Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02291722 1999-11-27
TEL N0:801-328-1627 tt598 P09
:.. , . -,
~.~r~~wy ~~~°9~1a
-. ~Pt~lu S '' ~ J ~~ 1999
PROCBSH !'OR TRgATIPIG SPENT, ALRALIHE DIGESTION
LIpUORS FRO! PAPER PULPING OPERATI0~1S, ACID PRODUCT
Haekaround of the Invention
yield: This invention is in the field of paper making,
specifically in the treating of alkaline digestion liquor for
separating contained lignins and other component solids from the
liquid component of Lhe normally waste, spent digestion liquor
resulting lrom the production of paper pulp used in the
2 0 manuf acture of paper .
State of the l4rrt: Paper making requires a source of
cellulose f ibere . Common raw materials as a fiber source are
hardwoods and softwoods as well as those of annual vegetable
origin, such as wheat and rice straw, bagasae (sugar cane stalks
after processing), hemp, and jute. Rag materials, as well as
recycled fibers. can also be used. Iiawever, wood has been a
primary source of cellulose fibers for paper making.
Before use, the wood or other raw material must be processed
to release the cellulose fibers. This operation is called
pulping". At present, commercial pulping operations are of
three principal types: mechanical, full chemical, and
semi.chemical. The processes with which the invention is
eoncorned are full chemical and aemichemical pulping.
Full chemical and semichemical pulping employ chemical
'S reagents to effect separation of the cellulosic fibers from other
components. Wood chips or other raw materials are cooked with
suitable chemicals in aqueous solution, usually at elevated
temperatures and pressures. The object is to dissolve the
ozganic binders holding the celluloaic fibers, termed "ligninsH,
so comprising up to Z6s of wood, for example, along with other types
of organic molecules, such ac saceharide molecules, and other
extraneous compounds, leaving the cellulose fibers intact.
Though there is some cellulose degradation, the objective can bQ
realised to a commercially satisfactory degree through the use
CA 02291722 1999-11-26
WO 98/54400 - PCTlUS97/09418
2
of a variety of chemical reagents. Pulp yields from wood using
such processes are usually about 50~ of the wood weight.
Lignins have been studied extensively and are said to
consist of the noncarbohydrate portion of the cell walls of plant
materials. Originally, the lignin content of plant materials was
defined as the residue after hydrolysis with strong acid
following removal of waxes, tannins, and other extractives,
including resins and tall oils . Lignins are amorphous, have high
molecular weight, and are predominantly aromatic in structure.
In general, the monomeric units comprising lignins can be
referred to as p-hydrocycinnamyl alcohols. More specifically,
according to The Merck Index, lignins comprise coniferyl,
p-coumouryl, and sinapyl alcohols. Their precise composition
vary with the method of isolation and with the species, age,
growing conditions, etc . , of the plant. Lignins are more or less
completely removed by chemical pulping, but are essentially not
removed at all by mechanical pulping.
Digestion liquors obtained from alkaline pulping usually
contain not only all the lignins in the source material, but
substantial amounts of cellulose or carbohydrate monomers, other
carbohydrates, and, from annual plant materials, such as rice
straw, a significant percent by weight of silica. Such used or
spent digestion liquors, normally waste, pose problems that are
unique in alkaline pulping operations.-Waste liquor streams from
other operations during the paper making process pose different
problems, such as removal of the resins and tall oils found in
gymnosperm trees.
The lignin solids precipitate when the spent alkaline
digestion liquid is acidified and pose a particular problem,
because they are polymerized by acidification, producing an
amorphous gum (see The Merck Index, p. 864, (1989), S. Budavari,
Editor). No easy, inexpensive, or commercially practical method
SU8ST1TUTE SHEET (RULE 26)
CA 02291722 1999-11-26
WO 98154400 PCT/US97/09418
3
of separating the lignins from the alkali waste liquors has
' heretofore been known.
