Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02292604 1999-12-02
WO 98/57933 PCTNS98/11821
PYRROLIDINE CARBOXYLIC ACID DERIVATIVES AS ENDOTHELIN ANTAGONISTS
This is a continuation-in-part application of US Serial No. 08/877,187 filed
June 17, 1997.
Technical Field
The present invention relates to compounds which are endothelia
antagonists, processes for making such compounds, synthetic intermediates
employed in these processes and methods and compositions for antagonizing
endothelia.
Background of the Invention
Endothelia {ET) is a 21 amino acid peptide that is produced by endothelial
cells. ET is produced by enzymatic cleavage of a Trp-Val bond in the precursor
peptide big endothelia {Big ET). This cleavage is caused by an endothelia
converting enzyme (ECE). Endothelia has been shown to constrict arteries and
veins, increase mean arterial blood pressure, decrease cardiac output,
increase
cardiac contractility ~yjt~, stimulate mitogenesis in vascular smooth muscle
cells
in vi r , contract non-vascular smooth muscle including guinea pig trachea,
human
urinary bladder strips and rat uterus j_n v~,, increase airway resistance 'ur
vivo,
induce formation of gastric ulcers, stimulate release of atrial natriuretic
factor ~yj~
and i-n vivo, increase plasma levels of vasopressin, aldosterone and
catecholamines, inhibit release of renin i n vi r and stimulate release of
gonadotropins in yes.
It has been shown that vasoconstriction is caused by binding of endothelia to
its receptors on vascular smooth muscle (Nature 411 (1988), FEBS Letters ?~1
440 (1988) and Biochem. Biophys. Res. Commun. ,~,~ 868 (1988)). An agent
which suppresses endothelia production or an agent which binds to endothelia
or
which inhibits the binding of endothelia to an endothelia receptor will
produce
beneficial effects in a variety of therapeutic areas. In fact, an anti-
endothelia
antibody has been shown, upon intrarenal infusion, to ameliorate the adverse
effects of renal ischemia on renal vascular resistance and glomerular
filtration rate
(Kon, et al., J. Clin. Invest. $,~ 1762 (1989)). In addition, an anti-
endothelia antibody
attenuated the nephrotoxic effects of intravenously administered cyclosporin
(Kon,
et al., Kidney Int. ~7 1487 (1990)) and attenuated infarct size in a coronary
artery
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
2
Clozel et al. (Nature 365: 759-761 (1993)) report that Ro 46-2005, a
nonpeptide ET-A/B antagonist, prevents post-ischaemic renal vasoconstriction
in
rats, prevents the decrease in cerebral blood flow due to subarachnoid
hemorrhage
(SAH) in rats, and decreases MAP in sodium-depleted squirrel monkeys when
dosed
orally. A similar effect of a linear tripeptide-like ET-A antagonist, BQ-485,
on arterial
caliber after SAH has also been recently reported (S.Itoh, T. Sasaki, K. Ide,
K.
Ishikawa, M. Nishikibe, and M. Yano, Biochem. Biophys. Res. Comm. , 195: 969-
75
(1993). These results indicate that agents which antagonize ET/ET receptor
binding
will provide therapeutic benefit in the indicated disease states.
Two structurally related endothelin receptors have been cloned, sequenced
and characterized (Hosada, K.; Nakao, K.; Arai, H.; Suga, S.; Ogawa, Y.;
Mukoyama, M.; Shirakami, G.; Saito, Y.; Nakanishi, S.; Imura, H. FEBS Lett.
1991,
187, 23-26: Sakamoto, A.; Yanagisawa, M.; Sakurai, T.; Takuwa, Y.; Yanagisawa,
H.; Masaki, T. Biochem. Biophys. Res. Commun. 1991, 178, 656-663). Each binds
the three endothelin isopeptides with differing affinities; the ETA
receptor displays affinity for ET-1 and ET-2 over ET-3, while the ETg
receptor is non-isopeptide selective. Originally described as a vasodilatory
receptor
due to its mediation of nitric oxide release (DeNucci,G.; Thomas, R.;
D'Orleans-
Juste, P.; Antunes, E.; Walder, C.; Warner, T. D.; Vane, J. R. Proc. Natl.
Acad. Sci.
U.S.A. 1988, 85, 9797-9800), it is now apparent that the ETg receptor is
responsible
for a greater diversity of physiologic function. Current research suggests a
role for
ETg mediated responses in certain disease states including established
pulmonary
hypertension (McCulloch, K. M.; MacLean, M. R.; J. Cardiovasc. Pharmacol.
1995,
26(Suppl. 3), S169-S176), contractile dysfunction associated with benign
prostatic
hyperplasia (Webb, M. L.; Chao, C.-C.; Rizzo, M.; Shapiro, R. A.; Neubauer,
M.; Liu,
E. C. K.; Aversa, C. R.; Brittain, R. J.; Treiger, B. Mol. Pharmacol. 1995,
47, 730-737;
Webb, M. L.; Meek, T. D. Med. Res. Rev. 1997, 17, 17-67), myocardial
infarction
(Vitola, J. V.; Forman, M. B.; Holsinger, J. P.; Kawana, M.; Atkinson, J. B.;
Quertermous, T.; Jackson, E. K.; Murray, J.J. J. Cardiovasc. Pharmacol. 1996,
28,
774-783), and atherosclerosis (Dagassan, P. H.;
Breu, V.; Clozel, M.; Kunzli, A.; Vogt, P, Turina, M.; Kiowski, Clozel, J. -P.
J.
Cardiovasc. Pharmacol. 1996, 27, 147-153). Our group has previously reported
the
discovery of a series of pyrrolidine-3-carboxylic acids which which bind
potently and
selectively to the ETA receptor subtype (Winn, M.; von Geldern, T. W.;
Opgenorth, T.
J.; Jae, H. -S.; Tasker, A. S.; Boyd, S. A.; Kester, J. A.; Mantei, R. A.;
Bal, R. B.;
Sorensen, B. K.; Wu-Wong, J. R.; Chiou, W. J.; Dixon, D. B.; Novosad, E. L;
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
3
Hernandez, L.; Marsh, K. C. J. Med. Chem. 1996, 39, 1039-1048). The compounds
claimed in the current invention differ from those previously described and
are
unique in that they bind potently and selectively to the ETg subtype, blocking
the
- actions of the endothelins on these receptors. As such, they may find
utility in the
treatment of diseases that are mediated by the ETg receptor.
Disclosure of the Invention
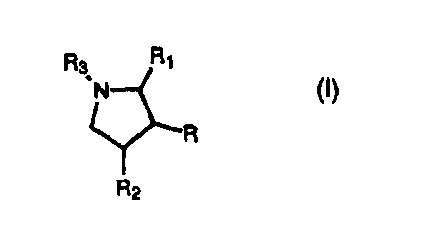
In accordance with the present invention there are compounds of the formula
(I):
Ra R1
~N
~R
R2
wherein
R is -(CH2)m-W wherein m is an integer from 0 to 6 and W is
(a) -C(O)2-G wherein G is hydrogen or a carboxy protecting group,
(b) -POgH2,
(c) -P(O)(OH)E wherein E is hydrogen, loweralkyl or arylalkyl,
(d) -CN,
(e) -C{O)NHR1~ wherein R17 is loweralkyl,
(f) alkylaminocarbonyl,
(g) dialkylaminocarbonyl,
(h) tetrazolyl,
(i) hydroxy,
{j) alkoxy,
(k) sulfonamido,
(() -C{O)NHS(O)2R~g wherein R16 is loweralkyl, haloalkyl, aryl or
dialkylamina,
(m) -S(O)2NHC(O)R16 wherein R16 is defined as above,
HO 0
NH
(n) O ,
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
4
sS' o
(o) Ho 0
OH
~N
r
(p) o ,
O
- NH
(q) O
.~~ Nw O
N
~~H
(r) O
N~ O
~S= O
N
(S) H ,
~~ N
~~ CFa
N
(t) H , Or
' ~ ~ NHSOpCF3
(U)
R1 and R2 are independently selected from hydrogen, foweralkyl, alkenyl,
alkynyl,
alkoxyalkyl, alkoxycarbonylalkyl, hydroxyalkyl, haloalkyl, haloalkoxyalkyl,
alkoxyalkoxyafkyl, thioalkoxyalkoxyalkyl, cycloalkyl, cycloalkylalkyl,
aminocarbonylalkyl, alkylaminocarbonylalkyl, dialkylaminocarbonylalkyl,
aminocarbonylalkenyl, alkylaminocarbonylalkenyl, dialkylaminocarbonylalkenyl,
hydroxyalkenyl, aryl, arylalkyl, aryloxyalkyl, arylalkoxyalkyl, heterocyclic,
(heterocyclic)alkyl and (Raa)(Rbb)N-Rcc' wherein Raa is aryl or arylalkyl, Rbb
is
hydrogen or alkanoyl and R~~ is alkylene, with the proviso that one or both of
R1 and
R2 is other than hydrogen;
R3 is R4-C(O)-R5- or R6-S(O)2-R~- wherein R5 is (i) a covalent bond, (ii)
alkylene,
(iii) alkenylene, (iv) -N(R2p)-R8- or -R$a N(R2o)-R$- wherein Rg and R8a are
independently selected from the group consisting of alkylene and alkenylene;
and
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
R2p is hydrogen, loweralkyl, alkenyl, haloalkyl, alkoxyalkyl, haloalkoxyalkyl,
cyicoalkyl or cycloalkylalkyl or (v) -O-R9- or -R9a-O-R9- wherein R9 and R9a
are
independently selected from alkylene;
- R~ is (i) a covalent bond, (ii) alkylene, (iii) alkenylene or (iv) -N(R21)-
Rip- or
5 -R~oa-N(R21)-R10- wherein Rip and Rloa are independently selected from the
group
consisting of alkylene and alkenylene and R21 is hydrogen, loweralkyl,
alkenyl,
haloalkyl, alkoxyalkyl, haloalkoxyalkyl, aryl or arylalkyl;
wherein R4 and R6 are
R11
R13~ H
R 14 ~~ ~ N\
R15
(i) R12 ,
wherein Rte and Rt2 are independently selected from the
group consisting of loweralkyl, cyano, aikoxy, halo, haloalkyl and phenyl and
R~3, R~4, and R15 are independently selected from the group consisting of
hydrogen,
loweralkyl, hydroxy, amino, alkoxy, aryl, heterocyclic, halo, carboxy, vitro,
alkyisuffonyl, arylsulfonyl, thioalkoxy, thioaryloxy, or cyano; or
(ii) heterocyclic(amino),
or a pharmaceutically acceptable salt thereof.
A preferred embodiment of the invention is a compound of formula (II)
Ra R1
~N
rrrr R
R2
(II)
wherein the substituents -R2, -R and -Rt exist in a trans,trans relationship
and R, Rt,
R2, and R3 are as defined above.
A more preferred embodiment of the invention is a compound of formula (() or
(II) wherein R3 is R4-C(O)-R5- wherein R4 is as defined above and R5 is
alkylene or
R3 is R6-S(O)2-R7- wherein R~ is alkylene and Rg is defined as above.
An even more preferred embodiment of the invention is a compound of
- formula (I) or (II) wherein R is -C(O)2-G wherein. G is hydrogen or a
carboxy protecting
group or R is tetrazolyl or R is -C(O)-NHS(O)2R~6 wherein R16 is loweralkyl,
haloalkyl
-30 or aryl, R1 and R2 are independently selected from
CA 02292604 1999-12-02
WO 98/57933 PCTNS98/11821
s
(i) loweralkyl, (ii) cycloalkyl, (iii) substituted and unsubstituted aryl, and
(iv)
substituted or unsubstituted heterocyclic, and R3 is R4-C(O)-R5- wherein R4 is
as
defined above and R5 is alkylene or Rg is Rg-S(O)2-R~- wherein R~ is alkyiene
and
R6 is defined as above.
A yet more preferred embodiment of the invention is a compound of formula {I)
or (II) wherein R is -C(O)2-G wherein G is hydrogen or a carboxy protecting
group,
tetrazolyl or -C{O)-NHS{O)2R16 wherein R16 is loweralkyl, haloalkyl or aryl,
R1 is (i)
alkoxyalkyi, (ii) cycloalkyl, (iii) phenyl, (iv) pyridyl, (v) furanyl or (vi)
substituted or
unsubstituted 4-methoxyphenyl, 4-fluorophenyl, 3-fluorophenyl, 4-ethoxyphenyl,
4-
propoxyphenyl, 4-isopropoxyphenyl, 4-trifluoromethylphenyl, 4-
pentafluoroethylphenyl, 2-fluoro-4-ethoxyphenyl, 3-fluoro-4-methoxyphenyl, 3-
fluoro-
4-ethoxyphenyl, 3-fluoro-4-propoxyphenyl, 3-methoxy-4-propoxyphenyl, 2-
fluorophenyl, 4-methoxymethoxyphenyl, 4-(2-methoxyethoxy)phenyl, 4-(2-
ethoxyethoxy)phenyl, 4-(2-isopropoxyethoxy)phenyl, 4-hydroxyphenyl, 1,3-
benzodioxolyl, 1,4-benzodioxanyl or dihydrobenzofuranyl wherein the
substituent is
selected from alkoxy, alkoxyalkoxy and carboxyalkoxy, R2 is substituted or
unsubstituted 1,3-benzodioxolyl, 7-methoxy-1,3-benzodioxolyl, 1,4-
benzodioxanyl, 8-
methoxy-1,4-benzodioxanyl, dihydrobenzofuranyl, benzofuranyl, 4-methoxyphenyl,
dimethoxyphenyl, fluorophenyl or difluorophenyl and R3 is R4-C(O)-R5- wherein
R4 is
as defined above and R5 is alkylene or R3 is R6-S(O)2-R~- wherein R~ is
alkylene
and Rg is defined as above.
Another yet more preferred embodiment of the invention is a compound of
formula (I) or (II) wherein R is -C(O)2-G wherein G is hydrogen or a carboxy
protecting
group, tetrazolyl or -C(O)-NHS(O)2R~6 wherein R16 is loweralkyl, haloalkyl or
aryl, R1
is (i) alkoxyalkyl, (ii) cycloalkyl, (iii) phenyl, (iv) pyridyl, (v) furanyl
or (vi) substituted or
unsubstituted 4-methoxyphenyl, 4-fluorophenyl, 3-fluorophenyl, 4-ethoxyphenyl,
4-
propoxyphenyl, 4-isopropoxyphenyl, 4-trifluoromethylphenyl, 4-
pentafluoroethylphenyl, 2-fluoro-4-ethoxyphenyl, 3-fluoro-4-methoxyphenyl, 3-
fluoro-
4-ethoxyphenyl, 3-fluoro-4-propoxyphenyl, 3-methoxy-4-propoxyphenyl, 2-
ffuorophenyl, 4-methoxymethoxyphenyl, 4-(2-methoxyethoxy)phenyl, 4-(2-
ethoxyethoxy)phenyl, 4-{2-isopropoxyethoxy)phenyl, 4-hydroxyphenyl, 1,3-
benzodioxolyl, 1,4-benzodioxanyl or dihydrobenzofuranyl wherein the
substituent is
selected from alkoxy, alkoxyalkoxy and carboxyalkoxy, R2 is substituted or
unsubstituted 1,3-benzodioxolyl, 7-methoxy-1,3-benzodioxolyl, 1,4-
benzodioxanyl, 8-
methoxy-1,4-benzodioxanyl, dihydrobenzofuranyl, benzofuranyl, 4-methoxyphenyl,
dimethoxyphenyi, fluorophenyl or difluorophenyl and R3 is R4-C(O)-R5- wherein
R4 is
as defined above and R5 is alkylene.
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
7
A still more preferred embodiment of the invention is a compound of formula
(I)
or (II} wherein R is -C(O)2-G wherein G is hydrogen or a carboxy protecting
group,
tetrazolyl or -C(O}-NHS(O)2R16 wherein R16 is loweralkyl or haloalkyl, R1 is
substituted or unsubstituted 4-methoxyphenyl, 4-fluorophenyl, 2-fluorophenyl,
4-
ethoxyphenyl, 4-propoxyphenyl, 4-isopropoxyphenyl, 3-fluoro-4-methoxyphenyl, 3-
fluoro-4-ethoxyphenyl, 2-fluoro-4-ethoxyphenyl, 3-fluoro-4-propoxyphenyl, 3-
methoxy-4-propoxyphenyl, 4-trifluoromethylphenyl, 4-pentafluoroethylphenyl, 4-
methoxymethoxyphenyl, 4-(2-methoxyethoxy)phenyl, 4-(2-ethoxyethoxy)phenyl, 4-
(2-
isopropoxyethoxy)phenyl, 4-hydroxyphenyl, 1,3-benzodioxolyl, 1,4-benzodioxanyl
or
dihydrobenzofuranyl wherein the substituent is selected from alkoxy,
alkoxyalkoxy
and carboxyalkoxy, R2 is 1,3-benzodioxolyl, 7-methoxy-1,3-benzodioxolyl, 1,4-
benzodioxanyl, dihydrobenzofuranyl, benzofuranyl, 4-methoxyphenyl,
dimethoxyphenyl, fluorophenyl or difluorophenyl and R3 is R4-C(O)-RS- wherein
R4 is
as defined above and R5 is alkylene.
A most highly preferred embodiment of the invention is a compound of formula
(I) or (II) wherein R is -C(O)2-G wherein G is hydrogen or a carboxy
protecting group,
R1 is substituted or unsubstituted 4-methoxyphenyl, 4-fluorophenyl, 2-
fluorophenyl,
3-fluoro-4-ethoxyphenyl, 3-fluoro-4-methoxyphenyl, 4-ethoxyphenyl, 4-
propoxyphenyl, 4-isopropoxyphenyl, 2-fluoro-4-ethoxyphenyl, 3-fluoro-4-
propoxyphenyl, 3-methoxy-4-propoxyphenyl, 4-methoxymethoxyphenyl, 4-(2-
methoxyethoxy)phenyl, 4-(2-ethoxyethoxy}phenyl, 4-(2-isopropoxyethoxy)phenyl,
4-
hydroxyphenyl, 1,3-benzodioxolyl, 1,4-benzodioxanyl or dihydrobenzofuranyl
wherein the substituent is selected from alkoxy, afkoxyalkoxy and
carboxyalkoxy, R2
is 1,3-benzodioxolyl, 7-methoxy-1,3-benzodioxolyl, 1,4-benzodioxanyl,
dihydrobenzofuranyl, benzofuranyl, 4-methoxyphenyl, dimethoxyphenyl,
fluorophenyl or difluorophenyl and R3 is R4-C(O)-R5- wherein R4 is
R»
Rya\-
H
Rya \~ ~ N\
R~5
R~2
wherein R11 and R~2 are independently selected from loweralkyl, and R~3,
R14, and R15 are independently selected from the group consisting of hydrogen,
loweralkyl, hydroxy, amino, alkoxy, aryl, heterocyclic, halo, carboxy, vitro,
alkylsulfonyl, arylsulfonyl, thioalkoxy, thioaryloxy, or cyano and R5 is
alkylene.
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
8
A most highly preferred embodiment of the invention is a compound of formats
(I) or (II) wherein R is -C(O)2-G wherein G is hydrogen or a carboxy
protecting group,
R1 is substituted or unsubstituted 4-methoxyphenyl, 4-fluorophenyl, 2-
fluorophenyl,
3-fluoro-4-ethoxyphenyl, 3-fluoro-4-methoxyphenyl, 4-ethoxyphenyl, 4-
propoxyphenyl, 4-isopropoxyphenyl, 2-fluoro-4-ethoxyphenyl, 3-fluoro-4-
propoxyphenyl, 3-methoxy-4-propoxyphenyl, 4methoxymethoxyphenyl, 4-(2-
methoxyethoxy)phenyl, 4-(2-ethoxyethoxy)phenyl, 4-(2-isopropoxyethoxy)phenyl,
4-
hydroxyphenyl, 1,3-benzodioxolyl, 1,4-benzodioxanyf or dihydrobenzofuranyl
wherein the substituent is selected from alkoxy, alkoxyalkoxy and
carboxyalkoxy, R2
1 o is 1,3-benzodioxolyl, 7-methoxy-1,3-benzodioxolyl, 1,4-benzodioxanyl,
dihydrobenzofuranyl, benzofuranyl, 4-methoxyphenyl, dimethoxyphenyl,
fluorophenyl or difluorophenyl and R3 is R4-C(O)-R5- wherein R4 is
R»
R~3~ H
_/
Rya ~\ ~ N\
R~5
Rt2
wherein R1~ and R~2 are independently selected from the
group consisting of lower alkyl, alkoxy and halo, and R13, R14, and R15 are
independently selected from the group consisting of hydrogen, loweralkyl,
hydroxy,
amino, alkoxy, aryl, heterocyclic, halo, carboxy, vitro, alkylsulfonyl,
arylsulfonyl,
thioalkoxy, thioaryloxy, or cyano and R5 is alkylene.
Another most highly preferred embodiment of the invention is a compound of
formula (I) or (II) wherein R is -C(O)2-G wherein G is hydrogen or a carboxy
protecting
group, R1 is substituted or unsubstituted 4-methoxyphenyl, 4-fluorophenyl, 2-
fluorophenyl, 3-fluoro-4-ethoxyphenyl, 3-fluoro-4-methoxyphenyl, 4-
ethoxyphenyl, 4-
propoxyphenyl, 4-isopropoxyphenyl, 2-fluoro-4-ethoxyphenyl, 3-fluoro-4-
propoxyphenyl, 3-methoxy-4-propoxyphenyl, 4-methoxymethoxyphenyl, 4-(2-
methoxyethoxy)phenyl, 4-(2-ethoxyethoxy)phenyl, 4-(2-isopropoxyethoxy)phenyl,
4-
hydroxyphenyl, 1,3-benzodioxolyl, 1,4-benzodioxanyl or dihydrobenzofuranyl
wherein the substituent is selected from alkoxy, alkoxyalkoxy and
carboxyalkoxy, R2
is 1,3-benzodioxolyl, 7-methoxy-1,3-benzodioxolyl, 1,4-benzodioxanyt,
dihydrobenzofuranyl, benzofuranyl, 4-methoxyphenyl, dimethoxyphenyl,
fluorophenyl or difluorophenyl and R3 is R4-C(O)-R5- wherein R4 is
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
9
R»
R~3
_/ H
Rya \\ ~ N\
R~s
R~2
wherein R11 and R12 are independently selected from the group consisting of
methyl, ethyl, and isopropyl, and R13, R14, and R15 are independently selected
from
the group consisting of hydrogen, loweralkyl, hydroxy, amino, alkoxy, aryl,
heterocyciic, halo, carboxy, nitro, alkylsulfonyl, arylsulfonyl, thioalkoxy,
thioaryloxy, or
cyano and R5 is alkylene.
Another most highly preferred embodiment of the invention is a compound of
formula (I) or (ll) wherein R is -C(O)2-G wherein G is hydrogen or a carboxy
protecting
group, R1 is substituted or unsubstituted 4-methoxyphenyl, 4-fiuorophenyl, 2-
fluorophenyl, 3-fluoro-4-ethoxyphenyl, 3-fluoro-4-methoxyphenyl, 4-
ethoxyphenyl, 4-
propoxyphenyl, 4-isopropoxyphenyl, 2-fluoro-4-ethoxyphenyl, 3-fluoro-4-
propoxyphenyl, 3-methoxy-4-propoxyphenyl, 4-methoxymethoxyphenyl, 4-(2-
methoxyethoxy)phenyl, 4-(2-ethoxyethoxy)phenyl, 4-(2-isopropoxyethoxy)phenyl,
4-
hydroxyphenyl, 1,3-benzodioxolyl, 1,4-benzodioxanyl or dihydrobenzofuranyl
wherein the substituent is selected from alkoxy, alkoxyalkoxy and
carboxyalkoxy, R2
is 7 ,3-benzodioxolyl, 7-methoxy-1,3-benzodioxolyl, 1,4-benzodioxanyl,
dihydrobenzofuranyl, benzofuranyl, 4-methoxyphenyl, dimethoxyphenyl,
fluorophenyl or difluorophenyl and R3 is R4-C(O)-R5- wherein R4 is
R»
R13~ H
_/
Rya \\ ~ N\
R ~~
Rt2
wherein R11 and R~2 are independently selected from the group consisting of
methyl, ethyl, and isopropyl, and R~3, R14, and R15 are independently selected
from
the group consisting of hydrogen, loweralkyl, hydroxy, amino, alkoxy, aryl,
heterocyclic, halo, carboxy, vitro, alkylsulfonyl, arylsulfonyl, thioalkoxy,
thioaryloxy, or
cyano and R5 is methylene.
The present invention also relates to processes for preparing the compounds
of formula (I) and (II) and to the synthetic intermediates employed in these
processes.
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
The present invention also relates to a method of antagonizing endothelia in a
mammal (preferably a human) in need of such treatment, comprising
administering to
the mammal a therapeutically effective amount of a compound of formula (I) or
(If).
The invention further relates to endothefin antagonizing compositions
5 comprising a pharmaceutical carrier and a therapeutically effective amount
of a
compound of formula (I) or (II).
The compounds of the invention comprise two or more asymmetrically
substituted carbon atoms. As a result, racemic mixtures, mixtures of
diastereomers,
as well as single diastereomers of the compounds of the invention are included
in the
10 present invention. The terms "S" and "R" configuration are as defined by
the IUPAC
1974 Recommendations for Section E, Fundamental Stereochemistry, Pure Appl.
Chem. (1976) 45, 13 - 30.
The term "carboxy protecting group" as used herein refers to a carboxylic acid
protecting ester group employed to block or protect the carboxylic acid
functionality
75 while the reactions involving other functional sites of the compound are
carried out.
Carboxy protecting groups are disclosed in Greene, "Protective Groups in
Organic
Synthesis" pp. 152-186 (1981 ), which is hereby incorporated herein by
reference. In
addition, a carboxy protecting group can be used as a prodrug whereby the
carboxy
protecting group can be readily cleaved in vivo , for example by enzymatic
hydrolysis,
to release the biologically active parent. T. Higuchi and V. Stella provide a
thorough
discussion of the prodrug concept in "Pro-drugs as Novel Delivery Systems",
Vol 14
of the A.C.S. Symposium Series, American Chemical Society (1975), which is
hereby incorporated herein by reference. Such carboxy protecting groups are
well
known to those skilled in the art, having been extensively used in the
protection of
carboxyl groups in the penicillin and cephalosporin fields, as described in
U.S. Pat.
No. 3,840,556 and 3,719,667, the disclosures of which are hereby incorporated
herein by reference. Examples of esters useful as prodrugs for compounds
containing carboxyl groups can be found on pages 14-21 of "Bioreversible
Carriers
in Drug Design: Theory and Application", edited by E.B. Roche, Pergamon Press,
New York (1987), which is hereby incorporated herein by reference.
