Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02304199 2000-03-15
WO 99/14591 PCT/CA98/00874
Dissolved Solids Analyzer
Field of the Invention
This invention relates to the application of conductivity and UV measurements
for on-line
measurements of an amount of dissolved solids in a liquid sample. More
particularly, an
aspect of the invention relates to the determination of the amount of
dissolved solids in a
pulp and paper mill process water or effluent using a combination of
conductivity and UV
absorbance measurements.
Background of the Invention
On-line measurements of the amount of dissolved solids of paper mill process
waters,
such as whitewater, graywater, and effluents, can provide the necessary
feedback for
optimizing retention, flocculation, and water flow in the paper mill. At
present, on-line
measurements do not provide the detail necessary for optimal control. This is
particularly
the case when measuring the total amount of dissolved solids in a liquid
sample.
The importance of the management of the composition of industrial water
streams is
described by Simons, NLK Consultants, and Sandwell Inc., in a 1994 publication
"Water
Use Reduction in the Pulp and Paper Industry", Canadian Pulp and Paper
Association,
Montreal. The excessive build-up of dissolved solids in a process water stream
may
decrease process efficiency and increase corrosion, foaming, odour, pitch,
precipitation,
and scaling. A counter-current flow of water to pulp streams is a commonly
used method
to efficiently use water in pulp processing and papermaking to optimize the
removal of
dissolved solids. In order to prevent production problems related to the build-
up of
dissolved solids in process water it is necessary to efficiently remove and
minimize the
variation of dissolved solids in liquid samples. Garver et al. in a Journal
entitled Tappi
Vol. 80 Number8, pages 163-173, 1997 teach that the temporal or spatial
variation in the
amount of dissolved solids in a water stream may lead to manufacturing
problems
including precipitation, deposition, scaling and pitch formation.
1
SUBSTiTUTE SHEET (RULE 26)
CA 02304199 2000-03-15
WO 99/14591 PCT/CA98/00874
One standard method for the examination of water and wastewater employed by
the
American Health Association measures the total amount of dissolved solids
directly by
gravimetric analysis after evaporation of a known volume of liquid after
filtration.
The empirical estimation of dissolved solids using a conductivity measurement
is an
established technique employing a calibration between the dissolved solids and
a
conductivity measurement. This method is widely used as a relative measure of
dissolved
inorganic salts and many conductivity/TDS (Total Dissolved Solids) meters are
available
on the market. The relationship between dissolved solids and conductivity
differs for
each type of ion depending on the charge and size of the ion. Empirical
constants to
convert conductivity (mS cm") to dissolved solids (mg L"1) may vary
considerably, i.e.
between 0.55 and 0.9 depending on ion type, concentration and temperature,
American
Public Health Association, Standard Methods for the Examination of Water and
Wastewater, American Public Health Association, American Water Works
Association,
Water Pollution Control Federation, Washington D.C. 1992, pp. 2-47. However,
the
amount of dissolved solids measured by conductivity is only reliable when
specific
inorganic salts dominate the dissolved solids present in the water.
Conversely,
conductivity measurements present a poor measure of the amount of dissolved
solids
when substances with little or no ionic charge contribute substantially to the
amount of
dissolved solids.
The principle disadvantages of using conductivity as a measure of the amount
of
dissolved solids are related to inaccuracies arising from the differences in
the specific
conductivity of different ions, association or chelation of positive or
negative ions
resulting in inactive ions, and the poor detection of organic acids and
organic neutral
substances. In a paper mill situation the relative ratio of dissolved
inorganic salts to
dissolved organic material varies dramatically depending on the location in
the pulp
processing sequence. For example, in a lignin retaining pulp brightening
process, such as
sodium hydrosulfite bleaching, the variation in the amount of dissolved solids
may be
largely related to the amount of bleach applied and the residual sulfur
species resulting
from hydrosulfite decomposition.
2
SUBSTITUTE SHEET (RULE 26)
CA 02304199 2000-03-15
WO 99/14591 PCT/CA98/00874
The patent literature describes applications using conductivity measurements
to control
water introduction, counter-current flow or sewer flow in pulp or paper
processing. The
objective of the control of the amount of dissolved solids using conductivity
measurements has been to improve the washing, separation and removal of solids
and to
minimize scaling and deposition. Rosenberger (U.S. Patent No. 4,096,028)
discloses
feed-forward control of the amount of dissolved material in a counter-current
flowing
liquid using conductivity measurements and flow rates. Sexton (U.S. Patent No.
4,046,621) disclosed a feed backwards method for the control of pulp treatment
using
conductivity measurements. Heoksema et al. disclose an apparatus for
conductivity
measurements of pulp washing liquors from a drum type washer. Lisnyansky and
Blaecha taught a control strategy for optimizing the efficiency of counter-
current flow
pulp washing based on a dilution factor or soda wash.
In a counter-current flow pulp treatment or washing not only the removal of
dissolved
ions may be controlled by a conductivity measurement but the accumulation of
the water
may also be measured and controlled. The benefits of maintaining a low or
constant
amount of dissolved solids are related to solubility equilibria which
influence the
extraction of unwanted material from pulp and also govern the deposition and
precipitation reactions leading to unwanted scale and deposits.
The absorbance from selected wavelengths of the UV may be used as a measure of
the
relative quantity of extractives and lignin or carbohydrate derived
components.
Marcoccia et al. (U.S. Patent No. 5,547,012) teach a method of control of
kraft pulping by
controlling the amount of dissolved organic material in a continuous digestor.
Sloan (U.S. Patent No. 4,886,576) teaches a method for using the UV absorbance
of
lignin dissolved during digester cooking for control of pulp cooking
parameters and
refiner energy. Manook et al. (U.S. Patent No. 5,420, 432 or Cdn. Patent No.
2,106,472)
disclose an organic pollutant monitor based on UV absorbance measurements for
the
determination of the amount of organic matter.
3
SUBSTITUTE SHEET (RULE 26)
CA 02304199 2000-03-15
WO 91/17305 by Paulonis et al. discloses a method and an apparatus for
determining the
concentrations of each of three components, namely sodium hydroxide, sodium
sulfide,
and sodium carbonate, that are intermixed in a homogeneous solution. The
method
taught by Paulonis et al. involves a mathematical relationship between the
concentration
of each of the components and detectable characteristics. The characteristics
measured
by Paulonis et al. are refractive index, absorbance, and conductivity.
