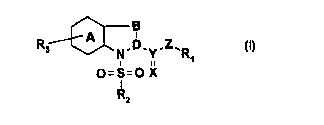Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02304451 2000-03-24
~WO 99/15501 PCT/EP98105300
-1-
SPECIFIC IMMUNOPHILIN LIGANDS AS ANTIASTHMATICS,
ANTIALLERGICS, ANTIRHEUMATICS, IMMUNOSUPPRESSANTS,
ANTIPSORIATICS AND NEUROPROTECTANTS
The invention relates to novel specific immunophilin ligands of the formula
B
R3 A N.D.Y-Z'R
O=S=O X
"2
The radicals R1, R2, R3, X, Y, Z, A, B and D have the following meaning:
R, - hydrogen, (C~-C~2)-alkyl or (C2-C6)-alkyloxy groups, where the alkyl
group is straight-chain or branched and can be substituted by a
mono- or bicyclic heteroaryl having 1-4 heteroatoms, preferably N, S
and O, such as morpholine, piperazine, piperidine, pyridine,
isoquinoline, quinoline, pyrimidine, oxazole, oxadiazole, isoxazole,
pyrazole, pyrrole, indole, indazole, phthalazines, thiophene, furan and
imidazole, or can be mono- or polysubstituted by a phenyl ring. This
phenyl ring itself can be mono- or polysubstituted by halogen,
(C~-C6)-alkyl, (C3-C~)-cycloalkyl, by carboxyl groups, carboxyl groups
esterified with straight-chain or branched (C~-C6)-alkanols, carbamoyl
groups, trifluoromethyl groups, hydroxyl groups, methoxy groups,
ethoxy groups, benzyloxy groups [lacuna] .. amino groups, which
themselves are in turn substituted by benzyl, benzoyl [lacuna] acetyl.
R~ can additionally be the amine radical of the following amino acid
methyl esters: histidine, leucine, valine, serine(Bzl), threonine,
pipecolic acid, 4-piperidinecarboxylic acid, 3-piperidinecarboxylic acid,
s-NHZ-lysine, s-Z-NH-lysine, s-(2CI-Z~NH-lysine, 2-pyridylalanine,
phenylalanine, tryptophan, glutamic acid, arginine(Tos), asparagine,
citrulline, homocitrulline, ornithine, thiazolecarboxylic acid, proline,
REPLACEMENT PAGE (RULE 26)
CA 02304451 2000-03-24
-2-
2-indolinecarboxylic acid, octahydroindolinecarboxylic acid,
tetrahydroisoquinolinecarboxylic acid, 5-aminovaleric acid,
8-aminooctanoic acid.
R2 = hydrogen, (C~-C~Z~alkyl or (C2-Cs~alkyloxy groups, where the alkyl
group is straight-chain or branched and can be substituted by a
mono- or bicyclic heteroaryl having 1-4 heteroatoms, preferably N, S
and O, such as morpholine, piperazine, piperidine, pyridine,
isoquinoline, quinoline, pyrimidine, oxazole, oxadiazole, isoxazole,
pyrazole, pyrrole, indole, indazole, phthalazines, thiophene, furan and
imidazole, or can be mono- or polysubstituted by a phenyl ring. This
phenyl ring itself can be mono- or polysubstituted by halogen,
(C~-Csralkyl, (C3-C~)-cycloalkyl, by carboxyl groups, carboxyl groups
esterified with straight-chain or branched (C~-Cs)-alkanols, carbamoyl
groups, trifluoromethyl groups, hydroxyl groups, methoxy groups,
ethoxy groups, benzyloxy groups [lacuna] amino groups, which
themselves are in turn substituted by benzyl, benzoyl or acetyl, or can
be substituted by mono- bi- or tricyclic aryl- or heteroaryl having 1-4
heteroatoms, preferably N, S and O, or by carboxy-(C~-C~2)-alkyl,
2U carboxycyclopentane, carboxycyclohexane or benzoyl which can be
mono- or polysubstituted by halogen, methoxy groups, amino groups,
carbamoyl groups, trifluoromethyl groups, carboxyl groups, or
carboxyl groups esterified with straight-chain or branched (C~-C6)-
alkanols.
R2 = amino-(C~-C~2~alkyl or amino-(C2-C6)-alkyloxy groups, where the
alkyl group is straight-chain or branched and can be substituted by a
mono- or bicyclic heteroaryl having 1-4 heteroatoms, preferably N, S
and O, such as morpholine, piperazine, piperidine, pyridine,
isoquinoline, quinoline, pyrimidine, oxazole, oxadiazole, isoxazole,
pyrazole, pyrrole, indole, indazole, phthalazines, thiophene, furan and
imidazole, or can be mono- or polysubstituted by a phenyl ring. This
phenyl ring itself can be mono- or polysubstituted by halogen,
(C~-C6)-alkyl, (C3-C~)-cycloalkyl, by carboxyl groups, carboxyl groups
esterified with straight-chain or branched (C~-C6)-alkanols, carbamoyl
groups, trifluoromethyl groups, hydroxyl groups, methoxy groups,
ethoxy groups, benzyloxy groups or amino groups, which themselves
REPLACEMENT PAGE (RULE 26)
CA 02304451 2000-03-24
..
-3-
are in tum substituted by benzyl, benzoyl or acetyl, or can be
substituted by mono- bi- or tricyclic aminoaryl- or aminoheteroaryl
having 1-4 heteroatoms, preferably N, S and O, or by carboxy-
(C~-C12)-alkyl, carboxycyclopentane, carboxycyclohexane or benzoyl
which can be mono- or polysubstituted by halogen, methoxy groups,
amino groups, carbamoyl groups, trifluoromethyl groups, carboxyl
groups, or carboxyl groups esterified with straight-chain or branched
(C,-C6)-alkanols.
R3 = H, F, OR4, Br, NHR4.
R4 = hydrogen, (C3-C~)-cycloalkyl, (C,-C6)-alkyl or carboxy-(C~-Cs~alkyl,
where the alkyl group can be straight-chain or branched and can be
substituted by a mono- bi- or tricyclic carbonylaryl or carbonylhetero-
aryl having 1-4 heteroatoms, preferably N, S and O, where aryl or
heteroaryl itself can be mono- or polysubstituted by halogen, (C~-C6)-
alkyl, (C3-C~)-cycloalkyl, by carboxyl groups, carboxyl groups
esterified with straight-chain or branched (C,-C6)-alkanols, carbamoyl
groups, trifluoromethyl groups, hydroxyl groups, methoxy groups,
ethoxy groups, benzyloxy groups or amino groups, which themselves
are in turn substituted by benzyl, benzoyl or acetyl.
A = without a ring, aromatic, non-aromatic, aromatic heterocyclic having
1-2 heteroatoms, preferably N, S, O, non-aromatic heterocyclic
having 1-2 heteroatoms, preferably N, S, O.
B=CHZ
D=CH
B-D = CH=C
X = O, S, H2
Y = S, C, single bond
Z = S, O, NR5
RS = hydrogen, (C~-C~2)-alkyl or (C2-Csralkyloxy groups, where the alkyl
group is straight-chain or branched and can be substituted by a
mono- or bicyclic heteroaryl having 1-4 heteroatoms, preferably N, S
and O, such as morpholine, piperazine, piperidine, indole, indazole,
REPLACEMENT PAGE (RULE 26)
CA 02304451 2000-03-24
-4-
phthalazine, thiophene, furan, imidazole, or can be mono- or
polysubstituted by a phenyl ring. This phenyl ring can itself be mono-
or polysubstituted by halogen, (C~-C6)-alkyl, (C3-C~)-cycloalkyl, by
carboxyl groups, carboxyl groups esterified with straight-chain or
branched (C~-Cs~alkanols, carbamoyl groups, trifluoromethyl groups,
hydroxyl groups, methoxy groups, ethoxy groups, benzyloxy groups
[lacuna] amino groups, which themselves are in turn substituted by
benzyl, benzoyl [lacuna] acetyl.
