Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02304879 2000-03-23
New tetracycles, process for their production and
pharmaceutical preparations containing these compounds
The present invention concerns new tetracycles, a
process for their production and pharmaceutical
preparations containing these compounds.
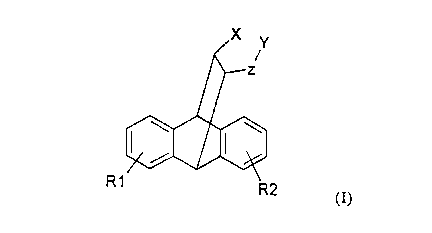
The invention concerns tetracyclo[6.6.2.02~~.09~14]hexadeca-
2(7),3,5,9(14),10,12-hexaene derivatives of the general
formula I
R1
R2
(I),
in which
R1 and R2 are the same or different and denote hydrogen
or a halogen atom,
X denotes hydrogen and
Y denotes a group -NR3R4 or a group -N+CH3R3R4 or
X and Y together form a group ~CH2-NR5 and
CA 02304879 2000-03-23
- 2 -
Z represents a~CH2 group or a~C=NH group in which
R3 denotes hydrogen, a lower alkyl group and
R4 denotes hydroxyl, carbamoyl, amidino, heteroaryl,
N-aralkylheteroaryl or a lower alkyl group or
R3 and R4 together with the nitrogen atom to which they
are bound, form a heterocyclic ring which can
be optionally broken by a further heteroatom
and which can be optionally substituted once
or several times,
R5 represents hydrogen, an amidino group or a
heterocyc1e which is optionally broken by
one or several heteroatoms,
provided that if R1 and R2 simultaneously denote hydrogen
(a) R4 does not denote a lower alkyl group or
(b) R5 does not denote hydrogen or
(c) R3 and R4 together do not form an unsubstituted
piperidine ring or morpholine ring
as well as physiologically tolerated salts, esters,
optically active forms and racemates thereof as well as
derivatives which can be metabolized in vivo to form
compounds of the general formula I as well as the use of
these compounds to produce pharmaceutical preparations.
The compounds of formula I have valuable pharmacological
properties and in particular they can inhibit the
activity of phospholipases.~~They are therefore suitable
for the treatment of acute and chronic, allergic, non-
CA 02304879 2000-03-23
- 3 -
allergic and traumatic inflammatory diseases such as for
example rheumatoid arthritis, osteoarthritis, ulcerative
colitis, acute pancreatitis, contact dermatitis,
inflammatory and allergic respiratory diseases, septic
shock, allergic shock, serum disease, auto immune
diseases, graft-versus-host reactions, host-versus-
graft-diseases, ischaemic or thrombotic diseases such as
coronary infarction or cerebral infarction.
Some of the compounds of formula I with pharmacological
activity have already been made known. The Dutch
application 6 412 205 CChem. Abstr. 63, 14787) describes
11-aminomethyl-9,10-dihydro-9,10-ethanoanthracenes with
antiemetic and anaesthetic action. Corresponding
substances are published in J. Med. Chem. 10, 86 (1967)
with anticholinergic, hypotensive, antihistaminic and
local anaesthetic action. However, an anti-inflammatory
action and in particular an inhibition of phospholipases
has not been previously described.
If not stated otherwise, lower alkyl in the residues R3,
R4 and as a substituent denotes alone or in combination
with aryl or amino, a straight-chained or branched alkyl
chain with 1 to 6 carbon atoms.
Preferred residues are a methyl, ethyl, propyl, iso-
propyl, n-butyl, isobutyl, tert.-butyl, n-pentyl or 3-
pentyl residue.
Aryl is understood as a phenyl or naphthyl residue which
can be optionally substituted by halogen or lower alkyl.
The phenyl residue is preferred.
Halogen is understood as fluorine, chlorine, bromine or
iodine, preferably chlorine.
CA 02304879 2000-03-23
- 4 -
The heteroatoms mentioned for the residues R3 and R4 and
R5 are understood as N, O, S and preferably N or O.
N-aralkylheteroaryl is understood as an aralkyl residue
bound via the N-atom to the heterocyc1e.
The heteroaryl groups specified for R4 are understood as
a pyridinyl, piperidinyl, pyridazinyl, pyrimidinyl,
pyrazinyl or piperazinyl residue. A pyridinyl,
piperidinyl or imidazolinyl residue is preferred, in
particular a 3-pyridinyl or 4-pyridinyl or 3-piperidinyl
or 4-piperidinyl or 4,5-dihydro-imidazol-2y1 residue.
