Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02291029 1999-11-24
WO 98/55571 PCT/EP98/03184
- 1 -
BAR COMPOSITIONS COMPRISING NOVEL CHELATING SURFACTAI~,TS AND
RELATED PROCESS FOR MANUFACTURE OF SUCH BARS
The present invention relates to personal wash beauty
bar compositions, particularly compositions comprising (1)
novel EDTA-derived chelating anionic surfactants, preferably
in combination with other types of anionic surfactants; and
(2) one or more amphoteric surfactants. The invention
relates to the incorporation of the novel EDTA-derived
chelating surfactants into specific bar skin cleansing
formulation bases.
Through careful balancing of the anionic, amphoteric
and optional nonionic surfactants, and through specific
handling of the novel chelating surfactants during
processing, ultra formulation mildness to skin is achieved
without sacrificing other desired user properties, such as
rich and creamy lather.
Hydrophobically modified ethylenediaminetriacetic acid
(EDTA) chelating surfactants, salts thereof, and methods for
making these compounds are taught, for example, in U.S.
Patent Nos. 5,177,243, 5,191,081 and 5,191,106 (all assigned
to Hampshire Chemical Corp.)
U.S. Patent No. 5,250,728 to B. A. Parker et al.,
(assigned to Hampshire Chemical Corp.), teaches a novel
route of synthesizing the hydrophobically modified
' ethylenediaminetriacetic acid.
__ _ _. _ _ _ _.
"" CA 02291029 1999-11-24
C6480
- 2 -
U.S. Patent No. 5,284,972 to B. A. Parker et al.
(assigned to Hampshire Chemical Corp.) teaches a synthetic
route leading to the salts of the hydrophobically modified
ethylenediaminetriacetic acid which are the novel chelating
surfactants applied by the subject invention.
WO-A-95/07337 (Procter & Gamble) describes high sudsing
detergent compositions containing N-alkoxy polyhydroxy fatty
acid amides, which are provided by the addition of secondary
carboxylate surfactants.
WO-A-95/13356 (Procter & Gamble) describes a personal
cleansing bar comprising 10-70 sodium cocoylisethionate,
4.5-50 parts magnesium soap and 4-15 liquid polyol having at
least two alcohol groups attached to separate carbon atoms,
and is water soluble and liquid at room temperature.
Inform, Vol. 6, No. 10 (-October, 1995, J. Crudden and
B. Parker) teaches the physical and physiological properties
of the novel chelating surfactants. Mild skin cleansers and
mild shampoos are among the potential applications, as
discussed in the articles.
Although these novel EDTA-derived surfactants are
ultra-mild to skin, inclusion of the surfactants into a
personal washing bar is fraught with difficulties. For
example, the lather produced by the chelating surfactant
alone is not as satisfactory as that of a conventional
anionic detergent (e. g., sodium lauryl ether sulfate).
Further, aqueous solutions of the EDTA surfactants at the
concentrations relevant to the personal washing have a
viscosity which is too low to deliver the desired sensory
cues.
AMENDED Sf-IEET
CA 02291029 1999-11-24
C648~ r~- ~ _
2a
By this invention, applicants have formulated these
chelating surfactants into specific skin cleansing
formulations using specific routes of processing such that
lather performance and other desired user properties are not
S sacrificed.
BRIEF SUMMARY OF THE INVENTION
The present invention comprises personal wash bar
compositions comprising:
.. },., .. .. ,
' c
AMEfVDED St+EET
__ ___ _____ _. ,._.___ _. _T_______.
CA 02291029 1999-11-24
C6480 ' ;.
- 3 -
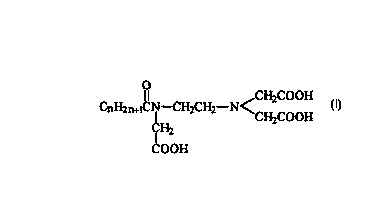
(1) 1 to 40% by wt. of hydrophobically modified
salts) of ethylenediaminetriacetic acid (I)
O
I I CH2COOH
CnH2~~CN-CH,CH2-NCH COOH
CH2 2
COON
(I)
wherein n is from 1 to 40;
(2) 1 to 40% by wt. of one or more synthetic (non-
soap) anionic surfactants other than the EDTA
derived anionics described in (1) (for lather
enhancement);
(3) 1 to 20% by wt. of one or more amphoteric and/or
zwitterionic surfactants (to reduce skin
irritation and enhance lather)
(4) 0 to 10% by wt. of one or more nonionic
surfactants;
(5) 20 to 85% by wt. total composition of a
structurant selected from the group consisting
of
alkylene oxide components having a molecular
weight of from about 2,000 to about 25,000; Cg to
C22 free fatty acids; C2 to C2p alkanols; paraffin
waxes; and water soluble starches;
(6) 0 to 20% by wt.-of fatty acid soaps;
wherein no rhore than 1% ~f composition comprises
inorganic salts with multivalent counterions (e. g.,
aluminum chloride).