Soda and sulfate pulping are both known in the art as being
alkaline pulping processes. The soda process employs caustic
soda (sodium hydroxide), whereas the sulfate process employs
sodium sulfide in addition to caustic soda. The sodium sulfide
used in the sulfate process results in a stronger cooking liquor
and accounts for stronger pulp and faster cooking in the sulfate
process as compared with soda pulping. The term ~~kraft pulping"
is an alternative to the term sulfate pulping. For the purposes
of this invention, there is no practical difference between the
lignin-laden, spent liquors that result from either the sulfate
or the soda process or from semichemical or other pulping
processes which make use of alkaline agents in conjunction with
mechanical means to make pulp, except there is often a relatively
great amount of silica in soda process digestion liquor as
compared with sulfate (kraft) process digestion liquor. The
common link between pulp-making processes with which the
invention is concerned is that spent digestion liquors employing
alkali, whether buffered or not, become laden with solids and
with organic matter, usually referenced to as total organic
carbon [TOC], primarily lignins, and that both the inorganic and
organic constituents must be recovered or otherwise processed to
accommodate environmental concerns as well as to recycle
inorganic digestive chemicals. All cooking or pulping reagents
employing alkali, especially caustic soda and sodium sulfide, are
expensive and the inorganic waste materials are usually too toxic
to release spent liquor to the environment.
The sulfate, i.e., kraft, process and the semikraft process
are normally used when wood is the raw material. The active
pulping ingredients, sodium hydroxide and sodium sulfide, make
up an obviously strongly alkaline solution. Standard in the
kraft pulping process is the provision of a liquor-recovery cycle
SUBSTITUTE SHEET (RULE 2fij
CA 02291722 1999-11-26
WO 98/54400 PCT/US97/09418
4
in which the organic constituents in the spent digestion liquor
(primarily residual lignins and carbohydrates) are burned for
steam generation and for recovery of the inorganic, alkaline,
pulping chemicals in molten form, they being then solubilized by
the addition of water to form so-called "green" liquor, which is
further processed for reuse.
The traditional waste digestion liquor recovery cycle
applied most frequently to kraft or semikraft process digestion
liquors comprises the step of evaporating digestion waste liquor,
the so-called "black liquor", to a high concentration, i. e., to
so-called "concentrated black liquor" or "black kraft liquor",
which is usually up to 70% solids by weight. Organic sulfur
compounds are found in the black liquor from the sulfate process
in association with sodium sulfide (NazS). Sodium carbonate
( NaZCOz ) , sodium sulfate ( NaZSO, ) , and silica ( Si02 ) are also
present. Total solids are usually about I5 percent by weight in
black liquor after separation from fiber pulp following
digestion.
The term "black liquor" is often also applied to other
lignin-laden, used or spent digestion liquors, the compositions
of which vary with the reagent chemicals used, the raw material,
and the particular mill concerned.
The soda process is normally applied to raw materials of
annual vegetable origin, such as cereal,~e.g. wheat and rice,
straw. Such materials normally contain a relatively high
percentage of silica, which is solubilized in the digestion
liquor. This poses additional separation problems, because, as
well known in the art, separating out silica by acidification of
the alkaline spent digestion liquor produces a gelatinous or
gummy mass that cannot be separated from the liquor in a
practical manner. The elevated silica content of liquor derived
by pulping such fiber sources, as much as one per cent by weight,
as compared to the relatively low silica content from wood fiber
SUBSTITUTE SHEET (RULE 26)
CA 02291722 1999-11-26
WO 98/54400 PCT/US97/09418
sources, generally precludes practical application of separation
' and recovery methods presently known to those skilled in the art .
In the usual kraft recovery process in which silica is a
negligible factor, after the black liquor is evaporated to about
5 70% by weight solids, other procedures, such as vacuum flashing,
may be performed to increase even more the preparation of solids
for burning. The high-solids-content, kraft black liquor is fed
into a reducing recovery furnace provided as part of the usual
kraft pulping plant for chemical and energy recovery. The usual
reducing recovery furnace requires a large capital investment,
and its capacity frequently limits production from a typical
kraft pulping plant.
David M. Whalen described a simple method for precipitating
lignin from kraft black liquor in vol. 58, No. 5, May 1975, of
the TAPPI Journal, pages 110-112 (see also Whalen et al. U. S.
Patent No. 3,546,200 of December 8, 1970). In that method, kraft
black liquor is added slowly and with stirring to a mixture of
an organic liquid, such as chloroform, and enough mineral acid
to bring the final pH to about 3. The process was successful on
a laboratory scale, but the large amounts of organic liquid
required made the process impractical on a commercial. scale. A
more efficient way of separating out the organic constituents,
primarily lignins, is still needed.
