Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02294300 1999-12-14
WO 99105096 PCT/US98/15386
UROKINASE INHIBITORS
Cross Reference to Related Applications
S This application is a continuation-in-part of U.S. Application Ser. No.
08/901,040, filed July 25, 1997, pending.
Background of the Invention
The present invention provides naphthamidine compounds which inhibit the
urokinase
t0 enzyme, pharmaceutical compositions containing these compounds and medical
methods of
treatment using these compounds.
Technical Field
Urokinase (urinary-type plasminogen activator or uPA (International Union of
1S Biochemistry classification number: EC3.4.21.31)) is a proteolytic enzyme
which is highly
specific for a single peptide bond in plasminogen. Plasminogen activation
(cleavage of this
bond by the urokinase enzyme) results in fonmadon of plasmin, a potent general
protease.
Many cell types use urokinase as a key initiator of plasmin-mediated
proteolytic
degradation or modification of extracellular support structures such as
extracellular matrix
20 (ECM) and basement membrane (BM). Cells exist, move and interact with each
other in tissues
and organs within the physical framework provided by ECM and BM. Movement of
cells
within ECM or across BM requires local proteolytic degradation or modification
of the
structures and allows cells to invade adjacent areas previously unavailable
prior to the
degradation or modification.
25 Cellular invasiveness inflated by urokinase is central to a variety of
normal and disease-
state physiological processes (Blasi, F., Vassalli, J. D., and Dano, K. J.
Cell Biol. 104:801-
804, 1987; Dano, K., Anderson, P. A., Grondahl-Hansen, J., Kristensen, P.,
Nielsen, L. S.,
and Skriver, L. Adv. Cancer Res. 44:139-266, 1985; Littlefield, B. A. Ann. N.
Y. Acad. Sci.
622: 167-175, 1991; Saksela, O., Biochim. Biophys. Acta 823: 35-65, 1985;
Testa, J. E. and
30 Quigley, J. P. Cancer Metast. Rev. 9:353-367, 1990). Such processes
include, but are not
limited to, angiogenesis (neovascularization), bane restructuring, embryo
implantation in the
uterus, infiltration of immune cells into inflammatory sites, ovulation,
spermatogenesis, tissue
remodelling during wound repair and organ differentiation, fibrosis, tumor
invasion, metastatic
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98115386
spread of tumor cells from primary to secondary sites and tissue destruction
in arthritis.
Amiloride, for example, a known urokinase inhibitor of only moderate potency,
has been
reported to inhibit tumor metastasis in vivo (Kellen, J. A., Mirakian, A.
Kolin, A. Anticancer
Res. 8:1373-1376, 1988) and angiogenesis/capillary network formation in vitro
(Alliegro, M.
C. and Glaser, B. M. J. Cell Biol. I IS[3 Pt 2]: 402a, 1991).
Inhibitors of urokinase, therefore, have mechanism-based anti-angiogenic, anti-
arthritic,
anti-inflammatory, anti-retinopathic (for angiogenesis-dependent
retinopathies), contraceptive
and tumoristatic uses.
I~ Summary of the Invention
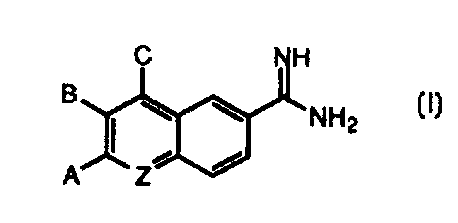
In its principle embodiment, the present invention provides a compound or a
pharmaceutically acceptable salt, ester or prodrug thereof, of formula (I)
C NH
\ \
'NHZ
A Z-
(I), wherein
Z is selected from the group consisting of
( 1 ) nitrogen;
(2) methine; and
(3) methine substituted with -hfRlR2;
A is selected from the ~~roup consisting of
( 1 ) hydrogen and
(2) -LARA>
B is selected from the group consisting of
(1) hydrogen and
(2j -LBRB and
C is selected from the group consisting of
(lj hydrogen and
(2) -LCRC,
with the proviso that at least one of A, B or C is other than hydrogen; and
-2-
SU8ST1TUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTJUS98115386
with the proviso that when A is other than hydrogen, at least one of B or C is
other than
hydrogen,
wherein for A, B, and C, Lp, Lg and L~ are independently selected from the
group
consisting of
( a covalent
1 ) bond,
(2) -(CH2)m-
(3) -NR1-,
(4) -NRZC(X)NR3-,
(5) -C(X)-,
n (6) -NR2C(X}-,
(7) -C(X)NR2-,
(8) -CH=CH-,
(9) -C=C-,
(1()) -O-,
~ ~ ( -S(O)t-,
1 1
)
( 12) -C-_-C(CH~)nNR~C(X)-,
( 13) -C(X)NR2(CH2)nC=C-,
( 14) -(CHZ)nNS02-,
( 15) -NR2S02(CH2)nC---C-,
2u ( -C=C(CH2)nNR~SOZNR3-,
16)
(17) -NR2SO~NR3(CH2)nC-C-,
(18) -S02NR2-,
(ly) -NR2S02-,
(20) -NR2S02NR3-,
25 (21 -N=N-,
)
(22) -C(X)N(OR2)-,
(23) -N(ORZ)C(X)-,
(24) -HC=CH(CH2)nNR2C(X)-,
(25) -(CH2)nNR2C(X)CH=CH-,
3t) (26}-CH=CH{CH2}nNS02-,
(27) -NR2S0~(CH2)nCH=CH-,
(28) -{CH2)nNR2S02NR3-,
(29) -NR2S02NR3(CH2)nCH=CH-,
(30) -NR2C(O)O-,
-3-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98115386
(31 ) -OC(O)NR2-,
(32) -CH=NO-,
(33) -ON=CH- and
(34} W , wherein W is selected from the group consisting of
(a) -O-,
(b) -S-,
(c} -NRi- and
(d) -(CH2)m-,
wherein each functional group is depicted with its right-hand end being the
end which is
attached to the naphthyl or quinolyl ring and its left-hand end being the end
which is
attached to Rp, Rg or R~;
RA> Rg and R~ are independently selected from the group consisting of
( I ) aryl;
l5 (2) arylalkoxy, wherein the alkylene group is of one to six carbon atoms;
(3) alkyl of one to ten carbon atoms;
(4) alkenyl of two to ten carbon atoms;
{5} alkoxycarbonyl of one to six carbon atoms;
(6) alkynyl of two to ten carbon atoms;
(7) halogen;
(~) -NRIR2>
(9) heterocycle;
( 10) cycloalkenyl of four to twelve carbon atoms;
( I 1 } cycloalkyl of three to twelve carbon atoms:
(12) -NRIC(O)NR2R3; and
( 13) -NR1C(O)Rsp, wherein Rsp is alkyl of one to six carbon atoms;
wherein, at each occurence, R E is selected from the group consisting of
(I) hydrogen;
(2) an N-protecting group;
3u (3) alkyl of one to six carbon atoms;
(4) alkenyl of two to six carbon atoms;
(5) alkynyl of two to six carbon atoms:
(f~) aryl;
-4-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98/15386
(7) arylalkyl, wherein the alkyiene group is of one to six carbon atoms;
(8) cycloalkyl of three to eight carbon atoms and
(9) cycloalkylalkyl, wherein the cycloalkyl group is of three to eight carbon
atoms, and the alkylene group is of one to ten carbon atoms; and
wherein, at each occurence, R2 and R3 are independently selected from the
group consisting of
(1) hydrogen;
(2) alkyl of one to six carbon atoms;
(3) alkenyl of two to six carbon atoms;
(4) alkynyl of two to six carbon atoms;
1t> (5) aryl;
(fi) arylalkyl, wherein the alkylene group is of one to six carbon atoms
(7) cycloalkyl of three to eight carbon atoms and
(H) c;ycloalkylalkyl, wherein the cycloalkyl group is of three to eight carbon
atoms, and the alkylene group is of one to ten carbon atoms; and
IS wherein, at each occurence, X is selected from the group consisting of
O and
(2) S; and
wherein, at each occurence,
m is one to five,
2U n is zero to four and
t is zero to two; and
wherein, at each occurence, the alkyl, alkenyl, alkynyl, aryl, heterocycle,
cycloalkyl,
and cycloalkenyl groups are optionally substituted.
The present invention also relates to a method of inhibiting urokinase in a
mammal,
'S particularly humans, by administering a therapeuticaiIy effective amount of
a composition
comprising a compound of formula (1).
The present invention also relates to pharmaceutical compositions which
comprise a
therapeutically effective amount of a compound of formula (1j in combination
with a
pharmaceutically acceptable carrier.
3I)
Detailed Description of the Invention
As used throughout this specification and the appended claims, the following
terms have
the meanings specified:
-5-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98115386
The term "alkyl," as used herein, represents a monovalent group derived from a
straight
or branched chain saturated hydrocarbon by the removal of a single hydrogen
atom and is
exemplified by methyl, ethyl, n- and iso-propyl, n-, sec-, iso- and tert-
butyl, neopentyl and the
like and may be optionally substituted with one, two, three or four
substituents independently
a selected from the group consisting of ( 1 ) alkoxy of one to six carbon
atoms; (2) alkylsulfinyl of
one to six carbon atoms; (3) allcylsulfonyl of one to six carbon atoms; (4)
amino; (5) aryl; (6)
arylalkoxy, wherein the alkylene group is of one to six carbon atoms; (7)
aryloyl; (8) azido; (9)
carboxaldehyde; ( 10) cycloalkyl of three to eight carbon atoms; ( I 1 ) halo;
( 12) heterocycle; ( 13)
(heterocycle)oxy; ( 14) (heterocycle)oyl; ( 15} hydroxy; ( 16) N-protected
amino; ( I7) vitro; ( I 8}
to oxo; ( 19) spiroalkyl of three to eight carbon atoms; (20) thioalkoxy of
one to six carbon atoms;
(21 ) -CO?R2; (22) -C(O)NR2R3; (23) -S02R4, wherein R4 is selected from the
group
consisting of (a) alkyl, (b) aryl and (c) arylalkyl, wherein the alkylene
group is of one to six
carbon atoms; (24) -S02NRSR~, wherein R5 and R~, are independently selected
from the group
consisting of (a) hydrogen, (b) alkyl, (c) aryl and (d) arylalkyl, wherein the
alkylene group is of
n one to six carbon atoms; (25) -NR7RH, wherein R~ and Rg are independently
selected from the
group consisting of (a) hydrogen; (b) an N-protecting group; (c) alkyl of one
to six carbon
atoms; (d) alkenyl of two to six carbon atoms; (e) alkynyl of two to six
carbon atoms; (f) aryl;
(g) arylalkyl, wherein the alkylene group is of one to six carbon atoms; (h)
cycloalkyl of three
to eight carbon atoms and (i) cycloalkylalkyl, wherein the cycloalkyl group is
of three to eight
?u carbon atoms, and the alkyIene group is of one to ten carbon atoms, with
the proviso that no
two groups are bound to the nitrogen atom through a carbonyl group or a
sulfonyl group.
The term "alkanoyl," as used herein, represents an alkyl group, as defined
herein,
attached to the parent molecular group through a carbonyl group, as defined
herein, and is
exemplified by formyl, acetyl, propionyl, butanoyl and the like.
25 The term "alkenyl," as used herein, represents monovalent straight or
branched chain
groups containing a carbon-carbon double bond derived from an alkene by the
removal of one
hydrogen atom and is exemplified by ethenyl, 1-propenyl, 2-propenyl, 2-methyl-
1-propenyl, 1-
butenyl, 2-butenyl and the like and may be optionally substituted with one,
two, three or four
substituents independently selected from the group consisting of ( 1 ) alkoxy
of one to six carbon
3U atoms; (2) alkylsulfinyl of one to six carbon atoms; (3) alkylsulfonyl of
one to six carbon
atoms; (4) amino; (5) aryl; (6) arylalkoxy, wherein the alkylene group is of
one to six carbon
atoms; (7) aryloyl; (8) azido; (9) carboxaldehyde; ( 10) cycloalkyl of three
to eight carbon atoms;
( 11 ) halo; ( 12) heterocycle; ( 13) (heterocycle)oxy; ( 14)
(heterocycle)oyl; ( I 5) hydroxy; ( I b) N-
protected amino; ( 17) vitro; ( 18) oxo; ( 19) spiroalkyl of three to eight
carbon atoms; (20)
-b-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTILTS98/15386
thioalkoxy of one to six carbon atoms; (2I ) -C02R2; (22) -C(O)NR2R3; (23) -
SOZR4, wherein
R4 is selected from the group consisting of (a) alkyl, (b) aryl and (c)
arylalkyl, wherein the
alkylene group is of one to six carbon atoms; (24) -SOZNRSRf,, wherein R5 and
R6 are
independently selected from the group consisting of (a) hydrogen, (b) alkyl,
(c) aryl and (d}
arylalkyl, wherein the alkylene group is of one to six carbon atoms; (25) -
NR~Rg, wherein R~
and Rg are independently selected from the group consisting of (a) hydrogen;
(b) an N-
protecting group; (c) alkyl of one to six carbon atoms; (d) alkenyl of two to
six carbon atoms;
(e) alkynyl of two to six carbon atoms; (f) aryl; (g) arylalkyl, wherein the
alkylene group is of
one to six carbon atoms; (h) cycloalkyl of three to eight carbon atoms and (i)
cycloalkylalkyl,
tU wherein the cycloalkyl group is of three to eight carbon atoms, and the
alkylene group is of one
to ten carbon atoms, with the proviso that no two groups are bound to the
nitrogen atom
through a carbonyl group or a sulfonyl group.
The term "alkoxy," as used herein, represents an alkyl group attached to the
parent
molecular group through an oxygen atom.
The term "alkoxyalkyl" as used herein, represents an alkyl group to which is
attached an
alkoxy group.
The term "alkoxycarbonyl," as used herein, represents an ester group; i.e. an
alkoxy
group, attached to the parent molecular group through a carbonyl group and is
exemplified by
methoxycarbonyl, ethoxycarbonyl and the like.
The term "alkylene." as used herein, represents a saturated divalent
hydrocarbon group
derived from a straight or branched chain saturated hydrocarbon by the removal
of two
hydrogen atoms, and is exemplified by methylene, ethylene, isopropylene and
the like.
The term "alkylsulfinyl," as used herein, represents an alkyl group attached
to the parent
molecular group through an -S(O}- group.
The term "alkylsulfinylalkyl," as used herein, represents an alkyl group, as
defined
herein, substituted by a sulfinyl group.
The term "alkylsulfonyl," as used herein, represents an alkyl group attached
to the
parent molecular group through an -S02- group.
The term "alkylsulfonylalkyl," as used herein, represents an alkyl group, as
defined
3U herein, substituted by a sulfonyl group.
The term "alkynyl," as used herein, represents monovalent straight or branched
chain
groups of two to six carbon atoms containing a carbon-carbon triple bond
derived from an
alkyne by the removal of one hydrogen atom and iS exemplified by ethynyl, I-
propynyl, and
the like and may be optionally substituted with one, two, three or four
substituents
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98/15386
independently selected from the group consisting of ( 1 ) alkoxy of one to six
carbon atoms; (2)
alkylsulfinyl of one to six carbon atoms; (3) alkylsulfonyl of one to six
carbon atoms; (4)
amino; (S) aryl; (6) arylalkoxy, wherein the alkylene group is of one to six
carbon atoms; (7)
aryloyl; (li) azido; (9) carboxaldehyde; ( I 0) cycloalkyl of three to eight
carbon atoms; ( I 1 ) halo;
S ( 12) heterocycle; ( 13} (heterocycle)oxy; ( 14) (heterocycle}oyl; ( 15)
hydroxy; ( 16) N-protected
amino; ( 17) vitro; ( 18) oxo; ( 19) spiroallcyl of three to eight carbon
atoms; (20) thioalkoxy of
one to six carbon atoms: (21 ) -C02R2; (22) -C(O)NR2R3; (23) -S02R4, wherein
R4 is selected
from the group consisting of (a) alkyl, (b) aryl and (c) arylalkyl, wherein
the alkylene group is
of one to six carbon atoms; (24) -S02NR5R6, wherein R5 and R6 are
independently selected
from the group consisting of (a) hydrogen, (b) alkyl, (c) aryl and (d)
arylalkyl, wherein the
alkylene group is of one to six carbon atoms; (25) -NR~Rg, wherein R7 and Rg
are
independently selected from the group consisting of (aj hydrogen; (b) an N-
protecting group;
(c) alkyl of one to six carbon atoms; (d) alkenyl of two to six carbon atoms;
(e) alkynyl of two
to six carbon atoms; (f) aryl; (g) arylatkyl, wherein the alkylene group is of
one to six carbon
atoms; (h) cycloalkyl of three to eight carbon atoms and (i) cycloalkylalkyl,
wherein the
cycloalkyl group is of three to eight carbon atoms, and the alkylene group is
of one to ten
carbon atoms, with the proviso that no two groups are bound to the nitrogen
atom through a
carbonyl group or a sulfonyl group.
The term "amino," as used herein, represents an -Nl-12 group.
2() The term "aminoalkyl," as used herein, represents an alkyl group, as
defined herein,
substituted by an amino group.
The term "aryl," as used herein, represents a mono- or bicyclic carbocyclic
ring system
having one or two aromatic rings and is exemplified by phenyl, naphthyl, 1,2-
dihydronaphthyl,
1,2,3,4-tetrahydronaphthyl, fluorenyl, indanyl, indenyl and the like and may
be optionally
substituted with one, two, three, four or five substituents independently
selected from the
group consisting of ( 1 ) alkanoyl of one to six carbon atoms; (2) alkyl of
one to six carbon
atoms; (3) alkoxy of one to six carbon atoms; {4j alkoxyalkyl, wherein the
alkyl and alkylene
goups are independently of one to six carbon atoms; (5) alkylsulfinyl of one
to six carbon
atoms; (6) alkylsulfinylalkyl, wherein the alkyl and alkylene groups are
independently of one to
six carbon atoms; (7) alkylsulfonyl of one to six carbon atoms; (8)
alkylsulfonylalkyt, wherein
the alkyl and alkylene groups are independently of one to six carbon atoms;
(9) aryl; ( 10)
arylalkyI, wherein the alkyl group is of one to six carbon atoms; ( I 1 )
amino; ( 12) aminoalkyl of
one to six carbon atoms; ( 13) aryl; ( 14) arylalkyl, wherein the alkylene
group is of one to six
carbon atoms; ( I S) aryloyl; ( 16) azido; ( 17) azidoalkyl of one to six
carbon atoms; ( 18)
_g-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98/15386
carboxaldehyde; ( 19) (carboxaldehyde)alkyl, wherein the alkylene group is of
one to six carbon
atoms; (20) cycloalkyl of three to eight carbon atoms; (2 I ) cycloalkylalkyl,
wherein the
cycloalkyl group is of three to eight carbon atoms and the alkylene group is
of one to ten carbon
atoms; (22) halo; (23) haloalkyl of one to six carbon atoms; (24) heterocycle;
(25)
(heterocycle)oxy; (26) (heterocycle)oyl; (27) hydroxy; (28) hydroxyalkyl of
one to six carbon
atoms; (29) nitro; (30) nitroalkyl of one to six carbon atoms; (31 ) N-
protected amino; (32) N-
protected aminoaIkyl, wherein the alkylene group is of one to six carbon
atoms; (33) oxo; (34)
thioalkoxy of one to six carbon atoms; (35) thioalkoxyalkyl, wherein the alkyl
and alkylene
groups are independently of one to six carbon atoms; (36) -(CH2)qC02R2,
wherein q is zero to
~o four; (37) -(CH2)yC(O)NR2R3; (38) -(CH2)qS02R4, wherein R4 is selected from
the group
consisting of (a) alkyl, (b) aryl and (c) arylalkyl, wherein the alkylene
group is of one to six
carbon atoms; (39) -(CHZ)qS02NR5R6, wherein R5 and R~, are independently
selected from the
group consisting of(a) hydrogen, (b) alkyl, (c) aryl and(d) arylalkyl, wherein
the alkylene
group is of one to six carbon atoms; (40) -(CH2)qNR7R~, wherein R~ and RR are
independently
~5 selected from the group consisting of (a) hydrogen, (b) an N-protecting
group, (r) alkyl of one
to six carbon atoms, (d) alkenyl of two to six carbon atoms, (e) alkynyl of
two to six carbon
atoms, (f) aryl, (g) arylalkyl, wherein the alkylene group is of one to six
carbon atoms, (h)
cycloalkyl of three to eight carbon atoms and (i) cycloaikylalkyl, wherein the
cycloalkyl group
is of three to eight carbon atoms, and the alkylene group is of one to ten
carbon atoms, with the
2c) proviso that no two groups are bound to the nitrogen atom through a
carbonyl group or a
sulfonyl group; (41 ) oxo; (42) perfluoroalkyl; (43) perfluoroalkoxy; (44)
aryloxy;
(45) cycloalkoxy; (46) cycloalkylalkoxy; and (47) arylalkoxy.
The term "arylalkyl." as used herein, represents an aryl group attached to the
parent
molecular group through an alkyl group.
25 The term "arylalkoxy," as used herein, represents an arylalkyl group
attached to the
parent molecular group through an oxygen atom.
The term "aryloxy," as used herein, represents an aryl group which is attached
to the
parent molecular group through an oxygen atom.
The term "aryloyl," as used herein, represents an aryl group which is attached
to the
ao parent molecular group through a carbonyl group.
The term "azido," as used herein, represents an -N3 group.
The term "azidoaIkyl," as used herein, represents an alkyl group, as defined
herein,
substituted by an azido group.
The term "carbonyl," as used herein, represents a C=O group.
-9-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98115386
The term "carboxaldehyde," as used herein, represents a -CHO group.
The term "(carboxaldehydejalkyl," as used herein, represents an alkyl group,
as defined
herein, substituted by a carboxaldehyde group.
The term "carboxy," as used herein, represents a -C02H group.
s The term "carboxyalkyl," as used herein, represents an alkyl group, as
defined herein,
substituted by a carboxy group.
The term "cycloalkyl," as used herein represents a monovalent saturated cyclic
hydrocarbon group and is exemplified by cyclopropyl, cyclobutyI, cyclopentyl,
cyclohexyl,
cycloheptyl, bicyclo[2.2. I jheptyl and the like. The cycloalkyl groups of
this invention can be
Il> optionally substituted with ( 1 ) alkanoyl of one to six carbon atoms; (2)
alkyl of one to six
carbon atoms; (3) alkoxy of one to six carbon atoms; (4) alkoxyalkyl, wherein
the alkyl and
alkylene goups are independently of one to six carbon atoms; (5) alkylsulfinyl
of one to six
carbon atoms; (6) alkyisulfinylalkyl, wherein the alkyl and alkylene groups
are independently
of one to six carbon atoms; (7) alkylsulfonyl of one to six carbon atoms; (8)
alkylsulfonylalkyl,
t5 wherein the alkyl and alkylene groups are independently of one to six
carbon atoms; (9) aryl;
( 10) arylalkyl, wherein the alkyl group is of one to six carbon atoms; ( 1 1
j amino; (12)
aminoalkyl of one to six carbon atoms; ( 13) aryl; ( 14) arylalkyl, wherein
the alkylene group is
of one to six carbon atoms; ( 15) aryloyl; ( 16) azido; ( 17) azidoalkyl of
one to six carbon atoms;
( 1 R) carboxaldehyde; ( 19) (carboxaldehyde)alkyl, wherein the alkylene group
is of one to six
2() carbon atoms; (20) cycloalkyl of three to eight carbon atoms; (21 )
cycloalkylalkyl, wherein the
cycloalkyl group is of three to eight carbon atoms and the alkylene group is
of one to ten carbon
atoms; (22) halo; (23) haloalkyl of one to six carbon atoms: (24) heterocycle;
(2S)
(heterocycle)oxy; (2fi) (heterocycle)oyl; (27) hydroxy; (28) hydroxyalkyl of
one to six carbon
atoms; (29) vitro; (30) nitroa(kyl of one to six carbon atoms; X31 ) N-
protected amino; (32) N-
25 protected aminoalkyl, wherein the alkylene group is of one to six carbon
atoms; (33) oxo; (34)
thioalkoxy of one to six carbon atoms; (35) thioalkoxyalkyl, wherein the alkyl
and alkylene
groups are independently of one to six carbon atoms; (36j -(CH2)yC02R2,
wherein q is zero to
four; (37) -(CH2)qC(O)NR2R3; (38) -(CH2)qS02R4, wherein R4 is selected from
the group
consisting of (a) alkyl, (b) aryl and (c) arylalkyi, wherein the alkylene
group is of one to six
3o carbon atoms; (39) -(CH2)qS02NR5Rb, wherein R5 and R6 are independently
selected from the
group consisting of(a) hydrogen, (b) alkyl, (c) aryl and (d) arylalkyl,
wherein the allcylene
group is of one to six carbon atoms; (40) -(CHZ)~NR~Rg, wherein R~ and Rg are
independently
selected from the group consisting of (a) hydrogen, (b) an N-protecting group,
(c) alkyl of one
to six carbon atoms, (d) alkenyl of two to six carbon atoms, (e) alkynyl of
two to six carbon
-10-
SU9STITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98/15386
atoms. (f) aryl, (g) arylalkyl, wherein the alkylene group is of one to six
carbon atoms, (h)
cycloallcyl of three to eight carbon atoms and (i) cycloalkylalkyl, wherein
the cycloalkyl group
is of three to eight carbon atoms, and the alkylene group is of one to ten
carbon atoms, with the
proviso that no two groups are bound to the nitrogen atom through a carbonyl
group or a
S sulfonyl group; (41 ) oxo; (42) perfluoroalkyl; (43) perfluoroalkoxy; (44)
aryloxy;
(45) cycloaikoxy; (46) cycloalkylalkoxy; and {47) arylalkoxy.
The term "cycloalkenyl," as used herein represents a monovalent cyclic
hydrocarbon
having at least one carbon-carbon double bond. The cycioalkenyl groups of this
invention can
be optionally substituted with (I) alkanoyl of one to six carbon atoms; (2)
alkyl of one to six
icy carbon atoms; (3) alkoxy of one to six carbon atoms; (4) alkoxyaIkyl,
wherein the alkyl and
alkylene goups are independently of one to six carbon atoms; (5) alkylsulfinyl
of one to six
carbon atoms; (6) alkylsulfinylaIkyl, wherein the alkyl and alkylene groups
are independently
of one to six carbon atoms; (7) alkylsulfonyl of one to six carbon atoms; (8)
alkylsulfonylalkyl,
wherein the alkyl and alkylene groups are independently of one to six carbon
atoms; (9) aryl;
l5 ( 10) arylalkyl, wherein the alkyl group is of one to six carbon atoms; (
11 ) amino; ( 12)
aminoalkyl of one to six carbon atoms; ( I3) aryl; ( 14) arylalkyl, wherein
the alkylene group is
of one to six carbon atoms; ( 15) aryloyl; ( 16) azido; ( 17) azidoalkyl of
one to six carbon atoms;
(t!i) carboxaldehyde; (19) (carboxaldehyde)alkyl, wherein the alkylene group
is of one to six
carbon atoms; (2()) cycloalkyl of three to eight carbon atoms; (21 )
cycloalkylalkyl, wherein the
'o cycloalkyl group is of three to eight carbon atoms and the alkylene group
is of one to ten carbon
atoms; (22) halo; (23) haloalkyI of one to six carbon atoms; (24) heterocycle;
(25)
(heterocycle)oxy; (26) (heterocycle)oyl; (27) hydroxy; (28) hydroxyalkyl of
one to six carbon
atoms; (29) vitro; (30) nitroalkyl of one to six carbon atoms; (31 ) N-
protected amino; (32) N-
protected aminoalkyl, wherein the alkylene group is of one to six carbon
atoms; (33) oxo; (34)
25 thioalkoxy of one to six carbon atoms; (35) thioalkoxyalkyl, wherein the
alkyl and alkylene
groups are independently of one to six carbon atoms; (3E>) -(CH2)~C02R2,
wherein d is zero to
four; (37) -(CH2)qC(O)NR2R3; (3IS) -(CH2)yS02R4, wherein R4 is selected from
the group
consisting of (a) alkyl, (b) aryl and (c) arylalkyl, wherein the alkylene
group is of one to six
carbon atoms; (39) -(CH2)~SO2NRSR~,, wherein R5 and R6 are independently
selected from the
30 group consisting of(a) hydrogen, (b) alkyl, (c) aryl and(d) arylalkyl,
wherein the alkylene
group is of one to six carbon atoms; (40) -(CH2)qNR~Rg, wherein R7 and Rg are
independently
selected from the group consisting of (a) hydrogen, (b) an N-protecting group,
(c) alkyl of one
to six carbon atoms, {d) alkenyl of two to six carbon atoms, (e) alkynyl of
two to six carbon
atoms, (f} aryl, (gj arylalkyl, wherein the alkylene group is of one to six
carbon atoms, (h)
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98l15386
cycloalkyl of three to eight carbon atoms and (i) cycloalkylalkyl, wherein the
cycloalkyl group
is of three to eight carbon atoms, and the alkylene group is of one to ten
carbon atoms, with the
proviso that no two groups are bound to the nitrogen atom through a carbonyl
group or a
sulfonyl group; (41 ) oxo; (42) perftuoroalkyl; (43) perfIuoroalkoxy; (44.)
aryloxy;
S (45) cycloalkoxy; (46) cycloalkylalkoxy; and (47) arylalkoxy.
The term "cycloallcoxy," as used herein represents a cycloalkyl group, as
defined
herein, attached to the parent molecular group through an oxygen atom.
The term "cycloalkylalkoxy," as used herein, represents an alkoxy group, as
defined
herein, to which is attached a cycloalkyl group.
to The term "cycloalkylalkyl," as used herein, represents a cycloalkyl group,
as defined
herein, attached to the parent molecular group through an alkyl group.
The term "haloalkyl," as used herein, represents an alkyl group, as defined
herein,
substituted by one, two, or three halogen atoms and is exemplified by
chloromethyl,
bromoethyl, trifluoromethyl and the like.
15 The term "halogen," as used herein, represents F, Cl, Br and I.
The term "heterocycle," as used herein, represents a 5-, 6- or 7-membered ring
containing one, two or three heteroatoms independently selected from the group
consisting of
nitrogen, oxygen and sulfur. The 5-membered ring has zero to two double bonds
and the 6-
and 7-membered rings have zero to three double bonds. The term "heterocycle"
also includes
2U bicyclic, tricyclic and tetracyclic groups in which any of the above
heterocyclic rings is fused to
one or two rings independently selected from the group consisting of an aryl
ring, a
cyclohexane ring, a cyclohexene ring, a cyclopentane ring, a cyclopentene ring
and another
monocyclic heterocyclic ring such as indolyl, quinolyl, isoquinolyl,
tetrahydroquinolyi,
benzofuryl, benzothienyl and the like. Heterocyc(ics include pyrrolyl,
pyrrolinyl, pyrrolidinyl,
2s pyrazolyl, pyrazolinyl, pyrazolidinyl, imidazolyl, imidazolinyl,
imidazolidinyl, pyridyl,
piperidinyl, homopiperidinyl, pyrazinyl, piperazinyl, pyrimidinyl,
pyridazinyl, oxazoiyl,
oxazolidinyl, isoxazolyl, isoxazolidinyl, morpholinyl, thiomorpholinyl,
thiazolyl, thiazolidinyl,
isothiazolyl, isothiazolidinyl, indolyl, quinolinyl, isoquinolinyl,
benzimidazolyl,
benzothiazolyl, benzoxazolyl, furyl, thienyl, thiazolidinyl, isothiazolyl,
isoindazoyl, triazolyl,
3U tetrazolyl, oxadiazolyl, uricyl, thiadiazolyl, pyrimidyl,
tetrahydrofuranyl, dihydrofuranyl,
tetrahydrothienyl, dihydrothienyl, dihydroindolyl, tetrahydroquinolyl,
tetrahydroisoquinolyl,
pyranyl, dihydropyranyl, dithiazolyl, benzofuranyl, benzothienyl and the like.
Heterocyclic
groups also include compounds of the formula
-12-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99!05096 PCTlUS98/15386
'~~ F~
~G
~ , wherein F is selected from the group consisting of -CH2-, -CH20- and -O-,
and
G is selected from the group consisting of -C(O)- and -(C(R')(R"))~ -, wherein
R' and R" are
independently selected from the group consisting of hydrogen or alkyl of one
to four carbon
atoms, and v is one to three and includes groups such as 1,3-benzodioxolyi,
1,4-benzodioxanyl
and the like. Any of the heterocycle groups mentioned herein may be optionally
substituted
with one, two, three, four or five substituents independently selected from
the group consisting
of ( I) alkanoyl of one to six carbon atoms; (2) alkyl of one to six carbon
atoms; (3) alkoxy of
one to six carbon atoms; (4) alkoxyalkyl, wherein the alkyl and alkylene goups
are
independently of one to six carbon atoms; (5) alkylsulfinyl of one to six
carbon atoms; (6)
to alkylsulfinylalkyl, wherein the alkyl and alkylene groups are independently
of one to six carbon
atoms; (7) alkylsulfonyl of one to six carbon atoms; (8) alkylsulfonylalkyl,
wherein the alkyl
and alkylene groups are independently of one to six carbon atoms; (9) aryl; (
10) aryialkyl,
wherein the alkyl group is of one to six carbon atoms; ( 1 1 ) amino; ( 12)
aminoalkyl of one to six
varbon atoms; (13) aryl; (I4) arylalkyl, wherein the alkylene group is of one
to six carbon
t 5 atoms; ( I S) aryloyl; ( 16) azido; ( 17) azidoalkyl of one to six carbon
atoms; ( 18)
carboxaldehyde; ( 19) (carboxaldehyde)alkyl, wherein the alkylene group is of
one to six carbon
atoms; (20) cycloalkyl of three to eight carbon atoms; (21 ) cycloalkylalkyl,
wherein the
cycloalkyl group is of three to eight carbon atoms and the alkylene group is
of one to ten carbon
atoms; (22) halo; (23) haloalkyl of one to six carbon atoms: (24} heterocycle;
(25)
2tt (heterocycle)oxy; (2f~) (heterocycle}oyl; (27) hydroxy; (28) hydroxyalkyl
of one to six carbon
atoms; (29) vitro; (30) nitroalkyl of one to six carbon atoms; (31) N-
protected amino: (32) N-
protected aminoalkyl, wherein the alkylene group is of one to six carbon
atoms; (33) oxo; (34)
thioalkoxy of one to six carbon atoms; (35) thioalkoxyalkyl, wherein the alkyl
and alkylene
~~roups are independently of one to six carbon atoms; (36) -(CH~)~C02R2,
wherein d is zero to
25 four; (37) -(CH2)qC(O)NR2R3; (38} -(CHZ)~S02R4, wherein R4 is selected from
the group
consisting of (a) alkyl, (b) aryl and (c) arylalkyl, wherein the alkylene
group is of one to six
carbon atoms;(39) -(CH2)qS02NRSR6, wherein RS and R~, are independently
selected from the
group consisting of(a) hydrogen, (b) alkyl, (c) aryl and(d) arylalkyl, wherein
the alkylene
group is of one to six carbon atoms; (40) -(CH2)qNR~Rg, wherein R7 and Rg are
independently
3tl selected from the group consisting of (a) hydrogen, (b) an N-protecting
group, (c) alkyl of one
to six carbon atoms, (d) alkenyl of two to six carbon atoms, (e) alkynyl of
two to six carbon
-13-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98115386
atoms, (f) aryl, (g) arylalkyl, wherein the alkylene group is of one to six
carbon atoms, (h)
cycloalkyl of three to eight carbon atoms and (i) cycloalkylalkyl, wherein the
cycloalkyl group
is of three to eight carbon atoms, and the alkylene group is of one to ten
carbon atoms, with the
proviso that no two groups are bound to the nitrogen atom through a carbonyl
group or a
sulfonyl group; (4I ) oxo; (42) perfluoroalkyl; (43) perfluoroalkoxy; (44)
aryloxy;
(45) cycloalkoxy; (46) cycloalkylalkoxy; and (47) arylalkoxy.
The term "(heterocycle)oxy," as used herein, represents a heterocycle group,
as defined
herein, attached to the parent molecular group through oxygen.
The term "(heterocycle)oyl," as used herein, represents a heterocycle group,
as defined
herein, attached to the parent molecular group through a carbonyl group.
The term "hydroxy" as used herein, represents an -OH group.
The term "hydroxyalkyl," as used herein, represents an alkyl group, as defined
herein,
substituted by one to three hydroxy groups, with the proviso that no more than
one hydroxy
group may be attached to a single carbon atom of the alkyl group and is
exemplified by
1 S hydroxymethyl, dihydroxypropyl and the like.
The term "methine" as used herein, represents a =C(H)- group.
The term "N-protected amino," as used herein, refers to an amino group, as
defined
herein, to which is attached an N-protecting or nitrogen-protecting group, as
defined herein.
The term "N-protected aminoalkyl," as used herein, refers to an alkyl group,
as defined
2U herein, which is substituted by an N-protecting or nitrogen-protecting
group, as defined herein.
The term "nitro," as used herein, represents an -N02 group.
The term "nitroalkyl," as used herein, represents an alkyl group substituted
by an -NOZ
group.
The terms "N-protecting group" or "nitrogen protecting group" as used herein,
represent
25 those groups intended to protect an amino group against undersirable
reactions during synthetic
procedures. Commonly used N-protecting groups are disclosed in Greene,
"Protective Groups
In Organic Synthesis," (John Wiley & Sons, New York ( I9S 1 )), which is
incorporated herein
by reference. N-protecting groups comprise aryl groups such as formyl, acetyl,
propionyl,
pivaloyl, t-butylacetyl, 2-chloroacetyl, 2-bromoacetyl, trifluoroacetyl,
trichloroacetyl, phthalyl,
30 o-nitrophenoxyacetyl, a-chlorobutyryl, benzoyt, 4-chlorobenzoyl, 4-
bromobenzoyl, 4-
nitrobenzoyl and chiral auxiliaries such as protected or unprotected D, L or
D, L-amino acids
such as alanine, leucine, phenylalanine and the like; sulfonyl groups such as
benzenesulfonyl,
p-toluenesulfonyl and the like; carbamate forming groups such as
benzyloxycarbonyl, p-
chlorobenzyloxycarbonyl, p-methoxybenzyloxycarbonyl, p-nitrobenzyloxycarbonyl,
2-
- I 4-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99!05096 PCT/US98/15386
nitrobenzyloxycarbonyl, p-bromobenzyloxycarbonyl, 3,4-
dimethoxybenzyloxycarbonyl, 3,5-
dimethoxybenzyloxycarbonyl, 2,4-dimethoxybenzyloxycarbonyl, 4-
methoxybenzyloxycarbonyl, 2-vitro-4,5-dimethoxybenzyloxycarbonyl, 3,4,5-
trimethoxybenzyloxycarbonyl, 1-(p-biphenylyl}-I-methylethoxycarbonyl, a,a-
dimethyl-3,5-
dimethoxybenzyloxycarbonyl, benzhydryloxycarbonyl, t-butyloxycarbonyl,
diisopropylmethoxycarbonyl, isopropyloxycarbonyl, ethoxycarbonyl,
methoxycarbonyl,
alIyloxycarbonyl, 2,2,2,-trichloroethoxycarbonyl, phenoxycarbonyl, 4-
nitrophenoxy carbonyl,
fluorenyl-9-methoxycarbonyt, cyclopentyloxycarbonyl, adamantyloxycarbonyl,
cyclohexyloxycarbonyl, phenylthiocarbonyl and the like; arylalkyl groups such
as benzyl,
triphenyfmethyl. benzyloxymethyl and the like and silyl groups such as
trimethylsilyl and the
like. Preferred N-protecting groups are formyl, acetyl, benzoyI, pivaloyl, t-
butylacetyl, alanyl,
phenylsulfonyl, benzyl, t-butyfoxycarbonyl (Boc) and benzyloxycarbonyl (Cbz).
The term "oxo." as used herein, represents =O.
The term "perfluoroalkyl," as used herein, represents an alkyl group, as
defined herein,
5 wherein each hydrogen radical bound to the alkyl group has been replaced by
a fluoride radical.
Perfluoroalkyl groups are exemplified by trifluoromethyl, pentafluoroethyl,
and the like.
The term "perfluoroalkoxy," as used herein, refers to a perfluoroalkyl group,
as defined
herein, attached to the parent molecular group through an oxygen atom.
The term "pharmaceutically acceptable salt," as use herein, represents those
salts which
2t) are, within the scope of sound medical judgement, suitable for use in
contact with the tissues of
humans and lower animals without undue toxicity, irritation, allergic response
and the like and
are commensurate with a reasonable benefit/risk ratio. Pharmaceutically
acceptable salts are
well known in the art. For example, S. M Berge, et al. describe
pharmaceutically acceptable
salts in detail in J. Pharmaceutical Sciences, 1977, fi6:1-19. The salts can
be prepared in situ
25 during the final isolation and purification of the compounds of the
invention or separately by
reacting the free base group with a suitable organic acid. Representative acid
addition salts
include acetate, adipate, alginate, ascorbate, aspartate, benzenesulfonate,
benzoate, bisulfate,
borate, butyrate, camphorate, camphersulfonate, citrate,
cyclopentanepropionate, digluconate,
dodecylsulfate, ethanesulfonate, fumarate, glucoheptonate, glycerophosphate,
hemisulfate,
3o heptonate, hexanoate, hydrobromide, hydrochloride, hydroiodide, 2-hydroxy-
ethanesulfonate,
lactobionate, lactate, laurate, lauryl sulfate, malate, maleate, malonate,
methanesulfonate, 2-
naphthalenesulfonate, nicotinate, nitrate, oleate, oxalate, palmitate,
pamoate, pectinate,
persulfate, 3-phenyfpropionate, phosphate, picrate, pivalate, propionate,
stearate, succinate,
sulfate, tartrate, thiocyanate, toluenesulfonate, undecanoate, vaIerate salts
and the like.
-15-
SUHSTiTUTE SHEET (RUSE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98/15386
Representative alkali or alkaline earth metal salts include sodium, lithium,
potassium, calcium,
magnesium and the like, as well as nontoxic ammonium, quaternary ammonium, and
amine
rations, including, but not limited to ammonium, tetramethylammonium,
tetraethylammonium,
methylamine, dimethylamine, trimethylamine, triethylamine, ethylamine and the
like.
The term "pharmaceutically acceptable ester," as used herein, represents
esters which hydrolyze
in vivo and include those that break down readily in the human body to leave
the parent
compound or a salt thereof. Suitable ester groups include, for example, those
derived from
pharmaceutically acceptable aliphatic carboxylic acids, particularly alkanoic,
alkenoic,
cycloalkanoic and alkanedioic acids, in which each alkyl or alkenyI group
preferably has not
to more than 6 carbon atoms. Examples of particular esters includes formates,
acetates,
propionates, butyates, acrylates and ethylsuccinates.
The term "pharmaceutically acceptable prodrugs" as used herein, represents
those
prodrugs of the compounds of the present invention which are, within the scope
of sound
medical judgement, suitable for use in contact with with the tissues of humans
and lower
o animals with undue toxicity, irritation, allergic response, and the like,
commensurate with a
reasonable benefit/risk ratio, and effective for their intended use, as well
as the zwitterionic
forms, where possible, of the compounds of the invention.
The term "prodrug," as used herein, represents compounds which are rapidly
transformed in vivo to the parent compound of the above formula, for example,
by hydrolysis
2o in blood. A thorough discussion is provided in T. Higuchi and V. Stella,
Pro-drugs as Novel
Delivery Systems, Vol. 14 of the A.C.S. Symposium Series, Edward B. Roche,
ed.,
Bioreversible Carriers in Drug Design, American Pharmaceutical Association and
Pergamon
Press, 1987, and ludkins, et al.Synthetic Communications, 26(23), 4351-4367
(1996), each of
which is incorporated herein by reference.
25 The term "spiroalkyl," as used herein, represents an alkylene diradical,
both ends of
which are bonded to the same carbon atom of the parent group to form a
spirocycliv group.
The term "sulfonyl," as used herein, represents an -SOZ-group.
The term "thioalkoxy," as used herein, represents represents an alkyl group
attached to
the parent molecular group through a sulfur atom.
30 The term "thioalkoxyalkyl," as used herein, represents an alkyl group
substituted by a
thioalkoxy group.
Asymmetric or chiral centers may exist in the compounds of the present
invention. The
present invention contemplates the various stereoisomers and mixtures thereof.
Individual
stereoisomers of compounds of the present invention are prepared synthetically
from
- I 6-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98/15386
commercially available starting materials which contain asymmetric or chiral
centers or by
preparation of mixtures of enantiomeric compounds followed by resolution well-
known to
those of ordinary skill in the art. These methods of resolution are
exemplified by (1) attachment
of a racemic mixture of enantiomers, designated { ~ ), to a chiral auxiliary,
separation of the
resulting diastereomers by recrystallization or chromatography and liberation
of the optically
pure product from the auxiliary or (2) direct separation of the mixture of
optical enantiomers on
chiral chromatographic columns. Enantiomers are designated herein by the
symbols "R" or
"S," depending on the configuration of subsitiuents around the chiral carbon
atom.
Geometric isomers may also exist in the compounds of the present invention.
The
tc~ present invention contemplates the various geometric isomers and mixtures
thereof resulting
from the arrangement of substituents around a carbon-carbon double bond and
designates such
isomers as of the Z or E configuration, wherein the term "Z" represents
substituents on the
same side of the carbon-carbon double bond and the term "E" represents
substituents on
opposite sides of the carbon-carbon double bond.
IS
Preferred Embodiments
Preferred compounds of the present invention have formula (I), wherein
A and C are hydrogen and
2U B is -LgRg, wherein
-Lg- is -O-, and
Rg is alkyl of two to six carbon atoms, and
wherein the alkyl group is substituted.
More preferred embodiments of the present invention have formula (I), wherein
25 A is -LARp and
B and C are hydrogen,
wherein -LA- is selected from the group consisting of
( 1 ) a covalent bond,
(2) -{CH2)m-,
30 (3) -NR2C(X)-,
(4) -C(X)NR2-,
(5) -NR2C(X)NR3-,
{6) -C=C-,
(7) -CH=CH-,
_17_
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98/15386
(8) -C(X)NR2(CH2)nC=C_
(9) -C(X)-,
( 10) _O_,
( 11 ) -OC(O)NR~- and
( 12) W ; and wherein
Rp is selected from the group consisting of
( 1 ) amino;
(2) aryl;
is (3) alkyl of one to ten carbon atoms;
(4} arylalkyl, wherein the alkylene group is of one to ten carbon atoms;
(5) cycloalkyl of three to eight carbon atoms;
(6) arylalkoxy, wherein the alkylene group is of one to ten carbon atoms and
(7) heterocycle, wherein the heterocycle is selected from the group consisting
of
( 1 ) furanyl,
(2) thienyl and
(3) imidazolyl; and
wherein, at each occurence, R2 is selected from the group consisting of
( 1 ) hydrogen and
(2) alkyl of one to six carbon atoms; and
wherein, at each occurence,
m ~s two,
n ~s one,
R ~ and R3 are hydrogen,
W and X are O,
aryl is phenyl,
the alkyl group and the aryl group are optionally substituted and
the alkenyl group is substituted.
Srill more preferred compounds of the present invention have formula (I),
wherein
A and B are hydrogen;
-18-
SUBSTITUTE SHEET (RULE 2S)
CA 02294300 1999-12-14
WO 99105096 PCT/US98/15386
C is -LCR~;
-L~- is selected from the group consisting of
( 1 ) a covalent bond,
(2) -OC(O)NR2-,
(3) -S02NR2-,
(4) -C(X)NR2-,
{5) -NRI- and
to (6) -O-;
R~ is selected from the group consisting of
( 1 ) alkyl of one to six carbon atoms;
(2) aryl:
I5 (3) arylalkyl, wherein the alkylene group is of one to six carbon atoms and
(4) hererocycle, wherein the heterocycle is selected from the group consisting
of
( 1 ) furanyl;
(2) pyrimidinyl; and
I w Fv
G
(3) ~ ~ , wherein F is -O-, G is -(C(R')(R"))~-, R' and R" are hydrogen and v
is
20 one;
X is O; and
wherein, at each occurence,
R I and R2 are H,
aryl is phenyl and
''S the alkyl is optionally substituted.
Still more preferred compounds of the present invention have formula (I)
wherein
A is -LpRp,
B is -LgRg,
C is hydrogen,
30 -Lp- and -Lg- are -O-, and
Rp and Rg are alkyl of one to six carbon atoms.
Still more preferred compounds of the present invention have formula (I)
wherein
- I 9-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98115386
A is -LpRp,
B is -LgRg,
C is -L~R~,
-Lp-, -Lg-, and -L~- are -O- and
Rp, Rg, and R~ are alkyl of one to six carbon atoms.
Still more preferred compounds of the present invention have formula (I)
wherein
A is hydrogen;
B is -LgRg;
C is -L~RC;
lU -Lg- is -O-;
-LC- is selected from the group consisting of
( 1 ) a covalent bond,
(2) -O-,
(3) -CH=CH-,
t5 (4) -NRt- and
(S) -NR2C(O)O-;
Rg is selected from the group consisting of
( 1 ) alkyl and
(2) arylalkyl, wherein the alkylene group is of one to six carbon atoms;
2t) R~ is selected from the group consisting of
( 1 ) alkyl of one to six carbon atoms;
(2) alkenyl of one to six carbon atoms;
(3) halogen;
(4) aryl and
25 (5) heterocycle, wherein
the heterocycle is selected from the group consisting of
(I) benzofuranyl;
(2) tetrahydrofuranyl;
(3) pyrimidinyl;
3t) (4) pyrazolyl;
(5) furanyl;
(6) pyrimidinyl;
(7) thiazolyl and
-20-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98/15386
F~
G
(8) ~ ~ , wherein F is -O-. G is -(C(R')(R"))v -, R' and R" are hydrogen and v
is
one; and
wherein, at each occurence,
aryl is phenyl and
alkyl, aryl and heterocycle are optionally substituted.
Preferred compounds falling within the scope of formula (I) include:
7, 8-dimethoxy-2-naphthalenecarboximidamide mono(trifluoroacetate) salt;
6, 7, 8-trimethoxy-2-naphthalenecarboximidamide mono(trifluoroacetate) salt ;
fi, 7-dimethoxy-2-naphthalenecarboximidamide mono(trifluoroacetate) salt ;
tU 2-[(7-aminoiminomethyl-2-methoxy-1-naphthalenyl)oxy)acetamide
mono(trifluoroacetate) salt;
7-benzyloxy-X-iodo-2-naphthalenecarboximidarnide mono(trifluoroacetate) salt;
methyl [(7-aminoiminomethyl-2-methoxy-I-naphthalenyl)oxyJacetate
mono{trifluoroacetate)
salt;
2-[(7-aminoiminomethyl-2-methoxy-I-naphthalenyl)oxyJ-yl-acetic acid
mono(trifluoroacetate)
1 S salt;
N-[4-(aminomethyl)phenylJ-h-aminoiminomethyl-2-naphthalenecarboxamide
bis(trifluoroacetate) salt;
N-[4-(amino)phenyl]-fi-aminoiminomethyl-2-naphthalenecarboxamide
bis(trifluoroacetate) salt;
I-[(7-aminoiminomethyl-2-methoxy-1-naphthalenyl)oxy]-3-hydroxypropane
2cl mono(trifluoroacetatej salt;
phenylmethyl [7-{aminoiminomethyl)-1-naphthalenyl)carbamate
mono(trifluoroacetate) salt;
N-[7-(aminoiminomethyl)-1-naphthalenyl)acetamide mono(trifluoroacetate) salt;
methyl [7-(aminoiminomethyl)-1-naphthalenyl)carbamate mono(trifluoroacetatej
salt;
methyl 3-[[7-(aminoiminomethyl)-1-naphthalenyl]amino-3-oxopropanoate
25 mono(trifluoroacetate) salt;
N-[7-(aminoiminomethylj-1-naphthalenyi]-2-(phenylmethoxyjacetamide
mono(trifluoroacetate)
Salt;
N-[7-(aminoiminomethyl )- I -naphthalenyl J-1,3-benzodioxole-5-carboxamide
mono(trifluoroacetate) salt;
3U N-[7-(aminoiminomethyl)-1-naphthalenyl]benzenemethanesulfonamide
mono(trifluoroacetate)
salt;
-21-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 - PCT/US98/15386
1-[(7-aminoiminomethyl-2-methoxy-1-naphthalenyl)oxy]-3-brornopropane
mono{hydrochloride) salt;
3-[(7-aminoiminomethyl-2-methoxy-I-naphthalenyl)oxy]propene
mono(trifluoroacetate) salt;
1-[(7-aminoiminomethyl-2-methoxy-1-naphthalenyl)oxy]-3-phenylpropane
mono(hydrochloride) salt:
1-( (7-aminoiminomethyl-2-methoxy-1-naphthalenyl foxy]-3-[ I -(3,4-
dimethoxy)phenyl]-
propane mono(hydrochloride) salt;
7-methoxy-8-{2-furanyl)-2-naphthalenecarboximidamide mono(trifluoroacetate)
salt;
methyl f~-(aminoiminomethyl)-4-[(methoxycarbonyl)anuno]-2-
naphthalenecarboxylate
IU mono(trifluoroacetate) salt;
(E)-(7-methoxy-8-[2-(Phenyl)ethenyl])-2-naphthaleneimidamide
mono{trifluoroacetate) salt
6-(4-phenylbutynyl)-2-naphthalenecarboximidarnide mono(trifluoroacetate) salt;
7-(2-hydroxyethoxy)-8-iodo-2-naphthalenecarboximidamide mono(trifluoroacetate)
salt;
7-(2-hydroxyethoxy)-2-naphthalenecarboximidanude mono(trifluoroacetate) salt;
fi-(4-methyl-1-pentynyl)-2-naphthalenecarboximidamide mono(trifluoroacetate)
salt;
f~-(5-phenylpentynyl)-2-naphthalenecarboximidamide mono(trifluoroacetate>
salt;
6-(3-phenyl-I-propynyl)-2-naphthalenecarboximidamide mono(trifluoroacetate)
salt;
fi-(phenylethynyl)-2-naphthalenecarboximidamide mono(trifluoroacetate) salt;
3-amino-N-[3-[6-(aminoiminomethyl)-2-naphthalenyl J-2-propynyl]benzamide
20 mono{trifluoroacetate) salt;
4-amino-N-(3-(6-aminoiminomethyl-2-naphthalenyl)-2-propynyl]benzamide
mono(trifluoroacetate) salt;
(S)-2-amino-N-[ 1-[(6-aminoiminomethyl-2-
naphthalenyl)carbonyl]cyclohexyl]propionamide
bis(trifluoroacetate) salt:
~5 fi-methoxy-K-benzyloxy-2-naphthalenecarboximidamide mono(trifluoroacetate)
salt:
2-[(7-aminoiminomethyl-3-methoxy-I-naphthalenyl)oxyJacetamide
mono(trifluoroacetate) salt;
N-(6-aminoiminomethyl-2-naphthalenyl)-N'-phenylurea mono(trifluoroacetate)
salt;
(E)-6-[2-(phenyl)ethenyl]-2-naphthalenecarboximidamide mono(trifluoroacetate)
salt;
6-[2-(phenyl)ethyl]-2-naphthalenecarboximidamide mono(trifluoroacetate) salt;
3U 7-propoxy-!~-iodo-2-naphthalenecarboximidamide mono(trifluoroacetate) salt;
( ~ )- 6-(3-phenyloxiranyl)-2-naphthalenecarboximidamide
mono(trifluoroacetate) salt;
(E)-6-[2-(2-thienyl)ethenyl]-2-naphthalenecarboximidamide
mono(trifluoroacetate) salt;
6-(3-oxobutyl)-2-naphthalenecarboximidamide mono{trifluoroacetate) salt;
6-(3-methoxyphenyl)-2-naphthalenecarboximidamide mono(trifluoroacetate) salt;
-22-
SUisSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98115386
N-[3-(methyl)phenyl]-6-aminoiminomethyl-2-naphthalenecarboxamide
mono(trifluoroacetate)
salt;
6-(2-formylphenoxy)-2-naphthalenecarboximidamide mono(trifluoroacetatej salt;
6-(2-formylphenyl)-2-naphthalenecarboximidamide mono{trifluoroacetate) salt;
6-[2-(hydroxymethyl)phenyl]-2-naphthalenecarboximidamide
mono(trifluoroacetate) salt;
6-(3-oxo-1-butenyl)-2-naphthalenecarboximidamide mono(trifluoroacetate) salt;
7-methoxy-K-(1H-pyrazol-4-yl)-2-naphthalenecarboximidamide
mono(trifluoroacetate) salt;
7-methoxy-8-iodo-2-naphthalenecarboximidamide mono(trifluoroacetate) salt;
N-phenyl-6-aminoiminomethyl-2-naphthalenecarboxamide mono(methanesulfonate)
salt;
lOf 4-[{6-aminoiminomethyl-2-naphthalenyl)oxy]-N-methylbenzeneacetamide
mono(trifluoroacetate) salt;
6-[2-(methylthio)phenyl]-2-naphthalenecarboximidamide mono(methanesulfonate)
salt;
fi-[2-(2-thiomethoxoxyethyl)phenyl]naphthalene-2-carboximidamide
mono{methanesulfonate)
salt;
t5 7-methoxy-?i-(3-furanyl)-2-naphthalenecarboximidamide
mono(methanesulfonate} salt;
7-rnethoxy-H-(2-benzofuranyl)naphthalene-2-carboximidamide
mono(methanesulfonate) salt;
(E)-H-[2-( 1,3-benzodioxol-5-yl)ethenyl ]-2-naphthalenecarboximidamide
mono(methanesulfonate) salt;
( ~ )-7-methoxy-H-(tetrahydro-3-furanyl)-2-naphthalenecarboximidamide
2O mono(methanesulfonate) salt;
f~-[[4-(2-aminoethyl)phenyl]ethynyl]-2-naphthalenecarboximidamide
mono(trifluoroacetate}
salt;
7-methoxy-R-[2-pyrimidinyl(oxy)]-2-naphthalenecarboximidamide
mono(trifluoroacetate) salt;
7-methoxy-~-[2-thiazoyl(oxy))naphthalene-2-carboximidamide
mono(trifluoroacetate) salt;
S 7-methoxy-H-(4-nitrophenoxy)-2-naphthalenecarboximidamide
mono(trifluoroac;etate) salt;
7-methoxy-li-pentafluorophenoxy-2-naphthalenecarboximidamide
mono(trifluoroacetate) salt;
7-methoxy-X-[N-2-pyrimidinyl(amino)j-2-naphthalenecarboximidamide
mono(trifluoroacetate)
salt;
N-(6-aminoiminomethyl-2-naphthalenyl)-N'-benzylurea mono(trifluoroacetate)
salt;
3c~ N-(6-aminoiminomethyl-2-naphthalenyl)-N'-methylurea mono(trifluoroacetate)
salt;
N-(b-aminoiminomethyl-2-naphthalenyl)-N'-isopropylurea mono(trifluoroacetate)
salt;
N-(6-aminoiminomethyl-2-naphthalenyl)-N'-phenyl-N'-methylurea
mono(trifluoroacetate) salt;
fi-aminonaphthalene-2-carboximidamide mono(trifluoroacetate) salt;
N-(6-aminoiminomethyl-2-naphthalenyl)-N'-cyclohexylurea mono(trifluoroacetate}
salt;
-23-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/L3S98/15386
N-(6-aminoiminomethyl-2-naphthalenyl)-N'-benzyloxyurea mono(trifluoroacetate)
salt;
l,l-dimethylethyl [4-[[(6-cyano-2-naphthalenyl)amino]carbonyl]phenyl]carbamate
mono(trifluoroacetate) salt
N-[6-(aminoiminomethyl)-2-naphthalenyl]-4-(aminomethyl)benzamide
mono(trifluoroacetatej
salt;
ethyl (6-(aminoiminomethyl)-2-naphthalenyl]carbamate mono{trifluoroacetate)
salt;
l,l-dimethylethyl [4-[[[fi-aminoiminomethyl)- 2-
naphthalenyl)amino]carbonyl]amino]phenyl]
carbamate mono(trifluoroacetate) salt;
(E)-6-[2-(phenylthio)ethenyl]-2-naphthalenecarboximidamide
mono(trifluoroacetate) salt;
lip (E)-6-[2-(2-furanyl)ethenyl]-2-naphthalenecarboximidamide
mono(trifluoroacetate) salt;
(E)-6-[2-(1H-imidazol-1-yl)ethenyl]-2-naphthalenecarboximidamide
mono(trifluoroacetate) salt;
(E)-4-[2-(6-aminoiminomethyl-2-naphthalenyl)ethenyl]benzenesulfonamide
mono(trifluoroacetate) salt;
(E)-4-[2-(6-aminoiminomethyl-2-naphthalenyl)ethenyl]benzoic acid
mono(trifluoroacetate) salt;
os 4-[7-(aminoiminomethyl)-2-methoxy-I-naphthalenyt]dihydro-2(3H)-furanone
rnono(trifluoroacetate) salt;
7-methoxy-k-(1-acetyl-1H-pyrazolyl)-2-naphthalenecarboximidamide
mono{trifluoroacetate)
salt;
7-methoxy-8-( 1-(methylsulfonyl)-1H-4-pyrazolyl]-2-naphthalenecarboximidamide
20 mono(trifluoroacetate) salt;
(E)-4-[2-(6-aminoininomethyl-2-naphthalenyl)ethenyl]benzamide
mono(trifluoroacetate) salt;
6-[2-(4-aminophenyl)ethoxy]-2-naphthalenecarboximidamide
mono(trifluoroacetate) salt;
methyl [3-methoxy-6-(aminoiminomethyl)-4-naphthalenyl]carbamate
mono(trifluoroacetate)
salt;
25 7-methoxy-8-[2-pyrimidinyl(amino)]-2-naphthalenecarboximidamide
bis(trifluoroacetate) salt;
fhthalenecarboxamide, mono(trifluoroacetate) salt;
fi-(4-aminophenyl)-2-naphthalenecarboximidamide, bis(trifluoroacetate) salt;
methyl 2-[4-[[[6-(aminoiminomethyl)-2-naphthalenyl]carbonyl]amino]-
phenoxy]acetate,mono(trifluoroacetate) salt;
3c) {E)-6-[2-[(3-hydroxymethyl)phenyl]ethenyl)-2-naphthalenecarboximidamide,
mono(trifluoroacetate) salt;
6-(2-phenyl-1-cyclopropyl)-2-naphthalenecarboximidamide,
mono(trifluoroacetate) salt;
(E)-6-[2-[4-(aminomethyl)phenyl]ethenyl]-?-naphthalenecarboximidamide,
bis(trifluoroacetate) salt;
-24-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98115386
(E)-6-[2-[4-( I ,2-dihdyroxyethyl)phenyl]ethenyl]-2-
naphthalenecarboximidamide,
rnono(trifluoroacetate) salt;
(E)-6-[2-[4-( 1 R-amino-2-hydroxyethyl}phenyl)ethenylJ-2-
naphthalenecarboximidamide,
bis(trifluoroacetate) salt;
7-methoxy-8-(2-pyrimidinylamino)-2-naphthalenecarboximidamide,
bis(trifluoroacetate) salt;
(E)-6-[2-[[4-(dimethylamino)methyl]phenyl]ethenyl]-2-
naphthalenecarboximidamide,
bis(tllfluoroacetate) salt;
(E)-6-[2-[4-(hydroxymethyl}phenyl]ethenyl]-2-naphthalenecarboximidamide.