The addition of 1 to 40% by wt. total composition of
the novel EDTA-derived surfactants will lead to
significantly enhanced mildness in such compositions without
sacrificing the surfactancy of the EDTA-derived surfactants.
p,6~A~PiDED Sh~EE1
CA 02291029 1999-11-24
WO 98/55571 PCT/EP98/03184
- 4 -
In a second embodiment, the application relates to a
process for making the composition mentioned above by
(1) dispersing an acid form of EDTA into the
a
structurant(s) at a temperature between about 80'
to 120°C;
(2) adding sufficient caustic (e.g., NaOH) to
neutralize the EDTA acid surfactant (molar ratio
of caustic to EDTA acid from about 1:1 to 1:3);
and
(3) mixing the EDTA/structurant solution with
remaining compounds at temperature of 80° to 120'C.
DETAILED DESCRIPTION OF THE INVENTION
In one embodiment, the present invention relates to
novel personal washing bar compositions, particularly
compositions in which the surfactant system comprises 1 to
40% wt. total composition of the salt or salts of
hydrophobically modified ethylenediaminetriacetic acid, and
additionally comprises one or more anionic surfactants and
one or more amphoteric surfactants, wherein no more than 1%
of said compositions comprise salts with multivalent
counterions (high levels are associated with lather
depression). Using precise formulation windows, it is
possible to incorporate EDTA-derived surfactants into bar
compositions retaining the benefits of mildness and without
sacrificing latherability.
In a second embodiment, the invention relates to a
process for forming such bar compositions while retaining
mildness and lathering, and acceptable bar properties by
ensuring that EDTA acid is first dispersed into structurant
and subsequently adding sufficient caustic to neutralize the
EDTA acid.
__ . ___.~ _ .__.~.._.
'- CA 02291029 1999-11-24
C6480 ,
- 5 -
The compositions and processing are defined in greater
detail below:
In the first embodiment of the invention, the personal
wash bar compositions comprise:
(1) 1 to 40% by wt. total composition salt or salts of
hydrophobically modified ethylenediaminetriacetic acid;
the hydrophobically modified ethylenediaminetriacetic
acids have general structure as follows:
'' ' ~" -" ~ CH~COOH
CnH2n.,.lCN-CH2CH2-N'CH~COOH
CH,
I -
COOH
(I)
where n is from 1 to 40.
.,
If unsaturation occurs, the hydrophobically modified
group may be CnH2n-1 where n is 2 to 40 and if further
unsaturation occurs, the group may be CnH2n-3 where n is 3 to
40 and so forth. The salts are the salts of one or more of
the carboxylic acid groups. These compounds and methods of
their preparation are. described, for example, in U.S. Patent
No, 5,284,972 to Parker et al., hereby incorporated by
reference into the subject application;
(2) 0.1% to 40% by wt. total composition one or more
anionic surfactants other than the hydrophobically
modified EDTA-derived compounds described above;
inclusion of the such anionic surfactants
(i.e., lathering surfactants) is required
AMENDED SKEET
- CA 02291029 1999-11-24
:.-
C6480 ~ - ~,
- 6 -
because the EDTA-derived surfactants alone do
not deliver satisfactory lather performance;
(3) 1 to 20% by wt. total composition one or more
amphoteric and/or zwitterionic surfactants;
inclusion of the amphoteric and zwitterionic
surfactants is a criticality and required because
the amphoteric surfactants reduce the skin
irritation potential of the anionic surfactants in
(2) and enhance the lather performance; and
(4) 0 to 10% by wt. total composition one or more
nonionic surfactants.
Finally the formulat~ioii's of the invention comprise no
more than 1% wt. total composition of inorganic and organic
salts of Calcium (Ca2+), Magnesium (Mg2+), Aluminum (A13+)
and other multivalent metal counterions, and mixtures
thereof; preferably said salts are excluded from the total
composition;
the restriction on the concentration of said salts is
important because such salts tend to diminish the lather
performance of the EDTA-derived surfactants.
Examples of the multi-valence salts include, but are
not limited to, Calcium Chloride, Magnesium Chloride,
Aluminum Chloride, Magnesium sulfate, Magnesium Stearate,
Calcium Laurate, etc.
By using these specific formulation ingredients in
specific formulation windows (e. g., 1% to 40% EDTA-derived
surfactants), it is possible to make a composition
comprising the mild surfactants without sacrificing
lathering ability. To further insure this, the formulations
should be made using the process encompassed by the second
embodiment of the invention described in more detail below.
AMENDED SHEET
CA 02291029 1999-11-24
WO 98/55571 PCT/EP98/03184
_ 7 _
The various formulation components are described in
greater detail below:
The EDTA-Derived Anionic Surfactants (Component (1))
The salt and/or salts of the hydrophobically modified
ethylenediaminetriacetic acid (EDTA) are salts(s) of the N-
acyl EDTA surfactants described in US Patent No. 5,177,243,
5,191,081, 5,191,106, 5,250,728, and 5,284,972, all of which
are incorporated by reference into the subject application.
The synthesis, physical and physiological properties of the
EDTA-derived surfactants are also summarized in an article
published recently (Inform, Vol. 6 no. 10, Oct. 1995).