The heterocyclic ring systems mentioned for R3 and R4
together with the N-atom to which they are bound are
understood as a pyrrolidine, pyrrole, pyrazole,
imidazole, pyridine, pyridazine, pyrimidine, pyrazine,
pyran, piperidine, piperazine or morpholine ring. A
pyrrolidine, morpholine or piperidine residue are
preferred.
Substitutents of the heterocyclic ring system which can
be formed by R3 and R4 together are, in addition to
common substitutents, preferably benzamido, benzylamino,
amino, monoalkylamino or dialkylamino. A single
substitution in the 4 position is preferred.
Heterocyc1e in the case of the residue R5 denotes
pyrimidine, pyridazine, pyrazole, pyrazine, imidazole,
indazole or purine. However, the imidazole residue is
particularly preferred.
Particularly preferred residues for R1 and R2 are
CA 02304879 2000-03-23
- 5 -
hydrogen and chlorine. A particularly preferred residue
for R3 is hydrogen or methyl, R4 particularly preferably
denotes carbamoyl, amidino, N-benzylaminopyridine,
piperidine, pyridine, methyl, hydroxy or imidazolyl. R3
and R4 together particularly preferably denote 4-
benzamidinopiperidine, 4-benzyl-aminopiperidine, 4-
aminopiperidine, 4-dimethylaminopiperidine, pyrrolidine,
piperidine or morpholine. R5 particularly preferably
denotes hydrogen, imidazole or amidino.
In addition to the compounds mentioned in the examples,
the invention concerns in particular all substances
which have any possible combination of the substituents
mentioned in the examples.
The compounds of formula I are produced by known methods
as described in the literature (e. g. in standard works
such as Houben-Weyl, "Methoden der Organischen Chemie,
Georg Thime Verlag", Stuttgart; Organic Reactions, John
Wiley & Sons, Inc., New York) and in the literature
references cited in the examples and namely under
reaction conditions that are known and suitable for the
said reactions. One can also use known variants that are
not mentioned here in detail. Furthermore a compound of
formula I can be converted by known methods into another
compound of formula I.
The process according to the invention for the
production of compounds of formula I is characterized in
that, in a known manner, a compound of the general
formula I in which
a) R1, R2, X, Y and Z have~~he stated meaning and R4 or
R5 represent hydrogen, is converted by reaction with
CA 02304879 2000-03-23
- 6 -
an activated carbonic acid derivative or with an
agent transferring the imidazoline group into a
compound of formula I in which R4 or R5 denote
carbamoyl, amidino or imidazolinyl, or
b) R1 and R2 have the stated meaning, X denotes
hydrogen, Y represents hydrogen or a nucleofuge group
and Z represents a carbonyl group, is converted by
reaction with a primary or secondary amine and
subsequent reduction into a compound of formula I in
which Z represents a CH2 group and Y represents NR3R4
or
c) R1 and R2 have the stated meaning, X denotes
hydrogen, Z represents a cyano group and Y is absent,
is reacted with hydroxylamine or a derivative thereof
to form a compound of formula I in which Z denotes a
;C=NH group and R4 denotes hydroxyl or
d) R1 and R2 have the stated meaning and X-Y-Z
represents a group CO-NH-CO, is converted by reduction
into a compound of formula I in which X-Y-Z denotes a
group CH2-NH-CH2
and subsequently, if desired, a carbonyl group is
reduced to a CH2 group, an arylmethyl group is cleaved
off or a tertiary nitrogen atom is quarternized by
alkylation
and optionally a base is converted into a
pharmacologically acceptable salt or the free compound
is produced from a salt.
.,
CA 02304879 2000-03-23
- 7 -
Compounds of formula I can occur as enantiomers and as
racemates. The invention concerns the pure enantiomers
as well as racemic mixtures.
Inorganic isocyanates and isourea derivatives which
carry a nucleofuge group come into consideration as
activated carbonic acid derivatives.
The agents transferring an imidazoline group are for
example 1H-imidazolines which carry a nucleofuge group
in the 2 position.
Nucleofuge groups are for example halogen atoms, the
azido group, alkoxy groups, aryloxy groups, alkylthio
groups and arylthio groups.
Complex metal hydrides such as sodium borohydride and
lithium aluminium hydride are preferably used as
reducing agents.