However, there is
no mention in WO 91/17305 to combine UV and conductivity measurements in such
a
manner as to include either a product or a ratio of the two characteristics
into the
mathematical relationship for determining the concentration of the components.
The
present invention demonstrates that using a product or a ratio between
conductivity and
absorbance provides a better measure for the determination of dissolved
solids. Further,
the method and the apparatus disclosed by Paulonis et al. teach a method and
an
apparatus for analyzing inorganic components. The present invention discloses
a method
for measuring both organic and inorganic dissolved solids from a same sample.
GB-A 2,282,880 by Owens discloses an ultraviolet absorbency-based monitor for
measuring the amount of organic pollution in a liquid. Furthermore, U.S.
Patent No.
5,420,432 to Manook et al. discloses an ultraviolet absorbance-based organic
pollutant
monitor. EP-A 0,559,305 by Richardson et al. teaches a method for
simultaneously
monitoring the concentration of multiple performance indicators in an aqueous
system by
analyzing the spectrum of the aqueous system between 200 nm and 2500 nm and
applying chemometric algorithms to simultaneously determine the concentration
of the
performance indicators. GB-A 2,282,880, U.S. Patent No. 5,420,432 and EP-A
0,559,305 all disclose UV absorption measurements in an aqueous sample for the
purpose
of determining dissolved organic components within the sample. The present
invention
discloses an apparatus and a method for determining both organic and inorganic
dissolved solids in a sample. Further, in accordance with an embodiment of the
present
invention the dissolved matter is determined from a mathematical relationship
including
at least a product or a ratio of the measured absorbance and conductivity.
This is not
suggested in the above-cited prior art references.
3a
AMENDED SHEET
CA 02304199 2000-03-15
WO 99/14591 PCT/CA98/00874
Papermaker's demands for high speed and efficiency, flexible manufacturing,
stringent
quality standards, and environmental compatibility coupled with new
developments in
on-line process control are driving the development of new sensor technology
for the
paper machine wet-end. The need for better means for providing wet-end
chemistry
control is emphasized by recent reports that only 10% of the world's 150
newsprint paper
machines operate at above 88% efficiency and over 60 % operate under in the
low
efficiency range of below 82.5%. (Mardon, J., Chinn, G. P., O'Blenes, G.,
Robertson, G..
Tkacz, A. Pulp and Paper Canada, 99(5) 43-46. (1998).
Nazair and Jones teach that wet-end variability arising from practical
determinants and
disturbances leads to variations in molecular and colloidal interactions that
result in
practical consequences in terms of the process and the product (Nazair, B. A;
Jones, J. C.
(Paper Technology 32(10) 37-41. 1991. Optimizing wet-end chemistry- the
practicalities.). Practical determinants include the type of fumish, fillers,
chemical being
used, addition rates, addition points, refining, pH, temperature and
consistency.
Disturbances include broke, machine breaks, quality of materials, machine wear
and
seasonality. These variations may deleteriously effect system cleanliness,
runability, first
pass retention and product quality factors including formation, sizing,
uniformity,
strength, porosity and defects. The high capital cost of paper machines
demands
maximization of paper machine efficiency and quality. The papermaker will
attempt to
minimize system-input variation and counteract variation in practical
determinants and
disturbances so as to minimize variation and degradation of process efficiency
and
product quality.
The consequences of poor control of the variation, total level and composition
of
dissolved substances have been recognized by numerous authors. Gill teaches
the
importance of variation control of dissolved and colloidal substances in the
paper
machine wet-end. "Dissolved and colloidal substances (DCS) are released from
the water
phase from contaminated pulps or broke, and form deposits at the wet-end,
press section,
machine fabrics and rolls. These deposits cause: downtime; defective products;
sheet
breaks; frequent fabrics change." William E. Scott address problems related to
wet-end
chemistry control. Principles of Wet End Chemistry. Tappi Press, Atlanta,
1996. p 3.
4
SUBSTITUTE SHEET (RULE 26)
CA 02304199 2000-03-15
WO 99/14591 PCT/CA98/00874
"Deposits and scale usually arise from out-of-control wet end chemistry.
Typical
examples include chemical additive overdosing, charge imbalances, chemical
incompatibility and the shifting of chemical equilibria. All of these
phenomena can lead
to the formation of precipitates or colloidal aggregates that produce deposits
and scale.
While there are numerous approaches to treating the symptoms of deposits the
best
approach is to determine what is out of control and fix it."
One simple measure of the variability of the wet-end system chemistry is the
level of
dissolved organic and inorganic solids in the paper machine white water
system. Tools.
that have become available for wet-end chemistry monitoring include retention
monitoring, turbidity and electrokinetic potential (streaming current, cation
charge
demand, and zeta-potential) instruments. On-line instrumentation for
monitoring and
controlling the inorganic and organic dissolved and colloidal solids in a
paper mill is at
present limited to conductivity measurement or on-line charge measurement.
While off-
line total dissolved solids, turbidity, pitch counts, COD and TOC measurements
may be
used. In summary, the presently available means for on-line monitoring of wet-
end
chemistry fall short of providing reliable measurement of dissolved organic
and inorganic
solids.
Chemicals can provide control of the levels of DCS and deposit formation can
be
eliminated or reduced to tolerable levels by careful control of water flow and
addition of
chemicals for either dispersing or adsorbing and coagulating dissolved and
colloidal
substances. (Gill, R. S. Paper Technology, 37, July/August, 1996. 23-31.
Chemical
control of deposits-scopes and limitations.)
It is an object of the present invention to provide a method and an apparatus
for on-line
measurement of the amount of dissolved solids in a liquid sample, such as in a
pulp or
paper mill process water or effluent.
It is another object of the invention to provide an analyzer for total
dissolved solids by
combining conductivity and UV measurements of a liquid sample. In combination,
these
measurements are used to determine the total dissolved solids in a liquid
using a
5
SUBSTITUTE SHEET (RULE 26)
CA 02304199 2000-03-15
WO 99/14591 PCT/CA98/00874
mathematical relationship for expressing the relationship between variables.
Furthermore, additional mathematical relationships are provided for estimating
the
relative contribution of inorganic and organic dissolved components, or ionic
and non-
ionic components.