The hydrophobically modified ethylenediaminetriactive
acids have general structure as follows:
O
CnH2~~CN-CH2CH2-N~CH2COOH
i H2 2
COOH
(I)
where n is from 1 to 40.
If unsaturation occurs, the hydrophobically modified
group may be CnHzn_1 where- n is 2 to 40 and if further
unsaturation occurs, the group may be CnH2n_j where n is 3 to
40 and so forth. The salts are the salts of one or more of
the carboxylic acid groups. These compounds and methods of
their preparation are described, for example, in U.S. Patent
No, 5,284,972 to Parker et al., hereby incorporated by
reference into the subject application.
-' CA 02291029 1999-11-24
C6480
_ g _
The counterions which may be used for the EDTA derived
surfactants of the subject invention include but are not
limit to Sodium (Na+), Potassium (K+), ammonium (NH4+),
monoethanolamine, diethanolamine, triethanolamine, N-
Propylamine, isopropylamine, and tris(hydroxymethyl
aminomethane). As noted, multivalent counterions should be
avoided.
Examples of the N-acyl EDTA surfactants used by the
current invention include, under the names given by B.
Parker et al. (Inform, Vol. 6 no. 10, Oct. 1995), sodium
lauroyl ED3A, Potassium ~oc.oyl.ED3A, triethanolamine
myristoyl ED3A, and sodium Oleoyl ED3A.
The EDTA-derived surfactants comprise 1% to 40% of the
total composition. In addition, the surfactant should
comprise at least 5%, preferably 8%, more preferably 10% of
the total anionic surfactants in the composition.
Other Anionic Surfactants (Component (2))
The anionic surfactant other than EDTA-derived
surfactant may be any such synthetic, non-soap anionic
surfactant well known to the person skilled in the art.
The anionic component will comprise from 0.1 to ~0% by
weight of the composition, preferably 5 to 30%, most
preferably 8 to~25% by weight of the composition.
Zwitterionic and Amphoteric Surfactants (Component (3))
Any zwitterionic or amphoteric surfactant well known to
the person skilled in the art may be used.
A~~EPIDED Sti~EET
i
:,_ ~-. -:.~ -_ _ :-_._ __. ..
-- CA 02291029 1999-11-24
C6480 ;. .'
- 9 -
The amphoteric/zwitterionic comprises 0.1 to 20% by
weight, preferably 0.5% to 15%, more preferably 1.0 to 10%
by wt. of the composition.
Optional Nonionic Surfactants (Component (4))
In addition to one or more anionic and amphoteric
and/or zwitterionic, the surfactant may optionally comprise
any nonionic surfactant well known by the person skilled in
the art.
Nonionic comprises 0 to 10% by wt. of the composition.
. ;. - .. . . .
Other Optional Ingredients
In addition, the compositions of the invention may
include optional ingredients as follows:
Organic solvents, such as ethanol; auxiliary thickeners,
such as carboxymethylcellulose, magnesium aluminum silicate,
hydroxyethylcellulose, methylcellulose, carbopols, glucamides,
or Antil~R~ from Rhone Poulenc; perfumes; sequestering agents,
such as tetrasodium ethylenediaminetetraacetate (EDTA), EHDP
or mixtures in an amount of 0.01 to 1%, preferably 0.01 to
0.05%; and coloring agents, opacifiers and pearlizers such as
zinc stearate, magnesium stearate, Ti02, EGMS (ethylene glycol
monostearate) ow Lytron 621 (Styrene/Acryl~te copolymer); all
of which are useful in enhancing the appearance or cosmetic
properties of the product.
The compositions may further comprise antimicrobials
such as 2-hydroxy-4,2'4' trichlorodiphenylether (DP300);
preservatives such as dimethyloldimethylhydantoin (Glydant
XL1000), parabens, sorbic acid etc.
AMENDED SKEET
CA 02291029 1999-11-24
WO 98/55571 PCT/EP98/03184
- 10 -
The compositions may also comprise coconut acyl mono-
or diethanol amides as suds boosters, and strongly ionizing
salts such as sodium chloride and sodium sulfate may also be
used to advantage.
Antioxidants such as, for example, butylated
hydroxytoluene (BHT) may be used advantageously in amounts
of about 0.01 or higher if appropriate.
Cationic conditioners which may be used include
Quatrisoft LM-200 Polyquaternium-24, Merquat ~-polymer; and
JaguartR~ type conditioners from Rhone-Poulenc; and
Salcare~-type conditioners from Allied Colloids.
Polyethylene glycols which may be used include:
Polyox WSR-205 PEG 14M,
Polyox WSR-N-60K PEG 45M, or
Polyox WSR-N-750 PEG 7M.
PEG with molecular weight ranging from 300 to 10,000
Dalton, such as those marketed under the tradename of
CARBOWAX SENTRY~R~ by Union Carbide.
Another ingredient which may be included are exfoliants
such as polyoxyethylene beads, walnut shells and apricot
seeds
The structurant of the invention can be a water soluble
or water insoluble structurant.
