Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02295495 2000-01-05
WO 99/02764 PCT/IB98/01045
A DRAINED CATHODE CELL FOR THE PRODUCTION OF ALUMINIUM
Field of the invention
The invention relates to cells for the production
of aluminium by the electrolysis of an aluminium compound
dissolved in a molten electrolyte, for example alumina
dissolved in a molten fluoride-based electrolyte.
It concerns in particular an aluminium production
cell of drained configuration in which the aluminium pool
protecting the carbon cathodes is no longer required
because the carbon cathodes are protected by an aluminium
wettable coating or are not made of carbon and are drained
whereby the aluminium produced which is formed on the
drained surface is collected and tapped.
The invention also concerns a method of producing
aluminium in this drained cathode cell.
Backaround of the Invention
The technology for the production of aluminium by
the electrolysis of alumina, dissolved in molten cryolite
containing salts, at temperatures around 950 C is more than
one hundred years old.
This process, conceived almost simultaneously by
Hall and Heroult, has not evolved as much as other
electrochemical processes, despite the tremendous growth in
the total production of aluminium that in fifty years has
increased almost one hundred fold. The process and the cell
design have not undergone any great change or improvement
and carbonaceous materials are still used as electrodes and
cell linings.
The electrolytic cell trough is typically made of a
steel shell provided with an insulating lining of
refractory material covered by prebaked anthracite-graphite
CA 02295495 2000-01-05
WO 99/02764 PCT/IB98/01045
- 2 -
or all graphite carbon blocks at the cell floor bottom
which acts as cathode and to which the negative pole of a
direct current source is connected by means of steel
conductor bars embedded in the carbon blocks. The side
walls are also covered with prebaked anthracite-graphite
carbon plates or silicon carbide plates.
The anodes are still made of carbonaceous material
and must be replaced every few weeks. The operating
temperature is still approximately 950 C in order to have a
sufficiently high rate of dissolution of alumina which
decreases at lower temperatures and to have a higher
conductivity of the electrolyte.
The carbonaceous materials used in Hall-Heroult
cells as cell lining deteriorate under the existing adverse
operating conditions and limit the cell life.
Another major drawback, however, is due to the fact
that irregular electromagnetic forces create waves in the
molten aluminium pool and the anode-cathode distance (ACD),
also called interelectrode gap (IEG), must be kept at a
safe minimum value of approximately 50 mm to avoid short
circuiting between the aluminium cathode and the anode or
reoxidation of the metal by contact with the C02 gas formed
at the anode surface, leading to a lower current
efficiency.
The high electrical resistivity of the electrolyte,
which is about 0.4 ohm. cm., causes a voltage drop which
alone represents more than 40% of the total voltage drop
with a resulting high energy consumption which is close to
13kWh/kgAl in the most modern cells. The cost of energy
consumption has become an even bigger item in the total
manufacturing cost of aluminium since the oil crisis, and
has decreased the rate of growth of this important metal.
In the second largest electrochemical industry
following aluminium, namely the caustic and chlorine
industry, the invention of the dimensionally stable anodes
(DSA ) based on noble metal activated titanium metal,
which were developed around 1970, permitted a revolutionary
progress in the chlorine cell technology resulting in a
CA 02295495 2000-01-05
WO 99/02764 PCT/IB98/01045
- 3 -
substantial increase in cell energy efficiency, in cell
life and in chlorine-caustic purity. The substitution of
graphite anodes with DSA increased drastically the life
of the anodes and reduced substantially the cost of
operating the cells. Rapid growth of the chlorine caustic
industry was retarded only by ecological concerns.
In the case of aluminium production, pollution is
not due to the aluminium produced, but to the materials and
the manufacturing processes used and to the cell design and
operation.
However, progress has been reported in the
operation of modern aluminium plants which utilize cells
where the gases emanating from the cells are in large part
collected and adequately scrubbed and where the emission of
highly polluting gases during the manufacture of the carbon
anodes and cathodes is carefully controlled.
While progress has been reported in the use of
carbon cathodes to which have been applied coatings or
layers of new aluminium wettable materials which are also a
barrier to sodium penetration during electrolysis, very
little progress has been achieved in design of cathodes for
aluminium production cells with a view to improving the
overall cell efficiency, simplifying assembly of the
cathodes in the cell, simplifying the removal and disposal
of used cathodes, as well as restraining movement of the
molten aluminium in order to reduce the interelectrode gap
and the rate of wear of its surface.
