Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02295856 2000-O1-10
PCT/AU98/00540
Received 19 October 1998
- 1 -
S PRODUCING LIQUID PRODUCTS CONTAINED IN CANS, BOTTLES AND
OTHER SUITABLE CONTAINERS
The present invention relates to a method of
producing a liquid product packed in cans or bottles or any
other suitable container.
The present invention relates particularly,
although by no means exclusively, to a method of producing
a carbonated beverage product, such as beer, packed in cans
or bottles or any other suitable container.
One particular, although by no means exclusive,
application of the present invention is a method of
producing beer in cans or bottles or any other suitable
containers which has a smooth, non-bitter, taste and
excellent foaming characteristics and the following
discussion of the prior art is in this context.
Australian patent application 55602/86 entitled
"Carbonating in Bottles and Cans" in the name of Gatehouse
Technical Ventures Limited describes that foam is an
important element in the consumer appeal of most beers and
of some other carbonated beverages. More particularly, the
Gatehouse patent application describes that:
"The most important means by which foam is
produced by any of these liquids is the release
of carbon dioxide from super-saturated solution.
Super-saturation arises when a previously-closed,
pressurised container is opened to atmosphere or
AMENDED SHEET - IPEA/AU
CA 02295856 2000-O1-10
PCT/AU98/00540
Received 19 October 1998
- 2 -
when the liquid contents are discharged from
within it through a tap or similar device.
Bubbles of carbon dioxide gas are then released
by turbulent flow, by nucleation on solid
surfaces or particles, or by diffusion into
existing gas bubbles.
In the case of beers and other carbonated
beverages, bubbles aggregate to produce foam
which rests on top of the beverage in the
drinking-glass (or other drinking container).
More bubbles are released, and foam consequently
produced, as the beverage is drawn into and flows
within the mouth, producing a variety of sensory
impressions including viscosity. As the beverage
is tipped from the glass, foam clings to its
walls, giving an attractive pattern known as
lacing . ~~
It is known that carbonation causes beer to have
carbon dioxide bite and, whilst this taste is regarded
favourably by some sections of the consumer market, there
are other sections of the market that regard the taste as
undesirable.
It is known to add nitrogen to beer as an
alternative means of producing foam in beer. It is also
known that nitrogen causes beer to have a smoother, less
bitter, taste.
There are a number of known options for
introducing nitrogen into beer.
One option is to dissolve nitrogen in beer prior
to filling into cans or bottles. This option is described
in a number of patent applications and patents including,
by way of example, Australian patents 642219 and 642714 in
AMENDED SI-~ET - IPEA/AU
CA 02295856 2000-O1-10
PCT/AU98/00540
Received 19 October 1998
- 3 -
the name of The BOC Group plc and International application
PCT/SE95/01449 (WO 96/17529) in the name of Tetra Laval
Holdings & Finance S A. In each of these patents and
patent application, the main reason for adding nitrogen gas
to cans is to generate super-atmospheric pressure in the
head-spaces of the cans to prevent deformation of the cans
during normal handling of the cans.
The addition of nitrogen gas to non-carbonated
liquid products prior to filling into cans or bottles is
also described in a number of patents and patent
applications including, by way of example, Australian
patent 642789 in the name of The BOC Group plc, UK patent
application 2134496 in the name of Asahi Breweries Ltd, and
US patent 4347695 in the name of General Foods Corporation.
The Gatehouse patent application describes the
option of dissolving nitrogen in beer prior to filling into
cans or bottles in the following negative terms:
"if nitrogen is dissolved in the beverage in a
reservoir before a filling operation carried out
in currently used equipment for filling small
containers with carbonated beverages, most of the
nitrogen is removed by 'gas washing' because, due
to the much lower solubility of nitrogen than
carbon dioxide in the liquid, any bubbles
liberated by liquid movement entrain nitrogen."
Another option for introducing nitrogen into beer
is to add nitrogen to beer at a filling station.