Summary of the Invention
In making the present invention I have realized that, with
a better way of separating out the lignins from the usual
digestive liquor, prior art kraft-pulp-producing plants with
their high cost recovery furnaces, can still be used in the
normal way, but a significant advantage can be achieved, because
pulp production from such a plant is not limited by what the
recovery furnace can handle. Instead, the excess spent digestion
liquor can be processed in accordance with the invention which
SUBS'TTTUTE SHEET iRULE 26)
CA 02291722 1999-11-26
WO 98/54400 PCT/US97/09418
6
does not require that the capacity of the standard recovery
furnace be increased.
Thus, it was a principal object of the invention to provide
a better way of separating out the lignins from the usual spent
and waste digestion liquor.
This objective has been accomplished by the addition of a
relatively inexpensive, water soluble, surface active, polymeric
agent, sometimes referred to hereinafter as ~~polymer°, to the
spent, waste, alkaline digestion liquor before or during the
acidification of such digestion liquor and is applicable to the
spent, waste digestion liquors from both the commonly used kraft
sulfate process and the soda process, whether or not diluted and
without the use of heat or pressure.
The polymers used have molecular weights ranging from about
five million to about twenty-five million, and may be anionic
polymers, such as copolymers of acryiamide and acrylic acid (or
sodium acrylate), or partially hydrolyzed polyacrylamide and
homopolymers or copolymers of sulfonic acid and acrylamide, which
are available as commercial products, such as Percol 919 and
Percol 156 from Allied Colloids, Inc., and Nalco 7877 from Nalco
Chemicals Company. On the other hand, they may be nonionic
polymers based on polyacrylamide chemistry or polyethylene
oxides, such as Percol 351, Percol 802, and PEO (polyethylene
oxide) available from Allied Colloids, Inc.; or may be cationic
polymers of different charge densities, such as Percol 368,
Percol 292, and Percol 2802. Nonionic polymers are preferred.
The polymer or a mixture of polymers is added in an amount
to bring concentration thereof to within the range of about 0 . 05 %
to about 1.0% by weight in the liquor. To achieve such
percentage range, the polymer or mixture of polymers is added in
amount from about 0.1 to about 5.0 pounds/ton of dry organic
material in the digestion liquor. The preferred level of
addition is about one pound per ton.
SUBSTIME SHEET (RUL.E 26)
CA 02291722 1999-11-26
WO 98/54400 PCT/US97/09418
7
As the acidification of such digestion liquors can lead to
the generation of gases, depending on the alkaline salt used in
the pulping process, it is preferred to add a different water
soluble, surface active agent, or combination of agents, of low
molecules weight, such as a fatty acid or soap of fatty acid, a
polysilicone, or a succinate, which has a carbon chain containing
from about eight carbon atoms to about eighteen carbon atoms,
primarily to enhance the action of the polymer, but,
incidentally, to control foaming. It has been found that the
presence of such an agent in the range of from about 0.01 to
about 1.0 pound per ton of the spent digestion liquor treated has
a significant beneficial effect in separation of solids or near
solids from the liquid component of the digestion liquor. The
preferred level of addition is in the low range of about 0.1
pound per ton of the liquor. The result is the recovery of a
substantially clear or very low colored liquid component of the
original digestion liquor, which "clarified" liquid is
essentially free of dissolved, higher molecular weight, organic
solids (primarily lignins and dissolved carbohydrates). Total
organic carbon ( TOC ) in the clarified liquid may be as low as
about 0.01% by weight.
Addition of the indicated other surface active, or
defoaming, agent to the digestion liquor prior to acidification
produces superior results. A clarified liquid component having
a residual total organic carbon content on the order of about
0.02 to about 0.01% by weight can easily be achieved. This
residual TOC represents a small amount of simple carbohydrate and
a very, very small amount of residual lignins, if any.
After the water soluble, surface active, polymer and the
additional surface active agent, or defoamer, are in place, the
digestion liquor is acidified to a pH below 7, preferably to at
least about 3. The lignins agglomerate and at least tend to
float to the top of the liquor as a non-gelatinous, non-gummy,
SUBSTITUTE SHEET (RULE 26)
CA 02291722 1999-11-26
WO 98/54400 PCT/US97/09418
8
solid fraction. The higher the solids concentration of the
digestion liquor, the greater the tendency of the coagulant to
float or to actually float at the surface of the liquor. There,
the lignins can be easily separated by mechanical, gravity
separation, as by screening the upper portion of the liquor or
by filtering. In a spent digestion liquor not as concentrated,
where the solids concentration is less than about 15 % by weight,
the lignins still coagulate, i.e., they form a non-gelatinous,
non-gummy solid fraction, but neither fall out of the liquor nor
actually float on top. In this condition, i.e., tending to
float, they can still be separated by a mechanical, gravity
separation step, such as filtration.