U.S. Patent 3,202,600 (Ransley) proposed the use of
refractory borides and carbides as cathode materials,
including a drained cathode cell design wherein a wedge-
shaped consumable carbon anode was suspended facing a
cathode made of plates of refractory boride or carbide in
V-configuration.
U.S. Patents 3,400,061 (Lewis et al) and 4,602,990
(Boxall et al) disclose aluminium electrowinning cells with
sloped drained cathodes arranged with the cathodes and
facing anode surfaces sloping across the cell. In these
cells, the molten aluminium flows down the sloping cathodes
CA 02295495 2000-01-05
WO 99/02764 PCT/IB98/01045
- 4 -
into a median longitudinal groove along the centre of the
cell, or into lateral longitudinal grooves along the cell
sides, for collecting the molten aluminium and delivering
it to a sump.
U.S. Patent 4,544,457 (Sane et al) proposed a
drained cathode arrangement in which the surface of a
carbon cathode block was covered with a sheath that
maintained stagnant aluminium on its surface in order to
reduce wear. In this design, the cathode block stands on
the cell bottom.
U.S. Patent 5,203,971 (de Nora et al) discloses an
aluminium electrowinning cell having a partly refractory
and partly carbon based cell lining. The carbon-based part
of the cell bottom may be recessed in respect to the
refractory part, which assists in reducing movement of the
aluminium pool.
An improvement described in U.S. Patent 5,472,578
(de Nora) consisted in using grid-like bodies which could
form a drained cathode surface and simultaneously restrain
movement in the aluminium pool.
U.S. Patent 5,316,718 and WO 93/25731 (both in the
name of Sekhar et al) proposed coating components with a
slurry-applied coating of refractory boride, which proved
excellent for cathode applications. These publications
included a number of novel drained cathode configurations,
for example including designs where a cathode body with an
inclined upper drained cathode surface is placed on or
secured to the cell bottom.
In U.S. Patent 5,362,366 (de Nora et al), a double-
polar anode-cathode arrangement was disclosed wherein
cathode bodies were suspended from the anodes permitting
removal and reimmersion of the assembly during operation,
such assembly also operating with a drained cathode.
U.S. Patent 5,368,702 (de Nora) proposed a novel
multimonopolar cell having upwardly extending cathodes
facing and surrounded by or in-between anodes having a
relatively large inwardly-facing active anode surface area.
CA 02295495 2000-01-05
WO 99/02764 PCT/IB98/01045
- 5 -
In some embodiments, electrolyte circulation was achieved
using a tubular anode with suitable openings.
WO 96/07773 (de Nora) proposed a new cathode design
for a drained cathode, where grooves or recesses were
incorporated in the surface of blocks forming the cathode
surface in order to channel the drained product aluminium.
In summary, since commercial production of
aluminium begun the cells have characteristics which have
permitted an increase in the total production and a
reduction of cost.
Aluminium is present in the electrolyte as a
suspension of small particles, soluble in small amounts,
and reacts with the anode gas which contains mainly C02
formed by the reaction of oxygen with carbon. This is
facilitated by the fact that the bubbles of C02 which form
on the anode escape with difficulty from under the anode,
through the electrolyte, before the gas is collected and
purified to recover fluorides and eliminate other dangerous
polluting impurities.
The reaction between aluminium and C02 which
reduces considerably the current efficiency of the process
is facilitated by the movement of the electrolyte due to
the intermittent escape of big bubbles formed, and by the
movement of the aluminium pool maintained on top of the
carbon cathode to protect the cathode from chemical
corrosion by the formation of aluminium carbide. Such
movement of the aluminium pool, due to the electromagnetic
forces which become violent when the current distribution
is not uniform, additionally leads to erosion of the
cathode surface.
Attempts have been made to improve the situation
such as by decreasing the size of the active surface of
each anode - see the above-mentioned U.S. Patent 5,368,702
(de Nora) - in order to have a more uniform current
distribution.
Of course, an improvement could be obtained if the
active surface of the cathode and of the anode would be at
a slope to facilitate the escape of the bubbles of the
CA 02295495 2000-01-05
WO 99/02764 PCT/IB98/01045
- 6 -
released gas. Moreover, to have a cathode at a slope and
obtain an efficient operation of the cell would be possible
only if the surface of the cathode were aluminium-wettable
so that the production of aluminium ions would take place
on a film of aluminium. So far, attempts to achieve this
have failed.
