Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02288055 1999-10-22
WO 98/47473 1 PCT/EP98/02243
Use of Unsaturated Aldehydes in Dying Fibers Containing Keratin
This invention relates to the use of unsaturated aldehydes for
coloring keratin-containing fibers, more especially human hair.
In general, keratin-containing fibers, for example hair, wool or pelts,
are dyed either with substantive dyes or with oxidation dyes which are
formed by oxidative coupling of one or more primary intermediates with one
another or with one or more secondary intermediates. Primary and
secondary intermediates are also known as oxidation dye precursors.
The primary intermediates normally used are primary aromatic
amines containing another free or substituted or amino group in the para or
ortho position, diaminopyridine derivatives, heterocyclic hydrazones, 4
aminopyrazolone derivatives and 2,4,5,6-tetraaminopyrimidine and
derivatives thereof.
Special representatives are, for example, p-phenylenediamine, p-
toluylenediamine, p-aminophenol, o-aminophenol, 1-(2'-hydroxyethyl)-2,5-
diaminobenzene, N,N-bis-(2-hydroxyethyl)-p-phenylenediamine, 2-(2,5-di-
aminophenoxy)-ethanol, 1-phenyl-3-carboxyamido-4-amino-5-pyrazolone,
4-amino-3-methylphenol, 2,4,5,6-tetraaminopyrimidine, 2-hydroxy-4,5,6-tri-
aminopyrimidine, 4-hydroxy-2,5,6-triaminopyrimidine, 2,4-dihydroxy-5,6-
diaminopyrimidine, 2-dimethylamino-4,5,6-triaminopyrimidine, 2-hydroxy-
ethylaminomethyl-4-aminophenol, 4,4'-diaminodiphenylamine, 4-amino-3-
fluorophenol, 2-aminomethyl-4-aminophenol, 2-hydroxymethyl-4-amino-
phenol, bis-(2-hydroxy-5-aminophenyl)-methane, 1,4-bis-(4-aminophenyl)-
diazacycloheptane, 1,3-bis-(N-(2-hydroxyethyl)-N-(4-aminophenylamino))-
2-propanol, 4-amino-2-(2-hydroxyethoxy)-phenol and 4,5-diaminopyrazole
derivatives according to EP 0 740 741 or WO 94108970, for example 4,5-
diamino-1-(2'-hydroxyethyl)-pyrazole.
The secondary intermediates used are generally m-phenylene-
CA 02288055 1999-10-22
_ WO 98/47473 2 PCT/EP98/02243
diamine derivatives, naphthols, resorcinol and resorcinol derivatives,
pyrazolones and m-aminophenols. Particularly suitable secondary inter-
mediates are 1-naphthol, pyrogallol, 1,5-, 2,7- and 1,7-dihydroxynaphtha-
lene, o-aminophenol, 5-amino-2-methylphenol, m-aminophenol, resorcinol,
resorcinol monomethyl ether, m-phenylenediamine, 1-phenyl-3-methyl-5-
pyrazolone, 2,4-dichloro-3-aminophenol, 1,3-bis-(2,4-diaminophenoxy)-
propane, 4-chlororesorcinol, 2-chloro-6-methyl-3-aminophenol, 2-methyl
resorcinol, 5-methyl resorcinol, 2,5-dimethyl resorcinol, 2,6-dihydroxypyri-
dine, 2,6-diaminopyridine, 2-amino-3-hydroxypyridine, 2,6-dihydroxy-3,4-
diaminopyridine, 3-amino-2-methylamino-6-methoxypyridine, 4-amino-2-
hydroxytoluene, 2,6-bis-(2-hydroxyethylamino)-toluene, 2,4-diamino-
phenoxyethanol, 1-methoxy-2-amino-4-(2-hydroxyethylamino)-benzene, 2-
methyl-4-chloro-5-aminophenol, 6-methyl-1,2,3,4-tetrahydroquinoxaline,
3,4-methylenedioxyphenol, 3,4-methylenedioxyaniline, 2,6-dimethyl-3-
aminophenol, 3-amino-6-methoxy-2-methylaminophenol, 2-hydroxy-4-
aminophenoxyethanol, 2-methyl-5-(2-hydroxyethylamino)-phenol and 2,6-
dihydroxy-3,4-dimethyl pyridine.
Although intensive colors with good fastness properties can be
obtained with oxidation dyes, the color is generally developed under the
influence of oxidizing agents, such as H202 for example, which in some
cases can result in damage to the fibers. In addition, some oxidation dye
precursors or certain mixtures of oxidation dye precursors can occasionally
have a sensitizing effect in people with sensitive skin. Although
substantive dyes are applied under more moderate conditions, their
disadvantage is that, in many cases, the colors obtained have inadequate
fastness properties.
DE-AS 28 30 497 discloses hair colorants which contain
salicylaldehyde in combination with oxidation dye precursors, for example
2,5-diaminotoluene, 4-aminophenol or 2,4-diaminotoluene, but which can
have a more or less high sensitizing potential (in combination with the
CA 02288055 1999-10-22
WO 98/47473 3 PCT/EP98/02243
hydrogen peroxide additionally present). DE AS 29 32 498 also discloses
H202-containing hair colorants which contain benzaldehydes, for example
2-hydroxy-3-methoxybenzaldehyde or 4-hydroxy-3-methoxybenzaldehyde,
in combination with oxidation dye precursors. US patents 5,034,014 and
5,199,954 describe hair color formulation examples which contain p-
dimethylaminobenzaldehyde or p-dimethylaminocinnamaldehyde, for
example in combination with the sensitizing p-phenylenediamine.
US 4,391,603 relates to oxidant-free hair colorants which contain
substituted benzaldehydes. The hair colorants disclosed in this document
are substantive colorants with which the shades, depths of color and color
fastness values of hair colorants containing oxidation dyes precursors
cannot be achieved.
The use of the aromatic aldehydes described in detail hereinafter for
coloring keratin-containing fibers was not previously known.
