Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02291612 1999-11-29
WO 98/55176 PCT/SE98/01029
A TRANSURETHRAL DEVICE
The present invention relates to a transurethral device for delivery of a drug
to the urethra
of a human or animal body comprising a drug carrying element which presents an
elongate
shaft having a free forward end for insertion into the urethra of the human or
animal body
in a forward direction, the elongate shaft being provided with a channel
having an opening
in the outer surface of the elongate shaft for transport of the drug to the
urethra, and is
particularly, although not exclusively, concerned with a transurethral device
of the
aforementioned type for use in the treatment of erectile dysfunction such as
impotence,
io priapism and Peyronie's disease in living male human or animal bodies.
Impotence in living male human bodies is the condition in which a male human
body is
unable to attain or sustain an erect penis for the satisfactory engagement of
sexual
intercourse. There are numerous documented causes for impotence, for example
is impotence caused by vasculogenic disorders such as insufficient blood flow
to the arteries
in the penis or excessive venal outflow. One aspect of the present invention
is directed to
erectile dysfunction such as impotence which is treatable by the local
application of an
erectile dysfunction treatment drug such as an impotence treatment drug to the
urethra.
2o Prior International patent application publications W095/26158 and
W096/28I42 make
known various types of transurethral device for treating erectile dysfunction
such as
impotence, priapism and Peyronie's disease in a living male human body by
delivering an
erectile dysfunction treatment drug into the urethra of the male human body.
For example,
W095/26158 and W096/28142 make known transurethrai devices which comprise a
2s transurethral insertion element having a drug carrying component part and a
plunger
component part movable in the drug carrying part. The drug carrying component
part
comprises an elongate shaft portion for insertion into the urethra of the male
human body
and a handle. portion which is connected to the rear end of the shaft portion.
In some cases
a sheath is provided for sheathing the insertable portion of the elongate
shaft.
CA 02291612 1999-11-29
WO 98/55176 PCT/SE98/01029
2
In one such transurethral device the drug carrying component part is provided
with an
open-ended axial bore which comprises a rearward section of a first width
which passes
through the handle portion and a forward section of a second width less than
the first width
which passes through the elongate shaft. The device is pre-assembled with the
erectile
dysfunction treatment agent, typically in the form of a solid pellet, in place
in the forward
section of the axial bore in the drug carrying part.
The plunger component part comprises an enlarged head portion and an elongate
shaft
portion which projects forwardly from the enlarged head portion. The plunger
component
io part is adapted to be inserted forwardly into the bore in the drug carrying
component part to
a withdrawn position in which the enlarged head portion is partially located
in the rearward
section of the bore such that a rearward section of the enlarged head portion
projects
rearwardly from the bore and the elongate shaft portion projects forwardly
into the forward
section of the axial bore such that the free forward end of the elongate shaft
portion is
~s spaced rearwardly of the erectile dysfunction treatment drug.
A sheath is provided for this device which comprises an elongate main body
having axially
spaced front and rear faces and which is provided with an elongate recess
which extends
forwardly from an open end in the rear face to a position intermediate the
rear and forward
2o faces. The elongate shaft portion of the drug carrying component part is
releasably
receivable in the recess presented by the sheath to protect the elongate shaft
portion from
structural damage and/or contamination prior to insertion thereof into the
urethra of the
male human body.
2s In use, the user holds the handle of the drug carrying component part in
his fingers and
removes the sheath to reveal the elongate shaft portion of the drug carrying
component
part. The elongate shaft portion of the drug carrying component is then
inserted into the
urethra of the user and the plunger component part depressed forwardly from
the
withdrawn position into the drug carrying component part to cause the free
forward end of
3o the elongate shaft of the plunger component part to discharge the erectile
dysfunction
CA 02291612 1999-11-29
WO 98/55176 PCT/SE98/01029
3
treatment drug forwardly from the axial bore in the drug carrying component
part into the
urethra.
In an alternative transurethral device the axial bore in the drug carrying
component is
capped-off with a flexible membrane to the rearward face of which the forward
end of the
plunger component part is attached. Forward movement of the plunger component
part
causes the membrane to present a convex surface over the forward opening of
the axial
bore in the drug carrying component part to enable an erectile dysfunction
treatment agent
in the form of a suppository to be received thereon. Rearward movement of the
plunger
~o component part draws the membrane into the bounds of the axial bore in the
drug carrying
component part and causes the membrane to change to a concave configuration
around the
suppository to secure the suppository to the drug carrying component part. In
use, the
elongate shaft of the drug carrying component part is inserted into the
urethra and the
plunger component part moved forwardly to release the suppository into the
urethra.
is
While the aforementioned prior transurethral devices successfully provide for
the delivery
of an erectile dysfunction treatment drug for, for example, the attainment and
sustainment
of a penile erection in the case of impotence, Applicants have identified
several areas in
which such devices suffer drawbacks.
zo
First, the devices are pre-assembled with the erectile dysfunction treatment
agent in place
in the drug carrying component part. This complicates the overall
manufacturing process
for the devices and counts against the devices being reused.