The removed solids are washed for removal of residual salts
and are thereafter dewatered and dried. At no point does the
process result in a slimy gelatinous mass or amorphous gummy
fraction of acid lignins or of acidified silica, as has
previously occurred upon acidification of black liquor of either
the kraft or the soda process. Moreover, by the process of this
invention, a significant advantage is achieved by using the
aforedescribed procedure as an alternate method of treating
excess digestion liquor produced by the usual kraft pulping plant
when operated at over the design capacity of the recovery furnace
for handling the residual solids to derive energy and recover
chemicals therefrom. Instead of requiring the expenditure of
large sums to expand the capacity of the recovery furnace of an
existing kraft processing plant, excess production of waste
digestion liquor can be handled by the present process and by use
of a relatively inexpensive supplemental furnace, such as a
fluidized bed furnace, as an economically acceptable capital
investment. After separation of the solid fraction from the
liquid fraction of the diverted waste digestion liquor, the clear
residual liquid component is returned to the flow stream entering
the plant. This means that the usual recovery furnace of the
SUBSTITUTE SHEET (RULE 25)
CA 02291722 1999-11-26
WO 98/54400 PCT/US97/09418
9
kraft pulp processing plant need not be the limiting factor in
pulp production for existing sulfate (kraft) processing plants.
In the soda process, inorganic chemicals dissolved in waste
' digestion liquor would normally not be further processed.
However, lignins with silica dissolved in the waste liquor are
separated together to produce a solid product, having value, and
a waste liquid that can be safely returned to the environment.
The Drawings
The best mode presently contemplated for carrying out the
invention commercially is illustrated in the accompanying
drawings, in which:
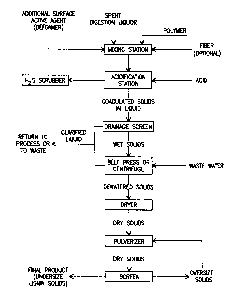
FIG. 1 is a schematic flow sheet showing, in general, steps
preferably employed in performing the basic method of the
invention; and
FIGS. 2-5 are graphs showing results of respective tests of
the process of the invention using acidification at different pH
values.
Detailed Description of the Illustrated Embodiments
The details as to which water soluble, surface active,
polymeric agents are preferred and how much should be added to
a quantity of spent digestion liquor for best results are
presented above and will not be repeated here. However, it will
be apparent to those skilled in the art that selection of the
preferred polymer and the amount to be used will depend on the
source of the lignin, i.e., on the particular raw material being
pulped and to the particular process employed. For example, for
hardwood and the kraft pulping liquor employed, the nonionic
polymer PERCAL 351, available from Allied Colloids, Suffolk,
Virginia, is superior. Other kraft pulping liquor, as from
softwoods, and soda pulping liquor, as from cereal straw, may
require use of different polymers and different amounts thereof
SUBSTIME SHEET (RULE 26)
CA 02291722 1999-11-26
WO 98/54400 PCT/US97/09418
depending on the particular raw material being pulped, the degree
of chemical and of mechanical pulping, and other considerations
known to those skilled in the art.
Turning to the drawings, the flow sheet of Fig. 1
5 illustrates in general a preferred embodiment of the basic
process of the invention for treating spent, waste digestion
liquors and is indicative of the lignins and other products
recovered therefrom.
The spent digestion liquor laden with lignins is passed into
10 a mixing station where the polymer is added and preferably also
the additional surface active, or defoaming, agent. This station
can be a static station or a continuous flow type. Optionally,
but preferably, fiber is added as well to aid coagulation
( formation of the solid fraction ) and its separation from the
i5 remaining liquid fraction.