Compounds of the general formula I can contain one or
several chiral centres and can then be present in a
racemic or in an optically active form. The optical
isomers can be resolved into enantiomers by well-known
methods. Wherever appropriate the described methods
refer to the resolution of final stages and/or
precursors. Either diastereomeric salts are formed from
the racemic mixtures by reaction with an optically
active acid such as e.g. D- or L-tartaric acid, mandelic
acid, malic acid, lactic acid or camphorsulfonic acid or
an optically active amine such as e.g. D- or L-a-phenyl-
ehtylamine, ephedrine, qui~idine or cinchonidine which
4
can be separated by crystallization, or the optical
isomers are separated by HPLC. Another method of
CA 02304879 2000-03-23
_ g
separating optical isomers is an enzymatic separation
during the synthesis.
Suitable pharmacologically acceptable salts are in
particular salts with non-toxic inorganic or organic
acids such as hydrochloric acid, sulfuric acid,
phosphoric acid, hydrobromic acid, acetic acid, lactic
acid, citric acid, malic acid, benzoic acid, salicylic
acid, malonic acid, malefic acid, succinic acid or
diaminocaproic acid as well as optionally alkaline
salts, alkaline earth salts and ammonium salts.
The salts are obtained in the usual manner e.g. by
neutralizing the compounds of formula I with the
appropriate acids or lyes. They are usually purified by
reprecipitation from water/acetone.
In order to produce pharmaceutical preparations the
compounds of the general formula I are mixed in a known
manner with suitable pharmaceutical carrier substances,
aroma substances, flavourings and dyes and for example
formed into tablets or dragees or suspended or dissolved
in water or oil e.g. olive oil with the addition of
appropriate auxiliary substances.
The substances of the general formula I can be
administered orally or parenterally in a liquid or solid
form. Water is preferably used as an injection medium
which contains the usual stabilizers, solubilizers
and/or buffers for injection solutions. Such additives
are for example tartrate or borate buffer, ethanol,
dimethylsulfoxide, complexing agents (such as ethylene
diaminetetraacetic acid), high molecular polymers (such
as liquid polyethylene oxide) to regulate viscosity or
CA 02304879 2000-03-23
- 9 -
polyethylene derivatives of sorbitol anhydrides.
Solid carrier substances are for example starch,
lactose, mannitol, methylcellulose, talcum, highly
dispersed silicic acid, higher molecular polymers (such
as polyethylene glycols).
Suitable preparations for oral application can if
desired contain flavourings and sweeteners. For an
external application the substances I according to the
invention can also be used in the.form of powders and
ointments. For this they are for example mixed with
physiologically tolerated diluent powders or common
ointment bases.
The administered dose depends on the age, health and
weight of the recipient, the extent of the disease, the
type of additional treatments that may be carried out at
the same time, the frequency of the treatments and the
type of desired effect. The daily dose of the active
compound is usually 0.1 to 50 mg/kg body weight.
Normally 0.5 to 40 and preferably 1.0 to 20 mg/kg/day in
one or several applications are effective in order to
obtain the desired results.
In addition to the substances mentioned in the examples,
the following compounds are preferred within the sense
of the invention:
1-{3,10-Dichloro-tetracyclo[6.6.2.02~~.0~~14]hexadeca-
2(7),3,5,9(14),10,12-hexaen-15-yl-methyl}-4-amino-
piperidine hydrochloride
.,
CA 02304879 2000-03-23
- 10 -
1-benzyl-4-{3,10-Dichloro-tetracyclo[6.6.2.02~~.09~14J_
hexadeca-2(7),3,5,9(14),10,12-hexaen-15-yl-methyl-
amino}-piperidine hydrochloride
4-~3,10-Dichloro-tetracyclo[6.6.2.02-~.09~14Jhexadeca-
2(7),3,5,9(14),10,12-hexaen-15-yl-methyl-amino}-
piperidine hydrochloride
1' -Tetracyclo [ 6 . 6 . 2 . 02 - 7 . O9-14 ] hexadeca-2 ( 7 ) , 3 , 5 , 9 (
14 ) , -
10,12-hexaen-15-yl-methyl}-1,4'-bipiperidine
1'-~3,10-Dichloro-tetracyclo[6.6.2.02~~.0g~14]hexadeca-
2(7),3,5,9(14),10,12-hexaen-15-yl-methyl}-1,4'-
bipiperidine
The following examples illustrate the invention without,
however, limiting it thereto.
