H +
22 4-amino-5-(3- 1-(4- 3- IR (KBr) 3480,
trifluoromethylphenyl)-7-dimethylaminophtrifluoromethyl-1610, 1580,
1560.
(4- enyl)-ethanonebenzaldehyde1360. 1200
cm-i;
dimethylaminophenyl)pyri MS m~= 410
do[2,3-d]pyrimidine; (M+H +
-60-
CA 02286909 1999-10-12
WO 98/46605 PGT/US98/07207
23 4-amino-5-(3- 1-(4- 3-amido- IR (KBr) 3480,
carboxamidophenyl)-7-(4-dimethylaminophbenzaldehyde1610, 1580,
1380,
dimethylaminophenyl)pyrienyl)-ethanone 1200 cm 1;
MS m/z
do[2,3-dJpyrimidine; ,~6 + +
24 4-amino-5-(3- 1-(4- 3-cyano- IR (KBr) 3460,
cyanophenyl)-7-(4- dimethylaminophbenzaldehyde3400, 2210.
1610,
dimethylaminophenyl)pyrienyl)-ethanone 1580, 1554.
1360
do[2,3-d]pyrimidine; cm-1; MS m/_
367
M+H +
25 4-amino-5-(3- 1-(4- 3-benzyloxy-IR (KBr) 3470,
benzyloxyphenyl)-7-(4-dimethylaminophbenzaldehyde1640, 1580,
1550,
dimethylaminophenyl)pyrienyl)-ethanone 1515, 1357.
1250
do[2,3-d]pyrimidine; cm-1; MS m/z
448
M+H +
26 4-amino-5-(3- 1-(4- 3-methoxy- IR (KBr) 3470,
methoxyphenyl)-7-(4-methoxyphenyl)-benzaldehyde1640, 1580.
1550,
methoxyphenyl)pyrido[2,ethanone 1515, 1357,
1250,
3-d]pyrimidine; 1240, 1180
cm-1;
MS m/z 359
M+H +
27 4-amino-5-(3- 1-(4- 3-bromo- IR (KBr) 3478,
bromophenyl)-7-(4- butoxyphenyl)-benzaldehyde1610, 1580,
1560,
butoxyphenyl)pyrido[2,3-ethanone 1515, 1355,
1255.
d]pyrimidine; 1240, 1180
cm-I;
MS m/z 449
M+H +
28 4-amino-5-(3-(2- 1-(4- 3-(2-pyridyl)-IR (microscope)
pyridyi)phenyl)-7-(4-dimethylaminophbenzaldehyde3476.1609.
1580,
dimethylaminophenyi)pyrienyl)-ethanone 1560, 1358
cm-1;
do[2,3-d]pyrimidine; MS m/z 419
M+H +
29 4-amino-5-(3- I-(4- 3-methyl- Iit (microscope)
methylphenyl)-7-(4-dimethylaminophbenzaldehyde3400. 1640,1600.
dimethylaminophenyi)pyrienyl)-ethanone 1580, 1540cm-1;
do[2,3-dJpyrimidine; MS m/z 356
M+H +
30 4-amino-5-(3- 1-(4- 3-chloro- IR (microscope)
chlorophenyl)-7-(4-dimethylaminophbenzaldehyde3400, 1600.
1580,
dimethylaminophenyl)pyrienyl)-ethanone 1540 cm-1:
MS m/z
do[2,3-d]pyrimidine; 376 M+ +
31 4-amino-5-(3- 1-(4- 3-fluoro- IR (microscope)
fluorophenyl)-7-(4-dimethylaminophbenzaldehyde3480, 1640,
1580,
dimethylaminophenyl)pyrienyl)-ethanone 1560cm-1:
MS m/z
do[2,3-d)pyrimidine; 360 M+H +
32 4-amino-5-(3- 1-(4- 3-bromo- IR (microscope)
bromophenyl)-7-(4- methoxyphenyl)-benzaldehyde3485.1607.1575,
methoxyphenyl)pyrido[2,ethanone 1550, 1515.
1350,
3-d]pyrimidine; 1255. 1240,
1180,
1030 cm-1;
MS m/.
407 M+H +
-61-
CA 02286909 1999-10-12
WO 98/46605 PCT/iJS98/07207
33 4-amino-5-(3- 1-(4- 3-methoxy- iR (microscope)
methoxyphenyl)-7-(4.bromophenyl)-benzaldehyde 3450, 1640,
1573,
bromophenyl)pyrido[2,3-ethanone 1555, 1496,
1350,
d]pyrimidine; 1260 cm-1;
MS m/z
407 M+H +
34 4-amino-5-(3- 1-phenyl- 3-bromo- IR (lcBr)
3480,
bromophenyl)-7-phenylethanone benzaldehyde 1640, 1580,
1560,
pyrido [2,3-d]pyrimidme; 1480, 1350,
700
cm-1; MS m/z
377
M+H +
35 4-amino-5-(3- 1-(4- 3-bromo- IIt (microscope)
bromophenyl)-7-(4- ethylphenyl)-benzaldehyde 3480, 1645,
1580
ethylphenyl)pyrido[2,3-ethanone (broad),1490,1380
d]pyrimidine; cm-1; MS mlz
405
M+H +
36 4-amino-5-(3- 1-(4- 3-bromo- lR (KBr) 3480,
bromophenyl)-7-(4- bromophenyl)-benzaldehyde 1610, 1575,
1540,
bromophenyl)pyrido[2,3-ethanone 1350 cm-1;
MS mlz
d]pyrimidine; 455 M+ +
37 4-amino-5-(3- 1-(4- 3-bromo- IR (microscope)
bromophenyl)-7-(4- cyanophenyl)-benzaldehyde 3480, 2230,
1618,
cyanophenyl)pyrido[2,3-ethanone 1580, 1555,
1545,
d]pyrimidine; 1350 cm-1;
MS mh
402 M+H +
38 4-amino-5-(3- 1-(4- 3-bromo- IR (microscope)
bromophenyl)-7-(4- hydroxyphenyl)-benzaldehyde 3481, 3060
(broad),
hydroxyphenyl)pyrido[2,3ethanone 1645, 1580,
1560,
-d]pyrimidine; 1544, 1360,
1240
1155 cm-1;
MS mlz
393 M+H +
39 4-amino-S-(3- 1-(4- 3-iodo- IR (microscope)
iodophenyl)-7-(4- dimethylaminophbenzaldehyde 3500, 3040,
1640,
dimethylaminophenyl)pyrienyl)-ethanone 1600, 1580,
1560
do[2,3-d]pyrimidine; cm-1; MS m/z
468
M+H +
40 4-amino-5-(3- 1-(4- 3-ethoxy- Iit (microscope)
ethoxyphenyl)-7-(4-dimethylaminophbenzaldehyde 3460, 3250,
1640,
dimethylammophenyl)pyrienyl)-ethanone 1600, 1580.
1560
do[2,3-d]pyrimidine; cm-1; MS m/z
386
M+H +
41 4-amino-5-(3- 1-(4- 3- IR (microscope)
trifloromethyoxyphenyl)-dimethylaminophtrifluoromethoxy3480, 1710,
1610,
7-(4- enyl)-ethanone-benzaldehyde1580, 1560,
1540
dimethylaminophenyl)pyri cm-1; MS mlz
426
do[2,3-d]pyrimidine; M+H +
42 4-amino-5-(3.5- 1-(4- 3,5-dichloro-IR (microscope)
dichlorophenyl)-7-(4-dimethylaminophbenzaldehyde 3500, 3040,
1640.
dimethylaminophenyl)pyrienyl)-ethanone 1600, 1580.
1560
do[2,3-d]pyrimidine; cm-1; MS m/z
411
M+H +
-62-
CA 02286909 1999-10-12
WO 98/4b605 PCT/US98/07207
43 4-amino-5-(3-bromo-4-1-(4- 3-bromo-4- I1t (microscope)
fluorophenyl)-7-(4-dimethylaminophfluoro- 3440. 3015,
1633,
dimethylaminophenyl)pyrienyl~ethanonebenzaldehyde1607,1583
cm-1;
do[2,3-d]pyrimidine; MS m/z 438
M+ +
44 4-amino-5-(3- 1-(4- 3-hydroxy- llt (microscope)
hydroxyphenyl)-7-(4-dimethylaminophbenzaldehyde3450. 1640.
i 1610.
dimethylaminophenyl)pyrienyl)-ethanone 1580,1560
cm-1;
do[2,3-d]pyrimidine; MS m/z 358
M+H +
45 4-amino-5-(3- 1-(4- 3-bromo- IR (microscope)
bromophenyl)-7-(4- morpholinylphenbenzaldehyde3483, 1607,
1578,
morpholinylphenyl)pyridoyl)-ethanone 1561, 1518,
1355,
[2,3-d]pyrimidine; 1228 1120
cm-1;
MS m/z 462
M+H +
46 4-amino-5-(3- 1-(4- 3-bromo- 1R (microscope)
bromophenyl)-7-(4- piperidinylphenybenzaldehyde3486, 1606,
1561,
piperidinylphenyl)pyrido[1)-ethanone 1540, 1519,
1353,
2,3-d]pyrimidine; 1231, 1199,
1128
cm-1; MS m/z
460
M+H +
47 4-amino-5-(3- 1-(4-(imidazol-1-3-bromo- IR (KBr) 3481,
bromophenyl)-7-(4- yl)phenyl}- benzaldehyde1580, 1555,
1525,
(imidazol-1- ethanone 1482, 1352.
1303,
yl)phenyl)pyrido[2,3- 1053 cm-1;
MS m/z
d]pyrimidine; 443 M+H +
48 4-amino-5-(3- 1-(4- 3-bromo- IR (KBr) 3470,
bromophenyl)-7-(4- chlorophenyl)-benzaldehyde1635, 1580,
1560,
chlorophenyl)pyrido[2,3-ethanone 1500, 1350,
1090
d]pyrimidine; cm-1; MS m/z
411
M+H +
49 4-amino-5-(3- 1-(4- 3-bromo- IR (KBr) 3484,
bromophenyl)-7-(4- isopropylphenyl)benzaldehyde1610, 1579,
1560,
isopropylphenyl)pyrido[2,-ethanone 1550. 1483,
1357
3-d]pyrimidine; cm-1; MS m/z
419
M+H +
50 4-amino-5-(3- 1-(4- 3-bromo- Itt (microscope)
bromophenyl}-7-(4- trifluorophenyl)-benzaldehyde3481, 3289.
1616,
trifluorophenyl)pyrido[2,3ethanone 1579, 1547,
1324,
-d]pyrimidine; 1312, 1122,
1070
cm-1; MS m/z
445
M+H +
51 4-amino-5-(3- 1-(4- 3-bromo- IR (KBr) 3481,
bromophenyl)-7-(4- diethylaminophebenzaldehyde1607, 1578,
1561,
diethylaminophenyl)pyridnyl)-ethanone 1533. 1353,
1200,
0[2,3-d]pyrimidine; 1155 cm-1;
MS mlz
448 M+H +
52 4-amino-5-(3- 1-(3,4,5- 3-bromo- IR (KBr) 3485.
bromophenyl)-7-(3,4,5-trimethoxyphenybenzaldehyde1579, 1548,
1507,
trimethoxyphenyl)pyrido[1)-ethanone 1340, 1129
cm-1~
2,3-d]pyrimidine; MS ml~ 467
M+H +
-63-
CA 02286909 1999-10-12
WO 98/46b05 PCT/US98/07207
53 4-amino-5-(3-(3- 1-(4- 3-(3- 1R (KBr)
3425,
methoxybenzyl)phenyl)-7-dimethylaminophmethoxybenzyl)-1613, 1580,
1558,
(4- enyl)-ethanonebenzaldehyde 1537 cm-1;
MS m/z
dimethylaminophenyl)pyri 478 ~+~+
'
.
do[2,3-d]
midine;
54 4-amino-5-(3- 1-(4- 3- IR (KBr)
~ 3469,
methoxyethyoxyphenyl)-dimethylaminophmethoxyethoxy-1610. 1580,
1560,
~-(4- enyl)-ethanonebenzaldehyde 1357 cm-1;
MS m/z
dimethylaminophenyl)pyri 416 (M+H)+
'
.
do[2,3-d]
midine;
55 4-amino-5-(3,4- 1-(4- 3,4- IR (KBr)
3466,
methylenedioxyphenyl)-7-dimethylaminophmethylenedioxy-16245, 1579,
1560
(4- enyl)-ethanonebenzaldehyde cm-1; MS
m/z 386
dimethylaminophenyl)pyri (M+~+
'
.
do[2,3-d]
midine;
56 4-amino-5-(3- 1-(4- 3-bromo- IR (KBr)
3480,
bromophenyl)-7-(4- ethoxyphenyl)-benzaldehyde 160'7, 1579,
1560,
ethoxyphenyl)pyrido[2,3-ethanone 1517, 1360,
1238,
d]pyrimidine; 1180 cm-1;
MS m/z
421 (M+H)+.
57 4-amino-5-(3- 1-phenyl- 3-bromo- IR (KBr)
3470,
bromophenyl)-7-(2'-ethanone benzaldehyde 1579, 1560,
1547,
thiophene)pyrido[2,3- 1429, 1361
cm-1~
d]pyrlmldlrie; MS m/z 383
M+H +
58 4-amino-5-(3- 1-(4- 3-bromo- IR (microscope)
bromophenyl)-7-(4- fluorophenyl)-benzaldehyde 3476.1600.1580,
fluorophenyl)pyrido[2,3-ethanone 1555, 1515,
1350,
d]pyrimidine; 1230 cm-1;
MS m/z
395 M+H +
59 4-amino-5-(3- 1-(4- 3- IR (KBr)
3436.
dimethylaminophenyl)-7-dimethylaminophdimethylamino-1601. 1580.
1563,
(4- enyl)-ethanonebenzaldehyde 1534, 1200
cm-1;
dimethylaminophenyl)pyri MS m/z 385
do[2,3-d]pyrimidine; M+H +
60 4-amino-5-phenyl-7-(4-1-(4- benzaldehyde IR (K>3r)
3400,
dimethylamlnophenyl)pyridimethylaminoph 1600. 1580,
1560,
do[2,3-d]pyrimldine;enyl)-ethanone 1530, 1200
cm-1;
MS m/z 342
+H +
61 4-amino-5-(3,4,5- 1-(4- 3,4,5- IR (I~r)
33460,
trimethoxyphenyl)-7-(4-dimethylaminophtrimethoxy- 1607.1578,
dimethylammophenyl)pyrienyl)-ethanonebenzaldehyde 1127cm-1;
MS m/z
do[2,3-d]pyrimldine; 432 M+H +
62 4-amino-5-(3- 1-(4- 3-bromo- IR (microscope)
bromophenyl)-7-(4- nitrophenyl)-benzaldehyde 3485.1618.1580,
nitrophenyl)pyrido[2,3-ethanone 1550, 1520,
1340,
d]pyrimidine; 860 cm-1;
MS m/z
422 M+H +
-64-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
63 4-amino-5-(3- 1-(4- 3-bromo- 1R (KBr) 3480,
bromophenyl)-7-(4- iodophenyl)- benzaldehyde1610, 1575,
1570,
iodophenyl~yrido[2,3-ethanone ' 1540. 1350,
1000
d]pyrimidine; cm-1; MS.m/z
503
M+ +
64 4-amino-5-(3- 1-(3,4- 3-bromo- IR (KBr) 3485,
bromophenyl)-7-(3,4-methylenedioxypbenzaldehyde1607, 1575.
1545,
methylenedioxyphenyl)pyrhenyl)-ethanone 1500, 1440,
1350,
ido[2,3-d]pyrimidine; 1255. 1038
cm-1;
MS m/z 421
M+H +
65 4-amino-5-{thiophen-2-1-(4- thiophene-2-IR (KBr) 3480,
yl)-7-(4- morpholinylphencarboxaldehyde1607, 1580,
1560,
morpholinylphenyl)pyridoyl)-ethanone 1226 cm-1;
MS m/z
(2,3-d]pyrimidine; 390 M+H +
66 4-amino-5-(3,5- 1-(thiophen-2-3,5-dimethoxy-IR (KBr) 3450,
dimethoxyphenyl)-7-yl)-ethanone benzaldehyde1640, 1600,
- 1580,
(thiophen-2-yle)pyrido 1560 cm-1;
MS m/z
[2,3-d]pyrimidine; 365 M+H +
67 4-amino-5-(3- 1-(4- 3-bromo- IIt (KBr)
3481,
bromophenyl)-7-(4- carboxamidophebenzaldehyde1674, 1611,
1577,
carboxamidophenyl)pyridnyl)-ethanone 1558,1352
cm-1;
0[2,3-d]pyrimidine; MS m/z 420
M+H +
68 4-amino-5-(3- 1-(4-(2- 3-bromo- IR (KBr) 3478,
bromophenyl)-7-(4-(2-methoxy)ethoxybenzaldehyde1607, 1580,
1560,
methoxy)ethoxyphenyl)pyphenyl)-ethanone 1515, 1357,
1260,
rido[2,3-d]pyrimidine; 1235, 1180,
1113
cm' 1; MS
m/z 451
M+H +
69 4-amino-5-(3,5- 1-(4- 3,5-dimethoxy-IR (Kgr) 3450,
dimethoxyphenyl)-7-(4-morpholinylphenbenzaldehyde1608, 1580,
1555,
morpholinylphenyl)pyridoyl)-ethanone 1541, 1230,
1210,
[2,3-d]pyrimidine; 1160 cm-i;
MS m/z
444 M+H +
70 4-amino-5-(3- 1-(thiophene-2-3- iit (KBr)
3486,
trifluoromethylphenyl)-7-yl)-ethanone trifluoromethyl-1620, 1580,
1560,
(thiophene-2-yl)pyrido benzaldehyde1325,1123cm-1;
[2,3-d)pyrimidine; MS m/z 373
M+H +
71 4-amino-5-(3- 1-(4- 3-bromo- Itt (KBr)
3450,
bromophenyl)-7-(4- aminophenyl)-benzaldehyde1632, 1605,
1580,
aminophenyl)pyrido[2,3-ethanone 1365 cm-i;
MS m/z
d]pyrimidine; 393 M+H +
72 4-amino-5-(3-bromo-4-1-(thiophene-2-3-bromo-4- IFt (KBr)
3480,
fluorophenyl)-7- yl)-ethanone fluoro- 1640, 1580,
~ 1560,
(thiophene-2-yl)pyrido benzaldehyde1500cm-1;
MS m/z
[2,3-d]pyrimidine; 401 M+H +
73 4-amino-5-(3-bromo-4-1-(2-furanyl)-3-bromo-4- IR (KBr) 3460,
fluorophenyl)-7-{2-ethanone fluoro- 1600. 1580,
1560,
furanyl)pyrido [2,3- benzaldehyde1500cm-1;
MS m/z
d]pyrirrudine;
385 M+H +
-65-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98107207
74 4-amino-5-(3,5- 1-(4- 3,5-dimethoxy-llt (KBr)
3460,
dimethoxyphenyl~7-(4-iodophenyl)-benzaldehyde 1604, 1575,
1556,
iodophenyl)pyrido[2,3-ethanone 1541, 1207,
1160
d]pyrimidine; cm-1; MS m/z
485
+H +
75 4-amino-5-(3,5- 1-(4- 3,5-dimethoxy-IR (KBr) 3459.
dimethoxyphenyl)-7-(4-imidazolylphenylbenzaldehyde ~ 1604. 1580,
1556,
imidazolylphenyl)pyrido[2)-ethanone 1524, 1484,
1304,
,3-d]pyrimidine; 1159, 1056
cm-1:
MS m/z 425
(M+H +
76 4-amino-5-(3,5- I-(4-(thiophene-3,5-dimethoxy-IR (KBr) 3457,
dimethoxyphenyl)-7-(4-2-yl)phenyl)-benzaldehyde 1602, 1579,
1557,
(thiophene-2- ethanone 1207, 1159
cm-1~
yl)phenyl)pyrido[2,3- MS m/z 441
d]pyrimidine; M+H +
77 4-amino-5-(3,5- 1-(4-(3- 3,5-dimethoxy-IR (KBr) 3452.
dimethoxyphenyl)-7-(4-pyridyl)phenyl)-benzaldehyde 1604, 1578.
1558,
(3- ethanone 1287, 1206,
1159
pyridyl)phenyl)pyrido[2,3 cm-1~ MS m/.
436
-d]pyrimidine; M+H +
78 4-amino-5-(3- I-(4-(4- 3-bromo- IR (KBr) 3475.
bromophenyl)-7-(4.-{4-methylpiperidinybenzaldehyde 1607, 1577,
1558,
methylpiperidinyl)phenyl)1)phenyl)- 1540, 1356,
1232
pyrido[2,3-d]pyrin-iidine;ethanone cm-1; MS m/_
475
M+H +
79 4-amino-5-(3- 1-(4- 3-bromo- IR (KBr) 3486.
bromophenyl)-7-(4- pyrrolidinylphenbenzaldehyde 1608, 1577,
1560,
pyrrolidinylphenyl)pyrido[yl)-ethanone 1533, 1353.
1196
2,3-d]pyrimidine; cm-1; MS m/.
446
+H +
80 4-amino-S-(4- 1-(4- 4- IR (KBr) 3327.
bromothiophen-2-yl)-7-{4-dimethylaminophbromothiophene-1604, 1578,
1548,
dimethylaminophenyl)pyrienyl)-ethanone2- 1521, 1367,
1350,
do[2.3-d]pyrimidine; carboxaldehyde1202, 820
cm-1;
MS m/z 426
M+H +
81 4-amino-5-(4- 1-(4- 4- nt (KBr) 3460,
bromothiophene-2-yl)-7-morpholinylphenbromothiophene-1606, 1578,
1558,
(4- yl)-ethanone2- 1541, 1517,
1232,
morpholinylphenyl)pyrido carboxaldehyde824 cm-1;
MS m/z
[2,3-d)pyrimidine; 468 M+H +
82 4-morpholinyl-5-(3-1-(4- 3-bromophenyl-IIt (microscope)
bromophenyl)-7-(4- dimethylaminophbenzaldehyde 3340. 1603,
1580,
dimethylaminophenyl)pyrienyl)-ethanone 1540 cm-1;
MS m/z
do[2,3-d]pyrimldine; 490 M+H +
83 4-amino-5-(5- I-(4- 5- IR (KBr) 3460.
bromothiophene-2-yl)-7-morpholinylphenbromothiophene-1606, 1580.
1558,
{4- yl)-ethanone2-yl- 1541, 1517,
1233
morpholinylphenyl)pyrido benzaldehyde cm-1; MS m/_
468
[2,3-d]pyrimidine; M+H +
-66-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
84 4-amino-5-(4- 1-(4- 4-bromo- 1R (microscope)
bromophenyl)-7-(4- dimethylaminophbenzaldehyde 3480, 3320,
1603,
dimethylarninophenyl)pyrienyl)-ethanone 1580, 1540,
820
do[2,3-d]pyrimidine; cm-1; MS m/z
420
(M+H +
85 4-amino-~-(3- 1-(4- 3-bromo- ~ llt (microscope)
bromophenyl)-7-(4- (acetyiamino)phebenzaldehyde 34801600.1580.