Water soluble structurants include moderately high
molecular weight polyalkylene oxides of appropriate melting
CA 02291029 1999-11-24
WO 98/55571 PCT/EP98/03184
- 11 -
point (e.g., 40° to 100°C, preferably 50° to 90°C)
and in
particular polyethylene glycols or mixtures thereof.
Polyethylene glycols (PEG'S) which are used may have a
molecular weight in the range 2,000 to 25,000, preferably
3,000 to 10,000. However, in some embodiments of this
invention it is preferred to include a fairly small quantity
of polyethylene glycol with a molecular weight in the range
from 50,000 to 500,000, especially molecular weights of
around 100,000. Such polyethylene glycols have been found
to improve the wear rate of the bars. It is believed that
this is because their long polymer chains remain entangled
even when the bar composition is wetted during use.
If such high molecular weight polyethylene glycols (or
any other water soluble high molecular weight polyalkylene
oxides) are used, the quantity is preferably from 1$ to 5~,
more preferably from 1~ to 1.5~ to 4~ or 4.5o by weight of
the composition. These materials will generally be used
jointly with a large quantity of other water soluble
structurant such as the above mentioned polyethylene glycol
of molecular weight 2,000 to 25,000, preferably 3,000 to
10,000.
Water insoluble structurants also have a melting point
in the range 40-100°C, more preferably at least 50'C, notably
50°C to 90°C. Suitable materials which are particularly
envisaged are fatty acids, particularly those having a
carbon chain of 12 to 24 carbon atoms. Examples are lauric,
myristic, palmitic, stark, arachidic and behenic acids and
mixtures thereof. Sources of these fatty acids are coconut,
topped coconut, palm, palm kernel, babassu and tallow fatty
acids and partially or fully hardened fatty acids or
distilled fatty acids. Other suitable water insoluble
structurants include alkanols of 8 to 20 carbon atoms,
CA 02291029 1999-11-24
WO 98/55571 PCT/EP98/03184
- 12 -
particularly cetyl alcohol. These materials generally have
a water solubility of less than 5 g/litre at 20°C.
Soaps (e.g., sodium stearate) can also be used at
levels of about 1~ to 15~. the soaps may be added neat or
made in situ by adding a base, e.g., NaOH, to convert free
fatty acids.
The relative proportions of the water soluble
structurants and water insoluble structurants govern the
rate at which the bar wears during use. The presence of the
water-insoluble structurant tends to delay dissolution of
the bar when exposed to water during use and hence~retard
the rate of wear.
Another optional ingredient is oil/emollient which may
be added as a benefit agent to the bars compositions.
Various classes of oils are set forth below.
Vegetable oils: Arachis oil, castor oil, cocoa butter,
coconut oil, corn oil, cotton seed oil, olive oil, palm
kernel oil, rapeseed oil, safflower seed oil, sesame seed
oil and soybean oil.
Esters: Butyl myristate, cetyl palmitate, decyloleate,
glyceryl laurate, glyceryl ricinoleate, glyceryl stearate,
glyceryl isostearate, hexyl laurate, isobutyl palmitate,
isocetyl stearate, isopropyl isostearate, isopropyl laurate,
isopropyl linoleate, isopropyl, myristate, isopropyl
palmitate, isopropyl stearate, propylene glycol monolaurate,
propylene glycol ricinoleate, propylene glycol stearate, and
propylene glycol isostearate.
CA 02291029 1999-11-24
WO 98/55571 PCT/EP98/03184
- 13 -
Animal Fats: Acytylated lanolin alcohols, lanolin,
lard, mink oil and tallow.
Fatty acids and alcohols: Behenic acid, palmitic acid,
stearic acid, behenyl alcohol, cetyl alcohol, eicosanyl
alcohol and isocetyl alcohol.
Other examples of oil/emollients include mineral oil,
petrolatum, silicone oil such as dimethyl polysiloxane,
lauryl and myristyl lactate.
The emollient/oil is generally used in an amount from
about 1 to 20~, preferably 1 to 15~ by wt. of the
composition. Generally, it should comprise no more than 20%
of the composition.
Preferably, the compositions of the invention should
comprise no more than 10, and should more preferably be free
of inorganic or organic salts of multivalent metal
counterions. Such metal counterions are defined as having
valence of +2 or higher and include counterions such as
calcium, magnesium and aluminum. Examples of such salts
include, for example, aluminum chloride, magnesium chloride,
calcium chloride and magnesium laurels. G~thile not wishing
to be bound by theory, it is believed essential to keep the
amount of such counterions low or absent so that they don't
interfere with lather performance of EDTA-derived anionic
surfactant.
In a second embodiment of the invention, the invention
, relates to a process of making the composition of the
invention to ensure that EDTA-derived surfactant is
. incorporated, provides desired mildness characteristics and
that latherability is not at the same time compromised.
CA 02291029 1999-11-24
.:.
C6480 = : . ; . .
- 14 -
More specifically, process comprises:
(a) dispersing an acid form of EDTA into molten
structurant system at temperature between 80°C and
120°C; and
(b) adding sufficient caustic to neutralize the EDTA
surfactant.