The Gatehouse patent application describes as an
invention a method of producing cans and bottles containing
beer in accordance with this option which comprises the
steps of
AMENDED SHEET - IPEA/AU
CA 02295856 2000-O1-10
PCT/AU98/00540
Received 19 October 1998
- 4 -
(i) partially filling a can or bottle with a
predetermined quantity of beer;
(ii) adding a predetermined quantity of liquid
nitrogen to the container or bottle; and
(iii)sealing the can or bottle.
The Gatehouse patent application describes that
the addition of nitrogen to beer in amounts of up to 1.14
grams of liquid nitrogen per litre of beer was found to
progressively improve foaming properties of beer.
A further option for introducing nitrogen into
beer is by means of inserts, commonly referred to as
"widgets", than are positioned in cans and store nitrogen
gas when the cans are sealed and release the gas as small
bubbles when the cans are subsequently opened. The small
bubbles produce foam in the beer. The cost of the widgets
and difficulties locating the widgets in cans in a high
throughput commercial line have limited the use of the
widgets.
A particular objective of the present invention
is to provide an improved method of producing cans or
bottles or other suitable containers containing beer.
A more general objective of the present invention
is to provide an improved method of producing cans or
bottles or other suitable containers containing a
carbonated or a non-carbonated liquid product.
According to one aspect of the present invention
there is provided a method of producing a liquid product
packed in cans or bottles or other suitable containers
which includes:
AMENDED SHEET - IFEA/AU
CA 02295856 2000-O1-10
PCT/AU98/00540
Received 19 October 1998
- 5 -
(i) injecting nitrous oxide and one or more than
one of nitrogen and carbon dioxide into the
liquid product;
(ii) pressurising the liquid product to increase
the solubility of nitrous oxide and one or
more than one of nitrogen and carbon dioxide
in the liquid product; and
(iii)filling the liquid product into cans or
bottles or other suitable containers and
thereafter sealing the cans or bottles or
other suitable containers.
1S The cans or bottles or other suitable containers
may be made from any suitable material. By way of example,
suitable materials include, metal, glass and PET.
In one embodiment the method further includes
depressurising the liquid product of step (ii) prior to
filling the liquid product into cans or bottles or other
suitable containers in step (iii).
In another embodiment the method further includes
partially depressurising the liquid product of step (ii)
prior to filling the liquid product under the reduced
pressure into cans or bottles or other suitable containers
in step (iii).
In one embodiment the method includes chilling
the liquid product to a predetermined temperature prior to
step (i) of injecting nitrous oxide and one or more than
one of nitrogen and carbon dioxide into the chilled liquid
product.
In another embodiment the method includes
chilling the liquid product to a predetermined temperature
AMENDED SI-SET - IPEA/AU
CA 02295856 2000-O1-10
PCT/AU98/00540
Received 19 October 1998
- 6 -
after step (i) of injecting nitrous oxide and one or more
than one of nitrogen and carbon dioxide into the liquid
product.
Preferably the predetermined temperature is in
the range of -1°C-8°C.
More preferably the temperature range is -1°C-
4°C.
It is preferred particularly that the temperature
range be -1°C-1°C.
Preferably step (i) of injecting nitrous oxide
and one or more than one of nitrogen and carbon dioxide
into the liquid product in step (i) is carried out under
pressure.
Preferably the pressure is at least 2 atmospheres
absolute.
Any suitable combination of nitrous oxide and one
or more than one of nitrogen and carbon dioxide, may be
injected into the liquid product in step (i).
Specifically: nitrous oxide and nitrogen; nitrous oxide and
carbon dioxide; and nitrous oxide, nitrogen and carbon
dioxide; may be injected in step (i).
In a situation where the liquid product is beer,
it is preferred that each of nitrogen, carbon dioxide, and
nitrous oxide be injected in step (i) into the beer.
In one embodiment the nitrous oxide, nitrogen and
carbon dioxide are injected as gases.
The nitrogen, carbon dioxide and nitrous oxide
may be injected into the liquid product as a gas mixture or
AMENDED SHEET - IPEA/AU
CA 02295856 2000-O1-10
PCT/AU98/00540
Received 19 October 1998
as separate gases.