(acetylamino)phenyl)pyridnyl)-ethanone 1520cm-1:
MS rr~:
0[2,3-d)pyrimidine; 434 M+H +
86 4-amino-5-(3- 1-(4- 3-bromo- IR (microscope)
bromophenyl)-7-(4- dimethyiaminophbenzaldehyde 3300, 1606.
1600,
dimethylaminophenyl)pyrienyl)-ethanone 1580, 1560
cm-1;
do[2,3-d]pyrirrudine; MS m/z 421
(M+H +
87 4-amino-5-(3,5- 1-(5- 3,5-dimethoxy-IR (microscope)
dimethoxyphenyl)-7-(5-pyrimidinylphenbenzaldehyde 3458. 1602,
1579,
pyrimidinylphenyl)pyrido[yl)-ethanone 1558.1460.1414,
2,3-d]pyrimidine; 1364. 1196.
1058
cm-1; MS m/z
437
M+H +
88 4-(4-fluorophenyl)amino)-1-(4- 3-bromo- IR (KBr) 3410,
5-(3-bromophenyl)-7-(4-dimethylaminophbenzaldehyde 1605, 1570.
1525.
dimethylammophenyl)pyrienyl)-ethanone 1503 cm-1:
MS m/z
do[2,3-d]pyrimidine; 514 +H +
89 4-amino-5-(4- 1-(4- 4- llt (KBr)
3470,
bromothiophene-2-yl)-7-pyrrolidinylphenbromothiophene-1609. 1577,
1555,
(4- yl)-ethanone 2- 1520. 1409,
1386,
pyrrolidinylphenyl)pyrido[ carboxaldehyde1350.1196.821
2,3-d]pyrimldine; cm-1; MS m/z
452
M+H +
90 4-amino-5-(4- 1-(thiophene-2-4- IR (KBr) 3308,
bromothiophene-2-yl)-7-yl)-ethanone bromothiophene-1606.1578.1543.
(thiophene-2- 2- 1526, 1427,
1359
yl)pyrido[2,3- carboxaldehydecm-1; MS m/z
389
d]pyrimidine; M+H +
91 4-amino-5-(3- 1-(5- 3-bromo- IR(microscope)
bromophenyl)-7-(5- (dimethylamino)tbenzaldehyde 3490, 1581,
1556,
(dimethylamino)thiophenehiophene-2-yl)- 1501, 1481,
1407,
-2-yl)pyrido[2,3- ethanone 1373. 1072
cm-1;
d]pylimidine; MS m/z 426
M+H +
92 4-amino-5-(3-bromo-5-1-(4- 3-bromo-5-iodo-IR (KBr) 3493,
iodophenyl)-7-(4- (dimethylamino)benzaldehyde 1608, 1562.
1533.
(dimethylamino)phenyl)pyphenyl)-ethanone 1364, 1350,
1200
rido[2,3-d]pyrimidine; cm-1: MS mlz
546
M+H +
93 4-amino-5-(3,5- 1-(4- 3,5- IR (KBr) 3484,
di(trifluoromethyl)phenyl)(dimethylamino)di(trifluoromethy1607, 1580.
1554.
-7-(4- phenyl)-ethanone1-benzaldehyde1386. 1280
cm-1;
(dimethylamino)phenyl)py MS m/z 478
rido[2,3-d]pyrimidine; M+H i+.
-67-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
94 4-amino-5-(3,5- 1-(4- 3,5- IR (KBr) 3500
,
di(trifluoromethyl)phenyl)morphoiinylphendi(trifluoromethy1643. 1602,
1578,
-7-(4- yl)-ethanone 1-benzaldehyde1554,1280 cm-1;
morpholinylphenyl)pyrido MS m~z 520
[2,3-dJpyrimidine; (M+~+.
95 4-amino-~-(3,5- 1-(4- 3,5-dibromo-IR (KBr) 3440
,
dibromophenyl)-7-(4-(dimethylamino)benzaldehyde~ 1608. 1570,
1559,
(dimethylamino)phenyl)pyphenyl)-ethanone I 1536 cm-1~
MS miz
rido[2,3-d]pyrimidine; 475 M+H~+
96 4-amino-5-(3,5- 1-(4- 3>5-dibromo-llt (KBr) 3480
,
dibromophenyl)-7-(4-molpholinylphenbenzaldehyde1607, 1560,
1540,
morpholinylphenyl)pyridoyl)-ethanone 1225 cm-1~
MS m/z
[2,3-d]pyrimidine; ~
_ 540 M+H
97 4-amino-5-(4- 1-(4-{4- 4- +
gz
(KBr) 3460
,
bromothiophene-2-yl)-7-methylpiperidinybromothiophene-1608. 1576,
1557,
(4-(4- 1)phenyl)- 2- 1540, 1513,
1384,
methylpiperidinyl)phenyl)ethanone carboxaldehyde1353, 1240,
823
pyrido[2,3-d]pyrimidine; cm-1; MS m/z
481
M+H +
98 4-amino-5-(3,5- 1-(4- 3,5-dibromo-Ilt (KBr) 3486,
dibromophenyl)-7-(4-(dimethylamino)benzaldehyde1608, 1570,
1559,
(dimethylamino)phenyl)pyphenyl)-ethanone 1536, 1360,
1350,
rido[2,3-d]pyrimidine; 1200, 823 cm-1;
MS m/ z 498
+ +
99 4-amino-5-(3- 1-(4- 3-bromo- IR
~
bromophenyl)-7-(3- (dimethylamino)benzaldehyde160 579 8 548,
(dimethylamino)phenyl)pyphenyl)-ethanone 1483, 1357
cm-1~
rido[2,3-d]pyrimldine; MS miz 420
+ +
100 4-amino-5-(3- 1-{4- 3-bromo- .
IR
(KBr) 3486,
bromophenyl)-7-(4- methylsulfonylpbenzaldehyde1600, 1580,
1550,
methylsulfonylphenyl)pyrihenyl)-ethanone 1490 cm-1~
MS m/z
do [2,3-d]pyrimidine; '
5 M+ +
101 4-amino-5-(3- 1-(3- 3-bromo- .
~
(KBr) 3486,
bromophenyl)-7-(3- methoxyphenyl)-benzaldehyde1605, 1578,
1550,
methoxyphenyl}pyrido[2,ethanone 1492. 1346,
1263
3-d]pyrimidine; cm-1; MS m/z
407
M+H +
.
102 4-amino-5-(3- 1-(4- 3-bromo- IR (KBr) 3485,
bromophenyl)-7-(4- (methylthio)phenbenzaldehyde1607, 1578,
1566,
(methylthio)phenyl)pyridoyl)-ethanone 1538, 1350,
1094,
[2,3-d]pyrimidine; 795 cm-1; MS
m~z
103 4-amino-5-(3- 1-(3,4- 3-bromo- ~3 M+H +.
(KBr) 3482
,
bromophenyl)-7-(3,4-dichlorophenyl)-benzaldehyde1634, 1576,
1545,
dichlorophenyl)pyrido[2,3ethanone 1488, 1342
cm-n
-d]pyrirrudine; MS mi_ 445
(M+H +
-68-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
104 4-amino-5-(3- 1-(4-(N-methyl-3-bromo- lIt (I~r) 3478,
bramophenyl)-7-(4-(N-N- benzaldehyde1672, 1639,
1603,
methyl-N- formylamino)phe 1579, 1547,
84i
formylamino)phenyl)pyridnyl)-ethanone cm-1; MS m/z
434
0[2,3-d]pyrimidine; ~M+~+.
105 4-amino-5-(3- ~ 1-{4- 3-bromo- IR (ICl3r)
3488,
bromophenyl)-7-(4-methylaminophebenzaldehyde1637, 1607.
1587.
methylaminophenyl)pyridnyl)-ethanone 1360 cm-1~
MS m~~
.
0[2,3-d]pyrimidine; 480 (Li+H
+
106 4-amino-S-(3-bromo-4-1-(4- 3-bromo-4- IR (KBr) 3489,
fluorophenyl)-7-(4-methylsulfonylpfluoro- 1578, 1560,
1496,
methylsulfonylphenyl)pyrihenyl)-ethanonebenzaldehyde1311, 1151,
775
do[2,3-d]pyrimidine; cm-1: MS mi_
473
M+ +
107 4-amino-5-(3- 1-(3-amino-4-3-bromo- IR (microscope)
bromophenyl)-7-(3-methoxyphenyl)-benzaldehyde3431, 1629,
1606,
amino-4- ethanone 1583, 1274
cm-1;
methoxyphenyl)pyrido[2, MS rr~z 422
3-d]pynmidine; M+ +
108 4-amino-5-(3- 1-(3-bromo-4-3-bromo- >It (microscope)
bromophenyl)-7-(3-(dimethylamino)benzaldehyde3470, 1638.
1570,
bromo-4- phenyl)-ethanone 1560. 1538,
1480,
(dimethylamino)phenyl)py 1345 cm-1;
MS m/z
rido[2,3-d]pyrimidine; 498 +H +
109 4-amino-5-(3- 1-(3-bromo-4-3-bromo- IR (microscope)
bromophenyl)-7-(3-(dimethylamino)benzaldehyde3438.1640..1605,
methyl-4- phenyl)-ethanone 1580. 1555,
1368
(dimethylamino)phenyl)py cm-1: MS m~:
434
rido[2,3-d]pyrimidine; M+ +
110 4-amino-5-(3- 1-(4-(N-methyl-3-bromo- IR (l~r) 3443,
bromophenyl)-7-(4-(N-N- benzaldehyde1699. 1635,
1606,
methyl-N- trifluoroacetylam 1201 cm-1~
MS m/z
trifluoroacetylamino)phenino)phenyl)- 502 (M+H)+
.
yl)pyrido[2,3- ethanone
d] ' midine;
I 4-amino-5-(3- 1-(4- 3-bromo- IR (KBr) 3438,
11
bromophenyl)-7-(4-(dimethylamino)-benzaldehyde1638. 1592,
1365
(dimethylamino)-3-3-fluorophenyl)- cm-i: MS mn
438
fluorophenyl)pyrido[2,3-ethanone (M+~+
'
.
d]
midine;
112 4-amino-5-(3- 1-(4-(N-ethyl-N-3-bromo- IR (l~r) 3477,
bromophenyl)-7-(4-(N-formylamino)phebenzaldehyde1672. 1604,
1580,
ethyl-N- nyl)-ethanone 1562. 1353
cm-1~
formylamino)phenyl)pyrid MS m;= 448
'
0[2,3-d]pyrirrudine; M+H)+
.
113 4,4-bis(acetylamino)-5-(3-1-(4-(N-methyl-3-bromo- lIt (KBr) 3434.
bromophenyl)-7-(.~-(N-N- benzaldehyde1667. 1635,
1600,
methyl-N- acetylamino)phe 1200 cm-1;
biS m/_
acetylamino)phenyl)pyridonyl)-ethanone 532 (Vi+H)+
'
.
[2,3-d]
rrudine;
-69-
CA 02286909 1999-10-12
WO 98/46605 PCTNS98/07207
114 4-amino-5-(3- 1-(4-{N-acetyl-3-bromo- IR (Ki3r)
3443,
bromophenyl)-7-(4-(N-N- benzaldehyde 1667, 1635,
1600,
acetyl-N- methylamino)phe 1200 cm-1;
Ms m/z
methylamino)phenyl)pyridnyl)-ethanone 532 (M+H)+.
0[2,3-d] 'midine;
115 4-amino-5-(3- 1-(4-(N- 3-bromo- Ilt (KBr)
3441,
bromophenyl)-7-(4-(N-ethylamino)phenbenzaldehyde 1633.1603.1572,
ethylamino)phenyl)pyrido[yl)-ethanone 1368 cm-i;
MS mi=
2,3-d]pyrimidine; 420 M+H +
I
116 4-amino-5-(3- 1-(-(N-methyl-3-bromo- IR (KBr) 3439,
bromophenyl)-7-(4-(N-N-(2- benzaldehyde 1636, 1601,
1529,
methyl-N-(2- methoxyethyl)am 1361 cm-1:
MS mi_
methoxyethyl)amino)phenino)phenyl)- 464 (M+H)+.
yl)pyrido[2,3- ethanone
d] ' midine;
117 4-amino-5-(3- 1-(-(N- 3-bromo- IR (KBr) 3430.
bromophenyl)-7-(4-(N-isopropylamino)benzaldehyde 1632, 1600,
1578.
isopropylamino)phenyl)pyphenyl)-ethanone 1530, 1357
cm-1;
rido[2,3-d]pyrirrudine; MS m~z 434
M+H +
118 4-amino-5-(3- 1-(4-N-ethyl-N-3-bromo- IR(microscope)
bromophenyl)-7-(4-N-(2- benzaldehyde 3488, 1657.
1604,
ethyl-N-(2- methoxyethyl)am 1579,1552,
1118
methoxyethyl)amino)phenino)phenyl)- cm-1; MS m~_
506
yl)pyrido [2,3- ethanone (M+H)+.
d] 'midine;
119 4-amino-5-(3- 1-(4-N-(3- 3-bromo- IR (KBr) 3201,
1
bromophenyl)-7-(4-N-(3-methoxypropionbenzaldehyde 1679, 1617,
methoxypropionyl)-N-yl)-N-isopropyl- 1597,1576,
1539,
isopropyl- amino)phenyl)- 1177. 1117
cm-1;
amino)phenyl)pyrido[2,3-ethanone MS miz 521
d] 'midine; M+H +
120 4-amino-5-(3- 1-(4-N-(2- 3-bromo- IIt (KBr)
3475,
bromophenyl)-7-(4-N-{2-(dimethylamino)benzaldehyde 1681, 1579,
1351.
(dimethylamino)ethyl)-N-ethyl)-N- cm-1: MS mi_
491
formylamino)phenyl)pyridformylamino)phe (M+H)+.
'
0[2,3-d] n 1)-ethanone
midine;
121 4-amino-5-(3- 1-(4-(N-(2- 3-bromo- IR (KBr) 3431,
bromophenyl)-7-(4-(N-(2-(dimethylamino)benzaldehyde 1634, 1601,
1573,
(dimethylamino)ethyl)amiethyl)amino)phe 1359 cm-1;
MS m/z
no)phenyl)pyrido[2,3-nyl)-ethanone 463 (M+H)+.
'
d]
mldine;
122 4-amino-5-(3- 1-(4-(N-methyl-3-bromo- IR (KBr) 3475.
bromophenyl)-7-(4-(N-N-(2- benzaldehyde 2220.1660.1604,
methyl-N (2 cyano)ethylamin 1580.1560,
1352
cyano)ethylamino)phenyl)o)phenyl}- cm-1; MS mi_
459 ~
pyrido[2,3-d]pyrimidine;ethanone M+H +
123 4-amino-5-(3- 1-(4-(N-methyl-3-bromo- IR (KBr) 3475.
bromophenyl)-7-(4-{N-N-(3- benzaldehyde 1663, 1604.
methyl-N-(3- methoxy)propion 1578,1559,
1352
methoxy)propionylamino)ylamino)phenyl}- 1114 cm-1:
MS m~=
phenyl)pyrido[2,3- ethanone 478 (M+H)+.
'
d]
rrudine;
-70-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
124 4-amino-5-(3- 1-(3-methyl-4-3-bromo- llt (KBr)
3486,
bromophenyl)-7-(3-(N-formyl-N- benzaldehyde 1677, 1607,
1579,
methyl-4-(N-formyl-N-methylamino)phe 1549,1351
cm-1;
methylamino)phenyl)pyridnyl)-ethanone MS m~z 448
0[2,3-d]pyrimidine: M+H +
12~ 4-amino-5-(3- 1-(3-methyl-4-3-bromo- IR (KBr) 3433.
bromophenyl)-7-(3-(N- benzaldehyde 1635, 1605,
1585,
methyl-4-(N- methylamino)phe 1359 cm-1;
MS miz
methylamino)phenyl)pyridnyi)-ethanone 420 (M+H)+.
'
0[2,3-d]
midine:
126 4-amino-5-(3- I-(4-(4- 3-bromo- IR (microscope)
bromophenyl)-7-(4-(4-methoxy-2- benzaldehyde 3473, 3063,
1710,
methoxy-2- butyl)phenyl)- 1671, 1582,1564,
butyl)phenyi)pyrido[2,3-ethanone 1352 cm-1;
MS mn
d]pyrimidine; 593 M+H +
127 4-amino-5-(3- 1-(4-(N-methyl-3-bromo- IR (microscope)
bromophenyl)-7-(4-(N-N-(2-(N- benzaldehyde 3443, 1638,
1606,
methyl-N-(2-(N- phthalimidyl)acet 1582,1359cm-1;
phthalimidyl)acetyl)amino)yl)amino)phenyl) MS miz 463
phenyl)pyrido[2,3--ethanone (M+H)+.
d] rrudine;
_
I28 4-amino-5-(3- 1-(3-methyl-4-3-bromo- IR (microscope)
bromophenyl)-7-(3-(N-methyl-N- benzaldehyde 3484, 1701,
1610,
methyl-4-(N-methyl-N-(trifluoroacetyl)a 1579,1559,
1221,
(trifluoroacetyl)amino)phemino)phenyl)- 1205,1151
cm-1;
nyl)pylido[2,3- ethanone MS m~z 516
d] 'midine; M+H +
129 4-amino-5-(3- 1-(3-methyl-4-3-bromo- lit (KBr)
3484,
bromophenyl)-7-(3-(N-acetyl-N- benzaldehyde 1663, 1607,
methyl-4-(N-acetyl-N-methylarnino)phe 1574,1547,
1354
methylamino)phenyl)pyridnyl)-ethanone cm-1; MS m~z
462
0[2,3-d]pyrimidine; M+H +
130 4-amino-5-(3- 1-(6- 3-bromo- IR (KBr) 3428,
bromophenyl)-7-(6-dimethylamino-benzaldehyde 1652. 1635,
1606,
dimethylamino-3- 3-pyridinyl)- 1585, 1365
cm-1;
pyridinyl)pyrido[2,3-ethanone MS m~z 421
d]PYTimidine; M+H +
131 4-amino-5-(3- I-(4- 3-cyano- IR (KBr) 3479,
cyanophenyl)-7-(4-methylsulfonylpbenzaldehyde 1638, 1576,1559,
methylsulfonylphenyl)pyrihenyl)-ethanone 1303,1147
cm-1-
'
do[2,3-d]pyrimidine; MS miz 402
M+H +
132 4-amino-5-(3- 1-(4-(N-methyl-3- Iit (KBr)
3418,
cyanophenyl)-7-(4-(N-N-formylamino)-cyanobenzaldehy2230. 1688,
1674,
methyl-N-formylamino)-phenyl)-ethanonede 1584, 1554,
1114
phenyl)pyrido[2,3- cm-1; MS m~=
381
d]PYi'imidine; M+H +
133 4-amino-5-(3- 1-(6-(N-methyl-3-bromo- IR (KBr) 3474,
-
bromophenyl)-7-(6-(N-N-formylamino)-benzaldehyde 1676. 1577,
1561.
methyl-N-formylamino)-3-pyridinyl)- 1353, 1130cm-1;
3-pyridinyl)pyrido[2,3-ethanone MS m~z 435
d]pyrimidine; M+H +
-71-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
134 4-amino-5-(3- 1-(6- 3-bromo- IR (KBr) 3487,
bromophenyl)-7-(6-morpholinyl-3-benzaldehyde3396, 1601,
1580,
morpholinyl-3- pyridinyl)- 1558, 1234cm-i;
p ethanone MS m/
p 463
din
y
l~yrido[2,3-
d] M +
~ +H
n
l
135 4-amino-5-(3- 1-(6-(N-methyl-3-bromo- IR (KBr) 3476,
bromophenyl)-7-(6-(N-N- benzaldehyde3307, 1702,
1683.
methyl-N- methoxyethylami 1605, 1560,
methoxyethylamino)-3-no)-3-pyridinyl)- 1116cm-1; MS
m~z
pyridinyl)pyrido[2,3-ethanone 465 (M+H)+.
'
d]
midine;
136 4-amino-5-(3- 1-(6- 3-bromo- IR (KBr) 3487,
bromophenyl)-7-(6-pyrrolidinyl-3-benzaldehyde3396, 1601,
1580,
pyrrolidinyl-3- pyridinyl)- 1558, 1234
cm-l
'
pyridinyl)pyrido[2,3-ethanone MS mi2 447
d]pyrimidine; M+H +
137 4-amino-5-(3- 1-(2- 3-bromo- Irt (microscope)
bromophenyl)-7-(2-(dimethylamino)-benzaldehyde34421640.1604,
(dimethylamino)-5-5-pyrimidinyl)- 1577, 1536,
1408,
p ethanone 3
' ~, 1;
yrimi
'dinyl)pyrido[2,3-
] MS m~
y 422
n
d midine;
M+ +
138 4-amino-5-(3- 1-(2-(N- 3-bromo- IR (microscope)
bromophenyl)-7-(2-(N-methoxyethyl-N-benzaldehyde3439, 1640,
1606,
methoxyethyl-N-methylmethyl amino)-5- 1587, 1556.
1537,
amino)-5- pyrimidinyl)- 1374, 1347
cm-i~
'
pyrimidinyl)pyrido[2,3-ethanone MS miz 466
d} 'midine; (M+H +
139 4-amino-5-(3- 1-(2-(N-formyl-3-bromo- IR (microscope)
bromophenyl)-7-(2-(N-N-methyl benzaldehyde3472, 1687,
1583,
formyl-N-methyl amino)-5- 1565, 1459,
amino)- 1353,
5-pyrimidinyl)pyrido[2,3-pyrimidinyl)- 1142, 988 cm-1;
d]pyrimidine; ethanone MS m/z 436
M+H +
140 4-amino-5-(3- 1-(2-(N- 3-bromo- IR (microscope)
bromophenyl)-7-(2-(N-methylamino)5-benzaldehyde3483, 1605,
1550,
methylamino)5- pyrimidinyl)- 1346 cm-1;
MS m/z
pyrimidinyl)pyrido[2,3-ethanone
408 (M+H)+.
'
d]
midine;
141 4-amino-5-(3- 1-(2- 3-bromo- IR (KBr) 3468,
bromophenyl)-7-(2-(1-pyrrolidinyl-5-benzaldehyde1600, 1581,
1552,
pyrrolidinyl)-5- pyrimidinyl)- 1527, 1482,
1330
pyrimidinyl)pyrido[2,3-ethanone cm-1; MS m/Z
448
d]pyrimidine; M+H +
142 4-amino-5-(3- 1-(2- 3-bromo- IR (microscope)
?
bromophenyl)-7-{2-(1-morpholinyl-5-benzaldehydecm-1~ MS m~z
463
molpholinyl)-5- pyrimidinyl)- (M+H)+
.
pyrlmidinyl)pyrido[2,3-ethanone
d] 'midine;
-72-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
143 4-amino-5-(3- 1-(6-(2-oxo-3-3-bromo- 1R (microscope)
bromophenyl)-7-(b-(2-oxazolidinyl)-3-benzaldehyde 3473, 1762,
1583,
oxo-3-oxazolidinyl)-3-pyridinyl~ 1571. 1562,
1491,
pyridinyl)pyrido[2,3-ethanone 1477, 1402,
1348,
d]pyrimidine; 1217 cm-1;
MS m/z
463 (M+H +
144 4-amino-~-(3- 1-(2-pyridyl)-3-bromo- IR (microscope)
bromophenyl)-7-(2- ethanone benzaldehyde 3427, 3017,
1601,
pyridyl)pvrido[2,3- 783 cm-1;
MS m/z
d]pyrittudine; 351/353 M+H
+
.
