Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02293399 2004-11-01
78348-2
Systems and Methods for Modifying Ice Adhesion Strength
Field of the Invention
The invention relates to methods and apparatus for
modifying ice adhesion strength between ice and selected
materials. More particularly, the invention relates to
systems and methods which apply electrical energy to the
interface between ice and such materials so as to either
increase or decrease the ice adhesion strength to facilitate
desired results.
Background
Ice adhesion to certain surfaces causes many
problems. For example, excessive ice accumulation on
aircraft wings endangers the plane and its passengers. Ice
on ship hulls creates navigational difficulties, the
expenditure of additional power to navigate through water
and ice, and certain unsafe conditions. The need to scrape
ice that forms on automobile windshields is regarded by most
adults as bothersome and recurring chore; and any residual
ice risks driver visibility and safety.
1
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
1 Icing and ice adhesion also causes problems with helicopter blades, and with
2 public roads. Billions of dollars are spent on ice and snow removal and
control. Ice also
3 adheres to metals, plastics, glasses and ceramics, creating other day-to-day
difficulties.
4 Icing on power lines is also problematic. Icing adds weight to the power
lines
which causes power outages, costing billions of dollars in direct and indirect
costs.
6 In the prior art, methods for dealing with ice adhesion vary, though most
7 techniques involve some form of scraping, melting or breaking. For example,
the aircraft
8 industry utilizes a de-icing solution such as Ethyl Glycol to douse aircraft
wings so as to
9 melt the ice thereon. This process is both costly and environmentally
hazardous; however,
lo the risk to passenger safety warrants its use. Other aircraft utilize a
rubber tube aligned
11 along the front of the aircraft wing, whereby the tube is periodically
inflated to break any
12 ice disposed thereon. Still other aircraft redirect jet engine heat onto
the wing so as to
13 melt the ice.
14 These prior art methods have limitations and difficulties. First, prop-
propelled
aircraft do not have jet engines. Secondly, rubber tubing on the front of
aircraft wings is
16 not aerodynamically efficient. Third, de-icing costs are extremely high, at
$2500-$3500
17 per application; and it can be applied up to about ten times per day on
some aircraft!
18 The above-referenced problems generally derive from the propensity of ice
to stick
19 and form onto surfaces. However, ice also creates difficulties in that it
has an extremely
low coefficient of friction. Each year, for example, ice on the roadway causes
numerous
21 automobile accidents, costing both human life and extensive property
damage. If
22 automobile tires gripped ice more efficiently, there would likely be fewer
accidents.
2
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
l It is, accordingly, an object of the invention to provide systems and
methods which
2 modify ice adhesion strength beneficially.
3 A further object of the invention is to provide systems for reducing ice
adhesion on
= 4 vehicle surfaces such as aircraft wings, ship hulls and windshields to
facilitate ice removal.
Still another object of the invention is to provide systems for increasing the
6 coefficient of friction between ice-clad roads and automobile tires, and
between ice and
7 other objects such as shoe soles and cross-country skis.
8 These and other objects will become apparent in the description which
follows.
9 Summary of the Invention
Certain of above-referenced problems would be lessened if the ice adhesion
11 strength were decreased between the ice and the surface upon which the ice
forms. For
12 example, if the adhesion strength between the ice and an aircraft wing were
decreased
13 sufficiently, wind pressure, buffeting or light manual brushing would
remove the ice from
14 the wing. Similarly, scraping an automobile windshield so as to be free of
ice would be
much less difficult if the ice adhesion strength between the ice and the
windshield were
16 lessened.
17 Other above-referenced problems would be lessened if the ice adhesion
strength
18 between ice and surfaces in contact with the ice were increased. For
example, if the ice
t9 adhesion strength were increased between automobile tires and icy roadways,
then there
would be less slippage and fewer accidents.
21 Ice has certain physical properties which allow the present invention to
selectively
22 modify the adhesion of ice to conductive (and semi-conductive) surfaces.
First, ice is a
3
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
I protonic semiconductor, a small class of semiconductors whose charge
carriers are protons
2 rather than electrons. This phenomenon results from hydrogen bonding within
the ice.
3 Hydrogen bonding occurs because the hydrogen atoms of water molecules in ice
share
4 their electrons with an oxygen atom. Thus, the nucleus of the water molecule
- uniquely a
single proton - remains available to bond with adjacent water molecules.
6 Similar to typical electron-based semiconductors, ice is electrically
conductive.
7 While this electrical conductivity is generally weak, the conductivity can
be altered by
8 adding chemical agents that donate or accept extra charge-carrying
particles, i.e., protons
9 in the case of ice.
Another physical property of ice is its evaporability. Evaporability of a
substance
11 is a function of vapor pressure at the substance surface. In most
materials, vapor pressure
12 drops rapidly at the liquid-to-solid interface. In ice, however, there is
virtually no change
13 in vapor pressure at the liquid-to-solid interface. The reason for this is
that the surface of
14 ice is covered with a liquid-like layer ("LLL").
The LLL has important physical characteristics. First, the LLL is only
nanometers
16 thick. Second, it ranges in viscosity from almost water-like, at
temperatures at or near to
17 freezing, to very viscous at lower temperatures. Further, the LLL exists at
temperatures as
0
18 low as -100 C, and thus practically exists for most temperatures around the
planet.
19 The LLL is also a major factor of ice adhesion strength. For example, if
one brings
the smooth surface of ice in contact with the smooth surface of an airplane
wing, the
21 actual area of contact between the two surfaces is on the order of one-
thousandth of the
22 total interface area between the two surfaces. The LLL functions as a
wetting substance
23 between the surfaces - the principal behind almost all adhesives - and
substantially
4
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
1 increases the effective contact area between the surfaces. This increase in
contact area
2 strongly affects ice adhesion.
3 The combination of the semiconductive properties of ice and the LLL allows-
one
4 to selectively manipulate ice adhesion strength between ice and other
surfaces. Generally,
water molecules within a piece of ice are randomly oriented. On the surface,
however, the
6 molecules are substantially oriented in the same direction, either outward
or inward. As a
7 result, all their protons, and hence the positive charges, either face
outward or inward.
8 While the exact mechanism is unknown, it is likely that the randomness of
water
9 molecules transitions to an ordered orientation within the LLL. However, the
practical
lo result of the ordering is that a high density of electrical charges, either
positive or negative,
t t occurs at the surface. Accordingly, if a charge is generated on the
surface coming on
12 contact with ice, it is possible to selectively modify the adhesion between
the two surfaces.
13 As like charges repel and opposites attract, an externally applied
electrical bias at the
14 interface of the ice and the other surface either reduces or enhances the
adhesion between
the ice and the surface.
16 In one aspect, the invention provides a power source connected to apply a
DC
17 voltage across the interface between ice and the surface upon which the ice
forms. By
18 way of example, the conductive surface can be an aircraft wing or a ship's
hull (or even the
t9 paint applied to the structure). A first electrode connects with the
surface; a
nonconductive or electrically insulating material is applied as a grid over
the surface; and
21 a second electrode is formed by applying a conductive material, for example
conductive
22 paint, over the insulating material, but without contacting the surface.
The surface area of
23 the second electrode should be small as compared to the overall surface
area protected by
5
CA 02293399 1999-12-09
WO 98/57851 PCTIUS98/12421
1 the system. By way of example, the surface area under protection (i.e., that
area sought to
2 be "ice-free") should be at least about ten times larger than the surface
area of the second
3 electrode.
4 One or more wires connect the second electrode to the power source; while
one or
more wires connect the first electrode to the power source. Ice forming over
the surface
6 and the conductive grid second electrode completes the circuit. A voltage is
then applied
7 to the circuit, selectively, which controllably modifies the ice adhesion
strength of the ice
8 with the surface.
9 A voltage regulator subsystem also preferably connects with the circuit so
as to
adjustably control the voltage applied across the interface and so as to
achieve control over
11 the ice adhesion strength. By way of example, ice made from different
concentrations of
12 ions can modify the optimum voltage for which the ice adhesion strength is
at a minimum;
13 and the voltage regulator subsystem thereby provides a mechanism by which
the minimum
14 can be changed selectively.
Other subsystems preferably connect with the circuit to provide other
features, for
16 example to detect whether water or ice completes the circuit. In one
aspect, the power
17 source is a DC supply (e.g., a battery) which provides voltage to the
circuit and which
18 connects to the deicing electrodes. In another aspect, a DC ammeter
connects with the
19 circuit to measure the DC conductivity of the ice (i.e., the semi-
conductive layer which
"shorts" the two electrodes when formed over the surface and any part of the
grid second
21 electrode). In another aspect, an AC supply connects with the circuit to
generate AC
22 voltages between about 10kHz and 100kHz, selectively. According to another
aspect, an
23 AC ammeter also connects with the circuit to measure the AC conductivity of
the ice at --
6
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
1 frequencies within the 10-1001cHz range. In still another aspect, a current
comparator
2 compares the AC and DC conductivities.
3 These aspects thus provide circuitry which can, for example, distinguish
whether
4 the semi-conductive layer formed over the surface is ice, which might be
dangerous, or
surface water. The AC conductivity (in the above-mentioned range) and DC
conductivity
6 of water are substantially the same. With respect to ice, however, the AC
conductivity and
7 DC conductivity differ by two to three orders of magnitude. This difference
in
8 conductivity is measured by the respective ammeters and is compared in the
current
9 comparator. When the difference in conductivity is greater than a
predetermined set point,
the current comparator signals an icing alarm. At this point, for example, the
voltage
11 regulator subsystem can operate to apply a DC bias to the circuit - and
thus to the
12 interface - at a desired field strength which sufficiently reduces the ice
adhesion strength.
13 According to one aspect of the invention, when ice is detected on an
aircraft wing, the
14 icing alarm initiates a feedback loop within the system which (a) measures
ice
conductivities, (b) determines appropriate bias voltages to reach minimum (or
near
16 minimum) ice adhesion conditions, and (c) applies a bias voltage to the ice-
wing interface
17 to facilitate ice removal.
1 s Those skilled in the art should appreciate that the above-described system
can be
19 applied to many surfaces where it is desired to reduce ice adhesion
strength, such as on
car windshields, ship hulls and power lines. In such cases, if the surface
material is
21 weakly conductive, it is desirable to "dope" the surface material such that
it is sufficiently
22 conductive. Doping techniques are known to those in the art. Automobile
tires, for
23 example, can be doped with iodine to make the rubber conductive. Automobile
glass,
7
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
1 likewise, can be doped with either ITO or fluoride doped Sn02 to make the
windshield an
2 acceptable semiconductor.
3 However, in another aspect, the above described system and circuit are -also
4 applicable to situations where it is desirable to increase the ice adhesion
strength. In this
aspect, for example, when the icing alarm detects ice, the system activates
the feedback
6 loop to regulate applied DC voltages to the interface so as to increase ice
adhesion.
7 Situations and surfaces which can benefit from this system include, for
example, the
8 bottom soles of a person's shoe (or shoes) and car tires on icy roads.
9 In still another aspect, the invention can include a variable ice
adhesion/voltage
1o control subsystem which increases and then decreases ice adhesion strength
between ice
1 i and a surface, selectively. By way of example, cross country skis (or
telemarking skis)
12 ideally have higher friction when climbing an incline (or when descending
an incline, in
13 certain situations) and have lower friction when "skiing" down an incline.
According to
14 one aspect of the invention, the ice adhesion system and circuit described
herein is
attached in circuit with the skis and the operator can adjustably control ski
friction
16 selectively.
17 In another aspect, the invention provides methodology for reducing the
adhesion
18 strength between an automobile windshield (or any window) so that ice is
easily removed.
i9 In this method, the window or windshield is doped so as to make the window
a semi-
conductor (i.e., so that the window is conductive to electricity). A first
electrode is
21 attached to the window; and a second grid electrode is suspended over the
windshield and
22 without contacting the windshield. By way of example, the second electrode
can be
23 applied to an electrically insulating grid between the grid electrode and
the windshield.