According to a specific object of the invention an on-line measurement and
control
system for dissolved substances in paper mill process waters is provided.
Environmental
concerns and demanding manufacturing processes afford the development of
sensors. In
accordance with the invention the amount of dissolved solids is measured as a
function of
both UV absorbance and conductivity of the sample. High levels of dissolved
solids and
variation in the amount of dissolved solids leads to runability problems of
paper
machines. Thus, to improve the manufacturing process in a pulp and paper mill
better
control of the amount of dissolved solids in process water, such as white
water, is needed.
Summary of the Invention
A method for determining an amount of dissolved matter in a liquid sample is
provided in
accordance with the invention, comprising the steps of:
(a) irradiating at least a portion of the liquid sample with light of at least
a first
wavelength within a range of wavelengths in an ultraviolet region, wherein
said range of
wavelengths is for allowing an absorption measurement of said liquid sample;
(b) measuring an absorption of the first wavelength by the liquid sample;
(c) measuring a conductivity of the liquid sample; and
(d) computing the amount of dissolved matter in the liquid sample from a first
relationship between the measured absorption of the first wavelength by the
liquid sample
and the measured conductivity of the liquid sample using a suitably programmed
processor.
In accordance with another aspect of the invention an apparatus is provided
for
determining an amount of dissolved matter in a liquid sample comprising:
6
SUBSTITUTE SHEET (RULE 26)
CA 02304199 2000-03-15
WO 99/14591 PCT/CA98/00874
(a) an ultraviolet detection unit for measuring an absorption of at least a
first
wavelength within a range of wavelength in an ultraviolet region, said
ultraviolet
detection unit for measuring the absorption by the liquid sample;
(b) a conductivity unit for measuring a conductivity of the liquid sample; and
(c) a suitably programmed processor for determining a first relationship
between
the absorption of the first wavelength by the liquid sample and the
conductivity of the
liquid sample for computing the amount of dissolved solids in the liquid
sample.
In accordance with the invention there is further provided a method for
controlling an
amount of dissolved solids in a process water from pulp and paper processing
using one
of a counter-current flow process and a discrete chemical treatment process
comprising
the steps of:
(a) measuring an absorbance of the process water at a first wavelength within
a
range of wavelength in an ultraviolet region;
(b) measuring the conductivity of the process water; and
(c) determining the amount of dissolved solids in the process water from a
first
relationship in dependence upon the measured absorbance and the measured
conductivity.
Brief Description of the Drawings
Exemplary embodiments of the invention will now be described in accordance
with the drawings in which:
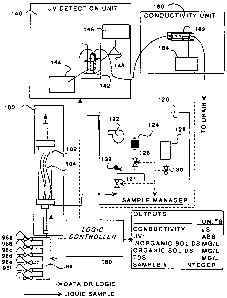
Figure 1 is a schematic diagram of the Dissolved Solids Analyzer;
Figure 2 presents a scatterplot of the amount of total dissolved solids versus
the product
of UV absorbance and conductivity;
Figure 3 shows a scatterplot presenting normalized data from the dissolved
solids
analyzer;
7
SUBSTITUTE SHEET (RULE 26)
CA 02304199 2000-03-15
WO 99/14591 PCT/CA98/00874
Figure 4 presents a plot of conductivity, UV absorbance and TDS as measured
from white
water in deinked pulp High Density (HD) storage;
Figure 5 presents a plot of conductivity, UV absorbance and TDS as measured
from white
water in TMP pulp High Density (HD) storage;
Figure 6 presents a plot of conductivity, UV absorbance and TDS as measured
from white
water in the paper machine 5 headbox;
Figure 7 presents a matrix plot showing the relationship between TDS, UV
absorbance,
conductivity, and the product of UV absorbance and conductivity;
Figure 8 presents mill data obtained with the dissolved solids analyzer
showing
conductivity and UV components over a period of time;
Figure 8a shows mill data obtained with the total dissolved solids analyzer
and presents a
plot of the product (UV absorbance*conductivity) and the ratio
(conductivity/UV) as a
function of time;
Figure 9 shows a graph of the turbidity versus the ratio of the UV absorbance
to the
conductivity;
Figure 10 shows a scatterplot of the turbidity and the ratio of the UV
absorbance to the
conductivity over a period of time;
Figure 11 shows a plot of UV absorbance of centrifuged and filtered TMP white
water in
dependence upon the amount of cationic polymer;
Figure 12 presents a plot of the variation of UV absorbance as a function of
added
cationic polymer;
Figure 12a shows a plot of UV absorbance vs. pH;
8
SUBSTITUTE SHEET (RULE 26)
CA 02304199 2000-03-15
Figure 13 presents a detailed diagrarn showing potential points for
application of the
Dissolved Solids Analyzer in an integrated pulp and paper mill;
Figure 14 presents a block diagram showing elements of measurement and the
control of
dissolved solids in an integrated newsprint mill; and
Figure 15 is a plot showing the components of the TDS equation broken down
into the
UV contribution, signifying the organic portion of the TDS, and the
conductivity
contribution, signifying the inorganic portion of the TDS.
Detailed Description of the Invention
The method and the apparatus in accordance with the invention provides for on-
line
measurements of dissolved solids in a liquid sample. This invention is
particularly useful
for determining or estimating the amount of dissolved solids in pulp or paper
mill process
water or effluents. Referring now to Figure 1, a schematic diagram of the
dissolved
solids analyzer is shown. The flow of the liquid sample is shown as solid
black lines and
the data flow is shown as dashed lines. A liquid sample is introduced into the
sample
manifold 98 by opening an inlet valve 96. In a preferred embodiment, the
apparatus in
agreement with the invention has a plurality of inlet valves 96a-f, as shown
in Figure 1,
for receiving samples from a plurality of processes in an integrated pulp and
paper mill.
Valves 96a-f are in communication with a logic controller 180 for controlling
the
delivery of liquid samples to the sample manifold 98. The sample manifold 98
is in
communication with a filtration unit 100. The function of the filtration unit
100 is to
filter the liquid sample in a reproducible manner for removing particulates
therefrom.
The liquid sample is passed through a Minworth SystemsTM (MSL) filtration unit
102
with "Zeeweed" TM hollow fiber microfilter 104 manufactured by Zenon LabsTM.