Only recently has it become possible to coat carbon
cathodes with a slurry which adheres to the carbon and
becomes aluminium-wettable and very hard when the
temperature reaches 700-800 C or even 950-1000 C, as
disclosed in the aforementioned U.S. Patent 5,316,718 and
WO 93/25731 (both in the name of Sekhar et al). Though
application of these coating to drained cathode cells has
been proposed, so far the commercial-scale application of
this technology has been confined to coating carbon bottoms
of cells operating with the conventional deep pool of
aluminium. Further design modifications in the cell
construction could lead to obtaining more of the potential
advantages of these coatings.
While the foregoing references indicate continued
efforts to improve the operation of molten cell
electrolysis operations, none suggest the invention and
there have been no acceptable proposals for improving the
efficiency cell, and at the same time facilitating the
implementation of a drained cathode configuration.
Ob-iects of the Invention
One object of the present invention is to provide a
drained cathode cell for the production of aluminium which
has characteristics which make the cell efficient from the
point of high current efficiency but also from the point of
view of reduced energy consumption.
Another object of the invention is to overcome
problems inherent in known designs of drained cathode cells
used in the electrowinning of aluminium wherein electrolyte
circulation is induced by anodically-released gases with
feeding of an alumina-rich melt at the lower part of the
anode-cathode gap.
CA 02295495 2000-01-05
WO 99/02764 PCT/IB98/01045
- 7 -
Yet another object of the invention is to provide a
drained cathode cell in which the product aluminium can be
better moved and collected.
A further object of the invention is to enhance the
circulation of electrolyte in a drained cathode cell by
using non-carbon oxygen-evolving anodes designed to favour
the escape of the anodically-produced gas while promoting
circulation of the electrolyte.
A yet further object of the invention is to provide
a drained cathode cell in which the cell sidewall also acts
as an active cathode whereby the cell can operate at low
current densities.
An even further object of the invention is to
implement a drained cathode cell design that can operate
without formation of a crust of solidified electrolyte,
possibly by operating at high current densities from 1 to 2
Amp/cm2.
Another object of the invention is to provide a
cell of drained cathode configuration having non-carbon
non-consumable anodes of shapes which permit the rapid
escape of bubbles when they are still small.
Yet another object of the invention is to provide a
cell of drained cathode configuration wherein a small
inter-electrode distance of several centimeters (typically
3cm or less) can be maintained while reducing contact
between the produced aluminium and the anodically-released
gases, by avoiding a deep pool of aluminium with waves and
by facilitating release of the bubbles of anodically-
produced gas.
Summary of the Invention
The invention proposes a drained cathode cell for
the production of aluminium by the electrolysis of an
aluminium compound dissolved in a molten electrolyte, in
which the active cathode surfaces are dimensionally stable
and have an aluminium-wettable surface and are at a slope,
and in which anode surfaces parallel to the cathode
surfaces are spaced by a reduced anode-cathode gap and are
CA 02295495 2000-01-05
WO 99/02764 PCT/IB98/01045
- 8 -
configured to induce an upward release of the anode gas and
an upward circulation of the electrolyte with a downward
draining of the aluminium produced.
The drained cell of the invention is further
characterized by the fact that it u-tilizes one or more of
the following features :
1) An alumina-rich melt is fed at the lower part of
the anode-cathode gap.
2) The anode-cathode gap between the sloping anode and
cathode surfaces is up to 3 cm, possibly about 2 cm.
3) The entire cell bottom or part of it is at a slope
so that the aluminium can be better moved and collected.
Preferably, the slope is such that it is sufficient to
ensure an efficient release of bubbles of the anodically-
released gas before these bubbles become too big, thereby
avoiding or considerably reducing reaction with particles
of aluminium.
4) The cell bottom is sloped without moving the center
of the cell (i.e. one end is raised, the other end is
lowered).
5) The tapping of the aluminium is made from a storage
located inside or outside the cells, at an end of the cell
or at the side, or in the middle.
6) The active cathode surface is made dimensionally
stable by a slurry-applied coating of aluminium-wettable
refractory material which controls the sodium penetration.
7) The active cathode surface as well as the remaining
bottom of the cells is protected by an aluminium-wettable
titanium diboride coating, or titanium diboride plates, or
a fiber cloth or a porous sheath filled with a titanium
diboride slurry.
8) The cell side wall also acts as an active cathode
and is protected by an aluminium-wettable titanium diboride
coating, or titanium diboride plates, or a fiber cloth or a
CA 02295495 2000-01-05
WO 99/02764 PCT/IB98/01045
- 9 -
porous sheet filled with a titanium diboride slurry, on
which aluminium is also formed.
9) The entire side face inside the cell, whether it is
in contact with aluminium or cryolite or anodically-
generated gases, is lined with an aluminized titanium
diboride coating, or titanium diboride plates, or a carbon
fiber cloth or foraminous copper filled with a titanium
diboride slurry.