mono(trifluoroacetate) salt;
lU 4-[[6-(aminoiminomethyl)-2-naphthalenyl]ethynyl]-L-phenylalanine,
mono(trifluoroacetate) salt;
6-(3-formylphenyi)-2-naphthalenecarboximidamide, mono(trifluoroacetate) salt;
(E)-fi-[2-( 1,2,3,4-tetrahydro-fi-isoquinolinyl)ethenylj-2-
naphthalenecarboximidamide,
bis(trifluoroacetate) salt;
n (E)- fi-[2-[3-(2-hydroxyethyl)phenyl]ethenyl]-2-naphthalenecarboximidamide,
mono(trifluoroacetate) salt;
6-(aminoiminomethyl)-N-(2,3-dihydro-1 H-inden-S-yl)-2-naphthalenecarboxamide,
mono(trifluoroacetate) salt;
f-[(4-aminophenyl)ethynyl]-2-naphthalenecarboximidamide, bis(trifluoroacetate)
salt;
2U I,1-dimethylethyl [2-[3-[[6-(aminoiminomethyl)-2-naphathalenyl)ethynyl]-6-
methoxyphenyl)ethyl]carbamate, mono(trifluoroacetate) salt;
I,1-dimethylethyl [[4-[[6-(aminoiminomethyl)-2-naphathalenyl]ethynylJ-
phenyl Jmethyl]carbamate, mono(trifluoroacetate) salt;
f~-[[4-(aminomethyl)phenylJethynyl]-2-naphthalenecarboximidamide,
bis(trifluoroacetate) salt;
'_, ~-[[3-(2-aminoethyl)-4-methoxyphenylJethynyl)-2-
naphthalenecarboximidamide,
bis(trifluoroacetate) salt;
fi-[[4-(hydroxymethyl)phenyl]ethynyl]-2-naphthalenecarboximidamide,
mono(trifluoroacetate) salt:
6-[( I ,2,3,4-tetrahydro-6-isoquinolinyl)ethynyl j-2-
naphthalenecarboximidamide,
3o bis(trifluoroacetate) salt;
f~-(aminoiminomethyi)-N-(4-methylphenyl)-2-naphthalenecarboxamide,
mono(trifluoroacetate) salt;
I,l-dimethylethyl [[4-[[[fi-(aminoiminomethyl)-2-naphathalenyl]aminoJ-
carbonyl)phenylJmethylJcarbarnate, mono(trifluoroacetate) salt;
-25-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98115386
N-[6-(aminoiminomethyl)-2-naphthalenyl)benzamide, mono(trifluoroacetate) salt;
1,1-dimethylethyl [[4-[[[6-(aminoiminomethyl)-2-naphathalenyl]amino]carbonyl]-
cyclohexyll]methyl]carbamate, mono(trifluoroacetate) salt;
N-(6-(aminoiminomethyl)-2-naphthalenyl]-N'-(4-aminophenyl)urea,
bis(trifluoroacetate) salt;
N-[6-(aminoiminomethyl)-2-naphthalenyl]-4-4-
(aminomethyl)cyclohexanecarboxamide,
bis(trifluoroacetate) salt;
N-[6-(aminoiminomethyl)-2-naphthalenyl]-N'-[(4-aminomethyl)phenyl] urea,
bis(trifluoroacetate) salt;
6-(aminoiminomethyl)-N-(4-ethylphenyl)-2-naphthalenecarboxamide, acetate salt;
tU C~-(aminoiminomethyl)-N-(2-naphthalenyl)-2-naphthalenecarboxanude,
mono(tlifluoroacetate) salt;
6-(5-phenyl-2-oxazolyl)-2-naphthalenecarboximidamide, mono(trifluoroacetate)
salt;
6-(5-phenyl-2-thiazoIyl)-2-naphthalenecarboximidamide, mono(trifluoroacetate)
salt;
E~-(aminoiminomethyl)-N-( 1,2,3,4-tetrahydro-f~-quinolinyl)-2-
naphthalenecarboxamide,
t 5 bis(trifluoroacetate) salt;
6-[amino(hydroxyimino)methyl]-N-phenyl-2-naphthalenecarboxamide;
6-[4-[(hydroxymethyl)phenyl]methoxy]-2-naphthalenecarboximidamide,
methanesulfonate salt;
6-(2-pyridinylethynyl)-2-naphthalenecarboximidamide, mono(trifluoroacetate)
salt;
N-[4-(aminocarbonyl)phenyl)-6-(aminoiminomethyl)-2-naphthalenecarboxamide,
20 mono(trifluoroacetate) salt;
6-(aminoiminomethyl)-N-(2-thiazolyl)-2-naphthalenecarboxamide,
monohydrochloride
6-(aminoiminomethyl)-N-(6-methoxy-3-pyridinyl)-2-naphthalenecarboxamide,
monohydrochloride
fi-(aminoiminomethyl)-N-( 1,3-benzodioxol-5-yl)-2-naphthalenecarboxamide,
2> mono(trifluoroacetate) salt;
f~-(aminoiminomethyl)-N-( 1,2,3,4-tetrahydro-2,4-dioxo5-pyrimidinyl)-
2-naphthalenecarboxamide, monohydrochloride
6-(aminoiminomethyl)-N-(3,5-difluorophenyl}-2-naphthalenecarboxamide,
mono(trifluoroacetate) salt;
30 E~-(aminoiminomethyl)-N-(1H-pyrazol-3-yl}-2-naphthalenecarboxamide,
mono(trifluoroacetate) salt;
fi-(aminoiminomethyl)-N-(5-methyl-3-isoxazolyll)-2-naphthalenecarboxamide,
mono(trifluoroacetate) salt;
f~-(aminoiminomethyl)-N-(pyrazinyl)-2-naphthalenecarboxamide,
mono(trifluoroacetate} salt;
-26-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98/15386
f~-(aminoiminomethyl)-N-(6-methyl-2-pyridinyl)-2-naphthalenecarboxamide,
mono(trifluoroacetate) salt;
6-(aminoiminomethyl)-N-(3,4,5-trimethoxyphenyl)-2-naphthalenecarboxamide,
monohydrochloride
6-(aminoiminomethyl)-N-(3-methyl-2-pyridinyI)-2-naphthalenecarboxamide,
bis(trifluoroacetate) salt;
fi-(aminoiminomethyl}-N-(5-bromo-2-thiazolyll)-2-naphthalenecarboxamide,
mono(trifluoroacetate) salt;
6-(aminoiminomethyl)-N-(5-methyl-2-pyridinyl)-2-naphthalenecarboxamide,
tU mono(trifluoroacetate) salt;
6-(aminoiminomethyl)-N-(4-methyl-2-thiazolyl)-2-naphthafenecarboxamide,
mono(trifluoroacetate) salt;
6-(aminoiminomethyl)-N-(6-quinolinyl)-2-naphthalenecarboxamide,
bis(trifluoroacetate) salt;
15 6-(aminoiminomethyl)-N-( 1 f-1-indazol-6-yl)-2-naphthalenecarboxamide,
bis(trifluoroacetate) salt;
6-(aminoiminomethyl)-N-( I H-indazol-5-yl)-2-naphthalenecarboxamide,
bis(trifluoroacetatej salt;
f~-(aminoiminomethyl)-N-( 1 H-indol-5-yl)-2-naphthalenecarboxamide,
2U mono(trifluoroacetate) salt;
6-(aminoiminomethyl)-N-(5-pyrimidinyl)-2-naphthalenecarboxamide,
mono(trifluoroacetate) salt;
E~-(aminoiminomethyl)-N-(3-pyridazinyl)-2-naphthalenecarboxamide,
mono(trifluoroacetate) salt:
25 fi-(aminoiminomethyl)-N-(5-bromo-2-pyridinylj-2-naphthalenecarboxamide,
mono(trifluoroacetate) salt:
f~-(aminoiminomethyl)-N-[ 3-( 1-methylethoxy)phenyl ]-2-
naphthalenecarboxamide,
mono(trifluoroacetate) salt;
6-(aminoiminomethyl)-N-( 1 H-imidazolyl)-2-naphthalenecarboxamide,
3o bis(trifluoroacetate) salt;
6-[2-[4-(hydroxymethyl)phenylJ-1-cyclopropyl]-2-naphthalenecarboximidamide,
mono(trifluoroacetate) salt;
N-(ethoxycarbonyl)-6-(2-phenyl- I -cyclopropyl)-2-naphthalenecarboximidamide
6-(aminoiminomethyl)-N-(2-methyl-6-quinolinyl)-2-naphthalenecarboxamide,
-27-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98l15386
bis(trifluoroacetate) salt;
6-(aminoiminomethyl)-N-(3-propoxyphenyl)-2-naphthalenecarboxamide,
mono(trifluoroacetate) salt;
6-(aminoiminomethyl)-N-[3-( 1-ethylpropoxy jphenyl]-2-naphthalenecarboxamide,
mono(trifluoroacetate) salt;
6-(aminoiminomethyl)-N-[3-(cyclopentyloxy)phenyl]-2-naphthalenecarboxamide,
mono(trifluoroacetate) salt;
6-(aminoiminomethyl)-N-(3-phenoxyphenyl)-2-naphthalenecarboxamide,
mono(trifluoroacetate) salt;
l 6-(aminoiminomethyl)-N-[3-(phenylmethoxy)phenyl]-2-naphthalenecarboxamide,
mono(trifluoroacetatej salt;
6-(aminoiminomethyl)-N-(3-ethoxyphenyl)-2-naphthalenecarboxamide,
mono(trifluoroacetate) salt;
f~-(aminoiminomethyl)-N-(4-nitrophenyl)-2-naphthalenecarboxamide,
I5 rnono(trifluoroacetate) salt;
fi-(aminoiminomethyl)-N-[3-(cyclobutylmethoxy)phenyl]-2-
naphthalenecarboxamide,mono(trifluoroacetate) salt;
6-[amino(ethoxycarbonyl)iminoJ-N-[3-( I-methylethoxy)phenyl]-2-
naphthalenecarboxamide;
6-(aminoiminomethyl)-4-[5-(ethylsulfonylj-3-furanyl]-N-phenyl-2-
naphthaienecarboxamide,
20 monohydrochloride;
methyl [7-(aminoiminomethyl)-2-methoxy-1-naphthalenyl)carbamate,
mono(trifluoroacetate) salt;
7-methoxy-8-(2-pyrimidinylamino)-2-naphthalenecarboximidamide,
bis(trifluoroacetate) salt;
7-methoxy-8-[(phenylmethyl)amino]-2-naphthalenecarboximidamide,
25 mono(trifluoroacetate) salt;
7-methaxy-8-(phenylamino)-2-naphthalenecarboximidamide, mono(trifluoroacetate)
salt;
7-methoxy-H-[(4-methoxyphenyl)aminoJ-2-naphthalenecarboximidamide,
mono(trifluoroacetate) salt;
(E)-3-[7-(aminoiminomethyl)-2-methoxy-1-naphthalenyl)-2-propenamide,
30 mono(trifluoroacetate) salt;
7-methoxy-8-(3-oxo- I -cyclopenten- I -yl)-2-naphthalenecarboxi midamide,
mono(trifluoroacetate) salt;
methyl 4-[[[7-(aminoiminomethyl)-1-(2-pyrimidinylamino)-2-
naphthalenyl]oxy]methylJbenzoate, mono(trifluoroacetate) salt;
-28-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98/15386
4-[[[7-(aminoiminomethyl)-1-(2-pyrimidinylamino)-2-naphthalenyl ]oxy]methyl]
benzoic acid, mono(trifluoroacetate) salt;
7-methoxy-8-(pyrazinyloxy)-2-naphthalenecarboximidamide, dimethanesulfonate
salt;
7-methoxy-8-(phenylthio)-2-naphthalenecarboximidamide, methanesulfonate;
7-methoxy-8-(pyrazinylamino)-2-naphthalenecarboximidamide,
bis(trifluoroacetate) salt;
methyl 5-[7-[(aminoiminomethyl)-2-naphthalenyl]oxy]pentanoate,
mono(trifluoroacetate) salt;
5-[[6-(aminoiminomethyl)-2-naphthalenyl]oxy]pentanoic acid,
mono(trifluoroacetate) salt;
methyl 4-[[[7-amino(hydroxyimino)methyl]-2-naphthalenyl]oxy]methyl]benzoate;
methyl 2-[[6-(aminoiminomethyl)-2-naphthaIenyl]oxy]acetate,
mono(trifluoroacetate) salt;
1U 7-[2-(4-morpholinyl)ethoxy]-2-naphthalenecarboximidamide,
bis(trifluoroacetate) salt;
2-[[6-(aminoiminomethyl)-2-naphthalenyl]oxy]acetic acid,
mono(trifluoroacetate) salt;
methyl 4-[fi-(aminoiminomethyl)-2-naphthalenyl]oxy]methyl]benzoate,
mono(trifluoroacetate) salt;
methyl [7-(aminoiminomethyl}-1-naphthalenyl]methylcarbamate,
mono(trifluoroacetate) salt;
n propyl [7-(aminoirninomethyl)-1-naphthalenyl]carbamate,
mono(trifluoroacetate) salt;
N-[7-(aminoiminomethyl}-1-naphthalenyl]-N'-methylurea, mono(trifluoroacetate)
salt;
ethyl [7-(aminoiminomethyl)-1-naphthalenyl)carbamate, mono(trifluoroacetate)
salt;
N-[7-(aminoiminomethyl)-1-naphthalenyl)propanamide, mono(trifluoroacetate)
salt;
N-[7-(aminoiminomethyl)-1-naphthalenyl)-2-methoxyacetamide,
mono(trifluoroacetate) salt;
2u N-[7-(aminoiminomethyl)-1-naphthalenyl]urea, mono(trifluoroacetate) salt;
N-[7-(aminoiminomethyl)-1-naphthalenyl]-2-hydroxyacetamide,
mono(trifluoroacetate) salt;
H-(2-pyrimidinylamino)-2-naphthalenecarboximidamide, bis(trifluoroacetate)
salt;
H-amino-2-naphthalenecarboximidanude, bis(trifluoroacetate) salt;
)i-(2-pyridinylamino)-2-naphthalenecarboximidamide, bis(trifluoroacetate)
salt;
?5 N-hydroxy-H-(2-pyrimidinylamino)- 2-naphthalenecarboximidamide,
mono(trifluoroacetate)
salt;
fi-(aminoiminomethyl)-4-(3-furanyl )-N-[4-(trifluoromethyl)phenyl]-2-
naphthalenecarboxamide.
mono(trifluoroacetate) salt;
6-(aminoiminomethyl)-4-(3-furanyl)-N-(4-pyridinyl)-2-naphthalenecarboxamide,
3U dihydrochloride;
f~-(aminoiminomethyl)-4-(3-furanyl)-N-( 1 H-pyrazol-3-yl)-2-
naphthalenecarboxamide,
dihydrochloride;
6-(aminoiminomethyl)-4-(3-furanyl)-N-(3-pyridinyl)-2-naphthalenecarboxamide,
dihydrochloride;
-29-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO_, 99/0509( _ PCTIUS98/15386,
methyl (7-(aminoiminomethyl)-3-[[[4-(aminomethyl)phenyl]amino]carbonyl]-1-
naphathalenyl]carbamate, bis(trifluoroacetate) salt;
6-(aminoiminomethyl)-4-(3-furanyl)-N-(2-pyridinyl)-?-naphthalenecarboxamide,
dihydrochloride;
fi-(aminoiminomethyl)-4-(3-furanyl)-N-phenyl-2-naphthalenecarboxamide,
monohydrochloride
6-(aminoiminomethyl)-4-[ t -(methylsulfonyl)-1 H-pyrazol-4-yl]-N-phenyl-2-
naphthalenecarboxamide, monohydrochloride;
h-(aminoiminomethyl)-4-[5-(methylthio)-3-furanyl)]-N-phenyl-2-
naphthalenecarboxamide,
monohydrochloride;
U) 6-(aminoiminomethyl)-N-[4-(aminomethyl)phenyl]-4-(2-pyrimidinylamino)-2-
naphthalenecarboxamide, tris(trifluoroacetate) salt;
6-(aminoiminomethyl)-N-phenyl-4-(2-pyrimidinylarnino)-2naphthalenecarboxamide,
mono(trifluoroacetatej salt;
N-[(4-(aminomethyl)phenyl]-6-[amino(hydroxyimino jmethyl]-4-(2-
pyrimidinylamino)-2-
t S naphthalenecarboxamide, bis(trifluoroacetate) salt;
6-(aminoiminomethyl)-N-[4-(hydroxymethyl)phenyl]-4-(2-pynmidinylaminoj-
2-naphthalenecarboxamide, mono(trifluoroacetate) salt;
methyl [3-[[[4-(aminomethyl)phenyl]amino]carbonyl]-7-[4-
amino(hydroxyiminojmethyl]-I-
naphthalenyl]carbamate, bis(trifluoroacetate) salt;
2(> fi-(aminoiminomethyl)-N-phenyl-4-(tetrahydro-3-furanyl)-2-
naphthalenecarboxamide,monohydrochloride;
f~-[amino(,hydroxyimino)methyl]-N-phenyl-4-(2-pyrimidinylamino)-2-
naphthalenecarboxamide
6-{aminoiminomethyl)-4-[5-(ethylthio)-3-furanyl]-N-phenyl-2-
naphthalenecarboxamide,monohydrochloride;
25 fi-(aminoiminomethyl)-4-[5-(propylthio)-3-furanyl]-N-phenyl-2-
naphthalenecarboxamide,monohydrochloride;
fi-(aminoiminomethyl)-N-phenyl-4-(2-pyrrolidinyl )-2-naphthalenecarboxamide,
mono(trifluoroacetate) salt;
6-(aminoiminomethyl)-4-[5-(propyisulfonyl)-3-furanyl]-N-phenyl-2-
naphthalenecarboxamide,
act monohydrochloride;
6-(aminoiminomethyI)-4-[5-[methylthio)methyl]-3-furanyl]-N-phenyl-2-
naphthalenecarboxamide, monohydrochloride;
fi-(aminoiminomethyl)-4-[5-(methoxymethyl)-3-furanyl]-N-phenyl-2-
naphthalenecarboxamide,
monohydrochloride;
-30-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99!05096 PCT/US98115386
6-(aminoiminomethyl)-4-[5-(methylsulfonyl)-3-furanyl]-N-phenyl-2-
naphthalenecarboxamide,
mono(trifluoroacetate) salt; and
6-(aminoiminomethyl}-4-[5-(ethythio)tetrahydro-3-furanyl]-N-phenyl-2-
naphthalenecarboxamide, monohydrochloride.
Determination of Urokina a inhibition
The efficacy of the compounds of this invention as urokinase inhibitors was
determined
by measuring the inhibition of the urokinase enzyme Abbokinase (Abbott
Laboratories, Abbott
Park, IL) on substrate S-2444, of formula pyroGlu-Arg-pNA-HCl (DiaPharma
Group, Inc.
1o Distributor of Chromogenix) at 200 ~M.
The assay was performed in a 96 well polystyrene, flat bottom plate in a 50 mM
Tris/0.15 M NaCI + 0.5 % Pluronic F-68 (Sigma P-5556), pH 7.4 (with HCI)
buffer. The
compounds of this invention, 10 mM in DMSO, were diluted with DMSO to eight
half log
concentrations, for example: 1200 pM, 40() ~M, 120 pM, 40 ~M, 12 ~tM, 4 ~tM, 1
~tM
tS and 0.4 pM. Four concentrations were chosen, then 5 ~I of each were diluted
to a total
assay volume of 200 X11. The final compound concentrations in the assay,
according to the
above example, were 30 p.M, 10 ~M, 3 ~tM, 1 pM, 0.3 ~M, 0.1 ~tM, 0.03 p.M and
0.01
pM, respectively. The substrate S-2444 was used at 200 pM in the assay.
Several vials
were reconstituted as directed on the vial, aliquoted and stored frozen. The
enzyme was
20 further diluted in assay buffer and 10 ~tl was used in the assay. Enzyme
concentration in
the assay was 2-3 nM. The assay was performed as follows: 175 pL of buffer was
pipetted into the polystyrene plate, 5 ~L solution of a compound of this
invention in
DMSO was added, the mixture was vortexed, 10 ~L of enzyme in buffer was added,
the
mixture was vortexed, i 0 ~tL of substrate in water was added, the mixture was
vortexed,
25 and the plate was placed in a Spectromax ~ (Molecular Devices Corporation,
Sunnyvale,
Ca) plate reader to follow the course of the reaction for 15 min at 405 nm.
The Spectromax
~ calculated the reaction rates which were used to calculate percent
inhibition of the
compounds of this invention versus the reaction rate of the enzyme in the
absence of any
inhibitor. The Ki 's of the inhibitors were calculated from the percent
inhibition and
3U previously established Km. The compounds of this invention inhibit
urokinase as shown
by the data for representative examples in Table 1.
Ta 1 1
Inhibitory Potency of Representative Compounds Against Urokinase
-3I-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98I15386
Exam Ie ICSp (~,M)
1 6.6 I
2 9.8
3 36
4 p,$
2.5
6 2.3
3.5
0.1
I.l
3.2
14 0.33
2.5
16 0.03
I ~ 4.26
1 ~ 0.42
19 2.21
0.803
21 I .7
22 1.7
23 4.0
24 4.9
2.1
26 0.04
2~ 0.93
2~ 2.1
2'~ 2.5
3.6
-32-
SUBSTITUTE SHEET (RULE 28)
CA 02294300 1999-12-14
WO 99105fl96 PCTIIJS98/15386
31 2.93 I
32 4.6
33 2.4
34 3.5
35 3.97
36 1.75
37 2.34
6.35
39 12.2
40 0.31
41 2.38
42 2.08
43 2.2
44 0. 3 5
45 2.94
46 2.4
47 4.8
4~ 1.3
49 3.3
5() 6.13
51 4.7
52 4.7
53 2.96
54 2.7
55 0.9
56 2.9
57 3.4
5~ 2.53
59 0.41
-33-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98/15386
60 0.72
61 0.73
62 0.64
__ ,
63 0.37
64 0.56
65 0.54
66 3. I 3
67 2.78
68 1.74
69 1.38
70 2.57
71 2.39
72 4.30
73 3.3
74 I .61
75 2.09
76 0.96
77 0.23
78 3.57
7'~ 0.96
80 ! .93
81 3.21
82 3.08
83 2.24
84 10.0
85 1.38
86 3.6
I 87 0.63
88 2.73
-34-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO_ 99105096 PCTIUS98/I5386,
9 6.5
90 0.07
91 0.05
92 0.04
g 3 2.36
95 1.73
9 6 0.86
97 1.31
9 8 0.24
9 9 3.02
100 3.16
1 01 0.8
1 02 0.34
1 03 0.57
104 1.2
1 05 0.84
1 06 0.76
1 a7 2.34
1 08 0.996
1 09 2.85
110
111 4.17
1 1 2 0.45
1 1 3 0.403
1 1 4 0.344
1 1 5 0.063
-35-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98/15386
1 1 6 0.045
1 1 7 0.278
1 1 8 0.121
1 1 9 4.41
1 20 0.93
1 21 0.89
1 22 0.33
1 23 1 .24
124 0.12
1 25 0.23
1 26 0.87
1 27 0.085
1 29 4.84
131 4.18
132 O.g6
1 33 0.044
1 34 0.064
1 35 1 .91
1 36 1 .67
1 37 0.82
1 38 0.46
1 39 2.64
1 40 0.46
141 0.00117
i 42 0.54
1 43 0.36
-36-
SU6STITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98/15386
1 44 1 .26 I
I
1 45 2.59
1 46 0.372
147 0.213
148 O,g1
149 3,g
150 0.16
1 52 0.083
153 0,877
1 54 0.035
1 55 2.33
156 0,1g
157 3.12
158 O,Og
1 59 2.23
1 60 2.62
1 61 1 .59
1 62 1 .33
1 63 0.03
1 64 0.45
165 0.00068
1 66 0.002
1 67 0.052
1 68 0.003
1 70 0.695
1 71
>30
-37-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WQ 99/05096 PCT/US98/15386
1 74 0.1 7
1 76 S.Oi 1
1 77 14.9
1 79 1 .61
1 80 0.097
1 81 8.92
i 82 >30
1 84 0.375
185 3.19
1 86 2.98
1 87 0.61 3
1 88 0.22
i 89 1 .3
i 90 0.565
1 91 0.887
1 92 1 .6
1 93 0.85
1 94 0.56
1 95 1 .3
1 96 0.62
1 97 2.03
1 98 0.504
1 99 2.3
200 0.037
201 0.077
202 0.792
-38-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98115386
203 0.624
204 0.331
205 0.337
206 5.73
207 4,12
208 0.96
209 0.82
21 0 0.78
21 1 0.072
212 5.09
213 4,5g
21 4 2.559
215 1.1
21 6 >30
21 7 0.67
21 8 0.086
219 0.103
220 0.03
221 0.52
222 0.346
223 0.07
224 1 .773
225 0.1 04
226 >30
227 0.015
228 0.025
-39-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99!05096 PCT/US98/15386
229 0.1 17
230 0.1 03
231 0.015
232 0.123
Pharmaceutical Compositions
The present invention also provides pharmaceutical compositions which comprise
compounds of the present invention formulated together with one or more non-
toxic
pharmaceutically acceptable carriers. The pharmaceutical compositions may be
specially
formulated for oral administration in solid or liquid form, for parenteral
injection or for rectal
administration.
The pharmaceutical compositions of this invention can be administered to
humans and
other animals orally, rectally, parenterally , intracisternally,
intravaginally, intraperitoneally or
In topically (such as powders, ointments or drops), bucally or as an oral or
nasal spray. The term
"parenteral" administration, as used herein, refers to modes of administration
which include
intravenous, intramuscular, intraperitoneal, intrasternal, subcutaneous and
intraarticular
injection and infusion.
Pharmaceutical compositions of this invention for parenteral injection
comprise
15 pharmaceutically acceptable sterile aqueous or nonaqueous solutions,
dispersions, suspensions
or emulsions as well as sterile powders for reconstitution into sterile
injectable solutions or
dispersions just prior to use. Examples of suitable aqueous and nonaqueous
carriers, diIuents,
solvents or vehicles include water, ethanol, polyols (such as glycerol,
propylene glycol,
polyethylene glycol and the like), and suitable mixtures thereof, vegetable
oils (such as olive
20 oilj and injectable organic esters such as ethyl oleate. Proper fluidity
can be maintained, for
example, by the use of coating materials such as lecithin, by the maintenance
of the required
particle size in the case of dispersions and by the use of surfactants.
These compositions may also contain adjuvants such as preservative, wetting
agents,
emulsifying agents and dispersing agents. Prevention of the action of
microorganisms may be
25 ensured by the inclusion of various antibacterial and antifungal agents,
for example, paraben,
chlorobutanol, phenol sorbic acid and the like. It may also be desirable to
include isotonic
agents such as sugars, sodium chloride and the like. Prolonged absorption of
the injectable
-40-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98115386
pharmaceutical form may be brought about by the inclusion of agents (such as
aluminum
monostearate and gelatin) which delay absorption .
In some cases, in order to prolong the effect of the drug, it is desirable to
slow the
absorption of the drug from subcutaneous or intramuscular injection. This may
be
s accomplished by the use of a liquid suspension of crystalline or amorphous
material with poor
water solubility. The rate of absorption of the drug then depends upon its
rate of dissolution
which, in turn, may depend upon crystal size and crystalline form.
Alternatively, delayed
absorption of a parenterally administered drug form is accomplished by
dissolving or
suspending the drug in an oil vehicle.
11) lnjectable depot forms are made by forming microencapsule matrices of the
drug in
biodegradable polymers such as polylactide-polyglycolide. Depending upon the
ratio of drug to
polymer and the nature of the particular polymer employed, the rate of drug
release can be
controlled. Examples of other biodegradable polymers include poly(orthoestersj
and
poly(anhydrides). Depot injectable formulations are also prepared by
entrapping the drug in
~ 5 liposomes or microemulsions which are compatible with body tissues.
The injectable formulations can be sterilized, for example, by filtration
through a bacterial-
retaining filter or by incorporating sterilizing agents in the form of sterile
solid compositions
which can be dissolved or dispersed in sterile water or other sterile
injectable media just prior to
use.
20 Solid dosage forms for oral administration include capsules, tablets,
pills, powders and
granules. In such solid dosage forms, the active compound is mixed with at
least one inert,
pharmaceutically acceptable excipient or carrier such as sodium citrate or
dicalcium phosphate
and/or a) fillers or extenders such as starches, lactose, sucrose, glucose,
mannitol and silicic
acid; b) binders such as carboxymethylcellulose, alginates, gelatin,
polyvinylpyrrolidone,
s sucrose and acacia; c) humectants such as glycerol; d) disintegrating agents
such as agar-agar,
e;alcium carbonate, potato or tapioca starch, alginic acid, certain silicates
and sodium carbonate;
e) solution retarding agents such as paraffin; f) absorption accelerators such
as quaternary
ammonium compounds; g) wetting agents such as cetyl alcohol and glycerol
rnonostearate; h)
absorbents such as kaolin and bentonite clay and i) lubricants such as talc,
calcium stearate,
30 magnesium stearate, solid polyethylene glycols, sodium lauryl sulfate and
mixtures thereof. In
the case of capsules, tablets and pills, the dosage form may also comprise
buffering agents.
Solid compositions of a similar type may also be employed as fillers in soft
and hard-filled
gelatin capsules using such excipients as lactose or milk sugar as well as
high molecular weight
polyethylene glycols and the like.
-41-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99!05096 PCTIUS98115386
The solid dosage forms of tablets, dragees, capsules, pills, and granules can
be
prepared with coatings and shells such as enteric coatings and other coatings
well known in the
pharmaceutical formulating art. They may optionally contain opacifying agents
and may also be
of a composition such that they release the active ingredients) only, or
preferentially, in a
certain part of the intestinal tract, optionally or in delayed fashion.
Examples of embedding
compositions which can be used include polymeric substances and waxes.
The active compounds may also be in micro-encapsulated form, if appropriate,
with one
or more of the above-mentioned excipients.
Liquid dosage forms for oral administration include pharmaceutically
acceptable
1u emulsions, solutions, suspensions, syrups and elixirs. In addition to the
active compounds,
the liquid dosage forms may contain inert diluents commonly used in the art
such as water or
other solvents, solubilizing agents and emulsifiers such as ethyl alcohol,
isopropyl alcohol,
ethyl carbonate, ethyl acetate, benzyl alcohol, benzyl benzoate, propylene
glycol, 1,3-butylene
glycol, dimethyl formamide, oils (in particular, cottonseed, groundnut, corn,
germ, olive,
15 castor, and sesame oils), glycerol, tetrahydrofurfuryl alcohol,
polyethylene glycols and fatty
acid esters of sorbitan and mixtures thereof.
Besides inert diluents, the oral compositions may also include adjuvants such
as wetting agents,
emulsifying and suspending agents, sweetening, flavoring and perfuming agents.
Suspensions, in addition to the active compounds, may contain suspending
agents such as
2o ethoxylated isostearyl aicohols, polyoxyethylene sorbitol and sorbitan
esters, microcrystalline
cellulose, aluminum metahydroxide, bentonite, agar-agar, and tragacanth and
mixtures thereof.
Compositions for rectal or vaginal administration are preferably suppositories
which can be
prepared by mixing the compounds of this invention with suitable non-
irritating excipients or
e:arriers such as cocoa butter, polyethylene glycol or a suppository wax which
are solid at room
25 temperature but liquid at body temperature and therefore melt in the rectum
or vaginal cavity and
release the active compound.
Compounds of the present invention can also be administered in the form of
liposomes.
As is known in the art, liposomes are generally derived from phospholipids or
other lipid
substances. Liposomes are formed by mono- or multi-lamellar hydrated liquid
crystals which
3a are dispersed in an aqueous medium. Any non-toxic, physiologically
acceptable and
metabolizable lipid capable of forming liposomes can be used. The present
compositions in
liposome form can contain, in addition to a compound of the present invention,
stabilizers,
preservatives, excipients and the like. The preferred lipids are the
phospholipids and the
phosphatidyl cholines (lecithins), both natural and synthetic. Methods to form
liposomes are
-42-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98/15386
known in the art, for example, Prescott, Ed., Methods in Cell Biology, Volume
XIV,
Academic Press, New York, N.Y. (197b), p. 33 et seq.
Dosage forms for topical administration of a compound of this invention
include
powders, sprays, ointments and inhalants. The active compound is mixed under
sterile
conditions with a pharmaceutically acceptable carrier and any needed
preservatives, buffers or
propellants which may be required. Opthalmic formulations, eye ointments,
powders and
solutions are also contemplated as being within the scope of this invention.
Actual dosage levels of active ingredients in the pharmaceutical compositions
of this
invention may be varied so as to obtain an amount of the active compounds)
that is effective to
! o achieve the desired therapeutic response for a particular patient,
compositions, and mode of
administration. The selected dosage level will depend upon the activity of the
particular
compound, the route of administration, the severity of the condition being
treated and the
condition and prior medical history of the patient being treated. However, it
is within the skill
of the art to start doses of the compound at levels lower than required for to
achieve the desired
! 5 therapeutic effect and to gradually increase the dosage until the desired
effect is achieved.
Generally dosage levels of about 1 to about 50, more preferably of about 5 to
about 20 mg, of
active compound per kilogram of body weight per day when administered orally
to a
mammalian patient. If desired, the effective daily dose may be divided into
multiple doses for
purposes of administration, e.g. two to four separate doses per day.
Preparation of Compounds of this Invention
The compounds of this invention may be prepared by a variety of synthetic
routes.
Representative procedures are outlined in the following Schemes 1-6.
'S Abbreviations
Abbreviations which have been used in the descriptions of the schemes and the
examples that follow are: THF for tetrahydrofuran; DMF for N,N-
dimethylformamide; OEt2 for
diethyl ether; EDC for 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide
hydrochloride; NMM
for N-methylmorpholine; LDA for lithium diisopropylamide; TFA for
trifluoroacetic acid;
DMSO for dimethylsulfoxide; DMAP for 4-(N,N-dimethylamino)pyridine; HATU for
O-(azabenzotriazole-1-yl)-N,N,N',N'-tetramethyluroniumhexafluorophosphate; Boc
for tert-
butylcarbonyioxy; DDQ for 2,3-dichloro-5,6-dicyano-1,4-benzoquinone; Bn for
benzyl; DPPA
for diphenylphosphoryl azide; DCC for dicyclohexylcarbodiimide; EDC for 1-(3-
dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride; SEM for 2-
-43-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTJUS98115386
(trimethylsilyl)ethoxymethyl ; dppf for l,1'-bis(diphenylphosphino)ferrocene;
and dba for
dibenzylideneacetone. Starting materials, reagents and solvents were purchased
from Aldrich
Chemical Company (Milwaukee, Wi).
As shown in Scheme 1, naphthalenecarbonitriles 4, 5_ and fi were prepared by
treating 3-
cyanopropionaldehyde diethyl acetal 2 with a strong base such as lithium
diisopropylamide then
treating the resulring anion with the appropriately substituted benzaldehyde
I_ followed by
cyclization and aromatization of the corresponding cyanohydrins ~ with a Lewis
acid such as
sulfuric acid.
t0
hem I
C C OH
B ~ CHO H CO~CN B ~ CN
~'3
A ~ OCH3 A ~ OCH3
3 OCH3
1 2
A, B and C are hydrogen and -LARA, -L~RB, -L~R~
-LA-, -LB- and -L~- are -O-
RA, R~ and R~ are alkyl
C
B ~ ~ CN
A
4: A is hydrogen; B and C are OCH3
5: A, B and C are OCH3
f~: A and B are OCH3, and C is hydrogen
t5 As shown in Scheme 2, selective demethylation of 4 with a Lewis acid such
as A1CI3 or
BBr3, preferably AlCl3, provided 7. 7 was treated with a base such as
potassium carbonate,
sodium hydride or cesium fluoride followed by RC-X, wherein X is a leaving
group, to provide
-44-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98/15386
X (-L~- is -O-). Alternatively, treatment of 7 with trifluoromethanesulfonic
anhydride or I,I,I-
trifluoro-N-phenyl-N-[(trifluoromethyl)sulfonyl]methanesulfonamide provided 9
which may
Rc-B, ~ ~ B,
be treated with Rc-B(OH)2, O , Rc-I, or R~ ~ wherein Rc is
unsubstituted or substituted aryl or heterocycle, in the presence of a
palladium catalyst,
S preferably Pd(II)C12(dba) or Pd(Ph3P)4, and base, preferably cesium fluoride
or potassium
carbonate, to provide 10. Alternatively, 9 may be treated with R~-NR 1 R2,
wherein R~ is
unsubstituted or substituted aryl or heterocycle, and at least one of R1 or R~
is hydrogen, in the
presence of a strong base, such as potassium t-butoxide, and a catalyst, such
as Pd(II)C12(dba),
to provide 1 I.
Scheme 2
OH LcRc
\ ~ CN HsC ~ ~ ~ CN
4
H / / ~ H / /
7 $
OTf LcRc
H3C I ~ ~ CN H3C ~ ~ ~ CN
H / / ~ H / /
y
R~ is unsubstituted or substituted aryl
or heterocycie
10: -L~- is a covalent bond
1 I : -L~- is -NR1_
iS As shown in Scheme 3, selective O-triflation of 12 followed by protection
of the amino
group of the resulting 13 with acid-labile carbobenzlyoxy provided 14.
Conversion of _14 to _15
was achieved with KCN in the presence of a palladium catalyst, preferably
-45-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98115386
tris(dibenzylideneacetone)dipalladium(0)-chloroform adduct, and deprotection
of,~ to provide
16 was accomplished with acid, preferably 30~1e HBr in acetic acid. Treatment
of 15 with
acylating agents R~C(O)Cl and base, preferably triethylamine,
diisopropylethylamine or
potassium carbonate provided intermediate 17.
S
Scheme 3
NH2 NH2 NHCbz NHCbz
/ \ OH \ \ OTf / \ OTf / \ CN
12 13 14 1~
NH2. HBr ~cRc
/ \ CN / \ CN
\ / ~ \ I /
16 l
-L~- is -C(O)NRI- or -OC(O)NR~- and
R~ is unsubstituted or substituted alkyl,
alkeneyl, hererocycle or aryl
1~~ As shown in Scheme 4, selective demethylation of the 8-methoxy group of 18
with a
Lewis acid such as AlCl3 or BBr3, preferably AICl3, was followed by
reprotection of phenol 19
by alkylation with Bn-X, wherein X is Cl, Br or I, in the presence of a base
such as potassium
carbonate, sodium hydride or cesium t7uoride. 2() was prepared by
deprotonation of this
intermediate with a strong, non-nucleophilic base swh as lithium, sodium or
potassium
n diisopropylamide or alkoxide followed by treatment with an alkyl formate,
preferably ethyl
formate to provide enol 2(l. Treatment of 20 with hydroxylamine provided
isoxazole 21 which
may be opened with lithium, sodium or potassium alkoxide, preferably sodium
methoxide, to
provide 22. Carbonyl reduction with concomitant alkene formation with achieved
with sodium
borohydride to provide 23; and aromatization with DDQ and catalytic
debenzylation with
2U hydrogen and a palladium catalyst, preferably palladium on carbon, provided
24. 24 was
-4H-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98115386
alkylated by treatment with a base such as potassium carbonate, sodium hydride
or cesium
fluoride followed by treatment R~-X.
~heme 4
~cRcO OH O C O OH C O-;
\ -."~ I \ ~ I \ / _~ I \
A / A / A / A /
Ik 19 20 21
A is -LARA, and C is -L~R~, wherein C is -L~R~, wherein L~ is -O- and
-LA- and -L~- are -O- and RA is alkyl R~ is arylalkyl
C O C OH
\ C~ , \ \ C ~ ~ \ \ CN
A A / A / /
'2 23 24
A is -LARA, wherein
-LA- is -O- and RA is alkyl
As shown in Scheme 5, monohydrolysis of 25 with one equivalent of base such as
lithium, sodium or potassium hydroxide provided the acid-ester 2-fi. Treatment
of 2fi with
m thionyl chloride or oxalyl chloride/DMF followed by treatment with ammonia
provided amide
27. Treatment of 27 with a dehydrating agent such as thionyl chloride or
phosphorus
oxychloride provided nitrile 2lS.
Regioselec;tive nitration of 2H with nitric acid/potassium nitrate followed by
reduction of
the vitro group with a palladium catalyst, preferably palladium on carbon,
provided intermediate
t 5 31, which was treated with R~C(O)Cl or R~OC(O)Cl and base, preferably
diisopropylethylamine or potassium carbonate, to provide 37.
Hydrolysis of 2K with one equivalent of lithium, sodium or potassium hydroxide
to
form carboxylic acid 29 followed by treatment with DPPA or thionyl chloride
then sodium azide
and hydrolysis of the intermediate isocyanate 32 with acid, preferably
sulfuric acid, provided
2o amine 33. Alternatively, treatment of 32 with a primary or secondary amine
provided urea 34
-47-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98/15386
(-LA- =-NR~C(X)R~-, wherein X is O).
2~ may be coupled to primary or secondary amines, and 33 may be coupled to
carboxylic acids to form amides 35 and 36, respectively. In either case, the
amines and
carboxylic acids are coupled using a dehydrating agent such as DCC, EDC or
HATLI.
Scheme 5
\ \ C02C~ ( \ \ C02~ I \ \ CONH2
H3CO2C / / H3CO2C / / H3CO2C / /
25 26 27
\ \ CN [ \ \ CN , \ \ CN
RALA / / ~ O2C / / -' RALA / /
29 30
-LA-is a covalent bond and
R~ is alkoxycarbonyl
\ \ CN \ \ CN
--
O=C=N / / I
RA LA
3? _34
NH2
CN
\ \
RADA / / I \ \ CN
31 H2N / /
33
-48-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTlUS98/15386
LcRC
~ CN
/ /
RALA
-L~- is -C(X)NR1- or -OC(O)NRI- and
RC is unsubstituted or substituted alkyl,
alkeneyl, hererocycle or aryl;
XisO
CN
29 --~
Rata,
-LA- is -NR1C(X)_
wherein X is O
CN
33
/
RA LA
36
-LA- is -C(X}NR1-
wherein X is O
S As shown in Scheme 6, intermediates wherein -LA- is -C---C- or -C=C- were
prepared
by treatment of of 38 with a triflaring agent, preferably- t1-
ifluoromethanesulfonyl anhydride, to
form 39 followed by coupling of the appropriate substituted alkenes or
unsubstituted or
substituted alkynes using a palladium catalyst, preferably palladium (II)
acetate, to form 4U.
-49-
SUBSTITUTE SHEET (RUE.E 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98/15386
Sch m
\ \ CN \ \ CN I \ \ CN
/ /
HO / / Tf0 / / RAt_A
3R 39 40
S
As shown in Scheme 7, conversion of the nitrite intermediates to the
carboximidamide
urokinase inhibitors 41 was achieved by three methods: (1) treatment of the
intermediate
carbonitriles with a non-nucleophilic base such as lithium, sodium or
potassium
bis(trimethylsilylamide), preferably lithium bis(trimethylsilylamide} followed
by hydrolysis
tc~ with acid, preferably HCI; (2) treatment of the nitrite with acid,
preferably HCI, followed by
treatment with ammonium acetate; and (3) treatment of the nitrite with H2S
followed by
treatment with ammonia gas in methanol. Of the three methods, the
H2S/NH3/methanol method
is preferred. The compounds of the invention were precipitated as
hydrochloride or methane
sulfonate salts or were purified by reversed phase medium pressure
chromatography to form
15 mono- or bis- trifluoroacetate salts.
Scheme 7
C C NH
\ \ CN g ~ \ \ NH2
/ / ~ A / /
41
Synthetic Methods
The foregoing may be better understood by reference to the following examples
which
illustrate the methods by which the compounds of the invention may be prepared
and are not
intended to limit the scope of the invention as defined in the appended
claims.
2S
Example 1
-50-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98/15386
7 8-Dimethoxy-2-nanhthalenecarboximidamide mono(trifluoroacetate) a~
Exam lp a 1 A
7. 8-Dimethoxy-2-narJhthalenecarbonitrile
A solution of freshly prepared LDA in THF at -78 °C was treated
dropwise with 3-
cyanopropionaldehyde diethyl acetal (3.0 g) in THF (5 mL), stirred for 1 h,
treated with 2,3-
dimethoxybenzaldehyde (3.2 g) in THF (5 mL), warmed to room temperature over
90 min,
treated with water (40 mL), concentrated and extracted with chloroform. The
organic layer was
washed with brine, dried (MgS04) and concentrated to provide 1.5 g of a yellow
oil.
tU MS (DCI/NH3) m/e 341 (M+H20)+.
A solution of the oil in methanol (5 mL) was added dropwise to 20% aqueous
sulfuric
acid ( 100 mL) at 90 °C. The solution was heated for 90 min, cooled to
room temperature and
extracted with chloroform. The combined organic extracts are washed with
brine, dried
{MgS04) and concentrated to provide I.0 g of a brown solid which was purified
by flash
a 5 chromatography on silica gel with 3:1 hexane/ethyl acetate to provide 800
mg of the title
compound.
MS (DCI/NH3) m/e 231 (M+H20)+.
Example 1 B
20 7.8-Dimethoxv-2-naphthalenecarboximidamide monoltrifluoroacetatel salt
A solution of Example 1 (200 mg) in THF (5 mL) at 0 °C was treated with
lithium
bis(trimethyisilylamide) (1.0 M in hexane, I.1 mL), stirred for 18 h at room
temperature,
treated with 10% HCl ( 10 mL), stirred for 24 h at room temperature,
concentrated and purified
by medium pressure liquid chromatography on a 30 cm x 2 cm C-18 column (40
micron, J.T
25 Baker) with UV detection at 250 nM with solvent mixtures in a gradient
ranging from 90%A
(0.1 % aq TFA)/lO~loB (methanol) to 10%A/90%B over 160 min at a flow rate of 5
mL/min
(fractions were collected every 2 min for i00 min and analyzed by TLC (10:1:1
acetonitrile/water/acetic acid) for product) to provide 100 mg of the title
compound.
tH NMR (300 MHz, DMSO-d6} 8 4.41 {s, 3H), 4.62 (s, 3H), 7.41 (d, 1H), 7.43
(dd, 1H),
30 7.60 (d, 1 H}, 7.80 (d, 1 H), 8.49 (d, 1 H), 9.31 (bs, ZH), 9.48 (bs, 2H);
MS (DCI/NH3) m/e 231 (M+H)+.
Anal. calcd for C13H14N2O2~TFA: C, 52.33; H, 4.39; N, 8.14. Found: C, 51.91;
H, 4.35; N,
8.05.
-51-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98/15386
Exam
6. 7. 8-trimethoxy-2-naphthalenecarboximidamid monoftrifluoroacetatel salt
Example 2A
6. 7. 8-trimethoxv-2-nat~hthalenecarbonitrile
The title compound was prepared as described in Example 1 A but substituting
2, 3, 4-
trimethoxybenzaldehyde for 2, 3-trimethoxybenzaldehyde.
MS (DCIlNH3) m/e 261 (M+H20)+.
W Exam In a 2B
6. 7. 8-trimethoxy-2-nat~hthalenecarboximidamide mono(trifluoroacetate) Salt
The title compound was prepared and purified as described in Example 1B to
provide
100 mg of the title compound.
~H NMR (DMSO-d6, 300 MHz) S 3.9I (s, 3H), 3.98 (s, 3H), 4.06 (s, 3H), 7.36 (s,
IH),
1s 7.75 (dd, 1 H) 7.99 (d, 1 H), 8.49 (d, 1 H), 9.18 (bs, 2H), 9.38 (bs, 2H);
MS (DCI/NH3) m/e 261 (M+H)+.
Anal. catcd for C~4H16N203~TFA: C, 51.34; H, 4.58; N, 7.48. Found: C, 50.91;
H, 4.25; N,
7.35.
Ex m 1
fi 7-dimethoxv-2-na~hthalenecarboximidamide monoftrifluoroacetate salt
Example 3A
fi~7-dimethox -2-naphthalenecarbonitrile
25 The title compound was prepared as described in Example 1 A but
substituting 3,4-
dimethoxybenzaldehyde for 2, 3-dimethoxy-benzaldehyde to provide 1.3 g of the
title
compound.
MS (DCI/NH3) m/e 231 (M+H20)+.
Example 3B
6. 7-dimethoxv-2-naohthalenecarboximidamide mono(trifluoroacetate) salt
The title compound was prepared and purified as described in Example 1 B to
provide
100 mg of the title compound.
-52-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98115386
1H NMR (DMSO-d~,, 300 MHz) 8 3.92 (s, 3H), 3.94 (s, 3H), 7.41 (s, 1H), 7.44
(s, 1H),
7.69 (dd, 1H), 7.93 (d, 1H), 8.49 (d, 1H), 9.18 (bs, 2H), 9.38 (bs, 2H);
MS (DCI/NH3) m/e 231 (M+H)+.
Anal. calcd for C13H14N2O2~TFA: C, 52.33; H, 4.39; N 8.14. Found: C, 52.15; H,
4.20; N
8.10.
Example 4
2-f (7-Aminoiminomethvl-2-methox -~phthalenyl)oxylacetamide
mono(trifluoroacetate) salt
m Example 4A
7-Methoxv-R-hydroxvnaphthalene-2-carbonitriIe
A solution of Example 1 A ( 1 g) in methylene chloride ( 100 mL) at 0
°C was treated with
A1CI3, stirred for 4 h at 0 °C and at room temperature for I 8 h,
poured into water (200 mL)
containing 6N HCl (20 mL), stirred for I h and diluted with methylene chloride
(100 mL). The
t5 layers were separated, and the organic layer was washed with brine and
dried (MgS04) to
provide 81 U mg of the title compound as an off-white solid.
MS (DCI/NH3) m/e 217 (M+H20)+.
Example 4B
20 1 Ll-Dimethvlethvl 2-I(7-Cvano-2-methoxv-1-naphthalenyl)oxylacetate
A slurry Example 4A (100 mg), K2C03 (70 mg), t-butyl bromoacetate
( 120 mgj and tetrabutylammonium iodide (25 mg) in DMF (3 mL) was stirred for
18 h at room
temperature, diluted with water (20 mL) and extracted with ethyl acetate. The
organic extract
was washed with saturated NaHC03 and brine, dried (Na2S04) and concentrated to
provide
25 200 mg of the title compound as a clear oil.
MS (DCI/NH3j m/e 331 (M+H~O)+.
Example 4C
21(7-Aminoiminomethvl-2-methoxv-1-naphthalenyl)oxviacetamide
mono(trifluoroacetatel salt
30 Example 4B ( 100 mg) in methanol (S mL) at 0 °C was treated with
HCl(g), stirred for
18 h at room temperature and concentrated to provide an off-white solid. The
solid was treated
with 2N NH3 in methanol (10 mL), heated at 50 °C for 3.5 h, cooled and
concentrated to a
yellow solid which was purified as described in Example 1 B to provide 10 mg
of the title
compound.
-53-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98115386
1H NMR (300 MHz, DMSO-d6) 8 3.93 (s, 3H), 4.79 (s, 2H), 7.55 (d, 2H), 7.65
(dd, 1H),
7.72 (d, 1 H}, 7.85 (d, 1 H), 8.09 (d, 1 H), 8.7 (d, I H), 9.03 (bs, 2H), 9.45
(bs, 2H);
MS (DCI/NH3) m/e 2?4 (M+H)+.
Anal. calcd for Ct4H15N303'TFA: C, 49.62; H, 4.16; N, 10.85. Found: C, 49.33;
H, 4.03;
s N, 10.50.
Example 5
7-Benzvloxv-8-iodo-2-naohthalenecarboximidamide mono(trifiuoroacetate) salt
1o Example SA
7-Benzvloxy-8-iodo-2-naphthalenecarbonitrile
The title compound was prepared as described in Example 43A but substituting
benzyl
bromide for propyl iodide.
MS (DCI/NH3) m/e 403 (M+NH4}+.
15 Example SB
7-Benzvloxv-8-iodo-2-naphthalenecarboximidamide mono(trifluoroacetate) salt
The title compound was prepared from Example SA according to the procedure of
Example I B.
1H NMR (300 MHz, DMSO-d6) 8 9.30 (br, 4H}, 8.44 {s, 1H), 8.I2 (d, IH), 7.71
(d, 2H),
20 7.67 (dd, 1 H), 7.57 (d, 2H), 7.45-7.34 (m, 3H), 5.45 (s, 2H);
MS (DCI/NH3) m/e 403 (M+H)+.
Anal. calcd for C~gH15N~0I~TFA: C, 46.53; H, 3.12; N, 5.43. Found: C; 46.55;
H, 3.10; N,
5.19.
'-5 Example 6
Methvl I(7-aminoiminomethvI-2-methox -y 1-naphthalen I)oxylacetate
mono(trifluoroacetate)
salt
Example 6A
30 Methvl f(7-cvano-2-methoxv-1-naphthalen 1 oxviacetate
A solution of Example 4A (600 mg), Cs2C03 (500 mg), t-butyl bromoacetate (120
mg}
and tetrabutylammonium iodide (2S mg) in DMF ( I S mL) was stirred for 18 h at
room
temperature, diluted with water (20 mL) and extracted with ethyl acetate. The
organic extract
-S4-
SU9STiTUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99!05096 PCT/US98J15386
was washed with saturated NaHC03 and brine, dried (Na2S04) and concentrated to
provide
800 mg of the title compound as a clear oil.
MS (DCI/NH3) m/e 331 (M+H20)+.
Example 6B,
Methyl f(7-aminoiminomethvl-2-methoxy-1-naphthalen~rllox~rlacetate
mono(trifluoroacetate~
Example 6A (100 mg) in methanol (30 mL) at 0 °C was treated with
HCI(g), stirred for
18 h at room temperature and concentrated to provide an off-white solid. The
solid was treated
~0 with ammonium acetate (100 mg) in methanol (10 mL), heated at 40 °C
for 15 h, cooled and
concentrated to a yellow solid which was purified as described in Example IB
to providel0 mg
of the title compound.
I H NMR (300 MHz, DMSO-d6) 8 3.65 (s, 3H), 3.93 (s, 3H}, 4.79 (s, 2H), 7.65
(dd, 1 H),
7.72 (d, 1 H}, 7.85 (d, I H), 8.09 (d, 1 H), 8.7 (d, 1 H), 9.03 (bs, 2H),
~ 5 9.45 (bs, 2H);
MS (DCI/NH3) m/e 289 (M+H)+.
Anal. calcd for C~SHt6N204~TFA: C> 50.75; H, 4.26; N, 6.96. Found: C, 50.42;
H, 4.03; N,
6.77.
20 Exam lie 7.
f(7-aminoiminomethvl-2-methox -~-naphthaien Iv )oxvlacetic acid
mono~trifluoroacetatel salt
A solution of Example 6B ( 100 mg) and LiOH~HzO ( I 50 mg) in 1: I THF/H20 ( I
0 mL)
at 5 °C was stirred 45 min and concentrated to provide an off-white
solid. The solid was
dissolved in 1 N HCl (20 mL), stirred 48 h at room temperature and filtered.
The resulting
?5 white solid was purified as described in Example 1B to provide 20 mg of the
title compound.
~ H NMR (300 MHz, DMSO-d~) 8 3.93 (s, 3H), 4.79 (s, 2H), 7.65 (dd, 1 H), 7.72
(d, I H),
7.85 (d, IH), 8.09 (d, 1H), 8.7 (d, 1H), 9.23 (bs> 2H), 9.45 (bs, ZH);
MS (DCI/NH3) m/e 275 (M+H)+.
Anal. calcd for C~4H14N204~TFA: C, 49.49; H, 3.89; N, 7.21. Found: C, 47.53;
H, 3.71; N,
~0 6.83.
Example 8
N-f4-(Aminomethvl)~henvll-6-aminoiminomethyl-2-nanhthalenecarboxamide
bis~trifluoroacetate) salt
-55-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US9811538ø
Examlhe ~A
2 6-Nanhthalenedicarboxvlic acid monomethvl ester
A suspension of dimethyl 2, 6-naphthalenedicarboxylic acid
(39.b g, 162 mmole) in dioxane (1.20 L) was heated at 70-80 °C until
all solid dissolved,
slowly treated with 1 equivalent of KOH in methanol, stirred for additional 30
minutes at 70 °C,
cooled to room temperature, filtered and washed with dioxane and diethyl
ether, dissolved in
water, treated with 1 N HCl until the aqueous layer was acidic to pH paper,
filtered, washed
with water and dried under vacuum to provide 33 g of a white powder.
MS (DCI/NH3} m/e 231 (M+H)+.
Example 8B
6-(Chlorocarbonvl)-2-naphthalenecarboxylic acid methyl ester
A suspension of Example 8A ( 15 g> 65 mmole) in toluene ( 190 mL) was treated
with
t 5 thionyl chloride (20 mL, 276 mmole) then DMAP ( 15 mg), heated at reflux
for 1 h and heated
at 85 °C for an additional 35 min. The condenser was replaced with a
distilling head and 60 mL
of solvent was removed. The thick precipitate which formed while cooling to
room temperature
was triturated with hexane and filtered to provide 14.8 g of white solid.
MS (DCI/NH3) m/e 249 (M+H)+.
Example 8C
6-(Aminocarbonvll-2-naphthalenecarboxylic acid meth l~r
A solution of Example 8B ( 15 g, 60.3 mmole) in methylene chloride (400 mL) at
room
temperature was treated with dry ammonia gas to precipitate the product. The
mixture was
stirred for an additional 15 min and filtered. The solid was washed with water
and dried under
vacuum to yield 13.3 g of a white powder.
MS (DCI/NH3) m/e 230 (M+H)+.
Example 8D
6-Cvano-2-naphthalenecarboxvlic acid meth 1 a tPr
A suspension of Example 8C (31 g, 135 mmole) in trimethyl phosphate (450 mL)
was
treated with triphosgene (27 g, 136 mmole), stirred for 20 min at room
temperature and heated
in an oil bath at 80 °C for 1 h. The product precipated from the
solution while cooling to room
-56-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05U96 PCTIUS98115386
temperature. The thick slurry was treated with water and fettered, and the
white solid was
thoroughly washed with water and dried under vacuum to provide 26.3 g of the
title compound.
MS (DCI/NH3) m/e 212 (M+H)+.
E~ple E
6-Cyano-2-nanhthaienecarboxylic acid
Example SD (1.9 g, 9 mmole) in 1:1 THF/H20 (40 mL) was treated with LiOH~H20
( 1.9 g, 45 mmole), stirred 90 min at room temperature and concentrated to a
thick slurry. The
slurry was dissolved in water (20 mL), acidified to pH 2 with solid citric
acid and extracted
with ethyl acetate. The combined organic extracts were washed with brine,
dried (Na2S04) and
concentrated to provide 1.6 g of the title compound as a white solid.
MS (DCI/NH3) m/e 197 (M+H)+.
Exam lie 8F
tert-Butoxvcarbonvlamino-4-aminomethylaniline
4-Aminomethylaniline (2 g) in 2:1 THF/H20 (30 mL) was treated with
Boc anhydride (3.93 g), stirred at room temperature for 18 h, diluted with
water and
concentrated to white slurry. The slurry was dissolved in ethyl acetate,
washed with water and
brine, dried (Na2S04) and concentrated to provide 2.4 g of a yellow solid.
MS (DCI/NH3) m/e 223 (M+H)+.
Exam~he AG
N-14-(aminomethyllphen 1~-cyano-2-naphthalenecarboxamide
A solution of Example 8E (200 mg) and hunig's base (180 ~L) in DMF (S mL) at 5
°C
?a was treated with HATU ( 193 mg), stirred for 1 h at 5 °C, treated
with
Example 8F (120 mg) and diisopropylethylamine ( 100 ~tL) in DMF (5 mL),
stirred for 8 h at
room temperature, diluted with ethyl acetate ( 10() mL), washed sequentially
with 1 N H3P04,
saturated sodium bicarbonate and brine, dried (Na2S04) and concentrated to
provide a yellow
oil which was purified on silica gel with 1 % methanol/methylene chloride to
provide 200 mg of
the title compound.