This
system has automated continuous cleaning and back-flushing. The filter 102
works for
white water at temperatures of 45 C and below. Those skilled in the art will
appreciate
that another filtration system may serve in place of the Zenon LabsTM hollow
fiber
microfilter. In order to obtain a reproducible measurement the filter 102 is
chosen to be
9
ANlENDED SNEET
CA 02304199 2000-03-15
of the cross-flow type or tangential-flow type with the flow across the
membrane being
20-100 times the flow through the membrane. Furthermore, the filter should be
regularly
backed-pulsed with the filtrate to ensure minimal accumulation of suspended
solids on
the filter surface. Filtering through a filter cake leads to unreliable
ultraviolet (UV)
measurements of a pulp or paper mill process water because an accumulating
filter cake
consisting of pulp fiber, fines and colloids will result in the selective
removal of some
dissolved substances. Filters which utilize the cross-flow principle to
minimize filter
cake formation are for example tubular membrane filters by KochTM Membrane
Systems,
Inc. and sintered metal filters by MottTM Industries. However, the liquid
samples may
also be manually introduced into the system through a sample port.
After the liquid sample is filtered in the filtration unit 100 it is directed
to the sample
manager 120. The sample manager consists of a valve 121 for delivering the
filtered
liquid sample to the UV detection unit 140 and the conductivity unit 160, a
valve 126 for
delivering fresh water from the fresh water reservoir 124 to the UV detection
unit 140
and the conductivity unit 160, a valve 130 for delivering a cleaning fluid
from the
cleaning fluid reservoir 128 to the UV detection unit 140 and the conductivity
unit 160, a
pump 122 for delivering the liquid sample, the fresh water or the cleaning
fluid from the
sample manager 120 to the UV detection unit 140 and the conductivity unit 160,
and a
pressure sensor 132. Valves 121, 126, 130, and the pressure sensor 132 are in
communication with a logic controller 180. The pressure sensor 132 provides
feedback
to the logic controller 180 for controlling a cleaning cycle.
The pump 122 delivers the liquid sample to the UV detection unit 140. In the
UV
detection unit 140, the liquid sample is passed through a flow-through cell
142. This
flow-through cell 142 is irradiated with UV light provided by a UV light
source 144
located on one side of the flow-through cell 142. A light detector 146,
located on another
side of the flow-through cell 142, measures the absorbance of UV light as it
traverses the
liquid sample. The light detector 146 is connected to a wavelength selector
148 and the
logic controller 180. The raw data of UV light absorbance by the liquid sample
is passed
from the light detector 146 to the logic controller 180 for further data
processing. In a
AMENDED SHEET
CA 02304199 2000-03-15
preferred embodiment a variable wavelength UV-visible spectrophotometer is
used, such
as a ShimadzuTM UV-visible HPLC detector set, or a D-starTM DFW-20/21
detector. The
UV detector may be purchased as an assembled unit or manufactured within an
integrated
dissolved solids detection system. Many single (fixed) wavelength or
selectable
wavelength UV-visible spectrophotometers are commercially available. However,
the
most important components of the UV detection unit 140 are:
i) the light detector 146, such as a silicon photovoltaic detector (SiemensTM)
or a
photomultiplier;
ii) the wavelength selector 148, such as a monochromator or a 280 run
interference filter for 280 nm (OrielTM, Edmond ScientificTM);
iii) the UV light source 144, such as a deuterium lamp or a xenon arc light
source,
examples include McPhersonTM, EGGTM, Ocean OpticsTM, ILCTM;
iv) the flow-through cell 142, such as a 1 mm quartz or SuprasilTM flow-
through
cell (HelmaTM, 170.000)
The UV detection unit is in its preferred embodiment temperature controlled
with high
quality power supplies for the UV light source and the light detector.
The preferred wavelength for measuring the UV absorbance is 280 ( 2) nm.
However, a
wavelength range between 205-380 nm produces suitable results.
After passing through the UV detection unit 140 the liquid sample is delivered
to the
conductivity unit 160. This conductivity unit 160 consists of a conductivity
probe 162
and a conductivity analyzer 164. The conductivity probe 162 is a flow-through
contact
probe with a cell constant of 1. The specifications for the conductivity
analyzer 164 are
as follows:
Ranges: Conductivity (switchable)
0 to 19.99 mS/cm
0 to 1999.9 S/cm
0 to 199.9 S/cm
Temperature range 0 to 100 C
Resolution: Conductivity 1 S/cm
Temperature 0.1 C
Accuracy: Conductivity +1-0.5%
Temperature 0.5 C
Temperature compensation 0 to 100 C
11
AMENDED SHEET
CA 02304199 2000-03-15
Excitation frequency 1 kHz
Reference temperature 0 to 100 C
Cell constant 0.2 (programmable)
Examples of suitable conductivity analyzers that can be used in the apparatus
shown in
Figure 1 are GLI Model C33TM, the ICTM Controls conductivity analyzer, the
HoneywellT"" 9782 Analyzer, and the HachTM Mode1471 conductivity analyzer. The
conductivity unit 160 is connected to the logic controller 180 and the raw
data obtained
from conductivity measurements of the liquid sample are delivered from the
conductivity
analyzer 164 to the logic controller 180 for further data processing.
Conductivity, also called specific conductance (K), is the conductance
compensated for
the area of the electrodes A and the distance between the electrodes 1. These
constants
that are related to the measurement process rather than the intrinsic property
of the
medium are often lumped together as a cell constant
B- 1
A
Specific conductance measurement for pulp and paper process waters often will
average
around 1000 S cm"1, and may range between 400 - 40000 S cm"t. The cell
constant for
paper machine white water should be between 1.0 and 10Ø The conductance may
be
written as G = x~=~ or the conductivity may be written as K=~
The proper units for conductivity are S cm". Conductivity measurements are
typically
made using an AC current cycling between 60-1000 Hz with plantinized platinum
electrodes and a modified Wheatstone bridge. Non-contact, toroidal
conductivity probes
are sometimes used to avoid electrode fouling under heavy fouling conditions.
Conductivity is temperature sensitive and measurements are normally
temperature
compensated.
Alternatively, if desired, the conductivity unit is placed between the sample
manifold 98
and the filtration unit 100 as the conductivity measurement is not influenced
by the
filtration process.