8
CA 02293399 2006-01-26
78348-2
The insulator and second electrode are preferably
transparent so users can see through the window. As ice
forms on the windshield, a current path is created from the
second electrode and the window (and hence the first
electrode). Ice thus "shorts" the circuit when formed over
the window and any part of the grid. A voltage is then
applied across the electrodes so that the ice adhesion
strength is reduced, as discussed herein. The area of the
electrodes is preferably much less than the overall area of
the interface between the ice and the windshield.
In still another aspect, a method is provided for
increasing the coefficient of friction between an automobile
tire and an icy road. An AC, high voltage power source is
connected with an automobile such that a voltage potential
is imparted to the interface between the road and the tire.
Typically, the AC has a frequency of between about 1kHz and
1000kHz. The tire is manufactured with a conductive
material disposed therein, such as carbon, or doped such as
with iodine, so that current flows through the tire. A
voltage potential is then selected and applied to increase
the adhesion strength of the ice relative to the tire,
increasing the tire's traction on the icy road.
A broad aspect of the invention provides a system
for modifying ice adhesion strength of ice adhered to a
surface, comprising: an electrode electrically insulated
from the surface, a DC source coupled to the surface and the
electrode to generate a DC bias to an interface between the
ice and the surface, the DC bias having a voltage which
modifies the ice adhesion strength selectively as compared
to the ice adhesion strength with substantially zero bias
voltage at the interface.
9
CA 021293399 2004-11-01
78348-2
Other useful background to the invention may be
found with reference to the following papers: Petrenko, The
Effect of Static Fields on Ice Friction, J. Appl. Phys.
76(2), 1216-1219 (1994); Petrenko, Generation of Electric
Fields by Ice and Snow Friction, J. Appl. Phys. 77(9), 4518-
4521 (1995); Khusnatdinov et al., Electrical Properties of
the Ice/So1id Interface, J. Phys. Chem. B, 101, 6212-6214
(1997); Petrenko, Study of the Surface of Ice, Ice/So1id and
Ice/Liquid Interfaces with Scanning Force Microscopy,
J. Phys. Chem. B, 101, 6276-6281 (1997); Petrenko et al.,
Surface States of Charge Carriers and Electrical Properties
of the Surface Layer of Ice, J. Phys. Chem. B, 101, 6285-
6289 (1997); and Ryzhkin et al.,
9a
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
1 Physical Mechanisms Responsible for Ice Adhesion. J. Phys. Chem. B, 101,
6267-6270
2 (1997).
3 The invention is next described further in connection with preferred
embodiments,
4 and it will become apparent that various additions, subtractions, and
modifications can be
made by those skilled in the art without departing from the scope of the
invention.
6 Brief Description of the Drawings
7 A more complete understanding of the invention may be obtained by reference
to
8 the drawings, in which:
9 FIGs. 1A - 1D graphically show spatial distributions of electrical charge
densities
p(x) near ice-air and ice-metal interfaces;
I I FIGs. 2A-2C illustrate effects of DC bias on ice adhesion to a liquid
metal
12 (Mercury), smaller contact angles O indicating stronger adhesion;
13 FIG. 3 schematically illustrates an ice manometer used in measurements of
ice-
14 mercury interfacial energy such as illustrated in FIG. 2;
FIG. 4 graphically illustrates experimental results of DC bias versus ice-Hg
16 interfacial energy for ice doped with 0.5% NaCI, T=-10 C;
17 FIG. 5 shows a graph illustrating electrostatic energy of a screening layer
of ice
18 per surface unit, We, vs. surface potential, Vs (T =-10 C);
19 FIG. 6 shows a graph illustrating adhesive energy of an ice-metal interface
per
surface unit, Wa, as a function of distance, z, curves 1, 2, and 3
corresponding to perfect
-----------------
CA 02293399 1999-12-09
WO 98/57851 PCT/LJS98/12421
1 occupancy by D defects, H30+ ions, and protons, respectively (fixed
occupancy curves)
2 and curve 4 depicting equilibrium dependence of adhesion energy on distance
for proton
3 surface states (T = -I 0 C);
4 FIG. 7 shows a graph illustrating the occupancy coefficient of surface
states for
D defects, f, as a function of surface states energy, Es (T =-10 C);
6 FIG. 8 schematically shows an evaluation system constructed according to the
7 invention and used to measure effects of DC bias on ice adhesion to
stainless steel;
8 FIG. 9 graphically illustrates shear stress vs. time for an ice-stainless
steel
9 interface measured with the system of FIG. 8 with no voltage applied to the
mobile steel
electrode, the ice samples grown from 0.5% solution of NaCl in distilled water
and tested
11 at -10 C under constant strain rate of 100 m/min;
12 FIG. 10 graphically illustrates shear stress vs. time for an ice-stainless
steel
13 interface measured with the system of FIG. 8 with +6.6 V applied to the
mobile steel
14 electrode, the ice sample grown from 0.5% solution of NaCI in distilled
water and tested at
-10 C under constant strain rate of 100 m/min;
16 FIG. 11 graphically illustrates shear stress vs. time for an ice-stainless
steel
17 interface measured with the system of FIG. 8 with -1.8 V applied to the
mobile steel
18 electrode, the ice sample grown from 0.5% solution of NaCI in distilled
water and tested at
19 -10 C under constant strain rate of 100 m/min;
FIG. 12 graphically illustrates the effect of +6.6 V on the interfacial
strength using
2 t data of FIGs. 9 and 10; FIGs. 12A and 12B graphically illustrate
experimental data of -
22 10 C ice doped with 0.5% NaCI to assess relative strength of an ice/steel
interface; FIG.
11
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
1 12C illustrates how production of gas bubbles at an ice/metal interface
functions as an
2 interfacial crack to reduce interfacial strength;
3 FIG. 13 shows a graph of experimental results including AWA = 0(W;ia -
W;nig)
4 and the current I versus DC bias V, ice being grown from water doped with
0.5% NaCI, T
2
=-10 C, and WA(0) = 400 10 mJ/m ;
6 FIG. 14 shows a graph of experimental results with AWA = A (Wi/a - WVHg) and
7 the current I versus DC bias V, ice being grown from water doped with 0.18%
HF, T=-
2
8 10 C, and WA(0) = 360 15 mJ/m
9 FIG. 15 shows a graph of experimental results with AWA = 0(W;/a - W;/lig)
and
1o the current I versus DC bias V, ice being grown from water doped with 0.2%
KOH, T=-
2
t 1 10 C, and WA(0) = 293 25 mJ/m ;
12 FIG. 16 shows a graph of experimental results, including current versus
time for
13 an ice specimen grown from a 0.2% solution of KOH, T=-10 C, where -1 V was
applied
14 to the mercury;
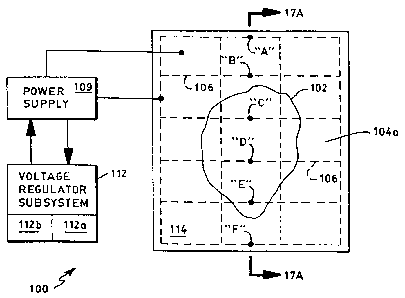
FIG. 17 illustrates one system constructed according to the invention for
16 modifying ice adhesion to a generic conductive (or semiconductor) material;
17 FIG. 17A shows a cross-sectional view (not to scale) of the system of FIG.
17;
18 FIG. 18 illustrates one system of the invention for decreasing the ice
adhesion
i 9 strength of ice that forms on an aircraft wing;
12
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
1 FIG. 19 illustrates a conductive paint/insulating lacquer grid constructed
according
2 to the invention and for forming over an aircraft wing;
3 FIG. 20 illustrates one other embodiment of the invention for modifyingr ice
4 adhesion strength of ice adhered to an aircraft wing;
FIG. 21 schematically illustrates a system constructed according to the
invention
6 for modifying ice adhesion strength between an automobile tire and an icy
road;
7 FIG. 22 shows one other system constructed according to the invention for
s imparting a voltage to the interface between an automobile tire and an icy
surface for
9 increasing the coefficient of friction therebetween;
FIG. 23 illustrates a system for modifying ice adhered to a car windshield;
and
i l FIG. 23A shows an alternative embodiment;
12 FIG. 24 shows one embodiment of the invention for reducing ice adhesion to
13 power lines; and FIG. 24A illustrates a cross-sectional view (not to scale)
of a power line
14 constructed according to the invention;
FIG. 25 shows an embodiment of the invention for altering the ice adhesion,
16 selectively, to a ski so as to increase or decrease friction to snow and/or
ice;
17 FIG. 26 illustrates a shoe heel and sole constructed according to the
invention for
18 increasing ice/snow adhesion to the sole and/or heel to increase shoe
traction;
19 FIG. 27 illustrates one system of the invention for removing ice and snow
from
power lines through application of a coating on the power line; and
13
CA 021293399 2004-11-01
78348-2
FIG. 28 illustrates application of a ferroelectric
coating onto a non-active surface, in accord with the
invention, to remove ice therefrom.
Detailed Description of the Drawings
The invention includes systems and methods which
modify ice adhesion strength to materials such as metals and
semiconductors by application of a DC bias to the interface
between the ice and the materials. The invention can thus
be used to reduce and in some cases eliminate the adhesion
of ice onto such materials.
In certain embodiments, the invention modifies the
electrostatic interactions which form the bonding between
ice and metals. These interactions are effectively changed
(either reduced or enhanced) by application of a small DC
(direct current) bias between the ice and the metals.
Experimentation and theoretical calculations have
shown that ice surfaces have high density electrical charges
of 10-2C/m2 to 3= 10-2C/m2 . See, Petrenko et al., Generation
of Electric Fields in Ice and Snow Friction, J. App1. Phys.,
77(9):4518-21 (1995); Petrenko, A Study of the Surface of
Ice, Ice/Solid and Ice/Liquid Interfaces with Scanning Force
Microscopy, J. Phys. Chem. B, 101, 6276 (1997); and Dosch
et al., Surface Science 366, 43 (1996). This charge density
originates from the strong polarization of water molecules
in the ice subsurface layer. These phenomena are further
illustrated in FIG. 1.
FIGs. lA-1D illustrate a relationship between
molecular polarization P and space charge density p as a
function of distance from an ice-air interface (FIGs. lA-1C)
or an ice-metal interface (FIG 1D). In FIG. 1D, an
electrical charge induced in a metal is equal in magnitude
14
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
I FIG. 1 refers to distance x relative to "L" as the screening length. FIG. 1A
also illustrates
2 water molecule polarization P (along the vertical axis 12a) near the
surface; while FIG. 1B
3 shows charge density p (along the vertical axis 12b) of the polarization
charge p(x) _-
4 dP/dx without screening. FIG. 1C illustrates charge density p (along the
vertical axis 12c)
of the polarization P but with additional screening by minority charge
carriers. FIG. 1D
6 graphs charge density p (relative to the vertical axis 12d) within ice near
the ice-metal
7 interface (data 14a) and within metal or dielectric material (data 14b) near
the same
8 interface.
9 The interaction between ice surface charges and the charge induced in a
solid
affects the strength of an ice-solid interface. By estimation, the
electrostatic attraction
I 1 (negative pressure Pel) of two plane surface charges is given by:
:
12 Pe1= ~"2 (1)
13 where Eo is the dielectric permittivity of the vacuum, and E is the
electric field strength in
14 the space between the charges. Since the charge distribution shown in FIG.
ID determines
the contact potential Vc of the two materials, we can estimate E as VC/L,
where L is the
16 distance between the plane charges located in the ice and in the solid. V.
for ice-metal
17 interfaces varies from a few tenths of a volt to about 1 V. See, Buser et
al., Charge
18 Separation by Collision of Ice Particles on Metals: Electronic Surface
States, Journal of
19 Glaciology, 21(85): 547-57 (1978), which is incorporated herein by
reference.