10) The main active cathode surface has a slope. This
slope is for example from 5 to 60 to horizontal,
preferably from 5 to 45 .
11) The cathode slope is obtained using the cross-
section of the cathode blocks, as disclosed in PCT
publication W096/07773 (de Nora).
12) The cathode slope is obtained by providing a wedge-
shaped member ("wedge"), which is solid or is made of
vertical plates spaced apart, on a flat cathode bottom, the
wedge being made of carbon or of "heavy" materials, i.e.
having a specific weight greater than the molten aluminium
and cryolite, or incorporating internal ballast, this wedge
also being coated with an aluminium-wettable titanium
diboride coating, see for example U.S. Patent 5,472,578 (de
Nora ) .
13) The cathode is made of a solid body of titanium-
diboride-based material made by consolidating preformed
titanium diboride, for example as described in PCT
publication W07/08114 (Sekhar et al) or by micropyretic
reaction for example as described in U.S. Patents 5,217,583
and 5,316,718 (both in the name of Sekhar et al), possibly
coated with an aluminium-wettable titanium diboride
coating, for example as described in U.S. Patent 5,534,119
(Sekhar et al), or coated with titanium diboride plates, or
a fiber cloth or a porous sheet filled with a titanium
diboride slurry.
14) The anode is a consumable carbon anode.
15) The anode is a non-carbon oxygen evolving anode.
CA 02295495 2000-01-05
WO 99/02764 PCT/IB98/01045
- 10 -
16) The non-carbon oxygen evolving anode comprises an
assembly of plates, rods, elongated members such as strips,
with a cross-section and spacing so as to favour gas
escape, for example rods in so-called "spaghetti"
configuration.
17) The non-carbon oxygen evolving anode is a double-
faced structure with louvers or other openings in its
surface for directing the anodically-produced gas inside
the anode structure.
18) The anode has a cross-section to favour escape of
the anodically-produced gas and circulation of the
electrolyte.
19) The conventional wedge of ramming paste located
between the bottom of the side walls and the edges of the
cell bottom is eliminated. This permits the anodes to be
near to the side wall, and the side wall can operate as a
cathode i.e. facing a vertical part of the anodes. (See
point 5). The cell can thus operate with low current
density.
20) With cathodes in sloping wedge configuration, the
alumina is fed to the lowest point of the wedge. This
facilitates circulation of the electrolyte enriched with
dissolved alumina.
21) The cell side wall is provided with sufficient
internal and/or external insulation that the cell operates
without formation of a crust of frozen electrolyte.
22) The cell operates at a high current density, from
0.5 to 2 Amp/cm2.
23) An aluminium-wettable coating is applied to
components of the cell as a slurry containing an "active"
powder of aluminium-wettable material (like titanium
diboride), wherein fibers are added to the slurry :
a) The fibers are made of electrically-conductive
and non-conductive materials such as carbonaceous, metals,
carbides, nitrides, borides, alumina etc.
CA 02295495 2000-01-05
WO 99/02764 PCT/IB98/01045
- 11 -
b) The active aluminium-wettable powder is titanium
diboride and similar materials, preformed or formed in
situ.
c) The fibers form a woven or non-woven cloth.
Further features of the invention are set out in
the claims.
Brief Description of the Drawinas
The invention will be further decribed with
reference to the accompanying schematic drawings, in
which :
Fig. 1 is a cross-sectional view through part of a
drained cathode aluminium production cell according to the
invention;
Fig. 2 is a plan view of the anode of the cell of
Fig. 1;
Fig. 3 is a side elevational view of the part of
the anode of Fig. 2;
Fig. 3A is a cross-section along line 3A-3A of Fig.
3;
Fig. 4 is a cross-sectional view through part of
the drained cathode aluminium production cell of Fig. 1.
Fig. 5 is a cross-sectional view through part of
another drained cathode aluminium production cell according
to the invention.
Figs 6, 7 and 8 are longitudinal cross-sectional
views through part of three further embodiments of drained
cathode cells; and
Fig. 8A shows a detail of Fig. 8.
Detailed Descriotion
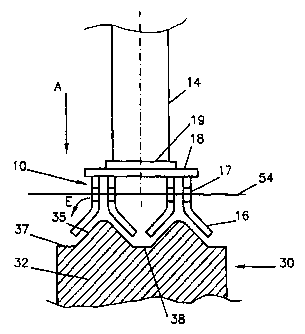
Fig. 1 shows part of a drained-cathode aluminium
production cell comprising a plurality of non-carbon
oxygen-evolving anodes 10 suspended over a cathode 30
CA 02295495 2006-11-22
WO 99/02764 PCTIIB98/01045
- 12 -
comprising a cathode mass 32 having inclined cathode
surfaces 35 and coated with an aluminium-wettable coating
37, for example a slurry-applied titanium diboride coating
according to U.S. Patent 5,316,718 (Sekhar et al).