From this station, the conditioned liquor is passed to
another station for its acidification with an acid, preferably
sulfuric acid or phosphoric acid. Organic acids may be used in
combination with mineral or inorganic acids as may be appropriate
for process needs. Hydrogen sulfide gas will be generated upon
acidification if sodium sulfide is contained in the alkaline salt
used for pulping. Preferably, this station is enclosed and has
conducting means for conducting such gas to a hydrogen sulfide
gas scrubber, as indicated. Upon acidification, particulariza-
tion (coagulation into smaller or larger aggregations) of the
lignins also commences. These tend to float to the surface of
the spent digestion liquor, but when the lignin concentration is
high, as when there is a total solids concentration in amounts
of around 15% or above, they actually float and can be screened
off and into a dewatering station, such as a drainage belt.
Much of the liquid is separated from the solids by passing
through the apertures of the drainage belt. The dewatering
station preferably further includes a belt press or centrifuge,
SUBSTITUTE SHEET (RULE 26)
CA 02291722 1999-11-26
WO 98/54400 PCT/US97/09418
11
where even more liquid is removed. A fresh water wash can be
' used to displace salts carried in the solids. The liquid removed
at the dewatering station is preferably passed to an evaporator.
Depending on the concentration of lignins as reflected in
the total solids concentration (TSC), the exact nature of the
separable, particulate, solid fraction, primarily lignins, that
form varies. As used herein, the term "particulate" refers
generally to the constituent particles of the solid fraction.
The non-gelatinous, non-gummy condition of these particles, i.e.
solid fraction that forms in the liquid fraction after treatment
according to the invention, is readily separated by gravity from
the clarified liquid fraction. In lower concentrations of the
digestion liquor, the particulate matter agglomerates and stays
suspended in the clarified liquor solution, tending to float.
It remains suspended in the clarified liquid fraction. As used
herein, the term "coagulate" also refers to this formation of a
readily separable, insoluble, particulate, solid fraction that
tends to float. In any event, one of the advantages of the
invention is that in two or three steps consisting of the
addition of polymer, with or without the addition of the
indicated defoamer, in conjunction with or preferably followed
by acidification, is that an insoluble non-gelatinous, non-gummy
particulate solid fraction is substantially instantaneously
produced and either tends to float or if the concentration of the
digestion liquor is high, actually does float at the surface of
the liquid fraction. No other operating parameters, such as
temperature, pressure, or shearing forces, are necessary.
If phosphoric acid is used for acidification,
crystallization is preferably employed to separate a crystallized
salt, such as disodium phosphate, which can be sold. Steam given
off from the evaporator can be put to use. The liquor clarified
of lignins is preferably evaporated to a salt concentration of
35% to 40% and is returned to the process.
SUBSTIME SHEET (RULE 26)
CA 02291722 1999-11-26
WO 98/54400 PCT1US97/09418
12
One measure\of efficiency of separation is indicated by the
percent TOC in the remaining liquid fraction. A low TOC value,
known in the art to be expressed as percent by weight, indicates
all organics are removed except for residual, low molecular
weight, soluble organic compounds that are not ordinarily subject
to precipitation. A high TOC value indicates precipitatable
solids remain in the liquid fraction after a treatment process
and the separation is incomplete and inefficient to a certain
degree. Values of percent TOC by weight above 0.2 (undiluted)
in the supernatant liquor in treating kraft or other digestion
liquor can be considered indicative of less than optimal
separation. Color and clarity of the remaining liquid fraction
are also indicative of completeness of removal of lignins.
Ideally, it should be clear and substantially colorless or a very
pale yellow.
Highly efficient separation of less concentrated, spent
digestion liquors, i.e., TOC values of about two percent or less,
and the presence of silica, are dependent upon the order of
addition in the treatment, that is, by adding polymer solution
and the indicated other surface active agent, or defoamer, to the
spent digestion liquor prior to the acidification. This order
of addition results in separation of lignins and other organic
components as agglomerated masses easily separable from the
clarified digestion liquor by simple mechanical, gravity separa-
tion methods, such as screening, belt-pressing, centrifugation,
and filtration.
The separated solid fraction can be subjected to various
dewatering processes with efficiency and ease heretofore unknown
in the prior art by commercially feasible and inexpensive
separating procedures. Again mechanical gravity separating
procedures for dewatering the separated solid fraction include
screening, belt pressing, centrifugation, and filtration. The
much improved efficiency of such procedures over the prior art
SUBSTITUTE SHEET (RULE 28)
CA 02291722 1999-11-26
WO 98/54400 - PCT/US97/09418
13
is shown by the ease with which the solid fraction can be air
' dried. A further advantage of the invention is that the air
dried, solid fraction is of very low moisture content compared
to the lignin-containing solid fraction of the prior art. After
air drying, the solid fraction can have a moisture content as low
as 5% by weight and is ready for shipment or for use as a fuel.