Example i
N-~Tetracyclo [ 6 . 6 . 2 . OZ- ~ . O9-14 ] hexadeca-
2(7),3,5,9(14),10,12-hexaen-15-yl-methyl}-urea
1.21 g (15 mmol) potassium cyanate is added to a solution
of 2.85 g (10 mmol) {tetracyclo[6.6.2.02-~.09-19]hexadeca-
2(7),3,5,9(14),10,12-hexaen-15 yl-methyl}-amine
hydrochloride (J. Org. Chem. 42, 1131 (1977)) in 200 ml
hot water and heated to reflux for 1 h. After cooling
2.4 g (86 ~ of theory) of the title compound with a
melting point of 182 - 184°C is isolated by filtration.
CA 02304879 2000-03-23
- 11 -
Example 2
N-{Tetracyclo[6.6.2.02~~.09~14]hexadeca-
2(7),3,5,9(14),10,12-hexaen-15-yl-methyl}-guanidine
A mixture of 3.8 g (16.4 mmol) {tetracyclo[6.6.2.02~~.09~14]-
hexadeca-2(7),3,5,9(14),10,12-hexaen-15-yl-methyl}-amine
and 3.1 g (18 mmol) S-methyl-isothiourea hydrobromide in
30 ml n-propanol is heated for 5 h to reflux under
nitrogen, cooled, admixed with diethyl ether, extracted
with water, the extract is alkalinized, extracted with
ethyl acetate and the organic phase is dried, concentrated
by evaporation and triturated with diethyl ether. 2.0 g
(44 % of theory) of the title compound with a melting point
of 128-130°C is isolated.
Example 3
1-{Tetracyclo[6.6.2.02-~.0914]hexadeca-2(7),3,5,9(14),10,12-
hexaen-15 yl-methyl}-4-benzamido-piperidine
A mixture of 4.8 g (20 mmol) {tetracyclo[6.6.2.02-~.09-14~-
hexadeca-2(7),3,5,9(14),10,12-hexaen-15-yl}-carbaldehyde
(Bull. Soc. Chim. France 1964, 550), 4.0 g (20 mmol) 4-
benzamido-piperidine, 100 ml toluene and 0.2 g p-toluene-
sulfonic acid is heated for 2 h under reflux on a water
separator. Subsequently it is concentrated, taken up in
100 ml methanol, 0.96 g sodium borohydr.ide is introduced
twice and each time it is heated for 1 h to reflux. It is
concentrated, the residue is taken up in ethyl acetate,
washed with water, the organic phase is dried and
chromatographed on silica c~el. 5.8 g (69 % of theory) of
the title compound with a melting point of 168 - 170°C is
eluted with isohexane/ethyl acetate 3:1.
CA 02304879 2000-03-23
- 12 -
Euample 4
1-{Tetracyclo[6.6.2.02~~.0914]hexadeca-2(7),3,5,9(14),10,12-
hexaen-15-yl-methyl}-4-benzylamino-piperidine hydrochloride
A solution of 5.4 g (12.8 mmol) of the compound of example
3 in 50 ml tetrahydrofuran is added dropwise to a
suspension of 1.5 g lithium aluminium hydride in 100 ml
tetrahydrofuran, it is subsequently heated for 3 h to
reflux, admixed with sodium chloride solution, filtered and
the filtrate is dried and concentrated. After adding excess
ethereal hydrogen chloride solution to the methanolic
solution, 5.1 g (98 % of theory) of the title compound is
isolated as a crude product.
Example 5
1-{Tetracyclo [ 6 . 6 . 2 . 02 ~ ~ . 09- la ] hexadeca-2 ( 7 ) , 3 , 5 , 9 (
14 ) , 10 , 12-
hexaen-15-yl-methyl}-4-amino-piperidine hydrochloride
5.0 g (12.3 mmol) of the compound of example 4 is
hydrogenated in 50 ml methanol over 1 g l0 % palladium
carbon at 50°C and 1 bar hydrogen pressure. It is filtered,
concentrated and chromatographed on silica gel. 2.9 g of
the target compound is eluted with ethyl acetate/methanol
1:1. After trituration with acetone, 2.3 g (60 % of theory)
of the title compound with a melting point of 225 - 230°C
remains.
CA 02304879 2000-03-23
- 13 -
EBample 6
1-Benzyl-4-{tetracyclo[6.6.2.02-x.09.14]hexadeca-
2(7),3,5,9(14),10,12-hexaen-15-yl-methylamino}-piperidine
hydrochloride
The title compound is obtained as a crude product in 80 %
yield in an analogous manner to example 3 from
{ tetracyclo [ 6 . 6 . 2 . 02 ~ ~ . 09.14 ] hexadeca-2 ( 7 ) , 3 , 5 , 9 ( 14
) , 10 , 12
hexaen-15-yl}-carbaldehyde and 4-amino-1-benzyl-piperidine.