Representative
carboxy protecting groups are C~ to Cg alkyl (e.g., methyl, ethyl or tertiary
butyl and
the like); haloalkyl; alkenyl; cycloalkyl and substituted derivatives thereof
such as
cyclohexyl, cyclopentyl and the like; cycioalkylalkyl and substituted
derivatives
thereof such as cyclohexylmethyl, cyclopentylmethyl and the like; arylalkyl,
for
example, phenethyl or benzyf and substituted derivatives thereof such as
alkoxybenzyl or nitrobenzyl groups and the like; arylalkenyl, for example,
phenylethenyl and the like; aryl and substituted derivatives thereof, for
example, 5-
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
11
indanyl and the like; dialkylaminoalkyl (e.g., dimethylaminoethyl and the
like);
alkanoyloxyalkyl groups such as acetoxymethyl, butyryloxymethyl,
valeryloxymethyi,
isobutyryloxymethyl, isovaleryloxymethyl, 1-(propionyfoxy)-1-ethyl, 1-
(pivaloyloxyl)-1--
ethyl, 1-methyl-1-{propionyloxy}-1-ethyl, pivaloyloxymethyl,
propionyloxymethyl and
the like; cycloalkanoyloxyalkyl groups such as cyclopropylcarbonyloxymethyl,
cyclobutylcarbonyloxymethyl, cyclopentylcarbonyloxymethyl,
cyclohexylcarbonyloxymethyl and the like; aroyloxyalkyl, such as
benzoyloxymethyl,
benzoyloxyethyl and the like; arylalkylcarbonyloxyalkyl, such as
benzylcarbonyloxymethyl, 2-benzylcarbonyloxyethyl and the like;
alkoxycarbonylalkyl, such as methoxycarbonylmethyl,
cyclohexyloxycarbonylmethyl,
1-methoxycarbonyl-1-ethyl, and the like; alkoxycarbonyloxyalkyl, such as
methoxycarbonyloxymethyl, t-butyloxycarbonyloxymethyl, 1-ethoxycarbonyloxy-1-
ethyl,
1-cyclohexyloxycarbonyloxy-1-ethyl and the like; alkoxycarbonylaminoalkyl,
such as
t-butyloxycarbonylaminomethyl and the like; alkylaminocarbonylaminoaikyl, such
as
methylaminocarbonylaminomethyl and the like; alkanoylaminoalkyl, such as
acetylaminomethyl and the like; heterocycliccarbonyloxyalkyl, such as 4-
methylpiperazinylcarbonyloxymethyl and the like; dialkylaminocarbonylalkyl,
such as
dimethylaminocarbonylmethyl, diethylaminocarbonylmethyl and the like; (5-
(loweralkyl)-2-oxo-1,3-dioxolen-4-yl)alkyl, such as (5-t-butyl-2-oxo-1,3-
dioxolen-4-
yl)methyl and the like; and {5-phenyl-2-oxo-1,3-dioxolen-4-yl)alkyl, such as
(5-
phenyl-2-oxo-1,3-dioxolen-4-yl)methyl and the like.
The term "N-protecting group" or "N-protected" as used herein refers to those
groups intended to protect the N-terminus of an amino acid or peptide or to
protect an
amino group against undersirable reactions during synthetic procedures.
Commonly
used N-protecting groups are disclosed in Greene, "Protective Groups In
Organic
Synthesis," (John Wiley & Sons, New York (1981 )), which is hereby
incorporated by
reference. N-protecting groups comprise acyl groups such as formyl, acetyl,
propionyl, pivaloyl, t-butylacetyl, 2-chioroacetyl, 2-bromoacetyl,
trifluoroacetyl,
trichloroacetyl, phthaiyl, o-nitrophenoxyacetyl, a-chlorobutyryl, benzoyl, 4-
chlorobenzoyl, 4-bromobenzoyl, 4-nitrobenzoyl, and the like; sulfonyl groups
such as
benzenesulfonyl, p-toluenesulfonyl and the like; carbamate forming groups such
as
benzyloxycarbonyl, p-chlorobenzyloxycarbonyl, p-methoxybenzyloxycarbonyl, p-
nitrobenzyloxycarbonyl, 2-nitrobenzyloxycarbonyl, p-bromobenzyloxycarbonyl,
3,4-
dimethoxybenzyfoxycarbonyl, 3,5-dimethoxybenzyloxycarbonyl, 2,4-
dimethoxybenzyloxycarbonyl, 4-methoxybenzyloxycarbonyl, 2-vitro-4,5-
CA 02292604 1999-12-02
WO 98/57933 PCT/US98111821
12
dimethoxybenzyloxycarbonyl, 3,4,5-trimethoxybenzyloxycarbonyl, 1-(p-
biphenylyl)-1-
methylethoxycarbonyl, a,a-dimethyl-3,5-dimethoxybenzyloxycarbonyl,
benzhydryloxycarbonyl, t-butyloxycarbonyl, diisopropylmethoxycarbonyl,
isopropyloxycarbonyl, ethoxycarbonyl, methoxycarbonyl, allyloxycarbonyl,
2,2,2,-
trichioroethoxycarbonyl, phenoxycarbonyl, 4-nitrophenoxycarbonyl, fluorenyl-9-
methoxycarbonyl, cyclopentyloxycarbonyl, adamantyloxycarbonyl,
cyclohexyloxycarbonyl, phenylthiocarbonyl and the like; alkyl groups such as
benzyl,
triphenylmethyl, benzyloxymethyl and the like; and silyl groups such as
trimethylsilyl
and the like. Preferred N-protecting groups are formyl, acetyl, benzoyl,
pivaloyl, t-
butylacetyl, phenylsulfonyl, benzyl, t-butyloxycarbonyl (Boc) and
benzyloxycarbonyl
(Cbz).
The term "alkanoyl" as used herein refers to an alkyl group as defined herein
appended to the parent molecular moiety through a carbonyl (-C(O)-) group.
Examples of alkanoyl include acetyl, propionyl and the like.
The term "alkanoylamino" as used herein refers to an alkanoyl group as
previously defined appended to an amino group. Examples alkanoylamino include
acetamido, propionamido and the like.
The term "alkanoylaminoalkyl" as used herein refers to R43-NH-R44- wherein
R43 is an alkanoyl group and R44 is an alkylene group.
The term "alkanoyloxyalkyl" as used herein refers to R3o-O-R31- wherein R3o
is an alkanoyl group and R3t is an alkylene group. Examples of
alkanoyloxyalkyl
include acetoxymethyl, acetoxyethyl and the like.
The term "alkenyl" as used herein refers to a straight or branched chain
hydrocarbon radical containing from 2 to 15 carbon atoms and also containing
at
least one carbon-carbon double bond. Alkenyi groups include, for example,
vinyl
(ethenyl}, allyl (propenyl), butenyl, 1-methyl-2-buten-1-yl and the like.
The term "alkenylene" denotes a divalent group derived from a straight or
branched chain hydrocarbon containing from 2 to 15 carbon atoms and also
containing at least one carbon-carbon double bond. Examples of alkenylene
include
-CH=CH-, -CH2CH=CH-, -C(CHg)=CH-, -CH2CH=CHCH2-, and the like.
The term "alkenyloxy" as used herein refers to an alkenyl group, as previously
defined, connected to the parent molecular moiety through an oxygen (-O-)
linkage.
Examples of alkenyloxy include allyloxy, butenyloxy and the like.
The term "alkoxy" as used herein refers to 8420- wherein R42 is a loweralkyl
group, as defined herein. Examples of alkoxy include, but are not limited to,
methoxy,
ethoxy, propoxy, iso-butoxy, tert-butoxy, and the like.
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
13
The term "alkoxyalkoxy" as used herein refers to R8o0-R8~0- wherein R8a is
loweralkyl as defined above and R8~ is alkylene. Representative examples of
alkoxyaikoxy groups include methoxymethoxy, ethoxymethoxy, t-butoxymefhoxy and
the like.
The term "alkoxyalkoxyalkoxy" as used herein refers to 8820-8830-Rg40-
wherein R82 is loweralkyl as defined above and Rg3 and Rg4 are alkylene.
Representative examples of alkoxyalkoxyalkoxy groups include
methoxyethoxymethoxy, ethoxymethoxymethoxy, t-butoxymethoxymethoxy and the
like.
The term "(alkoxyalkyl)sulfonyl" as used herein refers to Rg5-O-Rg6-S(O)2-,
wherein Rg5 is loweralkyl and Rgg is alkylene.
The term "alkoxyalkoxyalkyl" as used herein refers to an alkoxyalkoxy group
as previously defined appended to an alkyl radical. Representative examples of
alkoxyalkoxyalkyl groups include methoxyethoxyethyl, methoxymethoxymethyl, and
the like.
The term "alkoxyalkyl" as used herein refers to an alkoxy group as previously
defined appended to an alkyl radical as previously defined. Examples of
alkoxyalkyl
include, but are not limited to, methoxymethyl, methoxyethyl, isopropoxymethyl
and
the like.
The term "alkoxycarbonyl" as used herein refers to an alkoxyl group as
previously defined appended to the parent molecular moiety through a carbonyl
group. Examples of alkoxycarbonyl include rnethoxycarbonyl, ethoxycarbonyl,
isopropoxycarbonyl and the like.
The term "alkoxycarbonylalkenyl" as used herein refers to an alkoxycarbonyl
group as previously defined appended to an alkenyl radical. Examples of
alkoxycarbonylalkenyl include methoxycarbonylethenyl, ethoxycarbonylethenyl
and
the like.
The term "alkoxycarbonylalkyl" as used herein refers to R34-C(O)-Rg5- wherein
Rg4 is an alkoxy group and R35 is an alkylene group. Examples of
alkoxycarbonylalkyl include methoxycarbonylmethyl, methoxycarbonylethyl,
ethoxycarbonylmethyl and the like.
The term "alkoxycarbonylaminoalkyl" as used herein refers to
Rg8-C(O}-NH-R39- wherein R38 is an alkoxy group and R39 is an alkylene group.
Examples of alkoxycarbonyfaminoalkyl include methoxycarbonylaminoethyl and the
like.
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
14
The term "aikoxycarbonyloxyalkyl" as used herein refers to R36-C(O)-O-R3~_
wherein Rgg is an alkoxy group and R3~ is an alkylene group. Examples of
alkoxycarbonyloxyalkyl include (ethoxycarbonyloxy}methyl and the like.
The term "(alkoxycarbonyl)thioalkoxy" as used herein refers to an
alkoxycarbonyl group as previously defined appended to a thioalkoxy radical.
Examples of (alkoxycarbonyl)thioalkoxy include methoxycarbonylthiomethoxy,
ethoxycarbonylthiomethoxy and the like.
The terms "alkyl" and "loweralkyl" as used herein refer to straight or
branched
chain alkyl radicals containing from 1 to 15 carbon atoms including, but not
limited to,
methyl, ethyl, n-propyl, iso-propyl, n-butyl, iso-butyl, sec-butyl, t-butyl, n-
pentyl, 1-
methylbutyl, 2,2-dimethylbutyl, 2-methylpentyl, 2,2-dimethylpropyl, n-hexyl
and the
like.
The term "alkyiamino" as used herein refers to R51NH- wherein R51 is a
loweralkyl group, for example, ethylamino, butyfamino, and the like.
The term "(alkylamino)alkoxy" as used herein refers R52NH-R53-O- wherein
R52 is loweralkyl and R53 is alkylene.
The term "alkylaminocarbonyl" as used herein refers to an alkylamino group,
as previously defined, appended to the parent molecular moiety through a
carbonyl
(-C(O)-) linkage. Examples of alkylaminocarbonyl include methylaminocarbonyl,
ethylaminocarbonyl, isopropylaminocarbonyl and the like.
The term "alkylaminocarbonylalkenyl" as used herein refers to an alkenyl
radical to which is appended an alkylaminocarbonyl group.
The term "alkylarninocarbonylalkyl" as used herein refers to a loweralkyi
radical to which is appended an alkylaminocarbonyl group.
The term "alkylaminocarbonylaminoalkyl" as used herein refers to
R4p-C(O)-NH-R41- wherein R4p is an alkylamino group and R41 is an alkylene
group.
The term "alkylene" denotes a divalent group derived from a straight or
branched chain saturated hydrocarbon having from 1 to 15 carbon atoms by the
removal of two hydrogen atoms, for example -CH2-, -CH2CH2-, -CH(CH3}-, -
CH2CH2CH2-, -CH2C(CH3)2CH2- and the like.
The term "alkylsulfonyl" refers to an alkyl group appended to the parent
molecular moiety through a sulfonyl group -S(O)2-. Examples of alkyisulfonyl
include
methylsulfonyl, ethylsulfonyl, isopropylsulfonyl and the like.
The term "(alkylsulfonyl)amino" as used herein refers to an alkyl group as
previously defined appended to the parent molecular moiety through a
sulfonylamino
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
{-S(O)2-NH-) group. Examples of (alkylsulfonyl)amino include
methylsulfonylamino,
ethylsulfonylamino, isopropylsulfonylamino and the like.
The term "(alkylsulfonyl)alkoxy" as used herein refers to an alkylsulfonyl
group
. as previously defined appended to the parent molecular moiety through a
alkoxy
5 group. Examples of (alkylsulfonyl)alkoxy include methylsulfonylmethoxy,
ethylsulfonylethoxy, isopropylsulfonylisopropoxy and the like.
The term "(alkylthio)alkoxy" as used herein refers to R54-S-R55-O-, wherein
RSq. is loweralkyl and R55 is alkylene.
The term "alkynyl" as used herein refers to a straight or branched chain
10 hydrocarbon radical containing from 2 to 15 carbon atoms and also
containing at
least one carbon-carbon triple bond. Examples of alkynyl include -C--_C-H, H-C-
-_C-
CH2-, H-C-_-C-CH(CHg)-, CH3-C= C-CH2-, and the like.
The term "aminocarbonyl" as used herein refers to H2N-C(O)- .
The term "aminocarbonylalkenyl" as used herein refers to an alkenyl radical to
15 which is appended an aminocarbonyl (NH2C(O)-) group.
The term "aminocarbonylalkoxy" as used herein refers to H2N-C(O)-
appended to an alkoxy group as previously defined. Examples of
aminocarbonylalkoxy include aminocarbonylmethoxy, aminocarbonylethoxy and the
like.
The term "aminocarbonyialkyl" as used herein refers to a loweralkyl radical to
which is appended an aminocarbonyl (NH2C(O)-) group.
The term "aroyloxyalkyl" as used herein refers to R32-C(O)-O-R33- wherein R32
is an aryl group and R33 is an alkylene group. Examples of aroyloxyalkyi
include
benzoyloxymethyi, benzoyloxyethyl and the like.
The term "aryl" as used herein refers to a mono- or bicyclic carbocyclic ring
system having one or two aromatic rings including, but not limited to, phenyl,
naphthyl, tetrahydronaphthyl, indanyl, indenyl and the like. Aryl groups can
be
unsubstituted or substituted with one, two or three substituents independently
selected from loweralkyl, halo, haloalkyl, haloalkoxy, hydroxyalkyl,
alkenyloxy,
alkoxy, alkoxyalkoxy, alkoxycarbonyl, alkoxyalkoxyalkoxy, (cycloalkyl)alkoxy,
cycloalkoxy, (alkylamino)alkoxy, (alkylthio)alkoxy, alkoxycarbonylalkenyl,
(alkoxycarbonyl)thioalkoxy, thioalkoxy, amino, alkylamino, dialkylamino,
(diaikylamino)alkyl, (dialkylamino)alkoxy, aminocarbonyl, aminocarbonyialkoxy,
alkanoylamino, arylalkoxy, aryloxy, mercapto, cyano, vitro, carboxaldehyde,
carboxy,
carboxyalkenyl, carboxyaikoxy, carboxamide, alkylsulfonyl,
(alkylsulfonyl)amino,
(alkylsulfonyl)alkoxy, (alkoxyalkyl)sulfonyl, cyanoalkoxy,
(heterocyclic)alkoxy,
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
16
hydroxy, hydroxalkoxy, phenyl and tetrazolylalkoxy. In addition, substituted
aryl
groups include tetrafluorophenyl and pentafluorophenyl.
The term."arylalkenyl" as used herein refers to an alkenyl radical to which is
appended an aryl group, for example, phenylethenyl and the like.
The term "arylalkoxy" as used herein refers to 8450- wherein R45 is an
arylalkyl group, for example, benzyloxy, and the like.
The term "arylalkoxyalkyl" as used herein refers to a loweralkyl radical to
which is appended an arylalkoxy group, for example, benzyloxymethyl and the
like.
The term "arylalkyl" as used herein refers to an aryl group as previously
defined, appended to a loweralkyl radical, for example, benzyl and the like.
The term "aryloxy" as used herein refers to R46O- wherein R46 is an aryl
group,
for example, phenoxy, and the like.
The term "arylalkylcarbonyloxy" as used herein refers to a R62C(O)O- wherein
R62 is an arylalkyl group.
The term "arylalkylcarbonyloxyalkyl" as used herein refers to a loweralkyl
radical to which is appended an arylalkylcarbonyioxy group.
The term "aryloxyalkyl" refers to an aryloxy group as previously defined
appended to an alkyl radical. Examples of aryloxyalkyl include phenoxymethyl,
2-
phenoxyethyl and the like.
The term "carboxaldehyde" as used herein refers to a formaldehyde radical,
-C(O)H.
The term "carboxamide" as used herein refers to NH2-C(O)-.
The term "carboxy" as used herein refers to a carboxylic acid radical, -
C(O)OH.
The term "carboxyalkenyl" as used herein refers to a carboxy group as
previously defined appended to an alkenyl radical as previously defined.
Examples
of carboxyalkenyl include 2-carboxyethenyl, 3-carboxy-1-propenyl and the like.
The term "carboxyalkoxy" as used herein refers to a carboxy group as
previously defined appended to an alkoxy radical as previously defined.
Examples
of carboxyalkoxy include carboxymethoxy, carboxyethoxy and the like.
The term "cyanoalkoxy" as used herein refers to an alkoxy radical as
previously defined to which is appended a cyano (-CN) group. Examples of
cyanoalkoxy include 3-cyanopropoxy, 4-cyanobutoxy and the like.
The term "cycloalkanoyloxy" as used herein refers to Rso-C(O)-O- wherein Rgo
is a cycloalkyl group.
The term "cycloalkanoyloxyalkyl" as used herein refers to a loweralkyl radical
to which is appended a cycloalkanoyioxy group.
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
17
The term "cycloalkyl" as used herein refers to an aliphatic ring system having
3
to 10 carbon atoms and 1 to 3 rings including, but not limited to,
cyclopropyl,
cyclopentyl, cyclohexyl, norbornyl, adamantyl, and the like. Cycloalkyl groups
carp he
unsubstituted or substituted with one, two or three substituents independently
selected from loweralkyl, haloalkyl, alkoxy, thioalkoxy, amino, alkylamino,
dialkylamino, hydroxy, halo, mercapto, vitro, carboxaldehyde, carboxy,
alkoxycarbonyl and carboxamide.
The term "cycloalkyloxy" herein refers Rg1-O- wherein Rg1 is a cycloalkyl
group. Examples of cycloalkyloxy include cyclohexyloxy and the like.
The term "(cycloalkyl)alkoxy" herein Rg3-R64-O- wherein Rg3 is a cycloalkyl
as defined above and is appended to the parent molecular moiety through an
alkoxy
radical wherein Rg4 is an alkylene group. Examples of (cycloalkyl)alkoxy
include
(cyclopropyl)ethoxy and the like.
The term "cycloalkylalkyl" as used herein refers to a cycloalkyl group
appended to a loweralkyl radical, including but not limited to
cyclohexylmethyl.
The term "dialkylamino" as used herein refers to (R56)(Rs~)N- wherein R56 and
R5~ are independently selected from loweralkyl, for example diethylamino,
methyl
propylamino, and the like.
The term "(dialkylamino)alkyl" as used herein refers to a loweralkyl radical
to
which is appended a dialkylamino group.
The term "(dialkylamino)alkoxy" as used herein refers to an alkoxy radical to
which is appended a dialkylamino group.
The term "dialkylaminocarbonyl" as used herein refers to a dialkylamino
group, as previously defined, appended to the parent molecular moiety through
a
carbonyl (-C(O)-) linkage. Examples of dialkylaminocarbonyl include
dimethylaminocarbonyl, diethylaminocarbonyl and the like.
The term "dialkylaminocarbonylalkenyl" as used herein refers to an alkenyl
radical to which is appended a dialkylaminocarbonyl group.
The term "dialkylaminocarbonylalkyl" as used herein refers to R58-C(O)-R59-
wherein R58 is a dialkylamino group and R59 is an alkylene group.
The term "halo" or "halogen" as used herein refers to I, Br, CI or F.
The term "haloalkenyl" as used herein refers to an alkenyl radical to which is
appended at least one halogen substituent.
The term "haloalkoxy" as used herein refers to an alkoxy radical as defined
above, bearing at least one halogen substituent, for example,
2-fluoroethoxy, 2,2,2-trifluoroethoxy, trifluoromethoxy,
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
18
2,2,3,3,3-pentafluoropropoxy and the like.
The term "haloalkoxyalkyl" as used herein refers to a loweralkyl radical to
which is appended a haloalkoxy group.
The term "haloalkyl" as used herein refers to a lower alkyl radical, as
defined
above, to which is appended at least one halogen substituent, for example,
chloromethyl, fluoroethyl, trifluoromethyl or pentafluoroethyl and the like.
The term "heterocyclic ring" or "heterocyclic" or "heterocycle" as used herein
refers to any 3- or 4-membered ring containing a heteroatom selected from
oxygen,
nitrogen and sulfur; or a 5-, 6- or 7-membered ring containing one, two or
three
nitrogen atoms; one oxygen atom; one sulfur atom; one nitrogen and one sulfur
atom;
one nitrogen and one oxygen atom; two oxygen atoms in non-adjacent positions;
one
oxygen and one sulfur atom in non-adjacent positions; or two sulfur atoms in
non-
adjacent positions. The 5-membered ring has 0-2 double bonds and the 6- and 7-
membered rings have 0-3 double bonds. The nitrogen heteroatoms can be
optionally quaternized. The terms "heterocyclic" or "heterocycle" also
includes
bicyclic groups in which any of the above heterocyclic rings is fused to a
benzene
ring or a cycloalkane ring or another heterocyclic ring (for example, indolyl,
dihydroindolyl, quinolyl, isoquinolyl, tetrahydroquinolyl,
tetrahydroisoquinolyl,
decahydroquinolyl, decahydroisoquinolyl, benzofuryl, dihydrobenzofuryl or
benzothienyl and the like). Heterocyclics include: aziridinyl, azetidinyl,
pyrrolyl,
pyrrolinyl, pyrrolidinyl, pyrazolyl, pyrazolinyl, pyrazolidinyl, imidazolyl,
imidazolinyl,
imidazolidinyl, pyridyl, piperidinyl, homopiperidinyl, pyrazinyl, piperazinyl,
pyrimidinyl, pyridazinyl, oxazolyl, oxazolidinyl, isoxazolyl, isoxazolidinyl,
morpholinyl,
thiomorphofinyl, thiazolyl, thiazolidinyl, isothiazolyl, isothiazolidinyl,
indolyl,
quinolinyl, isoquinolinyl, benzimidazolyl, benzothiazolyl, benzoxazolyl,
oxetanyl,
furyl, tetrahydrofuranyl, thienyl, thiazolidinyl, isothiazolyl, triazolyl,
tetrazolyl,
isoxazolyl, oxadiazolyl, thiadiazolyl, pyrrolyl, pyrimidyl and benzothienyl.
x'
i.
Heterocyclics also include compounds of the formula / ~ where X* is
-CH2- or -O- and Y* is -C(O)- or j-C(R")2-]~ where R" is hydrogen or C1-C4-
alkyl and
v is 1, 2 or 3 such as 1,3-benzodioxolyl, 1,4-benzodioxanyl and the like.
Heterocycfics also include bicyclic rings such as quinuclidinyl and the like.
Heterocyclics can be unsubstituted, monosubstituted, disubstituted, or
trisubstituted with substituents independently selected from hydroxy, halo,
oxo (=O},
alkylimino (R*N= wherein R* is a loweralkyl group}, amino, alkylamino,
dialkylamino,
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
19
alkoxy, alkoxyalkoxy, haloalkyl, cycloalkyl, aryl, phenyl, arylalkyl, -COOH, -
S03H,
alkoxycarbonyl, nitro, cyano and Ioweralkyl. In addition, nitrogen containing
heterocycles can be N-protected.
The term "(heterocyclic)alkoxy" as used herein refers to a heterocyclic group
as defined above appended to an alkoxy radical as defined above. Examples of
(heterocyclic)alkoxy include 4-pyridylmethoxy, 2-pyridyimethoxy and the like.
The term "(heterocyclic)alkyl" as used herein refers to a heterocyclic group
as
defined above appended to a loweralkyl radical as defined above. Examples of
(heterocyclic)alkyl include 2-pyridylmethyl and the like.
The term "heterocyclic(amino)" refers to R77_NH- wherein R77 is an aromatic
heterocyclic group as defined above which is appended to an amino group. The
aromatic heterocycle is substituted with substituents R75 and R76 which are
both
bonded to the atoms of the aromatic heterocycle which are directly adjacent to
the
nitrogen. R75 and R7g are substituents independently selected from hydroxy,
halo,
oxo (=O), alkylimino (R*N= wherein R* is a loweralkyl group), amino,
alkylamino,
dialkylamino, alkoxy, alkoxyalkoxy, haloalkyl, cycloalkyl, aryl, phenyl,
arylalkyl, -
COOH, -S03H, alkoxycarbonyl, nitro, cyano and loweralkyl . The aromatic
heterocycle may also be optionally substituted with a third substituent which
is
selected from the group hydroxy, halo, oxo (=O), alkylimino (R*N= wherein R*
is a
loweralkyl group), amino, alkylamino, dialkylamino, alkoxy, alkoxyalkoxy,
haloalkyl,
cycloalkyl, aryl, phenyl, arylalkyl, -COOH, -S03H, alkoxycarbonyl, vitro,
cyano and
loweraikyl. Examples of heterocyclic(amino) include 2,4-diethylpyridine-3-
amino,
2,4-diethylthiophene-3-amino, 2,4-diethylpyridine-2-amino, and the like.
The term "heterocycliccarbonyloxyalkyl" as used herein refers to
R47-C(O)-O-R4$- wherein R47 is a heterocyciic group and R4$ is an alkylene
group.
The term "hydroxy" as used herein refers to -OH.
The term "hydroxyaikenyl" as used herein refers to an afkenyl radical to which
is appended a hydroxy group.
The term "hydroxyalkoxy" as used herein refers to an alkoxy radical as
previously defined to which is appended a hydroxy (-OH) group. Examples of
hydroxyalkoxy include 3-hydroxypropoxy, 4-hydroxybutoxy and the like.
The term "hydroxyalkyl" as used herein refers to a loweralkyl radical to which
is appended a hydroxy group.
The term "mercapto" as used herein refers to -SH.
The terms "methylenedioxy" and "ethylenedioxy" refer to one or two carbon
chains respectively attached to the parent molecular moiety through two oxygen
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
atoms. In the case of methylenedioxy, a fused 5 membered ring is formed. In
the
case of ethylenedioxy, a fused 6 membered ring is formed. Methylenedioxy
substituted on a phenyl ring results in the formation of a benzodioxolyl
0
radical. . Ethyienedioxy substituted on a phenyl ring results in the
0
5 formation of a benzodioxanyl radical o
The term "substantially pure" as used herein means 90% or more of the
specified compound.
The term "tetrazolyl" as used herein refers to a radical of the formula
H
1~-- N
~,-~.~,N
or a tautomer thereof.
10 The term "tetrazolylalkoxy" as used herein refers to a tetrazolyl radical
as
defined above appended to an alkoxy group as defined above. Examples of
tetrazolylalkoxy include tetrazolylmethoxy, tetrazofylethoxy and the like.
The term "thioalkoxy" as used herein refers to R~eS- wherein R7o is
loweralkyl.
Examples of thioalkoxy include, but are not limited to, methylthio, ethylthio
and the
15 like.
The term "thioalkoxyalkoxy" as used herein refers to R~1 S-R~20- wherein R~~
is loweralkyl as defined above and R72 is alkylene. Representative examples of
thioalkoxyalkoxy groups include CH3SCH20-, CH3CH2SCH20-, t-BuSCH20- and
the like.
20 The term "thioafkoxyalkoxyalkyl" as used herein refers to a
thioalkoxyalkoxy
group appended to an alkyl radical. Representative examples of
thioalkoxyalkoxyalkyl groups include CH3SCH2CHZOCH2CH2-, CH3SCH20CH2-,
and the like.
The term "trans,trans" as used herein refers to the orientation of
substituents
R2 N~ Rs
R'~,.
(Ri and R2) relative to the central substituent R as shown R'
CA 02292604 1999-12-02
WO 98157933 PCT/US98/11821
21
The term "trans,cis"as used herein refers to the orientation of substituents
(R1
R2.... N ~ Rs
R'~~.
and R2) relative to the central substituent R as shown R'
R21~~N ~ Rs
.\.
R. R ~
or . This definition encompasses both the case where R and R2 are
cis and R and R1 are traps and the case where R2 and R are traps and R and R1
are
Cis.