This in situ neutralization process is necessary to
avoid gelling of the EDTA derived surfactant. The gelling,
which occurs in an aqueous solution, prevents a homogeneous
mixing of the ingredients.
The following examples are intended to illustrate
further the invention and are not intended to limit the
invention in any way.
All percentages are intended to be percentages by
weight unless stated otherwise.
EXAMPLES
Protocol of Skin Mildness Evaluation
Mildness Assessments: Zein dissolution test was used
to preliminary screen the irritation potention of the
formulations studied. In an 2278 (8 oz). jar, 30 mLs of an
aqueous dispersion of a formulation were prepared. The
dispersions- sat-.in a 45°C bath until fully dissolved. Upon
equilibration at room temperature, 1.5 gms of zero powder
were added to each solution with rapid stirring for one
hour. The solutions were then transferred to centrifuge
tubes and centrifuged for 30 minutes at approximately 3,000
rpms. The undissolved zero was isolated, rinsed and allowed
to dry in 60°C vacuum oven to a constant weight. The
p~ENOED SHEET .
CA 02291029 1999-11-24
WO 98/55571 PCT/EP98/03184
- 15 -
percent zein solubilized, which is proportional to
irritation potential, was determined gravimetrically.
The Lather Volume Measurement: The lather performance
was studied by a cylinder shaking test. Forty grams of a
test solution was put in a 250 ml PYREX cylinder with cap.
Foam was generated by shaking the cylinder for 0.5 minute.
After the foam settled for 2.5 minutes, the foam height was
measured.
An In-Vitro Test of the Skin Mildness of Na-LED3A
The skin irritation potential of Na-LED3A was
investigated by the zein dissolution test. As shown in
Table 1, Na-LED3A dissolved significantly less amount of
zero than commonly used anionic surfactants, such as sodium
cocoyl isethionate and sodium lauryl ether (3E0) sulfate.
The result indicates that the sodium lauroyl EDTA is an
ultra-mild anionic surfactant to skin.
. . ..... ....... ..n... :::::..~:::::.::~-.-...-.-..-.-.-
.-.-.-.-....-.....:.:..
..... .. .. ..... ..................:.-_-_..__..-._...._._.
..........,........................
.. ...............................,-:.:._ ___
::::....:::::::::::::.~::::::::::.::::::::::
__...
:;: ;.~:..:~.:~;
N.,rr.............~a.r:..:.....:~..,,r:,.,:.::~...::~;:.:,.::.:.::::-.::::::::
.................
::, .::: :.::::::::::::::::::.:.::::.~:::.<.::::::::
..................
. .. .. ..:........ . . . :..:::: .....
..... . ... . .. ..:.. ... :::::n.:::::-
:::.::.:::;:::::..:.:::::::::::::.:::::.:::n:.:::
rr:.:.~::..r ...f, ,r, t~.v..:.. ::::?::::.
., .: ...,.::...~.... . .. :
! .nf.::x. ...:...
..........v........ . . ..r.........n...
:::.:tt:::.:::.att..,.:...:.:::.::at:::..
:.. .t 9 ..../.. ...........:.... .:....
.:..\.. .; :.. :..........
t::r.:: :a: rt f .: .C: ..... ,...t.....:.....:
tra~'t::. .. .:.~d.~.~r: .. :5.................;................. ...
;.......:..:..;.::....
t:,... ...
:.'.:: r.: n . .
......:.......:.....................
. . -..;:: ... a:.:::at.:.. ....
....: ..:: .: .. 't:4:::~::'4:'a:.:n::'nvt;::
. .nf /~::: . . . .:r. : ': '.::.: t: t:::u.;::i::
.:...a:n.: : .. ~ ~t:.;- :::.:.::,.::
:..r..}!v. ...: .:...c~'r/.x.:::.n.:.~:.~..
... : . : .. . . ..::
:'' .. . ....
: .....
..: r~.xvfi ~': :v::::::::::... .......:;::
. . . : :: : .. .v::::: ~ ~ ...
: . i. ~ w::
~ . ...
1 ~
n : ~.-.
ft : . :~
~ ~ ; :.,
/ . . ~.
> :::4:
~ ~ ~
~~~ ::.;
~ ~
~~~~
~~
~
~
~~
. . .
. ...
::u.;:::::i:::v::av%:C:v:.::::::.::::::::
.ItZ!..r...........................................
,..:. .
.........................................
. ..; .I: .
.i.F.$ :::
.; .tf .::: ! ....................
.n. .....
t .....................:......
. . ::. ~ .
'-.. ... ::::
.
.
:
.f. ~: nnr :n v . ... . H:...J......
.. fi..t
. . ::... : .. .. .........................................
v.::f:.!: nw::F..:::.: ::::
>.a.:::::.~::::.~::.:::.:.~.~..~.::....::::::::::..:::.:::::..:..
n:...........................................