The problem addressed by the present invention was to provide
colorants for keratin fibers, more especially human hair, which would be at
least equivalent in quality to conventional oxidation hair dyes in regard to
depth of color, grey coverage and fastness properties, but which would not
necessarily have to contain oxidizing agents, such as H202 for example. In
addition, the colorants according to the invention would have very little, if
any, sensitizing potential.
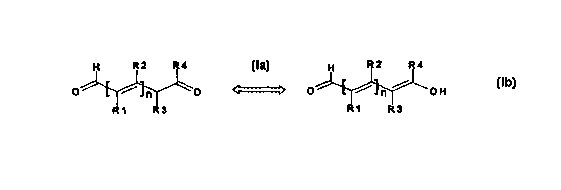
The present invention relates to the use of unsaturated aldehydes
corresponding to the following tautomeric resonance formulae la and Ib:
H R2 R4 H .R2 R4
O ~ ~ O ~ 'O ~ ~ ~ OH
R1 R3 R1 R3
(la) (Ib)
~ in which R', R2, R3 and R4 independently of one another represent
CA 02288055 1999-10-22
_ WO 98/47473 4 PCT/EP98/02243
hydrogen, halogen, a C~~ alkoxy, C» alkyl, aryl or C~~-alkoxy-C~~-alkyl
group and n is the number 1 or 2; R' and R2, R' and R3, R2 and R3 and R2
and R4 can form a 5- to 7-membered ring together with the rest of the
molecule where n = 1,
and the corresponding mono-, bis- or w-alkoxyacetals for coloring keratin-
containing fibers, more especially human hair.
It has surprisingly been found that the aldehydes corresponding to
formulae la and Ib are eminently suitable for coloring keratin-containing
fibers, even in the absence of oxidizing agents. They give colors with
excellent brilliance and depth of color and lead to a wide variety of shades.
In principle, however, oxidizing agents may be still be present.
In the context of the invention, keratin-containing fibers are
understood to include wool, pelts, feathers and, in particular, human hair.
In principle, however, the colorants according to the invention may also be
used to color other natural fibers such as, for example, cotton, jute, sisal,
linen or silk, modified natural fibers such as, for example, regenerated
cellulose, nitro, alkyl or hydroxyalkyl or acetal cellulose and synthetic
fibers
such as, for example, polyamide, polyacrylonitrile, polyurethane and
polyester fibers.
The unsaturated aldehydes corresponding to formulae la and Ib are
preferably selected from the group consisting of glutaconaldehyde, 1-
formyl-3-hydroxymethylene-1-cyclohexene, 1-formyl-3-hydroxymethylene-
1-cycloheptene and 7-hydroxy-2,4,6-heptatrienal and their substituted
derivatives and physiologically compatible salts thereof, more particularly
the alkali metal and ammonium salts, the tetrabutyl ammonium salts and
Na salts and mixtures thereof being particularly preferred.
The compounds corresponding to formula la and Ib are known from
the literature or are commercially available.
The aldehydes corresponding to formulae la and Ib are present in
CA 02288055 1999-10-22
WO 98/47473 5 PCT/EP98/02243
the colorants in quantities of preferably 0.03 to 65 mmol and more
preferably 1 to 40 mmol, based on 100 g of the colorant as a whole. A
single compound with the formula la or Ib or a mixture of several
compounds may be used. They may be used as substantive dyes or in the
presence of compounds which intensify the coloring effect of the aldehydes
used in accordance with the invention, such as oxidation dye precursors.
Colorants containing aldehydes of formula la or Ib as their sole
coloring component are preferably used for colors in the yellow and red
range. Colors with even greater brilliance and further improved fastness
properties, above all in the yellow, orange, brown and black range and in
the red and blue range, are obtained when the aldehydes of formulae la
and Ib are used together with compounds containing a primary or
secondary amino group, for example aniline derivatives, with nitrogen-
containing heterocyclic compounds, for example primary heteroaromatic
amines, aromatic hydroxy compounds or CH-active compounds. These
are, on the one hand, compounds which, on their own, have only a weak
coloring effect on keratin-containing fibers and which only produce brilliant
colors in conjunction with the aldehydes of formulae la and Ib. However,
they also include compounds which are already used as oxidation dye
precursors.
The present invention also relates to a preparation for coloring
keratin-containing fibers, more especially human hair, which contains
A. at least one unsaturated aldehyde corresponding to formula la or Ib and
B. at least one compound containing a primary or secondary amino group
or hydroxy group selected from primary or secondary aliphatic or
aromatic amines, nitrogen-containing heterocyclic compounds, amino
acids, oligopeptides made up of 2 to 9 amino acids and aromatic
hydroxy compounds and/or at least one CH-active compound.
CA 02288055 1999-10-22
_ WO 98/47473 6 PCT/EP98/02243
The above-mentioned compounds of component B may be used in a
quantity of 0.03 to 65 mmol and are preferably used in a quantity of 1 to 40
mmol, based on 100 g of the colorant as a whole.
Several different aldehydes of formulae la and Ib may even be used
together in the colorants. Similarly, several different compounds of
component B may also be used together. This embodiment also
encompasses the use of substances which represent reaction products of
unsaturated aldehydes corresponding to formula la and Ib with the
compounds B.
