Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
1
METHOD AND APPARATUS FOR TARGETED DRUG DELIVERY INTO A
LIVING PATIENT USING MAGNETIC RESONANCE IMAGING
BACKGROiIND OF THE INVENTION
1. Field of the Invention
This invention relates to i:he design, construction, and use of magnetic
resonance (MR) imaging to identify areas within a patient where changes in a
molecular environment ~rre occurring, as from chemical concentration changes
effected by medical procedures. The invention also describes a drug delivery
device for targeted drug delivery into a patient using magnetic resonance (MR)
imaging combined with conventional catheter placement techniques, particularly
including neurosurgical or neuroradiologic techniques used in intracranial
drug
delivery.
2. Back~around of the ~~rt
Although endosc;opic, arthroscopic, and endovascular therapies have
produced significant advances in healthcare, the diagnostic accuracy and
clinical
utility of these procedures is ultimately "surface limited" by what the
surgeon can
see through the device i~aelf or otherwise visualize during the course of the
procedure. Magnetic Rcaonance (MR) imaging, by comparison, overcomes this
limitation by enabling the surgeon to noninvasively visualize tissue planes
beyond the surface of the tissue under direct evaluation.
Moreover, MR imaging c,nables differentiation of normal from abnormal
tissues, and can display critical structures such as blood vessels in three
dimensions. Thus, high-speed MR-guided therapy offers an improved
opportunity to maximize the benefits of minimally invasive procedures.
Prototype high-speed M:R imagers which permit continuous real-time
visualization of tissues during surgical and endovascular procedures have
already
been developed. Recent publications in the medical literature have described a
number of MR-guided interventions including needle biopsies, interstitial
laser
therapy, interstitial cryotherapy ~~nd interstitial focused ultrasound
surgery.
The standard current pro cedure for drug treatment of various focal
neurological disorders, neurova~;cular diseases, and neurodegenerative
processes
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
requires neurosurgeons or interventional neuroradiologists to deliver drug
agents
by catheters or other tubular devices directed into the ccrebrovascular or
cerebroventricular circulation, or by direct injection of the drug agent, or
cells
which biosynthesize the drug agent, into targeted intracranial tissue
locations.
The blood-brain barrier and blood-cerebrospinal fluid barrier almost entirely
exclude large molecules like proteins, and control entry of smaller molecules.
Small molecules (< 200 daltons) which are lipid-soluble, not bound to plasma
proteins, and minimally ionized, such as nicotine, ethanol, and some
chemotherapeutic agents, readily enter the brain. Water soluble molecules
cross
the barriers poorly or not at all. Delivery of a drug into a ventricle
bypasses the
blood-brain barrier, and allows for a wide distribution of the drug in the
brain
ventricles, cisterns, and spaces due to the normal flow pathways of
cerebrospinal
fluid in the brain. However, following intracerebroventricular injection, many
therapeutic drug agents, particularly large-molecular weight hydrophobic
drugs,
I 5 fail to reach their target receptors in brain parenchyma because of
metabolic
inactivation and inability to diffuse into brain tissues, which may be up to
18
mm from a cerebrospinal fluid surface. To optimize a drug's therapeutic
efficacy,
it should be delivered to its target tissue at the appropriate concentration.
A
number of studies reported in the medical literature, for example, Schmitt,
Neuroscience, 13, 1984, pp. 991 - 1001, have shown that numerous classes of
biologically active drugs, such as peptides, biogenic amines, and enkephalins
have specific receptor complexes localized at particular cell regions of the
brain.
Placing a drug delivery device directly into brain tissue thus has the notable
advantage of initially localizing the injected drug within a specific brain
region
containing receptors for that drug agent. Targeted drug delivery directly into
tissues also reduces drug dilution, metabolism and excretion, thereby
significantly improving drug efficacy, while at the same time it minimizes
side
effects.
An important issue in targeted drug delivery is the accuracy of the
navigational process used to direct the movement of the drug delivery device.
Magnetic resonance imaging will likely play an increasingly important role in
optimizing drug treatment of neurological disorders. One type of MR unit
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
designed for image-guided therapy is arranged in a "double-donut"
configuration,
in which the imaging coil is split axially into two components. Imaging
studies
are performed with this system v~ith the surgeon standing in the axial gap of
the
magnet and carrying out procedures on the patient. A second type of high-speed
MR imaging system combines high-resolution MR imaging with conventional
X-ray fluoroscopy and digital subtraction angiography (DSA) capability in a
single hybrid unit. Both of these new generations of MR scanners provide
frequently updated images of the anatomical structures of interest. This real-
time
imaging capability makes it possible to use high-speed MR imaging to direct
the
movement of catheters and other drug delivery vehicles to specific tissue
locations, and thereby observe th,e effects of specific interventional
procedures.
A prerequisite for MRI-guided drug delivery into the brain parenchyma,
cerebral
fluid compartments, or cerebral vasculature is the availability of suitable
access
devices.
U.S. Pat. 5,571,089 to Crocker et al. and U.S. Pat. 5,Si4,092 to Forman
et al. disclose endovascular drug delivery and dilatation drug delivery
catheters
which can simultaneously dilate and deliver medication to a vascular site of
stenosis. U.S. Pat. 5,171,217 to March describes the delivery of several
specific
compounds through direct injection of microcapsules or microparticles using
multiple-lumen catheters, such as disclosed by Wolinsky in U.S. Pat.
4,824,436.
U.S. Pat. 5,580,575 to LJnger et al. Discloses a method of administering drugs
using gas-filled Iiposorr,~es comprising a therapeutic compound, and inducing
the
rupture of the liposomes with ultrasound energy. U.S. Pat. 5,017,566 to Bodor
discloses redox chemical systems for brain-targeted drug delivery of various
hormones, neurotransm fitters, and drugs through the intact blood-brain
barrier.
U.S. Pat. 5,226,902 to Elae et al. and U.S. Pat. 4,973,304 to Graham et al.
disclose drug delivery devices, in which biologically active materials present
within a reversibly permeable hydrogel compartment can be delivered into
tissues by various endogenous and exogenous stimuli. U.S. Pat. 5,167,625 to
Jacobsen et al. discloses implantable drug delivery system utilizing multiple
drug
compartments which are activated by an electrical circuit. U.S. Pat. 4,941,874
to
Sandow et al. discloses a device for the injection of implants, including drug
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
4
implants that may used in the treatment of diseases. U.S. Pat. 4,892,538
4,892,538, 5,106,627, 5,487,739 and 5,607,418 to Aebischer et al. disclose
implantable drug therapy systems for local delivery of drugs, cells and
neurotransmitters into the brain, spinal cord, and other tissues using
delivery
devices with a semipermeable membrane disposed at the distal end. U.S. Pat.
5,120,322 to Davis et al. describes the process of coating the surface layer
of a
stmt or shunt with lathyrogenic agent to inhibit scar formation during
reparative
tissue formation, thereby extending exposure to the drug agent. U.S. Pat.
4,807,620 to Strul and 5,087,256 to Taylor are examples of catheter-based
devices which convert electromagnetic Rf energy to thermal energy. Technology
practiced by STS Biopolymers (Henrietta, NY) allows incorporation of
pharmaceutical agents into thin surface coatings during or after product
manufacture. The invention disclosed by STS Biopolymers allows for the drugs
to diffuse out of the coating at a controlled rate, thereby maintaining
therapeutic
drug levels at the coating surface while minimizing systemic concentrations.
The coating can incorporate natural or synthetic materials that act as
antibiotics,
anticancer agents, and antithrombotics, according to the issued patent. U.S.
Pat.
5,573,668 to Grosh et al. discloses a microporous drug delivery membrane based
on an extremely thin hydrophilic shell. U. S. Pat. 5,569,197 to Helmus et al.
discloses a drug device guidewire formed as a hollow tube suitable for drug
infusion in thrombolytic and other intraluminal procedures.
A number of articles published in the medical literature, for example,
Chandler et al., Ann. N.Y. Acad. Sci., 531, 1988, pp. 206-212, Bouvier et al.,
Neurosurgery 20(2), 1987, pp.286-291, Johnstonetal., Ann.N.Y.Acad.Sci.,
531,1988, pp.57-67, and Sendelbeck et al., Brain Res., 328, 1985, pp. 251-258
describe implantable pump systems designed for continuous or episodic delivery
of therapeutic drugs into the central nervous system via systemic,
intrathecal,
intraccrebroventricular, and intraparenchymal injection or infusion.
The patented inventions referenced above provide useful methods for
introducing, delivering, or applying a drug agent to a specific target tissue,
but
each invention also has inherent problems. For example, some catheter systems
which provide endovascular drug delivery require temporary blocking of a
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
segment of the vessel, thereby transiently disrupting brain perfusion.
Microencapsulated coatings on catheters permit longer exposure of the tissue
to
the drug agent, but the physical limitations imposed by microcapsules restrict
the
volume and concentration of drug, that can be effectively applied to any
tissue
area. Exposed coatings on catheters which contain drug agents usually require
some type of sheath that must be removed from the catheter before the drug can
be released from the coating. The; sheath and any catheter components required
to physically manipulate the shearh greatly increase the profile of the
catheter,
and thereby limit the variety of applications for which the drug delivery
system
can be employed. Furthermore, tile binders or adhesives used in catheter
coatings may themselves significantly dilute the concentration of the
therapeutic
agent. Finally, thermal and light energy required to melt and bond coatings
such
as macroaggregated albmmin, to reduce tissue mass by ablation, and to inhibit
restenosis by cytotoxic irradiation may also cause damage to blood vessels.