In another embodiment the nitrous oxide and
carbon dioxide are injected as gases and the nitrogen is
injected as a liquid.
Preferably, the liquid product supplied to step
(i) is a carbonated liquid product. Depending on the
concentration of carbon dioxide, the method may include
stripping excess carbon dioxide from the liquid product
prior to step (i).
Preferably the liquid product is pressurised to
at least 2 atmosphere absolute in step (ii).
More preferably the liquid product is pressurised
to at least 5 atmosphere absolute in step (ii).
It is preferred particularly that the liquid
product be pressurised to 7-8 atmosphere absolute in step
(ii).
Preferably the liquid product is held under
pressure in step (ii) for at least 2 minutes.
Preferably the liquid product is held under
pressure in step (ii) for less than 10 minutes.
In a situation where the liquid product is beer,
the nitrogen is added principally to generate small bubbles
which produce foam when sealed cans or bottles are opened.
The carbon dioxide and nitrous oxide are more
soluble than nitrogen and therefore are not as effective as
nitrogen in generating foam - although a portion of both
gases will contribute to producing foam when the cans or
bottles are opened.
AMENDED SHEET - IPEA/AU
CA 02295856 2000-O1-10
PCT/AU98/00540
Received 19 October 1998
_ g _
The principal purpose of adding carbon dioxide to
beer is to ensure that beer does not go "flat" shortly
after being poured from the can or bottle into a glass or
other container.
The principal purpose of nitrous oxide is to take
away the adverse effect of carbon bite caused by carbon
dioxide.
In addition to the above, each of nitrogen,
carbon dioxide and nitrous oxide contributes to producing a
super atmospheric pressure in the head spaces of the cans
or bottles or other suitable containers to withstand
deformation during normal handling of the sealed cans or
bottles or other suitable containers.
Preferably the method further includes injecting
liquid nitrogen into the head spaces of the cans or bottles
or other suitable containers after filling the cans or
bottles or other suitable containers with the liquid
product and prior to sealing the cans or bottles or other
suitable containers.
According to the present invention there is also
provided a liquid product contained under pressure in a
sealed can or bottle or other suitable container, which
liquid product includes nitrogen, carbon dioxide, and
nitrous oxide which are released as gaseous phases and
cause foaming of the liquid product when the can or bottle
or other suitable container is opened.
The applicant has carried out a series of
experiments/trials producing and thereafter testing liquid
products, such as beer, in sealed cans - as described in
the preceding paragraph. The applicant found that the cans
of liquid products exhibited excellent foaming
AMENDED SHEET - IPEA/AU
CA 02295856 2000-O1-10
PCT/AU98/00540
Received 19 October 1998
_ g _
characteristics and taste. The applicant also found in a
number of instances that the foaming characteristics were
enhanced by shaking the cans prior to opening the cans and
pouring out the liquid products. This is a surprising
result in relation to carbonated liquid products, such as
beer, because usually even minor amounts of shaking
generate excessive amounts of foaming and are undesirable
on this basis.
Preferably the sealed can or bottle or other
suitable container contains 0.01-4 volume of nitrous oxide
per unit volume of the liquid product.
More preferably the sealed can or bottle or other
suitable container contains 0.3-1.2 volumes of nitrous
oxide per unit volume of the liquid product.
More preferably the sealed can or bottle or other
suitable container contains 0.4-1.2 volumes of nitrous
oxide per unit volume of the liquid product.
Preferably the sealed can or bottle or other
suitable container contains 0.1-3.5 volume of carbon
dioxide per unit volume of the liquid product.
More preferably the sealed can or bottle or other
suitable container contains 0.5-2.6 volumes of carbon
dioxide per unit volume of the liquid product.
More preferably the sealed can or bottle or other
suitable container contains 0.9-1.5 volumes of carbon
dioxide per unit volume of liquid product.