The term "cis,cis" as used herein refers to the orientation of substituents
(R1
and R2) relative to the central substituent R as shown
RZ ~.,,~N ~ Rs
R' R
Representative compounds of the invention include:
trans,trans- 2-(4-Methoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1~-((2,4,8-
trimethyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
traps,traps-2-(3-Fluoro-4-methoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-((2,4,6-
trimethyl}phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
traps,traps-2-(4-Propoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-({2,4,6-
trimethyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
traps,traps-2-(4-Methoxyphenyl)-4-{1,3-benzodioxol-5-yl)-1-((2,6-
diethyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
traps,traps-2-(4-Propoxyphenyl}-4-(1,3-benzodioxol-5-yl)-1-((2,6-
diethyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
traps.traps-2-(3-Fluoro-4-methoxyphenyl}-4-(1,3-benzodioxol-5-yl)-1-((2,6-
diethyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
traps,traps-2-(3-Fluoro-4-ethoxyphenyl}-4-(1,3-benzodioxol-5-yl)-1-((2,6
diethyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
traps,traps-2-(3-Fluoro-4-methoxyphenyi)-4-(7-methoxy-1,3-benzodioxol-5-yl)-1-
((2,6-diethyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
22
traps,traps-2-(3-methoxy-4-propoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-{(2,6-
diethylphenyl)aminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
traps,traps-2-(4-ethoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-((2,6-
diethylphenyl)aminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
traps,traps-2-(4-methoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-((2,6-
dimethylphenyl)aminocarbonylmethyl}-pyrrolidine-3-carboxylic acid,
traps,traps-2-(4-propoxyphenyl)-4-(7-methoxy-i ,3-benzodioxol-5-yl)-1-((2,6-
diethylphenyl)aminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
traps,traps-2-(3-methoxy-4-propoxyphenyl)-4-(7-methoxy-1,3-benzodioxol-5-yl)-1-
((2,6-diethylphenyl)aminocarbonylmethyi)-pyrrolidine-3-carboxylic acid,
traps,traps-2-{4-methoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-((2,6-
dibromo)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
[2R, 3R,4S]2-{4-Methoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-(N-(2,6-
diethyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
traps,traps-2-(4-methoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-{{2,6-
dimethoxy)phenylaminocarbonylmethyl)-pyrroiidine-3-carboxylic acid,
traps,traps-2-(4-methoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-((4-bromo-2,6-
diethyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
traps,traps-2-(4-methoxyphenyl)-4-{1,3-benzodioxol-5-yl)-1-((2-ethyl-6-
methyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
traps,traps-2-{4-methoxyphenyl)-4-(1,3-benzodioxol-5-yl}-1-((2,4,6-
triethyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
j2R,3R,4SJ2-(4-Propoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-(N-(2,6-
diethyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
traps,traps-2-(4-methoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-((2,6-
diisopropyl)phenyiaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
traps, traps-2-(4-methoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-((2,6-diethyl-4-
methyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
j2R,3R,4Sj-2-(4-ethoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-((2,6-
diethyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
traps,traps-2-(4-methoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-((4-carboxy-2,6-
diethyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
traps,traps-2-(4-methoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-((4-vitro-2,6
diethyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
traps,traps-2-(4-methoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-((2-isopropyl-6-
methyl)phenylaminocarbonylmethyl}-pyrrolidine-3-carboxylic acid,
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
23
traps,traps-2-(4-Methoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-(N-(2-ethyl-6-
methoxy)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
traps, traps-2-(4-isopropoxyphenyl)-4-( 1,3-benzodioxo I-5-yl)-1-(2,6-
diethylphenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
traps,traps-2-{2-fluoro-4-propoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-(2,6-
diethylphenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
traps,traps-2-(4-(2-Methoxyethoxy)phenyl)-4-(1,3-benzodioxol-5-yl)-1-(2,6-
diethylphenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
traps,traps-2-(4-(2-Ethoxyethoxy)phenyl)-4-(1,3-benzodioxol-5-yl)-1-(2,6-
diethylphenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
[2R,3R,4S]-2-(4-(2-Methoxyethoxy)phenyl)-4-(1,3-benzodioxol-5-yl)-1-(2, 6-
diethylphenylaminocarbonyimethyl)-pyrrolidine-3-carboxylic acid,
[2R,3R,4S]-2-(4-(2-Ethoxyethoxy)phenyl)-4-(1,3-benzodioxol-5-yl)-1-(2, 6-
diethylphenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
traps,traps-2-{4-(2-isopropoxyethoxy)phenyl)-4-(1,3-benzodioxol-5-yl)-1-(2,6-
diethylphenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
or a pharmaceutically acceptable salt thereof.
Preferred compounds of the invention are selected from the group consisting
of:
traps,traps-2-(4-Methoxyphenyl)-4-(1,3-benzodioxol-5-yl}-1-{{2,6-
diethyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid;
traps,traps-2-(4-Propoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-((2,6-
diethyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid;
traps,traps-2-(3-Fluoro-4-methoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-((2,6-
diethyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid;
traps,traps-2-(3-Fluoro-4-ethoxyphenyl}-4-(1,3-benzodioxol-5-yl)-1-((2,6-
diethyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid;
traps,traps-2-(3-Fluoro-4-methoxyphenyl)-4-(7-methoxy-1,3-benzodioxol-5-yl)-1-
((2,6-diethyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid;
traps,traps-2-(3-methoxy-4-propoxyphenyl}-4-(1,3-benzodioxol-5-yl)-1-((2,6-
diethyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid;
traps,traps-2-(4-ethoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-((2,6-
diethyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid;
traps,traps-2-(4-propoxyphenyl)-4-(7-methoxy-1,3-benzodioxol-5-yl)-1-((2,6-
diethyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
24
traps,traps-2-(3-methoxy-4-propoxyphenyl)-4-(7-methoxy-1,3-benzodioxol-5-yl)-1-
{(2,6-diethyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid;
[2R,3R,4S]2-(4-Methoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-(N-(2,6-
diethyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
[2R,3R,4Sj2-(4-Propoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-(N-(2,6-
diethyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid; and
[2 R,3 R,4S]-2-(4-ethoxyphenyl)-4-{ 1,3-benzodioxol-5-yl)-1-((2,6-
diethyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
traps, traps-2-(4-isopropoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-(2,6-
diethylphenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
traps,traps-2-(2-fluoro-4-propoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-(2,6
diethylphenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
traps,traps-2-(4-(2-Methoxyethoxy)phenyl)-4-(1,3-benzodioxol-5-yl)-1-(2,6-
diethylphenylaminocarbonyimethyl)-pyrrolidine-3-carboxylic acid,
traps,traps-2-(4-(2-Ethoxyethoxy)phenyl)-4-(1,3-benzodioxol-5-yl)-1-(2,6-
diethylphenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
[2R,3R,4S]-2-(4-(2-Methoxyethoxy)phenyl)-4-(1,3-benzodioxol-5-yl)-1-(2, 6-
diethylphenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
[2R,3R,4S]-2-(4-(2-Ethoxyethoxy)phenyl)-4-(1,3-benzodioxol-5-yi)-1-(2, 6-
diethylphenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
traps, traps-2-(4-(2-isopropoxyethoxy)phenyl)-4-(1,3-benzodioxol-5-yl)-i -(2,6-
diethylphenylaminocarbonyimethyl)-pyrrolidine-3-carboxylic acid,
or a pharmaceutically acceptable salt thereof.
Most preferred are the compounds:
traps,traps-2-(4-Propoxyphenyl)-4-{1,3-benzodioxol-5-yl)-1-{(2,6-
diethyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid;
[2R,3R,4S]2-(4-Methoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-(N-(2,6-
diethyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid;
[2R,3R,45']2-(4-Propoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-{N-(2,6-
diethyl)phenylaminocarbonyimethyl)-pyrrolidine-3-carboxylic acid; and
[2 R,3 R, 4S]-2-(4-ethoxyphenyl)-4-( 1,3-benzodioxol-5-yl)-1-{(2,6-
diethyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid;
traps,traps-2-(4-(2-Methoxyethoxy))-4-(1,3-benzodioxol-5-yl)-1-{2,6-
diethylphenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
traps,traps-2-(4-(2-Methoxyethoxy)phenyl)-4-(1,3-benzodioxol-5-yl)-1-(2,6-
diethylphenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
traps, traps-2-(4-(2-Ethoxyethoxy)phenyl)-4-(1,3-benzodioxol-5-yl)-1-(2,6-
diethylphenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
(2R,3R,4S]-2-(4-(2-Methoxyethoxy)phenyl)-4-(1,3-benzodioxol-5-yl)-1-(2, 6-
diethylphenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
5 [2R,3R,4S]-2-{4-(2-Ethoxyethoxy)phenyl)-4-(1,3-benzodioxol-5-yl)-1-(2, 6-
diethylphenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
- traps,traps-2-{4-(2-isopropoxyethoxy)phenyl)-4-(1,3-benzodioxol-5-yl)-1-(2,6-
diethylphenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid,
or a pharmaceutically acceptable salt thereof.
Methods for preparing the compounds of the invention are shown in Schemes
I-VII.
Scheme I illustrates the general procedure for preparing the compounds of the
invention when m is 0 and W is -C02H. A [3-ketoester 1_, where E is loweralkyl
or a
carboxy protecting group, is reacted with a nitro vinyl compound 2_, in the
presence of
a base (for example, 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) or sodium
ethoxide
or sodium hydride and the like) in an inert solvent such as toluene, benzene,
tetrahydrofuran or ethanol and the like. The condensation product ~ is reduced
(for
example, by hydrogenation using a Raney nickel or platinum catalyst). The
resulting
amine cyclizes to give the dihydro pyrrole 4. Reduction of 4 {for example,
sodium
cyanoborohydride or catalytic hydrogenation and the like) in THF solvent or
the like
gives the pyrrolidine compound 5_ as a mixture of cis-cis, trans,trans and
cis,trans
products. Chromatographic separation removes the cis-cis isomer leaving a
mixture
of the trans,trans and cis,trans isomers which is further elaborated. The cis-
cis
isomer can be epimerized (for example, using sodium ethoxide in ethanol or DBU
in
toluene) to give the trans,trans isomer and then carried on as described
below. The
pyrrolidine nitrogen is (1 ) acylated or sulfonylated with R3-X (R3 is R4-C(O)-
or
R6-S(O)2- and X is a leaving group such as a halide (CI is preferred) or X
taken
together with R4-C(O)- or R6-S(O)2- forms an activated ester including esters
or
anhydrides derived from formic acid, acetic acid and the like, aikoxycarbonyl
halides,
N-hydroxysuccinimide, N-hydroxyphthalimide, N-hydroxybenzotriazole, N-hydroxy-
5-
norbornene-2,3-dicarboxamide, 2,4,5-trichlorophenol and the like) or (2)
alkylated
with R3-X where X is a leaving group (for example, X is a halide (for example,
CI, Br
or I) or X is a leaving group such as a sulfonate (for example, mesylate,
tosylate,
triflate and the like)) in the presence of a base such as diisopropyl
ethylamine or
triethylamine and the like to give the N-derivatized pyrrolidine f which is
still a
mixture of trans,trans and cis,trans isomers. Hydrolysis of the ester ~ (for
example,
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
26
using a base such a sodium hydroxide in EtOH/H20) selectively hydrolyzes the
trans,trans ester to give a mixture of 7 and 8_, which are readily separated.
Many of the [3-ketoester starting materials employed in the preparation of the
compounds of the present invention are commercially available. They may also
be
prepared using the methods indicated in Scheme VIII. In the method of Scheme
VIII(a), an aromatic, heteroaromatic, or a- quarternary methyl ketone is
deprotonated
(e.g., with sodium hydride or lithium diisopropylamide) and treated with a
reagent
capable of transferring a carboalkoxy group (e.g., diethyl carbonate, methyl
chioroformate, or di-tert-butyldicarbonate). Alternatively, as shown in Scheme
VIII(b),
a carboxylic acid may be activated (e.g., with carbonyldiimidazole or oxalyl
chloride)
and treated with an acetate equivalent (e.g., ethyl lithioacetate, magnesium
methylmalonate, or Meldrum's acid followed by thermal alcoholysis).
A preferred embodiment is shown in Schemes II and III. A benzoyf acetate
such as 26 or 4-(2-methoxyethoxy)benzoyl acetate is reacted with a vitro vinyl
benzodioxolyl compound 27 using 1,8-diazabicyclo[5.4.0]undec-7-eve (DBU) as
the
base in toluene to give compound 28. Catalytic hydrogenation using Raney
nickel
leads to reduction of the vitro group to an amine and subsequent cyclization
to give
the dihydropyrrole 29. The double bond is reduced with sodium cyanoborohydride
to
give the pyrrolidine compound 30 as a mixture of cis-cis, trans,trans and
cis,trans
2o isomers. Chromatography separates the cis-cis isomer, leaving a mixture of
the
trans,trans and cis,trans isomers (31 ).
Scheme III illustrates the further elaboration of the trans,trans isomer. The
mixture (31) of trans,trans and cis,trans pyrrolidines described in Scheme IV
is
reacted with Br-CH2C(O)NHR4 in acetonitrile in the presence of
ethyldiisopropylamine to give the alkylated pyrrolidine compound 32, still as
a
mixture of trans,trans and cis,trans isomers. Sodium hydroxide in ethanol-
water
hydrolyzes the ethyl ester of the trans,trans compound but leaves the ethyl
ester of
the cis,trans compound untouched, thus allowing separation of the trans,trans
carboxylic acid 33 from the cis,trans ester 34.
Scheme IV illustrates the preparation of compounds where W is other than
carboxylic acid. Compound 55, which can be prepared by the procedures
described
in Scheme I, is converted (for example, using peptide coupling condition, e.g.
N-
methylmorpholine, EDCI and HOBt, in the presence of ammonia or other amide
forming reactions) to give carboxamide 5~. The carboxamide is dehydrated (for
example, using phosphorus oxychloride in pyridine) to give nitrite 57. Nitrite
57
under standard tetrazole-forming conditions (sodium azide and triethylamine
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
27
hydrochloride or trimethylsilylazide and tin oxide) is reacted to give
tetrazole ~8,.
Alternatively nitrite ~7 is reacted with hydroxylamine hydrochloride in the
presence ofi
a base (for example, potassium carbonate, sodium carbonate, sodium hydroxide,
triethylamine, sodium methoxide or NaH) in a solvent such as DMF, DMSO, or
dimethylacetamide to give amidoxime ,~. The amidoxime 5,~ is allowed to react
with
a methyl or ethyl chloroformate in a conventional organic solvent {such as,
chloroform, methylene chloride, dioxane, THF, acetonitrile or pyridine) in the
presence of a base (for example, triethylamine, pyridine, potassium carbonate
and
sodium carbonate) to give an O-acyl compound. Heating of the O-acyl amidoxime
in
an inert solvent (such as benzene, toluene, xylene, dioxane, THF,
dichloroethane, or
chloroform and the like) results in cyclization to compound ~. Alternatively
reacting
the amidoxime ~ with thionyl chloride in an inert solvent (for example,
chloroform,
dichloromethane, dioxane and THF and the like) affords the oxathiadiazole
fuel.
Scheme V illustrates a method for synthesizing pyrrolidines by an azomethine
ylide type [3+2]-cycloaddition to an acrylate. General structures such as
compound
70 are known to add to unsaturated esters such as 71 to provide pyrrolidines
such as
compound 72 (O. Tsuge, S. Kanemasa, K. Matsuda, Chem. Lett. 1131-4 (1983), O.
Tsuge, S. Kanemasa, T. Yamada, K. Matsuda, J. Org. Chem. ~ 2523-30 (1987), and
S. Kanemasa, K. Skamoto, O. Tsuge, Bull. Chem. Soc. Jpn. ,~2 1960-68 (1989)).
Silylimine ~ is reacted with acrylate 74 in the presence of trimethylsilyl
triflate and
tetrabutylammonium fluoride to give the desired pyrrolidine 7~ as a mixture of
isomers. This method can be modified to provide the N-acetamido derivatives
directly by reacting ~ and 74 with the appropriate bromoacetamide (for
example,
dibutyl bromoacetamide) in the presence of tetrabutylammonium iodide and
cesium
fluoride to give compound ~.
Scheme VI illustrates a method for producing an enantiomerically pure
pyrrolidine ~Q, which can be further elaborated on the pyrrolidine nitrogen.
Intermediate racemic pyrrolidine ester 77 (for example, prepared by the
procedure
described in Scheme V) is Boc-nitrogen protected (for example, by treatment
with
Boc20) and then the ester is hydrolyzed (for example, using sodium or lithium
hydroxide in ethanol and water) to give t-butyl carbamoyl pyrrolidine
carboxylic acid
. The carboxylic acid is converted to its (+)-a-methylbenzylamine salt, which
can
be recrystallized (for example from ethyl acetate and hexane or chloroform and
hexane) to afford the diastereomerically pure salt. This diastereomerically
pure salt
can be neutralized (for example, with sodium carbonate or citric acid) to
afford
enantiomerically pure carboxylic acid 7~. The pyrrolidine nitrogen can be
deprotected (for example, using trifluoroacetic acid) and the ester reformed
by the
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
28
use of ethanolic hydrochloric acid to give salt ~0. Alternatively one can use
ethanolic
HCI to cleave the protecting group and form the ester in one step. The
pyrrolidine
nitrogen can be further elaborated (for example, by treatment with the 2,6-
diethylbenzamide of bromoacetic acid in acetonitrile in the presence of
diisopropylethylamine) to give optically active compound 81. The use of (-)-a-
methylbenzylamine will give the opposite enantiomer. Other optically active
amines
may also be employed.
A preferred process is shown in Scheme VII. Nitro vinyl compound (88) is
reacted with ~i-keto ester 89 in the presence of a base such as sodium
ethoxide and
the like or a trialkylamine such as triethylamine or diisopropylethylamine and
the like
or an amidine such as DBU and the like in an inert solvent such as THF,
toluene,
DMF, acetonitrile, ethyl acetate, isopropyl acetate or methylene chloride and
the like
at a temperature of from about 0° C to about 100° C for a period
of time from about 15
minutes to overnight to give compound ~. Reduction of the vitro group followed
by
cyclization was effected for example by catalytic hydrogenation with a
hydrogen
pressure of from about atmospheric pressure to 300 p.s.i. over from about 1
hour to
about 1 day of compound 90 in an inert solvent such as THF, ethyl acetate,
toluene,
ethanol, isopropanol, DMF or acetonitrile and the like, using a hydrogenation
catalyst
such as Raney nickel, palladium on carbon, a platinum catalyst, such as
platinum
oxide, platinum on carbon or platinum on alumina and the like, or a rhodium
catalyst,
such as rhodium on carbon or rhodium on alumina and the like, and the like
affords
intermediate nitrone 91 a or a mixture of nitrone 97 a and imine 91 b. The
reaction
mixture comprising the nitrone or nitrone/imine mixture is treated with an
acid such as
trifluoroacetic acid or acetic acid or sulfuric acid or phosphoric acid or
methanesulfonic acid and the like, and the hydrogenation is continued to give
pyrrolidine compound ~2 as the cis,cis-isomer. Epimerization at C-3 is
effected by
treatment of compound 92 with a base such as sodium ethoxide, potassium t-
butoxide, lithium t-butoxide or potassium t-amyloxide and the like or a
trialkylamine
such as triethyiamine or diisopropylethylamine and the like or an amidine such
as
DBU and the like in an inert solvent such as ethanol, ethyl acetate, isopropyl
acetate,
THF, toluene or DMF and the like at a temperature of from about -20° C
to about 120°
C to give the trans,trans compound ~3. Compound ~ itself can optionally be
resolved into enantiomers prior to reacting with X-R3. The substantially pure
(i.e., at
least 95% of the desired isomer) optically active (+)-isomer of compound 9~ is
obtained by treatment of a mixture of the (+)-isomer and the (-)-isomer of 93
with S-
(+)-mandelic acid, D-tartaric acid or D-dibenzoyl tartaric acid or the like in
a solvent
such as acetonitrile, ethyl acetate, isopropyl acetate, ethanol or isopropanol
and the
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
29
like. The (+)-isomer of 9,,~ selectively crystallizes as the salt, leaving the
(-)-isomer of
9~ in solution. Alternatively, the substantially pure (i.e., at least 95% of
the desired
isomer) optically active (-)-isomer of compound 9~ can be selectively
crystallized by
reaction of a mixture of the (+)-isomer and the (-)-isomer of ~ with L-
tartaric acid, L-
dibenzoyl tartaric acid or L-pyroglutamic acid and the like, leaving the
desired (+)-
isomer of compound ~3 in solution.
Compound ,9~ (racemic or optically active) is reacted with X-R3 (where X is a
leaving group (for example, a halide or a suifonate) and R3 is as previously
defined)
using a base such as diisopropylethylamine, triethylamine, sodium bicarbonate
or
potassium carbonate and the like in an inert solvent such as acetonitrile,
THF,
toluene, DMF or ethanol and the like at a temperature of from about 0°
C to about
100° C to give the intermediate ester ~4. The ester can be isolated or
converted in
situ to the carboxylic acid (95) using hydrolysis conditions such as a base
such as
sodium hydroxide or lithium hydroxide or potassium hydroxide and the like in a
solvent such as ethanol-water or THF-ethanol and the like.
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
Scheme I
O R ~ N02
R~ ~ 2 C02E
C02E + Rt
1 2 R2
N02
$
[H]
H
N~
N
$ R2 Rt R2
Rt
C02E C02E
Mixture of
Cis-Cis
Traps-Traps
Cis-Traps
X R3 ,Rs
R2 Rt 7
,Rs C02H
N
R 2 R t Traps-Traps
S C02E [ H20] +
R3
Mixture of
N~
Traps-Traps R 2 "' R t
Cis-Traps C02E $
Cis-Traps
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
31
Scheme II
0
CH30 O ~ ~ N02 O
/ v DBU \ COOEt
Et02C -----
CH30 ~ \ O
Np2 I
H2
O ~H
O
O N ~ N
OCH3 O ' ._.
OCH3
COOEt
NaCNBH3 COOEt
Mixture of
Cis-Cis
Trans-Trans Chromatographic separation
Cis-Trans
Cis-Cis + Mixture of Trans-Trans and Cis-Trans
CA 02292604 1999-12-02
WO 98/57933 PCT/iJS98/11821
32
Scheme III
O
N,H
O \ / ,..,. \ / pCH3 O
COOEt O ~NHR4
Cis-Trans
BrCH2CONHR4 p N
and ~ \ ~ \ ~ pCHs
~O N H iPr2NEt COOEt
O \ / OCH3 Trans-Trans and Cis-Trans
COOEt
Trans-Trans
NaOH , H20, EtOH
O
O
NHR4
O O ~NHR4
N r N _
O - +
\ ~ OCH3 O \ / ..,. \ / OCH3
COOH COOEt
Trans-Trans Cis-Trans
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
33
Scheme IV
NiR3 ~Ra
N
R2~R R
~
~ 2
-- R1
-r
(CH2)m (CHp)m
C02H CONH2
NiRs N~R3
R2~ R2~
R1 Rt
E
(CH2)m (CH2)m
CN
N
NH
N=N
~Z
~$
NiR3 NiRs NiRs
R2
R1 R2 R R2 R
1 ~ 1
(CH2)m ~ (CHp)m --~ (CH2)m
N
H ~ r H2N NOH w N
O H N, r
O S-O
O
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
34
Scheme V
Rt C02Et
R3~ J I R~
N+ + Rs.
CH2 - R2 N~C02Et
R2
Me3Si~N I \ ~ I ~ \ C02Et
v _OCH3 + O /
74
OCH3
H I RaH t'1~ N C02Et
O
U ~ ~ O
O
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
Scheme v!
OCH3
OC H3
/ \
1. Boc20
C02Et 2. NaOH, EtOH ~cN --C02H
H20
_ /
O
OJ
(~) 11 (t) .Z~
1. (+) a-methylbenzylamine
2. recrystallize from EtOAc/hexane
3. 1 N H3P04
OCH3 OCH3
/ \ / \
HCI ~ HN
--COZEt BocN -..C02H
HCI
/ ~ EtOH /
O
y- O
(+) 8 0
(+) 7~
R4HNC(O)CH2Br
EtN(iPr)p, CH3CN
OCH3
/ \
H
RQ'
O - C02H
-- O
(+) $ ~ pJ
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
36
Scheme VII
R2~ N02
+ EO R~
$$ $$
O
i
N N
R2 1 R~ R2 1 R~ 02N O
C02E C02E R2 R~
C02E
NH NH
R2 R~ --~. R2~R~
C02E C02E
/R3 /R3
N N
R2~~R~ ~ R2 V _Ri
Cv02H - C02E
'~4
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
37
SCHEMEVIIi.
Scheme Vllla.
0 0
CH3 ---~ COOR
Aryl, Heteroaryl, or
a-quarternary
Scheme Vlllb.
O
R-cooH R~cooR
CA 02292604 1999-12-02
WO 98/57933 PCT/US98I11821
38
Compounds which are useful as intermediates for the preparation of
compounds of the invention are:
N
~(CH2)m
R2 I
W
wherei n
misOto6;
W is (a) -C(O)2-G where G is hydrogen or a carboxy protecting group, (b) -
P03H2,
(c) -P(O)(OH)E where E is hydrogen, loweralkyl or arylalkyl,
(d) -CN,
(e) -C(O)NHR1~ where R17 is loweralkyl,
(f) alkyiaminocarbonyl,
(g) dialkylaminocarbonyl,
(h) tetrazolyl,
(i) hydroxy,
(j) alkoxy,
(k) sulfonamido,
(I) -C(O)NHS(O)2R16 where R16 is loweralkyl, haloalkyl, phenyl or
dialkylamino,
(m) -S(O)2NHC(O)Ris,
HO O
NH
(n) O
SS O
(o) Ho 0
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
39
OH
~N
O
(P) ,
0
NH
O
(q) o ,
.~~ Nw
O
N
~~H
(r) O ,
~O
~S= O
N
(S) H >
N~ N
~~ C Fa
N
(t) H , or
' ~ ~ NHS02CF3
(u) ; and
R1 and R2 are independently selected from hydrogen, loweralkyl, alkenyl,
alkynyi,
alkoxyalkyl, alkoxycarbonylalkyl, hydroxyalkyl, haloalkyl, haloalkoxyalkyl,
alkoxyalkoxyalkyl, thioalkoxyalkoxyalkyl, cycloalkyl, cycloalkylalkyl,
aminocarbonylalkyl, alkylaminocarbonylalkyl, dialkylaminocarbonylalkyl,
aminocarbonylalkenyl, alkylaminocarbonylalkenyl, dialkylaminocarbonylalkenyl,
hydroxyafkenyl, aryl, arylalkyl, aryloxyalkyl, arylalkoxyalkyl, heterocyclic,
(heterocyclic)alkyl and
(Raa)(Rbb)N-Rcc wherein Raa is aryl or arylalkyl, Rbb is hydrogen or alkanoyl
and Roc
is alkylene, with the proviso that one or both of R1 and R2 is other than
hydrogen;
or a salt thereof;
and a compound of the formula:
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
H Rt H Fig
N
N
rrrrrr (CH2)m
~uun (CH2)m
R2 W R2
Or W
(IV) (V)
wherein n is 0 or 1;
5 misOto6;
W is {a) -C(O)2-G where G is hydrogen or a carboxy protecting group,
(b) -POgH2,
(c) -P(O){OH)E where E is hydrogen, loweralkyl or arylalkyl,
(d) -CN,
10 (e) -C(O)NHR1~ where R~~ is loweralkyl,
(f} alkylaminocarbonyl,
(g} dialkylaminocarbonyl,
(h) tetrazolyl,
{i) hydroxy,
15 (j) alkoxy,
(k) sulfonamido,
(I) -C(O)NHS(O)2Rig where R16 is loweralkyl, haloalkyl, phenyl or
dialkylamino,
(m) -S(O}2NHC(O)Rls,
HO O
NH
20 (n) o
SS o
(o} Ho 0
OH
~ N
i
(p) O
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
41
0
0
(4) ° ,
.~~ Nw O
N
~~H
(r) O ,
N~ O
~S=O
'' N
(S) H ,
N~ N
~~- CF3
N
(t) ~ H , Or
' ~ ~ NHS02CF3
(u) ; and
R1 and R2 are independently selected from hydrogen, loweralkyl, alkenyl,
alkynyl,
alkoxyalkyl, alkoxycarbonylalkyl, hydroxyalkyl, haloalkyl, haloalkoxyalkyl,
alkoxyalkoxyalkyl, thioalkoxyalkoxyalkyl, cycloalkyl, cycloalkylalkyl,
aminocarbonylalkyl, alkylaminocarbonylalkyl, dialkylaminocarbonylafkyl,
i0 aminocarbonylalkenyl, alkylaminocarbonylalkenyl,
dialkylaminocarbonylalkenyl,
hydroxyalkenyl, aryl, arylalkyl, aryloxyalkyl, arylalkoxyalkyl, heterocyclic,
(heterocyclic)alkyl and
(Raa)(Rbb)N-Rcc' wherein Raa is aryl or arylalkyl, Rbb is hydrogen or alkanoyl
and i~cc
is alkylene, with the proviso that one or both of R~ and RZ is other than
hydrogen;
or a salt thereof.