Taking L,& lnm (the main screening length in the doped ice illustrations
above),
21 E= 3.2 (the high-frequency dielectric constant of ice) and Vc = 0.5 V (the
typical
22 magnitude of a contact potential), equation (1) provides that Pei ;t~ 3.3
Mpa, a magnitude
CA 021293399 2004-11-01
78348-2
Taking L- 1 nm (the main screening length in the
doped ice illustrations above), E= 3.2 (the high-frequency
dielectric constant of ice) and Vc = 0.5 V (the typical
magnitude of a contact potential), equation (1) provides
that Pel ~ 3.3 MPa, a magnitude comparable with, but
exceeding, the macroscopic tensile strength of ice
at 1.5 MPa. See, Schulson et al., A Brittle to Ductile
Transition in Ice Under Tension, Phil. Mag., 49, 353-63
(1984).
More sophisticated calculations of the
electrostatic interaction energy between ice surface charges
and metals are shown below, utilizing real space-charge
distributions and charge relaxation calculations.
Specifically, it is shown below that this interaction energy
is 0.01 to 0.5 J/m2 at -10 C. The lower limit 0.01 J/mZ
corresponds to pure ice; while the upper value 0.5 J/m2
corresponds to heavy doping. These values are comparable
with other experimental results, described below, which
utilized scanning force microscopy ("SFM"). The SFM results
determined an electrostatic interaction energy of
0.08 0.012 J/m2; and experiments on ice/mercury interfaces
return 0.150 +/- 0.015 J/mz for that electrostatic part of
ice/metal adhesion.
Since electrostatic interactions contribute to ice
adhesions, the adhesion strength between ice and a
conductive material (e.g., a metal or semiconductor) is
changed by an external DC bias applied across the ice-
material interface.
To determine the effect of DC bias on ice
adhesion, the interface was modeled as a liquid-solid
interface instead of a solid-solid interface. Indeed, the
interfacial energy which determines the adhesion is reliably
16
CA 02293399 2004-11-24
78348-2
meaeured in a contact-angle experiment when one material is
a liquid and the other a solid, as in the water-metal
situation. A similar technique is thus employed fox an ice-
metal interface if the metal is in the liquid phase.
Mercury, for example, with its melting point at -38_83 C,
low chemical activity, and ease in preparing a clean
surface, is well suited to prove the model; and the effects
of small DC biases on the adhesion of ice to Mercury is
illustrated in FIGe. 2A-2C.
16a
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
1 FIG. 2A shows an initial adhesion of Mercury 18 to ice 20, the adhesion
strength
2 being represented by Oo. Accordingly, Oo represents adhesion strength
without applied
3 voltage (i.e., V = 0). FIG. 2B, on the other hand, illustrates the resultant
adhesion strength
4 O1 which occurs with the application of -1.75V supplied by a DC voltage
source 22. The
source 22 can be, for example, a battery or other voltage source known in the
art. Wiring
6 24 connects the source 22 to the Mercury 18 and to the ice 20 to complete
the circuit. FIG.
7 2C illustrates another adhesion strength 02 which results from an applied
voltage of -5V
8 provided by the source 22. It is noteworthy that 02 < Oo < O1 even though
the applied
9 voltage varies from OV (FIG. 2A) to -1.75V (FIG. 2B) to -5V (FIG. 2C),
indicating a
lo significant change of adhesion strength through a small range of negative
voltage
11 differentials. Adhesion strength O i shows a relatively "weak" adhesion as
compared to 02
12 or even Oo. Adhesion strength OZ, on the other hand, is relatively "strong"
as compared to
13 O, and Oo.
14 To measure the surface tension of the ice-mercury interface 16 of FIG. 2,
an ice
manometer 26 (schematically shown in FIG. 3) was used. The DC power supply 22'
was
16 used for the source 22 of FIG. 2. A DC ammeter 28 was placed in the
manometer circuit
17 26 to measure current flow. The source 22' connects in circuit to the
Mercury 18' and to a
18 mesh electrode 30 connected with the ice 20'. Accordingly, the circuit 26
is completed by
19 the current flow through the Mercury 18' and the ice 20'. The Mercury 18'
is in fluid
communication with the ice 20' through a small capillary 32 of selected
diameter. As the
21 DC bias changes, the ice adhesion between the Mercury 18' and the ice 20'
changes and
22 forces due to gravity adjusts the height "h" of the Mercury 18' within the
ice 20' (i.e.,
23 within the capillary 32 extending upwards into the ice 20').
17
CA 02293399 1999-12-09
WO 98/57851 PCTIUS98/12421
I Specifically, the equilibrium position h of the Mercury 18' in the capillary
32 is:
2 h= 2'( WiJa - Wi/Hg)/grp (2)
3 where g is gravity acceleration, r is the capillary radius, p is the density
of mercury, W;/a is
4 the surface energy of the ice-air interface, and W;/Hg is the surface energy
of the ice-Hg
interface. When h is measured, equation (2) is used to calculate Wi/Hg, and,
thereby, the
6 adhesion strength of ice to the liquid metal (Mercury). In FIG. 3, the
capillary's radius r
7 was 0.25 or 0.5 mm during testing.
8 Additional experimentation such as within the configurations of FIGs. 2 and
3
9 include 99.9998% pure electronic grade Mercury and polycrystalline ices
grown from:
1 o very pure deionized water; distilled water; untreated tap water; and
deionized water doped
11 with small concentrations of NaCl or KOH or HF. The experiments were
performed inside
12 a cold room in the temperature range -20 C to -5 C, 2 C (most testing was
conducted at
13 -10 C and with a relative humidity of 89-91%). For doped ices, it was noted
that DC bias
14 had a strong effect on the ice-Mercury interfacial energy. The magnitude
and sign of the
energy change 0(W;ia - Wi/Hg) depends upon the bias polarity and magnitude and
on the
16 type and concentration of the dopant. FIG. 4, for example, shows 0(W;/a -
W;nHg) versus
17 bias V measured at T=-10 C for ice doped with 0.5% NaC1. As illustrated,
the bias can
18 reduce or enhance adhesion of ice to Mercury: at approximately -1.75V, a
minimum
19 adhesion strength was reached; while the adhesion strength increased from -
2V to -6V.
2o The effect of interfacial energy is more pronounced for NaCI concentrations
above 0.05%.
21 With lower concentrations of NaCl, or with ice grown from tap water, the
adhesion
22 strength varied little and was weakly reproducible when a low DC bias was
applied. With
23 ice doped with 0.5% NaCI, on the other hand, the mercury moved immediately
after the
18
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
t voltage bias is applied; and the effect was completely reversible, i.e.,
W;mg, was restored,
2 after the bias was shut off. These results are reproducible and easy to
observe. The
3 maximum change in h was 12mm for a capillary radius r = 0.25 mm.
4 Measurements of current-voltage characteristics also show that it is the
voltage, not
the current, that causes the changes in adhesion strength discussed above.
Typical
6 experimentation, for example, produced current strengths in tens of pA; and
the estimated
-6
7 rate of the temperature change was less than 10 C/s. In ice doped with KOH
or HF, the
8 application of a DC bias caused a near-symmetrical decrease in W;mg, which
was
9 comparable in magnitude with that found on NaCI-doped ice. Application of an
AC
1 o voltage up to 40V in amplitude and in the frequency range 10Hz to 10kHz
did not produce
11 any noticeable changes in Wi/Hg. In pure deionized or distilled water, the
application of a
12 DC bias up to 40V also did not produce noticeable changes in Wi/Hg. It thus
takes 1 kV to
13 3 kV to change the adhesion of very pure ice to a metal. Different
reactions of pure and
14 doped ice to a DC bias are attributed to their differences in screening
length and electric
relaxation time.
16 The above experimentation confirms the important role played by electrical
double
17 layers on ice-metal interfaces in ice adhesion. Although the absolute
magnitude of WVHg
18 can slightly differ in the case of solid Mercury, the electrostatic
interactions are essentially
19 the same in both cases (for liquid Hg and solid Hg). It was also shown by
experimentation
that ice adhesion to a metal is efficiently modified by application of a small
potential
21 difference between the ice and metal. Variations of adhesion strength also
occur for a DC
22 bias applied to ice containing different impurities, to different solid
metals, and at different
23 temperatures.
19
CA 021293399 2004-11-01
78348-2
The inventor has also studied an electrostatic
model of ice adhesion based on the existence of the surface
states of protonic charge carriers on the surface of ice.
At distances greater than one intermolecular distance the
model gives an order of magnitude for the adhesive energy
which is significantly greater than both chemical bonding
energy and van der Waals forces. It also provides an
understanding of the time- and temperature-dependent
phenomena that explain the difference between adhesive
properties of ice and water, the physical mechanisms of
bonding between ice and other solids, and the nature and
strength of molecular bonding between ice and various
solids.
It is reasonable to classify bonding mechanisms
into one of three groups: a covalent or chemical bonding
mechanism, a dispersion of or fluctuation in electromagnetic
interaction (van der Waals forces), or a direct
electrostatic interaction. See, e.g., Israelachvili,
Intermolecular and Surface Forces, 2nd ed., Academic Press:
London, Ch. 2(1991). The first mechanism corresponds to
chemical reactions and the formation of interfacial
compounds. In covalent or chemical bonding, the adhesive
energy results from lowering of the quantum-mechanical
energy of the system due to overlap of the wave functions of
the interacting solids. Such an interaction is essential
only at a distance on the order of 0.1 - 0.2 nm. In
addition, this type of adhesion is very sensitive to the
chemical nature of adhesive solids. In a perfect contact,
the chemical bonding mechanism can provide adhesive energy
of <- 0.5 J/m2, a value considered the lowest value of
adhesion energy for the chemical bonding mechanism.
In contrast to chemical bonding, van der Waals
forces are long-range and act between all substances. These
CA 021293399 2004-11-01
78348-2
forces are defined only by the macroscopic characteristics
of a solid (dielectric function at different frequencies),
and for this reason they are rather insensitive to
experimental conditions. See, e.g., Mahanty et al.,
Dispersion Forces, Academic Press, London, Chapter 9 (1976);
Barash et al., The Dielectric Function of Condensed Systems,
Eds. Keldysh, et al., Elsiever Science, Amsterdam, Chapter 9
(1989).
In addition to chemical bonding and dispersion
forces, two solids that contain noncompensated or spatially
separated charges also generate electrostatic forces. Its
importance and importance to adhesion have recently been
rediscovered. See, Stoneham et al., J. Phys. C: Solid State
Physics, 18, L543 (1985); and Hays, Fundamentals of
Adhesion, Ed. Lee, Lee, Plenum Press, New York, Chapter 8
(1991).
Model of Adhesion Properties of Ice
A model is next development to describe the
electrical properties of the surface of ice. The model
reveals a connection between ice adhesion and other
properties of ice. The model is compared with van der Waals
forces, the chemical bonding mechanism, and with
experimental results.
The main conclusion of the model discussed below
is that electrostatic interaction plays a significant, if
not the major, role in ice adhesion. One important
parameter in the model is that of the ordering of water
molecules adjacent to the ice-solid interface or, in other
words, that of the appearance of the surface states for
protonic charge carriers. This reduces the problem to one
of simulating water molecule behavior at the solid surface.
21
CA 021293399 2004-11-01
78348-2
However, the below description will assume that there exist
surface states that can be occupied by protonic point
defects. The occupancy of these surface states is defined
by the interplay between the coulomb energy of captured
charge carriers and the energy depth of
21a
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
I the surface states. Then, either the occupancy coefficient of a surface
state (in the
2 nonequilibrium case) or the energy depth of the surface state will be taken
as a parameter.