12
AAIENDED SHEET
CA 02304199 2000-03-15
The logic controller 180 is a programmable unit which drives the components of
the
apparatus presented in Figure 1 in a predetermined sequence. This logic
controller 180
provides six 24 V DC outputs for controlling the valves 96a-f, 121, 126, 130
and the
pump 122 as well as six analog inputs/outputs for the light detector 146, the
pressure
sensor 132 and the conductivity analyzer 164. An example for a possible logic
controller
to be used in the invention is the Allen BradleyTM 5/03 PLC. A smaller logic
controller,
such as the Allen Bradley MicrologixTM 1000 also fulfills the requirements for
the logic
controller 180. However, the system logic and the data acquisition system
could be
custom designed and manufactured.
In one embodiment the raw data obtained from the light detector 146 and the
conductivity
analyzer 164 are directly delivered to a FoxboroT'" Distributed Control System
(D.C.S.).
There they can be accessed through the Aspen Technologies'T"' Process
Management
Information System (PMIS) using a Process ExplorerTM software.
After a liquid sample has been passed through the apparatus shown in Figure 1
for
determining the amount of dissolved solids in the liquid sample it is
advantageous to
perform a cleaning cycle. The logic controller 180 is opening/closing valve
126 for
flushing the apparatus with fresh water, valve 130 for flushing the apparatus
with a
cleaning fluid and valve 121 for preventing the liquid sample from being
delivered to the
UV detection unit 140 and the conductivity unit 160 when a cleaning cycle is
performed.
The pressure sensor 132 provides the feedback to the logic controller 180 for
controlling
the cleaning cycle, i.e. it provides the logic controller with the information
which valves
are to be opened/closed. Pump 122 delivers the fresh water or the cleaning
fluid to the
UV detection unit 140 and the conductivity unit 160.
In the specification the determination of dissolved matter can be expressed as
either an
exact quantity of measured/computed (via a UV and conductivity product) of
dissolved
matter or alternatively the relative quantity can be expressed in form of a UV
conductivity ratio.
13
AMENDED SHEET
CA 02304199 2000-03-15
WO 99/14591 PCT/CA98/00874
In accordance with the invention the total amount of dissolved solids (TDS) in
the liquid
sample is determined from a mathematical relationship combining the UV
absorbance
and the conductivity measurements of the liquid sample. There is an excellent
correlation
between the total amount of dissolved solids (TDS) and a combination of
conductivity
and UV measurements. Several mathematical relationships appear to give good
results
for accurately predicting the TDS from a UV and conductivity measurement. The
empirical relationship is set and may be updated by multilinear correlation of
the UV
absorbance and conductivity with measured TDS. Typically, one of the following
mathematical relationships for white water filtered at 0.45 microns is used:
Paper Machine 5 White water
TDS = 851.97 + 2.03 7* Conductivity* UV2.0 Multi lp e R= 0.887
Paper Machine 5 White water
TDS = 2303.59*UV2E0+ 0.918*Conductivity + -0.422* UV280* Conductivity
The relationship between TDS and UV absorbance and conductivity is relatively
constant
over extended periods in the paper mill. Using the data from paper machine
3white water
(3ww), paper machine 4 white water (4ww), and paper machine 5 white water
(5ww) for
the period between April 9-28, 1996 the following relations are obtained:
For 3ww, 4ww, 5ww coznbined
TDSpred= 788.79+0.19899*UVa,O*Conductivity R=0.9028
TDSP,,=177.77+0.5398*Conductivity+266.09*UVZ,, R=0.9033
For 3ww
TDSaed= 810+0.1855*UVz,,*Conductivity R=0.865
TDSpma 259+0.5301*Conductivity+191.55*UVZ90 R=0.865
For 4ww
TDSpma 774+0.203 3 5*UV,90* Conductivity R=0.930
TDS~a 109.25+0.5161 *Conductivity+259.62*UV_$a R=0.9563
For 5ww
TDSpma 788.8+0.1814*UV,80*Conductivity R=0.857
TDSp.d 547+0.59508*Conductivity+94.66*UVZ90 R=0.844
14
SUBSTITUTE SHEET (RULE 26)
CA 02304199 2000-03-15
WO 99/14591 PCT/CA98/00874
This is presented in Figure 2 showing a scatterplot of the amount of total
dissolved solids
versus the product of UV absorbance and conductivity. Figure 3 shows a
scatterplot
presenting normalized data, i.e. divided by the average, from the dissolved
solids analyzer
showing relative conductivity, UV measurements, the product of UV
*conductivity, and
the ratio UV/conductivity. The product of UV*conductivity (UVCONDNO) shows the
greatest relative variation and thereby provides a more comprehensive measure
of
accumulation of dissolved matter as compared to the individual UV and
conductivity
measurements. The ratio of the UV and conductivity measurements may deviate
from its
normal value when the relative contribution of the inorganic and organic
components is
shifted. Figure 3 shows the gradual accumulation of dissolved components over
a period
of time.
Figures 4-6 show three dimensional plots demonstrating the variation of TDS as
a
function of UV and conductivity. The variation in UV absorbance is greatest in
the
Thermomechanical pulp (TMP) line as shown in Figure 5 and the variation in
conductivity is greatest in the de-inked pulp line as shown in Figure 4. The
dominate
contributions to the TDS are wood extractives and hemicellulose components
from TMP
and dissolved inorganic fillers and process chemicals from the de-inked pulp.
Although
these graphs look substantially different for the different testing zone the
principle
variation is in the amount of variation of the UV or conductivity measurement.
The
multiple regression of TDS against conductivity, UV and the interaction
between the two
(conductivity* UV) is similar but with some variation of the weighting of the
conductivity and UV as a function of sampling zone.
Regression models for UV, conductivity, TDS data shown in Figures 4-6. At each
sensor
location the relationship between the TDS and the measured conductivity and UV
absorbance of the filtered liquid samples has to be established and
periodically tested.
Several possible multiple regression models are shown for the different
furnish over a
three month period at the Avenor-Thunder Bay integrated pulp and paper mill. A
comparison of the different models at the various locations provides some
indication of
how robust each of the models will be to variation in the furnish composition.