The cathode mass 32 is advantageously a composite
alumina-aluminium-titanium diboride material, for example
produced by micropyretic reaction of Ti02, B203 and Al.
Such composite materials exhibit a certain plasticity at
the cell operating temperature and have the advantage that
they can accommodate for thermal differences during cell
start up and operation, while maintaining good conductivity
required to effectively operate as cathode mass.
Alternatively the cathode mass 32 can be made of
carbonaceous material, for example packed carbon powder,
graphitized carbon, or stacked plates or slabs of carbon
imbricated with one another and separated by layers of a
material that is impermeable to the penetration of molten
aluminium. When the cathode is made of carbon, the cathode
slope can be obtained using the cross-section of the
assembled cathode blocks, the sloping top surface of the
assembled cathode blocks forming the active cathode
surface, as further described in international patent
application WO 96/07773 (de Nora).
Advantageously, the cathode mass 32 is supported in
a metal cathode holder shell or plate 31 (see Fig. 4) as
disclosed in Applicant's international patent application
W098/53120,- to which current is supplied by one or
more current collector bars extending through the electric
and thermic insulation in the bottom of the cell, or
through the sides of the cell.
As shown, the inclined active cathode surfaces 35
are arranged in a series of parallel rows of approximately
triangular cross-section, extending along (or across) the
cell. These surfaces 35 are inclined at an angle of for
example 30 to 60 to horizontal, for instance about 45 .
This slope is such that the produced aluminium drains
efficiently, avoiding the production of a suspension of
particles of aluminium in the electrolyte 54.
CA 02295495 2000-01-05
WO 99/02764 PCT/1B98/01045
- 13 -
Between the adjacent inclined surfaces 35 is a
trough 38 into which aluminium from the surfaces 35 can
drain. Conveniently, the entire aluminium production cell
is at a slope longitudinally, so the aluminium collected in
the troughs 38 can drain to one end of the cell where it is
collected in a storage inside or outside the cell.
The anodes 10 are suspended above the cathode 30
with a series of active inclined anode surfaces on plates
16 facing corresponding inclined cathode surfaces 35
leaving a narrow anode-cathode space, which can be less
than 3cm, for example about 2cm. The active parts of the
anodes are formed by plates 16 which for example are made
of nickel-iron-aluminium or nickel-iron-aluminium-copper
with an oxide surface as described in U.S. Patent No.
5,510,008 (de Nora et al). As shown in Fig. 1, these plates
16 are arranged in facing pairs forming a roof-like
configuration.
The sloping inner active faces of the anode plates
16 assist in removing the anodically-evolved gases,
principally oxygen. The chosen slope - which is the same as
that of the cathode surfaces 35, for example about 45 - is
such that the bubbles of anodically-released gas are
efficiently removed from the active anode surface before
the bubbles become too big. The risk of these gas bubbles
interacting with any particles of aluminium in the
electrolyte 54 is thus reduced or eliminated.
Each anode 10 comprises an assembly of metal
members that provides an even distribution of electric
current to the active anode plates 16. For this, the active
anode plates 16 are suspended from transverse plates 18
fixed under a central longitudinal plate 19 by which the
anode is suspended from a vertical current lead-in and
suspension rod 14, for example of square cross-section.
For example, each anode 10 is made up of four pairs
of active anode plates 16 held spaced apart and parallel to
one another and symmetrically disposed around the current
lead-in rod 14. As shown in Fig. 1, each active anode plate
16 is bent more-or-less about its center at about 45 , the
opposite plates 16 of each pair being spaced apart from one
CA 02295495 2000-01-05
WO 99/02764 PCT/IB98/01045
- 14 -
another with their bent lower ends projecting outwardly, so
they fit over the corresponding inclined cathode surfaces
35.
As seen in plan in Fig. 2, pairs of transverse
plates 18 which each carry two pairs of the active anode
plates 16 are symmetrically disposed about the current
lead-in rod 14 so that, overall, the active anode plates 16
are equally distributed about the axis of the current lead-
in rod 14. On each side of the current lead-in rod 14, two
side-by-side pairs of active anode plates 16 are carried by
two transverse plates 18 spaced apart lengthwise along the
plates 16/19.