145 4-amino-~-(3- 1-(3-pyridyl)-3-bromo- IR (microscope)
bromophenyl)-7-(3- ethanone benzaldehyde 3434, 3042,
1634,
pyridyl)pyrido[2,3- 1372 cm-1;
MS m~z
d]pyrimidine; 351/353 M+H
+
.
146 4-amino-5-(3-(thiophen-2-1-(4- 3-(thiophen-2-IR (microscope)
yl)phenyl)-7-(4- dimethylaminophyl)-benzaldehyde3482. 2922,
1578,
dimethylaminophenyl)pyrienyl)-ethanone 1356 cm-1;
MS m/z
do[2,3-d]pyrimidine; 420/422 M+H
+
147 4-amino-5-(3-(furan-2-1-(4- 3-(furan-2-yl)-IR (microscope)
yl)phenyl)-7-(4- dimethylaminophbenzaldehyde 3479, 3104,
1559,
dimethylaminophenyl)pyrienyl)-ethanone 1356 cm-1;
MS m/z
do[2,3-d]pyrimidine; 420/422 M+H
+
148 4-amino-~-(3-(3- 1-(4- 3-(3- IR (microscope)
methoxyphenyl)phenyl)-7-dimethylaminophmethoxyphenyl)-3477. 2924,
1579,
(4- enyl)-ethanonebenzaldehyde 1356 cm-1;
MS m/z
dimethylaminophenyl)pyri 420/422 (M+H)+.
'
do[2,3-d
rrudine;
149 4-amino-5-phenyl-7-(4-1-(4- benzaldehyde IR (microscope)
dimethylammophenyl)pyridimethylaminoph 3477, 3298,
1580,
do[2,3-d]pyrimidine;enyl}-ethanone 1355 cm-1;
MS m/z
315 +H +
150 4-amino-~-(3- 1-(4- 3-chloro- IR (microscope)
chlorophenyl)-7-(4-(morpholinyl)phbenzaldehyde 3480. 3056,
1579,
(morpholinyl)phenyl)pyridenyl)-ethanone 1356 cm-1
~ MS m/z
~
0[2,3-d]pyrirrudine; 391 M+H
+
151 4-amino-~-(3-bromo-4-1-(4- 3-bromo-4- IR (microscope)
fluorophenyl)-7-(4-(morpholinyl)phfluoro- 3491, 3044,
1560,
(morpholinyl)phenyl)pyridenyl)-ethanonebenzaldehyde 1230 cm-1;
MS m/z
0(2,3-d]pyrlrrudine; 453 +H +
152 4-amino-5-(3- 1-{4- 3-chloro- liz (microscope)
chlorophenyl)-7-(4-iodophenyl)- benzaldehyde 3478. 3280,
1539,
iodophenyl)pyrido[2,3-ethanone 1350 cm-1;
MS m/z
d]pynmidine; 432 M+H +
153 4-amino-~-(3- 1-(4-(thiophen-3-chloro- IR (microscope)
chlorophenyl)-7-(4-2-yl)phenyl)-benzaldehyde 3484.3055.1560,
(thiophen-2-
ethanone 1354 cm-1;
MS m/z
yl)phenyl)pyrido[2,3- 459 (M+H)+:
'
d]
midine;
154 4-amino-~-(3- 1-(4-(5- 3-chloro- IR (microscope)
chlorophenyl)-7-(4-(5-pyrimidinyl)phenbenzaldehyde 3477. 3040,
1578.
pyrimidinyl)phenyl)pyridoyl)-ethanone 1351 cm-1~
MS m~z
.
[2,3-d)pyrimidine; 459 M+H
+
-73-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
155 4-amino-5-(3-bromo-4-1-(4- 3-bromo-4- IR (microscope)
fluorophenyl)-7-(4-iodophenyl)-fluoro- 3444. 3048,
1607,
iodophenyl)pyrido[2,3-ethanone benzaldehyde 1356 cm-1;
MS m/z
d]pynmidine; 494/496 +
. +
156 4-amino-5-(4- 1-(4- 4- IR (microscope)
bromothiophene-2-yl)-7-methoxyphenyl)-bromothiophene-3460. 3300,
2900-
(4- ethanone 2- 3100, 1700,
1580,
methoxyphenyl)pyrido[2, carboxaldehyde1510 cm-1;
MS m~z
3-d] 'midine; 413 (M+H)+.
Example 157
4-amino-S-(3-bromo~henvllmethyl-7-(4-(dimethvlamino,}phenyl)pyridol2 3-
dlpyrimidine
hydrochloride
A mixture of 3-cyano-4-(3-bromophenyl)methyl-6-(4-
(dimethyl)aminophenyl)pyridine-2-amine ( 1.58 g) and ammonium sulfate (40 mg)
in triethyl
orthoformate was heated at reflex for 2 hours. The reaction mixture was cooled
and added
to a mixture of 8 g of ammonia in 150 mL of ethanol. After 16 hours at 25
°C, the reaction
was heated at reflex for two hours, and the solvent was removed in vacuo. The
residue was
purified by chromatography, then converted to the hydrochloride salt by
treatment with
ether/HCI, followed by drying to give the title compound.
The 3-cyano-4-(3-bromophenyl)methyl-6-(4-(dimethyl)aminophenyl)pyridine-2-
amine was prepared by a four-step procedure as follows:
step 157a: Rret~aration of 3-bromophenylacetaldel~de (the "R3 reagent")
To a solution of ethyl 3-bromophenylacetate (10.2 g, US patent 2,624,731
(1950)}
in 230 mL of dichloromethane was added 42 mL of 1M Dibal-H in toluene at -78
°C with
stirring. After 40 minutes at -78 °C, 10 mL of methanol was added, and
the reaction
allowed to warm to room temperature and partitioned between 50 mL of
dichloromethane
and 1200 mL of saturated aqueous potassium sodium tartrate. The organic layer
was dried
over sodium sulfate and the aldehyde used immediately in the next step without
purification.
step 157b~ preparation of a-(triphenvlnhos~honium)-4-
(dimethylamino)phenylethan 1 one
chloride
Following the procedure of Fukui et al. (J. Org. Chem. 33: 3594-3507 (1968)),
a-
bromo-(4-dimethylaminophenyl)ethan-1-one (the "R4 reagent", CAS #37904-72-6;
Cherri.
Abst. ( 1956), 864) was treated with triphenylphosphine in triethylamine and
acetonitrile.
The a-bromo-(4-dimethylaminophenyl)ethan-1-one was prepared by bromination
with
bromine in hydrobromic acid according to the method of Suzuki et al (J. Pharm.
Soc. Japan,
-74-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
(1955), X5:54. Removal of solvent and recrystallization from methanol/ethyl
acetate/toluene gave the title product as a white powder.
stev 157c: nreoaration of I-(4-(dimethvlamino)nhenvl)-4-l3-bromonhenvl)-but-2-
en-1-one
20 g of a-(triphenylphosphonium)-4.(dimethylamino)phenylethan-1-one chloride
(from step b) was partitioned between dichloromethane and 50 mL of 2N NaOH.
The
organic phase was dried over sodium sulfate and concentrated in vacuo. The
residue was
mixed with 3-bromophenylacetaldehyde (from step a) for 24 hours at 25
°C. The mixture
was purified by chromatography to give 8.35 g (61 %) of a cis/trans mixture of
the title
compound. The cis/trans mixture was taken to the next step without separation
of the
isomers.
stev 157d: preparation of 3-cyano-4-(3-bromo~henvl)methvl-6-(4-
(dimethvl)aminophenyl~pvridine-2-amine
A mixture of I-(4-(dimethylamino)phenyl)-4-(3-bromophenyl)-but-2-en-I-one
chloride (3.85 g, from step c), ammonium acetate (2.6 g) and malononitrile
(739 mg) in 3
mL of dimethoxyethane and 22 mL of ethanol was heated at 115 °C for 5
hours, then cooled
and worked up by partitioning between dichloromethane and water. The residue
obtained
on concentration of the organic phase was purified by flash chromatography to
give the title
compound.
Examples 158-~4
Following the procedures of Example 157, except substituting the appropriate
reagents for the R4 and R3 reagents of Example 157 as indicated in Table 3
below,
compounds of Examples 158-174 were prepared. The treatment with aqueous HCl
was
omitted, and the free bases were obtained except as indicated.
In Examples 167-174, the fvrmamide or formamidine acetate (added periodically
until the reaction was complete) treatment was replaced by treatment with
triethyl
orthoformate at reflux in the presence of a catalytic amount of ammonium
sulfate, followed
by cooling to 25 °C and addition of excess ammonia in ethanol. After 24
hours, the
precipitated amidine compound was filtered and washed with hexanes, then dried
under
vacuum. The amidine compound was then heated in 1,2-dichlorobenzene at 120-180
°C for
I-8 hours. The reaction mixture was cooled to room temperatureand purified by
chromatography, and the product was recrystallized if necessary (chloroform in
methanol).
-75-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
Table 3
Examples 158-187
Ex. Name R4 Reagent R3 Reagent Analytical Data
No.
(for 7- (for 5-
osition) osition
158 4-amino-5-(2- 1-(4- 3-phenyl- IR (KBr) 3340,3240-
'
phenylethyl)-7-(4-diethylaminophepropionaldehyde2800.1600,1580,1540;
diethylaminophenyl)pynyl)-ethanone H. Res. MS mn
rido[2,3-d] 'midine 398.2343 (M+H)+.
159 4-amino-5-(2- 1-(4- 3-methyl- IR (KBr)
methylpropyl)-7-(4-diethylaminophebutanaldehyde3550,3410,3320,
3240-
diethylaminophenyl)pynyl)-ethanone 2800,1605,1580,1560
rido[2,3-d]pyrimidine H. Res. MS m/z~
350.2357 M+ +
160 4-amino-5- but 1- 4 pentanaldehyde
1 -7-
( y )
(4- diethylaminophe ~5~300,3200-
diethylaminophenyl)pynyl)-ethanone 2800,1660,1610.1580.1
rido[2,3-d]pyrimidine 540 H. Res. MS
m~z
350.2354 M+ +
161 4-amino-5-(2-(4-1-(4- 4-(4- IR (lcBr)
bromophenyl)ethyl)-7-diethylaminophebromophenyl)-3500,3300,3200-
(4- nyl)-ethanonepropionaldehyde30,1650,1615.1580
diethylaminophenyl)py H. Res. MS m~z
rido[2,3-d] 'midine 478.1429 (M+H)+.
162 4-amino-5-(butyl)-'7-* ~ (fir)
(4- 3400,3350,3200-
dimethylaminophenyl) 2900,1650,1620,1580,1
pyrido[2,3- 570 H. Res. MS
m/z
d] dine 322.2032 (M+H)+.
163 4-amino-5-(2-(3-1-(4- 3-cyanophenyl-IR (KBr) 2850-
cyanophenyl)methyl)-dimethylaminophacetaldehyde3550,2220,1610,1580,1
~-(4- enyl)-ethanone 560,1540 MS m/z
381
dimethylaminophenyl) (M+H)+.
pyrido[2,3-
d] 'dine
164 4-amino-5-(2-(N-1-(4- 3-(N- IR (KBr) 3000-
carbobenzyloxy)aminodimethylaminophcarbobenzyloxy)3500,1710,1690,1650,1
ethyl)-7-(4- enyl)-ethanone- 590 H. Res. MS
m/z
dimethylaminophenyl) aminopropionald443.2184 (M+H)+.
pyrido[2,3- ehyde
d] 'dine
165 4-amino-5- 1-(4- cycloheptane-IR (KBr)
(cycloheptyl)-7-(4-dimethylaminophcarboxaldehyde3500.3250,3100,2950,2
dimethylaminophenyl)enyl)-ethanone 850,1620,1575
H. Res.
pyrido(2,3- MS m/z 362.2349
d] 'dine (M+H)+.
166 4-amino-5-(2-(5-** IR (KBr) 3200-
chloro-2-(thiophen-3- 3450,2950-
yl)phenylmethyl)-7-(4- 3100,1605.1580,1550
dimethylaminophenyl) H. Res. MS miz
pyrido[2,3-
472.1363 (M+H)+.
d] 'dine
-76-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
167 4-amino-5-(pentyl)-7-1-(4- hexanal IR (KBr)
(4- diethylaminophe 3430,3320,3240-
diethylaminophenyl)-nyl)-ethanone 2800,1580,1560,1540,1
pyrido[2,3- 350; mp. 211-214;
MS
d]pyrimidine mlz 364 (M+H)+;
H.
Res. MS ml~
364.2506
M+H'+
~
168 4-amino-5-hexyl-7-(4-1-(4- _ ~r)
heptanal
diethylaminophenyl)-diethylaminophe 3440,3310,3240-
pyrido [2,3- nyl)-ethanone 2800,1580,1560,1540,1
d]pyrimidine 350; mp. 215-217;
MS
mlz 378 (M+H)+;
H.
Res. MS m/z
378.2654
M+H +
169 4-amino-5-(2-(3-1-(4- 3-(3- 1R (KBr) 3640-
bromophenyl}ethyl)-7-diethylaminophebromophenyl}-3240,3200-
(4- nyl)-ethanonepropionaldehyde2800,1580,1555.1535,1
diethylaminophenyl)- 345; mp. 201-202;
MS
pyrido[2,3- m/z 476/478
(M+H)+;
d]pyrimidine H. Res. MS m/z
476.1448 M+
+
170 4-amino-5-((2- 1-(4- 2-(2- IR (KBr) 3640-
bromophenyl)methyl}-diethylaminophebromophenyl)-3240,3240-
7-(4- nyl)-ethanoneacetaldehyde 2800.1580,1555,1540,1
diethylaminophenyl)- 350; mp. 130-133;
MS
pyrido[2,3- m/z 462/464
(M+H)+;
d]pyrimidine H. Res. MS mlz
462.1297 M+
+
171 4-amino-5- 1-(4- cyclopropanecarIR (KBr)
cyclopropyl-7-(4-dimethylaminophboxaldehyde 3490,3290,3240-
dvnethylaminophenyl)enyl)-ethanone 2760,1610,1580,1540,1
-pyrido[2,3- 375; mp. 235-237;
MS
d] ~dlrie m/z 462/464
(M+H)+;
172 4-amino-5-cyclohexyl-1-(4- cyclohexanecarbIIt (xBr) 3640-
7-(4- dimethylaminophoxaldehyde 3000.2980-
dimethylaminophenyl)enyl)-ethanone 2760,1610,1580,1540,1
-pyrido[2,3- 345; mp. 231-234;
MS
d] dine m/z 462/464
(M+H)+;
173 4-amino-5-((2-bromo-1-(4- 2-(2-bromo-5-IR (KBr) 3460,3220-
5- dirnethylaminophchlorophenyl)-2760,1610,1575,1535,1
chlorophenyl)methyl)-enyl)-ethanoneacetaldehyde 365; mp. 185-187;
MS
7-(4- m/z 462/464
(M+H)+;
diethylaminophenyl)-
pyrido[2,3-
d] 'dine
174 4-amino-5-methyl-7-1-(4- acetaldehyde 1R (KBr) 3640-
(4- dimethylaminoph 3250.3250-
diethylaminophenyl)-enyl)-ethanone 2760.1610,1585,1560,1
pyrido[2,3- 350; mp. 238-246:
MS
d] ~dlrie m/z 462/464
(M+H)+;
* prepared from the compound of Example 157 by reaction with Pd(PPh3)4 and
zinc
cyanide in DMF under Suzuki reaction conditions.
-77_
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
** prepared from the compound of Example 173 by reaction with 2-
thiopheneboronic acid,
Pd(PPh3)4 and aqueous sodium carbonate under Suzuld reaction conditions.
Examples 175-188
Following the procedures of Example 1, except substituting the appropriate
reagents
for the R4 and R3 reagents of Example 1 as indicated in Table 4 below,
compounds of
Examples 175-188 were prepared. The treatment with aqueous HCl was omitted,
and the
free bases were obtained except as indicated.
Table 4
Examples 175-188
Ex. Name R4 Reagent R3 Reagent Analytical Data
No.
(for 7- (for 5-
osition) osition
175 4-amino-5-(2,3- 1-(4- 2,3- IR (KBr) 3500-
methylenedioxyphenyldimethylaminophmethylenedioxy-2500,1595,1580,1375;
-7-(4- enyl)-ethanonebenzaldehyde mp.290-305;
dimethylaminophenyl)
-pyrido[2,3-
d] 'dine
_
176 4-amino-5-(3-fluoro-1-(4- 3-fluoro-5- IR (KBr) 3500,3440-
5- dimethylaminophtrifluoromethyl-3240,3200-
trifluoromethylphenyl)enyl)-ethanonebenzaldehyde 2800,1610,1580,1560,1
_7_(4- 540,1370; mp.
293-296;
dimethylaminophenyl) MS m/z 428 (M+H)+;
-pyrido[2,3- H. Res. MS mlz
d]pyrimidine 428.1509 (M+H)+.
177 4-amino-5-(2- 1-(4- 2-bromo- IR (KBr) 3480,3440-
bromophenyl)-7-(4-dimethylaminophbenzaldehyde 3240,3200-
dimethylaminophenyl)enyl}-ethanone 2800,1610,1575,1555,1
-pyrido[2,3- 535,1355
d]pyrimidine mp. 261-263;
MS m/z
420/422 (M+H)+;
H.
Res. MS m/z
420.0823
(M+H)+.
178 4-amino-5-(3,5- I-(4- 3,5-dimethyl-IR (KBr) 3480,3440-
dimethylphenyl)-7-(4-dimethylaminophbenzaldehyde 3240.3200-
dimethylaminophenyl)enyl)-ethanone 2800,1610,1575.1555,1
-pyrido[2,3- 535,1360: mp.
284-286;
d]pyrimidine MS mlz 370 (M+H)+;
H. Res. MS m/z
370.2036 (M+H)+.
_78_
CA 02286909 1999-10-12
WO 98/46605 PCT/IJS98/07207
179 4-amino-5-(3,4- 1-(4- 3,4-dichloro-IR (KBr) 3490,3440-
dichlorophenyl)-7-(4-dimethylaminophbenzaldehyde 3240,3200-
dimethylaminophenyl)enyl)-ethanone 2800.1610,1575,1560,1
-pyrido[2,3- 535,1355- mp.
288-291;
d]pyrimidine MS mlz 410/412
(M+H)+: H. Res.
MS
m/= 410.0948
!M+H +
180 4-amino-5-(4-fluoro-1-(4- 4-fluoro-3- Bt (KBr) 3500,3440-
.
3- dimethylaminophtrifluoromethyl-3240,3200-
trifluoromethylphenyl)enyl)-ethanonebenzaldehyde 2800.1610.1580,1560,1
_7_(4_ 540,1505,1360;
mp.
dimethylaminophenyl) 254-257; MS
m/z 428
-pyrido[2,3- (M+H)+: H. Res.
MS
d]pyrimidine m/. 428.1487
{M+H)+.
181 4-amino-5-(3-bromo-1-(4- 3-bromo-5- 1R (KBr) 3470,3440-
5-methoxyphenyl)-7-morpholinylphenmethoxy- 3240,3200-
(4- yl)-ethanonebenzaldehyde 28001605,15801560;
morpholinylphenyl)- mp. 257-260;
MS m/z
pyrido[2,3-
492/494 (M+H)+.
d] 'dine
182 4-amino-5-(3-bromo-1-(4- 3-bromo-5- 1R (lc>3r) 3470,3440-
5-methoxyphenyl)-7-pyrrolidinylphenmethoxy- 3240,3200-
(4- yl)-ethanonebenzaldehyde 28001610,15801560,1
~
pyrrolidinylphenyl)- 540.1355; mp.
d
250;
pyrido[2,3- MS m/z 476/478
d]pyrimidine (M+H)+.
183 4-amino-5-(3-bromo-1-(4- 3-bromo-5- Ift (KBrj 3470,3440-
5-methoxyphenyl)-7-piperidinylphenymethoxy- 3240,3200-2800,1565;
(4-piperidinylphenyl)-1)-ethanone benzaldehyde mp. 224-244;
MS m/z
pyrido[2,3- 490/492 (M+H)+;
d] 'dine
184 4-amino-5-(3-bromo-1-(4- 3-bromo-5- IR (KBr) 3470,3420-
5-methoxyphenyl)-7-dimethylaminophmethoxy- 3240,3200-
(4- enyl)-ethanonebenzaldehyde 2800.1610,157515551
dimethylaminophenyl) 535.1355: mp.
262-266;
-pyrido[2,3- MS m/z 450/452
d]pyrimidine (M+H)+: H. Res.
MS
mlz 450.0944
M+H +
185 4-amino-5-(3- 1-(4- 3-methylthio-IR (KBr) 3460.3420-
methylthiophenyl)-7-dimethylaminophbenzaldehyde 3240.3200-
(4- enyl)-ethanone 2800,1605.1575,1560.1
dimethylaminophenyl) 535.1355: mp.
184-220;
-pyrldo[2,3- MS m/. 388 (M+H)+;
d]pyrimidine H. Res. MS m/z
388.1586 (M+H)+.
-79-
CA 02286909 1999-10-12
WO 98!46605 pr~rirtcoum~~m
186 4-amino-5-(3-bromo-1-(thiophene-2-3-bromo-5- IIt (KBr) 3470,3350-
5-methoxyphenyl)-7-yl)-ethanone methoxy- 2200,1700,1640,1580,1
(thiophene-2-yI)- benzaldehyde 5,1
2
7o~mp.
~~1
36
pyrid o ~
[2,3- ~~
~
9
d]pyrimidine 413/415 (M+H)+;
H.
Res. MS m/z
413.0069
(M+H)+.
187 4-amino-5-(2,3- 1-(4- 2,3-dimethoxy-IR (KBr) 3480,3440-
dimethoxyphenyl)-7-dimethylaminophbenzaldehyde 3240,3200-
(4- enyl)-ethanone 2800,1610,1580,1550,1
dimethylaminophenyl) 530,1360; mp.
222-225;
-pyrido[2,3- MS m/z 402 (M+H)+;
d]pyrimidine H. Res. MS m/z
***
402.1922 (M+H)+.
188 4-amino-5-(3- 1-(4- 3- IR (KBr)3490,3400-
methylsulfonylphenyl)dimethylaminophmethylsulfonyl-2800.1610,1580,1555,1
-7-(4- enyl)-ethanonebenzaldehyde 535,1355; mp.
dimethylaminophenyl) 245270; MS m/z
420
-pyrido[2,3- (M+H)+; H. Res.