Model A,
SUBSTITUTE SHEET (RULE 26)
CA 02304199 2000-03-15
WO 99/14591 PCT/CA98/00874
with an intercept and linear coefficients on cach variable is poor because of
the variability
in the intercept. Model B is reasonable and correct. Model C accounts for the
interaction
between the conductivity and UV variables. This interaction, quite high for
the deinked
pulp can be important from a control point of view in that it indicates that
when both
conductivity and UV increase there will be deposition of the dissolved
substances. An
important aim of a control strategy is to minimize the interaction of the
various dissolved
components. Model D is a simple one parameter model based on the product of
the UV
absorbance and conductivity. This model is probably the most robust model over
time
because it only involves one coefficient. In some instances, it is a benefit
in having a
simple model.
Head Box
A. TDS = 258 + 0.729*conductivity + 1718*UV280
B. TDS = 0.921 *conductivity + 1905* UV280
C. TDS = 1.010*conductivity + 2347*UV280 - 0.675*UV280*conductivity
D. TDS = 943 + 1.808 * UV280* conductivity
Thermomechanical Pulp
A. TDS = 426.8 + 0.792*conductivity + 1505*UV280
B. TDS = 0.947*conductivity + 1906*UV280
C. TDS = 1.194*conductivity + 2219*UV280 - 0.663*UV280*conductivity
D. TDS = 1318.8 + 1.322*UV280*conductivity
Deinked Pulp
A. TDS = 777 + 0.6576*conductivity + 362.8*UV280
B. TDS = 1. 03 6* conductivity + 11309* UV2B0
C. TDS = 1.238*conductivity + 3357* UV280 - 2.262*UV280*conductivity
D. TDS = 1370 + 1.05*UV280*conductivity
Fractions of inorganic and organic dissolved solids can be determined.
Inorganic
dissolved solids contribute mainly to the conductivity and organic dissolved
solids
contribute mainly to the UV absorbance. Using the coefficients of the TDS
equation the
portion of TDS that is derived from the conductivity (inorganic) or the UV
absorbance
16
SUBSTITUTE SHEET (RULE 26)
CA 02304199 2000-03-15
WO 99/14591 PCT/CA98/00874
(organic) may be derived. This provides a good relative measure of the
portions of
inorganic and organic components that contribute to the total amount of
dissolved solids.
The amount of organic dissolved solids is determined from their contribution
to the UV
term to the predicted dissolved solids terms. Thus for the above equation:
2303.59 * UV280
TDSu,~,,,,~ = 2303.59 * UV280 + 0.918 * Conductivity - 0.422 * UV280 *
Conductivity
The amount of inorganic dissolved solids is determined from their contribution
to the
conductivity term to the predicted dissolved solids term. Thus for the above
equation:
TDS = 0.918 * Conductivity
"'"'"~""` 2303.59 * UV280 + 0.918 * Conductivity - 0.422 * UV,80 *
Conductivity
Multiple R = 0.923
This is presented in Figure 15 showing the components of the TDS equation
broken down
into the UV contribution, signifying the organic portion of the TDS, and the
conductivity
contribution, signifying the inorganic portion of the TDS. Figure 15 further
shows that
there are periods when the TDS are relatively constant but the inorganic and
organic
portions are diverging.
The product of UV absorbance and conductivity often provides the best single
measure of
the amount of total dissolved solids. This is seen in Figure 7 presenting a
matrix plot
showing the relationship between TDS, UV absorbance, conductivity, and the
product of
UV absorbance and conductivity. The measurements were taken from paper machine
white water. The ordinate (y-axis) of each plot shows the relative intensity
of the
measurement shown in the bar chart to the right of the plot. The abscissa (x-
axis) is a
measure of the intensity of the measurement shown in the bar chart above the
scatter plot.
It is apparent that both conductivity (graph A) and UV absorbance (graph B)
provide only
a rough measure of the total amount of dissolved solids. Furthermore, the
correlation
between conductivity and UV absorbance (graph D) is poor. However. the
combined
17
SUBSTITUTE SHEET (RULE 26)
CA 02304199 2000-03-15
WO 99/14591 PCT/CA98/00874
measurement of UV absorbance and conductivity and using the product of both
measurements, UV*conductivity, shows a very good correlation to the TDS and
hence
provides the best indirect measurement of the total amount of dissolved solids
(graph C).
The product of UV absorbance and conductivity extenuates extremes in variation
of TDS
better than a sum weighted by multiple regression coefficients. The ratio of
UV
absorbance to conductivity provides a good measure of the relative change in
the
composition of dissolved organic and inorganic components. For example, in an
instance
where the ionic strength increases significantly, or when one or more (high
valent)
1.0 cations, such as Ca2+, Mg2+, A13+, and Fe2+, increases significantly, it
is expected that
dissolved and colloidal substances will be destabilized, i.e. they
precipitate. At pH 5.0
model dispersions of spruce pitch are destabilized at 0.1 M NaCI and 0.001 to
0.01 M
CaC12. The DLVO (Derjaguin, Landau, Verwey, and Overbeek) theoretical
description
of these effects is often used as a model that interprets charged particle
interaction in
terms of screening of charge-charge interactions by high ionic strength.
Counter-ion
condensation, or strong binding of a layer of usually high valent cations on
negatively
charged colloids and macromolecules leads to charge-neutralization that
destabilizes
dissolved and colloidal substance dispersions. In the event of such a
destabilization the
turbidity will first increase due to coagulation of small charged particles or
aggregation
and agglomeration of dissolved and colloidal substances with inorganic cations
or
polymeric cations. At a critical concentration the particle size will require
precipitation
and fixation of colloidal components.
Now turning to Figure 8, it is clear that the measures of UV absorbance and
conductivity
are not independent. The measurements are sometimes, but not always covariant
and the
components of the dissolved matter which are measured by UV absorbance and
conductivity interact with each other in solution or on surfaces. Figure 8
presents mill
data from the dissolved solids analyzer showing conductivity and UV
components. In
this case there is significant covariance. Under these circumstances one
measurement
cannot substitute for the other because of the deviation from covariance. The
deviation
from covariance appears on August 15 when furnish rich in UV absorbing
components
was introduced. The deviation on August 18 is due to the more rapid response
of the
18
SUBSTITUTE SHEET (RULE 26)
CA 02304199 2000-03-15
WO 99/14591 PCT/CA98/00874
water system to removing UV absorbing components compared to conductive
components. Both chemical and statistical arguments provide important insight
into why
the combined use of the two measurements is more effective in prediction of
properties of
a process water. From a chemical point of view, some of the UV absorbing
components
contribute to the conductivity. The positive covariance between conductivity
and UV
absorbance is a measure of the trend for one measurement to increase the
other.