Suitable compounds containing a primary or secondary amino group
are, for example, primary aromatic amines, such as N,N-dimethyl-, N,N-di-
ethyl-, N-(2-hydroxyethyl)-N-ethyl-, N,N-bis-(2-hydroxyethyl)-, N-(2-meth-
oxyethyl)-, 2,3-, 2,4-, 2,5-dichloro-p-phenylenediamine, 2-chloro-p-
phenylenediamine, 2,5-dihydroxy-4-morpholinoaniline dihydrobromide, 2-,
3-, 4-aminophenol, 2-aminomethyl-4-aminophenol, 2-hydroxymethyl-4-
aminophenol, o-, m-, p-phenylenediamine, o-, m-toluylenediamine, 2,5-
diaminotoluene, -phenol, -phenethol, 4-amino-3-methylphenol, 2-(2,5-di-
aminophenyl)-ethanol, 2,4-diaminophenoxyethanol, 2-(2,5-diamino-
phenoxy)-ethanol, 4-methylamino-, 3-amino-4-(2'-hydroxyethyloxy)-, 3,4-
methylenediamino-, 3,4-methylenedioxyaniline, 3-amino-2,4-dichloro-, 4-
methylamino-, 2-methyl-5-amino-, 3-methyl-4,-amino-, 2-methyl-5-(2-
hydroxyethylamino)-, 6-methyl-3-amino-2-chloro-, 2-methyl-5-amino-4-
chloro-, 5-(2-hydroxyethylamino)-4-methoxy-2-methylphenol, 1,3-diamino-
2,4-dimethoxybenzene, 2-, 3-, 4-aminobenzoic acid, -phenylacetic acid,
2,3-, 2,4-, 2,5-, 3,4-, 3,5-diaminobenzoic acid, 4-, 5-aminosalicylic acid, 3-
amino-4-hydroxy-, 4-amino-3-hydroxybenzoic acid, 2-, 3-, 4-amino-
benzenesulfonic acid, 3-amino-4-hydroxybenzenesulfonic acid, 4-amino-3-
hydroxynaphthalene-1-sulfonic acid, 6-amino-7-hydroxynaphthalene-2-
sulfonic acid, 7-amino-4-hydroxynaphthalene-2-sulfonic acid, 4-amino-5-
hydroxynaphthalene-2,7-disulfonic acid, 3-amino-2-naphthoic acid, 3-
CA 02288055 1999-10-22
WO 98/47473 7 PCT/EP98/02243
aminophthalic acid, 5-aminoisophthalic acid, 1,3,5-, 1,2,4-triaminobenzene,
1,2,4,5-tetraaminobenzene, 2,4,5-triaminophenol, pentaaminobenzene,
hexaaminobenzene, 2,4,6-triaminoresorcinol, 4,5-diaminopyrocatechol,
4,6-diaminopyrogallol, 3,5-diamino-4-hydroxypyrocatechol, aromatic
anilines and phenols containing another aromatic radical corresponding to
formula II:
R5 R9
R6 . R9
X
R7
10 (II)
(III
in which R5 is a hydroxy group or an amino group which may be substituted
by C» alkyl, C~.~ hydroxyalkyl, C~.~ alkoxy or C~.~ alkoxy-C~~-alkyl groups,
R6, R', R8, R9 and R'° represent hydrogen, a hydroxy group or an
amino
group which may be substituted by C,.~ alkyl, C~~ hydroxyalkyl, C,~ alkoxy,
C» aminoalkyl or C~.~ alkoxy-C~.~-alkyl groups or a sulfonic acid group and
X is a direct bond, a saturated or unsaturated optionally hydroxy-
substituted carbon chain containing 1 to 4 carbon atoms, a carbonyl,
sulfonyl or amino group, an oxygen or sulfur atom or a group
corresponding to formula III:
Z-(CH2-Q-CHZ-Z')o (I I I )
in which
Q is a direct bond, a CH2 or CHOH group,
Z and Z' independently of one another represent an oxygen atom, an NR"
group, where R" is hydrogen, a C~.~ alkyl or a hydroxy-C~.~-alkyl group, the
group O-(CH2)p-NH or NH-(CH2)p~-O, where p and p' = 2 or 3, and
CA 02288055 1999-10-22
_ WO 98/47473 8 PCT/EP98/02243
o is a number of 1 to 4,
such as for example 4,4'-diaminostilbene, 4,4'-diaminostilbene-2,2'-disul-
fonic acid monosodium or disodium salt, 4,4'-diaminodiphenyl methane,
-sulfide, -sulfoxide, -amine, 4,4'-diaminodiphenylamine-2-sulfonic acid, 4,4'-
diaminobenzophenone, -diphenyl ether, 3,3',4,4'-tetraaminodiphenyl,
3,3'4,4'-tetraaminobenzophenone, 1,3-bis-(2,4-diaminophenoxy)-propane,
1,8-bis-(2,5-diaminophenoxy)-3,6-dioxaoctane, 1,3-bis-(4-aminophenyl
amino)-propane, -2-propanol, 1,3-bis-[N-(4-aminophenyl)-2-hydroxyethyl
amino]-2-propanol, N,N-bis-(2-(4-aminophenoxy)-ethyl]-methylamine, N
phenyl-1,4-phenylenediamine.
The compounds mentioned above may be used both in free form
and in the form of their physiologically compatible salts, more especially as
salts of inorganic acids, such as hydrochloric acid or sulfuric acid.
Suitable nitrogen-containing heterocyclic compounds are, for exam
ple, 2-, 3-, 4-amino-, 2-amino-3-hydroxy-, 2,6-diamino-, 2,5-diamino-, 2,3
diamino-, 2-dimethylamino-5-amino-, 2-methylamino-3-amino-6-methoxy-,
2,3-diamino-6-methoxy-, 2,6-dimethoxy-3,5-diamino-, 2,4,5-triamino-, 2,6
dihydroxy-3,4-dimethyl pyridine, 2,4-dihydroxy-5,6-diamino-, 4,5,6-tri
amino-, 4-hydroxy-2,5,6-triamino-, 2-hydroxy-4,5,6-triamino-, 2,4,5,6-tetra
amino-, 2-methylamino-4,5,6-triamino-, 2,4-, 4,5-diamino-, 2-amino-4-
methoxy-6-methyl pyrimidine, 3,5-diaminopyrazole, -1,2,4-triazole, 3-
amino-, 3-amino-5-hydroxypyrazole, 2-, 3-, 8-aminoquinoline, 4-amino-
quinaldine, 2-, 6-aminonicotinic acid, 5-aminoisoquinoline, 5-, 6-amino-
indazole, 5-, 7-aminobenzimidazole, -benzothiazole, 2,5-dihydroxy-4-
morpholinoaniline and indole and indoline derivatives, such as 4-, 5-, 6-, 7-
aminoindole, 5,6-dihydroxyindole, 5,6-dihydroxyindoline and 4-hydroxy-
indoline. The compounds mentioned above may be used both in free form
and the form of their physiologically compatible salts, for example as salts
of inorganic acids, such as hydrochloric acid or sulfuric acid.