Surfactant ~ Zein Dissolved
1~ Na Cocoyl Isethionate 34
1~ Na Lauryl Ether (3E0) 41
Sulfate
l~ Na LED3A 8
When Na LED3a is incorporated in typical bar
formulations (Example 5), the percent zero dissolved is also
significantly reduced as shown below in Table 2.
CA 02291029 1999-11-24
WO 98/55571 PCT/EP98/03184
- 16 -
... -......... ......... .. . .., .....-...
-
..,,.,....:.:::.~::..~::::....:::::::.::.:.:.:::.:::.::.~::::::::::::::.:
----.... ..
::::::.:..::.
~::f ~
. .~:i:.i::i.''.v::;:
~.. f ::::ifii;
,r.. . r
't .,f:aa:::~::..~:.
'.xN. r wrr.-' :a:,;.,.:,...
N :..3'r~.:
f.,..:: x "..
' iiF::>:': :: Kw:i::'i'::4i'~r
1 "' ' .'<x>:nx, .,. rr. f ~'~'W.m:::.v::
'x4...:t .: v:.:
'::~~j'.'Yc<' .. fY:i ''
r ...i:: . .h............
ii~'' a . . . h.$':..:hv:::::nv.vfv::,~':.:'.':
:.:x:.
.f/...::ttsi' . :a :.c:~:::~u:.::::::::: :-. . ~ , .~..:::::::.::..:
. IS' ':'~ : v:5.: 4 . . .. . :.:::::
0 i ~...::: . .S~ ' r: : '~f.:.:.Sa , ::.::._:::::..::::.
. ~' .~ ....
h 'J. . . T .
'/.: . r ' t"%.~. : tx~."r ' :..a: .. ...: :::.::.
:r ::..''i.:'t:
: . .. . f.~.,..,: .. Jrr.'' . ,. .: ; w~:::::::::::
.... . ..... : .. . .... . ;:' . ,. ~~3
:: .;. .; .. _:: ::., ::..:."..,. :~
:a:~: ' . :~ :
. r'.',~: '
f ,::
' :
. .: :
*. '
..
~f... .:t.. .
.
~
~
~
.
:
W#;
:
~
~
~
.~
,
, ,
. .
, .. .
; . :
. .. ~.
: .:
.. r .. : :
~: x:
: :.... ... ',:
v,~ ..:.::r.: tx: . ....~..... .v.:.~.iS;:
: ... . :.:.. ::.$:::::..........
: . .. . .:. , . : ..; _.:::
:: ::::: ~::
,~: ..................
f:....t~. ~ : .... ' .... '.........:
:~. .........
~'. .. ::'~ i:
: F i:. w:::n
.dff:': . . : ..:......i'.'i . ~ ~
~ .... ...... ... .......... ............................
/.: ...... n . ..k ......1. :.:
....... ...........................:
..
:
:
:
:: v
~: :
~
Jff
r
. ...
.........
...
.....
.
.
.
...~
...n......:
.
..::~ :.::X.::.,t::.::::.:::::.::::v::v..:...:....n.:...................
~..:.........
Comflosition %, Zein Dissolved
Comparative Formulation A (no Na-LED3A 15.3
Formulation B 7.3
Formulation C 6.2
Formulation Processing
the Preparation of the EDTA Derived Surfactants:
Sodium lauroyl EDTA (named as Na-LED3A) was obtained through
neutralizing N-lauroyl-N, N'N'-ethylenediaminetriacetic
(Hampshire, under the trade name of LED3A) using 50o sodium
hydroxide (NaOH) solution. LED3A was first. dispersed and
mixed in molten polyethylene glycol 8000 at a temperature
between 80°C and 120°C. A precalculated amount of sodium
hydroxide solution (50%) was slowly added to neutralize the
LED3A. After adequate mixing, the remaining ingredients
were added. This in-situ process was used to avoid the
gelling of EDTA-derived surfactants in an aqueous solution
(gelling occurs at concentrations between 40% and 79o by
weight in water) which prevents a homogeneous mixing of the
bar material.
Formulation Processing: Formulations shown in the
examples of this invention were prepared in 400 mL beakers
in a 100°C oil bath. Mixing was accomplished with a
variable speed overhead motor. Batch size was varied from
100-250 gms. All chemicals used except the EDTA derived
surfactants were commercial materials and used as supplied.
CA 02291029 1999-11-24
WO 98/55571 PCT/EP98/03184
- 17 -
The lather performance of the Na-LED3A aqueous solution
is not as satisfactory as those commonly used anionic
surfactants, such as sodium lauryl ether (3E0) sulfate. As
shown in Table 3, the lather volume of the 2.5~ Na-LED3A is
significantly less than that of SLES. However, by adding
relatively low levels of SLES and Cocoamidopropyl betaine as
coactives to the Na-LED3A solution, the lather performance
was greatly improved. This example demonstrates the
necessity of inclusion of anionic and amphoteric surfactants
into an EDTA-derived surfactant based skin cleanser.
. .. .. . . ..... .. ........... . ......~.~~..:: . :::
:::::::.:::.::::::.:::.~:::::.:::::
.. . ........ ..... ........:..~. : .,:..::.:.....:
.:.}:?.:...