U.S. Pat. 5,470,307 to Lindall discloses a low-profile catheter system
with an exposed coating .containing a therapeutic drug agent, which can be
selectively released at a remote ti:>sue site by activation of a
photosensitive
chemical linker. In the invention disclosed by Lindall, the linker is attached
to
the substrate via a complementary chemical group, which is funetionalized to
accept a complementary bond to l:he therapeutic drug agent. The drug agent is
in
turn bonded to a molecular lattice to accommodate a high molecular
concentration per unit area and the inclusion of ancillary compounds such as
markers or secondary emitters. A:~though U.S. Pat. 5,470,307 to Lindall
describes
significant improvements over previous catheter-based drug delivery systems,
there are nonetheless some problems. First, in common with other currently
used endovascular access devices, such as catheters, microcatheters, and
guidewires, the catheter tip is difficult to see on MRI because of inadequate
contrast with respect to surrounding tissues and structures. This makes
accurate
localization difficult and degrade: the quality of the diagnostic information
obtained from the image. Also, tlhe mere observation of the location of the
catheter in the drug delivery system does not reliably or consistently
identify the
position, movement and/or efficient delivery of drugs provided through the
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
6
system. Thus, one objective of this invention is to provide for an MR-
compatible and visible device that significantly improves the efficacy and
safety
of drug delivery using MR guidance.
Any material that might be added to the structure of a pliable catheter to
make it visible must not contribute significantly to the overall magnetic
susceptibility of the catheter, or imaging artifacts could be introduced
during the
MR process. Moreover, forces might be applied to such a catheter by the
superconducting magnetic manipulation coils of a nonlinear magnetic
stereotaxis
system which might be used in the practice of the present invention. In either
case, the safety and efficacy of the procedure might be jeopardized, with
resulting increased risk to the patient. Also, an MR-visible catheter must be
made of material that is temporally stable and of low thrombolytic potential
if it
is to be left indwelling in either the parenchyma) tissues or the cerebral
vasculature. Examples of such biocompatible and MR-compatible materials
which could be used to practice the invention include elastomeric hydrogel,
nylon, teflon, polyamide, polyethylene, polypropylene, polysulfone, ceramics,
cermets steatite, carbon fiber composites, silicon nitride, and zirconia,
plexiglass,
and poly-ether-ether-ketone. It is also important that drug delivery devices
used
under MR guidance are MR compatible in both static and time-varying magnetic
fields. Although the mechanical effects of the magnetic field on ferromagnetic
devices present the greatest danger to patients through possible unintended
movement of the devices, tissue and device heating may also result from radio-
frequency power deposition in electrically conductive material located within
the
imaging volume. Consequently, all cables, wires, and devices positioned within
the MR imager must be made of materials that have properties that make them
compatible with their use in human tissues during MR imaging procedures.
Many materials with acceptable MR-compatibility, such as ceramics, composites
and thermoplastic polymers, are electrical insulators and do not produce
artifacts
or safety hazards associated with applied electric fields. Some metallic
materials, such as copper, brass, magnesium and aluminum are also generally
MR-compatible, viz. large masses of these materials can be accommodated
within the imaging region without significant image degradation.
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
7
Guidewires for the catheter or drug delivery system are usually made of
radiopaque material so that their precise location can be identified during a
surgical procedure through fluoroscopic viewing. Exemplary of guidewires used
under X-ray viewing is the guide:wire disclosed by LeVeen, U.S. Pat. No.
4,448,195, in which a ra.diopaquc: wire can be identified on fluoroscopic
images
by metered bands placed at predetermined locations. The U.S. Patent No.
4,922,924, awarded to Ciambale et al. discloses a bifilar arrangement whereby
radiopaque and radiotransparent filaments are wrapped on a mandril to form a
bifilar coil which providles radiopaque and radiotransparent areas on the
guide
wire. U.S. Pat. No. 5,375,596 to Twiss et al. discloses a method for locating
catheters and other tubular medical devices implanted in the human body using
an integrated system of wire transmitters and receivers. U.S. Pat. No.
4,572,198
to Codrington also provides for conductive elements, such as electrode wires,
for
systematically disturbing the magnetic field in a defined portion of a
catheter to
yield increased MR visibility of that region of the catheter. However, the
presence of conductive elements in the catheter also introduces increased
electronic noise and the possibility of Ohmic heating, and these factors have
the
overall effect of degrading the quality of the MR image and raising concerns
about patient safety. Thins, in all of these examples of implantable medical
probes, the presence of MR-incompatible wire materials causes large imaging
artifacts. The lack of clinically adequate MR visibility and/or imaging
artifact
contamination caused b;y the device is also a problem for most commercially
available catheters, mic~rocatheters and shunts. MRI enables image-guided
placement of a catheter or other drug delivery device at targeted intracranial
loci.
High-resolution visual images denoting the actual position of the drug
delivery
device within the brain would beg extremely useful to the clinician in
maximizing
the safety and efficacy of the procedure. Drug delivery devices, such as
catheters, that are both 1VIR-visible and radio-opaque could be monitored by
both
X-ray fluoroscopy and 1VIR imaging, thus making intra-operative verification
of
catheter location possible.
Initial attempts ~:o position and visualize endovascular devices in MR
imaging were based on passive susceptibility artifacts produced by the device
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
8
when exposed to the 15 MR field. Magnetic susceptibility is a quantitative
measure of a material's tendency to interact with and distort an applied
magnetic
field. U.S. Pat. 4,827,931 to Longmore and U.S. Patents No. 5,154,179 and
4,989,608 to Ratner disclose the incorporation of paramagnetic material into
endovascular devices to make the devices visible under MR imaging. U.S. Pat.
No. 5,211,166 to Sepponen similarly discloses the use of surface impregnation
of
various "relaxants", including paramagnetic materials and nitrogen radicals,
onto
surgical instruments to enable their MR identification. However, these patents
do not provide for artifact-free MR visibility in the presence of rapidly
alternating magnetic fields, such as would be produced during echo-planar MR
imaging pulse sequences used in real-time MR guidance of intracranial drug
delivery procedures. Nor do these patents teach a method for monitoring with
MR-visible catheters the outcomes of therapeutic interventions, such as, for
example, drug delivery into brain tissues, cerebral ventricles, or
subarachnoid
space. Ultrafast imaging sequences generally have significantly lower spatial
resolution than conventional spin-echo sequences. Image distortion may include
general signal loss, regional signal loss, general signal enhancement,
regional
signal enhancement, and increased background noise. The magnetic
susceptibility artifact produced by the device should be small enough not to
obscure surrounding anatomy, or mask low-threshold physiological events that
have an MR signature, and thereby compromise the physician's ability to
perform
the intervention. These relationships will be in part dependent upon the
combined or comparative properties of the device, the particular drug, and the
surrounding environment (e.g., tissue).
An improved method for passive MR visualization of implantable
medical devices has recently been disclosed by Weme ( Ser. No. 08/554446) ITI
Medical Technologies (Application Pending). This invention minimizes MR
susceptibility artifacts, and controls eddy currents in the electromagnetic
scattering environment, so that a bright "halo" artifact is created to outline
the
device in its approximately true size, shape, and position. In the method of
the
invention disclosed by ITI, an ultra thin coating of conductive material
comprising 1-10% of the theoretical skin depth of the material being imaged -
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
9
typically about 250,000 angstroms - is applied. By using a coating of 2,000-
25,000 angstroms thickness, ITI :has found that the susceptibility artifact
due to
the metal is negligible due to the low material mass. At the same time, the
eddy
currents are limited due to the ultra-thin conductor coating on the device. A
similar method employing a nitinol wire with Teflon coat in combination with
extremely thin wires of a stainless steel alloy included between the nitinol
wire
and Teflon coat, has recently been reported in the medical literature by Frahm
et
al., Proc. ISMRM, 3, 19!7, 193 1. Exemplary of methods for active MR
visualization of implanted medical devices is U.S. Pat. No. 5,211,165 to
Dumoulin et al., which discloses an MR tracking system for a catheter based on
transmit / receive microc;oils positioned near the end of the catheter by
which the
position of the device can be tracked and localized. Applications of such
catheter-based devices in endovascular and endoscopic imaging have been
described in the medical literature, for example, Hurst et al., Mag. Res.
Med.,
24, 1992, pp. 343-357, I<;antor et al., Circ. Res., 55, 1984, pp. 55-60;
Kandarpa
et al., Radiology, 181, 1991, pp. 99; Bomert et al., Proc. ISMRM, 3, 1997, p.
1925; Coutts et al., Proc. ISMRr~I, 3, 1997, p. 1924; Wendt et al., Proc,
ISMRM, 3, 1997, p. 1926; Lang~;aeter et al., Proc. ISMPM, 3, 1997, p. 1929;
Zimmerman et al., Proc. ISMRW, 3, 1997, p. 1930; and, Ladd et al., Proc.
ISMPM, 3, 1997, p. 1937.
In the treatment of neurological diseases and disorders, targeted drug
delivery can significantly improve therapeutic efficacy, while minimizing
systemic side-effects of the drug therapy. Image-guided placement of the tip
of a
drug delivery catheter directly into specific regions of the brain can
initially
produce maximal drug concentration close to the loci of tissue receptors
following injection of the drug. .At the same time, the limited distribution
of
drug injected from a sin~~le catheter tip presents other problems. For
example,
the volume flow rate of drug delivery must be very low in order to avoid
indiscriminate damage to brain cells and nerve fibers. Delivery of a drug from
a
single point source also limits the distribution of the drug by decreasing the
effective radius of penetration of the drug agent into the surrounding tissue
receptor population. Another aspect of this invention is therefore to overcome
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
these inherent limitations of single point source drug delivery by devising a
multi-lumen catheter with multiple drug release sources which effectively
disperse therapeutic drug agents over a brain region containing receptors for
the
drug, or over an anatomically extensive area of brain pathology.