Suitable amino acids are any naturally occurring and synthetic
CA 02288055 1999-10-22
WO 98/47473 9 PCT/EP98/02243
amino acids, for example the amino acids obtainable by hydrolysis from
vegetable or animal proteins, for example collagen, keratin, casein, elastin,
soya protein, wheat gluten or almond protein. Both acidic and alkaline
amino acids may be used. Preferred amino acids are arginine, histidine,
tyrosine, phenyl alanine, DOPA (dihydroxyphenyl alanine), ornithine, lysine
and tryptophane.
The oligopeptides may be naturally occurring or synthetic oligo-
peptides and the oligopeptides present in polypeptide or protein
hydrolyzates providing they are sufficiently soluble in water for use in the
colorants according to the invention. Examples of such polypeptides are
glutathione and the oligopeptides present in the hydrolyzates of collagen,
keratin, casein, elastin, soya protein, wheat gluten or almond protein.
These oligopeptides are preferably used together with compounds
containing a primary or secondary amino group or with aromatic hydroxy
compounds.
Suitable aromatic hydroxy compounds are, for example, 2-, 4-, 5-
methyl resorcinol, 2,5-dimethyl resorcinol, resorcinol, 3-methoxyphenol,
pyrocatechol, hydroquinone, pyrogallol, phloroglucinol, hydroxyhydro-
quinone, 2-, 3-, 4-methoxy-, 3-dimethylamino-, 2-(2-hydroxyethyl)-, 3,4-
methylenedioxyphenol, 2,4-, 3,4-dihydroxybenzoic acid, -phenylacetic acid,
gallic acid, 2,4,6-trihydroxybenzoic acid, -acetophenone, 2-, 4-chloro-
resorcinol, 1-naphthol, 1,5-, 2,3-, 2,7-dihydroxynaphthalene, 6-dimethyl-
amino-4-hydroxy-2-naphthalene sulfonic acid, 3,6-dihydroxy-2,7-naphtha-
lene sulfonic acid.
Examples of CH-active compounds are 1,2,3,3-tetramethyl-3H-
indolium iodide, 1,2,3,3-tetraamethyl indolium-p-toluene sulfonate, 1,2,3,3-
tetramethyl-3H-indolium methane sulfonate, 2,3-dimethylbenzothiazolium
iodide, 2,3-dimethylbenzothiazolium-p-toluene sulfonate, rhodanine, rhoda-
nine-3-acetic acid, 1-ethyl-2-quinaldinium iodide, 1-methyl-2-quinaldinium
iodide, barbituric acid, thiobarbituric acid, 1,3-dimethyl thiobarbituric
acid,
CA 02288055 1999-10-22
_ WO 98/47473 10 PCT/EP98/02243
diethyl thiobarbituric acid, oxindole, 3-indoxyl acetate, coumaranone and 1-
methyl-3-phenyl-2-pyrazolinone.
In one particularly preferred embodiment, component B is selected
from the group consisting of N-(2-hydroxyethyl)-N-ethyl-, 2-chloro-p-phenyl-
enediamine, N,N-bis-(2-hydroxyethyl)-p-phenylenediamine, 4-aminophenol,
p-phenylenediamine, 2-(2,5-diaminophenyl)-ethanol, 2,5-diaminotoluene,
3,4-methylenedioxyaniline, 3-amino-2,4-dichloro-, 2-methyl-5-amino-, 3-
methyl-4-amino-, 2-methyl-5-(2-hydroxyethylamino)-, 2-methyl-5-amino-4-
chloro-, 6-methyl-3-amino-2-chloro-, 2-aminomethyl-4-aminophenol, 2-
hydroxymethyl-4-aminophenol, 3,4-methylenedioxyphenol, 3,4-diamino-
benzoic acid, 2,5-diamino-, 2-dimethylamino-5-amino-, 3-amino-2-methyl-
amino-6-methoxy-, 2,3-diamino-6-methoxy-, 3,5-diamino-2,6-dimethoxy-,
2,6-dihydroxy-3,4-dimethyl pyridine, 2-hydroxy-4,5,6-triamino-, 4-hydroxy-
2,5,6-triamino-, 2,4,5,6-tetraamino-, 2-methylamino-4,5,6-triaminopyrimi-
dine, 3,5-diaminopyrazole, 3-amino-5-hydroxypyrazole, 5,6-dihydroxyindole
and 5,6-dihydroxyindoline and the physiologically compatible salts thereof
formed with preferably inorganic acids.
Oxidizing agents, for example H202, need not present. However, it
may be desirable in some cases to add hydrogen peroxide or other
oxidizing agents to the preparations according to the invention to obtain
shades which are lighter than the keratin-containing fibers to be colored.
Oxidizing agents are generally used in a quantity of 0.01 to 6% by weight,
based on the solution applied. A preferred oxidizing agent for human hair
is H202.
The colorants according to the invention produce a broad range of
shades in the range from yellow-orange to brown-black; their fastness
properties are good and the sensitizing potential very low.