... ~a.... . v:: \;c : Y:'/r%?:
,.,..: i::: .:. av
,. .,~
.::.:<...:.:~pt~
.:. ::? :
..y~,y~ "'' s': ?t. :::: Y':?-'.. ::...f..:...,.~...
\~'8k?"i Y ....
7 '' i::5'':.~4.
':S%' : ...lf : .~'j.:v:...
x ..:t-::: ....::.. >
..f:'~ "~kk' ~p~ ~g ,y
" t:~ . . x...: ~ ~. .
~. ~:3:5.::~
_._."~V-.... rrf?-....- ... .f........... y
': . .i~:. . ''.. ..H.::;
.. Y :'~7tr;Y.:'~ .. :'':'.?:::?~:.
n q'. J..w:.~.... ,r?.::.,,,.
x.'tr. . . ~ . j:
. 'f. : .t?n?-n'Y . Y"ffv:/vtv.:v. : ::i~'~i:'':i?ski?.y v.
'':::. ~~~, r..:. . ?::i, . :j .:. : ~//, ~ :~.?:f.:.
r v kv
:. . ~. ~
r5. ! ~7~.~
:~::' ' ~
?~
<
: .
' ' ~~~~~
'
'
'
r'
~
'
~
'
'
~
'
~
'
'
i .
. ~
~
., . :. ; :. . . ~
. .
: ...r. : :i:
..??:' : .
. :.r 17y
. .. . . . .
fY .. : ~:::
F . .. ..::.. :.
: . : ? :...........
. :..::.....
.; .Y.;; ,x -. -:: . :.
f.: .:... ... .
-: . .: .. ............
...' ~ .
~~
?~
~
~
.
......
.. .,~.~ ..~::.. ?.~:~~f.~
~.,~: . .. ::..................................................
..... .............::.5~....... :.......................:..
: : :.:: :: :.::.
:...........................................................,......
.. : r-.~.:... .............,~,,.<._........._............",~.
_
Comnos i t ions ~,~ather
Volume (ml)
2.5~ wt. Na-LED3A* 124
2.5~ wt. Sodium Lauryl Ether (3E0) Sulfate 170
2.5~ wt. Na-LED3A, 211
1.0~ wt. Sodium Lauryl Ether (3E0) Sulfate
0.5~ wt. Cocoamidopropyl Betaine
* An EDTA-derived anionic surfactant defined in Example
1.
Table 4 shows the lather volumes of bar formulations
which are composed of Na-LED3A. This example demonstrates
that acceptable lather performance is achieved when the
chelating surfactant is incorporated in bar formulations.
CA 02291029 1999-11-24
WO 98/55571 PCT/EP98/03184
- 18 -
. .. . ...... .. . .... .
...... ..... ............_.........
. . ......... ...................
m h . . ... .
..............:.....::.:.:::::::::::.~::::.....:::.:.:::
. .. .... .. .:...:., .. ., ::.::::.~::::::. .~::::::::
. .. ~...~..~ ~. .... ....... .:T.:.,.
.
.. .: ... ..... .. . .... ......,......~.::
. .
< c':~ t'
'f.:f .v ;., ::..
:f . .~../n:v. .:. ..nT.:. n f. v'~
k.::. :r: :f::;.:::::n:;;.:::;:.:
... >::
..,. ::...
6.:.'f f"f. . .:\ ~'::.:::~~o-....: ....
:: _o-i':... ..
Nf ..:
.. h . . t.. fi ffX. v
:"f'......f
.; .,;.,::. :.. :':::if ,:::. . .:~.:::
. ' x. ,.. .e: ...,;
:3 r ;:.:.~..::.. : ~.:. ..r...:l::%:''<:
G% ..
:i:.::i . .
.. o-..:.h ':":: ...:~...... 7:'
.::...:
f,.,, 5 . .'.'.. . '.'i 'i .'.'~'.'y~
4!.. .':...fv . ~ : ::;!.. ' : 7: ii. f. >::.'' ;:'..
":." ; . .'v :'w'::.:: ..:;3:tr::if:
. .. b
.; . . ,. .. . ~7'
3 '
. :~~
r ~
f .
t ~~
~
. r :~' ~~::::
. f ~
~~ '~''
'' ' '
~ '
~ ~~
~ '
, . .. ,.
y ; :.
..: .y .
. :,
. ,~.;
::: ,:"
. . ,
, ~;:. , ,: ... .... .
y :
" :;;;
' ' . .
: . .....
4L ' f: . . ., .. ....... . .....:.,..:
; .. . .. . :::.:
... l. .
.'.
;, . . . .. . . . ..... C .. ~
.. . ....:.....::........ ..
..a.. ..... ..:.. ... . .:.. .. .. ............:......................
.. ..: .::. ... :::~........................ . . . ....... .... . ..
. ......:. . . . .: .:...................................
...... ..:..... ...:.....::.......................,..,....
........:. .. ....:...:..., .':.c.~.,
:::::::.:::..c.~.:...:...........""""~."""",.........;.......,.................
....
z :::.......................:.:.
.....:..