The invention furthermore relates to the physiologically tolerable salts of
the compounds according to formula 1, the processes for the preparation of
the compounds according to formula I, and their pharmaceutical use.
Cyclosporin A (CsA) and FK 506 are immunosuppressant natural
substances derived from fungi which inhibit the Ca+2-dependent [sicJ signal
transmission pathway in some cell types. In T cells, both agents inhibit the
transcription of a number of genes, including the gene for IL-2, which is
activated by stimulation of the T cell receptors (TCR). FK 506 and CsA
both bind with high affinity to -soluble receptor proteins (G. Fischer et al.,
Nature 337, 476-478, 1989; M.W. Harding et al., Nature 341, 755-760,
1989). The FK 506 receptor was called FKBP, the CsA receptor cyclophilin
(Cyp). Both proteins catalyse the isomerization of cis- and traps-amide
bond rotamers of peptides and are also frequently called immunophilins.
The supramolecule of CsA-Cyp or FK 506-FKBP binds calcineurin (CN)
and inhibits its phosphatase activity. A cellular target molecule of CN was
recognized as the cytosolic, phosphorylated component of the transcription
factor NF-AT which, with inadequate CN activity for the action in the cell
nucleus, cannot be dephosphorylated and thus the active transcription
complex on the IL-2 promoter cannot be switched on (M.K. Rosen,
S.L. Schreiber, Angew. Chem. 104 (1992); 413-430; G. Fischer, Angew.
Chem. 106 ( 1994), 1479-1501;
The allergic, asthmatic disorders are based on an inflammatory reaction
which is controlled by T cells and their mediators. Corticosteroids are still
the agent of choice in the treatment of many allergic disorders. CsA and
FK 506 also proved in animal experiments and in clinical studies to be a
favorable therapeutic in bronchial asthma and underlying inflammations. In
REPLACEMENT PAGE (RULE 26)
CA 02304451 2000-03-24
-5-
animal experiments, it was possible to show the blockade of various
cytokines such as IL-2, IL-4 and IL-5, which cause allergically induced
inflammations.
Despite the multiplicity of attempts at the identification of novel active
immunophilin inhibitors, it was not possible until now to prepare or isolate
any more efficacious structures than CsA, FK 506, rapamycin or
derivatives of these natural substances. The high inhibitory potential of
CsA, FK 506 or rapamycin, however, is very considerably reduced by the
manifold side effects, in particular of the kidneys, and neurotoxicity.
(N.H. Sigal et al., J. Exp. Med. 173, 619-628, 1991). What lies behind this
fact is the non-specificity of the interaction between immunophilin ligands
and the cell-specific binding proteins. As a result, the known medicinal
therapeutic action of these immunosuppressants is considerably restricted.
Furthermore, the inadequate selectivity of the compounds proves
problematical, particularly in long-term therapy.
A further compound having immunosuppressant properties was discovered
during the screening of combinatorial substance mixtures (G. Quinkert,
i-i. Bang and D. Reichert, Helv. Chim. Acta 1996, 79, 1260). The structure
published there is an indoline-2-carboxamide which at 10 Nmol exhibited
an inhibition of IL-2 proliferation of 77% and at 1 Nmol an inhibition of IL-2
proliferation of 12%. New measurements at a concentration of 10 Nmol
showed an IL-2-dependent inhibition of proliferation of 29%.
The compounds described in this invention markedly stand out at the C
terminus and in their optical purity of the indolinecarboxylic acid from the
structure mentioned in the publication and additionally show a markedly
better antiasthmatic, antiallergic, antirheumatic, anti-inflammatory, anti
psoriatic and immunosuppressant activity.
A substance class which likewise contains indolinecarboxylic acid as a
central unit and exhibits immunosuppressant properties as well as anti-
asthmatic properties was described in the patent DE 196 16 509.1. The
substances described there differ significantly at the N terminus from the
substances described in this invention.
REPLACEMENT PAGE (RULE 26)
CA 02304451 2000-03-24
5.
-6-
The invention is based on the object of finding novel compounds having
useful pharmacological properties and making them available by targeted
synthesis.
A class of substance which binds immunophilins surprisingly specifically,
inhibits IL-2-dependent proliferation, and also inhibits the release of TNF-a
and GM-CSF and surprisingly blocks a Ca++-dependent signal transmission
pathway is represented by the compounds of the formula I according to the
invention. This class of compounds and their pharmaceutically acceptable
salts has [sic] a high affinity for immunophilins such as CypA, CypB, CypC
and FKBP12. Moreover, substances of the formula I inhibit various
cytokine syntheses, as ~nrell as a Ca++-dependent signal transmission
pathway.
-(hose compounds of the formula I which contain asymmetric carbon atoms
and therefore as a rule occur as racemates can be separated into the
optically active isomers in a manner known per se, for example using an
optically active acid. However, the possibility also exists of. employing
optically active starting substances to begin with, corresponding optically
active or diastereoisomeric compounds then being obtained as the final
product.
The invention thus comprises compounds of the formula I which contain an
asymmetric carbon atom, the R form, the S form and R, S mixtures, and,
the case of a number of asymmetric carbon atoms, the diastereoisomeric
forms.
Depending on the process conditions and starting substances, the
compounds of the formula I can be obtained as free compounds or in the
form of their salts. The salts obtained can be converted into the free bases
or acids in a manner known per se, for example using acids, alkali or ion
exchangers.
The compounds of the formula I liberated in this way can be converted into
the corresponding physiologically tolerable acid addition salts using
inorganic or organic acids or bases.
Both the free bases and their salts are biologically active. The compounds
of the formula I can be administered in free form or as a salt with a physio-
REPLACEMENT PAGE (RULE 26)
CA 02304451 2000-03-24
-7-
logically tolerable acid or base. Administration can be carried out orally,
parenterally, intravenously, transdermally or by inhalation.
The invention further relates to pharmaceutical preparations which contain
at least one compound of the formula I or its salts with physiologically
tolerable inorganic or organic acids or bases and, if appropriate, pharma
ceutically utilizable excipients and auxiliaries.
Suitable administration forms are, for example, tablets or coated tablets,
capsules, solutions or ampoules, suppositories, patches or powder
preparations which can be employed in inhalers.
The dose of the abovementioned pharmaceutical preparations depends on
the condition of the patient and on the administration form. The daily dose
of active compound is between 0.01 and 100 mg per kg of body weight per
day.