SUMMARY OF THE INVENTION
Magnetic Resonance Imaging (MRI) is used in combination with 1 ) an
MR observable delivery device or 2) an MR observable medical device which
can alter a water based molecular environment by performed medical operations,
10 the delivery device or medical device being used in the presence of MR
observable (in water, body fluid or tissue) compounds) or composition(s). MRI
images are viewed with respect to a molecular environment to determine the
position of the delivery or medical device (hereinafter collectively referred
to as
the "delivery device" unless otherwise specifically identified) and changes in
the
environment where the delivery device is present as an indication of changes
in
the molecular environment. As the delivery of material from the delivery
device
is the most significant event within the molecular envirom-nent in the
vicinity of
the delivery area, the changes in the molecular environment are attributable
to
the delivery of the MR observable compounds or compositions. Changes in
signal intensity within the MR images reflect the changes in the molecular
environment and therefore track the location of delivered materials, and are
indicative of delivery rates and delivery volumes in viewable locations. With
the
medical device, chemical composition within the molecular environment may
also be altered as by the removal of deposits of certain materials into the
liquid
(water) environment, where those materials can alter the MR response. Some
materials which may be removed by medical procedures will not affect the MR
response, such as calcium, but fatty materials may. Additionally, medical
treatments which stimulate natural activities of chemical producing systems
(e.g.,
the glands, organs and cells of the body which generate chemicals such as
enzymes and other chemicals with specific biological activity [e.g., dopamine,
insulin, etc.] can be viewed under direct MR observation and any changes in
CA 02289837 1999-11-12
WO 98/52064 PCTNS98/09942
11
chemical synthetic activity and/ or delivery can be seen because of molecular
environment changes which occur upon increased synthetic activity.
One recently established method of reading the data obtained from the
MR imaging is technically founded upon existing knowledge of Apparent
Diffusion Coefficients (ADC) i:n particular regions of the body. There is
significant published literature with respect to ADC values for specific
tissues in
various parts of animals, including various tissues of humans (e.g., Joseph V.
Hajnal, Mark Doran, e~; al., "MR Imaging of Anisotropically Restricted
Diffusion
of Water in the Nervous Systerr~: Technical, Anatomic, and Pathological
Considerations," .Iournal of Computer Assisted Tomography, 15(1): 1-18,
January/February, 199 l, pp. I- 1 8). It is also well established in the
literature
that loss of tissue structure through disease results in a decrease of the
ADC, as
the tissue becomes more like a homogeneous suspension. Clinical observations
of changes in diffusion behavior have been made in various tissue cancers,
multiple sclerosis, in stroke, where the reduction in diffusion precedes the
increase in T2, and in epilepsy. Thus, ADC values are specific for specific
types
of tissues. Accordingl:/, as different drugs/chemicals are introduced into a
tissue
volume under MR observation, the ADC resulting from each drug/chemical
interaction can be observed and the change in the ADC can be determined for
that drug/chemical interaction with that particular tissue/drug environment.
While the ADC is the preferred means within the present invention of
mapping the delivery of drug in tissue, other embodiments of the invention
allow
for additional tissue contrast parameters to track the delivery of a drug into
tissue. In other words, the delivery of a drug into tissue will cause other
MRI-
observable changes which can he mapped (as is done for ADC) and which can
be used to spatially track the delivery and extent of a drug into a tissue.
While
some of these observations may be larger in magnitude than others, any of the
effects can be used as a tracking; mechanism. The tissue contrast changes
apparent on an MR im~~ge can arise from ADC, from alterations in the BO
magnetic field (often referred to as magnetic susceptibility or ABO produced
by
the presence of a substance in said tissue), from alterations in local tissue
Tl
relaxation times, from local T2 relaxation times, from T2* relaxation times
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
12
(which can be created by susceptibility differences), from the magnetization
transfer coefficients (MTC is an effect produced by local communication
between free water protons and those of nearby macromolecular structures),
from
the ADC anisotropy observed in oriented matter, and also from local
differences
in temperature which will affect in varying degrees all of the included tissue
contrast parameters. In addition, the delivery of drug can also be tracked
from
magnetic filed frequency shifts caused by the drug or arising from agents
added
with unique frequency shifts from those of the local protons (such as that
created
from F- 19 or fluorine-19 agents found in or added to the drug).
MR imaging of the alterations in the BO magnetic field (also known as
imaging of the local magnetic susceptibility) can reveal the spatial
distribution of
a drug from the interaction of the drug with the otherwise homogeneous
magnetic field found in MRI. To enhance the alterations in the magnetic field
BO caused by the drug, small amounts of a BO-altering added agents can be
added to the drug during delivery. This can include iron oxide particles, or
materials comprising lanthanide-, manganese-, and iron-chelates. In addition,
vehicles containing differing gases (NZ, O2, COZ) will also alter the local
magnetic
field and thus produce a magnetic susceptibility effect which can be imaged.
The invention includes a device and a method for MR-guided targeted
drug delivery into a patient, such as intracranial drug delivery, intraspinal
drug
delivery, intrarenal drug delivery, intracardial drug delivery, etc. The MR-
visible
drug delivery device is guided to target entrance points to the patient such
as
periventricular, intracerebroventricular, subarachnoid, or intraparenchymal
tissues magnetic resonance imaging, or conventional methods of neurosurgical
or
neuroradiologic catheter manipulation. The drug delivery device has a linearly
arranged array of radiopaque and MR-visible markers disposed at its distal end
to
provide easily identifiable reference points for trackability and localization
under
susceptibility MR imaging and X-ray fluoroscopy guidance. Additionally, active
MR visualization of the drug delivery device is achieved by means of R-F
microcoils positioned along the distal axis of the device. MR visibility can
be
variably adjustable based on requirements related to degree of signal
intensity
change for device localization and positioning, enhancement along the shaft of
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
13
the device, enhancement around the body of the device, visibility of the
proximal
and distal ends of the device, degree of increased background noise associated
with device movement, and other factors which either increase or suppress
background noise associated with the device. Since the tip of the drug
delivery
device can be seen on TrIR and ~~-ray images and thus localized within the
brain,
the multiple point source locations of drug delivery are therefore known and
can
be seen relative to the tip or the shaft of the device.
Targeted delivery of drug agents is performed utilizing MR-compatible
pumps connected to variable-length concentric MR-visible dialysis probes each
with a variable molecular weight cut-off membrane, or by another MR-
compatible infusion de~~ice whie;h injects or infuses a diagnostic or
therapeutic
drug solution. Imaging of the injected or infused drug agent is performed by
MR
diffusion mapping using the R-1~ microcoils attached to the distal shaft of
the
injection device, or by imaging an MR-visible contrast agent that is injected
or
infused through the wal'~ls of the dialysis fiber into the brain. The delivery
and
distribution kinetics of injections or infusions of drug agents at rates
between I
ul/min to I 000 ul/min ~~re monitored quantitatively and non-invasively using
real-time contrast-enhanced magnetic susceptibility MR imaging combined with
water proton directional diffusion MR imaging.
One aspect of the present invention is to provide a non-invasive,
radiation-free imaging ;system for tracking a drug delivery device to a target
intracranial location.
Another aspect of the present invention is to provide an imaging system
for visualizing the distal tip of t:he drug delivery device at the target
intracranial
location.
A third aspect of this invention is to provide for an MR-compatible and
visible device that significantly improves the efficacy and safety of
intracranial
drug delivery using MR guidance.
A fourth aspect of the present invention is to provide for interactive MR
imaging of injected or iinfused MR-visible drug agents superimposed upon
diagnostic MR images of the local intracranial anatomy.
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
14
A fifth aspect of the present invention is provide an MR imaging method
for quantitative monitoring of the spatial distribution kinetics of a drug
agent
injected or infused from a drug delivery device into the central nervous
system,
in order to determine the efficacy of drug delivery at various intracranial
target
sites.
A sixth aspect of the present invention is to provide an MR imaging
method to evaluate how the spatial distribution kinetics of a drug agent
injected
or infused from a drug delivery device into the central nervous system is
influenced by infusion pressure, slow rate, tissue swelling and other material
properties of the brain, and by the physicochemical nature of the drug agent
infused.
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is a schematic of the drug delivery device illustrating an
1 S exemplary method of practicing the present invention.
FIG. 2 is a cross-sectional view of the preferred embodiment of the drug
delivery device, shown on a platform located above an anatomically targeted
site
in the brain. The view shows the disposition of a pump or reservoir containing
the injectable material in relation to the other components of the device.
FIGS. 3A and 3B illustrate the preferred arrangement of the individual
delivery catheters within the assembly of the multi-lumen delivery device.
FIG. 4 is a further cross-sectional view of the preferred embodiment of
the device which shows the disposition of R.F microcoil elements along the
distal shaft of the delivery device.
FIG. 5 is an elevated cross-sectional view of the preferred embodiment of
the device showing the disposition of the individual tubular probes at the
distal
tip of the delivery device.
FIG. 6A, 6B and 6C are side elevational views of the preferred
embodiment of the device illustrating the relationship between the R-F
microcoils and individual tubular components of the distal tip of each drug
delivery catheter.