In their vertical upper parts, the active anode
plates 16 have a series of apertures 17 of sufficient
height that the level of the molten electrolyte 54
intersects these apertures 17 about mid-way along (as shown
in Fig. 1), allowing for passage of the anodically-released
gases and circulation of the electrolyte 54 induced by gas-
lift. As shown in the left hand part of Fig. 3, these
apertures 17 are of oblong shape equally spaced apart from
one another along the length of the plates 16, but other
shapes are possible, for example circular or oval and
possibly with unequal spacing. For example, circular
apertures 17 are illustrated in the right hand part of
Fig. 3.
In a variation, the illustrated active anode plates
16 could be replaced by a series of bent vertical rods, or
a grid structure having through-spaces for gas release.
Fig. 4 shows part of the drained-cathode aluminium
production cell of Fig. 1, comprising a plurality of non-
carbon oxygen-evolving anodes 10 suspended over a cathode
30 comprising a cathode mass 32A,32B having inclined
cathode surfaces 35 and coated with an aluminium-wettable
coating 37, for example a slurry-applied titanium diboride
coating according to U.S. Patent 5,316,718 (Sekhar et al).
The lower part 32B of the cathode mass is
advantageously a composite alumina-aluminium-titanium
diboride material, for example produced by micropyretic
CA 02295495 2006-11-22
WO 99/02764 PCT/IB98/01045
- 15 -
reaction of Ti02, B203 and Al. Such composite materials
exhibit a certain plasticity at the cell operating
temperature and have the advantage that they can
accommodate for thermal differences during cell start up
and operation, while maintaining good conductivity required
to effectively operate as cathode mass.
The top part 32A of the cathode mass can be made of
carbonaceous material, for example packed carbon powder,
graphitized carbon, or stacked plates or slabs of carbon
imbricated with one another and separated by layers of a
material that is impermeable to the penetration of molten
aluminium. The cathode slope can be obtained using the
cross-section of the assembled cathode blocks, the sloping
top surface of the assembled cathode blocks forming the
active cathode surface, as further described in
international patent application WO 96/07773 (de Nora).
As illustrated, each carbon block making up the top
part 32A of the cathode mass has in its bottom surface two
metal current conductors 42 for evenly distributing
electric current in the blocks. At its edges, the top part
32A of the cathode mass is surrounded by a mass of ramming
paste 32C which could alternatively be replaced by silicon
carbide plates.
The lower part 32B of the cathode mass is supported
on a metal cathode holder shell or plate 31 as disclosed in
Applicant's international patent application W098/53120,
to which current is supplied by one or more current
collector bars extending through the electric and thermic
insulation 40 in the bottom of the cell, or through the
sides of the cell.
Above the active parts of the anodes 10 is supported a
horizontal removable insulating cover 60 which rests above
the level of the electrolyte 54 and which is located
underneath an outer cover 70. This cover 60 is made in
sections which are removable individually with the
respective anodes 10, optionally leaving gas-release gaps
63' around the anode rods 14.
In operation, the described cell can operate at a
current density from 0.5 to 2 Amp/cm2 of the projected
CA 02295495 2000-01-05
WO 99/02764 PCT/IB98/01045
- 16 -
surface area of the active anode plates 16. Due to the
slope of the active surfaces of the anode plates 16, for
example at about 45 , the bubbles of oxygen generated
during electrolysis on these sloping surfaces escape by
moving rapidly up, and are released from the top of the
active sloping surfaces while the size of the bubbles
remains small. This upward escape of the tiny bubbles of
oxygen creates a lift in the molten electrolyte 54 adjacent
to the inclined anode surfaces.
As indicated in Fig. 1, the level of the molten
electrolyte 54 intersects the apertures 17 about half-way
up, so that anodically-released gas (oxygen) can escape by
passing through these apertures 17. Also, the molten
electrolyte 54 circulated upwardly by gas lift can pass out
through the apertures 17, from where it circulates down
outside the inclined surface of the anode plates 16, as
indicated by arrow E in Fig. 1.
To replenish alumina consumed during electrolysis,
a supply of fresh alumina is periodically fed to the space
outside the bottom of the anode-cathode gap, as indicated
by arrow A. This fresh alumina is then entrained in the
flow of electrolyte 54 into the anode-cathode gap so that
the electrolyte 54 in this gap never becomes depleted of
alumina during operation.