3 Ice includes polar water molecules that strongly interact with any solid
substrate
4 which has dielectric permittivity different from that of ice. In addition,
there is theoretical
and experimental evidence for the existence of a surface charge in ice. This
surface charge
6 can also interact with the substrate. Here we assume that the surface charge
originates
7 from the capture of protonic charge carriers by the ice surface. The
captured defects are
8 presumably D defects, H30+ ions, or protons. Positive ions are smaller in
size than
9 negative ones, because they have fewer electrons or do not have them at all,
and exist as
protons. Thus we can use the image charge theory for smaller distances, where
the
1 t potential energy of the charge and its image may be less than the charge
energy within the
12 ice. For negative ions of larger size it is more difficult to reach this.
At thermal
13 equilibrium the occupancy of surface states is not perfect because the gain
in energy due to
14 captured charge carriers is compensated for by the rise in electrostatic
energy. However,
the electrostatic energy itself can be reduced significantly by charge
redistribution inside
16 the substrate (by induced charges). This could lead to perfect occupancy of
the surface
17 states and rather high adhesion energy (close to the electrostatic energy).
18 The spatial distribution of charge carriers in the subsurface layer of ice
is described
19 below. The first integral of Poisson's equation can be written in the
following form:
E= 60 .f(V) (3)
sso
21 where E and V are the electric field strength and electrostatic potential,
respectively (both
22 are functions of the space coordinate z); ao = eB =k= N; eB is the
effective charge of
22
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
t Bjerrum defects; N is the concentration of water molecules; X is the
screening length given
2 by ~okTleBN ; c and so are, respectively, the dielectric permittivities of
ice (~ 3.2) and of
3 a vacuum' and k and T are the Boltzmann's constant and temperature,
respectively. The
4 function f(V) is defined by the following equations:
f(V)= jIn(a(V)=a(-V)=bZ(V)=bz(-V)) (4)
exp( E; /2 kT) + (4/3) exp(e; V/kT)
6 a(V) (5)
exp(E;/2kT)+4/3
7 b(V) - exp(ER 12kT)+exp(eBV1kT) (6)
exp( E ,, /2 kT) + 1
8 Here we use Bjerrum defects as charge carriers being captured in the surface
states.
9 Equation (3) holds at any point of the ice crystal. Applying it to the ice
surface, we get the
lo relationship between the surface charge density 6s and the surface
potential V5: 6S = 60
t 1 f(VS).
12 Using equations (3) through (6), we can now calculate the electrostatic
13 contribution to the adhesion energy of ice. First, the electrostatic energy
of the screening
14 layer of ice as a function of the surface potential is calculated, since it
gives the upper limit
for the adhesion energy. Using the definition of electrostatic energy and
equation (3) we
16 get:
v
EEQ 2 ao ' dV Qo
17 W(V,.) = ~ E dx=-- ~I(V) ~tx=- ~f(V)dV (7)
0 2 2 0 dx 2 0
23
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
I The graph of We vs. VS is pictured in FIG. 5. Perfect occupancy by Bjerrum D
defects,
2 positive ionic defects H30+, or protons gives the values of the surface
potential Vs ;z~, 1.47
3 V, 2.50 V, and 5.13 V, respectively. According to FIG. 5, complete occupancy
of the
4 surface states by H30+ ions, Bjerrum defects, and protons correspond to an
upper limit of
adhesion energy of 0.8 J/m2, 0.32 J/m2, and 1.35 J/m2 , respectively. The
smaller values
6 are for imperfect occupancy. Using the relationship between the surface
charge density
7 and surface potential, energy vs. surface charge density is calculated.
8 Now let us consider a metallic plate at a distance d from an ice surface.
The non-
9 uniform charge distribution in the ice will induce a surface charge on the
metal and,
1o therefore, an electric field between the ice and the metal plate. The total
electrostatic
11 energy of the system per unit area can be written in the following form:
2
2 v
12 K'e(d>V)= ao d f(V)- a +a = j.r(V')dV' (8)
2eso vo 2 0
13 However, V in equation (8) is the surface potential of ice, which has to be
found from
14 minimization of the energy for each value of distance d. Surface charge
density can be
considered a constant, which arguably corresponds to a non-equilibrium
occupancy of the
16 surface states. Performing a minimization procedure for We (d,V),we arrive
at the
17 adhesion energy per unit area as a function of d:
18 Wa (d) - Wmin (d) - Wmin (00) (9)
24
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
i This function is pictured in FIG. 6 for the same cases of perfect occupancy
by the Bjerrum
2 D defects, positive ionic defects H30+, and protons, shown as data curves 1,
2, and 3
3 respectively.
4 Under equilibrium conditions, the surface charge density of ice increases
with a
decrease in the distance d because of screening of the ice surface charge by
an induced
6 charge on the metal plate. Indeed, in this case the coulomb energy of
captured charge
7 carriers decreases, so higher occupancy becomes possible. In considering
this case one
8 first has to sum up the electrostatic energy, the energy gain due to
occupancy of the
9 surface states, and the entropy contribution of the surface defects:
z z v
Ff(V) + 2= jf(V')dV'-e -E +eT = cr=In(~ )+(Q,,,-a)=ln(1-~ ) (10)
2 E0 60 ,,, ,,,
11 Here E. is the energy of surface states (assuming E. = -0.5 eV), am = e/S,
and S is the
12 surface area of one water molecule. The free energy F is then minimized
over V and a.
13 This procedure also assumes that the chemical potential of the ice bulk is
kept constant
14 and equals zero. Doing so for every value of d, we arrive at the
equilibrium free energy as
a function of the distance or equilibrium adhesion energy. This is also
pictured in FIG. 6
16 (curve 4, for protons).
17 A similar procedure enables us to find the equilibrium occupancy of the
surface
18 state or the surface potential of ice as a function of the energy of
surface states Eo or
19 temperature. Let us assume that the metallic plate is infinitely far from
the ice surface.
2o Then, to minimize the first positive element in equation (8), it is assumed
that 6= ao f(V).
21 F then becomes a function of only one parameter, either V or a. It is
somewhat easier to
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
1 perform the final minimization over V, but the results can also be
recalculated as a
2 function of a. The occupancy coefficient of the surface states with D
defects plotted
3 versus surface state energy is shown in FIG. 7. The surface state energy
level is measured
4 with respect to the chemical potential of D defects in the bulk.
As can be seen from the results of FIGs. 5-7, typical values of the adhesion
energy
6 are located between 1.3 J/m2 and 0.08 J/m2, depending on the type of charge
carriers and
7 the energy of their surface states. This magnitude is comparable to, or even
higher than,
8 the experimentally measured adhesion energy of ice-metal interfaces at -20
C. In fact, the
9 adhesion energy is as high as the chemical bonding mechanism; however, in
contrast to
1o the latter, the electrostatic mechanism remains significant up to a larger
distance (about 10
11 = roo; roo = 0.276 nm). Thus, at distances larger than roo, the
electrostatic mechanism is
12 significantly more important than the chemical bonding mechanism.
Accordingly, at
13 distances greater than roo, the electrostatic energy exceeds that of the
van der Waals forces
14 if the Hamaker constant equals 3- 10 20 J. Note that the last estimation
concerns an ice-ice
(or water-water) interface, but not an ice-metal interface, as do curves 1, 2,
3, and 4 in
16 FIG. 6. The van der Waals interaction between ice and metal, which is also
long-ranged,
17 can also be considered.
18 The adhesive energy thus equals 0.01 J/m2 even at z,& 90 - roo for the
maximum
19 density of a surface charge, indicating long range character. The adhesive
energy for a
2o non-equilibrium detaching experiment should be higher than that for an
attaching one. The
21 latter can be explained by efficient screening of electrostatic energy by a
metallic plate
22 when ice and metal are in contact. The behavior of adhesion energy with
distance in
23 equilibriurri experiments is thus readily understood. At small distances a
metallic plate -
26
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
I screens the electrostatic energy and there is high adhesive energy because
the occupancy
2 of surface states is high. However, when the distance increases, the
electrostatic energy
3 also increases, leading to lower occupancy coefficients and a lower surface
charge density.
4 By way of example, compare curves 3, 2, and 1 of FIG. 6. These curves are
equivalent to
the more rapid decay of free energy with distance than in the case of constant
occupancy.
6 The behavior of the occupancy coefficient (for the model of the surface
states for
7 D defects) as a function of the surface state energy, Es, is also considered
The occupancy
8 coefficient is close to zero when Es 1:t~ 0.1 eV, FIG. 7. One reason that
the charge carriers
9 are captured into the surface states with positive energy has to do with the
entropy gain in
the free energy. For the same reason, defects exist in the ice bulk. Note that
for the bulk
11 D defects, the "creation energy" equals 0.34 eV per defect, and this energy
is significantly
12 greater than 0.1 eV. Eventually this leads to an "occupancy coefficient"
for the bulk states
-7
13 on the order of 3- 10
14 Time-dependent phenomena can also be associated with ice adhesion, and are
inherent in the above-described model. In order to enter or leave the surface
state, defects
16 have to overcome some electrostatic barrier, and this leads to non-
equilibrium situations
17 and time-dependent phenomena.
t 8 One important element of this model is the electrostatic attraction
between the ice
19 surface charges and the charges induced in metals, a mechanism also
applicable to an ice-
insulator interface except for the difference in magnitude of the induced
charges. A charge
21 q on the ice surface induces the "image charge" -q in a metal; while the
same charge q will
22 induce a smaller "image" charge q' in the insulator according to the
following
23 relationship:
27
CA 021293399 2004-11-01
78348-2
R _R ~+1 (11)
where e is a dielectric permittivity of the insulator. In
most solid dielectrics, c is much larger than one and the
induced charges are comparable with charges induced in
metals. A smaller F results in smaller electrostatic
related adhesion. By way of example, Teflon, has a
permittivity c = 2.04; and is well known for its low
adhesion to ice.
It is useful to consider why ice is more adhesive
than water. Due to higher concentrations of charge carriers
in water, the screening of the surface charge in water (if
it is present) is more effective than in ice (the
corresponding initial electrostatic energy is much less than
in ice). Thus the screening of the electric field due to
the substrate cannot lower the energy significantly. Note
that at temperatures close to the melting point of ice, a
thin liquid layer may appear on an ice-solid interface. See
Dash et al., Rep. Prog. Phys. 58, 115 (1995). The model can
thus be updated to include the effect of surface premelting
on ice adhesion.
The above-described electrostatic model of ice
adhesion shows a relationship between the electrical
properties of the surface of ice and ice adhesion. The
model gives a correct order of magnitude for the adhesive
energy. The electrostatic interaction between ice and
metals supplies energy which is significantly higher than
chemical bonding energy and the van der Waals forces at
distances greater than intermolecular ones. The model also
provides an intuitive way to understand the time- and
temperature-dependent phenomena that help explain the
difference in adhesive properties of ice and water.
28
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
1 EFFECT OF DC BIAS ON ICE ADHESION TO STAINLESS STEEL
2 We now consider the effect of DC bias on ice adhesion to solid metals. For
3 experimentation purposes, we used the system 50 shown in FIG. 8. The space
betweeri-the
4 steel tubes 52 was filled with 0.5% solution of NaCI in water and the system
50 was then
placed into a cold room with temperature -10 C. Multiple systems 50 were also
filled with
6 saline water. The water salinity was close to salinity of ordinary ocean
water. All the
7 samples were kept for three hours inside the cold room before testing, a
time sufficient for
8 water to freeze and for formed ice to relax from intemal stress. Maximum
shear strength
9 of the ice-steel interface 54 was measured when the samples were loaded (via
the load cell
l0 56, with applied force 58) with a constant strain rate of 100 m/min. DC
bias in the range
11 from -21 V to +21 V was applied and maintained between the stainless steel
tubes 52 at
12 the beginning of loading. Teflon caps 60 permitted movement of the inner
tube 52a
13 relative to the ice. A DC power supply 63 provided the DC bias during
experimentation.
-4 The system 50 was supported by a platform 64. An insulating ball 66
thermally and
electrically decoupled the load cell 56 from the rest of the system 50.
16 During mechanical tests, the electric current, load and temperature were
recorded
17 on a computer hard drive. A data acquisition board DAS-1800 and Lab View
software
18 were used for data recording.