Compositions Lather Volume (ml)
Formulation A > 250
Formulation B > 250
Formulation C > 250
ThP Defoaming Effect of Multi-Valence Salts to the EDTA-
~~rived Surfactants
Organic and inorganic salts containing multi-valence
salts, such as Aluminum Chloride, Magnesium Chloride,
Calcium Chloride, Calcium stearate, Magnesium laurate, etc.
are often used in personal washing products. However, these
multi-valence salts can interact with the EDTA-derived
surfactants and cause defoaming if the salt concentration is
above to wt. total composition. As shown in Table 2, 2.50
salts significantly defoamed the EDTA-derived surfactant.
Therefore, preferably, these multi-valence salts are
excluded from the skin cleansing compositions claimed by
this invention.
CA 02291029 1999-11-24
WO 98/55571 PCT/EP98/03184
- 19 -
* An EDTA-derived anionic surfactant defined in
Example 1.
Skin Cleansincr Composition
All amounts are given in percentage of weight.
Formulations (A), (B) and (C) used sodium cocoyl isethionate
and Na-LED3A as the major anionic detergent with amphoteric
cocoamidopropyl betaine as a coactive. The formulations
provide rich, creamy, and slippery lather that was rinsed
off easily.
........................................
...............................~.................
......... ...... . ...............................
.................................
......
............................................................................
............................
..... ......... ................ ........:.............. ............
.... .. .....................,. .....:.-
..:...........................:......
..:................ ........ ..... .. .......:.............
... ....................... ....... .. ::::::.~:::::::::::::.
.....:.. .. ..: . . ........ .. .. .........
... ..... ...:...:.................. :.:.~::::::....
.:....:.. ............... ....:........:......::.
w:::::::?:r.................
:..:.......................:........
.......:::..:...: h:.. ... . ..:.........:. .............n.............
.: . . ., .... ... ..............:. .. ...
..... ....... .........:. ......... ...........................:...
...........:....... .... .......................
......................
...:J.. .... ...., ... .................
.,..,~:..,:n ~....::...:. J.; a fi:: . . ...............
,x,.; .::?.:7: w:\: ::n ..n:..n::::nv. . .... ......:
. :..i. ..;, 'i..: ~ .......:::Jr .:.
..........i:.?:.:.:.................
..:~..n... ..:'':.~ :::::::: :v :;w::x.. ..................:
:v::: v: :v: : r,.w:'u.-:::::::::::: ........................:
:vn. ..........,......:::::.:
..4h .... ..1... ...: ..... :ry:::
:. n a ...: ...J ....:........: ...:.:......:..... .............x
. . .. . . .. .r .........s.J ..:....... ..:......:mv:
.............
.............:...............:.......
. .... . . . r..:....... ..J....... . .. ......................
. . ... ......... ................ . .... (C)
. ..... .... u. ..s.:s:. . ........ ::..:::w:::::
.. :......:..... ........:............ u.::?::
..C...:.l.n:: .........:.....:......:.:J ::
5.:..k::...:........?~ ............. ........................
.-t :.::.... . . . ... . ... .:t.......:.................
.......................
.......,............. (B)
... ......... ............ ...........
. J~...... .......... ..?.. .........:...a........
. . . .:. . ............... ................................
....: .. . ..a
...,......:...........:.1:........:......:....:..:....:.........
............ . . .. ..:. ...............:.....
.............................
....:c.....:..." ...................
...:..... .. .... ....... .................
........... . . . . . .:. ................
....:............. ........ .....
... ........ .. >:. .... ............:.
. ...............,:.......:.................:.............
. . . . . .:.. . .. .............:......
...........:........ ......:
..; ..: .y. fy(..: q. : b::::::::. ~:::
rrs:vs.: :vnvv:.sw::: :..:.::: ::w:::::.:v:x:::.
_::::: ~~~~~. v.~':?::::::::::.a.:::::
~: T:: nw::._ n_. ~: ~ ::w:::::
:,r.?: . .: ..:nr.:.
.................:.....................................................
. .. .. .:... ...............r.....:..................x.........
......$F....:..........7.......................................................
..........................................................,........,...........
..............
Formulation (A)
Sodium cocoyl isethionate (From DEFI*) 27.0 13.5 7.0
Polyethylene glycol 8000 32.0 32.0 32.0
I
Cocoamidopropyl betaine 5.0 5.0 5.0
Palmitic-stearate acid (From IGEPON or 16.5 16.5 16.5
DEFI)
Sodium stearate 5.0 5.0 5.0
Maltodextrin 6.0 6.0 6.0
Na-LED3A** 0 13.5 20.0
Sodium isethionate 2.2 2.2 2.2
Coconut acid 1.1 1.1 1.1
Water 5.0 5.0 5.0
*DEFI: directly esterified fatty acid i.sethionate, which
is a mixture containing about 74$ by weight of sodium
aryl isethionate, 23o stearic-palmitic acid and small
amounts of other materials, manufactured by Lever
Brother Co., U.S.
*Na-LED3A: an EDTA-derived surfactant defined by Example
1.