Preferred intermediates include compounds of formula (III), (IV) and (V)
wherein
miszeroorl;
W is -C02-G wherein G is hydrogen or a carboxy protecting group,
and Ri and R2 are as defined above; or
the substantially pure (+)- or (-)-isomer thereof.
Particularly preferred intermediates are compounds of formula (Ill), (IV) and
(V)
wherein
m is 0;
W is -C02-G wherein G is hydrogen or a carboxy protecting group;
CA 02292604 1999-12-02
WO 98/57933 PCT/US98111821
42
and Ri is (i) alkoxyalkylalkyl, (ii) cycloalkyl, (iii) phenyl, (iv) pyridyl,
(v) furanyl or (vi)
substituted or unsubstituted 4-methoxyphenyl, 4-fluorophenyl, 2-fluorophenyl,
4-
trifluoromethylphenyl, 4-ethoxyphenyl, 3-fluoro-4-methoxyphenyl, 3-fluoro-4-
ethoxyphenyl, 4-propoxyphenyl, 4-isopropoxyphenyl, 2-fluoro-4-ethoxyphenyl, 4-
{2-
methoxyethoxy)phenyl, 4-(2-ethoxyethoxy)phenyl, 4-(2-isopropoxyethoxy)phenyl,
3-
fluoro-4-propoxyphenyl, 3-methoxy-4-propoxyphenyl, 4-pentafluoroethylphenyl, 4-
methoxymethoxyphenyl, 4-hydroxyphenyl, 1,3-benzodioxolyl, 7-methoxy-1,3-
benzodioxolyl, 1,4-benzodioxanyl or dihydrobenzofuranyl wherein the
substituent is
selected from alkoxy, alkoxyalkoxy and carboxyalkoxy and R2 is 1,3-
benzodioxolyl,
1,4-benzodioxanyl, dihydrobenzofuranyl, benzofuranyl, 4-methoxyphenyl,
dimethoxyphenyf, fluorophenyl or difluorophenyl; or
the substantially pure (+)- or (-)-isomer thereof.
The foregoing may be better understood by reference to the following
examples which are provided for illustration and not intended to limit the
scope of the
inventive concept. The following abbreviations are used: Boc for tert-
butyloxycarbonyi, Cbz for benzyloxycarbonyl, DBU for 1,8-
diazabicyclo[5.4.0]undec-
7-ene, EDCI for 1-(3-dimethylaminopropyl-3-ethylcarbodiimide hydrochloride,
EtOAc
for ethyl acetate, EtOH for ethanol, HOBt for 1-hydroxybenzotriazole, Et3N for
triethylamine, TFA for trifluoroacetic acid and THF for tetrahydrofuran.
Example 1
trans.trans- 2-{4-MethoxYphenyl)-~1.3-benzodioxol-5-y~-1-jf2.4.6-
trimethyl)phenylaminocarbonylmet~l)-pvrrolidine-3-carboxylic acid
Example 1 A
Ethvl 2-f4-methoxvbenzovl)-4-nitromethy, I-3-j1.3-benzodioxole-5~r1)butyrate
To ethyl (4-methoxybenzoyl)acetate (23.0 g, 0.104 mol), prepared by the
method of Krapcho et aL, Org. Syn. 47, 20 (1967), and 5-(2-nitrovinyl)-1,3-
benzodioxole (17.0 g, 0.088 mol) dissolved in 180 mL of toluene and heated to
80 °C
was added 1,8-diazabicyclo[5,4,0] undec-7-ene (DBU, 0.65 g) with stirring. The
mixture was heated until all the vitro starting material dissolved. The
solution was
stirred without heating for 30 minutes and then an additional 0.65 g of DBU
was
added. After stirring an additional 45 minutes, thin layer chromatography (5%
ethyl
acetate in methylene chloride) indicated the absence of vitro starting
material.
Toluene (200 mL) was added, and the organic phase was washed with dilute
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
43
hydrochloric acid and NaCI solution. The organic phase was dried over sodium
sulfate and then concentrated under reduced pressure. The residue obtained was
chromatographed on silica gel eluting with 3:1 hexane-ethyl acetate to give
21.22 g
of the desired product as a mixture of isomers and 9.98 g. of recovered ethyl
(4-
methoxybenzoyl)acetate.
Examlhe 1 B
Ethvl 2-l4-methoxvohenvl)-4-(1 3-benzodioxol 5 yl) 4 5 dih~rdro 3H ~yrrole 3
carboxylate
The compound resulting from Example 1 A (21 g} in 500 mL of ethanol was
hydrogenated under 4 atmospheres of hydrogen pressure using a Raney nickel
2800
catalyst (51 g). (The Raney nickel was washed with ethanol three times before
use.)
The catalyst was removed by filtration, and the solution was concentrated
under
reduced pressure. The residue obtained was chromatographed on silica gel
eluting
with 8.5% ethyl acetate in methylene chloride to give 12.34 g of the desired
product.
Exam~he 1 C
Ethvl 2-(4-methoxvohenvl-4-(1 3-benzodioxol-5 yl) ~oyrrolidine 3 carboxvlate)
as a mixture of cis-cis~ traps trans~ and cis traps isomers
The compound resulting from Example 1 B (11.89 g, 0.324 mol) was dissolved
in 27 mL of tetrahydrofuran and 54 mL of ethanol. Sodium cyanoborohydride
(2.35
g, 0.374 mol) and 5 mg bromocresoi green were added. To this blue solution was
added dropwise a solution of 1:2 concentrated HCl in ethanol at such a rate
that the
color was kept at light yellow-green. After the yellow color persisted without
additional HCI, the solution was stirred an additional 20 minutes. The
solution was
concentrated in vacuo and then partitioned between chloroform and an aqueous
potassium bicarbonate solution. The organic phase was separated, dried over
sodium sulfate, and concentrated under reduced pressure. The residue was
chromatographed on silica gel eluting with 85:15 ethyl acetate-hexane to give
5.96 g.
of a mixture of 64% trans,trans-compound and 34% cis,trans-compound. Further
elution with pure ethyl acetate gave 0.505 g of an unknown solid followed by
3.044 g
of pure cis,cis-compound.
Exam I~e 1 D
N-(2.4.6-Trimethyl henyl) ~romoacetamide
To a stirred solution of 2,4,6-trimethylaniline (i g, 7.40 mmol) in methylene
chloride (25 mL) at -50 °C was added successively N,N-
diisopropylethylamine (i.58
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
44
mL, 8.14 mmol, 1.1 eq) and bromoacetyl bromide (0.72 mL, 7.40 mmol, 1 eq) such
that the temperature did not exceed -40 °C. On completion of the
addition, the
cooling bath was removed, and the reaction mixture was allowed to warm to room
temperature. After stirring for a further 30 minutes, the mixture was diluted
with ether
(70 mL) and poured into 1 N sodium bisulfate solution. The phases were
separated,
and the upper layer was washed successively with water and brine. The organic
phase was dried (Na2S04) and the solvent evaporated to half volume, at which
point
the product crystallized. The crystals were removed by vacuum filtration to
afford the
title compound (1.51 g, 80%).
Exami~le 1 E
trans.trans-2-(4-Methoxyl henyl)-X1.3-benzodioxol-5- I~r )-1-((2 4 6
trimethvl)ahenvlaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid
The mixture of 64% trans,trans- and 34% cis,trans-pyrrolidines (the mixture
resulting from Example 1 C) (5.72 g, 15.50 mmol), ethyldiisopropylamine (4.20
g,
32.56 mmol), and the compound resulting from Example 1 D (19.0 mmol) in 30 mL
of
acetonitrile was heated at 50 °C for 1 hour. The solution was
concentrated in vacuo.
The residue was dissolved in toluene, shaken with potassium bicarbonate
solution,
dried over sodium sulfate and concentrated in vacuo to give the product as a
mixture
of trans,trans- and cis,trans- ethyl esters.
This mixture was dissolved in a solution of 50 mL of ethanol and 15 mL of
water containing 5.00 g of sodium hydroxide and stirred for 3 hours at room
temperature. The solution was concentrated in vacuo and 60 mL of water added.
The mixture was extracted with ether to remove the unreacted cis,trans- ethyl
ester.
The aqueous phase was treated with hydrochloric acid until slightly cloudy. It
was
then further neutralized with acetic acid to give the crude acid product. The
crude
product was filtered and purified by dissolving it in tetrahydrofuran, drying
over
sodium sulfate, concentrating in vacuo, and crystallizing from ether to give
the title
compound. ~H NMR (300MHz, CDC13) 8 8.22 (1 H, bs), 7.78 (2H, d, J=8Hz), 6.95
(5H,
m), 6.82 (1 H, bd, J=8Hz}, 6.77 (1 H, d, J=8Hz}, 5.96 (2H, s), 3.97 (1 H, bd,
J=lOHz),
3.81 (3H, s), 3.70 (1 H, ddd, 6, 5&3Hz), 3.57 (bdd, 10&3Hz), 3.45 (1 H, d,
J=16Hz),
3.13 (2H, m}, 2.24 (3H, s}, 2.06 (6H, s). MS (DCI, NH3} m/e 517 (M+H+). Anal.
Calc
for C3oH32N2O6 ~ 0.5H20: C, 68.56, H, 6.33, N 5.33. Found: C, 68.84, H, 6.20,
N,
5.31
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
Example 2
r ns raps-2-l3-FI pro-4-methoxyphenvll 4 l~ ~ hpnzodioxol 5 yrl~ 1 (j2 4 6
rim h I h n I min r n Im I - rroli in - rbox li i
The title compound was prepared by the procedures described in Example 1.
5 ~ H NMR (300MHz, CDC13) b 8.22 (1 H, bs), 7.21 (1 H, dd, J=12&2Hz), 7.12 (1
H, bd,
J=8Hz), 6.95 (1 H, t, 8Hz), 6.90 (2H, bs), 6.84 (1 H, d, J=2Hz), 6.80 (1 H,
dd, J=8&3Hz),
6.76 (1 H, d, J=8Hz), 5.93 (2H, s), 3.96 (1 H, d, J=lOHz), 3.89 (3H, s), 3.70
{1 H, ddd, 6,
5&3Hz), 3.56 (1 H, dd, 11 &5Hz), 3.45 (1 H, d, J=l6Hz), 3.10 (1 H, t, J=1
OHz), 3.07 (1 H,
dd, 8&6Hz), 3.02 {1 H, d, J=16Hz), 2.17 (3H, s), 2.07 (6H, s). MS (DCI, NH3)
m/e 535
10 (M+H+). Anal. Calc for C3pH3~ FN206 ~ 0.75H20 : C, 65.74, H, 5.98, N 5.11.
Found:
C, 65.96, H, 5.88, N, 5.16
Examlhe 3
traps.traps-2-(4-Propoxvohenyl)-4-all 3-benzodioxol 5 yl) 1 ( 2 4 6
15 trimethvl)ohenvlaminocarbonylmethyl) pyrrolidine 3 carboxylic acid
The title compound was prepared by the procedures described in Example 1.
1H NMR (300MHz, CDC13) 8 8.21 (1 H, bs), 7.38 (2H, d, J=8Hz), 6.90 (2H, d,
J=8Hz),
6.89 (2H, d, 3Hz), 7.83 (1 H, dd, J=8&2Hz), 6.75 (1 H, d, J=8Hz), 5.94 (1 H,
d, J=3Hz),
5.93 (1 H, d, J=3Hz), 3.96 {1 H, d, J=1 OHz), 3.85 (2H, q, J=7Hz), 3.70 (1 H,
ddd, 6,
20 5&3Hz), 3.58 (1 H, dd, 11 &5Hz), 3.48 (1 H, d, J=16Hz), 3.15 (1 H, dd,
8&6Hz), 3.13
(1 H, t, J=1 OHz), 2.99 (1 H, d, J=16Hz), 2.25 (3H, s), 2.05 (6H, s), 1.81
(2H, sext,
J=7Hz), 1.04 {3H, t, J=7Hz). MS (DCI, NH3) m/e 545 (M+H+). Anal. Calc for
C32H3sN2~s ~ 0.33H20: C, 69.79, H, 6.71, N 5.09, Found: C, 69.78, H, 6.73, N,
4.81
25 Exam lip a 4
~rans.traps-2-(4-Methoxvoheny~)-~1 3-benzodioxol 5 yl) 1 (~(2 6
diethvllohenvlaminocarbon !1r methylLpyrrolidine 3 ~arboxvlic acid
The title compound was prepared by the procedures described in Example 1.
1 H NMR (300MHz, CDCi3) 8 8.24 (1 H, bs), 7.39 (2H, d, J=8Hz), 7.21 (1 H, dd,
8&6Hz),
30 7.11 (2H, d, J=8Hz), 6.92 (2H, d, 8Hz), 7.89 (1 H, d, J=3Hz), 7.82 (1 H,
dd, J=8&2Hz),
6.75 (1 H, d, J=8Hz), 5.94 (1 H, d, J=3Hz), 5.93 (1 H, d, J=3Hz), 3.96 (1 H,
d, J=lOHz),
3.82 (3H, s), 3.70 (1 H, ddd, 6, 5&3Hz), 3.56 (1 H, dd, 11 &5Hz), 3.45 (1 H,
d, J=16Hz),
3.15 (1 H, dd, 8&6Hz), 3.13 {1 H, t, J=1 OHz), 3.01 (1 H, d, J=16Hz), 2.42
(4H, q,
J=7Hz), 1.08 (6H, t, J=7Hz). MS (DCI, NH3) m/e 559 {M+H4+), 531 (M+H+). Anal.
~35 Calc for C3~ H34N2O6: C, 70.17, H, 6.46, N 5.28. Found: C, 69.88, H, 6.42,
N, 5.09.
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
46
Example 5
trans.trans-2-(4-Propoxy henyl}-4-(1 3-benzodioxol-5-y~~-1-(f2 6-
diethvll henylaminocarbon I~yrly-~yrrolidine-3-carboxylic acid
The title compound was prepared by the procedures described in Example 1.
~ H NMR (300MHz, CDC13) S 8.27 {1 H, bs), 7.37 (2H, d, J=8Hz), 7.21 (1 H, dd,
8&6Hz),
7.11 (2H, d, J=8Hz), 6.90 (2H, d, 8Hz), 7.86 (1 H, d, J=3Hz), 7.83 (1 H, dd,
J=8&2Hz),
6.75 (1 H, d, J=8Hz), 5.93 (1 H, d, J=3Hz), 5.92 (1 H, d, J=3Hz), 3.96 (1 H,
d, J=l OHz),
3.85 (2H, q, J=7Hz), 3.70 (1 H, ddd, 6, 5&3Hz), 3.55 (1 H, dd, 11 &5Hz), 3.48
(1 H, d,
J=16Hz), 3.15 (1 H, dd, 8&6Hz), 3.13 (1 H, t, J=lOHz), 3.01 (1 H, d, J=l6Hz),
2.43 (4H,
q, J=7Hz), 1.82 (2H, sext, J=7Hz), 1.08 (6H, t, J=7Hz) 1.04 (3H, t, J=7Hz). MS
(DCI,
NH3) m/e 559 (M+H+). Anal. Calc for C33H3aN2~6 ~ 0.25H20: C, 70.38, H, 6.89, N
4.97. Found: C, 70.49, H, 6.85, N, 4.68.
Example 6
traps.traps-2-(3-Fluoro-4-methoxyphenyl}-~1 3-benzodioxol-5-yl~~(2 6
diethyl)ohenvlaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid
The title compound was prepared by the procedures described in Example 1.
~ H NMR (300MHz, CDC13) 8 8.21 (1 H, bs), 7.22 (1 H, dt, J=8&2Hz), 7.20 (1 H,
d,
J=8Hz), 7.17 (1 H, dt, J=8&2Hz), 7.10 (2H, d, J=8Hz), 6.96 (1 H, t, J=8Hz),
6.83 (1 H,
dd, J=8&2Hz), 6.80 (1 H, d, J=3Hz), 6.76 (1 H, d, J=8Hz), 5.94 (1 H, d,
J=3Hz), 5.93
(1 H, d, J=3Hz), 3.97 (1 H, d, J=lOHz), 3.90 (3H, s), 3.72 (1 H, ddd, 6,
5&3Hz), 3.58 (1 H,
dd, 11 &5Hz), 3.46 (1 H, d, J=l6Hz), 3.14 (1 H, t, J=lOHz), 3.12 (1 H, dd,
8&6Hz), 3.05
{1 H, d, J=l6Hz), 2.45 (4H, q, J=7Hz), 1.09 (6H, t, J=7Hz). MS (DCI, NH3) m/e
549
(M+H+). Anal. Calc for C31 H33FN20g ~ 0.5H20: C, 66.78, H, 6.15, N 5.02.
Found:
C, 66.81, H, 5.89, N, 4.87.
Example 7
traps.traps-2-(3-Fluoro-4-ethoxyphenyl)-4-j1 3-benzodioxol-5-~l-1-((2 6
diethvllohenvlaminocarbonylmethyl}-pyrrolidine-3-carbo~rlic acid
The title compound was prepared by the procedures described in Example 1.
~H NMR (300MHz, CDCIg) b 8.23 (1 H, bs), 7.23 (1 H, d, J=2Hz), 7.20 (1 H, dd,
J=8&3Hz), 7.11 (3H, m), 6.96 (1 H, t, J=8Hz), 6.83 (1 H, dd, J=8&2Hz), 6.80 {1
H, d,
J=3Hz), 6.76 (1 H, d, J=8Hz), 5.93 (1 H, d, J=3Hz), 5.92 (1 H, d, J=3Hz), 4.11
(2H, t.
J=7Hz), 3.97 (1 H, d, J=lOHz), 3.72 (1 H, ddd, 6, 5&3Hz}, 3.55 (1 H, dd, 11
&SHz), 3.47
(1 H, d, J=l6Hz), 3.14 (1 H, t, J=lOHz), 3.12 (1 H, dd, 8&6Hz), 3.04 (1 H, d,
J=16Hz),
2.45 (4H, q, J=7Hz), 1.47 (3H, t, J=7Hz), 1.09 (6H, t, J=7Hz). MS (DCI, NH3)
m/e 563
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
47
(M+H+). Anal. Calc for C32H35FN20g ~ 0.15TFA: C, 66.92, H, 6.11, N 4.83.
Found:
C, 67.19, H, 5.75, N, 4.69.
E~,-I
ran r n - -4- I -4- -1 t -1-
h r li ' r i
The title compound was prepared by the procedures described in Example 1.
~ H NMR (300MHz, CDC13) 8 8.24 (1 H, s), 7.25 (1 H, t, J=3Hz), 7.21 (1 H, bd),
7.14 (1 H,
m), 7.08 (2H, d, J=8Hz), 6.96 (1 H, t, J=8Hz), 6.56 (1 H, d, J=3Hz), 6.50 (1
H, d, J=3Hz),
5.93 (1 H, d, J=2Hz), 5.91 (1 H, d, J=2Hz), 3.97 (1 H, d, J=1 OHz), 3.90 (3H,
s), 3.72 (1 H,
ddd, 6, 5&3Hz), 3.58 (1 H, dd, 11 &5Hz), 3.46 (1 H, d, J=16Hz), 3.14 (1 H, t,
J=1 OHz),
3.12 (1 H, dd, 8&6Hz), 3.05 (1 H, d, J=l6Hz), 2.45 (4H, q, J=7Hz), 1.09 (6H,
t, J=7Hz).
MS (DCI, NH3) m/e 579 (M+H+). Anal. calcd for C32H35FN207 ~ 1.5H20: C, 63.65,
H, 6.31, N 4.64. Found: C, 64.00, H, 6.29, N, 4.26.
trans.trans-2-(3-methoxv-4-uro o~xypheny~-~,j1 3-ben~nr~~nYm 5 ylL((~
diethvllohenvlaminocarbonylmet y1),-pyrrolidine-3-carboxy i acid
The title compound was prepared by the procedures described in Example 1.
~ H NMR (300MHz, CDC13) b 8.22 (1 H, s), 7.21 (1 H, m), 7.12 (2H, d, J=lOHz),
7.02
(1 H, dd, J=9&3Hz), 6.93 (1 H, d, J=2Hz), 6.88 (1 H, d, J=2Hz), 6.85 (1 H, m),
6.82 (1 H,
d, J=2Hz), 6.75 (1 H, d, J=9Hz), 5.95 (1 H, d, J=2Hz), 5.93 (1 H, d, J=2Hz),
3.97 (2H, q,
J=9Hz), 3.84 (3H, s), 3.72 (2H, m), 3.60-3.45 (2H, m), 3.15 (2H, m), 3.03 (1
H, d
J=l8Hz), 2.43 (4H, q, J=9Hz), 1.87 (2H, m), 1.08 (6H, t, J=9Hz), 1.04 (3H, t,
J=9Hz).
MS (DCI, NH3) m/e 589 (MH+). Anal.calcd. for C34H40N2~7' 0.45 H20: C, 68.43,
H,
6.91, N, 4.69. Found: C, 68.45, H, 6.91, N, 4.62.
Example 10
~rans.trans-2-f4-ethoxypyl~,(1 3-ben~nr~~nYr,i-5-yp~(2 6
diethvlloheny La_minncarbony the -a~y~lidine-~-carbox4y ' acid
The title compound was prepared by the procedures described in Example 1.
1 H NMR (300MHz, CDC13) 8 8.26 (1 H, bs), 7.36 (2H, d, J=9Hz), 7.21 (1 H, m),
7.11
(2H, d, J=lOHz), 6.90 (2H, d, J=9Hz), 6.86 (1 H, d, J=2Hz), 6.83 (1 H, dd,
J=8&2Hz),
6.73 (1 H, d, J=9Hz), 5.94 (1 H, d, J=2Hz), 5.92 (1 H, d, J=2Hz), 4.10-3.90
(3H, m), 3.71
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
48
(1 H, m), 3.60-3.40 (2H, m), 3.15 (2H, m), 3.02 (1 H, d J=18Hz), 2.43 (4H, q,
J=9Hz),
1.42 (3H, t, J=9Hz), 1.08 (6H, t, J=9Hz). MS (DCI, NH3) m/e 545 (MH+). Anal.
calcd.
for C32H3sN2~s~ 0.5 H20: C, 69.42, H, 6.74, N, 5.06. Found: C, 69.52, H, 6.52,
N,
4.89.
Exam,~le 11
trans.trans-2-(4-methoxy lohenyl)~-4-,(1.3-benzodioxol-5yl)-~(2 6-
~limethyl)phenvlaminocarbonylmeth~rlLpyrrolidine-3-carboxylic acid
The title compound was prepared by the procedures described in Example 1.
~ H NMR (300MHz, CDC13) 8 8.32 (1 H, bs), 7.37 (2H, d, J=9Hz), 7.08 (3H, m),
6.91
(2H, d, J=9Hz), 6.88 (1 H, d, J=2Hz), 6.82 (1 H, dd, J=8&2Hz), 6.75 (1 H, d,
J=9Hz),
5.95 (1 H, d, J=2Hz), 5.93 (1 H, d, J=2Hz), 3.95 (1 H, d, J=1 OHz), 3.81 (3H,
s), 3.72 (1 H,
m), 3.55 (1 H, dd, J=10&5Hz), 3.46 (1 H, d J=l8Hz), 3.13 (2H, m), 3.00 (1 H,
d,
J=l8Hz), 2.10 (6H, s). MS (DCI, NH3) m/e 502 (MH+). Anal. calcd. for
C29H3oN2~s~
0.5 H20: C ,68.09, H, 6.11, N, 5.48. Found: C, 67.98, H, 6.02, N, 5.33.
Example 12
traps.traps-2-!4-prol~oxylnhenyl -~4-(7-methoxy-1.3-benzodioxol-5-yl)-1-((2 6-
diethyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid
The title compound was prepared by the procedures described in Example 1.
~ H NMR (300MHz, CDC13) 8 8.30 (1 H, bs), 7.36 (2H, d, J=9Hz), 7.21 (1 H, m),
7.09
(2H, d, J=lOHz), 6.91 (2H, d, J=9Hz), 6.59 (1 H, d, J=2Hz), 6.51 (1 H, d,
J=2Hz), 5.93
(1 H, d, J=2Hz), 5.91 (1 H, d, J=2Hz), 3.93 (3H, m), 3.80 (3H, s), 3.72 (1 H,
m), 3.60-
3.50 (2H, m), 3.15 (2H, m), 3.02 (1 H, d J=18Hz), 2.43 (4H, q, J=9Hz), 1.82
(2H, m),
1.08 (6H, t, J=9Hz), 1.05 (3H, t, J=9Hz). MS (DCI, NH3) m/e 589 (MH+). Anal.
calcd.
for C34H40N2~7' 0.25 H20: C, 68.84, H, 6.88, N, 4.72. Found: C, 68.80, H,
6.59, N,
4.52.
Examl ip a 13
traps. traps-2-!3-methoxv-4-aronoxyphenyl)-4-(7-methoxy-1 3-benzodioxol-5-yl)-
1
l2.6-diethyl)phenylaminocarbonyfmethyl) pvrrolidine-3-carbo~(ic acid
The title compound was prepared by the procedures described in Example v .
1 H NMR (300MHz, CDCIg) b 8.22 (1 H, s), 7.21 (1 H, m), 7.09 (2H, d, J=lOHz),
7.02
(1 H, dd, J=9&3Hz), 6.93 (1 H, d, J=2Hz), 6.87 (1 H, d, J=9Hz), 6.61 (1 H, d,
J=2Hz),
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
49
6.53 (1 H, d, J=2Hz), 5.93 (1 H, d, J=2Hz), 5.91 (1 H, d, J=2Hz), 3.97 (3H, q,
J=9Hz),
3.84 (3H, s), 3.82 (3H, s), 3.70 (1 H, m), 3.60-3.45 (2H, m), 3.'15 (2H, m),
3.02 (1 H, d
J=l8Hz), 2.42 (4H, q, J=9Hz), 1.85 (2H, m), 1.08 (6H, t, J=9Hz), 1.05 (3H, t,
J=9Hz).
MS (DCI, NH3) m/e 619 (MH+). Anal. calcd. for C3~H42N2Og ~ C, 67.94, H, 6.84,
N,
4.53. Found: C, 67.65, H, 6.98, N, 4.44.
- Exam I_p a 14
traps.traps-2-l4-methoxypgnyl)-4-~1 3 benzodioxol 5 I) 1
?6
i r m n I min r n I h I - rr li in - rb x lic i
The title compound was prepared by the procedures described in Example 1.
~H NMR (300MHz, CDC13) b 8.58 (1 H, bs), 7.58 (2H, d, J=9Hz), 7.40 (2H, bd,
J=lOHz), 7.02 (1 H, t, J=9Hz), 6.91 (2H, d, J=9Hz), 6.86 (1 H, m), 6.76 (1 H,
d, J=9Hz),
5.93 (2H, s), 3.98 (1 H, bd, J=1 OHz), 3.81 (3H, s), 3.73 (2H, m), 3.55 (1 H,
bd, J=15Hz),
3.13 (2H, m), 3.01 (1 H, bd, J=l8Hz). MS {DCI, NH3) m/e 633 (MH+). Anai.
calcd. for
C27H24BrzN2O6~ 0.3 H20: C, 50.85, H, 3.89, N, 4.39. Found: C, 50.45, H, 3.48,
N,
4.22.