Examples of compounds of the formula I which may be mentioned are:
Example 1 (2S)-1-[((2S)-1-(4-Acetylamino)phenylsulphonyl)indolin-2-yl~
2U carbonyl]-N-(2-methoxyethyl)indoline-2-carbamide
Example 2 (2R~1-[((2S)-1-(4-Acetylamino)phenylsulphonyl)indolin-2-yl)-
carbonyl)-N-(2-methoxyethyl)indoline-2-carbamide
Example 3 (2S)-1-[((2R)-1-(4-Acetylamino)phenylsulphonyl)indolin-2-yl)-
carbonyl]-N-(2-methoxyethyl)indoline-2-carbamide
Example 4 (2R)-1-[((2R)-1-(4-Acetylamino)phenylsulphonyl)indolin-2-yl~
carbonyl]-N-(2-methoxyethyl)indoline-2-carbamide
Example 5 (2R,S~1-[((2R,S~1-(4-Acetylaminophenylsulphonyl)indolin-2-
yl)carbonyl]-N-(2-methoxyethyl)indoline-2-carbamide
Example 6 (2S)-1-[((2S)-1-(4-Aminophenylsulphonyl)indolin-2-yl)-
carbonyl]-N-(2-methoxyethyl)indoline-2-carbamide
REPLACEMENT PAGE (RULE 26)
CA 02304451 2000-03-24
-8-
Example 7 (2S~1-[((2Sr1-(4-Aminophenylsulphonyl)prolyl)carbonyl]-N-
(2-methoxyethyl)indoline-2-carbamide
Example 8 (2S)-1-[((2S)-1-(4-Aminophenylsulphonyl)pipecolyl)carbonyl]-
N-(2-methoxyethyl)indoline-2-carbamide
Example 9 (2S)-1-[((2S)-1-(4-Methylphenylsulphonyl)prolyl)carbonyl]-N-
(2-methoxyethyl)indoline-2-carbamide
Example 10 (2S)-1-[(8-Quinolinylsulphonyl)]-N-(2-methoxyethyl)indoline-
carbamide
Example 11 1-[(2S)-1-(4-Acetylaminophenylsulphonyl)indolin-2-yl~
carbonyl]-N-leucine
Example 12 (S~Na {(2S)-1-[4-(Acetylamino)phenylsulphonyl]indolin-2-yl}-
carbonyl-NE (benzyloxycarbonyl)lysine methyl ester
Example 13 Methyl (E)-({(2S)-1-[4-(Acetylamino)phenylsulphonyl]indolin-
2-yl)carbonyl)-4-(aminophenyl)acrylate
Example 14 (2S)-1-[((2S~1-(1-Naphthalenylsulphonyl)indolin-2-yl)carbonyl]-
N-(2-methoxyethyl)indoline-2-carbamide
Example 15 (2S~1-[((2Sr1-(2-Naphthalenylsulphonyl)indolin-2-yl)carbonyl]-
N-(2-methoxyethyl)indoline-2-carbamide
Example 16 (2S~1-[((2S)-1-(4-Methylphenylsulphonyl)indolin-2-yl)carbonyl]-
N3-(N-propylimidazole)indoline-2-carbamide
Example 17 (2S~1-[((2S~1-(4-Methylphenylsulphonyl)indolin-2-yl)carbonyl]-
NZ-(N-ethylmorpholine)indoline-2-carbamide
Example 18 (2S}-1-[((2S)-1-(4-Methylphenylsulphonyl)indolin-2-yl)carbonyl]-
N2-(ethyl-2-pyridine)indoline-2-carbamide
REPLACEMENT PAGE (RULE 26)
CA 02304451 2000-03-24
_g_
Example 19 (2S}-1-[((2S~1-(4-Methylphenylsulphonyl)indolin-2-yl)carbonyl]-
(-4-pyridine)indoline-2-carbamide
Example 20 Methyl (2R)-[4-(acetylamino)phenylsulphonyl]indoline-2-
carboxylate
Example 21 (2R~[4-(Acetylamino)phenylsulphonyl]indoline-2-carboxylic
acid
Example 22 Methyl (2RS)-1-({(2RS)-1-[4-(acetylamino)phenylsulphonyl]-
indolin-2-yl}carbonyl)indoline-2-carboxylate
Example 23 (2RS~1-({(2RS~1-[4-(Acetylamino)phenylsulphonyl]indolin-2-yl}-
carbonyl)indoline-2-carboxylic acid
REPLACEMENT PAGE (RULE 26)
CA 02304451 2000-03-24
1 '
-10-
According to the present invention, the compounds of the formula I can be
prepared by the following processes.
1 St process:
B
A N.D.Y.ON + N'Z'R, A N.D.Y.Z.R
'~ R~ ~ ~~ ~ --
Boc X III BoC X
II IV
d~ ;O
A B + O S~Rx A 8
.D. .Z, -~ .D. .Z
R' H X Rt ~ R3 N Y ~fl~
or VIII O=S_-O X
V I
In the first process, the compounds of the formula I according to the
invention, in which R~, Rz, R3, A, B, D, X, Y and Z have the meaning
mentioned, are prepared by reacting a carboxylic acid derivative of the
formula II, in which R3, A, B, D, X, Y and Z have the meaning mentioned,
with an amine, alkanol, halogen compound or tosylate III to give an amide,
ester or ether IV, in which R~, R3, A, B, D, X, Y and Z have the meaning
mentioned, reacting this derivative IV, after deprotection with acid, to give
an intermediate V, in which R~, R3, A, B, D, X, Y and Z have the meaning
mentioned, and reacting in a continuing reaction with a compound VI, in
which R2 has the meaning mentioned, or with a compound VIII (see 2~d
process), in which R2, R3, A, B, D, X, Y and Z has [sic] the meaning
mentioned, to give the target compound I.
REPLACEMENT PAGE (RULE 26)
CA 02304451 2000-03-24
-11-
2"d process:
a. , o
A B + O ~S\~ 8 + N.Z.R, B
~O.y.ON~ ~D. .OH ~ A .D. _Z,
N Y ~ y
H X
VI O=S_O X or R' O;S X
V ~' O
YII VOI I
In the second process, the compounds of the formula I according to the
invention, in which R~, R2, R3, A, B, D, X, Y and Z have the meaning
mentioned, are prepared by reacting a carboxylic acid derivative of the
formula VII, in which R3, A, B, D, X, Y and Z have the meaning mentioned,
with a sulphonyl chloride VI, in which R2 have [sic] the meaning mentioned,
and reacting in a continuing reaction with a compound III, in which R~ has
the meaning mentioned, or with a compound V, in which R~, R3, A, B, D, X,
Y and Z has [sicJ the meaning mentioned, to give the target compound I.
for the preparation of the physiologically tolerable salts, the compounds of
'35 the formula I are reacted in a known manner with inorganic or organic
acids, such as, for example, hydrochloric acid, hydrobromic acid,
phosphoric acid, sulphuric acid, acetic acid, tartaric acid, citric acid,
fumaric
acid, malefic acid, lactic acid or embonic acid, or with inorganically [sic]
or
inorganic [sicJ bases.
Pharmaceutical preparations contain at least one compound of the general
formula I or its salts with physiologically tolerable inorganic or organic
acids
or bases and, if appropriate, pharmaceutically utilizable excipients and
auxiliaries.
The compounds of the formula I can be administered orally, parenterally,
intravenously, transdermally or by inhalation, in free form or as a salt with
a
physiologically tolerable acid or base.
Suitable administration forms are, for example, tablets or coated tablets,
capsules, solutions or ampoules, suppositories, patches or powder
preparations which can be employed in inhalers.
REPLACEMENT PAGE (RULE 26)
CA 02304451 2000-03-24
-12-
The dose of these abovementioned pharmaceutical preparations depends
on the condition of the patient and on the administration form. The daily
dose of active compound is between 0.01 and 100 mg per kg of body
weight.
The compounds of formula (I) according to the invention are distinguished
by immunophilin binding and inhibit its isomerase activity. This prolyl
isomerase activity is tested according to an enzyme test which is
customary worldwide: G. Fischer, H. Bang, A. Schellenberger, Biochim.
8iophys. Acta, 791, 87-97, 1984; D.H. Rich et al., J. Med. Chem. 38, 4164-
4170, 1995).
Without the peptidyl cis-trans isomerase activity of the immunophilins being
affected in each case, such compounds surprisingly specifically inhibit the
TNF-a, GM-CSF, IL-2, IL-4 and IL-5 proliferation of mast cells,
macrophages and activated T cells. The compounds according to the
invention can be employed, like cyclosporin A (Sandimrnun~, CsA), FK 506
or rapamycin (Tacrolimus) as immunosuppressants (R.Y. Galne et al., Br.