In another preferred embodiment, the colorants according to the
invention contain typical substantive dyes, for example from the group of
nitrophenylenediamines, nitroaminophenols, azo dyes, anthraquinones or
CA 02288055 1999-10-22
. WO 98/47473 11 PCT/EP98/02243
indophenols, in addition to the compounds present in accordance with the
invention and optionally other oxidation dye precursors in order further to
modify the color tones. Preferred substantive dyes are the compounds
known under the International names or commercial names of HC Yellow
2, HC Yellow 4, HC Yellow 5, HC Yellow 6, Basic Yellow 57, Disperse
Orange 3, HC Red 3, HC Red BN, Basic Red 76, HC Blue 2, HC Blue 12,
Disperse Blue 3, Basic Blue 99, HC Violet 1, Disperse Violet 1, Disperse
Violet 4, Disperse Black 9, Basic Brown 16 and Basic Brown 17 and also 4-
amino-2-nitrodiphenylamine-2'-carboxylic acid, 6-nitro-1,2,3,4-tetrahydro-
quinoxaline, hydroxyethyl-2-nitrotoluidine, picramic acid, 2-amino-6-chloro-
4-nitrophenol, 4-ethylamino-3-nitrobenzoic acid and 2-chloro-6-ethylamino-
1-hydroxy-4-nitrobenzene. The preparations according to the invention in
this embodiment contain the substantive dyes in a quantity of, preferably,
0.01 to 20% by weight, based on the colorant as a whole.
In addition, the preparations according to the invention may also
contain naturally occurring dyes such as, for example, henna red, henna
neutral, henna black, camomile blossom, sandalwood, black tea, black
alder bark, sage, logwood, madder root, catechu, sedre and alkanet.
Other dye components present in the colorants according to the
invention may be indoles and indolines and physiologically compatible salts
thereof. Preferred examples are 5,6-dihydroxyindole, N-methyl-5,6-di-
hydroxyindole, N-ethyl-5,6-dihydroxyindole, N-propyl-5,6-dihydroxyindole,
N-butyl-5,6-dihydroxyindole, 6-hydroxyindole, 6-aminoindole and 4-
aminoindole; 5,6-dihydroxyindoline, N-methyl-5,6-dihydroxyindoline, N-
ethyl-5,6-dihydroxyindoline, N-propyl-5,6-dihydroxyindoline, N-butyl-5,6-
dihydroxyindoline, 6-hydroxyindoline, 6-aminoindoline and 4-aminoindoline.
With regard to the dyes suitable for use in the hair coloring and tinting
formulations according to the invention, reference is also specifically made
to Ch. Zviak's work The Science of Hair Care, Chapter 7 (pages 248-250;
Substantive Dyes) and Chapter 8, pages 264-267; Oxidation Dye
CA 02288055 1999-10-22
WO 98/47473 12 PCT/EP98/02243
Precursors), published as Vol. 7 of the Series "Dermatology" (Editors: Ch.
Culnan and H. Maibach), Marcel Dekker Inc., New York/Basel, 1986 and to
the "Europ~ische Inventar der Kosmetik-Rohstoffe" published by the
Europ~ische Gemeinschaft and available in diskette form from the
Bundesverband Deutscher Industrie- and Handelsunternehmen fur
Arzneimittel, Reformwaren and Kiirperpflegemittel e.V., Mannheim,
Germany.
The compounds of component B and optionally other oxidation dye
precursors or the substantive dyes present, if any, do not have to be single
compounds. Instead, the hair colorants according to the invention - due to
the processes used for producing the individual dyes - may contain small
quantities of other components providing they do not adversely affect the
coloring result or have to be ruled out for other reasons, for example
toxicological reasons.
The colorants according to the invention produce intensive colors
even at physiologically compatible temperatures of <45°C. Accordingly,
they are particularly suitable for coloring human hair. For application to
human hair, the colorants are normally incorporated in a water-containing
cosmetic carrier. Suitable water-containing cosmetic carriers are, for
example, creams, emulsions, gels or even surfactant-containing foaming
solutions, for example shampoos or other formulations suitable for
application to the keratin-containing fibers. If necessary, the colorants may
even be incorporated in water-free carriers.
The colorants according to the invention may also contain any of the
known active substances, additives and auxiliaries typical of such
formulations. In many cases, the colorants contain at least one surfactant,
both anionic and zwitterionic, ampholytic, nonionic and cationic surfactants
being suitable in principle. In many cases, however, it has been found to
be of advantage to select the surfactants from anionic, zwitterionic or
nonionic surfactants.
CA 02288055 1999-10-22
WO 98/47473 13 PCT/EP98/02243
Suitable anionic surfactants for the preparations according to the
invention are any anionic surface-active substances suitable for use on the
human body: Such substances are characterized by a water-solubilizing
anionic group such as, for example, a carboxylate, sulfate, sulfonate or
phosphate group and a lipophilic alkyl group containing around 10 to 22
carbon atoms. In addition, glycol or polyglycol ether groups, ester, ether,
amide groups and hydroxyl groups may also be present in the molecule.
The following are examples of suitable anionic surfactants - in the form of
the sodium, potassium and ammonium salts and the mono-, di- and
trialkanolammonium salts containing 2 or 3 carbon atoms in the alkanol
group:
- linear fatty acids containing 10 to 22 carbon atoms (soaps),
- ether carboxylic acids corresponding to the formula R-O-(CH2-CH20)X-
CH2-COOH, in which R is a linear alkyl group containing 10 to 22
carbon atoms and x = 0 or 1 to 16,
- acyl sarcosides containing 10 to 18 carbon atoms in the acyl group,
- acyl taurides containing 10 to 18 carbon atoms in the acyl group,
- acyl isethionates containing 10 to 18 carbon atoms in the acyl group,
- sulfosuccinic acid mono- and dialkyl esters containing 8 to 18 carbon
atoms in the alkyl group and sulfosuccinic acid monoalkyl polyoxyethyl
esters containing 8 to 18 carbon atoms in the alkyl group and 1 to 6
oxyethyl groups,
- linear alkane sulfonates containing 12 to 18 carbon atoms,
- linear a-olefin sulfonates containing 12 to 18 carbon atoms,
- a-sulfofatty acid methyl esters of fatty acids containing 12 to 18 carbon
atoms,
- alkyl sulfates and alkyl polyglycol ether sulfates corresponding to the
formula R-O(CH2-CH20)X-S03H, in which R is a preferably linear alkyl
group containing 10 to 18 carbon atoms and x = 0 or 1 to 12,
CA 02288055 1999-10-22
. WO 98/47473 14 PCT/EP98/02243
- mixtures of surface-active hydroxysulfonates according to DE-A-37 25
030,
- sulfated hydroxyalkyl polyethylene and/or hydroxyalkylene propylene
glycol ethers according to DE-A-37 23 354,
- sulfonates of unsaturated fatty acids containing 12 to 24 carbon atoms
and 1 to 6 double bonds according to DE-A-39 26 344,
- esters of tartaric acid and citric acid with alcohols in the form of
addition
products of around 2 to 15 molecules of ethylene oxide and/or
propylene oxide with fatty alcohols containing 8 to 22 carbon atoms.