During electrolysis, ionic aluminium is converted
to metallic aluminium on the aluminium-wettable surface 37
of the inclined cathode surfaces 35. Because of the slope
of this cathode surface, for example at about 45 , the
aluminium produced drains as a thin film and is collected
in the troughs 38. This downflow of molten aluminium takes
place under gravity and is not interfered with by the
upward flow of gas and entrained electrolyte 54 adjacent to
the inclined surfaces of the anode plates 16. The formation
of a suspension of tiny particles of aluminium is minimized
or avoided.
As a result, the inclined active surfaces of the
anode plates 16 and the inclined active cathode surfaces 35
can be spaced apart with a small anode-cathode gap, less
CA 02295495 2006-11-22
WO 99/02764 PCT/IB98/01045
- 17 -
than 3cm and possibly only 2cm, while maintaining a high
efficiency of the electrolysis.
Fig.5 illustrates part of another cell according to
the invention including an anode structure of modified
design, the same references being used to designate the
same elements as before, or their equivalents, which will
not be described again in full.
In the cell of Fig. 5, above a cathode cell bottom 20
enclosed in an outer steel shell 21 and containing the
cathode 30 is suspended a series of non-carbon
substantially non-consumable oxygen evolving anodes 10,
each anode 10 comprising a series of inclined active lower
plates 16 suspended by a vertical current lead-in rod 14
via current distribution members 18.
In this example, the current distributi.on members
18 are formed by a series of side-by-side inclined metal
plates 16 connected by cross-plates, not shown. The active
parts of the anodes are formed by the inclined plates 16
which for example are made of nickel-iron-aluminium or
nickel-iron-aluminium-copper with an oxide surface as
described in U.S. Patent No. 5, 510, 008 (de Nora et al) .
These plates 16 are arranged in facing pairs forming a
roof-like configuration. The sloping inner active faces of
the anodes 10 assist in removing the anodically-evolved
gases, principally oxygen.
The illustrated anode 10 has three pairs of
inclined plates 16 in roof-like configuration. However, the
anode 10 can include any suitable number of these pairs of
inclined plates.
Instead of being full, the plates 16 could be
replaced by a series of rods or fingers spaced apart from
one another and also inclined. In this case, the
anodically-evolved gases can escape between the rods or
fingers.
In the embodiment of Fig. 5, the cathode 30
comprises a metal cathode carrier 31 in the form of a shell
or dished plate to which current is supplied by current
distribution bars 42 which in this case are horizontal and
lead through the side of the cell. Alternatively, the
CA 02295495 2000-01-05
WO 99/02764 PCT/IB98/01045
- 18 -
current collector bars 42 could be vertical and extend
through the bottom of the cell. The inner shell 31 has a
flat bottom and inclined side walls 33, and forms an open-
topped container for a cathode mass 32 which advantageously
is a composite alumina-aluminium-titanium diboride
material, for example produced by micropyretic reaction of
Ti02, B203 and Al and which wraps around the edges of the
cathode carrier 32's inclined side walls 33.
Advantageously, an air or gas space (not shown) can
be provided between the underside of the cathode carrier
shell 31 and the top of the bricks 40, in the spaces left
between the horizontal current distribution bars 42 wherein
a plurality of additional spacers such as girders are
provided. This space under the central flat part of the
cathode carrier 31 acts as a thermic insulating space by
means of which it is possible to adjust the temperature of
the cathode 30 (shell 31 and cathode mass 32) by supplying
a heating or cooling gas to the space. For example, during
cell start up, the cathode 30 can be heated by passing hot
gas through the space. Or during operation, the surface of
the cathode mass 32 can be cooled to make the electrolyte
54 contacting it form a protective paste.
The central part of the top of the cathode 32 mass
has a flat surface which can be inclined longitudinally
along the cell and leads down into a channel or a storage
for draining molten aluminium, situated at one end of the
cell. On top of the cathode mass 32 is a coating 37 of
aluminium-wettable material, preferably a slurry-applied
boride coating as described in U.S. Patent 5,316,718
(Sekhar et al). As shown in Fig, 4, on top of the cathode
mass 32 are arranged a plurality of active cathode bodies
39 having inclined surfaces also coated with the aluminium-
wettable coating 37 and which face the inclined faces of
the active anode plates or rods 16.
Above each anode 10, resting on the current
distribution members 18, it is possible to place a thermic
insulating cover (not shown). With this anode-cathode
arrangement, when the anode 10 is lowered to its operating
position the inclined active plates or rods 16 of the anode
10 are held with a small spacing above the inclined cathode
CA 02295495 2000-01-05
WO 99/02764 PCT/IB98/01045
- 19 -
surface 35. In this operating position of the anodes, such
thermic insulating cover can be held level with or slightly
below the top of the cell sidewalls 22 and just above the
level of the electrolyte 54.