Examlhe 1515
f2R.38.45'12-l4-Methoxvohenvll-4-(1 3-b nzodioxol 5 yl~(N ~(2 6
ie h I h n I min c r on Im th I - rr li ine-3- rb x li ci h drochl rid I
Examcle 15A-E
AI ernative r r i n f h i r n - r n - - 4-m h x hen I -4- 1 en dioxol-
5-ylLpyrrolidine-3-carboxyl~,~g
Examl I~ a 15A
~(2-Nitrovinyl)-1 3-benzodioxole
To piperonal (15.55 kg, 103.5 mol) under mechanical stirring and under
nitrogen was added ammonium acetate (13.4 kg, 173.8 mol), acetic acid (45.2
kg),
and nitromethane (18.4 kg, 301.4 mol) sequentially. The mixture was warmed to
70
°C. After about 30 minutes, the yellow product began to crystallize.
The reaction
temperature was raised to '80 °C and stirred for about 10 hours until
minimal
piperonal remains. The somewhat thick reaction mixture was cooled to 10
°C and
filtered. The precipitate was washed with acetic acid (2 x 8 kg) and then
water (2.x 90
kg). The product was dried under a nitrogen purge and then in a vacuum oven at
50
°C for 2 days to afford 15.94 kg (80%) of the title compound as a
bright yellow solid.
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
Example 15B
Ethyl (4-methoxybenzoyl)acetate
To potassium t-amylate (25 wt %, 50.8 kg, 99.26 mol) in toluene (15.2 kg)
5 cooled to 5 °C under mechanical stirring and under nitrogen was added
a mixture of
4-methoxyacetophenone (6.755 kg, 44.98 mol) and diethyl carbonate (6.40 kg,
54.18
mol) in toluene over 1 hour maintaining the temperature below 10 °C.
The reaction
mixture was heated to 60 °C for 8 hours until no 4-methoxyacetophenone
was
detected by HPLC. The mixture was cooled to 20 °C and quenched by
adding to a
10 mixture of acetic acid (8 kg} and water (90 kg) over 30 minutes while
maintaining the
temperature at <20 °C. The layers were separated, and the organic layer
was
washed with 5% sodium bicarbonate solution (41 kg) and concentrated to 14.65
kg.
The temperature is maintained below 50 °C during the distillation. The
yellow
product concentrate was assayed by HPLC against an external standard and the
15 yield was found to be 9.40 kg (94%).
Example 15C
Ethvl 2-(4-methoxybenzoyl}-4-nitromethyl-3-l1 3-benzodioxole-5-yl)butyrate
To the compound resulting from Example 15A (7.5 kg, 37.9 mol) suspended in
20 THF (56 kg) with mechanical stirring under nitrogen was added the compound
resulting from Example 15B (8.4 kg, 37.9 mol). The mixture was cooled to 17
°C,
sodium ethoxide (6.4 g, 0.095 mol) was added, and the reaction was stirred for
30
minutes. After about 15 minutes, the nitrostyrene was completely dissolved.
Sodium
ethoxide (6.4 g, 0.095 mol) was added, and the mixture was stirred at 25
°C until
25 HPLC shows less than 1 area % ketoester remaining. The reaction was
concentrated to 32.2 kg which was determined by HPLC assay to be 14.9 kg
(95%).
Example 15D
Ethvl cis.cis-2-(4-methoxyphenyl)-4-(1 3-benzodioxol-5-yl)-l~yrrolidine-3-
carboxvlate
30 Raney nickel (20.0 g), from which the water had been decanted, was charged
to a stirred hydrogenator equipped with a thermocouple. THF (20 mL), the crude
compound resulting from Example 15C (40.82 g, 0.0482 mol}, and acetic acid
(2.75
mL, 0.0482 mol) were added sequentially. The mixture was put under a hvdroaen
atmosphere at 60 psi until the hydrogen uptake slowed dramatically. TFA was
35 added, and the mixture was hydrogenated at 200 psi until HPLC shows no
residual
imine and <2 area % nitrone. The catalyst was filtered away and washed with
100
mL of methanol. The filtrate was assayed by HPLC and found to contain 13.3 g
(75%
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
51
yield) of the cis, cis-pyrrolidine compound. The filtrate was concentrated and
chased
with additional THF {200 mL) to give a final volume of 100 mL. The mixture was
neutralized with 2 N NaOH solution (50 mL), diluted with water (200 mL), and
extracted with ethyl acetate (2 x 100 mL). The combined nearly colorless ethyl
acetate layers were assayed against an external standard by HPLC to be13.0 g
(73%) of the title compound.
Exam Ip a 15E
E~hvl traps traps-2-(4-methoxyahenvl)-4 (1 3 benzodioxol 5 yl) pyrrolidine 3
carboxylate
The solution of the compound resulting from Example 15D (38.1 g, 0.103 mol)
was chased with ethanol (200 mL) to a final volume of 100 mL and sodium
ethoxide
(3.40 g, 0.050 mol) was added. The mixture was heated to 75 °C. When
HPLC
shows <3% of the cis,cis isomer remaining, the mixture was cooled to room
temperature. The product was assayed by HPLC against an external standard and
found to contain 34.4 g (90% yield) of the title compound. The crude compound
solution was concentrated and the residue taken up in isopropyl acetate (400
mL).
The organic layer was washed with water (2 x 150 mL) and then extracted with
0.25
M phosphoric acid solution (2 x 400 mL). The combined phosphate layers were
stirred with ethyl acetate (200 mL) and neutralized to pH 7 with solid sodium
bicarbonate (21 g). The organic layer was separated and found to contain 32.9
g
(87%) of the title compound.
Exam I~p a 15F
Ethv! ~2R 3R 4512-(4-Methoxy~henyl)-~1 3-benzodioxol 5 ylLp~rrrolidine 3
carboxylate
The racemic amino ester from Example 1 (32.9 g) was dissolved in 50 mL of
acetonitrile. (S)-(+)-Mande(ic acid (2.06 g, 0.0136 mmol) was added and
allowed to
dissolve. The mixture was seeded with the product and allowed to stir at room
temperature for 16 hours. The reaction mixture was cooled to 0 °C and
stirred for 5
hours. The product was filtered and dried in a vacuum oven with a nitrogen
purge for
1 day at 50 °C. The resultant salt (20.0 g, 0.0383 moi) was suspended
in ethyl
acetate (150 mL) and 5% sodium bicarbonate solution {150 mL). The mixture was
stirred at room temperature until the salt dissolved and carbon dioxide
evolution had
ceased. The organic layer was separated and concentrated.
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
52
Example i 5G
~2R.3R.45'j~4-Methoxyphenyl)-4 j1.3-benzodioxol-5-y~-1 ~~2 6-
dieth~rl)lohenylaminocarbonylmeth~rl) ~yrrolidine-3-carboxylic acid
The title compound was prepared from the compound of Example 15F
according to the procedures of Example 1 E.
Example 15H
f2R.3R.4512-(4-Methoxvnhenvl)-4-(1.3-benzodioxol-5-yl)-~N-(2 6
diethvllQhenylaminocarbonylmethvl)-pyrrolidine-3-carboxylic acid hydrochloride
salt
The compound of Example 15G {450 mg) was dissolved in 10 mL of
isopropanol. A slight excess of saturated HCI in ethanol was added, and the
resultant solution was stirred for 10 min. The solvents were removed in vacuo,
and
the excess HCI was chased with isopropanol. The residue was taken up in ether
and
filtered, leaving 448 mg of the title compound as a white solid. MS {DCI/NH3)
m/e
531 (M+H)+. Anal calcd for C3i H35N20gCl : C, 65.66 H, 6.22; N, 4.94. Found:
C,
65.72; H, 6.39; N, 4.85.
Exam~he 16
gaps.traps-2-{4-methoxyphenyl)-4-(1.3-benzodioxol-5-vl)-1-~(,{2 6
dimethoxy);~ahenylaminocarbonylmetf~yl)-ovrrolidine-3-carboxlrlic acid
Exam !lo a 16A
2.6-Dimethoxxaniline
To a stirred solution of 2,6-dimethoxybenzoic acid {2.00 g, 11.0 mmol) in 1,2-
dichloroethane (45 mL) at ambient temperature was successively added N-
methylmorpholine (1.45 mL, 13.2 mmol) and diphenylphosphoryl azide (2.60 mL,
12.1 mmol). After heating the mixture for 2 hours at 75 °C, cuprous
iodide (150 mg)
and benzyl alcohol (2.27 mL, 22.0 mmol) were added and heating was continued
overnight. Solvents were removed in vacuo and the residue was chromatographed
on silica gel, eluting with 4:1 hexane-ethyl acetate to give the intermediate
carbamate
(1.50 g, 48 % yield) as a white, crystalline solid. The solid was dissolved in
methanol
(15 mL) and added to a flask purged with nitrogen containing 10% palladium-on-
charcoal {500 mg). The mixture was placed under a balloon of hydrogen and
stirred
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
53
4 hours at ambient temperature. The mixture was filtered through a pad of
Celite and
solvents were removed in vacuo to give the title compound (800 mg, 48% yield}.
Exam Ip a 16B
trans.traps-2-l4-methoxvohenyl)-4-(1 3-benzodioxol-5 vll 1 ((2 6
dimethoxvlohenvlaminocarbonylmethyl)-pyrrolidine 3 carboxylic acid
The title compound was prepared by the procedures described in Example 1,
substituting the compound of Example 16A for 2,4,6-trimethylaniline in Example
1 D.
~ H NMR (300MHz, CDC13) 8 8.18 {1 H, bs), 7.39 (2H, bd, J=9Hz), 7.17 (1 H, t,
J=9Hz),
6.99 (1 H, d, J=2Hz), 6.90 (2H, d, J=9Hz), 6.86 (1 H, d, J=2Hz), 6.75 {1 H, d,
J=9Hz),
6.56 (2H, d, J=9Hz), 5.93 (2H, s), 3.88 (1 H, bd, J=1 OHz), 3.81 (3H, s), 3.71
(6H, s),
3.70 (2H, m), 3.49 (1 H, bd, J=lSHz), 3.03 (2H, m), 2.85 (1 H, bd, J=l8Hz).
NMR (DCI,
NH3) mie 535 (MH+). Anai. calcd. for C29H3oN20s- 0.75 AcOH: C, 63.20, H, 5.74,
N,
4.83. Found: C, 63.18, H, 5.34, N, 4.79.
Exam Ip a 17
traps.traps-2-(4-methoxvc~henylL,(1 3-benzodioxol-5-yl)-1 ~({4 bromo 2 6
diethyl)ohenvlaminocarbonylmethYl)-I~yrrolidine 3 carboxylic acid
Exam~he 17A
4-Bromo-2.6-diethyianiline
To a stirred solution of 2,6-diethylaniline (10.0 g, 67.0 mmol) in acetic acid
(50
mL) at ambient temperature was added bromine (10.4 mL, 201 mmol). The reaction
was stirred overnight at ambient temperature. The reaction mixture was diluted
with
diethyl ether (200 mL) and washed with 5% sodium bisulfite (4 x 50 mL) and
brine.
The organic phase was dried with sodium sulfate and the solvents were removed
in
vacuo. The residue was chromatographed on silica gel, eluting with 9:1 hexane-
ethyl acetate to give the title compound (3.28 g, 21 % yield).
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
54
Examl I~ a 17B
trans.trans-2-(4-methoxyphenyl -X1.3-benzodioxol-5 ~)-1-~~4-bromo-2.6
diethyl)phen~aminocarbonylmethyl)-~oyrrolidine-3-carboxylic acid
The title compound was prepared by the procedures described in Example 1,
substituting the compound of Example 17A for 2,4,6-trimethylaniline in Example
1 D.
1 H NMR (300MHz, CDC13) 8 8.21 (1 H, bs), 7.38 (2H, d, J=9Hz), 7.23 (2H, s),
6.92
(2H, d, J=9Hz), 6.88 (1 H, d, J=2Hz), 6.82 (1 H, dd, J=8&2Hz), 6.75 (1 H, d,
J=9Hz},
5.93 (i H, d, J=2Hz), 5.91 (i H, d, J=2Hz), 3.95 (1 H, d, J=9Hz), 3.82 (3H,
s), 3.72 (1 H,
m), 3.52 (1 H, m), 3.45 (1 H, d J=18Hz), 3.14 (2H, m), 3.00 (1 H, d, J=18Hz),
2.39 (4H,
q, J=9Hz), 1.07 (6H, t, J=9Hz). MS (DCI, NH3) m/e 609 (MH+). Anal. calcd. for
Cg1 H33BrN20g : C, 61.09, H, 5.46, N, 4.60. Found: C, 60.80, H, 5.35, N, 4.54.
Example 18
traps.traps-2-(4-methoxyphenyl)-4-(1.3-benzodioxol-5-yl)-1-,~2-ethyl-6-
methyl)phenylaminocarbonylmethyl)-pyrrolidine-3-carbolic acid
The title compound was prepared by the procedures described in Example 1.
~ H NMR (300MHz, CDC13) b 8.32 {1 H, bs), 7.38 (2H, d, J=9Hz), 7.20-7.10 (3H,
m),
6.92 (2H, d, J=9Hz), 6.87 (1 H, d, J=2Hz), 6.82 (1 H, dd, J=8&2Hz), 6.76 (1 H,
d,
J=9Hz), 5.94 (1 H, d, J=2Hz), 5.92 (1 H, d, J=2Hz), 3.95 (1 H, d, J=9Hz), 3.82
(3H, s),
3.73 {1 H, m), 3.55 (1 H, dd, J=12&6), 3.47 (1 H, d J=18Hz), 3.14 (2H, m),
3.02 (1 H, d,
J=l8Hz), 2.44 (2H, q, J=9Hz), 2.10 (3H, s), 1.10 (3H, t, J=9Hz). MS (DCI, NHg)
m/e
517 (MH+). Anal. calcd. for C3pH32N2~s~ 0.5 H20: C, 68.56, H, 6.33, N, 5.33.
Found:
C, 68.58, H, 6.29, N, 5.13.
Example 19
traps.traps-2-{4-methoxyphenyl)-4-(1.3-benzodioxol-5-yl)-1- (2.4.6-
trieth~)~phenylaminocarbonyimethyl)r-pyrrolidine-3-carboxylic acid
Exam Ip a 19A
Ethyl traps.traps-2-(4-methoxyphenyl)-X1.3-benzodioxol-5-yl)~-~,~2 4 6
triethvl)ohenylaminocarbonylmethyrlLpyrrolidine-3-carbo~late
To a mixture (purged with nitrogen) of [1,1'-bis(diphenylphosphino)ferrocene]
dichloropalladium (II) (1:1 complex with dichloromethane} (13 mg) and cesium
carbonate (307 mg, 0.942 mmol) in anhydrous N,N-dimethylformamide (2 mL) at
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
ambient temperature was added ethyl traps,traps-2-(4-methoxyphenyl)-4-(1,3-
benzodioxol-5-yl)-1-(4-bromo-2,6-diethylphenyl)aminocarbonylmethyl)-
pyrrolidine-3-
carboxylate {200 mg, 0.314 mmol, prepared in Example 17) in anhydrous
tetrahydrofuran (8 mL). After stirring the mixture for 10 minutes at ambient
5 temperature, 1.0 M triethylborane (0.471 mL, 0.471 mmol) in tetrahydrofuran
was
added. The reaction was stirred overnight at 65 °C under nitrogen. The
reaction
mixture was diluted with ethyl acetate (100 mL) and washed with water {2 x 30
mL)
and brine. The organic phase dried with sodium sulfate, and the solvents were
removed in vacuo . The residue was chromatographed on silica gel eluting with
3:1
10 hexane-ethyl acetate to give the title compound (110 mg, 60 % yield).
Examlhe 19B
traps.traps-2-{4-methoxyphe_nyl)-4-(1.3-benzodioxol-5-yl)-1-{j2.4.6-
triethvl)ahenyrlaminocarbon~lmethyl~,pvrrolidine-3-carboxylic acid
The title compound was prepared by the procedures described in Example 1 E.
~H NMR (300MHz, CDC13) 8 8.22 (1 H, bs), 7.38 {2H, d, J=9Hz), 6.95 (2H, s),
6.91
(2H, d, J=9Hz), 6.84 (1 H, d, J=2Hz), 6.82 (1 H, dd, J=8&2Hz), 6.75 (1 H, d,
J=9Hz),
5.93 (1 H, d, J=2Hz), 5.91 (1 H, d, J=2Hz), 3.95 (1 H, d, J=1 OHz), 3.82 (3H,
s), 3.71 (1 H,
m), 3.52 (1 H, dd, J=9&2Hz), 3.46 (1 H, d J=l8Hz), 3.13 (2H, m), 3.00 (1 H, d,
J=l8Hz), 2.60 (2H, q, J=9Hz), 2.40 (4H, q, J=9Hz), 1.22 (3H, t, J=9Hz), 1.08
(6H, t,
J=9Hz). MS (DCI, NH3) m/e 559 (MH+). Anal. calcd. for C33H3gN2Og ~ 0.25 H20:
C,
70.38, H, 6.89, N, 4.97. Found: C, 70.18, H, 7.14, N, 4.63.
Exam Ie~20.
~2R 3R 4SI2-(4-Propoxy,phenyl~-4-(1 3-benzodioxol-5-vl)-1-(N-~~(2 6-
diethyl)hhenvlaminocarbonylmethyl)-avrrolidine-3-carboxylic acid
Example 20A
t2R 3R 4~2-~4-Pro~o_xviahenyl,)-4-{1 3-benzodioxol-5-girl)-1-tert-
butoxycarbonvl-
pyrrolidine-3-carboxylic acid
The racemic amino ester from Example 3 (8.00 g) was combined with 4.45 g of
di-tert-butyldicarbonate in 100 mL of THF; 10 mL of triethylamine was added,
and the
resultant solution was stirred at ambient temperature for 3 hrs. The solvents
were
removed in vacuo; the residue was taken up in EtOAc and washed sequentially
with
aqueous 1 N H3P04, bicarb, and brine. The crude product was dissolved in 30 mL
of
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
56
ethanol; 12 mL of 2.5 N NaOH solution was added, the mixture was stirred
overnight
at ambient temperature, then warmed to 50 °C for 2 hrs. The solvents
were removed
in vacuo; the residue was partitioned between water and ether. The aqueous
extract
was acidified with aqueous 1 N H3P04 and extracted twice with EtOAc. The
organic
extracts were washed with brine and dried over Na2S04 to give 9.2 g of
trans,trans-
2-(4-propoxyphenyl)-4-(1,3-benzodioxol-5-yl)-1-tert-butoxycarbonyl-pyrrolidine-
3-
carboxylic acid. This material was dissolved in 30 mL of EtOAc, and 1.3 mL of
(R)-
(+)-a-methylbenzylamine was added. The solution was stirred for 10 min; the
solvents were removed in vacuo, 50 mL of ether were added, and the resultant
solution was seeded. After standing overnight, the solvents were removed in
vacuo;
the residue was taken up in 70 mL of ether and filtered. The solid product was
recrystallized from EtOAc / ether. The crystalline material was stirred
vigorously in a
two-phase mixture of 1 N H3P04 and EtOAc; the organic layer was decanted, then
washed with brine and dried over Na2S04.
Example 20B
Ethvl f2R.3R.4Sj2-(4-Propoxyl~henyl)-4-(1 3-benzodioxol-5-yl)-pyrrolidine-3-
carboxylate
The compound of Example 20A was dissolved in ethanol and cooled in an ice
bath. Gaseous HCI was bubbled through the solution until saturated; the
resultant
solution was warmed to ambient temperature and allowed to stir overnight under
a
blanket of nitrogen. The solvents were removed in vacuo; the residue was taken
up
in bicarb and extracted with EtOAc. The organic layer was decanted, then
washed
with brine and dried over Na2S04.
Exam I~e 20C
f2R.38.45"12-(4-Proooxyphenyl)-4-(1 3-benzodioxol-5-girl)-1-(N-(2 6-
diethvfphenvllaminocarbon~rimethyl)-pvrrolidine-3-carbox~rlic acid
The title compound was prepared from the compound of Example 20B
according to the procedures of Example 1 E. MS (DCI/NH3) m/e 559 (M+H)+. Anal
calcd for C33H38N206~ 0.2 H20: C, 70.49; H, 6.88; N, 4.98. Found: C, 70.52; H,
6.78; N, 4.85.
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
57
Examlhe 21
trans.trans-2-(4-methoxy henyl~-4-(1 3-benzodioxol-5-yl~((2 6
diisooro~yl)phenylaminocarbonylmethyl)-pyrrolidine-3-carbox~rlic acid
The title compound was prepared by the procedures described in Example 1.
~ H NMR (300MHz, CDC13, 8) 8.29 (1 H, bs), 7.39 (2H, d, J=9Hz), 7.29 (1 H, m),
7.15
(2H, d, J=9Hz), 6.93 (2H, d, J=9Hz), 6.85 (1 H, d, J=2Hz), 6.83 (1 H, dd,
J~&2Hz),
6.74 (1 H, d, J=9Hz), 5.93 (1 H, d, J=2Hz), 5.91 (1 H, d, J=2Hz), 3.96 (1 H,
d, J=lOHz),
3.83 (3H, s), 3.73 (1 H, m), 3.55 (1 H, dd, J=12&6), 3.50 (1 H, d J=l8Hz),
3.14 (2H, m),
3.01 (1 H, d, J=l8Hz), 2.84 (2H, m), 1.16 (6H, d, J=8Hz), 1.05 (6H, d J=8Hz).
MS
(DCI, NH3) m/e 559 (MH+). Anal. calcd. for C33H3gN20g~ 0.5 H20: C; 69.82, H,
6.92,
N, 4.93. Found: C, 69.69, H, 6.63, N, 4.89.
Example 22
trans.traps-2-l4-methoxylph2nyl)-4-(1 3-benzodioxol-5-yl)-1;x(2 6-diethyl-4-
methvllahenvlaminocarbonylmethyl)-p~rrrolidine-3-carbo~rlic acid
The title compound was prepared by the procedures described in Example 19.
iH NMR (300MHz, CDC13) 8 8.20 (1 H, bs), 7.38 (2H, d, J=9Hz), 6.92 {4H, m),
6.86
(1 H, d, J=2Hz), 6.82 (1 H, dd, J=8&2Hz), 6.75 (1 H, d, J=9Hz), 5.93 (1 H, d,
J=2Hz),
5.91 (1 H, d, J=2Hz), 3.95 (1 H, d, J=1 OHz), 3.81 (3H, s), 3.72 (1 H, m),
3.55 (1 H, dd,
J=9&2Hz), 3.45 (1 H, d J=l8Hz), 3.13 (2H, m), 3.00 (1 H, d, J=l8Hz), 2.39 (4H,
q,
J=9Hz), 2.28 (3H, s), 1.07 (6H, t, J=9Hz). NMR (DCI, NH3) 545 m/e (MH+). Anal.
calcd. for C3zH3sNz~s ~ 0.5 H20: C, 69.42, H, 6.74, N, 5.06. Found: C, 69.43,
H, 6.57,
N, 4.94.
Exam f~,e 23
(2R.3R.4S1-2-(4-ethoxy henyl)-4-(1 3-benzodioxol-5-yl,)-1-~(2 6-
diethvllahenvlaminocarbonylmethyly-l~yrrolidine-3-carboxylic acid
The title compound was prepared from the racemic amino ester of Example 10
according to the procedures of Example 20. 1 H NMR (300MHz, CDC13) 8 8.32 (1
H,
bs), 7.38 (2H, d, J=9Hz), 7.21 (1 H, m), 7.12 (2H, d, J=1 OHz), 6.90 (3H, m),
6.83 (1 H,
dd, J=8&2Hz), 6.74 (i H, d, J=9Hz), 5.94 (1 H, d, J=2Hz), 5.92 (1 H, d,
J=2Hz), 4.05
~35 (2H, m), 3.96 (1 H, d, J=lOHz), 3.72 (1 H, m), 3.53 (1 H, dd, J=10&3Hz),
3.47 (1 H, d,
J=l8Hz), 3.13 (2H, m), 3.02 (1 H, d J=lBHz), 2.44 (4H, q, J=9Hz), 1.42 (3H, t,
J=9Hz),
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
58
1.08 (6H, t, J=9Hz). MS (DCI, NH3) m/e 545 (MH+). Anal. caicd. for C32H3sN2~s~
0
H20: C, 69.42, H, 6.74, N, 5.06. Found: C, 69.67, H, 6.73, N, 4.98.
Example 24
tr n r ns- - 4-m x n I - - 1 i I- I _ 4-
i h I h I min n Im I - rr li in - r i
The title compound was prepared by the procedures described in Example 19.
1 H NMR (300MHz, DMSO) 8 7.68 (2H, bs), 7.54 (2H, d, J=9Hz), 7.27 (2H, m),
6.93
(2H, d, J=9Hz), 6.83 (2H, m}, 5.98 (2H, s), 3.92 {1 H, d, J=9Hz), 3.76 (3H,
s), 3.62 (1 H,
m), 3.45-3.00 (2H, m), 3.00-2.80 (3H, m), 2.44 (4H, q, J=9Hz), 1.04 (6H, t,
J=9Hz).
NMR (DCI, NH3) m/e 575 (MH+). Anal. calcd. for C32H34N2~8' 0.5 H20: C, 65.85,
H,
6.04, N, 4.80. Found: C, 66.03, H, 5.84, N, 4.67.
Exarr~nle 25
~rans.traps-2-(4-methoxychenvl)-4-(1 bPn~nr~~r,Yol 5 yl~(.(-4 vitro
i h I h n I min r Im h rr li in - r x li i
~xam~ .25A
2.6-Diet yl-4-nitroaniline
To a stirred solution of 2,6-diethylaniline (5.0 g, 34 mmol) in concentrated
sulfuric acid (30 mL) at 0 °C was added dropwise concentrated nitric
acid (15.9 M,
2.10 mL, 34 mmo!). The cooling bath was removed, and the reaction was stirred
for 3
hours at ambient temperature. After pouring the reaction mixture into ice, the
solution
was neutralized using 4 N sodium hydroxide and extracted with methylene
chloride
(3 x 50 mL). The extracts were dried with sodium sulfate, and solvents were
removed
in vacuo to give the title compound.
3o Example ~~B
traps traps-2-(4-m-vohenvl)-4-(1 3 benzodioxnl ~ ~.n ~ ~~4 vitro
h I r n r 'n -
The title compound was prepared by the procedures described in Example 1,
substituting the compound of Example 25A for 2,4,6-trimethylaniline in Example
1 D.
~ H NMR (300MHz, CDCIg) 8 8.38 (1 H, bs), 7.77 (1 H, d, J=9Hz), 7.38 (2H, d,
J=9Hz),
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
59
7.24 (1 H, d, J=9Hz), 6.92 (2H, d, J=9Hz), 6.88 (1 H, d, J=2Hz), 6.82 (1 H,
dd,
J-8&2Hz), 6.75 (1 H, d, J=9Hz), 5.93 (1 H, d, J=2Hz), 5.91 {1 H, d, J=2Hz),
3.97 (1 H, d,
J=9Hz), 3.83 (3H, s), 3.74 (1 H, m), 3.48 (2H, m), 3.18 (2H, m), 3.04 (1 H, d,
J=l8Hz),
2.63 (2H, m), 2.44 (2H, q, J=9Hz), 1.10 (3H, t, J=9Hz), 1.08 (3H, t, J=9Hz).
MS (DCI,
~5 NH3) m/e 576 (MH+). Anal. calcd. for C31 H33N308~ 0.75 H20: C, 63.20, H,
5.90, N,
7.13. Found: C, 63.30, H, 5.81, N, 7.14.
Example 26
traps.traps-2-(4-methoxvohenvll-4-(1 3-benzodioxol-5-yl -L1,-_(!2 isol r~olQyl
n
methvllahenvlaminocarbonylmethylL~yrrolidine-3-carboxylic acid
The title compound was prepared by the procedures described in Example 1.
1H NMR (300MHz, CDC13) 8 8.35 (1 H, bs), 7.39 (2H, d, J=9Hz), 7.18 (2H, m),
7.07
(1 H, dd, J=9&2Hz), 6.92 (2H, d, J=9Hz), 6.86 (1 H, d, J=2Hz), 6.82 (1 H, dd,
J=8&2Hz),
. 6.75 (1 H, d, J=9Hz), 5.94 (1 H, d, J=2Hz), 5.92 (1 H, d, J=2Hz), 3.96 (1 H,
d, J=l OHz),
3.83 (3H, s), 3.72 (1 H, m), 3.50 (2H, m), 3.15 (2H, m), 3.02 (1 H, d,
J=l8Hz), 2.86
(1 H, m}, 2.09 {3H, s), 1.16 (3H, d, J=8Hz), 1.07 (3H, d J=8Hz). MS (DCI, NH3)
m/e
531 (MH+). Anal. calcd. for C31H3aN206~ 0.5 H20: C, 69.00, H, 6.54, N, 5.19.