2s Second, the devices are site specific in their delivery of the treatment
agent in the urethra.
This concentration of the treatment agent in a specific location or site in
the urethra can
lead to pain being experienced by the patient.
Third, to facilitate insertion of the elongate shaft portion of the drug
carrying component
3o part of the previously proposed transurethral devices it is recommended
that before
insertion of the elongate shaft portion of the drug carrying component part
into the urethra
CA 02291612 1999-11-29
P 1740-1 WO PC~f/ SE 98/01 029
~.1 ? -09-1999
4
the user first pass urine to wet the urethra. Clearly this is not always
practical. Avoidance
of this step, however, can obviously lead to pain on insertion of the elongate
shaft portion
of the drug carrying component part into the urethra. Alternately, the user
can be provided
with a separate supply of lubricant for application to the outer surface of
the elongate shaft
of the drug carrying component part of the transurethral insertion element
after
unsheathing thereof. Again, this is not always convenient or practical and
moreover the
lubricant tends to end up on the hands and clothes of the user. More
importantly, this
additional step introduces the possibility of contamination of the elongate
shaft portion of
the drug carrying component part of the transurethral insertion element.
The present invention proposes to deal with the aforementioned drawbacks of
the prior art.
According to a first aspect of the invention there is provided a transurethral
device for
delivery of a drug to the urethra of a human or animal body comprising a drug
carrying
element which presents an elongate shaft having a free forward end for
insertion into the
urethra of the human or animal body in a forward direction, the elongate shaft
being
provided with a channel having an opening in the outer surface of the elongate
shaft for
transport of the drug into the urethra, and a sheath for sheathing at least
the insertable
length of the elongate shaft, characterised in that the device further
comprises means
adapted for drawing the drug into the channel in the elongate shaft from a
fluid supply of
the drug and that the sheath contains the fluid supply of the drug. The sheath
acts to
reduce the risk of contamination of the shaft prior to use of the device and
enables the drug
to be drawn into the channel of the elongate shaft just prior to use of the
device, the drug
carrying element then being withdrawn from the sheath for delivery of the drug
to the
urethra.
AMENDED SHEET
CA 02291612 1999-11-29
P 1740-1 WO PC~I/ SE 98/01 029
:'! 7 -09- 1999
s
By "insertable length" is meant the length of the elongate shaft which is to
be inserted into
the urethra.
In a preferred embodiment of the invention the means adapted for drawing the
drug into
s the channel in the elongate shaft from the fluid supply of the drug is
adapted for drawing a
metered dose of the drug into the channel from the fluid supply.
In an embodiment of the invention said means adapted for drawing the drug into
the
channel in the elongate shaft comprises plunger means adapted to move in the
channel
between a rearward position and a forward position and biasing means for
biasing the
plunger means to the rearward position, the plunger means being movable
forwardly from
the rearward position to the forward position against the biasing action of
the biasing
means upon application of a predetermined condition to the plunger means with
removal of
the predetermined condition after the plunger means has been moved forwardly
from the
1 s rearward position against the biasing action of the biasing means causing
the plunger
means to be biased back to the rearward position by the biasing means whereby
the drug is
able to be drawn into the channel in the elongate shaft through the opening
when the
elongate shaft is dipped in the fluid supply of the drug to a level above the
opening.
In an embodiment of the invention the fluid supply of drug in the sheath is so
arranged in
the sheath that at least a substantial portion of the outer surface of the
insertable length of
the elongate shaft is able to be coated with the drug prior to insertion into
the urethra. A
better distribution of the drug in the urethra is thus obtained as compared to
the case where
only the drug dose carned in the channel in the elongate shaft is delivered.