The described cell of Fig. 5- employs inclined non-
carbon oxygen-evolving anodes 10 facing a dimensionally-
stable drained cathode 30 with inclined aluminium-wettable
operative surfaces 35/37, enabling the cell to operate with
a narrow anode-cathode gap, say about 3cm or less
(particularly because of the improved gas release with the
inlined anode-cathode surfaces), instead of about 4 to 5 cm
for conventional cells. This smaller anode-cathode gap
means a substantial reduction in the heat produced during
electrolysis, leading to a need for extra insulation to
prevent freezing of the electrolyte.
In operation of the cell of Fig. 4 or Fig. 5, it is
advantageous to preheat each anode 10 before it is
installed in the cell in replacement of an anode 10 that
has become disactivated or requires servicing. In
particular, this inhibits the formation of an electrolyte
crust which could lead to part of an anode being
disactivated until the electrolyte crust has melted.
Figs 6 to 8 show three further embodiments of
drained cathode cells with consumable carbon anodes 10'.
In the cell of Fig. 6, the cathode is made up of a
series of carbon blocks 82 of generally rectangular cross-
section assembled together side-by-side on a layer of
refractory insulating material 40. These carbon blocks 82
are joined by ramming paste or glue. Each carbon block 82
has a centrally-located current collector bar 42 extending
transverse to the cell.
On the flat top face of blocks 82 are arranged
wedge-shaped carbon bodies 83 having sloping top surfaces
84 inclined at about 5 to horizontal. As illustrated,
these top surfaces 84 are oppositely inclined to one
another to provide a series of shallow V-shaped recesses
forming the active cathode surfaces.
CA 02295495 2000-01-05
WO 99/02764 PCT/IB98/01045
- 20 -
These bodies 83 are also joined by ramming paste or
glue to the blocks 82, advantageously using a TiB2-
containing slurry, as described in U.S. Patent
N . 5,320,717 (Sekhar).
The exposed inclined top surfaces 84 of the bodies
83 are coated with an aluminium-wettable refractory
coating, preferably the slurry-applied TiB2 as described in
U.S. Patent N . 5,534,119 (Sekhar et al).
The lower active faces of the anodes 10' have
corresponding V-shaped inclined surfaces facing the
inclined active cathode surfaces 84. The anode surfaces
have exactly the same angle of inclination as the cathode
surfaces, e.g. about 5 . The anode-cathode gap is held at a
reduced value, about 3cm or less. This is sufficient to
promote efficient removal of the bubbles of anodically-
generated gas. This also promotes an upward (and sideward)
circulation of the electrolyte 54 in the anode-cathode gap,
whereas the produced aluminium is drained to the center of
the V-shaped recesses and collected by inclining the cell
to one side, where the aluminium is collected.
Fig. 7 illustrates a similar design, but where the
cathode blocks 82 are of trapezoidal cross-section and have
integral inclined surfaces 84, arranged alternately to form
the shallow V-shaped recesses. In this case, the sloping
cathode surfaces 84 are provided by the modified cross-
sectional shape of the carbon blocks 82.
Fig. 8 illustrates a modification of the drained
cathode cell of Fig. 6 wherein the solid cathode wedges 83
are replaced by wedge-shaped members made of a series of
side-by-side spaced-apart plates 85 connected by cross-bars
86. As illustrated, each wedge-shaped cathode member is
made up of eight vertical plates 85 joined by two cross-
bars 86. However, any suitable number of plates 85 can be
connected by any suitable number of cross-bars 86, of round
cross-section or any other suitable cross-section.
These plates 85 can be made of carbon, in which
case they are secured to the cathode blocks 82 or loaded
with ballast. Advantageously, however, the plates 85 can be
CA 02295495 2000-01-05
WO 99/02764 PCT/IB98/01045
- 21 -
made of a refractory material, such as alumina, having a
specific weight greater than molten aluminium. In both
cases, the entire surface of the wedge-shaped plates 85, or
at least their top parts including the sloping surfaces,
will be coated with an aluminium-wettable refractory
material, preferably slurry-applied TiB2.
In this design, the produced aluminium can drain in
the spaces between the plates 85. The height of the lower
end of the wedge-shaped plates 85 is such that it is
possible to allow a fluctuation of the level of the
produced aluminium to facilitate tapping of the aluminium
by a batch process. As before, the cell floor is
advantageously inclined to promote collection of the
aluminium at the side/end of the cell.
Of course, the cells of Figs 6, 7 and 8 could
employ non-carbon oxygen-evolving anodes instead of carbon
anodes.
,,;.