Figure 8a shows a plot of the product (UV absorbance*conductivity) and the
ratio
(conductivity/UV) as a function of time. The results were obtained on-line
with the Total
Dissolved Solids Analyzer measuring paper machine white water. The plot
clearly shows
that the product UV*conductivity provides the best single measurement of
overall change
in the amount of dissolved solids as described above. Furthermore, the plot
also shows
that the ratio of conductivity/UV provides a measure of the relative mixture
of dissolved
inorganic components.
Known components that will contribute to both UV and conductivity include
resin acids,
such as dehydroabietic acid, phenolic components such as gallic acid and acid
lignin-
carbohydrates complexes containing glucuronoxylan or arabinoglucuronoxylan.
Other
acidic, UV-absorbing lignin or carbohydrate derived components may be fonmed
during
an oxidative chemical process such as peroxide bleaching. During alkaline
pulping and
bleaching the peeling reaction results in the formation of saccharinic acid
moieties on
carbohydrate components.
Furthermore, some of the UV absorbing components also interact, associate or
chelate the
cations from the solution thus decreasing the availability of free ions to
contribute to
conductivity. From a statistical point of view, in any multi-factor analysis
of variance,
factors A and B interact if the effect of factor A is not independent of the
level of factor
B. The model z' = A x x + B x y + C x x x y, where z' is the predicted
dependent variable
(dissolved solids) and x and y are dependent variables. The beta values for
coefficients
A, B and C provide a measure of the relative importance of the individual
terms x and y
and the interaction term xy. For example, when a sample TDS is regressed
against the
conductivity and UV absorbance the following results are obtained:
19
SUBSTITUTE SHEET (RULE 26)
CA 02304199 2000-03-15
WO 99/14591 PCT/CA98/00874
Stepwise Forward Multiple Regression: Summary for Dependent Variable: TDS
R= .99897481 R2= .99795066 Adjusted R2= .99767121
F(3,22)=3571.1 p<.00000 Std.Error of estimate: 74.838
BETA St. Err.of B St. Err. of t(22) p-level
BETA B
UV absorbance .785572 .091505 2921.407 340.2923 8.58499 .000000
(280 nm)
Conductivity .746419 .131612 1.214 .2141 5.67134 .000011
S cm''
Interaction Term -.534150 .116628 -2.063 .4504 -4.57994 .000146
UV*conductivity
In these results, the absolute value of the BETA term provides a measure of
the relative
importance of each term. Note that in this water sample the UV and the
conductivity
contribute comparably to the TDS. The interaction term shows that the
interaction
between the two is nearly as important, but of an opposite sense as either one
of the
single terms. The negative interaction term is also consistent with a chemical
interaction
of inorganic and organic components that occurs at high concentrations and
leads to
chelation, agglomeration and precipitation.
The interaction between different components in the white water may be
measured using
the product of UV absorbance and conductivity. When multiple regression is
used to fit
the TDS to a UV absorbance and conductivity measurement the Beta value
obtained for
the UV*conductivity product provides an expression for the interaction of the
different
components. The more negative the Beta value is, the more likely it is that
mixing
variation of one of the components will result in scaling, deposits or
precipitation.
The UV absorbance provides a direct measure of lignin and a representative
measure of
extractives and carbohydrate components dissolved in process waters. UV
absorbance is
a well-known measure of the amount of lignin present. However, lignin and most
lignan
extractive structures have a shoulder at 280 nm that is relatively invariant
with ionization
of phenolic hydroxyl constituents. The extinction coefficient at 280 nm for
lignin from
TMP has been determined to be 17.8 L g'I cm". Thus the UV absorbance
correlates well
with the TDS at different places in the paper machine even while the overall
composition
SUBSTITUTE SHEET (RULE 26)
CA 02304199 2000-03-15
WO 99/14591 PCT/CA98/00874
varies. UV absorbing dehydroabetic acid is a dominate resin acid extractive
liberated
from spruce wood during mechanical pulping. The relative portions of different
wood
extractives liberated from pulp do not vary substantially with variations in
the total
organic carbon (TOC) caused by recirculation of the process water.
Substantial_swings in
pH do change the relative portions of different extractives and for this
reason the
calibration between UV and TOC must be location specific in a paper mill. UV
lignin
measurements correlate well with the biological oxygen demand (BOD) and the
chemical
oxygen demand (COD) from thermomechanical pulp wood material. It is now
generally
accepted that unpurified hemicellulose components are directly attached to
lignin
moieties. Hence the UV absorbance of the components attached to lignin
provides a
measure of the hemicellulose components. Thus the UV absorbance correlates
well with
the TDS at different places in the paper machine even while the overall
composition
varies.
Substances contributing to either UV absorbance and conductivity are known to
be
detrimental to papermaking. The overall build-up of dissolved solids can
interfere with
paper machine operations. Salts and electrolytes screen electrostatic
interactions and
reduce the effectiveness of cationic polymers. Also, anionic organic
substances are
known to lead to deposits, and reduce the paper machine runability.
The combination of anionic trash (hemicellulose, resin acids, fatty acids) as
determined
by UV absorbance and electrolytes as measured by conductivity are required to
optimize
the efficiency of cationic polymers added for fixation and retention. Turning
to Figure 9,
a graph is presented showing that the ratio of the UV absorbance to the
conductivity
measurements correlates well with the turbidity measurement. Figure 10
presents a
scatterplot of turbidity and the ratio of the UV absorbance to the
conductivity
measurements over a period of approximately 2 months. This scatterplot shows
clearly
that the ratio of the UV absorbance to the conductivity trends well with the
turbidity
measurements. The relationship between the ratio of dissolved substances and
the
turbidity caused by colloidal particles is an indirect manifestation of the
shift in the
dissolved-colloidal equilibria caused by an increase in the amount of
dissolved organic
material contributing to colloids and a decrease in the amount of electrolytes
in the water
21
SUBSTITUTE SHEET (RULE 26)
CA 02304199 2000-03-15
WO 99/14591 PCT/CA98/00874
that may destabilize the colloidal substances. Turbidity has been used in the
past as a
means for controlling the addition of cationic fixing aids and flocculants.
TOC (total
organic carbon) has been used as a means to control the addition of cationic
polymer and
as indicated above the UV absorbance provides a representative measure of the
TOC.