It is preferred particularly that the sealed can
or bottle or other suitable container contains 1.2-1.5
volumes of carbon dioxide per unit volume of liquid
product.
AMENDED SHEET - IPEA/AU
CA 02295856 2000-O1-10
PCT/AU98/00540
Received 19 October 1998
- 10 -
Preferably the sealed can or bottle or other
suitable container contains 0.1-1.8 volume of nitrogen per
unit volume of the liquid product.
More preferably the sealed can or bottle or other
suitable container contains 0.5-1.2 volumes of nitrogen per
unit volume of the liquid product.
More preferably the sealed can or bottle or other
suitable container contains 0.8-1.2 volumes of nitrogen per
unit volume of the liquid product.
It is preferred particularly that the sealed can
or bottle or other suitable container contains 1-1.2
volumes of nitrogen per unit volume of the liquid product.
Preferably the internal pressure of the sealed
bottle or container is greater than 3 atmosphere absolute
at ambient temperature.
More preferably the internal pressure is 4-5
atmospheres absolute.
Preferably the sealed can or bottle or other
suitable container does not include a "widget" or other
device for storing nitrogen, carbon dioxide and nitrous
oxide for release when the can or bottle is opened.
Preferably the liquid product is beer.
According to another aspect of the present
invention there is also provided a method of producing a
carbonated liquid product, such as beer, packed in cans or
bottles or other suitable containers which includes:
(i) placing a predetermined quantity of a
AMENDED SHEET - IPEA/AU
CA 02295856 2000-O1-10
PCT/AU98/00540
Received 19 October 1998
- 11 -
carbonated liquid product in the cans or
bottles or other suitable containers;
(ii) adding nitrogen, and optionally one or more
other additives which promote foaming, to
the liquid product in the cans or bottles or
other suitable containers; and
(iii)sealing the cans or bottles or other
suitable containers.
It is preferred that the nitrogen be added as
liquid nitrogen.
Preferably nitrous oxide is added as a foaming
agent.
Preferably, when the nitrogen and carbon dioxide
are the only foaming agents in the liquid product, the
nitrogen is added to step (ii) in an amount of more than
1.14 grams of nitrogen per litre of the liquid product.
According to another aspect of the present
invention there is also provided a method of producing a
carbonated liquid product, such as beer, packed in cans or
bottles or other suitable containers, which includes:
(i) dissolving nitrogen, and optionally one or
more other additives which promote foaming,
into a carbonated liquid product to form a
nitrogen-containing liquid product;
(ii) filling a can or bottle or other suitable
container with a predetermined quantity of
the nitrogen-containing liquid product; and
(iii)sealing the can or bottle or other suitable
AMENDED SHEET - IPEA/AU
CA 02295856 2000-O1-10
PCT/AU98/00540
Received 19 October 1998
- 12 -
container.
It is preferred that the nitrogen be added as a
gas.
Preferably nitrous oxide is added as a foaming
agent.
Preferably the method includes chilling the
carbonated liquid product prior to dissolving nitrogen in
step (i).
Preferably, when nitrogen carbon dioxide are the
only foaming agents in the liquid product, the nitrogen is
added in an amount of more than 1.14 grams of nitrogen per
litre of lilquid product.
The applicant has found that the addition of
nitrogen to beer that contains less than the conventional
level of carbonation in amounts greater than 1.14 grams of
nitrogen per litre of beer increases significantly the
foaming characteristics of the beer and causes the beer to
have a smoother, less bitter, taste compared to beer having
no nitrogen addition, the conventional level of
carbonation, and no other foaming agents.
It is preferred that the liquid product be beer
although it is emphasised that the invention is not
restricted to beer and extends to any other liquid product
and to any non-carbonated liquid product.
The term "foaming agent" is understood to mean
any agent, in gaseous or liguid form, that promotes foaming
in a liguid product.
The term "foaming agent" includes, by way of
example only, nitrogen, carbon dioxide, nitrous oxide,
AMENDED SHEET - IPEA/AU
CA 02295856 2000-O1-10
PCT/AU98/00540
Received 19 October 1998
- 13 -
helium, and argon.