Preferred anionic surfactants are alkyl sulfates, alkyl polyglycol ether
sulfates and ether carboxylic acids containing 10 to 18 carbon atoms in the
alkyl group and up to 12 glycol ether groups in the molecule and, in
particular, salts of saturated and, more particularly, unsaturated C~22
carboxylic acids, such as oleic acid, stearic acid, isostearic acid and
palmitic acid.
In the context of the invention, zwitterionic surfactants are surface-
active compounds which contain at least one quaternary ammonium group
and at least one -COO~-~ or -S03~-~ group in the molecule. Particularly
suitable zwitterionic surfactants are the so-called betaines, such as N-alkyl-
N,N-dimethyl ammonium glycinates, for example cocoalkyl dimethyl
ammonium glycinate, N-acylaminopropyl-N,N-dimethyl ammonium
glycinates, for example cocoacylaminopropyl dimethyl ammonium
glycinate, and 2-alkyl-3-carboxymethyl-3-hydroxyethyl imidazolines
containing 8 to 18 carbon atoms in the alkyl or acyl group and
cocoacylaminoethyl hydroxyethyl carboxymethyl glycinate. A preferred
zwitterionic surfactant is the fatty acid amide derivative known by the CTFA
name of Cocamidopropyl Betaine.
Ampholytic surfactants are surface-active compounds which, in
addition to a .Cs_~8 alkyl or acyl group, contain at least one free amino
group
and at least one -COOH or -S03H group in the molecule and which are
CA 02288055 1999-10-22
WO 98/47473 15 PCT/EP98/02243
capable of forming inner salts. Examples of suitable ampholytic surfactants
are N-alkyl glycines, N-alkyl propionic acids, N-alkyl aminobutyric acids, N-
alkyl iminodipropionic acids, N-hydroxyethyl-N-alkyl amidopropyl glycines,
N-alkyl taurines, N-alkyl sarcosines, 2-alkyl aminopropionic acids and alkyl
aminoacetic acids containing around 8 to 18 carbon atoms in the alkyl
group. Particularly preferred ampholytic surfactants are N-cocoalkyl amino-
propionate, cocoacyl aminoethyl aminopropionate and C~2_~8 acyl
sarcosine.
Nonionic surfactants contain, for example, a polyol group, a poly-
alkylene glycol ether group or a combination of polyol and polyglycol ether
groups as the hydrophilic group. Examples of such compounds are
- products of the addition of 2 to 30 moles of ethylene oxide and/or 0 to 5
moles of propylene oxide to linear fatty alcohols containing 8 to 22
carbon atoms, to fatty acids containing 12 to 22 carbon atoms and to
alkylphenols containing 8 to 15 carbon atoms in the alkyl group,
- 02_22 fatty acid monoesters and diesters of products of the addition of 1
to 30 moles of ethylene oxide to glycerol,
- C&22 alkyl mono- and oligoglycosides and ethoxylated analogs thereof,
- products of the addition of 5 to 60 moles of ethylene oxide to castor oil
and hydrogenated castor oil,
- products of the addition of ethylene oxide to sorbitan fatty acid esters,
- products of the addition of ethylene oxide to fatty acid alkanolamides.
Examples of cationic surfactants suitable for use in the hair
treatment formulations according to the invention are, in particular,
quaternary ammonium compounds. Preferred quaternary ammonium
compounds are ammonium halides, such as alkyl trimethyl ammonium
chlorides, dialkyl dimethyl ammonium chlorides and trialkyl methyl
ammonium chlorides, for example cetyl trimethyl ammonium chloride,
stearyl trimethyl ammonium chloride, distearyl dimethyl ammonium
chloride, lauryl dimethyl ammonium chloride, lauryl dimethyl benzyl
CA 02288055 1999-10-22
WO 98/47473 16 PCT/EP98/02243
ammonium chloride and tricetyl methyl ammonium chloride. Other cationic
surfactants suitable for use in accordance with the invention are the
quaternized protein hydrolyzates.
Also suitable for use in accordance with the invention are cationic
silicone oils such as, for example, the commercially available products Q2-
7224 (manufacturer: Dow Corning; a stabilized trimethyl silyl amodimethi-
cone), Dow Corning 929 Emulsion (containing a hydroxylamino-modified
silicone which is also known as Amodimethicone), SM-2059 (manufacturer:
General Electric), SLM-55067 (manufacturer: Wacker) and Abil~-Quat
3270 and 3272 (manufacturer: Th. Goldschmidt; diquaternary polydimethyl
siloxanes, Quaternium-80).
Alkyl amidoamines, particularly fatty acid amidoamines, such as the
stearyl amidopropyl dimethyl amine obtainable as Tego Amid~S 18, are
distinguished not only by their favorable conditioning effect, but also and in
particular by their ready biodegradability.
Quaternary ester compounds, so-called "esterquats", such as the
the dialkyl ammonium methosulfates and methyl hydroxyalkyl dialkoyl-
oxyalkyl ammonium methosulfates marketed under the trade name of
Stepantex~, are also readily biodegradable.