19 Since ice adhesion is very sensitive to salt concentration, the
concentration was
measured in melt of the samples after the tests. Before and after, the
surfaces of the
21 stainless steel tubes 52 were washed with a mild abrasive-containing
washer, rinsed first in
22 distilled water, methanol, and again in distilled water. The cleaning
procedures and the
23 control of salt concentration are important for data reproducibility.
29
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
1 To determine if an application of DC power (from the supply 63) causes a
change
2 in ice temperature, a thermocouple (not shown) was placed in the ice 62
between the steel
3 tubes 52 in several tests. Within the precision of these tests ( 0.05 C),
no change in
4 temperature was noted.
FIG. 9 shows results of a typical load versus time diagram when an ice-steel
6 interface is tested under zero DC bias. As one can see, the load reaches its
maximum
7 value and then drops down when the interface is broken. The residual
resistance of the
8 sample to the constant strain rate is due to viscous sliding of the steel on
the salted ice.
9 Still, application of a DC bias can significantly change both the maximum
strength of the
l0 interface and the residual resistance of ice-steel specimens.
11 FIG. 10 shows results of a typical mechanical test performed on an ice-
steel
12 interface when +6.6 V is applied to the inner (mobile) tube 52a. FIG. 11
shows results
13 similar to that of FIG. 10 when -1.0 V is applied to the mobile electrode.
FIGs. 9 and 10
14 are combined in FIG. 12 to illustrate the effect of DC bias on interfacial
strength. The
results of such tests are summarized in Table I below, which illustrates that
for voltages
16 tested, a significant decrease in tma., was observed. This effect was
particularly large for V
17 = +6.6 volts.
18
19
Table 1: Maximum interfacial strength zma,, and residual shear strength tifeS
of ice-
21 steel interfaces at T=-10 C and ice doped with 0.5% NaCI.
22
DC bias (volts) -cmax (kPa) zres (kPa)
0 64 6 21 2
6.6 37t7 13f3
-1.0 45 5 12 2
-1.8 48 7 19 3
23
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
1 In most recent tests, as shown in FIGs. 12A and 12B, we found that the
relative
2 strength of an ice/steel interface can be reduced by almost one order of
magnitude when V
3 =-21 V is applied to the electrode. ao is the interfacial strength at V= 0
and a corresponds
4 to V# 0. To explain such a dramatic drop in ice adhesion, factors other than
the
electrostatic interaction are involved. Namely, when DC current flows through
ice,
6 gaseous hydrogen (H2) and oxygen (02) accumulate at the ice/steel interfaces
in the form
7 of small bubbles, due to ice electrolysis. As illustrated in FIG. 12C, these
bubbles 67 play
8 a role in development of interfacial cracks which appear when the interface
(between the
9 ice 69 and metal 71) is loaded, reducing the maximum interfacial strength.
ADDITIONAL TESTING AND COMMENTARY ON ADHESION OF ICE TO
11 MERCURY
12 As previously discussed, FIGs. 1 and 2 show a strong and reversible effect
of a
13 small DC bias (-6V to +6V) on ice adhesion to Mercury. The effect was
observed in ices
14 doped with KOH, HF, and NaCl and was absent in very pure ice grown from
deionized
water. AC voltage of up to 40V did not cause any noticeable changes in ice
adhesion.
16 This section reports further on the effect of a low DC bias applied across
an ice-Hg
17 interface on the interfacial energy and on the work of adhesion. This
section also reports
18 the fraction of ice-metal interfacial energy that is due to long-range
electrostatic
19 interactions.
As reported above, a liquid-solid interface was used instead of a solid-solid
one.
21 Indeed, the interfacial energy which determines the adhesion is reliably
measured in
22 contact-angle experiments when one material is liquid and the other is
solid, as in the
23 water-metal case. A similar technique can be employed for an ice-metal
interface if the_
31
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
I metal is in the liquid phase. Mercury, with its melting point of -38.83 C,
low chemical
2 activity, and easily prepared clean surface, is well suited for such
experiments.
3 Electronic grade 99.9998% pure mercury was used as well as polycrystalline
ices
4 grown from: 1) very pure deionized water; 2) distilled water; 3) untreated
tap water; or 4)
deionized water doped with small concentrations of laboratory grade NaCI, KOH,
or HF.
6 Most of the experiments were carried out inside a large cold room at T=-10 C
and
7 relative humidity 89% to 91%. Some experiments were performed at
temperatures of -
8 5 C, -15 C, and -20 C. The temperature control was t0.2 C.
9 To measure the surface tension of an ice-Mercury interface, two techniques
were
1 o employed. For demonstration purposes, the first technique was a
conventional contact-
11 angle method with a drop of mercury on a flat, smooth ice surface,
schematically shown in
12 FIG. 2. Before the contact-angle measurements were made, the ice surface
was smoothed
13 by a microtome machine and polished on an optically smooth quartz plate.
14 The second technique used the manometer system of FIG. 3, which is more
precise
and reproducible in the case of the ice-Mercury interface. Pure or doped water
was placed
16 into a quartz tube 31 and frozen inside the cold room at T=-10 C. The
quartz tube 31 had
17 an internal diameter of 10mm and contained a stainless-steel cylindrical
mesh electrode 30
18 and a thin stainless-steel wire stretched along the tube's axis. After the
water was frozen,
t9 the wire was carefully pulled out, producing a thin circular capillary 33
with very smooth
walls. The capillary's radius, r, was either 0.5 or 0.25mm. Prior to the
surface tension
21 measurements, the capillary was filled with liquid mercury from the mercury
tank 19. In
22 order to work with a fresh mercury surface during the measurements, the
mercury 18' was
23 regularly pulled back to the mercury tank 19 and then pushed down to the
capillary 33. We
32
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
1 measured the difference, h, in the mercury levels in the capillary and in
the tank for an
2 advancing and retracting mercury front. Two main factors have limited the
precision of the
3 technique. First, due to hysteresis in adhesion, even for a fresh mercury
surface, we
4 observed a small difference in h measured for an advancing and retracting
front, Oh At~
0.5mm. Second, due to the granular structure of the ice, which had a typical
grain size of
6 lmm, the image of the mercury inside the capillary was not sharp; this
introduced
7 additional error of about 0.2 to 0.3mm. The resulting error, shown in
diagrams and in the
8 text, corresponds to the standard deviation of our tests.
9 In equilibrium, the mercury level difference h is given (again) by equation
(2).
lo When h is measured, equation 2 is also used to calculate Wi/a - Wi/Hg, and
hence the work
11 WA of ice adhesion to a liquid metal:
12 WA = (Wi/a - Wi/[-lg) + WHg/a (12)
-2
13 where WHg/a is the energy of the Hg/air interface. WHg/a = 493 mJ'm at -10
C. See,
14 Jasper, J. Phys. Chem. Ref. Data, 1, 841 (1972). The contact angle of
mercury on ice, 0,
can be calculated from these experimental data and as a function of DC bias
as:
16 0= acos((Wi/a - Wi/xg)/ WHgIa) (13)
17 Experimental Results
18 On the doped ices, a small DC bias had a strong effect on the ice-Mercury
19 interfacial energy. The magnitude and sign of the energy change A(Wi/a -
Wi/Hg) depends
on the polarity and magnitude of the bias and on the type and concentration of
dopants.
21 The effect of different biases on the shape of a mercury drop on ice doped
with NaCI is
33
CA 02293399 1999-12-09
WO 98/57851 PCTIUS98/12421
I shown schematically in FIG. 1. Table 2 shows 0, calculated using equation
(13), for ice
2 20' doped with different impurities and under different DC biases applied
between the
3 mercury and the mesh electrode.
4
Table 2: Contact angle 0 of mercury on ice grown from
6 water containing different dopants and under different DC
7 biases applied between the mercury and the mesh electrode,
8 positive voltage corresponding to a positive potential on
9 mercury.
11 Contact Angle 0
12 Ice Donant OV -1.75V -5V
13 0.5% NaCI 101f3.5 116f4 77f4
14 0.2% KOH 113f9
0. i 8 /a H 105f6
16
17 FIG. 13 graphically shows the change in the work of adhesion OWA = 0(W;ia -
18 W;h.ig) versus bias V, measured by the "manometer" of FIG. 3, at T=-10 C,
for ice
19 grown from water doped with 0.5% NaCl. As shown, the bias can reduce or
enhance
adhesion of ice to mercury. When the bias does not exceed 6V, the effect
becomes very
21 pronounced for NaCl concentrations above 0.05%. The positive bias
corresponds to a
22 positive potential on the mercury. Because of the minimum in the WA(V)
dependence,
23 which is seen at -1.75V after a negative potential of <- 2V is applied, the
mercury column
24 first moves down and then up.
At lower concentrations of NaCI (<0.05%) or with ice grown from tap water, the
26 effect was smaller, while in ice doped with 0.5% NaCl, the mercury begins
to move
27 immediately after the bias is applied. In most pure ice grown from
deionized water, a DC
28 bias of up to 40V did not produce any noticeable change in ice adhesion to
mercury. When
29 doped ice was used the effect was completely reversible; i.e., W;/Hg was
restored after the
3o bias was shut off. Nevertheless, on several occasions, as mentioned above,
hysteresis was
34
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
I observed in the motion of mercury. The maximum observed change in h was 12mm
for r
2 0.25mm. FIGs. 14 and 15 show the effect of a DC bias on OWA of ice doped
with HF and
3 KOH, respectively.
4 The effect of a DC bias on adhesion of doped ices to mercury was also
observed at
-5 C, -15 C, and -20 C, but most of the measurements were performed at -10 C.
The
6 reason for this is that-5 C doped ice contains many tiny liquid inclusions,
while-20 C ice
7 often cracks inside the apparatus.
8 Measurements of current-voltage characteristics showed that it was the
voltage, not
9 the current, that caused the changes in ice adhesion (see FIGs. 13 - 15).
For example,
lo AWA passes through minima of the same magnitude and located at the same
voltage in ice
11 samples whose conductivity differed by a factor of 20. Electrical heating
did not play any
12 role in the effect either, because for voltages below the threshold of ice
electrolysis ( 2V)
13 the current was measured in singles and tens of A and the estimated rate
of the
-6
14 temperature change was less than 10 C/s. Thus, the effect of electrical
heating was
neglected.
16 Due to the low solubility of all impurities in solid ice, dopants dissolved
in water
17 are ejected by the growing ice front and are finally concentrated in the
grain boundaries
18 and on the ice surface, increasing their electrical conductivity. In the
results of this section,
19 the measured DC current is a sum of the bulk, surface, and grain-boundary
currents.
In electrochemistry the peaks of current, shown in FIG. 16, when the bias was
21 "on" and "off' are usually explained in terms of build-up and decay of an
electric double
22 layer at electrolyte/metal interfaces. The large currents (?1mA) used for
IVI > 2V were not
CA 02293399 1999-12-09
WO 98/57851 PCTIUS98/12421
1 stable, but steadily decayed over time. To plot them versus voltage, current
was measured
2 twenty seconds after the bias was switched "on". To prevent the accumulation
of electrode
3 polarization, the polarity of the bias was reversed every time. Thus, the
measurements
4 were performed in a sequence of +0.2V, -0.2V, +0.4V, -0.4V, etc.
The application of AC voltage, up to 40V in amplitude and in the frequency
range
6 IOHz to 10kHz, did not produce any noticeable changes in WA. As mentioned
above, in
7 pure deionized water, the application of a DC bias of up to 40V did not
produce noticeable
8 changes in WvHg. It takes 1kV to 3kV to change the adhesion of very pure ice
to a metal.
9 The different reactions of pure and doped ices to a DC bias are attributed
to the differences
io in their electrical conductivities. Accordingly, certain embodiments of the
invention utilize
t 1 electrical "feedback" to gauge ice conductivity, in real time, and to
select the DC bias
12 based on this measurement, to minimize the adhesion strength for a given
ice-material
13 interface. Those skilled in the art should appreciate that adhesion
strength can also be
14 increased in real time and based upon the same feedback, if desired; or
both.