MS (DCI/NH3) m/e 419 (M+H20)+.
Example 8H
-57-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98/1538k
N-f4-(aminomethvl) henvll-6-aminoininomethyl-2-nanhthalenecarboxamide
bis(trifluoroacetatel salt
The title compound was prepared from Example 8G (200 mg) by the procedure and
purification from Example SB to provide 37 mg of the title compound.
S tH NMR (300 MHz, DMSO-d6) b 4.08 (m, 2H), 7.45 (d, 2H), 7.88 (d, 2H), 7.95
(dd, 1H),
8. I 8 (dd,1 H), 8.20 (bs, 3H), 8.23 {d, l H), 8.35 (d, l H), 8.58 (s, l H),
8.70 (s, 1 H), 9.39 (s,
2H), 9.55 (s, 2H), 10.68 (s, IH);
MS (DCI/NH3) m/e 319 (M+H)+.
Anal. calcd for C19H17N40~TFA: C, 50.56; H, 3.69; N, 10.25; Found: C, 50.18;
H, 3.59; N,
ttj 10.11.
Exam 1~ 9
N-f4-(amino)phenvll-6-aminoiminometh 1-2-naphthalenecarboxamide
bis(trifluoroacetate) salt
t5 Exam In a 9A
N-f4-(amino)phenyll-6-cvano-2-naphthalenecarboxamide
The title compound was prepared according to the procedure described for
Example 8G
but substituting 1,4-diaminobenzene for Example 8F. MS (DCI/NH3) m/e 288
(M+H)+.
Example 9B
N-f4-(amino)nhenvll-6-aminoiminomethyt-2-naphthalenecarboxamide
bis(trifluoroacetatel salt
The title compound was prepared with Example 9A ( 100 mg) following the
procedure
and purification from Example 6B.
tH NMR (300 MHz, DMSO-d~,) ~ 7.15 (d, 2H), 7.75 (d, 2H), 7.95 (dd, 1H), 8.18
(dd, 1H),
?5 8.23 (d, 1 H), 8.35 (d, 1 H), 8.58 (s, 1 H), 8.70 (s, 1 H), 9.25 (s, 2H),
9.48 (s, 2H), 10.58 (s,
1 H);
MS (DCI/NH3) m/e 305 (M+H)+.
Anal. calcd for ClgH~6Nq.0~2TFA: C, 49.63; H, 3.41; N, 10.52; Found: C, 46.57;
H, 3.62;
N, 10.66.
Example 10
1-f(7-Aminoiminomethvl-2-methoxy-I-naphthalenvlloxyl-3-hydroxwro ane
mono(trifluoroacetatel salt
-58-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98/15386
Exam I
I-f(7-Cvano-2-methoxv-1-nanhthalen Iloxvl-3-lf2-tetrahvdro-2H-pyranviloxy~pro
ane
monoltrifluoroacetatel salt
A suspension of Example 4A (200 mgj and Cs2C03 (0.32 g) in DMF (15 mL) was
treated with 2-(3-bromopropyl)-tetrahydro-2H-pyran (0.25 g), stirred at room
temperature for
18 h then diluted with water ( 100 mL) and ethyl acetate (50 mL). The organic
layer was
separated, washed with 10% citric acid, water and brine, dried (MgS04) and
concentrated to
provide 320 mg of an oil.
MS (DCI/NH3) m/e 323 (M+H)+.
lU
Example 10B
1-1l7-Aminoiminomethyl-2-methox -~ hthalenyllox r~l-~-hydrox ro~ne
monoltrifluoroacetate) salt
Example l0A {0.3 g} was processed and purified according to the procedure of
Example
1i IB to provide 110 mg of an off-white solid.
~H NMR (300 MHz, DMSO-d~) 8 1.97 (q, 2H), 3.67 (t, 2H), 4.2t) (t, 2H), 7.61-
7.70 (m,
3H), 7.84 (d, l H), 8.08 (d, 1 H), 8.50 (d, 1 H);
MS (DCI/NH3) m/e 275 (M+H)+.
Anal. caIcd for C13H13N302~TFA~0.75H20: C, 50.81; H, 5.14; N, 6.97. Found: C,
SI.23;
2U H, 5.28; N, 6.97.
Exam l~e l I
8-amino-2-naphthalenecarbonitrile h~drobromide
25 Example 11 A
2-Trifluoromethanesulfo~loxx-8-aminonaphthelene
A solution of 8-amino-2-naphthol (10 g) and triethylamine (12 mL) in dioxane
(200 mL)
was treated with N-phenyltrifluoromethane sulfonimide (25 g) in dioxane (80
mL), stirred for 4
h and concentrated. The resulting dark brown solid was triturated with hexane
and filtered to
3U provide 12 g of the title compound as a brown solid.
MS (DCI/NH3) m/e 292 (M+H)+.
>~xamnle 1 I B
2-Trifluoromethanesulfon~y-8-carbonylben~yloxyaminonanhthelene
-59-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98115386
A solution of Example I 1 A (2 g) in I 0% aq Na2C03 (20 mL) and dioxane (250
mL)
was treated with benzylchloroformate (2 mL) in dioxane (20 mL), stirred at
room temperature
for 5 h then extracted with ethyl acetate. The organic layer was dried (MgS04)
and
concentrated, and the crude product was chromatographed on silica gel with 7:
I hexane/ethyl
acetate to provide 2.5 g (86%) of the title compound.
MS (DCI/NH3) m/e 443 (M+NH4)+_
Example I 1 C
8-lN-carbonvlbenzy~x )-2-nanhthalenecarbonitrile
Tris(dibenzylideneacetone)dipalladium(0)-chloroform adduct ( 120 mg),
I,1'-bis(diphenyfphosphino)-ferrocene (260 mg), potassium cyanide (766 mg),
Example 1 IB (2.5 g) and N-methyl-2-pyrrolidione (5 mL) were combined
sequentially, stirred
at room temperature until a yellow reaction complex formed then warmed to 80
°C for 40 min.
The dark brown reaction mixture was cooled to room temperature and
chromatographed on
I~ silica gel with 9:1 hexane/ethyl acetate to provide I.5 g of the title
compound as a colorless
solid.
MS (DCI/NH3) m/e 292 (M+NH4)+.
Example 1 l D
$-Amino-2-naphthalenecarbonitrile hydrobromide
Example 1 I C ( 1.4 g) was treated with a solution of 30% HBr in acetic acid
(5 mL) and
stirred at room temperature for 6 h. The reaction mixture was treated with
diethyl ether and
filtered to provide I.I g of the title compound as a yellow solid.
MS (DCI/NH3) m/e 186 (M+NH4)+.
?5
Example 12
General Acviation Procedure
A suspension of Example l OD { 1 equivalent), triethylamine ( 1 equivalent)
and DMAP
(0.25 equivalents) in methylene chloride (0.3M) was treated dropwise with the
appropriate acid
3c) chloride ( 1.1 equivalents) in methylene chloride (0.3 M), stirred at room
temperature for 30 min
and treated with water (SO mL). The organic layer was dried (MgS04),
concentrated and used
in following reactions without further purification.
Example I3
-60-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98115386
General Amidine ~nthesis Procedure
A solution of crude acylation products from Example 12 (ca. 100 mg) at room
temperature in 10:1 pyridineariethylamine ( 10 mL) was treated with hydrogen
sulfide for 5 min,
stirred at room temperature for 18 h, diluted with water (50 mL) and extracted
with ethyl
acetate. The ethyl acetate was dried (MgS04) and concentrated. The residue was
dissolved in
acetone (30 mL), treated with methyl iodide (2 mL), refluxed for 1 hour,
evaporated to dryness,
redissolved in methanol (25 mL), treated with ammonium acetate (100 mg),
stirred for 18 h at
room temperature, concentrated and purified as described in Example 1 B to
provide Examples
14-20 as white solids.
Exam l~ a 14
8-(carbonvlbenzvloxy)amino-2-naphthalenecarboximidamide
mono(trifluoroacetate),sal
I H NMR (DMSO-d~, 300 MHz) 8 5.14 (s, 2H), 7.36-7.50 (m, SH}, 7.67-7.90 (m,
4H), 8.14
(d, 1 H), 8.67 (s, 1 H), 9.08 (s, 2H), 9.37 (s, 2H), 9.78 (s, 1H);
t, MS (DCI/NH3) m/e 320 (M+H)+.
Anal. calcd for C19HISN302~1.STFA~O.SH20: C, 52.91; H, 3.94; N, 8.41. Found:
C, 52.86;
H, 4.07; N, 8.18.
Example I S
N-f7-(aminoiminomethvl)-1-naphthalenvl)acetamide mono(trifluoroacetate) salt
I H NMR (DMSO-d6, 300 MHz) 8 4.19 (s, 3H), 7.66-7.88 (m, 3H), 8.12-8.16 (m,
2H), 8.69
(s, 1 H), 8.98 (d, 1 H), 9.16 (s, 2H), 9.47 (s, 2H), 10.14 (s, I H);
MS (DCI/NH3) m/e 228 (M+H)+.
Anal. calcd for C14H12N30~1.2TFA~().25H20: C, 50.18; H, 4.02; N, 11.40. Found:
C,
?s 50.62; H, 4.47; N, 10.90.
Example 16
Methvl f7-(aminoiminometh 1)-Y~1-n_aphthalenvl)carbamate
mono(trifluoroacetate) salt
IH NMR (DMSO-d6, 300 MHz) 8 3.88 (s, 3H), 7.67-7.85 (m, 4H), 8.14 (d, 1H), 8.6
(s,
I H), 8.28 (s, 3H), 9.67 (s, I H);
MS (DCI/NH3) m/e 244 (M+H)+.
Anal. calcd for C13H13N302~TFA: C,50.43; H, 3.95; N, 11.76. Found: C, 50.12;
H, 4.05;
N, I 1.61.
-6 I -
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98/15386
Example 17
Methy_1~j7-(aminoiminomethyl)-1-na hthalenyllaminol-3-oxo r~oyonate
monoltrifluoroacetate) alt
1H NMR (DMSO-d6, 300 MHz) 8 3.69 (s, 2H), 3.71 (s, 3H), 7.69 (m, 4H), 8.18 {d,
1H),
8.58 (s, 1 H), 9.11 (s, 2H), 9.48 (s, 2H);
MS (DCI/NH3) m/e 286 (M+H)+.
Anal. calcd for C15H14N3O3~I.1TFA~H20: C, 48.18; H, 4.26; N, 9.80. Found: C,
48.30; H,
4.09; N, 9.58.
o Exam lp a 18
N-f7-(aminoiminomethvl)-1-naphthalenvll-2-(phenvlmethoxy~acetamide
monoltrifluoroacetate_)
S L
l H NMR (DMSO-d6, 300 MHz) 8 4.29 (s, 2H)> 4.73 (s, 2H), 7.33-7.48 (m, SH),
7.69 (m,
4H), 8.17 (d> 1 H}, 8.47 (s, 1 H), 9.21 (br, 4H),
I 0.0 (s, 1 H);
MS (DCIlNH3) m/e 334 (M+H)+.
Anal. caIcd for C2pH1gN302~1TFA~H20: C, 56.77; H, 4.76; N, 9.03. Found: C,
56.84; H,
4.49; N, 8.93.
Example 19
N-f7-(aminoiminomethyl)-1-naphthalenvll-1 3-benzodioxole-5-carboxamide
mono(trifluoroacetatel salt
~ H NMR (DMSO-d6, 300 MHz) b 6.19 ( 1 H, 2H), 7.12 (d, 1 H), 7.65-7.79 (m,
SH), 7.97 (d,
1 H), 8.20 (s, 1 H), 8.53 (s, 1 H), 9.2 (br s, 3H),
zs 10.35 (s, 2H);
MS (DCI/NH3) m/e 334 (M+H)+.
Anal. calcd for C~gH14N302~TFA: C, 55.82; H, 3.68; N, 9.30. Found: C, 55.69;
H, 33.61;
N, 9.23.
Examl 1~ a 20
N-17-(aminoiminomethvl)-1-nanhthalenvllbenzenemethanewlfonamide
mono(trifluoroacetate)
1H NMR (DMSO-d6, 300 MHz) 8 4.60 (s, 2H), 7.32-7.33 (m, SH), 7.67-7.70 (m,
2H), 7.82
(d, l H), 7.92 (d, I H), 8.17 (s, 1 H), 8.70 (s, 1 H),
-62-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98115386
9.14 (s, 2H), 9.35 (s, ZH), 9. I 9 (s, I H);
MS (DCI/NH3) m/e 340 (M+H)+.
Anal. calcd for C~gH17N302S~TFA~H20: C, 50.95; H, 4.28; N, 8.91; Found: C,
50.76; H,
3.70; N, 8.65.
Example 21
I-f (7-aminoiminomethvl-2-methoxy-I-nanhthalenyl)oxyl-~-bromopro
mono(h~drochloride) salt
1o Exam In a 21A
I-f(7-Cvano-2-methoxv-1-naphthalenyl)oxy -'~-bromopronane mono(hvdrochloridel
salt
The title compound was prepared from Example 4A, 1,3-dibromopropane and the
procedure of Example 10A.
MS (DCI/NH3) m/e 337 (M+NH4)+.
Example 21B
1-f(7-Aminoiminomethyl-2-method-I-na~hthalenyl oxyl-3-bromoRropane
mono(hydrochloride) salt
The title compound was prepared from Example 21 A and the procedure of Example
1 B.
After HCl hydrolysis, the solution was cooled to 0 °C, and the white
solid which precipitated
was filtered and dried to provide the title compound.
1 H NMR (300 MHz, DMSO-d6) 8 2.35 (m, 2H), 3.86 (t, 2H), 4.00 (s, 3H), 4.25
(t, 2H),
7.65 (dd, 1 H), 7.70 (d, 1 H), 7.90 (d, 1 H), 8.10 (d, 1 H), 8.55 (s, i H), 9.
IS (br s, 2H), 9.42
(br s, 2H);
MS (DCI/NH3) m/e 337 (M+H)+.
Anal. calcd for C~SHI~BrN202~HC1~0.75H20: C, 46.53; H, 5.08; N, 7.23. Found:
C, 46.65;
H, 5.11; N, 7.16.
Example 22
3U 3-f(7-Aminoiminomethvl-2-methoxv-1-na hty halenylloxy ronene
monoltrifluoroacetate, salt
Ex mlhe 22A
3-f(7-Cvano-2-methox -v 1-nanhthalenyl oxylpro~ nee
-63-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98/15386
The title compound was obtained as a biproduct from the procedure of Example
21A.
MS (DCI/NH3) m/e 257 {M+NH4)+.
Example 22B
3-1(7-Aminoiminomethyl-2-methox -y I-na hthalenylloxylpr~;nene
mono(trifiuoroacetatPl salt
The title compound was prepared from Example 22A and the procedure and
purification
in Example 1 B.
1H NMR (300 MHz, DMSO-d6) 8 4.00 (s, 3H}, 4.70 (d, 2H), 5.22 (d, 1H), 5.42 (d,
1H),
6.18 (m, 1 H), 7.62 (dd, I H), 7.85 (d, I H), 8.10 (d, 1 H), 8.50 (s, 1 H),
9.12 (br s, 2H), 9.45
l0 (br s, 2H);
MS (DCI/NH3) m/e 257 (M+H)+.
Anal. calcd for C~SH16N2~2'TFA-0.25H20: C, 54.47; H, 4.71; N, 7.47. Found: C,
54.61;
H, 4.38; N, 7.40.
l5 Exams 1~ a 23
1-1(7-Aminoiminomethvl-2-methoxy-1-naphthalen 1~)oxyl-3-phenyl,propane
mono(hydrochioride) salt
Exam 1~ 23A
2U 1-f(7-Cyano-2-methoxy-1-naphthalenyl)oxyl-3-ohen~propane
The title compound was prepared from Example 4A, 1-bromo-3-phenylpropane, and
the
procedure of Example 10A.
MS (DCI/NH3) m/e 335 (M+NH4)+.
25 Exam lie 23B
1-1~7-Aminoiminomethvf-2-methoxy-1-naphthaie~l)oxyl-3=phenylpropane
monolhvdrochloride) salt
The title compound was prepared from Example 23A and the procedure of Example
21 B.
30 ~ H NMR (300 MHz, CD30D) 8 2.21 (m, 2H), 2.94 (t, 2H), 4.00 (s, 3H), 4.22
(t, 2H), 7.18
{m, 1 H), 7.28 (m, 4H), 7.62 (m, 2H), 7.79 (d, 1 H), 8.02 (d, 1 H), 8.62 (s, 1
H);
MS (DCI/NH3} m/e 335 (M+H)+.
HRMS (FAB) calcd m/e for C21H23N202'HCI: 335.1760 (M+H)+. Found m/e 335.1762.
-64-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99!05096 PCT/US98/15386
Exam! 1~ a 24
I-fl7-Aminoiminomethyl-2-methox -v I-na~hthalenvl)oxyl-3-f I-!3 4
dimethox~ henyllpropane monolhydrochloride) salt
Example 24A
I-Bromo-3-l3.4-dimetho~~hen~pro ane
The title compound was prepared from 3-(3,4-dimethoxyphenyl}-1-propanol as
described in Journal of the American Chemical Society, 95 , 8749 ( 1973),
which is
incorporated herein by reference, to provide the title compound.
W MS (DCI/NH3) m/e 276 (M+NI-I4)+.
Example 24B
1-l!7-Cvano-2-methoxv-1-nanhthalen lv loxvl-3-ll-(3 4-dimethoxy~phenyl ro ane
The title compound was prepared from Examples 4A and 24A and the procedure of
l S Example 10A.
MS (DCI/NH3) m/e 395 (M+NH4)+.
Exam I
!-Il7-Aminoiminomethvl-2-methoxy-1-naphthalen~l)oxvl-3-( 1-!3 4-
20 dimethoxv)phenYlll~r~ane monolh~drochloride) salt
The title compound was prepared from Example 24B and the procedure of Example
21B.
~ H NMR (300 MHz, DMSO-d6) 8 2.11 (m, 2H), 2.80 (t, 2H), 3.74 (s, 6H), 3.98
(s, 3H),
4.15 (t, 2H), 6.75-6.92 (m, 3H), 7.65 (dd, 1 H), 7.70 (d, I H), 7.86 (d, 1 H),
8.10 (d, 1 H},
25 R.55 (s, 1H), 9.15 (br s, 2H), 9.53 (br s, 2H) ;
MS (DCI/NH~) m/e 395 (M+H}+.
Anal. calcd for C23H26N204~HC1O).SH20: C, 62.79; H, 6.42; N, 6.37. Found: C,
63.08; H,
6.38; N, 6.17.
3O Examl I~ a 25
7-Methoxv-R-!2-furanvl)-2-naDhthalenecarborimidamide monoltrifluoroacetate~
salt
Exam lie 25A
7 _Methoxv-li-trifluoromethanes Ifonvloxy-2-narzhthaIenecarbonitrile
-65-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98/15386
A solution of Example 4A (310 mg) in methylene chloride (5 mL) at 0 °C
was treated
with I ,1,1-trifluoro-N-phenyl-N-[(trifluoromethyl)sulfonyljmethanesulfonamide
(614 mg) and
triethylamine (240 mL), stirred for 18 h at room temperature, diluted with
methylene chloride
( 100 mL), washed with water and 20% aq NaOH, dried (MgS04) and concentrated
to provide
560 mg of the title compound as a white solid.
MS (DCI) m/e 349 (M+H20}+.
Exam l
Furan-2-boronic acid
ir) A solution of furan (5.3 mL, 73 mmole) in diethyl ether (67 mL) at -20
°C was treated
with n-butyllithium (2.5 M in hexanes, 26 mL, 65 mmole), stirred for 2 hours
at -20 °C and
transferred by cannula to a -20 °C solution of triisopropyl borate (33
mL, 147 mmole) in diethyl
ether (17 mL). The thick mixture was warmed to room temperature, treated with
3N HCl (200
mL) and extracted with diethyl ether. The extracts were washed with 1N KOH,
and the KOH
t5 layer was cooled to 0 °C and acidified with 6N HCI. The acidic
solution was extracted with
diethyl ether, and the acidic ether extracts were washed with brine, dried
(MgS04) and
concentrated to provide the title compound.
1 H NMR (300 MHz, DMSO-d6) 8 6.45 (dd, 1 H), 7.05 (t, 1 H), 7.80 (dd, 1 H),
8.19 (s, 2H).
Exam In a 25C
7-Methoxv-R-(2-furanyi)-2-naphthalenecarbonitrile
Example 25A ( 1. l0 mmol) was combined with Pd(OAc)2 (0.1 1 mmol) and I, l'
bis(diphenylphosphino)ferrocene (0.22 mmol) in DMF {5 mL,), stirred for 10
min, treated with
Example 25B ( I .32 mmol) and Cs2C03 (3.3 mmol), heated at $5 °C for 6
h, cooled to room
temperature arid chromatographed on silica gel with l0~lo ethyl acetate/hexane
to provide the title
compound.
MS (DCI/NH3) m/e 250 (M+H}+.
Exam 1e~25D
8-(2-Furanvl)-7-methoxv-2-naphthalenecarboximidamide mono(trifluoroacetate)
salt
The title compound was prepared from Example 25C and the procedure and
purification
in Example 1 B.
-66-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98I15386
t H NMR (300 MHz, DMSO-d6) 8 3.97 (s> 3H), 6.73 (m, 1 H), 6.80 (m, I H), 7.64
(dd, 1 H),
7.78 (d, 1H), 7.91 (m, IH), 8.16 (d, 1H), 8.20 (d, 1H), 8.30 (s, IH), 9.08 (br
s, 2H), 9.40
(br s, 2H);
MS (DCI/NH3) m/e 267 (M+H)+.
Anal. calcd for C16H14N202~TFA: C, 56.85; H, 3.98; N, 7.37. Found: C, 56.68;
H, 3.67; N,
7.35.
Example 26
methyl 6-(aminoiminomethvl)-4-f (methoxvcarbonyllaminol-2-
naphthalenecarboxylate
1o mono(trifluoroacetate) salt
Example 26A
2-Cvano-1-nitro-6-carboxynaphthalene methyl ester
A solution of 2-cyano-6-methylnaphthoate (5.2 g) in concentrated sufuric acid
(75 mL)
1 S at 0 °C was treated with potassium nitrate ( 1.03 ed) in one
portion, stirred for 10 min, poured
onto ice (500 g) and extracted with ethyl acetate. The ethyl acetate layer was
washed with water,
1 N NaOH and brine, dried (MgS04), treated with silica gel and filtered.
Concentration of the
ethyl acetate to ca. 200 mL precipitated the product. The mixture was heated
until all solid
dissolved, treated with MeOH (20 mL) and ether (20 mL) and stirred overnight.
The resulting
2t) solid was filtered and washed with methanol to provide 2.31 g of the title
compound as a
cream-colored solid. The mother liqueuor was evaporated, treated with
methylene chloride
(250 mL) then solid silica gel, filtered and concentrated. Crystalization from
ethyl
acetate/methanol provided an additional 1.6 g of product for a total yield:
3.91 g (62%).
MS (DC1/NH~) m/e 257 (M+H)+.
2S
Example 26B
2-Cvano-1-amino-6-carboxynaphthalene methyl ester
A solution of Example 26A (lg, 3.9mmole) and 10% Pd on carbon (112 mg) in
ethyl
acetate (80 mL) was stirred under 1 atm of hydrogen for 9 h, purged with
nitrogen for 1 h,
30 filtered and evaporated to provide 810 mg (92%) of the title compound as a
yellow solid.
MS (DCI/NH3) m/e 227 (M+NH4)+.
Example 26C
6-Cvano-4-f(methoxvcarbonyl)aminol-2-naphthalenecarboxylic acid methyl ester
-67-
SUBSTITUTE SKEET (RULE 26j
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98/15386
A solution of Example 26B (2.50 mmol) in methylene chloride (40 mL) was
treated
sequentially with diisopropylethylamine (2 mL) and methylchloroformate (195
~L, 2.52
mmole), stirred for 2 h, treated with methanol { 10 mL), stirred for an
additional 10 minutes,
diluted with methylene chloride (60 mL), washed with water and brine, dried
(MgS04) and
evaporated. The residue was purified on silica gel using 10% ethyl
acetate/hexane to provide
280 mg (59%) of light yellow solid.
MS (DCI/NH3) m/e 285 (M+H)+.
Example 26D
t0 Methyl 6-(aminoiminomethyl)-4-1(methoxy arbonyl)amino]-2-
naphthalenecarboxvlate
mono(,~,rifluoroacetate) salt
The title compound was prepared using Example 26C ( 125 mg, 0.44 mmol) and the
procedure in Example 40D to provide 35mg of a white solid.
tH NMR (DMSO-d~,) 8 3.7R (s, 3H), 3.95 (s, 3H), 7.R9 (dd, 1H), 8.37-R.40 (m,
3H), 8.53
t 5 (s, 1 H), 8.740 (s, 1 H) 9.18 (br s, 2H), 9.45 (br s, 2H), 9.90 (s, 1 H),
8.42 (s, 1 H), 8.63 (d,
1 H), 9.18 (br s, 4H), 10.58 (s, 1H);
MS (DCI/NH3) m/e 302 (M+H)+.
Anal. calcd for C15HI5N304~TFA~1.SH20: C, 46.16; H, 4.33; N, 9.50. Found: C,
45.96;
46.16; H, 4.06; N, 9.12.
Example 27
(E)-17-Methoxv-R-f2-lPhenyl)ethenyll l-2-naphthaleneimidamide
mono(trifluoroacetate) salt
Example 27A
LE)-(7-Methoxv-R-f2-(Phenyl)ethenyll l-2-nat~hthalenecarbonitrile
Example 25A and styrene boronic ester, prepared according to the procedure of
Journal
of the American Chemical Society, ~ 5249 ( 1975), which is incorporated herein
by reference,
was processed according to the procedure described in Example 26B to provide
the title
compound.
MS (DCI/NH3) m/e 303 (M+NH4)+.
ExamnIe 27B
(E)-17-Methoxv-R-f2-(Phenyl)ethenvll l-2-naphthaleneimidamide
mono(trifluoroacetatel salt
The title compound was prepared from Example 27A and the procedure of Example
1B.
-68-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98/15386
1H NMR (300 MHz, DMSO-d6) 8 3.98 (s, 3H), 7.28 (t, 2H), 7.39 (t, 2H), 7.64 (m,
SH),
8.00 (d, 1 H), 8.10 (d, 1 H), 8.62 (s, 1 H), 9.22 (br s, 2H), 9.42 (br s, 2H};
MS (DCI/NH3) m!e 303 (M+H)+.
Anal. calcd for C2pH1gN20~TFA: C, 63.46; H, 4.60; N, 6.73. Found: C, 63.10; H,
4.73; N,
6.43.
Example 28
6-(4-Phenvlbutynyl)-2-nanhthalenecarboximidamide mono(trifluoroacetate) salt
Example 28A
6-Hvdrox -~naphthalenecarbonitrile
A solution of 6-bromo-2-naphthol (25.0 g, 112 mmol) and copper(I) cyanide ( 11
g, 123
mmol) in DMF (30 mL) was heated at 135 °C for 18 h, cooled, diluted
with ethyl acetate (50
mL), triturated with 10% aq sodium hydroxide and filtered through Celite~. The
filtrate was
2S acidified to pH 2 and extracted with ethyl acetate. The combined extracts
were concentrated,
dissolved in ethanol ( 150 mL) and triturated with water to precipitate 14.01
g of the title
compound.
MS (DCI/NH3) m/e 170 (M+H)+.
2t) Example 28B
6-(Trifluoromethanesulfonyloxv)-2-naphthalenecarbonitrile
A solution of Example 28A (14.01 g, 82.8 mmol) and triethylamine (9.2 g, 91.1
mmol)
in methylene chloride (40 mL} at 0 °C was treated dropwise with
trifluoromethylsulfonic
anhydride (28 g, 99.4 mmol), warmed to 25 °C for 48 h, concentrated,
redissolved in ethanol
25 (50 mL) and triturated with water to precipitate 8.4 g of the title
compound.
MS (DCI/NH3) m/e 319 (M+NH4)+.
Example 28C
6-(4-Phenvlbutvnvl)-2-na~hthalenecarbonitrile
30 The title compound was prepared from Example 28B, 4-phenyl-1-butyne and the
procedure of Example 57B.
MS (DCI/NH3) m/e 299 (M+NH4)+.
Example 28D
-69-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98/15386
6-(4-Phenvlbutvnvl)-2-nanhthalenecarbQximidamide mono(trifluoroace~ tel alt
The title compound was prepared from Example 28C and the procedure of Example
1B.
1H NMR (300 MHz, DMSO-d6) 8 2.80 (t, 2H), 2.95 (t, 2H), 7.22 (m, 1H), 7.36 (m,
4H),
7.58 {d, 1H), 7.82 (d, 1H), 8.05 (d, 1H), 8.10 (d, 2H), 8.45 (s, 1H), 9.10 (br
s, 2H), 9.42
(br s, 2H);
MS (DCI/NH3) m/e 299 (M+H)+.
Anal. calcd for C2lHigN2~TFA~0.75H20: C, 64.86; H, 4.85; N, 6.58. Found: C,
64.78; H,
4.64; N, 6.03.
to Examp]e 29
7-(2-Hvdroxvethoxvl-8-iodo-2-naphthalenecarboximidamide mono(trifluoroacetate)
salt
Example 29A
3-II(1.1-Dimethvlethvl)dimethvlsilyl ~xyl-1-propano( 4-nitrobenzenesulfonate
is A solution of 3-t-butlydimethylsiloxy-I-propanol, prepared by the method of
McDougal, et al. JOC, 1986, 51, 3388, which is incorporated herein by
reference, (7.6 g, 40
mmol) and diisopropylethylamine (10.4 mL, 60 mmol} in methylene chloride (200.
mL) at 0 °C
was treated with p-nitrophenylsulfonyl chloride (9.7 g, 44 mmol), stirred for
3 h, poured into
saturated NaHC03 and exacted with diethyl ether. The extracts were washed with
brine, dried
20 (Na2S04), and concentrated. The residue was chromatographed on silica gel
with 5% ethyl
acetate/hexanes to provide 6.00 g of the title compound.
MS (DCI/NH3) m/e 395 (M+NH4)+.
Example 29B
25 7-12-fl(1.I-Dimethviethvlldimethylsilylloxylethoxyl-8-iodo-2-
naphthalenecarbonitriIe
The title compound was prepared in a manner analogous to that of Example 43A
but
substituting Example 29A for propyl iodide.
MS (DCl/NH3) m/e 468 (M+H)+.
3o Example 29C
7-(2-Hvdroxvethoxy)-8-iodo-2-nat~hthalenecarbonitrile
The title compound was prepared in a manner analogous to that of Example 53F
but
substituting Example 29B for Example 53E.
MS (DCI/NH3) m/e 357 (M+H)+.
-70-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98/15386
Exam 1~9D
7-l2-Hvdroxvethoxvl-8-iodo-2-narzhthalenecarboximidamide
mono(trifluoroacetate) salt
The title compound was prepared from Example 29B according to the procedure of
Example 1 B.
t H NMR (300 MHz, DMSO-d6) 8 1.96 (m, 2H), 3.69 (t, ZH), 4.33 (t, 2H), 4.58
{br, I H),
7.63 (d, 1 H), 7.66 (dd, 1 H}, 8.12 {dd, 2H), 8.42 (s, 1 H), 9.20 (s, 2H),
9.53 (s, 2H);
MS (DCI/NH3) m/e 245 (M+H)+;
Anal. calcd for Ct3H12N202I~TFA~0.21H20: C, 53.07; H, 4.85; N, 7.74.
i0 Found: C, 53.07; H, 4.75; N, 7.65.
Exam 1R a 30
7-(2-Hvdroxvethoxv)-2-naDhthalenecarboximidamide monoftrifluoroacetate) salt
Example 30A_
7-(2-Hvdroxyethoxy)-2-n~yhthalenecarbonitrile
Example 29B ( I20 mg, 0.26 mmol), palladium(II)Cl2dppf (46 mg, 0.03 mmol) and
diisopropylamine (263 mg, 2.6 mmol) were heated in a sealed tube for 2 h at
I00 °C, cooled to
room temperature, diluted with ethyl acetate, washed with water, dried
(Na2S04), and
2o concentrated. The residue was purified on silica gel with 15% ethyl
acetate/hexanes to provide
85 mg of the title compound.
MS (DCI/NH3) m/e 342 (M+H)~.
Example OB
7-(2-Hvdroxvethox )-y 2-na hn thalenecarboximidamide mono(trifluoroacetate)
salt
2S The title compound was prepared from Example 29B according to the procedure
of
Example 1 B.
~ H NMR (300 MHz, DMSO-d6) 8 1.96 (m, 2H}, 3.69 (t, 2H}, 4.33 (t, 2H), 4.58
(br, 1 H),
7.63 (d, 1 H), 7.66 (dd, 1 H), 8.12 (dd, 2H}, 8.42 (s, 1 H), 9.20 (s, 2H),
9.53 (s, 2H);
MS (DCI/NH3) m/e 228 (M+H)+;
3o Anal. calcd for Ct4H15N202~TFA: C, 53.78; H, 4.51; N, 7.84.
Found: C, 53.60; H, 4.30; N, 7.81.
Exam le 1
6-(4-Methvl-1-Dentvnvl)-2-nanhthalenecarboximidamide monoltriflunrnace~tel
cair
-71-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98/15386
exam lp a 31 A
6-l4-Methvl-1-pen nyll-2-nanhthalenecarbonitrile
The title compound was obtained from Example 28B, 4-methyl-I-pentyne and the
procedure of Example 57B.
MS (DCI/NH3) m/e 251 (M+NH4)+.
Exam Ip a 31 B,
6-(4-Methvl-1-Dentynvl)-2-naphthalenecarboximidamide mono(trifluoroacetate)
salt
to The title compound was prepared from Example 3IA and the procedure of
Example IB.
~H NMR (300 MHz, DMSO-d6) 8 1.05 (d, 6H), 1.90 (m, 1H), 2.2U (d, 2H}, 7.62
(dd, 1H),
7.82 (dd, 1 H), 8.09 (d, 1 H), 8.12 (d, 1 H), 8.18 (s, 1 H), 8.48 (s, 1 H},
9.12 (br s, 2H), 9.42
(br s, 2H);
MS (DCI/NH3) rn/e 251 (M+H)+.
1S Anal. calcd for Cl~HtgN2~TFA: C, 62.63; H, 5.26; N, 7.69. Found: C, 64.85;
H, 5.32; N,
7.46.
Example 32
6-l5-Phenvlpentvnvl)-2-naphthalenecarboximidamide monoftrifluoroacetate) salt
Example 32A
6-f 5-Phenylpentynyl)-2-naphthalenecarbonitrile
The title compound was obtained from Example 28B, 5-phenyl-I-pentyne and the
procedure of Example 57B.
MS (DCI/NH3) m/e 313 (M+Nl-I4}+.
Example 32B
6-(5-Phenvhentvnvl)-2-nanhthalenecarboximidamide mono(trifluoroacetate) Salt
The title compound was prepared from Example 32A and the procedure of Example
IB.
3u ~ H NMR (300 MHz, DMSO-d6) 8 I .90 (m, 2H), 2.80 (t, 2H), 3.39 (t, 2H),
7.19-7.37 (m,
SH), 7.62 (dd, IH), 7.82 {dd, 1H), 8.08 (d, 1H), 8.15 (d, IH),
8.18 {s, I H), 8.48 (s, 1 H), 9.15-9.45 (br d, 4H);
MS (DCI/NH3) m/e 313 (M+H)+.
-72-
SUBSTITUTE SKEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98/I5386
Anal. calcd for C22H20N2~'1'FA: C, 67.60; H, 4.96: N, 6.57. Found: C, 67.32;
H, 5.21; N,
6.27.
Example 33
6-(3-Phenvl-1-Droovnvl)-2-naphthalenecarboximidamide monoltrifluoroacetate)
salt
Example 33A
6-(3-Phenyl-1-p~vnyl)-2-n ~hthalenecarbonitrile
The title compound was obtained from Example 28B, 3-phenyl-1-propyne and the
procedure of Example 57B.
MS (DCI/NH3) m/e 285 (M+NH4)+.
Example 33B
6-(3-Phenyl-1-propvnyl)-2-naphthalenecarboximidamide mono(trifluoroacetate)
salt
5 The title compound was prepared from Example 33A and the procedure of
Example 5B.
~H NMR (300 MHz, DMSO-d~,) 84.00 (s, 2H), 7.28-7.50 (m, 5H), 7.70 (dd, 1H),
7.85 (dd,
1 H), 8.09 (d, 1 H), 8.15 (d, 1 H), 8.21 (s, 1 H), 8.49 (s, 1 H), 9.21 (br s,
2H), 9.45 (br s, 2H);
MS (DCI/NH3) m/e 285 (M+H)+.
Anal. calcd for C2pH 16N2~TFA~0.25H20: C, 65.59; H, 4.38; N, 6.95. Found: C,
65.43; H,
3.95; N, 6.70.
Example 34
6-(Phenvlethynyl)-2-naphthalenecarboximidamide mono(trifluoroacetate) salt
'S Example 34A
6-(Phenyleth~nyl)-2-naphthalenecarbonitrile
The title compound was obtained from Example 28B, phenylacetylene and the
procedure of Example 57B. MS (DCI/NH3) m/e 271 (M+NH4)+.
Example 34B
6-(Phenvlethvnvl)-2-navhthalenecarboximidamide mono(trifluoroacetate) salt
The title compound was prepared from Example 34A and the procedure of Example
1 B.
~ H NMR (300 MHz, DMSO-d6) 8 7.49 (t, 3H), 7.62 (m, 2H), 7.80 (dd, 1H), 7.86
(dd, 1 H),
8.15 (d, 1H), 8.19 (d, 1H), 8.38 (s, 1H}, 8.52 (s, 1H), 9.38 (br s, 4H);
-73-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTlUS98/15386
MS (DCI/NH3) m/e 271 (M+H)+.
Anal. calcd for Ct9Ht4N2~TFA: C, 65.62; H, 3.93; N, 7.29. Found: C, 65.64; H,
4.1 l; N,
7.21.
Exam Ip a 35
3-Amino-N-f3-16-(aminoiminomethyl)-2-naphthalen ly 1-2-propvnyllbenzamide
monoftrifluoroacetate) salt
Example 35A
6-(3-Amino-1-propynyl -2-naphthalenecarbonitrile
The title compound was obtained from Example 28B, propargyl amine and the
procedure of Example 41 A.
MS (DCI/NH3) m/e 207 (M+NH4)+.
is Example 35B
3-Amino-N-f 3-(6-cyano-2-naphthalenvl)-2-propynyllbenzamide
A solution of Example 35A ( 100 mg, 0.49 mmole), 3-aminobenzoic acid (73 mg,
0.53
mmolej, EDC (141 mg, 0.74 mmole) and DMAP (89mg, 0.74 mmole), in THF (5.5 mL)
was
stirred at room temperature for 2.5 h and concentrated. The residue was
dissolved in methylene
chloride, washed with 1 N HCI, water, saturated NaHC03, and brine, dried
(MgS04)
concentrated and purified by flash chromatography on silica gel with 2%
ethanol/methylene
chloride to provide the title compound.
MS (DCI/NH3) m/e 326 (M+H)+.
2S Example 35C
3-Amino-N-f3-f6-(aminoiminometh l~~hthale~ll-2-propynyllbenzamide
mono(trifluoroacetate) salt
The title compound was prepared from Example 35B and the procedure of Example
1B.
~ H NMR {300 MHz, DMSO-d~,) 8 4.32 (d, 2H), 5.69 (br s, 2H), 6.58 (d, 2H),
7.62 (m, 3H), 7.82 (d, 1 H), 8.08 (d, 1 H), 8.14 (d, 1 H), 8.20 (s, 1 H), 8.43
(s, 1 H), 8.60 (t,
1 H), 9.19 (br s, 2H), 9.42 (br s, 2H);
MS (DCI/NH3) m/e 343 (M+H)+.
Anal. calcd for C2lHtgN40~TFA~0.25H20: C, 59.93; H, 4.26; N, 12.16. Found: C,
59.86;
H, 3.97; N, 11.93.
-74-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTlUS98/15386
Exam_ lp a 36
4-Amino-N-f3-f6-aminoiminomethyl-2-naphthalenvl)-2-prop~n~vllbenzamide
mono(trifluoroacetate) salt
Example 36A
4-Amino-N-f3-(6-cyano-2-na hthaleny_112-~lzvn~]~enzamid~
Example 35A and 4-aminobenzoic acid were subjected to the conditions described
in
Example 35B to afford the title compound.
t0 MS (DCI/NH3) m/e 326 (M+H)+.
Exam le 3 ~B
4-Amino-N-f3-(6-aminoiminomethyl-2-nalahthalenvil-~-prQRynvllbenzamide
mono(trifluoroacetate) ,alt
I S The title compound was prepared from Example 36A and the procedure of
Example SB.
tH NMR (300 MHz, DMSO-d6) 8 4.38 (d, 2H), 6.89 (m, IH), 7.20 (m, 2H),
7.22 (s, 1 H), 7.63 (dd, 1 H), 7.82 (dd, 1 H), 8.09 (d, 1 H), 8.12 (d, 1 H),
8.20 (s, 1 H), 8.46
(s, 1 H), 8.95 (t, 1 H), 9.19 (br s, 2H), 9.42 (br s, 2H);
MS (DCI/NH3) m/e 343 (M+H)+.
2() Anal. calcd for C2tH16N40~2.STFA: C, 49.27; H, 3.19; N, 8.54. Found: C,
49.27; H, 3.33;
N, 8.89.
Exam i~ a 37
(S)-2-Amino-N-f 1-f(6-aminoirtinomethyl-2-
naphthalenvl)carbonyllcxcfohexvll~r_opionamide
'S bis(trifiuoroacetate) salt
Example 37A
6-ffl-Aminocyclohex lyethynvll-2-naphthalenecarbonitrile
The title compound was obtained from Example 28B, 1-ethynylcyclohexylamine and
the
30 procedure of Example 41 A.
MS (DCI/NH3) m/e 275 (M+NH4)+.
Exam In a 37B
(S)-2-Amino-N-f 1-f(6-cvano-2-naohthalenyl)carbonyllcyclohexvl~propionamide
-75-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTlUS98/15386
Example 37A and N-(t-butoxycarbonyl)-L-alanine were subjected to the
conditions
described in Example 35B to provide the title compound.
MS (DCI/NH3) m/e 446 (M+H)+.
S exam le 7
(S)-2-Amino-N-fI-f( -aminoiminomethyl-2-naa hthalenyl~carbonyllc~rclohex ~~11,
roRionamide
bisltrifluoroacetate) salt
The title compound was a rearrangement product of Example 37B resulting from
the
procedure of Example SB.
t0 tH NMR (300 MHz, DMSO-d~,) b 1.24 (d, 3H), 1.40-1.62 (m, 8H}, 2.15-2.26 (s,
IH), 2.29-
2.38 {s, IH), 3.51 (d, 1H), 3.78 (d, 1H}, 3.82 (s, 1H),
7.90 (dd, 2H), 8.09 (dd, 1H), 8.18 (d, 1H), 8.37 (d, 1H), 8.55 (s, 1H), 8.78
(s, IH), 9.31
(s, 2H), 9.50 (s, 2H);
MS (DCI/NH3) m/e 381 (M+H)+.
I5 Anal. calcd for C~3H2gN402~2TFA~2H20: C, 49.39: H, 5.22; N, 8.53. Found: C,
49.15; H,
4.79; N, 8.70.
Ex mple 3R
6-methoxv-8-benzvloxv-2-naphthalenecarboximid~mide monoftrifluoroacetate, salt
Exam le 8A
8-Hvdroxv-6-methoxy-3 4-dihydro-2H-nanhthalen-1 one
A solution of 6, 8-dimethoxy-3, 4-dihydro-2H-naphthalen-I-one (IS g, 72.8
mmole),
prepared according to the procedure of J. Chem. Soc., London 2782 (1955),
which is
incorporated herein by reference, in methylene chloride ( I50 mL) at 0
°C was treated
portionwise with AIC13 (14.3 g, 107 mmole), stirred for 20 h at room
temperature, poured onto
ice with stirring and extracted with methylene chloride when the ice melted.
The extracts were
washed with water and brine, dried (MgS04) and concentrated to provide 13.8 g
of the title
compound.
3~ MS (DCI/NH3) m/e 193 (M+H)~.
Examho a 38B
$-Benzvloxv-6-methoxv-3 4-dihy ro-2H-n phthalen 1 one
-76-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98I15386
A mixture of Example 38A (2.5 g, 13 mmole), benzyl bromide (2.1 mL, 17.8
mmole),
K2C03 powder ( 14.3 g, 100 mmole), and 2-butanone (88 mL) was stirred at
reflux for 4 h,
treated with additional benzyl bromide ( 1.0 mL, 8.5 mmole), stirred at reflux
for an additional 3
h, cooled to room temperature, filtered and concentrated. The residue was
dissolved in
methylene chloride, washed with 1N HCI, water and brine, dried (MgS04) and
concentrated.
The crude product was purified on silica gel with
30% ethyl acetate/hexanes to provide the title compound.
MS (DCI/NH3) m/e 283 (M+H)+.
no Exam Ie~38C
3.4-Dihvdro-2-(hvdroxvmethvlenel-6-methoxy-R-(ohe~lmethoxy) ll2Hl
naphthalenone
Sodium metal ( 1.29 g, 55.9 mmole) was added portionwise to a mixture of
ethanol (4.2
mL} and benzene ( 15 mL). The mixture was stirred at reflux for 1.5 h, cooled
to 0 °C and
treated dropwise with ethyl formate (5.6 mL, 70 mmole) then dropwise with of a
solution of
IS Example 3HB (6.7g, 23.8 mmole) in benzene (20 mL), stirred at room
temperature for 2 h,
cooled to 0 °C, treated sequentially with ice/water and 6N HCl (75 mL)
and extracted with ethyl
acetate. The extracts were washed with brine, dried (MgS04) and concentrated
to provide the
title compound.
MS (DCI/NH3} m/e 311 (M+H)+.
Example 38D
4 5-Dihvdro-7-methox ~-~9-(~henvlmethoxy)nanhthf2 1-dlisoxazole
f2.1-dlisoxazole
A suspension of Example 38C (7.5 g, 24.3 mmole), hydroxylamine hydrochloride
(4.11
2S g, 57.6 mmole) and acetic acid (63 mL) was stirred at 1 10 °C for 7
min, cooled to room
temperature, diluted with water and extracted with methylene chloride. The
extracts were
washed with water and brine, dried (MgS04), and concentrated. The crude
product was
purified by flash chromatography on silica gel with 30% ethyl acetate/hexanes
to provide the
title compound.
3o MS (DCI/NH3) m/e 308 (M+H)+.
Exam 1
8-Benzvloxv-2-cvano-6-methoxy-3 4-dih dronaphthalen 1 one
_77_
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98/15386
Sodium methoxide, prepared from sodium metal (0.17 g, 7.35 mmol} in methanol
(3.9
mL), was treated dropwise with a solution of Example 38D (I.S g, 4.9 mmole) in
benzene (SO
mL), stirred at room temperature for 4.5 h, treated sequentially with water
and 1 N HCl and
extracted with ethyl acetate. The extracts were washed with brine, dried
(MgS04) and
concentrated to provide the title compound.
MS (DCI/NH3) m/e 308 (M+H)+.
Example 38F
2-Cvano-6-methoxy-8-Benzyloxv-3 4-dihvdronaphthalene
to A suspension of Example 38E (2.6 g, 8.6 mmole) in absolute ethanol (2S mL)
at room
temperature was treated portionwise with NaBH4 ( 1.6 g), stirred for 20 min at
room
temperature and for 20 min at reflux, cooled to room temperature, treated with
water (20 mL)
and concentrated. The residue was dissolved in methylene chloride, washed with
water and
brine, dried (MgS04), filtered and concentrated to provide 2.6 g of an orange
foam. The foam
~5 was stirred at reflux for 20 min with p-toluenesulfonic acid monohydrate
(0.52 g, 2.7 mmole)
in benzene (52 mL), cooled to room temperature, diluted with ethyl acetate,
washed with water
and brine, dried (MgS04) and concentrated to provide the title compound.
MS (DCI/NH3) m/e 309 (M+NI-14)+.
20 Exam lp a 38G
2-Cyano-6-methoxv-8-benzvloxynar?hthalene
A solution of Example 38F (0.4 g, 1.4 mmole), 2,3-dichloro-S,6-dicyano-1,4-
benzoquinone (0.79 g, 3.S mmole) in benzene (40 mL) was stirred at reflux for
4 hours, treated
with additional 2,3-dichloro-S,6-dicyano-1,4-benzoquinone (0.4 g, 1.8 mmole),
stirred at
25 reflux for an additional S h, cooled to room temperature, diluted with
ethyl acetate, washed with
saturated NaHC03 and brine, dried (MgS04} and concentrated to provide the
title compound.
MS (DCI/NH3) m/e 290 (M+H)+.
Example 38H
30 8-Benzvloxv-6-methoxv-2-nanhthalenecarboximidamide monoftrifluoroacetate)
alt
The title compound was prepared from Example 38G and the procedure of Example
I B.
~H NMR (300 MHz, DMSO-d6} 8 4.38 (d, 2H), 6.89 (m, 1H), 7.20 (m, 2H), 7.22 (s,
1H),
7.63 (dd, IH), 7.82 (dd, 1H), 8.09 (d, IH), 8.12 (d, IH), 8.20 (s, IH), 8.46
(s, 1H), 8.95
(t, I H), 9. I9 (br s, 2H), 9.42 (br s, 2H);
_78_
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98115386
MS (DCI/NH3) m/e 307 (M+H)+.
Anal. calcd for CigH1gN202~TFA: C, 60.00; H, 4.56; N, 6.66. Found: C, 59.93;
H, 4.46; N,
6.51.
Example 39
2-f (7-Aminoiminomethvl-3-methoxv-1-naphthale~l)oxvlacetamide
mono(trifluoroacetate) salt
Example 39A
6-Methoxy-8-hydroxy_2=,na~hthalenecarbonitrile
A mixture of Example 38G ( 1.62 g, 5.6 mmole) and 10% dry Pd/C (0.50 g) in
methanol ( 150 mL) was hydrogenated in a Pan shaker at room temperature under
4 atm for 30
h. The mixture was filtered and concentrated to provide the title compound.
MS (DCI/NH3) m/e 217 (M+NH4)+.
1 S Example 39B
2-f(7-Cvano-3-methox -1-naphthale~l~)oxylacetamide
Example 39A and 2-bromoacetamide were subjected to the conditions described in
Example SA to provide the title compound.
MS (DCI/NH3) m/e 274 (M+NH4)+.
Example 39C
2-f(7-Aminoiminomethvl-3-methoxv-1-na~hthalenyl)oxylacetamide
mono(trifluoroacetate) salt
The title compound was prepared from Example 39B and the procedure of Example
1 B.
~ H NMR (300 MHz, DMSO-d6) 8 3.93 (s, 3H), 4.70 (s, 2H), 6.70 (d, I H), 7.09
(d, 1 H),
z5 7.65 (s, 2H), 7.82 (dd, 1H), 7.99 (d, !H), 8.70 (s, 1H), 9.05 (s, 2H), 9.38
(s, 2H);
MS (DCI/NH3) m/e 274 (M+H)+.
Anal. calcd for C~4H15N3O3~TFA: C, 49.62; H, 4.16; N, 10.85. Found: C, 49.68;
H, 4.24;
N, 10.61.
=~~ Example 40
N-(6-aminoiminomethvl-2-nanhthalenyl)-N'-phenylurea mono(trifluoroacetate)
salt
Example 40A
6-Cvano-2-nanhthalenecarbonyl chloride
-79-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98/15386
A suspension Example 8E (4.4 g, 22.3 mmol) in toluene (100 mL) was treated
with
thionyl chloride (6.0 mL) and DMAP (5 mg), heated at 55 °C for lh,
treated with additional
thionyl chloride (3 mL), warmed to 95 °C for 1 h, cooled to room
temperature, stirred in hexane (75 mL) for 2.5 h and fltered to provide 3.62
of the tide
compound as a white powder. The filtrate was concentrated and triturated with
ether to provide
an additional 1.02 g of the title compound.
MS (DCI/NH3) m/e 2I5 (M+H)+.
Example 40B
2-Cvano-6-naRhthoyl azide
A solution of Example 40A ( 1.65 g, 7.65 mmole) in acetone (600 mL) at room
temperature was treated with a solution of sodium azide (3 g, 46 mmole) in
water ( 10 mL),
stirred for 1.5 h and diluted with water (60 mL). The resulting solid was
filtered, washed with
water and dried to provide 4.24 g of the title compound as a white powder.
MS (DCI/NH3) m/e 240 (M+NH4)+.
Example 40C
N-(6-cvano-2-na hthale~i)-N'- henylurea
A solution of Example 40B (221.2 mg, 1 mmole) in toluene ( 18 mL) was heated
at 85
°C for 1 h then at 95 °C for 1.5 h, cooled to room temperature,
treated with aniline (240 ~L,
2.63 mmole), stirred for 25 min and treated with ether ( 10 mL). The resulting
solid was
collected, washed with ether and dried under vacuum to yield 230 mg of white
powder.
MS (DCI/NH3) m/e 305 (M+NH4)+.
Example 40D
N-(6-aminoiminomethvl-2-naphthalenyl)-N,'-phenvlurea mono(trifluoroacetate)
salt
A solution of Example 40C (148 mg, 0.5 mmole) in 10:1 pyridineariethylamine
(10
mL) was treated with H2S for 5 min, stirred at room temperature for 18 h and
concentrated.
The resulting solid was dissolved in acetone ( 15 mL), treated with
iodomethane (0.8 mL, 12.8
mmole), stirred for 2 h, diluted with ether ( 10 mL), filtered, washed with
ether and dried under
vacuum. The resulting solid was dissolved in methanol, treated with 2N NH3 in
methanol (2
mL), warmed to 50 °C for 4 h and concentrated. The product was purified
according to the
procedure described in Example 1 B to provide 62 mg of the title compound.
1H NMR (300 MHz, DMSO-d6) S 7.00 (t, IH), 7.31 (dd , 2H), 7.52 (d, 1H),
_80_
SUBSTITUTE SHEET (RULE 26j
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98/15386
7.65 (dd, IH), 7.76 (dd, IH, 8.02 (d, 2H), 8.30 (s, IH), 8.39 (s, IH), 9.05
(br s, 2H), 9.11
(s, IH), 9.33 (br s, 2H), 9.42 (s, IH);
MS (DCI/NH3) m/e 305 (M+H)+;
Anal. calcd for C1gH16N40~TFA: C, 57.42; H, 4.10; N, 13.39. Found: C, 57.50;
H, 4.05;
s N, I 3.08.
Exam a 41
(El-6-f2-lPhenvI)ethenvll-2-naphthalenecarboximidamide mono(trifluoroacetate)
sal
Example 41 A
El-6-I2-(Phenyl)ethenvll-2-na~hthalenecarbonitrile
A solution of Example 28B (350 mg, I .16 mmol), styrene ( 157 mg, 1.51 mmol),
palladium (II) acetate (26 mg, 0.12 mmol), triphenylphosphine (61 mg, 0.23
mmol),
triethylamine (2 mL) and acetonitrile ( I mL) in a sealed tube with minimal
head volume was
l5 heated at 100 °C for 19 h, diluted with ethyl acetate {20 mL),
washed with water, dried
(MgS04) and concentrated with silica gel (4 g). The mixture was
chromatographed on silica gel
with 10% ethyl acetate/hexane to provide 160 mg of the title compound.
MS (DCI/NH3) m/e 273 (M+NH4)+.
Example 41 B
E)-6-f2-(Phenvllethenvll-2-nanhthalenecarboximidamide mono(trifluoroacetate)
salt
The title compound was prepared from Example 41 A from the procedure of
Example
I B.
1H NMR (300 MHz, DMSO-d~) 8 7.33 (t, IH), 7.4 (t, 2H), 7.5 (d, 2H), 7.69 (d,
IH), 7.70
2~ (d, 1 H), 7.81 (dd, 1 H), 8.03 (dd, 1 H), 8. I 0 (d, 1 H), 8. I 3 (d, 1 H),
8.17 (s, 1 H), 8.44 (s,
I H), 8.97 (s, 2H), 9.41 (s, 2H);
MS (DCI/NH3) m/e 273 (M+H)+ ;
Anal. calcd for C~yH~6N2~TFA: C, 65.28; H, 4.43; N, 7.25. Found: C; 64.95; H,
4.60; N,
6.42.
Exam lp a 42
6-I2-lPhenyl)ethvll-2-nanhthalenecarboximidamide mono(trifluoroacetate) salt
Example 42A
_81 _
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98115386
6-f2-lPhenyllethyll-2-naphthalenecarbonitrile
A mixture of Example 57B (80 mg, 0.31 mmol) and palladium on carbon (20%
water,
50 mg) in methanol (5 mL) was stirred under 1 atm of hydrogen for 0.5 h,
filtered and
concentrated to provide 72 mg of the title compound.
S MS (DCI/NH3) m/e 275 (M+NH4)+.
Ex mp e1 42B
6-f2-lPhenvllethvll-2-nanhthalenPC-arboximidamide monoltrifluoroacetate salt
The title compound was prepared from Example 42A and the procedure of Example
1B.
t0 IH NMR (300 MHz, DMSO-d6) s 3.03 (m, 2H), 7.23 (m, SH), 7.60 (dd, IH), 7.76
(dd,
1 H), 7.85 (s, 1 H), 8.03 (t, 2H), 8.42 (s, 1 H), 8.99 (s, 2H), 9.39 (s, 2H);
MS (DCI/NH3) m/e 275 (M+H)+.
Anal. calcd for CI9HIgN20~1.33TFA: C, 61.29; H, 4.59; N, 6.61. Found: C;
61.56; H, 4.62;
N, 5.21.
IS
Example 43
7-Pronoxv-R-iodo-2-naphthalenecarboximidamide monoltrifluoroacetate) salt
Example 43A
20 7-pro oiL-v-R-iodo-2-naphthalenecarbonitrile
Example 53A (65 mg, 0.25 mmol) in DMF (2 mL} was treated with propyl iodide
(40
mL), stirred at 65 °C for 1 h, diluted with water and extracted with
diethyl ether. The organic
extracts were dried (MgSO4) and concentrated, and the residue was purified on
silica gel with
10% ethyl acetate/hexanes to provide 160 mg of the title compound.
25 MS (DCI/NH3) m/e 355 (M+H)+.
Exam lie 43B
7-Pronoxv-R-iodo-2-nanhthaienecarboximidamide monoltrifluoroacetat 1 salt
The title compound was prepared from the product in Example 43A according to
the
30 procedure of Example I B.
I H NMR (300 MHz, DMSO-d6) b 1.09 (t, 3H), 1.82 (m, 2H), 4.23 (t, 2H), 7.62
(d, 1 H),
7.65 (dd, 1 H), 8.12 (dd, 2H), 9.15 (s, 2H), 9.42 (s, 1 H), 9.53 (s, 2H);
MS (DCI/NH3} m/e 355 (M+H)+.
-82-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98115386
Anal. calcd. for C14H15N20I~TFA~0.26C~Hg: C, 43.49; H, 3.70; N, 5.69. Found:
C; 43.50;
H, 3.59; N, 5.75.
Example 44
S ( ~ )- 6-(3-Phenvloxiran lY )-2-naphthalenecarboximidamide
mono(trifluoroacetate) salt
Example 44A
( ~ )-6-(3-Phen loxiranyl -2-naphthalenecarbonitrile
A solution of Example 41 A (69 mg, 0.27 mmol) and m-chIoroperbenzoic acid (70
mg,
0.41 mmol) in methylene chloride (3 mL) was stirred at 25 °C for 3
days, concentrated, loaded
on a silica gel column (pretreated with 0.1 % triethylamine in ethyl acetate)
and eluted with 10%
ethyl acetate/hexane) to provide 72 mg of the title compound.
MS (DCI/NH3) m/e 289 (M+NH4)+.
Example 44B
{ ~ )- 6-(3-Phenvloxiranyl)-2-naphthaienecarboximidamide
mono(trifluoroacetate) salt
The title compound was prepared with Example 44A from the procedure of Example
1B.
t H NMR (300 MHz, DMS~-d6) 8 4.24 (d, 1 H), 4.35 (d, 1 H), 7.43 (m, SH), 7.67
(dd, I H),
7.83 (dd, 1 H), 8. I2 (s, 1 H), 8.13 (d, 1 H), 8.16 (d, 1 H), 8.50 (s, I H),
9.03 (s, 2 H), 9.44 (s,
2H);
MS (DCI/NH3) m/e 289 (M+H)+.
Anal. calcd for C~9H16N20~ l.3 TFA: C, 64.52; H, 4.55; N, 6.51. Found: C;
64.35; H, 4.60;
N, 5.87.
Example 45
(E)-6-f 2-(2-Thienyl)ethenyl l-2-n~hthalenecarboximidamide
mono(trifluoroacetate) salt
3o Example 45A
2-V inylthiophene
A suspension of methyltriphenylphosphonium bromide (19.13 g, 53.5 mmol) in THF
( 100 mL) was treated dropwise with 2M butyllithium in THF ( 17.8 mL) then
dropwise with 2-
-83-
SUBSTITUTE SHEET (RULE 26j
CA 02294300 1999-12-14
WO 99105096 PCTIUS98/15386
carboxythiophene (5 g, 44.6 mmol), stirred for 30 min then distilled at 74-78
°C to provide the
title compound.