Med. J. 282, 934-936, 1981 ), for the treatment of autoimmune disorders
(R.H. Wiener et al., Hepatology 7, 1025, Abst. 9, 1987; L. Fry, J:
Autoimmun. 5, 231-240, 1992, G.J. Feutren, J. Autoimmun. 5, 183-195,
1992, EP 610,743), allergic inflammations (P. Zabel et al., Lancet 343,
1984), [lacuna] antiasthmatics (C. Bachert, Atemw.-Lungenkrkh. 20, 59,
1994), [lacuna] insulin-dependent diabetes mellitus (C.R. Stiller, Science,
223, 1362-1367, 1984), sepsis, as a neuroprotectant or for
neuroregeneration in multiple sclerosis, Alzheimer's and Parkinson's
disease (US 5 614 547, JP 08 333 334, Nature Medicine, 3, 4, 1997),
antirheumatics, psoriasis (SANDORMA, 4, 1995) and also in combination
with known immunophilin ligands such as CsA, FK 506 or rapamycin
(M.J. Wyvratt, N.H. Sigal, Perspectives in Drug Discovery and Design,
Immunosuppression, 2, 1, 1994; WO 92121313, US 5 330 993).
The invention is illustrated in greater detail below with the aid of working
examples. The abbreviations used here are:
AcOEt ethyl acetate
Boc tert-butyloxycarbonyl
(Boc)20 tert-butyloxycarbonyl [sic] anhydride
REPLACEMENT PAGE (RULE 26)
CA 02304451 2000-03-24
-13-
CN calcineurin
CsA cyclosporin A
Cyp cyclophilin
DMAP N,N-dimethylaminopyridine
EA elementary analysis
EE ethyl acetate [sic]
FKBP FK 506 binding protein
HPLC high-pressure liquid chromatography
in OPV in an oil pump vacuum
soln solution
MeOH methanol
PPlase peptidyl-proline cis-trans
isomerase
in RE in a rotary evaporator
in vac. in vacuo
RT room temperature
rac racemic
ent enantio
-tFA trifluoroacetic acid
Z benzyloxycarbonyl
REPLACEMENT PAGE (RULE 26)
CA 02304451 2000-03-24
-14-
General procedure stage fsicl for the preparation of carboxamides of the
general formula IV:
[lacuna] (3.3 mmol) of the Boc-protected carboxylic acid, 1 eq (3.3 mmol)
of the appropriate amine and 1.5 eq (4.9 mmol) of 2-chloro-1-methyl
pyridinium iodide and 2.5 eq (8.1 mmol, 1.13 ml) of TEA were dissolved or
suspended together in DCM, and the mixture was stirred for 30 min and
refluxed for 6 h. The solvent was distilled off on a rotary evaporator and the
residue was taken up in AcOEt. This suspension was washed twice each
with aqueous KHS04 soln, with aqueous NaOH soln and once with
aqueous, satd NaCI soln, dried over Na2S04 and purified by
chromatography on silica gel using AcOEt/hexane or using CH2C12/MeOH
mixtures.
General procedure for the preparation of sulphonamides of the general
formula VIII:
100 mmol of the amino acid was [sic] suspended in water and treated with
300 mmol of NaOH and with 110 mmol of sulphonyl chloride and the
mixture was heated at 90°C for 4 h. After cooling, it is [sic]
acidified with
aqueous 2N HCI, and the precipitated product was filtered off with suction
and dried at 40°C.
General procedure for the preparation of compounds of the general
formula I:
4.7 mmol of Boc-protected carboxamides [sic] of the general formula IV
were stirred at room temp. for 2 h in DCM/TFA 4:1. The solvt and excess
TFA were removed in vac. The oily residue was stirred at 35°C for
24 h in
120 ml of DCM with a (7 mmol) sulphonamide [sic] of the general formula
VI, with 11.7 mmol of TEA and 7.7 mmol of Mukaiyama reagent. The
solvent was distilled off on a rotary evaporator and the residue was taken
up in AcOEt. This suspension was washed twice each with aqueous
KHS04 soln, with aqueous NaOH soln and once with aqueous satd NaCI
soln, dried over Na2S04 and purified by chromatography on silica gel using
AcOEt/hexane or using CH2CI2/MeOH mixtures.
The following compounds of the general formula I were prepared according
to these general procedures:
REPLACEMENT PAGE (RULE 26)
CA 02304451 2000-03-24
-15-
Example 1 (2S)-1-[((2Sr1-(4-Acetylamino)phenylsulphonyl)indolin-2-yl)-
carbonyl)-N-(2-methoxyethyl)indoline-2-carbamide
M.p.: 224-227°C (dec.) (AcOEt/PE).
TLC: DCM/MeOH 95:5; Rf 0.35.
'H NMR (270 MHz, (D6) DMSO): 2.01 (s, 3H); 3.08 (s, 3H); 3.09-3.83
(m, 8H); 4.70-5.45 (m, 2H); 6.93-7.41 (m, 7H); 7.62-8.16 (m,
5H); 8.45-8.73 (m, 1 H); 10.44 (s, 1 H).
EA: cal. for C29H3oN406S C 61.91 H 5.37 N 9.96;
found: C 60.42 H 5.15 N 9.64.
Example 2 (2R)-1-[((2S~1-(4-Acetylamino)phenylsulphonyl)indolin-2-yl)-
carbonyl]-N-(2-methoxyethyl)indoline-2-carbamide
M.p.: 223-227°C (dec.) (AcOEt/PE).
TLC: DCM/MeOH 95:5; Rf 0.35.
'H NMR (270 MHz, (D6) DMSO): 2.0 (s, 3H); 3.09 (s, 3H); 3.10-3.70
(m, 8H); 4.77-5.51 (m, 2H); 6.89-7.39 (m, 7H); 7.60-8.21 (m,
5H); 8.31-8.59 (m, 1 H); 10.39 (s, 1 H).
MS (ESI+): cal. for C29H3oN406S, M = 562.65: found: M+ = 563.72.
example 3 (2S~1-[((2R)-1-(4-Acetylamino)phenylsulphonyf)indolin-2-yl)-
carbonyl)-N-(2-methoxyethyl)indoline-2-carbamide
M.p.: 224-227°C (dec.) (AcOEt/PE).
TLC: DCM/MeOH 95:5; Rf 0.35.
'H NMR (270 MHz, (D6) DMSO): 2.02 (s, 3H); 3.00 (s, 3H); 3.15-3.80
(m, 8H); 4.72-5.40 (m, 2H); 6.98-7.44 (m, 7H); 7.66-8.22 (m,
5H); 8.48-8.70 (m, 1 H); 10.52 (s, 1 H).
MS (ESI+): cal. for C29H3oN406S, M = 562.65; found: M' = 563.71.
Example 4 (2R)-1-[((2R)-1-(4-Acetylamino)phenylsulphonyl)indolin-2-yl)-
carbonyl]-N-(2-methoxyethyl)indoline-2-carbamide
M.p.: 221-225°C (dec.) (AcOEt/PE).
TLC: DCM/MeOH 95:5; Rf 0.35.
'H NMR (270 MHz, (D6) DMSO): 2.08 (s, 3H); 3.00 (s, 3H); 3.11-3.76
(m, 8H); 4.72-5.39 (m, 2H); 6.88-7.47 (m, 7H); 7.62-8.17 (m,
5H); 8.41-8.68 (m, 1 H); 10.43 (s, 1 H).
MS (ESI''): cal. for CZ9H3oN40sS M = 562.65 found: M+ = 563.7.
REPLACEMENT PAGE (RULE 26)
CA 02304451 2000-03-24
-16-
Example 5 (2R,S~1-[((2R,S~1-(4-Acetylaminophenylsulphonyl)indolin-2-
yl)carbonyl]-N-(2-methoxyethyl)indoline-2-carbamide
M.p.: 220-225C (dec.) (AcOEt/PE).