One example of a quaternary sugar derivative suitable for use as a
cationic surfactant is the commercially available product Glucquat~100
(CTFA name: Lauryl Methyl Gluceth-10 Hydroxypropyl Dimonium
Chloride).
The compounds containing alkyl groups used as surfactants may be
single compounds. In general, however, these compounds are produced
from native vegetable or animal raw materials so that mixtures with
different alkyl chain lengths dependent upon the particular raw material are
obtained.
The surfactants representing addition products of ethylene and/or
propylene oxide with fatty alcohols or derivatives of these addition products
CA 02288055 1999-10-22
- WO 98/47473 17 PCT/EP98/02243
may be both products with a "normal" homolog distribution and products
with a narrow homolog distribution. Products with a "normal" homolog
distribution are mixtures of homologs which are obtained in the reaction of
fatty alcohol and alkylene oxide using alkali metals, alkali metal hydroxides
or alkali metal alcoholates as catalysts. By contrast, narrow homolog
distributions are obtained when, for example, hydrotalcites, alkaline earth
metal salts of ether carboxylic acids, alkaline earth metal oxides,
hydroxides or alcoholates are used as catalysts. The use of products with
a narrow homolog distribution can be of advantage.
Other active substances, auxiliaries and additives are, for example,
- nonionic polymers such as, for example, vinyl pyrrolidone/vinyl acrylate
copolymers, polyvinyl pyrrolidone and vinyl pyrrolidone/vinyl acetate
copolymers and polysiloxanes,
- cationic polymers, such as quaternized cellulose ethers, polysiloxanes
containing quaternary groups, dimethyl diallyl ammonium chloride
polymers, acrylamide/dimethyl diallyl ammonium chloride copolymers,
dimethyl aminoethyl methacrylate/vinyl pyrrolidone copolymers quater
nized with diethyl sulfate, vinyl pyrrolidone/imidazolinium methochloride
copolymers and quaternized polyvinyl alcohol,
- zwitterionic and amphoteric polymers such as, for example, acrylamido-
propyl/trimethyl ammonium chloride/acrylate copolymers and octyl
acrylamide/methyl methacrylate/tert.butyl aminoethyl methacrylate/2-
hydroxypropyl methacrylate copolymers,
- anionic polymers such as, for example, polyacrylic acids, crosslinked
polyacrylic acids, vinyl acetate/crotonic acid copolymers, vinyl
pyrrolidone/vinyl acrylate copolymers, vinyl acetate/butyl maleate/
isobornyl acrylate copolymers, methyl vinyl ether/maleic anhydride
copolymers and acrylic acid/ethyl acrylate/N-tert.butyl acrylamide
terpolymers,
- thickeners, such as agar agar, guar gum, alginates, xanthan gum, gum
CA 02288055 1999-10-22
WO 98/47473 18 PCT/EP98/02243
arabic, karaya gum, carob bean flour, linseed gums, dextrans, cellulose
derivatives, for example methyl cellulose, hydroxyalkyl cellulose and
carboxymethyl cellulose, starch fractions and derivatives, such as
amylose, amylopectin and dextrins, clays such as, for example,
bentonite or fully synthetic hydrocolloids such as, for example, polyvinyl
alcohol,
- structurants, such as glucose and malefic acid,
- hair-conditioning compounds, such as phospholipids, for example soya
lecithin, egg lecithin and kephalins, and also silicone oils,
- protein hydrolyzates, more particularly elastin, collagen, keratin, milk
protein, soya protein and wheat protein hydrolyzates, condensation
products thereof with fatty acids and quaternized protein hydrolyzates,
- perfume oils, dimethyl isosorbide and cyclodextrins,
- solubilizers, such as ethanol, isopropanol, ethylene glycol, propylene
glycol, glycerol and diethylene glycol,
- antidandruff agents, such as Piroctone Olamine and Zinc Omadine,
- other substances for adjusting the pH value,
- active substances, such as panthenol, pantothenic acid, allantoin,
pyrrolidone carboxylic acids and salts thereof, plant extracts and
vitamins,
- cholesterol,
- UV filters,
- consistency factors, such as sugar esters, polyol esters or polyol alkyl
ethers,
- fats and waxes, such as spermaceti, beeswax, montan wax, paraffins,
fatty alcohols and fatty acid esters,
- fatty acid alkanolamides,
- complexing agents, such as EDTA, NTA and phosphonic acids,
- swelling and penetration agents, such as glycerol, propylene glycol
monoethyl ether, carbonates, hydrogen carbonates, guanidines, ureas
CA 02288055 1999-10-22
WO 98/47473 19 PCT/EP98/02243
and primary, secondary and tertiary phosphates, imidazoles, tannins,
pyrrole,
- opacifiers, such as latex,
- pearlescers, such as ethylene glycol mono- and distearate,
- propellents, such as propane/butane mixtures, N20, dimethyl ether,
C02 and air and
- antioxidants.
To produce the colorants according to the invention, the constituents
of the water-containing carrier are used in the usual quantities for this
purpose. For example, emulsifiers are used in concentrations of 0.5 to
30% by weight while thickeners are used in concentrations of 0.1 to 25%
by weight, based on the colorant as a whole.
It can be of advantage to the coloring result to add ammonium or
metal salts to the colorants. Suitable metal salts are, for example,
formates, carbonates, halides, sulfates, butyrates, valerates, caproates,
acetates, lactates, glycolates, tartrates, citrates, gluconates, propionates,
phosphates and phosphonates of alkaline earth metals, such as potassium,
sodium or lithium, alkaline earth metals, such as magnesium, calcium,
strontium or barium, or of aluminium, manganese, iron, cobalt, copper or
zinc, sodium acetate, lithium bromide, calcium bromide, calcium gluconate,
zinc chloride, zinc sulfate, magnesium chloride, magnesium sulfate,
ammonium carbonate, chloride and acetate being preferred. These salts
are preferably present in a quantity of 0.03 to 65 mmol and more preferably
in a quantity of 1 to 40 mmol, based on 100 g of the colorant as a whole.