MS (DCI/NH3) m/e 111 (M+H}+.
Exam 1e~45B
fEl-6-f 2-l2-Thien Ily ethenyll-2-naphthalenecarbonitrile
The title compound was prepared from the product of Example 45A and the
procedure
of Example 41 A.
MS (DCI/NH3) m/e 279 (M+NH3)+.
Ex n Ir~45C'
(E)-6-f2-f2-Thienvl)ethenLrll-2-nanhthalene~arh ximidamide
mono(trifluoroac .rarPl calr
The title compound was prepared from Example 45B and the procedure of Example
1B.
~H-NMR (300 MHz, DMSO-d6) S 7.12 (dd, 2H), 7.15 (d, 1H), 7.32 (d, 1H), 7.6 (d,
1H),
7.74 (d, 1 H), 7.80 (dd, 1 H), 7.9-8.1 (m, 3H), 8.14 (s, 1 H), 8.43 (s, 1 H),
9.03 (s, 2H), 9.42
(s, 2H};
MS (DCI/NH3) m/e 279 (M+H)+;
Anal. calcd. for C1~H14N202S~TFA: C, 53.77; H, 3.56; N, 6.60. Found: C; 54.88;
H, 3.66;
N, 6.45.
Exam_ Ip a 46
-l3-Oxobutvll-2-naphthalenecarboximidamide monoftrifluoroac tate~ salt
Example 46A
6-f3-Oxobutvl)-2-naphthalenecarbonitrile
The title compound was prepared from Example 28B, 1-buten-3-of and the
procedure of
Example 41 A.
MS (DCI/NH3) m/e 241 (M+NH4)+.
Exam Il~ a 46B
6-(3-Oxobutvll-2-nat~hthalenec~rboximidami P mono(tri uoroa~etate) salt
The title compound was prepared from Example 46A and the procedure of
ExamplelB.
-84-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO, 99/05096 _ PCT/US98/15386
1 H NMR (300 MHz, DMSO-d6) 8 2.13 (s, 1 H), 2.94 (m, 4H), 7.57 (dd, 1 H) 7.78
(dd> 1 H),
7.85 (s, 1 H), 8.01 (d, 1 H), 8.05 (d, 1 H), 8.43 (s, 1 H), 8.48 (m, 2H}, 9.06
(s, 2H), 9.40 (s,
2H);
MS (DCI/NH3) m/e 241 (M+H)+;
S Anal. caicd. for C 1 gH 16N20~ I .3TFA: C, 54.3 I ; H, 4.48; N, 7. I 9.
Found: C; 54.33; H, 4.35;
N, 7.27.
Example 47
6-(3-Methoxwhenyl)-2-naphthalenecarboximidamide mono~trifluoroacetate) salt
io
Example 47A
6-l3-Methoxvphen ly )-2-na~hthalenecarbonitriIe
A solution of Example 28B (300 mg, 1 mmol), palladium (II) acetate (22 mg, 0.
I
mmol) and 1-I'-bis(diphenyphosphino)ferrocene (111 mg, 0.2 mmol} was stirred
in DMF (3
15 mL) for 15 min, treated with Cs2C03 (813 mg, 2.5 mmol) and 3-
methoxyphenylboronic acid
(228 mg, 1.5 mmol), stirred for 20 min at 80 °C, cooled, treated with
pH 7 buffer ( 10 mL} and
extracted with diethyl ether. The ether extracts were dried (MgS04),
concentrated and purified
on silica gel with 10% ethyl acetate/hexane to provide 140 mg of the title
compound as a white
solid.
20 MS (DCI/NH3) m/e 277 (M+NH4)+.
Example 47B
6-(3-Methoxvnhenvl)-2-nanhthalenecarboximidamide mono(trifluoroacetate) salt
The title compound was prepared from Example 47A and the procedure of Example
1 B.
25 t H NMR (300 MHz, DMSO-d6) 8 3.88 (s> 3H), 7.03 (m, I H), 7.44 (m, 3H),
7.84 (dd, 1 H},
11.05 (dd, 1 H), 8.19 (d, 1 H), 8.21 (d, I H), .4 t (s, 1 H), 8.5 i (s, 1 H),
9. I 1 (s, 2H), 9.45 (s,
2H);
MS (DCI/NH3) rn/e 277 (M+H)+;
Anal. calcd for C~gH~6N20~TFA~0.2H20: C, 61.03; H, 4.45; N, 7.I2. Found: C;
61.03; H,
30 4.11; N, 6.8b.
Example 48
N-f3-(methvl)phenvll-6-aminoiminomethvl-2-naphthalenecarboxamide
mono(trifluoroacetate_)
salt
-85-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98/15386
Examtile 48A
N-f3-(meth~phenyll-~~~ -c~ano-2-naphthalenecarboxamide
The title compound was prepared from 3-methyl phenylis.ocyanate, Example 55C
and
the procedure from Example 55C.
MS (DCUNH3) m/e 287 (M+H)+.
Exam le 4 B
N-f3-(methvi)phenvll-6-aminoiminometh 1-v 2-naphthalenecarboxamide
mono(trifluoroacetate)
The title compound was prepared from Example 48A and the procedure of Example
1 B.
1H NMR (300 MHz, DMSO-d6) 8 2.34 (s, 3H), 6.96 (d, 1H), 7.27 (t, IH), 7.62 (d,
IH),
7.66 (s, 1 H), 7.91 (dd, 1 H), 8.15 (dd, I H), 8.29 (d, 1 H), 8.31 (d, 1 H),
8.54 (s, 1 H), 8.68
(s, iH), 9.15 (s, 2H), 9.49 (s, 2H), 10.46 (s, 1H);
MS (DCI/NH3) m/e 304 (M+H)+;
Anal. calcd for C19H17N30~TFA~0.12C-7Hg: C, 61.23; H, 4.46; N, 9.81. Found: C;
61.12;
H, 4.42; N, 9.43.
Example 49
6-(2-FormvlDhenoxv)-2-nanhthalenecarboximidamide monoltrifluoroacetate) salt
Example 49A
6-f2-Formylphenoxv)-2-naphthalenecarbonitrile
A solution of 2-hydroxybenzaldehyde (72 mg, 0.59 mmol),
6-bromo-1-cyanonaphthalene ( 150 mg, 0.65 mmol), and Cs2C03 (248 mg,
0.76 mmol) in DMF ( 10 mL) was heated at 90 °C for 2 days, treated with
water and extracted
with ethyl acetate. The combined organic extracts were dried (MgS04) and
concentrated, and
the crude product was purified by column chromatography with 10% ethyl
acetate/hexane to
provide 4f? mg of the title compound.
MS (DCI/NH3) m/e 291 (M+NHa)+.
Exam Ip a 49B
6-l2-FormvlDhenoxv)-2-naphthalenPcarboximidamide mono(trifluoroacetate) cult
The title compound was prepared with Example 49A and the procedure of Example
1 B.
-86-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98115386
~ H-NMR (300 MHz, DMSO-d6) 8 7.19 (d, 1 H), 7.44 (t, 1 H), 7.56 (s, 1H), 7.60
(d, 1 H),
7.79 (m, 2H), 7.94 (dd, 1 H), 8.01 (d, 1 H), 8.2 (d, 1 H), 8.S I (s, 1 H),
9.03 (s, 2H), 9.41 (s,
2H), 10.35 (s, 1H);
MS (DCI/NH3) m/e 291 (M+H)+.
S Anal. calcd. for ClgH~4N202~TFA~ 1.7H20: C, SS.16; H, 4.27; N, 6.43. Found:
C; SS.17;
H, 3.92; N, 5.94.
Exam 1e1~50,
6-(2-Formvlphenvl)-2-naphthalenecarboximidamide mono(trifluoroacetate) salt
to
Example SOA
6-(2-Formvlnhenyl)-2-navhthalenecarbonitrile
The title compound was prepared from Example 28B, 2-formylphenylboronic acid
and
the procedure of Example 47A.
15 MS (DCI/NH~) m/e 27S (M+N3-14)+.
)Jxamnle SOB
6-f2-Formvlphenvl)-2-nanhthalenecarboximidamide mono(trifiuoroacetate) salt
The tide compound was prepared from Example SOA and the procedure of Example
1B.
20 1H NMR (300 MHz, DMSO-d6) 8 7.71-7.64 (m, 2H), 7.79 (d, 1H), 7.81 (s, IH),
7.88 (dd,
I H),7.9 (d, 1 H), 8.16 (d, I H), 8.23 (t, 2H), 8.56 (s, 1 H), 9.05 (s, 2H),
9.48 (s, 2H), 9.92
(s, 1 H);
MS (DCI/NH3) m/e 27S (M+H)+;
Anal. calcd for CIHH14N20~TFA: C, 61.86; H, 3.89; N, 7.21. Found: C; 61.98; H,
3.59; N,
25 6.88.
Example 51
6-f2-(Hvdroxymethvllohenvll-2-navhthalenecarboximidamide
mono(trifluoroacetate) salt
Example S 1 A
~-12-(Hvdroxvmethvl)1-2-naphthalenecarbonitrile
Example SOA (98 mg, 0.38 mmol) and sodium borohydride ( 1 S mg, 0.80 mmol)
were
dissolved in methanol ( 10 mL) and stirred for O.S h. The solution was
concentrated, and the
_87_
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98115386
residue was purified on silica gel with 30% ethyl acetate/hexane to provide 90
mg of the title
compound.
MS (DCI/NH3) m/e 277 (M+NH4)+.
Example 1B
6-f2-(Hvdroxvmethvl)phenyll-2-naphthalenecarboximi~iamide
mono(trifluoroacetatel salt
The title compound was prepared from Example S 1 A and the procedure of
Example 1 B.
1H NMR (300 MHz, DMSO-d6) 8 9.46 (s, 2H), 9.06 (s, 2H), 8.54 (s, 1H),
8.16 (t, 2H), 8.07 (s> IH), 7.85 (dd, IH), 7.74 (dd, IH), 7.63 (d, IH), 7.49-
7.34 (m, 3H),
4.46 (s, 2H);
MS (DCI/NH3) m/e 277 (M+H)+;
Anal. calcd. for C1gH16N20~ 1.44TFA: C, 56.93; H, 3.99; N, 6.36. Found: C;
56.94; H,
3.88; N, 6.46.
S Exam~2
613-Oxo-1-bntenvll-2-nanhthalenecarboximidamide mono(trifluoroacetatel salt
Example 52A
6-l3-Oxo-1-buten 1W-2-naphthalenecarbonitrile
The title compound was prepared from methyl acrylate, Example 28B and the
procedure
of Example 41 A. MS (DCI/NH3) m/e 222 (M+H)+.
Exam Ie~52B
6-(3-Oxo-1-butenvl)-2-naohthalenecarboximidamid monoltrifluoroacetatel alt
The title compound was prepared from Example 52A and the procedure of Example
IB.
1 H NMR (300 MHz, DMSO-d6} 8 9.46 (s, ZH), 9.13 (s, 2H), 8.48 (s, 1 H), 8.38
(s, 1 H),
8.18 (d, I H), 8.15 (d> 1 H), 8.01 (dd, 1 H), 7.85 (dd, 1 H), 7.82 (d, 1 H),
7.03 (d, I H), 2.40
(s, 1H);
MS (DCI/NH3) m/e 239 (M+H)+.
Anal. calcd for Cj5H1q.N20~1.58TFA: C, 52.13; H, 3.75; N, 6.69. Found: C;
52.09; H, 3.63;
N, 6.64.
Examl la a 53
7-Methoxv-8-( 1 H-DVrazol-4 yl)-2-na~hthalenecarboximidamide
bis(trifluoroacetate) salt
_88_
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98I15386
Example 53A
7-hvdroxv-8-iodo-2-n~hthalenecarbonitriIe
A mixture of 7-cyano-2-naphthol (22.3 g, 131.8 mmol), sodium carbonate (29.3
g, 277
mmol) and I2 (31.8 g, 125.2 mmol) in water (500 mL) and THF (80 mL) at 0
°C was stirred at
room temperature for 3 h, acidified with 1 M HCl and extracted with ethyl
acetate. The extracts
were washed with saturated Na2S203 and brine, dried (Na2S04) and concentrated.
The
product was recrystallized from ethyl acetate to yield 33 g of the title
compound.
MS (DCI/NH3) m/e 313 (M+NH4)+.
Exam l S B
7-Methoxy-8-iodo-2-naphthalenecarbonitrile
Example 53A (36.7 g, 124.2 mmol) in methanol (500 mL) and ethyl acetate (300
mL)
was treated over 3 h with 2M trimethylsilyldiazomethane in hexane (260 mL),
stirred for 24 h,
t 5 concentrated and recrystallized from ethyl acetate to provide 36.4 g of
the title compound.
MS {DCI/NH3) m/e 327 (M+NH4)+.
Example 53C
4-Iodo-I-ff2-(trimethylsilyl)ethox lv meth lv 1-1H-pyrazole
A slurry of NaH ( 1.94 g, 48.5 mmol) in THF (40 mL) at 0 °C was treated
with a
solution of 4-iodopyrazole (8.97 g, 46.2 mmol) in THF (20 mL), stirred for I
h, treated with
SEM chloride (9.00 mL, 50.8 mmol), stirred at room temperature for 1 h, poured
into water
and extracted with ethyl acetate. The extracts were washed with brine, dried
(MgS04) and
concentrated. The residue was chromatographed on silica gel with 10% ethyl
acetate/hexanes to
provide 14.4 g of the title compound.
MS (DCI/NH3) m/e 325 (M+H)+.
Exam le D
I -ff2-~Trimethv~i_Ivl)ethox l~methyll-IH-pyrazol4-yllboronic acid
Example 53C (12.97 g, 40 mmol) in THF (250 mL) at -78 °C was treated
with 2.5 M
butyliithium in hexanes ( 17.6 mL, 44 mmol), stirred at -78 °C for IO
min, treated with trimethyl
borate ( 11.36 mL, 100 mmol), warmed to room temperature, treated with 3M HCl
(400 mL)
and extracted with ethyl acetate. The extracts were concentrated, and the
residue was dissolved
-89-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98115386
in 1 M NaOH (500 mL), extracted with diethyl ether, acidified with
concentrated HCI and
extracted with ethyl acetate. The extracts were washed with brine, dried
(Na2S04), and
concentrated. The residue was chromatographed on silica gel with ethyl acetate
to provide 2.20
g of the title compound.
S MS (DCI/NH3) m/e I99 (M-B(OH)2)+.
Example 5
7-Methoxv-8-f I-1f2-(trimethvlsilvl)ethoxylmethyll-IH-pvrazol-4=yll-2-
nanhthalenecarbonitrile
Examples 53B (I.55 g, 5 mmol) and 53D (1.45 g, 6 mmol) were subjected to the
procedure
described in Example 47A to provide 1.64 g of the title compound.
MS (DCI/NH3) m/e 380 (M+H)+.
Examyle 53F
_7-Methoxv-8-l l H-pvrazol-4-yl)-2-naphthalenecarbonitrile
A solution of Example 53E ( 1.84 g, 4.85 mmoI) in THF
( 10 mL) was treated with 1 M tetrabutylammonium fluoride in THF (24 mL),
refluxed for 6 h
and concentrated. The residue was chromatographed on silica gel with 1: I
ethyl acetate/hexanes
to provide 0.88 g of the title compound.
MS (DCI/NH3) m/e 267 (M+NH4)+.
Exam 1e~53G
7-Methoxv-8-(1 H-nvrazol-4-vl)-2-naphthalenecarboximidamide
bis(trifluoroacetate) salt
The title compound was prepared from Example 53F and the procedure of Example
I B.
~ H NMR (300 MHz, DMSO-d6) 8 3.89 (s, 3H), 7.60 (dd, 1 H), 7.71 (d, 1 H), 7.92
(s, 2H),
8.05 (d, I H), 8.12 (d, 1 H), 8.29 (s, 1 H), 9.33 (s, 2H), 9.34 (s, 2H);
MS (DCI/NH3) m/e 267 (M+H)+ ;
Anal. calcd. for C15H14N40~2.8TFA: C, 42.30; H, 2.90; N, 9.59. Found: C;
42.54; H, 3.1 l;
N, 9.03.
Example 54
7-Methoxv-8-iodo-2-nanhthaleneca_rhnxirrudamide mono(trifluoroacetate) salt
The title compound was prepared from Example 53B and the procedure of Example
1 B.
-90-
SU9ST1TUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98/15386
~H NMR (300 MHz, DMSO-d6) 8 4.01 (s, 3H), 7.65 (m, 2H), 8.12 (d, IH), 8.15 (d,
IH),
8.42 (s, 1 H), 9.14 (s, 2H), 9.52 (s, 2H);
MS (DCI/NH3) m/e 327 (M+H)+.
Anal. calcd for C12H12N20I~ I.2TFA: C, 37.28; H, 2.87; N, 6.04. Found: C;
37.35; H, 2.47;
N, 5.93.
Example 55
N-nhenvl-6-aminoiminomethvl-2-nanhthalenecarboxamide mono(methanesulfonate)
salt
Example 55A
2-T ifluoromethanesulfonvloxy-6-bromonaphthalene
A solution of 6-bromo-2-naphthol (4.96 g, 22.25 mmol),
N-phenyltrifluoromethanesulfonate (7.95 g, 22.25 mmol), and
diisopropylethyIamine (7.75
mL, 44.5 mmol) in methylene chloride (25 mL) were stirred for 3 h at room
temperature,
t S poured into water and extracted with diethyl ether. The extracts were
washed with brine, dried
(MgS04), and concentrated. The residue was chromatographed on silica gel with
3% ethyl
acetate/hexane to provide 7.89 g of the title compound.
MS (DCI/NH3j m/e 354 and 356 (M+H)+.
20 Example SSB
l-Bromo-2-naphthalenecarbonitriie
Example SSA (7.89 g, 22.2 mmol) was combined with Zn(CN)2 ( I .33 g,
I 1.33 mmol) and Pd(PPh3)4 (256 mg, 0.22 mmol) in DMF (50 mL), heated at
90° C for 3 h,
cooled to room temperature, treated with saturated NaHC03 and extracted with
diethyl ether.
25 The extracts were washed with brine, dried over (MgS04), and condensed. The
residue was
chromatographed on silica gel with 5% ethyl acetate/hexanes to provide 2.67 g
of the title
compound.
MS (DCI/NH3) m/e 231 and 233 (M+H)+.
3o Example 55C
N-phenyi-6-cyano-2-naphthalenecarboxamide
A solution of Example 55B (224 mg, 0.965 mmol) in THF (3 mL) and hexanes ( 1
tnL)
at -100° C was treated with 2.5 M butyllithium in hexanes (0.386 mL,
0.965 mmol), stirred at
-100° C for 5 min, treated with phenyl isocyanate (0.115 mL, 1.06
mmol), warmed to room
-91-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTlUS98/15386
temperature, treated with pH 7 buffer (0.5 mL) and concentrated. The residue
was
chromatographed on silica gel with 20% ethyl acetate/hexanes as eluent, to
provide 54 mg of the
title compound:
MS (DCI/NH3) m/e 273 (M+H}+.
Example 55D
N-nhenvl-6-aminoiminomethyl-2-naphthalenecarboxamide mono(methanesulfonate)
salt
A solution of Example 55C (52 mg, 0.191 mmol) in THF (2 mL) was treated with 1
M
lithium bis(trimethylsilyl)amide in THF (0.6 mL), stirred for 18 h, treated
with 2M HCl (4
mL}, stirred for another 24 h, made basic with saturated Na2C03 and extracted
with ethyl
acetate. The extracts were washed with brine, dried (Na2S04) and concentrated.
The crude
product was dissoved into a minimal amount of methanol (ca. l mL), treated
with
methanesulfonic acid (1 drop), diluted with diethyl ether (400 mL) and
filtered to provide 15 mg
of the title compound.
t5 t H NMR (300 MHz, DMSO-d6) 8 2.32 (s, 3H), 7.15 (dd, I H), 7.40 (dd, 2H),
7.83 (d, 2H),
7.90 (dd, I H), 8.17 (dd, 1 H), 8.25 (d, 1 H), 8.34 (d, 1 H), $.57 (s, 1 H),
8.70 (s, 1 H), 9.09
(br s, 2H), 9.51 (br s, 2H);
MS (DCI/NH3) m/e 290 (M+H)+.
Anal. calcd for CIgH~6N30~1.1 CH3S03H: C, 57.96; H, 4.95; N, 10.61. Found: C,
58.03;
H, 4.48; N, 10.36.
Examlhe 5656
4-ff6-Aminoiminomethyl-~-naphthalen I~ox~-methvlbenzeneacetamide
mono(trifluoroacetate) salt
Example 5 A
N-methyl-3-hydrox'~~lacetamide
A solution of 3-hydroxyphenylacetic acid (1.00 g, 6.57 mmol) and oxalyl
chloride
(0.63 mL, 7.22 mmol) in methylene chloride (20 mL) was treated dropwise with
pyridine (0.6
mL, 7.37 mmol), stirred for 90 min, poured into 40% aqueous methylamine (30
rrtL), stirred
for IS min, concentrated, dissolved into 1 M HCl and extracted with ethyl
acetate. The extracts
were washed with brine, dried (MgS04) and concentrated. The residue was
chromatographed
on silica geI with ethyl acetate to provide 260 mg of the title compound.
-92-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98/15386
MS (DCI/NH3) m/e 166 (M+H)+.
Example 56B
4-j(6-Cyano-2-naphthalenyi)oxxl-N-methylbenzeneacetamide
A mixture of Example 56A (245 mg, 1.48 mmol), Example 55B (344 mg, 1.48 mmol)
and Cs2C03 (530 mg, 1.63 mmol) in DMF (3 mL) was stirred for 72 h at
120 °C, cooled and chromatographed on silica gel with 1:1 ethyl
acetate/hexanes to provide 54
mg of the title compound.
MS (DCI/NH3) m/e 317 (M+H)+.
Example 56C
4-f (6-Aminoiminomethvl-2-naphthalenyl)oxyl-N-methylbenzeneacetamide
mono(trifluoroacetate) salt
The title compound was prepared from Example 56B and the procedure of Example
55D.
~ H NMR (300 MHz, DMSO-d6) 8 2.33 (s, 3H), 2.58 (d, 3H), 3.42 (s, 2H), 7.05
(m,
2H),7.12 (d, 1 H), 7.40 (dd, 1 H), 7.45 (m, 2H), 7.79 (dd, 1 H), 7.98 (q, 1
H), 8.02 (d, 1 H),
8.15 (d, 1H), 8.49 (s, 1H), 8.99 (br s, 2H), 9.39 (br s, 2H);
MS (DCl/NH3) m/e 334 (M+H)+.
2o Anal. calcd for C~9H1~N302~ 1.5CH3S03H: C, 54.08; H, 5.28; N, 8.80. Found:
C, 53.80;
H, 5.37; N, 8.52.
Example 57
fi-12-(Methvlthio)nhenvll-2-naphthalenecarboximidamide mono(methanesulfonate)
salt
Example 57A
2-Cyanonaphthalene-6-boronic acid
A solution of Example 55B (6.37 g, 27.45 mmol) in THF (220 mL) and hexanes (50
mL) at
-100 °C was treated with 2.5 M butyllithium in hexanes ( 1 1.0 mL, 27.5
mmol), stirred at -100
3U °C for 10 min, treated with trimethyl borate (7.8 mL, 68.6 mmol),
warmed to room
temperature, treated with 3M HCl (400 mL) and extracted with ethyl acetate.
The extracts were
concentrated and the residue was dissolved into 1 M NaOH (500 mL), extracted
with diethyl
ether, acidified with 12M HCl and extracted with ethyl acetate. The extracts
were washed with
-93-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98/15386
brine, dried (Na2S04), and concentrated. The residue was dissolved into
minimal methanol
and ethyl acetate and triturated with hexanes to yield 2.74 g of the title
compound.
MS (DCI/NH3) m/e 215 (M+NH4)+.
Exam ly a 57B
6-f 2-(Methylthio)phenvll-2-navzhthalene~arbonitrile
A solution of 2-bromothioanisole (0.147 mL, 1.10 mmol), Pd(OAc)2 (24 mg, 0.11
mmol) and
1,1'-bis(diphenylphosphinoferrocene) (120 mg, 0.22 mmol) in DMF (5 mL) was
stirred for 10
min, treated with Example 57A (260 mg, 1.32 mmol) and CszC03 ( 1.07 g, 3.3
mmol), heated
at 85 °C for 6 h, cooled to room temperature and chromatographed on
silica gel with 10% ethyl
acetate/hexanes to provide 155 mg of the title compound.
MS (DCI/NH3) m/e 231 (M+NH4)+.
Exam le 57
is 6-f2-(Methylthio) hen Il-2-naohthalenecarboximidamide
mono(methanesulfonate) salt
The title compound was prepared from Example 57B and the procedure of Example
SSD.
1 H NMR (300 MHz, DMSO-d6) 8 2.32 (s, 3H), 2.40 (s, 3H), 7.34 (m, 2H), 7.45
(m, 2H),
7.82 (dd, 2H), 7.95 (dd, 1 H), 8.06 (s, 1 H), 8.15 (d, 1 H), 8.20 (d, 1 H),
8.55 (s, 1 H), 9.03
(br s, 2H), 9.56 (br s, 2H);
MS (DCI/NH3) m/e 293 (M+H)+.
Anal. calcd for CIgHI~N2S~CH3S03H: C, 58.18; H, 5.18; N, 7.12. Found: C,
57.97; H,
5.31; N, 6.97.
Example 58
f~-f2-(2-Thiomethoxoxvethvl)phen,ylinaDhthalene-2-carboximidamide
monolmethanesulfonate)
Example ,S~iA
2-l2-Bromoethvllbromobenzene
A solution of 2-bromophenethyl alcohol (5.05 g, 25.1 mmol) and pyridine (3.65
mL, 45.2
mmol) in acetonitrile (60 mL) was treated with Ph3PBr2 ( 13.8 g, 32.65 mmol),
stirred at 0° C
for 2 h, diluted with hexanes and filtered through a plug of silica gel with
25% diethyl
ether/hexanes to provide 6.0 g of the title compound.
-94-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98/15386
MS (DCIJNH3) m/e 263 (M+H)+.
Example 58B
2-(2-Thiomethox-yethyl)bromobenzene
A solution of Example 58A (990 mg, 3.75 mmol) and sodium thiomethoxide (290
mg,
4. I2 mmol) in DMF (5 mL) was heated at 90° C for 5 h, cooled and
chromatographed on silica
gel with 1 % ethyl acetate/hexanes to provide 646 mg of the title compound.
MS (DCI/NH3) m/e 231, 233 (M+H)+.
to Example 58C
6-(2-(2-Thiomethoxyethyl)phenyll-2-naphthalenecarbonitrile
The title compound was prepared from Example 58B (300 mg, 1.30 mmol), Example
57A (260
mg, 1.32 mmol) and the procedure described in Example 57B.
MS (DCI/NH3) m/e 321 (M+NH4)+.
IS
Example 58D
6-12-(2-Thiomethoxoxvethvl)ohenvllnaphthaiene-2-carboximidamide
mono(methanesulfonate~
st
The title compound was prepared from Example 58C and the procedure from
Example
2o SSD.
IH NMR (300 MHz, DMSO-d6) 8 I.78 (s, 3H), 2.31 (s, 3H), 2.55 (m, 2H), 2.85 (m,
2H),
7.30-7.48 (m, 4H), 7.66 (dd, 1 H), 7.85 (dd, I H), 8.04 (s, I H), 8.18 (d, 1
H), 8.20 (d, 1 H),
8.55 (s, 1H), 9.01 (br s, 2H), 9.43 (br s, 2H);
MS (DCI/NH3) m/e 321 (M+H)+.
25 Anal. calcd for CzpH2oN2S2~ I .35CH3S03H: C, 56.96; H, 5.69; N, 6.22.
Found: C, 57.08;
H, 5.49; N, 6. i 4.
Example 59
7-Methoxv-8-(3-furanvl)-2-naahthalenecarboximidamide
mono(methanesulfonateZsalt
Example 59A
7-Methoxv-8-l3-furanyl)-2-naphthalenecarbonitrile
The title compound was prepared from Example 53B, furan-3-boronic acid (873
mg,
7.80 mmol) and the procedure of Example 57B.
-95-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98/15386
MS (DCI/NH3) m/e 267 (M+NH4)+.
Example 59B
7-Methoxv-8-(3-furanvl)-2-naphthaienecarboximidamide mono(methanesulfonate)
salt
The title compound was prepared from Example 58C and the procedure from
Example
55D.
1H NMR (300 MHz, DMSO-d6) 8 2.34 (s, 3H), 3.91 (s, 3H), 6.76 (s, 2H), 7.62
(dd, IH),
7.74 (d, 1 H), 7.87 (dd, I H), 7.96 (s, I H), 8.12 (d, I H), 8. I 5 (d, I H),
8.25 (s, 1 H), 8.96 (br
s, 2H), 9.35 (br s, 2H);
n> MS (DCI/NH3) m/e 267 (M+H)+.
Anal. calcd for Ci~,H~4N202~CH3S03H: C, 55.77; H, 5.00; N, 7.63. Found: C,
55.73; H,
4.61; N, 7.4$.
Example 60
7-Methoxv-8-(2-benzofuranyl)naphthalene-2-carboximidamide
monolmethanesuifonate) salt
Example 60A
7-Methoxv-8-(2-benzofuranvi)-2-naphthalenecarbonitrile
The title compound was prepared from Example 53B ( 166 mg, 0.5() mmol),
benzofuran-2-
2o boronic acid ( 1 13 mg, U.70 mmol) and the procedure of Example 57B.
MS (DCI/NH3) m/e 317 (M+NH4)+.
Example COB
7-Methoxv-8-(2-benzofuranyl)naphthalene-~-- c:arboximidamide
mono(methanesulfonate) salt
'_', The title compound was prepared from Example 60A (72 mg, ().240 mmol) and
the procedure
from Example 55D.
~H NMR (3(l0 MHz, DMSO-d~;) b 2.30 (s, 3H), 3.98 (s, 3H), 7.24 (s, 1H), 7.36
(m, 2H),
7.67 (m, 2H), 7.75 (m, I H), 7.84 (d, 1 H), 8.21 (d, 1 H), 8.30 (d, 1 H), 8.32
(s, 1 H), 8.88 (br
s, 2H), 9.39 (br s, 2H);
3U MS (DCI/NH3) m/e 317 (M+H)+.
Anal. calcd for C2pH 1~N2O2~ 1.3CH3S03H: C, 57.98; H, 4.84; N, 6.35. Found: C,
57.79;
H, 4.78; N, 6.22.
Example 6I
-96-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98115386
(E)-H-f 2-( I ,3-Benzodioxol-5-vl)ethenyll-~-nar~hthalenecarboximidamide
mono(methanesulfonate salt
Exam le 1 A
(E)-S-f 2-( 1.3-Benzodioxol-5- 1)ethenvll-2-na~hthalenecarbonitrile
Example 53B (75 mg, 0.243 mmol), PdCI2(dppf) (20 mg, 0.024 mmol), 3,4-
methylenedioxystyrene (43 mg, 0.291 mmol) and diisopropylethylamine (0.170 mL,
0.97
mmol) in N-methylpymolidinone (2 mL) were stirred at 90° C for 18 h,
cooled to room
temperature and chromatographed on silica gel with 20°lo ethyl
acetate/hexanes to provide 46 mg
m of the title compound.
MS (DCI/NH3) mle 347 (M+NH4)+.
Example 61 B
(E)-H-f 2-( I ,3-Benzodioxol-5- llethenyl l-2-naphthalenecarboximidamide
t5 mono(methanesulfonate) salt
The title compound was prepared from Example 61 A (43 mg, 0.131 mmol) and Ehe
procedure from Example 55D.
~H NMR (300 MHz, DMSO-d~,j 8 2.32 (s, 3H), 4.01 (s, 3H), 6.07 (s, 2H}, 6.96
(d, 2H),
7.10 (d, 2H), 7.32 (d, 2H), 7.45 (s, 1 H), 7.56 (d, 1 H), 7.66 (d, 2H), 7.72
(d, 1 H), 8.06 (s,
2c> 1 H), 8.03 (d, 1 H), 8.12 (d, 1 H), 8.66 (s, 1 H), 8.96 (br s, 2H), 9.44
(br s, 2H);
MS (DCI/NH3) m/e 347 (M+H)+.
Anal. calcd for C2t H I~N~03~ I .1 CH3S03I-l: C, 58.72; H, 4.99; N, 6.2().
Found: C, 58.77;
H, 5.07; N, 5.99.
--'s Example 62
( ~ )-7-Methoxy-!~-(tetrahydro-3-furanyl -. )?-naphthalenec:arboximidamide
mono(methanesulfonate) salt
Example 62A
3O (~ }-7-methoxv-H-f3-hvdroxv-1-(hvdroxvmet~l)-1-hropenyll-2-
naphthalenecarbonitrile
A solution of Example 53B (3.09 g, 10 mmol), PdCI? (120 mg, 1 mmol), cis-2-
butene-1,4-dioi
( 1.23 mL, I 5 mmol) and NaHCO~ ( I .() I g, 12 mmol) in N-methylpyrrolidinone
( 10 mLj was
stirred at 130 °C for I h, cooled to room temperature and
chromatographed on silica gel with
-97-
SUBSTITUTE SHEET (RULE 2B)
CA 02294300 1999-12-14
WO 99105096 PCT/US98/15386
30°lo ethyl acetate/hexanes to provide 2.19 g of the title compound as
a mixture of
diastereomers.
MS (DCI/NH3) m/e 269 (M+H)+.
Exam lp a f 2B
( ~ )-7-Methoxy-8-(tetrahvdro-3-furanyl)-2-naphthalenecarbonitrile
Example 62A ( 140 mg, 0.52 mmol) in methylene chloride (3 mLj at 0 °C
was treated with
triethylsilane (0.166 mL, 1.04 mmol) and BF3~OEt2 (0.096 mL, 0.78 mmol),
stirred at room
temperature for 4 h, concentrated and chromatographed on silica gel with 25010
ethyl
lU acetate/hexanes to provide 100 mg of the title compound.
MS (DCI/NH3) m/e 271 (M+NH4)+.
Exam Ie~62C
(~ )-7-Methoxy-H-(tetrahydro-3-furanyl)-2-na~hthalenecarboximidamide
t S mono(methanesulfonate) salt
The title compound was prepared from Example 62B (96 m~, 0.379 mmol) and the
procedure from Example 55D.
1 H NMR (300 MHz. DMSO-d~,) 8 2.20 (m, 1 H), 2.33 (m, 1 H), 2.39 (s, 3H), 3.99
(s, 3H),
3.90-4.03 (m, 3H ), 4.1 1 (m, 1 H), 4.42 (m, 1 H), 7.64 (d, 1 H), 7.68 (d, 1
H), 8.01 (d> 1 H),
2U 8.1 () (d, 1 H), 8.70 (s, 1 H), 9.01 (br s, 2H), 9.41 (br s, 2H),
MS (DCI/NH3) m/e 271 (M+H)+.
Anal. calcd for C~~,H1gN202~1.2CH3S03H: C. 53.57; H, 5.96; N, 7.26. Found: C,
53.67;
H, 5.7X; N. 6.72.
Exam l
f~-Il4-(2-Aminoethyl)phenyl lethvnvll-2-naphthalenecarboximidamide
mono(trifluoroacetate)
salt
Example 63A
3U 6-(Trimeth~silyleth~n~l)-2-naphthalenecarbonitrile
Example 28B and trimethylsilylacetylene were submitted to the conditions
described in
Example 42C to provide the title compound.
MS (DCI/NH3) m/e 267 (M+NH4)+.
_98_
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98115386
Example 63B
6-Ethynvl-2-naphthalenecarbonitrile
A mixture of Example 63A (t).4 g, 1.6 mmole) and K2C03 (U.4 g, 3.2 mmole) in
methanol ( 16 mL) was stirred at room temperature for 18 h, concentrated,
treated with water
and extracted with methylene chloride. The organic layer was washed with U.5
NHCI and
brine, dried (MgS04) and evaporated to provide the title compound.
MS (DCI/NH3) m/e 195 (M+NH4)+.
Examlale 63C
1u 4-Bromo-lN-tert-butoxycarbo ~l~ henethvlamine
4-Bromophenethylamine and di-t-butyldicarbonate were subjected to the
conditions
described in Synthesis, 48, 1986 to provide the title compound.
MS (DCI/NH3) m/e 319 (M+NH4}+.
S Example 63D
6-f 14-(2-N-tert-butoxycarbonvlaminoeth~lphe~l lethynyll-2-
na~hthalenecarbonitrile
The title compound was obtained with Examples 63B and C from the procedure
described in Example 57B to provide the title compound.
MS (DCI/NH3) m/e 414 (M+NH4)+.
2()
Exam l~E
6-I14-(2-Aminoethvl)z~henvllethvnvll-2-naphthalenecarboximidamide
mono(trifluoroacetate~
salt
The title compound was prepared with Example 63D and the procedure of Example
5B.
25 ~ H NMR (3U() MHz, DMSO-d~,) b 2.9U (t, 2H), 3.U9 (m, 2H) 7.36 (d, 2H),
7.60 (d, 2H),
7.76 (d, 2H), 7.76 (dd, IH), 7.85 (s, 2H), 7.87 (dd, 1H). 8.13 (d. 1H), 8.18
(d, 1H), 8.31
(s, I H), 8.5U (s, I H}, 9.18 (s, 2H), 9.45 (s, 2H);
MS (DCI/NH3) m/e 314 (M+H)+.
Anal. calcd for C21H19N3~2TFA~H20: C, 53.67; H, 4.14; N, 7.15. Found: C,
53.37; H,
3t~ 3.93; N, 7.17.
Exam lp a 64
7-Methoxv-8-12-pvrimidinvl(oxv)1-~-naphthaIenecarboximidamide
mono(trifluoroacetatel
-99-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98/I5386
Example 4A
7-Methoxv-8-f 2-Rvrimidinyl(oxy)1-2-naphthalenecarbonitrile
Example 4A ( 125 mg, 0.627 mmol) and 2-chloropyrimidine ( 143 mg, 1.25 mmol)
were
subjected to the procedure described in Example 6A to provide 101 mg of the
title compound.
MS (DCI/NH3) m/e 278 (M+H)+.
Example 64B
7-Methoxv-8-f2-twrimidinvltox )1-2-naphthalenecarboximidamide
mono(trifluoroacetate)
The title compound was prepared with Example 64A and the procedure of Example
1 B.
1 ~H NMR (300 MHz, DMSO-d~,) b 2.51 (s, 3H), 3.83 (s, 3H), 7.18 (t, 1H), 7.70
(dd, 1H),
7.80 (d, 1 H), 8.05 (d, 1 H), 8.19 {d, 1 H), 8.34 (s, 1 H), 8.62 (d, 2H), 9.07
(br s, 2H), 9.45
(br s, 2H);
MS (DCI/NH3) m/e 295 (M+H)+.
Anat. calcd for C?pH ~~N4O4~ 1.33TFA: C, 40.48; H, 2.60; N, 8.35. Found: C,
40.25; H,
~s 2.94; N, 8.92.
Exam 1» a 65
7-Methoxv-8-f2-thiazovi(oxv)lnaphthalene-2-carboximidamide
mono(trifluoroacetate) salt
?« Example 65A
7-Methox~r-8-f2-thiazovl(oxy)1-~-naphthalenecarbonitrile
A mixture of Example 4A (250 mg, 1.25 mmol), 2-bromothiazole (225 mL, 2.50
mmol) and
CsF (2t)9 mg, 1.38 mmol) in DMSO (4 mL) was stirred at 120 °C for 4
days, cooled and
chromatographed on silica gel with 30% ethyl acetate/hexanes to provide 162 mg
of the title
?a compound.
MS (DC1/NH3) m/e 283 (M+H)+.
Example 65B
7-Methoxv-8-f2-thiazovl(oxvllna~hthalene-2-carboximidamide
mono(trifluoroacetate) salt
3tl The title compound was prepared with Example 65A and the procedure of
Example 1 B.
~H NMR (300 MHz, DMSO-d6) b 3.98 (s, 3H) 7.25 (m, 2H), 7.73 (dd, 1H), 7.86 (d,
1H),
8.12 (d, 1 H), 8.22 (d, 1 H), 8.35 9.09 (bs, 2H), (s, 1 H), 9.48 (bs, 2H).
MS (DCI/NH3) m/e 300 (M+H)+.
Anal. calcd for C~5H~3N30~S~TFA: C, 49.40; H, 3.41; N, 10.70. Found: C, 49.10;
-100-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 - PCT/U598/15386
H, 3.40; N, 10.69.
Exam I
7-Methoxv-8-(4-nitroDhenoxy)-~-naphlhalenecarboximidamide
mono(,).rifluoroacetate) salt
S
Example 66A
7-Methoxv-8-(4-vitro henoxv)-2-naohthalenecarbonitrile
The title compound was prepared from Example 4A (125 mg, 0.627 mmol), 1,4-
dinitrobenzene (i43 mg, 1.25 mmol) and the procedure described in Example 65A
to provide
I~) 227 mg of the title compound.
MS (DCI/NH3) m/e 338 (M+NH4)+.
Examl la aIa a h6B
7-Methoxv-8-(4-nitroDhenoxv)-2-naphthalenecarboximidamide
mono(trifluoroacetate) salt
15 The title compound was prepared with Example 66A and the procedure of
Example
SSD.
I H NMR (300 MHz, DMSO-d~,) 8 9.43 (br s, 2H), 8.94 (br s, 2H), 8.25 (m, 4H),
H.15 (d,
I H), 7.88 (d, I H), 7.72 (dd, 1 H), 7.05 (d, 2H}, 3.91 (s, 3H), 2.30 (s, 3H};
MS (DCI/NH3) m/e 338 (M+H)+.
2u Anal. calcd for CIgH15N304~1.75CH3S03H: C, 46.93; H, 4.39; N, 8.31. Found:
C, 47.17;
H, 4.32; N, 8.I2.
Exam Ie~67
7-Methoxv-H-DentatluoroDhenox -?-naphthalenecarboximidamide
mono(trit7uoroacetatel Salt
?S
Example 67A
7-Methoxv-8-pentafluorol henoxv-2-nanhthalenecarbonitrile
Example 4A ( 100 mg, 0.50 mmol) and hexafluorobenzene ( I 15 mL, 1.00 mmol)
were
subjected to the procedure described in Example 65A to provide I50 mg of the
title compound.
3o MS (DCI/NH3) m/e 383 (M+NH4)+.
Example fi7B
7-Methoxv-8-pentafIuoroDhenoxy-2-naphthalenecarboximidamide
mono(trifluoroacetate) salt
-101-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTJUS98/15386
The title compound was prepared with Example 67A and the procedure of Example
55D.
~ H NMR (300 MHz, DMSO-d6} 8 2.31 (s> 3H), 3.82 (s. 3H), 7.87 (dd, 1 H}, 7.88
(d, I H),
8.02 (d, I H), 8.20 (d> 1 H), 8.65 (s, 1 H), 9.04 (br s, 2H). 9.4? (br s, 2H);
S MS (DCI/NH3) m/e 383 (M+H)+.
Anal. calcd for CigH11N2F50~~1.2CH3S03H: C, 46.67; H, 3.19; N, 5.68. Found: C,
46.55;
H, 3.00; N, 5.58.
Exam I~e 68
7-Methoxv-R-fN-2-nhenvlaminoll-2-naphthalenecarboximidamide
mono(trifluoroacetate~salt
Example 68A
7-Methoxv-H-IN-2-phenvl(amina) 1-2-naphthalenecarbonitrile
A solution of Example 25A (3()9 mg, l.()0 mmol), aniline (0.109 mL, 1.2 mmol),
~ 5 NaO~Bu ( 1 I 5 mg, I .2 mmol), Pd2(dba)3 ( 10 mg, 0.01 mmol) and dppf ( 17
mg, 0.03 mmol} in
toluene (5 mL) was stirred for 3 h at I()0 °C, cooled and
chromatographed on silica gel with
10% ethyl acetate/hexanes to provide 175 mg of the title compound.
MS (DCI/NH3) m/e 275 (M+H)+.
?O Example 6RB
7-Methoxv-H-(N-2-phenvlamino)-2-naphthalenecarbo~cimidamide
mono(trifluoroacetate~ salt
The title compound was prepared with Example 68A and the procedure of Example
SSD.
~H NMR (3()() MHz, DMSO-d~,) ~ 3.95 (s, 3H), 5.92 (bs, 1H), 6.6i (d, 2H), 6.94
(t, 1H),
?5 7.1 fi (dd. 2H), 7.45 (dd. 1 H). 7.4H (d, 1 H), 7.7fi (d. 1 H), 7.)if; (d.
1 H), K.13 (d. 1 H), 9.()H
(bs, 2H), 9.31 (bs, 2H).
MS (DCI/NH3) m/e 292 (M+H)+.
Anal. Calcd for CigH~7N30~TFA: C, 59.26; H, 4.4H; N, 10.37. Found: C, 59.20;
H, 4.32; N, 10.1 S.
31)
Example f 9
N-(6-Aminoiminometh 1-v 2-naphthalenyl)-N'-benzv)uren mono(trifluoroac.etate~
valt
Example 69A
-102-
SUBSTtTUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 _ PCT/US98/15386
N-(6-Cvano-2-na_phthalenvl)-N'-benzylurea
The title compound was prepared with Example 40A, benzylamine and the
procedure from
Example 40B.
MS (DCI/NH3) m/e 302 (M+H)+.
i
Example 69B
N-(6-Aminoiminomethvl-2-nalahthalenyl)-N'-benzylurea mono(trifluoroacetate)
salt
The title compound was prepared with Example 69A and the procedure from
Example 40D.
~ H NMR (300 MHz, DMSO-d6) 8 4.35 (d, 2H), 6.91 (t, I H}, 7.35-7.24 (m, SH),
7.59 (dd>
1> 1 H), 7.72 (dd, 1 H), 7.95 (d, 1 H), 7.96 (d, 1 H), 8.22 (d, 1 H), 8.35 (d,
1 H), 8.92 (br s, 2H),
9.13 (s, 1 H), 9.32 (br s, 2H).
MS (DCI/NH3) m/e 319 (M+H)+.
Anal. calcd for C~9H1RN40~TFA: C, 50.57; H, 4.24; N, 15.72. Found: C, 50.34;
H, 4.15;
N, 15.54.
IS
Example 70
N-(6-Aminoiminomethvl-2-naohthalenyl)-N'-methylurea mono(trifluoroacetate)
salt
Example 70A
2o N-(6-Cyano-2-naphthalenyl)-N'-meth, Iv urea
The title compound was prepared with Example 40A (22I.2 mg, I .00 mmole) and
methylamine (2.3 mL, 2.34 mmol) in THF ( 10 mL) according to the procedure
from Example
40B.
MS (DCI/NH3) m/e 226 (M+H)+.
?5
Example 70B
N-(6-Aminoiminometh 1-v 2-naphthalenyl)-N'-methylurea mono(trifluoroacetate)
salt
The title compound was prepared with Example 70A and the procedure of Example
40D.
3U ~ H NMR (300 MHz, DMSO-df,} b 2.69 (d, 3H), 6.32 (q, 1 H), 7.60 (dd, 1 H),
7.73 (dd, 1 H), 7.93 (d, 1 H), 7.95 (d, 1 H, 8.19 (d, 1 H), 8.49 (d, I H),
9.09 (s, 1 H), 9. I 5 (br.
s, 4H);
MS (DCI/NH3) m/e 243 (M+H}+.
-103-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTlUS98115386
Anal. calcd for C ~ ~H 14N40~TFA: C, 50.57; H, 4.24; N, 15.72. Found: C,
5Q.34; H, 4.15;
N, 15.54.
Example 71
N-(6-Aminoiminomethyl-2-naphthalenyl)-N'-isoprop~rlurea mono(trifluoroacetate)
Salt
Example 71 A
N-(6-Cyano-2-naphthalen 1 -N'-isopropvlurea
The title compound was prepared with Example 40A, isopropylamine and the
procedure
it) from Example 40B.
MS (DCI/NH3) m/e 254 (M+H)+.
Example 71 B
N-(fi-Aminoiminomethvl-2-naphthalenyl)-N'-is~rop,~rlurea
mono(trifluoroacetate) salt
1, The title compound was prepared with Example 71 A and the procedure of
Example 5B.
~ H NMR (300 MHz, DMSO-d~) 8 1. I 3 (d, 6H), 3.76-3.114 (m, 1 H), 6.28 (d, I
H), 7.55 (dd,
1 H), 7.72 (dd, 1 H), 7.94 (d, 1 H), 7.95 (d, 1 H). 8. I 9 (d, i H), H.34 (d,
1 H), 8.85 (s, 1 I-I),
9.3 (br s, 2H), 9.0 (br s, 2H);
MS (DCI/NH3) m/e 271 (M+H)+.
2U Anal. calcd for C ~ 5H ~ gN40~TFA: C, 53.12; H, 4.98; N, 14.58. Found: C,
15.13; H, 4.84;
N, I 4.50.
Example 72
N~h-Aminoiminomethyl-2-naphthalenyl>-N'-phenyl-N'-methylurea
mono(trifluoroacetate) salt
~5
Example 72A
N-(6-Cvano-2-naphthalenvl)-N'-phe~l-N'-methylurea
The title compound was prepared with Example 40A, N-methyl-N-phenylamine and
the
procedure from Example 40B.
3c~ MS (DCI/NH3) m/e 302 (M+H)+.
Example 72B
N-(6-Aminoiminomethyl-2-naphthalenyl)-N'-phenyl-N'-methylurea
mono(trifluoroacetate) salt
The title compound was prepared with Example 72A and the procedure of Example
40D.
-104-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98/15386
1H NMR (300 MHz, DMSO-d~,} 8 3.33 (s, 3H), 7.25-7.47 (m, SH}, 7.7I-7.77 (m,
2H), 7.95
(two overlaping doublets, ZH), 8.16 (d, I H). 8.35 (d, 1 H), 8.64 (s, I H),
8.96 (br s, 2H),
9.34 {br s. 2H);
MS (DCI/NH3) m/e 319 {M+H)+.
S Anal. calcd for C2pH~7N40~TFA: C, 58.33; H, 4.43; N, 1 1.96. Found: C.
58.38; H, 4.69;
N, 1 I .82.
Example 73
6-Aminonaphthalene-2-carboximidamide mono(trifluoroacetate) salt
Example 73A
6-Phenvlcarbamovl-2-naphthalenecarbonitrile
The title compound was prepared from Example 40A, phenol and the procedure
from
Example 40B.
MS (DCI/NH~) m/e 289 (M+H)+.
Example 73B
6-Aminonaphthalene-2-carboximidamide mono(trifluoroacetate) salt
The title compound was prepared from Example 73A and the procedure of Example
2c 4()D.
~H NMR (3()0 MHz, DMSO-d~,) 8 6.01 (br s, 2H), 6.86 (d, 1H), 7.06 (dd, 1H),
7.58-7.67
(m, 2H), 7.74 (d, 1H), 8.2I (d, IH), 8.74 (br s, 2H), 9.16 (br s, 2H);
MS (DCI/NH~) m/e 196 (M+H)+.
Anal. calc:d for C~~H1~~N3~TFA: C, 52.18; H, 4.04; N, 14.04. Found: C, 51.92:
H, 3.87; N,
25 13.80.
Example 74
N-(6-aminoiminomethyl-2-nanhthaIenyl)-N'-c cy lohexvlurea
mono(trifluoroacetate) salt
Example 74A
N-(6-Cvano-2-naphthalen i -N'-cyciohex lurea
The title compound was preparedwith Example 40A, cyclohexylamine and the
procedure from Example 40B.
MS (DCl/NH3) m/e 294 (M+H)+.
-105-
SU8ST1TUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 _ PCT/iJS98/15386
Exam 1R a 74B
N~(6-aminoiminomethvl-2-na hthalenyl)-N'-cvcloherylurea mono(trifluoroacetate)
alt
The title compound was prepared with Example 74A and the procedure of Example
40D.
~H NMR (300 MHz, DMSO-d~,) 8 1.14-1.39 (m, SH), 1.54-1.58 (m, 1H). 1.65-1.72
(m,
2H), 1.81-1.86 (m, 2H), 3.46-3.52 (m, 1 H), 6.36 (d, 1 H), 7.55 (dd, 1 H),
7.72 (dd, 1 H),
7.93 (d, 1 H), 7.95 (d, 1 H), 8.18 (d, 1H), 5.35 (d, i H), 8.87 (s, 1 H), 9.00
(br s, 2H), 9.28
(br s, 2H):
MS (DCI/NH3) m/e 31 1 (M+H)+.
m Anal. calcd for C19H21N40~TFA: C, 56.60; H, 5.46; N, 13.20. Found: C, 56.61;
H, 5.72;
N, 13.03.
Exam lie 75
N-(6-aminoiminomethvl-~-naphthalenyl)-N'-benzyloxyurea mono(trifluoroac;etate)
salt
i5
Example 75A
N-(6-cyano-2-naphthalenyll-N'-benzyloxyurea
The title compound was prepared with Example 40A, O-benzylhydroxylamine and
the
procedure from Example 40B.
2a MS (DCI/NH3) m/e 318 (M+H)+.
Example 75B
N-(6-arninoiminomethvl-2-naphthalenyl)-N'-benzylo~urea mono(trifluoroacetate)
salt
The title compound was prepared with Example 75A and the procedure of Example
40D.
''s ~ H NMR (300 MHz, DMSO-d~) b 4.87 (s, 2H), 7.25-7.42 (m, 3H), 7.45-7.51
(m, 2H), 7.75
(dd, 1H), 7.75 (dd, 1H), 7.97 (d, 2Hj, 8.30 (d, 1H), 8.38 (d, 1H), 8.97 (br s,
2H), 9.21 (s,
IH), 9.35 (br s, 2H), 9.77 (s, IH);
MS (DCI/NH3) m/e 335 (M+H)+.
Anal. calcd for C19H1~N402~TFA: C, 56.25; H, 4.27; N, 12.49. Found: C, 56.26;
H, 4.39;
3U N. 12.30.
Example 76
l , l-Dimethvlethvl I4-f f l6-aminoiminomethyl-2-
naphthalenvl)aminolcarbonvllphenyllcarbamate
mono(trifluoroacetate) Bait
-I06-
SUBSTITUTE SHEET (RULE 26~
CA 02294300 1999-12-14
WO 99105096 PCTIUS98/15386
Example 7C~A
fi-Amino-2-na~hthalenecarbonitriie
Sulfuric acid (45mL) was treated with Example 40B (6.5 g), stirred for 30 min,
warmed
s to room temperature for 20 min, poured onto ice, diluted with water to
approximately 500 mL,
cooled to 0 °C and treated with 50°lo aq sodium hydroxide such
that the temperature did not
exceed 35 °C. The light solid which precipitated was filtered, washed
with water to pH 7, dried
under vacuum and purified on silica gel with 20% ethyl acetate/ hexanes to
provide 3.3 g of the
title compound. MS (DCI/NH3) m/e 1 fig (M+H)+.
IU
Example 7f B
1.1-Dimethvlethvl (4-1f(6-cyano-2-nap halenvllaminolcarbo~llphenyllcarbamate
mono(trifluoroacetatel salt
The title compound was prepared from Example 7f~A, 4-N-Boc-aminomethylbenzoic
1 S acrid, the procedure from Example 35B with methylene chloride in place of
THF.
MS (DCI/NH3) m/e 417 (M+H)+.
Example 7f~C
I-Dimeth lyethy114-ff(fi-aminoiminomethyl-2-
naohthalenvl)aminolcarbonvllphenvllcarbamate
2U mono(trifluoroacetate) salt
The title compound was prepared with Example 76B and the procedure of Example
40D.
IH NMR (300 MHz, DMSO-d(,) 8 3.30 (s, 9H), 4.22 (d, 2H), 7.42 (d. ZH), 7.49
(t, 1H),
7.79 (dd, 1 H), 7.95-8.00 (m, 3H), 8.09 (d, 2H), 8.42 (s, 1 H), 8.63 {d, 1 H),
9. I 8 (br s, 4H),
10.58 (s, 1 H);
MS m/e 434 (M+H)+.
Anal. calcd for C~4H2~NSOyTFA: C, 59.5E~; H, 5.(l0; N, 10.29. Found: C, 58.55;
H, 4.ii5;
N, 10.41.
Examlhe 7777
3o N-lfi-(Aminoiminomethvl)-2-naphthalenvll-4-(aminomethvl)benzamide
mono(trifluoroacetate)
salt
Exam In a 77A
-107-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98/15386
N-f6-(Arninoiminomethvll-2-na hthalenvll-4-(aminornethvI)benzamide
monoltrif7uoroacetate)
A solution of Example 76B (35 mg, 0.07 mmole) in 1:1 TFA/methylene chloride
was
stirred at room temperature for 1 h then concentrated. The residue was
dissolved in water ( I2
mL), filtered through a 0.45 filter and concentrated. The solid was suspended
in diethyl ether
and filtered to yield 27 mg of the title compound as a white solid.
~ H NMR (300 MHz, DMSO-d6) 8 4.17 (q, 2H), 7.65 (d, 2H}, 7.50 (dd, I H),
7.99 (dd, 1H), 5.06-8.12 (m, 4H), 8.30 (br s, 2H), 8.44 (d, 1H), 8.64 (d, 1H),
9. I 3 (br s, 2H), 9.40 (br s, 2H), 10.70 (s, 1 H);
lU MS (DCIJNH3) m/e 319 (M+H)+.
Anal. calcd for C~yH1gN40~2.25TFA~0.5H20: C, 48.34; H, 3.67; N, 9.59. Found:
C, 48.45;
H, 3.74: N, 9.45.
Example 78
1S Ethvl l6-(aminoiminomethvl)-2-naphthalenyljcarbamate monoltrifluoroacetate)
salt
Example 75A
Ethvl (6-cvano-2-nanhthalenyllcarbamate mono(trifluoroacetate) salt
The title compound was prepared with Example 40A, ethanol and the procedure
from
2o Example 40B.
MS (DCI/NH3) m/e 241 (M+H)+.
Exam Ie~78B
Ethvl ffi-(aminoiminomethvl)-2-naphthalenXllcarbamate mono(trifluoroacetate)
salt
~s The title. compound was prepared with Example 75A and the procedure of
Example
40D.
~H NMR (300 MHz, DMSO-d~,) 8 1.29 (t, 3H), 4.19 (q, 2H), 7.69 (dd, IH),
7.76 (dd, 1 H), 5.1 (d, 2H), 5.23 (d, 1 H), 8.35 (d, I H}, 9.()3 (br s, 2H),
9.33 (br s, 2H),
10.1 I (s, I H);
3O MS (DCIlNH3) rn/e 25R (M+H)+.
Anal. calcd for C~4H 15N30?~TFA: C, 51.76; H, 4.34; N, 1 1.32. Found: C, S
1.32; H, 4.15;
N, 10.93.
Example 79
-108-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 - PCTIUS98/15386
1,1-dimethvlethvl f4-fff6-aminoiminomethvll-~-
nanhthale~l)aminolcarbonyllaminolph null
carbamate mono(trifluoroacetate) salt
Exam In a 79A
1,1-Dimethvl f4-fff(6-cyano-2-
naphthalen~rl)aminolcarbonvllaminolphenyllcarbamate
The title compound was prepared with Example 40B, 4-(N-tert-
butoxycarbonylamino)-
aminobenzene and the procedure from Example 40C.
MS (DCI/NH3) m/e 403 (M+H)+.
Example 79B
1,1-dimethvlethvl f4-fff6-aminoiminomethyl)- 2-
naphthalenyl)aminolcarbonvllamino~hen,L
carbamate mono(trifluoroacetatel calr
The title compound was prepared with Example 79A and the procedure of Example
40D.
l; ~ H NMR (30() MHz, DMSO-d~,) ~ 1.22 (s, 9H), 7.38 (s, 4H), 7.62 (dd, 1 H),
7.75 {dd, 1H), H.(>() (d, 2H), k.27 (d, 1H), H.3H (d, 1H}, H.77 (a, 1H), H.90
(br s, 2H), 9.16
(s, 1 H), 9.20 (s, 1 H), 9.33 (br s, 2H);
MS (DCI/NH3) m/e 420 (M+H)+.
Anal. calcd for C23H2SN503~2TFA: C, 56.28; H, 4.91; N, 13.13. Found: C, 56.1H;
H, 5.07;
2U N, 12.44.
Example SO
(E)-6-f2-(Phenvlthio)ethenvll-2-naphthalenecarboximidamide
mono(trifluoroacetate) salt
'S Example R0A
(E)-f~-f 2-~Phenvlthio)ethenyll-~-naphthalenecarbonitrile
The title compound waa prepared from Example SSB, phenylvinyl sulfide and the
procedure of Example 57B.
MS (DCI/NH3j m/e 305 (M+NH4)+.
Example ROB
(E)-6-f2-(Phenvlthio)ethenvll-~-nalahthalenecarboximidamide
mono(trifluoroacetate) salt
The title compound was prepared from Example 80A and the procedure of Example
1 B.
- I 09-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO. 99/05096 PCT/US98115386
~ H NMR (300 MHz, DMSO-d~,) 8 6.91 (d, 1 H), 7.52-7.33 (m, SH), 7.50 (d, 1 H),
7.75- 7.83
(m, 1H), 7.98-8.89 (m, 1H), 8.08-8.80 (m, 3H), 8.44 (m, IH), 9.03 (s, 2Hj,
9.40 (s, ZH);
MS {DCI/NH3) m/e 305 (M+H)+.