TLC: DCM/MeOH 95:5; Rf 0.35.
'H NMR (270 MHz, (D6) DMSO): 2.05 (s, 3H); 3.10 (s,
3H); 3.06-3.75
(m, 8H); 4.72-5.40 (m, 2H); 6.91-7.41 (m, 7H);
7.68-8.26 (m,
5H); 8.46-8.79 (m, 1 H); 10.41 (s, 1 H).
EA: cal. for C29H3oN406S x'/. H20
(567.15) C 61.41 H 5.42 N 9.87;
found: C 61.23 H 5.51 N 9.63.
Example 6 (2S~1-[((2S)-1-(4-Aminophenylsulphonyl)indolin-2-yl)-
carbonyl]-N-(2-methoxyethyl)indoline-2-carbamide
TLC: DCM/MeOH 95:5; Rf 0.16.
'H NMR (270 MHz, (D6) DMSO): 2.0 (s, 3H); 3.02-3.8 (m, 8H); 4.72-
5.35 (m, 2H); 6.15 (s, NH2); 6.91-7.41 (m, 7H); 7.68-8.26 (m,
5H); 8.3-8.7 (m, 1 H).
Example 7 (2S)-1-[((2S)-1-(4-Aminophenylsulphonyl)prolyl)carbonyl]-N-
(2-methoxyethyl)indoline-2-carbamide
TLC: DCM/MeOH 95:5; Rf 0.12.
'H NMR (270 MHz, (D6) DMSO): 1.7 (m, 2H); 1.9 (m, 2H); 2.05 (s,
3H); 3.0-3.74 (m, 6H); 4.5 (m, 1 H); 5.1 (m, 1 H); 6.05 (s, NH2);
7.0-7.65 (m, 7H); 8.2 (m, 1 H).
Example 8 (2S)-1-[((2S)-1-(4-Aminophenylsulphonyl)-4-piperidinyl)-
carbonyl]-N-(2-methoxyethyl)indoline-2-carbamide [sic]
TLC: DCM/MeOH 95:5; Rf 0.14.
'H NMR (270 MHz, (Ds) DMSO): 1.55 (m, 2H); 1.85 (m, 2H); 2.03 (s,
3H); 2.3-2.4 (m, 2H); 2.85-3.65 (m, 6H); 4.1 (m, 1 H); 5.15 (m,
1 H); 6.05 (s, NH2); 7.0-7.65 (m, 8H); 8.4 (m, 1 H).
Example 9 (2S)-1-[((2S)-1-(4-Methylphenylsulphonyl)prolyl)carbonyl]-N-
(2-methoxyethyl)indoline-2-carbamide
TLC: DCM/MeOH 95:5; Rf 0.42.
REPLACEMENT PAGE (RULE 26)
CA 02304451 2000-03-24
-17-
'H NMR (270 MHz, (D6) DMSO): 1.7 (m, 2H); 1.9 (m, 2H); 2.05 (s,
3H); 3.0-3.74 (m, 6H); 4.54 (m, 1 H); 5.1 (m, 1 H); 7.0-7.75 (m,
8H); 8.25 (m, 1 H).
Example 10 (2S~1-[(8-Quinolinylsulphonyl)]-N-(2-methoxyethyl)indoline-
carbamide
TLC: DCM/MeOH 95:5; Rf 0.46.
'H NMR (270 MHz, (D6) DMSO): 3.08 (s, 3H); 3.09-3.83 (m, 4H); 6.15
(m, 1 H); 6.65-8.65 (m, 10H); 9.15 (s, 1 H).
Example 11 1-[(2Sr1-(4-Acetylaminophenylsulphonyl)indolin-2-yl)-
carbonyl]-N-leucine
MS (ESI+): cal. for C23H2~N405S, M = 471.56; found: M+ = 471.9,
M++Na+ = 495.3
Example 12 (S)-Na {(2S)-1-[4-(Acetylamino)phenylsulphonyl]indolin-2-yl}-
carbonyl-NE (benzyloxycarbonyl)lysine methyl ester
M.p.: 189-192°C (AcOEt/PE).
TLC: DCM/MeOH 95:5; Rf 0.3.
'H NMR (270 MHz, DMSO): 1.2-1.45 (m, 4H); 1.6-1.76 (m, 2H); 2.08
(s, 3H); 2.78-3.21 (m, 5H); 3.63 (s, 3H); 4.25 (m, 1 H); 4.8-
4.92 (m, 1 H); 5.02 (s, 2H); 6.93-7.45 (m, 9H); 7.65-7.75 (m,
4H); 8.40 (m, 1 H); 10.33 (s, 1 H)
EA: cal. for C32H36N4O8S (636.83) C 60.36 H 5.70 N 8.8;
found: C 60.27 H 5.93 N 8.92.
Example 13 Methyl (E)-(f(2S~1-[4-(Acetylamino)phenylsulphonyl]indolin-
2-yl}carbonylr4-(aminophenyl)acrylate
M.p.: 167-171 °C (AcOEt)
TLC: DCM/MeOH 95:5; Rf 0.63.
'H NMR (270 MHz, DMSO): 2.11 (s, 3H); 3.05-3.11 (m, 1 H); 3.27-3.36
(m, 1 H); 3.71 (s, 3H); 4.95 (dd, J1 = 4.1, J2 = 13, 1 H); 6.68 (d,
J = 16.1, 1 H); 7.00-7.83 (m, 12H); 10.37 (s, 1 H); 10.48 (s,
1 H).
EA: cal. for C2~H25N306S x 1 /8 H20
(521.83): C 62.14 H 4.87 N 8.01;
found: C 62.28 H 5.10 N 7.72.
REPLACEMENT PAGE (RULE 26)
CA 02304451 2000-03-24
-18-
Example 14 (2S)-1-[((2S~1-(1-Naphthalenylsulphonyl)indolin-2-yl)carbonyl]-
N-(2-methoxyethyl)indoline-2-carbamide
TLC: DCM/MeOH 95:5; Rf 0.35.
'H NMR (270 MHz, (D6) DMSO): 2.05 (s, 3H); 3.10 (s,
3H); 3.0-3.7 (m,
8H); 4.8-5.2 (m, 2H); 6.93-7.41 (m, 7H); 7.7-8.4
(m, 9H).
Example 15 (2S~1-[((2S~1-(2-Naphthalenylsulphonyl)indolin-2-yl)carbonyl]-
N-(2-methoxyethyl)indoline-2-carbamide
M.p.: 224-227C (dec.) (AcOEt/PE).
TLC: DCM/MeOH 95:5; Rf 0.35.
'H NMR (270 MHz, (D6) DMSO): 2.0 (s, 3H); 3.06 (s,
3H); 3.1-3.8 (m,
8H); 4.75-5.5 (m, 2H); 6.93-7.41 (m, 7H); 7.7-8.4
(m, 9H).
Example 16 (2S~1-[((2S~1-(4-Methylphenylsulphonyl)indolin-2-yl)carbonyl]-
N3-(N-propylimidazole)indoline-2-carbamide
TLC: DCM/MeOH 95:5; Rf 0.26.
'H NMR (270 MHz, (Ds) DMSO): 1.95 (m, 2H); 2.3 (s, 3H); 3.~1 (m,
2H); 4.05 (m, 2H); 4.64 (m, 1 H); 5.1 (m, 1 H); fi.9-7.85 (m,
15H); 8.2 (m, 1 H).
Example 17 (2S)-1-[((2S~1-(4-Methylphenyisulphonyl)indolin-2-yl)carbonyl]-
N2-(N-ethylmorpholine)indoline-2-carbamide
TLC: DCM/MeOH 95:5; Rf 0.24.