Example 7
4-{Tetracyclo[6.6.2.02~~.09~1q]hexadeca-2(7),3,5,9(14),10,12-
hexaen-15-yl-methylamino}-piperidine hydrochloride
The title compound with a melting point of 226 - 228°C is
obtained in an 89 % yield in an analogous manner to that
described example 5.
Euample 8
1-{Tetracyclo[6.6.2.02-~.09-19]hexadeca-2(7),3,5,9(14),10,12-
hexaen-15-yl-methyl}-4-dimethylamino-piperidine
hydrochloride
The title compound is obtained in a 65 % yield as an
amorphous solid in an analogous manner to that described in
example 3 from {tetracyclo[6.6.2.02-7.09-14]hexadeca-
2(7),3,5,9(14),10,12-hexaen-15-yl}-carbaldehyde and 4-
dimethylamino-piperidine.
CA 02304879 2000-03-23
- 14 -
Example 9
4-{Tetracyclo[6.6.2.0z~~.O9-14]hexadeca-2(7),3,5,9(14),10,12-
hexaen-15-yl-methylamino]-pyridine
The title compound is obtained in an analogous manner to
that described in example 4 in a yield of 71 % of theory
with a melting point of 164 - 166°C by reduction of 4-
{tetracyclo[6.6.2.02~~.09~14]hexadeca-2(7),3,5,9(14),10,12-
hexaen-15-yl-carbonylamino}-pyridine and subsequent
trituration with diethyl ether.
The starting material used above can be obtained as
follows:
A mixture of 5.4 g (20 mmol)
{tetracyclo[6.6.2.02-7.09'14]hexadeca-2(7),3,5,9(14),10,12-
hexaen-15-yl}-carbonyl chloride) (J. Am. Chem. Soc. 94,
1193 (1972)) and 4.7 g (50 mmol) 4-aminopyridine in 50 ml
tetrahydrofuran is stirred for 1 h at room temperature and
2 h under reflux, it is subsequently filtered and the
filtrate is chromatographed on silica gel. After elution
with ethyl acetate, one obtains 2.5 g {tetracyclo-
[6.6.2.02~~.09~14]hexadeca-2(7),3,5,9(14),10,12-hexaen-15-yl-
carbonylamino}-pyridine (38 % of theory) with a melting
point of 217-219°C.
Example 10
4-{3,10-Dichloro-tetracyclo[6.6.2.02-7.09-14]hexadeca-
2(7),3,5,9(14),10,12-hexaen-15-yl-methylamino}-pyridine
The title compound with a melting point of 322 - 325°C is
CA 02304879 2000-03-23
- 15 -
obtained in a 61 % yield in an analogous manner to that
described in example 9 by reducing the carbonylamino
compound that can be obtained from {3,10-dichloro-
tetracyc lo- [ 6 . 6 . 2 . 02 ~ ~ . 09 ~ 1 ~ ] hexadeca-2 ( 7 ) , 3 , 5 , 9 (
14 ) , 10 , 12 -
hexaen-15-yl}-carbonyl chloride (Tetrahedron 28, 1435
(1972)) and 4-amino-pyridine.
Example 11
{3,10-Dichloro-tetracyclo[6.6.2.02~~.09~14]hexadeca-
2(7),3,5,9(14),10,12-hexaen-15-yl-methyl}-dimethylamine
The title compound with a melting point of 88 - 90°C is
obtained in a 64 % yield in an analogous manner to that
described in example 9 by reducing the carbonylamino
compound that can be obtained from {3,10-dichloro-
tetracyclo [ 6 . 6 . 2 . 02 ~ ~ . O9-1q ] -hexadeca-2 ( 7 ) , 3 , 5 , 9 ( 14 )
, 10 , 12-
hexaen-15-yl}-carbonyl chloride and dimethylamine.
Example 12
N-{3,10-Dichloro-tetracyclo[6.6.2.02-~.O9~lq]hexadeca-
2(7),3,5,9(14),10,12-hexaen-15-yl-methyl}-piperidine
The title compound is obtained as an amorphous solid in a
40 % yield in an analogous manner to that described in
example 9 by reducing a carbonylamino compound obtainable
from {3,10-dichloro-tetracyclo[6.6.2.02-x.09-19]hexadeca-
2(7),3,5,9(14),10,12-hexaen-15-yl}-carbonyl chloride and
piperidine.