Once dried, the lignins may be passed through a pulverizer and
screened. Undersize lignins can be used as fuel. oversize
lignins can be passed through the pulverizer again.
Initially, the process of the invention was evaluated by
obtaining two identical samples of a concentrated kraft digestion
liquor . One was acidif led with sulfuric acid after treatment
with the polymer. The other was acidified in the normal manner
as a control without the polymer. It was noted that the
I5 precipitate that formed in the polymer-treated sample coagulated
and floated to the top and was easily separated from the
clarified liquid component of the digestion liquor. The
precipitate in the untreated sample was in a slimy, gelatinous
form and was not easily separated. In addition, the polymer-
treated sample produced 75% more dried precipitate than the
control sample.
The following table shows the organic and inorganic (ash),
as well as total solids distribution between precipitate and
filtrate for both samples:
30
SUBSTITUTE SHEET (RULE 26)
CA 02291722 1999-11-26
WO 98/54400 PCT/US97/09418
14
TABLE SHOWING COMPONENT DISTRIBUTION BETWEEN
PRECIPITATE AND FILTRATE IN COMPARATIVE TESTS
Precipitate
Control Polymer-treated
% of Total Organics 44.4 63.7
% of Total Ash 13.1 20.0
% of Total Solids 30.1 46.9
Filtrate
Control Polymer-treated
% of Total Organics 55.6 36.3
% of Total Ash 86.9 80.0
% of Total Solids 70.0 53.1
Additional tests were performed as in the following example:
EXAMPLE 1
TREATMENT OF CONCENTRATED KRAFT LIQUOR WITH POLYMER
FOLLOWED BY ACIDIFYING
Spent digestion liquor from a kraft pulping process was
diverted after its separation from fiber on a washing drum, the
composition factors being total organic carbon (TOC) of 7.9
percent and pH of 12.3. While at ambient temperature, polymer
solution was added in an amount yielding about 30 ppm of the
polymer in the liquor. The mixture was then acidified with
sulfuric acid, causing the formation of a solid precipitate
containing primarily lignins, which floated to the surface of the
residual liquid as non-gelatinous, non-gummy, agglomerated masses
of coagulant. These masses of precipitated solids were then
easily separated from the clarified liquid by filtering, but
could not be separated by screening . Analysis revealed 2 .15 % TOC
in the clarified liquor fraction.
SUBSTITUTE SHEET (RULE 26)
CA 02291722 1999-11-26
WO 98/54400 PCT/US97/09418
EXAMPLE 2
' TREATMENT OF CONCENTRATED KRAFT LIQUOR WITH POLYMER AND
ANOTHER SURFACE ACTIVE AGENT, DEFOAMER,
' PRIOR TO ACIDIFICATION
5 Digestion liquor was diverted from the same source as in
Example 1 and was treated at ambient temperature by
simultaneously adding the same amount of the same polymer
solution plus a fatty acid at two parts per 1000 parts of the
liquor. After one minute of standing, the liquor was acidified
10 to a pH of 3.5 to 4.0 with 50 percent strength sulfuric acid
added slowly over a period of one minute. Lignin solids in the
acidified liquor similarly formed non-gelatinous, non-gummy,
agglomerated masses which floated at the surface of the residual
liquid, but were readily separated from the liquid fraction on
15 a 10 mesh screen. The clarified liquor fraction was clear and
almost colorless. Analysis revealed 0.17 percent TOC.
EXAMPLE 3
TREATMENT OF DILUTE KRAFT DIGESTION LIQUOR
WITH POLYMER
Digestion liquor was again diverted from the same source but
was diluted with an equal quantity of tap water. The composition
of the diluted liquor was approximately 7.5% solids and 3.9% TOC
with a pH of about 12.3. To this dilute liquor at ambient
temperature, the same polymer solution as in the foregoing
examples was added to bring the amount of polymer in the diluted
liquor to 30 ppm. After one minute of standing, the mixture was
acidified to a pH of 3.5 to 4.0 with 50 per cent strength
sulfuric acid again added slowly over a period of one minute.