Preferably, the
2s drug has lubricating properties so as to facilitate insertion of the
elongate shaft into the
AMENDED SHEET
CA 02291612 1999-11-29
P 1740-1 WO PClp SE 98/01 029
~1 ? -D 9-1999
6
urethra. For example, the drug is so selected as to have inherent lubricating
properties, for
example the drug may comprise a therapeutic agent selected from the group
consisting of
the alfa-2 receptor antagonist Atipamezole, nitrates, long- and short-acting a
blockers,
calcium blockers, ergot alkaloids, chloropromazine, haloperidol, yohimbine,
natural and
synthetic vasoactive prostaglandins and their analogs, vasoactive intestinal
peptides,
dopamine agonists, opioid antagonists, sildenafil, prazosin, alprostadil or
mixtures thereof
where the device is to treat erectile dysfunction such as impotence, or
include a lubricant
component, for example cellulose.
In an embodiment of the invention the plunger means is movable forwardly
against the
biasing action of the biasing means on application of a predetermined force
thereto.
In an embodiment of the invention the opening is a forward opening in the free
forward
end of the elongate shaft, the drug carrying element has a rearward end and
the channel
extends from the forward opening to a rearward opening in the rearward end of
the drug
carrying element. In this case it is therefore convenient for the plunger
means to be a
plunger element which extends forwardly into the channel from the rearward
opening.
In an embodiment of the invention the channel is stepped into a forward
section of a first
diameter which extends rearwardly from the forward opening and a rearward
section of a
second diameter greater than the first diameter which extends forwardly from
the rearward
opening and the plunger element comprises a rearward section adapted to
slidingly fit in
the rearward section of the channel such that in the rearward position of the
plunger
element the rearward end of the plunger element rearward section protrudes
from the
rearward opening of the channel and the forward end of the plunger element
rearward
section is disposed in the rearward section spaced rearwardly from the
shoulder between
AMENDED SHEET
CA 02291612 1999-11-29
PC~y SE 98/01 029
P 1740-1 WO
~1 ~ -D9- 1999
7
the forward and rearward sections of the channel and a forward section which
extends
forwardly from the forward end of the plunger element rearward section into
the forward
section of the channel. The predetermined force is thus able to be applied to
the protruding
part of the plunger element to move the plunger element forwardly against the
biasing
action of the biasing means.
In an embodiment of the invention the biasing means is a resilient member
positioned
between the forward end of the plunger element rearward section and the
shoulder between
the rearward and forward sections of the channel, the resilient member being
compressed
on forward movement of the plunger element in the channel. The resilient
member may be
a spring.
In an embodiment of the invention the forward end of the plunger element
rearward section
abuts the shoulder between the rearward and forward sections of the channel in
the forward
1 S position of the plunger element. Alternately, in the forward position of
the plunger element
the forward end of the plunger element rearward section is prevented from any
further
appreciable forward movement by the resilient member. These delimiting
features in
combination with selection of biasing means of a predetermined biasing action
enables a
predetermined metered dose of the drug to be drawn into the channel in the
elongate shaft.
For a compact construction of the device forward movement of the plunger
element against
the biasing action of the biasing means when the drug has been drawn into the
channel
causes the drug to be ejected from the forward opening. There is therefore no
need for the
device to be provided with separate drug ejection means.
The transurethral device in accordance with the invention is particularly,
although not
exclusively, for use in the delivery of an erectile dysfunction treatment drug
to the urethra
of a living male human or animal body, for example an impotence treatment
drug.
AMENDED SHEET
CA 02291612 1999-11-29
P 1740-1 WO PC~I/ SE 98/01 029
;1 7 -D9- 1989
8
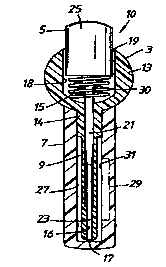
By way of example an embodiment of the present invention will now be described
with
reference to the accompanying Figure which is a side cross-sectional view of
an assembled
transurethral device 10 for treating erectile dysfunction such as impotence in
a male human
patient or body. The transurethral device 10 comprises a transurethral
insertion element
for insertion in the urethra of the male human body having a drug carrying
part 3 and a
plunger part 5 and a sheath 7 for storage of the transurethral insertion
element prior to use
thereof.
AMENDED SHEET
CA 02291612 1999-11-29
WO 98/55176 PCT/SE98/01029
9
Preferably, the parts of the transurethral device 10 are moulded as one-piece
bodies from a
plastics material.
The drug carrying part 3 presents an elongate shaft 9 having a free forward
end adapted to
be insertable into the urethra of the male human body, a finger grip 13 for
the male human
body to hold the transurethral insertion element and a collar 14 which
connects the forward
end of the finger grip 13 to the rear end of the elongate shaft 9. An axial
bore 15 extends
through the drug carrying part 3 between a forward open end 17 at the forward
end of the
elongate shaft 9 and a rear open end 19 in the rear end of the finger grip 13.