MS
d 'dine m/z 420.1493
M+ +
Exam lp a 189
4-acetylamino-5-f3-bromophenyl)-7-f4-dimeth ly aminonhenyl)gyridol2 3-
dluyrimidine
A suspension of 4-amino-5-(3-bromophenyl)-7-(4-
dimethylaminophenyl)pyrido[2,3-d)pyrimidine (from Example 15, 0.28 g, 0.67
mole) in
pyridine (3 mL) was treated with acetic anhydride {0.10 g, 1.0 mmol) and the
reaction
mixture was stirred for 4 hours at 25 °C. The volatiles were removed
under reduced
pressure, and the residue was purified by flash chromatography (Si02,
EtOAc/hexanes) to
provide the title compound (0.23 g, 73% theoretical): IR (ICBr) 3368, 3048,
1695, 1567;
MS mlz 462/464 (M+H)+.
Examples 190-198
Following the procedures of Example 189, except substituting the appropriate
acylating reagent for the acetic anhydride of Example 189 as indicated in
Table 5 below,
compounds of Examples 190-198 were prepared.
Table 5
Examples 190-198
fix. ivame Acylating Analytical Data
N o . I ( Reagent
-80-
CA 02286909 1999-10-12
WO 98/46605 PCTJUS98/07207
190 4-formylamino-5-(3- acetic anhydrideIR (KBr) 3382,
and 3047,
bromophenyl)-7-(4- formic acid 1704.1570;
dimethylaminophenyl~ MS m/z 448/450
'do[2,3-d 'dine--190 (M+I~+.
191 4-(methoxyacetyl)amino-5-(3-methoxyacetyl IR (KBr) 3344,
3044,
bromophenyl)-7-(4- chloride 1731, 1561:
MS min
diethylaminophenyl)-pyrido[2,3- 492/494 (M+1~+.
p d] 'dine
192 4-trifluoroacetylamino-5-(3-trifluoroaceticIR (KBr) 3426,
3072,
bromophenyl)-7-(4- anhydride 1610, 1578:
MS m~z
dimethylaminophenyl)- 516/518 (M+1~+.
ido[2,3-d] 'dine
193 4-pentanoylamino-5-(3- pentanoyl chlorideIlt (KBr) 3408,
2954,
bromophenyl)-7-(4- 1699, 1569;
MS miz
dimethylaminophenyl)- 504/506 (M+1-0+.
ido[2,3-d] 'dine
194 4-benzoylamino-5-(3- benzoic anhydrideIR (KBr) 3420,
3056,
bromophenyl)-7-(4- 1606, 1583;
MS miz
dimethylaminophenyl)- 524/526 (M+I~+.
'do[2,3-d] imidine
195 4-(N-BOC-glycyl)amino-5-(3-N-BOC-glycyl- iZt (KBr) 3362,
2975,
bromophenyl)-7-(4- imidazole 1719, 1570:
MS m/z
dimethylaminophenyl)- 577/579 (M+1-0+.
'do[2,3-d] 'dine
196 4-(N-phthalimidylglycyl)amino-5-N-phthalimidyl-Iit (KBr) 3408,
2927,
(3-bromophenyl)-7-(4- glycyl-chloride1719, 1570;
MS m~z
dimethylaminophenyl)- 607/609 (M+I~+.
'do[2,3-d] 'dine
197 4-(ethoxycarbonyl)amino-5-(3-diethyl dicarbonatelIt (KBr) 3405,
2987,
bromophenyl)-7-(4- 1738, 1569:
MS m/z
dimethylaminophenyl)- 492/494 (M+~+.
'do[2,3-d 'dine
198 4-(ethylaminocarbonyl)amino-5-ethyl isocyanateIR (KBr) 3405,
3053,
(3-bromophenyl)-7-(4- 1701, 1548:
MS m/z
dimethylaminophenyl)- 491/493 (M+I~+.
ido[2,3-d] imidine
Exam lp a 199
4-allvlamino-~-(3-bromophenyl)-7-l4-dimethylaminophenylZpyrido f2 3-dl
pyrimidine
The product was prepared by treating a solution of 4-chloro-5-(p-
dimethylaminophenyl)-7-(p-bromophenyl)pyrido[2,3-d]pyrimidine in CH2Cl2-TEA
with
allylamine and heating the resulting mixture at reflux for 1 hour. The
volatiles were
removed under reduced pressure, and the residue was purified by flash
chromatography
(Si02, EtOAc/hexanes) to provide the title compound IR (KBr) 3437, 1564, 1355,
1195;
l0 MS m/z 460/462 (M+H)+.
The 4-chloro-5-(p-dimethylaminophenyl)-7-(p-bromophenyl)pyrido [2.3-
d]pyrimidine was prepared as follows.
-81-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
A sample of 4-(4-bromophenyl)-3-cyano-6-(4-(dimethylamino)phenyl)pyridine-2-
amine (from Example 1, S.0 g, 12.7 mmol) in 20 mL of H2S04 was heated at 80
°C for 30
minutes. Ice was added, and the reaction mixture was neutralized with aqueous
NaOH.
The resulting crude 3-carboxamide was collected by filtration, triturated with
EtOAc-
S hexanes, then dried under reduced pressure (4.95 g, 95% theoretical). A
solution of the
carboxamide (4.25 g, 10.3 mmol) in triethylorthoformate (20 mL) was treated
with p-
toluenesulfonic acid (catalytic) and the reaction mixture was warmed at 80
°C for 4 hours.
The volatiles were removed and the crude bicyclic 4-hydroxyl-5-(p-
dimethylaminophenyl)-
7-(p-bromophenyl)pyrido[2,3-d]pyrimidineproduct was suspended in POCl3 (15 mL)
then
warmed at 100 °C for 2 hours. The POC13 was removed under reduced
pressure to provide
crude 4-chloro-5-(p-dimethylaminophenyl)-7-(p-bromophenyl)pyrido[2,3-
d]pyrimidine.
The invention therefore relates to intermediate compounds of formula III
wherein X is
selected from hydroxyl or halogen and the remaining variables are the same as
in formula I
or II.
Example 200
4-(2-(N.N-dimethvlamino)ethvlamino)-5-(4-bromophenvl)-7-(4-
dimethvlaminophenvl)
pyrido 12.3-dl pvrimidine trihydrochloride
The product was prepared by treating a solution of 4-chloro-5-(p-
dimethylaminophenyl)-7-(p-bromophenyl)pyrido[2,3-d]pyrimidine (prepared as in
Example
199) in CH2C12-TEA with the 2-(dimethylamino)ethylamine and heating the
resulting
mixture at reflux for 1 hour. The volatiles were removed under reduced
pressure, and the
residue was purified by flash chromatography (Si02, EtOAc/hexanes) to provide
the title
compound. The product was treated with excess 2M HCl (aq) followed by
lyophilization to
give the product as the trihydrochloride salt; IR (KBr) 3385, 1561, 1356,
1197; MS m/z
491/493 (M+H)+.
Example 201
4-(4-(N.N-dimethvlaminolbut~rlamino)-5-(3-bromophenvl)-7 ~4
dimethvlaminonhenvl) pyrido f 2 3-dl pvrimidine tetrahvdrochloride
The product was prepared by treating a solution of 4-amino-5-(p-
dimethylaminophenyl)-7-(p-bromophenyl)pyrido[2,3-d]pyrimidine in CH2C12-TEA
with the 4-(dimethylamino)butylamine and heating the resulting mixture at
reflux for 1
hour. The volatiles were removed under reduced pressure, and the residue was
purified by flash chromatography (Si02, EtOAc/hexanes). The product was
treated
with excess 2M HCl (aq) followed by lyophilization to give the product as the
-82-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
tetrahydrochloride salt; IR (KBr) 3439, 1567, 1356, 1196; MS m/z 519/521
(M+H)+.
Example 202
4-(N-allvl-N-formvlamino)-5-l4-dimethvlaminophenvl)-7-
~p-bromophenvl)pyrido12,3-d]pyrimidine
A sample of the compound from Example 190 above, 4-formylamino-5-(2-
phenylethyl)-7-(4-diethylaminophenyl)-pyrido[2,3-d]pyrimidine (0.27 g, 0.6
mmol)
in 3 mL of a 4:1 mixture of THF and DMF at 0°C was treated with NaH
(60%
dispersion, 36 mg, 0.9 mmol) and the solution was stirred for 0.5 hour. Allyl
bromide (0.29 g, 2.4 mmol) was added, and the reaction mixture was stirred for
an
additional 0.5 hour. Aqueous workup followed by flash chromatography provided
the
title compound: LRMS m/z 488/490. IR (cm') 3428, 2910, 1696, 1551, 1362,
IS
1193.
Example 203
4-diacetylamino-5-(4-dimethvlamino~henyl)-7-(p-bromophenyl)
pvridof 2.3-d]pvrimidine
This compound was isolated as a minor product from the reaction mixture of
Example 190 above: LRMS m/z 504/506. IR (crri') 2922, 1726, 1550, 1360, 1197.
Example 204
4-amino-5-(3-bromophenyl)-7-(5-amino-2-~,vridyl)nvridof 2.3-dlwrimidine
A solution of 5-aminopyridine-2-ethanone ( 1.15 g, 8.45 mmol), 3-
bromobenzaldehyde ( 1.70 g, 9.2 mmol), malononitrile (0.61 g, 9.2 mmol), and
ammonium acetate ( 1.15 g, 15 mmol) in 25 mL of benzene was heated at reflux
with
azeotropic removal of water. After 6 hours the reaction mixture was
concentrated, and
the desired intermediate (1.82 g, 49%) was isolated following flash
chromatography
(Si02, EtOAc-CH2Cl2). LRMS m/z 366/368. The intermediate was suspended in 15
mL of formamide, and the reaction mixture was heated at 180 °C for 4
hours. .The
solution was cooled to 25 °C, 10 mL of 4M HCl (aq) was added, and the
mixture was
stirred for 1 hour. The aqueous solution was neutralized with NaOH (aq), and
the
precipitate was collected by filtration. The title compound (1.3 g, 68%) was
isolated
following flash chromatography of the precipitate: LRMS m/z 393/395; IR (cm-1
)
3481, 3161, 1620, 1573, 1483, 1359.
The 5-aminopyridine-2-carboxaldehyde starting material was prepared as
follows:
-83-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
204a. 5-amino-2-bromogyridine
A solution of 2-bromo-5-nitropyridine (5.1 g, 25 mmol) in 50 mL of a 10:1
mixture
of acetic acid and water was treated with iron powder (7.8 g, 140 mmol) in
several portions
over 20 minutes. After an additional 30 minutes the volatiles were removed
under reduced
S pressure, and the residue was quenched with 5% aqueous sodium carbonate. The
aqueous
solution was extracted with methylene chloride, and the combined organic layer
was dried
(sodium sulfate) then concentrated in vacuo to provide the desired product as
a white solid
(4.25 g, 98%).
204b. 5-aminopvridine-2-ethanone
A sample of 5-amino-2-bromopyridine (4.25 g, 24 mmol), PdCl2(PPh3)2 (0.34 g, 2
mole%), CuI (0.09 g, 2 mole%), and trimethylsilylacetylene (3.0 g, 31 mmol)
were
dissolved in 100 mL of a 4:1 mixture of triethylamine and acetonitrile, and
the reaction
mixture was stirred 24 hours at 25 °C. The reaction mixture was
concentrated, and the
IS residue was dissolved in 100 mL of a 10:1 mixture of acetone and water.
Hg(02CCF3)2
( 11.1 g, 26 mmol) and H2S04 (72 mmol) were added to the reaction mixture, and
the
solution was heated at reflux for 2 hours. The reaction mixture wac cnniP~i m
7S °r anrt
neutralized with saturated aqueous sodium carbonate. The aqueous layer was
extracted with
methylene chloride, then the combined organic layer was dried (Na2S04) and
concentrated
in vacuo. Flash chromatography (Si02, EtOAc-Hexanes) provided the title
compound:
LRMS m/z 137 (M = H+); IR (cm-1) 3428, 1668, 1646, 1582, 1358, 1274.
Example 205
Following the procedure of Example 204, 5-dimethylaminopyridine-2-
ethanone was reacted with bromobenzaldehyde, malononitrile, and ammonium
acetate
to give the title compound. The residue was triturated with excess HCl/ether,
the
volatiles were removed under reduced pressure, and the title compound was
dried
under high vacuum: LRMS m/z 421/423. IR (cm-1) 3245, 1664, 1545, 1395.
The 5-dimethylaminopyridine-2-carboxaldehyde starting material was prepared
as follows:
205a..3-N,N-dimethvlaminopyridine
A solution of 3-aminopyridine (9.4 g, 0.10 mol) in a l: l mixture of formic
acid (96%) and formaldehyde (37% aqueous solution) was heated at reflux for 18
hours. The volatiles were removed under reduced pressure and the residue was
-84-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
neutralized with saturated aqueous NaHC03. The aqueous layer was extracted
with
CH2C12, then the combined organic layer was dried (Na2S04) and concentrated
under
reduced pressure. Flash chromatography (Si02, EtOAc-Hexanes) provided the
title
compound: ( 11.1 g, 91 %), LRMS m/z 123 (M + H+).
- 205b. 2-bromo-5-N . N-dimethxlamin~ '~mdine
A solution of 3-N,N-dimethylaminopyridine (5.88 g, 48.1 mmol) in 150 mL
of CH2C12 at 0 °C was treated with 2,4,4,6-tetrabromo-2,5-
cyclohexadienone (20.7
g, 50 mmol) in several portions over 30 minutes. After 2 hours at 0 °C
the reaction
mixture was concentrated, and the desired 2-bromo-5-N,N-dimethylaminopyridine
was isolated following flash chromatography (16.5 g, 82%): LRMS m/z 201/203.
204c. 5-N . N-dimethyjar~n' pyridine-2-ethanone
Following the procedure of Example 203b, 2-bromo-5-N,N-
dimethyiaminopyridine, except converting the compound to the trihydrochloride
salt
by treatment with HCl/ether, was converted to the title compound: LRMS m/z
165; IR
(cm-1) 3480, 1666, 1581, 1368, 1272.
Exam In a 206
4-amino-5-(3-bromonhenyl)-7-(5-dimethylamino-2-R azinyi~
pxridof2.3-dl~"vrimidine hydrochloride
Following the procedure of Example 204, 5-dimethylaminopyrazine-2
ethanone was reacted with bromobenzaldehyde, malononitrile, and ammonium
acetate
to give the title compound. The residue was triturated with excess HCl/ether,
the
volatiles were removed under reduced pressure, and the title compound was
dried
under high vacuum: LRMS m/z 422/424. IR (cm ') 3310, 1630, 1525, 1444, 1375.
The 5-dimethylaminopyrazine-2-carboxaldehyde starting material was prepared
as follows:
206a. 5-dimethylaminoRyrazine-2-eth~~one
A solution of 5-hydroxypyrazine-2-carboxylic acid (4.0 g, 28.5 mmol) in 50
mL of thionyl chloride and 0.1 mL of DMF was heated at reflux for 8 hours. The
volatiles were removed under reduced pressure, and the residue was dissolved
in 20
mL of toluene. This solution was added to a solution of dimethyl malonate
(4.75 g,
36 mmol), MgCI, (2.09 g, 22 mmol) and tziethyl amine .(7.08 g, 70 mmol) in 100
mL
of toluene. The reaction mixture was stirred for 1 hour at 25 °C,
quenched by addition
of water, and the product was extracted with methylene chloride. The solvent
was
-85-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
removed, the crude intermediate was dissolved in 25 mL of a 25:1 mixture of
DMSO
and water, and the resulting solution was warmed at 150 °C for 2 hours.
The reaction
was quenched by addition of water, and the product was extracted with
methylene
chloride to provide 2-acetyl-5-chloropyrazine (LRMS mlz 156). This
intermediate was
treated with aqueous dimethylamine at room temperature for 30 minutes to
afford 5-
dimethylaminopyrazine-2-ethanone-(LRMS m/z 166): LRMS m/z 422/424; IR (cm-')
3310, 1630, 1525, 1444, 1375.
Example 207
4-amino-5-f3-bromonhenvl)-7-(2-oxobenzoxazolin-6-yI)pyridof2 3-dlpyrimidine
Following the procedure of Example 204, 2-oxobenzoxazolin-6-ethanone_was
reacted with bromobenzaldehyde, malononitrile, and ammonium acetate to prepare
the
title compound: LRMS m/z 434/436; IR (crri') 3095, 1760, 1579, 1481, 1350.
The 2-oxobenzoxazolin-5-ethanone_starting material was prepared as follows:
207a. 2-oxobenzoxazolin-6-ethanone
DMF (9 mL) was added dropwise to A1C13 (58.7 g, 440 mmol) over 20
minutes and the resulting suspension was stirred 15 minutes at 25 °C.
Acetic
anhydride (7.14 g, 70 mmol) and 2-benzoxazolinone (6.0 g, 44. mmol) were added
and the reaction mixture was warmed at 80 °C and stirred for 4 hours.
The mixture
was cooled to 25 °C and poured into ice/HzO. The resulting precipitate
was collected
by filtration and dried under vacuum to provide the title compound (6.4 g, 81
%,
LRMS m/z 177).
Example 208
4-amino-5-f3-bromophenyl)-7-f 1-methyl-2-oxobenzoxazolin-6-,
pvridoT2.3-dlpyrimidine
Following the procedure of Example 204, 1-methyl-2-oxobenzoxazolin-5-
ethanone was reacted with bromobenzaldehyde, malononitrile, and ammonium
acetate
to prepare the title compound: LRMS m/z 448/450; IR (crri') 3440, 1782, 1605,
1458,
1350.
The 1-methyl-2-oxobenzoxazolin-5-ethanone-starting material was prepared as
follows:
208x. 1-methyl-2-oxobenzoxazolin-5-ethanone
-86-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
A solution of 2-oxobenzoxazolin-5-ethanone_(from Example 206x, 2.50 g,
14.1 mmol) in 20 mL of a 4:1 mixture of THF and DMF at 0 °C was treated
with NaH
(60 % dispersion, 0.8 g, 20 mmol) and the mixture was stirred 20 minutes at 0
°C.
Methyl iodide (3.97 g, 28 mmol) was added and the reaction mixture was warmed
to
25 °C and stirred for 15 minutes. Saturated aqueous NaHC03 was added
and the
aqueous layer was extracted with CH,CI=. The desired product (2.55 g, 94%,
LRMS
m/z 191), was isolated following flash chromatography (Si02, EtOAc-CHZCIz).
Example 209
4-amino-5-((5-chloro-2-(3-methoxyphenvl'phenvl)methvl)-7-(4-
dimethylaminophenyllyvridof 2.3-dlpvrimidine
The title compound was prepared from the compound of Example 173 by
reaction with 3-methoxyphenylboronic acid, Pd(PPh3)4 and aqueous sodium
carbonate under Suzuki reaction conditions. IR (KBr) 3550-3250,3240-
2760,1580,1560,1540,1350; H. Res. MS m/z 496.1902 (M+H)+.
Example 210
4-amino-5-((2-bromophenyi)methyl)-7-(4-dimethylaminophenvl)pvridof2 3-
dlpvri_midine
Following the procedures of Example 157, except substituting 1-(4-
dimethylaminophenyl)-ethanone for the R4 reagent and 2-(2-bromophenyl)-
acetaldehyde for the R3 reagent of Example 157, the title compound was
prepared as
shown in Table 6.
Tahle. fi
Ex. Name R4 Reagent R3 Reagent Analytical
No. (for (for Data
7- osition) 5- osition
210 _ 1-(4- 2-(2- IR (KBr);
4-amino-5-((2- MS
bromophenyl)methyl)-dimethylaminophebromophenyl)- m/z 434,436
7-(4- nyl)-ethanoneacetaldehyde (M+H)+,
dimethylaminophenyl)
pyrido[2,3-
d] 'dine
Example 211
4-amino-5-(2-((thiophene-2-yl)phenyl)meth.,yl)-7-(4-
diethvlaminophenyl)pyridof2 3
dlpvrimidine
The title compound was prepared from the compound of Example 173 by
reaction with 2-thiopheneboronic acid, Pd(PPh3)4 and aqueous sodium carbonate
under Suzuki reaction conditions. IR (KBr) 3640-3240, 3240-2800, 1580, 1560,
1540, 1350; H. Res. MS m/z 466.2070 (M+H)+.
_87_
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
Example 212
4-amino-5-(2-((thio~hene-3-vl)phenyl)methyl)-7-(4-diethylaminophenvl)pyridof2
3
dlpyrimidine
The title compound was prepared from the compound of Example 173 by
reaction with 3-thiopheneboronic acid, Pd(PPh3)4 and aqueous sodium carbonate
under Suzuki reaction conditions. IR (KBr) 3640-3240, 3240-2800, 1580, 1560,
1540, 1350; H. Res. MS m/z 466.2057 (M+H)+.
Examples 213-222
Following the procedures of Example 1, except substituting the appropriate
reagents
for R4 and R3 as indicated in Table 7 below, compounds of Examples 212-222
were
prepared.
1 s Table 7
Examples 213-222
Ex. Name R4 Reagent R3 Reagent Analytical
(for (for
No. 7- osition) 5- osition Data
213 4-amino-5-(3- 1-(4-(N-formyl-N-3-bromo- IR (KBr) 3490,
bromophenyl)-7-(4-(2- benzaldehyde 1689, 1120,
800
(N-formyl-N-(2- methoxy)ethylamin cm-1; MS m/z
methoxyethyl)amino)po)phenyl)- 478/480 (M+H)+.
henyl)pyrido[2,3-ethanone
d] 'midine;
214 4-amino-S-(3- * ~ fir)
bromophenyl)-7-(4- 3330,2925,
1675,
(N-(2- 800 cm-1;
MS m/z
methoxyethyl)amino)p +
451/453 (M+H)
henyl)pyrido[2,3- .
d] 'midine;
215 4-amino-5-(3- 1-(4-(N-methyl-N-3-bromo- IR (KBr) 3440,
bromophenyl)-7-{4-((2- benzaldehyde 1600, 1160,
810
(N-methyl-N-((2- dimethylamino)eth cm-1; MS m/z
dimethylamino)ethyl)ayl}amino)phenyl)- 477/479 (M+H)+
.
mino)phenyl)pyrido[2,ethanone
3-d] 'midine;
216 4-amino-5-(3- ** IR (KBr) 3480.
bromophenyl)-7-(4-{2- 1520. 710
cm-1~
methoxy)acetylamino) MS m/. 464,466
ethyl)amino)phenyl)py (M+H)+.
rido[2,3-d] imidine;
_88_
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
217 4-amino-5-(3- *** IR (KBr) 3475,
bromophenyl)-7-((4- 1690, 1355,
800
formylamino)phenyl)p cm-1; MS m/z
yrido[2,3- 420/422 (M+H)+.
d] mldine;
218 4-amino-5-(3- **** IR (KBr) 3452.
~
bromophenyl)-7-(4-(2- 1605, 1250,
590
(dimethylamino)acetyl cm-1; MS m!z
amino)phenyl)pyrido[ 477/479 (M+H)+.