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
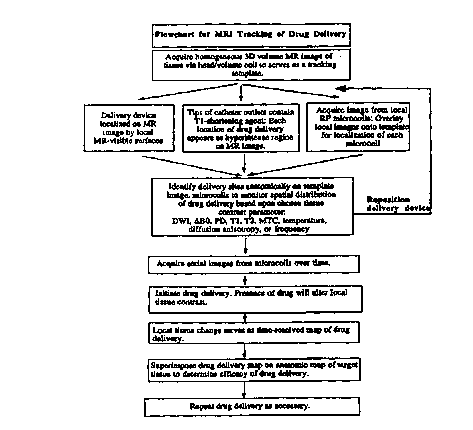
FIG. 7 is a flowchart of the MR imaging methods used to establish the
position and orientation of the delivery device, and to track the spatial
distribution kinetics of a material injected or infused from the delivery
device
into tissue.
5 FIG. 8 illustrates how the method of the invention is used to track the
spatial distribution kinetics of different drug agents based on their signal
intensity decay profiles i:ollowinl; injection into a homogeneous cavity in
the
brain extracellular compartment.
FIG. 9 illustrates how the method of the invention is used to track the
10 spatial I 0 distribution kinetics of different drug agents based on their
respective
signal intensity decay profiles folllowing injection into the heterogeneous
extracellular space of thc: brain.
FIG. 10 illustrates how the method of the invention is used to track the
spatial distribution of a drug agent that is injected into heterogeneous brain
tissue
15 comprised of 5 nerve cellls and nerve fibers.
FIG. 11 illustrate, how th:e method of the invention is used to track the
spatial distribution of a drug agent injected into the region of a brain
tumor.
DETAILED DESCRIPTION ()F THE INVENTION
One of the significant difficulties with delivery of materials such as
drugs, hormones, or neurotransmitters to living tissue is assuring that the
materials are delivered to the tarl;et receptor location in the intended
amount.
Many materials which are delivered to a patient, even though beneficial in the
treatment of a specific condition. may be moderately or even strongly noxious
or
damaging to healthy tis:;ue. It is therefore one object of conventional
materials
application treatment to maximi~:e dosage to a desired location and to
minimize
dosage to locations which do not. require the delivery of the material.
Additionally, newer medical treatments may include procedures which remove
unwanted deposits of materials vvith an expectation that the removal will be
assisted by physical removal (by a withdrawal system) or natural bodily
function
removal (e.g., the circulatory system), or which may attempt to stimulate the
body to produce natural chemicals (of which a patient may be deficient) at an
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
16
increased rate (e.g., electrical stimulation to increase the production of
dopamine). Because these procedures are usually highly invasive, it would be
extremely desirable to have a real time indication of immediate, transient and
persistent effectiveness of the procedure. Where undesired deposits or
collections of materials are being dispersed, it would be desirable to
visualize the
actual movement of materials to assist in collecting them (e.g., through
catheters)
or tracking them to assure that they are not again depositing or collecting
(as in
intravenous or cerebrospinal fluid blockage), or moving in segments which are
too large and could cause blockage in other parts of the body as they are
carried
about. Unfortunately, with in vivo delivery of materials, particularly
extremely
small doses in small volumes delivered by small instrumentation into tissue
regions protected by the blood-brain barrier, or the brain-cerebrospinal fluid
barrier, or into visually inaccessible areas, it has not been possible to
observe real
time distribution of the material delivery, or the dispersion or distribution
of the
material at the injection or infusion site within the tissue. Where even small
variations or miscalculations about the location of the target sight and the
delivery device can significantly affect the delivery of material and the
effectiveness of the delivered material, real time observation of the material
delivery is even more critical than in topical or gross (e.g., massive
systemic
injection) delivery events. There has been no truly effective observation
system
for such delivery prior to the present invention.
The basic operation of the present invention therefor involves the initial
MR imaging observation of a molecular environment of a patient (e.g., a
particular area or region of a patient, such as tissue, particularly such
tissue as
that present in organs or systems of animal bodies and especially the human
body, including, but not limited to the intracranial compartment and the
various
anatomic regions of the brain, including the cerebral ventricles, cisterns,
epidural
and subdural spaces, sinuses, and blood vessels, the spinal cord, including
disks,
nerves and associated vascular system, the heart and the coronary vascular
circulation, liver and the hepatic vascular circulation, kidney and the renal
vascular circulation, spleen and the splenic vascular system, gastrointestinal
system, special senses, including the visual system, auditory system, and
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
17
olfactory system endocrine system including the pituitary gland, thyroid
gland,
adrenal gland, testes, and ovaries, with observation of an MR image signal
intensity at a given time and/or state (e.g., prior to material introduction
or at
some defined stage of material diffusion into the molecular environment. In an
example of the method of the invention, the distribution of the material in
the
tissue is determined by releasing an amount of the material through a drug
delivery device positioned in the tissue, allowing the material to diffuse in
the
tissue, and analyzing the: resulting MR signal intensity. On a continual basis
or
at some subsequent time interval later (e.g., a pulsed interval, preselected
interval, random interval, frequent or sporadic intervals), the MR image of
the
molecular state within the same ~;eneral area is observed. Changes in the
characteristics, properties or quality of the image, such as the image signal
intensity within the area are presumptively (and in most cases definitively)
the
result of the introduction of material into the original molecular environment
and
alteration of the MR response for regions of the envirom-nent where delivered
material concentration has changed. By repeating observation of the MR image
signal intensity within am area at least once (e.g., first taking the initial
observation at a material) concentration state at a time Ti, and at least one
subsequent observation ~~f MRIobservable changes such as in the signal
intensity
qualities at a time T2), tl~e change in MR image signal intensity qualities
can be
related to the change in :material concentration between times T, and T2,
whether
that change is from a starting point of zero concentration or from an existing
concentration level. The observations therefore relate to the actual delivery
of
material into the molecular environment in an observable, and to some lesser
degree, quantifiable manner.
The change in the signal, e.g., the change in the amplitude of the MR
signal in the visible image may I>e altered by:
a) a change in the aI>parent diffusion coefficient (ADC) of tissue
water protons;
b) a change in tissue magnetic susceptibility (BO);
c) a change in Tl tissue relaxivity (T1);
d) a change in T2 ti~~sue relativity (T2};
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
18
e) a change in tissue magnetization transfer coefficients (MTC);
fj a change in tissue chemical shift frequency;
g) a change in tissue temperature; or
h} a combination of any one or more of a) - g) alone or with other
effects.
The MR signal is dephased by the random motion of diffusing water
molecules, and the presence of the delivered material locally affects the
degree to
which the amplitude of the signal is altered by the dephasing. If the amount
of
active ingredient to be delivered is quite small, or the effect of that
material on
the alteration of the amplitude is minimal, the delivered material may be
associated with a larger amount of a second material or another more NM signal
responsive material, which are preferably selected on a basis of similarity in
diffusion rates through like materials or at least comparable (mathematically
relatable) diffusion rates. In this manner, using such a taggant material, the
diffusion of the delivered material may be assumed to relate to the
diffusion/delivery of the taggant material. Unlike other observational
techniques, these taggant materials may be readily provided as non-toxic,
inexpensive taggant materials since there is such a wide variety of materials
which could be so used, and their only functional requirements would be
diffusion rate and non-toxicity. Many dyes commonly used in medical
procedures could be used for this purpose.
The availability of an MR-visible drug delivery device combined with
NM-visible chemical or drug agents would make it possible to obtain near real-
time information on drug delivery during interventional procedures in an intra-
operative MR system, as well as for pre-operative and post-operative
confirmation of the location of the drug delivery device. Medical and surgical
applications would include vascular surgery and interventional radiology,
cardiac
surgery and cardiology, thoracic surgery and radiology, gastrointestinal
surgery
and radiology, obstetrics, gynecology, urology, orthopedics, neurosurgery and
neurointerventional radiology, head and neck surgery and radiology, ENT
surgery and radiology, and oncology. In addition to direct tissue injection,
the
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
19
method of the invention applies to drug delivery via intraluminal,
intracavitary,
laparoscopic, endoscopic, intravenous, intraarterial applications.
There is currently considerable interest in the therapeutic use of small
ions as well as macromolecules i.n the treatment of various neurologic
diseases.
To be effective, such molecules must be able to reach target tissue receptors.
Many molecules that are used in therapeutic drugs are large in size, for
example,
neuroleukin, a neuromodulator drug tested for treatment of amyotrophic lateral
sclerosis is about 56 kDa, bethan.echol chloride used in treatment of
Alzheimer's
Disease is about I 96 kD~a and nerve growth factor is about 13 kDa. While the
importance of large molecular weight molecules in direct parenchyma) drug
therapy is growing, littlc; is knov~m about the time course and the spatial
range of
their actions, since dynamic visualization methods for studying macromolecular
diffusion have not been available;.
Diffusion of dru;; and/or water protons in a complex medium, such as a
brain cell microenvironrnent, is influenced by numerous factors. Materials
injected into the brain or spinal cord do not move unimpeded through the
aggregations of neurons, glia, capillaries, and nerve fibers. The distribution
of a
drug volume in the brain cell mic;roenvirom-nent following injection directly
into
brain tissue is governed by a number of factors including the physicochemical
characteristics of the dmg, capillary uptake, metabolism, excretion, size of
the
extracellular space (the ~~olume fraction), and geometry of the brain cell
microenvironment (tortuosity). 'f he degree to which each of these factors
influences the distribution of a p<rrticular drug agent within the brain or
spinal
cord is an important determinant of the effectiveness of drug treatment of
diseases of the central nervous system.