CA 02304879 2000-03-23
- 16 -
Example 13
N-{6,13-Dichloro-tetracyclo[6.6.2.02-~.09~19]hexadeca-
2(7),3,5,9(14),10,12-hexaen-15-yl-methyl}-piperidine
The title compound is eluted as an amorphous by-product in
a 15 % yield during the chromatographic purification of the
previous compound.
Example 14
N-{6,13-Dichloro-tetracyclo[6.6.2.02-7.09-14]hexadeca-
2(7),3,5,9(14),10,12-hexaen-15-yl-methyl}-pyrrolidine
The title compound is obtained as an oil in a 28 % yield in
an analogous manner to that described in example 9 by
reducing the carbonylamino compound obtainable from {3,10,
dichloro-tetracyclo [ 6. 6. 2 . 02-~ . 09~ 19 ] hexadeca-
2(7),3,5,9(14),10,12-hexaen-15-yl}-carbonyl chloride and
pyrrolidine.
Example 15
N-~3,10-Dichloro-tetracyclo[6.6.2.02-7.09-i4]hexadeca-
2(7),3,5,9(14),10,12-hexaen-15-yl-methyl}-morpholine
The title compound with a melting point of 175 - 177°C is
obtained in a 41 % yield in an analogous manner to that
described in example 9 by reducing the carbonylamino
compound obtainable from {3,10-dichloro-
tetracyclo [ 6 . 6 . 2 . 02 ~ ~ . O9~ 14 ] ~exadeca-2 ( 7 ) , 3 , 5 , 9 ( 14 )
, 10 , 12-
hexaen-15-yl}-carbonyl chloride and morpholine.
CA 02304879 2000-03-23
- 17 -
Example 15
N-{3,10-Dichloro-tetracyclo[6.6.2.02~~.09-14]hexadeca-
2(7),3,5,9(14),10,12-hexaen-15-yl-methyl}-morpholine
The title compound with a melting point 175 -177°C is
obtained in a 41 % yield in an analogous manner to that
described in example 9 by reducing the carbonylamino
compound obtainable from X3,10-dichloro-
tetracyclo[6.6.2.02~~.09~14]hexadeca-2(7),3,5,9(14),10,12-
hexaen-15-yl}-carbonyl chloride and morpholine.
Example 16
1-{3,10-Dichloro-tetracyclo[6.6.2.0z-~.09~19]hexadeca-
2(7),3,5,9(14),10,12-hexaen-15-yl-methyl}-4-dimethyl-amino-
piperidine hydrochloride
The title compound with a melting point of 295°C (decomp.)
is obtained in a 70 % yield in an analogous manner to that
described in example 9 by reducing the carbonylamino
compound obtainable from X3,10-dichloro-tetracyclo-
6 . 6 . 2 . 02 ~ ~ . O9- 14 ] hexadeca-2 ( 7 ) , 3 , 5 , 9 ( 14 ) , 10 , 12 -
hexaen-15-
yl}-carbonyl chloride and 4-dimethylamino-piperidine and
subsequent precipitation of the hydrochloride.
Example 17
~ 3 ,10-Dichloro-tetracyclo [ 6 . 6 . 2 . 02- ~ . O9~ 1 ~ ] hexadeca-
2(7),3,5,9(14),10,12-hexaen-15-yl-methyl}-trimethylammonium
iodide
,
A mixture of 0.5 g (1.5 mmol) of the compound of example
CA 02304879 2000-03-23
- 18 -
11, 10 ml acetone and 0.47 ml {7.5 mmol) methyl iodide is
stirred for 8 h at room temperature and the precipitate is
subsequently removed by filtration. 0.5 g (70 % of theory)
of the title compound with a melting point of 172 - 175°c
remains.
Euample 18
N-{3,10-Dichloro-tetracyclo[6.6.2.02~~.09-14]hexadeca-
2(7),3,5,9(14),10,12-hexaen-15-yl-methyl}-N-methyl-
piperidinium iodide
The title compound with a melting point of 254 - 256°C is
obtained in a 43 % yield in an analogous manner to that
described in example 17 from the compound of example 12 and
methyl iodide.