2,3-d] dine;
219 4-amino-5-(3- 1-(4-(2-oxo-3-3-bromo- IR (KBr) 3480,
bromophenyl)-7-(4-(2-oxazolidinyl)phenybenzaldehyde 1750, 1400,
700
oxo-3- 1)-ethanone cm-1; MS m!z
oxazolidinyl)phenyl)p 462/464 (M+H)+.
yrido[2,3-
d] 'midine;
220 4-amino-5-(3- 1-(6-(2-propyl)-3-3-bromo- IR (KBr) 3474,
bromophenyl)-7-(6-(2-pyridinyl)- benzaldehyde 3098, 1636,
1566,
propyl)-3- ethanone 1499, 1352,
1282
pyridinyl)pvrido[2,3- cm-1:MS m!z
393
d]pyrimidine (M+H)+.
trip drochloride
221 4-amino-5-(3- 1-{3-methyl-4-3-bromo- IR (KBr) 3440,
bromophenyl)-7-(3-pyrrolidinylphenylbenzaldehyde 1640, 1607,
1586,
methyl-4- )-ethanone 1370 cm-1;
MS m/z
pyrrolidinylphenyl)pyr 433 (M+H)+.
ldo[2,3-d]pyrimidine
dih drochloride
222 4-amino-5-(3- 1-{6-imidazolyl-3-3-bromo- ilt (KBr)
3028,
bromophenyl)-7-(6-pyridinyl)- benzaldehyde 1641, 1607.
1595,
imidazolyl-3- ethanone 1375cm-1;
MS m/z
p 417 (M+H)+.
Pid
~la~yrido[2,3-
d
y
trih drochloride
*prepared by deformylation of Example 213 with dilute HCl in methanol.
**prepared by acylation of Example 213 with 2-methoxyacetyl chloride/pyridine.
***prepared by formylation of the 7-(3-bromophenyl)-2-cyano-5-(4-
aminophenyl)pyridine-2-amine intermediate.
****prepared by acylation of Example 213 with the 2-(dimethylamino)acetyl
chloride.
Examples 223-225
Following the procedures of Example 157, except substituting the appropriate
reagents for the R4 and R3 reagents of Example 157 as indicated in Table 8
below,
compounds of Examples 223-225 were prepared.
-89-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
Table 8
Examples 223-225
Ex. Name R4 Reagent R3 Reagent Analytical Data
No.
(for 7- (for 5-
osition) osition
223 4-amino-5- 1-(4- 2-phenyl- IR (KBr)
phenylmethyl-7-(.~-diethylaminopheacetaldehyde3450.3380,2850-
diethylaminophenyl)pynyl)-ethanone 3200,1605,1580,1560,1
rido[2,3-dJpyrimidine 540; H. Res.
MS mi_
384.2176 M+H)+.
224 4-amino-5-(2-{3-* gt mgr) X00_
aminopropynyl)phenyl 3450.2050,2120,1650,1
methyl)-7-{4- 605.1540; MS
miz 437
diethylaminophenyl)py (M+H)+.
rido[2,3-dJ 'midine
225 4-amino-5-(1-{2-1-(4- 2-(2- 1R (KBr) 3520,3250-
bromophenyl)ethyl)-7-dimethylaminophbromophenyl)-3500,2850-
(4- enyl)-ethanone)propionaldehyd3150,1605,1580,1560,1
dimethylaminophenyl) a 540; H. Res.
MS miz
3 448.1137 (M+F-0+.
p
yrido[2
-
'me
d
a
*prepared from the compound of Example 170 by reaction with propargylamine,
CuI and
Pd(PPh3)4 under Suzulci reaction conditions.
Examples 226-228
Following the procedures of Example 1, except substituting the appropriate
reagents
for R4 and R3 as indicated in Table 9 below, compounds of Examples 226-228
were
prepared.
Ta le 9
Examples 226-228
Ex. Name R4 Reagent R3 Reagent Analytical
No. (for (for Data
7-position) 5- osition
226 4-amino-5-(4- 1-(4- 3-bromo- 1R (1c13r)
3456,
dimethylaminophenyl)bromophenyl)- benzaldehyde 3053, 16600.
1556
-7-(4- ethanone cm-1; MS mi=
420
bromophenyl)pyrido[2 (M+H)+.
'
,3-d]
midine
227 4-amino-5-(2-furanyl)-1-(4-(N- furan-2- IR (KBr) 3460.
7-(4-(N- molpholinyl)phenycarboxaldehyde1600, 1580.
1457
morpholinyl)phenyl)-1)ethanone cm-1~ MS mi=
374
pyrldo[2,3- (M+H)+.
'
d]
dine
-90-
CA 02286909 1999-10-12
WO 98/46605 PCTlUS98/07207
228 4-amino-5-(3- 1-(5-(2- 3-bromo- IR (KBr) 3442
bromophenyl)-7-(2-(dimethylamino)pybenzaldehyde 1640. 1604,
1577,
dimethylamino-5-rimidinyl)~thanon 1536, 1408,
1367,
pyrimidinyl)pyrido[2,a 1348 cm-1;
MS m/z
3-d]pyrimidine 422 M+ -~
Exam lp a 229
4-amino-~-(3-bromophenyl)-7-(4-(ureido),phenyl)~yridof2 3-dlnvrimidine
A solution of 4-amino-5-(3-bromophenyl)-7-(4-aminophenyl)pyrido[2,3-
d]pyrimidine (Example 71, 310 mg, 0.79 mmol) in 2 mL of acetic acid was
treated with
sodium cyanate (~6 mg, 0.87 mmol), and the reaction mixture was stirred for 30
minutes at
25 °C. The solution was concentrated and the residue was suspended in
aqueous NaHC03.
The crude product was collected by filtration, then purified by flash
chromatography. The
product was dissolved in methanol and treated with excess 2M aqueous HCl to
provide the
hydrochloride salt: LRMS m/z 435/437. IR (cm') 3442, 2212, 3186, 3059, 1681,
1582,
1525, 1358.
Examvle 230
4-amino-5-( 1-phenylmethvl-3-3-~iperidin~l)-7-(4-dieth~laminophen~?pyridof 2 3
dl~yrimidine
Following the procedures of Example 157, except substituting 1-(4-diethylamino-
phenyl)-ethanone for the R4 reagent and 1-phenylmethylpiperidine-3-
carboxaldehyde
(prepared as described by Gilligan et al., J. Med. Chem., ,x:4344-4361 (1992))
for the R3
reagent thereof, the title compound was prepared. The treatment with aqueous
HCl was
omitted, and the free base was obtained. IR (KBr) 3440, 3100-2800-1640, 1605,
1595,
1535 crri l; MS m/z 467 (M+H)+; mp 218-220 °C.
Examples 231-243
Following the procedures of Example 1, except substituting the appropriate
reagents
for R4 and R3 as indicated in Table 10 below, compounds of Examples 230-243
were
prepared. In some cases, the treatment with aqueous HCl was omitted, and the
free bases
were obtained.
-91-
CA 02286909 1999-10-12
WO 98!46605 PCT/US98/07207
T I 10
E~camnles 231-243
Ex. Name R4 Reagent R3 Reagent Analytical
N (for 7- Data
o (for 5-
.
osition) osition
231 4-amino-5-(3- I-(6-(3-methyl-3-bromo- IR (KBr) 3484,
163s
,
bromophenyl)-7-(6-(3-5-isoxazolyl))-3-benzaldehyde ls~a, 1s62.
1352
methyl-5-isoxazolyl))-3-pyridin 1 ~m-1; Ms m/Z
) 4s9
pyridinyl)pyrido[2,3-ethano a (M+H)+.
d] 'midine;
232 4-amino-5-(3- 1-(6-chloro-3-3-bromo- ~ (tcl3r)
34~s, 1608,
bromophenyl)-7-(6- pyridinyl)- benzaldehyde 1s~4, 1s42
om-1;
chloro-3- ethanone Ms m/z 414
pyridinyl)pyrido[2,3- (M+H)+.
d] 'midine;
233 4-amino-5-(3- 1-(6-methoxy-3-3-bromo- 1R (tcBr)
3484, 163s,
bromophenyl)-7-(6- pyridinyl)- benzaldehyde ls6o. 1348
cm-1;
methoxy-3- ethanone MS m/z 409
pyridinyl)pyrido[2,3- (M+H)+.
d] 'midine;
234 4-amino-5-(3- 1-(6-(1,2,4- 3-bromo- IR (KBr) 3494,
1612,
brornophenyl)-7-(6-triazol-4-yl)-3-benzaldehyde 159, 1467,
1359,
(1,2,4-triazol-4-yl)-3-pyridinyl)- 121, 1233
cm-1;
pyridinyl)pyrido[2,3-ethanone Ms .nrz aas
d] 'midine; (M+H)+.
235 4-amino-5-(3- I-(2- 3-bromo- IR (KBr) 3434,
1637,
bromophenyl)-7-(2- morpholinyl-5-benzaldehyde l6os, lsgs,
133s.
morpholinyl-5- pyrlmidinyl)- cm-1; MS m/z
463
pyrimidinyl)pyrido[2,3-ethanone (M+H)+.
d] 'midine;
236 4-amino-S-(2-thiazolyl)-7-1-(4- 2-thiazole- IR (KBr) 3400,
1637.
(4-pyrroiidinylphenyl)-pyrrolidinylphencarboxaldehydel6og, 1s32.
~m-1
pyrido[2,3-d]pyrimidine;yl)-ethanone Ms mlz 3~6
M+H +.
237 4-amino-5-(3- I-(6-pyrazolyl-3-3-bromo- at (tcsr)
344, ts8o,
bromophenyl)-7-(6- pyridinyl)- benzaldehyde ts6~, 1492,
139s,
pyrazolyl-3-pyridinyl))-ethanone ~m-1: Ms m/z
444
'do[2,3-d] 'midine; (M+H)+.
238 4-amino-5-(3- 1-(4-(1-methyl-3-bromo- 1R (r) 3400,
166s,
bromophenyl)-7-(4-(1-ureido)phenyl)-benzaldehyde l3so cm-1;
Ms miZ
methyl-ureido)phenyl)-ethanone 450 (M+H)+
'
do[2,3-d) 'midine;
239 4-amino-5-(3- 1-(4-(N-methyl-3-bromo- IR (ICBr)
347s, 1s78,
bromophenyl)-7-(4-(N-N-(2- benzaldehyde lss3. 1482,
~ 1396.
~ methyl-N-(2- pyrirru~yl)~ cm-1; Ms m/z
484
pyrimidinyl)amino)phenylo)phenyl)- (M+H)+.
i )- rido[2,3-d] imidine;ethanone
-92-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
240 4-amino-5-(3- 1-(3-fluoro-4-3-bromo- IR (KBr) 3448,
1600,
bromophenyl)-7-(3- (N- benzaldehydets2s, 1476,
cm-1;
fluoro-4-(N-formyl-N-methylamino)phe Ms rnz 4s4
methylamino)phenyl)-nyl)-ethanone (Ht+H)+.
pyrido[2,3-d]pyrimidine;
241 4-formylamino-5-(3-1-(3-fluoro-4-3-bromo- ttt (KBr)
3465, 1607,
bromophenyl)-7-(3- (N- benzaldehydets46, l3so,
om-1;
fluoro-4-(N-formyl-N-methylamino)phe vts miz 481
methylamino)phenyl)-nyl)-ethanone (M+H)+.
pyrido[2,3-d]pyrimidine;
242 4-amino-5-(3- 1-(4-(N-methyl-3-bromo- IR (lCBr)
3470, 16s0,
bromophenyl)-7-(4-(N-N- benzaldehydets7o, 1338,
cm-1;
methyl-N- methylsulfonyl- ncs miz 4a4
methylsulfonylamino)-amino)phenyl)- yvt+H)+.
phenyl)pyrido[2,3- ethanone
d] 'midine;
243 4-amino-5-{3- 1-(6-(N-methyl-3-bromo- IR (KBr) 3460,
1680,
bromophenyl)-7-{6-(N-N- benzaldehydets8o, 1330
cm-1;
methyl-N- methylsulfonyl- nts miz 4gs
methylsulfonylamino)-3-amino)-3- (M+H)+.
pyridinyl)pyrido[2,3-pyridinyl)-
d] ' midine; ethanone
~~ separated dy ctiromatograpny nom the same reaction mixture; tormylatlon
occurs dunr~g
the cyclization step
Exam lp a 244
4-amino-5-(3-bromophenyl)-7-(1-methyl-5-indolinyl)pyrido12.3-dl~~rimidine
dihydrochloride
A sample of 4-(3-bromophenyl)-3-cyano-6-(1-methyl-5-indolinyl)pyridine-2-
amine was heated at reflux in formamide. The reaction was monitored by TLC,
and
when the reaction was complete the mixture was cooled to room temperature. The
product was allowed to precipitate, then recovered by filtration and washed
with
water. Additional product was extracted from the filtrate. The product was
purified
by column chromatography eluting with 10% MeOH/CHZC12 and converted to the
hydrochloride salt by treatment with ether/HCI. The salt was isolated and
dried under
vacuum to give the title compound. LRMS m/z 432/434; IR (crri') 3500, 3400,
3300,
3200-2800, 1610, 1580, 1560, 1540.
The 4-(3-bromophenyl)-3-cyano-6-(1-methyl-5-indoiyl)pyridine-2-amine
starting material was prepared as follows:
244a. 5-bromo-1-methylindoline
-93-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
Acetic acid (60 mL) was added to a mixture of 5-bromo-1-methylindole (10 g,
47.6 mmol) and sodium cyanoborohydride (8 g). After one hour at 15 °C,
the reaction
was basified with aqueous NaOH and extracted with toluene. The organic phase
was
dried over MgS04 and concentrated to a powder under vacuum. This material was
purified by flash chromatography to give the title compound, 8.62 g (82 %): MS
212,
214 [M+H]+.
244b. 5-acetyl-1-methvlindoline
A mixture of 5-bromo-1-methylindoline (8.6 g, 40.7 mmol),
to trimethylsilylacetylene ( 12 mL), palladium bis-triphenylphosphine
dichloride (600
mg), CuI (620 mg) and triethylamine (16 mL) in acetonitrile (20 mL) was heated
at 75
°C for 3 days, then cooled and concentrated in vacuo. The residue was
dissolved in
120 mL of 1:1 ethyl acetate/hexane, and the solids were removed by filtration.
The
solvent was removed and a sample of the residue (5 g) was dissolved in 90%
aqueous
15 acetone (44 mL). To this solution was added sulfuric acid (2.2 g), and
Hg(OCOCF3)2 (9 g). The reaction was heated at reflux for 20 minutes, cooled,
made
basic with aqueous sodium hydroxide and extracted with ethyl acetate. The
organic
layer was dried over MgS04 and concentrated to an oil, which was purified by
flash
chromatography to give 850 mg of the title compound: MS 176 [M+H]+.
244c. 4-(3-bromophen lY )-,3:,c~rano-6-ll-methyl-5-indolinvl)Ryridine-2-amine
Prepared by condensation of 1',1'-dicyano-3-bromostyrene (prepared by
condensation of 3-bromobenzaldehyde with malononitrile in ethanol in the
presence of
a catalytic amount of glycine) and the 5-acetyl-1-methylindoline (the R4
reagent) with
. ammonium acetate in ethanol. The reaction mixture was heated to reflux in a
vessel
fitted with a Dean-Stark apparatus. After 3.5 hours, the mixture was cooled,
and the
solvent was removed. The residue was purified by flash chromatography, eluting
with methylene chloride, to give the title compound (588 mg, 30% yield; MS m/z
394
(M+H)+.
Example 245
4-amino-5-(3-bromophenvl)-7-( 1-methyl-5-benzimidazol~l~pvridof2 3-dlp,
rimidine
tetrahydrochloride
The title compound was prepared according to the procedure of Example 1,
except
substituting 1-methyl-5-acetyl-benzimidazole (prepared according to the
procedure of D. J.
Evans et. al., J. Chem. Soc. Perkin Trans. Il, 1978, 865) for the 4-
-94-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
dimethylaminobenzaldehyde (the R3 reagent) therein. IR (KBr) 3650-3230, 3230-
2000,
1635, 1605, 1590, 1555, 1365 cm' 1; MS m/z 431/433, 431.0605 {M+H)+.
Example 246
4 4-amino-5-(3-bromophenvll-7-l6-dimethvlamino-3-pyridazinyl)pyrido12,3-
dlvvrimidine
tetrahvdrochloride
246a. 6-( I -butoxy~ethenyl)-3-chlorop~rridazine
l0 To a solution of 20 g (200 mmol) of butyl vinyl ether in 80 mL of THF at -
78 °C
was added 130 mL of a 1.7 M solution of t-butyl lithium in pentane over about
20 minutes.
The yellow suspension was stirred while allowing to warm to 0 °C. THF
(150 mL) was
added, and the mixture cooled to -78 °C and a solution of 23 mL (200
mmol) of trimethyl
borate in 50 mL of THF was added. The reaction was warmed to 20 °C, 20
mL of methanol
was added, and the solution concentrated in vacuo. The residue was diluted
with 400 mL of
dioxane, and 20.9 g ( 140 mmol) of 3,6-dichloropyridazine, 2.31 g of
Pd(PPh3)4, and 200
mL of 2 M- aqueous sodium carbonate was added. The reaction was heated to
reflux over
one hour, then cooled and filtered to remove solids. The filtrate was
concentrated in vacuo
and partitioned between ethyl acetate and 1 M sodium hydroxide. The organic
phase was
dried over Na2S04, concentrated in vacuo, and purified by flash chromatography
to give
6.3 g (21 %) of the title compound. MS { M + ]+ 213, 215.
246b. 1-(6-chlorQpvridazin-3-yllethanone
A mixture 6.3 g of the compound from Step 246a in 40 mL of dimethoxyethane, 10
mL of water, and 4 mL of 12 M HCl was stirred for 20 minutes, then 125 mL of
water was
added, and the reaction was neutralized with 12 g of NaHC03. The reaction was
extracted
with ethyl acetate, dried over Na2S04, and concentrated in vacuo to give a
yellow solid, 4.7
g.
246c. 1-(3-(6-ldimeth~lamino,~vridazin-3-xl))ethanone (the R4 rea ent
A solution of 1.57 g (10 mmol) of 1-(6-chloropyridazin-3-yl)ethanone (from
Step
246b) in 15 mL of dimethoxyethane was treated with 50 mmol of 40% aqueous
dimethylamine. After one hour, the reaction was partitioned between CH2Cl2 and
water.
The organic phase was dried over CH2C12 , and concentrated in vacuo to give
the title
compound.
-95-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
246d. 3-acetyl-6-~ylami no~pvridazine
The title compound was prepared by condensing l,l-dicyano-(3-(3-
bromophenyl)propene (the R3 reagent) with the compound from Step 246c (the R~
reagent)
and ammonium acetate in ethanol according to the procedure of Example 1574.
246e. 4-amino-5-f3-bromophenyl)-7-f6-dimethvlamino-3=pvridazinvl,~pvridof2 3
dlnvrimidine tetrahydrochloride
The title compound was prepared from the compound of Step 246d according to
the
procedure of Example I57, except substituting formamide for the ammonium
sulfate and
triethyl orthoformate thereof.
Examples 247-248
Following the procedures of Example 246, except in step (c) substituting the
appropriate reagents for methylamine as indicated in the Table 1 IA below,
compounds of
Examples 247-248 were prepared.
Table 11 A
Exam les 247-248
Ex. Name reagent of Analytical
N ste c Data
o
,
247 4-amino-5- morpholine IR (KBr)
3600-3200,
(3bromophenyl)-7-(6- 3000, 1630,
1605,
mOrphOhriyl-3- 1590, 1550
cm-1;
pyridazinyl)pyrido[2,3- MS miz 464/466,
d~pyrilriidlne 464.0829
(M+H)+;
dih drochloride
248 4-amino-5-(3- pyrrolidine IR (KBr)
3600-3250.
bromophenyl)-7-{6- 3100-2800,
1640,
pyrrolidinyl-3- 1605. 1560
cm-I;
pyridazinyl)pyrido(2,3- Ms miz 448/450.
d]Pyt irnidine (M+H)+;
dih drochloride
Examples 249-251
Following the procedures of Example 244, except in step (c) first substituting
the
appropriate reagent for R4 as indicated in Table 11B below for the R4 reagent
of Example
244 step c, and secondly performing the condensation with ammonium acetate
substituting
dichloroethane as the solvent in place of the ethanol solvent in Example 244
step c; the
compounds of Examples 249-251 were prepared. In some cases, the hydrochloride
salts
were not prepared.
-96-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
Table 11 B
Examples 249-260
~ ~ Name R4 Reagent a~Yt~cal
D
, (for 7- a
N
o
osition)
249 4-amino-5-(3- 2-acetyl-5- IR (KBr)
348, 3os8.
bromophenyl)-7-(S- morpholinyl- Is62. 1542.
I3~8.
morpholinyl-2- pyrazine 1306 ~m-I:
Ms Min
pyrazinyl)pyrido[2,3- 464/466,
(M+H)+;
d]pyrimidine
dih drochloride
250 4-amino-S-(3- 2-acetyl-5-(N-(2-IR (KBr)
3482, 3299,
bromophenyl)-7-(S-(N-(2-methoxyethyl)-3053, 1612,
1540,
methoxyethyl)-N- N-methylamino)-1310 ~m-1;
Ms m
methylamino)-2- pyrazine 466/46s,
(M+H)+;
pyrazinyl)pyrido[2,3-
d]pyrimidine
dih drochloride
2S 4-amino-S-(3- 1-((4- nt (KBr)
1 3040, 1680,
'
bromophenyl)-7-(4- acetylphenyl)-1640, 1605,
1580,
(morpholinylmethyl)-methyl)- 1400 cm-1;
MS m~Z
phenyl)pyrido[2,3- morpholine 466/468,
(M+H)+;
d]pyrimidine
h drochloride
Example 252
4-amino-S-(3-bromophenyl)-7-lS-IN.N-bisf2-methox~vl)amino)-2-
pvridinyl)Ryridof2 3
dlpyrimidine trih3rdrochloride
step 2S2a. 1-(S-bromo-2-p~ric~yl)ethanone. ethylene ketal
A solution of dibromopyridine {5.2 g, 21.95 mmol), tributyl(1-ethoxyvinyl)tin
(9.11
g, 25.24 mmol), Pd2(dba)3 (0.7 g, 0.8 mmol), and (2-furyl)3P (0.37 g, 1.6
mmol) in SO
mL of toluene/THF (S:1 ) was warmed at reflux for 10 hours. The reaction
mixture was
concentrated, and the crude product was purified by elution through a short
column of silica
gel. The resulting enol-ether compound, ethylene glycol (2.79 g, 4S mmol), and
p-toluene
sulfonic acid (0.1 g) were dissolved in SO mL of toluene and the solution was
warmed at
reflux for 10 hours. The reaction mixture was quenched by the addition of
saturated
aqueous NaHC03, and the aqueous layer was extracted with CH2C12. The combined
organic layer was dried (Na2S04), concentrated under reduced pressure, and the
resulting
crude product was purified by flash chromatography to provide the title
compound (3.68 g,
79%).