Found:
C, 69.27, H, 6.67, N, 5.21.
Example 27
traps.traps-2-f4-Methoxvohenyl)-4-(1 3-benzodioxol-5-arll-1-{N (2 ethyl 6
methoxvlc~henvlaminocarbonyrl~n~thyl)-pyrrolidine-3-carboxylic acid
Example 27A
~thvl 3-oxo-4-f3-methoxy-2-vitro henkl~rg~ i nat_e
Potassium ethyimalonate (3.68 g) was combined with 2.29 g of magnesium
chloride in 12 mL of DMF; the reaction mixture was heated at 60 °C for
4 hrs. The
resultant mixture was cooled to ambient temperature. Simultaneously, 3-methoxy-
2-
nitrobenzoic acid (3.4 g) was dissolved in 12 mL of DMF; 3.06 g of 1,1-
carbonyldiimidazole was added (gas evolves), and the resultant solution (after
stirring at ambient temperature for 4 hrs) was added to the malonate mixture.
The
resultant slurry was stirred at ambient temperature for 14 hrs. Solvents were
removed in vacuo; the residue was taken up in EtOAc and washed sequentially
with
1 N H3P04, bicarb, and brine, and was concentrated in vacuo..
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
Example 27B
2-Nitro-3-(1-hydroxvethyl)anisole
The compound of Example 27A (3.2 g) was dissolved in 50 mL of
5 concentrated sulfuric acid and stirred at ambient temperature for 48 hrs.
The reaction
mixture was poured onto 300 mL of ice and extracted twice with EtOAc. The
organic
extracts were washed sequentially with water, bicarb, and brine, and were
concentrated in vacuo. The crude product was heated neat at 160 °C for
3 hrs. The
resultant dark brown residue was extracted with EtOAc. The organic extracts
were
10 concentrated. The crude product was dissolved in 15 mL of ethanol; sodium
borohydride (450 mg) was added, and the resultant solution was stirred at
ambient
temperature for for 2 hrs. The solvents were removed in vacuo; the residue was
taken up in 10% aqueous HCI and stirred for 15 min. The mixture was extracted
with
EtOAc; the organic extracts were washed sequentially with bicarb and brine,
and
15 were concentrated in vacuo. The crude product was purified by flash
chromatography on silica gel, eluting with 1:1 EtOAc/hexanes, to give 1.08 g
(32%
overall) of the title compound as a colorless oil.
Example 27C
20 2-Ethyl-6-methoxvani line
The compound of Example 27B (310 mg) was dissolved in 10 mL of THF; 1.5
mL of H3P04 was added, followed by 50 mg of 10% palladium-on-charcoal. The
resultant mixture was purged with nitrogen, then placed under a balloon of
hydrogen,
25 and stirred overnight. Bicarb was added carefully, and the mixture was
filtered
through a pad of Celite. The filtrate was extracted with EtOAc; the organic
extracts
were washed with bicarb and brine, and were concentrated in vacuo. The crude
product was purified by flash chromatography on silica gel, eluting with 1:1
ether/hexanes, to give 102 mg (43% yield) of the title compound as a colorless
oil.
Example 27D
traps.traps-2-(4-Methoxvohenvl)-4-{1 3-benzodioxol 5 yl) 1 (N (2 ethyrl 6
methoxv)phenvlaminocarbonylmethyl) pyrroli ine 3 carboxylic acid
The title compound was prepared according to the procedures of Example ,
substituting the compound of Example 27C for 2,4,6-trimethylaniline. j H NMR
(300
MHz, CD30D) 8 1.10 (t, J=8 Hz, 3H), 2.48 (d, J=8 Hz, 2H), 3.4-3.9 {m, 7H),
3.73 (s,
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
61
3H), 3.84 (s, 3H), 5.93 (s, 2H), 6.80 (d, J=8 Hz, 1 H), 6.86 (d, J=8 Hz, 2H),
6.93 (dd,
J=2,8 Hz, 1 H), 7.03 (bd d, J=9 Hz, 2H), 7.07 (d, J=2 Hz, 1 H), 7.23 (t, J=8
Hz, 1 H), 7.54
(bd d, J=9 Hz, 2H). MS (APCI) m/e 533 (M+H)+. Anal calcd for C30H32N2p7 . 0.7
TFA: C, 61.59 H, 5.38; N, 4.57. Found: C, 61.27; H, 5.44; N, 4.61.
ExamQle 28
traps.traps-2-l4-iso-Proooxypyj)-4-(1 3-be~~~dioxol-5-vI,L1 (2 6
diethvlohenvlaminocarbr,~,Qylmethy[)-~yL~idine-3-carboxy ' ar~r~
The title compound was prepared by the procedures described in Example 1.
1H NMR (300MHz, CDC13) 8 7.51 (2H, bd d, J--9Hz), 7.22 (1 H, dd, J--8,9Hz),
7.13
(1 H, s), 7.11 (1 H, dd, J--1,BHz), 7.05 (1 H, d, J--2Hz), 6.99 (2H, bd d, J--
9Hz), 6.91
(1 H, dd, J--2,8Hz), 6.78 (1 H, d, J=8Hz), 5.93 (1 H, d, J=3Hz), 5.92 (1 H, d,
J=3Hz),
4.64 (1 H, septet, J--7Hz), 3.80 (3H, m), 3.55 (2H, m), 2.47 (4H, q, J=7Hz),
1.33 (6H,
dd, J--2,7Hz), 1.09 (6H, t, J=7Hz). MS (ESI+) m/e 559 (M+H+). Anai. Calc for
C33H3gN20g ~ 0.7 TFA: C, 64.71, H, 6.11, N 4.39. Found: C, 64.54, H, 5.78, N,
4.21.
Exam~~~
traps.traps-~(2-Fluoro-4-Prooo~yl~,~r,yIL4-~1 3-benzodioxol y~)~,(2 6
diethvlohenylaminocarbonvlmethYJ,)-~yrrolidine 3 carboxyr ' arid
The title compound was prepared by the procedures described in Example 1.
1 H NMR (300MHz, CDC13) 8 1.06 (t, 3H, J=7Hz), 1.09 (t, 6H, J=7Hz), 1.83 (m,
2H),
2.44 (q, 4H, J=7Hz), 3.4-3.9 (m, 5H), 3.99 (t, 2H, J=6Hz}, 5.95 (dd, 2H,
J=1,2Hz), 6.8-
6.9 (m, 4H), 7.03 (d, 1 H, J=2Hz), 7.11 {d, 1 H, J=8Hz), 7.13 {s, 1 H), 7.22
(dd, 1 H,
J=7,9Hz), 7.63 (t, 1 H, J=9Hz). MS (ESI+) m/e 577 (M+H+). Anal. Calc for
C33H37N20gF~ 1.OTFA: C, 60.87, H, 5.55, N 4.06. Found: C, 60.74, H, 5.61, N,
3.97.
Examlhe 3030
traps.traps-2-(4-(2-Methoxvethoxy,)~~ I Pnyl}-~1 3-benzodiox9l 5 yl~,1 (2 6
diethvlohenvlaminocarbon,~rfmethyl)-~y r i ine ~ ~art,r,xylic acid
The title compound was prepared by the procedures described in Example 1.
1 H NMR (300MHz, CDC13) 8 1.08 (t, J=7Hz, 6H), 2.43 (q, J=7Hz, 4H), 3.00 (d,
J=11 Hz, 1 H), 3.05-3.15 (m, 2H), 3.44 (s, 3H), 3.46 (d, J=11 Hz, 1 H), 3.45-
3.55 (m,
1 H), 3.65-3.75 (m, 1 H), 3.75-3.80 (m, 2H), 3.93 (d, J=7Hz, 1 H), 4.12-4.17
(m, 2H),
. 5.94 (dd, J=2Hz, 4Hz, 2H), 6.75 (d, J=8Hz, 1 H), 6.82 (dd, J=2Hz, 9Hz, 1 H),
6.87 {d,
J=2Hz, 1 H), 6.95 (d, J=BHz, 1 H), 7.10 (d, J=6Hz, 2H), 7.19-7.24 (m, 1 H),
7.37 (d,
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
62
d=8Hz, 2H), 8.29 (s, 1 H). MS (APCI+) m/e 575 (M+H+). Anal. Calc for
C33H3sN2O7:
C, 68.97 H, 6.67, N 4.87. Found: C, 68.92, H, 6.83, N, 4.77.
Examl le OA
Ethvl f4-(2-methoxyethoxy benzoy~-acetate
Methyl 4-hydroxybenzoate was reacted with 1-bromo-2-methoxyethane, and
potassium carbonate in dimethylformamide. The resultant ester was hydrolyzed
to
the acid with NaOH in alcohol. This acid was reacted with carbonyl diimidazole
in
THF; the resulting imidazolide was reacted with the potassium salt of the mono
ethyl
ester of malonic acid, and magnesium chloride, to give the title compound as a
colorless oil.
1 ~ Examl Ip a 30B
traps.traps-2-(4-(2-Methoxvethoxy~,~ heny! -L4-(1 3 be~zodioxol 5 yIL,1 "~2 6
diethvlohenvlaminocarbonylmeth~)-IQyrrrolidine '~ ~arboxviic acid
The title compound was prepared by the procedures described in Example 1,
employing the b-ketoester described above as starting material. 1 H NMR
(300MHz,
CDC13) d 1.08 (t, J=7Hz, 6H), 2.43 (q, J=7Hz, 4H), 3.00 (d, J=11 Hz, 1 H),
3.05-3.15
(m, 2H), 3.44 (s, 3H), 3.46 (d, J=11 Hz, 1 H), 3.45-3.55 (m, 1 H), 3.65-3.75
(m, i H),
3.75-3.80 (m, 2H), 3.93 (d, J=7Hz, 1 H), 4.12-4.17 (m, 2H}, 5.94 (dd, J=2Hz,
4Hz, 2H},
6.75 (d, J=8Hz, 1 H), 6.82 (dd, J=2Hz, 9Hz, 1 H), 6.87 (d, J=2Hz, 1 H), 6.95
(d, J=8Hz,
1 H), 7.10 (d, J=6Hz, 2H), 7.19-7.24 (m, 1 H), 7.37 (d, d=8Hz, 2H), 8.29 (s, 1
H). MS
(APCI+) m/e 575 (M+H+). Anal. Calc for C33H38N2O7: C, 68.97 H, 6.67, N 4.87.
Found: C, 68.92, H, 6.83, N, 4.77.
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
63
The following compounds of Examples 31- 857 can be prepared using the
methods described in the above examples.
31 32 33
H
N
I / H
34 35 36
37 38 39
CH3
H
N~
H I / IOI N ..uCOOH
F "1
' ' O
J
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/I 1821
64
40 41 42
CH3
H
N~N
w~~COOH
I/ O
F / \
O
- of
43 44 45
CH3. CH3
H H
I I \ " N .,nCOOH I / O
" N wnCOOH
i O
CI
F / \ O / \
J
46 47 48
3
\ \
I \
F i CI I , H I , OH
0
J
49 so 51
CH3
H
\ \ N
I , ~N w~~COOH
"1
/\
0
- of
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
52 53 54
CH3 CH3
~ l
H H
~,nCOOH
\ N " N I \ N If N .,nCOOH
I / O , O
O r ~ O
F OJ O
55 56 57
OCH3 O~
H H
N
I / jO( N .mCOOH I ~ N~N
.,nCOOH
/ O
O
_ OJ
58 59 60
U'
H \ l H
N~
N .,aCOOH I % IOI N .,nCOOH I
CI / O
rvJ rvJ
10 61 62 g3
I~ I~ I~
F / C / /
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
66
64 65 66
o-~
~ /
I ' v i' N ~~nCOOH H ~H
i O
O
I
J
67 68 69
o-~
a~ H
!I N ~ N~N
I / ° .,nCOOH 'i I .,nCOOH
F ~ °
O F
of J
70 71 72
b
~H I ~ -~ I w
v i
73 74 75
o-~ °~ o"
~ / ~ I ~
H a
N N
..nCOOH I ~ ~N ~ N
wnCOOH ~~uCOOH
O ~ o I
cc
° / \ o / ~ o
J of of
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
67
76 77 78
~ i H w
F C~ ~ i
79 80 81
H
N
~~nCOOH
I~
/\
0
F- J
82 83 84
o--~ _
H ~ / ~ /
~ b~
N I1 N ..nCOOH ~ \ " N ~~»COOH
C ~ O / O
O
85 gg 87
o-~
H
\ N~ N ~~nCOOH
O
/ \
O
J
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
68
88 89 90
o-~
H ~I ~I
( / ~N "nCOOH ( ~ ~~N
.~~iCOOH
O
/ \ O / \ O
CH3 of - of
91 92 93
°'~-
H
N
j( .mCOOH
( / O
\
O
- of
94 95 96
o-~ o-~
~ I ~ I
I " N ~ \ ~~N .~uCOOH
/ O .mCOOH / O
F
F / \ F
_ O O
of J
97 98 99
o'L o-L
v I v I
H H
( , N OII .,nCOOH ( \ N II N (
.~~iCOOH
/ O F
F / \ O / \
O
_ of _ of
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
69
100 101 102
°''~..
H a
.,~~cooH
I~
_ _ ~ / \
0
- of
103 104 105
°'
F I / I / O .,nCOOH
/ \
O
- of
106 107 10g
109 110 111
o-~
~ /
.,nCOOH H I
F
O
O
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
112 113 114
OCH3
\ /
H F
OH I , 'O' N ~~~~COOH
O / ' O
J cH3o _ of
5 115 116 117
I~ a I
I ~ ~H
F C~ i i
118 119 120
CH3 CH3
\ / \ /
~~~~COOH
H F " N ~~nCOOH
F )H
I/ o
FI/ o
/
10 ~ J i
121 122 123
CH3 3
\ /
H ~F
y N~
I ~ IOI N w~iCOOH CH
O O
J J
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
71
124 125 126
CH3
H ~F
wnCOOH
O
\ O
J
127 128 129
CH3
H~ 'F
N II N H
wnCOOH
/ O
F
O >
J
130 131 i32
CH3
\ N ~ ~ N " N .,nCOOH
F / C / O
/_\ O
F
133 134 135
OCH3
N~ wF
/ H ~ \ '1 N .,nCOOH
i O
F
O
i
1
O
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
72
136 137 138
r
H \ I
N F
~N .,~~COOH
O
- of
139 140 141
r
\ I \
H ~F H F
N~ N
" N .,uCOOH ~ \ ~N .,nCOOH
F ~ O C / O I i
O / ~ O
of - of
142 143 144
°'\
H
~F
y N
I , H I , ~N ~~nCOOH
F "1
0
cH3 of
145 146 147
o-~\
\ /
w ~ F
~N .,~~cooH
F
O
- of
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
73
148 149 150
H~/''~ \ IF
\ N II N ~ \
O ..~iCOOH ~ /
r
- of
151 152 153
o'~ o''~ o
~ I ~ I ~ I
H~ ~F N~ ~ a ~F
\ V II N .,nCOOH ~ \ II N ..nCOOH ~ \ II N .,nCOOH
F / O C~ / O / O
F r ~ ~ F r ~ J F r
O
154 155 156
o-~
~ F
fI N .~nCOOH
/ O
O
J
157 158 159
o-~ o-~
v / v I
~F ~ H ~F
N ..nCOOH I \
~~N ~mCOOH
O / F / O
r ~J r ~J
F D
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
74
160 161 162
°~ °"
~ /
'F ~J F
~N
wtCOOH ~ / ~N ~~nCOOH
H ~ , O
O
F_ J
163 164 165
o-~
~ ~F
W N f1 N
F ~ , O ~~~iCOOH
166 167 168
~, a
F~
169 170 171
°-v
H
F
CA 02292604 1999-12-02
WO 98157933 PCT/US98111821
172 173 174
175 176 177
F
5
178 179 180
o-~
I
H ~F
~~N .,~iCOOH
/ O
F' ~
O
J
10 181 182 183
o-~ o--~
r r
~ I ~ I ~ I
H H H ~F
~ 'F 'F N
IOI N .,nCOOH
~~N ~~nCOOH ~ \ ~ ~~nCOOH
F / O
F i ~J J i ~J
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
76
184 185 186
187 188 189
F
190 191 192
o-~
H \ I F \ I
~ ~F
I " N ~~nCOOH
O F
~~nCOOH
C / O
O
193 194 195
I ~ ~
F I
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
77
i I \
CI
205 206 207
°~-
\
\ ~ ~~~~COOH
F
O
J
196 197 198
o-~.o_
H
N N
~~~~COOH
_ O
CI-l~ OJ
199 200 201
202 203 204
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
78
208 209 210
I \
ci / I /
211 212 213
o-
H
\ N~N ~mCOOH H
F ~ / O
O
_ OJ
214 215 21C
H
217 218 219
' o'
I/ I \
~~N
~~~~COOH
F / O
O
O
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
79
220 221 222
_o-~~
H
I ' N~N .,uCOOH
C / O
_ / \ O
223 224 225
H
w w
F I ~ CI
226 227 228
229 230 231
o-
H H
~~nCOOH
~ i o
/ \
o
- of
.o~
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
232 233 234
o~ '~°v °~.
~/
N
~~~iCOOH
O
J
235 236 237
5
.o
238 239 240
0
CH
O
J
10 241 242 243
°'~-o' o~o~ y
vl vl
~~N
~~~iCOOH ~ / O
N ~~nCOOH
i
I / ~ O f / ~ O
J F- J
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
81
244 245 246
- o\
w b
I/
247 248 249
o-
~I
H
~~nCOOH
O
O
250 251
O~OMe
~Me
H
W N II N
~~~~COOH
/ O
_ O
O
252 253
a
H
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
82
254 255
~-0M a
H
O
256 257
o-~
o'LoMe o~oM~
\/ \I
H H
N .~~iCOOH
~N ~~~iCOOH
O
O
CI
O
O
CH3 of CH~o of
258 259
o-\o
\ I
~N .~nCOOH
\ O
of
'~OMe
260 261
O~~OMe
H \ / \ /
~~N
II N ..nCOOH
wnCOOH
i O
CI ~ O
O
of J
'~OME
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/i1821
83
262 263
o-~
o~oMe
\ /
v~N ~nCOOH
0
F /
J
264 265
'\..oME
266 267
H
I w a~ I w N1>~
F ~ O
268 269
0
~~.~OMe ~'~pME
H \ /
~ ~~N
I \ N " N wnCOOH
I ~~nCOOH
/ O
CI ~ O
O / ~ O
_ of _ of
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
84
270 27i
Me
272 273
0--~~
~~M9 ~OMe
H
~N wnCOOH
CI I ~ O
\ O
F - of
274 275
~OMe
O~\
O~.OMe
" N .~nCOOH
i O
~ O
O
i0 276 277
0
~OMe O
~OMe
H
N N
~~nCOOH
c
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
5
o~
\ //
H
N'~
[O~ N .,nCOOH
CI
O
CH3 of
10 286 287 288
-o~
\ I \ I
H
~~N .,nCOOH ~ \ N~N .,nCOOH
F
J
278 279
280 281 282
_o~
H \ I H \ I
W N~N ~ N N
/ O .,nCOOH ~ / ~ .,nCOOH
C
~. -~ J
283 284 285
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
86
289 290 291
v I v I
H
N
j( ~~nCOOH I / ~ ~~nCOOH I
I ~ O
O F
O
_OJ _OJ
292 293 294
H H
N ~ N
~~nCOOH (
I~
F
O
- of
295 296 297
/
H H
~~N ~~~~COOH I \ N " ~~~iCOOH
CI ~ O / O
O / ~ O
J _ of
298 299 300
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/1182I
87
301 302 303
°~ o-v ,
\ /
I \ N II N .,nCOOH
i 0
F
O
F _ OJ
304 305 306
o-\"
H \,
N .,n000H
I~ o
~ 0
0
307 308 309
\ /
H
N
I , I / ~N wnCOOH
F
O
' °J
310 311 312
F
7
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
88
313 314 315
o~ o~
\/ \1
H H
N' ~
N ~~nCOOH \ II N .,nCOOH
O ( / O
O / ~ O
CH3 of J
316 317 318
\ /
N'
N .,~iCOOH
CI I i O i i
O
J
319 320 321
'o~ o~
\ I D \ II
N IOI N .,nCOOH ~ \ N fI N ."iCOOH
F i / O
F / ~ ~ F /
O
of
322 323 324
'o~ Io
H \ I H \ I
.,nCOOH ~ \ N I) N .,nCOOH
F / O C~ r O
/_\ O / \ O
of J
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
89
325 326 327
.,
~ l
~~nCOOH
O
\ O
- of
328 329 330
7
I ~ b I ~ b
331 332 333
H
CI
334 335 336
H
CA 02292604 1999-12-02
WO 98/57933 PCTIUS98/11821
337 338 339
/ \ I
H \ H
II N \ N~N .~nCOOH
~~~~COOH ~ / O
O
r \ O / \ O
_ OJ CH3O _ of
340 341 342
H \ I H \ I
N ~ N II
I / O °~~COOH ~ / O N ~~nCOOH
CI
/ ~ O v ~ / ~ O
CH30 OJ CHa OJ
343 344 345
°~ °~
H \ I H \ I
N~N
N .mCOOH ~ \ .,nCOOH
F / O C~ / O
r vJ r vJ
O
346 347 348
o~
H \ /
\ ~~N .mCOOH
O
F r ~ O
J
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
91
349 350 351
°"'~ °~
v I
H
N
.~nCOOH 1 ~ ~ ~N ~~nCOOH
F v
O ~ / \ O
J of
352 353 354
o-a
~I ~I
H N~ a
N~N ~,nCOOH ~ / ~O~ N .,nCOOH ~ j ~ .,nCOOH
CI
/ \ O / \ O / \ O
J J J
355 356 357
~ I ~ I
a~ a~
N .mCOOH ~ \ I' N .,nCOOH
F I / O CI / O
O / \ O
J F- J
358 359 360
o-a
H
~ N N
F ~ ~ C ~ r ~ ."~COOH
O
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
92
361 362 363
o-~
w /
~~N
O ~~~iCOOH I /
F
O
364 365 366
H
W N II N
~~nCOOH
/ O
/_\ O
CH3o of
367 368 369
H
370 371 372
-I I /
CI
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
93
373 374 375
H
Iw N ~w
F
376 377 378
H
CI
379 380 381
H
N
I i
382 383 384
H
v
I , ~N w~~COOH
F
O
F- J
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
94
385 386 387
w
li I~
F
388 389 390
0
i
\ ! H \ /
I , ~~N w~COOH I , 'O' N w~~COOH
1 ~\ 1 /\
J
391 392 393
NMe2
H H \ I
I / I / H I / ~N .~nCOOH
/ \
O
J
394 395 396
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
397 39$ 399
~N~
r
\ /
H
W N II N
~mCOOH
O
400 401 402
\ /
~ a~N .,~~COOH
O
O
J
403 404 405
/J
b
CA 02292604 1999-12-02
WO 98/57933 PCT/US98111821
96
406 407 408
H
~ N
I, I~
409 410 411
CH3
O
\ l H \ I H \ I
a
~N .,nCOOH I ~ N II N ..nCOOH ~ ' N I' N .,nCOOH
O / O
O / \ O / \ O
O ~O ~O
412 413 414
o-~
H \ /
N H
H I / I ~ N II N .,nCOOH
/ O
F F /
O
J
415 416 417
o-~
l
F ~ a N F W a~N
wnCOOH ~ ..nCOOH
F / O
F
F / ~ o
- of
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
97
418 419 420
H \ /
\ ~~N .,nCOOH
O
O
J
421 422 423
H
N' /
~O
424 425 426
427 428 429
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
98
430 431 432
°~ °'~-~
H H
I ~ N II N .,nCOOH
I \ ~~N ~~nCOOH
O F / O
/ ~J / ~J
F O F O
433 434 435
o-
I ~ al~N .,~~cOOH
~~N ~~~~cooH
O
F / O
/
F_ J
436 437 438
,.
/ a ~I
N ~ II N .,nCOOH
I .,~iCOOH I / O
r~~ r
O
439 440 441
_ ,
v !
H
N~
I , 'O' N wnCOOH
O'
CA 02292604 1999-12-02
WO 98/57933 PCT/US9811I821
99
442 443 444
vl vl
H
~~nCOOH
.~~~COOH I / O
I~ ~ F
o \ o
cH3 of cH3 J
445 446 447
~o~
448 449 450
451 452 453
,'
/ _
N .r~COOH I \ ~N .,yppH
F ~ CI
\ O / \ O
J F- J
CA 02292604 1999-12-02
WO 98/5'7933 PCT/US98111821
100
454 455 456
0
o~
H
N
7H
F
0
457 458 459
o -~ '~ '~ o
o-
\ ~~N ~~nCOOH
I i O
O
J
460 461 462
H H
N
j(
N~ ~ ~ O
463 464 465
H
N
,a
0
CF
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
101
466 467 468
L
w b
F
469 470 471
H H
N ~ N
I ~ F I ~
472 473 474
H
N
CI
475 476
o-
L
~ /
H
N
F ~ / Ipl N .~~~COOH I
"1
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
102
477 478
o-
H
.mCOOH
i O
_~ O
O
H
N
OO
479 480
H
y N y
481 482 483
o-~~
H
N 'I N wnCOOH
I i O
O
CH3 of
484 485 486
H
I w N1~ I w
0
CA 02292604 1999-12-02
WO 98/57933 PCTNS98/11821
103
487 488 489
w b
490 491 492
'°
H
O
493 494 495
~I
.~~~cooH
O
a
O
496 497 498
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
104
499 500
H
N~N .,nCOOH
F CI
O
J
501 502
~ I ~ I
H ~ ~~N
N~ .,~~COOH
N .,nCOOH ~ , O
O
'O
~H3 - of
503 504
~o~
H
\ N~N .,nCOOH
O
O
CH3 of
505 506 507
~/
~ N N
O ~ ~ .~~~COOH
/_v
OJ
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
105
508 509 510
' ~ '
~/ ~/
H
N II N .."OpOH \ II N .."COON
i O ~ i O
_ of _ of
511 512 513
N
I i I / O
514 515 516
o-~~
H W
N
jO( ~ ~N ,~nCOOH
0
517 518
'~'° o.
/ p ~/
H ~
1V ~ II N ..nCOOH
O .~~iCOOH ~ ~ O
r ~~ / ~ J
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/I1821
106
519 520
521 522
o-
~/
~~N ~~uCOOH
O
523 524
525 526
H
N'~
[JO
CF
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
107
527 528
529 530
''~o~ o-L
o-
~I ~I
a~ b~
N .,nCOOH ~ IOI N .,nCOOH
i O
O / ~ O
- of
W,..o
o-
~/
.,~~COOH
0
0
J
o~
o'
531 532
o-~
~I
N .~nCOOH
O
O
F- J
w p~r
CI I / O
533 534
H
w ~r
~ o
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
108
535 536
~I
~N .nCOOH
O
O
O
537 538
o-~
~/
~~ N
~~~~COOH
O
O
539 540
o-L~
~ /
~ ~ b~N
o .~~~COOH
F CI
O
J
541 542 543
_o~sv
H
w N I! N
~~~~COOH
O
O
J
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
109
544 545 546
~ ~s~
H H
N ~~nCOOH
~r O
\ O
- pJ
547 548 549
s~
550 551 552
O'~s~ '~s~
H \ / H
N ~~nCOOH
O ~~nCOOH CI ~ / O
O / \ O
F-pJ F- J
553 554 555
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
110
556 557 558
0.1 _~ °'~-s\
\ I
~~N ~mCOOH
/ O
/
O
J
559 560 561
...,
\ I
H
N~N wnCOOH
O
O
J
562 563 564
°
_ \
\ I
H H
/ O ~~~iCOOH
" N \ N II
C I / O
O
CH3
565 566 567
O., n"
\ I
H
w N II N
~~~iCOOH
O
O
J
CA 02292604 1999-12-02
WO 98/57933 PCTNS98/11821
111
568 569 570
o~ n
571 572 573
Op n" n"
.S~
F
574 575 576
o" Q~oz
H
I1 N ..nCOOH
C / O
O
O
577 578 579
"~s°2
vl
H
N
fO1 ~ .a~COOH
O
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
112
580 581 582
-~s, s,
c
583 584
~s~ ~s~
585 586
'~sr
587 588
w
ci
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
113
589 590 591
H H
N ~ N'
~/ ~/
- F
592 593 594
_°~S.