Despite the fact ):hat the average spacing between brain cells may be no
more than 20 nm, the mean free path of an ion at the typical ionic strength of
the
mammalian nervous system is only about 0.01 nm. In ways similar to altering
the local ADC of the water protons, presence and transport of a drug through a
tissue will also alter the magnetic; susceptibility, Tl, T2, MTC, water proton
diffusion anisotropy, chemical shift frequency, and temperature of the protons
within each imaged vox~~l. This represents the distance traveled between
CA 02289837 1999-11-12
WO 98/52064 PCT/US98I09942
collisions with other molecules. Almost all these collisions actually take
place
with water molecules since the concentration of water is 55 M. Thus ions
intrinsic to the brain rarely encounter cell membranes and generally behave as
though they were in a free medium. However, the diffusivity properties becomes
5 much more complicated when the boundary has a complex geometry, or when
macromolecular interactions involve exogenous solutions injected into tissues.
In complex media such as brain tissue, diffusion obeys Ficks Law,
subject to two important modifications. First, the diffusion coefficient, D,
is
reduced by the square of the tortuosity factor to an apparent diffusion
coefficient
10 ADC* = D / tortuosity factor 2 because a diffusing material encounters
membranous obstructions as it executes random movements between cells.
Second, the source strength is divide by the volume fraction of the
extracellular
space so that a given quantity of released material becomes more concentrated
than it would have been in a free medium.
15 In most media, tortuosity and volume fraction are essentially
dimensionless factors which depend only on the geometrical constraints imposed
by local structures. In brain tissue, however, a third factor, non-specific
uptake,
is present in the diffusion equation as a term, k', for loss of material
across the
cell membranes. In fact k' can be expressed as P (S) / volume fraction, where
P
20 is the membrane permeability and (S) is the volume average of the membrane
surface area. Complex local boundary conditions imposed by cell membranes
can thus be removed by averaging the local diffusion equations and boundary
conditions over some characteristic volume of tissue a few micrometers in
extent. Thus in the case where a substance is injected from a point source at
a
rate of q moles/sec in a free medium, the source term becomes q/tortuosity in
a
complex medium while the diffusion coefficient ADC is modified to be
ADC/volume fraction 2 in the new equation, which is the apparent diffusion
coefficient.
Knowledge of the properties of the brain extracellular microenvironment
is thus essential to understanding the role of diffusion in delivering
metabolic or
therapeutic agents to brain or spinal cord cells. Diffusion has been
determined
employing radioactive or fluorescent tracers, in which the concentration
profiles
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
21
of the tracer are monitored over time, and its diffusivity is inferred from
the data.
Microscopic displacements can be seen with tracers on the scale of
millimeters.
Spatially resolved methods, such as infrared spectroscopy or Rayleigh
scattering,
have been used allowing resolution in the micrometer range. Such tracer
techniques have been successfully applied in biological systems, such as the
brain. However, because of the inherent invasiveness of using exogenous
tracers, such technique:. cannot lue used in vivo with humans.
Techniques have also been developed for determining the diffusion
characteristics of small molecules in local regions of the brain using
radiotracers,
microiontophoresis, or pressure microinjection combined with ion-selective
microelectrodes. The applicatia~ns of these methods to intracranial drug
delivery
have been described in the medical literature, for example, Lux et at., Exp.
Brain Res., 17, 1973, pp. 190-2.05, GardnerMedwin, Neurosci. Res. Progr.
Bull., 1980,18, pp. 208-226, Nicholson et al., J. Physiol., 1981, 321, pp. 225-
257, Nicholson et al., Ie~rain Res., 1979, 169, pp. 580-584. However, these
techniques have several key limitations. First, these techniques provide a
measurement at only a single paint in the tissue so that spatial patterns of
diffusion cannot be determined. Second, ion-selective microelectrodes can only
be used with a few small ions. 'Third, since radiotracer techniques rely on
postmortem counting of particles in fixed and sectioned tissues, they provide
limited spatial resolution and no dynamic information.
Several previous studies have obtained estimates of the ADC of large
fluorescent molecules i:rom digitized images of fluorescent molecules as they
diffused away from blood vessels. However, the complicated geometry of the
source and inability to precisely characterize the emitted flux, substantially
limit
the clinical utility of the information. Similarly, new optical imaging
methods,
in which a uniform distribution of fluorescent tracer is first established in
the
sample and then a regi~an is photobleached with a strong laser, has serious
limitations because the: laser beam can also damage the tissue area being
imaged.
Studies with optical fluorescence methods suggest that molecules as large as
70
kDa can pass through the brain extracellular microenvironment. Below some
limit between 1 O and ~~0 kDa, molecular diffusion is not restricted any more
than
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
22
with much smaller molecules. Similar constraints have been found for diffusion
in the brain intracellular microenvironment, whereby all molecules diffuse at
least three times slower than in aqueous solution, suggesting a similar
tortuosity
in the intracellular envirom-nent.
An integrative optical imaging technique disclosed by Tao and
Nicholson, Biophysical J., 1993; 65, pp. 2277-2290 yields an apparent
diffusion
coefficient from digitized images, and enables precise determination of the
diffusion characteristics of fluoresently labeled compounds of high molecular
weight. The generalized equations disclosed by Nicholson and Tao have two
nondimensional factors that incorporate the structure of the tissue into the
imaging solution. The first factor, the tortuosity, accounts for the hindrance
to
extracellular diffusion that arises from the obstructions presented by cell
membranes. The second structural factor is the volume fraction, which is
defined as the ratio of the volume of the brain extracellular microenvironment
to
the total volume of tissue averaged over some small reference domain. The
method disclosed by Nicholson and Tao ("Hindered diffusion of high molecular
weight compounds in brain extracellular microenvironment measured with
integrative optical imaging." Biophysical J. 1993; 65:2277-2290) does not,
however, yield a direct measurement of the molecular distribution in a three-
dimensional sample, and furthermore requires use of large fluorescent markers
which are not suitable for repeated injections in human patients.
An alternative approach to measuring diffusivity of therapeutic drug
injections is to monitor the diffusion process itself, i.e. the random motions
of an
ensemble of particles. Einstein showed that the diffusion coefficient measured
in
nonequilibrium concentration cell experiments is the same quantity that
appears
in the variance of the conditional probability distribution, P(r/ro, t), the
probability of finding a molecule at a position r at a time t, which was
initially at
a position ro. For free diffusion, this conditional probability distribution
obeys
the same diffusion relation. Thus, MR imaging parameters which reflect the
differences in relative water proton-diffusion path lengths may serve to
enable
imaging differentiation between tissue water protons and protons in
macromolecular solutions that are injected into brain tissues. Molecular water-
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
23
proton diffusion is caused by thermally induced random Brownian motion. As
the protons continually ~~ollide with their microenvironments, their average
random traveled pathlength <L>, along one direction (e.g. along the magnet-
bore
direction) is described according to Einstein as: <L2> = 2 TD where over an
observation time of T (seconds) the displacement is expressed by a "diffusion
coeff cient, D" in Trim ?,/S or CM2/S. The diffusion process is continuous, so
that the average displacement of any population of water protons increases
with
MR imaging time. However, thc~ diffusion behavior of protons can be hindered
by impermeable or semi-permeable barriers, such as cell membranes, and
macromolecules, which may themselves contain populations of diffusing
protons. For tissue water protons diffusing within a tissue matrix, the
observed
diffusion rate and direction will reflect the molecular and macromolecular
barriers or hindrances that the diffusing protons encounter during their
translational processes. One example of the application of this concept in
human
neurobiology is that myelinated nerve fibers in the brain and spinal cord
preferentially dispose the diffusion of water protons along, rather than
across,
myelin tracts thereby giving rise, to diffusional anisotropy MR imaging
properties
(Moseley et al., Mag. l2es. Med., 19, 199 1, pp. 321-326, Moseley et al.,
Topics
Mag. Res. Med. , 3, 199 1, pp., 5068).
Although noted for its effects on high-resolution, high-field MR spectra
more than 25 years ago, molecular (water proton) diffusion has just recently
been
shown to have an important im~~act in clinical MR neuroimaging applications.
While TI and T2 relaxation timfa reflect frequency-dependent rotational and
proton exchange processes, diffusion is caused solely by molecular or proton
displacements or translations. Molecular size, shape, microenvironment, and
temperature all influen~~e the dii:fusion rate of molecules. Generally, larger
molecules will translate (diffuse;) more slowly than smaller molecules, such
as
water protons, and the differences in diffusion rates between different
populations of molecules can be: distinguished by signal intensity differences
on
diffusion-weighted MF; images, particularly MR images which employ large
diffusion gradients (b values). 'Thus, the measurable diffusion of smaller
versus
large molecules with MR imaging can be used as an in vivo tracer to probe the
CA 02289837 1999-11-12
WO 98/52064 PCTNS98/09942
24
structural orientation of the tissues into which the drug agent has been
injected.
Advances in diffusion-weighted MR imaging have been made possible by major
technical improvements in MR scanner hardware and software. High-speed MR
echo-planar imaging now enables subsecond diffusion-sensitive imaging of
water proton behavior in brain and spinal cord.
Thus, MR-visible molecules may exist in a variety of environments in
brain tissue, which modify the way in which the molecules can move. First, the
space in which the molecules can move may be small (e.g., intracellular) or
large
(e.g., an enlarged extracellular space). Second, the amount of dissolved
compounds and proteins may alter the viscosity of the substance injected into
the
tissue. The random movement of the molecules is characterized by its diffusion
coefficient ADC as the mean square distance moved for unrestricted isotropic
(i.e. same in all directions) diffusion (for example a large sample of pure
water}.