Lignins therein formed a non-gelatinous, non-gummy, suspended,
particulate solid fraction that was readily filtered from the
remaining liquid fraction. The clarified liquid was clear and
almost colorless. Analysis revealed 0.80 per cent TOC.
SUBSTITUTE SHEET (RULE 26)
CA 02291722 1999-11-26
WO 98/54400 - PCT/US97/09418
16
EXAMPLE 4
TREATMENT OF DILUTE KRAFT DIGESTION LIQUOR WITH POLYMER
AND DEFOAMER SURFACE ACTIVE AGENT
Dilute spent digestion liquor at ambient temperature
diverted from the same stage of an alkaline pulp-making plant and
of the same diluted composition as that in Example 3 was treated
by adding enough polymer solution to bring the amount of polymer
in the mixture to 30 ppm and simultaneously adding a fatty acid
to bring its amount two parts per 1000 parts of the mixture.
After one minute of standing, the liquor was acidified to pH of
3.5 to 4.0 with 50 percent strength sulfuric acid added slowly
over a period of one minute. A solid fraction containing lignins
was formed characterized by large masses of agglomerated
coagulant that readily separated from supernatant liquid on a 10
mesh screen. The liquid fraction after screening was clear and
almost colorless. Analysis revealed 0.10 percent TOC.
From the foregoing examples with respect to kraft digestion
liquor, it can be seen that when a surface active defoamer agent
is added, the coagulant flocs produced during co-precipitation
stick together as a separable mass. Conversely, when the
defoamer surface active agent is not employed, a ffiner,
particulate, solid fraction is formed that cannot be separated
by a 10 mesh screen, but is nevertheless easily separable by
filtering. Such solid fraction is neither gelatinous nor gummy,
as are precipitated fractions obtained by prior art methods . The
beneficial effect of the additional surface active defoamer agent
is the formation of screen-separable, lignin-containing,
coagulant masses. Screening is more efficient than filtration,
and is an unexpected result from the apparent co-precipitation
action of the two surface active agents. Furthermore, the
differences in TOC between Examples 1 and 2, and again between
Examples 3 and 4, show substantially improved separation by use
of surface active defoamer agent.
SUBSTITUTE SHEET (RULE 26)
CA 02291722 1999-11-26
WO 98/54400 PCT/US97/09418
17
The following example with respect to soda digestion liquor
' shows how the process of the invention similarly eliminates the
prior art gelatinous nature of the lignins solids, despite the
high silica content of the soda digestion liquor.
It has also been found that, upon addition of polymer to
spent soda digestion liquor containing a considerable amount of
silica, followed by acidification to a pH of about 3 in
accordance with the invention, a suspended, particulate, solid
fraction and a residual, clarified, liquid fraction are formed.
The particulate precipitate is a combination primarily of lignins
and silica. Heretofore, silica-laden, spent, digestion liquor
of the soda pulping process posed difficult separation and waste
disposal problems. However, the combined precipitate of lignins
and silica obtained by the herein disclosed process is
substantially as readily separable as the precipitate obtained
from the low silica content, kraft digestion liquor. This
represents a further unexpected result and a further advantage
of the invention.
The following example is typical:
EXAMPLE 5
TREATMENT OF SPENT, SEMI-CHEMICAL, SODA DIGESTION LIQUOR
FROM PULPING A CEREAL STRAW MATERIAL
A sample of waste digestion liquor was obtained .from a
semi-chemical, soda pulping process. Its measured composition
was about 15 percent by weight solids and 3.7 percent by weight
TOC at a pH of 12.3. Silica content was determined by analysis
to be 144 parts per million by weight in the liquor. While at
ambient temperature, a polymer solution was added in amount to
produce 30 ppm in the liquor; subsequently, after one minute of
standing, the polymer-treated liquor was allowed to flow into a
flume simultaneously with recycled, clarified liquor to which 50
per cent strength sulfuric acid had been added such that the pH
SUBSTITUTE SHEET (RULE 26)
CA 02291722 1999-11-26
WO 98/54400 PCT/US97/09418
18
of the clarified liquor was in the range of 3.5 to 4Ø Lignins
and silica were separated as non-gelatinous, non-gummy,
agglomerated coagulant solids in a receiving vessel into which
the mixed liquor flowed. The supernatant liquid component from
filtering was clear and almost colorless, with 0.04 percent TOC
and a silica content of only 15 parts per million by weight.