Anal. calcd for CIc~HI6N~S~I.1TFA: C, 59.55; H, 4.03; N, 6.57. Found: C,
59.53; H, 4.12;
S N, 6.60.
Example 81
(E)-6-f2-(2-Furanvllethenvll-2-naphthalenecarboximidamide
mono(trifluoroacetate) salt
»~ Example 81 A
2-Vinvlfuran
A solution of methyl(triphenylphosphonium)bromide (26.78 g, 75 mmol) in
toluene (80
mL) was treated with butyllithium in hexanes (27.5 mL, 68.75 mmol) then
furfural (6 g, 62.5
mmol). stirred for 0.5 h and distilled at 69-72 °C to provide the title
compound as a clear,
1 S e:oiorless liquid with some toluene contaminant.
MS (DCl/NH3j m/e 83 (M+H)+.
Example 81 B
(E)-6-12-(2-Furanyl)ethen I -2-naphthalenecarbonitrile
2o The title compound was prepared from Examples SSB and 81 A and the
procedure of
Example 57B.
MS (DCI/NH3) m/e 263 (M+NH4j+.
Example 81 C
25 (E)-6-I2-(2-Furanvl)ethenvll-2-naphthalenecarboximidamide
mono(trifluoroacetate) salt
The title compound was prepared from Example X 1 B and the procedure of
Example 1 B.
~H NMR (30() MHz, DMSO-d~,} 8 6.61 (dd, lHj, 6.66 (d, IHj, 7.19 (d, 1H), 7.38
(d, 1H),
7.77 (d, 1 H). 7.80 (dd, 1 H), 8.14-7.97 (m, 3H), 8.44 (s, 1 H), 9.05 (s, 2H),
9.42 (s, ZH);
MS (DCI/NH3) m/e 263 (M+H)+.
3u Anal. calcd for C~7Ht3N~0~1.2TFA: C, 58.49; H, 3.85; N, 7.04. Found: C,
58.45; H, 3.78;
N, 7.36.
Example 82
(E)-6-12-(1H-Imidazol-1-vl)ethenvll-~-naphthalenecarboximidamide
mono(trifTuoroacetate) salt
-110-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98/I5386
Exam lp a R2A
(E)-6-f 2-( 1 H-Imidazol-1-yl )ethenvll-2-naa~hthalenecarbonitrile
The title compound was prepared from Example SSB, 1-vinylimidazole and the
procedure of Example 42C.
MS (DCI/NH3) m/e 263 (M+NH4)+.
Exam lp a 82B
E)-6-12-f IH-Imidazol-1-vl)ethenvll-2-naphthalenecarboximidamide
mono(trifluoroacetatel salt
~U The title compound was prepared from Example 82B and the procedure of
Example
40D.
1 H NMR (300 MHz, DMSO-d6) 8 9.44 (s, 2H), 9.14 (s, 2H), li.15 (d, I H), 8.17-
~i.05 (m,
4H), 7.93 (d, 1 H), 7.84 (dd, 1 H), 7.59 (s, 1 H), 7.49 (d, 1 H);
MS (DCI/NH3j m/e 263 (M+H)+.
l, Anal. calcd for C~~,H13N.~~2.7TFA: C, 45.28; H, 2.97; N, 9.91. Found: C,
45.33; H, 3.52;
N. 9.79.
Examl 1~ a H3
(E)-4-f 2-(6-Aminoiminometh l-2-nanhthalenvl)ethe~llbenzenesulfonamide
mono(trifluoroacetate) salt
Examlole R3A
4-vinylsulfonamide
A solution of thionyl chloride (7.5 mL) and 4-t-butylcatachol (45 mg, 0.3
nunoi) in
?~ DMF (9 mLj at () °C was treated with 4-vinylbenzene sulfonic acid
sodium salt (3 g, 14.6
rnmol), stirred for 6 h, stored at -1 () °C for 3 days, poured into ice
water and extracted with
benzene. The organic layer was washed with water, dried (Na2S04), filtered and
concentrated
to provide 4-vilylsulfonyl chloride as a clear, colorless oil. A portion of
the chloride (1 g, 4.95
mmol) was disslolved in THF ( 10 mL), cooled to 0 °C, treated dropwise
with concentrated
3O ammonium hydroxide until gas evolution ceased and extracted with ethyl
acetate. The
combined extracts were dried (Na2S04), and concentrated to provide 707 mg of
the title
compound as a pale yellow solid.
MS (DCI/NH3j m/e 201(M+NH4)+.
SUBSTtTUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98115386
Example 83B
(E)-4-f2-(6-Cvano-2-naphthalenyl)ethenyllbenzenesulfonamide
The title compound was prepared from Example SSB, Example 83A and the
procedure
of Example 57B.
MS (DCI/NH3) m/e 352 (M+NH4)+.
Example 83C
(E)-4-f 2-(6-Aminoiminomethvl-2-naphthalenyl)ethenyllbenzeneeulfonamide
mono(trifiuoroacetateo calr
W The title compound was prepared from Example 83B and the procedure of
Example
40D.
t H NMR (300 MHz, DMSO-d~,) b 9.43 (s, 2H), 9.05 (s, 2H), 8.46 (s, 1 H), 8.21
(s, 1 H),
8.16-7.95 (m, 3H), 7.86 (s, 2H}, 7.84-7.67 {m, 2H), 7.62 (d, 1H), 7.4-7.36 (m,
2H);
MS (DCI/NH3) m/e 352 (M+Hj+.
t5 Anal. calcd for Ct9H~-7N~02S~1.SC2F302H: C, 50.84; H, 3.59; N, 8.11. Found:
C, 50.83;
H, 3.89; N. 7.88.
Exam lp a 84
E_)-4-12-l6-Aminoirninomethvl-2-naphthalenyl)ethenyllbenzoic acid
monoltrifluoroacetate) salt
2U
Example 84A
(E)-4-f2-(6-Cyano-2-naphthalen 1 ethenyllbenzoic acid
The title compound was prepared from Example SSB, 4-vinylbenzoic acid and the
procedure of Example 57B.
~5 MS (DCI/NH3j m/e 300 (M+H)+.
Example 84B
E)-4-f2-(6-Aminoiminomethyl-2-naphthalenvl)ethenyllbenzoic acid
mono(trifluoroac;etate) salt
The title compound was prepared with Example 84A and the procedure of Example
3u 40D.
iH NMR (300 MHz, DMSO-d~,) ~ 7.62 - 7.58 (m, 2H), 7.90 (d, 2H), 7.98 (d, 1H),
8.12 -
8.04 (m, 3H), 8.20 (s, 1 H), 8.56 (s, 1 H), 9.07 (bs, 2H), 9.35 (bs, 2H).
MS (DCI/NH3) m/e 317 (M+H)+.
Anal. calcd for C2pHl~,N~02~TFA: C, 61.40; H, 3.98; N, 6.51. Found: C, 61.10;
-1 12-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 _ PCTIUS98115386
H, 3.63; N, 6.45.
Example 85
4-f7-(Aminoiminomethvl)-2-methoxv-1-na htp halenylldihvdro-2(3H)-furanone
monoftrifluoroacetate) salt
Exam lp a 85A
4-(7-Cvano-2-methoxy-1-nanhthalenvl)dihydro-2(3H)-furanone
Example 62A (269 mg, 1.00 mmol) and pyridinium chlorochromate (360 mg, 1.67
mmol) in
lu methylene chloride ( 15 mL) were stirred at room temperature for 24 h,
filtered through Celite~
and concentrated. The residue was chromatographed on silica gel with 20% ethyl
acetate/
hexanes to provide 170 mg of the title compound.
MS (DCI/NH3) m/e 285 (M+NH4)+.
Exam to a H5B
4-l7-lAminoiminomethvl)-2-methoxy-1-n_aphthalenvl ldihydro-2(3H)-furanone
mono(trifluoroacetate) salt
The title compound was prepared from Example 85A and the procedure of Example
40D.
2v} ~ H NMR (300 MHz, DMSO-d6) S 2.96-2.75 (m, 2H), 3.96 (s, 3H), 4.33 (m, 1
H), 4.66 (t,
1 H), 8.85 (m, 1 H), 7.68 (dd, 1 H), 7.73 (d, I H), 8.08 (d, 1 H), 8.12 (d, 1
H), 8.67 (s, 1 H),
9.14 (s, 2H), 9.43 (s, 2H);
MS (DCI/NH3) m/e 285 f M+H)+.
Anal. calcd for CI~,H1~N~03~1.1TFA: C, 53.72; H, 4.24; N, 6.91. Found: C,
53.75; H, 4.26;
'?5 N, 6.94.
Example 86
7-Methoxv-8-(I-acetyl-1 H-nvrazolvl)-2-naphthalenecarboximidamide
mono(trifluoroacetate)
io
Example 86A
7-Methoxv-8-( 1-acetyl-1 H-Ryrazolyll-2-na~hthalenecarbonitrile
A solution of Example 53F (90 mg, 0.361 mmol) in THF (2 mL) was treated with a
0.5 M
solution of potassium bis(trimethylsilyl)amide in toluene (0.866 mL, 0.433
mmol), stirred for 5
-113-
SUBSTITUTE SHEET (RULE 2fi)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98/15386
min, treated with acetyl chloride (38 mL, 0.542 mmol), stirred for 10 min and
concentrated.
The crude product was chromatographed on silica gel with 2590 ethyl
acetate/hexanes to yield
67 mg of the title compound.
MS MS (DCI/NH3) m/e 309 (M+NH4)+.
Example 86B
7-Methoxv-8-(1-acetyl-1H-ovrazoivl)-2-naphthalenecarboximidamide
mono(trifluoroacetate)
The title compound was prepared with Example 86A and the procedure of Example
1c~ 40D.
~H NMR (300 MHz, DMSO-d~) 8 3.89 (s, 3H), 7.59 (d, 1H), 7.92 (s, 2H),
X.06 (d, I H), 8.12 (d, I H), 8.28 (s, 1 H), 8.94 (s, 2H), 9.34 (s, 2H);
MS (DCI/NH3) m/e 309 (M+H)+.
Anal. calcd far C«H~~,N40y1.9TFA: C, 47.59; H. 3.44; N, 10.67. Found: C,
54.03; H,
i s 4.06; N, 13.26.
Example S7
7-Methoxv-8-f 1-(methvlsulfonvl)-1 H-4-pyrazolyl -2-naphthalenecarborimidamide
mono(trifluoroacetate) salt
?u
Example 87A
7-Methoxv-8-f I-(methvlsulfonyl)-1 H-4-n~rrazolyll-2-naphthalenecarbonitrile
The title compound was prepared from Example 53F (19() mg, 0.762 mmol),
methanesulfonyl chloride (().(lHH mL, 1.14 mmol) and the procedure of Example
86A to
?s provide 122 mg of the title compound.
MS (DCI/NH~) m/e 345 (M+NH4)+.
Example 87B
7-Methoxv-8-fl-(methvlsulfonvl)-1H-4-p azol Ivl-2-n~,phthalenecarboximidamide
mono(trifluoroacetate) salt
The title compound was prepared from Example 87A and the procedure of Example
4(1D.
-114-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98/15386
1H NMR (300 MHz, DMSO-d6) 8 2.75 (s, 3H), 3.98 (s, 3H) 7.64 (dd, IH), 7.78 (d,
1H),
8.15 (s, I H), 8.18 (s, I H), 8.21 (s, 1 H), 8.24 (s, 1 H), 8.62 (s, 1 H),
8.97 {s, 2H), 9.40 (s,
2H);
MS (DCI/NH3) m/e 345 (M+H)+.
s Anal. calcd for C16H16N403S~ 1.4TFA: C, 44.75; H, 3.47; N, 1 I.09. Found: C,
44.59; H,
3.86; N, 11.38.
Example 88
(E)-4-f2-(6-Aminoininomethyl-2-naDhthalenyl)ethenvllbenzamide
mono(trifluoroacetate) salt
IU
Example 88A
(E)-4-f 2-(6-Cyano-2-naphthalenvl )ethenyllbenzamide
Example 85A (16() mg, 0.54 mmol) in thionyl chloride (4 ml) was refluxed for
0.5 h,
cooled to (1 °C, treated with concentrated aqueous ammonia until gas
evolution ceased, diluted
15 with ethyl acetate, heated to dissolve residual solids, washed with water,
dried (MgS04) and
concentrated to provide 1 (>(1 mg of the title compound as an orange solid.
MS (DCI/NH3) rn/e 316 (M+NH4)+.
Example 88B
2c) (E)-4-f2-(6-Aminoininomethyl-2-naphthalenyl)ethenvllbenzamide
mono(trifluoroacetatel salt
The title compound was prepared with Example 88A and the procedure of Example
1 B.
~ H NMR (300 MHz, DMSO-d6) S 7.34 (br, 1 H), 7.51 (d, 1 H), 7.56 (d, 2H), 7.73
(d, 2H),
7.82 (m, 2H), 7.9(1 {d, 2H), 7.96 (br, 1 H), 8.09 (q, 3H ), 8.17 (s, 1 Hj,
8.43 (s, 1 H), 9.01 (s,
2H), 9.4(I (,, 2H):
25 MS (DCI/NH3 ) m/e 316 (M+H)+.
Anal. calcd for C2trH pN30~ 1.1 TFA: C, 60.()9; H, 4.1 1; N, 9.44. Found: C,
60.22; H, 4.13;
N, 8.79.
Exa die 89
6-f2-(4-Aminonhenv!)ethoxvl-2-naphthalenecarboximidamide
mono(trifluoroacetate) salt
Example 89A
6-f2-(4-Aminovhenyl)ethoxy -2-n~hthalenecarbonitrile
-1 t5-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 _ PCT/US98115386
A solution of Example 4A, (300 mg), Cs2C03 ( 1.2 g ), 4-aminophenethylbromide
{470
mg) and tetrabutylammonium iodide ( 10 mg j in DMF (5 ml) was stirred for 18 h
at room
temperature, diluted with water and extracted with ethyl acetate. The organic
extract was
washed with saturated aq NaHC03 and brine, dried (Na2S04) and concentrated to
provide 200
s mg of the title compound as a dark brown oil.
MS (DCI/NH3) m/e 306 (M+NI-~)+.
Example 89B
6-I2-(4-Aminophenvl)ethoxvl-2-naphthalenecarboximidamide
mono(trifluoroacetate) salt
1n The title compund was prepared with Example 89A according to the procedure
of
Example SB.
1 H NMR (300 MHz, DMSO-d~,) 8 3.15 (t, 2H), 3.6 (bs, 3H), 4.35 (t, 2H), 6.93
(d, 2H),
7.24 (d. 2H) 7.38 (dd, 1 H). 7.55 (d. 1 H), 7.78 (dd, 1 H), 7.98 (dd, 1 H),
8.21 (d, I H), 8.4
(d, 1H), 9.21 (bs, 2H1, 9.39 (bs, 2H);
I i M S m/e 30 fi ( M+H')+.
Anal. calcd for C~9H19N30~2TFA: C, 51.79; H, 3.97; N, 7.88; Found: C, 50.99;
H, 4.6H; N,
7.59.
Exam I
21~ Methvl f 3-methoxv-6-(aminoiminomethyl)-4-na hthalen I lcarbamate
mono(trifluoroacetate) salt
Example 90A
7-Methoxv-2-trifluoromethanesulfonyloxv naphthalene
A solution of 7-methoxy-2-naphthol (3.24 g, 18 mmole) in DMF {20 mL) and
methylene chloride (2() mL> was treated with N-phenyl
trifluoromethanesulfonimide ((~.f~ g, 18
mmole> and triethylacnine (5.2 mL, 37 mmole), stirred 20 h at room
temperature, diluted with
CH2Cl2 ( 10() mL) , washed sequentially with distilled water, 20 % KOH and
brine, dried
(MgS04) and concentrated to provide the title compound as a clear oil.
MS (DCI/NH3): m/e 272 (M + NH4)+.
Example 90B
7-Methox -2-naphthalenecarbonitrile_
Example 90A ( 12 mmole), zinc cyanide ( 12 mmole}, Pd(OAc)2 (0.3 mmole) and
triphenylphosphine (1.2 mmole} in DMF (40 mL) was heated for 6 h at 85
°C, diluted with
-116-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 - PCTIUS98115386
ethyl acetate (200 mL), washed with saturated NaHC03, brine, dried (MgS04) and
concentrated to a dark oily residue. Purification of the residue on silica gel
with 1:1 hexane:
methylene chloride then CH2C12 provided 1.S g of the title compound as a white
solid.
MS (DCI/NH3) m/e 201 (M + NH4)+.
Example 90C
7 -Methox~8-nirro-2-naphthalenecarbonitri~
Example 90B (3 g, 16.4 mmole) in acetic anhydride (30 mL,) at 0 °C was
treated with
fuming HN03 (1.2 mL), and the resulting thick slurry was diluted with water
(20 mL), stirred
IU 20 min then filtered and dried in vacuo to provide 3.69 g of the title
compound as a yellow
solid.
MS (DCI/NH3) m/e 246 (M+NH4)+.
Example 90D
7-Methoxy-H-amino-~-n~phthonitrile
Example 90 C (3.69 g, 16.1 mmole) and 1 O~lc Pd/C (0.4 g) in ethyl acetate (
100 mL)
was stirred under a hydrogen atmosphere for 2 h at room temperature, filtered
and concentrated
to provide 3 g of the title compound as a yellow solid.
MS (DCI/NH3) rn/e 217 (M+NH4)+.
2U
Example 90E
Methvl f3-methox~~ano-4-na hthalenyllcarbamate
Example 90D (R 1 mg, 0.41 mmol) in dioxane (7 mL) and 10% NaOH ( 15 mL) was
treated with methyl chloroformate (1 l2 mg. 0.9H mmoij, stirred for 2 h,
diluted with ethyl
5 acetate, washed with water, dried (MgS04) and concentrated to provide 105 me
of the title
compound. MS (DCI/NH~) m/e 274 (M+NH3)+.
Example 90F
Methvl f3-methoxv-6-(aminoiminomethyl -1 4-n~phthalenyllcarhamate
mono(trifluoroacetate) salt
3u The title compound was prepared from Example 9()E according to the
procedure of
Example 40D. tH-NMR (300 MHz, DMSO-d~,) 8 9.48 (s, 2H), X.99 (s, 2H), 8.93
(br, 1H),
H.34 (s, 1 H), H.12 (d, 1 H), 8.04 (d, I H), 7.72 (d, 1 H), 7.65 (dd, 1 H),
3.95 (s, 3H);
MS (DCI/NH~) m/e 274 (M+H)+;
-117-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98/15386
Anal. calcd for CIqHiSN3O3~l.HTFA: C. 44.07; H, 3.53; N, 8.74. Found: C,
44.14; H, 3.20;
N, 8.53.
Example 91
7-Methoxv-8-(2-nvrimidinvl(amino)1-2-naphthalenecarboximidamide
bis(trifluoroacetate) salt
Example 91 A
7-Methoxv-8-(2-nvrimidinyllamino)1-2-n~hthalenecarbonit~ile
A solution of Exampie 90D (23U mg, 1.2 mmole), 2-chloropyrimidine (280 mg, 2
lu mmole), sodium-tent-butoxide ( 12() mg, I .2 mmole), Pd(dba)3~CHCl3 and
dppf in toluene (5
mL) was heated in a sealed tube for 18 h at 100 °C, diluted with ethyl
acetate ( 100 mL), washed
with brine, dried (MgS04j and concentrated to provide 100 mg of a brown oil.
MS (DCI/NH3) m/e 294 (M+NH4)+.
15 Example 91 B
7-Methoxv-8-(2-nvrimidinyl(amino)1-~-naphthalenecarboximidamide
bis(tritluoroacetate) salt
The title compound was prepared in a manner analogous to that of Example 40D.
IH-
NMR (30() MHz, DMSO-d6) b 9.43 (s, 2H), 9.1 1 (s, ZH), 8.46 (s, 1H), 8.16 (br,
3H), 8.15
ld, 1 H), lS.04 (d, 1 H), 7.82 (dd, 1 H), 7.75 (d, 1 H), 7.54 (s, i H) 7.50
(d, 1 H), 4.08 (d, 2H);
?u MS (DCI/NH3) m/e 294 (M+H)+.
Anal. calcd for C1~,HISN50~3.8TFA: C, 39.01; H, 2.61; N,9.64; Found: C, 39.01;
H, 3.06;
N, 9.63.
Example 92
6-(aminoiminomethvl )-N-(4-(hydrorymet~l)phenyl l-~-naphthaienecarboxamide
'-S mono(trifluoroacetate)(salt)
Example 92A
4-amino-benzyloxv-rert-butyldimethylsilyl ether
A solution of 4-aminobenzyl alcohol (1 g, 8.1 mmolj in DMF (20 mL) was treated
with
3u imidazole (0.54 g, X. I mmol) and tent-butyl dimethylsilyl chloride ( 1.22
g, 8.12 mmol), stirred
overnight at room temperature, diluted with ethyl acetate ( 100 mL), washed
with 1 N H3P04,
saturated NaHCO~ and 10~c NaCI, dried (Na2S04) and concentrated to an oil
which was
purified on silica gel with 3: 1 hexanes: ethyl acetate to provide 0.5 g of a
clear oil.
MS m/z 238 (M+H)+.
-1 18-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98I15386
Example 92B
Example 92A (0.3. 1.1 mmol) and 6-carboxy-2-naphthonitrile, Example 8E (0.2 g,
1
mmol) were processed as described in Example 95C to provide 100 mg of the
desired
compound.
MS m/z 434 (M+NH4)+.
Example ~2C
A solution of Example 92B in 1 M tetrabutyl ammonium fluoride THF solution (2
mL)
m was stirred for I hour at room temperature, quenched with 10~/o NH4Cl
solution (50 mL) and
diluted with ethyl acetate ( 100 mL). The layers were separated, and the
organic layer was
washed with 10% NaCI, dried (MgS04) and concentrated to provide a light brown
oil which
was triturated with methylene chloride and filtered to provide 0.1 g of the
desired compound as
a white solid.
li MS m/z 320 (M+NH4)+.
Example 92D
6-laminoiminomethvl)-N-f4-(hydroxymeth~phenyl -~-na~hthalenecarboramide
mono(trifluoroacetate)(Salt~
20 Example 92C (0.1 g, 0.33 mmol) was processed and purified according to the
procedure in Example 95D to provide 15 mg of the desired compound.
MS m/z 320 (M+H)+;
~H NMR 30() MHz, (DMSO-d~,): 8 1().45 (s, 1H), 9.45 (bs. 4H), 8.75 (s, 1H),
8.59 (s, 1H),
H.32 (d, E H), H.22 (d, 1 H), 8.1 H (dd, 1 H ), 7.92 (dd, 1 H), 7.85 (d, 2H),
7.45 (d, 2H), 4.2()
S (~.2H);
Anal. calc'd for C~yH~7N30?~TFA: C, 5k.20; H, 4.19; N, 9.7(1. Found: C. 57.H0;
H, 3.91;
N, 9.35.
Example 93
3n 6~4-aminoDhenvl)-2-naphthalenecarboximidamide bis(trifluoroacetate) salt
Ex' mple 93A
6-cyano-2-naphthalene boronic acid (0.3 g, 1.64 mmol), 4-iodoaniline (0.36 g,
1.64
mmol), Palladium[1, I'-Bis (diphenylphosphino)-ferrocene~ dichloride (0.13 g,
0.164 mmol)
-119-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98/15386
and CsF (0.75 g, 4.92 mmol) are mixed together in DMF ($ mL) heated 20 hours.
at HO °C.
The mixture is diluted with ethyl acetate ( 100 tnL) washed with 1 N H3P04,
saturated
NaHCO~, 10% NaCI, dried over anhydrous sodium sulfate. The drying agent
filtered, solvent
removed under vacuum leaving a brown solid. The solid is purified on silica
gel eluting with 3:
1 hexanes: ethyl acetate. The fractions corresponding to the desired compound
are concentrated
under vacuum leaving a yellow solid. 0.2 g, 75%.
MS (M+NH4+): 262.
Exam~he 93B
U) 6-(4-aminophenvl)-2-naphthalenec~rboxitnidamide bis(trifluoroacetateo salt
The desired compound is obtained from the material prepared in Example 93A
(0.1 g,
0.41 mmol) using the procedure described in Example 94D Yield: 35 mg, 53%
MS (M+H)+262;
is t1-I NMR 30t) MHz, (DMSO-d~~): 89.45 (bs, 2Hj, 9.35 (bs, 2H), 8.45 (d, 1H),
8.22 (s, IHj,
8.15 (d, 1 H j, 8.1 t) (d, I H), 7.99 (dd, I H), 7.79 (dd, I H), 7.65 (d, 2H),
6.95 (d, 2H), 4.80
(bs, 3H);
Anal. c:alc'd: C?tHI~N~O~,F~: C, 51.54, H, 3.50, N, 8.59, Found: C, 51.95, H,
3.84.
'« Example 95
methyl 2-I4-lllfi-(aminoiminomethyl)-2-
na_phthalenvllcarbonvllaminolphenoxylacetate
mono(trifluoroacetate) salt
Example 95A
25 4-Acetamidophenol ( 5 ~, 33 mmol) is dissolved in THF ( I (?() mL) treated
with Cesium
c:urbonate (10.25 g, 33 mmol) and Methyl bromoacetate (3.4 mL, 36 mmol) and
stirred 24
hours at room temperature. The reaction mixture is diluted with water ( 100
tnL) and
concentrated under vacuum. The residue is dissolved in ethyl acetate ( 100 mL)
washed with I
N H3P04 (20 mL), saturated NaHCO~ (20 mL), 10% NaCI (20 mL) and dried over
anhydrous
3U Na2S04. The drying agent is filtered and the solvent removed under vacuum
leaving the desired
compound as a white solid, 6.8 g, (92%).
MS (M+NH4+): 241.
Example 95B
-120-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98/15386
The material obtained in Example 95A is treated with 2 N HCl (75 mL) and
refluxed for
3 hours. The clear mixture is cooled to room temperature then concentrated
under vacuum to an
off white solid as the desired compound. 6 g, 92~1c.
MS (M+NH4+): 195.
S
Exam lp a 95C
6-carboxy-2-naphthonitrile (0.1 g, .51 mmol) is dissolved in DMF (5 mL) cooled
in an
ice bath to 5 °C. To the homogeneous mixture is added
Diisopropylethylamine (0.18 mL, 1.05
mmol) and O- (7-Azabenzotriazol-1-yl)-N, N, N', N'-tetramethyluronium
hexafluorophosphate
n> (HATU). The resultant slurry is stirred at 5 °C 45 minutes. To this
slurry is added the material
obtained in Example 95B (0. I 2 g, .56 mmol) and the mixture is stirred at
room temperature
overnight. The next day, the reaction mixture is diluted with ethyl acetate (
100 mL) washed
with 1 N H~P04 (20 mL), saturated NaHC03 (2() mL), 10°lo NaCI, dried
over anhydrous
Na2S04 filtered and solvent removed under vacuum yielding the desired compound
as a brown
15 solid. ().2H g, 65%.
MS (M+NH4)+: 375.
Example 95D
methyl 2-14-if ff-laminoiminomethvl)-~-
naphthalenyllcarbonvllamino~henoxylacetate
?~~ mono(trifluoroacetate) salt
The material obtained in Example 95C (0.25 g, .75 mmol) is dissolved in
methanol
saturated with HCl (g) (30 mLj stirred 15 hours at room temperature. The
solvent removed
under vacuum and the resultant yellow solid is treated with 2 M NH3/methanol
(20 mLj. This
solution is refluxed 6 hours, cooled, solvent removed under vacuum and the
resulting brown
s solid is purified by reverse phase HPLC. The desired compound is obtained
from
lyophilization. 19.3 mg, 2()c'/c.
MS (M+H)+: 375
~ HNMR 300 MHz, (DMSO-d~,): 8 10.45 (s, 1 H), 9.45 (bs, 4H), 5.65 (d, 1 H),
8.59 (s, 1 H),
5.15 (d, 1 H), 5.1 U (d, 1 H), 5.05 (d, I H), 7.92 (d, 1 H), 7.75 (d, 2H),
6.95 (d, 2H), 4.50 (s,
3U 2H), 3.75 (s, 3H),
Anat. calc'd: C23H~1N30~,F3: C, 56.10, H, 4.3, N, 5.53, Found: C, 55.80, H,
3.93, N, 5.33.
Example 96
(E)-E-12-1(3-hvdroxvmethvllDhen Il~ethe_null-~-naphthalenecarboximidamide
-121-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99(05096 PCT/US98I15386
mono(trifluoroacetatel(salt)
Example 96A
The above was prepared from 3-iodobenzyl alcohol using the procedure in
Example
s 41 A.
MS (DCI/NH3) m/z (M+NH3)+ 303.
Example 96B
(E)-6-(2-1 (3-hvdroxvmethvl)phenyl(ethenyll-2-naphthalenecarboximidamide
monoltrifluoroacetate)(~lt~
The above was prepared from Example 96A using method from Example 40D.
MS (DCI/NH3) rn/z (M+H)+ 303;
~ H-NMR (300 MHz, DMSO-d~) b 9.18 (br, 4H), 8.45 (s, 1 H), 8.17 (s, I H), 8.13-
8.04 (m,
3H). 7. H 1 (dd, 1 H ), 7.64 (s. 1 H), 7.57 (d, 2H), 7.51 (d, I H) 7.39 (t, I
H), 7.28 (d, I H),
I s 5.27 (t, 1 H ), 4.55 (d, 2H);
Anal. calc'd for C22H19N~03F3 3/10 TFA: C, 60.64; H, 4.35; N, 6.28. Found: C,
60.53; H,
4.87; N, 6.57.
Example 97
20 6-(2-phenyl-1-cyclonropvl)-?-naphthalenecarboYimidamide
monoftrifluoroacetate)(salt)
Example 97A
Copper (I) chloride (43 mg, 0.4 mmol), powdered zinc (26 mg, 0.4 mmol) were
suspended in 1 mL dioxane for 18 hours. The product from Example 41 B (60 mg,
U.2 mmoi)
25 was added and stirred and heated at 95 °C for 2() hours. The
reaction mixture was concentrated
on silica gel and purified by silica gel chromatography to give the desired
compound.
MS (DCI/NH3) tn/z (M+NH;;)+ 287.
Exam Ip a ~7B
3U 6-(2-nhenvl-I-cvclooropvll-2-naphthalenecarbo~cimidamide
monoltrifluoroacetate)(salt)
The above was prepared from Example 1 using method from Example 1B.
MS (DCI/NH3) m/z (M+H)+ 287;
-122-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98/15386
~H-NMR (300 MHz. DMSO-d~) 8 9.41 (s, 2H), 9.16 (s, 2H), 8.46 (s, 1H), 7.83 (d,
2H),
7.62 (s. I H), 7.58 (dd, 1 H), 7.34 (dd, 1 H), 7.35-7.29 (m, 3H), 7.25-7.17
(m, 2H) 2.38-2.28
(m, 2H), 1.61 (t, 2H);
Anal. calc'd for C22H19N202F3 1/10 TFA: C, 65.00; H, 4.70; N, 6.84. Found: C,
65.22: H,
5.23; N, 5.10.
Example 98
(E)-6-f 2-(4-(aminomethyl)nhenyllethenvll-2-naphthalenecarboximidamide
bis(trifluoroacetate)(salt)
Example 98A
The desired compound was prepared using ethene under 500 atm pressure in a
manner;
Analagous to that of Example 4I A.
MS (DCI/NH3) m/z (M+NH~)+ 197.
Example 98B
4- (Aminomethyl)-iodobenzene hydrochloride ( 1 g, 3.7 mmol) and Boc anhydride
( l .22
g, 5.6 mmol) were mixed with l U~7o NaOH ( 15 cnL), ethyl acetate (20 mL) and
stirred 2 hours.
The organic; layer was washed with 5% sodium bicarbonate (2x, 10 mL), dried
(magnesium
2U sulfate), and concentrated to give I .22 g of desired compound.
MS (DCI/NH3) m/z (M+NH3)+ 351.
Example 98C
The desired compound was prepared using the product from Example s 98A and 98B
in
a manner analagous to that of Example 41 A.
MS (DCI/NH3) m/z (M+NH3)+ 4()2.
Example 98D
(E)-6-l2-f4-(aminomethvl)phenyl lethenyll-2-naphthalenecarboximidamide
bis(trifluoroacetate)(Salt)
The above product was prepared in a manner analogous to that of Example 40D
with the
addition of trifluoroacetic acid in methylene chloride to remove the Boc
group.
MS (DCI/NH3) m/z (M+H)+ 302;
-123-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98/15386
I H-NMR (300 MHz, DMSO-d~,) 8 9.43 (s. 2H), 9. I 1 (s, 2H), 8.46 (s, 1 H),
8.16 (br, 3H},
8.15 (d. 1 H), 8.04 (d, I H), 7.82 (dd, 1 H}, 7.75 (d, I H), 7.54 (s, 1 H)
7.50 (d, 1 H), 4.08 (d,
2H);
Anal. calc'd for C24H21N304F62/5 TFA: C. 50.46; H, 3.63; N, 6.99. Found: C;
50.37; H,
s 3.86; N, 7.05.
Example 99
methvl 17-(aminoiminomethvl)-2-methoxy-1-naphthalenvl )carbamate
mono(trifluoroacetate)(salt)
1 The desired compound was prepared using material prepared as described in
Example
90D and utilizing the procedures described in Example 9I A and Example 40D.
MS (DCUNH3) m/z (M+H)+ 306;
~H-NMR (300 MHz, DMSO-d~,) 8 9.33 (s, 2H), 8.98 (s, 2H), 8.63 (s, 1H), 7.99
(d, 1H),
7.60 (dd. 1 H), 7.58 (s, 1 H), 7.54 (d, 1 H), 7.35-7.20 (m, 5H ), 4.52 (s,
2H);
I S Anal. calc'd for CZ 1 H2t~N3O3F3 13/5 TFA: C, 44.03; H, 3. I 9: N, 5.89.
Found: C; 43.97: H,
3.55: N. f~.1 ().
Example 100
7-methoxv-8-(2-pvrimidinylamino)-2-naphthalenecarboximidamide
bisltrifluoroacetate)(salt)
2U The desired compound was prepared using material prepared as described in
Example
90D and utilizing the procedures described in Example 91 A and Example 40D.
MS (DCI/NH3) m/z (M+H)+ 322;
IH-NMR (300 MHz, DMSO-d~,) b 9.35 (s, 2H), 8.90 (s, 2H), 8.34 (s, 1H), 8.1 I
(d, 1H),
7.90 (d, 1H), 7.72 (d, 1H), 7.60 (dd. 1H), 7.47 (s, 2H), 6.70 (d, 2H) 6.49 (d.
2H), 3.~8 (s,
'?, _3H), 3.64 (s, 3H);
Anal. calc:'d for C~ I H2~~N,;04F~ 1 /1 () TFA: C, 56.90: H, 4.53; N, 9.38.
Found: C; 56.88; H,
4.41; N, 9.43.
Example 101
3(> 7-methoxv-8-((phenvlmethvllaminol-~-naphthalenecarboximidamide
monoftrifluoroacetate)(salt)
Example 1 () l A
-124-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US9811538(
4-Bromostyrene (4.8 g, 26.2 mmol) was dissolved in 100 mL THF and cooled to -
78
°C. Butyl lithium (2.5 M in hexanes. 28.8 mmol) was added dropwise and
stirred 5 minutes.
Iodine in THF was added dropwise until an orange/red color persisted.
Concentrated aqueous
ammonium chloride (20 mL) was added and the reaction was warmed to room
temperature,
s diluted with ether, washed with 10~o Na2S205 solution ( 1 x, 50 mL), and
brine ( 1 x, 50. mL),
dried (magnesium sulfatej, and concentrated to give the desired compound.
MS (DCI/NH3) m/z 122.
Exam le 1 I B
m The product from I()4A (2.35 g, 10.2 mmol), 1.6 mL 60°l0, N-
methylmorphiline-N-
oxide/water solution, 3.75 mL acetone, 0.1 mL water were stirred 1 hour. 20 mL
Osmium
ten-oxide/tert-butanol solution (0.02 mmol/mL} was added and stirred at 0
°C for 20 hours. The
reaction was concentrated on silica gel and purified by silica gel
chromatography to give the
desired compound.
t, MS (DCI/NH3) m/z (M+NH~)+ 282.
Example 101 C
The desired compound from Example 104B is coupled using Method 41A.
MS (DCI/NH3) m/z (M+NH3)+ 333.
2Il
Example 1 O 1 D
7-methoxv-8-((phenvlmethyl)aminol-~-n~phthalenecarborimidamide
mono(trifluoroacetate)lsalt)
The above was prepared from Example 104C using method described in Example
40D.
?5 MS (DCI/NH3) m/z (M+H)+ 333;
~ H-NMR (300 MHz, DMSO-d~) 8 9.42 (s, 2H), 9.12 (s, 2H), 8.45 (s, 1 H), 8.15-
8.05 (m,
4H), 7.81 (dd, lHj, 7.63 (d, 2H), 7.48 (d, 2H), 7.39 (d, 2H), 4.56 (t, IH),
3.45 (d, 2H);
Anal. calc'd for C23H21N204F3 2/5 TFA: C, 58.19; H, 4.39; N, 5.71. Found: C,
58.17; H,
4.41; N, 5.87.
3c~
Example 102
7-methoxv-8-(nhenvlamino)-~-naohthaIenecarboximidamide
monoltrifluoroacetate)lsaIt)
Exam In a 102A
-125-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99!05096 PCT/US981I5386
Ten-butylcarbamate (3.62 g, 15.7 mmol) was dissolved in 63 mL propanol. I I 8
mL
NaOH/water solution (0.4 N), tej-t-butyl hypochlorite (5.5 mL, 47.8 mmol). and
(DHQD)2PHAL (612 mg, 0.63 mmol) in 50 mL propanol were added and stirred 10
minutes.
The product from Example 2 (3.62 g, I5.7 mmol}, and KZOs04~2 water (211 mg,
0.63 mmol)
S were added and stirred 24 hours. The reaction was concentrated and
recrystallized from
ethanol/hexanes to give the desired compound.
MS (DCI/NH3} m/z (M+NH3)+ 3H 1.
Example 102B
The above was prepared from Example t02 using the method described in Example
41 A.
MS (DCI/NH3) m/z (M+H)+ 415
Example 102C
is 7-methoxv-H-(Dhenvlamino)-2-naohthalenecarboximidamide
mono~trifluoroacetate)lsalt)
The above was prepared from Example 102B using the method described in Example
94D.
MS (DCI/NH3) m/z (M+H)+ 264;
~ H-NMR (3(l() MHz, DMSO-d6) 8 9.42 (s, 2H), 9.07 (s, 2H), R.45 (s, 1 H), 8.33
(br, 3H),
2~~ H.16-8.03 (m, 4H) 7.75 (d, ZH), 7.56 (s, 2H), 7.49 (d, 2H), 5.49 {br 1 h),
4.2X (br 1H),
3.62 (m, 2H};
Anal. calc'd for C~5H23N~OSF~,~S TFA: C, 37.30; H, 2.51; N, 3.74. Found: C;
37.06; H,
3.12; N, 4.42.
'S Example I()3
7-methoxv-h-f (4-metho~cyphenyl)amino!-2-naphthalenecarboximidamide
mono(trifluoroacetate)lsalt)
Example 103A
3U 4-Bromobenzaldehyde (600 mg, 3.24 mmol), 16.2 mL dimethylamine in THF (32.4
mmol), and sodium triacetoxyborohydride (I.24 g, 5.S mmol) were suspended in
dichloroethane ( t 0 mL). The reaction mixture was concentrated, diluted with
water acidified to
pH=2 and extracted with ether (3x, 20 mL). The aqueous solution was basified
with
- I 26-
SUBSTITUTE SHEET (RULE 26j
CA 02294300 1999-12-14
WO 99/05096 PCT/US98/15386
NaOH/water to pH=12 and extracted with methylene chloride (3x, 30 mL,
acidified with
HCI/methanol and concentrated to give the desired compund.
MS (DCI/I~fH3) m/z (M)+ 214.
Example 103B
The above was prepared from Example 107A using method from Example 4IA.
MS (DCI/NH3) m/z (M+H)+ 313.
Example 103C
l t) 7-methoxv-8-I(4-methoxyphenyllamino 1-~-nanhthalenecarboximidamide
mono(trifluoroacetatel(salt)
The above was prepared from Example 103 using method from Example 40D.
MS (DCI/NH3) m/z (M+H)+ 294;
iH-NMR (300 MHz, DMSO-d~,) 8 9.43 (s, 2H}, 9.11 (s, 2H), 8.46 (s, IH), 8.18-
8.06 (m,
t5 4H), 7.H4 (d, 4I-I), 7.60 (s, 2H), 7.56 (s, 1H), 4.53 (s, 2H), 3.05 (s,
6H);
Anal. calc'd for C2~,H25N304F~ 7/5 TFA: C, 4)i.46; H, 3.73; N, 5.91. Found: C,
48.36; H,
4.25; N, 6.19.
Exam le 1 )4
20 (E)-t~-f2-f4-(1, 2-dihdvroxveth~phenyllethenyll-~-
naohthalenecarboximidamide
mono(trif3uoroacetate)lsalt)
Example 104A
The above was prepared from 4-bromobenzyl alcohol and the compound prepared in
25 Example 9~iA using the method from Example 41 A.
MS (DCl/NH3) m/z (M+NH3)-~ 303.
Example 104B
(E)-6-f2-f4-(1, 2-dihdvroxvethyl)phenyl ethenyll-2-
naphthalenecarboximidamide_,
3O mono(trifluoroacetate)(salt)
The above was prepared from Example 104A using method from Example 40D.
MS (DCI/NH3) m/z (M+H)+ 303;
t H-NMR (300 MHz, DMSO-d6) 8 9.00 (br, 4H}, 8.44 (s, 1 H), 8.15-8.01 {m, 4H),
7.81 (dd,
1 H), 7.64 (d, 2I-i), 7.48 (d, 1 H), 7.36 (d, 2H), 5.21 (br, I N) 4.53 (s,
2H);
-127-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 _ PCT/US98115386
Anal. calc'd for C?2Ht9N203F3 4/5 TFA: C, 55.84; H, 3.93; N, 5.52. Found: C,
55.60; H,
3.93; N, 6.41.
Example 105
S (E)-6-f2-f4-(1R-amino-2-hvdroxveth~,~henyll then ly_1-2-
naohthalenecarborimidamide
bis(trifluoroacetate)(salt~,
Example 105A
Using the procedure described for Example 121 A, and substituting N-BOC-p-
lt) iodophenylalanine (BACHEM Bioscience Inc.} for 4-iodoaniline, the desired
compound was
obtained.
MS {DCI/NH3) m/z 458 (M+NH4)+;
tH NMR (300 MHz, CDC13) 8 1.35 (s, 9H), 2.9() (t, 1H), 3.09 (dd, 1H), 4.15 (m,
IH), 7.20
td, 1H), 7.36 (d, 2H). 7.56 (d, 2H), 7.78 (d, 1H), 7.X5 (d, 1H), 8.12 (d, 1H),
8.17 (d, lHj,
15 8.32 (s, 1H), 8.62 (s, 1H).
Example 105B
Using the product obtained in Example 105A and the procedure described in
Example
40D the desired compound was obtained.
2t~ MS (ESI) m/z 458 (M+H)+;
~ H NMR (300 MHz, DMSO} 8 I .35 (s, 9H}, 2.90 (dd, 1 H), 3.10 (dd, 1 H), 4.13
(m, 1 H),
7.10 (d, 1 H), 7.36 (d, 2H}, 7.55 (d, 2H), 7.78 (dd, 1 H}, 7.85 (dd, 1 H),
8.13 (d, 1 H), 8.19
(d, 1 H), 8.30 (s, 1 H), 8.50 (s, I H), 9.22 (s, 2H), 9.42 (s, 2H).
'S Example 105C
IE)-6-12-f4-( 1 R-amino-2-hvdroxvethvl)phenvl lethenyl l-~-
naphthalenecarboximidamide
bis(triouoroacetate)(sal~
Using the product obtained in Example 1()5B and the procedure described for
Example
124D, the desired compound was obtained.
3o MS (ESI) m/z 358 (M+H)+;
t H NMR (300 MHz, DMSO) b 3.(12 (m, I H), 3.19 (dd, 1 H), 3.63 (t, 1 H), 7.39
(d, 2H), 7.58
(d, 2H), 7.76 (d, l H), 7.88 (d, 1 H), 8.15 (d, I H), 8.19 (d, lHj, 8.30 (s, 1
H), 8.51 (s, 1 H),
9.41 (s, 2H), 9.80 (s, 2H);
-128-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99!05096 PCTJUS9811538~
Anal. calc'd for C2q.H2~F3N304~H20: C, 58.90; H. 4.53; N, 8.59. Found: C,
58.75: H. 4.22;
N, 8.28.
Example 106
S 7-methoxv-R-(2-pvrimidinylamino)-2-naphthalenecarboximidamide
bis(trifluoroacetate)(salt)
Example I06A
Sodium borohydride (0.22 g, 5.8 mmol) was added to a suspension of (4-
bromobenzoyl)methanol (2.5 g, 11.6 mmol, Maybridge Chem. Co.) and 25 mL abs.
ethanol.
1u The reaction mixture was stirred at reflux for 1 hour. After cooling to
room temperature, the
ethanol was evaporated under vacuum, and water was added to the residue. The
mixture was
extracted with CH2Cl2. The extracts were washed with saturated aqueous sodium
chloride,
dried over MgS04, filtered, and evaporated under vacuum to afford the desired
compound.
MS (DCI/NH3) m/z 234/236 (M+NH4)+;
o s ~ H NMR (3()0 MHz, CDCl3) 8 2.10 (t, 1 H), 2.62 (d, 1 H ), 3.63 (m, 1 H},
3.78 (m, 1 H), 4.81
(m, 1 H), 7.25 (d, 2H), 7.50 (d, 2H).
Example 106B
Using the product obtained in Example 106A and the procdure described in
Example A-
2U 22621?i-A, the desired compound was obtained.
MS (DCl/NH3) m/z 331 (M+NH4)+;
~ H NMR (300 MHz, CDC13) 43.45 (t, I H}, 4.59 (q, 1 H), 4.76 (t, 1 H), 5.36
(d, 1 H), 7.42 {d,
ZH), 7.59 (d, 2H), 7.78 (dd, 1 H), 7.85 (dd. 1 H), !x.10 (d, 1 H), 8.15 (d, 1
H}, 8.30 (s, 1 H),
8.61 (s, 1 H).
2;
Example 1 ()6C
7-methoxv-8-(2-pvrimidinylamino)-2-naphthalenecarboximidamide
bis(trifluoroacetate)falt)
Using the product obtained in Example 106B, and the procedure described in
Example
40D, the desired compound was obtained.
aU MS (ESI) m/z 331 (M+H)+;
~H NMR (3()0 MHz, DMSOj S 3.45 (t, 1H), 4.59 (q, IH), 4.7R (t, 1H), 5.38 (d,
1H), 7.42
(d, 2H), 7.59 (d, 2H), 7.78 (dd, 1 H), 7.84 (dd, 1 H), $. I 2 (d, I Hj, 8.18
(d, 1 H), 8.31 (s,
I H), 8.50 (s, 1 H}, 9.20 (s, 2H), 9.43 (s, 2H);
- I 29-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98115386
Anal. calc'd for C2~HJ9F~N204~H20: C, 59.74; H, 4.SH; N, 6.06. Found: C,
59.95; H, 4.I7;
N, 6.13.
Example 107
s (E)-~-I2-II4-(dimethvlamino)methyl~phenyrllethen 1 -~-
naphthalenecarboximidamide
bis(trifluoroacetate)(salt~
Example IO7A
Using the procedure described for Example I2IA, and substituting 3-
m benzyloxybromobenzene CChem. Ber.124 ( 1 ), 163, 1991 ) for 4-iodoaniline,
the desired
compound was obtained.
MS (DCI/NH3) m/z 377 (M+NH4)+;
J H NMR (3()O MHz, CDCl3) 8 5.1 I (s, 2H), 7.()2 (d, 1 H), 7.20 (m, 2H), 7.29
(d, 1 H), 7.31
(d, IH), 7.42 (m, 3H), 7.60-7.75 (m, 3H}, 7.H9 (t, 2H), H.()S (s, 1H), H.21
(s, IH).
Example 1 (77B
(E)-6-12-114-(dimethvlamino)meth~lphenyl ethenyl-!-~-
naphthalenecarboximidamide
bis(trifluoroacetate)(saIt~
Using the product obtained in Example 1()8B, and the procedure described in
Example
20 4UD, the desired compound was obtained.
MS (ESI) m/z 377 (M+H)+;
) H NMR (3()0 MHz, D MSO) 8 5. I 8 (S, 2H), 7.12 (dd, 1 H), 7.22 (d, 1 H),
7.2$ (m, 1 H),
7.4(J (t, 3H), 7.45 (t, 3H), 7.79 (dd, 1H), 7.H5 (dd, IH), H.16 (d, 1H), x.20
(d, IH), H.35 (s,
1H), H.5(J (s, 1H), 9. 3(J (s, 1H);
?5 Anal. calc'd for C~~H21F~N~03~().25 HBO: C, 67.94; H, 4.3H; N, 5.66. Found:
C, 67.H(); H,
4.4K; N, 5.43.
Example 1 ()S
(E)-6-f 2-I4-(hvdroxymethvl)phenvl lethenvl l-2-na~phthalenecarboximidamide
~~) mono(trifluoroacetate)(salt)
Using the product obtained in Example I08A and the procedure described in
Example
94D the desired compound was obtained.
MS (ESI) m/z 2R7 (M+H)+;
-130-
SUBSTITUTE SHEET (RULE 2fi)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98115386
~ H NMR (300 MHz, DMSO) 8 6.89 (m, I H), 6.98 (t, 1 H), 7.03 (d, 1 H), 7.29
(t. 1 H), 7.78
(dd, IH), 7.88 (dd, 1H), 8.13 (d, 1H), 8.I7 (d, 1H), 8.32 (s, 1H). 8.50 (s.
1H), 9.40 (s,
SH);
Anal. calc'd for C~iHtSF3N~03~().5 H20: C, 61.62; H, 3.94; N, 6.84. Found: C,
61.29; H,
3.8I; N, 6.59.
Example 109
4-f I6-(aminoiminomethyl)-2-naphthalenyllethynvll-L-phenylalanine
mono(trifluoroacetate)(salt)
1u
Example 109A
To a solution of the product from Example 8D (2.13 g, 10.08 mmol> and LiBH4
(121
mg, 5.55 mmol) in THF (5 mL) was added toluene (2 mL), and the THF was boiled
off using a
short-path distillation apparatus over several hours. The reaction was then
heated at 70°C for 2
n hours, cooled, quenched with 1 M HCI, and extracted with 2x ethyl acetate.
The extracts were
washed with water and brine, dried over Na~S04, and condensed. The crude
product was
chromatographed on Si02 using SO~Io ethyl acetate/hexanes as eluent, to yield
1.12 g (6I%) of
the desired compound.
MS (DCI (NH3)) m/z 201 (M+NH4)+;
20 ~ H NMR (300 MHz, CDC13) 8 8.22 (s, 1 H), 7.90 (m, 3Hj, 7.61 (m, 2H), 4.92
(d, 2H), 1.84
(t, 1H).
Exam,.ple 109B
To a solution of the product from Example 109A (2.I2 g, I 1.57 mmol) and Liar
(1.1 1
5 g, 12.73 mmol) in DMF ( 1()0 mL) was added PBr3 ( 1.21 mL, 12.73 mmoi) at
0°C, and the
reaction was warmed to room temperature, and stirred for 1 hours. The reaction
was then
duenched with pl-I 7 buffer, and extracted with 3x diethyl ether/hexanes. The
extracts were
washed with 2x water and 2x brine, dried over Na~S04, and condensed, to yield
2.72 g (96%)
of the desired compound.
3o MS (DCI (NH3)) m/z 185 (M+NH4-gr)+;
1 H NMR (30(1 MHz, CDCl3) S 8.22 (s, I H), 7.92 (s, 1 H), 7.9() (s, 2H), 7.62
(dd, 2H), 4.64
(s, 2H).
Example 109C
-131-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98/15386
To a solution of NaH (60~1o in mineral oil, 44 mg, 1. I mmol) in DMF (5 mL)
was
added 4-ethylphenol ( 122 mg, 1.0 mmol j, and the reaction was stirred at room
temperature for
20 minutes. The product from Example 109B (270 mg, 1.1 mmol) was then added,
and the
reaction was stirred for 10 minutes. The crude reaction mixture was
chromatographed on Si02
:: using hexanes as eluent, to yield 220 mg (77%) of the desired compound.
MS (DCl (NH3)) m/z 305 (M+NH4)+;
~ H NMR (300 MHz, CDC13) 8 $.22 (s, 1 H), 7.95 (s, 1 H), 7.93 (s, 1 H), 7.91
(s, 1 H), 7.66
(dd, 1H), 7.61 (dd, 1H), 7.15 (d, 2H), 6.94 (d, 2H), 5.22 (d, 2Hj, 2.60 (q,
2H), 1.21 (t,
3H).
Example 109D
4-If6-(aminoiminometh 1)-2-na hthalen~lleth5rn I~l-L-,phenvlalanine
r~r ono(trifluoroacetat )lsalt~
The desired compound was prepared from Example 109C and the procedure of
Example
is 55D.
MS (DCI/NH3) m/z 305 (M+H)+;
~H NMR (30() MHz, DMSO-d~,) 8 1.15 (t. 3H), 2.14 (s, 3Hj, 2.56 (q, 2H), 5.30
(s, 2H),
6.9?S (d, 2H), 7.14 (d, 2H}, 7.74 (dd, 1 H), 7.H2 (dd, 1 H), H.15 (m, 3H),
H.4S (s, 1 H}, 9_.01
(br s, 2H), 9.62 (br s, 2H);
2n Anal. valc'd for C2pH2pN20~1.4 CH4S03: C, 58.56; H, S.SS; N, 6.3K. Found:
C, 5H.55; H,
5.56; N, 6.39.
Example 1 1 1
6-(3-formviohenvl)-2-nanhthalenecarboximidamide mono(trifluoroacetate)(salt)
2S
Example 1 1 1 A
The product from Example 2$B (334 mg, 1.1 1 mmol j, palladium acetate (25 mg,
0.1 1
mmol), dppf (123 mg, 0.22 mmol) were dissolved in degassed DMF (5 mLj and
stirred at
room temperature for 1/2 hour. Cesium carbonate (902 mg, 2.H mmol) and 2-
3o formylphenylboronic acid (251 mg, 1.27 mmol) were added and stirred under
nitrogen at RO °C
for I hour, poured into pH 7 buffer, extrated with diethyl ether (3x, 20 mL),
and dried. The
desired compound was purified by chromotography eluting with 10~'/o ethyl
acetate/ hexanes.
MS (DCI/NH3) m/z (M+NH3)+ 275.
-132-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98115386
Example 1 1 1 B
6-f3-formvl~henvl)-2-nar?hthalenecarboximidamide mono(trifluoroacetarP)fealt)
The above product was prepared in a manner analogous to that of Example IB.
MS (DCI/NH3) m/z (M+H)+ 274;
s ~ H-NMR (300 MHz, DMSO-d~,) b 10.16 (s, 1 H}, 9.47 (s, 2H), 9.10 (s, 2H),
8.54 (s, I Hj,
8.52 (s, 1 H), 8.41 (s, 1 H), 8.28-8.23 (m, 3H), 8.12 (dd, 1 H), 8.00 (dd, 1
H), 7.87 (dd, 1 H),
7.80 (t. 1 H);
Anal. calc'd for C2~)HISN~03F3 2/5 TFA: C, 59.28: H, 3.70; N, 6.34. Found: C;
59.36; H,
3.89; N, 7.21.
Examl 1~ a I 12
(E)-6-f2-(1 ~ 3 4-tetrahydro-6-isoquinolin 1 a h n_yll-2-
naphthalenecarboximidami a
~sltrifluoroacetate)(,~alt)
Example 1 12A
The above was prepared from Example 127 using method described in Example 41
A.
MS (DCI/NH3} m/z (M+H)+411.
Example 1 12B
20 (E)-6-f2-(1. 2, 3. 4-tetrahvdro-6-isoduinolin 1)teethe,nyll-~-
naphthalenecarboximidamide
bisltrifluoroacetate)(salt)
The desired compound was prepared by the method described in Example 40D.
MS (DCI/NH3) m/z (M+H)+ 328;
~ H-NMR (300 MHz, DMSO-d~,) 8 9.36 (s> 2H), 9.25 (s, 2H), 9.10 (d, 2H), 8.41
(s, I H),
?5 7.99 (t. 2H). 7.89 (d, IH), 7.7H (d, IH), 7.71 (dd, 1H), 7.56 {m. 4H). 7.43
(s, 1H), 3.11
(br, 2H) 2.16 (br 2H), 1.78 (br, 2H);
Anal. calv'd for C2~,H23N304Fr, 3/5 TFA: C, 52.31; H, 3.1i 1; N, 6.72. Found:
C, 52.13; H,
4.42; N, 7.23.
ExamrJle 113
(E)- 6-f2-f3-(2-hvdroxvethvl)phenyllethenyll-2-naphthalenecarboximidamid
mono(trifluoroacetatel(salt)
Example 1 13A
-133-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98/15386
The above was prepared from 2-bromo-3 (hydroxyethyl)alcohol and the compound
prepared in Example 98A using method described in Example 41 A.
MS (DCI/NH3) m/z (M+NH3)+ 317.
Example I 13B
(E)- 6-12-f3-!2-hvdroxvethvl) henvllethenyl -~-na hoo thalenecarboximidamide
monoltrifluoroacetate)lsaltl
The above was prepared from Example 1 13A using method described in Example
40D.
MS (DCI/NH~) m/z (M+H)+ 3I7;
n> >H-NMR (300 MHz, DMSO-d~,) 8 8.9 (br, 4H), 8.46 (s, IH), 8.17 (s, IH), 8.I3-
8.03 (m,
3H), 7.H2 (dd, 1 H), 7.54 (s, 2H), 7.49 (s, 2H), 7.33 (t, 1 H) 7.1 H {d. I H),
4.71 (t, I H), 3.66
(m, 2H), 2.7$ (t, 2H);
Anal. calc'd for C23H~1 N203F3 3/10 TFA: C, 61.41; H, 4.66; N, 6.09. Found: C,
64. l Ii; H,
4.92; N, C~.51.
IS
Example 114
h-laminoiminomethvl)-4-!~i-furanvl)-N-14-ltrifluoromethyllphenvll 2
n~hthalenec.arboxamide
mono( trifluoroacetate)(salt)
't~ Example 1 14A
The product from Example 152B (100 rng, (1.36 mmol), 4-
(trifluoromethyl)aniline {86
rng, 0.53 mmol), and DMAP (5 mg, 0.04 mmol) were dissolved in THF (5 mL) and
stirred for
24 hours. The reacaion mixture was concentrated on silica gel and purified by
chromotography
(Biotage Flash 40) using ethyl acetate/hexanes.
25 MS (ESI) m/z (M+H)+4()f~.
Exam~e I 14B
fi-(aminoiminomethvl)-4-!'i-furanvl)-N-f4-(trifluorometh~~phenyll 2
nanhthalenecarboramide
mono(trifluoroacetate)ls It)
3U
The above was prepared from Example I 14A using method described in Example 1
B.
MS (CI) m/z (M+H)+ 424;
-134-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT1US98115386
1 H-NMR (300 MHz, DMSO-d~,) b 10.91 (s, I H), 9.51 (s, 2H). 9.1 1 ( s, 2H),
8.69 (s, I H),
H.62 (s, 1 H), 8.43-8.35 (m, 2H), R.1 S (d, 1 H), X.06 (d. 2H), 7.911 (t. 1
H), 7.92 (dd, 1H),
7.78 (dd, 2H) 7.14 (m, 1 H);
Anal. caIc'd for C25HI7N304F~ 1/10 TFA: C, 55.37; H, 3.15; N, 7.70. Found: C,
55.44; H,
s 3.15; N, 7.31.
Example 115
6-(atninoiminomethvl)-4-(3-furanyl )-N-(4-pyridinvl)-~-naphthalenecarboxamide
dihvdrochloride
Example 1 15A
The above product was prepared in the manner of Example 114A.
MS (ESI) m/z (M+H)+ 340.
I S Example 1 I SB
6-(aminoiminomethvl)-4-(3-furanyl)-N-(4-pvridin l)-~-naphthalenecarboxamide
dihydrochloride
The above was prepared from Example 1 1 SA using method described in Example 1
B.
MS (AP/CI) m/z (M+H)+ 357;
2c> I H-NMR (300 MHz, DMSO-d~,) 8 12.43 (s. 1 H), 9.69 (s, 2H), 9.40 (s, ZI-
i), 8.94 (s, 1 H),
H.H I (d, 2H), 8.65 (s, 1 H), B.SH-H.56 (m, 2Hj, 8.49 (s, 1 H), 8.42 (d, 1 H),
8.30 (m, 1 H)
7.97-7.95 (m, 2H), 7.27 (s, I H);
Anal. calc'd for C~~H1gN40~C12 37/IO HCI: C, 44.65 H, 3.88 N, 9.92. Found: C,
44.72: H,
3.70: N, 9.51.
05
Example 1 16
6-(aminoiminomethyl)-4-l3-furanvl)-N-( 1 H-pyrazol-~ yl) 2
naphthalenecarboxamide
dihydrochloride
Example I 16A
The above product was prepared in the manner of Example 1 14A.
MS (ESIj m/z (M+H)+ 329.