ADC is high in pure water, and lower by about a factor of 10 in tissue. As
tissue
becomes destroyed by disease processes, ADC is expected to rise toward its
free
water value. Diffusion-weighted imaging, in which field gradients are applied
to
attenuate the signal from rapidly diffusing water, shows increased image
intensity in areas of low ADC. Similarly, the presence of a drug in tissue, or
its
transport through tissue extracellular, intercellular or intracellular
microenvirom-
ments, will also alter the magnetic susceptibility, T1, T2, MTC, water proton
diffusion anisotropy, chemical shift frequency, and temperature of protons
within
each imaged voxel.
The medical treatment and the medical device used in the practice of the
present invention, even when a delivery device, may also be a diagnostic
device
rather than only a treatment device. For example, there are numerous diseases
which alter the thickness of specific layers or coverings within the body,
such as
the myelin around nerves. The present invention provides a diagnostic tool to
the degree that alterations in the thickness or existence of coatings such as
myelin will alter the transport of chemical from one part of the body to
another.
Where, as in certain myelin deficiency diseases such as Multiple Sclerosis,
the
effect on the myelin is progressive and not uniform, the administration of
chemicals into an area under MR imaging guidance according to the present
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
invention can enable viewing of the variations in the rate of migration or
transport of these obser<~able chemicals to different areas of the myclinated
nerve. The degree of advance of the disease can thus be observed, and it is
possible to diagnose or <:ven quantify the stage of the disease more acutely
and
5 comparatively within a given patient. According to that method, a chemical
material would be introduced into the patient, and the relative movement of
that
chemical through supposedly similar structures in the area could be observed.
Significant differences in penetration rates and/or concentrations of these
chemicals through similar tissue material (e.g., the myelin) would be
indicative
10 of different properties (e;.g., thicf;ness, hydrophilicity, porosity, etc.)
which would
be symptomatic of a disease. The observation would therefore provide data that
could support or prove a clinical diagnosis of a disease which is known to
affect
the specific properties observed.
FIGS. 1 and 2 illustrate an MR-compatible drug delivery device made in
15 accordance with the most prefewed embodiment of the present invention. A
variable-length concentric MR-visible mufti-lumen catheter 4 is formed by
extruding a tubular assembly wil:h both porous 4b and non-porous 4a tubular
components, The non-p~~rous tubular component 4a is made of MR-visible
elastomeric hydrogel, v,~rious polymeric compositions including
20 polyvinylchloride, poly,~crylonitrile, polyvinylidene fluoride,
polystyrene,
polyurethane, and polya~mides, or other similar low friction material intended
to
minimize abrasive dam~~ge to thc~ brain during insertion. One or more of the
tubing conduits 2, 2a, 2~b in the rnulti-lumen catheter are connected to a
pump 3,
3a, 3b or other temporary reservoir 1, 1 a, 1 b, which circulates a
therapeutic drug
25 solution or MRsensitive; contras: agent through a dialysis fiber into a
target tissue
or pathological lesion.
The distal terminus of each porous tubular component 4b has a dialysis
probe 17 with a variable molecular weight cut-off membrane 18 which permits
unimpeded movement of cerebrospinal fluid, small ions, and small molecular
weight drugs, but is substantially impermeable to blockage by cellular
material,
said semipermeable membrane having a molecular weight exclusion of
approximately 100-200 kD. The dialysis membranes can be made of regenerated
CA 02289837 1999-11-12
WO 98!52064 PCT/US98/09942
26
cellulose hollow fiber tubing, as well as various polymeric compositions
including polyvinylehloride, polyacrylonitrile, polyvinylidene fluoride,
polystyrene, polyurethane, polyamides, cellulose acetates and nitrates,
polymethyImethacrylate, polysulfones, polyacrylates, and derivatives,
copolymers and mixtures thereof.
The inlet tubing of the dialysis probe is connected to a microinjection
pump 3 or reservoir 1 providing a flow of 0.1-10 ml/minute of drug solution or
sterile Ringer's solution perfuming the inside of the probe. The outlet tubing
2a
is connected to a section of plastic tubing leading to a collection vial 3a.
Regenerated cellulose hollow fiber dialysis tubing is cemented into the distal
end
of the plastic tubing with clear epoxy or other MR-compatible bonding
material.
The dialysis fiber (Spectra/Or; Spectrum Medical) or other similar
commercially
available semi-permeable membrane has a nominal molecular weight cut-off of
100-200 kD, an LD. (interior diameter) of 5-50 micrometers and a membrane
length of 1-10 mm. With further reference to FIGS. 1-3 of the drawings, the
outlet tubing 2a is incorporated into the probe into the dialysis chamber 1
via a
small perforation in the inlet tubing. The entire upper portion of the
assembly,
including the junction between the inlet tubing and plastic cannula, is sealed
with
epoxy. The outer tubing consists of 5-10 cm length of flexible fused silica
tubing (Polymicro Technologies). These probes are inexpensive and easy to
construct, and the small o.d. (Outside diameter) minimizes the tissue damage.
The concentric design makes it simple to implant the probe into different
intracranial locations. With reference to Figure 4, active MR visualization of
drug delivery is achieved by means of one or more RF microcoils 9, 9a, 10, l0a
positioned along the longitudinal axis of the device 4. Particularly preferred
is an
RF coil consisting of a circular loop of gold or other conductive material 9
positioned around the widest part of the drug delivery device, which would
project the field-of vision (FOV) furthermost into the tissue.
Depending on orientation of the coil with the magnetic BO, single
microcoils may be used separately or may be constructed in an array that may
be
used together to optimally image the surrounding tissue structure and
contrast.
In order to reduce the thickness of the R-F microcoil, the coil material is
sputter-
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
27
coated onto the surface of the drug delivery device. Preferred also for very
small
(nanoliter or microliter) injections is a solenoid volume R-F microcoil 9a,
which
by design is sensitive only to the; volume inside the coil, said imaging
volume
being directly related to the diameter of the R-F coil. Another preferred MR
imaging method which can be u;~ed to practice the invention is a combination
of
R-F microcoil and surface coil positioned on the surface of the patient's
head.
Also preferred is telescoping coil 10 inside of the catheter, expanding it
when
one wants to image and then withdraw the coil and move on. One may see
several cm with this idea. Another preferred method of MR imaging involves
the use of an oblong surface loop of wire at the end of a slanted drug
delivery
device or along the shank of the device, thereby yielding a long FOV. In each
of
these preferred embodiments of the invention, the transmitting coil would be
the
head or body volume RF coil inside of the MR imager. The R-F surface coil is
used only for detection purposes. In another preferred embodiment, a
preamplifier l Ob positioned near the distal end of the delivery device 4
serves to
amplify signals from th~~ R-F microcoils 9, 9a, 10, 10a.
With further refi~rence to FIGS. 3 and 4, the medical device used in the
preferred practice of the; present invention for delivery of materials may
vary
widely with respect to its structure, being highly dependent upon the
particular
procedural use to which it is being intended. 1-lowever, there are many
features
which can be common i:o all of the devices or which should at least be
considered in the various constn.~ctions. The simplest device could be a
single
delivery tube (catheter) which h;~s MR responsive material in or on the
composition of the tubing 19, preferably near the distal end or outlet of the
delivery tube for assisting in detection by the MR imaging system. The next
level of simplified construction 'would be the presence of MR coils or
microcoils
9, 9a, 10, l0a at or near the distal end of the catheter. This again, as
elsewhere
described, improves the: visibility of the viewable signal observable by the
MRI
system. More than one coil or nnicrocoil may be present, as the distribution
of
microcoils along a length of the catheter helps define the region within which
local signals are detected at efficient intensities. That is, each coil acts
as a
detector of local MR intensity, auld each coil supports a volume around the
coil
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
28
which is observable by MRI systems. The coils may add or integrate their
detectable volumes, defining a combined volume which can be efficiently
observed by the MR system. As different medical procedures are performed in
different environments, with different shapes and different variations in
densities, the coils may be located, sized, angled, or otherwise designed to
provide specific MR signals and/or responses tailored to the anticipated needs
of
a particular procedure. In general, the invention is best practiced by
employing
an array of R-F microcoils, such that an image is obtained for any orientation
of
the drug delivery device.
The device may also include numerous catheter elements and/or ports
and/or supplemental or independent functional elements. For example, as
illustrated in FIG. 3, at least two ports 21, 22 may be needed, one to carry
in on
chemical material and another to deliver a second distinct chemical material
which is or may become desirable during a medical procedure. For example, in
addition to a primary treatment chemistry being delivered, saline solutions or
specifically tailored solutions to dilute potential oversized deliveries could
be
desirable. Some treatments may require sequences of drug delivery or delivery
of various drugs which may not be storage stable prior to delivery to a
patient.
Separate ports 23, 24 would be desirable in those events. Additionally, ports
may be used to evacuate undesirable materials which are directly or indirectly
introduced by the medical procedure. The withdrawal port 25 may comprise a
tube with a port which can be attached to negative pressure with respect to
the an
opening in such a withdrawal port, thus being able to reduce liquid or small
particle solids volumes within the area of the procedure. Where the liquid
volume or solids are MR viewable, the MR viewable device may be directed
towards specific locations or areas and the ports targeted towards those
specific
areas. In addition, the various ports may be marked or designed to provide
distinct signals when viewed by MR systems so that they may be distinguished
during performance of the procedures. For example, MR insensitive materials
may be used to line a port 26 or materials with different distributions or
intensities of MR response may be used in the various ports to differentiate
the
elements while being observed during performance of procedures. For example,
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
29
where a withdrawal tube 27 has openings through which materials may be
withdrawn, the orientation of that opening within the device becomes
important.