Incremental changes in the UV absorbance of dissolved solids which coincide
with the
variation of a cationic polymer dose provide a good measure of the
interference of
dissolved anionic substances to the flocculation or fixing action of an added
cationic
polymer. This is showr- in Figures 11 and 12 which show results from
laboratory studies
and mill trials relating the variation of the UV absorbing dissolved
substances to the
addition of cationic polymer. Figure l 1 shows a plot of UV absorbance of
centrifuged
and filtered TMP white water in dependence upon the amount of cationic
polymer. This
graph shows clearly the effect of added cationic polymer on the measured
amount of
colloids and dissolved substances. Laboratory results, as presented in Figure
11, show
that dissolved matter is removed upon the addition of cationic polymer which
is used as a
retention aid or fixing agent. The removal of dissolved matter is indicated by
a decrease
in the measured values of UV absorbance. The results are compared to the
variation of
colloidal substances with the addition of cationic polymer. The colloidal
components are
not removed until a portion of the UV-absorbing dissolved matter reacts with
the
polymer. Figure 12 presents a plot of the variation of UV absorbance as a
function of
added cationic polymer. The results shown in Figure 12 represent mill trial
results upon
polymer addition at a medium consistency pump. The mill trial clearly shows
the
removal of dissolved material as measured by UV absorbance upon the addition
of
cationic polymer used as a retention aid or fixing agent. The results are
compared to the
variation of colloidal substances with the addition of cationic polymer. The
dissolved
matter reacts with the cationic polymer in the TMP white water but not in the
case of the
gray water for recycled newsprint that has low concentrations of dissolved,
wood-derived
organic material. The results of the trial presented in Figure 12 points out
the advantages
of using measurements of both dissolved and colloidal substances to control
the addition
of cationic polymer as a fixing agent.
22
SUBSTITUTE SHEET (RULE 26)
CA 02304199 2007-10-25
The UV absorbance of white water components shows a good correlation with the
dissolved
solids and hence UV measurements indicate the effects of changes in the pH of
a liquid sample.
The amount of dissolved solids is affected by altering the pH of the liquid
sample, i.e. if the pH is
lowered organic dissolved solids are precipitated and if the pH is increased
the inorganic
dissolved solids are precipitated. In application such as washing and pressing
the UV absorbance
provides and excellent measure of the effectiveness of the removal of
potentially soluble
substances. This is shown in Figure 12a presenting a plot of UV28o absorbance
vs. pH for results
obtained from a twin wire press.
The dissolved solids analyzer in agreement with the invention provides a
measure of the overall
change of dissolved solids in pulp or paper mill process waters or effluents.
The analysis of
different process steams provides a means to control both the overall level of
dissolved solids
using the product of the UV absorbance measurement and the conductivity
measurement and the
relative composition of the dissolved solids. The dissolved solids analyzer is
used in various areas
in pulp and paper processing, such as controlling dissolved solids in counter-
current flow
processes, controlling dissolved solids in pulp washing operations, reducing
deposition and
scaling, controlling dissolved solids in papermaking operations.
Referring now to Figure 13 a detailed diagram is presented showing potential
points for
application of the Dissolved Solids Analyzer (7, 7', 8', 10) in an integrated
pulp and paper mill. In
this figure, pulp flows are shown in dash lines, water flows are shown in
solid thin lines, and
sampling and analysis flows for the Dissolved Solids Analyzer are shown in
thick solid lines.
Dissolved solids are generated in pulp mills from pulping, bleaching, addition
of process
chemicals and washing. The dissolved solids in paper machine white water are
controlled in order
to maintain a constant level of dissolved solids. Dissolved solids enrichment
in pulp mills occurs
during processes 11-20 in the Thermomechanical Pulp (TMP) mill and 28-34 in
the deinking
mill. All purging is done in the pulp mills. Water and pulp storage areas
including 3, 21, 6 and 35
are expected to be substantially neutral to dissolved solids composition. Pulp
is ultimately
delivered to headbox 24 and paper machine 25 processes for paper
manufacturing. Valve 43 from
chip washers 11, impregnators (12) and chip heaters (not shown) are always
open. Valve 47 from
the Twin Wire Press (TWP), valve (46) from flotation (30), and valve 45 from
clarifier 37 are
23
CA 02304199 2007-10-25
always open. Valves 42 and 44 are open proportionally to respective pulp
production rates and
are supervisory controlled by Dissolved Solids Analyzers 7 and 7'. All fresh
water is introduced
in the paper mill. The valve 39 between fresh water 1 to Paper Mill (PM)
showers 27 is always
open. Valve 38 (fresh water to PM white water 6) is used to provide feedback
control to maintain
a set-point for the dissolved solids. The flow rates through valves 42 and 40
are determined by
water levels in tanks 4 and 5. The Dissolved Solids Analyzers 8 and 8' are
used to measure a
variation of dissolved solids across discrete chemical treatment processes 20
(hydrosulfite
bleaching) and 22 (cationic polymer addition), or 34 (hydrosulfite bleach) and
36 (cationic
polymer addition).
Figure 14 shows an example for an application of the Dissolved Solids
Analyzer. A block
diagram is presented showing elements of measurement and the control of
dissolved solids in an
integrated newsprint mill. Measurements of dissolved solids in the paper mill
and pulp mill
provide information to maintain the concentration and the composition of
dissolved solids by
varying the amount of fresh water and the relative counter-current flow to
each pulp mill. In this
figure, the pulp slurry flow is shown in dashed lines and the white water flow
is shown in solid
lines. The TDS Analyzer 200 as shown in Figure 14 is used to determine the
amount of dissolved
solids in a pulp slurry flow from a Groundwood Pulp Mill 210, a
Thermomechanical Pulp Mill
220, and a Recycled Pulp Mill 230. Further, the TDS analyzer is used to
determine the amount of
dissolved solids in a white water flow coming from a paper machine 250 to a
White Water Silo
240. The Thermomechanical Pulp Mill is mostly a source of organic dissolved
solids and the
Recycled Pulp Mill is mostly a source of inorganic dissolved solids. An
analysis of different
process streams provides a means for controlling both, the overall level of
dissolved solids using
the product of the LN absorbance measurements and the conductivity as well as
the relative
composition of the dissolved solids.
24