When ice was doped with NaCI or HF and a DC bias exceeded the electrolysis
16 threshold with positive potential on the Mercury, the appearance of a
yellowish oxide film
17 appeared on the mercury surface. The film disappeared in a few seconds
after the bias was
i 8 reversed. However, with a negative potential applied to the mercury, there
was no
19 noticeable color change in the stainless-steel mesh electrode. This electro-
corrosion of the
mercury surface may be responsible for the asymmetry of aWA versus V
dependencies
21 shown in FIGs. 13 and 14. In the case of ice doped with 0.2% KOH, there was
also no
22 noticeable color change of ice/Hg interfaces associated with electro-
corrosion.
36
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
1 Other possibilities exist for anomalies in the data. For example, a
stainless-steel-
2 doped ice-mercury sandwich behaves as a weak battery, generating a small
electromotive
3 force (EMF) with a negative potential on the mercury. This EMF was -0.18V
for ice
4 doped with 0.5% NaCI and --0.3V for ice doped with 0.2% KOH . Other physical
mechanisms could also contribute to the above-reported effects: 1)
electrostatic
6 interactions of electrical charges in an electric double-layer of the ice-
metal interface; 2)
7 electro-oxidation and electro-reduction (redox) of the metal surface; and 3)
exfoliation of
8 the ice/metal interface, caused by the gases liberated in the electrolysis
of ice. These are
9 briefly discussed below.
lo Electrostatic Interaction
11 Due to redox reactions, there are always potential differences, VC, between
a metal
12 electrode and an electrolyte (ionic conductor). Thus, the standard
potential Vo of Mercury
13 is +0.7958V at 25 C. The real potential between a Mercury electrode and a
particular
14 electrolyte depends on the electrolyte's pH, varying from about +0.9V in
very acidic
solutions to about +0.2V in very alkaline solutions. See, Oldham, Fundamentals
of
16 Electrochemical Science, Academic Press, New York, pp. 309-355 (1994). The
electric
17 double-layer on the interface associated with this contact potential Vc
consists of an
18 atomically thin, positive charge of density +X on the mercury and an ionic
space-charge -1,
19 in the subsurface layer of the electrolyte. The energy of the interfacial
electric field is
given by:
21 W ' ~ V N C(V )- Vl v Hs 2 2 (14)
37
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
I where C(V) is an "apparent" interfacial capacitance that itself depends on
Vc. The
2 electrostatic portion of the work of adhesion is then given by:
C{V = V 2
3 WA = 2 . (15)
4 When an external bias V is applied to the interface, WA' is given by:
s W f C(V )- (V, + V ) 2
A 2 (16)
6 which predicts a minimum in WA at V= -Vc. This type of dependence is seen in
FIG.
7 15, in the left side (V < 0) of FIG. 13, and in the part of FIG. 14 in which
-3V < V< 0.
8 The absolute value of WA can be compared with the prediction of equation
(16) and with
9 experimental observations. To estimate C, a time constant of izt~ I Os is
used with which
lo the current rises and decays in FIG. 16:
11 CR ~ (17)
12 where R is the resistance of a steel/ice/mercury sandwich; R;:z 1 V/50 A =
2= 10552; S is
13 the area of the ice-Hg interface. The two factor appears due to the
presence of the
14 stainless-steel/ice interface, which is assumed to be identical to the ice-
Hg interface. This
15 provides an order-of-magnitude rough estimate.
2
16 Equation (17) calculates C at an approximate value of 0.4F/m , which is
quite
17 typical for electrode capacitance when metals are immersed in concentrated
electrolytes.
38
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
2
i Substitution of C:zz 0.4F/m and the mercury standard potential Vc ;t~ 0.8V
into equation
2 (16) results in the following minimum of W;~ :
3 OWA =WA(0~-WA' tV,160mJl m2 (18)
2
4 This result is comparable with the above results (AWA = 100 - 150 mJ/m ).
Deviation of experimental results from equation (16) may be due to the other
effects
6 discussed herein.
7 The position of the minima in WA are -1.75V for HF- and NaC1-doped ices,
which
8 is about double the expected V. of Hg in an acidic electrolyte. But, the
applied bias V is
9 shared between the ice-stainless steel interface, ice-bulk, and ice-Hg
interface. If below the
threshold of ice electrolysis V is shared almost equally between two
interfaces, then the
11 observed minima are right in place at -2Vc ~z, -1.8V. Notice that on
freezing, NaCl enters
12 ice as Cl and H}, leaving Na{ and OH- outside ice, and making NaC1 doping
similar to
13 HCl doping. Since Vc of mercury is smaller in alkaline electrolytes, the
minimum of WA
14 for KOH-doped ice must be at a lower negative voltage, and it is (see FIG.
15).
Oxidation and Reduction
16 As noted above, a yellowish film was observed with the mercury oxide (which
is
17 red in bulk) when a positive potential was applied to the mercury in
contact with acidic
18 (HF- and NaCI-doped) ice. Most probably, this film destroys the nice
symmetry of WA(V)
19 dependence, predicted by equation (16) and seen in FIG. 15 for KOH-doped
ice.
Gases Released in Electrolysis
39
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
1 Gas release for IVI _ 2V can cause exfoliation of the ice-metal interface
and thus
2 decreases the work of adhesion, WA. Such a decrease is not seen in FIGs. 13-
15 (although
3 there may be some in FIG. 14 at V<-2V) even though a current of 1mA
generates
4 approximately 0.15 mm3/s of (H2+02) at atmospheric pressure. Perhaps the
gases easily
escaped upward along the ice-Mercury interface. Nevertheless, in the case of
an ice-solid
6 metal interface, the gases generated by ice electrolysis can crack the
interface, thus
7 reducing ice adhesion strength.
8 Other Interactions
9 Supposing that at the minima of WA(V) the electrostatic interaction between
space
charges sitting on the metal and on the ice is zero, the reminder, WA (0) -
AWmin, is equal
2 2
11 to 190 25mJ/m for an alkaline-ice/Hg interface and 290 10 mJ/m for an
NaCI-doped-
12 ice/Hg interface. Then, what is left may be attributed to Lifshitz-Van der
Waals and polar
13 Lewis acid-base interactions.
14 The effect of a relatively small DC bias (-6V < V < +6V) on ice adhesion to
Mercury is thus demonstrated. Depending on the bias polarity and magnitude,
the work of
16 adhesion can be decreased by as much as 37-42% or increased by up to 70%.
In this small
17 bias range, the effect was not observed on very pure ice or under AC
voltage. Electrostatic
18 interactions of the electrical charges in the interfacial electric double
layer is the most
19 plausible major mechanism of the phenomenon, with some contributions of
electrolytic
gas-release and metal oxidation.
21 FIG. 17 (and cross-sectional view 17A) illustrate a system 100 constructed
22 according to the invention. System 100 operates to reduce the adhesion of
ice 102 formed
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
1 onto the surface 104a of a material 104. The system 100 forms a circuit that
includes the
2 material 104, a conductive grid 106 (including illustrative points "A" - "F"
on the grid),
3 and a power supply 109. The grid 106 is suspended above the surface 104a so
that it
4 remains electrically insulated from the material 104.
In a preferred embodiment of the invention, the suspension of the grid 106
over the
6 surface 104a is obtained through use of an insulating grid 108 disposed
between the grid
7 106 and the surface 104a. FIG. 17A illustrates the grid 108 in greater
detail. The cross-
8 sectional view of FIG. 17A is not to scale so as to illustrate the
relationship of the
9 insulating grid 108 and the conducting grid 106. In reality, the thickness
(in the dimension
1 o of FIG. 17A) of the grids 106, 108 can be much smaller than an inch (even
as low as
11 0.010 to 0.020 inch); and can be considered as "coatings". By way of
example, the grid
12 108 can be made from a thin coating of electrically insulating paint; while
the grid 106 can
13 be made from a thin coating of electrically conductive paint. The grid 106
is connected so
14 as to function as a single electrode. The material 104 thus becomes a first
electrode of the
system 100; and the grid 106 becomes the second electrode in the circuit.
16 Grids 106, 108 can also be pliant and formable over the surface 104a, which
can
17 represent any shape even though a flat surface 104a is shown. By way of
example, the
18 material 104 can represent an aircraft wing or a car windshield; and the
grids 106, 108 are
19 conformal to the structure material 104.
When ice 102 forms onto the surface 104a, the circuit of system 100 is
completed
21 as the ice 102 operates as a semiconductor (as discussed above). When the
circuit is
22 completed, the power supply 109 provides a DC bias to the interface between
the ice 102
41
CA 02293399 1999-12-09
WO 98/57851 PCTIUS98/12421
1 and the material 104. The bias is typically less than a few volts; and thus
a battery can
2 function as the supply 109.
3 The magnitude of the bias depends upon the desired application. In the case
qf a
4 car windshield or an airplane wing, the bias is selected so that a minimum
(or near-
minimum) ice adhesion results, thereby facilitating the removal of the ice 102
from the
6 material 104.
7 However, in the case of a boot heel, for example (i.e., where the surface
104a is the
8 bottom of the sole of a shoe), the ice 102 represents ice beneath the heel;
and the bias is
9 selected so as to increase the normal ice adhesion strength between the ice
and the heel,
1o thereby increasing friction between the shoe and possibly preventing a slip
on the ice.
11 A voltage regulator subsystem 112 is also preferably connected in circuit
with the
12 system 100. As described in more detail below, the voltage regulator
subsystem 112
13 operates in feedback with the circuit and the supply 109 so as to decrease
or increase the
14 DC bias in an optimum fashion. By way of example, the subsystem can include
circuitry
and a microprocessor 112a to measure data from the circuit and to determine
the
16 conductivity (and/or temperature) of the ice 102. Such measurements are
used in turn by
17 the subsystem 112 to generate a signal which effectively changes the amount
of the DC
18 bias applied to the circuit. Specifically, in one embodiment, the power
supply 109 is
19 responsive to the signal to generate the right voltage at the ice-material
interface. The
value of the DC bias can be stored in memory 112b within the subsystem 112
such as
21 through a look-up table and based upon experimental data. For example, ice
with a
22 conductivity of "X" (as measured by the subsystem, in real time preferably)
in contact
23 with a material 104 of conductivity "Y" (known a priori, as the system 100
is installed
42
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
I with the material 104 for a given application) will be used through the look-
up table in
2 memory 112b to determine which voltage to apply to the ice-material
interface.
3 The grid electrode 106 is preferably spaced so as to ensure (as best as
possible) that
4 ice 102 formed onto the surface 104a will contact at least some portion of
the grid 106.
With reference to FIG. 17, for example, the ice 102 comes in contact with
several areas of
6 the grid 106, including at points "C" - "E". Accordingly, the circuit of
system 100 will be
7 completed as the ice 102 "shorts" at least one part of the grid to material
electrodes 106,
8 104, respectively.
9 The actual size of the spacing between conductive areas of the grid 106 -
for
1o example, the area 114 of FIG. 17 - should be sized for the specific
application. By way of
11 example, if the surface 104a is the surface of an aircraft wing, then the
spacing can be
12 relatively large, e.g., greater than one square foot. However, for a car
windshield, area 114
13 should be smaller, if desired, so that smaller ice deposits on the
windshield (such as in the
14 corners of the windshield) are likely to short to the grid 106.
FIG. 18 illustrates a system 130 constructed according to the invention. One
16 electrode of the subsystem 130 is the aircraft wing 132. The aircraft wing
132 is
17 electrically coupled to ground 134. A DC power supply 136 is electrically
coupled to a
18 DC ammeter 138. The DC ammeter 138 is electrically coupled to an inductor
140. The
19 inductor 140 is electrically coupled through wiring 141 to a conductive
paint 142 (or other
wing-conformal, conductive equivalent) which is applied to the insulating
layer 144 fixed
21 on the aircraft wing 132.