By lining the edges of the opening with material having unique MR
responsiveness within the device: 28, the position and orientation of the
opening
can be readily determined. Particularly preferred is a 2,000-5,000 angstrom
thick
coating of MR-visible material along the distal shaft of the device.
Where multiple catheters or ports or functional elements are combined
into a single device, the configuration of the different components should be
tailored for a particular procedure. The different components may be
associated
by various orientations. As illustrated in FIG. 3B, the most preferred is
generally
a central tube or tubes with other tubes forming a circular distribution
around the
central tube or tubes. An MR-visible guidewire may be inserted within the
device 4 to assist in positioning 'the device at a target anatomical location.
Particularly preferred is a guidewire or other structural support made of
Nitinol~rM
or other MR-compatible shape memory metal. This is the simplest geometry and
provides for smallest diameter siizing of the device. As illustrated in F1G.
SA,
other configurations such as parallel alignment of the elements in a strip-
tike
orientation, stacking of element:; in rows and columns, or mixtures of these
and
other configurations may also bf; useful. Other elements which may be included
within the device, in addition to or separate from the use of delivery and/or
withdrawal tubes 29, include thermal elements 30 (for providing heat),
radiation
carrying elements 31 (e.g., ultraviolet radiation, visible radiation, infrared
radiation, and even hard radiation carrying elements, such as optical fibers
or
other internal reflection radiation carrying systems), detection elements 32
(e.g.,
pH indicators, electronic activit~r indicators, pressure detectors, ion
detectors,
thermal detectors, etc.), and any other sensing or detection element which
would
be useful during medic,~l procedures. These individual elements are each
extendable to permit o~~timal positioning within the tissue would be
configured
as desired or needed fo:r the parf.icular procedure intended for the device.
Procedurally inert elements such as structural supports, reinforcing elements
or
coatings, back-up elements, and. the like, may also be present within the
device.
Particularly preferred as structural supports or reinforcing elements are
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
circumferential bands of Nitinol or other MR-compatible shape memory metals
which, when activated, can facilitate accurate directed placement of the
functional tip of the device.
One type of configuration which is presently considered as the preferred
5 embodiment of the invention is the use of a core of elements) surrounded by
a
sheath or distribution of additional elements. For example, with further
reference to FIGS. 3A and 3B, a central core element my comprise a single tube
for delivery of a material, a pair of tubes for delivery of two chemicals, a
delivery
and withdrawal tube, or a procedurally inert structural support element 11.
10 Around the central core element may be disposed multiple additional
elements
21-27, usually seeking as near to a circular distribution about the central
core as
geometries allow. The attempt at the circular distribution is primarily for
purposes of optimizing a small size for the diameter of the article, and is
not
necessarily a functional aspect to the performance of the device. With respect
to
15 FIG. 5, the MR responsive materials, including MR microcoils, may be
located
within the central core 33, around the central core 34 (beneath any next
layering
of elements), or over the elements surrounding the central core 34a. Where one
or more of the elements receive, transmit or are powered by electrical
signals, it
is desirable that these elements be electrically separated by either or both
of
20 physical separation or additional insulation to prevent mixing or cross-
transmission of signals between the distinct elements. Carrying and
withdrawing
tubes (as well as other elements) may also secondary functions. For example, a
carrying tube may be conductive (by being naturally conductive or by having a
conductive coating in or outside of the tube) and the electrical connection
may be
25 associated with an electronic element or component at the distal end of the
device. The tube may thereby act as a carrying tube and electrical connection
to
the electronic component or element. Structural or adhesive support materials
between different elements may also provide such functions.
The various individual elements within the device must be structurally
30 associated, especially away from the distal end, and during insertion, may
need
structural association at the distal end 11. The structural support or
structural
integrity may be provided by some physical means of attaching the various
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
31
elements. This may be done by adhesive materials between the individual
elements (which adhesive should be MR compatible), fusion of the various
elements, or by coextrusion of the tubes into a single unit (or single
component
of a multiple element device). The adhesive may be an organic or inorganic
adhesive. The distal end of the device may have the ends of the elements
temporarily or controllably bonded during insertion. This may be beneficial
because it may be desirable to hare the individual elements fan out or
separate
during a medical procedure, for e:Kample, as in the case of a target tissue or
area
of pathology which is anatomically extensive. The adhesive could be water
soluble (which would dissolve in a timely manner after insertion), solvent
soluble (with solvent delivered into the distal end during a preliminary
procedure, or radiation disruptable (e.g., a positive-acting resist adhesive
composition which is sensitive to UV, visible or IR radiation which may be
delivered through a radiation carrying port). Many other variations and
combinations of these considerations and constructions may be used within the
practice of the present invention.
With reference to FIG. 6a, in another embodiment the dialysis probe is
replaced by an MR-visible microcatheter 38, which is a single extrusion
catheter
made from one of several possible sizes of a polyethylene terephthalate
proximal
shaft, e.g. 30 ga. The 12 mm distal segment of the microcatheter drug delivery
device is made of elastomeric hydrogel or similar soft material which
minimizes
tissue damage during insertion. A plurality of semipermeable membranes 38b
are placed circumferenti;~lly at regular intervals along the distal segment of
the
microcatheter, thus enabling wide dispersion of an injected agent,
semipermeable
membrane consisting of a 0. I 8-0.22 ml millipore filter. The companion
microguidewire in this example is made of nitinol or similar memory metal
which enables directed f~lacement of the tip of the catheter. The
microguidewire
37 is threaded into a cle~u~ hub lu~~k-lock cap 39 made of poly-methel-pentene
or
similar MR-compatible plastic. l3oth the catheter and guidewire have a
linearly
arranged array of radiop~aque and MR-visible markers 40 disposed at the distal
end to provide easily identifiable reference points for trackability and
localization under MR imaging a.nd Xray fluoroscopy guidance. The
CA 02289837 1999-11-12
WO 98/52064 PCTNS98/09942
32
microcatheter can also be made from any of the well-known soft, biocompatible
plastics used in the catheter art such as Percuflex, a trademarked plastic
manufactured by Boston Scientific Corporation of Watertown, Massachusetts.
With further reference to FIG. 6a of the drawings, when the delivery device is
positioned intracranially, the distal markers will be identifiable in an MR
image
and by X-rays. In another preferred embodiment, two or more R-F microcoils
are placed along the distal shaft of the microcatheter.
With further reference to FIG. 6 of the drawings, the delivery device can
be employed to deliver pharmacologic therapies in order to reduce morbidity
and
mortality associated with cerebral ischemia, intracranial vasospasm,
subarachnoid hemorrhage, and brain tumors. In the method of the invention the
distal tip of the mufti-lumen catheter assembly is typically positioned a few
millimeters above the intracranial target structure using MR imaging. In one
preferred embodiment of the invention illustrated in FIGS. 6B and 6C, surface
modifications of the material components of the dialysis probe 18 enable timed-
release kinetics of MR-visible biologic response modifiers, including peptide
macromolecules. In another preferred embodiment of the invention, a pump or
other infusion or injection device circulates a solution containing a
therapeutic
drug or an MRvisible contrast agent through the walls of the dialysis fiber
into
the brain at rates between 0.01 microliter/min to 10 microliter/min. In
another
preferred embodiment of the invention, pressure ejection techniques well
described in the medical literature are used to deliver a predetermined amount
of
a therapeutic drug agent or MR-visible contrast through one or more of the
tubular components of the mufti-lumen device. In one specific preferred
embodiment of the invention, the catheter is backfilled with the drug or
contrast
agent, which is functionally connected to a PicospritzerT"'' (General Valve
Corp,
Fairfield, NJ) or a similar instrument that is able to deliver pulses of
nitrogen or
compressed air with a duration ranging from a few milliseconds to several
seconds at a pressure of 10-50 psi. Using such a pressure ejection mode of
drug
delivery, the concentration of the released substance in the vicinity of the
tip is
accurately defined by the concentration of the material in the delivery
device. A
binary solution can also be released, in that two therapeutic or diagnostic
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
33
compounds can be deli~~ered at l:he same time by pressure ejection of two
materials from two or more separate microcatheters.
In another embodiment of the invention, the MR-visible solution contains
stoically stabilized liposomes, with lipophilic or hydrophilic chelators, such
as
polyaminocarboxylic acids and their salts, such as DTPA on phosphatidyl
cthanolamine for steric acid embedded within the external bilayer, or double-
label liposomes that chc~late a T.?-sensitive metal ion within the internal
aqueous
space and another T1-sensitive metal ion on the outside membrane surface, or
liposomes which contain 100-1000 air bubbles, such as argon, carbon dioxide,
or
air, as a contrast agent. In another preferred embodiment, R-F microcoils 41a-
f
are positioned at the di~~tal ends of individual delivery tubes, said
microcoils
acting as local MR detectors.
With further reference to FIGS. 1 and 2, in a method of the inveniion, the
implantable MR-visible; multilu:men catheter includes in another tubing
conduit a
hydrocephalus pressure valve IC~ and self sealing port ID preferably made of
Nitino, Tm or other sirniiar MR-compatible material for regulating the flow of
cerebrospinal fluid through the catheter after placement of the catheter tip
into
cerebral ventricle or other intracranial fluid compartment under MR imaging
guidance.
With further reference to FIGS. I and 2, in the method of the invention,
the implantabie MR-visible multilumen catheter also includes in another tubing
conduit a metabolic biopsy microcatheter which is used to collect and measure
the number of small molecules present in the extracellular fluid, including
energy-related metabolites, such as lactate, pyruvate, glucose, adenosine, and
inosine, and excitatory amino acids, such as glutamate and aspartate, in a
separate reservoir 3b.