Example 1 I6B
-t35-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98/15386
6-(aminoiminomethvl)-4-('~-furanvl)-N-( 1 H-~yrazol-'~-vl)-2-
naphthalenecarboxamide
dihydrochloride
The above was prepared from Example I I6A using method described in Example I
B.
MS (CI) m/z (M-H)+ 344;
1 H-NMR (300 MHz, DMSO-d6) b i I .16 (s, 1 H), 9.52 (s, 2H), 9.10 (s, 2H),
8.69 {s, i H),
8.61 (s, 1H), 8.35 (m, 2H), 8.24 (s, IH), 7.96-7.88 (m, 3Hj, 7.69 (m, IH),
7.15 (s, IH)
6.69 (m, 1 H):
Anal. calc'd for C(9H(7N502C12 9/10 HC1: C. 50.63; H, 4.00; N, 15.54. Found:
C, 51.()5;
H, 4.62; N, 14.26.
Examl 1~ a I 17
6-(aminoiminomethvl )-4-(3-furanyl )-N-(3-pyridinyl l-2-nanhthalenecarboxamide
dihydrochloride
Example 117A
The above product was prepared in the manner of Example 1 14A.
MS (ESI) m/z (M+H)+ 340.
Example l I7B
2U 6-(aminoiminomethvl)-4-(3-furanyll-N-(3-pyridin l~-2-na-
phthalenecarboxamide
dihydrochloride
The above was prepared from Example 1 17B using method described in Example 1
B.
MS (C1 ) m/z ( M+H )+ 357;
~H-NMR (3()0 MHz, DMSO-df,) b 1().9() (s, IH), 9.59 (s, 2H), 9.26 (s, 2H),
9.03 (s, 2H),
25 h.74 (s, 1 H). ?S.F~3 (s, 1 H), H.42-8.2f~ (m, 3I-I), 8.22 (s, lI-I), 7.97-
7.91 (m, 2H), 7.47-7.43
(m, 2H), 7.17 (s, 1H);
Anal. calc'd for C21H1~N4O2C12 55/1() HCI: C, 40.00 H, 3.76 N, 8.89. Found: C,
40.09; H,
3.78; N, 8.44.
-t« Example 118
6-(aminoiminomethvl)-N-(2 ~-dihydro-1H-inden 5 yl) 2 naphthalenecarboxamide
mono(trifluoroacetate)(salt)
Example 1 18A
-136-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98115386
To a solution of the compound prepared in Example SE (303 mg, 1.4 mmol) in THF
(30 mL) and propylene oxide ( I S mL) was added two drops of Et3N followed by
the 5-
aminoindene (300 mg, 2.2 mmol). The reaction was stirred at room temperature
overnight. The
solvent was evaporated and the product was purified via crystallization from
ether to yield 226
S mg (S6%) of the product as white solid. Mass spectrum (CI+), 313 (M+1 )+.
Example I I8B
6-(aminoiminomethvll-N-(2 'i-dihydro-IH-inden-S-vI)-~-nay~hthalenecarboxamid~P
mono(trifluoroacetate)( ~It)
The compound prepared in Example 118A {20S mg, 0.66 mmol) in THF (20 mL) at
room temperature, was added butyl lithium ( 1 mL, 1 mmol) followed by
chlorotrimethyl silane
( 180p.L, 1.S mmol). After i0 minutes the mixture was charged with additional
butyl lithium (3
mL, 3 mmol). The reaction was stirred at room temperature, overnight. The
reaction mixture
was added a solution of 4 N HCl in dioxane stirred far an hour then added
water and
n evaporated. The product was purified by MPLC RP CIA with methanol-water and
0.1% TFA as
eluent chromatography. The yield of the product as TFA salt with 0.25%c water
as white solid
S 1 mg ( 17 %).
MS (ESI+) 330 (M+I)+;
~ H NMR (DMSO-d6) 1 ().45 (s, 1 H), 9.S 1 (s, 2H), 9.21 (s, 2H), 8.66 (s, 1
H), B.SS (s, I H),
2U 8.32 (d, J=B.SHz, 1H), 8.20 (Abq, J=9.OHz, 2H), 7.90 (dd, Jl=9.0Hz,
J~=I.SHz, 1H), 7.73
(s, 1H), 7.53 (dd, J(=8.0Hz, J2=I.SHz, IH), 7.22 (d, J=8.lHz, IH), 2.91-2.82
(m, 4H),
2.04 (quintet, J=7.3Hz, 2H);
Anal. calc'd for C21H1yN~O~TFA~0.2S H20 C: 61.67; H, 4.61; N, 9.38. Found: C:
61.63; H,
4.43; N, ~J.2S.
,5
Example I 19
methyl .5-I7-f(aminoiminomethvl)-~-naphthalen lv lorylpentanoate
mono(trifluoroac;etate~ (salt)
Example 1 19A
J« 7-hvdroxy-2-cyanonaphthalene
7-methoxy-2-cyanonaphthalene (2.79 g, 5.23 mmol) and tetrabutylammonium iodide
( 17 mg, 0.157 mmol) were combined in a mixture of benzene (3S mL) and
cyclohexanes ( 17.5
mL). The resulting solution was added to a rapidly stirring, cooled
(ice/water) suspension of
aluminum triiodide (6.21 g, 15.23 mmol) in a mixture of benzene (3S mL) and
cyclohexanes
-137-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 , PCTlUS98/15386
( 17.5 mL) under an inert atmosphere. After the addition, the resulting
suspension was heated at
reflux for 2.5 hours. The heating was removed and after cooling to near room
temperature, the
reaction mixture was cooled in an ice bath and quenched by the addition of
water ( l0U mL). The
resulting mixture was further diluted with 2 M aqueous sodium thiosulfate
solution (50 mL) and
S extracted with ethyl acetate (3 X 80 mL). The combined organic layers were
dried and
evaporated. The resulting solid was dissolved in a minimum of hot ethyl
acetate, diluted hot
with hexanes to the cloud point and placed in a refrigerator for 2 hours. The
desired compound
was collected by filtration, ( 1.99 g, 77% ).
MS (DCI (NH3)) mJz 187 (M+NH4)+.
Example 119B
The resulting product from Example 1 19A was treated with methyl 5-
bromovalerate in
an analogous manner as described in Example 1 19A.
~'~S (DCI (NH3)) m/z 3()1 (M+NH4)+.
IS
Example 1 19C
methyl 5-f 7-f (aminoiminomethyl)-2-nal hthalenyllox~pentanoate
mono(trifluoroacetate) (salt)
The resulting product from Example 1 19B (3X0 mg, 1.3412 mmol) was treated in
an
analogous manner as described in Example 94D to yield the desired compound
(369 mg, 73°l0).
2o MS (DC1 (NH3)) tn/z 301 (M+H)+;
1H NMR (300 MHz, DMSO-d6) b 1.7X5 (m, 4H), 2.425 (t, 2H), 3.600 (s, 3H), 4.150
(t,
2H}, 7.3RC) (dd, 1 H), 7.46() (d, 1 H), 7.640 (dd, 1 H), 7.9X0 (d, 1 H), K.070
(d, 1 H), 8.322 (d,
1 H), 9.230 (v br s, 3H);
Anal. calc'd for CpH2t~N~O~~C2HO~F~: C, 55.07; H, 5.1 l; N, 6.76. Found: C,
54.96; H,
'_'S 5.2~: N. 6.66.
Example 120
(E)-3-17-(aminoiminomethyl)-2-methox -1-naphthalenyl) ~ propenamide
mono(trifluoroacetate)(salt)
3«
Examlhe 120A
The product obtained from Example 53B and acrylamide were subjected to the
conditions described in Example 41 A to provide the desired compound.
MS (DCI/NH3) m/z 253 (M+H)+.
-13k-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98115386
Example 120B
(E)-3-f7-(aminoiminomethvl)-2-methox -~phthaleny~-2-propenamide
mono(trifluoroacetate)(salt)
s The product obtained from Example 120A was subjected to the conditions
described in
Example 94D to provide the desired compound.
MS (DCI/NH3) m/z 270 (M+H)+;
IH NMR (300 MHz, DMSO) 8 4.02 (s, 3H), 6.90 (d, IH), 7.22 (s, 1H), 7.62-7.70
(m, 2H),
7.74 (d, I H), 8.02 (d, l H), 8.1 1 (d, 1 H), 8.15 (d, I H), 8.58 (s, I H),
9.18 (s, 2H), 9.50 (s,
m 2H);
Anal. calc'd for Cl7Ht~F3N304~H20: C, 50.88; H, 4.52; N, 10.47. Found: C,
50.89; H,
4.32; N, 10.43.
Exam 1
6-f(4-arninovhenvl)ethvnvll-2-naphthalenecarboximidamide
bis(trifluoroacetate)(salt)
IS
Example 121 A
A mixture of the product obtained in Example 63B (130 mg, 0.73 mrnol), 4-
iodoaniline
(173 mg, 0.79 mmol), dichlorobis (triphenylphosphine)palladium (II) (25 mg,
().0325 mmol),
copper (I) iodide (2.7 mg, ().()I86 mmol), DMF (0.65 mL), and triethylamine
(1.95 mL) was
2o degassed with N2 and was stirred at 75°-80° for 1.5 hour. The
mixture was cooled to room
temperature, diluted with CH2CI2, washed with water, dried (MgS04), filtered,
and evaporated
under vacuum to afford an oil which was purified by flash chromatography,
eluting with 3: !
hexanes: ethyl acetate to afford the desired compound.
MS (DCI/NH~) m/z 269 (M+H)+.
?5
Example 121 B
fi-If4-aminophenvl)ethvnvll-2-naohthalenecarboximidamide
bis(trif7uoroacetate?(salt)
Using the product obtained in Example 121A and the procedure described in
Example
40D, the desired compound was obtained.
3o MS (DCI/NH3) m/z 286 (M+H)+;
iH NMR (30U MHz, DMSO) 8 6.80 (d, 2H), 7.29 (d, 2H), 7.70 (dd, 1H), 7.X5 (dd,
1H),
8.09 (d, 1 H), 8.14 (d, 1 H), 8.20 (s, I H), 8.45 (s, 1 H), 9.09 {s, 2H), 9.42
(s, 2H);
Anal. calc'd for C23H17Fr,N~04~0.25 H20: C, 53.34; H, 3.41; N, 8.1 I. Found:
C. 53.45; H,
3.70; N, 7.76.
-139-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98/15386
Example 122
1, 1-dimethvlethvl f2-f3-ff6-(aminoiminomethvIl-2-nanhathalenyllethvnyl]-f~
methoxwhenvllethyllcarbamate mono(trifluoroacetate)(sal~
Exam;r~le I22A
Using 5-bromo-2-methoxyphenethylamine hydrobromide (Transworld), and the
procedure described in Example 63C, the desired compound was obtained.
MS (DCl/NH3) m/z 330 (M+H)+.
Example 1228
Using the procedure described for Example 12I A, and substituting the product
obtained
in Example 122A for 4-iodoaniline, the desired compound was obtained.
MS (ESI) m/z 427 (M+H)+.
Example 122C
l, I-dimethvlethyl f2-f3-ff6-(aminoiminomethyl)-2-n~hathalen I~lethynyll~fi
methoxmhenvllethvllcarbamate mono(trifluoroacetate)(sal~
Using the product obtained in Example A-226638-B and the procedure described
in
2c> Example 40-D, the desired compound was obtained.
MS (DCI/NH3) m/z 444 (M+H)+;
~H NMR (30() MHz, DMSO) 8 1.38 (s, 9H), 2.70 (t, 2H), 3.15 (q, 2H), 3.115 (s,
3H), 6.89
(t, 1 H), 7.04 (d, 1 H}, 7.37 (d, I H), 7.49 (dd, 1 H). 7.75 (dd, I H). 7.~5
(dd, 1 H), R. I I (d,
1 H), X.16 (d. 1 H), $.30 (s, 1 H), 1;.4f~ (s, 1 H), 9.07 (s, 2H), 9.42 (s,
2H) ;
Anal. calc'd for C2yH3«F3N~Oy().2S HBO: C, 61.97; H, 5.47; N, 7.411. Found: C,
61.11; H,
5.14; N, 7.21.
Example 123
I, 1-dimethvlethvl ff4-ff6-(aminoiminomethyI)-~-
naphathalenyllethynvllohenyllmeth ~Lll,
3« carbamate mono(trifluoroacetate)(salt~
Example 123A
Using 4-bromobenzylamine, and the procedure described in Example 63C, the
desired
compound was obtained.
-140-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/OS096 PCT/US98115386
MS (DCI/NH3) m/z 303 (M+NH4)+.
Example 123B
Using the procedure described for Example 121A, and substituting the product
obtained
s in Example 123 for 4-iodoaniline, the desired compound was obtained.
MS (DCI/NH3) m/z 400 (M+NH4)+.
Example 123C
1, 1-dimethvlethvl [[4-[[6-faminoiminometh 1)-~-
naphathalenyliethynyllphenyllmeth rL1)
carbamate, mono(trifluoroacetate)(salt)
Using the product obtained in Example 123B and the procedure described in
Example
40D, the desired compound was obtained.
MS (DCl/NH3) m/z 400 (M+H)+;
1 H NMR (3()() MHz, DMSO) 8 1.40 (s, 9H), 4.1 H (d, 2H), 7.34 (d, 2H), 7.45
(t, 1 H), 7.5k
(d, 2H), 7.78 (dd, 1 H), 7.H6 (dd, 1 H), H.15 (d, 1 H), 8.19 (d, I H), 8.31
(s, 1 H), H.50 (s,
1 H), 9.1()-9.42 (s, 4H) ;
Anal. calc'd for C27H2~,F3N304: C, 63.15; H, 5.10; N, K.18. Found: C, 62.95;
H, 4.97; N,
H.09.
'~> Example i 24
6-[[4-(aminomethvl)phenyliethynvll-2-naphthalenecarboximidamide
bis(trifluoroacetate)(salt)
Trifluoroacetic acid (0.73 mL) was added dropwise to a suspension of the
product
obtained in Example 123C (80 mg, 0.2 mmol) and 1.5 mL CH2CI2. The reaction
mixture was
'a stirred for 0.25 hour at room temperature, then was evaporated to dryness
under vacuum.
Toluene was added and evaporated under vacuum several times to afford a tan
solid which was
purified by reverse phase chromatography, eluting with methanol / 0.1 plc
aqueous TFA to
afford the desired compound.
MS (APCI) m/z 300 (M+H)+;
3n iH NMR (300 MHz, DMSO) 84.10 (s, 2H), 7.55 (d, 2H), 7.70 (d, 2H), 7.79 (dd,
1H), 7.89
(dd, I H), H.16 (d, 1 H), 8.19 (d, 1 f-I), H.20 (s, 2H), 8.36 (s, 1 H), 8.53
(S, 1 H), 9.20 (s, 2H),
9.44 (s, 2H);
Anal. calc'd for C24H IyF~N304: C, 54.66; H, 3.63; N, 7.97. Found: C, 54.42;
H, 3.57; N,
7.76.
-14l-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98115386
Example 125
6-f f 3-l2-aminoethvll-4-methoxyphenvIlethynyll-2-naphthalenecarboximidamide
bis(trifluoroacetate)ls~lr~
S
Using the product obtained in Example 122C and the procedure described for
Example
124, the desired compound was obtained.
MS (ESI) m/z 344 (M+H)+;
~H NMR (300 MHz, DMSO) 8 2.90 (t, 2H), 3.06 (t, 2H), 3.88 (s, 3H), 7.1 I (d,
iH), 7.44
lu (d, 1 H), 7.~7 (dd. 1 H), 7.75 (dd, 1 H), 7.82 (s. 2H), 7.88 (dd, 1 H), 8.
I 2 (d, 1 H), 8.17 (d,
1H), 8.28 (s, IH), 8.50 (s, 1H), 9.20 (s, '?H), 9.45 (s, 2H);
Anal. calc'd for C26H23F6N~05~U.5 H20: C, 53.80; H, 4.17; N, 7.24. Found: C,
53.89; H,
4.31; N, 6.83.
> > Example 126
-II4-(hvdroxvmethvl)phen Iy~rhvnyll-2-naphthalenecarboximidamide
monoltrifluoroacetate)l alt)
Example 126A
2( Using the procedure described for Example 121A, and substituting 4-
bromobenzyl
alcohol for 4-iodoaniline, the desired compound was obtained.
MS (DCI/NH3j m/z 301 (M+NH4j+.
Example 126B
?S f~-I14-(hvdroxvmethvf )phenvl-lethyn~l I-2-naphthalenecarboximidamide
mono(trifluoroacetate)lsalt)
Using the product obtained in Example 126A and the procedure described in
Example
94B the desired compound was obtained.
MS (ESI) m/z 3()1 (M+H)+;
3U ~ H NMR (300 MHz, DMSO) 8 4.58 (d, 2H), 5.32 (t, 1 H), 7.41 (d, 2H), 7.59
(d, 1 H), 7.79
(dd, 1 H), 7.86 (dd, 1 H), 8.12 (d, 1 H), 8.18 (d, 1 H), 8.32 (s, 1 H), 8.50
(s, 1 H), 9.14 (s,
2H), 9.46 (s, 2H);
Anal. calc'd for C22H1~F3N203~0.5 H20: C, 62.41; H, 4.29; N, 6.62. Found: C,
62.56; H,
4.13; N, 6.65.
-142-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98/15386
Exam lie 127
6-f( 1. 2, 3, 4-tetrahvdro-6-isoquinolin Iv )ethynyll-2-
naphthalenecarboximidamide
bis(trifluoroacetate )(salt)
Exams a 127A
A solution of boron tribromide ( 1.2 mL, 12.5 mmol) and 12.5 mL CH2Cl2 was
added
dropwise to a -78° solution of 6-methoxytetrahydroisoquinoline ( I.0 g,
5.0 mole, Org. Synth.,
67. 60, 1988} and 38 mL CH2C12. The reaction mixture was stirred for 1 hour at
-78°, 1 hour at
1() 0°, and 0.25 hour at room temperature. The reaction mixture was
cooled to -78°, and 20 mL
methanol was added dropwise. The solution was stirred for 1 hour at room
temperature.
Solvent was evaporated under vacuum and the residue was dried under vacuum to
afford the
desired compound.
MS (DCI} m/z 150 (M+H)+.
IS
Example 127B
The produe;t obtained in Example 127A ( 1.15 g, 5.0 mmol) was subjected to the
conditions described in Example 63C. A 2.1 g portion of this material was
stirred at reflux for
1.5 hour with 6() mL methanol and 9 mL l0~lo aqueous NaOH. After cooling to
room
2o temperature, methanol was evaporated under vacuum. Water was added and the
solution was
acidified with 6 N HCI. The mixture was extracted with CH2CI2. The organic
layer was
washed with water, saturated aqueous sodium chloride, dried (MgS04), filtered,
and solvent
evaporated under vacuum to afford the desired compound.
MS (DCI/NH3) m/z 267 (M+NH4}+.
2S
Example 1270
Using the product from Example 127B and the procedure described in Example
28B,
the desired compound was obtained.
MS (DCI/NH3) m/z 399 (M+NH4)+.
3(l
Example 127D
Using the procedure described for Example 121A, and substituting the produca
obtained
in Example I27C for 4-iodoaniline, the desired compound was obtained.
MS (DCI/NH3) m/z 426 (M+NH4)+,
-143-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98115386
Example 127E
Using the product obtained in Example 127D and the procedure described in
Example
40D, the desired compound was obtained.
MS (ESI) m/z 426 (M+H)+;
~ H NMR (300 MHz, DMSO) b 1.45 (s, 9H), 2.82 (t, 2H), 3.59 (t, 2H), 4.58 (s,
2H), 7.29
(d, 1 H), 7.42 (d, 2H), 7.76 (dd, 1 H), 7.83 (dd, 1 H), 8.15 (d, 1 H), 8.19
(d, 1 H), 8.35 (s,
1 H), 8.51 (s, ( 1 H), 9.20 (s, 2H), 9.45 (s, 2H).
«> Example 127F
6-f ( 1. 2. 3. 4-tetrahvdro-f~-isoquinolin~l)ethynyll-2-
naphthalenecarboximidamide
bis(trifluoroacetate)( alt)
Using the product obtained in Example 127E and the procedure described in
Example
124D, the desired compound was obtained.
n MS (ES1) m/z 326 (M+H)+;
~ H NMR (300 MI-lz, DMSO) 8 3.03 (t, 2H), 3.42 (t, 2H), 4.35 (s, 2H), 7.35 (d,
1 H), 7.46
(d, ZH), 7.78 (dd, I H), 7.89 (dd, 1 H), 8.15 (d, 1 H), 8.19 (d, I H), 8.35
(s, 1 H), 8.52 (s,
1 H), 9.17 (s, 2H), 9.31 (s, 2H), 9.48 (s, 2H);
Anal. calc'd for C2~HZIF~,N304: C, 56.42; H, 3.82; N, 7.59. Found: C, 56.31;
H, 3.81; N,
2n 7.42.
Example 129
5-flfi-(aminoiminomethvl)-2-naohthalenylloxylpentanoic acid
mono(trifluoroacetate)(salt)
'S Example 129A
The resulting product from Example 3 19A (250 mg, 1.477 mmol) was treated with
methyl 5-bromovalerate in an analogous manner as described in Example I 19B to
yield the
desired compound (394 mg, 9410).
MS (DCI (NH3)) m/z 301 (M+NH4)+.
3~
Example 129B
5-ff6-(aminoiminomethvll-2-naphthalen~rlloxyjpentanoic acid
mono(trifluoroacetate)(snlt)
The resulting product from Example 129A (262 mg, 0.8723 mmol) was treated in
an
analogous manner as described in Example 94D to yield the ester amidine
product. The product
- I 44-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98115386
( 140 mg, 0.542 mmol} was dissolved in methanol ( 1 1 mL). To this was added a
solution of
lithium hydroxide (68.2 mg, 1.626 mmol) in water (3 mL) and the resulting
mixture was stirred
at room temperature under an inert atmosphere for 18 hours. The reaction was
evaporated and
the residue purified by reverse phase chromatography to yield the desired
compound ( 184 mg,
74%).
MS (DCI (NH3)) m/z 287 (M+H) +;
tH NMR (300 MHz, DMSO-d6} 8 1.71 I (m, 2H}, 1.817 (m, 2H), 2.320 (t, 2H),
4.144 (t,
2H), 7.377 (dd, 1 H), 7.472 (d, 1 H), 7.632 (dd, 1 H), 7.980 (d, 1 H), 8.()81
(d. 1 H), 8.329 (s
! H), 9.100 (br s, 2H), 9.390 (br s, 2H}, 12.100 (br s, 1 H);
m Anal. calc'd for C~6H1gN203~ (C2H02F3) 1.15~H200.65: C, 51.22; H, 4.80; N,
6.53.
Found: C, 51.30; H, 5.07; N, 6.12.
Exam,~le 131
7-methoxv-1;-( 3-oxo-1-cvclopenten-1-yl )-2-naphthalenecarboximidamide
t ~ mono(trifluoroacetate)(salt)
Example 131 A
The material prepared as described in Example 25A (0.5 g, I.5 mmol) and the 3-
tributylstannyl-2-cyclopentenone prepared as described by Labourde et al. Tet
Letters 31, (13),
2( 11(37-1 H40 ( 1990) are coupled via Pd catalyst as described by the method
of Stilie et al. .IACS
109, 54711-541;6 ( 1987) yielding after flash chromatography with 3: 1
hexanes/ethyl acetate a
white solid. 300 mg, 72%.
MS (DCI/NH3}: 21;1 (M+NH4).
Example I 31 B
7-methoxv-H-( 3-oxo- I -i:yc l openten-1-yl )-~-naphthalenecarbox imidamide
mono(trifluoroacetate)(salrl
The material prepared above (130 mg, .4 mmol) is prepared according to the
H2S/pyridine method described in Example 40D. The desired compound was
obtained as an
30 off-white solid after reverse phase chromatography. 52 mg, 45%.
MS (DCI/NH3): M+H+ 28 I
t H NMR (DMSO-d6): 9.45 (bs, 2H); 9.12 (bs, 2H), 8.25 -8.32 (m, 2H), 8.20 (dd,
1 H), 7.86
(d, 1 H), 7.75 (dd, 1 H), 6.42 (m, 1 H), 4.05 (s, 3H), 3.15 (m, 2H), 2.75 (m,
2H)
-145-
SUBSTITUTE SHEET (RUtE 26)
CA 02294300 1999-12-14
WO 99!05096 PCTIUS98/15386
Anal. calc'd for C1yH17N204F3~1.75 TFA: C, 57.87; H, 4.35; N. 7.10. Found: C,
51.37; H,
4.21; N, 7.14.
Example 132
~aminoiminomethyl)-N-(4-methylphenyl)-2-naphthalenecarboxamide
mono(trifluoroacetate)(salt)
Example 132A
p-Toluidine (0.11 g, 1 mmol), and the cyano ester prepared in Example 8E (0.2
g, 1
m mmol) are coupled according to the procedure described in Example RG,
providing an off-white
solid as the desired compound (0.16 g, 56%).
MS (ESl +, -): 287 (M+) ; 285 (M-).
Example 132 B
t s 6-(aminoiminomethyl)-N-(4-methxlphenyl )-2-n~hthalenecarboxamide
mono(trifluoroacetate)(salt~
The desired compound is prepared according to the procedure described in
Example 6B
and purified as described in Example 1 B, yielding a white solid (35 mg, 35%).
MS (ESI +): 304 (M+)
2s~ I HNMR (DMSO-d6j: 10.55 (s, 1 H); 9.45 (bs, 2H); 9.15 (bs, 2H); 8.65 (s, 1
H); 8.58 (s, I H);
R.32 (d, I H), 8.20 (d, 1 H), 8.19 (dd, 1 H); 7.9h (dd, 1 Hj, 7.75 (d, 2H),
7.12 (d, 2H), 2.35
(s, 3Hj;
Anal. calc'd for C2)H1~N3O~F3: C, 60.43; H, 4.35; N, 1().07 Found: C, 59.94;
H, 4.Ofi; N,
9. R(1.
,5
Example 133
methyl 4-1 f 17-(aminoiminomethyi )-1-(~-pvrimidinylamino )-2-naphthalenyl
foxy !methyl 1
benzoate, mono(trifluoroacetate)(salt)
~t> Example 133A
The material described in Example 91 A is treated with All3 according to the
procedure
described in Example I I9A and alkylated with 4-CarbomethoxyBenzylbromide
according to the
procedure described in Example 109B, yielding the desired compound as a white
solid, 100
mg, 8370.
-146-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98/15386
MS (ESI +, -): 41 I (M+) ; 409 (M-)
Example 1338
methyl 4-lff7-(aminoiminomethyl)-1-(2-pyrimidinylamino)-2-naphthalen lv
loxylmet~ll
benzoate, mono(trifluoroacetate)(salt)
The desired compound is prepared according to the procedure described in
Example
40D and purified according to the procedure described in Example 1 19B,
yielding a light yellow
solid, (49 mg, 5()~0).
MS (ESI +, -): 42R (M+); 426 (M-)
lu I H NMR (DMSO-d6): 9.45 (bs, 2H); 9. I S (s, 1 H); 8.97 (bs. 2H); 8.45 (dd.
I H); 8.38 (d,
1 H); 8.15 (d, I H ), 8.09 (d, I H), 7.95 (d, 2H); 7.76 (d, 1 H), 7.68 (dd, 1
H), 7.35 (d, 2H),
6.85 (d, 2H), 5.39 (s, 2H); 3.85 (s, 3H);
Anal. calc'd for C2~H22N505F~: C, 57.67; H, 4.10; N, 12.93 Found: C, 55.34: H,
3.88; N,
I 2.05.
IS
Exam lp a 134
4-1117-(aminoiminomethvll-1-(2-p rimidin_~rlamino)-2-naphthalenylloxyjmethyl~
benzoic acid, mono(trifluoroacetate)(saltl
20 The material prepared in Example 134 (40 mg) is dissolved in 1: 1
THF/water. To the
clear solution is added LiOH~water (9 mg), the resulting clear solution is
stirred 18 hours at
room temperature. The reaction mixture is concentrated to a yellow solid. The
solid is dissolved
in distilled water. acidified to pH 2 with 3 N HCI and stirred 2 hours at room
temperature. The
desired compound is isolated by filtration, dried under vacuum as a yellow
solid. Yield: 39 mg
25 (46~%)
MS (APCI): M+H+: 414
( H NMR (DMSO-dfi): 9.45 (bs, 2H); 9.15 (s, I H); 8.97 (bs, 2H); 8.45 (dd, 1
H); 8.38 (d,
1 H); 8. I S (d, 1 H ), 8.09 (d, 1 H), 7.95 (d, 2H); 7.76 (d, 1 H}, 7.68 (dd.
1 H), 7.35 (d, 2H),
6.85 (d, ZH), 5.39 (s, 2H);
3u Anal. calc'd for C25HZ~~N505F3C1~3 H20: C, 48.67; H, 4.25; N, I 1.35 Found:
C, 49.64; H,
4.44; N, 11.69.
Example 135
-147-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTlUS98115386
I, 1-dimethvlethyl ff4-f((6-laminoiminomethyl)-2-naphathalenyllaminolcarbonyll
phen ll~yllcarbamate, monoltrif(uoroacetate)lsalt)
Example 135A
The material prepared in Example 8B was added to cold (0°C) sulfuric
acid (45 mL).
Within I minutes at 0°C bubling started to form. Allowed to bubble at
0°C for 30 minutes than
slowly warmed to room temperature. Left at room temperature for 20 minutes.
then poured into
ice and diluted with water (to approx. 500 mL). The suspension was placed in
an ice bath and
carefully added a solution of 50% aqueuos sodium hydroxide so that the
temperature would not
I~r exceed 35°C. The light yellow solid was filtered than washed with
water till the wash became
neutral to pH paper (7.0). The product was dried under vacuum. The product was
purified on
silica gel column using 20°lo ethyl acetate 80% hexanes as eluent to
yield 3.3 g (67%) of light
yellow solid.
MS m/z ! f~9 (M+1 )+.
IS
Example 135B
A solution of Example 135A (135 mg, 0.8 mmol), 4-N-Boc-aminomethylbenzoic acid
(404 mg, l .6 mmol) and EDCI (307 mg, 1.6 mmol) in methylene chloride (25 mL),
at room
temperature, was added DMAP (3 mg) and stirred overnight. The reaction mixture
was diluted
2o with methylene chloride (60 mL) then washed 2% aqueous hydrochloric acid
(2x30 mL), water
(20 mL), 0.5 M aqeuos sodium hydroxide (2x50 mL) and brine. The organic phase
was dried
over magnesium sulfate and evapoorated. The product was purified via on silica
column usinga
gradient of 25% to 60% ethyl acetate in hexanes as eluent. Yield 175 mg (54%)
of white
powder.
~S
Example 135C
1, I-dimethvlethvl lf4-(ff6-laminoiminomethyl)-~-
naphathalenyllarninolcarbonvll
ohenyllmethvllcarbamate, mono(trifluoroacetate)lsalt)
The reaction was carried out in the same manner as described in Example 40D.
Yield
3() 1 10 mg (64%).
MS m/z 40R (M+( )+' 425 (M+l li)+
I H NMR: 3.30 (s, 9H), 4.22 (d, 2H, J=7.1 Hz), 7.42 (d, 2H, J=8.5Hz), 7.49 (t,
1 H,
J=7. I Hz), 7.79 (dd, 1 H, J 1=8.2Hz, J2=2.0Hz), 7.95-8.00 (m, 3H), 8.09 (d,
2H, J=H.4Hz),
$.42 (s> 1H), (i.63 (d, 1H, J=2.OHz), 9.lii (br s, 4H), 10.58 (s, 1H);
-148-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98/15386
Anal. c:alc'd for C~~,H2~F3N405: C, 58.55; H, 4.85; N, 10.41. Found: C, 58.64;
H, 5.11; N,
10.52.
Exam 1
S N-f6-laminoiminomethyl)-2-naphthalenyl)benzamide
monoltrifluoroacetate)(salt)
The desired compound is prepared as described in Example 135;
tH NMR (300 MHz, DMSO-d~,j 10.67 (s, IH), 9.25 (br.s, 4H), $.65 (d, J=I.SHz,
1H), 8.43
(d, J=l.4Hz, 1H), 8.10 (d, J=9.2Hz, 2H), 8.03-7.97 (m, 3H), 7.81-7.78 (m, 1H),
7.65-7.55
m (m, 3H).
MS (ESI/NH3) m/z 290 (M+H)+;
Anal. calc'd for C~gH~5N30~CF3COOH: C, 59.55; H, 4.00; F, 14.13; N, 10.42.
Found: C,
50.47; H, 3.88; F, 14.42; N, 10.39.
s Example 137
1, 1-dimethvlethvl ff4-lff6-laminoiminomet~l)-2-naphathalenvllaminolcarbonyll
cvclohexvlllmethvllcarbamate mono(trifluoroacetate)lsaltl
Example 137A
2e To a solution of the 6-Amino-2-naphthalenecarbonitrile prepared in Example
73 ( 100
mg, 0.6 mmol) in methylene chloride (35 mL) at room temperature, was added I-
carboxy-4-
(Boc-aminomethyl)cyclohexanes (2R0 mg, 1.1 mmol) followed by 1-ethyl-3- (3-
dimethylaminopropyt)carbodiimide hydrochloride (EDAC, 225 mg, 1.2 mmol). After
5 minutes
to the reaction mixture was added DMAP (20 mg, c:atalytic:). The reaction was
stirred at room
?~ temperature for 72 hours. The reaction mixture was diluted 7: 3 ethyl
acetate: hexanes then
filtered through silica gel and washed with the name solvent mixture.
The organic solvent was washed by aq. Acid (2% Hcl, 2x50 mL), water and aq.
Base
( 10C/e NaOH, SO mL). The solvent was dried over magnesium sulfate filtered
and evaporated.
Crystalization from ether/ hexanes afforded the product as white solid. Yield
166 mg (68%).
3n MS (DCI/NH3) m/z 40); (M+H)'~.
Example 1 ~7B
l, 1-dimethvlethv) ff4-fff6-(aminoiminometh 1)-2-naphathalenvIjaminolcarbonvll
cvclohexv(llmethvllcarbamate monoltrifluoroacetate~(salt)
-149-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98115386
A solution of the substrate (Example 137A) in Pyr: Et3N ( 1(?: 1, 20 mL) was
bubbled
HZS for 5 minutes. Stirred at room temperature overnight. The reaction mixture
was added
ethyl acetate ( 100 mL) followed by 1 % aq. KHS04 (60 mL) and separated:
washed organic
layer with water (2x50 mL), sodium bicarb. And brine, dried over magnesium
sulfate &
S evaporated. To a suspension of the resulting solid in acetone (25 mL) and
added MeI (1.0 mL).
Stirred at 50°C for 2 hours, all dissolved. Evaporated solvent to a
complete dryness and added
methanol (30 mL) and ammonium acetate ( 150 mg}. The mixture was stirred at
room
temperature overnight. Purification by Reverse Phase Cig MPLC. After
evaporation added
toluene & evaporated (2x40 mL). The resulting oil was treated with methanol
and ether and the
U product precipitated as white solid (72 mg, 43%).
MS (ESI/NH3) m/z 425 (M+H)+;
~H NMR (300 MHz, DMSO-d~,) 1().24 (s, IH), 9.05 (br.s, 4H), 8.49 (s, IH), 8.38
(d,
J=l.7Hz, 1 H), 8.03-8.00 (m, 2H), 7.77-7.74 (m, 2H), 6.93-6.91 (m, I H), 2.83-
2.79 (m,
2H), 2.4t)-2.30 (ml), 1.92-1.75 (m, 4H), 1.50-1.45 (m, 1H), 1.39 (s, 9H), 0.96-
0.91 (m,
S 4H);
Anal. valc'd for Cz4H;;~N.~03~CF3COOH: C, 57.98; H, 6.18; N, 1().40. Found: C,
57.63; H,
6.24; N, 10.21.
Example 138
20 N-16-(aminoiminomethvl)-?-naphthalenyll-N'-f4-aminopheny~urea
bis(trifluoroacetate)(salt)
Example 138A
To a solution of the compound prepared in Example 40B (194.2 mg, 1 mmol) in
dioxane ( 1 () mL) at room temperature, was added 4-phenylenediamine mono Boc
(416, 5 mg, 2
25 mmol). The product started to precipitate within minutes. After an hour the
reaction mixture was
added ether (5 mL) and the white solid product was filtered and washed with
ether to yield 350
mg (87C/c).
MS (DCI/NH~) m/z 403 (M+H)+.
> Exam_ple 138B
A solution of the corresponding nitrile prepared in Example 138A (105 mg, 0.36
mmolj
in Pyr: Et3N ( 10: 1, 20 mL) was bubbled H2S for 5 minutes and stirred at room
temperature
overnight. The reaction mixture was diluted with ethyl acetate ( 100 mL)
washed with aqueous
0.25 N HCl (25 mL) followed by water (2x50 mLj, saturated solution of sodium
bicarbonate,
-150-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98/15386
and brine, dried over magnesium sulfate & evaporated. To a solution of the
resulting solid in
acetone (25 rnL) was added MeI ( 1.() mL)and stirred at 50°C for 30
minutes- very strong
precipitate was observed. Added ether and filtered the yellow precipitated.
The yellow solid was
added methanol ( 10 mL} and ammonium acetate ( 150 mg) and stirred at room
temperature
S overnight. Purified as described inExample 1B. Yield of the white solid 69
mg.
MS (DCIINH3) m/z 420 (M+H)+.
Example I38C
N-16-laminoiminomethvl)-2-naphthalenyll-N'-(4-aminophenyl)urea
bisltrifluoroacetate)(salt)
lU The Boc protected substrate (Example 138B) was dissolved in methylene
chloride: T'FA
1: 1 (25 mL) and stirred at room temperature forl hour. Evaporated solvent
under vacuum
added toluene & evaporated again. Added water & little acetonitrile, filtered
& lyophilized. 36
mg of white solid.
MS (ESI/NH3) m/z 32() (M+H)+;
l5 ~ 1-1 NMR (300 MHz, DMSO-d~,} 9.26 (br.s, 2H), 9.2 I (br.s, 1 H), 8.85
(br.s, 2H), 8.31 (d,
J=l.7Hz. 1H), 8.18 (d, J=l.7Hz, IH}, 7.95-7.91 (m, 2H), 7.68 (dd, J1=fi.6Hz,
J2=2.0Hz,
1 H), 7.57 (dd, J I=9.2Hz, J2=l.4Hz, 1 H), 7.34 (d, J=8.8Hz, 21-i), 6.91 (d,
J=8.4Hz, 2H};
Anal. catc'd for C~gHI~N50~2~CF3COOH~H20: C, 46.73: H, 3.74; N, 12.39. Found:
C, C,
47.03: H, 3.55: N, 12.36.
2U
Example 139
N-16-laminoiminomethvl)-2-naphthalenvll-4-4-laminometh~ c~exanescarboxamide
bisltrifluoroacetate)(salt,}
25 A solution of the substrate prepared in Example 137 as TFA salt (45 mg) in
methylene
chloride: TFA 1: 1 (20 mL) was stirred at room temperature for 1 hour. The
solvent was
evaporated under vacuum, added toluene & evaporated (20 mLx2). Dissolved in
water, filtered-
0.45~ frit and lyophilized. 35 mg, white solid as bis TFA salt.
MS (ESI/NH~) m/z 325 (M+H)+;
30 ~H NMR (300 MHz, DMSO-d~,) 10.31 (s, 1H), 9.31 (br.s, 2H), 9.10 (br.s, 2H},
8.49 (s,
IH), 8.39 (s, 1H), 8.04-8.00 (m, 2H), 7.78-7.71 (m, 2H), 2.71 (d, J=7.OHz,
2H), 2.44-2.36
(m, 1H)1.96-1.85 (m, 4H), 1.61-1.42 (m, 3H), 1.()9-1.02 (m, 2H);
Anal. calc'd for C~yH~4N40~2~CF3COOH: C, 50.00; H, 4.74; N, 10.14. Found: C,
49.95;
H, 4.70; N, 09.96.
-151-
SU8ST1TUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98/15386
Exam l
N-f6-(aminoiminometh ly )-2-naphthalenvll-i'd'-f(4-aminomethyl) henyl urea
bis(trifluoroacetate)(salt~
s
Ex~m~le 140A
To a solution of the isocyanate prepared in Example 40B ( 140 mg, 0.72 mmol)
in
dioxane (8.0 mL) at room temperature, was added 4-N-Boc-aminomethylbenzoic
acid (320 mg,
1.44 mmol) and stirred for I hour. The product was precipitating during the
reaction. The
W mixture diluted with ether (15 mL), filtered and washed with ether to yield
215 mg {72%) of
white solid.
MS (DCI/NH3) m/z 417 (M+H)+.
Example 140B
n A solution of the nitrile (Example 14()A) ( 198 mg, (1.47 mmol) in 10: 1
pyridine:
triethylamine ( 10 ml.,) was treated with H2S for 5 min, stirred at room
temperature for I S h and
concentrated. The resulting solid was dissolved in acetone (15 mLj, treated
with iodomethane
(0.1i mL, 12.H mmol), stirred for 2 hours, diluted with ether ( 10 mL),
filtered, washed with
ether and dried under vacuum. The resulting solid was dissolved in methanol (4
mL) and was
?U added NH40Ac (200 mg, 2.6 mmol) at room temperature overnight. The product
was purified
according to the procedure described in Example IB to provide 112 mg (54%) of
the
corresponding amidine.
MS (DCI/NH3) m/z 434 (M+H)+.
Example 140C
N-ffi-(aminoiminomethyl)-~-naphthalenyll-N'-f (4-aminomethyl)~henyl lurea
bis(trifluoroacetate)(salt)
A solution of the substrate (Example 140B) in methylene chloride: TFA 1: 1 (20
mL)
was stirred at room temperature for 1 hour. The solvent was evaporated under
vacuum, added
3t) toluene & evaporated (20 mLx2). Dissolved in water, filtered- 0.45, frit
and lyophilized. 38.1
mg, white solid.
MS (ESi/NH3) m/z 334 (M+H}+;
t H NMR (300 MHz, DMSO-d6} 9.68 (s, 1 H), 9.45 (s, 1 H), 9.35 (br.s, 2H), 9.08
(br. s,
2H), 8.40 (d, J=l.7Hz, 1H), 8.31 (d, J=l.BHz, 1H), 8.10 (br.s, 3H), 8.04-7.99
(m, 2H),
-152-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98/15386
7.76 (dd, Jl=8.8Hz, J2=l.BHz, 1H), 7.67 (dd, J1=8.8Hz, J2=l.7Hz, IH), 7.58 (d,
J=8.4Hz, 2H), 7.39 (d, J=8.4Hz, 2H), 3.98 (br. s, 2H);
Anal. calc'd for ClyH~gN5012~CF3COOH~H20: C, 47.67; H, 4.00; F, 19.67; N,
12.09.
Found: C, 47.33; H, 3.70; F, 19.59; N, 11.71.
Example 141
methvl 17-(aminoiminomethvil-3-f f f4-laminometh~lDhenyllaminolcarbonyl~ l
~,vhathalenvllcarbamate bis(trifluoroacetate)(salt)
Example l 41 A
To a solution of the ester, prepared as described in Example 26, (747 mg, 2.63
mmol)
in dioxane ( 10 mL) was added acetone ( 1 mL) and an excess of sodium
hydroxide ( I N in
water, 10 mL). After 1 hour the mixture was added water (40 mL) and ethyl
acetate (85 mL)
then was acidified with lU~7c aq. HCI. The ethyl acetate layer was separa~ed
washed with water
(2x2() mL) then brine, dried over magnesium sulfate filtered and evaporated.
The product was
isolated as a light yellow solid.
MS m/z 271 (M+1 )t.
Example 141 B
To a suspension of naphthoic acid derivative, prepared in Example 141 A (270
mg, I .1
mmol) in methylene chloride (8.0 mL) was added diisopropylethylamine (DIEA,
485~.L, 2.8
mmol) followed by O- (azabenzotriazole-I-yl)-N, N, N', N'-
tetramethyluroniumhexafiuorophosphate (HATU, 527 mg, 1.39 mmol). After 10
minutes
added the 4-N-Boc-aminomethylaniline (370 mg, 1.7 mmol). After an hour, added
ethyl acetate
~s ( 120 mL) and washed organic: layer with 5~/c aq. citric acid (50 mL),
water (2x4() mL) and brine
(50 mL). The rxn was dried over magnesium sulfate filtered through small
amount of silica and
evaporated. Purification on silica eluting with ethyl acetate in hexanes. The
product, after
concentration, was added ethyl acetate and ether and filtered to yield 350.0
mg (7010) of yellow
solid.
MS m/z 447 (M+I )+.
Example 141 C
A suspension of the naph derivative (Example 141B) (300 mg, 0.67 mmol) in
ethyl
acetate (20 mL) was added 120 mg of the Pd catalysit then stirred at room
temperature, under
-153-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98/15386
hydrogen, at atmospheric pressure and stirred for I hour.. The crude was
carried on to the next
step without any purifications or analysis.
To a solution of the crude amine in dioxane {25 mL) and 10% aqeuos sodium
carbonate
(2.5 mL) was added the methyl chlorofotmate { I .0 rnL, large excess). After 2
hours the rxn
S was quenched with methanol then diluted with ethyl acetate (80 mL) and water
(50 mL). The
ethyl acetate Iayer was separated, dried over magnesium sulfate filtered and
evaporated. The
product was separated on silica column using a gradient of ethyl acetate in
hexanes (from 5 to
30% ethyl acetate in hexanes). Yield 140 mg of off white solid.
MS m/z 492 (M+18)+'
Example 141 D
The desired compound was prepared as described in Example 26.
M S m/z 492 (M+ 1 +.
Example 141 E
methvl f7-(aminoiminomethvl)-~-f114-(aminomethyl~phe~llaminolcarbonyll-1
naphathalenvllcarbamate bis(trifluoroacetate)(calt)
A solution of the substrate (Example 141 D) in a mixture of methylene
chloride: TFA 4:
1 (20 rnL) was stirred at room temperature for 15 minutes. The solvent was
concentrated under
2c) vacuum, and separated on RP C 18 MPLC. Yield, 21 mg off white solid.
MS m/z 392 (M+18')+
~ H NMR (DMSO): 3.783 (s, 3H), 4.03 (q, 2H, J=S.SHz), 7.47 (d, 2H, j=8.4Hz),
7.85 (d,
2H, j=8.4Hz), 7.94 (d, 1 H, j=8.8Hz), 8.15 (wide s, 2H), 8.31 (d, 1 H,
j=8.8Hz), 8.33 (s,
1 H). R.47 (s, I H). H.74 (s, 1 H), 9.29 (s, 2H), 9.47 (s, 2H), 9.90 (s, I H),
10.68 (s, 11-i).
~s Anal. calc'd for C25H25F~,N5O~ (base molecule + 2 TFA+1 H20): C. 47.04; H,
3.70; N,
10.52. Found: C, 47.1(): H, 3.95; N, 10.99.
Example 142
fi-(aminoiminomethvl)-N-(4-ethvlphenyl)-~-na~hthalenecarboramide acetate(salt)
3« Example 142A
The reaction was carried out in the same manner as described in Example 141 B.
Yield
374 mg (61 %) of white powder.
MS DCI/NH3): m/z 301 (M+NH4+).
-154-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98115386
Example 142B
6-(aminoiminomethvl)-N-(4-eth~~henyl)-2-naphthalenecarboxamide acetatelsalt)
The reaction was earned out in the same manner as described for the naphthyl
analog
prepared in Example 141 D, 313 mg. The solid that precipitated during the last
step was filtered
s and washed in ether to yield 71 mg ( 18%) of white solid, as acetate salt.
MS (ECI) m/z 301 (M+H)+;
~H NMR (300 MHz, DMSO-d6) 8 1.19 (t, J=7.4Hz, 3H), 2.60 (q, J=7.4Hz, 2H), 7.22
(d,
J=8.5Hz, 2H}, 7.72 (d, J=B.SHz, 2H), 7.94 (dd, JI=8.8Hz, JZ=l.7Hz, 1H), 8.08-
8.23 (m,
3H), 8.47 (d, J=I .7Hz, 1 H), 8.63 (s, 1 H), 10.43 (br. s, 1 H);
lU Anal. calc'd for C2~~H19N30~AcOH~ 0.5 H20: C, 68.38; H, 6.26; N, 10.87.
Found: C,
611.56; H, 6.21; N, 10.67.
Example 143
fi~aminoiminomethyl)-N-(2-naphthalenyl)-2-n~hthalenecarboxamide
monoltrifluoroacetate)(salt)
Example 143A
To a solution of the acid chloride prepared as described in Example KB (341
mg, 1.6
mmol) in methylene chloride (20 mL) was added a solution of the '?-Amino
naphthalene (249
2O mg, 1.74 mmol) in methylene chloride ( 10 mL) and propylene oxide ( 12 mL).
The rxn was
stirred at room temperature overnight. The reaction mixture was added ether
and the product
was filtered, washed with ether and hexanes and dried under vacuum. Yield 440
mg (86°~0).
MS (DCI/NH3) m/z 340 (M+NH4)+.
'-5 Example 143B
6-(aminoiminomethyl)-N-(2-naphthafenyl)-2-naphthalenecarboramide
mono(trifluoroacetate)(sal~
The desired compound is prepared as described in Example 141 B. Yield of white
solid
40 mg ( 10°l0).
30 MS (ESI) m/z 340 (M+H)+;
1H NMR (300 MHz, DMSO-d~) 7.43-7.55 (m, 2H), 7.86-7.96 (m, 5H), 8.20-8.28 (m,
2H),
H.()X-8.23 (m, 3H}, H.34 (d, J=8.8Hz, IH), H.50 (d, J=l.7Hz, IH}, 8.57 (d,
J=l.3Hz, 1H),
X.75 (s, 1 H}, 9.33 (br. s, 4H) 10.75 (s, I H);
-155-
SUBSTtTUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98115386
Anal. calc'd for C22H»N30~TFA~ 0.25 H20: C, 62.95: H, 4.07; N, 9.18. Found: C,
63.09;
H, 3.72; N, 8.99.
Example 144
S 6-l5-nheny!-2-oxazolvl)-2-naphthalenecarboximidamide
mono(triftuoroacetate)(salt)
Example 144A
To a suspension of the phenacyl amine hydrochloride (Aldrich) (415 mg, 2.42
mmol) in
a mixrure of methylene chloride (50 mL) and propylene oxide ( I 5 mL) at room
temperature was
to added a solution of the 2-nitrite-6-acidchloride (56() mg, 2.6 mmolj in
methylene chloride (30
mL) followed by DMF {3.0 mL). Thje reaction was stirred at room temperature
overnight. The
reaction mixture was added ether ( i 5 mL) and the product was filtered and
washed with
hexanes to yield 555 mg (73~1c) of white solid.
MS (DCI/NH3) m/z 3I5 (M+H)+.
1S
Examt3le 144B
A suspension of the substrate (Example 144A) (354 mg, 1.12 mmol) in
phosphorous
oxychloride (3.5 mL) was boiled for 1.5 hours. The reaction mixture was poured
into ice and
the mixture was added ethyl acetate (80 mL) and aqeuous solution of 10%
potassium carbonate
2e) ( l00 rnL). The organic layer was separated, washed with brine dried over
magnesium sulfate
and evaporated. Added ether and filtered 249 mg (75%).
MS (DCL/NH3) tn/z 297 (M+H)+.
Examvle 144C
25 fi-(5-yhenvl-2-oxazolvl)-2-naphthalenecarboximidamide
mono(trifluoroacetate)(salt~
To a solution of the substrate Example 1448 ( 132 mg, 0.44 mmol) in THF (20
mL) at
room temperature was added a solution of 1 N LiHMDS in hexanes ( 1.5 mL, 1.5
mmol j and
stirred overnight. Quenched with 4 N HCl in dioxane ( 1 mL). After 10 minutes
added a few
drops of water and stirred for additional 30 minutes. The solvent was
evaporated under vacuum
3p and the residue was purified on reverse phase chromatography. Yield 58 mg
of white solid
41 %).
MS (ESI/NH3) m/z 314 (M+H)+;
IH NMR (300 MHz, DMSO-d~) 9.50 (s, 2Hj, 9.19 (s, 2H), 8.86 (s, IH), 8.55 (s,
1H), 8.38-
8.26 (m, 3H), 7.98 (s, 1H), 7.96-7.89 (m, 3H), 7.58-7.54 (m, 2H), 7.47-7.42
(m, 1H);
-I56-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98115386
Anal. calc'd for C2~~H15N30~ 1.15CF3COOH: C, 60.26: H, 3.66: N, 9.45: F,
14.75. Found:
C, 60.1 I ; H, 3.H 1: N, 9.20; F, 14.81.
Exam lp a 145
s 6-(5-phenyl-2-thiazolyl)-2-naphthalenecarboximidamide
mono(trifluoroacetate)(salt)
Example 145A
A suspension of the substrate, prepared in Example 144A, (340 mg, 1.1 mmol)
and
Lawesson's reagent (60U mg, 1.48 mmol) was heated to R5°C for 4Shours.
Solvent was
m evaporated to dryness and the product was isolated via silica column using
10070 ethyl acetate in
hexanes as eluent. Yield: 200.0 mg (59%) of yellow solid.
MS (DC1/NH3) m/z 313 (M+H)+.
Exa ale 145B
t5 6-(5-phenyl-2-thiazolvl)-2-naphthalenecarboximidamide
mono(trifluoroacetatel(salt)
To a solution of the substrate, Example I45A (! HO mg, O.SH mmol) in THF (20
mL) at
room
temperature was added a solution of 1 N LiHMDS in hexanes (2.0 mL, 2.0 mmol)
and
stirred overnight. Quenched with 4 N HCl in dioxane ( 1 mL). After 10 minutes
added a few
20 drops of water and stirred for additional 30 minutes. The solvent was
evaporated under vacuum
and the residue was purified on MPLC RPC18. Yield 36 mg (19%) of white solid.
MS (ESI/NH~) m/z 330 (M+H)+;
~ H NMR (300 MHz, DMSO-d~,j 9.49 (s, 2H), 9.14 (s, 2H), X.71 (s, 1 H), k.52
(s, 1 H), 8.47
(s, 1H), x.35-H.22 (m, 3H), 7.90-7.7H (m, 3H), 7.55-7.50 (m, 2H), 7.46-7.43
(m, IH);
'_, Anal. calc'd for C2«H15N3S~CF3COOH~H~O: C, 57.26; H, 3.93; N, 9.11. Found:
C, 56.H3;
H, 3.55; N, X.79.
Example 146
6-(aminoiminomethyl)-4-(3-furanyl)-N-(2-pyridinyl)-~-naphthalenecarboxamide
3t) dihydrochIoride
Example I46A
The above product was prepared in the manner of Example 1 14A.
MS (ESI) m/z (M+H)+ 340.
-157-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98/15386
Example 1468
6-(aminoiminometh ly )-4-f3-furanyi -L(2-p~linvl)-2-naphthalenecarboxamide
dihvdrochloride
The above was prepared from Example 146A using method described in 1458.
MS (CI) m/z (M+H)+ 357;
I H-NMR (3UU MHz, DMSO-d6) 8 I 1.27 (s, I H), 9.56 (s, 2H), 9.17 (s, 2H), 8.76
(s, I H),
8.63 (s, 1 H), 8.45-8.24 (m, SH}, 7.96-7.89 (m, 3H), 7.25 (m, 2H), 7.18 (s, 1
H):
Anal. calc'd for CZ I H ~ gN402C12 19/ 10 HCI: C, 54.33 H, 4.75 N, 12.()7.
Found: C, 54.89;
1 H, 5.28; N, 9.81.
Exam 11~ a 147
6-(aminoiminomethvl)-N-( 1. 2. 3_ 4-tetrahydro-6-c~uinolinyl)-2-
n~hthalenecarboxamide~
bis(triff uoroacetate~salt}
IS
Examlale I47A
The reaction was carried out in exactly the same manner as described for
Example I l8A
using 6-aminoquinoline to yield the product in 72~~c. Mass spectrum (CI+) 324
(M+I )+.
21~ Example 1478
The reaction was carried out in exactly the same manner as described for
Example 1188
to yield the product in 45~% (off white solid). Mass spectrum (ESI+) 341
(M+I}+.
Example 147C
Z, 6-(aminoiminomethvl)-N-( 1. ?, 3, 4-tetrahydro-f~-duinolin~)-~-
naphthalenecarboYamide
bis(trifluoroacetate)(salt)
To a Suspension of the substrate, Example 1478 (261 mg, U.6 mmol) in degassed
methanol ( 15 mL) was added platinum oxide (IO mg, cat.). The reaction mixture
was charged
with hydrogen at atmospheric pressure and stirred vigorously overnight. The
next day the
3e) solution was filtered through celite to remove the catalyst, and the
product was purified over
rnplc with RPC I 8 silica using methanol (+ (). I ~/o TFA) and water (+ 0. I
ale TFA) as eluent.
Yield 122 mg of white solid and bis TFA salt.
MS (ESI+) 345 (M+I)+;
-158-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98/15386
IH NMR (DMSO-d6) 10.51 (s, 1H), 9.65 (s, 2H), 9.50 (s, ?H), 8.64 (s, IH), 8.52
(s, 1H),
8.22 (d. J=B.OHz, 1 H), 8.12 (Abq, J=9.OHz, 2H), 7.86 (d, J=9.0Hz, 1 H), 7.61
(s, I H),
7.56 (d, J=8.0Hz, 1H), 6.98 (d, J=B.SHz, IH), 3.28 (t, J=S.SHz. 2H), 2.75 (t,
J=6.3Hz,
2H), I .92-1.86 (m, 2H);
S Anal. calc'd for C21H20N40+2.25 TFA+().25 H20: C, 50.59 (50.46); H, 3.79
(3.79); N,
9.25 (9.25); F, 21.18 (20.83).
Example 148
7-methoxy-8-(pyrazinyloxy)-2-naphthalenecarboximidamide
dimethanesuIfonate(salt)
Example 148A
The product from Example 4A ( 125 mg, 0.627 mmol) was combined with
chloropyrazine ( 1 12 mL, I.25 mmol) and Cs2C03 (409 mg, 1.25 mmol) in N-
methylpyrrolidinone (4 mL), and the reaction was stirred at 130 °C for
1 hour. The reaction was
I5 pooled, and the crude mixture was chromatographed on Si0? using 409e ethyl
acetate/hexanes
as eluent, to yield 75 mg (43%) of the desired compound.
MS (DCI (NHS) m/z 2711 (M+H)+.
Example 148B
2U 7-methoxv-8-(pvrazinvloxv)-2-naphthalenecarboximidamide
dimethanesulfonate(salt)
The product from Example 148A (70 mg, 0.252 mmol) was subjected to the
procedure
described in Example 1B to yield the desired compound (106 mg, 71~7c). m.p.
155° C.
MS (DCI (NH3) m/z 295 (M+H)+;
~H NMR (300 MHz, DMSO) b 9.42 (br s, 2H), 9.()4 (br s, 2H), 8.72 (s, 1H), 8.38
(d, J=3
?s Hz, 1 H), 8.36 (m. 1 H), 8.21 (d. J=9 Hz, 1 H ), 8.09 (m, 1 H). 8.06 (d,
J=9 Hz, 1 H), 7.82 (d>
J=9 Hz, I f-I), 7.73 (dd, J=9, 2 Hz, 1 H), 3.83 (s, 3H), 2.38 (s, 3H);
Anal. calc'd for C~~H22N4S20~' 1.1 CH4S03: C, 38.74; H, 4.49; N, 9.46. Found:
C, 38.68;
H, 4.53; N, 9.34.
3o Example 149
7-methoxv-H-(phenylthio)-2-naphthalenecarboximidamide methanesulfonate
Example 149A
-159-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98/15386
To a solution of NaH (48 mg, 60%, 1.2 mmol) in HMPA (2 mLj was added PhSH
(0.133 mL, 1.3 mmol), and the reaction was stirred for 5 minutes. To this was
added the
product from Example 53B (309 mg, 1 mmol), and the reaction was heated at
100°C for 3
hours. The crude reaction mixture was chromatographed on Si02 using 20% ethyl
acetate/hexanes as eluent. The product was taken up in ethyl acetate { 10 mL)
and methanol ( 10
mL), and treated with a 2 M solution of TMSCHN2 (10 mL). The reaction was
stirred for 60
minutes and condensed. The crude product was chromatographed on Si02 using 15%
ethyl
acetate/hexanes as eluent, to yield 1 I5 mg (39%) of the desired compound:
MS {DCI (NH3) m/z 309 (M+NH4)+.
»3
Example 149B
7-methoxv-8-(nhenvlthio)-2-naphthalenecarboximidamide rnethanesulfonate
The desired compound was prepared from Example 149A and the procedure of
Example
SSD.
l5 MS (DCl/NH3) rn/z 309 (M+H)+;
~ H NMR (300 MHz, DMSO-d~,) 8 2.33 (s, 3H), 3.96 (s, 3H), 6.96 (d, 2H), 7.1 1
(dd, I H),
7.20 (d, l H), 7.22 (d, 1 H), 7.69 (dd, 1 H), 7.112 (d, 1 H ), 8.22 (d. 1 H),
8.33 (d, 1 H), 8.81 (s,
1 H), 9.() I (br s, 2H), 9.46 (br s, 2H);
Anal. calc'd for C«HI~,N~OS~1.15 CH4S03: C, 54.91; H, 4.96; N, 6.69. Found: C,
54.70;
2c> H, 5.15; N, 6.58.
Example 150
7-methoxv-~-(nvrazinvlamino)-2-naphthalenecarboximidamide
bis(trifluoroacetate)(salt)
''S The desired compound was prepared from the product prepared in Example 90D
using
the method described in Example 91 A, then converted to the amidine as
described in Example
40D.