The pH value of the ready-to-use coloring preparations is normally in
the range from 2 to 11 and preferably in the range from 5 to 9.
In order to color the keratin-containing fibers, more especially
human hair, the colorants are generally applied to the hair in the form of the
water-containing cosmetic carrier in a quantity of 100 g, left thereon for
about 30 minutes and then rinsed out or washed out with a commercially
CA 02288055 1999-10-22
WO 98/47473 20 PCT/EP98/02243
available shampoo.
The aldehydes corresponding to formulae la and Ib and the
compounds of component B may either be applied to the hair
simultaneously or even successively, in which case it does not matter
which of the two components is applied first. The ammonium or metal salts
optionally present may be added to the first component or to the second
component. A time of up to 30 minutes can be allowed to pass between
application of the first component and application of the second
component. The fibers may even be pretreated with the salt solution.
The aldehydes corresponding to formulae la and Ib and the
compounds of component B may be stored either separately or together
either in the form of a liquid or paste-like preparation (aqueous or water-
free) or as a dry powder. If the components are stored together in a liquid
preparation, the preparation in question should be substantially free from
water to reduce any risk of the components reacting. Where the reactive
components are stored separately, they are mixed thoroughly together only
shortly before application. Where the components are stored as a dry
powder, a defined quantity of warm water (50 to 80°C) is normally added
and a homogeneous mixture prepared before application.
Examples
Preparation of a coloring solution
A suspension of 10 mmol of an aldehyde corresponding to formula
la or Ib, 10 mmol of a reactant, 10 mmol of sodium acetate and 1 drop of a
20% fatty alkyl ether sulfate solution in 100 ml of water was prepared. The
suspension was briefly heated to around 80°C and filtered after
cooling,
after which the pH value was adjusted to 6.
One tress of 90% grey, non-pretreated human hair was placed in
this coloring solution for 30 minutes at 30°C. The colored tress was
then
CA 02288055 1999-10-22
WO 98/47473 21 PCT/EP98/02243
rinsed for 30 seconds with Juke-warm water, dried in a stream of warm air
(30-40°C) and then combed. The colors were visually evaluated in
daylight.
The particular shades and depths of color are shown in Table 1
below.
The depth of color was evaluated on the following scale:
- : very faint, if any, color
(+) : weak intensity
+ : medium intensity
+(+) : medium to strong intensity
++ : strong intensity
++(+) : strong to very strong intensity
+++ : very strong intensity
Table 1
Coloring with 2-chloro-1-formyl-3-hydroxymethylene cyclohexene
Component B Shade Depth of color
- Yellow ++
2,5-Diaminotoluene x H2S04 Dark blue +++
2,4,5,6-Tetraaminopyrimidine x H2S04Orange ++
1,8-Bis-(2,5-diaminophenoxy)-3,6-
dioxaoctane x 4HCI Grey-black ++(+)
2-Methylamino-3-amino-6-methoxy-
pyridine x 2HCI Dark brown ++(+)
2-(2,5-Diaminophenyl)-ethanol x Violet-blue +++
H2S04
2-Aminomethyl-4-aminophenol x 2HCI Violet-red ++(+)
4,4'-Diaminodiphenyldiamine x H2S04Grey-green ++(+)
2,6-Dimethoxy-3,5-diaminopyridine
i
CA 02288055 1999-10-22
WO 98/47473 22 PCT/EP98/02243
x 2HCI Black +++
N,N-bis-(2-hydroxyethyl)-p-phenylene-
diamine x H2S04 Dark brown ++(+)
Table 2
Coloring with glutaconaldehyde
tetrabutyl ammonium salt
Component B Shade Depth of color
Rust red ++(+)
2,5-Diaminotoluene x H2S04 Dark violet +++
2,4,5,6-Tetraaminopyrimidine x Gold-yellow ++(+)
H2S04
1,8-Bis-(2,5-diaminophenoxy)-3,6-
dioxaoctane x 4 HCI Dark brown ++(+)
2-Methylamino-3-amino-6-methoxy-
pyridine x 2 HCI Dark violet ++
2-(2,5-Diaminophenyl)-ethanol x Red +++
H2S04
2-Aminomethyl-4-aminophenol x 2HCIBlue-violet +++
4,4'-Diaminodiphenylamine x H2S04 Black +++
2,6-Dimethoxy-3,5-diaminopyridine
x 2HCI Black +++
Table 3
Coloring with glutaconaldehyde sodium salt
Component B Shade Depth of color
- Rust red ++
2,5-Diaminotoluene x HZS04 Dark red-brown ++(+)
2,4,5,6-Tetraaminopyrimidine x H2S04 Orange-yellow ++
1,8-Bis-(2,5-diaminophenoxy)-3,6-
I
CA 02288055 1999-10-22
WO 98/47473 23 PCT/EP98/02243
dioxaoctane x 4 HCI Dark grey ++(+)
2-Methylamino-3-amino-6-methoxy-
pyridine x 2HCI Dark grey-brown++(+)
2-(2,5-Diaminophenyl)-ethanol x H2S04 Violet-red ++(+)
2-Aminomethyl-4-aminophenol x 2HC1 Red ++(+)
N,N-Bis-(2-hydroxyethyl)-p-phenylene-
diamine x HCI Blue-black ++(+)
4,4'-Diaminodiphenylamine x H2S04 Dark olive-green+++
2,6-Dimethoxy-3,5-diaminopyridine
x 2HCI Ollive green ++
L-Histidine Light brown ++
L-Lysine Copper ++
L-Proline Orange-brown ++(+)
3-Methyl-1-phenyl-2-pyrazolinone Rust red ++(+)
Thiobarbituric acid Orange-brown ++(+)
Barbituric acid Brown-yellow ++
Oxindole Rust red ++
1-Ethylquinaldinium iodide Rust red ++(+)
Rhodanine Red-brown ++(+)