H H
N N II N .~nCOOH
/
/ O
O
O--i
595 596
o~
's
H
\ N~N .u~COOH
F I / O
O
597 598
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
114
599 600
-\s- ,
Oz z
I
ci /
601 602 603
0
~s-
z ' / Oz
N ( ~ N II N .,nCOOH I
O F / O
_ O
CH3 OJ CF
604 605 606
2
H
N
c I / ~ I /
c
607 608 609
2 ~ 2 2
N~ N
I / 'OI N w~~COOH I
Ci
O
- pJ
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
115
610 611
~ l ~ I o2
H H
w N N II
I / ~N ~"~COOH \ N ~~nCOOH
O
O / ~ O
J J
612 613
614 615
2
'l z
H
\ N~N ~~uCOOH
O
O
616 617 618 .
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
116
619 620 621
~oH
I
H
W W N N
C
~~~iCOOH
/
O
J
622 623 624
C
625 626 627
628 629 630
H ~OH
\ ~~N .~~~COOH
O
CI
O
F- J
OH OH .pH
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
117
631 632 633
13H
H
N
F
634 635 636
.OH
H
N
I
C
637 638 639
r
OOH
~ /
H
w N'~ N W
/ O wnCOOH ~ /
F
O
J
640 641 642
H
~ N
~/
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/i1821
118
643 644 645
0
~l
H
W N II N
~~~~COOH
F ~ / O
O
CH3 of
646 647 648
_ w ow
~I ~l
~~N ~mCOOH
.,y00H / O
O C
O / \ O
- of J
649 650 651
v I
b
~N .,~~COOH
O
F- J
652 653 654
o-~
H
N II N ..nCOOH
C ~ / O
/ \ O
J
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
119
655 656 657
/
o~ \
\I
N ..~~cooH
I~
/.. \
o-./
658 659 660
COOH
\I
H
V II N ..nCOOH
O C
/ \ O
J
661 662 663
COOH OOH COOH
H \ I H \ I H \ I
N
~ ~ N N
N ~mCOOH
I j IOI N .,nCOOH I i O
N ."~COOI
F
/. \ / ~ / \
O _ C
CHs O~ CHs
664 665 666
OOH OOH
\ / \ /
H H
~ W N II N
N »nCOOH ~~nCOOH
O
/ \ O
CH3 o J
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/I1821
120
667 668 669
OOH
' H
N
N .~nCOOH
CI
\ O
- of
67o s71 s72
COOH
H
N~
'O' N 'u~COOH
O
J
673 674 675
OOH
H
" N .~nCOOH
O
of
s7s 677 67s
OOH
H \
N~N ~mCOOH
Cl ~ O
~ O
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
721
679 680 681
6$2 683 684
ONHz
ONHZ
\ I \ I
~~'N
~N .~nCOOH ~ ~ ( / O
~~~COOH
O F / CI
CH3 O CH3O O
ss5 6ss ss7
688 689 690
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
122
691 692 693
H2 ONHZ ONH2
\ I \ /
H
N 'I wnCOOH
N .~nCOOH ~ \ N
~H C~ I i O / O
O /_\ O /_\ O
J F OJ F O
f 94 695 696
CONH2 CONH2
ONH2
\ / \ / \ /
H~ H H
L ~ N II N ~ N
.~nCOOH I ~ V II N ..nCOOH ~ \ ~N .~~COOH
O F ~ O CI ~ O
O / ~ O
O~ O
697 698 699
CONH2
H \ I
N~N .~nCOOH H
O
/ ~ O
O--J
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
123
700 701 702
N
H \ I H \ I
N~ ~ N II
OH ~ / IOI N .,nCOOH ~ N .~nCOOH
O
p /_\ O
- pJ ~H3o of
703 704 705
N
\ I \ I
H H
N ~
~N .~nCOOH ~ ~ N II N .,nCOOH
CI ~ O
O / ~ O
CH3 OJ CH3 OJ
706 707 708
n
N
\ I
H
H
N~N ~~uCOOH
O
/ \ O
J
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
124
709 710 711
772 713 714
N
fI N
i , p ~"~COOH
' O
715 716
717
H \ I H \ I
w N
I , ~ .~~COOH I / ~ ~~COOH
C
O
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
125
718 719 720
N-
H \ /
\ ~~N ~mCOOH
O
/ \
O
J
721 722 723
~N _ v
N-
\ / \ /
H H
N N
wnCOOH ~ I / ~N ~~nCOOH
I~
/ \ o / \ o
J ~H3 J
724 725 726
\ /
H
N
I ~N ~~nCOOH I W
/ \
_ O
CH3 of
CA 02292604 1999-12-02
WO 98157933 PCT/US98/11821
126
727 728 729
b
Iw ~ Iw H Iw
F ~ CI
730 731 732
733 734 735
~N-
N-
\ I \ I
~~N .,nCOOH I ~ N II N .,nCOOH I w
~ O
F
/ \ O ~ ~ O
F _ J _o~
736 737 738
H \ I H \ I
N~ N~
N .,nCOOH ~ / IOI N .,nCOOH I w
C i O i
/ ~ O / ~ O
CA 02292604 1999-12-02
WO 98/57933 PCTNS98/11821
127
739 740 741
~ / ~ /
N N
W N N
F I ~ o .,~~cooH c i ~ .~ I ~ ~ .~nCOOH
/ \ o / \ o
J J
742 743 744
,/
N~ N
I i IOI N ..nCOOH I
F
/ \
O
CH3o of
~N ~p~COOH
O
/ \
CH30 O'
745 74fi 747
H \ / H \ /
N' ~
N .,nCOOH I ~ V IOI N .,nCOOH
O
/ \ / \
_ O
CH3o o~ of
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
128
748 749 750
v / v /
N 'I N .,nCOOH ~ \ N '1 N .,nCOOH
CI ~ O / O
O O
J J
751 752 753
/
H
N~ N w
O .,nCOOH
F
\ O
F- J
754 755 756
H
H
~ N
i
~' 1
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
129
757 758 759
~ / ~
H
.,nCOOH I ' ~N .mCOOH
i O i O
/ \ O
o.~ J
760 761 762
~~o
_ o
H \ I H
N wnCOOH I \ " N wnC001
C / O / O
/ \ O / \ O
J J
763 764 765
_ o
J
H
.mCOOH
CHsO O
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
130
766 767 768
H
.~nCOOH
O
O
J
769 770 771
~~o
H
~~nCOOH
i O
\ O
J
772 773 774
'" ''o
b
I '~ ~ \ ~N ~~nCOOH
C / i O
O
O--i
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
131
775 776 777
0
~/
H
N ~~~~COOH
O
r ~
O
778 779
o~ow ~o w
I , '°' N ~~~COOH I
1
r~
°
J
780 781
782 783
~o~
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
132
784 785 786
~o~
H
787 788 789
H N O~
~i ~i
790 791
.~o~ ~ N ,°~°v
I ~ ~~ ~~~~cooH
F "1
°
J
792 793
~ /
H .O~OW
v °~
~nCOOH
O
C
\ O
F- J
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
133
794 795
H H
I ~ N ~ N
i O
796 797 798
H w0~0\
~ ~\
" N ~nCOOH
C I ~ O
O
799 800
V
OCH3 U2Y ~p
N \ / H
w N II N
wnCOOH I ~ O
N ~~nCOOH
O CI
O / ~ O
J J
801 802
H
~ N
i b~ I ~
CH3
3
CA 02292604 1999-12-02
WO 98/57933 PCTNS98/11821
134
803 804
o2s-'~ocH
\ /
H
' N~N wnCOOH
O
/_\ O
cH3 of
3 CH3
805 806 807
02s'~.~OCH3
H ~ /
' N
~ ' ~1~N .,~~coOH
/ / F / O
/ \ O
J
808 809 810
~1
O2 CHs o2 HOC
\ / \ /
H H
' N II N ' N II
O .,~~COOH ~ / O N ..~~COOH
CI
v ~ / ~ O v ~ / ~ O
J J
811 812
/ O
H<
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
135
813 814
02S~OCHa
H \ /
N~ ~ ~ N (I N .,//COON
O / O
O
815 816
Ha
F
817 818 819
\ /
H
N~
°~5"~C" ~ / II N .,nCOOH
o " ~o~ F
J
820 821 822
o2s-'~
\ /
H
/ N IOI N
.,nCOOH
/_\ O
cH3 of
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
136
823 824 825
O2~ O2~
~I
N ..nCOOH ~ ~ N II N ..nCOOH
C i / O
\ / \
CH3 of CH3 OJ
826 827 828
02~ oz~
~ I
.,nCOOH ~ ~~N .,nCOOH
F
/ \ O / \ O
J J
829 830 831
o2s~
J \ ~~N .~~iCOOH
F i O
/ \ O
F- J
832 833 834
~H
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/1182I
137
835 836 837
838 839 840
o2s--~
H \ /
I W N II N W
O .,nCOOH I /
F
O
J
841 842 843
02s-~-
\ /
~,ncooH
I/ o
~ o
- pJ
844 845 846
\I
I ~ a~N .,ncooH
/ I/ o
0
J
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
138
847 848 849
ozs~ o2s'~
~! ~I
H H
N ..vCOOH
/ p ..nCOOH I / O
CI
/ \ / \
J J
850 851 852
o2s~
H ~ I ~ I
.~nCOOH I \ ~N .mCOOH
F
O / \ O
J F- J
853 854 855
p2s'~' o
H I ~I
N II N .,nCOOH ~ ~ ~~N .,nCOOH
O ~ O
/ \ O / \ o
F _ J _ O
856 857
Ozs-L o2s-~-
/ H ~ /
N~
~~N .n~COOH ~ j IOl N ..uC001-I
CI
o~s~
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
139
Exam I~e 858
traps.traps-2-(4-Methoxyr)~~vl-4-(1 3-benzodioxol-5-yrl)-~! -2 6
diethvlc~heny~,)~aminoca.rhonyr~amino~~yrrrolidine3-carbox~yrlic a~~,
Example 858A
Ethvl traps.traps-2-(4-methoxy)phenyl-4-(1 3-benzodioxol-5-yl~-1-amino-
pyrrolidine
3-carboxvlate
To a solution of 1.OOg (2.71 mmol~, ethyl traps,traps-2-(4-methoxy}phenyl-4-
(1,3-
benzodioxol-5-yl)-1-pyrrolidine-3-carboxylate in 6 mL of acetonitrile was
added a
solution of 1.18 g (10.4 mmol) of hydroxylamine-O-sulfonic acid in 2 mL of
H20. The
homogeneous reaction mixture warmed spontaneously, and was stirred for 20
minutes, then concentrated in vacuo. The residue was dissolved in 15 mL of
ethyl
acetate and extracted with 0.7M NaHC03 solution (2 x 25 mL), then brine (1 x
10 mL),
dried over MgS04, filtered, and concentrated to a thick oil. This consisted of
a mixture
of the starting pyrrolidine and the corresponding 1-aminopyrrolidine. To a
solution of
892 mg (2.2 mmol) of this oil in 10 mL of ethyl acetate was added 700 mg (3.21
mmol) of di-tertbutoxycarbonic anhydride. The mixture was stirred at ambient
temperature for 30 min, then concentrated in vacuo. Silica gel chromatography,
eluting with 30% EtOAc/Hex, gave 360 mg of ethyl traps,traps-2-(4-
methoxy)phenyl-
4-(1,3-benzodioxol-5-yl)-1-(tart-butoxycarbonyl)amino-pyrrolidine-3-
carboxylate as a
colorless oil. The desired product was slightly more polar than the
tertbutoxycarbonylpyrrolidine. To 360 mg (0.768 mmol) of the Boc hydazine was
added 2 mL of trifluoroacetic acid. After stirring for 2h at ambient
temperature, the
solvent was removed in vacuo. The residue was taken up in 10 mL of 0.6M NaHC03
solution and extracted with ethyl acetate (3 x 3 mL). The combined ethyl
acetate
layers were back extracted with brine (1 x 3 mL), dried over MgS04, filtered,
and
concentrated to 249 mg of a nearly colorless oil.
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
140
Example 858B
traps.traps-2-(4-Methoxy)~phenyl-4 ~(1.3-benzodioxol-5-y~,)-1-((N-2.6-
diethylphenyl)~aminocarbonyl)amino-~~rrolidine3-carboxyrlic acid
To an ice cooled solution of 100 mg (0.670 mmol) of 2,6-diethylaniline in 2 mL
of THF
and 0.4 mL of N,N-diisopropyl-N ethylamine was added 66 mg (0.223 mmol) of
triphosgene. The suspension was stirred at 0 °C for 10 min, then a
solution of 249
mg (0.645 mmol) of ethyl trans,trans-2-(4-methoxy) phenyl-4-(1,3-benzodioxol-5-
yl)-
1-amino-pyrrolidine-3-carboxylate in 2 mL of THF was added. The mixture was
stirred for 1.5 h, then a 20 mL of 0.6M NaHC03 solution was added. The
suspension
was extracted with ethyl acetate (3 x 5 mL), dried over MgS04, filtered, and
concentrated to an oil which began to crystallize. This was taken up in a
small
amount of ethyl acetate, allowed to crystallize, and filtered to give 108 mg
(30% from
the hydrazine) of ethyl traps,traps-2-(4-Methoxy)phenyl-4-(1,3-benzodioxol-5-
yl)-1-
((N-2,6-diethylphenyl)aminocarbonyl) amino-pyrrolidine3-carboxylate.
Hydrolysis of
the ester was performed as described for other examples to give the title
compound
as a white solid. 'H NMR (300 MHz, ds DMSO) b 1.00 (t, J=7.4, 6H), 2.33 (br t,
J=7.OHz, 4H), 2.90 (t, J=9.9Hz, 1 H), 3.38-3.50 (m, 2H), 3.59 (m, 1 H), 3.75
(s, 3H), 4.11
(d, J=10.3Hz, 1 H), 5.98 (d, J=0.7Hz, 1 H), 5.99 (d, J=1.1 Hz, 1 H), 6.82 (m,
2H), 6.89 (d,
J=8.4Hz, 2H), 7.03 (d, J=7.OHz, 2H), 7.12 (m, 1 H), 7.33 (d, J=1.SHz, 1 H),
7.38 {s, 1 H),
7.53 (d, J=8.8Hz, 2H), 8.02 (s, 1 H), 12.3 (s, 1 H); MS {CDI, m/z) 532 (MH+).
Anal.
Calcd for C3~H33N3~6~ C~ 67.78, H, 6.26, N, 7.90. Found: C, 67.71, H, 6.42, N,
7.82.
f2R.3S.4S1-2f4-(2-methoxyethoxylpheny~-~1 3-benzodioxol-5-KI~-~2 6-
diethvlphenyiaminocarbon ly methyl)-pyrrolidine-3-carboxylic acid
CA 02292604 1999-12-02
WO 98/57933 PCTNS98/11821
141
Example 859A
~yl(2R.3S.4Sj-2(4-(2-methoxyrethoxy)phenyl)-4-(1.3-benzodioxol-5-y~-
~yrrolidine
3-carboxylate
Ethyl traps, traps-2[4-(2-methoxyethoxy)j-4-(1,3-benzodioxol-5-yl)-
pyrrolidine-3-
carboxylate, from example 30, was reacted with di-ten=butyl dicarbonate, and
the
resulting product was hydrolyzed with NaOH to give traps, traps -2-[4-(2-
methoxyethyl)J-4-( 1,3-be nzodioxol-5-yl)-1-(tert-butyloxycarbonyl)-pyrrolidi
ne-3-
carboxylic acid. This acid was resolved by salt formation with R-(+)- alpha
methyl
benzylamine. The resolved salt was washed with aqueous HCI to remove the
resolving agent, then heated with HCI in ethanol at 70 degrees C for 18 hours
to
produce ethyl [2R,3S,4S]-2[4-(2-methoxyethoxy)]-4-(1,3-benzodioxol-5-yl)-
pyrrolidine-3-carboxylate, which was purified by chromatography on silica gel,
eluting
with ethyl acetate.
Example 8598
f2R.3S.4Sj-2[4-(2-methoxyethoxy)I henyrlj-4-(1.3-benzoriioxol-~,vll-1-(2 6
diethylphenylaminocarbonylmethylL~yrrolidine-3-carboxyrlic acid
The title compound was prepared as described in Example 30, employing the
compound of Example 859A as starting material. 1 H NMR (300MHz, CDC13) 8 1.08
(t, J=7Hz, 6H), 2.43 (q, J=7Hz, 4H), 3.00 (d, J=11 Hz, 1 H), 3.05-3.15 (m,
2H), 3.44 (s,
3H), 3.46 (d, J=11 Hz, 1 H), 3.45-3.55 (m, 1 H), 3.65-3.75 (m, 1 H), 3.75-3.80
(m, 2H),
3.93 (d, J=7Hz, 1 H), 4.12-4.17 (m, 2H), 5.94 (dd, J=2Hz, 4Hz, 2H), 6.75 (d,
J=8Hz,
1 H), 6.82 (dd, J=2Hz, 9Hz, 1 H), 6.87 (d, J=2Hz, 1 H), 6.95 (d, J=8Hz, 1 H),
7.10 (d,
J=6Hz, 2H), 7.19-7.24 (m, 1 H), 7.37 (d, d=8Hz, 2H), 8.29 (s, 1 H).
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/I1821
142
Exam Ip a 860
traps, traps-2-(4-(2-Ethoxyethoxy))-4-( 1,3-benzodioxol-5-yl)-1-(2,6-
diethylphenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid
The title compound was prepared by the procedures described in Example 30. 1 H
NMR (300MHz, CD30D) 8 1.09 (t, J=7Hz, 6H), 1.23 (t, J=7Hz, 3H), 2.47 (q,
J=7Hz,
4H), 3.4-3.55 (broad, 2H), 3.62 (q, J=7Hz, 2H), 3.6-3.9 (broad, 4H), 3.82 (m,
2H),
4.16 (m, 2H), 4.4 (broad, 1 H), 5.93 (dd, J=1 Hz,2Hz, 2H), 6.77 (d, J=8Hz, 1
H), 6.90
{dd, J=2Hz, 8Hz, 1 H), 7.04 (m, 3H), 7.12 (m, 2H), 7.22 (dd, J=7Hz,9Hz, 2H),
7.44 (m,
2H). MS (ESI+) m/e 589 (M+H+). Anal. Calc for C34H40N207Ø5TFA: C, 65.11, H,
6.32, N 4.34. Found: C, 64.81, H, 6.36, N, 4.25.
Exams IQ a 861
traps, traps-2-(4-(2-Isopropoxyethoxy))-4-( 1,3-benzodioxol-5-yl)-I -(2,6-
diethylphenylaminocarbonylmethyl)-pyrrolidine-3-carboxylic acid
The title compound was prepared by the procedures described in Example 30. 1 H
NMR (300MHz, CD30D) 8 1.10 (t, J=7Hz, 6H), 1.18 (2, J=7Hz, 6H), 2.47 (q,
J=7Hz,
4H), 3.4-3.55 (broad, 2H), 3.6-3.9 (broad, 4H), 3.75 (hept, 1 H, J=7Hz), 3.82
(m, 2H),
4.17 (m, 2H), 4.4-4.5 (broad, 1 H), 5.93 (broad s, 2H), 6.78 (d, J=8Hz, 1 H),
6.90 (dd,
J=2Hz, 8Hz, 1 H), 7.05 (m, 3H), 7.12 (m, 2H), 7.20 (dd, J=7Hz,9Hz, 2H), 7.56
(m, 2H).
MS (ESI+) m/e 603 {M+H+). Anal. Calc for C35H42N207Ø6TFA: C, 64.79, H, 6.40,
N, 4.17. Found: C, 64.40, H, 6.53, N, 4.20.
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
143
Examl Ip a 862
traps. traps-2-(4-(2-Propoxy))-4-( 1,4-benzodioxan-b-yl)-1-(2,6-
diethylphenylaminocarbonylmethyl)
pyrrolidine-3-carboxylic acid
The title compound was prepared by the procedures described in Example 1. 1 H
NMR (300MHz, CD30D) 8 1.06 (t, J=7Hz, 3H), 1.09 (t, J=7Hz, 6H), 1.81 (m, 2H),
2.46
(q, J=7Hz, 4H), 3.3 (m, 2H), 3.4-3.5 (broad, 2H), 3.6-3.9 (broad, 4H), 3.95
(t, J=6Hz,
2H), 4.22 (s, 4H), 4.4 (broad, 1 H), 6.80 (d, J=8Hz, 1 H), 6.92 (dd, J=2Hz,
BHz, 1 H),
6.99 (m, 3H), 7.13 (m, 2H), 7.22 (dd, J=7Hz,9Hz, 2H), 7.44 (m, 2H). MS (ESI+)
m/e
573 (M+H+), 595 (M+Na+). Anal. Calc for C34H48N206Ø6TFA: C, 65.95, H, 6.38.
N 4.37. Found: C, 65.83, H, 6.40, N, 4.31.
Examr~le 863
traps. traps-2[(4-butoxyphenyl)~~-X1.3-benzodioxol-5yll-1-~2.6-
diethyl~ylaminocarbonvlmethylL~yrfolidine-3-carboxylic acid
The title compound was prepared by using the method of example 30 by
substituting
1-bromobutane for 1-bromo-2-methoxyethane. 1 H NMR (300MHz, CDC13) b 0.99 (t,
J=7Hz, 3H), 1.08 (t, J=7Hz, 6H), 1.42-1.57 (m, 2H), 1.73-1.82 (m, 2H), 2.42
(q, J=7Hz,
4H), 3.00 (d, J=11 Hz, 1 H), 3.05-3.15 (m, 2H), 3.46 (d, J=11 Hz, 1 H), 3.50-
3.55 (m, 1 H),
3.65-3.75 (m, 1 H), 3.93-4.00 (m, 3H), 5.94 (dd, J=2Hz, 4Hz, 2H), 6.75 (d,
J=BHz, 1 H),
6.82 (dd, J=2Hz, 9Hz, 1 H), 6.87 (d, J=2Hz, 1 H), 6.93 (d, J=8Hz, 1 H), 7.10
(d, J=7Hz,
2H), 7.19-7.24 (m, 1 H), 7.37 (d, H=8Hz, 2H), 8.30 (s, 1 H). MS (APCI+) m/e
573
(M+H+). Anal. Calc for C34H40N206: C, 71.31 H, 7.04 N, 4.89. Found: C, 71.05
H, 7.09 N, 4.83
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
144
Exams Ip a 864
traps, traps-2f4-i(2-methnxy Athoxy)ethoxy}~pj~nyl~-~1 3-ben~n~inYnl ~ y ~ 1
(2 6
diethvlohenylaminocarbony the)-~yrrolidine-3-carboxyr ' acid
The title compound was prepared by using the method of example 30 by
substituting
1-bromo-2-{2-methoxyethoxy)ethane for 1-bromo-2-methoxyethane. 1 H NMR
(300MHz, CDC13) 8 1.08 (t, J=7Hz, 6H), 2.43 (q, J=7Hz, 4H), 3.00 (d, J= 11 Hz,
1 H),
3.05-3.15 (m, 2H), 3.40 (s, 3H), 3.46 (d, J=11 Hz, 1 H), 3.44-3.55 (m, 1 H),
3.57-3.62 (m,
2H), 3.65-3.75 (m, 1 H), 3.70-3.75 (m, 2H), 3.88 (t, J=6Hz, 2H), 3.93 (d,
J=7Hz, 1 H),
4.15 (t, J=7Hz, 2H), 5.94 (dd, J=2Hz, 4Hz, 2H), 6.75 (d, J=8Hz, 1 H), 6.82
(dd, J=2Hz,
9Hz, 1 H), 6.87 (d, J=2Hz, 1 H), 6.93 (d, J=8Hz, 1 H), 7.10 (d, J=7Hz, 2H),
7.19-7.24 (m,
1 H), 7.37 (d, H=8Hz, 2H), 8.30 (s, 1 H). MS (APCI+) m/e 619 (M+H+). Anal.
Calc for
C35H42N20g: C, 67.94 H, 6.84 N, 4.53. Found: C, 67.49 H, 6.90 N, 4.41.
Example 865
traps. traps-2f(3-oropoxyphenyl))-4-{1 3-benzodioxol-5-vl) 1 (2 6
diethvlnhenylaminocarbonyfmethy~,)-pyrrolidine-3-carboxylic acir~
The title compound was prepared by using the method of example 30 by
substituting
1-bromopropane for 1-bromo-2-methoxyethane and methyl 3-hyroxybenzoate for
ethyl 4-hyroxybenzoate. 1 H NMR (300MHz, CDC13) 8 1.02 (t, J=7Hz, 3H), 1.08
{t,
J=7Hz, 6H), 1.75-1.87 (m, 2H), 2.43 {q, J=7Hz, 4H), 3.02 (d, J=11 Hz, 1 H),
3.09-3.20
(m, 3H), 3.48-3.56 (m, 2H), 3.65-3.75 (M, 1 H), 3.90 (t, J=7Hz, 2H), 3.98 (d,
J=8Hz,
1 H), 5.94 (dd, J=2Hz, 4Hz, 2H), 6.75 (d, J=8Hz, 1 H), 6.80-6.89 {m, 3H), 6.98-
7.05 (m,
2H), 7.10 (d, J=8Hz, 2H), 7.19-7.32 (m, 2H), 8.30 (s, 1 H). MS (APCI+) m/e 559
(M+H+). Anal. Calc for C33H3g N206: C, 70.94 H, 6.86 N, 5.01. Found: C, 70.65
H, 6.63 N, 4.92.
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
145
Example 866
traps.traps-2-l4-(2-methoxy)ether)lohenyl-4-~1.3-benzodioxol-5-vll-1-l,~N-2.6
diethyrlphenyl)aminocarbonyrl)methyl-pyrrolidine-3-carboxylic acid
~cample 866A
4-(2-methoxy)ethylbenzoic acid
To a 3-necked 50 mL flask fitted with two septa and a nitrogen balloon was
added 800 mg (20 mmol) of 60% NaH dispersed in mineral oil. The oil was
removed
by washing and decanting with hexanes (3 x 5 mL), using a pipette and keeping
a
positive pressure of N2 over the flask. Next, 5 mL of THF was added, the
suspension
was cooled with an ice bath, then a solution of 2.01 g (10.0 mmol) of 4-
bromophenethanol in 5 mL of THF was added via cannula. The ice bath was
removed, and the mixture was stirred for 10 min, then 700 mL (11 mmol) of
iodomethane was added. The mixture was stirred at ambient temperature for 1 h,
then 1 mL of H20 was added to quench the excess NaH. The reaction was poured
into 50 mL of H20, then extracted with diethyl ether (3 x 20 mL). The combined
ether
layers were back extracted with brine (1 x 20 mL), dried over MgSO,, filtered,
and
concentrated to 2.088 (97%) of a colorless oil. To a solution of 1.03 g (4.78
mmol) of
the above 4-(2-methoxy)ethylbromobenzene in 10 mL of THF was added 400 mg of
magnesium turnings, and a crystal of 12. The mixture was heated to reffux
under N2 for
5 min, then cooled to ambient temperature. The Grignard reagent was
transferred via
syringe to a 50 mL 3-necked flask under N2. A balloon of C02 was opened over
the
reaction, and the red color quickly faded to yellow. After stirring for 1 h at
ambient
temperature, the reaction was concentrated in vacuo. The residue was taken up
in
20 mL of H20 and acidified with 12M HCI to pH=1, then extracted with diethyl
ether (3
x 10 mL). The combined ether layers were back extracted with 2M NaOH (3 x 5
mL),
then the combined NaOH layers were extracted with diethyl ether (2 x 5 mL).