22 The insulating layer 144 and paint 142 are preferably arranged as a grid
pattern,
23 such as described in connection with FIG. 17 and shown further in FIG. 19.
In FIG. 19,
43
CA 02293399 1999-12-09
WO 98/57851 PCTIUS98/12421
I the conductive layer 142' on the wing 132' and the insulating layer 144'
(shown here as an
2 insulating lacquer) form a grid pattern 145. Accordingly, the power supply
136' connects
3 to the conductive paint 142' and to ground through the wing electrode 132'.
As ice forms
4 on the wing 132', the circuit is shorted by the ice and a DC bias is applied
at the ice-wing
interface so as to reduce ice adhesion and to facilitate ice removal.
6 Preferably, the total area covered by the insulating lacquer 144' does not
exceed
7 about 1% of the front edge 132a' of the wing 132'. The grid pattern 145 can
be sized and
8 arranged over the front 132a', as shown, or over the entire wing 132', or
over some other
9 area as a matter of design choice. A wing or aircraft manufacturer who has
historical or
l0 other data on typical ice deposits for the particular wing or aircraft can
thus apply the grid
11 145 over that particular region only, if desired.
12 The voltage applied between the wing 132 and 132' of FIGs. 18 and 19,
13 respectively, is generally adjusted to between one and six volts, with a
corresponding
14 current below 1A per m2 of the grid area.
Those skilled in the art should appreciate that a wide variety of commercially
16 available insulating lacquers 144' and conductive paints 142 exist; and
that a particular
17 brand should be chosen after testing of icing simulations. Furthermore, the
optimal
18 spacing of the grid 145 (i.e., to size the area 114 of FIG. 17) should also
be determined
19 experimentally or through analysis for a particular design.
With further reference to FIG. 18, the DC ammeter 138 can additionally couple
to
21 a feedback subsystem 150. The feedback subsystem 150 in turn electrically
couples to the
22 DC power supply 136 to "control" the DC bias applied to the wing-ice
interface,
23 depending upon characteristics such as ice conductivity and temperature. A
temperature
44
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
i sensor 152 thus also preferably connects with the circuit 130 to measure the
temperature
2 of the ice 154.
3 Further features of the system 130 can include an AC power supply 156
(operating
4 between about 10kHz and l00kHz) electrically coupled to an AC ammeter 158,
which in
turn electrically couples to the conductive paint 142. A current comparator
160 is
6 electrically coupled to both the AC ammeter 158 and the DC ammeter 138.
7 An icing alarm subsystem 162 can also be included with the system 130. The
8 current comparator 160 can for example couple to the icing alarm subsystem
144 and to
9 the feedback subsystem 150 so as to initiate certain events, such as
discussed below.
The DC ammeter can be used to measure the DC conductivity of the circuit 130.
i 1 The DC conductivity signal measurement is provided to the feedback
subsystem 150,
12 which in turn regulates the current supplied by the DC power supply 136,
and to the
13 current comparator 160.
14 The AC ammeter can be used to measure the AC conductivity of the circuit
130
within the applied frequency range of 10-100kHz, for example. The AC
conductivity
16 signal measurement is provided to the current comparator 160 (and
optionally to the
17 feedback 150 for A/D and data processing). A comparison between the AC and
DC
18 conductivities is used by the system 130 to distinguish between water and
ice, both of
19 which "short" and complete the circuit. Specifically, the ratio of the AC
to DC
conductivity is 2-3 orders of magnitude greater in the case of ice as compared
to water,
21 providing a signal measurement which readily distinguishes ice over water.
22 As ice forms on the wing 132, therefore, the current comparator 160 signals
the
23 feedback subsystem 150 which in turn commands the DC power supply 136 to
increase or
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
I decrease the DC bias at the ice-wing interface. The DC bias is selected at a
magnitude
2 (generally between one and six volts) so as to minimize ice adhesion
strength of the ice
3 154 on the wing 132.
4 Upon deicing of the wing 132, the signal differential received by the
current
comparator 160 drops below a preset value; and the current comparator 160
deactivates
6 the icing alarm 162. Simultaneously, the current comparator 160 signals the
feedback
7 subsystem 150 which in turn commands the DC power supply 136 to decrease the
bias to
8 the initial level.
9 In summary, the ammeters 138 and 158 are used to determine conductivity of
the
1 o material which shorts between the grid electrode 142 and the wing 132. As
shown, that
1 t material is ice 154. The system 130 thus distinguishes between ice and
water in an
12 automatic manner. The inductor 140 prevents AC voltage from entering the
"DC" parts of
13 the circuit, which should be accurately controlled to modify the ice
adhesion strength. The
14 feedback subsystem 150 can and preferably does include a microprocessor and
memory to
command and control the power supply 136 at a near-optimum DC bias based upon
16 feedback data such as ice temperature and ice conductivity (and/or ice
purity). The
17 feedback circuitry preferably increases or decreases DC bias voltages at a
level that
18 provides a density of about 0.1 mA/cm2 (or about 1 mA/in2 current density
at the ice-wing
19 interface) after receiving an ice alarm signal from the subsystem 162.
Accordingly, for a
current of about 10-30A, a total energy consumption of about 100-500 watts is
required
21 for a typical large airplane.
22 The "DC" parts of the circuit of FIG. 18 thus primarily operate to provide
DC bias
23 to the ice-wing interface, and, secondarily (if desired) to measure the DC
conductivity of
46
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
1 the ice 154. The "AC" parts of the circuit of FIG. 18 thus primarily operate
to measure the
2 AC conductivity. The remaining portions of the circuit of FIG. 18 thus
provide: (a) an
3 inductor to prevent signal coupling between the DC and AC parts; (b)
feedback and
4 measurement and control circuitry to control the applied DC bias based upon
detection of
ice (as compared to water) and/or measured feedback parameters such as ice
temperature
6 and conductivity.
7 FIG. 20 illustrates one other system 200 used to de-ice an aircraft wing
202. A DC
8 power source 201 supplies a DC bias to the wing 202, acting as the first
electrode (the
9 wing 202 is either conductive or coated with metal foil or conductive
paint), and to a
to conductive grid 204 that is electrically insulated from the wing 202. The
grid 204 is
11 insulated from the wing 202 by an insulating film 206 disposed between the
wing 202 and
12 the grid 204. The grid 204 acts as the second electrode in the circuit of
FIG. 20. When ice
13 210 forms on the wing 202, it bridges the circuit and the DC bias is
applied to the interface
14 between the wing 202 and the ice 210.
FIG. 21 illustrates a system 250 used to increase friction between an
automobile
16 tire 252 and ice 254 on the road 256. As shown, the tire 252 includes a
plurality of strips
17 252a that are conductively doped (such as with iodine) to transmit a
current. A DC power
18 supply 258 connects to the strips 252a through cabling 260 and generates a
DC bias
19 relative to ground 262. The DC voltage differential generated by the supply
258 is
2o between about 5V and 1000V (and is usually in the range IOV to I OOV). The
DC supply
21 can also include voltage regulation circuitry and feedback subsystems (as
discussed above)
22 to determine the conductivity of the ice 254 (or snow) and to adjust the DC
bias
23 accordingly. A non-contact temperature sensor (not shown) can also be used
to remotely
47
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
t sample the ice temperature, as known to those in the art, so that
temperature can also be
2 used as a feedback parameter.
3 Voltage is applied to the strips 252a by way of a electrical control
lever.266.
4 Alternating strips 252a can be driven with plus or minus potential through
the supply 258,
if desired, to acquire the highest adhesion for a given voltage range. The
lever 266 can be
6 moved up and out of the way when the system 250 is not in use (such as by an
electro-
7 mechanical controller). By connecting the supply 258 to the wheel axle 270,
a constant
8 distance is maintained between the lever 266 and the current source.
9 Those skilled in the art should appreciate that the strips 252a can be
welded onto
l o existing tires (or tire material) and that fewer or more strips 252a can
be used. Indeed, the
i l tire 252 can be entirely doped so as to become conductive wherein no
strips are required.
12 Those skilled in the art should appreciate that circuitry such as presented
in FIG.
13 18 can also be used in the tire system of FIG. 21. However, the DC and AC
voltages in
14 such an embodiment are applied to neighboring strips 252a (shown as + and -
respectively) so as to decouple the two signals. The DC voltage applied
between the
16 strips 252a is small (about IOV) before an ice alarm is received; but is
switched to high
17 voltage of 100V to 1000V after the signal.
18 FIG. 22 shows a system 300 that increases the friction between an
automobile tire
l9 302 and an icy road 304. The tire 302 is doped or manufactured so that
current can flow
through the rubber of the tire 302. An AC power source 306 is housed within
the car 308
21 and is connected to the tire 302 through appropriate wiring 309 (the wiring
is non-
22 interfering with the wheel rotation, such by connection through the axle).
The AC source
23 306 applies a high frequency (10-1000kHz), high voltage signal to the tire
302; and that
48
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
I signal imparts a substantially DC voltage between the tire 302 and the road
304. That
2 voltage is preferably of a magnitude which increases the friction between
the ice 310 and
3 the tire 302.
4 FIG. 23 shows a system 400 which includes a car window 402 connected in
circuit
with a DC source 404. The window material is doped (such as with ITO or
fluoride doped
6 S;02) to become one conducting electrode of the system 400. The other
electrode is a grid
7 406 formed by transparent conducting strips placed on the window 402 and
electrically
8 insulated by a insulating grid (not shown) between the grid 406 and the
window 402.
9 Preferably, a voltage regulator subsystem 408 such as described above
monitors factors
I o such as ice conductivity and temperature so as to determine when ice
bridges the gap
i l between the grid 406 and the window 402 (as compared to water) and further
to bias the
12 DC voltage applied to the interface between the ice and the window to a
point of near-
13 minimum ice adhesion. See, e.g., FIG. 4. The insulating grid beneath grid
406 is similar to
14 the layer 144' of FIG. 19.
Note that the grid 406 is connected together such that each point on the grid
406
16 resides at a constant potential, preferably.
17 An altemative window grid and electrode pattem is shown in FIG. 23A, which
18 includes a DC source 450 connected to a first electrode 452 (coupled to a
first grid) and to
19 a second electrode 454 coupled to a second grid interleaved with the first
grid. The system
of FIG. 23A is an alternative embodiment as compared to FIG. 23; and can
include
21 additional circuitry and control such as described herein.
22 The anti-icing grid of FIGs. 23 and 23A is preferably made of conductive
23 transparent coatings which are common in LCD and solar-cell technologies.
DC voltages
49
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
I of 1-2V are typically applied to the comb-like grids of transparent
conductive electrodes
2 on the window. The desired bias can depend upon the electrode material and
the
3 manufacturer. The electrodes can be either painted or vapor-deposited on the
windshield.
4 As discussed herein, it should be understood that car windshields can be
doped
with either ITO or fluoride doped S;02, for example, to become an acceptable
6 semiconductor (including transparency). Alternative transparent coatings
include doped
7 polyaniline. Lithium ion conductive glass might also be used. For automobile
tires,
8 copolymer-carbon deposits can be used within the rubber so as to conduct
electricity.
9 Iodine might also be used. Thin rubber films developed by CSIRO in Australia
might also
be used with the invention.
1l FIG. 24 illustrates a power line ice control system 500 constructed
according to
12 the invention. The system includes a power control module 502 (including
functionality
13 such as DC power supply, and preferably voltage regulation and DC and AC
ice detection
14 and measurement as described herein) that is connected by wiring 504 to
doped power line
wiring 506. The wiring 506 is shown in an illustrative cross-sectional view
(and not to
16 scale) in FIG. 24A. The wiring 506 thus includes a main power line 508 and
an insulating
17 layer 510 , both of which are known to those skilled in the art. A doped
outer layer 512
18 surrounds the insulating layer 510 to provide ice control DC bias in
circuit with the
19 module 502. A conductive grid 514 axially extends (with optional
circumferential
winding) along the length of the wiring 506 and is electrically insulated from
the layer 512
21 by an insulating grid 516 (also axially disposed) between the grid 514 and
the layer 512.