Euample 19
N-Hydroxy-{3,10-dichloro-tetracyclo[6.6.2.02~~.09~14]-
hexadeca-2(7),3,5,9(14),10,12-hexaen-15-yl}-carboximidamide
A mixture of 8.25 g (25 mmol) {3,10-dichloro-
tetracyclo [ 6 . 6 . 2 . 02 - ~ . O9-19 ] hexadeca-2 ( 7 ) , 3 , 5 , 9 ( 14 )
, 10 , 12-
hexaen-15-yl}-carbonitrile (Tetrahedron 28, 1435 (1972)),
5.3 g sodium carbonate, 7.0 g hydroxylamine hydrochloride,
100 ml ethanol and 25 ml water is refluxed for 20 h. It is
filtered and the precipitate is chromatographed on silica
gel. 0.75 g (9 % of theory) of the title compound with a
melting point of 203 - 205°C is isolated with ethyl
acetate.
The isomeric N-hydroxy-{6,13-dichloro-tetracyclo-
CA 02304879 2000-03-23
- 19 -
[6.6.2.02-~.09-14]hexadeca-2(7),3,5,9(14),10,12-hexaen-15-
yl}-carboximidamide can be detected as a by-product.
Euample 20
2-{3,10-Dichloro-tetracyclo[6.6.2.02~~.09~14]hexadeca-
2(7),3,5,9(14),10,12-hexaen-15-yl-methylamino}-4,5-dihydro-
1H-imidazole
A mixture of 4.55 g (15 mmol) {3,10-dichloro-
tetracyclo[6.6.2.02-7.09-19]hexadeca-2(7),3,5,9(14),10,12-
hexaen-15-yl-methyl}-amine, which is prepared from the
nitrile used in example 19 by reduction with lithium
aluminium hydride, and 3.36 g (17 mmol) 2-methylsulfanyl-
4,5-dihydro-1H-imidazole hydrobromide is heated at 15 mbar
for 30 min to 160°C and for 10 min to 180°C. After cooling,
acetone is added, it is filtered and the precipitate is
recrystallized from ethanol. 5.0 g (73 % of theory) of the
title compound with a melting point of 194 - 195°C is
isolated.
Example 21
2-{Tetracyclo [ 6 . 6 . 2 . OZ ~ ~ . O9~ 14 ] hexadeca-2 ( 7 ) , 3 , 5, 9 ( 14
) , 10, 12-
hexaen-15-yl-methylamino}-4,5-dihydro-1H-imidazole
The title compound with a melting point of 160 - 162°C is
obtained (57 % of theory) in an analogous manner to that
described in example 20 from {tetracyclo[6.6.2.02~~.09-14]-
hexadeca-2(7),3,5,9(14),10,12-hexaen-15-yl-methyl}-amine.
CA 02304879 2000-03-23
- 20 -
Example 22
N-{3,10-Dichloro-tetracyclo[6.6.2.02-x.09-14]hexadeca
2(7),3,5,9(14),10,12-hexaen-15-yl-methyl}-guanidine
The title compound with a melting point of 211 - 213°C
is obtained in a 58 % yield in an analogous manner to
that described in example 2 from {3,10-dichloro-
tetracyclo [ 6 . 6 . 2 . 02 - ~ . 09 ~ i4 ] hexadeca-2 ( 7 ) , 3 , 5 , 9 ( 14
) , -
10,12-hexaen-15-yl-methyl}-amine.
Example 23
2-{6,10-Dichloro-tetracyclo[6.6.2.02~~.0~~14]hexadeca-
2(7),3,5,9(14),10,12-hexaen-15-yl-methylamino}-4,5-
dihydro-1H-imidazole hydrobromide
The title compound with a melting point of 155 - 158°C
(decomp.) is obtained in a yield of 28 % of theory in an
analogous manner to that described in example 20 from
{6,10-dichloro-tetracyclo[6.6.2.02~~.09-1~]hexadeca-
2(7),3,5,9(14),10,12-hexaen-15-yl-methyl}-amine that is
obtainable by reduction of the corresponding nitrile.
Example 24
N-{6,10-Dichloro-tetracyclo[6.6.2.02-~.0~~14]hexadeca-
2(7),3,5,9(14),10,12-hexaen-15-yl-methyl}-guanidine
hydrobromide
The title compound with a melting point of 93 - 95°C
(decomp.) is obtained in a yield of 24 % of theory in an
analogous manner to that described in example 2 from
CA 02304879 2000-03-23
- 21 -
{6,10-dichloro-tetracyclo[6.6.2.02-~.09~14]hexadeca-
2(7),3,5,9(14),10,12-hexaen-15-yl-methyl}-amine.