Where the liquid product contains foaming agents
in addition to nitrogen and carbon dioxide, the amount of
the nitrogen added to the liquid product may be less than
1.14 grams per litre of the liquid pro$uct with the amount
of the nitrogen depending on the amount and foaming
properties of the other foaming agents) added to the
liquid product.
In one embodiment the beer and the cans or
bottles or other suitable containers are separately
sterilised and transferred under sterile conditions to a
filling station maintained under sterile conditions. At
the filling station, measured quantities of beer are filled
into the cans and bottles or other suitable containers,
thereafter an amount of liquid nitrogen, preferably greater
than 1.14 grams per litre of beer, is added to the cans and
bottles or other suitable containers, and finally the cans
and bottles or other suitable containers are sealed.
A particular advantage of this embodiment is that
the sterilisation of the beer and the cans and bottles or
other suitable containers involves no increase in the
internal pressure of the canned and bottled beer.
Alternatively the beer and the cans or bottles
or other suitable containers are transferred under non-
sterilised conditions to a filling station, measured
quantities of the beer are filled into the cans or bottles
or other suitable containers, thereafter an amount of
liquid nitrogen, preferably greater than 1.14 grams per
litre of beer, is added, and the cans or bottles or other
suitable containers are then sealed. Finally, in order to
pasteurise or sterilise the canned and bottled beer, the
cans and bottles or other suitable containers are exposed
to various means of heating. The heating of the beer
AMENDED SHEET - IPEA/AU
CA 02295856 2000-O1-10
PCT/AU98/00540
Received 19 October 1998
- 14 -
produces an increase in internal pressure. In order to
accommodate the pressure increase it is necessary to use
stronger cans or bottles or other suitable containers than
are used conventionally and/or to provide a larger head
S space than is used conventionally for a given volume of
beer to allow for volume expansion.
The present invention is described further by way
of example with reference to the accompanying drawings, of
which:
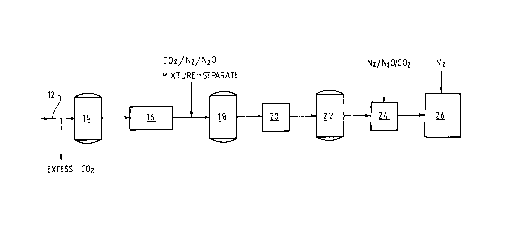
Figure 1 is one preferred embodiment of a method
of producing canned or bottled beer in accordance with the
present invention;
Figure 2 is another preferred embodiment of a
method of producing canned or bottled beer in accordance
with the present invention; and
Figure 3 is another preferred embodiment of a
method of producing canned or bottled beer in accordance
with the present invention.
The preferred embodiments described below relate
to producing beer. It is emphasised that the present
invention is not limited to producing beer and extends to
producing any carbonated and non-carbonated liQuid product.
With reference to Figure 1, carbonated beer
produced by conventional beer-making technology flows along
a line 12 and excess carbon dioxide (if any) is stripped
from the beer prior to the beer reaching the holding tank
14.
The beer flows from the holding tank 14 through a
chiller 16 in which the beer is chilled to a temperature in
a range of -1°C to 4°C.
AMENDED SHEET - IPEA/AU
CA 02295856 2000-O1-10
PCT/AU98/00540
Received 19 October 1998
- 15 -
Thereafter, any one or more of nitrogen gas,
carbon dioxide gas, and nitrous oxide gas are injected
under pressure of 2-3 atmospheres absolute into the chilled
beer as it flows from the chiller 16 to a holding tank 18.
The gases may be injected separately or as a gas
mixture.
It is preferred that a mixture of nitrogen,
carbon dioxide, and nitrous oxide gases be injected in the
chilled beer.
The amount of each gas injected into the chilled
beer should be within the broad range described above and
having regard to the levels of injection of the other
gases. As a general guideline, as the level of injected
carbon dioxide increases, the level of injected nitrous
oxide can decrease.