The bore 15 is
io stepped into a forward section 16 of a first width which extends rearwardly
from the
forward open end 17 through the elongate shaft 9 and a rearward section I 8 of
a second
width which is greater than the first width which extends forwardly from the
rear open end
19 through the finger grip 13. The forward section 16 of the bore I S is
adapted in use to
carry an erectile dysfunction treatment drug which is to be transferred to the
urethra of the
~ s male human body by the plunger part 5 as hereinafter to be described.
The plunger part 5 of the transurethral insertion element presents an elongate
shaft 21
having a free forward end 23 and an enlarged head 25 connected to the rear end
of the
elongate shaft 21. When the transurethral insertion element is assembled the
elongate shaft
20 21 and enlarged head 25 of the plunger part 5 are respectively received in
the forward
section 16 and rearward section 18 of the bore 15 in the drug carrying part 3.
The sheath 7 is provided with a bore 27 sized to releasably receive the
elongate shaft 9 and
collar 14 of the drug carrying part 3 until such time as the transurethral
insertion element is
2s to be used. The elongate shaft 9 is thus kept free from damage and/or
contamination. The
sheath 7 is further provided with a fluid supply of the erectile dysfunction
drug (not shown)
such that the drug surrounds substantially all of the elongate shaft 9 when
sheathed. The
collar 14 of the drug carrying part 3 acts as a sealing ring to prevent
leakage of the drug
from the bore 27 of the sheath 7. The drug has a composition which comprises a
3o therapeutic agent for treating erectile dysfunction and, for example, one
or more carriers
and/or enhancers for the therapeutic agent.
CA 02291612 1999-11-29
WO 98/55176 PCT/SE98/01029
While it is shown that the whole of the elongate shaft 9 of the drug carrying
part 3 is
sheathed in the sheath 7 it will be appreciated by those versed in the art
that if the length of
the elongate shaft 9 is longer than that portion to be inserted into the
urethra then it is
sufficient that just this insertable length be sheathed.
The transurethral insertion element further comprises biasing means in the
form of a spring
30 between the forward end of the enlarged head 25 of the plunger part 5 and
the step
between the forward and rearward sections 16, 18 of the bore 15 through the
drug carrying
~o part 3. The spring 30 acts to bias the plunger part 5 rearwardly to a
withdrawn position as
shown. Application of a forger pressure to the enlarged head 25 enables the
plunger part 5
to be depressed forwardly against the biasing action of the spring 30.
Release or relaxation of the finger pressure on the enlarged head 25 causes
the plunger part
~s 5 to be returned to the withdrawn position by the spring 30. The effect of
this reciprocal
movement of the plunger part 5 is to generate a pressure at the forward open
end 17 of the
bore I 5 in the drug carrying part 3 which draws a dose of the drug in the
fluid supply
thereof into the bore 15 through the forward open end 17.
2o By selecting a spring of a predetermined biasing strength a metered dose of
the drug can be
drawn into the bore 15 in the drug carrying part 3 by delimiting the forward
movement of
the plunger part 5, that is, the plunger part 5 would be depressed forwardly
from the
withdrawn position as far as possible against the action of the spring 30
before release.
Alternately, or in addition, a metered dose can be drawn into the bore 15 in
the drug
2s carrying part 3 by so constructing and arranging the transurethral
insertion element such
that depressing the plunger part 5 sufficiently forwardly causes the forward
end of the
enlarged head 25 to abut the step between the forward and rearward sections
16, I 8 of the
bore 15 in the drug carrying part 3. The device 10 is set up so as to draw in
a dose of the
drug of up to 100 mg, for example 50 mg, with the dosage comprising a
therapeutically
3o effective amount of the therapeutic agent which values are known in the
art. As an
example, therapeutically effective amounts for various erectile dysfunction
therapeutic
CA 02291612 1999-11-29
WO 98/55176 PCT/SE98/01029
11
agents are given in W095/26158 and W096/28142. Of course, the parameters of
the
device such as the properties of the spring 30 and hence the return distance
of the plunger
part S can be customised to draw in a different metered dose of the drug.
Once the drug has been drawn into the bore 15 through the forward open end 17
as
aforesaid the transurethral insertion element is withdrawn from the sheath 7
for insertion
into the urethra of the male body. Withdrawal of the transurethral insertion
element from
the sheath 7 results in the outer surface of the elongate shaft 9 of the drug
carrying part 3
additionally becoming coated with the drug.