Example 25
N-{ 6-Chloro-tetracyclo [ 6 . 6 . 2 . 02- ~ . O9~ 14 ] hexadeca-
2(7),3,5,9(14),10,12-hexaen-15-yl-methyl}-guanidine
hydrobromide
The title compound with a melting point of 151 - 153°C
(decomp.) is obtained in a yield of 32 % of theory in an
analogous manner to that described in example 22 from
{6-chloro-tetracyclo[6.6.2.02~~.09-19]hexadeca-
2(7),3,5,9(14),-10,12-hexaene-15-yl-methyl}-amine.
EBample 26
5,10-Dichloro-3a,4,9,9a-tetrahydro-4,9-o-benzeno-
benz[f]isoindole
A solution of 23.0 g (67 mmol) 5, I0-dichloro-3a,4,9,9a-
tetrahydro-4,9-o-benzeno-Benz[f]isoindole-1,3-divne in
290 ml tetrahydrofuran is added dropwise to a suspension
of 5.1 g (134 mmol) lithium aluminium hydride in 45 ml
diethyl ether, it is subsequently refluxed for 8 h,
admixed with sodium chloride solution, the organic phase
is concentrated and the residue is purified by
conversion into hydrochloride and subsequent release of
the base. 11.0 g (52 % of theory) of the title compound
with a melting point of 126 - 130°C is isolated.
The 5,10-dichloro-3a,4,9,9a=tetrahydro-4,9-o-benzeno-
benz-[f]isoindole-1,3-dione used as a starting substance
CA 02304879 2000-03-23
- 22 -
can be obtained as follows:
83 g (0.24 mol) 5,10-dichloro-3a,4,9,9a-tetrahydro-4,9-
o-benzeno-benz[f]isobenzofuran-1,3-dione (Bull. Soc.
Chim. France 1973, 190) is introduced into a mixture of
370 ml chloroform and 370 ml liquefied ammonia, it is
stirred for a further 3 h, filtered, the precipitate is
taken up in a large amount of water, heated to boiling
point, filtered and the filtrate is acidified. 77 g (88
% of theory) 3,10-dichloro-tetracyclo[6.6.2.02-x.09~19]_
hexadeca-2(7),3,5,9(14),10,12-hexaen-15,16-dicarboxylic
acid monoamide with a melting point of 274 - 276°C
precipitates.
27.5 g of the previously described amide in 400 ml
xylene is refluxed for 30 min on a water separator. The
precipitate that forms on cooling is removed by
filtration and washed with diethyl ether. 23.5 g (90 %
of theory) 5,10-dichloro-3a,4,9,9a-tetrahydro-4,9-0-
benzeno-benz[f]isoindole-1,3-dione with a melting point
of 279 - 281°C is obtained.
Example 27
5,10-Dichloro-2-(4,5-dihydro-1H-imidazol-2-yl)-
3a,4,9,9a-tetrahydro-4,9-o-benzeno-Benz[f] isoindole
The title compound with a melting point of 325 - 326°C
is obtained in a 41 % yield in an analogous manner to
that described in example 20 from 5,10-dichloro-
3a,4,9,9a-tetrahydro-4,9-o-benzeno-benz[f]isoindole and
2-methylsulfanyl-4,5-dihydro-1H-imidazole hydrobromide.
,
CA 02304879 2000-03-23
- 23 -
Example 28
5,10-Dichloro-3a,4,9,9a-tetrahydro-4,9-o-benzeno-
benz[f]isoindol-2-yl-carboximidamide
The title compound in with a melting point of 236 -
238°C is obtained a 27 % yield in an analogous manner to
that described in example 2 from 5,10-dichloro-
3a,4,9,9a-tetrahydro-4,9-o-benzeno-benz[f]isoindole and
S-methyl-isothiourea hydrobromide.
Example 29
Enzyme Assay and Pharmakological Tests
As a representative compound of this application, the
compound of Example 28 was tested in the PLA2-enzyme
assay and in the animal experiment.
1. In the PLA,-enzyme assay, the compound of Example 28
showed an inhibition of the cytosolical PLAz-enzyme
activity, but no inhibition of the secretorial PLA~-
activity.
2. In the animal experiment (rats), the compound of
Example 28, administered i.p., showed an inhibition
of the acute inflammatory carrageenin edema (EDso -
16 mg/kg) as well as an inhibition of the generalized
adjuvans arthritis.