-97-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
Stew 252b. 1-(5-lbis(2-methoxvethyl)amino)-2-g r~'idyl)ethanone
Following literature procedure (J. Org. Chem. 1996, 61, 720), a suspension of
the
compound from step 252a, bis(2-methoxyethyl)amine, t BuONa, Pd2(dba)3, and
BINAP
toluene was warmed at 80 °C for 8 hours. The reaction mixture was
quenched by the
addition of saturated aqueous NaHC03 and the aqueous layer was extracted with
CH2Cl2.
The combined organic layer was concentrated and the resulting residue was
dissolved in 20
mL THF/3 M HCl (4:1 ) and stirred for 4 h. The reaction mixture was
neutralized by the
addition of 2 M NaOH (aq) and the aqueous layer was extracted with CH2C1~. The
combined organic layer was dried, concentrated under reduced pressure, and the
crude
product was purified by flash chromatography to provide the title compound
Sten 252c. 4-amino-5-(3-bromophenyl)-7-(5-(N N-bis(2-methoxyethvl)aminol-2-
p~ridinvl)pvridof2.3-dl~,vrimidine trihvdrochloride
Following the procedures of Example 244, except in step (c) first substituting
the
t5 reagent from Step 252b for the R4 reagent of Example 244 step c, and
secondly performing
the condensation with ammonium acetate substituting dichloroethane as the
solvent in place
of the ethanol solvent in Example 244 step c, the free base of the title
compound was
prepared. The title compound was prepared from this by treatment with HCL in
ether. IR
(KBr) 3440, 1635, 1605, 1580, 1360 cm-1; MS m/z 466/468, (M+H)+.
Examples 253-260
Following the procedures of Example 244, except in step (c) first substituting
the
appropriate reagent for R4 as indicated in Table 11B below for the R4 reagent
of Example
244 step c, and secondly performing the condensation with ammonium acetate
substituting
dichloroethane as the solvent in place of the ethanol solvent in Example 244
step c, the
compounds of Examples 253-260 were prepared. In some cases, the hydrochloride
salts
were not prepared.
E Name R4 Reagent Analytical
x.
No.
(for 7- Data
osition)
253 4-amino-5-(3- 1-((4- IR (IC.Br)
3105,
bromophenyl)-7-(4- acetylphenyl)-1645, 1620,
(imidazolylmethyl)-methyl)imidazole1570, 1350
phenyl)pyrido[2,3- crri l; MS
m =
dJpyrirrudine 466/468,
trihydrochloride (M+H)+;
-98-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
Z$4 4-amino-$-(3- 1-($- IR (KBr) 3297,
3081,
bromophenyl)-7-(S-(1-morpholinyl-2-1646, 1564,
1494,
morpholinyl)-2- pyridyl~thanone1362 om-1;
Ms miz
pyndinyl)pyrido[2,3-* 463/465, (M+H)*;
d]pyrimidine
trih drochloride
2$$ 4-amino-$-(3- 1-(4- IR (KBr) 3308.
1645,
bromophenyl)-7-(4- ((dimethylamino)1590, 1560,
1375
((dimethylamino)methyl)-methyl)phenyl)-~m-1: Ms miz
phenyl)pyrido[2,3- ethanone 509/511, (M+H)+;
d]pyrimidine
dih drochloride
2$6 4-amino-$-(3- 1-{$-(4- IR (KBr) 3000,
1650.
bromophenyl)-7-($-(4-hydroxypiperidin1600. 1580,
lsso.
hydroxy-1-piperidinyl)-2-yl)-2- 1400 om-1;
Ms miz
pyridinyl)pyrido[2,3-pyridyl)ethanone477/479, (M+H)+;
d]pyrimidine **
dih drochloride
2$7 4-amino-$-(3- - $-acetyl-2- IR (KBr) 3477,
3060,
bromophenyl)-7-($-(N-pyridinemethana1678, 1638,
1566,
formyl-N-methylamino)-mine 1495, 1319
cm-1;
2-pyridinyl)pyrido[2,3- MS miz 435/437,
d]pyrimidine (M+H)'';
dih drochloride
2$8 4-amino-S-(3- 2-acetyl-$-(2-IR (KBr) 3085,
1562,
bromophenyl)-7-($-(2-propenyl)- 1485, 1357
cm-1;
propenyl)-2- pyridine Ms miz 41s/42o.
pyridinyl)pyrido[2,3- (M+H)+;
d] 'dine
2$9 4-amino-$-(3- 6-acetyl-3-(2-IR (KBr) 3440,
1770,
bromophenyl)-7-(3-(2-methoxyethyl)-1625.1605.1580,
methoxyethyl)-2-oxo-6-benzoxazol-2-1360 cm-1:
Ms m/z
benzoxazolyl)pyrido[2,3-one 492/494, (M+H)+;
d]pyrimidine
h drochloride
260 4-amino-$-(3- 4- IR (KBr) 3283,
3054,
bromophenyl)-7-(4-(1-(N-acetylbenzeneeth1678, 1631,
1647.
formylamino)- anar~ne 1352 cm-1:
MS m/z
ethyl)phenyl)pyrido[2,3- aas/4so, (M+H)+;
d] 'dine
rucpa.rea as m ~x. ~o~o, except sunsatunng morpnollne for the bls(Z-
methoxyethyl)amine thereof.
** Prepared as in Ex. 2$2b, except substituting 4-hydroxypiperidine for the
bis(2-methoxyethyl)amine thereof.
Exam l
4-amino-$-(3-pyg~yl)-7-(4-dimethvlamino)vhen~RvridQj2 3-dlp«rimidine
The compound was prepared by using the method generally described above in
Scheme 3 and the associated examples using 1-(4-dimethylaminophenyl)ethanone
as the R4
l0 reagent (7-position) and nicotinaldehyde as the R3 reagent ($-position). IR
(cm-1) 330$.8,
2922, 1606, 1$78, 1$3$, 1360. MS (M+H) 342.
-99-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
Example 262
4-(methvlamino)-5-(3-bromonhenvl)-7-l4-dimethylaminophenvl)~,vridof2 3 d~,pyru
hydrochloride
The title compound was prepared by using the method described in Example 200,
except substituting methyIamine for the 2-(dimethylamino)ethylamine thereof.
MS (M+H),
478 (1Br); IR (cm-1) 345, 3047, 2959, 1580, 1351, 1234.
Example 263
l0 4-(2-methoxvethvlamino)-5-(3-bromophenvl)-7-(4-
dimethvlaminonhenyl~yridof2 3-d~pvrimidine hydrochloride
The title compound was prepared by using the method described in Example 200,
except substituting 2-methoxyethylamine for the 2-(dimethylamino)ethylamine
thereof. MS
(M+H), 522 ( 1 Br); IR (cm-1 ) 34I 5, 2920, 1569, 1321, 1234.
Example 264
4-amino-5-(3-bromonhenvl)-7-(4-(I-methyl-2-imidazolyl?phenvl)pyridoT2 3
dlpvri_midine
trip vdrochloride
Step 264a. 1-(4-(1-Methylimidazol-2-yl)phenyl)ethanone
A solution of N-methyl imidazole (0.90 g, 11.0 mmol) in I2 mL of THF at -78
°C
was treated with n-BuLi (7.5 mL, 1.6 M solution in hexanes, 12.0 mmol) for 0.5
hours at -
78 °C. Next, ZnCl2 (20 mL, 1.0 M solution in Et20, 20 mmol) was added,
and the
solution was warmed to 25°C. To this solution was added Pd(PPh3)4 (70
mg, 0.06 mmol)
followed by 4-iodoacetophenone ethylene acetal (prepared from iodoacetophenone
and
ethylene glycol in the presence of an acid catalyst by standard procedures),
and the reaction
mixture was heated at reflux for 4 hours. The solution was then cooled to 25
°C and
quenched by the addition of saturated aqueous NaHC03 ( 10 mL). The aqueous
layer was
extracted with CH2C12, and the combined organic layer was concentrated under
reduced
pressure. The residue was dissolved in 30 mL of THF, 15 mL of 3 M aqueous HCl
was
added, and the mixture was stirred for 2 hours at 25 °C. The solution
was neutralized by the
addition of saturated aqueous NaHC03, and the aqueous layer was extracted with
CH2CI2.
The combined organic layer was dried (MgS04) then concentrated under reduced
pressure.
The crude product was purified by flash chromatography to provide the title
compound
3~ (0.89 g, 64%).
-100-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
Step 264b. 4-amino-5-(3-bromoR,henyl)-7-(4-(1-methyl-2-
imidazolvhghenvl)pyridof2.3-
dlpvrimidine trihvdrochloride
Following the procedures of Example 244, except in step (c) first substituting
the R4
reagent from Step 264a for the R4 reagent of Example 244 step c, and secondly
performing
the condensation with ammonium acetate substituting dichloroethane as the
solvent in place
of the ethanol solvent in Example 244 step c, the title compound was prepared.
MS (M+H)
458 ( 1 Br); IR (cm-1 ) 305 l, 2948, 1577, 1474, 1354.
Examples 265-267
Following the procedures of Example 244, except in step (c) first substituting
the
appropriate reagent for R4 as indicated in the Table below for the R4 reagent
of Example 244
step c, and secondly performing the condensation with ammonium acetate
substituting
dichloroethane as the solvent in place of the ethanol solvent in Example 244
step c, the
compounds of Examples 264-285 were prepared. In Ex. 266, the hydrochloride
salt was
not prepared.
Ex. Name R4 Reagent Analytical
No.
(for 7- Data
osition)
265 '1'~-5-(3- 1-(4- Ms (M+H),
460: >It
bromophenyl)-7-(4- (aminomethyl)ph(gym-1) 3024
2933,
(aminomethyl)phenyl)pyrienyl)ethanone1550, 1493,
1328
do[2,3-d) 'midine
266 4-'ammo-5-(3- 1-(2-bromo-4-Ives (M+H),
soo (2
bromophenyl)-7-(2- (dimethylamino)Br): >It (~,r~-1)
3049,
bromo-4- phenyl)ethanone29x9, 1536,
1468,
(dirnethylamino)phenyl)py 13 20
rido[2,3-d] 'dine
267 4-amino-5-(3- 1-(4- Ivts (M+H),
44s (1
bromophenyl)-7-(4- (dimethylaminoetBr)~ ~ (~-1)
3420,
(dimethylaminoethyl)phenhyl)phenyl)ethan3~0, 2980,
1635,
1) rid0 2,3- one 1610, 1590,
a 1435,
py 1415
a
]
'
a
4-amino-5-(3-bromophenyl)-7-(4-(3-(dime~ylamino)proRynyl henyl)~,3rridof2 '~-
A suspension of the compound of Example 63 (0.80 g, 1.59 mmol),
PdCl2(PPh3)2, CuI, and 3-dimethylaminoprop-1-yne in 20 mL of DMF/TEA (4:1) was
heated at 50 °C for 3 hours. The volatiles were removed under reduced
pressure, and the
-101-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
residue was purified by flash chromatography to provide the title compound
(0.50 g, 68 %).
MS (M+H), 459 (1Br); IR (cm-1) 3027, 2964, 1513, 1470, 1360.
Examples 269-271
Following the procedures of Example 268, except substituting the reagent
compound
shown in the table below for the 3-dimethylaminoprop-1-yne of Example 268, the
compounds shown in the table below were prepared.
Ex. Name Reagent Analytical
N Data
o
.
269 4-amino-5-(3- l, l-dimethyl-ms (lvt+H),
4s9
bromophenyl)-7-(4-(3-propargyl (1Br); ttt
amine (om-1)
amino-3- 3041, 2967,
1562,
methylbutynyl)phenyl)pyri 1484, 1319
do[2,3-dJ imidine
270 4-amino-5-(3- dimethyl ms (M+H),
486
bromophenyl)-7-(4- phosphite (1Br); IR
(cm-1)
dimethylphosphonatophen 3105, 2912,
162s,
yl)pyrld0[2,3- 1437, 1350
d] 'dine
271 4-amino-5-(3- methyl propargyltuts (M+H),
446
bromophenyl)-7-(4-(3-ether (1Br);1Ft
(m-1)
(methoxypropynyl)pyrido[ 3053, 2929,
1560,
2,3-dJ 'midine 1484, 1352
Exam In a 272
4-amino-5-(3-bromo henyl)-7-(4-carboxyphenyl)pyridof2 3-dlnvrimidine
A solution of 4-amino-5-(3-bromophenyl)-7-(4-cyanophenyl)pyrido[2,3-
d)pyrimidine (the compound of Example 37, (0.47 g, 1.17 mmol) in 15 mL of 6 M
HCl
(aqueous) was heated at 60 °C for 8 hours. The mixture was lyophilized
and the crude
product was purified by flash chromatography to provide the title compound
(0.14 g, 28%).
MS (M+H), 422 (1Br); IR (cm-1) 3064, 2628, 1692, 1403, 1273.
Example 273
4-amino-5-l3-bromophenvl)-7-(4-methyl-3-oxo-2H-4H-~vridof3 2-bl 1 4
oxazinyl)p3rridof2 3-d]pvrimidine
Sten 273a. 7-acet 1-~ 2H-pyridof3 2-bl-I 4-oxazin-3(4H)-one
A solution of 2H-pyrido[3,2-b]-1,4-oxazin-3(4H)-one (9.8 g, 65.27 mmol,
Aldrich) in 120 mL of THF/MeOH (5:1 ) was treated with 0.4 mL of concentrated
HCI
(aqueous) followed by N-bromosuccinimide ( 17.8 g, 100 mmol) in several
portions over 10
minutes. After 12 hours at 25 °C the reaction mixture was quenched by
the addition of
-I02-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
saturated aqueous NaHS03. The aqueous layer was extracted with CH2CI2 and the
combined organic layer was dried (Na2S04), concentrated under reduced
pressure, and
purified by flash chromatography to provide 7-bromo-2H-pyrido[3,2-b)-1,4-
oxazin-3(4H)-
one (8.4 g, 56%). A mixture of 7-bromo-2H-pyrido[3,2-b]-1,4-oxazin-3(4H)-one
(3.2 g,
14 mmol), tributyl(1-ethoxyvinyl)tin (6.1 g, 17 mmolj, Pd2(dba)3 (0.5 g, 0.56
mmol), and
(2-furyl)3P (0.3 g, 1.2 mmol) in 30 mL of toluene/THF (5:1 ) was warmed at
reflux for 10
hours. The reaction mixture was concentrated under reduced pressure, and the
residue was
dissolved in 50 mL of THF. 15 mL of 4 M HCl (aqueous) was added, and the
mixture was
stirred for 4 hours at 25 °C. The solution was neutralized by the
addition of NaHC03
(aqueous), and the aqueous layer was extracted with CH2C12. The combined
organic layer
was dried (Na2S0~, concentrated, and the crude product was purified by flash
chromatography to provide 7-acetyl-2H-pyrido[3,2-b]-1,4-oxazin-3{4H)-one (2.37
g,
88%). MS (M+H), 463 (1 Br); IR (cm-1) 3400, 3200-2800, 1700, 1640, 1605, 1590,
1395, 1380, 1345.
Sten 273b. 7-acetvl-4-methyl-2H-pyridof~ 2-bl-1 4-oxazin 3(4H1 one
The compound from step 273 a was treated with methyl iodide and NaH in l:l
THF/DMF for 6 hours at 0 °C to 25 °C. The reaction was quenched
with aqueous sodium
bicarbonate solution, the mixture was extracted with dichloromethane, and the
resiue was
purified by chromatogaphy to give the title compound. MS {M+H), 407.
Sten 273c. 4-amino-5-(3-bromophenyl)-7-(4-metal 3 oxo 2H 4H pyridof~ 2 bl 1 4
oxazin~pyridof2 3-dlpyrimidine
Following the procedure of Example 244 Step c, except first substituting 7-
acetyl-4-
methyl-2H-pyrido[3,2-b]-1,4-oxazin-3(4H)-one (the R4 reagent) from Step 273b
for the R4
reagent of Example 244 Step c, and secondly performing the condensation with
ammonium
acetate substituting dichloroethane as the solvent in place of the ethanol
solvent in Example
244 step c, the title compound was prepared. MS (M+H), 463 (1 Br); IR (cm-1)
3400,
3200-2800, 1700, 1640, 1605, 1590, 1395, 1380, 1345.
Exam lp a 274
4-amino-5-l3-bromonhenvll-7-(4-(2 (dimethvIaminolethyll ~ oxo 2H 4H pyridof3 2
bl
l~xazin-7-yllpyridof2 3-dlpvrimidine
S~t p 274a. 7-acetyl-4-dimethylaminoeth ly 2H ny~j3 2 bl 1 4 oxazin 3(4H) .one
-103-
CA 02286909 1999-10-12
WO 98/4bb05 PCT/US98/07207
The compound from Example 273 Step a was treated with 2-chloro-(N,N-
dimethyl)ethylamine HCl and K2C03 in aqueous acetone at reflux. The mixture
was diluted
with water and extracted with dichlorometttane, and the residue was purified
by
chromatogaphy to give the title compound.
Step 274b. 4-amino-5-(3-bromophenyl)-7-(4-(2-(dimethvlamino)ethyl)-3-oxo 2H 4H
pVIld013.2-bl-1 4-oxazin-7-vl)pyridof2 3-dlpvrimidine
Following the procedures of Example 244 Step c, except in step c first
substituting
7-acetyl-4-dimethylaminoethyl -2H-pyrido[3,2-b]-I,4-oxazin-3(41-x-one (the R4
reagent,
from Step 273b) for the R4 reagent of Example 244 Step c, and secondly
performing the
condensation with ammonium acetate substituting dichloroethane as the solvent
in place of
the ethanol solvent in Example 244 step c, the title compound was prepared. MS
(M+H),
519 (1 Br); IR (cm-1) 3440, 1685, 1630, 1605, 1580, 1395
Example 275
4-amino-5-(3-bromonhenyl)-7-(2 3-dihvdro-3-(dimethylaminoethyl)-2-
oxobenzoxazol 6
yl~pvridoT2.3-dlpyrimidine
Step 275a. 6-acet~rl-2-benzoxazohnone
Following the procedures of Example 273 Step a, except substituting 2-
benzoxazolinone (Aldrich) for the 2H-pyrido[3,2-b]-1,4-oxazin-3(41-x-one
thereof, the title
compound was prepared.
Step 275b. 6-acetyl-3-(dimethylaminoethyl)-2-benzoxazolinone
The compound from Example 275 Step a was treated with 2-chloro-(N,N-
dimethyl)ethylamine HCl and K2C03 in aqueous acetone at reflux. The mixture
was diluted
with water and extracted with dichloromethane, and the residue was purified by
chromatogaphy to give the title compound.
3o Sten 275c. 4-amino-5-(3-bromophenvl)-7-(2 3-dihydro-3-(dimethylaminoethyl)
2
oxobenzoxazol-6=yl)pvrido~2 3-dlpyrimidine
Following the procedures of Example 244 Step c, except in step c first
substituting
the compound from Step 275a for the R4 reagent of Example 244 Step c, and
secondly
performing the condensation with ammonium acetate substituting dichloroethane
as the
solvent in place of the ethanol solvent in Example 244 step c, the title
compound was
prepared. MS (M+H), 506 ( 1 Br); IR (cm-1 ) 3400, 3050, 1630, 1610, 1360.
-104-
CA 02286909 1999-10-12
WO 98/46605 PCT/ITS98/07207
Exampl 76
4-amino-5-l3-bromonhenyl_)-7-(4-methyl-3-oxo-2H-4H-benzo-1.4-oxazin_-7-
vl)pvridof2 3
dlpvrimidine
Step 276a. 6-acedvl-3-methyl-2-benzoxazolinone
The compound from Example 275 Step a was treated with methyl iodide and NaH in
1:1 THF/DMF for 6 hours at 0 °C to 25 °C. The reaction was
quenched with aqueous
sodium bicarbonate solution, the mixture was extracted with dichloromethane,
and the resiue
was purified by chromatogaphy to give the title compound.
Step 276b. 1-(3-h~droxv-4-methvlaminQphenvl)-ethanone
The compound from Step 276a ( 1.60 g, 8.37 mmol) was dissolved in acetone (70
mL) and treated with 1 M aqueous K2C03 solution (25 mL) with heating at reflux
overnight.
The mixture was neutralized with acid, then extracted with diethyl ether. The
solvent was
dried (MgS04) and removed under vacuum to give the title compound (2.01 g)
Step 276c. 7-acetyl-4-methyl-2H-4H-benzo-1,4-oxazin-3-one
The compound from Step 276b (2.01 g, 8.37 mmol) was dissolved in DMSO and
treated with sodium ethoxide (8.4 mmol) and bromoacetic acid ( 1.40 g, 8.4
mmol) at room
temperature overnight. The mixture was diluted with water and ether, and the
title
compound was isolated by filtration (0.48 g). MS (M+H), 206.
Step 276d. 4-amino-5-(3-bromophenyl)-7-(4-methyl-3-oxo-2H-H-benzo-1,4-oxazin-7-
vl)pvridof 2.3-dlpvrimidine
Following the procedures of Example 244 Step c, except in step c first
substituting
the compound from Step 276c for the R4 reagent of Example 244 Step c, and
secondly
performing the condensation with ammonium acetate substituting dichloroethane
as the
solvent in place of the ethanol solvent in Example 244 step c, the title
compound was
prepared. MS (M+H), 462 ( 1 Br); IR (cm-1 ) 3500, 2800-3200, 1690, 1645, 1610,
1590,
1385, 1355.
Example 277
4-amino-S-(3-bromophenyl)-7-(2 2 4-trimethyl-3-oxo-2H-4H-benzo-1 4-oxazin-7-
vl)pyridof 2.3-dlpyrimidine
-105-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
Steg 277a. 7-acetyl-2.2 4-trimethyl-2H-4H-benzo-1 4-oxazin-3-one
The compound from Step 276b (2.25 g, 9 mmol) was dissolved in DMSO and
treated with sodium ethoxide (9 mmol) and 2-bromo-2-methylpropanoic acid (1.76
g, 9
mmol) at room temperature overnight. The mixture was diluted with water, and
the mixture
was extracted with ether.ethyl acetate. The extract was dried (MgS04), the
solvent was
removed under vacuum, and the residue was purified by chromatography (silica
gel) to give
the title compound ( 1.33 g) MS (M+H), 234.
Step 277b. 4-amino-5-l3-bromophenyl)-7-(2.2,4-trimethyl-3-oxo-2H-4H-benzo-1 4-
oxazin-7-vl)pyridof2,3-dlpyrimidine
Following the procedures of Example 244. Step c, except in step c first
substituting
the compound from Step 277a for the R4 reagent of Example 244 Step c, and
secondly
performing the condensation with ammonium acetate substituting dichloroethane
as the
solvent in place of the ethanol solvent in Example 244 step c, the title
compound was
prepared. MS (M+H), 490 (1 Br); IR (cm-1) 3450, 2900-3100, 1680, 1645, 1610,
1515,
1385, 1365, 1165.