~o
The elongate shaft 9 of the drug carrying part 3 of the transurethral
insertion element is
then inserted into the urethra of the male body and the plunger part 5
depressed forwardly
to transfer the drug from the bore 15 to the urethra through the forward open
end 17.
is This arrangement leads to a more uniform distribution of the drug on the
walls of the
urethra as compared to the case if only the drug in the bore 15 were delivered
to the urethra
because the drug coated on the elongate shaft 9 is transferred to the urethra
wall rearward
of the delivery site for the drug in the bore 1 S. The metered dose of the
drug drawn into the
bore 15 of the elongate shaft 9 or the concentration of the therapeutic agent
in the drug can
2o be tailored if need be to take account of this additional source of drug
administered to the
urethra to ensure that an acceptable collective amount of the therapeutic
agent is
administered by the two different administration mechanisms employed by the
device 10.
Advantageously, the drug used has lubricating properties. Insertion of the
elongate shaft 9
2s into the urethra is thus facilitated by the drug coating thereon having
lubricating properties.
This can be achieved by selecting a drug having inherent lubricating
properties or by
forming the drug with a lubricant. Where the transurethral device 10 is to
deliver an
erectile dysfiulction treatment drug such as an impotence treatment drug the
alfa-2
antagonist Atipamezole (Orion Farmos), nitrates, long- and short-acting a
blockers,
so calcium blockers, ergot alkaloids, chloropromazine, haloperidol, yohimbine,
natural and
synthetic vasoactive prostaglandins and their analogs, vasoactive intestinal
peptides,
CA 02291612 1999-11-29
WO 98/55176 PCT/SE98/01029
12
dopamine agonists, opioid antagonists, sildenafil ("Viagra" sold by Pfizer),
prazosin,
alprostadil or mixtures thereof may be mentioned as therapeutic agents or
therapeutic agent
mixtures having inherent lubricating properties which could form a part of the
composition
of the drug. Alternately, a cellulose may be mentioned as a suitable lubricant
for inclusion
in the composition of the drug.
Rather than simply filling the bore 27 of the sheath 7 with a fluid supply of
the drug the
sheath 7 may instead be provided with a drug containing compartment 29. As can
be seen,
a boundary wall 31 of the drug containing compartment 29 also serves as a
portion of the
io boundary wall of the bore 27 in the sheath 7. The common boundary wall 31
is constructed
so as to be rupturable or otherwise openable on application of a predetermined
condition
thereto, in this case a predetermined pressure being applied to the outer
surface of the
sheath 7 which when transmitted to the common boundary wall 31 causes the
common
boundary wall 31 to be brought to an open disposition. Bringing the common
boundary
is wall 31 to the open disposition results in the drug being able to be
discharged from the
compartment 29 into the bore 27 in the sheath 7 and onto the adjacent outer
surface of the
elongate shaft 9 of the drug carrying part 3.
The drug can be coated over the whole of the outer surface of the elongate
shaft 9 of the
2o drug carrying part 3 by manipulation of the transurethral insertion element
relative to the
sheath 7. The collar 14 of the drug carrying part 3 again acts as a sealing
ring to prevent
leakage of the drug from the bore 27 of the sheath 7. Alternatively, the drug
containing
compartment 29 can be so dimensioned and juxtaposed to the bore 27 in the
sheath 7 that
discharge of the drug from the compartment 29 automatically coats the elongate
shaft 9 of
zs the drug carrying part 3 bounded by the bore 27. For example, the drug
containing
compartment 29 could be formed so as to bound substantially all of the bore
adjacent the
elongate shaft 9 of the drug carrying part 3.
While it is preferable that substantially all of the insertable length of the
elongate shaft 9 of
3o the drug carrying part 3 be coated, insertion of the elongate shaft 9 into
the urethra of the
CA 02291612 1999-11-29
WO 98/55176 PCT/SE98/01029
13
male human body will still be more comfortable even if only a part of the
insertable length
is coated, for example a forward section of the elongate shaft 9.
It can therefore be seen that the present invention provides a transurethral
device that is
s simple to fill with a dose of drug. The present invention also solves the
problem of the
general requirement to keep the prior art devices which use an erectile
dysfunction
treatment drug in the form of a solid pellet or suppository cool to compensate
for the heat
sensitivity of the drug form and the difficulty in dispensing the drug form if
it softens.
Moreover, the present invention provides a transurethral device which may be
reused.
io Embodiments of the invention, such as those described herein with reference
to the
accompanying Figures of drawings, are additionally easier to insert into the
urethra and
deliver a more uniform distribution of the drug into the urethra when compared
to prior art
devices.