MS (DCI/NH3) m/z (M+H)+ 294;
~ H-NMR (300 MHz, DMSO-d~,) 8 9.39 (s, 2H), 9.02 (s, 2H), 8.95 (s, 1 H), 8.40
(d, 1 H),
30 8.14 (d, 1 H), 8.03 (s, 2H), 7.92 (dd, I H), 7.84 (d, I H), 7.76 (d, 1 H)
7.66 (dd 1 H), 3.90 (s,
3H);
Anal. calc'd for C~pHi~N505Fr; I/2 TFA: C, 43.79; H, 3.07; N, 12.20. Found: C;
43.81; H,
3.22; N, 12.24.
-160-
SUBSTtTUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98/15386
Example 152
6-(aminoiminomethvl)-4-(3-furanvl)-N-phen 1-2-nanhthalenecarboxamide
monohydrochloride
Examlale 152A
To a solution of the product from Example 8D (5.50 g, 26.04 mmol) in CH2CI2 {
125
mL) was added dibromodimethylhydantoin (4.47 g, 15.62 mmol) and
trifluoromethanesulfonic
acid (2.5I mL, 2H.41 mmol), and the reaction was stirred at 23°C in the
dark for 18 hours. The
mixture was poured into aqueous NaHS03. the solution was made basic with
Na2C03, and
extracted with 3x ethyl acetate, and the extracts were washed with brine,
dried over Na2S04,
to and condensed. The product was recrystallized From ethanol/ethyl acetate,
to yield 5.~0 g (77%)
of the desired compound.
MS (DCI (NH3) m/z 307 (M+NH4)+.
Examlale 152B
t5 To a solution of the product from Example 152A (1.4U g, 4.H26 mmol) in THF
(40
mL), water (10 mL), and methanol (10 mL) was added LiOH~water (4()5 mg, 9.65
mmol), and
the reaction was stirred for 18 hours. The mixture was poured into 1 M HCI and
extracted with
3x ethyl acetate, and the extracts were washed with brine, dried over Na2S04,
and i;ondensed,
to yield 1.23 g (92%) of the desired compound.
20 MS (DCI (NH3)) m/z 295 (M+NI-14)+.
Example 152C
To a solution of the product from Example 152B (440 mg, 1.6() mmol), in
toluene (25
mL) was added oxalyl Chloride (0.140 mL, 1.6 mmol), and the reaction was
stirred at KO °C for
25 1?( hours. The toluene was boiled off until HC1 evolution ceased, and the
reaction was then
cooled. Aniline ( 1 mL, 1 1 mmol) was added. and the reaction was stirred for
10 min, nad
poured into 1 M HCI. The solution was extracted with 3x ethyl acetate, and the
extracts were
washed with brine, dried over Na2S04, and condensed, to yield 560 mg (99%) of
the desired
compound.
3U MS (DCI (NH3)) m/z 370 (M+NH4)+.
Example 152D
-161-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98/15386
The desired compound was prepared from Example 152C (408 mg, 1.16 mmol), furan-
3-boronic acid (182 mg, 1.62 mmol) and the procedure of Example 578, to yield
220 mg
(56%) of the desired compound.
MS (DCI (NH3)) m/z 356 (M+NH4)+.
Example 152E
6-(aminoiminomethyl)-4-f3-furanyl)-N-phenyl-2-nanhthalenecarboxamide
monoh,Lochloride
The desired compound was prepared from Example 152D and the procedure of
Example
40D.
W MS (DCI/NH3) m/z 356 (M+H)+;
~H NMR (300 MHz, DMSO-d~) 8 7.15 (m, 2H), 7.41 (dd, 2H), 7.83 (d, 2Hj, 7.91
(d, 1H),
7.99 (dd, 1 H), 8. I 9 (d, 1 H), 8.38 (s, 1 H), 8.41 (d, 1 H), 8.62 (s, 1 H),
8.69 (s, 1 H), 9.15 (br
s, 2H), 9.55 (br s, 2H);
Anal. calc'd for C22H~~N30y2.75 HC1: C, 57.99; H, 4.37; N, 9.22. Found: C,
57.85; H,
IS 4.84: N. 9.44.
Example 153
6-(aminoiminomethvl)-4-I 1-(methylsulfonyll-1 H=pvrazol-4-yll-N~hen
nanhthalenecarboxamide monohydrochloride
Example 153A
The desired compound was prepared from the product from Example 53D and the
product from Example 152A by the procedure of Example 47A.
MS (DCI/NH~) m/z 4()8 (M+H)+.
,;
Example 153B
The desired compound was prepared from the product from Example 153A, by the
procedure of Example 53F.
MS (DCI/NH3) m/z 278 (M+H)+.
3t1
Example 1530
The desired compound was prepared from the product from Example 153B, by the
procedure of Example 87A (86A).
MS (DCI/NH3) m/z 373 (M+NH4)+.
-162-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98115386
Example 153D
The desired compound was prepared from the product from Example 153C, by the
procedures of Example s 152B and 152C
MS (DCI/NH3} m/z 356 (M-S02Me+NH4)+.
Example 153E
6-(aminoiminomethyl)-4-fl-(meth ls~n l~l-IH-p~rrazol-4-vll-N-phenyl-2
naphthalenecarboxamide. monohydrochloride
1o The desired compound was prepared from Example 153D and the procedure of
Example
4UD.
MS (DCI/NH3) NONE ;
IH NMR (300 MHz, DMSO-d~) 8 3.99 (s, 3H), 7.14 (t, 1H), 7.40 (t. ZH), 7.54 (d,
2H),
7.91 (d, 1 H), 5.05 (s, 1 H), 5.15 (d, I H), 5.35 (m. 2H), X.65 (br s, 2H),
9.33 (br s, 2H),
9.fi 1 (br s, 2H), 10.56 (s, 1 H);
Anal. calc'd for C22HtyN5S03~2.75 HCI: C, 49.51; H, 4.11; N, 13.12. Found: C,
49.44; H,
3.53; N, 12.09.
Exat~ple 154
2U 6-(aminoiminomethvl)-4-jS-(methylthio)-3-furanvlll-N=phenyl-~-
nanhthalenecarboxamide
mono~rdrochloride
Example 154A
To a solution of 2-trimethylsilyl-3-bromofuran (4.17 g, 19.03 mmol) in THF (40
mL)
?S at -78°C was added a 1.5 M solution of LDA (12.7 mL, 19.03 mmol),
and the reaction was
stirred at -78°C for 1 hour. Methyl disulfide (1.89 mL, 20.93 mmol} was
then added, and the
reaction was allowed to warm to room temperature overnight. The reaction was
poured into
saturated aqueous NH4Cl solution, and extracted with 3x diethyl ether. The
combined extracts
were washed with brine, dried over Na2S04, and condensed. The crude material
was
30 chromatographed on Si02 using hexanes as eluent, to yield 3.02 g (60%) of
the desired
compound.
MS (DCI/NH3) m/z 265, 267 (M+H)+.
Example 154B
-163-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98115386
A solution of the product from Example I54A (2.68 g, 10.1 mmol) and a I M
solution
of TBAF (20.2 mL} in THF (20 mL) was stirred for 30 minutes. The reaction was
condensed
and The desired compound was chromatographed on Si02 using hexanes as eluent,
to yield
1.39 g (71 %) of 2-methylthio-4-bromofuran. This material was taken on
directly to the next
step. To a solution of this product (7 mmol) in THF (25 mL) at -78°C
was added a 2.5 M
solution of BuLi (2.fi mL. 7 mmol), and the reaction was stirred for 5
minutes. Bu3SnCl (1.90
mL, 7 mmol} was then added, the reaction was stirred for 30 min, and then
allowed to warm to
room temperature. The reaction was poured into saturated aqueous NH4C1
solution, and
extracted with 3x diethyl ether. The combined extracts were washed with brine,
dried over
1U Na2S04, and condensed. The crude material was chromatog~raphed on Si02
using hexanes as
eluent, to yield 1.24 g (30%) of the desired compound.
MS (DCI/NH3) m/z 404 (M+H)+.
Exam le 154
A solution of the product from Example 154B (920 mg, 2.28 mmol), the product
from
Example 152A (662 mg, 2.28 mmol}, and PdCl2 (PPh3)2 ( I61 mg, 0.23 mmol) in
CH3CN ( 15
mL) was stirred for 18 hours at 80°C. The reaction was condensed and
the crude material was
chromatographed on Si02 using 20% ethyl acetate/hexanes as eluent, to yield
671 mg (91 °lo) of
the desired compound.
2U MS (DCl/NH3) m/z 324 (M+H)+.
Example 154D
The desired compound was prepared from the product from Example 154C, by the
procedure of Example 152 B.
MS (DCI/NH3) m/z 310 (M+H)+.
Example 154E
The desired compound was prepared from the product from Example 154D, by the
procedure of Example 152C.
3u MS (DCI/NH3) m/z 402 (M+NH4)+.
Example 154F
6-(aminoiminomethvl)-4-15-(methvlthio)-3-furan 11~N-_phenyl-2-
naphthalenecarboxamide
mono~drochloride
-164-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98/15386
The desired compound was prepared from Example 1 S4E and the procedure of
Example
144C.
MS (DCI/NH3) m/z 402 (M+H)+;
1 H NMR (300 MHz, DMSO-d6) 8 2.54 (s, 3H), 7.15 (t, 1 H), 7.24 (s, I H), 7.40
(t, 2H), 7.84
(d, 2H), 7.92 (dd, 1H), 8.19 (d, 1H), 8.39 (d, 1H), 8.44 (s, 1H), 8.61 (s,
iH), 8.69 (s, IH),
9.35 (br s, 4H), 1().61 (s, 1H);
Anal. calc'd for C23H19N3S02~2.25 HCI: C, 57.14; H, 4.43; N, 8.69. Found: C,
57.13; H,
4.21; N, 8.56.
Exam lp a 15S
methyl f7-(aminoiminomethyl)-I-naphthalenvllmeth~rlcarbamate
mono(trifluoroacetatel (salt)
The desired compound is prepared according to the procedures described in
Example s
12 and 13. Yield: 40 mg (42~%) of white solid
t5 MS m/z: 258 (M+H)+
~H NMR (DMSO, 300 MHz): 3.28 (s, 3H), 3.82 (br, 3H), 7.66 (dd, 1 H, Jl=7.5 Hz,
J2=1.4
Hz), 7.78 (m, 1 H), 8. 89 (Dd, I H, J 1=8.8 Hz, J2=2.0 Hz), 8.05 (d, I H, 8.1
Hz), 8.24 (d, I
H, 8.8 Hz), 8.30 (s, 1 H), 9.09 (s, 2H), 9.52 (s, 2H);
Anal. calc'd for C14H15N302. 1.25 C2F302H~0.25 H20: C, 49.02; H, 4.18; N,
10.39.
Found: C, 48.81; H, 3.91; N, 10.1 S.
Exam IR a 156
~roovl f7-(arninoiminometh 1)-1-na hthalenyllcarbamate
mono(trifluoroacetate)(salt)
2S The desired compound is prepared according to the procedures described in
Example s 12 and
13. Yield: 52 mg (46~~c) of white solid
MS m/z: 272 (M+Hj+
~ H NMR (DMSO, 300 MHz): 0.97 (t, 3H, J 1=J2=7.1 Hz), 1.67 (Sextet, 2H, J=7.1
Hz), 4.19
(t, 3H, J 1=J2=6.8 Hz). 7.71 (d, I H, 8.S Hz), 7.83 (m, 3H), 8.14 (d, 1 H,
J=8.5 Hz), 8.67
3o (s, 1H), 9.22 (Br, 3H), 9.63 (s. 1H);
Anal. calc'd for C15H17N302. 0.25 C2F302H~0.75 H20: C, 49.18; H, 4.66; N,
9.83. Found:
C, 49.27; H, 4.87; N, IO.U2.
Example 1 S7
-16S-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98/15386
N-f7-(aminoiminomethyl_)-1-naphthalenyll-N'-meth~iurea
mono(trifluoroacetate~(salt)
The desired compound is prepared according to the procedures described in
Example s
12 and 13. Yield: 36 mg (S4%) of white solid
MS m/z: 243 (M+H)+
S ~H NMR (DMSO. 300 MHz): 2.80 (d, 1H), 6.45 (d, 1H), 7.70 (m, 2H), 7.82 (dd.
Jl=8.9
Hz. J2=2.1 Hz), 8.08 (dd, 7.4 Hz, J=1.3 Hz), 8.17 (d, 1 H, J=8.S Hz), 8.62 (s,
1 H), 8.72 (s,
1 H), 9.07 (s, 2H), 9.47 (s, 2H), (s, 2H);
Anal. calc'd for C13H14N4O. 1.S C2F302H~O.S H20: C, 45.50; H, 3.94; N, 13.27.
Found:
C, 45.22; H, 3.86; N, 13.12.
Example 1S8
ethyl 17-(aminoiminomethyl)-1-naphthalen rLl)carbamate
mono(trifluoroacetate)(salt)
The desired compound is prepared according to the procedures described in
Example s
12 and 13. Yield: 70 mg (76%) of white solid
~5 MS m/z: 2S8 (M+H)+
1H NMR (DMSO, 300 MHz): 1.30 (t, 3H, Ji=J2=7.4 Hz), 4.20 (q, 2H, J=7.0 Hz),
7.80 (m,
4H), 8.1 S (d, 8.S Hz), 8.68 (s, 1 H), 9.13 (s, 2H), 9.38 (s, 2H), 9.66 (s, 1
H);
Anal, calc'd for C~4H15N302~1.5 C2F302H: C, 47.67; H, 3.88; N, 9.81. Found: C,
47.97;
H, 3.85; N, 9.78.
Example 159
N-17-(aminoiminomethyl)-1-naphthalenvl)probanamide
monoltrifluoroacetatel(saltl
The desired compound is prepared according to the procedures described in
examples
12 and 13.
Yield: 2t) mg (23 % ) of white solid
MS m/z: 242 (M+H)+
1 H NMR (DMSO, 3()0 MHz): 1.18 (t, 3H, 7.4Hz), 3.38 (m, 2H), 7.89 (m, 3H),
7.81 (dd,
I H, J I=8.4Hz, J2=1.9 Hz), 7.87 (d, 1 H, 8.S Hz), 8.58 (s, 1 H), 9.02 (s,
2H), 9.47 (s, 2H),
9.97 (s, i H).
3u Anal. calc'd for C ~4H 15N30. 1. I S C2F302H . 0.25 H20: C, 51.94; H, 4.45;
N, 11.15;
Found: C. 52.02; H, 4.24; N, 10.76.
Exam le 16
-166-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98115386
N-f7-laminoiminomethvl)-I-nanhthalenvl)-2-methoxyacetamide
monoltrifluoroacetate)( alt)
The desired compound is prepared according to the procedures described in
Example s
12 and 13. Yield: 30 mg (30%) of white solid
MS m/z: 258 (M+H)+
s 1H NMR (DMSO, 300 MHz): 3.49 (s, 3H), 4.I8 (s, 2H), 7.80 (m, 3H), 7.93 (d, I
H, 7.7
Hz), 8.47 (d, I H, 8.5 Hz), 8.47 (s, 1 H), 9.10 (s, 2H), 9.46 (s, 2H), 9.99
(s, 2H);
Anal. calc'd for C14H15N302~1CZF302H~1.25 H20: C, 48.80: H, 4.73: N, 10.67.
Found: C,
48.53; H, 4.34; N, 10.54;
~ H NMR (DMSO, 300 MHz): 2.16 (s, 3H), 4.85 (s, 2H), 7.82 (m, 4H), 8.18 (d, I
H, J=8. I8
~0 Hz), 8.55 (s, 1 H), 9.10 (s> 2H), 9.44 (s, 2H), 10.24 (s, 1 H);
Anal. calc'd for C15H15N3O3~1C2F302H~1 H20: C, 48.93; H, 4.35; N, 10.07.
Found: C,
48.82: H, 4.27; N, 10.00.
Example 161
15 N-f7-(aminoiminomethvl)-1-naphthalenyllurea mono(trifluoroacetate)(salt)
The desired compound is prepared according to the procedures described in
Example s
12 and 13. Yield: 35 mg (54%) of yellownish solid
MS m/z: 229 (M+H)+
tH NMR (DMSO, 300 MHz): 6.22 (s, 2H}, 7.65 (m, 2H), 7.77 (dd, JI=8.8 Hz,
J2=I.SHz).
20 8.57 (s, 1 H), 8.79 (s, 1 H), 9.07 (s, 2H), 9.49 (br, 2H);
Anal. calc'd for C22Ht2N40. 1 CZF302H~0.75 H20: C, 40.90; H, 3.33; N, I 1.95.
Found: C,
40.95; H, 3.64; N, 12.31.
Exam! la a 162
25 N-17-(aminoiminomethvl)-1-naphthalen 1y~1~2-hydroxvacetamide
mono(trifluoroacetate)(saltl
The desired compound is prepared according to the procedures described in
Examples
12 and 13. Yield: 24 mg (37%) of white solid
MS m/z: 244 (M+H)+
~H NMR (DMSO, 300 MHz): 4.18 (s, 2H), 5.82 (br, 1H). 7.67 (m, 1H), 7.85 (m,
3H), 8.20
3U (d, 1 H), 9. I 8 (s, 2H), 9.42 (s, 2H), 9.89 (s, 1 H);
Anal. calc'd for C~3H13N302~1 C2F302H~0.75 H20: C, 48.59; H, 4.21; N, 11.33.
Found: C,
48.64: H, 3.89; N, I 1.25.
Example 163
-167-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98115386
8-l2-pyrimidinylamino)-2-naphthalenecarboximidamide
bis(trifluoroacetate)(salt)
The desired compound is prepared according to the procedures described in
Examples
91 A and l3.Yield: 28 mg (28%) of pale yellow solid
MS m/z: 264 (M+H)+
S 1H NMR (DMSO, 300 MHz): 6.90 (t, IH, J1=J2=4.7 Hz), 7.69 (d, J=7.8 Hz), 7.80
(1 H,
J=8. I Hz), 7.81 ( 1 H, dd, J 1=8.4 Hz, J2=2.1 Hz), 8.08 ( 1 H, dd, J 1=7.4Hz,
J2=0.6 Hz),
8.14 (1H, d, J=8.5 Hz), 8.49 (d, 2H), 8.75 (1H, d, 3=1.7 Hz). 9.06 (s, 2H),
9.37 (s, 2H),
9.6U (s, l H);
Anal. calc'd for Ct5H13N5. 2.25 C2F302H~0.25 H20: C, 44.67; H, 4.283.03; N,
13.36.
icy Found: C> 44.49; H, 2.80; N, 13.40.
Example 164
H-amino-2-naphthalenecarboximidamide bis~trifluoroacetate)(,sal>;~
To a solution of crude 8-amino-2-nitrile-naphthalene prepared as described in
Example
t 5 1 () at room temperature in 10: I pyridine: triethylamine ( 10 mL) was
bubbled hydrogen sulfide
for 5 minutes and stirred at room temperature overnight. The reaction mixture
was added water
(50 mL) and the product was extracted into ethyl acetate (3x30 mL) . The
combined organic
solvent was dried over magnesium sulfate, filtered and evaporated. The
resulting yellow
substance was taken up in acetone (30 mL} then added methyl iodide (2 mL). The
reaction
2t> mixture was boiled for 1 hour, then evaporated to dryness. To the
resulting yellow substance in
methanol (25 mL) was added ammonium acetate ( 100 mg) and stirred at room
temperature
overnight. The solvent was removed under vacuum and the product was purified
via reverse
phase chromatography using 0.1 ~lo TFA/water and 0.1 ~lo TFA/methanoE as
eluent.
MS m/z: 186 (M+H)+
25 t H NMR (DMSO, 300 MHz): 5.14 (br, 3H), 6.80 (dd, 1 H, J 1=7.8 Hz, J2=1
Hz), 7.17 (d,
I H, J=7.8 Hz}, 7.40 (dd, I H, J 1=J2=7.8 Hz), 7.70 (dd, 1 H, J 1=8.9 Hz,
J2=2. I Hz), 7.91
(d, IH, J=8.5 Hz), 8.65 (s, 1H), 8.95 (s, 2H), 9.23 (s, 2H);
Anal. calc'd for CI tHt 1N3~2.5 C2F302H~0.75 H20: C, 39.72; H, 3.13; N, 8.69.
Found: C,
40.01; H, 3.19: N, 8.88.
Exam I~ 1 fi5
6-(aminoiminomethvl)-N-14-(aminometh ~Lll~~ll-4-(~-~vrimidin~rlamino)-2
n~phthalenecarboxamide tris(trifluoroacetate)( a~)
-168-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98I15386
Example 165A
A suspension of 2-nitrite-6-methyester naphthalene Example 8D (5.0 g, 23 mmol)
in
concentrated sulfuric acid (50 mL) at -5 °C was added a solid potassium
nitrate. The colour of
reaction mixture was gradually changed into a dark brown. The reaction mixture
was stirred for
I 5 minutes and was poured into ice-water. Collected the precipitate and
washed with water. The
crude product was recrystallized from ethyl acetate and ethanol. Yield 4.3 g
(68%) of pale
yellow powder.
Example 165B
to To a solution of 2-nitrite-6-methylester-8-vitro-naphthalene, Example 165A
(3.5 g, 13.6
mmol) in a mixture of THF ( 100 mL) and ethyl acetate ( 120 mL) was added 10%
Pd-C (350
mg). The reaction mixture was placed under hydrogen at atmospheric pressure
(balloon) and
stirred at room temperature for 3 5 hour. The mixture was filtered through
celite and silica gel,
washed with ethyl acetate and evaporated. The resulting solid was washed with
ether (20 mL).
~ 5 Yield 2.20 g {7 I °lo) of yellow powder.
Example 165
A mixture containing 2- Nitrite-6-methylester-8-amino-naphthalene, Example
165B (2.8
g, 12.3 mmol), BINAP ( 116 mg, 0.186 mmol), Pd2 (DBA)3,64 mg, 0.061 mmol), Na0-
t-Bu
20 ( 1.667 g, 17.6 mmol), 2-Brornopyrimidine (2.363 g, 14.9 mmol) and Toluene
(80 mL) in an
oven-dried flask under nitrogen, was stirred at room temperature for 10
minutes. The reaction
mixture was heated to HO'C for 1 hour. At the end of the reaction (TLC,
hexanes+ethyl
acetate=4: 1 ), brine 9200 mL) was added. Extracted the mixture with CH2Cl2.
(4x250 mL).
Evaporated the solvent. Yield 3.~ g (93%) of pale yellow powder.
Example I65D
TO a suspension of 2-nitrite-6-methylester-8-N- (2-pyrimidine)-naphthalene,
Example
165C (5.2 g, 17.1 mmol ) in ethanol ( 150 mL) was added potassium carbonate
(3.54 g, 33.3
mmol j in water (200 mL). The resulting suspension was heated at 120 °C
for 2 hours, at that
3~ time all the suspension turned into a clear solution. The mixture is cooled
down, then acidified
with 2 N HCI. The resulting precipitate was collected by filtration to yield
4.5 g (90%) of pale
yellow powder. No further purification was required for the next step.
Example 165E
-169-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98/15386
To a cold (0°C) solution of 2-nitrite-8-N- (2-pyrimidine)-6-
carboxylic acid-
naphthaleneExampIe I65D (5.0 g, 17.2 mmol) in DMF ( 150 mL) was added DIEA
(7.6 mL,
42.6 mmol) and O- (7-Azabenzotriazol-1-yl)-N, N, N', N'-
tetramethyluroniumhexafluorophosphate (9.8 g, 25.7 mmol) followed by tert-
S Butoxycarbonylamino-4-aminomethylaniline (5.74 g, 20 mmol). The resuting
reaction mixture
was stirred for about 1 hour. The reaction was quenched with water (200 mL)
and the resulting
precipitate was collected by filtration to yield 3.26 g (38%) of pale yellow
powder.
Example 165F
t0 To a solution of the substrate, Example I65E (3.0 g, 6.07 mmol) in pyridine
(150 mL)
was added triethylamine (9 mL). H2S was passed for 10 minutes and the
resultiing mixture was
stirred at room temperature for 48 hour. 100 mL of water was added to the
reaction mixture and
the precipitate was Collected by filtration. Yield (3.0 g (93%) of yellow
solid.
S Example 1656
To a solution of the thioamide, Example 165F in acetone (200 mL) was added Mel
(6
mL) and the mixture was stirred at room temperature overnight. The mixture was
evaporated to
dryness to yield 1.2 g (78%) of yellow solid.
2U Example 165H
6-(aminoiminornethvl)-N-f4-(aminomethyl henyll-4-(2-p~rimidinvlamino)-2
n~,phthalenecarboxamide tris(trifluoroacetate)lsalt)
To a solution of the imidate ester, Example 1656, (1.5 g, 2.2 mmol) in
methanol (50
mL j was added ammonium acetate (0.5 g, 6.4 mmol) and stirred at room
temperature
25 overnight. After evaporation of the solvent the residue was treated with
ether (3x25 mL) and the
ether was decanterd out. The residue was dissolved in a mixture of 10: l: 1
acetonitrile: water:
acetic acid (50 mL). After addition of ether ( 100 ml.j, the Boc protected
product precipitated as
an acetate salt and was colIeted by filtration. The solid was added 1: I TFA:
methylene chloride
(50 mL) containing thioanisole (0.5 mL). The reaction mixture was stirred at
room temperature
30 overnight. The product was purified over RPCtg chromatography using water:
methanol with
0.1 °lo TFA as eluent. Yield after lyophilization 0.5 g (54%) of pale
yellow solid.
MS m/z: 412 (M+H)+
tH NMR (DMSO, 300 MHz): 4.02 (q, 2H, J=5.8), 6.94 (dd, 1H, JI2=J2=4.8 Hz),
7.45 (d,
2H, J=8.5 Hz), 7.84 (d, 2H, J=8.4 Hz), 7.92 (dd, IH, 31=8.5 Hz, J2=1.7 Hz),
8.13 (br,
- I 70-
SUBSTITUTE SHEET (RULE 26j
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98115386
3H), 8.30 (d. 1 H, J=8.9 Hz), 8.41 (s, 1 H}, 8.52 (d., 2H), 8.55 (s, 1 H), 8.8
i (s, I H), 9.19
(s, 2H), 9.43 (s, 2H), 9.75 (s, 1H), 10.62 (s, 1H):
Anal. calc'd for C23H21N70~2.5 C2F302H~1 H20: C, 43.49; H, 3.22; N, 11.83.
Found: C,
43.54; H, 3.34; N, 11.69.
S
Example 166
6-laminoiminomethvl)-N-phenyl-4-l2-pyrimidi~lamino)-2-nanhthalenecarboxamide
monoftrifluoroacgtate) (salt)
The same procedure as described in Examples 165 substituting aniline for 4-
amino
l0 benzyl amine.
MS m/z: 383 (M+H)+
~ H NMR (DMSO, 300 MHz): 6.93-6.96 (m, 1 H), 7.14 (dd, 1 H, J I=7.3 Hz, J2=7.4
Hz),
7.40 (dd, 2H, J1=J2=7.3 Hz}, 7.80 (, d, 2H, J=8.1 Hz), 7.91 (d, 1H, J=8.9 Hz),
8.30 (d,
1 H, J=9.0 Hz), 8.41 (s, 1 H), 8.52-8.54 (m, 3H), 8.80 (s, 1 H), 9.16 (s, 2H),
9.45 (s, 2H),
t 5 9.78 (s, 1 H), 1 ().55 (s, 1 H);
Anal. calc'd for C22HtgN~,0~2 CZF302H~0.25 H20: C, 50.78; H, 3.28; N, 13.31.
Found: C,
50.85; H, 3.28; N, 13.31.
Example 167
2o N-f(4-(aminomethvl)phenvll-6-lamino(hvdroxyimino)methyil-4-(2-
pyrimidinylamino)-
2-naphthalenecarboxamide bis(trifluoroac fate, (salt)
To a suspension of compound, prepared in Example 165 E (0.20 g, 0.40 mmol ) in
methanol (40 mL) and water (20 mL) was added hydroxylamine hydro-chloride ( 1
I 2 mg, I .75
mmol) and sodium carbonate (85 mg, 0.80 mmol), the reaction mixture was
stirred for 48
25 hours at room temperature, TLC showed no reaction. Th reaction mixture was
heated at reflux
for 1() hours and removed the most of the solvents, the precipitate was
collected by filtration,
gave 1.2 g of pale yellow solid. The solid was disoolved in 1: 1 TFA+CHzCl2
(30 mL) and
sitrred at room temperature for 24 hours. The solvents was removed under
vaccuum and the
residue was loaded to a R I 8 reverse phase column. The fraction was
lyophilized and yielded a
3tt pale yellow powder (80 mg, 66°~0).
MS m/z: 428 (M+H)+
t H NMR (DMSO, 300 MHz): 4.02 (q, 2H, J=6.1 Hz), 6.92 (dd, 1 H, J I=J2=5.1
Hz), 7.46
(d, 2H, 8.4 Hz), 7.85 (d, 2H, J=8.5 Hz), 7.88 (d, IH, 9Hz), 8.12 (br, 3H),
8.22 (d, 1H,
J=8.9 Hz), 8.40 (s,
-171-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98/15386
~H ), 8.48 (s, 1H), 8.51 (d, 2H, J=4.8 Hz), 8.64 (s, 1H), 9.74 (s, 2H), 10.61
(s> 2H);
Anal. calc'd for C23H2tN~02~2.9 C2F302H~1.25 H20: C, 44.31; H, 3.41; N, 12.56;
F, 21.17
Found: C, 44.08; H, 3.30; N, 12.50, F, 21.25.
Example 168
6-(aminoiminomethyl)-N-f4-(hvdroxymethyl)phenvll-4~2 ~yrimidinylamino)
2-naphthalenecarboxamide, mono(trifluoroacetate)(salt)
The desired compound is prepared as described in Example 165 using 4-amino
benzyl
alcohol instead of 4-amino benzylamine.
t0 MS m/z: 413 (M+H)+
iH NMR (DMSO, 300 MHz): 4.48 (s, 2H), 6.94 (dd, 1H, 2H, J=8.8 Hz), J1=J2=4.8
Hz),
7.32 (d, 2H, J=8.8 Hz), 7.90 (dd, 1 H, J 1=8.5 Hz, J2=1.7 Hz), 8.28 (d, 2H,
J=8.8 Hz), 8.41
(s, 1H), 8.54 (d, 2H, J=4.8 Hz), 8.55 (dd, 1H, J=1.3 Hz), 8.80 (s, 1H), 9.08
(s, 2H), 9.43
(s, 2H), 9.73 (s, 1 H), 10.48 (s, 1 H);
Anal. calc'd for C23H20N~,02: C, 49.06; H, 3.83; N, 12.81; F, 16.50. Found: C,
48.74; H,
3.86; N, 12.63, F, t 6.54.
Example 170
methyl f3-fff4-(aminometh~phenyllaminolcarbon~Jl-7-j4-amino(hydrox
imino)methyll-
1-naphthalenvllcarbamate, bis(trifluoroacetate)(saltl
Example 170A
To a suspension of the nitrile, Example 14I D (213 mg, 0.45 mmol) and
hydroxylamine
hydrochloride (338 mg, 4.86 mmol) in methanol (40 rnL) water (5 mL) was added
potassium
carbonate (53R mg, 3.9 mmol) stirred at room temperature overnight. The
solvent was
evaporated and the resulting solid was washed with ether and hexanes to yield
the product as
white solid 153 mg (62~1~ ).
MS (ECI) m/z 508 (M+H}+.
Example 1708
methyl f3-fff4-(aminomethyl)nhenvllaminolcarbonvll-7-f4-
amino(hydroxyimino)methyll
1-naphthalenyllcarbamate bis(trifluoroacetate)(salt)
The Boc protected substrate, Example 170A was added 3 mL of 4 N HCI in dioxane
and stirred at room temperature for 20 minutes. The solvent was evaporated
under vacuum and
-172-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98/15386
the product was separated on MPLC with a column of RP Clg using methanol-water
+0.1%TFA as eluent. Yield of white solid 117 mg (79%).
MS (ECI) m/z 408 (M+H)+;
~ H NMR (300 MHz, DMSO-d~,) 8 3.77 (S, 3H), 4.03 (s, 2H), 4.01 (q, J=5.9 2H),
7.46 (d,
J=B.SHz. 2H), 7.86 (d, J=8.SHz, 2H), 7.90 (dd, JI=8.5, Hz, J2=l.4Hz, IH), 8.09-
8.15 (m,
4H), 8.17 (d, J=8.4Hz, I H), 8.29 (s, 1 H), 8.42 (s> I H), 8.56 (s, 1 H), 9.80
(s, I H), 10.62
(s, 1H);
Anal. calc'd for C21 H21 N504~2.5 TFA~0.5 H20: C, 44.52; H, 3.52; F, 20.31; N,
9.98.
Found: C, 44.78; H, 3.57; F, 19.82; N, 9.87.
Exam I~e 171
6-Iamino(hydroxyimino)methyll-N- henyl-2-naDhthalenecarboxamide
The title compound is prepared as described by Judkins et al., Synthetic
Communications 26 (23), 4351-4367 ( 1996). The compound prepared in Example
55C (0.1
a g, .36 mmole) is dissolved in a 30: 1 mixture of Toluene: methanol to which
is added
hydroxylamine hydrochloride (3.6 mmole) and potassium tent-butoxide (3.6
mmole). The
resulting slurry is refluxed for I7 hr., cooled, solvents removed under
vacuum. The residue is
taken up in distilled water (30 ml) extracted with ethyl acetate (2 X 100 ml).
The combined
organic extracts are washed with 10% NaCI (50 rnl), dried over anhydrous
Na2S04. The
2o sample is filtered of drying agent and the solvent removed under vacuum
leaving a white solid
(65 mg). The material is purified by medium pressure reverse phase
chromatography as
described in Example 1. The title compound is obtained as white solid (45 mg)
MS (m/z) M + H+: 306
~ H NMR (DMSO-d6): 10.51 (s, 1 H), 9.32 (s, OH), 8.70 (s, 1 H), 8.57 (s, 1 H),
8.34 (d, I H),
25 8.25 (d, 1 H), 8.17 (dd, 1 H), 7.90 (dd, 1 H), 7.83 (d, 2H), 7.40 (dd, 2H),
7.15 (dd, 1 H),
6.25 (bs, 2H).
Analysis: calc'd for C2~H1~,N304 F3: C, 57.28, H, 3.85, N, 10.02; Found C,
56.89, H, 3.65,
N, 9.90.
=t~ Example 174
8-(2-DVridinvlamino)-2-nanhthalenecarboximidamide bisftriffuoroacetate) salt
Example 174A
-173-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98115386
To a solution of the product from 11 D ( 1 b8 mg, 1.00 mmol) in 5 mL toluene
was added
2-bromopyridine (0.105 mL, I.1 mmol), NaOtBu ( 135 mg, 1.4 mmol), Pd2dba3 (92
mg, 0.1
mmol), and P (o-tolyl)3 (122 mg, 0.4 mmol), and the reaction was stirred for
24 hours at 100
°C. The reaction was cooled, and the crude reaction mixture was
chromatographed on Si02
S using 50% ethyl acetate/hexanes as eluent to yield 80 mg (33%) of the
desired compound.
MS (DCI (NH3)) m/z 246 (M+H)+.
Example 174B
R_-12-yvridinvlamino)-2-naphthalenecarboximidamid bi ( 'fluoroacetate) salt
1o The product from Example 174A (121 mg, 0.493 mmol) was dissolved in THF (2
mL)
and 0.543 mL of a 1 M solution of LiN (TMS)2 in THF was added. The reaction
was stirred
for 5 min, and TMSCI (0.069 mL, 0.543 mmol) was added. After stirring for 30
min, another
1.09 mL of the 1 M solution of LiN (TMS)2 was added. The reaction was stirred
for 18 hours,
and 10 mL of a 2 M aq. HCl solution was added. The reaction was stirred for
another 24 hours.
IS and was basicified with saturated aq. Na2C03. The mixture was extracted
with 3x ethyl acetate,
and the extracts were washed with brine, dried over Na2S04, and condensed. The
crude
product was purified by reverse-phase HPLC to yield the desired compound (21
mg, 7%): m.p.
I 37-147 °C.
MS (DCI (NH3) m/z 263 (M+H)+;
2U 1 H NMR (300 MHz, DMSO) S 9.98 (br s, 1 H), 9.46 (br s, 2H), 9.27 (br s,
2H), 8.74 (s,
1 H), 8.21 (d, J=9 Hz, I H), 8.1 1 (d, J=6 Hz, 1 H), 8.02 (d, J=9 Hz, 1 H),
7.8 I -7.95 (m, 3H),
7.75 (dd, J=9, 9 Hz, 1H), 7.16 (m, 1H), 6.95 (m, 1H), 2.55 (s, 3H);
Anal. calc'd for CI~H14N4~3.IC2HF302: C, 43.30; H, 2.80; N, 9.10. Found: C,
43.14; H,
3.04; N, 9.90.
Example 176
fi-I4-l(hvdroxvmethvl)Dhenvllmethoxvl-2-nanhthalenecarboximidamide
methanesulfonatei~alt)
Example 176A
To a solution of NaH (b0% in mineral oil, 1.17 g, 29.3 mmol) in THF (50 mL)
was
added 4-bromobenzyl alcohol (5.22 g, 27.9 mmol) in THF (50 mL), and the
reaction was
stirred at room temperature for 20 minutes. p-Methoxybenzyl chloride (4.07 mL,
30 mmol)
was then added, and the reaction was stirred at 50°C for 2 hours. The
mixture was poured into
water and extracted with 3x diethyl ether, and the extracts were washed with
brine, dried over
-174-
SUBSTITUTE SHEET (RUtE 26)
CA 02294300 1999-12-14
WO 99!05096 PCTIU598/15386
Na2S04, and condensed. The crude reaction mixture was chrornatographed on Si02
using
hexanes as eluent, to yield 7.39 g (86%) of the desired compound.
MS (DCl (NH3)) m/z 326 (M+NH4)+;
!H NMR (300 MHz, CDCl3) 8 7.47 (d, 2H), 7.28 (d, 2H), 7.23 (d> 2H), 6.90 (d,
2H)> 4.48
(s, 3H), 4.47 (s, 2H), 3.91 (s, 3H).
Example 176B
To a solution of the product from Example 176A (6.80 g, 22.13 mmol) in THF (
100
mL) and hexanes (20 mL) at -100°C was added a 2.5 M solution of BuLi
(8.85 mL, 22.13
!0 mmol), and the reaction was stirred for 2 minutes. DMF (3.43 mL, 44.3 mmol)
was then
added, and the reaction was warmed to room temperature. The mixture was poured
into water
and extracted with 3x diethyl ether, and the extracts were washed with brine,
dried over
Na2S04, and condensed. The crude reaction mixture was taken up in methanol (
100 mL) and
NaBH4 ( 1.0 g, 26.2 mmol) was added in portions. A few drops of water were
added, and the
!5 mixture condensed. The residue was chromatographed on Si02 using 20% ethyl
acetate/hexanes as eluent, to yield 3.32 g (58%) of the desired compound.
MS (DCI (NH3)) m/z 276 (M+NH4)+;
!H NMR (300 MHz, CDCI3) 87.38 (s, 4H), 7.29 (d, 2H), 6.90 (d, 2H), 4.70 (s,
2H), 4.54
(s, 2H), 4.49 (s, 2H), 3.81 (s, 3H), 1.62 (s, 1H).
Example 176C
To a solution of the product from Example 176B ( I 93 mg, 0.747 mmol), the
product
from Example 2HA ( I 39 mg, 0.822 mmol), and Ph3P (216 mg, 0.822 mmol) in THF
( 10 mL)
at 0°C was added diethylazodicarboxylate (0.129 mL, 0.822 mmol), and
the reaction was
stirred for 90 minutes at room temperature. The crude reaction mixture was
condensed, and the
residue was chromatographed on Si02 using 10% ethyl acetate/hexanes as eluent,
to yield 225
mg (74%) of the desired compound.
MS (DCl (NH3)) m/z 427 (M+NH4)+;
!H NMR (30U MHz, CDC13) 8 8.15 (s, IH), 7.81 (d, 1H), 7.77 (d> IH), 7.58 (dd,
IH), 7.48
(d, 2H), 7.42 (d, 2H), 7.02-7.15 (m, 4H), 6.90 (d, 2H), 5.21 (s, 2H), 4.56 (s,
2H), 4.51 (s,
2H), 3.91 (s, 3H).
Examtale t 76D
-175-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98/1538(
To a solution of the product from Example 176C (220 mg, 0.537 mmol) in CH2C12
(40
mL) and water (7 mL) was added DDQ (244 mg, 1.07 mmol), and the reaction was
stirred for
90 minutes. The crude reaction mixture was taken up in CH2Cl2, washed with 2x
aqueous
NaHC03 and brine, dried over Na2S04, and condensed. The residue was
chromatographed on
Si02 using 50% ethyl acetate/hexanes as eluent, to yield 89 rng (57%) of the
desired
compound.
MS (DCI (NH3)) m/z 307 (M+NH4)+;
1 H NMR (300 MHz, CDCl3 ) 8 8.15 (s, 1 H}, 7.81 (d, 1 H), 7.77 (d, I H), 7.57
(dd, I H), 7.49
(d, 2H), 7.41 (d, 2H), 7.33 (dd, 1H), 7.21 (d, 1H), 5.21 (s, 2H), 4.74 (s,
2H), I.63 (br s,
io 1 H); .
Example 176E
6-f4-f(hvdroxvmethvl)ohenvlimethoxvl-2-naphthalenecarboximidamide methane
ulfonate~sal~
The desired compound was prepared from Example 176D and the procedure of
Example
~s SSD.
MS (DCI/NH3) m/z 307 (M+H)+;
~ H NMR (300 MHz, DMSO-d~,) 8 2.31 (s, 3H), 4.52 (s, 2H), 5.25 (s, 2H), 7.37
(d, 2H),
7.39 (dd, 1 H), 7.47 (d, 2H), 7.59 (d, 1 H), 7.79 (dd, 1 H}, 8.00 (d, 1 H),
8.03 (d, I H), 8.4I
(s, I H), 8.89 (br s, 2H), 9.34 (br s, 2H);
20 Anal. calc'd for C~9H1gN202~1.15 CH4S03: C, 58.06; H, 5.46; N, 6.72. Found:
C, 58.24;
H, 5.62; N, 6.59.
Example 177
N-hvdroxv-8-(2-vvrimidinylaminol- 2-naphthalenecarboximidamide
S mono(trifluoroacetate)(saEt~
The desired compound is prepared from the nitrile described in Example I63 and
utilizing the procedure described in Example 167. Yield as a white powder: 50
mg
MS m/z: 280 (M+H)+
30 ~ H NMR (DMSO, 300 MHz): 6.90 (dd, 1 H, J 1=J2=5.2Hz), 7.45 (m, 3H), 8.0
(d, 1 H, J=8.5
Hz), 8.14 (d, 1 H, J=8.4Hz), 8.49 (d, J=5.3 Hz), 8.61 (s, 1 H), 9.58 (s, 1 H);
Anal. calc'd for C15H13N50~2.0 C2F302H~0.5 H20: C, 44.20; H, 3.I2; N, 13.56.
Found: C,
44.24; H, 2.94; N, 13.49.
-176-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99J05096 PCTIUS98J15386
Exam lp a 179
6-(2-R~ylethynXll-2-naphthalenecarboximidamide mono(trifluoroacetate)( altl
Example 179A
Using the product obtained in Example 28B, 2-ethynylpyridine (Lancaster
Chemical
Corp.) and the procedure described in Example 121 A, the desired compound was
obtained.
MS (DCI/NH3) m/z 255 (M+H)+.
Examvle 179B
Using the product obtained in Example 179A and the procedure described in
Example
94D the desired compound was obtained.
MS (DCI) m/z 272 (M+H)+;
~ H NMR (300 MHz, D MSO) & 7.45-7.50 (m, 1 H), 7.72 (d, 1 H), 7.82 (dd, 1 H),
7.86-7.92
(m, 2H), 8.1 H (d, I H), 8.22 (d, 1 H), 8.42 (s, 1 H), 8.54 (s, 1 H), 8.68 (m,
1 H), 9.25 (s, 2H),
9.49 (s, 2H);
Anal. calc'd for C20H ~4F3N302~0.25 H20: C, 61.62; H, 3.75; N, 10.78. Found:
C, 61.72;
H, 3.64; N, 10.66.
Example 180
6-(aminoiminometh lv )-N-N-phenyl-4-(tetrahydro-3-furanyl)-2-
naphthalenecarboxamide
monohydrochloride
Examt~le 180A
The desired compound was prepared from the product from Example l 52 by the
procedure of Example 62A.
MS (DCI/NH3) m/z 340 (M-H20)~.
Example 180B
The desired compound was prepared from the product from Example 180A by the
3~> procedure of Example 62B.
MS (DCI/NH3) m/z 343 (M+H)+.
Exam le 180
-177-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98/15386
The desired compound was prepared from Example I80B and the procedure of
Example
SSD.
MS (DCI/NH3) m/z 360 (M+H)+;
~ H NMR (300 MHz, DMSO-d6) 8 2.20 (m, 1 H), 2.52 (m, 1 H), 3.85 (dd, 1H), 3.94
(ddd,
1H), 4.08 (ddd, lh), 4.26 (dddd, 1H), 4.39 (dd, 1H), 7.14 (t, 1H), 7.40 {t,
2H), 7.81 (d,
2H), 7.95 {d, 1 H), 8.08 (s, 1 H), 8.32 (d, 1 H), 8.59 (s, 1 H), 8.79 (s, 1
H), 9.25 (br s, 2H),
9.61 (br s, 2H);
Anal. calc'd for C22H21N302~2.6 HCI: C, 58.18; H, 5.24; N, 9.25. Found: C,
58.21; H,
4.91; N, 9.13.
Exam Ip a 181
6-famino(h droxyiminolmethyll-N-phenyl-4-(2-pvrimidinvlartin~l-2-
naphthalenecarboxamide
Prepared in a similar manner as described in Example 177 using the material
prepared in
Example 166.
t5 MS m/z: 399 (M+H)+
~ H NMR (DMSO, 300 MHz): 6.88 (dd. 1 H, 4.7Hz), 7.1 1 (dd, l H, J l=J2=7.SHz),
7.37 (dd,
2H, J 1=J2=7.5 Hz), 7.82 (d, 2H, J=8.8 Hz), 7.94 (dd, 1 H, J 1=8.SHz, J2=1.4
Hz), 8.04 (d,
1 H, J=8.8Hz), 8.31 (s, 1 H), 8.43 (dd, 1 H, J=14 Hz), 8.74 (d, 2H, J=4.7 Hz),
8.52 (s, 1 H),
9.55 (s, 1 H), 9.85 (s, 2H), 10.41 {s, 1 H);
2o Anal. calc'd for C22H~gN602~0.4 H20: C, 65.14; H, 4.67; N, 20.72. Found: C,
65.57; H,
4.45; N, 20.18.
Example 182
methvl 4-f f f 7-amino(hvdroxviminolmethyll-2-nanhthaIenvllo~imethvllbenzoate
25 The resulting product from Example 2I 3A ( 1 10 mg, 0.347 mmol),
hydroxylamine
hydrochloride (26.5 mg, 0.381 mmol), triethylamine (53 ftl, 0.381 mmol) and N,
N-
dimethylformamide (12 mL) were combined in a four inch glass pressure tube.
The tube was
sealed and heated for 24 hours at 80 °C. The tube was cooled to room
temperature and
additional hydroxylamine hydrochloride (48.1 mg, 0.693 mmol) and triethylamine
(96.6 ~,1,
30 0.693 mmol) in N, N-dimethylformamide (2 mL) was added. The tube was
resealed and heated
for 24 hours at 80 °C. The addition, as above, was repeated a second
time, the tube was
resealed and heated for 72 hours at 80 °C. The reaction mixture was
concentrated to a solide
residue which was purified by column chromatography on silica gel (60 g)
eluted with 15%
-178-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98115386
acetone in methylene chloride to yield the target compound as a white solid
(43 mg, 50% based
on recovered starting material).
MS {DCI (NH3)) m/z 351 (M+H)+;
IH NMR NMR (300 MHz, DMSO-d6) 8 3.860 (s, 3H), 5.349 {s, 2H), 5.860 (s, 2H),
7.280
(dd, 1H), 7.381 (d, 1H), 7.660 (d, 2H)> 7.688 (dd, IH), 7.805 (d, 1H), 7.850
(d, 1H), 8.010
(d> 2H), 8.070 (s, 1H), 9.724 (s, IH);
Anal. calc'd for CZpH1gN204~0.15 H20: C, 68.04; H, 5.22; N, 7.93. Found: C,
68.06; H,
5.05; N, 7.87.
to Example 184
N-I4-(aminocarbonyl)phen,yll-6-(aminc~iminometh I)-2-naphthalenecarboxamide
monoltrifluoroacetate) salt
Ex~mole 184A
A suspension of the product obtained in Example 8A (30U mg, 1.39 mmol) and 5
mL
dichloromethane was added dropwise to a 0° solution of 4-aminobenzamide
(207 mg, 1.52
mmol), triethylamine (0.44 mL, 3.2 mmol), and 10 mL dichloromethane. The
reaction mixture
was stirred for 0.5 hour at 0° and for I H hours at room temperature.
Excess ether was added
with stirring and the resultant solid was filtered, washed with 1 N HCI,
water, and was dried
under vacuum to afford the desired compound.
MS (DCI) m/z 333 (M+NH3)+.
E~mule 184B
N-I4-(aminocarbonvl)phenvll-6-(aminoiminomethyl)-2-naphthalenecarboxamide
monoltrifluoroacetate) salt
Using the product obtained in Example 184A and the procedure described in
Example
4C the desired compound was obtained.
MS (DCI) m/z 333 (M+H)+;
~ H NMR (300 MHz, DMSO) 8 10.76 (S, 1 H), 9.51 (S, 2H), 9.14 (S, 2H), 8.74 {S,
1 H),
3U 8.56 (S, I H), 8.33 (D, I H, J=8.8 Hz), 8.26 (D, 1 H, J=8.8 HZ Hz}, 8.16
(DD, 1 H, J=8.46,
1. I I Hz), 7.91 (M, 6H), 7.31 (S, 1 H};
Anal. calc'd for C2pH~7F3N404~I.5 H20: C, 53.28; H, 4.26; N, 11.83. Found: C,
53.47; H,
3.86; N, 11.96.
-179-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98/15386
Exam Ip a 185
methvl2-II6-(aminoiminomethyll-2-na ht~h_alenylloxvlacetate
mono(trifluoroacetate)(saltl
Example 185A
7-methoxy-2-cyanonaphthalene, (2.79 g, 5.23 mmol) and tetrabutylammonium
iodide
( 17 mg, 0.157 mmol) were combined in a mixture of benzene (35 mL) and
cyclohexanes ( 17.5
mL). The resulting solution was added to a rapidly stirring, cooled
(ice/water) suspension of
aluminum triiodide (6.21 g, 15.23 mmol) in a mixture of benzene (35 mL) and
cyclohexanes
( 17.5 mL) under an inert atmosphere. After the addition, the resulting
suspension was heated at
reflux for 2.5 hours. The heating was removed and after cooling to near room
temperature, the
reaction mixture was cooled in an ice bath and quenched by the addition of
water (100 mL). The
resulting mixture was further diluted with 2 M aqueous sodium thiosulfate
solution (50 mL) and
extracted with ethyl acetate (3 X 80 mL). The combined organic layers were
dried and
evaporated. The resulting solid was dissolved in a minimum of hot ethyl
acetate, diluted hot
~ 5 with hexanes to the cloud point and placed in a refrigerator for 2 hours.
The desired compound
was collected by filtration, ( 1.99 g, 77%).
MS (DCI (NH3)) m/z 187 (M+NI-I4)+.
xam lep 1858
The resulting product from Example 185A (217 mg, 1.283 mmol) was combined with
cesium carbonate (460 mg, 1.411 mmol) and tetrabuthylammonium iodide
(catalytic) in DMF (7
mL). To this was added t-butyl bromoacetate ( 193 ~tL, 1.283 mmol) and the
resulting mixture
was stirred 2 hours under an inert atmosphere. The reaction mixture was
diluted with water
( 100 mL) and extracted with ethyl acetate (2 X 50 mL). The combined organic
layers were dried
and evaporated. The residue was purified by column chromatography to yield the
desired
compound as an oil (332 mg, 91 %):
MS (DCI (NH3)) m/z 284 (M+H) +, 301 (M+NH4)+.
Examnfe 185C
3o methyl 2-f16-(aminoiminomethyl)-2-na thalenylloxvlacerate
mono(trifluoroacetatel( alt)
The product from Example 1858 (323 mg, 1.140 mmol) was dissolved in anhydrous
methanol (32 mL) under an inert atmosphere and cooled to 0 °C.
Anhydrous hydrogen chloride
was bubbled into the solution until it became saturated. The reaction was
stirred for 15 minutes
at 0 °C and saturated again with anhydrous hydrogen chloride. After
stirring for an additional 20
-180-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/LTS98I15386
minutes at 0 °C the solution was saturated one final time with
anhydrous hydrogen chloride and
stirred 18 hours while warming to room temperature. The reaction was
evaporated to a solid
and dried under high vacuum for 2 hours. The solid was slurried in methanol
(64 mL),
ammonium acetate (220 mg, 2.850 mmol) was added, and the mixture was heated at
reflux 2
hours. The reaction was evaporated and purified by reverse phase
chromatography to give the
desired compound (265 mg, 90%).
MS (DCI (NH3)) m/z 259 (M+H)+;
IH NMR (300 MHz, DMSO-d6) 8 3.735 (s, 3H), 4.995 (s, 2H), 7.430 (dd, 1H),
7.458 (s,
1 H}, 7.669 (dd, 1 H), 8.025 (d, 1 H}, 8.095 (d, 1 H), 8.325 (d, 1 H), 9.090
(br s, 1 H), 9.410
l0 (br s, 1 H;
Anal. calc'd for C14H~4N203~ (C2H02F3) 1.05: C, 51.16; H, 4.01; N, 7.41.
Found: C,
51.35; H, 3.98; N, 7.48.
Exam lie 1 R6
6-laminoiminomethyl)-N-(2-thiazolvl)-2-naphthalenecarboxamide
monohydrochloride
Example 186A
The above product was prepared in the manner of Example 8A using 2-
aminothiazole.
MS (APCI) m/z (M+H)+ 280.
2U
Example 186B
6-(aminoiminomethvl)-N-(2-thiazolvl)-2-nanhthalenecarboxamide
monohvdrochloride
The above was prepared from Example 1B.
MS (APCI) m/z (M+H)+ 297;
IH-NMR (3()0 MHz, DMSO-d6) 8 12.88 (s, IH), 9.59 (s, 2H), 9.30 (s, 2H), 8.87
(s, IH),
8.59 (s, 1 H), 8.32-8.22 (m, 4H), 7.93 (dd, J=1.8, 8.4 Hz, 1 H), 7.61 (d,
J=3.3 Hz, ! H),
7.33 (d, J=3.3 Hz, IH);
Anal. calc'd for C~5H~3N40C1S 2/5 HC1: C, 52.03; H, 3.89; N, 16.18. Found: C,
52.01; H,
3.88; N, 16.12.
Example 187
6-(aminoiminomethvl)-N-f6-methoxy-3-wndin lv )-2-na~hthalenecarboxamide
monohydrochloride
-181-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98/15386
Ex~arr ple 187A
The above product was prepared in the manner of Example 8A using 5-amino-2-
methoxypyridine.
MS (APCI) m/z (M+H)+ 304.
Example 187B
6-(aminoiminomethyll-N-(6-methoxv-3-pyridin lv )-2-nanhthalenecarboxamide,
monollydrochloride
The above was prepared as described Example I B (144D).
1U MS (CI) m/z (M+H)+ 321;
1H-NMR (300 MHz, DMSO-d6) 8 10.71 (s, 1H), 9/60 (s, 2H), 9.41 (s, 2H), 8.76
(s, 1H),
8.60 (s, 2H), 8.30 (d, J=9, IH), 8.25-8.12 (m, 4H), 7.93 (dd, J=1.8, 8.7 Hz,
1H), 6.89 (d,
J=9.0 Hz, I H), 3.87 (s, 3H);
Anal. calc'd for C2UH1~N404F3 1/2 TFA: C, 51.47; H, 3.60; N, 11.45. Found: C,
51.39; H,
a 3.88; N, 11.65.
Example I88
6-(aminoiminomethyl)-N-( 1. 3-benzodioxol-5-yl)-2-naphthalenecarboxamide
mono(trifluoroacetate)(sa~~
2U
Example 188A
The above product was prepared in the manner of Example 8A using 3, 4-
methylenedioxyaniline.
MS (APCI) m/z (M+H)+ 317.
2S
Example 188B
The above was prepared as described Example 1 B.
MS (CI) m/z (M+H)+ 334;
1 H-NMR (300 MHz, DMSO-d~,) 8 9.70 (s, 1 H), 8.20 (s, 2H), 9.96 (s, I H), 7.81-
87.63 (m,
3U 3H), 7.31 (dd, J=1.8> 8.4 Hz, IH), 7.11 (d, J=4.2 Hz, 1H), 6.91 (dd, J=4.5,
10.8 Hz), 6.4
(d, J=8.4 Hz, 1 H), 5.59 (s, 2H);
Anal. caIc'd for C21H1~N303F3 3/5 TFA: C, 55.44; H, 3.48; N, 8.77. Found: C,
55.44; H,
3.52; N, 8.85.
-I82-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98115386
Exam I~e 1 H9
6-(aminoiminomethyl)-N-( 1. 2. 3. 4-tetrahydro-2. 4-dioxo5-Ryrimidinvl)
2-na>?hthalenecarboxamide. monohydrochloride
Example 189A
The above product was prepared in the manner of Example 8A using 5-
aminouracil.
MS (APCI) m/z (M-H)+ 305.
Example 189B
l0 ~~aminoiminomethyl)-N-(I 2 3 4-tetrahvdro-2 4-dioxo -pyrimidin,
2-nat~hthalenecarboxamide, monohydrochloride
The above was prepared as described in Example 1 B.
MS (CI) m/z (M+H)+ 324;
~ H-NMR (300 MHz, DMSO-d6) 8 1 1.50 (s, 1 H), 10.90 (m, 1 H), 9.53 (s, 3H),
9.21 (s, 2H),
~5 8.70 (s, IH), 8.55 (s, 1H), 8.33 (d, J=8.4 Hz, 1H), 8.20 (d, J=8.7 Hz, 1H),
8.13-8.09 (m,
2H), 8.00 (s. 1 H), 7.88 (dd, J=I .8, 8.4 Hz, 1 H);
Anal. calc'd for C16H14N5~3C1 2/5 HC1: C, 51.49; H, 3.88; N, 18.76. Found: C,
SI.87; H,
4.01; N, 17.68.
2U Example 190
~aminoiminomethyl)-N-(3 5-difluor~henyl)-2-naphthalenecarboxamide
mono(trifluoroacetatel(salt)
Exam~ale 190A
25 The above product was prepared in the manner of Example 8A using 3, 5-
difluoroaniline.
MS (APCI) m/z (M-H)+ 307.
Example 190B
3U 6-(aminoiminomethyl)-N-(3 5-difluoro h~envll-2-naphthalenecarboxamide
monoltrifluoroacetatel~salt)
The above was prepared as described in Example 1 B.
MS (CI) m/z (M+H)+ 326;
-183-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99!05096 PCTIUS98/15386
IH-NMR (300 MHz, DMSO-d6) 8 10.93 (s, 1H), 9.53 (s, 2H), 9.34 (s, 2H), 8.71
(s, 1H),
8.58 (s, 1 H), 8.33 (d, J=8.4 Hz, 1 H), 8.26 (d, J=8.7 Hz, 1 H), 8.14 (m, 1
H), 7.92 (dd,
J=0.9, 8.1 Hz, 1 H), 7.61 (d, J=7.8 Hz, 2H}, 7.04-6.97 (m, 1 H);
Anal. calc'd for C2pH14N3O2F5 2/5 TFA: C, 54.92; H, 3.21; N, 9.42. Found: C,
54.96; H,
3.36; N, 9.37.
Example I91
6-(aminoiminomethvl)-N-(1H-pvrazol-3- 1)-2-naphthalenecarboxamide
mono(trifluoroacetate)(salt)
Example 191 A
The above product was prepared in the manner of Example 8A using 3-
aminopyrazole.
MS (APCI) m/z (M+H)+ 263.
Example 191 B
6-(aminoiminomethvll-N-( I H-pvrazol-3-vl)-2-naphthalenecarboxamide
mono(trifluoroacetate)f salt)
The above was prepared as desribed in Example 1 B.
MS (CI) m/z (M+H)+ 280;
IH-NMR (300 MHz, DMSO-d6) 8 I 1.13 (s, 1H), 9.55 (s, 2H), 9.37 (s, 2H), 8.75
(s, 1H),
8.55 (s, 1H), 8.23 (d, J=8.4 Hz, 1H), 8.12 (s, 2H), 7.90 (d, J=8.4 Hz, 1H)>
7.70 (s, IH),
6.69 (s, 1 H);
Anal. calc'd for C17H14N503F3 7/10 TFA: C, 46.53; H, 3.12; N, 14.70. Found: C,
46.47;
H, 3.16; N, 14.85.
Example 192
6-(aminoiminomethvl)-N-(5-methyl-'3-isoxazolyll)-2-naphthalenecarboxamide
mono(trifluoroacetate)(salt)
p Example 192A
The above product was prepared in the manner of Example 8A using 3-amino-5-
methylisoxazole.