Example 2?8
4-amino-5-cvclohexyl-7-(4-(2-dimethvlamino)ethyl)-2H-4H-benzo-3-oxo-1 4-oxazin-
7
vl)pvrido~2.3-dlpyrimidine
Step 278a. 1-(3-hydroxv-4-f2-(dimethylamino)ethyl,)phenyi)-ethanone
A sample of 6-acetyl-3-(dimethylaminoethyl)-2-benzoxazolinone (from Example
27~
Step b) was dissolved in acetone and treated with 1M aqueous K2C03 solution
with heating
at reflux overnight. The mixture was neutralized with acid, then extracted
with diethyl ether.
The solvent was dried (MgS04) and removed under vacuum to give the title
compound.
Step 278b. 7-acet~rl-4-ldimethvlamino)ethyl)-2H-4H-benzo-14-oxazin-3-one
A sample of the compound from Step 278a (8.94 g, 32 mmol) was dissolved in
DMSO and treated with sodium ethoxide (32 mmol) and bromoacetic acid (5.34 g,
32
mmol) at room temperature for 2 days. The mixture was diluted with water then
extracted
with ether. The extract was dried (MgS04), the solvent was removed under
vacuum, and
the residue was purified by chromatography (silica gel) to give the title
compound ( 1.94 g).
MS (M+H), 263.
-106-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
Sten 278c. 4-amino-5-cvclohexvl-7-(4-(dimethvlamino)ethyl)-2H-4H-benzo-3-oxo-
1.4-
oxazin-7-vl)pvridof 2.3-dlpyn~idine
Following the procedures of Example 244 Step c, except in step c first
substituting
1.1-dicyano-3-cyclohexylethene (prepared according to the method of Moison, et
al.
(Tetrahedron (1987), 43:537-542) by treating cyclohexane carboxaldehyde with
malononitrile in the presence of finely powdered magnesium oxide in
dichloromethane) for
the R3 reagent of Example 244 Step c, and substituting the compound from Step
278b for
the R4 reagent of Example 244 Step c, and also performing the condensation
with
ammonium acetate but also substituting dichloroethane as the solvent in place
of the ethanol
solvent in Example 244 step c, the title compound was prepared. MS (M+H) 447;
IR(cm-1)
3400, 2900, 1690, 1610, 1590, 1395.
Example 279
4-amino-5-(3-bromophenvl)-7-(5-( 1-methvlet~l)-2-gvridvl)~,vrido f 2,3-
dlpyrimidine
Step 279a. 1-(5-meth~vl-2-pyridvl)ethanone
A solution of 2-acetyl-5-bromopyridine ( 1.45 g, 7.9 mmol), 2-
propenyltrimethyltin
( 1.77 g, 8.7 mmol), Pd2(dba)3 (0.33 g, 0.36 mmol), and tri-2-furylphosphine
(0.17 g,
0.72 mmol) in 25 mL of benzene was warmed at 60 °C for 4 hours. The
reaction mixture
was concentrated and the coupled product was purified by flash chromatography
( 1.22 g, 96
%). The product was dissolved in 25 mL of EtOH and the solution was purged
with a
stream of H2. 10% Palladium on charcoal (50 mg) in 0.5 mL of EtOH was added
and the
reaction mixture was stirred for 12 h under an atmoshpere of H2. The reaction
mixture was
filtered and the resulting solution was concentrated under reduced pressure.
The tide
compound, 2, ( 1.04 g, 84%) was isolated following flash chromatography.
Step 279b. 4-amino-5-(3-bromophenvl)-7-(5-( 1-methvlethyl)-2-~yridyl~pvridof 2
3-
dlpyrimidine
Following the procedures of Example 244 Step c, except in step c substituting
the
compound from Step 279a for the R4 reagent of Example 244 Step c, and
performing the
condensation with ammonium acetate and also substituting dichloroethane as the
solvent in
place of the ethanol solvent in Example 244 step c, the title compound was
prepared. MS
(M+H) 421 (1Br); IR (cm-1) 3489, 2940, 1545, 1482, 1357.
Examples 280-281
Following the procedures of Example 244 Step c, except in step c substituting
the
compound shown below for the R4 reagent of Example 244 Step c, and performing
the
-107-
CA 02286909 1999-10-12
WO 98/46b05 PCT/IJS98/07207
condensation with ammonium acetate and also substituting dichloroethane as the
solvent in
place of the ethanol solvent in Example 244 step c, the compounds shown in the
table below
were prepared.
Ex. I Name R4 Reagent Analytical
No.
(for 7- Data
osition)
280 4-amino-5-(3- I-(5-piperidinyl-Ms (M+H),
460
bromophenyl)-7-(5- 2- (1Br); llt
(cm-1)
piperidin-I-ylpyrid-2-pyridyl)ethanone3064, 2937,
1556,
yi)pyrido[2,3-
* 1493, 1358
d] 'dine
281 4-amino-J-(1-(4- 1-(2- MS (M+H),
491 (i
bromophenyl)ethyl)-7-(6-morpholinyl-5-BTO IR (cm-1)
1685,
morpholinylpyrid-3-pyridyl)ethanonelsss, lsos,
1240,
yl)pyrido[2,3- ** lllo, 940
d] 'dine
* Prepared as in Ex. 252b, except substituting morpholine for the bis(2-
methoxyethyl)amine thereof.
** prepared by treatment of 5-acetyl-2-chloro-pyridine with morpholine in
refluxing ethanol.
Exam In a 282
4-amino-5-l3-bromophenyl)-7-~4-(lN-formvlamino)methy~ henylOvridof2 3-
dlpyrimidine
Sten 282a. 4-cvanoacetonhenone. acetal with 2 2-dimeth~r rolz, line g]xcol
A sample of 4-cyanoacetophenone (4.35 g, 30 mmol) was dissolved in 150 mL of
hexanes, and to this solution were added 2,2-dimethylpropylene glycol (3.44 g,
33 mmol)
and a catalytic amount { 10 mg) of p-toluene sulfonic acid. The reaction was
heated
overnight at reflux with a Dean-Stark trap, and an additional portion of
glycol (33 mmol)
was added. The reaction was continued for 3 hours, then cooled and the solvent
was
removed. The residue was dissolved in ethyl acetate, and this solution was
washed with
aqueous NaHC03, water and brine, and dried over MgS04. The solvent was removed
under vacuum to give the title compound (7.46 g).
step 282b. 4-(aminometh 1)~phenone acetal with 2 2-dime~lnro~,vlene g_lvcol
The compound from Step 282a (2.31 g, 10 mmol) was dissolved in ether (50 mL)
and stirred with lithium aluminum hydride (0.76 g, 20 mmol) at ambient
temperature
overnight. The reaction was quenched with MgS04~10 H20, and the mixture was
diluted
with ether. The mixture was filtered, and the filtrate removed to give the
title compound.
Step 282c. 1-(4-(BOC-~minomet~lvl~phenyllethanone
-108-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
The compound from Step 282b (1.18 g, 5 mmol) was dissolved in THF (20 mL),
1N HCl (20 mL) was added, and the mixture was stirred for 2 days. The
volatiles were
removed under vacuum, the residue was dissolved in THF (20 mL), and d-tibutyl
dicarbonate (2.18 g, 10 mmol) was added. The mixture was stirred at room
temperature
s over a weekend. The solution was diluted with water, and the mixture was
extracted with
ether and ethyl acetate. The organic extracts were direct (MgS04), and the
solvent was
remove under vacuum to give the title compound.
Step 282d. 4-amino-5-(3-bromophenyl)-7-(4-(N-
formvlaminolmethvllphenvl)pyridof2.3-
l0 dlpvrimidine
Following the procedures of Example 244, except in step c substituting the
compound from Step 282c for the R4 reagent of Example 244 Step c, and
performing the
condensation with ammonium acetate but also substituting dichloroethane as the
solvent in
place of the ethanol solvent in Example 244 step c, the title compound was
prepared. MS
15 (M+H) 434 ( 1 Br); IR (cm-1 ) 3440, 2700-3150, 1635, 1580, 1380.
Example 283
4-amino-5-(3-bromophenyl)-7-(4-( 1-(N-methylamino)-1-
methylethyl)phenvl)pvridof 2.3-
dlp~rimidine
Step 283a. 4-(1-amino-1-methyleth, 1)~acetophenone
CeCl3 ( 10 g, 34.9 mmol) was suspended in THF (60 mL), and the mixture was
cooled to -78 °C. Methyl lithium ( 1.4 M, 2 mL) was added, and the
mixture was stirred for
20 minutes. Then the compound from Example 282 Step a, (4-cyanoacetophenone
acetal
with 2,2-dimethylpropylene glycol, 2.31 g, 10 mmol) in 2 mL of THF was added.
After
stirring for 4 hours, the mixture was allowed to warm to room temperature
while stirring for
16 hours. The reaction was quenched with water and ammonium hydroxide,
filtered, and
the filtrate was extracted with dichloromethane. The solution was dried
(MgS04), and the
solvent was removed to give the title compound.
Step 283b. 4-(1-lN-BOC-amino)-1-methylethyl)acetophenone
The compound from Step 283a {2.32 g, 8.77 mmol) was treated sequentially with
HCl and di-t-butyl dicarbonate according to the procedure of Example 282 Step
c to give the
title compound (1.60 g). MS (M+H) 278.
-109-
CA 02286909 1999-10-12
WO 98146605 PCT/US98/07207
Steo 283c 4-amino-5-( 3-bromonhenvl)-7-(4-( 1-(N formvlamino) 1
methvlethvl)phenvl)~yridof2 3-dlpyrimidine
Following the procedures of Example 244 Step c, except in step c substituting
tit
compound from Step 283b for the R4 reagent of Example 244 Step c, and
performing the
condensation with ammonium acetate but also substituting dichloroethane as the
solvent in
place of the ethanol solvent in Example 244 step c, the title compound was
prepared. MS
(M+H)
462 ( 1 Br); IR (cm-1 ) 3440, 1640, 1605, 1580, 1380.
l0 Example 284
4-amino-5-(3-bromophenyl)-7-(4-(1-(N N-dimethylamino)-1-
methvlethvl)phenvl)pvridof2 3-dlpvrimidine
Step 284a. 4-(1-(dimethvlamino)-1-methvlethyl)acetonhenone
The compound from Step 283a (1.18 g, 5 mmol) was dissolved in 5 mL formic
acid, and 5 mL of formalin (37% ) was added. The mixture was heated at reflux
for 4
hours, then cooled and neutralized with 2N Na3C03. The mixture was extracted
with
dichloromethane. The solution was dried .(MgS04), and the solvent was removed
to give
the title compound (0.94 g). MS (M+H) 462 ( 1 Br); IR (cm-1 ) 3520, 1640,
1610, 1580,
1375.
Examples 285-286
Following the procedures of Example 157, except substituting the
appropriate reagents for the R3 and R4 reagents of Example 157 as indicated in
the Table below, compounds of Examples 285-286 were prepared. For
Example 286, treatment with aqueous HCl was omitted, and the free base was
obtained.
Examples 285-286
Ex. Name R3 Reagent R4 Reagent Analytical Data
No.
(for 5- (for 7-
osition position)
285 4-amino-~-(3- l,l-dicyano-(3-1-(N-acetyl-5-mp (hydrochloride
salt )
bromophenyl)-7-(N-(3- indolinyl)- >2~oc. 1R ~~mv>
3a4s.
acetyl-5- bromophenyl)prethanone 3loo-2soo. 1640.
l6os.
indoliny opene 144s, 1395.
1)pyrido[2,3- 1326.
_ t,luvts [M+H]-
dJ dine m/z 460.
462.
-110-
CA 02286909 1999-10-12
WO 98/46605 PCTNS98/07207
286 4-amino-5-cyclohexyl-l, l-dicyano-3-1-(6-chloro-3-mp 240-242 c.
iit (cm-
7-(6-chloro-3- cyclohexylethenepyridyl)- 1) 3s28, 3300,
3086,
pyridyl)pyrido[2,3- ethanone 2936, 2853, 1645,
d]pyrlmidine 1590,
ls7s, 1s65,1350.
L~Ms
Ht+H + m/z 340.
Examples 287-300
Following the procedures of Example 157, except substituting the appropriate
R3
- 5 and R4 reagents as indicated in the Table below and replacing the
formamide or formamidine
acetate treatment with treatment with triethyl orthoformate at reflux in the
presence of a
catalytic amount of ammonium sulfate, followed by cooling to 25 °C and
addition of excess
ammonia in ethanol, compounds of Examples 287-300 were prepared. . After 24
hours, the
precipitated amidine compound was filtered and washed with hexanes, then dried
under
vacuum. The amidine compound was then heated in 1,2-dichloroethane at reflux
for 1-8
hours. The reaction mixture was cooled to room temperature and purified by
chromatography, and the product was recrystallized if necessary. The treatment
with
aqueous HCl was omitted in some cases, and the free bases were obtained.
Examples 287-300
Ex. Name R3 Reagent R4 Reagent Analytical Data
No.
(for 5- (for 7-
osition osition)
287 4-amino-5-(1-(2- l,l-dicyano-2-1-(6- >It (cm-1) 26oo-3soo,
bromophenyl)ethyl)-7-methyl-(3-(2-dimethylamino-l6so, 1602,
1596, ls2o
(6-dimethylamino-3-bromophenyl)pr3-pyridyl)- cm-1. Lltlvls
(lvt+H)+
pyridyl)pyrido[2,3-opene ethanone m/z 449,4s1.
d 'dine
288 4-amino-5-(1-{2- l,l-dicyano-2-1-(6- mp (dihydrochloride
salt)
bromophenyl)ethyl)-7-methyl-(3-(2-morpholinyl-3-213-216 C. IR
(cm-1)
(6-morpholinyl-3-bromophenyl)prpyridyl)- 24oo-3soo, 1660,
1600.
pyridyl)pyrido[2,3-opene ethanone Q~s fHt+Hl+
m/z 491&
d] 'dine
289 4-amino-5-{1-(2- 1,1-dicyano-2-1-(6-(N-methyl-mp 2s2-2s3c.
rn (omv)
bromophenyl)ethyl)-7-methyl-(3-(2-N- 3sls. 3310,
3200-2800,
(6-(N-methyl-N- bromophenyl)prfotmyl)amino)-16~s, lsgs,
ls6o, ls4s,
form 1 amino)-3- o ene 3 d 1) 1340. Lwvts
Y ) P -PYr'i Y (Nt+t-~ m/z
- 462, 464.
phenyl)pyrido[2,3- ethanone
d] 'dine
290 4-amino-5-cyclohexyl-l,l-dicyano-3-1-(6- mp (dihydrocnloride
salt)
7-(6-morpholinyl-3-cyclohexylethenemorpholinyl-3-tog-21o. ilt
(cm-1)
3490, 3300,
pyridyl)pyrido[2,3- pyrldyl)- 3050-3250.
1620, lsgo.
d]pynmldlne ethanone lsso, 1490.
LRMS M+H + m/z
391.
-111-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
291 4-amino-5-((2- 1,1-dicyano-(3-1-(6- mp (dihydrochloride
salt )
bromophenyl)methyl)-(2- morphoiinyl-3-201-204 c. Bt
(cm-1)
7-(6-morpholinyl-3-bromophenyl)prpyridyl)- 2aso, isss
isb~
iso2
,
pyridyl)p opene ethanone ,
o[2,3- ,
d 1345 . LltMS
d~d (M+H]+
m/z 477, 479.
292 4-amino-5-{4- l,l-dicyano-3-1-(6- mp (dihydrochloride
salt)
tetrahydropyranyl)-7-(4- morpholinyl-3-213-216 c. nt
(om-1)
(6-morpholinyl-3-tetrahydropyranypyrldyl)- 3310, 3060, 29ss,
1587,
pyridyl)pyrido[2,3-1}ethene ethanone lss9, lso6. l3so.
d] ding * LRMS [M+H]+ m/z
393.
293 4-amino-5-cyclohexyl-l,l-dicyano-3-1-(6- mp (dihydro~hloride
salt)
7-(6-dimethylamino-3-cyclohexylethenedimethylamino-272-274 c. at
(cm ~t)
pyridyl)pyrido[2,3- 3-pyridyl)- 3s32.3294.3100.2930,
2853, 1606. 1586,
d]pyrlmidine ethanone 1s60,
1522. 1387. LRMS
M+H + m/z 349.
294 4-amino-5-(1- 1,1-dicyano-3-1-(6- mp (free base):
2235-22s
ethylpropyl)-7-(6-ethylpentene dimethylamino-c~ ~ (gym-1)
3480,
dimethylamino-3- 3-pyridyl)- 3-3470. 2800-3000,
1630, 1610, 1s80,
p ethanone is6s,
yridyl)p
o[2,3-
d is20. LRMS [M+H]+
d~d m/z
aa~~u
337.
295 4-amino-S- l , l -dicyano-3-1-(6- >R (om-~ ) 349s,
3320,
cyclopentyl-7-(6-cyclopentylethenmorpholinyl-3-3080, 29so, 164s,
1600,
morpholinyl-3- a p~dyl)_ lsoo, 1400, 13s0,
1240.
LRMS [M+H]' m/z
pyridyl)pyrido[2,3- ethanone 377.
d] 'dine
296 4-amino-5-cyclohexyl-1,1-dicyano-3-1-(2-chloro-3-t>z (om-~) 33os,
3lss,
7-(2-chloro-3- cyclohexylethenepyridyl)- 2930, 28s5, 1590,
1610,
1s90, ls4s, 134s.
pyridyl)pyrido[2,3- ethanone
Las [M+H]- m/z
d]pyrlmldine 340,
342.
297 4-amino-5-(3,5- 1,1-dicyano-3-1-(6- nt (om-~) 3310,
3100,
dimethylcyclohexyl}-(3,5- dimethylamino-29s0. 1605, 1590,
l5ss,
7-(6-dimethylamino-3-dimethylcyclohe3-pyridyl)- 1390, l3so. LRMs
pyridyl)pyndo[2,3-xyl)ethene ethanone [M+H]' m/z 377.
d] dine
298 4-amino-5-((N- l, l-dicyano-(3-1-(6- tlz (cm-1) 3s38,
3311,
(benzyloxycarbonyl}-(4- morpholinyl-3-3032, 292s Z8s2,
1696
4-piperidinyl)methyl)-(benzyloxycarbopyndyl)- lsgs, 1560. LltMs
7-(6-morpholinyl-3-nyl)piperidin-1-ethanone [M+H)+ m/z 540.
pyridyl)pyrido[2,3-yl)propene
d] 'dine
299 4-amino-5-cyclohexyl-1,1-dicyano-3-1-(6-bromo-3-m.p. 2so-2s2
c, IR (cm-
7-(6-bromo-3- cyclohexylethenepyridyl)- I) 3s30, 3298,
3093,
pyridyl)pyrido[2,3- ethanone 2932.28s6.1645,
lsg3,
d]pyrimidine 1s69. 1543, 1461,
1346.
LRMS M+H + 384.
300 4-amino-5-cyclohexyl-l,l-dicyano-3-1-(3- 386.
m. p. 223-224
c. IR
7-(3- cyclohexylethenecyanophenyl)-(gym-1) 3s2s,
3298,
cyanophenyl)pyrido[2, ethanone 307s, 2937, 223s.
164s.
1586, 1s48. 1567,
3-d]pynmidine 146.
LRMS [M+H]+ 332.
me ~,~-a~cyano-~-cyclonexyleulene was prepared according to the method
of Moison, et al. (Tetrahedron (19$7), x:537-542) by treating cyclohexane
carboxaldehyde with malononitrile in the presence of finely powdered magnesium
oxide in dichloromethane.
-112-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
The reagents for the following examples were prepared by this method
susbstituting the compound shown below for the cyclohexane carboxaldehyde used
to prepare the reagent of Example 290.
Example 292, tetrahydropyran-4-carboxaldehyde;
Example 294, 2-ethylbutanaldehyde;
Example 295, cyclopentane carboxaldehyde;
Example 297, 3,5-dimethylcyclohexane carboxaldehyde;
Example 298, N-(phenylmethoxylcarbony)piperidine-4-carboxaldehyde (this
material was prepared from N-(carbobenzyloxy)-4-(2-hydroxyethyl)piperidine
(Brehm et al., Helv.Chim.Acta, 70; (1987), 1981-1987 by treatment with TEMPO
(2,2.6,6-tetramethylpiperidinyloxy radical) and potassium bromide in
dichloromethane at 0 °C to which was added commercial bleach (Clorox)
containing
sodium bicarbonate).
Examples 301-305
Following the procedures of Example 246, except in step (c) substituting the
appropriate reagents for methylamine as indicated in the Table below to
prepare the correct
R4 reagent, and substituting the R3 reagent shown below for the R3 reagent of
Example 246
step d, the compounds of Examples 30I-305 were prepared. For Example 302 only,
the
2o condensation solvent was DMSO instead of ethanol and dimethoxyethane.
Examples 301-305
Ex. blame R3 reagent reagent of Analytical
N ste c Data
o
.
301 4-amino-5-(1-(2- 1,1-dicyano-(3-dimethylaminemp (dihydrochloride
bromophenyl)ethyl)-7-{6-(2- sau ) >22oc.
IR
dimethylamino-3- bromophenyl)pr (gym-') 3soo-2400.
dazln 1 do 2,3- o ene 1640, 1610,
PYn Y )PYn [ P 1580,
1370. LRMS
[M+H]'
d] die m/z 450, 452.
302 4-amino-5-(3- 1',1'-dicyano-imidazole mp
bromophenyl)-7-(6- (3-bromostyrenesodium salt (tetrahydrochloride
imidazolyl-3- salt ) >24oc.
IR
pyridazinyl)pyrido[2,3- i64o~ ib~o
X1590,
d]pyrimidine 1560, 1415,
1370.
LRMS [M+H]'
m/z
445, 447.
303 4-amino-5-(3- 1',1'-dicyano-azacycloheptanemp (dihydrochloride
bromophenyl)-7-(6- (3-bromostyrene Salt ) >19oc.
IR
(azacycloheptanyl)-3- (cm'') 3435.
3100-
2400, 1635,
pyridazinyl)pyrido[2 1610,
3-
, ls9o. lsso,
d] 1440,
rimidine
py 1370. LRMS
[M+Hl'
m/z 476, 478.
304 4-amino-5-(3- 1', l'-dicyano-N-methyl-N-( mp (dihydrochloride
1-
bromophenyl)-7-(6-(N-(3-bromostyrenemethylethyl))amiSalt ) >21oc.
IR
methyl-N-(1- ne (mv> 3435,
3100-
meth leth 1))amino)-3- 2400, 163s.
Y y 1610.
1590, 1550,
pyridazinyl)pyrido[2,3- 1410,
1370. LRMS
[M+H]-
d] 'dine
m/z 450, 452.
-113-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
305 4-amino-5-(1-(2- 1,1-dicyano-(3-morpholine Bt (cm-1)
3475,
bromophenyl)ethyl)-7-(6-(2- 3313, 3100,
l6so,'
morphofinyl-3- bromophenyl)pr
1620, 1580,
ls5s.
pyndazinyl)pyrido[2,3-opene lwvts iM+Hl+
z:c
d] 'dine 492, 494.