The clear and low coloration, clarified liquid from the
process of the invention is a novel product comprising the salt
ions formed as a result of interaction of the original alkali
charge and the added acid, in the range of about 3% to about
0.01% TOC by weight, depending on the starting spent liquor and
the procedures employed in clarification.
Likewise, the recovered lignin-containing, solid fraction
product is unique. The dewatered solid fraction comprises
lignins, polymer, and, if employed in the recovery process, the
added surface active defoaming agent, as well as some or
considerable silica. The amount of silica may be great depending
on the source material used. Besides the usual substances found
in lignins isolated by prior art methods, the dewatered, air
dried lignins as the solids fraction of the invention contains
from about 0.05 percent dry solids (or about 500 parts per
million) polymer. If the additional surface active defoaming
agent is used, the lignins-containing, solids fraction will
contain from about 0~ 0005 percent to about 0 . 05 percent by weight
of such additional agent. The dewatered and dried solids
fraction, if air dried, has a moisture content in the range of
about 5.0 percent to about 10.0 percent by weight. If dried by
heating, the solids fraction has a moisture content in the range
of about 1.0 percent to about 3.0 percent by weight.
Operating parameters for adapting the method disclosed
herein to the characteristics of particular alkaline waste
digestion liquors diverted from various stages of pulp-making are
well within the skill of those practiced in the art of pulp-
SUBSTtME SHEET (RULE 26~
CA 02291722 1999-11-26
WO 98/54400 PCT/US97109418
19
making. Some digestion processes are carried out at higher
alkalinity levels, and the degree of digestion may be less
severe, I.e., the percent of dissolved solids less, such as with
- semi-chemical pulping where mechanical separation of fibers from
the raw material is occurring simultaneously with chemical
separation. '~Deepness" as used in the industry refers to degree
of digestion, I.e., more severe chemical pulping is referred to
as "more deep" digestion and vice versa. Waste liquor from
semi-chemical or lesser intensity "deep" chemical pulping can be
processed somewhat differently in accordance with the skill of
the art guided by the present examples within the scope of the
invention.
As previously indicated, the foregoing procedure can be
advantageously applied to only a relatively minor part, typically
about 10.0 percent by volume, of a kraft digestion liquor from
the usual kraft pulping plant equipped with the standard, very
expensive, recovery furnace. In the case of spent digestion
liquor from a soda pulping plant, all of the digestion liquor
would be treated by the present invention. This is so, because
in the usual soda pulping process, the resulting digestion liquor
is not suitable for processing and no recovery furnace is part
of the standard equipment as it is in the kraft pulping plant.
It should be noted that Examples 3 and 4, wherein
concentrated black liquor from the kraft process was diluted by
the addition of an equal amount of water before processing in
accordance with the invention, show somewhat but not greatly
different results from those of Examples 1 and 2 in which there
was no dilution of the concentrated black liquor, but in which
conditions were otherwise quite similar. These examples
illustrate the fact that those skilled is the art should apply
the process experimentally to individual situations on the basis
of their knowledge and skills, thereby determining the best
approach so far as particular agents, quantities thereof, and
SUBSTITUTE SHEET (RULE 26)
CA 02291722 1999-11-26
WO 98/54400 PCT/US97/09418
other factors that can be varied for such individual situations
are concerned. This also applies to the handling of soda
digestion liquors.
Example 5 shows how adjusting physical arrangements for the
5 adding of polymers and for contacting the treated liquor with
acid to reduce pH may be significant in achieving efficient
lignin and silica separation.
The graphs of Figs. 2-5, are based on tests made on
respective filtrates (clarified liquids) for transmittivity and
10 TOD content vs pH of processed kraft digestion liquors, the
liquor of Figs. 2 and 3 being screen room wash water and that of
Figs. 4 and 5 being dilute waste water, both being derived from
waste, spent digestion liquor. Visible light transmittivity was
measured in each instance by the use of the same turbidity
15 measuring instrument, a nephelometer. These graphs show that the
process can be carried out at various pH values above and below
a pH of 3.
Whereas this invention is here illustrated and described
with reference to embodiments thereof presently contemplated as
20 the best mode of carrying out such invention in actual practice,
it is to be understood that various changes may be made in
adapting the invention to different embodiments without departing
from the broader inventive concepts disclosed herein and
comprehended by the claims that follow.
SUBSTITUTE SHEET (RULE 26)