With reference to FIG. i' to Fig. 1 1 of the drawings, in the method of the
invention, MR imagin~; is used to differentiate normal brain tissues from
various
pathologic conditions, including; solid brain tumor, abscess cavity, edema,
necrotic infarcts, rever:~ibly ischemic infarcts, demyelination, and
hemorrhage,
based on the characteristic ADC: of these tissue pathologies already well
established in the medical literature. In order to determine the delivery and
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
34
distribution kinetics of intracerebrovascular, intrathecal, and intra-
parenchyma)
injections or infusions of drug or contrast agents within the brain for
purposes of
creating a means of acquiring a metabolic" biopsy, a sequence of MR images are
collected over a period of time t, which is preferably < 100 min and > 10 sec.
The MR intensity distribution and spatial variation of the calculated ADC of
the
tissue volume undergoing MR imaging prior to drug delivery is compared with
the ADC in the same region following drug delivery in order to determine the
efficacy of drug delivery to the targeted intracranial loci.
Methods to obtain absolute measurements of ADC using MR imaging
have been described in the medical literature, for example, Moseley et al.,
Mag.
Res. Med., 19, 1991, pp. 321-326, and Moseley et al., Topics Mag. Res.
Med., 3, 1991, pp. 50-68). It is well established that if there is restriction
to
diffusion (e.g., from cell walls), then the measured ADC will decrease with
increasing diffusion time. Thus, an express objective of the present invention
is
to evaluate the efficacy of MR image-guided drug delivery by measuring
restricted diffusion with localized MR pulse sequences. In the method of the
present invention, modeling of restricted diffusion is used to estimate the
size of
the diffusion spaces and the permeability of the barriers to drug agents
injected
into the brain microenvironment. A conventional imaging sequence is repeated
with field gradients of increasing strength or duration. The signal decays
away
exponentially as a -bD , where b depends on the strength, duration and timing
of
the diffusion-sensitizing gradients. However, the diffusion gradients make the
sequence extremely sensitive to motion. Thus, in a preferred embodiment of the
invention, a navigator echo technique, or its variants, are used to suppress
the
contaminating effects of patient motion on the ADC measured with MR imaging.
In another preferred embodiment, high speed echoplanar imaging is used without
movement artifact. In a further preferred embodiment of the present invention,
localized measurements of the ADC, ABO, TI, T2, MTC, chemical shift
frequency, and temperature are acquired from images produced from single-shot
or mufti-shot stimulated echo (STEAM), gradient echo (GRE or FLASH), or fast
spin-echo (FSE) MRI sequences.
CA 02289837 1999-11-12
WO 98/52064 PCTNS98/09942
In one preferred embodiment of the imaging method of the invention, a
1.5 tesla, 80 - cm- bore MR imal;er with actively shielded gradients of at
least 20
mT/m is used to acquire; axial di:Ffusion-weighted echoplanar images through a
volume of brain tissue one slice ;at a time, with separate application of
diffusion
5 gradients in three ortho~;onal directions. Trapezoidal diffusion gradients,
equal
in magnitude and duration, are applied in the vertical (anterior-posterior)
direction, and phase-encoding gradients are applied in the horizontal (left-
right)
direction. A 5-cm field-of view and 200-kHz continuous readout sampling is
preferred, which requirea a plateau readout gradient of 12 mT/m. Also
preferred
I 0 are readout gradient trapezoids with 320-microsecond ramps and 640
microsecond plateaus, resulting :in 1.28-millisecond readout lobes and 82-
millisecond total readoc~t time. ~Che spin echo is placed coincident with the
zero-
phase-encoded gradient echo. To attain the preferred diffusion gradient of b =
600 s/mmZ, a spin-echo time of ~~0 milliseconds was used, and the center of k-
15 space is placed symmetrically. Diffusion-weighted images are preferably
acquired as 16 contiguous 1.5 mm slices at 1 slice per second in an
interleaved
order to minimize magr~etizatior~ transfer and slice cross-talk effects. At
least
four diffusion strength, preferably b = 10, 207, 414, and 621 S/mmz, should be
applied separately in each primary orthogonal direction. Reference scans are
20 acquired without phase~~encodin~gradients to allow correction of echo
position
and phase before Fourif;r transformation reconstruction, to minimize image
ghosts. Thus, in the preferred method of the invention, a total of 384
diffusion-
weighted echo-planar spans are acquired in approximately 6.4 minutes. The
resulting 128 x I28 images are reconstructed by two-dimensional Fourier
25 transformation. Nominal image resolution is mm x 2.1 mm x 5 mm, giving a 17-
microL nominal voxel.
With reference to FIGS. 8-11 of the drawings, in the most preferred
embodiment of the MR imaging; method of the invention, a therapeutic drug
agent is injected from a.n MR visible drug delivery device into the
30 intraparcnchymal extracellular space of the brain. The solution containing
the
macromolecular drug agent may either form a cavity or infiltrate the
extracellular
space depending on a number o:f factors. In either case, subsequent diffusion
is
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
36
governed by the volume fraction (extracellular or pore fraction), the
tortuosity of
the brain tissue (apparent increase in path length of the diffusing particle),
and
the diffusion coefficient of the substance itself. A finite and specified
concentration of the substance with a finite and specified volume is deposited
in
the tissue in a period that is effectively instantaneous (i.e. « time-scale of
subsequent diffusion measurements). The injected volume of substance can
exhibit at least two distinct behaviors disclosed by MR imaging in the method
of
the present invention.
In the first example, summarized in FIG. 8, the injected volume can form
a fluid filled cavity in the tissue, within which the volume fraction and
tortuosity
take the value of unity which corresponds to a free aqueous solution. Outside
this region, the brain tissue has a volume fraction and tortuosity. In this
example, diffusion as a function of distance from the injected substance can
be
represented as a series of curves denoting the concentration as a function of
I 5 distance from the center of the cavity at successive time intervals.
Different drug
agents will diffuse at different rates thereby yielding characteristic
individual
signal intensity delay curves on MR imaging. At the interface between the
fluid-
filled cavity and surrounding brain tissue two continuity conditions involving
flux and concentration apply. Since the amount of material leaving the first
region, per unit area of the interface, must be equal to the amount arriving
at the
second, the phase averages of the fluxes in the two regions must be equal.
In the second example, summarized in FIG. 9, the injected material does
not form a cavity but instead infiltrates the extracellular space. The
diffusion of
each agent is related to its molecular weight, molecular radius, and the
tissue
matrix structure into which the material is injected. Throughout the whole
brain
tissue, the diffusion behavior is governed by the volume fraction and
tortuosity
and no discontinuity exists. In the third example of the MR imaging method of
the invention summarized in FIG. IO, MR visualization of a drug agent injected
into a region of nerve fibers in the brain or spinal cord is performed with
diffusion-weighted anisotropic MR imaging. In the preferred method of
anisotropic imaging, a 3 x 3 matrix (tensor) is used, and the signal loss is
measured for at least six directions of diffusion gradient. The matrix can be
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
37
transformed to one that its independent of the directions along which the
gradients were applied, :end there:fore of the orientation of the patient in
the
magnet. In the preferred method, two measurements are of particular interest.
First, the trace of the tensor (i.e. the sum of the diagonal elements) is
relatively
uniform throughout normal brain, despite its anisotropic structure. It can be
thought of as the diffusion coefficient averaged over all directions. Second,
an
anisotropy index, such as the ratio of the diffusion coefficient in the most
freely
diffusible direction to that in the least freely diffusible, is highly
sensitive to the
directionality of the tissue structure. To measure high values in a
directional
I 0 structure the voxel size should bc; small enough so that there is no
averaging of
directions within the voxel. Loss of tissue structure is likely to decrease
the
anisotropy, as the tissue become~~ more like a homogenous suspension. Clinical
observations of changes in diffusion behavior have been made in multiple
sclerosis, in stroke, where the reduction in diffusion precedes the increase
in T2,
I S and in experimental epilepsy.
In the fourth example of the MR imaging method of the invention (FIG.
11 ) macromolecular transport of drugs in tumor tissue is hindered to a lesser
extent than in normal tissue, resulting in an altered ADC which enables the
visualization of injected drug in neoplastic versus normal tissues.
20 A catheter system for delivering fluid to a selected site within a tissue
comprises a pump for delivering the fluid and a catheter coupled to the pump.
The catheter comprises a first tubular portion that has a generally
cylindrical
lumen of a first internal diameter and is composed of a relatively impermeable
material. A second tubular portion that has an open end is disposed within the
25 lumen and a closed dist~~l end is disposed without the lumen. The second
tubular
portion is composed of a flexible, porous material having a preselected
microporosity that is operable to permit fluid to flow from the catheter into
the
tissue. The second tubular portion is selectively moveable with respect to the
first tubular portion. Alternatively, a catheter for delivering fluid to a
selected
30 site within a tissue comprises a first tubular portion that has a generally
cylindrical lumen of a first internal diameter and is composed of a relatively
impermeable material. ,~ second tubular portion that has an open end is
disposed
CA 02289837 1999-11-12
WO 98/52064 PCT/US98/09942
38
within the lumen and a closed distal end is disposed without the lumen. The
second tubular portion is composed of a flexible, porous material that has a
semipermeable membrane with pre-selected molecular weight exclusion that is
operable to permit fluid to flow from the catheter into the organism. The
second
tubular portion is selectively moveable with respect to the first tubular
portion.