The
basic layers were treated with 12M HCI until pH= 1, then the product was
extracted
with ethyl acetate (2 x 5 mL). The combined ethyl acetate layers were back
extracted
with brine (1 x 5 mL), dried over MgS04, filtered, and concentrated to 427 mg
(50%)
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
146
of 4-(2-methoxy)ethylbenzoic acid as a yellow solid. The yellow impurity did
not
interfere with any subsequent reactions.
Example 8668
ins.traps-2-(4-(2-methoxy}ethy!)~~,~nyl-4-(1 3-benzodioxol-5-yl~~(N-2 6-
diethvlnhenvl)aminocarbonyl)methyrl-pyrrolidine-3-carboxylic acid
Prepared according to the procedures described in Example 1. 'H NMR (300 MHz,
ds DMSO) 8 1.00 (t, J=7.5Hz, 6H), 2.40 (q, J=7.5Hz, 4H), 2.66 (t, J=8.3Hz, 1
H), 2.79 (t,
J=6.8Hz, 2H), 2.87 (d, J=15.6Hz, 1 H), 3.00 (t, J=9.2Hz, 1 H), 3.20 (d,
J=15.9Hz, 1 H),
3.24 (s, 3H), 3.42-3.47 (rn, 1 H), 3.53 (t, J=6.9Hz, 1 H), 3.60 (m, 1 H), 3.91
(d, J=9.5Hz,
1 H), 5.94 (s, 2H), 6.76 (d, J=7.8Hz, 1 H), 6.84 (dd, J=1.4Hz, 8.1 Hz, 1 H),
7.08 (d,
J=7.5Hz, 2H}, 7.17 (d, J=6.4Hz, 3H), 7.22 (d, J=1.4Hz, 1 H), 7.51 (d, J=8.1
Hz, 2H),
9.21 (s, 1 H); MS (CDI m/z) 559 (MH'); Anal. Calcd for C33H38N206~0.20 H3P04.
C,
68.54, H, 6.73, N, 4.84. Found C, 68.28, H, 6.46, N, 4.82.
f=xamnle 867
traps. traps-2[~2-methoxyethoxyyl i nyl]-4-j1 3-benzodioxol-5-yl}-~2 6
diethvlphenvlaminocarbonvlmethyl)pvrrolidine-3-carboxylic acid
The title compound was prepared by using the method of example 30 by
substituting methyl 3-hydroxybenzoate for methyl 4-hyroxybenzoate. 1 H NMR
(300
MHz, CDC13) S 1.08 (t, J=7Hz, &H), 2.43 (q, J=7Hz, 4H), 3.00-3.20 {m, 3H),
3.42 (s,
3H), 3.50 (d, J=11 Hz, 1 H), 3.53-3.59 (m, 1 H), 3.65-3.75 {m, 1 H), 3.75 (t,
J=6Hz, 2H),
3.98 (d, J=8Hz, 1 H), 4.11 (t, J=6Hz, 2H), 5.94 (dd, J=2Hz, 4Hz, 2H), 6.75 (d,
J=8Hz,
1 H), 6.80-fi.89 (m, 3H), 7.05-7.10 (m, 2H), 7.10 (d, J=8Hz, 2H), 7.19-7.32
(m, 2H),
8.30 (s, 1 H). MS (APCI+) m/e 575 (M+H+) Anal. Calc for C33H38N2O7: C, 68.97
H, 6.67 N, 4.87. Found: C, 68.78 H, 6.84 N, 4.72.
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
147
Exams Ip a 868
traps. traps-2[(2-methyl-4-pnoxy hen~1)1-4-(1.3-benzodioxol-5-yl)-~2.6-
diethylphenylaminocarbonylmethyl)-~yrrolidine-3-carboxvli
Example 868A
Ethyl (4-pro~oxy-2-methKlbenzoyrl)~ acetate
4-Hydroxy-2-methylacetophenone was reacted with 1-bromopropane and potassium
carbonate in dimethylformamide to give 4-propoxy-2-methylacetophenone. This
compound was reacted with diethyl carbonate, using the method described in
example 15B to provide the title compound.
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
148
Example 8688
traps. traps-2'[j2-methyl-4-~ r~o~xyRhenyl)]-4-(1 3-benzodioxol-5-y~_1~2 6-
diethvll henylaminocarbonylmethyl)-l;~ylrolidine-3-carboxylic acid
Prepared using the procedures described in Example 30, employing the compound
of Example 868A as starting material. 1 H NMR (300 MHz, CDC13) 8 1.02 (t,
J=7Hz,
3H), 1.08 {t, J=7Hz, 6H), 1.75-1.85 (m, 2H), 2.33 (s, 3H), 2.40-2.48 (q,
J=7Hz, 4H},
2.98 (d, J=11 Hz, 1 H), 3.06-3.18 (m, 2H), 3.39 (d, J=11 Hz, 1 H), 3.50-3.58
(m, 1 H),
3.65-3.75 (m, 1 H), 3.90 (t, J=7Hz, 2H), 4.28 (d, J=8Hz, 1 H), 5.94 (dd,
J=2Hz, 4Hz, 2H),
6.70-6.88 (m, 5H), 7.08-7.25 (m, 3H), 7.48 (d, J=8Hz, 1 H), 8.28 (s, 1 H). MS
(APCI+)
m/e 773 (M+H+). Anal. Calc for C34H40N2~6~ C~ 70.56 H, 7.79 N, 4.33. Found:
C, 70.16 H, 7.70 N, 4.26.
Exam Ip a 869
traps.traps-2-f4-f3-methoxv)aroinyll henyl-4-(1 3-benzodioxol-5-yl~(~N 2 6-
diethvlahenvl)aminocarbonyl)methyl-pyrrc~,iidine-3-carboxylic acid
Example 869A
4-(3-methoxy)~~pylbenzoic acid
To a mixture of lO.Og (46.5 mmol) of methyl 4-bromobenzoate, 325 mg (1.45
mmol) of
palladium(II)acetate, 15.1 g (51.0 mmol) of tetrabutylammonium chloride, and
13.78
(140 mmol) of potassium acetate was added 200 mL of DMF. The mixture was
degassed and back filled with N2 twice, then 10 mL of allyl methyl ether was
added.
The reaction was stirred at 50 °C for 6h, then at ambient temperature
for 66h. The
reaction was monitored by TLC (20% ethyl acetate/ hexanes). Additional Pd
catalyst
(319 mg) was added after this time, as some bromoester remained. After heating
at
50 °C under N2 for 2h, the reaction was poured into 1 L of water, and
this suspension
was divided into two portions for ease of handling. Each was extracted with
diethyl
ether (3 x 100 mL), then each set of ether layers was back extracted with
water (1 x
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
i49
100 mL), saturated aq. NaHC03 solution (1 x 100 mL), and brine (1 x 100 mL),
dried
over MgS04, filtered and concentrated in vacuo. The combined crude yield of
methyl
4-(3-methoxy-1-propenyl)benzoate and methyl 4-{3-methoxy-2-propenyl)benzoate
was 9.17 g. To a mixture of 8.57g (41.6 mmol) of the above and 400 rng of 10%
Pd-C
was added 75 mL of THF. The mixture was stirred at ambient temperature under 1
atm of H2 for 2h, then the catalyst was filtered away, and the solvent was
removed in
vacuo. Purification via a 15% ethyl acetate/hexanes column gave 3.35 g (39%)
of
methyl 4-(3-methoxypropyl)benzoate as a colorless oil. This material was
combined
with 20 mL of 1.39M NaOH in 5:1 ethanol:H20. The reaction was heated at reflux
for
30 min, then concentrated in vacuo. The residue was taken up in 25 mL of H20,
then
40 mL of 1 M HCI was added. The mixture was extracted with diethyl ether (3 x
25
mL), then the combined ether layers were back extracted with brine (1 x 25
mL), dried
over MgS04, filtered, and concentrated in vacuo to 3.03 g (97%) of 4-(3-
methoxy)propylbenzoic acid as a white solid.
Example 869
trans.trans~(4-( -methox~y~pro~yll henyl-4~j1.3-benzodioxol-5-y1-) 1-(fN-2 6
diethyl,~,pyj)aminocarbony~,)methylpyrrrolidine-3-carboxylic acid
Prepared according to the procedures of Example 1. 'H NMR (300 MHz, ds DMSO) 8
1.00 (t, J=7.5Hz, 6H), 1.80 (m, 2), 2.41 (q, J=7.5Hz, 4H), 2.62 (t, J=7.7Hz,
2H), 2.86 (t,
J=9.7Hz, 1 H), 2.93 (d, J=15.8Hz, 1 H), 3.11 (t, J=9.6Hz, 1 H), 3.18 (d,
J=16.2Hz, 1 H),
3.23 {s, 3H), 3.31-3.40 (m, 2H), 3.50 (m, 1 H), 3.53 (m, 1 H), 3.95 (d,
J=9.9Hz, 1 H), 5.98
(s, 2H), 6.80 {dd, J=1.SHz, 8.1 Hz, 1 H), 6.85 (dd, J=1.SHz, 8.1 Hz, 1 H),
7.10 {d,
J=7.OHz, 2H), 7.18 (m, 3H), 7.28 (d, J=1.SHz, 1 H), 7.53 (d, J=8.1 Hz, 1 H),
9.28 (s, 1 H);
MS (CDI m/z) 571 (MH+); Anal. Calcd for C34H40N206 0.05H3P04: C, 70.70, H,
7.00, N,
4.85. Found: C, 70.68, H, 6.91, N, 4.62.
As an indication that the compounds described herein act through binding to
endothelia receptors, the compounds have been evaluated for their ability to
displace
endothelia from its receptor.
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
150
Binding Assay
.~T Rece for
Preparation of membranes from Porcine cerebellum:
Porcine cerebellum was homogenized in 25 volumes (w/v) of 10 mM Hepes
(pH 7.4) containing 0.25 M sucrose and protease inhibitors (3 mM EDTA, 0.1 mM
PMSF, and 5 Ng/ml Pepstatin A) by 3-10 sec polytron at 13,500 rpm with 10 sec
intervals. The mixture was centrifuged at 1000xg for 10 min. The supernatant
was
collected and centrifuged at 30,OOOxg for 30 min. The precipitate was
resuspended
in Buffer A (20 mM Tris, 100 mM NaCI, 10 mM MgCl2, pH 7.4) containing the
aforementioned protease inhibitors and centrifuged again. The final pellet was
resuspended in Buffer A containing protease inhibitors and stored at -
80°C until
used. Protein content was determined by the Bio-Rad dye-binding protein assay.
[251]ET-3 binding to membranes:
Binding assays were performed in 96-well microtiter plates pretreated with
0.1 % BSA. Membranes prepared from cells were diluted 100 fold in Buffer B (20
mM Tris, 100 mM NaCI, 10 mM MgCl2, pH 7.4, with 0.2% BSA, 0.1 mM PMSF, 5
Ng/mL Pepstatin A, 0.025% bacitracin, and 3 mM EDTA) to a final concentration
of 0.2
mg/mL of protein. In competition studies, membranes (0.02 mg) were incubated
with
0.1 nM of [1251]ET-3 in Buffer B (final volume: 0.2 mL) in the presence of
increasing
concentrations of unlabeled ET-3 or a test compound for 4 hours at 25
°C. After
incubation, unbound ligands were separated from bound ligands by a vacuum
filtration method using glass-fiber filter strips in PHD cell harvesters
(Cambridge
Technology, Inc., MA), followed by washing the filter strips with saline (1
mL) for three
times. Nonspecific binding was determined in the presence of 1 NM ET-1. The
data
are shown in Table 1. The per cent inhibition at a concentration of 1 p.M is
shown.
The data show that the compounds of the invention bind to the endothelin
receptor.
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
151
Table 1
Binding Data
Inhibition % Inhibition
Example of Example of
ETg ETg
at 1 ~ at 1 ~IJv,
1 96.4 2 91.5
3 82.1 4 94.0
96.5 6 92.9
7 94.5 8 93.6
9 94.8 10 95.2
11 96.0 12 96.7
13 91.3 14 96.6
93.4 16 92.3
17 97.1 18 94.9
19 94.9 20 95.5
2i 97.1 22 95.3
23 99.1 24 93.3
95.7 26 98.0
27 98.8 28 97.2
29 94.7 30 97.4
858 98.3 859 95.6
860 93.0 861 96.7
862 92.8 863 92.7
864 96.3 865 92.1
866 92.0 867 93.5
868 96.1 869 98.9
5
The ability of the compounds of the invention to lower blood pressure can be
demonstrated according to the methods described in Matsumura, et al., Eur. J.
Pharmacol. 1~5 103 (1990) and Takata, et al., Clin. Exp. Pharmacol. Physiol.
1Q 131
(1983).
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
152
The ability of the compounds of the invention to treat congestive heart
failure
can be demonstrated according to the method described in Margulies, et al.,
Circulation 82 2226 (1990).
The ability of the compounds of the invention to treat myocardial ischemia can
be demonstrated according to the method described in Watanabe, et al., Nature
344
114 (1990).
The ability of the compounds of the invention to treat coronary angina can be
demonstrated according to the method described in Heistad, et al., Circ. Res.
54 711
(1984).
The ability of the compounds of the invention to treat cerebral vasospasm can
be demonstrated according to the methods described in Nakagomi, et al., J.
Neurosurg. 66 915 (1987) or Matsumura, et al., Life Sci. 49 841-848 (1991).
The ability of the compounds of the invention to treat cerebral ischemia can
be
demonstrated according to the method described in Hara et al., European. J.
Pharmacol. 197: 75-82, (1991 ).
The ability of the compounds of the invention to treat acute renal failure can
be
demonstrated according to the method described in Kon, et al., J. Clin.
Invest. 83
1762 (1989).
The ability of the compounds of the invention to treat chronic renal failure
can
be demonstrated according to the method described in Benigni, et al., Kidney
Int. 44
440-444 (1993).
The ability of the compounds of the invention to treat gastric ulceration can
be
demonstrated according to the method described in Wallace, et al., Am. J.
Physiol.
256 6661 (1989).
The ability of the compounds of the invention to treat cyclosporin-induced
nephrotoxicity can be demonstrated according to the method described in Kon,
et al.,
Kidney Int. 37 1487 (1990).
The ability of the compounds of the invention to treat endotoxin-induced
toxicity (shock) can be demonstrated according to the method described in
Takahashi, et al., Clinical Sci. 79 619 (1990).
The ability of the compounds of the invention to treat asthma can be
demonstrated according to the method described in Potvin and Varma, Can. J.
Physiol. and Pharmacol. C7 1213 (1989).
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
153
The ability of the compounds of the invention to treat transplant-induced
atherosclerosis can be demonstrated according to the method described in
Foegh, et
al., Atherosclerosis 7$ 229-236 (1989).
The ability of the compounds of the invention to treat atherosclerosis can be
demonstrated according to the methods described in Bobik, et al., Am. J.
Physiol. 258
C408 (1990) and Chobanian, et al., Hypertension 15 327 (1990).
The ability of the compounds of the invention to treat LPL-related lipoprotein
disorders can be demonstrated according to the method described in Ishida, et
al.,
Biochem. Pharmacol. 44 1431-1436 (1992).
The ability of the compounds of the invention to treat proliferative diseases
can
be demonstrated according to the methods described in Bunchman ET and CA
Brookshire, Transplantation Proceed. 2~ 967-968 (1991 ); Yamagishi, et al.,
Biochem.
Biophys. Res. Comm. 191 840-846 (1993); and Shichiri, et al., J. Clin. Invest.
87
1867-1871 (1991 ). Proliferative diseases include smooth muscle proliferation,
systemic sclerosis, cirrhosis of the liver, adult respiratory distress
syndrome,
idiopathic cardiomyopathy, lupus erythematosus, diabetic retinopathy or other
retinopathies, psoriasis, scleroderma, prostatic hyperplasia, cardiac
hyperplasia,
restenosis following arterial injury or other pathologic stenosis of blood
vessels.
The ability of the compounds of the invention to treat acute or chronic
pulmonary hypertension can be demonstrated according to the method described
in
Bonvallet et al., Am. J. Physiol. ?~ H1327 (1994). Pulmonary hypertension can
be
associated with congestive heart failure, mitral valve stenosis, emphysema,
lung
fibrosis, chronic obstructive pulmonary disease (COPD), acute repiratory
distress
syndrome CARDS), altitude sickness, chemical exposure, or may be idiopathic.
The ability of the compounds of the invention to treat plaletet aggregation,
and
thrombosis, can be demonstrated according to the method described in McMurdo
et
al. Eu. J. Pharmacol. ?~ 51 (1994).
The ability of the compounds of the invention to treat cancers can be
demonstrated according to the method described in Shichiri, et al., J. Clin.
Invest. 87
1867 (1991 ).
The ability of the compounds of the invention to treat adenocarcinoma can be
demonstrated according to the method described in Nelson, et al., Nature
Medicine,
1_, (9), 944 (1995).
The ability of the compounds of the invention to treat IL-2 (and other
cytokine)
mediated cardiotoxicity and vascular permeability disorders can be
demonstrated
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
154
according to the method described in Klemm et al., Proc. Nat. Acad. Sci. 92
2691
(1995).
The ability of the compounds of the invention to treat nociception can be
demonstrated according to the method described in Yamamoto et al., J.
Pharmacol.
Exp. Therap. 271 156 (1994).
The ability of the compounds of the invention to treat colitis can be
demonstrated according to the method described in Hogaboam et al (EUR. J.
Pharmacol. 1996, ~9, 261-269}.
The ability of the compounds of the invention to treat ischemia-repurfusion
injury in kidney transplantation can be demonstrated according to the method
described in Aktan et al (Transplant Int 1996, 9_, 201-207).
The ability of the compounds of the invention to treat angina, pulmonary
hypertension, Raynaud's disease, and migraine can be demonstrated according to
the method described in Ferro and Webb (Drugs 1996, 51,12-27).
The compounds of the present invention can be used in the form of salts
derived from inorganic or organic acids. These salts include but are not
limited to the
following: acetate, adipate, alginate, citrate, aspartate, benzoate,
benzenesulfonate,
bisulfate, butyrate, camphorate, camphorsulfonate, digluconate,
cyclopentanepropionate, dodecylsulfate, ethanesulfonate, gfucoheptanoate,
glycerophosphate, hemisulfate, heptanoate, hexanoate, fumarate, hydrochloride,
hydrobromide, hydroiodide, 2-hydroxy-ethanesuffonate, lactate, maleate,
methanesulfonate, nicotinate, 2-naphthalenesulfonate, oxalate, pamoate,
pectinate,
persulfate, 3-phenylpropionate, picrate, pivalate, propionate, succinate,
tartrate,
thiocyanate, p-toluenesulfonate and undecanoate. Aiso, the basic nitrogen-
containing groups can be quaternized with such agents as loweralkyl halides,
such
as methyl, ethyl, propyl, and butyl chloride, bromides, and iodides; dialkyl
sulfates
like dimethyl, diethyl, dibutyl, and diamyl sulfates, long chain halides such
as decyl,
lauryl, myristyl and stearyl chlorides, bromides and iodides, aralkyl halides
like
benzyl and phenethyl bromides, and others. Water or oil-soluble or dispersible
products are thereby obtained.
Examples of acids which may be employed to form pharmaceutically
acceptable acid addition salts include such inorganic acids as hydrochloric
acid,
sulphuric acid and phosphoric acid and such organic acids as oxalic acid,
malefic
acid, succinic acid and citric acid. Basic addition salts can be prepared in
situ during
the final isolation and purification of the compounds of formula (I), or
separately by
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
155
reacting the carboxylic acid function with a suitable base such as the
hydroxide,
carbonate or bicarbonate of a pharmaceutically acceptable metal cation or with
ammonia, or an organic primary, secondary or tertiary amine. Such
pharmaceutically
acceptable salts include, but are not limited to, cations based on the alkali
and
alkaline earth metals, such as sodium, lithium, potassium, calcium, magnesium,
aluminum salts and the like, as well as nontoxic ammonium, quaternary
ammonium,
and amine cations, including, but not limited to ammonium,
tetramethylammonium,
tetraethyiammonium, methylamine, dimethylamine, trimethylamine, triethyfamine,
ethylamine, and the like. Other representative organic amines useful for the
1o formation of base addition salts include diethylamine, ethylenediamine,
ethanolamine, diethanolamine, piperazine and the like.
The compounds of the invention are useful for antagonizing endothelin in a
human or other mammal. In addition, the compounds of the present invention are
useful (in a human or other mammal) for the treatment of hypertension, acute
or
chronic pulmonary hypertension, Raynaud's disease, congestive heart failure,
myocardial ischemia, reperfusion injury, coronary angina, cerebral ischemia,
cerebral vasospasm, chronic or acute renal failure, non-steroidal
antiinflammatory
drug induced gastric ulceration, cyclosporin induced nephrotoxicity, endotoxin-
induced toxicity, asthma, fibrotic or proliferative diseases, including smooth
muscle
proliferation, systemic sclerosis, cirrhosis of the liver, adult respiratory
distress
syndrome, idiopathic cardiomyopathy, lupus erythematosus, diabetic retinopathy
or
other retinopathies, psoriasis, scieroderma, prostatic hyperplasia, cardiac
hyperplasia, restenosis following arterial injury or other pathologic stenosis
of blood
vessels, LPL-related lipoprotein disorders, transplantation-induced
atherosclerosis or
atheroscferosis in general, platelet aggregation, thrombosis, cancers,
adenocarcinoma, IL-2 and other cytokine mediated cardiotoxicity and
permeability
disorders, colitis, migraine, and nociception.
Total daily dose administered to a host in single or divided doses may be in
amounts, for example, from 0.001 to 1000 mg/kg body weight daily and more
usually
0.1 to 100 mg/kg for oral administration or 0.01 to 10 mg/kg for parenteral
administration. Dosage unit compositions may contain such amounts of
submultiples
thereof to make up the daily dose.
The amount of active ingredient that may be combined with the carrier
materials to produce a single dosage form will vary depending upon the host
treated
and the particular mode of administration.
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
156
It will be understood, however, that the specific dose level for any
particular
patient will depend upon a variety of factors including the activity of the
specific
compound employed, the age, body weight, general health, sex, diet, time of
administration, route of administration, rate of excretion, drug combination,
and the
severity of the particular disease undergoing therapy.
The compounds of the present invention may be administered orally,
parenterally, sublingually, by inhalation spray, rectally, or topically in
dosage unit
formulations containing conventional nontoxic pharmaceutically acceptable
carriers,
adjuvants, and vehicles as desired. Topical administration may also involve
the use
of transdermal administration such as transdermal patches or iontophoresis
devices.
The term parenteral as used herein includes subcutaneous injections,
intravenous,
intramuscular, intrasternal injection, or infusion techniques.
Injectable preparations, for example, sterile injectable aqueous or oleagenous
suspensions may be formulated according to the known art using suitable
dispersing
or wetting agents and suspending agents. The sterile injectable preparation
may
also be a sterile injectable solution or suspension in a nontoxic parenterally
acceptable diluent or solvent, for example, as a solution in 1,3-propanediol.
Among
the acceptable vehicles and solvents that may be employed are water, Ringer's
solution, and isotonic sodium chloride solution. In addition, sterile, fixed
oils are
conventionally employed as a solvent or suspending medium. For this purpose
any
bland fixed oil may be employed including synthetic mono- or diglycerides. In
addition, fatty acids such as oleic acid find use in the preparation of
injectables.
Suppositories for rectal administration of the drug can be formulated
according
to the known art. The title compound was prepared by mixing the drug with a
suitable
nonirritating excipient such as cocoa butter and polyethylene glycols which
are solid
at ordinary temperatures but liquid at the rectal temperature and will
therefore melt in
the rectum and release the drug.
Solid dosage forms for oral administration may include capsules, tablets,
pills,
powders, and granules. In such solid dosage forms, the active compound may be
admixed with at least one inert diluent such as sucrose lactose or starch.
Such
dosage forms may also comprise, as is normal practice, additional substances
other
than inert diluents, e.g., lubricating agents such as magnesium stearate. In
the case
of capsules, tablets, and pills, the dosage forms may also comprise buffering
agents.
Tablets and pills can additionally be prepared with enteric coatings.
CA 02292604 1999-12-02
WO 98/57933 PCT/US98/11821
157
Liquid dosage forms for oral administration may include pharmaceutically
acceptable emulsions, solutions, suspensions, syrups, and elixirs containing
inert
diluents commonly used in the art, such as water. Such compositions may also
comprise adjuvants, such as wetting agents, emulsifying and suspending agents,
and sweetening, flavoring, and perfuming agents.
The compounds of the present invention can also be administered in the form
of liposomes. As is known in the art, liposomes are generally derived from
phospholipids or other lipid substances. Liposomes are formed by mono- or
multi-
lamellar hydrated liquid crystals that are dispersed in an aqueous medium. Any
non-
toxic, physiologically aceptable and metabolizable lipid capabale of forming
liposomes can be used. The present compositions in liposome form can contain,
in
addition to a compound of the present invention, stabilizers, preservatives,
excipients, and the like. The preferred lipids are the phospholipids and
phosphatidyl
cholines (lecithins), both natural and synthetic.
Methods to form liposomes are known in the art. See, for example, Prescott,
Ed., Methods in Cell Bioloav, Volume XIV, Academic Press, New York, N.Y.
(1976), p.
33 et seq.
A representative solid dosage form, for example, a tablet or a capsule,
comprises:
Compound of the invention: 35% w/w
Starch, Pregelatinized, NF50% w/w
Microcrystalline Cellulose, NF 10% w/w
Talc, Powder, USP 5% w/w
While the compounds of the invention can be administered as the sole active
pharmaceutical agent, they can also be used in combination with one or more
cardiovascular agents independently selected from diuretics, adrenergic
blocking
agents, vasodilators, calcium channel blockers, renin inhibitors, angiotensin
converting enzyme (ACE) inhibitors, angiotensin 11 antagonists, potassium
channel
activators and other cardiovascular agents.
Representative diuretics include hydrochlorothiazide, chlorothiazide,
acetazolamide, amiloride, bumetanide, benzthiazide, ethacrynic acid,
furosemide,
indacrinone, metolazone, spironolactone, triamterene, chlorthalidone and the
like or
a pharmaceutically acceptable salt thereof.
Representative adrenergic blocking agents include phentoiamine,
phenoxybenzamine, prazosin, terazosin, tolazine, atenolol, metoprolol,
nadolol,
CA 02292604 1999-12-02
WO 98157933 PCT/US98/1182I
158
propranolol, timolol, carteolol and the like or a pharmaceutically acceptable
salt
thereof.
Representative vasodilators include hydralazine, minoxidil, diazoxide,
nitroprusside and the like or a pharmaceutically acceptable salt thereof.
Representative calcium channel blockers include amrinone, bencyclane,
diltiazem, fendiline, flunarizine, nicardipine, nimodipine, perhexilene,
verapamil,
gallopamil, nifedipine and the like or a pharmaceutically acceptable salt
thereof.
Representative renin inhibitors include enalkiren, zankiren,
RO 42-5892, PD-134672 and the tike or a pharmaceutically acceptable salt
thereof.
Representative angiotensin II antagonists include
DUP 753, A-81988 and the like.
Representative ACE inhibitors include captopril, enalapril, lisinopril and the
like or a pharmaceutically acceptable salt thereof.
Representative potassium channel activators include pinacidil and the like or
a
pharmaceutically acceptable salt thereof.
Other representative cardiovascular agents include sympatholytic agents such
as methyldopa, cionidine, guanabenz, reserpine and the like or a
pharmaceutically
acceptable salt thereof.
The compounds of the invention and the cardiovascular agent can be
administered at the recommended maximum clinical dosage or at lower doses.
Dosage levels of the active compounds in the compositions of the invention may
be
varied so as to obtain a desired therapeutic response depending on the route
of
administration, severity of the disease and the response of the patient. The
combination can be administered as separate compositions or as a single dosage
form containing both agents.
When administered as a combination, the therapeutic agents can be
formulated as separate compositions which are given at the same time or
different
times, or the therapeutic agents can be given as a single composition.
The foregoing is merely illustrative of the invention and is not intended to
limit
the invention to the disclosed compounds, processes, compositions and methods.
Variations and changes which are obvious to one skilled in the art are
intended to be
within the scope and nature of the invention which are defined in the appended
claims.