'H NMR (270 MHz, (D6) DMSO): 1.5-1.7 (m, 4H); 1.9 (m, 4H); 2.25 (s,
3H); 2.75 (m, 2H); 3.65 (m, 2H); 4.72-5.35 (m, 2H); 6.91-7.71
(m, 12H); 8.4 (m, 1 H).
Example 18 (2S~1-[((2S~1-(4-Methylphenylsulphonyl)indolin-2-yl)carbonyl]-
N2-(ethyl-2-pyridine)indoline-2-carbamide
TLC: DCM/MeOH 95:5; Rf 0.19.
'H NMR (270 MHz, (D6) DMSO): 2.2 (s, 3H); 2.6 (m, 2H); 3.4 (m, 2H);
4.72-5.35 (m, 2H); 6.85-7.9 (m, 12H); 8.4 (m, 1 H).
Example 19 (2S~1-[((2S~1-(4-Methylphenylsulphonyl)indolin-2-yl)carbonyl)-
(4-pyridine)indoline-2-carbamide
TLC: DCM/MeOH 95:5; Rf 0.24.
REPLACEMENT PAGE (RULE 26)
CA 02304451 2000-03-24
-19-
'H NMR (270 MHz, (D6) DMSO): 2.4 (s, 3H); 3.25 (m, 2H); 4.72-5.35
(m, 2H); 6.85-8.1 (m, 16H); 8.4 (m, 1 H).
Example 20 Methyl (2Rr[4-(acetylamino)phenylsulphonyl]indoline-2-
carboxylate
M.p.: 172-176°C (AcOEt/PE).
TLC: DCM/MeOH 95:5; Rf 0.44.
'H NMR (270 MHz, (D6) DMSO): 2.22 (s, 3H); 3.0-3.22 (m, 2H); 3.83
(s, 3H); 4.63 (dd, J~ = 15.2, J2 = 5.3, 1 H); 6.88-7.31 (m, 4H);
7.52-7.70 (dd, J~ = 8.9, J2 = 8.9, 4H); 7.93 (s, 1 H).
EA: cal. for C~8H~8N205S x'/4 H20
(374.42): C 57.74 H 4.84 N 7.48;
found: C 56.82 H 4.99 N 7.15.
Example 21 (2R~[4-(Acetylamino)phenylsulphonyl]indoline-2-carboxylic
acid
M.p.: 198-202°C.
'TLC: DCM/MeOH 95:5; 1 % HoAc; Rf 0.20.
'~I NMR (270 MHz, CDCI3): 2.08 (s, 3H); 2.97-3.34 (m, 2H); 3.78 (s,
3H); 4.86-4.92 (dd, J = 15.5, 5.4, 1 H); 6.95-7.36 (m, 4H);
7.67-7.78 (dd, J = 9.0, 9.0, 4H); 10.33 (s, 1 H); 12.97 (s, 1 H).
Example 22 Methyl (2RSr1-({(2RS)-1-[4-(acetylamino)phenylsulphonyl]-
indolin-2-yl}carbonyl)indoline-2-carboxylate
M.p.: 213-215°C (AcOEt/PE).
TLC: DCM/MeOH 95:5; Rf 0.31.
'H NMR (270 MHz, DMSO): 2.07 (s, 3H); 3.02-3.46 (m, 4H); 3.76 (s,
3H); 5.18-5.69 (m, 2H); 6.95-7.40 (m, 7H); 7.71 (s, 4H); 10.33
(s, 1 H).
EA: cal. for C2~H25N306S x 1 H20
(537.59): C 60.32 H 5.09 N 7.82;
found: C 60.13 H 4.89 N 7.62.
Example 23 (2RS~1-({(2RS~1-[4-(Acetylamino)phenylsulphonyl]indolin-2-yl}-
carbonyl)indoline-2-carboxylic acid
M.p.: 190-192°C.
TLC: DCM/MeOH 95:5; Rf 0.23.
REPLACEMENT PAGE (RULE 26)
CA 02304451 2000-03-24
-20-
'H NMR (270 MHz, DMSO): 2.07 (s, 3H); 3.03-3.70 (m, 4H); 5.05-5.70
(m, 2H); 6.96-7.53 (m, 7H); 7.72 (4H); 7.95-8.09 (m, 1 H);
10.35 (s, 1 H).
EA: cal. for C26HZSNsOsS x 1/2 H20
(514.56): C 60.69 H 4.70 N 8.17;
found: C 60.64 H 4.81 N 8.03.
The listed examples 1-23 proved to be surprisingly strongly binding
immunophilin modulators which are suitable and able as the carrier-
immobilized formula [sic] to bind pathogenic immunophilins from fluids, in
particular body fluids.
To find strongly binding Cyp B or FKBP ligands of the formula I, the
immobilized ligands were subjected to an SDS-PAGE with cell
homogenate. Carrier-immobilized ligands which have a particular affinity
for the immunophilins bind these specifically with a high affinity.
The compounds of formula (I) according to the invention are distinguished
by immunophilin binding and inhibit its peptidyl-prolyl cis-trans isomerase
(PPlase) activity. For the initial screening (1 Nmol/l of substance), the
inhibition of human cyclophilin B is determined in the PPlase test. This
PPlase activity is tested for according to an enzyme test which is
customary worldwide: G. Fischer, H. Bang, C. Mech, Biomsd. Biochim.
Acfa, 43, 1101-1111; G. Fischer, H. Bang, A. Schellenberger, Biochim.
Biophys. Acfa, 791, 87-97, 7984; D.H. Rich et al., J. Med. Chem. 38, 4164
4170, 9995).