Example 306
4-amino-S-cvclohexyl-7-l6-(4-acetylR~erazinyi~pyr~'dyl)~yridof7 3 dlpvri_mid~e
A mixture of 679 mg (2 mlnol) of the compound from Example 298 and 1.28 g ( 10
mmol) of N-acetylpiperazine in 5 mL of DMSO was heated at 110 °C for 5
hours. On
cooling a precipitate was deposited, which was collected and washed with 20%
methanol
and dried to give 647 mg of the product as orange flakes: IR (cm-1 ) 3522,
3306, 3I 10,
2925, 2854, 1670, 1650, 1586, 1506. LRMS [M+H]+ m/z 432.
Examples 307-322
Follwing the procedure of Example 306, except substituting the reagent
shown in the table below for the N-acetylpiperazine of Example 306, the
compounds
shown in the table were prepared. The compounds were purified by HPLC
chromatography.
Ex. Name reagent Analytical Data
No.
_
307 4-amino-5- 1-acetyl-1,4- m.p. 169-171
c, llt (cm-
cyclohexyl-7-(6-(4-diazacycloheptane1) 3535 3309,
3096,
acetyl-1,4- 2930. 2854,
1638, 1605,
1587, 1s58,
diazacycloheptanyl)- 1513.
Ltu~ts 1M+H]+446.
3-
pyridyl)pylido[2,3-
d] 'dine
308 4-amino-~- 1-methyl-1,4- l.tuv~s [M+H]+419.
cyclohexyl-7-(6-(4-diazacycloheptane
methyl-1,4-
diazacycloheptanyl)-
3-
pyridyl)pyrido[2,3-
d] ' dine
309 4-amino-~- N-methyl-N-(2-(2-I.RMS [M+Hl+
441.
cyclohexyl-7-(6-(N-pyridyl)ethyl)amine
methyl-N-(2-(2-
pyridyl)ethyl)amino)
-3-
pyridyl)pyrido[2>3-
d] 'dine
-114-
CA 02286909 1999-10-12
WO 98/46605 PCT/~JS98/07207
3101 4-amino-5- N,N-dimethyl, L~ttvts [ivi+Hl+
N'- a21.
cyclohexyl-7-(6-2-methyl-1,2-
(N-{N',N'- ethylenediamine
dimethylaminoethyl)
-N-methylamino)-3-
pyridyl)pyrido[2,3-
d] 'dine
311 4-amino-5- azetidine LRMS [M+HI+
36i.
cyclohexyl-7-(6-
azetidinyl-3-
pyridyl)pyrido[2,3-
d] 'dine
312 4-amino-5- N-methyl-N-(3- Litlvis [M+H]+
aa~.
cyclohexyl-7-{6-(3-pyrrolidinyl)acetami
(N- de
rnethylacetamido)pyr
rolidinyl)pyridyl)pyr
ido[2,3-
d] 'dine
313 4-amino-5- pyrrolidine-2- LFtMS [M+H]+
419.
cyclohexyl-7-(6-(3-formamide
(formamido)pyrrolid
inyl)pyridyl)pyrido[
2,3-d ' midine
314 4-amino-5- 1-phenyl-1,38- t,xivts [M+H]+536.
cyclohexyl-7-(4-triazaspiro[4.5]deca
oxo-1-phenyl-1,3,8-n-4-one
triazaspiro[4.5[deca
n-8-yl)pyrido[2,3-
d 'dine
315 4-amino-5- 2- LxMS [M+H1+
a2o.
cyclohexyl-7-(6-(2-(methoxymethyl)pyr
(methoxymethyl)pyrrolidine
rolidin-1-
yl)pyridyl)pyrido[2,
3-d] 'midine
316 4-amino-5- N- Liztvts llvt+HI+
a21.
cyclohexyl-7-(6-(N-(methoxyethyl)prop
methoxyethyl-N-ylamine
propylamino)pyridyl
)pyrido[2,3-
d] 'dine
317 4-amino-5- 2-(methylamino)-c.ltMS livt+HI*
a29.
cyclohexyl-7-(N-dimethylacetaldehyd
methyl-N-(2,2- a
dimethoxyethyl)ami
no)pyrido[2,3-
d] dine
318 4-amino-5- N-(4-piperidyl)-Ltuvts [M+H]*
433.
cyclohexyl-7-(6-(4-dimethylamine
(dimethylamino)pipe
ridinyl)pyridyl)pyrid
0[2,3-d] 'midine
-115-
CA 02286909 1999-10-12
WO 98/46605 PCT/(TS98/07207
319 4-amino-S- piperidine-4- Ltt~tS [M+H)+
433.
cyclohexyl-7-(6-(4-formamide
(aminocarbonyl))pip
eridinyl)pyridyl)pyri
do[2,3-d) 'midine
320 I 4-amino-~- N 1 N 1-diethyl-N3-LR~~ts (M+H)+
a t9.
cyclohexyl-7-(N-methyl-1,3-
methyl-N-(3- propanediamine
(diethylamino)propy
1)aminopyrid-3-
yl)pyrido[2,3-
d] 'dine
32 4-amino-5- N-methyl-(4- LRtvts fM+H)+
) 4ai.
cyclohexyl-7-(6-(N-pyridyl)ethylamine
methyl-N-(4-
pyridyl)ethylamino)
pyrid-3-
yl)pyrido[2,3-
d] 'dine
322 4-amino-5- N-methyl-(3- LRMS (M+H)+ 42~.
cyclohexyl-7-(6-(N-pyridyl)methylamine
methyl-N-(3-
pyridylmethyl)amino
)pyrid-3-
yl)pyrido[2,3-
d] 'dine
Example 323
4-amino-5-fl-l2-bromophen 1 ethyl)-7-(1-methyl-5-indolyl)pyridof2 3
d]pyzimidine
The procedures of Example 157 were followed, except substituting 1',1'-dicyano-
3-
bromostyrene for the R3 reagent and 1-( 1-methyl-5-indolyl)-ethanone for the
R4 reagent .
After 24 hours, the precipitated amidine compound was filtered and washed with
hexanes,
then dried under vacuum. The amidine compound was then heated in 1,2-
dichloroethane at
reflux for 1-8 hours. The reaction mixture was cooled to room temperature and
purified by
chromatography, and the product was recrystallized if necessary. The treatment
with
aqueous HCl was omitted, and the free bases was obtained. IR (KBr) cm-1 3500,
1578,
1500; MS m/z 431 (M+H)+.
Exam )n a 324
4-amino-5-(1-(2-bromoDhenyl ethyl)-7-(1-methyl-2 3-dioxo 5 indolvl)nvridof2 3
dlpyrimidine
The title compound was prepared from the compound of Example 323 by oxidation
with Cr03 in sulfuric acid. IR (microscope) 3471, 1765, 1500 cm-l MS m/.
461(M+H)+.
-116-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
Examines 325-326
Fflllowing the procedures of Example 157, except substituting the appropriate
R3
and R4 reagents as indicated in the Table below, compounds of Examples 325-326
were
prepared. After 24 hours, the precipitated amidine compound was filtered and
washed with
hexanes, then dried under vacuum. The amidine compound was then heated in 1,2-
dichloroethane at reflux for 1-8 hours. The reaction mixture was cooled to
room
temperature and purified by chromatography, and the product was recrystallized
if
necessary. The treatment with aqueous HCl was omitted in some cases, and the
free bases
' were obtained.
Examples 325_326
Ex. Name R3 Reagent R4 Reagent Analytical Data
No.
(for 5- (for 7-
osition osition)
325 4-amino-5-(3- 1', I'-dicyano-3-1-(3-fluoro-4-(IR (microscope)
1- 3443,
bromophenyl)-7-(3-bromostyrene morpholinyl)phe3~. 1639, 1606.
1584,
fluoro-4-(I- nyl)-ethanone1520. 1362, 1245
cm-1;
morpholinyl)phenyl)p Ms miZ 4so (M+H)+.
yrido[2,3-
d] 'dine
326 4-amino-5-{3- 1', l'-dicyano-3-1-(4-hydroxy-3-IR (K$r) 3461.
1623,
bromophenyl)-7-{4-bromostyrene nitrophenyl)-1s79. ls4s, 1523.
1353
hydroxy-3- ethanone ~m-1; Ms miz
43s
nitrophenyl)pyrido[2,3 (M+H)+.
-d] 'dine
Example 327
Following the procedures of Example 244 Step c, except in step c substituting
the
compound resulting from the reaction of 2-acetyl-5-chloropyridine in refluxing
ethanol with
the precursor reagent compound (4-piperidinone ethylene ketal) shown below for
the R4
reagent of Example 244 Step c, and substituting dichioroethane as the solvent
in place of the
ethanol solvent in Example 244 step c, the compound shown in the table below
was
prepared.
Ex. Name precursor Analytical
N rea ent Data
o
.
327 4-amino-S-(3- 4-piperidinoneitt (microscope)
bromophenyl)-7-(6-(4,4-ethylene ketal3091, 1602,
1580.
. ethylenedioxypiperidinyl)- 1558, 1512.
1353.
3-pyridyl)pyrido[2,3- 1236, 1103
cm' 1:
d] 'dine Ms m/z 519
(M+H)+.
-I17-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
Example 328
4-amino-5-l3-bromonhenvl)-7-(6-l4-oxopiperidinvl)-3-pvrid~rl,},~yridol2 3-
dlpvrimidine
Treating the compound of Example 327 with dilute HCI, the title compound was
prepared. IR (microscope) 3438, 3051, 1645, 1605, 1558, 1450, 1371, 1240 cm-1;
MS
m/z 475 (M+H)+.
Examples 329-331
Following the procedures of Example 244 Step c, except in step c substituting
the
compound resulting from the reaction of 2-acetyl-5-chloropyridine in refluxing
ethanol with
the precursor reagent compound shown below for the R4 reagent of Example 244
Step c>
and substituting dichloroethane as the solvent in place of the ethanol solvent
in Example 244
step c, the compounds shown in the table below were prepared.
Examlales 329-331
Ex. Name precursor Analytical
No. rea ent Data
-
329 4-amino-5-(3- piperazine IR (I~r)
3489, 1674,
bromophenyl)-7-(6-(4- '
formylpiperazinyl)-3- lso3, 1233,
1004
pyridyl)pyrido[2,3- cm-1: MS
m/z 491
d] dine (M+H)+.
330 4-amiri0-5-(3- 1- IR (microscope)
bromophenyl)-7-(6-(4-methylpiperazine343x, 3osl,
ls4o
methylpiperazinyl)-3- cm-1: Ms
mlz 4~~
pyridyl)pyrido[2,3- (M+H)+.
d) 'dine
331 4-amino-5-(3- thiomorpholineIR (KBr)
3486, 1602,
bromophenyl)-7-(6- 1s81, ls6o.
lso2,
thiomorpholinyl-3- 1228 cm-1;
MS m/z
pyridyl)pyrido[2,3- 4~9 (M+H)+.
d) imidin
Example 332
4-amino-5-(3-bromophenyl)-7-l6-l4 4-dioxothiomorphoiinyl)-,3-nyridy~~yridof2 3
dlpyrimidine
The compound of Example 331 was treated with 4-chloroperbenzoic acid in
methanol and dichloromethane to give the title compound. IR (microscope) 3471,
1601,
1581, 1562, 1510, 1353, 1316, 1285, 1122 cm-1; MS m/z 511(M+H)+.
-118-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
Example 333
4-amino-5-(2-bromo~henvl)- -(6-mornholinvl-3-pvridyl)nvridof2 3-dlp'~midine
Step 333a. 1'.1'-dicyano-2-bromostyrene
The title compound was prepared by condensation of 2-bromobenzaldehyde with
malononitrile and Mg0 in dichloromethane by the standard procedure of
Broekhuis et al.
(Recl. J. R. Neth. Chem. Soe., 99: 6-12 (1980)).
Sten 333b. 5-acetvl-2-morpholin,~l, vridine
The title compound was prepared by the reaction of 5-acetyl-2-chloropyridine
with
morpholine in refluxing ethanol.
yep 333c. 4-(2-bromonhenyl)-3-cyano-6-morpholin~pvtidine-2-amine
The title compound was prepared by condensation of 1',1'-dicyano-2-
bromostyrene
with 5-acetyl-2-morpholinylpyridine and ammonium acetate in dichloroethane at
reflux.
After the reaction was complete (TLC), the mixture was cooled, and the solvent
was
removed. The residue was triturated with methanol to give the product
Step 33~ 4-amino-5-l2-brom_ophenvl)-7-(6-mor~hoiinvl-3-pvridyl)pyridof2 3-
2o dlpyrimidine
A sample of 4-(2-bromophenyl)-3-cyano-6-morpholinylpyridine-2-amine was heated
at 180-190 °C in formamide. The reaction was monitored by TLC, and when
the reaction
was complete the mixture was cooled to room temperature. The product was
allowed to
precipitate, then recovered by filtration and washed with water. Additional
product was
extracted from the filtrate. The product was purified by column chromatography
eluting
with 10% MeOH/CH2C12. IR (microscope) 3493, 1547, 1109cm-1; MS m/~ 464 (M+H)+.
Examules 334-336
Following the procedures of Example 333, except in Step a substituting the
precursor aldehyde reagent shown below for the 2-bromobenzaldehyde of Example
333
Step a, and carrying the product forward as in procedures 333 Stepbs b-d, the
compounds
shown in the table below were prepared.
-119-
CA 02286909 1999-10-12
WO 98/46605 , PCT/US98/07207
Examples 334-336
Ex. Name precursor Analytical
N aldehyde Data
o
.
rea ent
334 I 4-amino-~-(3-bromo-4-3-bromo-4- IR (microscope)
methoxyphenyl)-7-(6-methoxybenzaldehyde3486, 1600,
is7s,
morpholinyl-3- 1562, 1500,
1260,
pyridyl)pyrido[2,3- 1237 cm-1:
MS miz
d] 'dine 493 (M+H)+.
335 4-amino-S-(4- 4-bromobenzaldehyde11t (microscope)
bromophenyl)-7-(6- 3497, 1532,
moipholinyl-3- 1098cm-i;
MS m/z
pyridyi)pyrido[2,3- 464 (M+H)+.
d) 'dine
336 4-amino-5-(3- 3-chlorobenzaldehydeIR (microscope)
chlorophenyl)-7-(6- 3484, isoo,
morpholinyl-3- 1o34cm-i:
MS (F~,s)
pyridyl)pyrido[2,3- miz 587 (M+H)+.
d] 'dine
Example 337
4-amino-5-f3-bromophenvl)-7-(5-chloro-6-morpholinyl-3-pyridyl)pyridof2 3
dlpyrimidine
Following the procedures of Example 333, except in Step a substituting 3-
bromobenzaldehyde for the 2-bromobenzaldehyd, in Step b substituting 5-acetyl-
2,3-
dichloropyridine for the 5-acetyl-2-chloropyridine to give 5-acetyl-3-chloro-2-
morpholinylpyridine, and substituting 5-acetyl-3-chloro-2-morpholinylpyridine
for the 5-
acetyl-2-morpholinylpyridine in step c, then the carrying the product forward
as in Example
333 Step d, the title compound was prepared. IR (microscope) 3493, 1635, 1585,
1555,
1492, 1340, 1241, 1113 cm-1; MS m/~ 497 (M+H)+.
Example 338
4-amino-5-(3-bromophenyl)-7-(6-(N-oxidomorpholinyl)-3-pyrid3rI)pyridof2 3-
dlpyrimidine
The title compound was prepared by treating the compound of Example 134 with
hydrogen peroxide in acetic acid according to standard procedures. IR
(microscope) 3486,
1579, 1552, 1353, 1121. 1020 cm-1; MS m/z 479 (M+H)+.
-120-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
Example 339
4-amino-5-l3-bromophen_vl)-7-(6-(N-l2-h~yethoxveth~rl)amino)-3-~,
'~dyl)pyridof2.3
dlgvrimidine
Step 339a. 1'.1'-dicvano-3-bromostyrene
The title compound was prepared by condensation of 3-bromobenzaldehyde with
malononitrile and Mg0 in dichloromethane by the standard procedure of
Broekhuis et al.
(Recl. J. R. Neth. Chem. Soc., 99: 6-12 (1980)).
Sten 339b. S-acetvl-2-(N-(2-ethox~yl)amino)pyridine
The title compound was prepared by the reaction of 5-acetyl-2-chloropyridine
with
2-ethoxyethylamine in refluxing ethanol.
Step 339c. 4-(3-bromophenyl)-3-cyano-6-hl-(2-ethoxyeth~lamino~pvridine-2-amine
t5 The title compound was prepared by condensation of I',1'-dicyano-2-
bromostyrene
with 5-acetyl-2-morpholinylpyridine and ammonium acetate in dichloroethane at
reflux.
After the reaction was complete (TLC), the mixture was cooled, and the solvent
was
removed. The residue was triturated with methanol to give the product.
Step 339d. 4-amino-5-(2-bromovhenyl,~-7-(6-(N-(2-ethoxyethvl)amino)-3-
pvridvl)pvridof2,3-dlgyrimidine
A sample of the compound from Step 239d was treated according to the procedure
of
Example 233d to give the title compound. IR (microscope) 3301, 1610, 1579,
1543, 1346,
1304, 1120 cm-1; MS m/z 481 (M+H)+.
Example 340
4-amino-~-(3-bromophenyl)-7-(6-(N-(2-hydroxvethox3rethyl)-N-formylamino)-3
pyridvl)pyridof 2,3-dlpyrimidine
This compound was isolated by chromatography as a product of the reaction
described in Example 239 Step d. IR (microscope) 3306, 1679, 1596, 1577, 1548,
1493,
1352, 1125 cm-I; MS m/z 509 (M+H)+.
-121-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
Example 341
4-amino-5-(3-bromophenyl)-7-(6-(N-l2-h droxyethoxyethvl)-3:gvridvl-N
oxide)gvridof2,3-dlQvrimidine
The title compound was prepared by treating the compound of Example 341 with
hydrogen peroxide in acetic acid according to standard procedures. IR
(microscope) 3296,
1628, 1560, 1411, 1353 cm-l; MS m/z 497 (M+H)+.
Example 342
4-amino-5-(3-bromophenvl)-7-(6-(3-hvdroxv)morpholinvl)-3-p ry-yl)pyridof2 3
d~pyrimidine
The title compound was prepared from the compound of Example 328 by reduction
with (Lithium Aluminum Hydride, and subsequent workup acccording to standard
procedures). IR {microscope) 3349, 1510, 1116 cm-l; MS m/z 478 (M+H)+.
Example 343
1-(5-(4-amino-5-(3-bromo~henyl)pyridof2 3-dlnvrimidin-7-yl)-2-~ 1~-
'id~piperidine 4
phosphate, disodium salt
The title compound was prepared from the compound of Example 342 by treatment
with POCl3, and subsequent workup acccording to standard procedures. IR
(microscope)
3498, 1500, 1444 cm-1; MS m/z 5~6 (M+H)+.
Example 344
4-amino-5-f3-bromophenyl)-7-(6-(2-h dv rox,vlmoroholinvl)-3-pyridvl)pyridof2 3
dlpvrimidine
The title compound was prepared from the compound of Example 339 by oxidation
of the free hydroxy group to an aldehyde with TEMPO reagent. During workup of
the
mixture, the compound self-condensed to give the title compound. IR
(microscope) cm-1;
MS m/z 492 (M+CH30H-H20)+.
3o Example 345
4-amino-5-l3-bromonhenvl)-7-(4-methvlenvlnineridinvi)-3-nvridvl)pvridof2 3-
dlnvrimidine
The title compound was prepared from the compound of Example 328 by treatment
with methyl triphenylphosphine bromide at -78 °C in DMSO. After
quenching and warming
the mixture to room temperature, the title compound was extracted, then
purified by
chromatography. IR (microscope) 3055, 1602, 1559, 1508, 1440, 1344, 1174 cm-l;
MS
m/z 473 (M+H)+.
-122-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
Examrle 346
4-amino-5-(3-brom~yl)-7-(4-hvdroxy-4-(~drox~et)~vl)p3ncridinvl)-3
wridvl)pvridof 2.3-dlpvrimidine
The title compound was prepared from the compound of Example 345 by treatment
with OsO~ in DMSO at room temperature. After quenching, the title compound was
extracted, then purified by chromatography. IR (microscope) 3304, 1603, 1580,
1557,
1509, 1352, 1241 cm-l; MS m/z 507 (M+H)+.
Example 347
4-amino-5-(3-bromophenyl)-7-(6-(4.4-ethylenedioxy~iperidin l~~,vridvl)pyridof
2 3-
dlpvrimidine
Sten 347a. 1.1-dicyano-3-cyclohexvlethene
The 1,1-dicyano-3-cyclohexylethene was prepared according to the method of
Moison, et al. (Tetrahedron (1987), 43:537-542) by treating cyclohexane
carboxaldehyde
with malononitrile in the presence of finely powdered magnesium oxide in
dichloromethane.
Sten 347b. 2-acetvl-5-14,4-ethylenedioxypineridinvl)pyridine
A sample of 2-acetyl-5-chloropyridine was treated in refluxing ethanol with 4-
piperidinone ethylene ketal to give the title compound.
tep 347c. 4-amino-5-cvclohexvl-7-(6-(4 4-ethvlenedioxwineridinvll-3-
p n~'dvl)pvridof2.3-dlgvrimidine
Following the procedures of example 339 Step c, except substituting the
compounds
from Step 347a and 347b for the compounds of Steps 339a and 339b, and carrying
the
product forward according to the procedure of example 339 Step d, the title
compound was
prepared. IR (microscope) 2929, 1604, 1585, 1557, 1514, 1426, 1344, 1238, 1106
cm-l;
MS m/z 447 (M+H)+.
Example 348
4-amino-5-cvclohexyl-7-(6-(4-oxo-pi eridinyl)-3~yridvl)pyridof2 3-dlgvrimidine
The title compound was prepared from the compound of Example 347 by treatment
with dilute HCl in ethanol. The title compound was purified by chromatography.
IR
(microscope) 2928, 1715, 1603, 1585, 1559, 1507, 1344, 1226 cm-l; MS mlz 403
(M+H)+.
-123-
CA 02286909 1999-10-12
WO 98/46605 PCT/US98/07207
Example 349
4-amino-5-cvclohe~vl-7-l6-(4-methvlen~~ ridinvl)-3-g ry~'id",yl)nvridof2 3-
dlgmirnidine
The title compound was prepared from the compound of Example 348 by treatment
with methyl triphenylphosphine bromide at -78 °C in DMSO. After
quenching and warming
the mixture to room temperature, the title compound was extracted, then
purified by
chromatography. IR (microscope) 2929, 1604, 1584, 1557, 1506, 1342, 1239 cm-1;
MS
m/z 401 (M+H)+.
Example 350
4-N-liminomethyl)amino-5-cvclohexyl-7-(6-dimethylamino-3-pvrid~~vridof2 3-
dlpyrimidine
This compound was isolated from the reaction mixture of Example 293 as a side
product: IR (cm-1) 3289, 3089, 2930, 2841, 1674, 1606, 1559, 1531. LRMS [M+H]+
m/z 376.
-124-