22 When ice 520 forms on the wiring 506, the ice 520 shorts the circuit and a
DC bias applies
23 to the interface between the layer 512 and the ice. By regulating the bias
to the correct
24 magnitude, the removal of ice 520 from the wiring 506 is facilitated.
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
1 FIG. 25 shows a system 600 constructed according to the invention for
modifying
2 ice adhesion on a ski 602 so as to increase or decrease ski-to-snow/ice
friction selectively.
3 System 600 is shown with a view of the bottom 602a of the ski 602. A grid
604 (shown
4 illustratively and including an electrically conducting grid spaced from the
bottom 602a by
an insulating grid, such as described herein) is provided on the bottom 602a
as part of the
6 circuit of system 600. A battery 606 connects to the grid 604 and to the
bottom 602a and
7 provides the DC bias to the circuit. A controller 608 senses ice
conductivity (and
8 optionally temperature) and regulates the bias generated by the battery 606.
The ski
9 bottom 602a is made from semiconductor material or is doped or lacquered
with
1U conductive strips. In contact with snow or ice, the controller controls the
applied voltage
t i and, thereby, the friction between the ski 602 and snow and ice.
12 Those skilled in the art should appreciate that the controller (and/or
battery) are
13 shown illustratively in FIG. 25 as dotted lines. Their physical location is
a matter of
14 design choice and may be on the top surface of the ski or in a boot pack or
binding. In
addition, the controller can be made responsive to user input to alter the
friction in real
16 time. For example, skiers climbing up an incline (such as in cross-country)
can select
17 "increased friction" and the system 600 will respond to do so. The user can
also select
18 "decreased friction" and the controller commands a bias that minimizes the
ice adhesion
19 strength of ice/snow to the bottom 602a.
FIG. 26 shows yet another embodiment of the invention for modifying ice
21 adhesion strength for ice/snow in contact with a sole of a shoe 699.
Specifically, FIG. 26
22 shows a system 700 including a battery 702. For illustrative purposes only,
there are two
23 batteries 702 shown to illustrate two alternative electrode designs. In the
first design, at the
24 heel 699a (made conductive by techniques discussed herein and known to
those in the art),
51
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
1 the battery 702a connects to a conductive grid 704 such as described herein
(and spaced
2 from the conductive heel 699a). When in contact with snow or ice, the snow
or ice bridges
3 the circuit and a DC bias is applied to the ice-heel interface to increase
friction.
4 The other design of FIG. 26 is shown illustratively in that a grid electrode
is not
really required for a small surface such as a shoe 699. Rather, a single
electrode 706 may
6 be sufficient (note that as above the electrode 706 is spaced from the sole
by an insulating
7 layer 706a. Here, the sole of the shoe is conductive (or made conductive
through doping)
8 so that when snow or ice contact the electrode 706, the circuit is completed
and the
9 optimum DC bias is applied from the battery 702b so as to increase shoe
traction.
FIG. 27 shows one preferred embodiment of the invention suitable to reduce or
11 remove ice from power lines 700. The inset to FIG. 27 shows a cross-
sectional view of
12 the power line 700 constructed according to the invention. As known in the
art, the
13 normal power line 702 generates power at 60Hz but with very high E-fields
such as
14 10,000 volts per inch. In accord with the invention, a coating 704 is
applied over the line
702 at a thickness of "t".
16 In one embodiment, the coating 704 is a ferroelectric material, as known in
the art.
17 Ferroelectric materials are essentially ceramics that exhibit a very high
dielectric constant
18 (e.g., 10,000) and very high dielectric loss (e.g., tanS = 10) at certain
conditions, and a
19 relatively low dielectric constant (3-5) and small dielectric loss at other
conditions. One
condition that can change the constant is temperature. In a preferred aspect,
the material is
21 selected so that above freezing, the dielectric constant is low, and below
freezing
22 temperatures, the constant is high. When ambient temperature drops below
the freezing
52
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
1 point, the coating is intensively heated by the AC electric field due to the
high dielectric
2 constant and dielectric loss.
3 Those skilled in the art should appreciate that the above-described
embodiment:can
4 be self-regulating in keeping the coating temperature close to (or slightly
above) the
melting point. If the coating is overheated by the power line's electric
field, it
6 automatically undergoes a phase transformation from the ferroelectric to the
normal state,
7 at which point the coating stops absorbing the electric field energy. By
choosing a phase
8 transition temperature, therefore, the coating temperature can be adjusted
per user needs
9 and per the environmental conditions of the local area.
The coating 704 generates heat in the presence of an AC field such as
generated by
l 1 the line 702. Specifically, it exhibits hysteresis that generates heat
over the AC cycle; and
12 the coating thus generates heat due to the oscillating E-field of the line
702.
13 The thickness "t" is typically on the order of 1/100 of an inch, though
other
14 thicknesses can be applied depending upon coating materials and desired
heating. By
changing the thickness, for example, temperatures at the surface 704a can be
increased by
16 1-10 degrees, or more. The thickness "t" is chosen so that a desired amount
of heat is
17 generated (i.e., heat sufficient to generally melt ice and snow on the
surface 704a of the
18 line 700).
19 When the coating exhibits low dielectric constant and loss (i.e., when the
coating is
above "freezing" or some other desired temperature), much less heat is
generated by the
21 coating 704 and, thereby, much less energy is expended by the line 702.
53
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
I The coating 704 can also be constructed by ferromagnetic materials with the
same
2 or similar effect. In this case, the coating absorbs the energy of the
magnetic field
3 generated by a power line.
4 More particularly, when a ferroelectric material is placed in an oscillating
electric
field (AC), the material is heated by the field due to a dielectric loss. The
heating power
6 per cubic meter is:
7 W=WEEOtans E 2 (19)
41r
8 where s' is a relative dielectric permittivity (usually E' is approximately
104 for typical
9 ferroelectrics), so is a dielectric permittivity of free space (Eo = 8.85E-
12 F/m), w is an
l0 angular frequency of the AC field ((o = 27rf, where f is a usual frequency
for the power
i l line, e.g., 60Hz in conservative power lines), tanS is the tangent of
dielectric loss, and
12 ( EZ ) is the average of electric field squared.
13 Ferroelectrics are characterized with very large values of E' and tanS
below the so-
14 called Curie Temperature, T,,, and small s' and tan6 above Tc. Thus, the
dielectric loss (or
heating power of the AC electric field) is very high below and close to Tc;
and it drops by
16 a large factor (e.g., 106) above that temperature. This makes
ferroelectrics with Tc close to
17 or just above the melting temperature an optimum choice for a coating 704
such as
18 described above. Such coatings absorb the electric power when the outside
temperatures
l9 drop below the melting point, Tn,, and are heated by the field to a
temperature above TR, so
that they again transform into usual insulators (i.e., no longer absorbing the
electric field in
21 significant quantity).
54
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
I Accordingly, when such coatings are placed in an AC field, the ferroelectric
2 material maintains a constant temperature which is close to T. and just
above TR,. This
3 self-adjusting mechanism to prevent icing is very economic: the maximum
heating power
4 per one meter of the power line, or per m2 at any surface to be protected,
can be increased
or decreased by changing the coating thickness and/or by adding a neutral (not
6 ferroelectric) insulating paint or plastic to the coating. Examples of
suitable ferroelectric
7 materials according to the invention include:
8 Table 3: Ferroelectric materials
Name Formula T, (Kelvin)
Rochelle salt NaKC4H4O6 4H20 255-297
Deuterated Rochelle salt NaKC4H2 D206 4H20 251-308
TGSe (NH2CH2COOH)3 H2Se)4 295
Potassium tantalate niobate KTa2/3 Nb,/3 03 271
Anti momium nitrate NH4NO3 255, 305
Pb3MgNb2Og -273K (0 degrees C)
9
By way of example, consider the heating power calculations for Pb3MgNb2Og. In
11 this example, a middle range power line is considered with V2 = lOkV and
with a
12 wire diameter of lcm = 2*radius. The electric field strength on the wire
surface is:
CA 02293399 1999-12-09
WO 98/57851 PCTIUS98/12421
I E /~1 3x 105 ~m (20)
1nII*r
lr)
2 or 3kV/cm, where L is the distance between the wires (L=lm). Substitution as
above,i.e.,
3 EZ = 3E5 V/m, t) = 2n* 60Hz, E' = 104 and tan8 = 10, computes to W(1mm,
60Hz) _
4 4.5E5 watts/m3. A lmm thick film, for example, thus generates 450 watt/m2,
which is
more than sufficient for typical melting of ice.
6 When applied to power lines, the maximum power that can be dissipated in the
7 coating is limited by a capacitance C2 between the wires:
8 W. - W22 Vz (21)
9 For wires of 2cm thickness, with 1 m distance between wires, C2 - 1.21 E-11
F/m. For a
power line at V=350kV, Wm,,., = 300 Watt/m, which is sufficient energy to keep
a lm long
i 1 cable free of ice.
12 In addition to ferroelectrics, almost any semiconductor coating will
provide similar
13 effects. To reach the maximum performance of Equation (21), the coating
dielectric
14 conductivity a should satisfy the condition:
a;Z~ sEOw (22)
16 where s is the coating's dielectric constant, and go is that of free space.
For a 60Hz line
17 and E;t~ 10, aAt~ 3.4E-8 (ohm.m) -1. Such conductivity is very typical for
many undoped
18 semiconductors and low-quality insulators. Thus, such a coating is not
expensive (certain
t 9 paints qualify for these coatings). Moreover, the same temperature
"tuning" - described
56
CA 02293399 1999-12-09
WO 98/57851 PCTIUS98/12421
I above - can be achieved due to a strong temperature dependence of
conductivity of
2 semiconductor materials (e.g., an exponential dependence). Thus, the optimal
conditions
3 according to Equation (22) are satisfied only in a narrow temperature
interval, e.g., -10 C
4 T<_ 10 C, where the coating will melt ice, otherwise consuming little power.
Those skilled in the art should appreciate that other surfaces such as
described
6 herein can also be treated with these coatings. For example, applying such a
coating to an
7 airplane wing will also provide melting capability by subjecting the coating
to AC and,
8 particularly, by increasing that AC as in Equation (19) above. By way of
example, for
9 Pb3MgNb2Og, a frequency of 100kHz will heat a 1 mm thick coating to W(1 mm,
100kHz,
to 3E5V/m) = 750 kWatt/mZ.
11 FIG. 28 illustrates an embodiment of the invention utilizing such coatings
to de-ice
12 non-active surfaces (i.e., those surfaces without internal AC e-fields). In
FIG. 28, a
13 ferroelectric coating 800 is applied to a structure 802 (e.g., an aircraft
wing). Foil
14 electrodes 804a, 804b provide for application of AC power to the structure
802. The AC
power derives from a standard AC power supply 806. An ice detection system 808
(e.g.,
16 the detection system of FIG. 18), in circuit with the structure 802,
preferably informs the
17 power supply 806 of ice on the structure 802, whereinafter AC power is
applied. The AC
18 frequency and coating thickness are chosen to generate heat at the desired
quantities (e.g.,
19 so as to keep icing from forming on an aircraft wing).
The invention thus attains the objects set forth above, among those apparent
from
21 preceding description. Since certain changes may be made in the above
apparatus and
22 methods without departing from the scope of the invention, it is intended
that all matter
57
CA 02293399 1999-12-09
WO 98/57851 PCT/US98/12421
I contained in the above description or shown in the accompanying drawing be
interpreted
2 as illustrative and not in a limiting sense.
3 For example, those skilled in the art should appreciate that grid electrodes
such as
4 described in connection with FIG. 17 can also be applied to surfaces
including the roof of
a house, oil pipelines, driveways, and other areas prone to ice collection.
6 In view of the foregoing, what is claimed is:
58