The compounds of the general formula I according to the invention are
preincubated at 4°C for 15 min together with 10 nmol of Cyp B. The
enzyme reaction is started using the test peptide Suc-Ala-Ala-Pro-Phe-Nan
after addition of chymotrypsin and HEPES buffer. The change in extinction
at 390 nm is then monitored and analysed. The photometrically determined
change in extinction results from two subreactions: a) the rapid chymo-
tryptic cleavage of the trans peptide; b) the non-enzymatic cis-trans
isomerization, which is catalysed by cyclophilins. The corresponding
PPlase activity of the compounds of the general formula according to the
invention are [sic] shown in Table 1:
REPLACEMENT PAGE (RULE 26)
CA 02304451 2000-03-24
-21 -
Table 1:
Compound [10 Nmol) Inhibition
[%)
Example 1: 40
(2S)-1-[((2S)-1-(4-Acetylamino)phenylsulphonyl)indolin-2-yl)-
carbonyl]-N-(2-methoxyethyl)indoline-2-carbamide
Example 2: 40
(2R)-1-[((2S)-1-(4-Acetylamino)phenylsulphonyl)indolin-2-yl)-
carbonyl]-N-(2-methoxyethyl)indoline-2-carbamide
Example 3 40
(2S)-1-[((2R~1-(4-Acetylamino)phenylsulphonyl)indolin-2-yl)-
carbonyl]-N-(2-methoxyethyl)indoline-2-carbamide
Example 4 60
(2R)-1-[((2R)-1-(4-Acetylamino)phenylsulphonyl)indolin-2-yl)-
carbonyl]-N-(2-methoxyethyl)indoline-2-carbamide
Example 5 20-4.0
(2R,S)-1-[((2R,S)-1-(4-Acetylaminophenylsulphonyl)indolin-
2-yl)carbonyl]-N-(2-methoxyethyl)indoline-2-carbamide
Example 6 40
(2S)-1-[((2S)-1-(4-Aminophenylsulphonyl)indolin-2-yl)carbonyl]-
N-(2-methoxyethyl)indoline-2-carbamide
Example 7 40
(2S)-1-[((2S)-1-(4-Aminophenylsulphonyl)prolyl)carbonyl]-
N-(2-methoxyethyl)indoline-2-carbamide
Example 8 40-60
(2S)-1-[((2S)-1-(4-Aminophenylsulphonyl)pipecolyl)carbonyl]-
N-(2-methoxyethyl)indoline-2-carbamide
Example 9 40-60
(ZS)-1-[((2S)-1-(4-Methylphenylsulphonyl)prolyl)carbonyl]-
N-(2-methoxyethyl)indoline-2-carbamide
Example 10 20-40
(2S)-1-[(8-Quinolinylsulphonyl)]-N-(2-methoxyethyl)indoline-
carbamide
Example 11 40
1-[(2S)-1-(4-Acetylaminophenylsulphonyl)indolin-2-yl)carbonyl]-
N-leucine
REPLACEMENT PAGE (RULE 26)
CA 02304451 2000-03-24
-22-
Example 12 60
(S)-Na {(2S)-1-[4-(Acetylamino)phenylsulphonyl]indolin-2-yl}-
carbonyl-NE (benzyloxycarbonyl)lysine methyl
ester
Example 13 0-20
Methyl (E)-({(2S)-1-[4-(Acetylamino)phenylsulphonyl]indolin-
2-yl}carbonyl)-4-(aminophenyl)acrylate
Example 14 20
(2S)-1-[((2S)-1-(1-Naphthalenylsulphonyl)indolin-2-yl)carbonyl]-
N-(2-methoxyethyl)indoline-2-carbamide
Example 15 20
(2S)-1-[((2S)-1-(2-Naphthalenylsulphonyl)indolin-2-yl)carbonyl]-
N-(2-methoxyethyl)indoline-2-carbamide
Example 16 20-40
(2S)-1-[((2S)-1-(4-Methylphenylsulphonyl)indolin-2-yl)carbonyl]-
N3-(N-propylimidazole)indoline-2-carbamide
Example 17 40
(2S)-1-[((2S)-1-(4-Methylphenylsulphonyl)indolin-2-yl)carbonyl]-
Nz-(N-ethylmorpholine)indoline-2-carbamide
Example 18 40
(2S)-1-[((2S)-1-(4-Methylphenylsulphonyl)indolin-2-yl)carbonyl]-,
N2-(ethyl-2-pyridine)indoline-2-carbamide
Example 19 40
(2S)-1-[((2S)-1-(4-Methylphenylsulphonyl)indolin-2-yl)carbonyl]-
(4-pyridine)indoline-2-carbamide
Example 20 0-20
Methyl (2R)-[4-(acetylamino)phenylsulphonyl]indoline-
2-carboxylate
Example 21 0-20
(2R)-[4-(Acetylamino)phenylsulphonyl]indoline-2-carboxylic
acid
Example 22 30
Methyl (2RS)-1-({(2RS)-1-[4-(acetylamino)phenylsulphonyl]-
indolin-2-yl}carbonyl)indoline-2-carboxylate
Example 23 0-20
(2RS)-1-({(2RS)-1-[4-(Acetylamino)phenylsulphonyl]indolin-
2-yl}carbonyl)indoline-2-carboxylic acid
REPLACEMENT PAGE (RULE 26)
CA 02304451 2000-03-24
-23-
The formation of the supramolecule from CsA-Cyp B-calcineurin (Ca2+-
dependent phosphatase) appears to be responsible for the known
immunosuppressant effects of CsA. For the investigation on the interaction
with this supramolecule from CsA-Cyp B or CsA-Cyp B-calcineurin with cell
homogenates of a human T-cell line, the compounds of the general
formula I according to the invention were incubated with 3H-CsA
(100 nmol). After gel filtration on Superose 12, the radioactivity of the
eluted fractions was measured and compared with the untreated control.
The corresponding displacement of 3H-CsA by the compounds of the
general formula I according to the invention from the supramolecule
Cyp B-CsA and Cyp-CsA-calcineurin is shown in Table 2:
Table 2:
DisplacementDisplacement
Compound [10 Nmol] from Cyp-CsAfrom Cyp-CsA-
in [%] CaN in [%]
Example 1: 30 -85
(2S)-1-[((2S)-1-(4-Acetylamino)phenylsulphonyl)-
indolin-2-yl)carbonyl]-N-(2-methoxyethyl)indoline-
2-carbamide
Example 2: -gp
(2R)-1-[((2S)-1-(4-Acetylamino)phenylsulphonyl)-
indolin-2-yl)carbonyl]-N-(2-methoxyethyl)indoline-
2-carbamide
Example 3
(2S)-1-[((2R)-1-(4-Acetylamino)phenylsulphonyl)-
indolin-2-yl)carbonyl]-N-(2-methoxyethyl)indoline-
2-carbamide
Example 4 -gg
(2R)-1-[((2R)-1-(4-Acetylamino)phenylsulphonyl)-
indolin-2-yl)carbonyl]-N-(2-methoxyethyl)indoline-
2-carbamide
Example 5 10 -73
(2R,S)-1-[((2R,S)-1-(4-Acetylaminophenyl-
sulphonyl)indolin-2-yl)carbonyl]-N-(2-methoxy-
ethyl)indoline-2-carbamide
REPLACEMENT PAGE (RULE 26)
CA 02304451 2000-03-24
-24-
Example 6 50 -59
(2S)-1-[((2S)-1-(4-Arninophenylsulphonyl)indolin-
2-yl)carbonyl]-N-(2-methoxyethyl)indoline-2-
carbamide
Example 7 45 -65
(2S)-1-[((2S)-1-(4-Aminophenylsulphonyl)prolyl)-
carbonyl]-N-(2-methoxyethyl)indoline-2-carbamide
Example 8 42 -81
(2S)-1-[((2S)-1-(4-Aminophenylsulphonyl)-
pipecolyl)carbonyl]-N-(2-methoxyethyl)indoline-
2-carbamide
Example 9 39 -64
(2S)-1-[((2S)-1-(4-Methylphenylsulphonyl)prolyl)-
carbonyl]-N-(2-methoxyethyl)indoline-2-carbamide
Example 10 15 -54
(2S)-1-[(8-Quinolinylsulphonyl)]-N-(2-methoxy-
ethyl)indolinecarbamide
Example 11 8 12
1-[(2S)-1-(4-Acetylaminophenylsulphonyl)indoliri-
2-yl)carbonyl]-N-leucine
Example 12 27 14
(S)-Na {(2S)-1-[4-(Acetylamino)phenylsulphonyl]-
indolin-2-yl}carbonyl-NE (benzyloxycarbonyl)lysine
methyl ester
Example 13 1 g
Methyl (E)-({(2S)-1-[4-(Acetylamino)phenyl-
sulphonyl]indolin-2-yl}carbonyl)-4-(aminophenyl)-
acrylate