MS (APCI) m/z (M+H}+ 278.
-184-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98115386
Example 192B
6-laminoiminomethyi)-N-(5-methyl-3-isoxazol r~lll-2-nanhthalenecarboxamide
mQno(trifluoroacetate)( alt)
The above was prepared as described in Example 1 B.
s MS (CI) m/z (M+H)+ 295;
1 H-NMR (300 MHz, d6-DMSO) 8 I 1.62 (s, 1 H), 9.52 (s, 2H), 9.33 (s, 2H), 8.78
(s, 1 H),
8.55 (dd, 1H), 8.31-8.16 (m, 3H), 7.90 (dd, 1H), 6.81 {s, 1H), 2.44 (s, 3H)
Anal. calc'd for CIgH15N404F3: C, 52.95; H, 3.70; N, 13.72. Found: C, 52.73;
H, 3.64; N,
13.24.
t0
Example 193
6-(aminoiminomethvl)-N-(nvrazinyl)-2-naphthalenecarboxamide
mono(trifluoroacetate)(~~lt)
Example 193A
15 The above product was prepared in the manner of Example 8A using 2-
aminopyrazine.
MS (APCI) m/z (M-H)+ 275.
Example 193B
6-(aminoiminomethvl)-N-(pyrazinyl)-2-nanhthalenecarboxamide
mono(trifluoroacetate)(salt)
z0 The above was prepared as described in Example 1 B.
MS (CI) m/z (M+H)+ 292;
~ H-NMR (300 MHz, d~,-DMSO) b 11.41 (s, 1 H), 9.52 (s, 2H), 9.49 (s, 1 H),
9.27 (s, 2H),
8.83 (s, 1 H), 8.56 (s, 1 H), 8.53-8.46 (m, 2H), 8.30 (d, 1 H), 8.23 (s, 2H),
7.90 (dd, 1 H);
Anal. calc'd for C~~H~4NS03F3: C, 49.36; H, 3.16; N, 15.15 1/2 TFA. Found: C,
49.53; H,
25 3.22; N, i 4.87.
Exam-ple 194
6-(aminoiminomethvl)-N-(6-meth~pyridin 1)-2-naphthalenecarboxamide
monoltrifluoroacetate)( alt)
Example 194A
The above product was prepared in the manner of Example 8A using 2-amino-6-
methylpyridine.
MS (APCI) m/z (M+H)+ 288.
-185-
SUBSTITUTE SHEET (RUtE 2fi)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98115386
Example 194B
6-(aminoiminomethvl)-N-l6-methyl-2-p 'dinvl)-2-naDhthalenecarboxamide
mono(trifluoroacetate)(salt)
The above was prepared as described in Example 1 B.
MS (C1) m/z (M+H)+ 305:
~H-NMR (300 MHz, d6-DMSO) 8 11.02 (s, 1H), 9.53 (s, 2H), 9.37 (s, 2H), 8.80
(s, 1H),
8.55 (s, 1H)> 8.29 (d, 1H), 8.20 (s, 2H), 8.07 (d, 1H), 7.89 (d, 1H)> 7.78 (t,
1H), 7.08 (d,
1 H), 2.48 (s, 3H);
t0 Anal. calc'd for C20H17N403F3~ C, 48.74; H> 3.33: N, 10.20 715 TFA. Found:
C, 48.74; H,
3.59; N, 10. I 1.
Example 195
6-(aminoiminomethvl)-N-(3. 4, 5-trimethoxvphenyI)-2-n~~hthalenecarboxamide
monohydrochloride
Example 195A
The above product was prepared in the manner of Example 8A using 3> 4, 5-
trimethoxyaniline.
2o MS (APCI) m/z (M+H)+ 363.
Example 195B
6-(aminoiminomethvl)-N-(3. 4. 5-trimethoxvphenvl)-2-n~phthalenecarboxamide
mono~drochloride
25 The above was prepared as described in Example 1 B.
MS {CI) m/z (M+H)+ 380;
~ H-NMR (300 MHz, d~-DMSO) b 10.54 (s, 1 H), 9.61 (s, 2H), 9.34 (s, 2H), 8.72
(s, 1 H),
8.59 (s, 1H), 8.33-8.15 (m, 3H), 7.91 {dd, 1H), 7.30 (s, 2H), 3.80 (s, 9H)
Anai. calc'd for C21H22N304C1 63/10 HCI: C, 39.09; H, 4.42; N, 6.SI. Found: C,
38.94; H,
30 4.60: N, 7.61.
Exam lie 196
6-(aminoiminomethvl)-N-( -methyl-2-p 'din~rl)-2-nanhthalenecarboxamide
bis~trifluoroacetateO(salt)
-186-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98/15386
Example 196A
Using the procedure described for Example I84A and substituting 2-amino-3-
picolene
for 4-aminobenzamide, the desired compound was obtained.
MS (DCI) m/z 288 (M+H)+.
Example 196B
6-laminoiminomethvll-N-l3-methyl-2-nvridi ~1)-2-nanhthalenecarboxamide
bisftrifluoroacetate)(salt~
Using the procedure described in Example 1B and the product obtained in
Example
196A, the desired compound was obtained.
MS (DCI) m/z 305 (M+H)+;
~ H NMR (300 MHz, DMSO) 8 10.85 (s, 1 H), 9.52 (s, 2H), 9.22 (s, 2H), 8.76 (s,
1 H), 8.56
(s. I H), 8.35 (dd, 1 H, J=4.41, 1.10), 8.32 (d, 1 H, J=8.80), 8.22 (m, 2H),
7.90 (dd, 1 H,
J=8.83, 1.84), 7.80 (dd, I H, J=7.73, 1.11 ), 7.3 I (dd, 1 H, J=7.72, 4.78),
2.26 (s, 1 H);
Anal. calc'd for C22H1gF6N403~0.75 H20: C. 48.40; H, 3.60; N, 10.26. Found: C,
48.81;
H, 3.66; N, 10.43.
Exam l
6-f aminoiminomethyll-N-(5-bromo-2-thiazolyll)-2-nanhthalenecarboxamide
mono(trif?uoroacetate)(salt~
Example 197A
Using the procedure described for Example 184A and substituting 2-amino-5-
~5 bromothiazole for 4-aminobenzamide, the desired compound was obtained.
MS (DCI) m/z 358 (M+H)+.
Example 197B
6-faminoiminomethvll-N-(5-bromo-2-thiazolvll)-2-naphthalenecarboxamide
3o monoltrifluoroacetate)(salt~
Using the procedure described in Example 1B and the product obtained in
Example
197A, the desired compound was obtained.
MS (ESI+) m/z 375 (M+H)+;
-187-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98/15386
1H NMR (300 MHz, DMSO) 8 10.85 (s, 1H), 9.55 (s, 2H), 9.24 (s, 2H), 8.87 (s,
iH), 8.57
{d, I H, J=1.69), 8.31 (d, I H, J=8.47), 8.25 (d, 2H, J=1.01 ), 7.92 (dd, 1 H,
J=8.48, 2.04),
7.71 {s, 1 H);
Anal. calc'd for C~~HI2BrF3SN403~ 1.25 H20~0.25 TFA: C. 38.90; H, 2.75; N,
10.37.
Found: C, 38.97; H, 3.24; N, 10.66.
Example 198
6-(aminoiminomethvl)-N-(5-methyl-2-pvridinvl)-2-naphthalenecarboxamide
mono(trifluoroacetate)(salt)
to
Example 198A
Using the procedure described for Example 184A and substituting 2-amino-5-
picolene
for 4-aminobenzamide, the desired compound was obtained.
MS (ESI+) m/z 288 (M+H)+.
Example 1988
6-(aminoiminomethvl)-N-(5-methyl-2-p 'dinyl~-2-naphthalenecarboxamide
mo_no(trifluoroacetate)(salt)
Using the procedure described in Example 1 B and the product obtained in
Example
2U 198A, the desired compound was obtained.
MS (DCI) m/z 305 (M+H)+;
1 H NMR (300 MHz, DMSO) 8 1 I .0 l (s, 1 H), 9.50 (s, 2H), 9.16 {s, 2H), 8.79
(s, 1 H), 8.54
(s, 1 H), 8.30 (d, 1 H, J=9.19), 8.27 (d, I H, J=1.47), 8.20 (s. 2H), 8. I 5
(d, 1 H, J=8.83),
7.89 (dd. 1 H, J=8.46, 1.48), 7.72 (dd, I H, J=8.46, 1.84), 2.31 (s, 3H);
Anal. calc'd for C2pH17F3N403~0.25 H20~0.2 TFA: C, 54.98; H, 4.00; N, 12.57.
Found: C,
54.99; H, 3.59; N, 12.43.
Example 199
6-laminoiminomethvl)-N-(4-methyl-2-thiazolyl)-2-naphthalenecarboxamide
mono(trifluoroacetate)(salt)
Example 199A
Using the procedure described for Example I84A and substituting 2-amino-5-
methyl
benzothiazoIe for 4-aminobenzamide, the desired compound was obtained.
-188-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98115386
MS (ESI-) m/z 293 (M+H)-.
Example 199B
6-(aminoiminomethvll-N-(4-methyl-2-thiazolvl)-2-naphthalenecarboxamide
monoltrifluoroacetate)(salt)
Using the procedure described in Example 1 B and the product obtained in
Example
I 99A, the desired compound was obtained.
MS (ESI+) m/z 31 I (M+H)+;
~ H NMR (300 MHz, DMSO) 8 9.51 (s, 2H), 9.25 (s, 2H), 8.86 (s, 1 H), 8.55 (s,
1 H), 8.30
{d, 1H, J=8.48), 8.25 (d, 1H, J=2.03), 7.91 (dd, 1H, J=8.48, I.70), 6.87 (s,
1H), 2.34 (s,
3H);
Anal. calc'd for CigH~5F3N4S03~0.5 TFA: C, 47.40; H, 3.25; N, I I.64. Found:
C, 47.90;
H, 3.36; N, 11.71.
Example 200
6-(aminoiminomethvl l-4-f 5-(ethylthio)-3-furanyll-N-phenyl-2-
naphthalenecarboxamide
monohydrochloride
Example 200A
The desired compound was prepared from 2-trimethylsilyl-3-bromofuran and
diethyldisulfide by the procedure of Example 154A.
MS (DCI/NH3) m/z 279, 281 (M+H)+.
Example 200B
A solution of the product from Example 200A (8.60 g, 30.8 mmol) in THF (20 mL)
and a 1 M solution of TBAF (61.6 mL) was stirred for 24 hours. The reaction
was condensed
and chromatographed on Si02 using hexanes as eluent, to yield 3.32 g (52%) of
2-ethylthio-4-
bromofuran. The desired compound was prepared from this material by the
procedure of
Example 57A.
MS (DCI/NH3) m/z 127 (M-B (OH)z)+.
Example 200C
The desired compound was prepared from the product from Example 200B and the
product from Example 152C by the procedure of Example 57B.
- I 89-
SU9STITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98/15386
MS (DCI/NH3) m/z 416 (M+NH4)+.
E_xamnle 200D
6-faminoiminomethvl)-4-15-(ethvlthio)-3-furanyll-N-yhenyl-2-
naphthalenecarboxamide
monohvdrochloride
The desired compound was prepared from Example 200C and the procedure of
Example
144C.
MS (DCI/NH3j m/z 416 (M+H)+;
~ H NMR (300 MHz, DMSO-d6) 8 1.30 (t. 3H), 2.92 (q, 2H), 7.15 (t, 1 H), 7.34
(s, 1 H),
t0 7.40 (t, 2H), 7.83 (d, 2H), 7.92 (dd, 1H), 8.20 (d, 1H), 8.40 (d, 1H), 8.47
(s, 1H), 8.60 (s,
1 H), 8.69 (s, 1 H), 9.21 (br s, 2H), 9.58 (br s, 2H), 10.61 (s, 1 H);
Anal. calc'd for C24H21N3S02~1.5 HCI: C, 61.31; H, 4.82; N, 8.94. Found: C,
61.39; H,
4.89; N, 9.03.
is Example 201
6-(aminoiminomethvl)-4-f S-(pronylthio)-3-furanyll-N-phe~l-2-
naphthalenecarboxamide
monohydrochloride
Example 20I A
2o The desired compound was prepared from 2-trimethylsilyl-3-bromofuran and
dipropyldisulfide by the procedure of Example 1S4A.
MS {DCI/NH3) m/z 293, 29S (M+H)+.
Example 201 B
?S The desired compound was prepared from the product from Example 201 A by
the
procedure of 1 S4B.
MS (DCl/NH3) m/z 432 (M+H)+.
Example 201 C
3o The desired compound was prepared from the product from Example 201B and
the
product from Example 1 S2C by the procedure of Example I S4C.
MS (DCI/NH~) m/z 430 (M+NH4)+.
Example 201 D
-190-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98/15386
6-laminoiminomethvl)-4-f5-l r~o~ylthio)-3-furan l~phen 1-y_
2~naphthalenecarboxamide
rt~onohvdrochloride
The desired compound was prepared from Example 201C and the procedure of
Example
144C.
MS (DCI/NH3) m/z 430 (M+H)+;
~H NMR {300 MHz, DMSO-d6) 8 I.O1 (t, 3H), 1.66 (qt, 2H), 2.89 (t, 2H), 7.15
(t, 1H),
7.34 (s, 1H), 7.40 (t, 2H), 7.84 (d, 2H), 7.92 (dd, 1H), 8.20 (d, 1H), 8.40
(d, IH), 8.47 (s,
1 H), 8.60 (s, 1H), 8.69 (s, 1 H), 9.22 (br s, 2H), 9.58 (br s, 2H), 10.61 (s,
1 H);
Anal. calc'd for C25H23N3S02~ I .25 HC1: C, 63.20; H, 5. I4; N, 8.84. Found:
C, 63.24; H,
to 5.16; N, 8.93.
Example 202
6-laminoiminomethyl)-N-l6-quinolinvl)-2-naphthalenecarboxamide
bis(trifluoroacetate)(salt)
(5
Example 202A
To a solution of the acid chloride, Example 8B, (331 mg, 1.5 mmol) in THF ( 15
mL),
at room temperature, was added propylene oxide ( 10 mL), DMAP (5 mg), a drop
of
triethylamine and finally 6-aminoquinoline (288 mg, 2.0 mmol). After 4 hours
at room
2O temperature, added ethyl acetate ( 10 mL) and ether (20 mL) and filtered
the off-white solid
product. Yield 357 mg (7290).
MS (DCI/NH3) m/z 324 (M+H)+.
Examlale 202B
25 6-laminoiminomethvl)-N-(6-quinoiinvl)-2-n~phthalenecarboxamide
bis(trifl uoroacetate)lsalt)
The desired compound was prepared as described in Example 1 B.
MS (ESI+) m/z 341 (M+H)+, (ESI-) 339 (M-I)-;
~ H NMR (300 MHz, DMSO-d6) 8 10.98 (s, 1 H), 9.53 (s, 2H), 9.25 (s, 2H), $.93-
8.91 (m,
30 1 H), 8.77 (s, 1 H), 8.67 (d, J= I .BHz, 1 H), 8.58 (s, 1 H), 8.53 (d,
J=8.SHz, 1 H), 8.37-8.09
(m, SH), 7.93 (dd, J 1=B.SHz, J2=I .BHz, 1 H), 7.65-7.62 (m, 1 H);
Anal. calc'd for C=,H,6N40~2TFA: C, 52.83; H, 3.19; N, 9.86. Found: C, 52.62;
H, 2.94; N,
9.74.
-191-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98/15386
Exam Ip a 203
6-(aminoiminomethyl)-N-( 1 H-indazol-6-vl)-2-n~phthalenecarboxamide
bis(trifluoraacetat s It
S Example 203A
The desired compound was prepared as described for Example 202A but
substituting 6-
aminoquinoline for 6-aminoindazvle to provide 285 mg of the desired compound.
MS: ESI+: 313 (M+1); ESI-311 (M-I).
Example 203B
6-(aminoiminomethvl)-N-(1 H-indazol-6-yIl-2-nanhthalenecarboxamide
bis(trifluoroasetate)(taltl
To a suspension of the ammonium chloride ( 140 mg, 2.6 mmol) in Toluene (2 mL)
at
0°C was slowly added a solution of 2 N trimethylaluminium in toluene
(871~tL, 1.74 mmol).
is After 5 minute the reaction mixture was allowed to warm to room temperature
for 3U minutes.
To the solution of the aluminium reagent at room temperature was added the
nitrite, Example
203A from section (a) and the reaction mixture was heated to 100°C for
48 hours. The reaction
mixture was cooled down then was poured into a suspension of silica in
chloroform and stirred
for an hour. The silica was filtered then washed with methanol. The solvent
was concentrated
zo and purified by medium pressure liquid chromatography on a 30 cm x 2 cm C-
I8 column (40
micron, J.T Baker) with UV detection at 250 nM with solvent mixtures in a
gradient ranging
from 90%A (0.1% aq TFA)/IU%B (methanol) to 10%A/90%B over 160 minutes at a
flow rate
of 5 mL/min (fractions were collected every 2 minutes for 100 min, to provide
42 mg of the
desired compound.
25 MS (ESI+) m/z 330 (M+H)+, (ESI-) 328 (M-1)-;
~H NMR (300 MHz, DMSO-d6) 8 10.65 (s, IH), 9.47 (m, 4H), 8.65 (s, 2H), 8.50
(s, 2H),
8.25-8.23 (m, 2H), 8.17-8.10 (m, 2H), 7.94 (s, 1H), 7.86-7.84 (dd, J1=8.8Hz,
J2=l.6Hz,
1 H), 7.66 (d, J=8.SHz, I H), 7.37 (d, J=8.4Hz, 1 H);
Anal. calc'd for C19H15Ng 0~2 TFA: C, 49.56; H, 3.07; N, 12.56. Found: C,
49.68; H,
~a 3.10; N, 12.47.
Example 204
6-laminoiminomethvl )-N-( I H-indazol-5-yI)-2-naphthalenecarboxamide
bisltrifluoroacetat .ll.calt)
-192-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98115386
Example 204A
The desired compound was prepared as described for Example 202A but
substituting 6-
aminoquinoline for 5-aminoindazole to provide 362 mg of the desired compound.
MS: ESI+: 313 (M+1); ESI-311 (M-I).
Example 204B
6-(aminoiminomethvll-N-l I H-indazol- ;yl -2-na hthalenecarboxamide
bisltrifluoroacetate)(salt),
lU The desired compound was prepared as described for Example 203B to provide
55 mg
of the desired compound.
MS (ESI+) m/z 330 (M+H)+, (ESI-) 328 (M-I)-;
1 H NMR (300 MHz, DMSO-d~,) ~ 13.06 (s, I H), 10.58 (s, 1 H), 9.51 (s, 2H),
9.15 (s, 2H),
8.71 (d, j=l.9Hz, 1H), 8.56 (d, j=l.9Hz, 1H), 8.34-8.17 (m, 4H), 8.10 (s, IH),
7.90 (dd,
~5 J 1=B.SHz, J2=1.7Hz, 1 H), 7.63 {dd, J 1=8.9Hz, J2=I.7Hz, 1H), 7.56 (d,
J=8.8Hz, 1 H);
Anal. calc'd for CIyHiSNS O~TFA~ 1.75 H20: C, 53.11; H, 4.14; N, 14.75. Found:
C,
53.20; H. 3.99; N, 14.42.
Example 205
20 6-laminoiminomethyt)-N-l I H-indol-5-vll-~-nawhthalenecarboxamide
mono(trifluoroacetate)( altl
Example 205A
The desired compound was prepared as described for Example 202A but
substituting 6-
25 aminoquinoline for 6-aminoindole to provide 744 mg of the desired compound.
MS: (ESI)+: 329 (M+1)+ and (ESI)-: 327 (M-1)-.
Example 205B
fi--(aminoiminomethvl)-N-l 1 H-indol- -yl~nayhthalenecarboxamide
monoltrifluoroacetate)(saltl
The desired compound was prepared as described for Example 203B to provide 90
mg
of the desired compound.
MS (ES1*) m/z 329 (M+H)+, (ESI-) 327 (M-1)-;
-193-
SU6STiTUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98/15386
~H NMR (300 MHz, DMSO-d6) 8 11.09 (s, 1H), 10.40 (s, 1H), 9.5I (s, 2H), 9.15
(s, 2H),
8.71 (s, 1H), 8.55 (s, IH), 8.32 (d, J=8.4Hz, 1H}, 8.26-8.17 (m, ZH), 8.05 (d,
J=2.6Hz,
1 H), 7.90 (dd, J 1=8.4Hz, J2=1.BHz, 1 H), 7.46-7.35 (m, 3H), 6.45-6.43 (m, 1
H);
Anal. calc'd for C2pH16N4 O~TFA: C, 59.73; H, 3.87; N, 12.66. Found: C, 59.27;
H, 4.17;
N, 12.74.
Example 206
7-f2-(4-momholinvl)ethoxvl-2-naphthalenecarboximidamide
bis(trifluoroacetate)(salt)
1 p Example 206A
The nitrite was prepared as described in Example 119A using 2-
chloroethylmorpholine.
MS (DCI (NH3)j m/z 283 (M+H) +.
Example 206B
7-f2-(4-momholinvl)ethoxvl-2-naphthalenecarboximidamide
bis(trifluoroacetate)(salt)
The desired compound was prepared as described in Example 1 19B, as an off-
white
solid (50°lo yield).
MS (DCI (NH3)) m/z 300 (M+H)+;
~H NMR (300 MHz, DMSO-d6j 8 3.500 (br m, 4H), 3.700 (br m, 2H), 3.990 (br m,
4H),
2p 4.490 (br m, 2H), 7.435 (dd, 1 H), 7.530 (d, 1 H), 7.680 (dd, 1 H), 8.035
(d, 1 H), 8.100 (d,
1 H), 8.345 (d 1 H), 9.165 (br s, 2H), 9.420 (br s, 2H);
Anal. calc'd for CpH21N302~ (C2H02F3) 2.15: C, 46.98; H, 4.29; N, 7.72. Found:
C, 47.00;
H, 4.32; N, 7.77.
'S Example 207
6-(aminoiminomethyl)-N-phen 1-v 4-(2-pyrrolidinyl)-2-naphthalenecarboxamide
mono(trifluoroacetate)(salt)
Exam,~le 207A
3p The desired compound was prepared from the product from Example 152A, by
the
procedure of Example 68A.
MS (DCI/NH3) m/z 281 (M+H)+.
Example 207B
-194-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98115386
The desired compound was prepared from the product from Example 207A by the
procedure of Example 152B.
MS (DCI/NH3) m/z 267 (M+H)+.
Example 207C
A solution of the product from Example 207B (220 mg, 0.826 mmol),
diisopropylethyl
amine (0.288 mL, 1.65 mmol), and O- (7-azabenzotriazol-1-yl)-N, N, N', N'-
tetramethyluronium hexafluorophosphate (314 mg, 0.826 mmoI) in DMF ( 10 mL)
was stirred
for 30 minutes at 0°C. Aniline (0.083 mL, 0.909 mmol) was added, and
the reaction was
t0 stirred at room temperature for 4 hours. The reaction was poured into
saturated aqueous
Na2C03 solution, and extracted with 3x ethyl acetate. The combined extracts
were washed with
water and brine, dried over Na2S04, and condensed. The crude material was
recrystallized
from ethanol/hexanes to yield 212 mg (75%) of the desired compound.
MS (DCI/NH3) m/z 342 (M+H)+.
iS
Exam In a 207D
6-(aminoiminomethvl)-N-phenyl-4-!2-pyrrolidin I -2-n ~~phthalenecarboxamid~,
monoltrifluoroacetatel( altl
The desired compound was prepared from Example 207C and the procedure of
Example
20 t B .
MS (DCI/NH3) m/z 359 (M+H)+;
IH NMR (300 MHz, DMSO-d~,) 8 2.02 (t, 4H), 3.55 (t, 4H), 7.13 (t, IH), 7.38
(t, 2H), 7.41
(s, 1 H), 7.80 (m, 3H), 8.08 (s, I H), 8. I 8 (d, 1 H), 8.67 (s, I H), 9.10
(br s. 2H), 9.43 (br s,
2H), 10.41 (br s, IH);
25 Anal. calc'd for C22H22N40~ 1.0 C2HF302: C, 61.01; H, 4.91; N, I 1.86.
Found: C, 60.47;
H, 5.36; N, 7.39.
Example 208
6-faminoiminomethvl)-N-l5-Ryrimidinvll-2-nanhthalenecarboxamide
-t~l monoltrifluoroacetate)( alt?
Example 208A
The above product was prepared in the manner of Example 8A using 5-
aminopyrimidne.
- I 95-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98/15386
MS (APCI) m/z (M+H)+ 275.
~xamnle 208B
6-(aminoiminomethyI)-N-(5-pvrimidinyl~-~-naphthalenecarboxamide
mono(trifluoroacetate)(sal 7
The above was prepared as described in Example 1B.
MS (CI) m/z (M+H}+ 292;
~H-NMR (300 MHz, DMSO-d6) 8 10.99 (s, 1H), 9.52 (s, 2H), 9.27 (s, ZH), 9.24
(s, 2H),
8.98 (s, IH), 8.76 (s, 1H), 8.58 (s, IH), 8.36-8.18 (m, 3H), 7.92 (dd> J=1.5,
8.4 Hz, 1H);
Anal. calc'd for C~gH14N503F3 7/10 TFA: C, 47.97; H, 3.05; N, 14.40. Found: C,
47.78;
H, 3.05; N, 14.67.
Exam In a 2~9
6-laminoiminomethyl -N-('~-pyri a~inyl)-2-nanhthalenecarboxamide
5 mono(trifluoroacetatel(salt)
Example 209A
3-Amino-6-chloropyridazine ( I.OS g, 8.2 mmol) was dissolved in 10 mL methanol
with
2 mL ammonia/methanol. Palladium/carbon (200 mg, 10°~0) was added and
stirred under 1 atm
hydrogen for 4 hours. The reaction was filtered, concentrated, and used
without further
purification.
MS (CI) m/z (M+H)+ 96.
Example 209B
The above product was prepared in the manner of Example 12 using the product
from
Example 209A.
MS (APCI) m/z (M+H)+ 275.
Example 2090
6-(aminoiminomethyl)-N-(3-p ridazinyll-2-nanhthalenerarboxarnid~,
mono(trifluoroacetatP)(~,I ~,
The above was prepared from Example 209B as described in Example I B.
MS (CI) m/z (M+H)+ 292;
-19b-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS98115386
tH-NMR (300 MHz, d6-DMSO) 8 11.72 (s, iH}, 9.51 (s, 2H), 9.22 (s, 2H), 9.06
(m, IH),
8.86 (s> IH), 8.56 (s, IH), 8.45 (d, 1H), 8.31 {d, IH)> 8.23 (s, 2H), 7.90
(dd, 1H), 7.78
(dd> I H)
Anal. calc'd for C1gH14N503F3 1/2 TFA: C, 49.29; H, 3.16; N, 14.73. Found: C,
49.56; H,
3.23; N, 14.73.
Example 210
6-(aminoiminomethyl)-N-(5-bromo-2-p"vridin I -2-naphthalenecarboxamid~
mono(trifluoroacetate)(salt)
Example 210A
Using the product obtained in Example 8E, 2-amino-5-bromopyridine, and the
procedure described for Example 8G the desired compound was obtained.
MS (APCI+) tn/z 352 (M+H)+.
1S
Example 210B
6-(aminoiminomethvl)-N-(5-bromo-2-pyridin 11-2-naphthalenecarboxamide
mono(trifluoroacetate)(salt)
Using the procedure described in Example 1B and the product obtained in
Example
20 210A, the desired compound was obtained.
MS (DCI) m/z 369 (M+H)+;
tH NMR (300 MHz, DMSO) 8 I 1.30 (s, 1H), 9.51 (s, 2H), 9.17 (s, 2H), 8.80 (s,
1H), 8.57
(d, 1 H, J=2.57), 8.55 (s, 1 H), 8.31 (d, 1 H, J=8.45), 8.26 (d, 1 H, J=8.82),
8.19-8.24 (m,
2H, ), 8.14 (dd, I H, J=2.57, 9.19), 7.90 (dd, 1 H, J=1.83, 8.82;
25 Anal. calc'd for C19Hj4BrF~N403: C, 47.22; H, 2.92; N, 1 1.59. Found: C,
47.60; H, 3.01;
N, 11.30.
Example 211
6-(aminoiminomethvl)-N-13-(I-methvlethoxy)phen I -2-naphthalenecarboxamide
3o mono(trifluoroacetatel(salt)
Example 21 I A
The above product was prepared in the manner of Example 12 using 3-
isopropoxyaniline.
-197-
SUBSTITUTE SHEET (RUtE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98/15386
MS (APCI) m/z (M+H)+ 331.
Example 211 B
6-(aminoiminomethvl)-N-f3-l I-methylethoxy)phenyll-2-na",phthalenecarboxamide
mono(trifluoroacetatel(salt~
The above was prepared from Example 21 IA using method 1B.
MS (CI) m/z (M+H)+ 348;
t H-NMR (300 MHz, d6-DMSO) 8 10.50 (s, 1 H), 9.50 (s, 2H), 9.22 (s, 2H), 8.67
(s, 1 H),
8.56 (s, 1 H), 8.3I (d, 1 H), 8.25-8. I 3 (m, 2H), 7.90 (dd, I H), 7.51 (m, 1
H), 7.36 (m, I H),
7.26 (t, 1 H), 6.68 (dd, I H), 4.59 (m, 1 H), 1.30 (d, 6H)
Anal. calc'd for C23H2~N304F3 1/5 TFA: C, 57.96; H, 4.61; N, 8.66. Found: C,
57.99; H,
4.90; N, 8.68.
Example 212
t5 2-IT6-(aminoiminome~hvl)-2-na hthalen~lo~lacetic acid
mono(trifluoroacetate)(salt)
The product from Example 185C ( 140 mg, 0.542 mmoi) was dissolved in methanol
( 1 1
mL). To this was added a solution of lithium hydroxide (68.2 mg, 1.626 mmol)
in water (3
mL) and the resulting mixture was stirred at room temperature under an inert
atmosphere for 18
2o hours. The reaction was evaporated and the residue purified by reverse
phase chromatography
to yield the desired compound (102 mg, 52%).
MS {DCI (NH3)) m/z 245 (M+H)+;
t H NMR (300 MHz, DMSO-d6) b 4.875 (s, 2H), 7.420 (s, 1 H), 7.435 (dd, i H),
7.660 (dd,
1 H), 8.015 (d, I H), 8.100 (d, 1 H), 8.340 (d, 1 H), 9.125 (br s, 1 H), 9.420
(br s, 1 H;
?S Anal. calc'd for C13H~2N203~ (C2H02F3)t.3(t: C. 47.74; H, 3.42; N, 7.14.
Found: C, 47.93;
H, 3.36; N, 7.I7.
Example 213
methyl 4-f6-(aminoiminomethyl~2-na~hthalenyl loxvlmethvilbenzoate
3(~ monoltrifluoroacetate)(salt)
Example 2I A
The resulting product from Example 185A was treated with methyl 4-
(bromomethyl)benzoate in an analogous manner as described in Example 119B.
-198-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCT/US98115386
MS (DCI (NH3)) m/z 335 (M+NH4)+.
Example 213B
methyl4-16-(aminoiminometh l~aphthalen llox l~hyllbenzoate
mono(trifluoroacetate)(salt~
The resulting product from Example 213A (250 mg, 0.78$ mmol) was treated in an
analogous manner as described in Example 119C to yield the desired compound (
130 mg,
79%).
MS (DCI (NH3)) m/z 335 (M+H) +;
~u ~H NMR (300 MHz, DMSO-d6) 8 3.870 (s, 3H), 5.400 (s, 2H), 7.500 (dd, 1H),
7.540 (d,
1 H), 7.619 (dd, 1 H), 7.620 (d, 2H), 8.025 (d, 2H), 8.026 (d, I H), 8.090 (d,
1 H), 8.410 (d,
1 H), 9.260 (v br s, 3H);
Anal. calc'd for C~4H14N~03~C2H02F3~H20 0.70: C, 57.32; H, 4.46; N, 6.08.
Found: C,
57.33; H, 4.70; N, 5.95.
l5
Example 214
6-(aminoiminomethvl)-N-( 1 H-imidazolyl)-2-naphthalenecarboxamide
bis(trifluoroacetate)(salt)
2u Example 214A
Using the product obtained in Example 8E, 2-aminoimidazole, and the procedure
described for Example 8G the desired compound was obtained.
MS (ESI-) m/z 261 (M+H)-.
25 Example 2I4B
Using the procedure described in Example 1 B and the product obtained in
Example
214A, the desired compound was obtained.
MS (ESI+) m/z 280 (M+H)+;
~ H NMR (300 MHz, DMSO) 8 9.50 (s, 2H), 9.16 (s, 2H), 8.78 {s, I H), 8.53 (s,
1 H), 8.26-
30 8.31 (m, 2H), 8.20 (d, 1H, J=8.46), 7.88 (dd, 1H, J=1.84, 8.83), 6.95 (s,
2H);
Anal. calc'd for C17H~4F3N503~0.2 TFA~H20: C, 48.14; H, 3.76; N, 16.13. Found:
C,
48.54; H, 3.40; N, 16.02.
Example 215
-199-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98115386
6-12-f4-lhvdrox m~eth_yllphen_yll-1-cvclo~ropvll-2-naphthaleneca_rboximidamide
monoltrifluoroacetate)l alt)
Example I SA
The material prepared as described in Example 104 (210 mg, .52 mmol) is
dissolved in
THF (6 mL) and added dropwise to 10 mL diazomethane cooled to 0 °C then
added Pd (OAc)2
(9.8 mg). Vigorous bubbling occurs for 5 minutes. the resulting black slurry
is stirred 20 min,
filtered and solvent removed under vacuum leaving 0. I g clear oil.
MS (DCI/NH3): m/z {M+NH4+): 316.
IU
Examvle 215B
6-f2-14-(hvdroxvmethvl)phen Iv 1-I-cyclopropyll-2-nanhthalenecarboximidamide
monoltrifluoroacetate)(cal~
The desired compound is prepared as described in Example l, purified by
reverse phase
chromatography to give 19.9 mg of white solid.
MS (DCl/NH3) m/z (M+H)+ 316;
~H-NMR (300 MHz, DMSO-d6) b 9.41 (s, 2H), 9.16 (s, 2H), Ii.46 (s, lHj, 8.08
(d, 2H),
H.03 (d, 1 H), 7.85 (s, 1 H), 7.75 (dd, 1 H), 7.58 (dd, 1 H), 7.3-7.1 (m, 4H),
4.49 (s, 2H),
2.38-2.48 (m, 2H), 1.61-1.70 (m, 2H);
2o Anal. calc'd for C23H21N202F3 I H20: C, 62.10; H, 4.80; N, 6.29. Found: C,
62.00; H, 4,
7s; N, 6.zs.
Exam lp a 216
N-(ethoxvcarbonvl)-6-l2-phenyl-1-cyclo rop I~a~phthalenecarboximidamide
The sample described in Example 97 ( 130 mg, .45 mmolj is dissolved in DMF (3
mL)
cooled to 0 °C. and treated with triethylarnine (0.01 mL) and ethyl
chloroformate (0.05 mL).
The resulting solution is stirred three days at room temperature then diluted
with 100 mL ethyl
acetate washed with distilled water (20 mL), dried over anhydrous sodium
sulfate, filtered and
solvent removed under vacuum leaving a clear oil. The oil is purified by
silica gel
chromatography eluting with 2: 1 hexanes/ethyl acetate, lyophilized and the
desired compound
is isolated as a white powder (55 mg).
MS (DCI/NH3) m/z (M+H)+ 359;
-200-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99!05096 PCTIUS98/15386
t H-NMR (300 MHz, DMSO-d6} b 9.21 (s, 2H), 8.46 (s, I H), 7.83 (d, 2H), 7.62
(s, I H),
7.58 (dd, 1H), 7.34 (dd, 1 H), 7.35-7.29 (m, 3H), 7.25-7.17 (m, 2H) 4.1 (q,
2H), 2.38-2.28
(m, 2H), 1.61 (t, 2H), I.25 (t, 3H);
Anal. calc'd for C23H22N2O2 C, 77.07; H, 6.19; N, 7.82. Found: C, 76.63; H,
6.05; N,
7.45.
Example 217
6-(aminoiminomethvl)-N-(2-methvl-6 guinolinyl)-2-naphthalenecarboxamide
bis(trifluoroacetate)(salt)
t0
The desired compound is prepared in a similar manner as described in Example
8E and
144C.
MS m/z: 355 (M+H)+
~ H NMR (DMSO, 300 MHz): 10.95 (s, 1 H}, 9.5 I (s, 2H), 9.14 (s, 2H), 8.75 (s,
1 H), 8.65
(s, 1H), 8.57 (s, 1H), 8.35 (dd, 1H, J1=J2=8.5 Hz), 8.28 (dd. 1H,
Jl=J2=B.SHz), 8.19 (dd,
1 H, J I =J2=8.8 Hz), 8.1 S (dd. 1 H, J 1=J2=$.3 Hz). 8.04 (dd, 1 H, J
I=J2=8.8Hz}, 7.91 (dd,
1 H, J I=8.4 Hz, J2=8.8 Hz), 7. 60 (dd, 1 H, J 1=J2=8. I Hz). 5.99 (S, 3H);
Anal. calc'd for C22HtgN40~2.25 C2F302H~2 H20: C, 49.20; H, 3.78; N, 8.66; F,
I9.82.
Found: C, 49.02; H, 3.36; N, 8.66.
Example 218
6-(aminoiminomethvl)-N-(3-propox~henvl)-~-naphthalenecarboxamide
monoftrifluoroacetate)(salt)
'S Example 218A
3-Aminophenol ( 1 g, 7.2 mmol), triphenylphosphine (2.25 g, 8.6 mmol), and 1-
propanol (0.517 g, 8.6 mmol) were dissolved in 25 mL anhydrous THF.
Diethylazodicarboxylate ( 1.S g, 8.6 mmol) was added dropwise over I minute.
The solution
was allowed to stir I S minutes and poured slowly into hexanes while stirring.
Filtration through
3o silica gel/celite afforded the product as a viscous yellow oil.
MS (APCI) m/z (M+H)+ 152.
Example 218B
-201-
SUBSTtTUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US981I5386
The above product was prepared in the manner of Example i 2 using the product
from
Example 218A.
MS (APCI} m/z (M+H)+ 331.
Exam lep 2180
6-(aminoiminomethvl)-N-(3~~y Rhenyl)-2-naDhthalPnecarboxamide
monoltrifluoroacetate)(sal )
The above was prepared from Example 218 as described in Example 1B.
MS (CI) m/z (M+H)+ 348;
lu ~ H-NMR (300 MHz, d6-DMSO) 8 10.51 (s, 1H), 9.50 (s, 2H), 9.18 (s, 2H),
8.68 (s, 1 H),
8.55 (s, 1 H}, 8.32 (d, 1 H), 8.25-8. I 3 (m, 2H), 7.90 (dd, 1 H), 7.52-7.33
(m, 2H), 7.27 (t,
1 H), 6.73 (dd, 1 H), 3.94 (t, 2H), 1.75 m, 2H), 1.00 {t, 3H)
Anal. calc'd for C23H2ZN304F3: 1/20 TFA: C, 59.49; H, 4.77; N, 9.02. Found: C,
59.43; H,
4.94; N, 9.10.
IS
Example 219
6-(aminoiminomethvl)-N-f 3-( 1-ethylpropoxy)phen Iv 1-2-naphthalenecarboxamide
mono(trifluoroacetate)(salt~
20 Example 2I9A
The above product was prepared in the manner of Example 218A using 3-pentanol.
MS (APCI) m/z (M+H)+ 18().
Example 219B
25 The above product was prepared in the manner of Example 12 using the
product from
Example 219A.
MS (APCI) m/z (M+H)+ 359.
Exam 1 19
30 fi~aminoiminomethvl)-N-13-(1-eth~propoxylphenvll-2-naphthalenecarboxamide
mono(trifluoroacetate)( alt)
The above was prepared from Example 219B as described in Example 1 B.
MS (CI) m/z (M+H)+ 376;
-202-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/0509b PCT/US98/15386
IH-NMR (300 MHz, d6-DMSO) b 10.47 (s, IH}, 9.49 (s, 2H), 9.14 (s, 2H), 8.67
(s, 1H),
8.55 (s, I H), 8.31 (d, 1 H), 8.25-8.16 (m, 2H), 7.90 (dd, I H), 7.5 I (s, 1
H), 7.38 (m, 1 H),
7.26 (t, 1H), 6.72 (dd, IH), 4.18 (m, 1H), 1.65 (m, 4H), 0.93 (t, 6H)
Anal. calc'd for C25H26N304F3: C, 61.34; H, 5.35; N, 8.58. Found: C, 61.05; H,
5.42; N,
8.22.
Example 220
6-(aminoiminomethvl)-N-13-(cyclopentvlox~phenyll-2-naphthalenecarboxamide
mono(trifluoroacetate)(salt)
Example 220A
The above product was prepared in the manner of Example 218A using
cyclopentanol.
MS (APC1) m/z (M+H)+ 86.
t5 Example 220B
The above product was prepared in the manner of Example I 2 using the product
from
Example 220A.
MS (APCI) m/z (M+H)+ 357.
2u Example 220C
6-(aminoiminomethvl)-N-f 3-(cyclopentyloxv)phenyll-2-naphthalenecarboxamide
mono(trifluoroacetate)(salt)
The above was prepared from Example 220B as described in Example 1 B.
MS (CI) m/z (M+H)+ 374;
25 ~ H-NMR (300 MHz, d~-DMSO) 8 10.50 (s, 1 H), 9.51 (s, 2H), 9.30 (s, 2H),
8.68 (s, 1 H),
8.56 (s, I H), 8.32 (d, 1 H), 8.25-8.13 (m, 2H), 7.90 (dd, 1 H), 7.49 (m, I
H), 7.38 (m, 1 H),
7.26 (t, 1 H), 6.72 (dd, 1 H), 4.79 (m, 1 H}, I .96- I .08 (m, 8H}.
Anal. calc'd for C25H24N304F3 2/5 TFA: C, 60.68; H, 5.06; N, 8.49. Found: C,
60.68; H,
5.33; N, 8.65.
Example 221
6-(aminoiminomethyl)-N-(3-phenoxYphenyI)-2-naphthalenecarboxamide
mono(trifluoroacetate)(salt)
-203-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTlUS98115386
Examlale 221 A
The above product was prepared in the manner of Example 218A using 3-
phenoxyanline.
MS (APCI) m/z (M+H)+ 365.
Example 221 B
6-(aminoiminomethvl)-N-(3- henoxyphenvl)-2-naphthalPnecarboxamide
monoltrifluoroacetatel( alts
The above was prepared from Example 221A as described in Example 1B.
t0 t H-NMR (300 MHz, d6-DMS O) 8 10.61 (s, I H), 9.50 (s> 2H), 9.20 (s, 2H),
8.66 (s, 1 H),
8.54 (s, 1 H), 8.30 (d, 1 H), 8.22 (d, 1 H), 8.12 (dd, 1 H), 7.90 (dd, 1 H),
7.64-7.57 (m, 2H),
7.46-7.37 (m, 3H), 7.I7 (m, 1H), 7.06 (m, 2H), 6.79 (dd, IH),
MS (CI) m/z (M+H)+ 382;
Anal. calc'd for CZ~,H2pN304F3: C, 63.03; H, 4.07; N, 8.48. Found: C, 62.87;
H, 4.24; N,
8.08.
Exam~222
6-(aminoiminomethvl)-N-f3-(phenylmethox~phenyll-2-nanhthalenecarboxamide
monoltrifluoroacetate)(salt)
2~t Example 222A
The above product was prepared in the manner of Example 218A using 3-
benzyloxyaniline.
MS (APCI) m/z (M+H)+ 379.
Example 222B
6-(aminoiminomethvl)-N-f3-(nhenylmethoxyZphenvll-2-n~phthalenecarboxamide
monoltrifluoroacetate)haltl
The above was prepared from Example 222A as described in 1B.
MS (CI) m/z (M+H)+ 396;
3c) 1 H-NMR (300 MHz, d6-DMSO) 8 10.53 (s, 1 H), 9.50 (s, 2H), 9.22 (s, 2H),
8.68 {s, I H),
8.55 (s, 1H), 8.31 (d, 1H), 8.23 (d, IH), 8.14 (dd, IH), 7.90 (dd, 1H), 7.61-
7.27 (m, 8H),
6.80 (dd, IH), 5.13 (s, 2H)
Anal. calc'd for C27H22N304F3: C, 63.65; H, 435; N, 8.25. Found: C, 63.48; H,
4.27; N,
8.07.
-204-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98/15386
Exam 1
6-(aminoiminomethvl)-N-l3-ethoxpenyl -) 2-n~, halPnecarboxami~e
monoltrifluoroacetate)lsatrl
Example 223A
The above product was prepared in the manner of Example 218A using 3-
ethoxyaniline.
MS (APC1) m/z (M+H)+ 317.
t~ Example 223B
6-laminoiminomethvl)-N-f3-ethoxymhenyll-2-naphthalenecarboxamide
monoltrifluoroacetate)l alt(
The above was prepared from Example 223A as described in Example 1B.
MS (CI) m/z (M+H)+ 334;
~ H-NMR (300 MHz, d~,-DMSO) 8 10.52 (s, I H), 9.50 (s, 2H), 9.24 (s, 2H}, 8.68
(s, I H},
8.55 (s, 1 H), 8.32 (d, 1 H), 8.25-8.13 (m, 2H), 7.90 (dd, I H), 7.51 (m, 1
H), 7.38 (m, 1 H),
7.26 (t, 1 H), 6.72 (dd, 1 H), 4.04 (q, 2H), 1.34 (t, 3H)
Anal. calc'd for C22H2pN304F3: C, 59.06; H, 4.SI; N, 9.39. Found: C, 58.69; H,
4.54; N,
9.82.
?t>
Exam Ih a 224
6-laminoiminomethyl)-N-l4-nitro~n ly )-2-naphthalenecarboxamide
monoltrifluoroacetate)lsalt)
'S Example 224A
The above product was prepared in the manner of Example 218A using 4-
nitroaniline.
MS (APC1) m/z (M+H)+ 3I8.
Example 224B
3(> 6-laminoiminomethvl)-N-l4-nitronhenyl)-2-n~aphthalenecarboxamide
monoltrifluoroacetatPll~alt)
The above was prepared from Example 224A as described in Example 1 B.
MS (CI) m/z (M+H)+ 335;
-205-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98/15386
1H-NMR (300 MHz, d6-DMSO) 8 / 1.15 (s, IH), 9.55 (s, 2H), 9.22 (s, 2H), 8.77
(s, 1H),
8.58 (s, 1 H), 8.36-8.12 (m, 7H)
Anal. calc'd for C2pH15N40gF3 1/10 TFA: C, 52.54; H, 3.29; N, 12.10. Found: C,
53.58;
H, 3.37; N, 12.50.
Example 225
6-(aminoiminomethvl)-N-f 3-(cvclobutvlmethoxy~Dhenvll-2-naphthalenecarboxamide
mono(trifluoroacetate)lsalt)
Example 225A
The above product was prepared in the manner of Example 218A using
cyclobutylmethanol.
MS (APCI} m/z (M+H)+ 177.
5 Example 225B
The above product was prepared in the manner of Example 225A using the product
from Example 11
MS (APCI) m/z (M+H)+ 357.
Example 225C
6-(aminoiminomethyl)-N-f 3-(cvclobutylmethoxy?phenyll-2-naphthalenecarboxamide
monof trifluoroacetate)(salt)
The above was prepared from Example 225B as described in Example 1 B.
MS (CI) m/z (M+H)+ 374;
~ H-NMR (300 MHz, d~,-DMSO) 8 10.50 (s, 1 H), 9.50 (s, 2H), 9.20 (s, 2H),
8.611 (s, 1 H),
8.55 (s, 1H), 8.32 (d, 1H), 8.25-8.13 (m, 2H), 7.90 (dd, 1H), 7.51 (m, 1H),
7.38 (m, 1H),
7.26 (t, 1 H), 6.72 (dd, 1 H), 3.95 (d, 2H), 2. I 1-1.81 (m, 7H);
Anal. calc'd for C25H24N304F3 7/5 TFA: C, 59.09; H, 5.22; N, 8.27. Found: C,
59.02; H,
5.20; N, 8.55.
Example 226
6-(amino(ethoxvcarbonvl)iminol-N-f3-(1-methvlethoxv)phenvll-2
naphthalenecarboxamide
The above was prepared from Example 21 lA using method described in Example
216.
-206-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTIUS9811S386
MS (Cl) mlz (M+H)+ 420;
~H-NMR (300 MHz, d6-DMSO) 8 10.41 (s, IH), 9.24 (br, 2H), 8.67 (s, IH), 8.59
(s, IH),
8.12-7.96 (m, 4H), 7.47 (s, 1H), 7.36 (m, 1H), 7.25 (t, IH), 6.67 (dd, 1H),
4.58 (m, IH),
4.11 (q, ZH), 1.30 (m, 9H)
Anal. calc'd for C24H25N304 1/4 H20: C, 67.99; H, 6.06 N, 9.91. Found: C,
67.99; H,
6.07; N, 9.64.
Exam Ip a 227
6-(aminoiminomethvl)-4-f5-(ethvlsulfonyl)-3-furanvll-N-,~he~l-2-
n~phthalenecarboxamide
monohvdrochloridP
Example 227A
A solution of the product from Example 200C (670 mg, 1.68 mmol) and mCPBA (725
mg, 3.36 mmol) in CH2C12 (25 mL) was stirred for I hour. The reaction was
condensed and
chromatographed on Si02 using 50% ethyl acetate/hexanes as eluent, to yield
585 mg (81 %) of
the desired compound.
MS (DCI/NH3) m/z 448 (M+NH4)+.
Example 227B
6-(aminoiminomethvl)-4-f5-(ethvlsulfonyl)-3-furanyll-N=phenyl-2-
naphthalenecarboxamide
mono~drochloride
The desired compound was prepared from Example 227A and the procedure of
Example
13.
MS (DCI/NH3) m/z 448 (M+H)+;
~H NMR (300 MHz, DMSO-d~,) 8 1.29 (t, 3H), 3.50 (y, 2H), 7.16 (t, 1H), 7.42
(t, 2H), 7.83
(d, 2H), 7.95 (dd, I H), 8.05 (s, t H), 8.28 (s, 1 H), 8.43 (d, I H), 8.57 (s,
1 H), k.74 (s, 1 H),
8.79 (s, I H), 9.19 (br s, 2H), 9.59 (br s, 2H), 10.61 (s, 1 H);
Anal. calc'd for C24H22N3S04~ 1.0 HC1~ 1.0 H20: C, 57.43; H, 4.82; N, 8.37.
Found: C,
57.21; H, 5.04; N, 8.34.
Exam le 228
6-laminoiminomethvl)-4-f5-(nro~2vlsulfonyl)-3-furanyll-N-phenyl 2
nanhthalenecarboxamide
monohydrochloride
-207-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99105096 PCTICIS98115386
Examvle 228A
The desired compound was prepared from the product from Example 201C by the
procedure of Example 227A.
MS (DCI/NH3) m/z 462 (M+NH~)+.
Exam-ple 228B
6-(aminoiminomethvl)-4-f 5-(nrogvlsulfonyll-3-furan lvhN-phen I-v 2-
nanhthalenecarboxamide
monohvdrochloride
The desired compound was prepared from Example 228A and the procedure of
Example
to 13.
MS (DCI/NH3) m/z 462 (M+H)+;
1H NMR (300 MHz, DMSO-d6) 8 1.03 (t, 3H), 1. 75 (qt, 2H), 3.48 (q, 2H), 7.16
(t, 1H),
7.41 (t, 2H), 7.84 (d, 2H), 7.95 (dd, IH), 8.05 (s, 1H), 8.28 (d, 1H), 8.42
(d, 1H), 8.58 (s,
1 H), 8.77 (s, 1 H), 8.82 (s, 1 H), 9.28 (br s, 2H), 9.63 (br s, 2H), 10.66
(s, 1 H);
Anal. calc'd for C25H24N3S04~ 1.0 HCI~ I .5 H20: C, 57.20; H, 5.18; N, 8.00.
Found: C,
56.86; H, 5.08; N, 8.28.
Example 229
6-(aminoiminomethvli-4-f5-fmethylthio)methyll-3-furany,-N- henyl-2
naDhthalenecarboxamide monohvdrochloride
Example 229A
To a solution of 2-trimethylsilyl-3-bromofuran ( 10.41 g, 47.5 mmol) in THF
(100 mL)
at -7R°C was added a I .5 M solution of LDA (34.8 mL, 52.25 mmol), and
the reaction was
stirred at -78°C for 1 hour. DMF (4.41 mL, 57.0 mmol) was then added,
and the reaction was
allowed to warm to room temperature and stirred for 1 hour. The reaction was
poured into
saturated aqueous NH4CI solution, and extracted with 3x diethyl ether. The
combined extracts
were washed with brine, dried over Na2S04, and condensed. The crude material
was taken up
in methanol (200 mL) and NaBH4 ( 1.15 g, 24.0 mmol) was added in portions to
the stirred
solution. After 30 min, the solution was consensed> taken up in pH7 buffer,
and extracted with
3x ethyl acetate. The combined extracts were washed with brine, dried over
Na2S04, and
condensed. The crude product was chromatographed on Si02 using 30% ethyl
acetate/hexanes
as eluent, to yield 5.52 g (47%) of the desired compound.
MS (DCI/NH3) m/z 250 (M+H)+.
-208-
SU6STITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98/15386
Example 229B
To a solution of the product from Example 229A (5.52 g, 22.15 mmol) and LiCI (
1.03
g, 24.36 mmol) in DMF (60 mL) at 0 °C was added PC13 (2.12 mL, 24.36
mmol), and the
reaction was stirred at room temperature for 1 hour. The reaction was poured
into saturated
aqueous NH4Cl solution, and extracted with 3x diethyl ether/hexanes. The
combined extracts
were washed with 2x water, 2x brine, dried over Na2S04, and condensed to yield
4.70 g
(79%) of the desired compound.
t0 Example 229C
A solution of the product from Example 229B (4.70 g, 17.56 mmol) and MeSNa
(1.35
g, 19.3 mmol) in DMF (40 mL) was stirred at room temperature for 3 hours. The
reaction was
poured into saturated aqueous NaHC03 solution, and extracted with 3x Diethyl
ether. The
combined extracts were washed with brine, dried over Na2S04, and condensed.
The crude
~5 product was chromatographed on Si02 using 30alc ethyl acetate/hexanes as
eluent, to yield 4.00
g (82%) of the desired compound.
Example 229D
The desired compound was prepared from the product from Example 229C by the
2U procedure of Example 154B.
MS (DCI/NH3) m/z 30R (Bu3Sn+NH4}+.
Example 229E
The desired compound was prepared from the product from Example D and the
product
25 from Example 152C by the procedure of Example 154C.
MS (DCI/NH3) m/z 416 (M+NH4)+.
Exam 1e~229_F
6-laminoiminomethyl)-4-f 5-ff mgthvlthiolmethyll-3-furan~rll-N-phg~l-2-
30 nanhthalenecarboxamide monohvdrochloride
The desired compound was prepared from Example 229E and the procedure of
Example
144C.
MS (DCI/NH3) m/z 416 (M+H)+;
-209-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTIUS98/15386
1H NMR (300 MHz, DMSO-d6) b 2.15 (s, 3H), 3.87 (s, 2H), 7.14 (t, IH), 7.40 (t,
2H), 7.84
(d, 2H), 7.92 (dd, 1H), 8.I9 (d, IH), 8.32 (s, 1H), 8.39 (d, IH), 8.64 (s,
IH), 8.69 (s, IH),
9.31 (br s, 2H), 9.61 (br s, 2H), 10.62 (s, IH);
Anal. calc'd for C24H21N3S02'I.4 HCI: C, 61.79; H, 4.$4; N, 9.01. Found: C,
61.83; H,
4.82; N, 9.13.
Example 230
6-(aminoiminomethvl)-4-(5-(methoxvmethvl)-3-furan 1~-N~henyl-2-
naphthalenecarboxamide
mono~drochloride
Example 230A
The desired compound was prepared from 2-trimethylsilyl-3-bromofuran and
chloromethyl methyl ether by the procedure of Example 154A.
Example 230B
The desired compound was prepared from the product from Example 230A by the
procedure of Example I54B.
MS (DCI/NH3) m/z 308 (Bu3Sn+NH4)+.
2o Example 230C
The desired compound was prepared from the product from Example 230B and the
product from Example 152C by the procedure of Example 154C.
MS (DCI/NH3) m/z 400 (M+NH4)+.
2S Example 230D
6-(aminoiminomethvl)-4-15-(methoxymethyl)-3-furanvll-Nphenyl-2-
naphthalenecarboxamide
monohydrochloride
The desired compound was prepared from Example 230C and the procedure of
Example
144C.
30 MS (DCI/NH3) m/z 400 (M+H)+;
~ H NMR (300 MHz, DMSO-d~,) 8 3.38 (s, 3H), 4.49 (s, 2H), 7.14 (t, 1 H), 7.18
(t, 1 H), 7.40
(t, 2H), 7.84 (d, 2H), 7.92 (dd, 1 H), 8.19 (d, 1 H), 8.38 (d, 1 H), 8.42 (s,
I H), 8.64 (s, 1 H),
8.70 (s, lH), 9.32 (br s, 2H), 9.62 (br s, 2H), 10.68 (s, 1H);
-210-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCTlUS98115386
Anal. calc'd for C24H21N303'2.8 HC1: C, 57.48; H, 4.78; N, 8.38. Found: C,
57.40; H,
4.44; N, 8.38.
Example 23I
6-(aminoiminomethvl)-4-f5-lmethylsulfonyl)-3-furanyll-N- henyl-2-
naphthalenecarboxamide
mono(trifluoroacetate)(salt)
Example 231 A
The desired compound was prepared from the product from Example 152C by the
a> procedure of Example 227A.
MS (DCI/NH3) m/z 434 (M+NH4)+.
Example 231 B
6-(aminoiminomethvl)-4-f 5-(methylsulfonvl)-3-furanyll-N-phenyl-2-
n~hthalenecarboxamide
mono(trifluoroacetate)(salt~
The desired compound was prepared from Example 231 A and the procedure of
Example
I3.
MS (DCI/NH3) m/z 434 (M+H)+;
~H NMR (300 MHz, DMSO-d6) 8 3.44 (s, 3H), 7.16 (t, 1H), 7.40 (t, 2H), 7.82 (d,
2H),
2c) 7.91 (s, 1 H), 7.95 (dd, I H), 8.00 (s, 1 H), 8.36 (s, 1 H), 8.43 (d, 1
H), 8.57 (s, 1 H), 8.75 (s,
2H), 9.18 (br s, 2H), 9.53 (br s, 2H);
Anal. calc'd for C23H19N3S04~1.0 C2HF302: C, 54.84; H, 3.68; N, 7.67. Found:
C, 55.05;
H, 3.74; N, 7.75.
25 Example 232
6-(aminoiminomethvl)-4-f 5-(ethythio)tetrahydro-~i-furanvll-N-phenyl 2
naDhthalenecarboxamide monohydrochloride
Example 232A
3o The desired compound was prepared from the product from Example 152A by the
procedure of Example 62A.
MS (DCI/NH3) m/z 315 (M+NH4)+.
Example 232B
-Z I 1-
SUBSTITUTE SHEET (RULE 26)
CA 02294300 1999-12-14
WO 99/05096 PCT/US98/15386
A solution of the product from Example 232A ( I .62 g, 5.45 mmol), ethanethiol
(2.2
mL), and conc. HCl (0.80 mL) in CHCI3 (22 mL) was stirred at room temperature
for 3 hours
and condensed. The crude product was chromatographed on Si02 using 15% ethyl
acetate/hexanes as eluent, to yield I.20 g (6S%) of the desired compound.
MS (DCI/NH3) m/z 359 (M+NH4)+.
Example 232C
The desired compound was prepared from the product from Example 232B by the
procedure of Example 152B.
tt> MS (DCI/NH3) m/z 345 (M+NH4)+.
Example 232D
The desired compound was prepared from the product from Example 232C by the
procedure of Example 207C.
MS (DCI/NH3) m/z 420 (M+NH4)+.
Example 232E
fi-(aminoiminomethyl)-4-l5-(eth th~trahydro-3-furan, I~-N-phenyl-2
nanhthalenecarboxamide. monohydrochloride
2o The desired compound was prepared from Example 232D and the procedure of
Example
144C.
MS (DCI/NH3) m/z 420 (M+H)+;
tH NMR (300 MHz, DMSO-d6) 8 1.18 (dt, 3H), 2.42 (m, 1H), 3.50 (dq, 2H), 3.74
(m, 1H),
3.93 (m, 1 h), 4.39 (m, 1 H), 4.54 (m, i H), 5.38 (dd, O.SH), 5.42 (dd, O.SH),
7.14 (t, 1 H),
7.40 (t, 2H), 7.82 (d, 2H), 7.95 (d, 1 H), 8.06 (s, O.SH), 8.20 (s, 0.5H),
8.32 (d, 1 H), 8.60
(s, 1 H), 8.71 (s, O.SH), 8.80 (s, O.SH), 9.32 (br s, 2H), 9.62 (br s, 2H),
10.59 (br s, 1 H);
Anal. calc'd for C24H25N3~2S~ I.0 HCI: C, 63.22; H, 5.75; N, 9.21. Found: C,
62.93; H,
5.58; N, 9.01.
-212-
SUBSTITUTE SHEET (RULE 26)