The beer flows from the holding tank 18 to a
pressurisation station 20 at which the beer is pressurised
to at least 7 atmosphere absolute to increase the
dissolution of the injected gases into the beer.
The pressurised beer flows to a holding tank 22
and thereafter to a depressurisation station 24 at which
the pressure is reduced to atmospheric pressure or any
other suitable filling pressure and the beer is then filled
into cans at a filling station 26.
The pressurisation station 20 and the
depressurisation station 24 may be of any suitable
construction. Tyyically, the stations are tank or pipes.
The stations may be a single vessel.
The embodiment of the method shown in Figure 2 is
AMENDED SHEET - IPEA/AU
CA 02295856 2000-O1-10
PCT/AU98/00540
Received 19 October 1998
- 16 -
similar to that shown in Figure 1. The main difference is
that the beer supplied to the method is not carbonated. As
a consequence, injection of carbon dioxide gas after the
chiller 16 is necessary to produce required levels of
carbon dioxide.
With reference to Figure 3, carbonated beer
produced by conventional beer-making technology flows along
a line 32 to a chiller 36 and is cooled in the chiller to a
temperature in the r ~:nge of -1°C to 4°C .
The chilled beer flows from the chiller 36 to a
holding tank 38 and a gas mixture of nitrogen and nitrous
oxide, and optionally carbon dioxide, is injected into the
beer under pressure of 2-3 atmospheres absolute before it
reaches the holding tank 36.
The beer flows from the holding tank 38 to a
pressurisation station 40 at which the beer is pressurised
to 7-8 atmospheres absolute.
The method may include the optional steps of
passing the beer from the holding tank 38 through a second
chiller (not shown) to adjust the temperature of the beer
and injecting further nitrogen gas to the beer to reach a
required level of nitrogen in the beer before supplying the
beer to the pressurisation station 40.
The beer flows from the pressurisation station 40
to a depressurisation station 42 at which the beer is
depressurised to 3-5 atmospheres absolute.
The beer is then filled at a filling station 44
into cans or bottles or other suitable containers under
this pressure and liquid nitrogen is added to the head
space of each container prior to closing the containers.
AMENDED SHEET - IPEA/AU
CA 02295856 2000-O1-10
PCT/AU98/00540
Received 19 October 1998
- 17 -
The applicant has carried out a series of trials
of the preferred embodiment shown in Figure 3 on a
commercial filling line. The trials were successful and
produced canned beer with foaming characteristics that were
at least comparable to "widget" containing cans.
The method includes an option of providing
nitrogen gas, carbon dioxide gas, and nitrous oxide gas at
the depressurisation station 24 to maintain the levels of
these gases in the beer. Specifically, in a situation
where the depressurisation station includes a tank with a
head space, it is important to maintain the partial
pressure of nitrogen, carbon dioxide, and nitrous oxide in
the head space the same as the required partial pressure of
these gases in the beer.
In addition, the method includes an option of
introducing liquid nitrogen into the head space of cans or
bottles prior to sealing the cans or bottles.
Many modifications may be made to the preferred
embodiments described above without departing from the
spirit and scope of the present invention.
By way of example, whilst each preferred
embodiment chills the beer prior to injecting one or more
of nitrogen, carbon dioxide, and nitrous oxide into the
beer, the present invention is not limited to this
arrangement and gas injection can be made prior to chilling
the beer. Chilling the beer prior to gas injection is
particularly preferred for a range of reasons, including
avoiding the possibility of icing up of the chiller.
By way of further example, whilst each preferred
embodiment includes separate holding tanks and
pressurisation/depressurisation stations, the present
invention is not limited to this arrangement and extends to
AMENDED SHEET - ~EA/AU
CA 02295856 2000-O1-10
PCT/AU98/00540
Received 19 October 1998
- 18 -
any suitable arrangement. By way of example, a single tank
could be used in place of the holding tanks and the
pressurisation/depressurisation stations.
AMENDED SHEET - IPEA/AU