Example 14 -51
(2S)-1-[((2S)-1-(1-Naphthalenylsulphonyl)indolin-
2-yl)carbonyl]-N-(2-methoxyethyl)indoline-2-
carbamide
Example 15 -48
(2S)-1-[((2S)-1-(2-Naphthalenylsulphonyl)indolin-
2-yl)carbonyl]-N-(2-methoxyethyl)indoline-2-
carbamide
REPLACEMENT PAGE (RULE 26)
CA 02304451 2000-03-24
-25-
Example 16 41 -46
(2S)-1-[((2S)-1-(4-Methylphenylsulphonyl)indolin-
2-yl)carbonyl]-N3-(N-propylimidazole)indoline-2-
carbamide
Example 17 39 -52
(2S)-1-[((2S)-1-(4-Methylphenylsulphonyl)indolin-
2-yl)carbonyl]-N2-(N-ethylmorpholine)indoline-2-
carbamide
Example 18 34 -53
(2S)-1-[((2S)-1-(4-Methylphenylsulphonyl)indolin-
2-yl)carbonyl]-N2-(ethyl-2-pyridine)indoline-2-
carbamide
Example 19 42 -49
(2S)-1-[((2S)-1-(4-Methylphenylsulphonyl)indolin-
2-yl)carbonyl]-(4-pyridine)indoline-2-carbamide
Example 20 ~ 4 18
Methyl (2R)-[4-(acetylamino)phenylsulphonyl]-
indoline-2-carboxylate
Example 21 3 5
(2R)-[4-(Acetylamino)phenylsulphonyl]indoline-
2-carboxylic acid
Example 22 2 8
Methyl (2RS)-1-({(2RS)-1-[4-(acetylamino)phenyl-
sulphonyl]indolin-2-yl)carbonyl)indoline-2-
carboxylate
~xample 23 4 ~ 12
(2RS)-1-({(2RS)-1-[4-(Acetylamino)phenyl-
sulphonyl]indolin-2-yl)carbonyl)indoline-2-
carboxylic acid
The II-2 [sic] proliferation test is based on the incorporation of 3H-
thymidine
in T cells stimulated with OKT-3 (human anti-CD-3-antibodies) and is
carried out in the following manner:
100,000 T cells are inoculated into microtitre plates in 150 NI of culture
medium per well, stimulated by addition of OKT-3 (1 Ng/ml) and incubated
for 45 h in each case with one of the compounds of the general formula I
according to the invention. After this incubation time, 10 NI of the 3H-
thymidine solution (0.5. NCi) are pipetted into each well. The mixture is
REPLACEMENT PAGE (RULE 26)
CA 02304451 2000-03-24
-26-
then incubated in a 5% C02 atmosphere at 37°C for 6 h. After harvesting
the cells, the radioactivity is quantified in a [i-counter. The corresponding
CD3-induced inhibition of proliferation by the compounds of the general
formula I according to the invention are [sic] shown in Table 3:
REPLACEMENT PAGE (RULE 26)
CA 02304451 2000-03-24
-27-
Table 3:
CD3-induced
Compound [10 Nmol] inhibition
of
proliferation
in [%]
Example 1: 83
(2S)-1-[((2S)-1-(4-Acetylamino)phenylsulphonyl)indolin-2-yl)-
carbonyl]-N-(2-methoxyethyl)indoline-2-carbamide
Example 2: 85
(2R)-1-[((2S)-1-(4-Acetylamino)phenylsulphonyl)indolin-2-yl)-
carbonyl]-N-(2-methoxyethyl)indoline-2-carbamide
Example 3 84
(2S)-1-[((2R)-1-(4-Acetylamino)phenylsulphonyl)indolin-2-yl)-
carbonyl]-N-(2-methoxyethyl)indoline-2-carbamide
Example 4 85
(2R)-1-[((2R)-1-(4-Acetylamino)phenylsulphonyl)indolin-2-yl)-
carbonyl]-N-(2-methoxyethyl)indoline-2-carbamide
Example 5 85
(2R,S)-1-[((2R,S)-1-(4-Acetylaminophenylsulphonyl)indolin-
2-yl)carbonyl]-N-(2-methoxyethyl)indoline-2-carbamide
Example 6 84
(2S)-1-[((2S)-1-(4-Aminophenylsulphonyl)indolin-2-yl)-
carbonyl]-N-(2-methoxyethyl)indoline-2-carbamide
Example 7 7g
(2S)-1-[((2S)-1-(4-Aminophenylsulphonyl)prolyl)carbonyl]-
N-(2-methoxyethyl)indoline-2-carbamide
Example 8 71
(2S)-1-[((2S)-1-(4-Aminophenylsulphonyl)pipecolyl)carbonyl]-
N-(2-methoxyethyl)indoline-2-carbamide
Example 9 75
(2S)-1-[((2S)-1-(4-Methylphenylsulphonyl)prolyl)carbonyl]-
N-(2-methoxyethyl)indoline-2-carbamide
Example 10 59
(2S)-1-[(8-Quinolinylsulphonyl)]-N-(2-methoxyethyl)indoline-
carbamide
REPLACEMENT PAGE (RULE 26)
CA 02304451 2000-03-24
-28-
Example 11 61
1-[(2S)-1-(4-Acetylaminophenylsulphonyl)indolin-2-yl)-
carbonyl]-N-leucine
Example 12 ~ 7g
(S)-Na {(2S)-1-[4-(Acetylamino)phenylsulphonyl]indolin-2-yl}-
carbonyl-NE (benzyloxycarbonyl)lysine methyl
ester
Example 13 46
Methyl (E)-({(2S)-1-[4-(Acetylamino)phenylsulphonyl]indolin-
2-yl}carbonyl)-4-(aminophenyl)acrylate
Example 14 4g
(2S)-1-[((2S)-1-(1-Naphthalenylsulphonyl)indolin-2-yl)-
carbonyl]-N-(2-methoxyethyl)indoline-2-carbamide
Example 15 56
(2S)-1-[((2S)-1-(2-Naphthalenylsulphonyl)indolin-2-yl)-
carbonyl]-N-(2-methoxyethyl)indoline-2-carbamide
Example 16 54
(2S)-1-[((2S)-1-(4-Methylphenylsulphonyl)indolin-2-yi)-
carbonyl]-N3-(N-propylimidazole)indoline-2-carbamide
Example 17 57
(2S)-1-[((2S)-1-(4-Methylphenylsulphonyl)indolin-2-yl)-
carbonyl]-N2-(N-ethylmorpholine)indoline-2-carbamide
Example 18 5g
(2S)-1-[((2S)-1-(4-Methylphenylsulphonyl)indolin-2-yl)-
carbonyl]-NZ-(ethyl-2-pyridine)indoline-2-carbamide
Example 19 64
(2S)-1-[((2S)-1-(4-Methylphenylsulphonyl)indolin-2-yl)-
carbonyl]-(4-pyridine)indoline-2-carbamide
Example 20 I 15
Methyl (2R)-[4-(acetylamino)phenylsulphonyl]indoline-
2-carboxylate
Example 21 12
(2R)-[4-(Acetylamino)phenylsulphonyl]indoline-2-carboxylic
acid
Example 22 43
Methyl (2RS)-1-({(2RS)-1-[4-(acetylamino)phenylsulphonyl]-
indolin-2-yl}carbonyl)indoline-2-carboxylate
REPLACEMENT PAGE (RULE 26)
CA 02304451 2000-03-24
-29-
Example 23 ~ 46
(2RS)-1-({(2RS)-1-[4-(Acetylamino)phenylsulphonyl]indolin-
2-yl}carbonyl)indoline-2-carboxylic acid
Like CsA, FK 506 or rapamycin, the compounds of the general formula I
according to the invention show the blockade of cytokines such as TNF-a,
GM-CSF, IL-2, IL-4 and IL-5, which cause the allergically induced
inflammations in the case of disease, in animal experiments.
For the determination of the inhibition of cell division by the compounds of
the general formula I according to the invention, 50,000 human tumor cells
were cultured for 48 h in the presence of the compounds of the general
formula I according to the invention, provided with 10 NI of yellow
tetrazolium salt solution (MTT) and incubated in a C02 atmosphere at
37°C
for a further 4 h. The resulting violet coloration was analysed photo-
metrically at 570 nm. After addition of 100 NI of SDS solution each, the
colouration was quantified photometrically after overnight incubation. it was
not possible to establish a general cytotoxicity of the compounds of the
general formula I according to the invention.
REPLACEMENT PAGE (RULE 26)